Abstract
Study Objectives:
To compare causes of sleep disturbance and to compare self-reported sleep duration among groups of late premenopausal women and early perimenopausal women.
Methods:
In a longitudinal study of a community-based sample of healthy women 40 to 50 years of age, menstrual cycle and symptom data were collected every 2 months; anthropometric measures, a urine sample for follicle stimulating hormone (FSH), and the Pittsburgh Sleep Quality Index (PSQI) measures were collected every 6 months.
Results:
At 12 to 18 months, 206 women remained premenopausal and 69 women became perimenopausal. Poor sleep quality (PSQI score > 5) was experienced by 42% of the total cohort. Awakening to use the bathroom was the most frequent reason (81%) for sleep disturbance in the entire cohort, followed by feeling too hot (26%). However, premenopausal women were significantly more likely to awaken to use the bathroom than perimenopausal women (P = .047), and perimenopausal women were more likely than premenopausal women to awaken because of feeling too hot (P = .002). Women in early perimenopause reported shorter sleep duration (P = .007) and worse sleep quality (P = .05) than premenopausal women of similar age.
Conclusions:
Sleep disturbance is a significant issue for midlife women regardless of age or reproductive stage. Identification of salient factors that disrupt sleep, such as nocturia prior to menopausal transition or feeling too hot early in menopausal transition, will provide direction for developing tailored intervention strategies to improve sleep and quality of life.
Commentary:
A commentary on this article appears in this issue on page 1095.
Citation:
Jones HJ, Zak R, Lee KA. Sleep disturbances in midlife women at the cusp of the menopausal transition. J Clin Sleep Med. 2018;14(7):1127–1133.
Keywords: hot flashes, midlife women, menopause, nocturia, sleep disturbance, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research about sleep disturbance in midlife women has primarily focused on differences associated with the transition from perimenopause to postmenopause. Factors associated with sleep disturbance during the premenopause stage just prior to the menopausal transition have not been as extensively explored. Using descriptive and multivariate statistical analyses, causes of sleep disturbance and differences in sleep duration among late premenopausal women were compared to those of women in early perimenopause.
Study Impact: This study identifies some of the salient factors that contribute to poor sleep quality in the premenopausal and perimenopausal reproductive stages, such as nocturia and feeling too hot. Awareness of these potential risks factors for sleep disturbance can improve clinical assessment, preventive interventions, and treatments.
INTRODUCTION
Research suggests that sleep problems are more prominent in women than men, and become more prevalent with age, yet influence from sex-related physiological changes and hormone fluctuations remain largely unknown.1,2 The National Institutes of Health estimates that sleep disturbance is experienced by 16% to 42% of women in premenopause, 39% to 47% in perimenopause, and 35% to 60% in postmenopause.3 Vasomotor symptoms (hot flashes and night sweats) are common during menopause and often reported as the cause of awakenings.1,4 Lower urinary tract symptoms (LUTS) such as nocturia and incontinence are also common and associated with sleep disturbance in midlife women5,6 as well as short sleep duration in men and women.7 Age and body mass index (BMI) further complicate sleep disturbance due to strong associations with LUTS, sleep-disordered breathing, and vasomotor symptoms in both perimenopausal and postmenopausal women.5
Research on sleep in midlife women has primarily focused on comparing premenopause to postmenopause. Few have explored sleep disturbance as women transition from late premenopause to early perimenopause. The purpose of this study was to identify specific symptoms associated with sleep disturbance at these reproductive stages, what symptoms contribute to poor sleep quality, and whether sleep duration is affected. Sleep complaints specific to late premenopause were compared with complaints in early perimenopause to characterize sleep during this transition. We previously reported that LUTS was more prevalent in late premenopausal women than perimenopausal women of similar age and demographics,8 and vasomotor symptoms accompanying the menopausal transition are known to influence sleep quality. Therefore, we hypothesized that late premenopausal women would be more likely to experience poor sleep due to bladder symptoms and perimenopausal women would be more likely to experience poor sleep due to vasomotor symptoms (hot flashes and night sweats).
