Abstract
RutA is a novel flavoenzyme on the uracil catabolic pathway that catalyzes uracil ring opening by a unique amide oxidation reaction. Here we provide evidence that this reaction also involves the formation of a flavin-N5-oxide.
In the Rut pathway, uracil 1 is converted to 3-hydroxypropionate 3, ammonia, and carbon dioxide (Figure 1).1,2 RutA catalyzes the first step of this pathway in which uracil is converted to 3-ureidoacrylic acid 2. Previous mechanistic studies of this reaction identified RutA as the first example of a flavin hydroperoxide-mediated amide cleavage reaction (conversion of 1 to 9 in Figure 2).3 The mechanism of hydroperoxide reduction (9 to 2) remained unclear. The observation that chemically synthesized peracid 9 was readily reduced by NADH or DTT initially suggested that this reduction was not enzyme-catalyzed. This was further supported by the absence of a suitable cysteine residue at the active site of RutA that can participate in peroxide reduction by the well-characterized peroxiredoxin mechanism.4
Figure 1.
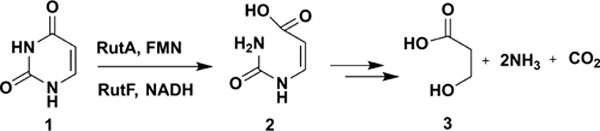
RutA catalyzes the first step in uracil catabolism. RutF is a flavin reductase required to generate the reduced cofactor for RutA.
Figure 2.
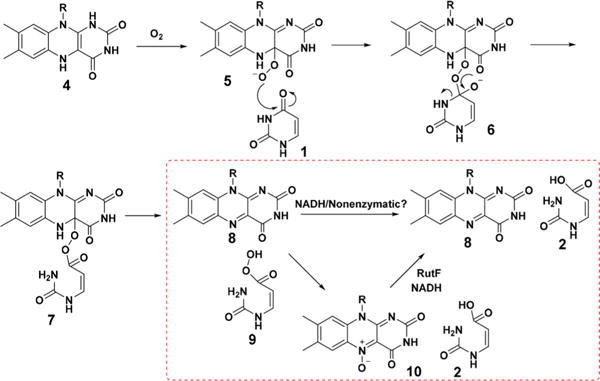
Mechanistic proposal for the RutA-catalyzed reaction. Conversion of 9 to 2 is highlighted in the box.
Flavin-N5-oxide 10 has recently been identified as a new flavin oxidation state in the EncM- and DszA-catalyzed reactions (Figure 3A,B).5–8 EncM catalyzes 1,3-diketone oxidation during the biosynthesis of polyketide antibiotic enterocin. Detailed mechanistic studies of this system revealed the formation of flavin-N5-oxide upon reaction of reduced flavin with molecular oxygen. Flavin-N5-oxide, thus formed, acts as an oxidizing agent to convert 1,3-diketone 13 to 1,2,3-triketone product 14. DszA catalyzes a novel oxidative C−S bond cleavage reaction in the dibenzothiophene catabolic pathway. In this reaction, flavin-N5-oxide is formed by the transfer of oxygen from the substrate hydroperoxide to oxidized flavin. This suggested the possibility that acyl hydroperoxide 9 might also undergo a similar reduction (Figure 2). In this communication, we describe the experimental validation of this hypothesis.
Figure 3.
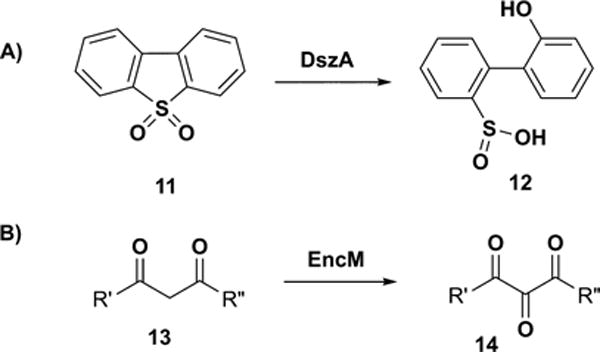
(A) DszA-catalyzed and (B) EncM-catalyzed reactions involve formation of a flavin-N5-oxide.
RutA was overexpressed in Escherichia coli BL21(DE3) and purified by Ni-affinity chromatography.3 The normal RutA activity was confirmed by incubating uracil in the presence of RutA, FMN, NADH, and E. coli flavin reductase (Fre)9 (to substitute for the RutF flavin reductase). To test for the formation of the FMN-N5-oxide, the RutA assay had to be performed in the absence of NADH. The flavin reductase/NADH was therefore replaced by the photochemical reduction of the flavin by EDTA.10 In this reaction mixture, FMN was photoreduced using EDTA under anaerobic conditions; stoichiometric amounts of uracil and RutA were added, and the resulting mixture was incubated in a glovebox for 30 min. The mixture was then exposed to air to initiate formation of the flavin hydroperoxide. After 1 h, the reaction was heat-quenched. High-performance liquid chromatography (HPLC) analysis demonstrated the consumption of FMN and uracil in a 1:1 ratio and the formation of a new FMN derivative at 20.3 min (Figure 4A).
Figure 4.
