Abstract
The SHANK2 gene codes for a protein involved in organising the postsynaptic density and disruptions have been associated with autism spectrum disorders (ASDs). ASDs are frequently comorbid with intellectual disability and anxiety disorders and emerging evidence suggests potentially common aetiologies. Here, we report the case of an 18-year-old man with ASD who presented with severe anorexia due to fear of food contamination, food avoidance and stereotypies attributable to underlying obsessive compulsive disorder (OCD). The patient was found to be heterozygous for c.2518C>T (p.Pro840Ser), a likely damaging coding variant in the proline rich region of SHANK2. Interestingly, the patient’s disordered eating behaviour began to improve only after high-dose fluoxetine was initiated to target OCD symptoms. Overall, this case highlights the utility of molecular genetic testing in clinical psychiatry and provides an example of how genetic information can inform clinicians in the treatment of complex neuropsychiatric syndromes.
Keywords: genetic screening / counselling, psychiatry, eating disorders, drugs: psychiatry, anxiety disorders (including ocd and ptsd)
Background
Based on current diagnostic definitions, autism spectrum disorders (ASDs) affect at least 15 per 1000 children and are characterised by difficulties with social interaction and communication as well as restricted and stereotyped patterns of interests and activities.1 2 Phenotypically, approximately 32% of all ASD cases have some degree of intellectual disability and another 25% have a borderline low IQ.1 Additionally, ASDs are frequently comorbid with other psychiatric disorders. Obsessive compulsive disorder (OCD) and specific phobias are most common, with a lifetime prevalence of greater than 40% among individuals with ASDs.3 4 Interestingly, this high rate of comorbidity, in addition to emerging molecular evidence, has led some to suggest potentially common aetiologies between ASDs and anxiety disorders.5
Regarding aetiology and pathophysiology, a number of different genes, pathways and brain regions have been implicated, but a growing body of evidence suggests that dysfunctional synaptic proteins are frequently involved.6 7 In particular, causative variants in the SHANK family of genes, which code for a group of scaffolding proteins containing multiple domains for protein-protein interaction including ankyrin repeats and an SH3 domain, have been repeatedly identified.8–10
SHANK2, or the SH3 and Multiple Ankyrin Repeat Domains 2 gene, is one member of the SHANK family and codes for a protein that localises to the postsynaptic density of excitatory synapses.11 12 The postsynaptic density is a specialised structure of the postsynaptic membrane that is crucially important for neuronal signalling. The SHANK2 protein is involved in organisation of this structure and, specifically, plays a role in organising the neurotransmitter receptor apparatus and the adhesion of the postsynapse to presynaptic terminals.13 Together with other proteins, SHANK2 is part of a structural matrix that anchors and clusters postsynaptic neurotransmitter receptors, synaptic cell adhesion molecules and components of intracellular signalling pathways opposite to the neurotransmitter release site and is thus critical to the function of the postsynaptic signal transduction machinery.10 13
Several animal models have been developed to explore the role of SHANK2 and its relationship to neuropsychiatric phenotypes.14–17 In particular, one mouse model lacking only exon 7 of Shank2 demonstrated impairments in social interaction, increases in stereotypical behaviours and hyperactivity.14 A mouse lacking exons 6 and 7 was separately shown to display ASD-like behaviours such as reduced social interactions and impaired spatial memory.16
Regarding the clinical relationship between SHANK2 and ASD, in a study of 396 patients with ASD and 184 with mental retardation, a de novo SHANK2 copy number variant (CNV) was found in two participants and none of the 5023 matched controls. In the same study, six different SHANK2 missense variants and a six basepair duplication were found among participants with ASD or mental retardation, but not found in any of the 659 controls.8 In another study of 851 individuals with ASD and 1090 controls, SHANK2 variants affecting highly conserved amino acids were identified in 3.4% of cases but only 1.5% of controls. Further, when transfected into neuronal culture, only the variants identified in cases resulted in decreased synaptic density.9 Overall, the authors of the above studies conclude that disruptions of the SHANK2 gene may lead to synaptic dysfunction and significantly increase the risk of ASD.8 9
In this report, we summarise the case of an 18-year-old man with known ASD and OCD who presented with severe anorexia nervosa (body mass index (BMI) 13.8) and was found to have a novel coding variant in the proline rich region of SHANK2.