METHODS
Sample
The sample for this analysis included late premenopausal and early perimenopausal women living in the San Francisco Bay area participating in the University of California San Francisco (UCSF) Midlife Women's Health Study. Women were included if they were white, African American, or Latina; between 40 to 50 years of age; still experiencing regular menstrual periods, and not planning a pregnancy. Women were excluded if they were undergoing hormone therapy or had any major health problem such as cancer, stroke, diabetes, or recent hospitalization or surgery.9 The details of recruitment and protocol were previously reported.9,10 The UCSF Committee on Human Research approved the study and all participants provided informed consent prior to data collection.
There were 347 women enrolled, and attrition during the first 2 years was less than 10%.10 Assessments occurred at enrollment and every 6 months through 1 year after hysterectomy, 1 year after initiating hormone therapy, or 1 year after the last menstrual period. Participants were followed for up to 5 years. During the second year, 30 women were excluded due to missing data at any two of the first four timepoints, and 7 were excluded because of hysterectomy, pregnancy, hormone initiation, or absence of menses for 12 months.
This analysis focuses on 275 participants with complete data for the first 12 months who had regular menses with low urinary follicle stimulating hormone (FSH) (< 2.5 IU/dL) (n = 206) or who had entered early perimenopausal transition in the next 12 months (n = 69) with elevations in FSH (> 2.5 IU/dL) and onset of irregular menstrual cycles.
Measures
Self-report demographic data and questionnaires were completed every 6 months and included the Pittsburgh Sleep Quality Index (PSQI) to assess sleep quality. Menstrual cycle data and symptom severity (urinary frequency, hot flashes, night sweats, etc.) were collected every 2 months by telephone using the Seattle Women's Health Survey.11 Weight and height were measured every 6 months to obtain body mass index (BMI) (kg/m2), and FSH level was obtained from first-morning urine samples frozen and analyzed in batch assays (Esoterix Endocrinology Services Laboratory, Calabasas Hills, California, United States). Reproductive stage was estimated with FSH levels and menstrual cycle regularity based on Stages of Reproductive Aging Workshop (STRAW) criteria.12
Menstrual Cycle Patterns and Symptoms
During telephone interviews every 2 months between visits, participants were asked about their previous menstrual cycle dates and characteristics as well as symptoms during the prior week. Urinary frequency, hot flashes, and night sweats were among the 52 symptoms read to them on the phone, and responses were recorded by the research team member, who was blinded to FSH laboratory values. Responses ranged from 0 (none), 1 (minimal or mild), 2 (moderate), to 3 (extreme) and were averaged to obtain a score from 0–3 for that 6-month period. For this analysis, responses were then dichotomized as either absent (0) or present at any level (> 0).
Pittsburgh Sleep Quality Index
The PSQI is a 19-item self-report assessment of sleep quality over the past month.13 The PSQI evaluates 7 components of sleep (quality, duration, onset latency, daytime dysfunction, sleep disturbance, use of sleep medications, and sleep efficiency). Scores range from 0 to 21, and a score above 5 indicates poor sleep quality. Cronbach alpha coefficients for the 7 components were 0.72 at enrollment and 0.78 at 12 months.
One item in the PSQI asks about number of hours per night typically spent sleeping during the past month, and this item was used to estimate habitual sleep duration (component 3). Sleep disturbance (component 5) is a computation of how often a person wakes up during the night (not in the past month = 0; < 1×/wk = 1; 1–2×/wk = 2; ≥ 3×/wk = 3) due to any of the following reasons: getting up to use the bathroom, not breathing comfortably, coughing or snoring loudly, feeling too cold, feeling too hot, bad dreams, pain, and “other” as described by the participant. A total score for sleep disturbance can range from 0 to 27, and this total is then recoded into a range of 0 to 3 range (component 5) as are the other 6 components.
Statistical Analysis
Means and standard deviations (SD) were used to describe the two reproductive groups and differences were tested using independent t tests or F tests for continuous variables. Associations between sleep quality and subjective sleep duration were tested with Pearson r or Spearman rho for continuous variables, and chi-square for categorical variables. For multiple regression analyses, race was dummy-coded and other variables were examined for normal distributions; no variables required transformation. All analyses were performed using SPSS version 22.0 software (IBM Corp, Armonk, New York, United States). Statistical significance was set at P < .05.