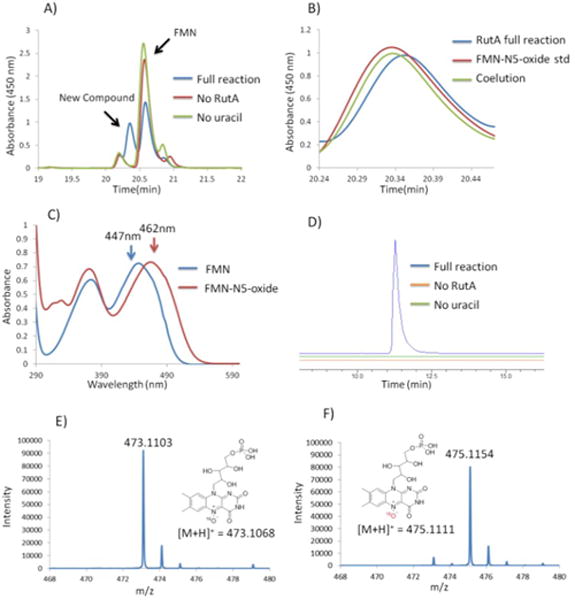
(A) HPLC analysis of the RutA reaction run in the absence of NADH showing the formation of a new compound at 20.3 min. (B) Co-elution of the new compound with synthetically prepared FMN-N5-oxide. (C) Comparison of the UV−vis spectra of FMN and FMN-N5-oxide formed in the RutA full reaction. (D) Extracted ion chromatograms for [M + H]+ = 473.1 Da demonstrate FMN-N5-oxide formation only in the full reaction mixture. (E) The exact mass of the new compound is consistent with the mass expected for the FMN-N5-oxide ([M + H]+ = 473.1 Da). (F) Exact mass of the new compound formed when the reaction is performed in the presence of 18O2.
This new compound co-eluted with an authentic standard of chemically synthesized FMN-N5-oxide,5,8 and its ultraviolet− visible (UV−vis) spectrum was identical to that of the standard (Figure 4B,C). Liquid chromatography−mass spectrometry (LC−MS) analysis revealed that the mass corresponding to FMN-N5-oxide is present only in the full reaction (Figure 4D,E). When the reaction was performed in the presence of 18O2, the expected 2 Da mass increase was observed (Figure 4F). These data unequivocally establish formation of FMN-N5-oxide in the RutA-catalyzed reaction.
Flavin-N5-oxide was proposed as a possible oxidizing intermediate in the early days of flavoenzymology but was then dismissed in favor of the flavin hydroperoxide. The unequivocal identification of this new flavin oxidation state in the EncM-catalyzed (enolate oxidation),5–7 DszA-catalyzed (sulfone monooxygenase),8 and RutA-catalyzed (amide monooxygenase) reactions suggests that the use of this new flavin oxidation state may be more prevalent than previously thought. EncM may have evolved the use of flavin-N5-oxide because the Baeyer−Villiger oxidation using flavin hydroperoxide is likely to compete with ketone Cα hydroxylation. We would therefore expect to find other examples of the EncM motif in flavoenzymes involved in hydroxylation of sites adjacent to electrophilic centers. This expectation is further supported by the versatility of oxoammonium ions as oxidants in organic synthesis.11–14 Our studies of DszA and RutA suggest that the reduction of substrate-derived hydroperoxide by oxidized flavin, is another catalytic motif likely to involve the formation of a flavin-N5-oxide (Figure S3).
Supplementary Material
Acknowledgments
Funding
This work was supported by the Robert A. Welch Foundation (A-0034) and by NIH (DK44083).
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.7b00493.
Detailed procedure for overexpression and purification of RutA, HPLC and LC−MS parameters, and chromatograms for all the enzymatic reactions (PDF)
ORCID
Sanjoy Adak: 0000-0003-2889-4272
Notes
The authors declare no competing financial interest.
References
- 1.Loh KD, Gyaneshwar P, Markenscoff Papadimitriou E, Fong R, Kim KS, Parales R, Zhou Z, Inwood W, Kustu S. Proc Natl Acad Sci U S A. 2006;103:5114–5119. doi: 10.1073/pnas.0600521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KS, Pelton JG, Inwood WB, Andersen U, Kustu S, Wemmer DE. J Bacteriol. 2010;192:4089–4102. doi: 10.1128/JB.00201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee T, Zhang Y, Abdelwahed S, Ealick SE, Begley TP. J Am Chem Soc. 2010;132:5550–5551. doi: 10.1021/ja9107676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall A, Karplus PA, Poole LB. FEBS J. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teufel R, Miyanaga A, Michaudel Q, Stull F, Louie G, Noel JP, Baran PS, Palfey B, Moore BS. Nature. 2013;503:552–556. doi: 10.1038/nature12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teufel R, Stull F, Meehan MJ, Michaudel Q, Dorrestein PC, Palfey B, Moore BS. J Am Chem Soc. 2015;137:8078–8085. doi: 10.1021/jacs.5b03983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teufel R, Agarwal V, Moore BS. Curr Opin Chem Biol. 2016;31:31–39. doi: 10.1016/j.cbpa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adak S, Begley TP. J Am Chem Soc. 2016;138:6424–6426. doi: 10.1021/jacs.6b00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xun L, Sandvik ER. Appl Environ Microbiol. 2000;66:481–486. doi: 10.1128/aem.66.2.481-486.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty S, Massey V. J Biol Chem. 2002;277:41507–41516. doi: 10.1074/jbc.M205432200. [DOI] [PubMed] [Google Scholar]
- 11.Bobbitt JM, Bruckner C, Merbouh N. Org React (Hoboken, NJ, U S) 2009;74:103–424. [Google Scholar]
- 12.Liu YC, Ren T, Guo QX. Chin J Chem. 1996;14:252–258. [Google Scholar]
- 13.Golubev VA, Miklyush RV. Zh Org Khim. 1972;8:1356–1357. [Google Scholar]
- 14.Ren T, Liu YC, Guo QX. Bull Chem Soc Jpn. 1996;69:2935–2941. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