Case presentation
Case report
The male proband is the first child of a mother who has no significant medical or psychiatric history. His father has no formal psychiatric history but is noted by the proband and family to be socially isolative with rigid behavioural patterns. His parents were non-consanguineous and both of Ethiopian descent. A three-generation family history was otherwise notable for a maternal uncle with polysubstance abuse, a maternal first cousin twice removed with schizophrenia and a paternal first cousin with attention deficit hyperactivity disorder.
With regard to the proband, there was no known exposure to alcohol, tobacco, illicit substances, known teratogens or infection in utero. The proband was born at 38 weeks gestation by vaginal delivery that required suctioning. He was born with a nuchal cord that was managed without complications. At birth, he weighed 2.95 kg.
Developmentally, while gross motor milestones were met on time, fine motor skills were delayed relative to accepted standards. Language was also delayed, with first words occurring at approximately age 3 years and sentences at age 3.5 years. Regarding social development, the proband is significantly impaired. He does not use expressive gestures or imitate others and shows little interest in interaction with peers. Behaviourally, he has developed numerous rigid routines and stereotypes. Additionally, by age 9 years, he had developed severe obsessions of contamination that were associated with repetitive hand washing, avoidance of public places and avoidance of specific foods. The proband was formally diagnosed with ASD and OCD during his first psychiatric admission at age 13 years. At that time, a dysmorphology assessment and brain MRI were both unremarkable. He also underwent an extensive neuropsychological evaluation, which was consistent with the diagnosis.
The proband has never used tobacco, alcohol or any illicit substances. He has never been sexually active.
He has no other significant past medical or surgical history and he has no allergies.
Eating disorder history
At age 16, the proband was admitted to the Johns Hopkins Child and Adolescent Psychiatry Service due to restricting behaviour characterised by decreased total intake and avoidance of specific foods. On admission, BMI was 14.1 (weight 43.1 kg, height 1.75 m). He was diagnosed with anorexia nervosa, restricting type and OCD. He was started on a standardised weight gain protocol. The treatment team also recommended starting psychopharmacological treatment for OCD but his family declined. After 9 days, his family elected to seek treatment at a different facility. BMI was 14.7 at the time of transfer.
Approximately 4 months later, the proband was admitted to the Johns Hopkins Eating Disorders Service after losing 6.8 kg over the course of 1 month in the setting of worsening food avoidance, school avoidance and stereotypies. On admission, BMI was 14.2. He was diagnosed with anorexia nervosa, restricting type, complicated by OCD. During this admission, the treatment team strongly recommended a comprehensive strategy involving a standard weight gain protocol and approach to managing disordered eating behaviour as well as treatment of OCD symptoms. However, the family expressed a strong preference to minimise pharmacotherapy and minimise the attention to OCD symptoms in favour of a treatment plan focused primarily on the proband’s disordered eating behaviour. After 63 days, he had gained only 1.54 kg (BMI 14.7) and was again discharged according to his family’s preference to seek treatment at a different facility.
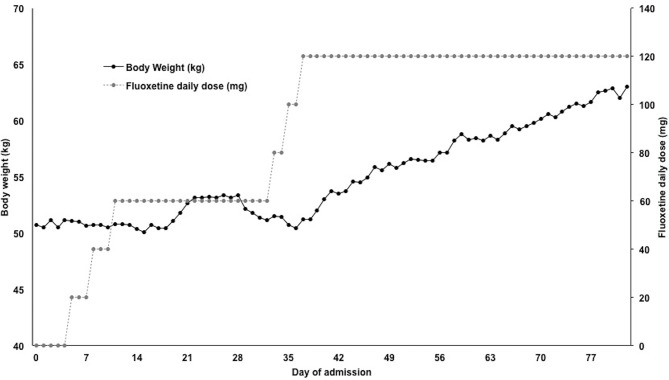
Twenty-four months later, the proband was readmitted to the Johns Hopkins Eating Disorders Service with a BMI of 13.8. At that time, the history was thought to be most consistent with Avoidant/Restrictive Food Intake Disorder. A brain MRI without contrast was unremarkable. He had an EEG which was normal. During the admission, he had significant difficulty eating meals in a social setting, was noted to spend multiple hours a day inspecting each bite of food for contamination and had profound exacerbation of his stereotypies during meals. Given the team’s concern that his disordered eating behaviour was a manifestation of his previously known OCD and that prior admissions focusing on eating disorder treatment only had not been successful, the proband and his family agreed to pharmacological treatment for OCD. Fluoxetine was started and titrated to 120 mg daily, which resulted in dramatic improvement in behaviour and weight gain (figure 1). At the time of discharge, his BMI was 19.1 with a weight of 56.93 kg.