RESULTS
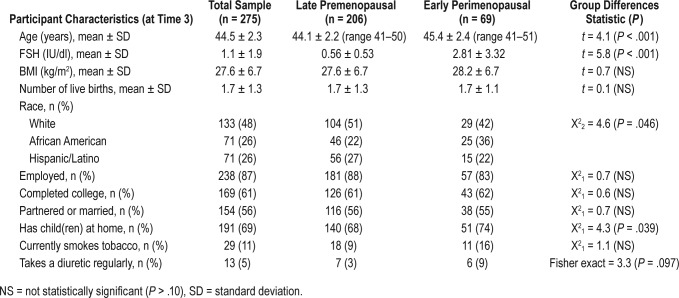
Ages ranged from 40 to 50 years at enrollment. Demographic and clinical characteristics at 12 months are shown in Table 1 by reproductive stage. The two groups were similar for most characteristics. Their mean age was significantly different by an average of only 1 year, and as expected, they differed in FSH values. The perimenopausal group was significantly (P = .039) more likely to have children living at home (74%) compared to the premenopausal group (68%).
Table 1.
Demographic and clinical characteristics by menopausal stage (n = 275).
Menstrual Cycle Symptom Experience
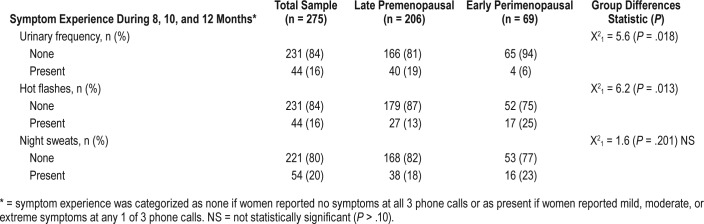
The reproductive stage groups were significantly different for two symptoms from the telephone interviews: urinary frequency and hot flashes. As seen in Table 2, premenopausal women were more likely to endorse urinary frequency (n = 40, 19%) compared to only 4 (6%) perimenopausal women (P = .018), yet use of diuretics was rare and only slightly higher in the perimenopausal group (Table 1). Conversely, hot flashes were more likely to be experienced by perimenopausal women (25%) compared to 13% of premenopausal women (P = .013). Approximately 20% of the total sample reported night sweats but there was no significant difference between the two groups (Table 2). No other menstrual cycle symptoms were significantly different by group.
Table 2.
Women's symptom experience (n = 275).
PSQI Sleep Quality
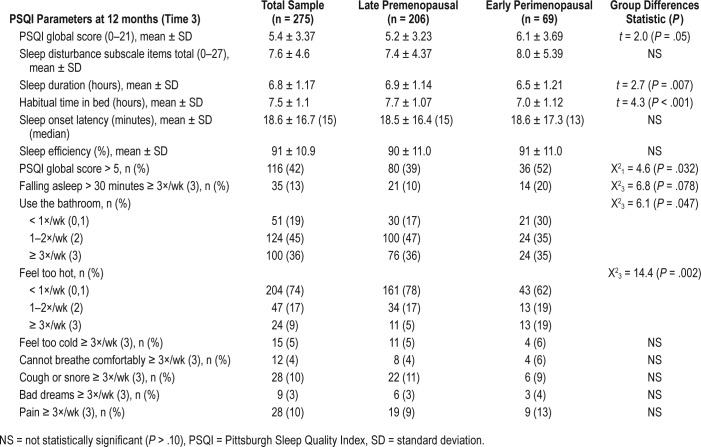
A higher PSQI score (worse sleep quality) was related to BMI (r = .156, P = .009), urinary frequency (rho = .117, P = .047), hot flashes, and night sweats (rho = .127, P = .032) but unrelated to FSH level or race. As seen in Table 3, the overall sample had a mean PSQI score of 5.4, and 42% had a PSQI score > 5. The score for perimenopausal women was significantly higher than the premenopausal group's score, and over half of the perimenopausal group (52%) had a PSQI score > 5 compared to 39% of the premenopausal group.
