Abstract
Introduction:
The purpose of this systematic review is to provide supporting evidence for a clinical practice guideline on the use of actigraphy.
Methods:
The American Academy of Sleep Medicine commissioned a task force of experts in sleep medicine. A systematic review was conducted to identify studies that compared the use of actigraphy, sleep logs, and/or polysomnography. Statistical analyses were performed to determine the clinical significance of using actigraphy as an objective measure of sleep and circadian parameters. Finally, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used to assess the evidence for making recommendations.
Results:
The literature search resulted in 81 studies that met inclusion criteria; all 81 studies provided data suitable for statistical analyses. These data demonstrate that actigraphy provides consistent objective data that is often unique from patient-reported sleep logs for some sleep parameters in adult and pediatric patients with suspected or diagnosed insomnia, circadian rhythm sleep-wake disorders, sleep-disordered breathing, central disorders of hypersomnolence, and adults with insufficient sleep syndrome. These data also demonstrate that actigraphy is not a reliable measure of periodic limb movements in adult and pediatric patients. The task force provided a detailed summary of the evidence along with the quality of evidence, the balance of benefits and harms, patient values and preferences, and resource use considerations.
Citation:
Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(7):1209–1230.
Keywords: actigraphy, circadian rhythm, sleep disorders, systematic review
INTRODUCTION
This systematic review is intended to provide supporting evidence for a clinical practice guideline on the use of actigraphy in patients with suspected or diagnosed sleep disorders or circadian rhythm sleep-wake disorders,1 and update the evidence review conducted for the previously published American Academy of Sleep Medicine (AASM) practice parameters on the use of actigraphy in these populations.2 The scientific literature summarized in prior practice parameters established the validity of actigraphy to assess sleep in healthy individuals and select groups of patients. The objective of this systematic review is to examine the clinical value of actigraphy in the assessment and treatment of patients with suspected or diagnosed sleep disorders and circadian rhythm sleep-wake disorders (CRSWDs). The review focuses exclusively on clinical grade devices approved by the FDA as an actigraph or equivalent device that uses an accelerometer to measure limb activity associated with movement during sleep for physiologic applications. The review does not cover consumer wearable devices,3 or other non-prescription devices directly marketed to consumers.
BACKGROUND
Actigraphy is a procedure that records and integrates the occurrence and degree of limb movement activity over time. Actigraphic devices can be worn on the wrist, ankle or waist, relatively unobtrusively over a period of days to weeks. For sleep applications, the devices are typically worn on the wrist or ankle. Mathematical algorithms are then applied to these data to estimate wakefulness and sleep. In addition to providing a graphical summary of wakefulness and sleep patterns over time (ie, temporal raster plots), actigraphy generates estimates of certain sleep parameters that are also commonly estimated by using sleep logs, or measured directly by polysomnography (PSG), the gold standard measure of sleep. The sleep parameters estimated by actigraphy, in common with standard sleep logs, include: sleep latency (SL); total sleep time (TST); wake after sleep onset (WASO); and sleep efficiency (SE; SE = TST / time in bed). Unlike PSG, actigraphy does not provide estimates of sleep architecture, as information related to the staging of non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep is generally not available, and requires electroencephalogram (EEG), electrooculography (EOG), and electromyography (EMG). Similarly, actigraphy does not provide information related to respiratory function.
Actigraphy devices available for clinical use generally include a piezoelectric or a microelectromechanical systems accelerometer. The devices have storage to enable transfer of the resulting values into an interface (usually via USB or serial port) and to program the timing mechanism. Many devices also have at least one event button that can be used by the wearer to document select events (eg, drowsiness, bed time). Some actigraphy devices also have light sensors for detecting white light or specific wavelengths of light.
Several factors have been identified as important for the reliable and valid use of actigraphy to measure certain sleep parameters.4 These include: (1) technical features of the device (eg, tri-axial versus dual or single axis accelerometers); (2) software driven data acquisition settings (eg, sampling rates and sensitivity settings); (3) location of device placement5; (4) the mathematical algorithms used to estimate sleep/wake6; (5) clinical features of the population being studied, (6) utilization of a standardized scoring approach to setting rest activity intervals; and (7) training of patients in data collection procedures.7 Standardized information on the technical aspects of actigraphy as well as analysis and interpretation procedures for clinical and research use have recently been published.8 It is important to note that the basic technology in products sold “direct to consumers” may differ significantly from what is available for clinical application. At the present time, data are not adequate to suggest that consumer products can be used as a replacement for clinical devices using validated sleep scoring algorithms, technologies, and procedures.
In clinical practice, patients or caregivers are sometimes asked to estimate and record certain sleep parameters and related information manually through daily sleep logs. Sleep logs provide critically important clinical information about the patient's subjective experience. However, when used as a sole assessment tool, sleep logs have some inherent and significant limitations, including: (1) they are subject to bias; (2) sometimes they cannot be completed accurately by patients with cognitive limitations or by infants and children; and (3) they may not be completed because they are cumbersome for many patients and caregivers. In contrast, actigraphy is a relatively passive, objective procedure that involves the use of a nonobtrusive monitor with a low device failure rate. Actigraphy is relatively inexpensive, patient adherence is typically good, and it can provide useful diagnostic information and data regarding treatment response. Actigraphy scoring software typically provides graphical detail about certain sleep parameters and patterns that can be communicated to patients and referring providers in simple, understandable terms.
The role of actigraphy may vary based on the specific sleep disorder and sleep assessment procedure. With respect to insomnia disorder, for example, actigraphy may be more useful as an adjunct to sleep logs (the reference standard for insomnia) or as a standalone procedure in special instances where reliable self-report is not feasible, such as young children ranging to identify sleep disruption in psychiatric, neurodevelopmental, medical, and sleep disorders. The sleep patterns of patients with insomnia are characterized by high night-to-night variability.9 Concurrent actigraphy and sleep log collection provides information about that variability as well as the degree and pattern of discrepancy between the 2 types of assessment (ie, objective versus subjective).10,11 Such information is useful for both diagnosis and treatment planning, for example, with respect to identifying and treating paradoxical insomnia.
In patients with suspected or diagnosed CRSWD, characterizing sleep across multiple 24-hour periods is essential for both adult and pediatric populations. Actigraphy-generated temporal raster plots can be extremely useful in visually depicting changing periodicities associated with circadian dysrhythmia, which can facilitate accurate diagnosis. This is true for multiple, specific CRSWDs, and also for differential diagnosis when the type of CRSWD is not clear based on clinical history alone. This is particularly critical as the treatment itself must be tailored to the precise CRSWD. For example, the timing of light exposure or melatonin administration is dependent upon precise estimates of intrinsic circadian phase. Actigraphy may also be a viable method for documenting disturbed sleep/ wake patterns in individuals with shift work sleep disorder. The ability of actigraphy software to show time-based relations and easily identify shifting trends in bedtimes and wake times make it an especially useful tool for the assessment of multiple CRSWDs.
Actigraphy may also play a role when administration of a home sleep apnea test (HSAT) is appropriate in adult populations.12 For gold standard sleep apnea assessment, PSG is used to measure the apnea-hypopnea index (AHI) as determined by the number of respiratory events × 60 divided by the TST in minutes. HSAT refers to a study performed to diagnose sleep-related breathing disorders such as obstructive sleep apnea (OSA), generally without direct determination of sleep versus wake or of sleep stages. The use of the respiratory event index (REI) was introduced to be used for HSATs that do not record sleep by EEG, EOG and EMG. The REI describes the total number of respiratory events scored × 60 divided by monitoring time. HSAT devices that do not have any mechanism for removing the wake time from the denominator in the calculation use total recording time (TRT) in determining the REI. Devices that use TRT in the index calculation are likely to underestimate the severity of the sleep-disordered breathing (SDB) and may result in increased false negatives. HSAT devices that use built-in actigraphy with the ability to eliminate wake and artifact time in estimating sleep time, therefore, may improve the diagnostic accuracy of the REI.
Actigraphy may be especially useful in documenting insufficient sleep both for the purpose of improving the interpretation of the Multiple Sleep Latency Test (MSLT) in adult and pediatric patients with suspected central disorders of hypersomnolence and for assessing insufficient sleep syndrome (ISS). Objective measurement may be especially important in facilitating treatment of the sometimes complex medical and occupational risks associated with ISS.
Some studies have sought to evaluate whether actigraphy worn on the ankles might provide a reasonable estimate of periodic limb movements in adult and pediatric patients, although it is increasingly clear that additional measures of arousal may be important in evaluating the clinical significance of periodic limb movement during sleep.
METHODS
Expert Task Force
The AASM commissioned a task force (TF) of sleep medicine clinicians with expertise in the use of actigraphy in patients with suspected sleep disorders to develop this systematic review. The TF was required to disclose all potential conflicts of interest (COI) according to the AASM's COI policy prior to being appointed to the TF, and throughout the development of this document. In accordance with the AASM's conflicts of interest policy, TF members with a Level 1 conflict were not allowed to participate. TF members with a Level 2 conflict were required to recuse themselves from any related discussion or writing responsibilities. All relevant conflicts of interest are listed in the Disclosures section.
PICO Questions
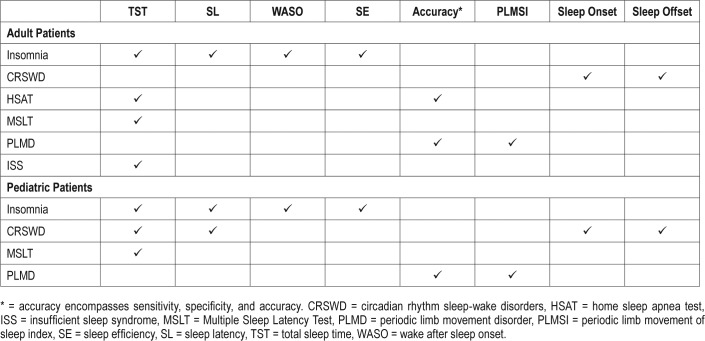
PICO (Patient, Intervention, Comparison, and Outcomes) questions were developed by the TF after a review of the existing AASM practice parameters on the use of actigraphy,2 and a review of relevant systematic reviews, meta-analyses, and guidelines published since June 2005. To develop the PICO questions, the TF identified sleep disorders for which actigraphy may provide clinically useful information (summarized in Table 1), and the clinically relevant outcomes that actigraphy provides for each sleep disorder (summarized in Table 2). The AASM Board of Directors approved the final list of questions before the literature searches were performed.
Table 1.
PICO questions.
Table 2.
“Critical” outcomes by patient population.
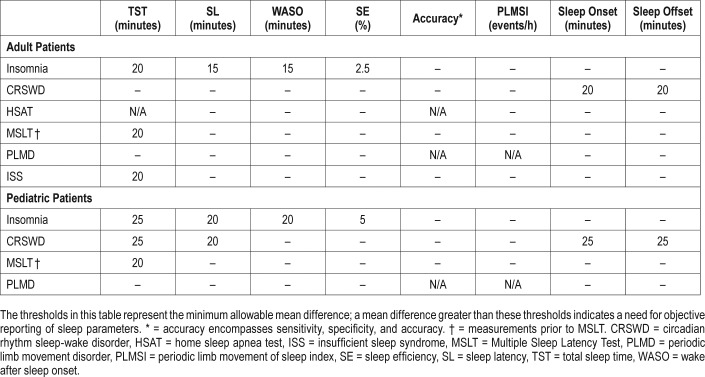
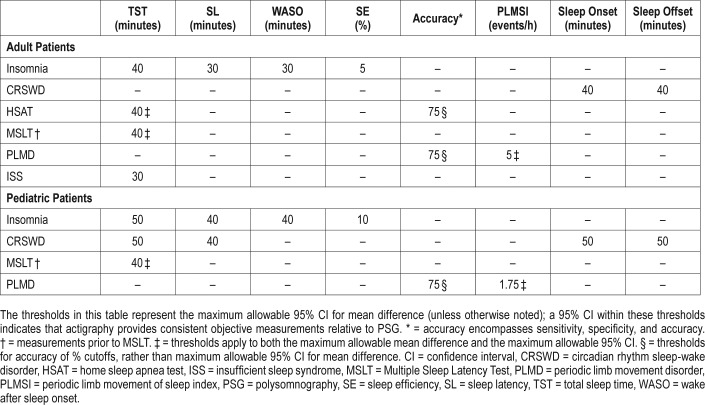
The TF compared actigraphy to both sleep logs and PSG to determine whether actigraphy provides information that is consistent with PSG and also distinct from patient-reported data. The TF set two different sets of clinical significance thresholds (CST) for each outcome and PICO to determine if the data provided by actigraphy was clinically significant. The first CSTs were set for comparisons of actigraphy to sleep logs and was defined as the minimum allowable mean difference between the measurements. When comparing actigraphy to sleep logs, a mean difference greater than these thresholds indicates a clinically meaningful difference and a need for objective reporting of sleep parameters. A summary of these CSTs is presented in Table 3; a graphical representation of these thresholds is presented in Figure 1. The second CSTs were set for comparisons of actigraphy to PSG and were defined as the maximum allowable 95% confidence interval (CI) for the mean difference (unless otherwise noted in Table 4). When comparing actigraphy to PSG, a 95% CI within these thresholds indicates that actigraphy provides a sufficiently narrow range of possible mean differences relative to PSG, and therefore provides consistent objective measurements for reporting of sleep parameters. A summary of these CSTs is presented in Table 4; a graphical representation of these thresholds is presented in Figure 2. The CSTs were established prior to analysis based on the clinical judgement and experience of the TF and informed by the literature. Larger CSTs were established for pediatric populations due to increased measurement error associated with caregiver report, and both PSG and self-report sleep diary alternatives pose additional challenges for some pediatric populations, such as those with developmental disabilities, which likely increase measurement error. In addition, there is more variability across pediatric patients based on age and other factors. The TF endeavored to balance the need for accuracy, care giver burden, and the differential sleep needs of pediatric groups relative to adults.