Figure 1.
Trends in body weight (black line, left axis) and fluoxetine dose (grey line, right axis) over the course of inpatient psychiatric admission. The data suggest an improvement in weight gain that correlated with high-dose fluoxetine treatment. Behaviourally, this treatment response was also associated with improvement in OCD-like behaviours. OCD, obsessive compulsive disorder.
Additionally, given the complex psychiatric phenotype with an apparent developmental origin, molecular genetic testing was pursued.
Investigations
Array CGH analysis
Genomic DNA was extracted from peripheral blood, and array comparative genomic hybridisation (array CGH) was performed at the Johns Hopkins Cytogenetics Laboratory. The array used was the Human Infinium CytoSNP-850K Beadchip containing over 850 000 markers (mean spacing 3.5 kb) (Illumina, USA). Data were analysed with CNV partition 2.4.4.0 (Illumina, USA).
Mitochondrial genome analysis
Mitochondrial genomic DNA was extracted from peripheral blood. Sequencing as well as deletion analysis was performed by Courtagen Laboratories.
Next-generation sequencing analysis
Genomic DNA was extracted from peripheral blood, captured with inversion probes and sequenced using next generation sequencing (NGS) on Illumina MiSeq sequencing system with 250 bp paired-end reads. Sequence results were mapped to USCS hg19 genomic reference. Deletion/duplication testing was performed via analysing NGS coverage data. Variants were scored based on ACMG guidelines for variant interpretation.18 All total, more than 1200 genes were sequenced using this method, focusing on genes related to neurodevelopmental disorders and/or nuclear mitochondrial disorders. Sequencing was performed by Courtagen Laboratories.
Outcome and follow-up
The proband underwent genetic testing to assess for underlying causes for his symptoms of ASD, OCD and disordered eating behaviour.
Results from the array CGH showed 46,XY with no abnormalities detected at the level of resolution analysed. Results from the mitochondrial sequencing were also non-diagnostic. However, two homoplasmic variants of unknown significance were found at m.13967C>T (p.Thr544Met) and m.4350C>T (synonymous). Both variants are rare with low evolutionary conservation in similar species and have both been reported in normal population databases.
Notable results from the NGS panels include a novel heterozygous c.2518C>T (p.Pro840Ser) variant in the SHANK2 gene (isoform SHANK2E, NM_012309.3). This variant is located in the proline-rich domain of SHANK2 where other disease-causing variants have been found.8 The proline to serine substitution is a marked change in polarity. Furthermore, this proline residue is evolutionarily conserved across all species assessed in the UCSC browser. PolyPhen-2 predicts this variant to be damaging with a score of 0.917 (sensitivity 0.81, specificity 0.94). Based on ACMG variant classification criteria, this has been categorised as ‘3’, variant of unknown significance.18
This c.2518C>T has not been previously reported and has not been found in any queried database of healthy controls. It is not present in 1000 Genomes, ExAC or gnomAD. Of note, the variant was paternally inherited and, while the father has no formal psychiatric history, he was repeatedly noted by the proband and family to be socially isolative with rigid behavioural patterns suggestive of ASD.