Table 3.
Sleep quality parameters by reproductive stage (n = 275).
The PSQI component 5 total score for the nine items comprising sleep disturbance was slightly higher in the perimenopausal group but did not significantly differ between groups.
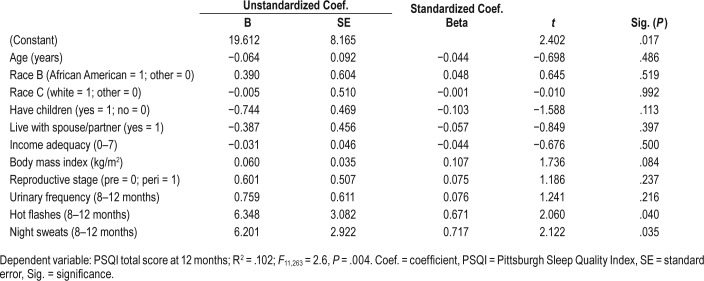
In a multivariate linear regression model, controlling for age, reproductive stage, and all other variables, having night sweats and having hot flashes were significant, but urinary frequency and BMI were no longer significant. The overall model (see Table 4) accounted for 10.2% of the variance in sleep quality (R2 = .102, F11, 263 = 2.6, P = .004).
Table 4.
Multivariate linear regression model for sleep quality.
PSQI Component 5: Reasons for Sleep Disturbance
Most reasons for awakening were similar for each group. However, as seen in Table 3, more premenopausal women (83%) awakened to use the bathroom (P = .047) and more perimenopausal women (38%) awakened from feeling too hot (P = .002). Very few (< 10%) awakened from inability to breathe comfortably, coughing, or snoring, and rates did not differ by reproductive stage. However, BMI was associated with awakenings due to coughing or snoring (rho = .300, P < .001) as well as difficulty initiating sleep, taking > 30 minutes to fall asleep (rho = .155, P = .009), pain (rho = .129, P = .030) and feeling too hot (rho = .121, P = .043).
PSQI Sleep Duration
Sleep duration was inversely related to FSH values (r = −.141, P = .017). There was a significant difference in duration by race (F2,273 = 13.2, P < .001), with African American women reporting 6.3 ± 1.13 hours sleep whereas Latinas reported 6.6 ± 1.22 hours and white women reported 7.1 ± 1.03 hours (data not shown). Duration was unrelated to other demographic and reproductive characteristics, and unrelated to urinary frequency or vasomotor symptoms.
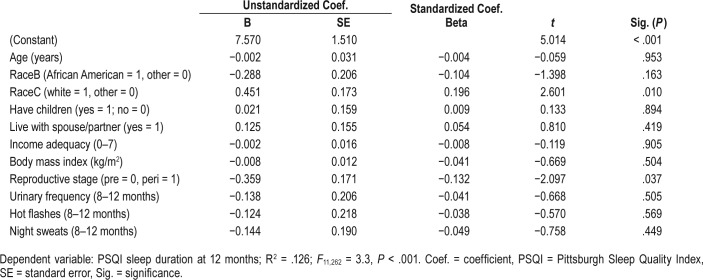
In multivariate linear regression analysis (Table 5), only race and reproductive stage were significant in accounting for variance in sleep duration. Controlling for age, BMI, urinary frequency, hot flashes, and night sweats and other clinical characteristics, longer sleep duration was associated with being white and being premenopausal. Urinary frequency, hot flashes, and night sweats did not contribute to the model. The overall model with 11 predictors was significant and accounted for 12.5% of the variance in sleep duration (R2 = .126, F11, 262 = 3.3, P < .001). Despite premenopausal women reporting longer time in bed, the two groups did not differ on PSQI component 2 (sleep onset latency) or component 4 (sleep efficiency).
Table 5.
Multivariate linear regression model for sleep duration (hours).