Table 3.
Clinical significance thresholds for the minimum allowable mean difference between actigraphy versus sleep log or caregiver report.
Figure 1. Hypothetical mean difference of actigraphy versus sleep log measurements (clinically significant).
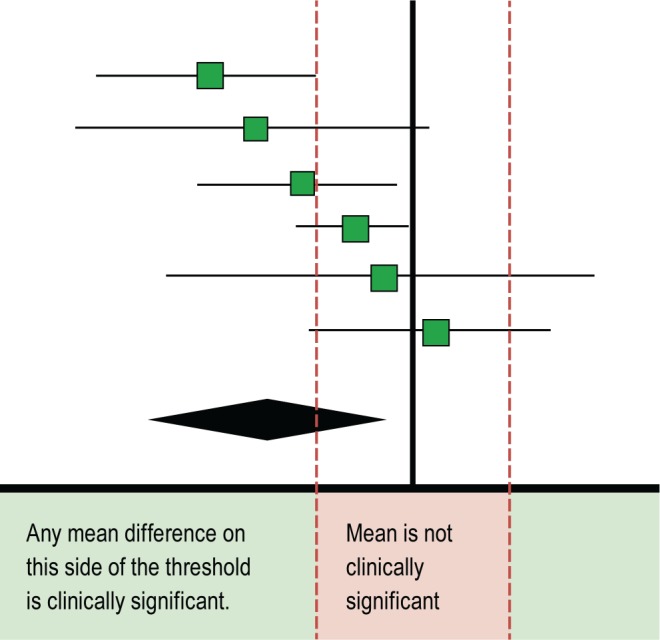
Table 4.
Clinical Significance Thresholds for the maximum allowable 95% CI of the mean difference between actigraphy versus PSG.
Figure 2. Hypothetical range of mean differences of actigraphy versus PSG measurements (clinically significant).
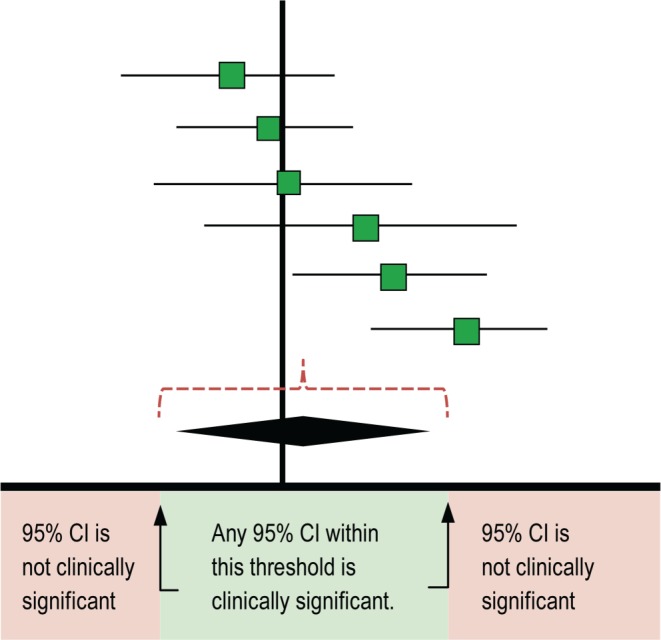
CI = confidence interval, PSG = polysomnography.
Literature Searches, Evidence Review and Data Extraction
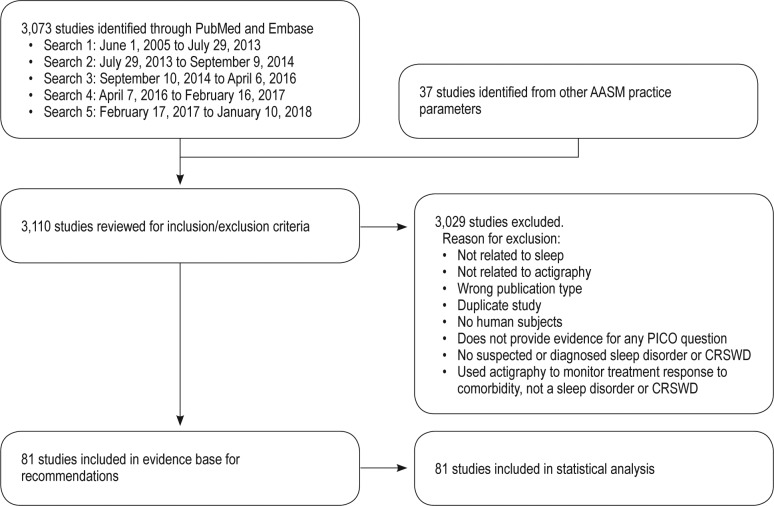
Literature searches were performed using the PubMed and Embase databases for individual questions. A combination of MeSH terms and keywords listed in the supplemental material were used. The databases were searched from June 1, 2005 through January 10, 2018 for any relevant literature published since the 2007 guideline literature search was performed. The articles that were cited in the 2007 AASM practice parameters were included if they met the study inclusion criteria. In addition, the task force reviewed all AASM guidelines published since 2006, to identify additional references that may be relevant to actigraphy. The limits of the searches (requiring all listed criteria to be met) were: humans, English, all adults (with the exception of questions 4, 5, and 7), and randomized controlled trial (RCT) or observational studies. A total of 3,073 citations were identified from both databases, and 37 studies were identified in the other AASM practice parameters.
Articles were included for review and possible data extraction if they focused on patient assessment or monitoring of treatment response for a sleep disorder with actigraphy, sleep logs and/or PSG; addressed at least one of the PICO questions; and included one of the outcomes of interest. Articles were excluded if they focused on actigraphy not related to sleep; were not RCTs or observational studies; were duplicates; involved non-human subjects; involved subjects without a suspected or diagnosed sleep or circadian rhythm sleep-wake disorder; used actigraphy to monitor treatment response of a comorbid condition; or used actigraphy as a measurement tool, but did not provide evidence for any PICO questions. Studies were also excluded if they did not present data for any of the critical outcomes and/or did not present data in a format suitable for statistical analysis. A total of 81 articles from the literature searches were accepted and considered for meta-analysis and evidence grading. Specific data elements of all accepted studies were extracted into evidence tables (not published) to address each clinical question. Upon review of these articles, 81 studies were determined to be suitable for meta-analysis and/or the Grading of Recommendations Assessment, Development and Evaluation (GRADE) process. An evidence base flow diagram is presented in Figure 3.
Figure 3. Evidence base flow diagram.
AASM = American Academy of Sleep Medicine, CRSWD = circadian rhythm sleep-wake disorder, PICO = Patient, Intervention, Comparison, and Outcomes.
Meta-Analysis and Interpretation of Clinical Significance
Meta-analyses were performed on outcomes of interest for each PICO question. Review Manager 5.3 software (The Cochrane Collaboration, London, United Kingdom) was used to compare the use of actigraphy versus sleep logs and actigraphy versus PSG for the assessment of sleep parameters and of treatment response in patients with various sleep disorders. All analyses were performed using the random effects model with results displayed as a forest plot. Meta-analyses were performed when at least 5 studies were available by pooling data across studies for each relevant outcome of interest for each PICO (studies for PICO 7 were grouped by patient population). When 3–4 studies were available, meta-analyses were performed at the discretion of the task force. For several questions, there was insufficient evidence to perform meta-analyses for certain comparisons and outcome measures. In these cases, studies are described individually.
For the assessment of sleep parameter estimates, the mean differences in baseline sleep parameter measurements from actigraphy, sleep logs and PSG were determined by pooling both intervention and non-intervention studies. (For simplicity, the term “baseline” is used in the text to describe all data extracted for the pre-intervention phase of interventional studies and the initial assessment time point for cross sectional studies.) For the assessment of treatment response, given the limited number of treatment outcome studies identified, the heterogeneity of intervention types and assessment time points, the task force was not able to evaluate whether actigraphy was sensitive to change relative to sleep logs or PSG. Instead, the TF analyzed the mean difference of posttreatment measurements from actigraphy, sleep logs and PSG. The pooled results for each continuous outcome measure are expressed as the mean difference between the intervention and comparator. The results of the meta-analyses are presented in the supplemental material.
Interpretation of clinical significance for the outcomes of interest was conducted in two different ways. First, by comparing the mean difference in measurements of actigraphy and sleep logs against their CSTs (Table 3). Next, by comparing the 95% CI of the mean difference of actigraphy versus PSG measurements to their CSTs (Table 4). For comparisons of actigraphy to sleep logs, the CST was defined as the minimum allowable mean difference between the measurements; a mean difference greater than the threshold demonstrates that actigraphy provides unique information from sleep logs, and objective measurements are warranted (see Figure 1, which shows an example of a clinically significant mean difference). For comparisons of actigraphy to PSG, the CST was defined as the maximum allowable 95% CI for the mean difference between actigraphy and PSG (unless otherwise noted in Table 4); a 95% CI within the threshold demonstrates that actigraphy provides a sufficiently narrow range of possible mean differences relative to PSG (regardless of the mean difference, unless otherwise noted in Table 4). A sufficiently narrow range of mean differences indicates that actigraphy provides consistent objective measurements relative to PSG, and may be useful as an objective measurement of sleep parameters (see Figure 2, which shows an example of a sufficiently narrow range of mean differences).
Detailed reviews of the evidence and clinical significance of the findings for all critical outcomes are provided for each PICO question.
GRADE Assessment for Developing Recommendations
The evidence was assessed according to the GRADE process for the purposes of making clinical practice recommendations.13,14 The TF considered the following four GRADE domains: quality of evidence, balance of beneficial and harmful effects, patient values and preferences, and resource use, as described below:
Quality of evidence: based on an assessment of the overall risk of bias (randomization, blinding, allocation concealment, selective reporting), imprecision (95% CI relative to the CST, sample size < 200), inconsistency and indirectness (study population), and risk of publication bias (funding sources), the TF determined their overall confidence that the estimated differences in measurements found in the body of evidence were representative of the true differences in measurements that patients would experience. The overall quality of the evidence was based on all outcomes that the TF deemed critical for decision making.
Benefits versus harms: based on any harms/side effects reported within the accepted literature, and the clinical expertise of the TF, the TF determined if the beneficial outcomes of using actigraphy outweighed any harms. Benefits versus harms compared to alternative measurement tools was also considered.
Patient values and preferences: based on the clinical expertise of the TF members and any data published on the topic relevant to patient preferences for actigraphy, the TF determined if patient values and preferences would be consistent across the majority of patients, and if patients would use actigraphy based on the body of evidence.
Resource use: based on the clinical expertise of the TF members, the TF determined if accessibility and costs associated with actigraphy compared favorably to alternative measurement tools. Information on both costs to patients and to the health care system were considered.
A summary of each GRADE domain is provided after the detailed evidence review for each PICO question.