Discussion
Here, we present the case of an 18-year-old man with a history of ASD and OCD who developed severe eating disordered behaviour that responded to high-dose fluoxetine. Genetic testing revealed a paternally inherited SHANK2 variant, c.2518C>T p.Pro840Ser. This novel variant is located in the proline-rich domain of SHANK2 and results in the substitution of a highly conserved proline residue to a polar serine residue which is likely damaging and may contribute to the proband’s complex phenotype. Interestingly, while his father had not been formally diagnosed with any neuropsychiatric disorder, the proband and multiple family members confirmed the father’s long history of social isolation and rigid behavioural patterns, and other studies have found that SHANK2 variants may display incomplete penetrance.8
The relationship between ASDs and SHANK2 variants has been well established, and multiple single nucleotide variants as well as small deletions and CNVs have been reported.12 19 Clinical data are further supported by several studies in model organisms that have been previously reviewed.15 17 In one knockout model, lack of Shank2 exon 7 results in impairments in social interaction, increased in stereotypical behaviours and hyperactivity.14 Similarly, mice lacking Shank2 exons 6–7 display reduction in social interactions as well as mild impairments in spatial memory.16
In addition to ASD, the proband developed OCD features during early childhood. A large body of literature has established the high rate of OCD among patients with ASDs. While the role of SHANK2 in OCD has not been well studied in humans, interestingly, two different Shank2 knockout mouse models have been found to exhibit repetitive behaviours and increased self grooming which are common behavioural phenotypes found in models of OCD.14 16 20–23
Clinically, one of the most interesting aspect of the proband’s phenotype was his profoundly disordered eating behaviour necessitating multiple inpatient psychiatric admissions for severe anorexia. Over time, it became evident that this disordered behaviour, characterised by difficulty eating meals in a social setting, spending multiple hours a day inspecting food for contamination and exacerbation of stereotypies during meals, likely represented a manifestation of OCD complicated by underlying ASD. While, to our knowledge, eating disordered behaviour has not been previously reported in individuals with SHANK2 variants, complex psychiatric phenotypes involving anorexia have been described in at least three patients with variants in the closely related SHANK3 gene.24 25
Further supporting an argument that the proband’s disordered eating behaviours are related to underlying OCD was his lack of improvement during his more than 2 months in a standardised anorexia treatment programme followed by dramatic improvement that correlated closely with titration of fluoxetine. It is now well recognised that selective serotonin reuptake inhibitors (SSRIs), particularly at high doses, represent a mainstay of treatment in OCD and this has been reviewed several times.26 27 It is also increasingly accepted that SSRIs should not be expected to augment the treatment of typical anorexia cases.28–30
Based on the evidence presented here, we suggest that the novel Pro840Ser SHANK2 heterozygous variant likely contributes (along with other genetic and environmental factors that were not the focus of this report) to the proband’s complex neuropsychiatric phenotype. Together, this case sheds light on a potentially novel genetic cause of ASD, OCD and disordered eating behaviour. Further, this case demonstrates the practical role for molecular genetic testing in clinical psychiatry and provides a clear example of how genetic information can inform the treatment of patients with complex neuropsychiatric syndromes.
Learning points.
The characteristic features of autism spectrum disorders (ASD) include disordered social interaction and communication, but ASDs are frequently comorbid with other neuropsychiatric syndromes including intellectual disability and anxiety disorders. Emerging evidence suggests potentially common aetiologies.
SHANK2, or the SH3 and Multiple Ankyrin Repeat Domains 2 gene, codes for a protein that is involved in organising the postsynaptic density. Disruptions of SHANK2 have been associated with ASD.
We identified a case of severe anorexia in the context of a complex neuropsychiatric phenotype including features of ASD and obsessive compulsive disorder (OCD). This phenotype was associated with a novel Pro840Ser SHANK2 variant.
In this case, the patient’s weight and disordered eating behaviour improved only after high-dose fluoxetine was initiated. This treatment response would not be expected in typical cases of anorexia.
This case identifies a potentially novel genetic cause of ASD, OCD and disordered eating behaviour. Further, this case provides an example of how genetic information can be used to guide treatment of patients with complex neuropsychiatric syndromes.
Acknowledgments
The authors are grateful to the proband and his parents for their participation in this study. We thank Courtagen Genetics (now known as Medicinal Genomics Corporation) for proving results of the mitochondrial genome analysis and next generation DNA sequencing. We thank the Johns Hopkins Cytogenetics Laboratory for providing the array CGH analysis and the Johns Hopkins DNA Diagnostic Laboratory for support with interpretation.