DISCUSSION
In this study, sleep quality, as indicated by the global PSQI score, was significantly worse for perimenopausal women. Others have reported an association between menopause status and sleep quality,14,15 and our findings support these associations, even in very early distinctions between late premenopause and early perimenopause. Although both groups had similar total sleep disturbance scores (PSQI component 5), premenopausal women were more likely to attribute awakenings to using the bathroom, and perimenopausal women were more likely to attribute awakenings to feeling too hot.
The most frequent reason for awakenings for the entire cohort was to use the bathroom. Nonetheless, it was reported more frequently among the premenopausal group than among the perimenopausal cohort As previously reported,8 LUTS such as frequency and nocturia were common in a subsample of this group when studied with a more detailed urologic questionnaire, with 73% reporting nocturia, defined as getting up to use the bathroom at least once per night.16 Potential factors contributing to the prevalence of LUTS in midlife women include medications, fluid intake, caffeine intake, infection, and pregnancy-related changes of the urinary tract.17 Decreasing estrogen levels associated with the menopausal transition can also increase nocturia and other LUTS in perimenopausal and postmenopausal women, as the decline in estrogen is associated with atrophy of urethral epithelium and decreased sensitivity of urethral smooth muscle.18 Mood disorders, such as anxiety, could also contribute to awakenings, which then result in emptying the bladder.19,20 The PSQI asks how often during a typical month the participant awakens to “use the bathroom”. Thus, this question captures awakening because of the need to urinate but also captures awakening for any reason and then feeling the need to urinate. The lack of specificity in this item makes our finding that this complaint is more frequent among women before they enter the menopausal transition even more clinically meaningful, as one would expect perimenopausal women to report nocturia even if awakened by other causes, yet they reported significantly less frequent nocturia than the premenopausal group. To better understand how nocturia interferes with sleep in premenopausal women, more research is needed on LUTS in general.
The only reason for sleep disturbance that was more prevalent on the PSQI for the perimenopausal group than for the late premenopausal group was feeling too hot. Similarly, the perimenopausal women were more likely to report hot flashes during the telephone surveys, without specifying time of occurrence yet they were not more likely to complain of night sweats. This distinction may indicate that “feeling too hot” at night is a sentinel symptom of early menopausal transition even before awareness of night sweats. The role of vasomotor symptoms in insomnia for midlife women has been historically difficult to characterize. For example, some researchers find a disparity between objective verification of hot flashes and subjective sleep quality, with complaints of vasomotor symptoms influencing sleep quality more than the actual number of hot flashes.21,22 Although it is common to awaken and perceive the need to urinate when an awakening occurs from any cause, the need to use the bathroom was not reported with greater frequency in this early perimenopausal women group than in the late premenopausal group. This suggests that awakenings due to nocturia are a less disruptive stimulus to perimenopausal women than feeling too hot. If feeling too hot is an early analog of awakening with a hot flash or night sweat, a potential mechanism could be withdrawal of vagal tone that accompanies a hot flash.23 Combined with the increase in sympathetic tone that accompanies an awakening, a subjectively more intense arousal and prolonged awakening would result. Thus, it becomes important to ascertain whether women in early menopausal transition are experiencing vasomotor symptoms early on to address this clinically significant and treatable reason for poor sleep prior to its significant effect on sleep quality. Based on findings from this study, clinicians should inquire about feeling too hot at night as part of an initial screening for potential vasomotor symptoms.
The late premenopausal women spent significantly more time in bed and had longer sleep duration than early perimenopausal women. However, the two groups did not differ on self-reported minutes to fall asleep, and both groups had high sleep efficiency. Controlling for significant correlates (age, race, BMI), premenopausal women had longer sleep duration compared to perimenopausal women, and African American and Latina women had shorter sleep duration compared to white women. These race differences in sleep duration are consistent with findings of shorter sleep duration in African American women.24,25 Mechanisms that contribute to this disparity remain poorly understood and warrant further study.25 Longer total sleep time was associated with being premenopausal but there was no correlation with the absolute urinary FSH value once covariates were controlled, a finding consistent with other studies that used polysomnography to measure sleep duration.26–28 Of interest, Sowers et al.,28 found that an increase in total sleep time was associated with a more rapid rise in serum FSH. We did not analyze rate of rise in FSH but rather used FSH to help define current reproductive stage and found longer total sleep time in women who were still premenopausal compared to women who had already begun the transition toward menopause. Therefore, our data cannot be directly compared with findings reported by Sowers at al.28 Findings do suggest, however, that more research is needed on the relationship between change in FSH levels and sleep duration.