Public Comment and Final Approval
Drafts of the systematic review and accompanying guideline were made available for public comment for a two-week period on the AASM website. AASM members, the general public and other relevant stakeholders were invited to provide feedback on the drafts. The TF took into consideration all the comments received and made decisions about whether to revise the draft based on the scope and feasibility of comments. The public comments and revised documents were submitted to the AASM Board of Directors who subsequently approved the final documents for publication.
The AASM expects this systematic review to have an impact on professional behavior, patient outcomes, and, possibly, health care costs. This review reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
THE USE OF ACTIGR APHY
The aims of the current systematic reviews and data analyses were to address 7 PICO questions pertaining to the use of actigraphy relative to sleep logs and/or PSG across a wide range of clinical populations, and in conjunction with HSAT and MSLT. While sufficient data were available for meta-analyses for most PICO questions, there are caveats that should be considered with respect to interpreting the results. With regard to sleep parameters, the TF noted variability across studies with respect to definitions and technical details such as algorithms and sensitivity threshold settings used or reported. As is common practice, many studies utilized information noted by the patient in a sleep log for the analysis and interpretation of actigraphy-estimated sleep parameters. This is important particularly with respect to determining bedtime (“lights off”) to calculate SL. Other studies relied completely on actigraphy algorithms to estimate SL, while some studies failed to report these details. The TF decided not to analyze the number of nightly awakenings as a sleep parameter of interest, since actigraphy typically identifies numerous isolated brief awakenings lasting less than a minute (eg, 30 seconds), which are common even in normal sleep and often not perceived, remembered or retrospectively reported by patients. Diary measures of awakenings likely reflect a distinct construct related to consolidated frank awakenings, which are not consistently defined or reported in standard software to date, making comparison across devices and sleep log estimates of questionable utility. The TF also cautions that generalizability of some of the meta-analytic findings may be limited due to a small number of studies meeting the inclusion/ exclusion criteria and/or patients across studies. Generalizability to the broad spectrum of sleep disorder patients seen in clinical settings may also be limited by heterogeneity across sleep disorder severity and subpopulations with clinical comorbidities, both of which may influence validity.
Below are detailed summaries of the evidence identified in the literature searches and the statistical analyses performed by the task force. Each evidence summary is accompanied by a discussion of the quality of evidence, balance of benefits and harms, patient values and preferences, and resource use considerations that contributed to the development of the recommendations, which are provided in the accompanying clinical practice guideline.1
Use of Actigraphy in the Evaluation of Insomnia in Adults
Our review of the literature identified 46 studies11,15–59 that used actigraphy concurrent with sleep logs and/or PSG in adults with suspected or diagnosed insomnia. Both non-intervention and intervention studies met the eligibility criteria and were included. The number of studies included in the analyses varied by sleep parameter and whether the comparison was to sleep logs or PSG. Overall, more studies were identified that provided comparisons of actigraphy to sleep logs than to PSG.
The data for examining the use of actigraphy for assessment were either based on a single night or drawn from the baseline periods of intervention trials with insomnia and represent sleep parameter values averaged over 1 to 2 weeks. Similarly, data for analyses examining the use of actigraphy to assess treatment response were either based on a single night or were drawn from sleep parameter values averaged over 1 to 2 weeks following treatment. The vast majority of the intervention studies reviewed involved 1 or more components of cognitive-behavioral treatment for insomnia. Due to the number of studies identified, they are not individually described here. A summary of the study characteristics can be found in the supplemental material.
The meta-analyses and figures are provided in the supplemental material, Figure S1a through Figure S8b. Summary of findings tables are provided in the supplemental material, Table S1a through S2b. A summary of the evidence for each outcome is provided below.
Total Sleep Time
A meta-analysis of 40 studies11,15–50,56,57,59 compared actigraphy to sleep logs for the assessment of TST (Figure S1a). The meta-analysis showed a clinically significant mean difference of 37.40 minutes higher (95% CI: 22.14 to 52.67 minutes higher) TST as assessed by actigraphy compared to sleep logs. This difference indicates actigraphy and sleep logs provide distinct information when assessing TST. The quality of evidence was moderate due to imprecision.
A meta-analysis of 15 studies15,16,18,20–22,24,27,33,39–41,43,46,56 compared actigraphy to PSG for the assessment of TST in patients with suspected or diagnosed insomnia. See supplemental material, Figure S1b. The meta-analysis showed a clinically significant range of possible mean differences of 35.12 minutes (95% CI: 8.07 minutes lower to 27.05 minutes higher) with an overall mean difference of 10.14 minutes. This range is narrow enough that actigraphy can be reliably used to provide an objective assessment of TST for the purpose of making clinical care decisions. The quality of evidence was high.
A meta-analysis of 3011,25–50,57–59 studies compared actigraphy to sleep logs for the assessment of treatment response in TST (Figure S5a). The meta-analysis demonstrated a clinically insignificant mean difference in TST measured by actigraphy of 8.10 minutes higher (95% CI: 9.23 minutes lower to 25.42 minutes higher) as compared to logs. This small difference indicates actigraphy and sleep logs provide similar measurements of treatment-related changes in TST. The quality of evidence was moderate due to imprecision.
A meta-analysis of 7 studies27,33,39–41,43,46 compared actigraphy to PSG for the assessment of treatment response in TST (Figure S5b). The meta-analysis demonstrated a clinically insignificant range of possible mean differences of 83.4 minutes (95% CI: 37.1 minutes lower to 46.3 minutes higher) with an overall mean difference of 4.6 minutes. This large range indicates actigraphy and PSG provide distinct information and should not be used interchangeably for the assessment of treatment-related changes in TST. The quality of evidence was moderate due to imprecision.
Sleep Latency
A meta-analysis of 36 studies11,15–22,24,26,28–32,34–42,44–50,53,55,56,59 compared actigraphy to sleep logs for the assessment of SL (Figure S2a). The meta-analysis showed a clinically significant mean difference in SL measured by actigraphy of 23.99 minutes lower (95% CI: 27.29 to 20.69 minutes lower) as compared to sleep logs. This difference indicates actigraphy and sleep logs provide distinct information when assessing SL. The quality of evidence was high.
A meta-analysis of 12 studies15,16,18,20–22,24,39–41,46,56 compared actigraphy to PSG for the assessment of SL (Figure S2b). The meta-analysis showed a clinically significant range of possible mean differences of 6.78 minutes (95% CI: 2.29 to 9.07 minutes lower) with a mean difference of 6.17 minutes. This range is narrow enough that actigraphy can be reliably used to provide an objective assessment of SL for the purpose of making clinical care decisions. The quality of evidence was high.
A meta-analysis of 27 studies11,26,28–32,34–42,44–50,53,55,58,59 compared actigraphy to sleep logs for the assessment of treatment response in SL (Figure S6a). The meta-analysis demonstrated a clinically insignificant mean difference in SL measured by actigraphy of 10.55 minutes lower (95% CI: 8.20 to 12.90 minutes lower) as compared to sleep logs. This small difference indicates actigraphy and sleep logs provide similar measurements of treatment-related changes in SL. The quality of evidence was high.
Four studies39–41,46 compared actigraphy to PSG for the assessment of treatment response in SL (Figure S6b). All studies reported a clinically significant range of possible mean differences, with the largest range of differences being 29.8 minutes (95% CI: 12.1 minutes lower to 17.7 minutes higher). This small range indicates actigraphy and PSG provide similar information for the assessment of treatment-related changes in SL. The quality of evidence was moderate due to imprecision due to small sample size.
Wake After Sleep Onset
A meta-analysis of 34 studies11,15–23,25,27,28,30–32,34–43,46–50,53,55,59 compared actigraphy to sleep logs for the assessment of WASO (Figure S3a). The meta-analysis showed a clinically insignificant mean difference in WASO measured by actigraphy of 5.65 minutes lower (95% CI: 14.81 minutes lower to 3.51 minutes higher) as compared to sleep logs. This difference indicates actigraphy and sleep logs do not provide distinct information when assessing WASO. The quality of evidence was high.
A meta-analysis of 12 studies15,16,18,20–22,27,39–41,43,46 compared actigraphy to PSG for the assessment of WASO (Figure S3b). The meta-analysis showed a clinically insignificant range of possible mean differences of 33.22 minutes (95% CI: 13.68 minutes lower to 19.54 minutes higher), with a mean difference of 1.5 minutes. This large range indicates actigraphy cannot be reliably used to provide an objective assessment of WASO that is comparable with PSG. The quality of evidence was downgraded to moderate due to imprecision.
A meta-analysis of 26 studies11,25,27,28,30–32,34–43,46–50,53,55,58,59 compared actigraphy to sleep logs for the assessment of treatment response in WASO (Figure S7a). The meta-analysis demonstrated a clinically insignificant mean difference in WASO measured by actigraphy of 11.47 minutes higher (95% CI: 0.58 minutes lower to 23.51 minutes higher) as compared to sleep logs. This small difference indicates actigraphy and sleep logs provide similar measurements of treatment-related changes in WASO. The quality of evidence was moderate due to imprecision.
A meta-analysis of 6 studies27,39–41,43,46 compared actigraphy to PSG for the assessment of treatment response in WASO (Figure S7b). The meta-analysis demonstrated a clinically insignificant range of possible mean difference in WASO measured by actigraphy as compared to PSG of 86.0 minutes (95% CI: 53.2 minutes lower to 32.8 minutes higher) with a mean difference of 10.2 minutes. This large range indicates actigraphy and PSG provide distinct information and cannot be used interchangeably for the assessment of treatment-related changes in WASO. The quality of evidence was moderate due to imprecision.
Sleep Efficiency
A meta-analysis of 34 studies11,15,16,18–20,23,25,28–43,46–51,53,55,57,59 compared actigraphy to sleep logs for the assessment of SE (Figure S4a). The meta-analysis showed a clinically significant mean difference in SE measured by actigraphy of 7.5% higher (95% CI: 5.1% to 10.0% higher) as compared to sleep logs. This difference indicates actigraphy and sleep logs provide distinct information when assessing SE. The quality of evidence was high.
A meta-analysis of 9 studies15,16,18,20,33,39–41,46 compared actigraphy to PSG for the assessment of SE (Figure S4b). The meta-analysis showed a clinically insignificant range of possible mean differences of 7.8% (95% CI: 4.9% lower to 3.0% higher), with a mean difference of 1%. This large range indicates actigraphy cannot be reliably used to provide an objective assessment of SE that is comparable with PSG. The quality of evidence was moderate due to imprecision.
A meta-analysis of 30 studies11,25,28–43,46–51,53–55,57,59 compared actigraphy to sleep logs for the assessment of treatment response in SE (Figure S8a). The meta-analysis demonstrated a clinically insignificant mean difference in SE measured by actigraphy of 2.1% higher (95% CI: 0.6% lower to 4.8% higher) as compared to sleep logs. This small difference indicates actigraphy and sleep logs provide similar measurements of treatment-related changes in SE. The quality of evidence was moderate due to imprecision.
A meta-analysis of 5 studies33,39–41,46 compared actigraphy to PSG for the assessment of treatment response in SE (Figure S8b). The meta-analysis demonstrated a clinically insignificant range of possible mean difference in SE measured by actigraphy as compared to PSG of 7.9% (95% CI: 0.2% to 8.1%), with a mean difference of 4.2%. This large range indicates actigraphy and PSG provide distinct information and cannot be used interchangeably for the assessment of treatment-related changes in SE. The quality of evidence was moderate due to imprecision.
Overall Quality of Evidence
The quality of evidence for actigraphy for both assessment and the evaluation of treatment response for critical clinical outcomes for insomnia was moderate to high depending on the outcome. The reason for downgrading the quality of evidence for some comparisons or outcomes was imprecision. Thus, the overall quality of evidence was moderate.
Benefits Versus Harms
Actigraphy may be useful to assess TST and SL in patients with suspected and diagnosed insomnia disorder and provides a consistent measure of SL, compared to PSG. Benefits include convenience and relatively low patient burden. Another convenience relative to PSG is that actigraphy requires considerably less time to prepare the patient and the patient can remove the actigraphy device as easily as taking off a watch. The ability of actigraphy to provide relatively low burden, and longitudinal assessment of sleep patterns and response to treatment is another benefit. Actigraphy-derived short sleep in patients with insomnia is associated with negative health outcomes (eg, cardiometabolic risk, hypertension, depression).60–64 Thus, actigraphy may provide additional benefits for certain patient subgroups, including those with suspected paradoxical insomnia or at risk for cardiometabolic and other medical and psychiatric comorbidities impacted by short sleep duration. These benefits must be weighed against the potential for harm. The TF determined that there were no clinically significant and undesirable outcomes associated with actigraphy. Therefore, the TF determined that if actigraphy is used in the context described in the recommendation and remarks, the risk of harm is minimized and the probability of clinical benefits increased.