Footnotes
Contributors: All authors contributed to the conception of this work, acquisition, analysis and interpretation of data, drafting of this manuscript and approved the final version of this manuscript. They also agreed to be held accountable for all aspects of this work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Christensen DL, Bilder DA, Zahorodny W, et al. Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. J Dev Behav Pediatr 2016;37(1):1–8. 10.1097/DBP.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 2.Nestadt G, Grados M, Samuels JF. "Genetics of OCD.". The Psychiatric clinics of North America 2010;33(no. 1):141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leyfer OT, Folstein SE, Bacalman S, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord 2006;36(7):849–61. 10.1007/s10803-006-0123-0 [DOI] [PubMed] [Google Scholar]
- 4.Szatmari P, Mérette C, Emond C, et al. Decomposing the autism phenotype into familial dimensions. Am J Med Genet B Neuropsychiatr Genet 2008;147B:3–9. 10.1002/ajmg.b.30561 [DOI] [PubMed] [Google Scholar]
- 5.Meier SM, Petersen L, Schendel DE, et al. Obsessive-compulsive disorder and autism spectrum disorders: longitudinal and offspring risk. PLoS One 2015;10(11):e0141703 10.1371/journal.pone.0141703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabrucker AM, Schmeisser MJ, Schoen M, et al. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol 2011;21(10):594–603. 10.1016/j.tcb.2011.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Grant SG. Synaptopathies: diseases of the synaptome. Curr Opin Neurobiol 2012;22(3):522–9. 10.1016/j.conb.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 8.Berkel S, Marshall CR, Weiss B, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 2010;42(6):489–91. 10.1038/ng.589 [DOI] [PubMed] [Google Scholar]
- 9.Leblond CS, Heinrich J, Delorme R, et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet 2012;8(2):e1002521 10.1371/journal.pgen.1002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilmatre A, Huguet G, Delorme R, et al. The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 2014;74(2):113–22. 10.1002/dneu.22128 [DOI] [PubMed] [Google Scholar]
- 11.OMIM (TM). Online Mendelian Inheritance in Man. Baltimore, MD: Johns Hopkins University, 2018. MIM Number: 603290. [Google Scholar]
- 12.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci 2000;113:1851–6. [DOI] [PubMed] [Google Scholar]
- 13.Ziff EB. Enlightening the postsynaptic density. Neuron 1997;19(6):1163–74. 10.1016/S0896-6273(00)80409-2 [DOI] [PubMed] [Google Scholar]
- 14.Schmeisser MJ, Ey E, Wegener S, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 2012;486(7402):256–60. 10.1038/nature11015 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Bey AL, Chung L, et al. Therapeutic approaches for shankopathies. Dev Neurobiol 2014;74(2):123–35. 10.1002/dneu.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won H, Lee HR, Gee HY, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012;486(7402):261–5. 10.1038/nature11208 [DOI] [PubMed] [Google Scholar]
- 17.Yoo J, Bakes J, Bradley C, et al. Shank mutant mice as an animal model of autism. Philosophical Transactions of the Royal Society B: Biological Sciences 2014;369(1633):20130143 10.1098/rstb.2013.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17(5):405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sealey LA, Hughes BW, Sriskanda AN, et al. Environmental factors in the development of autism spectrum disorders. Environ Int 2016;88:288–98. 10.1016/j.envint.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 20.van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 2011;14(3):302–17. 10.1007/s10567-011-0097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008;47(8):921–9. 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- 22.de Bruin EI, Ferdinand RF, Meester S, et al. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord 2007;37(5):877–86. 10.1007/s10803-006-0215-x [DOI] [PubMed] [Google Scholar]
- 23.Kalueff AV, Stewart AM, Song C, et al. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci 2016;17(1):45–59. 10.1038/nrn.2015.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vucurovic K, Landais E, Delahaigue C, et al. Bipolar affective disorder and early dementia onset in a male patient with SHANK3 deletion. Eur J Med Genet 2012;55(11):625–9. 10.1016/j.ejmg.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Denayer A, Van Esch H, de Ravel T, et al. Neuropsychopathology in 7 patients with the 22q13 deletion syndrome: presence of bipolar disorder and progressive loss of skills. Mol Syndromol 2012;3(1):14–20. 10.1159/000339119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koran LM, Hanna GL, Hollander E, et al. "Practice guideline for the treatment of patients with obsessive-compulsive disorder.". The American journal of psychiatry 2007;164:1. [PubMed] [Google Scholar]
- 27.Mancuso E, Faro A, Joshi G, et al. Treatment of pediatric obsessive-compulsive disorder: a review. J Child Adolesc Psychopharmacol 2010;20(4):299–308. 10.1089/cap.2010.0040 [DOI] [PubMed] [Google Scholar]
- 28.Attia E, Haiman C, Walsh BT, et al. Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry 1998;155(4):548–51. 10.1176/ajp.155.4.548 [DOI] [PubMed] [Google Scholar]
- 29.Holtkamp K, Konrad K, Kaiser N, et al. A retrospective study of SSRI treatment in adolescent anorexia nervosa: insufficient evidence for efficacy. J Psychiatr Res 2005;39(3):303–10. 10.1016/j.jpsychires.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 30.Walsh BT, Kaplan AS, Attia E, et al. Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 2006;295(22):2605–12. 10.1001/jama.295.22.2605 [DOI] [PubMed] [Google Scholar]