In summary, findings from this study highlight the prevalence and nature of sleep disturbance in late premenopause and early perimenopause, and differentiates symptoms that are most disruptive. The median age at menopause in the United States is 52.7 years,29 and the average age for the two groups in our sample was 45 years, suggesting that poor sleep commonly associated with menopause may occur earlier in midlife than previously thought. Clinicians should begin to screen women older than 40 years of age for sleep problems and associated risk factors such as daytime urinary frequency, urge or stress incontinence, nocturia, vasomotor symptoms, and the possible sentinel symptom of awakenings associated with “feeling too hot” early in transition to menopause. Because sleep problems may continue or increase during menopausal transition and into old age, clinicians should provide anticipatory guidance. For example, it would be beneficial to discuss factors that contribute to awakenings and provide education about preventive techniques. These techniques, such as decreasing fluid intake prior to bedtime and emptying the bladder before getting into bed even if there is no urge or feeling of fullness, can become healthy sleep hygiene behaviors into old age.
As women are susceptible to the development of other risk factors for sleep problems with the aging process,4 more research on reproductive stage and sleep problems is needed. A better understanding of how LUTS and vasomotor symptoms affect sleep in women prior to becoming postmenopausal is needed. Developing tailored and effective interventions based on reasons for nocturnal awakenings at an earlier point in women's lives30 will improve quality of sleep and quality of life for women into old age.
Limitations
These results add to our understanding of sleep disturbance in midlife women, but there are a few limitations to keep in mind. First, generalizability to all midlife women is limited, as women in these two reproductive stages differed in age by only 1 year and were from one geographic area in the United States. They were also in good health, recruited from the general community rather than health care settings, thus making it unlikely that sleep was affected by chronic illness. Second, mood disorders such as anxiety or depression can contribute to sleep disturbance, and this was not addressed in our analysis. The Center for Epidemiological Studies-Depression was used as a measure of depressive symptoms and is an effective screening tool in large epidemiologic studies. However, the PSQI and Center for Epidemiological Studies-Depression were highly correlated with significant overlap between items. Third, the data were focused on just one 6-month time period from a longitudinal study, yet this narrow window was necessary to focus on women transitioning from late premenopause to perimenopause. Finally, sleep duration and sleep quality were self reported, and not verified with polysomnographic recordings. However, sleep studies in a laboratory may only reflect 1 or 2 nights and the added burden of going to a laboratory can discourage participation unless sleep is problematic, which would also create a biased sample.
DISCLOSURE STATEMENT
This research was conducted at the University of California San Francisco. Each author had a part in the writing and approval of this manuscript. Dr. Jones was supported by funding from training grant T32 NR00788 and the National Black Nurses' Association. This study was funded by NIH grant #R01 NR04259 with a supplement from the Office of Women's Health. The authors report no conflicts of interest.