Patient Values and Preferences
Complaints of not getting enough sleep and difficulties falling and/or staying asleep are all primary reasons prompting seeking of medical care. Although SL, WASO, and SE are often the targets of treatment, TST is also a relevant outcome for some patients. One study65 showed patients with objective short TST (< 6 h/night) based on two weeks of actigraphy prior to treatment did not respond as well to CBT-I as did patients with normal TST (≥ 6 hours). Specifically, patients with short TST on objective evaluation reported significantly less improvement in terms of insomnia remission, SE, WASO, and total wake time compared to patients with normal sleep duration at six months after treatment. Thus, TST, SL, WASO, and SE are all sleep parameters that patients value. Patients may prefer actigraphy to completing daily sleep logs and/or undergoing overnight PSG, given the lower burden. Sleep logs require daily completion over multiple days. In contrast, PSG requires either an overnight stay in the sleep center or a home-based study. Although some individuals with insomnia often sleep better away from their home environment where conditioning often reinforces and perpetuates their insomnia, patients nonetheless may express concern and anxiety regarding their ability to sleep in the lab. For both sleep center and home-based studies, patients can experience burden and anxiety related to both the process of being prepared for the study and their ability to sleep while wearing testing-related equipment. PSG is not recommended for the routine assessment of insomnia, but may be indicated when other sleep disorders are suspected. The TF noted that the use of actigraphy (as reported in the studies evaluated) did not completely eliminate the need for patients to provide some daily self-report information, given that reported in and out of bed times were frequently used to set the sleep window used to score data from the actigraphy device. Some patients may prefer the combined approach of completing sleep logs and actigraphy. Some patients may object to actigraphy, because the wrist band has the potential to aggravate sensitive skin. Addressing the potential dermatological issues (different band, lining the band) may reduce or eliminate skin-related concerns for some patients. The TF determined that actigraphy provides outcomes that patients value with minimal undesired effects.
Resource Use
Actigraphy is more costly than sleep logs in terms of the technical and professional components of the service. However, these costs are relatively low and compare favorably to the technical and professional costs associated with PSG. Economic analyses comparing the cost-effectiveness of these devices for the assessment of insomnia or the evaluation of treatment response have not been conducted. The TF concluded actigraphy may be more cost effective if an objective measurement of sleep is needed.
Use of Actigraphy in the Evaluation of Insomnia in Pediatric Populations
Our review of the literature identified a total of 5 studies meeting inclusion criteria. Four studies66–69 reported mean differences between actigraphy and sleep logs for TST (3 studies),66,67,69 SL (3 studies),66,67,69 and WASO (3 studies).67–69 Data also included the review of one study70 of non-specific sleep disorders (including participants with insomnia) in children with autism. We also identified 4 intervention studies67–69,71 for meta-analysis that reported posttreatment actigraphy and sleep log estimates of TST (4 studies),67–69,71 SL (3 studies)67,69,71 and WASO (4 studies).67–69,71 We also reviewed post intervention data from the study of non-specific sleep disorders in children with autism, reported posttreatment data on TST and SL.70
Regarding studies reporting baseline data on TST and SL, one was a case-control study comparing young children (mean age = 6.6 ± 1.1 years) with insomnia to healthy controls, and healthy snorers.66 The other study reported data on TST, SL and WASO and was an RCT of group cognitive behavior therapy for insomnia in adolescents.67 A single arm pilot study of CBT-I in adolescents also reported baseline data for WASO only (ages 11–18).68 A second pilot study of modified CBT-I in adolescents with insomnia and depression reported data on TST, SL, and WASO.69 The study of non-specific sleep disorders provided baseline data on TST and SL and was an RCT testing the effects of a weighted blanket in children with autism whose parents reported sleep problems (mean age = 9, range 5–16 years).70
The studies reporting posttreatment data included an RCT of CBT-I with behavioral treatment for anxiety in children (mean age = 9.3 ± 1.9)71 two studies67,68 of CBT-I in adolescents, and a pilot study of modified CBT-I in adolescents with insomnia and depression69. Posttreatment data was also reviewed for the RCT testing the effects of a weighted blanket in children and adolescents with autism (mean age = 9, range 5–16 years).70 The meta-analyses and figures are provided in the supplemental material, Figure S9 through Figure S14. Summary of findings tables are provided in the supplemental material, Table S3 and Table S4. A summary of the evidence for each outcome is provided below.
Total Sleep Time
For baseline TST, all three studies66,69,70 met our clinical significance threshold of 25 minutes, indicating that actigraphy and sleep logs provide distinct information when assessing TST. Actigraphy estimated lower TST compared to sleep logs by a large mean difference of 119.8 minutes (95% CI: 114.4 to 25.2 minutes lower) in one study66, 27.0 minutes (95% CI: 4.1 to 49.9 minutes lower) in the second67, and 32.0 minutes (95% CI: 78.79 minutes lower to 14.79 minutes higher) in the third69. One additional study of children with autism also met clinical threshold, demonstrating that actigraphy estimated lower TST compared to sleep logs by a large mean difference of 79.0 minutes (95% CI: 49.2 to 108.9 minutes lower)70 (Figure S9 and Figure S28). The quality of evidence was moderate due to imprecision.
Assessment of treatment response with meta-analysis of four studies67–69,71 (n = 149) demonstrated that actigraphy TST did not meet the clinical significance threshold of 25 minutes. Actigraphy estimated lower TST compared to sleep logs by a mean difference of 19.14 minutes (95% CI: 46.41 minutes lower to 8.13 minutes higher). One additional study of children with autism70 did meet the clinical threshold, finding that actigraphy estimated lower TST compared to sleep logs by a large mean difference of 74.5 minutes (95% CI: 40.5 to 108.50 minutes lower) (Figure S12 and Figure S29). These studies indicate the actigraphy and sleep logs provide similar measurements. The quality of evidence was moderate due to the small sample size and imprecision.
Sleep Latency
For baseline SL, none of the three insomnia studies66,67,69 demonstrated that actigraphy-based estimates of SL met the clinical significance threshold of 20 minutes, suggesting they provide similar estimates. One study67 demonstrated a mean difference in SL of 10.0 minutes lower (95% CI: 0.04 to 20.0 minutes lower) compared to sleep logs, the second66 demonstrated a mean difference in SL of 2.9 minutes higher (95% CI: 1.4 to 4.4 minutes higher) compared to sleep logs, and the third69 demonstrated a mean difference of 4.0 minutes lower (95% CI: 23.7 minutes lower to 15.7 minutes higher). Additionally, the study of children with autism70 also failed to reach clinical significance, demonstrating a small mean difference of 6.60 minutes higher (95% CI: 9.7 minutes lower to 22.9 minutes higher) compared to sleep logs (Figure S10 and Figure S28). The quality of evidence was moderate due to imprecision.
With respect to treatment response, meta-analysis of 3 studies67,69,71 demonstrated that actigraphy and sleep logs yielded similar estimates of posttreatment SL with a small mean difference in SL of 2.94 minutes higher (95% CI: 13.10 minutes lower to 7.21 minutes higher) compared to sleep logs. Additionally, the study of children with autism70 also failed to meet clinical significance with a small posttreatment mean difference of 18.70 minutes higher (95% CI: 3.3 to 34.1 minutes higher) (Figure S13 and Figure S30). The quality of evidence was moderate due to the small sample size.
Wake After Sleep Onset
The baseline studies assessing WASO,67–69 demonstrated that all three met the clinical significance threshold of 20 minutes, suggesting that actigraphy and sleep logs provide distinct information when assessing WASO. One study67 demonstrated that actigraphy estimated a large mean difference in WASO of 23.0 minutes higher (95% CI: 12.8 to 33.2 minutes higher) compared to sleep logs, the second68 demonstrated that actigraphy estimated a mean difference in WASO of 46.0 minutes higher (95% CI: 35.7 to 56.3 higher) compared to sleep logs, and the third69 demonstrated a mean difference of 39.0 minutes higher (95% CI: 21.82 to 56.18 minutes higher) (Figure S11). The quality of evidence was moderate due to the small sample size.
With respect to treatment response, meta-analysis of 4 studies,67–69,71 demonstrated a clinically significant mean difference in WASO of 45.72 minutes higher (95% CI: 18.46 to 72.94 minutes higher) with actigraphy compared to sleep logs, suggesting that actigraphy and sleep logs provide distinct information when assessing posttreatment WASO (Figure S14). The quality of evidence was moderate due to imprecision and small sample size.
Sleep Efficiency
None of the accepted studies provided data on SE.
Overall Quality of Evidence
The overall quality of evidence was moderate due to the small sample sizes and imprecision. Given the heterogeneous nature of pediatric populations in the included studies, which ranged in age from 3 to19, a span involving changing sleep needs, insomnia symptom presentations and potential distinct insomnia causes, the generalizability of the findings is significantly limited.
Benefits Versus Harms
Potential benefits of actigraphy include increased sensitivity over sleep logs in identifying short sleep and increased WASO, and the ability to obtain reliable sleep parameter estimates when many pediatric patients may be unable to reliably report sleep parameters or when caregiver burden and accuracy is an issue. Potential harms of actigraphy are mild and include skin irritation. When evaluating potential benefits versus harm, the task force considered the vulnerability of this population, the relatively high prevalence of insomnia in pediatric populations72–74 and findings that sleep disturbance can impact growth and development, psychological and cognitive functions and may be an indicator of medical and psychiatric disorder.72,75–79 Although studies with PSG data were not identified meeting our eligibility criteria, PSG validation studies have demonstrated acceptable validity of actigraphy in infants and children, particularly in healthy normal subjects.80–83 Based on their clinical expertise, the task force determined that the potential benefits of actigraphy outweighed potential harms.
Patient Values and Preferences
Although minimal data exists related to patient values and preferences on the use of actigraphy versus sleep logs for assessing insomnia in pediatric populations, the task force's experience is that the use of actigraphy is favored by the majority of patients and caregivers with no significant uncertainty or variability due to: 1) the relatively unobtrusive nature and minor burden of this comparatively passive monitoring procedure; 2) the fact that monitoring sleep patterns over multiple days as required to assess insomnia, imposes a major burden to caregivers of young children unable to accurately report sleep parameters; 3) the utility of objective data monitoring to complement patient self-report and 4) the increased accuracy that actigraphy data adds to inform clinical diagnosis, decision making, and monitoring treatment response. However, families and caregivers sometimes express concern about out of pocket expenses related to inconsistent third-party reimbursements.
Resource Use
The cost of actigraphy is higher than paper sleep log monitoring, but much less expensive than PSG and other home sleep testing devices with multiple sensor technologies. Moreover, PSG and HSAT devices are not well tolerated over multiple consecutive monitoring periods. Minimal data exist evaluating the cost benefit, but potential savings to medical health care systems and third-party payers and employers is potentially high. Actigraphy has the potential to improve the accurate detection of insomnia and treatment. Policy interventions related to data obtained from actigraphy could result in a decrease in downstream health care expenses. At the present time, cost benefits of the use of actigraphy to assess pediatric insomnia and treatment response require systematic study.
Use of Actigraphy in the Evaluation of Circadian Rhythm Sleep-Wake Disorders in Adults
Our review of the literature identified 2 studies84,85 meeting inclusion criteria. A cross-sectional study84 compared craniopharyngioma patients, who are at risk for damage to the sleep-wake and circadian rhythm systems, to matched healthy controls. The study included actigraphy and sleep log assessment of sleep onset and sleep offset, as well as melatonin secretion.84 Another study85 assessed sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis. This patient population often exhibits poor sleep/wake, which may be linked to altered circadian rhythms. The figures are provided in the supplemental material, Figure S15 through Figure S18. Summary of findings tables are provided in the supplemental material, Table S5 and Table S6. A summary of the evidence for each outcome is provided below.
Sleep Onset
One study84 measured sleep onset time in patients with suspected CRSWD due to craniopharyngioma or consequent surgery. In this study,84 the mean difference in sleep onset time was 0.3 hours later (95% CI: 0.8 hours earlier to 1.4 hours later) with sleep logs compared to actigraphy (Figure S15). A second study85 evaluated the effects of a circadian rhythm intervention (light therapy) on hospitalized patients with liver cirrhosis and found that the difference in measurement of a treatment effect for actigraphy compared to sleep logs was 0.60 hours later (95% CI: 0.1 to 1.1 hours later). These differences crossed the clinical significance thresholds established by the TF, indicating that actigraphy and sleep logs may provide distinct measurements in some patients (Figure S17). The quality of evidence for sleep onset was very low due to small sample size and imprecision.
Sleep Offset
The two studies described above84,85 also assessed sleep offset time. One study84 reported a mean difference between actigraphy and sleep logs of 0.2 hours later (95% CI: 1.0 hours earlier to 0.6 hours later) for sleep offset time (Figure S16). The other study85 found a mean difference in the measured treatment effect between actigraphy and sleep logs of 0.4 hours earlier (95% CI: 0.9 hours earlier to 0.1 hours later) with actigraphy compared to sleep logs (Figure S18). These differences crossed the clinical significance thresholds established by the TF, indicating that actigraphy and sleep logs may provide distinct measurements in some patients. The quality of evidence for sleep onset was very low due to small sample size and imprecision.
Overall Quality of Evidence
The overall quality of evidence was very low due to small sample sizes and imprecision. The two available studies used concurrent measurement; however, the sample sizes in these studies were small. In addition, there was imprecision, with the 95% CI crossing the clinical significance threshold for assessment of treatment response as determined by the TF.
Benefits Versus Harms
The main benefit of actigraphy is that it can be worn in the home setting longitudinally and requires little or no effort for tracking sleep onset and sleep offset times by patients. There are minimal harms associated with the use of actigraphy. In some patient populations (eg, frail older adults in long-term care) where skin health is an issue, the risk of irritation under the device may be higher; however, this risk appears very low (< 1%) in studies recording actigraphy for up to 1 week. Based on their clinical expertise, the task force determined that the benefit of accurate assessment with minimal burden outweigh the potential harms associated with actigraphy devices. It should be noted, however, that the information provided by actigraphy, eg, sleep onset and offset patterns and sleep continuity parameters, is inherently limited with respect to assessing the underlying chronobiological complexity associated with CRSWDs.
Patient Values and Preferences
Indirect evidence suggests actigraphy is acceptable to patients with CRSWDs as shown by high patient acceptance of actigraphy in reviewed studies. Patients with CRSWDs may find it difficult to complete sleep logs for extended periods of time, and actigraphy may be a less cumbersome alternative. Also, given the useful information on sleep parameters that can be obtained with actigraphy, most patients are likely to use actigraphy in place of sleep logs alone. Laboratory PSG may also prevent assessment of “natural” sleep onset or sleep offset times in patients with very late or very sleep onset or sleep offset times. As a result, actigraphy is likely to provide more useful information to clinicians about sleep onset and sleep offset, and is likely to be more acceptable to patients than in-center assessment of these parameters with PSG.
Resource Use
Actigraphy is more expensive than sleep logs, and therefore may be more resource intensive. However, in the absence of a widely available objective method for assessment of circadian rhythms in the home environment, actigraphy is currently the most widely available tool for this purpose. Actigraphy is not routinely paid for by insurers for evaluation of sleep patterns in patients with suspected CRSWDs, and as a result, the cost to patients may be higher. The cost to the health care system with actigraphy monitoring may also be higher than sleep logs alone; however, some of the higher costs of diagnosis may be offset by reduced costs associated with fewer delays in identifying appropriate interventions (eg, light therapy) and avoiding inappropriate ones (eg, hypnotic medications) for patients with CRSWDs.
Melatonin Levels and Profiles
In addition to the above outcomes, the use of actigraphy is supported by multiple studies conducted to evaluate actigraphy-based estimates of sleep that included biological markers of circadian phase such as dim light melatonin onset (DLMO) and melatonin secretion profiles in patients with suspected or confirmed CRSWDs. The physiologic markers of circadian phase are considered gold standards. Studies with actigraphy and melatonin assessments included patients with advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD); the results of these studies informed the recent AASM clinical practice guidelines for the treatment of CRSWDs.86 Studies show that actigraphy can reflect changes in endogenous melatonin in patients with DSWPD,87–89 and after circadian interventions for patients with DSWPD, ASWPD and shift work sleep/wake phase disorder.87,90–93
Use of Actigraphy in the Evaluation of Circadian Rhythm Sleep-Wake Disorders in Pediatric Populations
Our literature review identified 3 studies94–96 meeting eligibility criteria for pediatric populations with CRSWD. TST actigraphy and sleep log data were available from baseline and posttreatment assessments and are included in the meta-analyses. The TF also reviewed TST data from a heterogenous study that included participants with suspected CRSWD, phase delay and/or insomnia.97 For SL, data were available from three studies94–96 for baseline and posttreatment assessment. Only 1 study96 reported baseline and posttreatment data on sleep onset and sleep offset. All of the studies were RCTs testing melatonin and/or light therapy for delayed sleep phase syndrome in children with a wide age range (2–21 years old). Most of the studies included both male and female participants who were largely school age children. One study94 included children primarily in their late adolescents/early adulthood. Two studies95,97 involved children with neurodevelopmental disorders. No studies meeting our inclusion criteria included PSG assessments. PSG validation studies81–83 have however, demonstrated acceptable validity of actigraphy in infants and children, particularly in healthy normal subjects. The meta-analyses and figures are provided in the supplemental material, Figure S19 through Figure S26. Summary of findings tables are provided in the supplemental material, Table S7 and Table S8. A summary of the evidence for each outcome is provided below.
Total Sleep Time
For baseline sleep parameters, meta-analysis of 3 studies94–96 demonstrated that the clinical significance criteria of 25 minutes was met, indicating that actigraphy and sleep logs provide distinct information when assessing TST. Meta-analysis demonstrated a large mean difference in TST of 47.4 minutes lower (95% CI: 99.4 minutes lower to 4.5 minutes higher) for actigraphy compared to sleep logs. This was not statistically significant, however (P = .07). One additional study97 of non-specific sleep disorders in children with developmental disorders, also met the clinical significance threshold for TST. This study demonstrated a large mean difference in TST of 96.6 minutes lower (95% CI: 65.2 to 128.0 minutes lower) for actigraphy compared to sleep logs97 (Figure S19 and Figure S27). The quality of evidence was low due to imprecision and the small sample size.
With respect to treatment response, meta-analysis of three studies94–96 demonstrated that actigraphy TST met the clinical significance threshold of 25 minutes, indicating that actigraphy and sleep logs provide distinct information when assessing posttreatment TST. Meta-analysis demonstrated a large mean difference of 52.7 minutes lower TST (95% CI: 20.8 to 84.6 minutes lower) for actigraphy estimates compared to sleep logs. The study of non-specific sleep disorders in children with developmental disorders,97 also met the clinical significance threshold for TST. This study demonstrated a large mean difference in posttreatment TST of 121.4 minutes lower (95% CI: 88.4 to 154.4 minutes lower) for actigraphy estimates compared to sleep logs (Figure S23 and Figure S29). Interventions included melatonin supplementation and/or bright light therapy. Taken together, these data indicate that actigraphy measures of TST yield lower estimates compared to sleep logs at baseline and posttreatment, suggesting that actigraphy may be more sensitive at detecting sleep loss in pediatric populations with CRSWD. The quality of evidence was low due to imprecision and small sample size.
Sleep Latency
Three studies94–96 reported baseline and posttreatment SL estimates. Meta-analyses for both baseline and posttreatment estimates of SL demonstrated that the small mean differences did not meet the clinical significance threshold of 20 minutes, indicating that actigraphy and sleep logs provide similar estimates. The mean difference for baseline SL was 3.0 minutes lower (95% CI: 14.9 minutes higher to 20.9 minutes lower) for actigraphy compared to sleep logs. Only one baseline study94 met the clinical significance criteria, demonstrating a mean difference in SL of 20 minutes lower (95% CI: 6.8 minutes lower to 33.12 minutes higher) for actigraphy estimates compared to sleep logs. The other two studies95,96 suggested actigraphy estimated slightly longer SL relative to sleep logs. One additional study of non-specific sleep disorders in children with developmental disorders97 met the clinical threshold reporting a large mean difference in SL of 24.8 minutes higher (95% CI: 9.71 minutes lower to 59.3 minutes higher) for actigraphy estimates compared to sleep logs (Figure S20 and Figure S28). The quality of evidence was low due to imprecision and small sample sizes.
When assessing response to treatment, the small mean difference for posttreatment SL of 1.1 minutes lower (95% CI: 11.1 minutes lower to 9.0 minute higher) for actigraphy compared to sleep logs, was not clinically significant, suggesting that actigraphy and sleep logs provide similar estimates. Only one arm of one study testing light therapy94 met the clinical significance threshold, reporting a mean difference in posttreatment SL of 24.0 minutes lower (95% CI: 37.9 minutes lower to 10.1 to higher) for actigraphy estimates compared to sleep logs (Figure S24). The quality of evidence was low due to imprecision and small sample size.
Sleep Onset
Only one study96 reported baseline sleep onset and the small mean difference between actigraphy and sleep logs estimates did not meet the clinical significance threshold of 25 minutes, suggesting that actigraphy and sleep logs provide similar estimates. This study96 found a mean difference in sleep onset of 0 minutes (95% CI: 0.24 minutes lower to 0.24 minutes higher) between actigraphy and sleep logs. This study96 also reported a mean difference in posttreatment sleep onset of 0 minutes (95% CI: 0.20 minutes lower to 0.20 minutes higher) between actigraphy and sleep logs (Figure S21 and Figure S25 respectively). The quality of evidence was very low due to imprecision and very small sample size.
Sleep Offset
Only one study96 was identified that reported baseline sleep offset. The mean difference between actigraphy and sleep log estimates met the clinical significance threshold of 25 minutes, suggesting actigraphy and sleep provide distinct estimates. This clinical trial of melatonin and light therapy in school aged children with likely delayed sleep phase syndrome demonstrated a large mean difference in baseline sleep offset of 1.4 hours lower (95% CI: 1.2 to 1.6 hours lower) for actigraphy estimates compared to sleep logs.96 With respect to treatment response, a large mean difference of 1.7 hours lower (95% CI: 1.5 to 1.9 hours lower) for actigraphy estimates compared to sleep logs was found (Figure S22 and Figure S26). The quality of evidence was very low due to imprecision and very small sample size.
Overall Quality of Evidence
The overall quality of evidence was low due to the small sample sizes, and imprecision. Given the heterogenous nature of pediatric populations included in the studies, which ranged in age from 2 to 21 years, a developmental span involving changing sleep and circadian rhythm patterns, the generalizability of the findings is significantly limited.
Benefits Versus Harms
As many pediatric patients are unable to accurately monitor and record their sleep and caregiver sleep logs are burdensome for caregivers and prone to error, actigraphy may be the only feasible means to assess certain sleep parameters over multiple nights. Based on their clinical expertise and the above reviewed data, the task force determined that the benefits that actigraphy provides outweigh potential minor harms. Benefits of actigraphy include a relatively unobtrusive, passive, and objective measure of sleep in pediatric populations. Alternative, more intensive home sleep testing devices, which also provide objective sleep parameter estimates using multiple and more obtrusive sensor technologies, may not be as well tolerated over multiple consecutive monitoring periods. The evidence reviewed above suggests that actigraphy, compared to sleep logs, provides distinct estimates for some key sleep parameters, notably TST. The finding that actigraphy may be more sensitive than sleep logs in detecting reduced sleep time in pediatric populations is an important potential benefit. The primary adverse effects associated with actigraphy monitoring are skin irritation, which is typically mild. When evaluating the benefits versus harms, the task force considered the vulnerability of this population and the relatively high prevalence of CRSWD in pediatric populations.73,75–78
Patient Values and Preferences
Although minimal data exists related to patient values and preferences on the use of actigraphy versus sleep logs for assessing CRSWD in pediatric populations, the task force's experience and opinion is that the use of actigraphy is favored by the majority of patients and caregivers. This is due to: (1) the relatively unobtrusive nature and minor burden of the monitoring procedure; (2) the fact that monitoring sleep patterns over multiple days is required to assess CRSWD, which imposes a major burden on caregivers of young children who may be unable to accurately report sleep parameters; (3) the utility of objective data monitoring to complement patient self-report and (4) the increased accuracy that actigraphy data provides to inform clinical diagnosis, decision making, and monitoring treatment response. Patients and caregivers sometimes express concern about out of pocket expenses related to inconsistent third-party reimbursements.