ABBREVIATIONS
- BMI
body mass index
- FSH
follicle stimulating hormone
- LUTS
lower urinary tract symptoms
- PSQI
Pittsburgh Sleep Quality Index
- SD
standard deviation
- SDB
sleep-disordered breathing
- STRAW
Stages of Reproductive Aging Workshop
REFERENCES
- 1.Kravitz HM, Avery E, Sowers M, et al. Relationships between menopausal and mood symptoms and EEG sleep measures in a multi-ethnic sample of middle-aged women: the SWAN sleep study. Sleep. 2011;34(9):1221–1232. doi: 10.5665/SLEEP.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lord C, Sekerovic Z, Carrier J. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathologie-biologie. 2014;62(5):302–310. doi: 10.1016/j.patbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–1013. [PubMed] [Google Scholar]
- 4.Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96–104. doi: 10.1016/j.psyneuen.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terauchi M, Hirose A, Akiyoshi M, Owa Y, Kato K, Kubota T. Prevalence and predictors of storage lower urinary tract symptoms in perimenopausal and postmenopausal women attending a menopause clinic. Menopause. 2015;22(10):1084–1090. doi: 10.1097/GME.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 6.Araujo AB, Yaggi HK, Yang M, McVary KT, Fang SC, Bliwise DL. Sleep related problems and urological symptoms: testing the hypothesis of bidirectionality in a longitudinal, population based study. J Urol. 2014;191(1):100–106. doi: 10.1016/j.juro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliwise DL, Holm-Larsen T, Goble S, Juul KV, van der Meulen E, Norgaard JP. Delay of first voiding episode is associated with longer reported sleep duration. Sleep Health. 2015;1(3):211–213. doi: 10.1016/j.sleh.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Jones HJ, Huang AJ, Subak LL, Brown JS, Lee KA. Bladder symptoms in the early menopausal transition. J Womens Health (Larchmt) 2016;25(5):457–463. doi: 10.1089/jwh.2015.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Guiterrez Y, Gilliss C, Lee KA. Physical activity, weight, and waist circumference in midlife women. Health Care Women Int. 2012;33(12):1086–1095. doi: 10.1080/07399332.2012.673658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilliss CL, Lee KA, Gutierrez Y, et al. Recruitment and retention of healthy minority women into community-based longitudinal research. J Womens Health Gend Based Med. 2001;10(1):77–85. doi: 10.1089/152460901750067142. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell ES, Woods NF. Symptom experiences of midlife women: observations from the Seattle Midlife Women's Health Study. Maturitas. 1996;25(1):1–10. doi: 10.1016/0378-5122(96)01047-x. [DOI] [PubMed] [Google Scholar]
- 12.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31(7):979–990. [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014;21(12):1301–1318. doi: 10.1097/GME.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 16.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology Urodyn. 2002;21(2):167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 17.Zachoval R, Krhut J, Sottner O, et al. [Nocturia, incidence, ethiology, diagnostics] Ceska gynekologie / Ceska lekarska spolecnost J Ev Purkyne. 2013;78(6):566–572. [PubMed] [Google Scholar]
- 18.Calleja-Agius J, Brincat MP. The urogenital system and the menopause. Climacteric. 2015;18(Suppl 1):18–22. doi: 10.3109/13697137.2015.1078206. [DOI] [PubMed] [Google Scholar]
- 19.Kang B, Doo M, Kim Y. Associations between self-reported sleep quality and duration and dietary consumptions, psychological symptoms, and obesity in Korean adults. Prev Nutr Food Sci. 2017;22(4):271–276. doi: 10.3746/pnf.2017.22.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcantara C, Patel SR, Carnethon M, et al. Stress and sleep: results from the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. SSM Popul Health. 2017;3:713–721. doi: 10.1016/j.ssmph.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19(7):742–748. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102(6):1708.e1701–1715.e1701. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Zambotti M, Colrain IM, Sassoon SA, Nicholas CL, Trinder J, Baker FC. Vagal withdrawal during hot flashes occurring in undisturbed sleep. Menopause. 2013;20(11):1147–1153. doi: 10.1097/GME.0b013e31828aa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 25.Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med. 2016;18:74–81. doi: 10.1016/j.sleep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 26.de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. doi: 10.1210/jc.2014-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampio L, Polo-Kantola P, Polo O, Kauko T, Aittokallio J, Saaresranta T. Sleep in midlife women: effects of menopause, vasomotor symptoms, and depressive symptoms. Menopause. 2014;21(11):1217–1224. doi: 10.1097/GME.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 28.Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 29.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gosling JA, Batterham PJ, Glozier N, Christensen H. The influence of job stress, social support and health status on intermittent and chronic sleep disturbance: an 8-year longitudinal analysis. Sleep Med. 2014;15(8):979–985. doi: 10.1016/j.sleep.2014.04.007. [DOI] [PubMed] [Google Scholar]