Resource Use
The cost of actigraphy is higher than paper sleep log monitoring, but much less expensive than PSG and other home sleep testing devices with multiple sensor technologies. Minimal data exist evaluating the cost benefit, but savings to medical health care systems and third-party payers and employers are potentially high. Actigraphy has the potential to improve the accurate detection of CRSWD: treatment and policy interventions related to these data could reduce downstream health care expenses. At the present time, however, cost benefits of the use of actigraphy to assess pediatric CRSWD and treatment response are unclear and require systematic study.
Use of Actigraphy in the Evaluation of Sleep-Disordered Breathing With Home Sleep Apnea Tests in Adults
Our review of the literature identified 6 studies56,98–102 which examined the concomitant use of actigraphy with HSAT in the evaluation of SDB. It is important to note that the TF was unable to identify a single study which directly addresses the PICO question, which ideally should include data on comparing the accuracies of REI determination with and without actigraphy accompanying HSAT use, and simultaneously compared that to AHI determined by PSG as gold standard. Five of the studies contained data on comparing estimated TST by actigraphy against measured TST by PSG in patient population with SDB. Only one study used a HSAT device with integrated actigraphy.101
The meta-analyses are provided in the supplemental material, Figure S31. A summary of findings table is provided in the supplemental material, Table S9. A summary of the evidence for each outcome is provided below.
Total Sleep Time
In order to determine the utility of adding actigraphy to HSAT, the first critical outcome examined the accuracy of TST estimation by actigraphy compared to PSG in patients with suspected or diagnosed SDB. Five studies56,98–100,102 were included in the meta-analysis. Of note, three of the studies56,98,102 did not study the use of HSAT but instead presented data on the comparison of TST between actigraphy and PSG in the setting of OSA and were therefore included in the meta-analysis. Actigraphy appeared to be less accurate in estimating TST as PSG-determined AHI increases, likely due to movements related to severe and frequent apneas. The overall results showed a mean difference in TST measured by actigraphy as compared to PSG of 14.54 minutes higher (95% CI: 49.77 minutes higher to 20.70 minutes lower) which indicated a sufficiently small mean difference, however, variability was significant, with a range of possible mean differences of 70 minutes. These results are consistent with other studies103–105 which have demonstrated the validity of actigraphy in estimating TST in the setting of SDB (Figure S31). The quality of evidence was moderate due to imprecision.
Accuracy
One study99 compared AHI values obtained by PSG versus AHI values calculated by simplified polygraphy (akin to a HSAT setup) with or without actigraphy-estimated TST in 20 subjects with SDB. Using actigraphy-estimated TST to calculate AHI improved both sensitivity (88% AHI-act versus 50% AHI-tib) and negative predictive value (92.5% AHI-act versus 75% AHI-tib) in the subset of patients with severe OSA (AHI > 30 events/h). However, for the diagnosis of moderate OSA (defined as AHI > 10 to 29 in this study) by simplified polygraphy, sensitivity and specificity were the same (at 100%) with or without actigraphy-estimated TST data.
Another study100 compared a biomotion sensor and actigraphy-estimated TST with standard PSG. In a post hoc analysis, the use of actigraphy-estimated TST resulted in a reduced number of misclassifications of SDB severity categorizations compared to using TRT (∼7% misclassifications with actigraphy versus ∼10% misclassifications using TRT).
In one other study,101 AHI/RDI thresholds of 10, 15, and 30 events/h were used to compare the accuracy of PSG versus an HSAT device with built-in actigraphy. Based on the manual analysis of two “observers,” the sensitivity ranged between 83.8% and 95.8%, and the specificity between 92% and 100% for the different AHI thresholds studied. This study showed increased sensitivity with the addition of actigraphy TST, compared to using recording time in HSAT, with the increased sensitivity primarily observed in patients with severe OSA (RDI ≥ 30 events/h).
Another study106 examined Taiwanese bus drivers who were studied for SDB. They used AHI thresholds of 5 and 15 events/h and showed an increase in AHI when measured with actigraphy-estimated TST as compared with recording time, but this was not statistically significant.106 The quality of evidence was low due to indirectness and small sample size.
Overall Quality of Evidence
The overall quality of evidence on the use of actigraphy with HSAT to estimate TST (monitoring time) during recording, in the absence of alternative objective measurements of TST, in adult patients suspected of SDB was low due to imprecision, indirectness and small samples size. The overall quality of evidence was also downgraded based on the indirectness of additional evidence from other sleep disorders supporting that TST estimated by actigraphy is reliably accurate when compared to PSG. The quality of evidence in assessing the accuracy of REI by the addition of actigraphy with HSAT was also downgraded due to small sample size.
Benefits Versus Harms
By providing an improved TST estimation (monitoring time) over total time in bed (TIB) or TRT, actigraphy may improve the diagnostic accuracy of HSAT in calculating respiratory event indices and thus the diagnostic accuracy of HSAT in detecting SDB in the evaluation of patients suspected or diagnosed with SDB. In addition, the TF considered the empirical concern that in patients with short sleep duration or chronic insomnia (TST < 6 hours), simply using TIB or TRT may increase the denominator in calculating the AHI, thereby underestimating the severity of OSA or missing the diagnosis completely. Hence, actigraphy may be particularly useful in such patients with short sleep duration or chronic insomnia to help improve the diagnostic accuracy of HSAT. The TF determined that only actigraphy integrated within HSAT devices should be used in the clinical settings as adding actigraphy separately to a HSAT study will be impractical to do so. The TF cautions the limitation in actigraphy use in cases with limited upper extremity mobility (eg, stroke patients). Based on their clinical expertise and the above reviewed data, the TF determined that there were no clinically significant and undesirable outcomes associated with actigraphy (integrated within HSAT devices).
Patient Values and Preferences
In adult patients with suspected sleep-related breathing disorder, currently there are no available studies to draw from in assessing patients' values and preferences on actigraphy incorporated within HSAT devices. However, patients will likely value the potentially more accurate assessment of SDB severity that could be obtained with the addition of actigraphy (integrated within HSAT devices) which can impact access to treatment (eg, based on REI cut-off requirements of third-party payers, job requirements, disability benefits, etc.). Actigraphy should carry minimal burden for the patients.
Resource Use
From a resource use perspective, it would be most appropriate to compare the use of actigraphy integrated within HSAT device versus HSAT without actigraphy. It is neither practical to separately collect actigraphy data and synchronize with the HSAT recording, nor feasible to obtain actigraphy testing separately during a HSAT study for billing purposes. Several HSAT systems already have integrated actigraphy. However, economic analyses comparing the cost-effectiveness on HSAT with integrated actigraphy for the assessment of SDB have not been conducted. The TF concluded using HSAT with integrated actigraphy function may be more cost effective by potentially improving the diagnostic accuracy of SDB by HSAT when compared with only using TIB or TRT.
Use of Actigraphy in the Evaluation of Central Disorders of Hypersomnolence With the Multiple Sleep Latency Test
The MSLT measures the physiologic sleep tendency of an individual during the habitual wake period,80 and is recommended in the diagnostic evaluation of narcolepsy and other central disorders of hypersomnolence.107 The MSLT can be influenced by a number of factors, including sleep duration leading up to the testing.108 An in-center sleep study with EEG, EMG and EOG recording is recommended as standard procedure for the night prior to the MSLT to identify any underlying clinical conditions that could result in sleep fragmentation and to document that the patient had a sufficient amount of sleep the night prior to the study.80 Although the overnight PSG will rule out acute insufficient sleep that might influence interpretation of the findings for diagnosing disorders of hypersomnolence, chronic insufficient sleep time may also negatively influence the MSLT study, and should be ruled out prior to the MSLT as well. Sleep-wake patterns over a period of time are most commonly assessed using sleep logs rather than actigraphy. Sleep logs, however may be subject to bias (eg, motivational factors) resulting in patients overestimating, or in some cases, underestimating their TST.
The figures are provided in the supplemental material, Figure S32a and Figure S32b. Summary of findings tables are provided in the supplemental material, Table S10a and Table S10b. A summary of the evidence for each outcome is provided below.
Total Sleep Time
In this review, we identified only one study108 that examined the nightly sleep duration by both actigraphy as well as sleep logs in the 2-week period prior to a MSLT in patients with excessive daytime sleepiness. It found that actigraphy compared to sleep logs estimated a large mean difference that was clinically significant of 86 minutes lower (95% CI: 58.4 to 113.6 minutes lower).108 See supplemental material, Figure S32a. These data demonstrate that actigraphy provides unique measurements compared to sleep logs, and may be helpful to ascertain nightly sleep duration prior to MSLT. When comparing the TST recorded by actigraphy and PSG on the night before the MSLT, the study reported a mean difference of 15.60 minutes, which is within the clinical significance threshold, however the 95% CI of 49.40 minutes (−40.30, 9.10) exceeded the clinical significance threshold.108 See supplemental material, Figure S32b. The quality of evidence was moderate due to imprecision and small sample size.
In addition, in their subgroup analysis, patients who had a mean sleep latency (MSL) of less than 8 minutes in the MSLT were found to have a mean nightly sleep duration of only 4.53 ± 1.37 hours by actigraphy, while patients who had a MSL of more than or equal to 8 minutes were found to have a mean nightly sleep duration of 6.10 ± 1.37 hours by actigraphy.108 This difference in mean nightly sleep duration between the two groups of patients was reported to be statistically significant.108 However, in terms of sleep logs-recorded mean nightly sleep duration, no significant difference was found between these two groups of patients (7.08 ± 0.70 hours for patients with MSL < 8 minutes versus 6.94 ± 0.93 hours for patients with MSL ≥ 8 minutes).108 Results from this study suggests that patients with a MSL < 8 minutes on the MSLT were more likely to overestimate their nightly sleep duration on sleep logs compared to actigraphy, suggesting that sleep logs may be unreliable in patients with a reduced SL on the MSLT.108 It is likely that some patients who were referred for an MSLT in the evaluation for hypersomnia disorders had unrecognized insufficient sleep syndrome. The task force noted that this study was limited by a military sleep center setting, a relatively small sample size, and patients consisted of mostly men (87%).
Overall Quality of Evidence
The overall quality of evidence on the use of actigraphy to monitor TST prior to MSLT testing in adult and pediatric patients with suspected central disorders of hypersomnolence was moderate. The quality of evidence was downgraded due to imprecision due to small sample size from one single study. The overall quality of evidence was also downgraded due to indirectness of evidence; that is, some evidence supporting this recommendation was based on studies evaluating the accuracy of TST versus sleep logs in patients with a variety of sleep disorders or complaints. Despite looking broadly at available literature, no pediatric data were currently available. However, the TF determined that the findings and recommendation reported here could be extended to the pediatric population, particularly in adolescents, where differentiating CRSWDs from hypersomnia conditions can be clinically challenging.
Benefits Versus Harms
The ability for actigraphy to provide longitudinal assessment of TST and sleep patterns in patients with suspected hyper-somnia disorder may improve the diagnostic accuracy of the subsequent MSLT and potentially reveal other sleep disorders or circadian rhythm sleep-wake disorders. Actigraphy is a noninvasive test that can be conducted over multiple nights, which is not feasible with PSG. The TF determined that there were no clinically significant and undesirable outcomes associated with actigraphy. Given the ICSD-3 diagnostic criteria on hypersomnia disorders recommending that insufficient sleep should be ruled out, the TF determined that there is evidence to suggest that actigraphy be used in combination with sleep logs prior to MSLT in adults suspected of central disorders of hypersomnolence to improve the diagnostic accuracy of the MSLT.
Patient Values and Preferences
Actigraphy is able to provide objective sleep duration data prior to MSLT which could improve the diagnostic accuracy of the MSLT compared to sleep logs alone. Patients with suspected hypersomnia condition will benefit from a more accurate diagnosis by use of actigraphy prior to MSLT. The TF determined that the vast majority of patients would want to receive a correct clinical diagnosis in the evaluation for hypersomnia disorders. However, minimal evidence exists to indicate how much patients value the main outcome. The use of actigraphy under consideration here requires patients to wear a wrist watch device continuously for up to two weeks prior to MSLT.
Resource Use
Actigraphy device is reusable and data can be collected from patient over a period of time prior to MSLT. In practical terms, actigraphy studies can be obtained over a period of 7–14 days, though currently there is no available data to determine the optimal length of study prior to MSLT. It is a relatively low cost medical diagnostic test.
Use of Actigraphy in the Evaluation of Insufficient Sleep Syndrome in Adults
Our review of the literature identified 11 studies109–119 permitting the comparison of actigraphy and sleep log estimates of TST for routine assessment in participants at risk for insufficient sleep syndrome. These studies included data from male and female participants, ranging in age between 18–57.9 years. The majority of the studies were within-subject, case control or quasi experimental designs evaluating workers with occupations involving extended shifts/duty hour schedules curtailing sleep opportunity relative to off duty hours. Occupations included pilots/astronauts (4 studies),109,113,116,117 medical interns/residents (3 studies),111,114,118 oil rig workers (1 study),112 tunnel workers (1 study),115 and ballet dancers (1 study)110. Three intervention studies113,116,119 meeting eligibility criteria were identified, which assessed post intervention TST. Due to the small number of intervention studies and heterogeneity in the sample characteristics, as well as the varying interventions deployed, we did not conduct meta-analyses evaluating treatment response. The meta-analyses and figures are provided in the supplemental material, Figure S33 and Figure S34. Summary of findings tables are provided in the supplemental material, Table S11 and Table S12. A summary of the evidence for each outcome is provided below.
Total Sleep Time
Meta-analysis of the 10 baseline assessment studies109–118 demonstrated that actigraphy estimated lower baseline TST relative to sleep logs by a large mean difference of 38.5 minutes (95% CI: 27.0 to 49.2 minutes lower), which exceeded the clinical significance threshold of 20 minutes. This finding suggests that actigraphy may be more sensitive than sleep logs in detecting short sleep in individuals at risk for ISS (Figure S33). The quality of evidence was high.
With respect to treatment response, only three studies113,116,119 were identified. Similar to the baseline assessment studies, two studies, one in pilots113 and the other astronauts,116 demonstrated that actigraphy estimated lower posttreatment TST compared to sleep logs by large mean differences of 57.00 minutes (95% CI: 26.6 to 87.4 minutes lower) and 26 minutes (95% CI: 12.0 to 40.0 minutes lower), respectively. The mean differences in both studies were clinically significant. Interventions included a behavioral counter fatigue intervention in airline pilots113 conducted in within subjects experimental study and sedative medications for on duty astronauts116 in an observational study. A study112 of offshore oil platform workers with difficulty adjusting to shiftwork, however, found that sleep logs tended to yield lower estimates of TST relative to actigraphy in this randomized cross-over experiment comparing light therapy and melatonin against placebo. The light therapy intervention arm demonstrated that posttreatment actigraphy estimated greater TST compared to sleep logs by a large mean difference of 38 minutes (95% CI: 76.7 minutes higher to .70 minutes lower), which was clinically significant.112 This mean difference, however, was not statistically significant. The melatonin intervention arm actigraphy estimated higher TST compared to sleep logs by a small mean difference of 5.5 minutes (95% CI: 37.11 minutes higher to 26.11 minutes lower), which was neither clinically nor statistically significant112 (Figure S34). These posttreatment data suggest that actigraphy estimates of posttreatment TST generally yield lower estimates of TST compared to sleep logs, though the direction of the differences may not be uniformly consistent and may be specific to a particular intervention types or subpopulations. The quality of posttreatment evidence was low due small sample size, imprecision, and heterogeneity of the studies.
Overall Quality of Evidence
The overall quality of the evidence was judged to be moderate due primarily to the three treatment response studies. Treatment response studies were downgraded because of heterogeneity, and imprecision, ie, one study had 95% CI crossing the clinically significance threshold. The evidence pertaining to the 10 assessment studies of baseline data was judged to be of high quality.
Benefits Versus Harms
The potential benefits of actigraphy assessment of TST in patients at risk for ISS are strong relative to the minor undesirable effects, which include as small risk of skin irritation. The majority of the studies demonstrate that actigraphy estimates of TST yield evidence of greater sleep loss compared to sleep log estimates. This indicates that actigraphy may be more sensitive in detecting insufficient sleep disorders compared to sleep logs. This is important because insufficient sleep is highly prevalent,120–123 associated with motor vehicle accidents, diminished work-related productivity and medical and psychiatric morbidity.60–64,122–127 The discrepancy of −38 minutes between actigraphy and sleep logs is clinically significant in that this differential degree of chronic sleep loss would be expected to impact sleep debt and be expected to be more robustly associated with physiologic and neurobehavioral risk factors of medical and psychiatric morbidity. Based on their clinical expertise, and the meta-analyses, the task force determined that the potential benefits of actigraphy outweighed its potential harms.
Patient Values and Preferences
Although minimal data exists related to patient values and preferences on the use of actigraphy versus sleep logs for assessing insufficient sleep, the task force's experience is that the use of actigraphy is favored by the majority of patients with no important uncertainty or variability due to: (1) the relatively unobtrusive nature and minor burden of this relatively passive monitoring procedure; (2) the utility of objective data monitoring to complement patient self-report; and (3) the increased accuracy that actigraphy data provides to inform clinical diagnosis, decision making, and monitoring treatment response. Patients sometimes express concern about out of pocket expenses related to inconsistent third-party reimbursements and variable co-pays.
Resource Use
The cost of actigraphy is higher than paper sleep log monitoring, but much less expensive than PSG and other home sleep testing devices with multiple sensor technologies. Minimal data exist evaluating the cost benefit, but potential savings to medical health care systems and third-party payers and employers are high. Actigraphy is expected to improve the accurate detection of insufficient sleep; treatment and policy interventions related to these data could reduce downstream health care expenses, lost productivity, reduced accidents and other deleterious effects of insufficient sleep. At the present time cost benefits of the use of actigraphy to assess treatment response are less certain due to limitations in the small number of well-designed outcome studies and mixed findings related to clinical significance.
Use of Actigraphy in the Evaluation of Periodic Limb Movement Disorder
A review of the literature to identify studies including both actigraphy and EMG and EEG during in-center PSG to estimate periodic limb movement frequency yielded 3 studies in adults128–130 and 2 in pediatric patients131,132 meeting our inclusion/exclusion criteria. One study128 compared two different actigraphy devices to EMG. A second study130 examined the reliability of actigraphy to measure limb movements in a population with suspected insomnia, SRBD, or daytime sleepiness; only patients with a pre-PSG diagnosis of RLS were used in our analyses. The small number of studies precluded meta-analysis. However, summary information for each study are shown in the supplemental material, Figure S35. A summary of findings tables is provided in the supplemental material, Table S13. A summary of the evidence for each outcome is provided below.
Accuracy
None of the included studies in adults provided information on the accuracy of PLMD diagnosis using current diagnostic criteria. One study129 of adults provided sensitivity/specificity using a PLMSI cutoff of 15 events/h on PSG and a PLMSI cutoff of 16 events/h on the actigraphy device, and reported sensitivity of 82.4% and specificity of 70.8%, a false-positive rate of 31.8 and a false-negative rate of 26.3. The PLMSI threshold of 16 events/h is not routinely used for diagnostic purposes in clinical practice. A study of children with sickle cell disease provided sensitivity/specificity data using a PLMSI cutoff of 5 events/h on PSG and a PLMSI cutoff of 5 events/h on actigraphy.132 They reported sensitivity of 100%, and specificity of 8% for raw actigraphy, and 25% after correcting the PLMSI for sleep time. The false-positive rate was 58% (53% after correcting for sleep time), and the false negative rate was 0% (with or without sleep time correction). These studies indicate the accuracy of actigraphy for the diagnosis of PLMD is inadequate. The quality of evidence was moderate due to small sample size.
Periodic Limb Movement Index
The correspondence between the PLMSI derived from actigraphy varied widely. One study128 compared EMG to two different actigraphy devices to EMG in patients with PLMSI > 5 events/h at baseline. They found that the average PLMSI using one device was 34.4 events/h (standard deviation [SD] = 30.7) measured on both legs with one device, and 63.6 events/h (SD = 39.3) measured on both legs with the second device, while the PLMSI based on EMG during laboratory PSG was 37.0 events/h (SD = 30.7). In a second study129 patients with suspected PLMD were studied, and EMG derived PLMSI was compared to one actigraphy device worn for 5 consecutive nights (4 nights at home). The mean PLMSI was 30.4 events/h (SD = 34.3) on actigraphy, compared to 21.0 events/h (SD = 28.9) as measured by EMG during laboratory PSG. In a third study of patients suspected of RLS,130 the mean PLMSI based on EMP during laboratory PSG was 51.2 events/h (SD = 34.1) while the mean PLMSI based on actigraphy was 47.71 events/h (SD = 35.42). However, the range of possible mean differences between EMG-derived and actigraphy-derived PMLSI was 58.14 events/h. In a study of pediatric patients,131 the mean PLMSI based on EMG was 4.0 events/h (SD = 1.3) for the left leg and 4.0 events/h (SD = 1.5) for the right leg, while the PLMSI based on actigraphy was 6.4 events/h (SD = 4.1) on the left leg and 7.9 events/h (SD = 3.9) on the right leg (Figure S35). In a second study132 of pediatric patients, Bland Altman analyses demonstrated that actigraphy overestimated the mean PLMSI, compared to EMG, by 8.1 events/h (SD = 10.7). These studies demonstrate that actigraphy does not accurately identify periodic limb movements, compared to the gold standard EMG. The quality of evidence was moderate due to imprecision.
Overall Quality of Evidence
The overall quality of evidence was moderate. The available studies used concurrent measurement; however, the evidence was drawn from only five studies with small sample sizes, and only two devices were studied. In addition, there was imprecision, with the 95% CI crossing the clinical significance threshold as determined by the TF for both adult and pediatric studies.
Benefits Versus Harms
The main benefit of actigraphy is that it can potentially be worn outside of the sleep center and may provide a simpler alternative for patients; however, the potential harms of misclassification of patients with and without PLMD outweighs the benefit of increased convenience. Given that actigraphy both over and under-estimated PLMSI compared to EMG during PSG, it cannot be viewed as a substitute for EMG during in-center PSG in the diagnosis of PLMD.
Patient Values and Preferences
While patients may prefer a simpler diagnostic tool, diagnostic accuracy is also important to patients. The TF concluded that most patients would prefer EMG during PSG over actigraphy.
Resource Use
Actigraphy may be less expensive than in-center PSG; however, actigraphy is not routinely covered by insurers for diagnosis of PLMD. As a result, the cost to patients may be higher for actigraphy compared to in-center PSG with EMG. Although data are limited, given the low diagnostic utility, there could also be added cost to the health care system from repeat diagnostic testing or use of inappropriate treatments, even if the cost was covered by insurers.
DISCUSSION AND FUTURE DIRECTIONS
Our review and analyses support the utility of actigraphy as a relatively low cost, objective measure of sleep patterns and certain estimated sleep parameters in both children and adults, across a wide range of sleep disorders, when conducted using validated algorithms with attention to sensitivity settings and standardized scoring procedures.
Overall, our meta-analyses indicated that actigraphy yields significantly distinct estimates of sleep patterns when compared to sleep logs, suggesting that, although the two measures are often correlated, they provide unique information contributing to the clinical understanding of patients with sleep disorders. With respect to specific sleep and CRSWDs, the utility of actigraphy in objective estimation of sleep and wake parameters across multiple consecutive 24-hour periods renders it a very useful tool for assessing circadian dysrhythmia. With respect to insomnia disorder, there is ample evidence of its validity and utility in assessing sleep continuity in conjunction with sleep logs both in terms of general diagnostic assessment as well as posttreatment assessment. Actigraphy is also especially useful to assess sleep continuity in patients who are typically unable to complete sleep logs reliably, including children and individuals with cognitive impairment. Finally, actigraphy may be especially useful in assessing TST in individuals at risk for ISS. The data in populations at risk for insufficient sleep suggest that actigraphy estimated shorter sleep duration compared to sleep log estimates and therefore may be especially useful in identifying short sleep, which contributes to increased medical and psychiatric morbidity, injuries and workplace accidents.60–64,122–127
Future scientific reports using actigraphy should uniformly publish detailed technical and scoring procedures including sensitivity settings, scoring algorithms, and scoring procedures so that future research can more fully establish validity, particularly in special patient populations. A major finding across disorders is that actigraphy generally yields distinct information from sleep log estimates, and in some cases, actigraphy estimates in comparison to those from sleep logs correspond more closely with PSG measures. More research that compares all 3 approaches across patients with different types of sleep and circadian rhythm sleep-wake disorders is warranted. Given that actigraphy and sleep logs often generate distinct parameter estimates for the same variables, there is an important research imperative to establish normative data that account for demographic and developmental factors such as age, sex, ethnicity, as well as disease type (eg, sleep disorders, healthy individuals, medical and psychiatric disorders).
A key strength of actigraphy is that it provides relatively unobtrusive monitoring of sleep patterns over long periods of time. In addition, the use of these devices to measure sleep behavior is becoming broadly applied, and the experience of the task force is that actigraphy is largely acceptable to patients with sleep disorders; however, data are needed to understand patient preferences based on sleep disorder, age, and other factors. Future research should also explore statistical models that capitalize on these micro-longitudinal data, evaluating day-to-day variation in sleep parameters and trajectories over time rather than relying exclusively on aggregated, mean level data. Sleep disorders such as chronic insomnia disorder and CRSWDs often involve considerable variability in symptoms and sleep parameters, which may be readily captured via actigraphy and analyzed using time series data analytic approaches. In addition, this information can be displayed graphically to patients, enabling them to understand diagnostic decisions and evaluate their own response to treatment. The review and meta-analyses that the TF performed highlighted some important gaps that would benefit from future investigation. In particular, the TF identified very few studies that have evaluated the relative benefit of actigraphy-based TST estimates used in conjunction with HSAT devices that do not determine actual sleep time by EEG, EOG and EMG. Similarly, more studies are needed to evaluate the use of actigraphy prior to MSLT in assessment for narcolepsy and other central disorders of hypersomnolence. In pediatric patients, more research is needed to establish whether actigraphy can reliably detect response to well-established treatments. A similar need exists to determine the sensitivity of actigraphy to behavioral interventions that target extension of habitual sleep duration and quality in individuals with ISS.
DISCLOSURE STATEMENT
The development of this paper was funded by the American Academy of Sleep Medicine. Drs. Martin and Carden serve on the AASM Board of Directors. Mr. Harrod and Mr. Heald are employed by the American Academy of Sleep Medicine. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The task force thanks and acknowledges the early contributions of William C. Sherrill Jr., MD. Dr. Sherrill served as a member of the task force during the initial stages of the systematic review and contributed to the development of the PICOs and search strategies and the initial evidence review. The task force also thanks Drs. Shalini Paruthi, Katherine Sharkey, and Adam Spira for serving as external reviewers of the document and providing valuable feedback.
REFERENCES
- 1.Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, Carden KA. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2018;14(7):1231–1237. doi: 10.5664/jcsm.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S, Deak MC, Gault D, et al. Consumer sleep technology: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2018;14(5):877–880. doi: 10.5664/jcsm.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell T, Ancoli-Israel S, Redline S, Stone KL Osteoporotic Fractures in Men (MrOS) Study Group. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7(4):357–367. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinkhan M, Berger K, Hense S, et al. Agreement of different methods for assessing sleep characteristics: a comparison of two actigraphs, wrist and hip placement, and self-report with polysomnography. Sleep Med. 2014;15(9):1107–1114. doi: 10.1016/j.sleep.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Sadeh A. Iii. Sleep assessment methods. Monogr Soc Res Child Dev. 2015;80(1):33–48. doi: 10.1111/mono.12143. [DOI] [PubMed] [Google Scholar]
- 7.Patel SR, Weng J, Rueschman M, et al. Reproducibility of a standardized actigraphy scoring algorithm for sleep in a US Hispanic/Latino population. Sleep. 2015;38(9):1497–1503. doi: 10.5665/sleep.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JM, Kay DB, Rowe M, McCrae CS. Sleep discrepancy, sleep complaint, and poor sleep among older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68(5):712–720. doi: 10.1093/geronb/gbt030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay DB, Buysse DJ, Germain A, Hall M, Monk TH. Subjective-objective sleep discrepancy among older adults: associations with insomnia diagnosis and insomnia treatment. J Sleep Res. 2015;24(1):32–39. doi: 10.1111/jsr.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creti L, Libman E, Baltzan M, Rizzo D, Bailes S, Fichten CS. Impaired sleep in chronic fatigue syndrome: how is it best measured? J Health Psychol. 2010;15(4):596–607. doi: 10.1177/1359105309355336. [DOI] [PubMed] [Google Scholar]
- 16.Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015;67(10):1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. 2015;24(3):296–304. doi: 10.1111/jsr.12259. [DOI] [PubMed] [Google Scholar]
- 18.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29(2):232–239. [PubMed] [Google Scholar]
- 19.Levenson JC, Troxel WM, Begley A, et al. A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. J Clin Sleep Med. 2013;9(2):125–131. doi: 10.5664/jcsm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallieres A, Morin CM. Actigraphy in the assessment of insomnia. Sleep. 2003;26(7):902–906. doi: 10.1093/sleep/26.7.902. [DOI] [PubMed] [Google Scholar]
- 21.McCall C, McCall WV. Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect? J Clin Sleep Med. 2012;8(1):59–65. doi: 10.5664/jcsm.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21(1):122–127. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straus LD, Drummond SP, Nappi CM, Jenkins MM, Norman SB. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress. 2015;28(1):8–16. doi: 10.1002/jts.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkelman JW, Benson KL, Buxton OM, et al. Lack of hippocampal volume differences in primary insomnia and good sleeper controls: an MRI volumetric study at 3 Tesla. Sleep Med. 2010;11(6):576–582. doi: 10.1016/j.sleep.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Lovato N, Lack L, Wright H, Kennaway DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. 2014;37(1):117–126. doi: 10.5665/sleep.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lengacher CA, Reich RR, Paterson CL, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psychooncology. 2015;24(4):424–432. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi: 10.5665/sleep.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarnefelt H, Lagerstedt R, Kajaste S, Sallinen M, Savolainen A, Hublin C. Cognitive behavioral therapy for shift workers with chronic insomnia. Sleep Med. 2012;13(10):1238–1246. doi: 10.1016/j.sleep.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007;30(2):203–212. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- 30.Taylor DJ, Zimmerman MR, Gardner CE, et al. A pilot randomized controlled trial of the effects of cognitive-behavioral therapy for insomnia on sleep and daytime functioning in college students. Behav Ther. 2014;45(3):376–389. doi: 10.1016/j.beth.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 31.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore. 2011;7(2):76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–1563. doi: 10.5665/sleep.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med. 2005;165(21):2527–2535. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 35.Taibi DM, Vitiello MV. A pilot study of gentle yoga for sleep disturbance in women with osteoarthritis. Sleep Med. 2011;12(5):512–517. doi: 10.1016/j.sleep.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 37.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 38.Harris J, Lack L, Kemp K, Wright H, Bootzin R. A randomized controlled trial of intensive sleep retraining (ISR): a brief conditioning treatment for chronic insomnia. Sleep. 2012;35(1):49–60. doi: 10.5665/sleep.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyagi S, Resnick NM, Perera S, Monk TH, Hall MH, Buysse DJ. Behavioral treatment of chronic insomnia in older adults: does nocturia matter? Sleep. 2014;37(4):681–687. doi: 10.5665/sleep.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mundt JM, Crew EC, Krietsch K, et al. Measuring treatment outcomes in comorbid insomnia and fibromyalgia: concordance of subjective and objective assessments. J Clin Sleep Med. 2016;12(2):215–223. doi: 10.5664/jcsm.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falloon K, Elley CR, Fernando A, 3rd, Lee AC, Arroll B. Simplified sleep restriction for insomnia in general practice: a randomised controlled trial. Br J Gen Pract. 2015;65(637):e508–e515. doi: 10.3399/bjgp15X686137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman L, Zeitzer JM, Kushida C, et al. Scheduled bright light for treatment of insomnia in older adults. J Am Geriatr Soc. 2009;57(3):441–452. doi: 10.1111/j.1532-5415.2008.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamadera W, Sato M, Harada D, et al. Comparisons of short-term efficacy between individual and group cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2013;11(3):176–184. doi: 10.1111/sbr.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato M, Yamadera W, Matsushima M, Itoh H, Nakayama K. Clinical efficacy of individual cognitive behavior therapy for psychophysiological insomnia in 20 outpatients. Psychiatry Clin Neurosci. 2010;64(2):187–195. doi: 10.1111/j.1440-1819.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 46.Friedman L, Benson K, Noda A, et al. An actigraphic comparison of sleep restriction and sleep hygiene treatments for insomnia in older adults. J Geriatr Psychiatry Neurol. 2000;13(1):17–27. doi: 10.1177/089198870001300103. [DOI] [PubMed] [Google Scholar]
- 47.Yeung WF, Chung KF, Tso KC, Zhang SP, Zhang ZJ, Ho LM. Electroacupuncture for residual insomnia associated with major depressive disorder: a randomized controlled trial. Sleep. 2011;34(6):807–815. doi: 10.5665/SLEEP.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovato N, Lack L, Kennaway DJ. Comparing and contrasting therapeutic effects of cognitive-behavior therapy for older adults suffering from insomnia with short and long objective sleep duration. Sleep Med. 2016;22:4–12. doi: 10.1016/j.sleep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Tang NK, Goodchild CE, Salkovskis PM. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav Res Ther. 2012;50(12):814–821. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Yeung WF, Chung KF, Zhang SP, Yap TG, Law AC. Electroacupuncture for primary insomnia: a randomized controlled trial. Sleep. 2009;32(8):1039–1047. doi: 10.1093/sleep/32.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alessi C, Martin JL, Fiorentino L, et al. Cognitive behavioral therapy for insomnia in older veterans using nonclinician sleep coaches: randomized controlled trial. J Am Geriatr Soc. 2016;64(9):1830–1838. doi: 10.1111/jgs.14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epstein DR, Sidani S, Bootzin RR, Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35(6):797–805. doi: 10.5665/sleep.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574–584. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 54.Kang SG, Kang JM, Cho SJ, et al. Cognitive behavioral therapy using a mobile application synchronizable with wearable devices for insomnia treatment: a pilot study. J Clin Sleep Med. 2017;13(4):633–640. doi: 10.5664/jcsm.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung KF, Yeung WF, Yu YM, et al. Acupuncture for residual insomnia associated with major depressive disorder: a placebo- and sham-controlled, subject- and assessor-blind, randomized trial. J Clin Psychiatry. 2015;76(6):e752–e760. doi: 10.4088/JCP.14m09124. [DOI] [PubMed] [Google Scholar]
- 56.Choi SJ, Kang M, Sung MJ, Joo EY. Discordant sleep parameters among actigraphy, polysomnography, and perceived sleep in patients with sleep-disordered breathing in comparison with patients with chronic insomnia disorder. Sleep Breath. 2017;21(4):837–843. doi: 10.1007/s11325-017-1514-5. [DOI] [PubMed] [Google Scholar]
- 57.Chung KF, Yeung WF, Yu BY, et al. Acupuncture with or without combined auricular acupuncture for insomnia: a randomised, waitlist-controlled trial. Acupunct Med. 2018;36(1):2–13. doi: 10.1136/acupmed-2017-011371. [DOI] [PubMed] [Google Scholar]
- 58.Shechter A, Kim EW, St-Onge MP, Westwood AJ. Blocking nocturnal blue light for insomnia: a randomized controlled trial. J Psychiatr Res. 2018;96:196–202. doi: 10.1016/j.jpsychires.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor DJ, Peterson AL, Pruiksma KE, Young-McCaughan S, Nicholson K, Mintz J. Internet and in-person cognitive behavioral therapy for insomnia in military personnel: a randomized clinical trial. Sleep. 2017;40(6) doi: 10.1093/sleep/zsx075. [DOI] [PubMed] [Google Scholar]
- 60.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–89. doi: 10.1016/j.jsmc.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diem SJ, Blackwell TL, Stone KL, et al. Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am J Geriatr Psychiatry. 2016;24(3):248–258. doi: 10.1016/j.jagp.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27(10):1235–1242. doi: 10.1093/ajh/hpu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song Y, Dzierzewski JM, Fung CH, et al. Association between sleep and physical function in older veterans in an adult day healthcare program. J Am Geriatr Soc. 2015;63(8):1622–1627. doi: 10.1111/jgs.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2014;18(4):293–297. doi: 10.1016/j.smrv.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Bathgate CJ, Edinger JD, Krystal AD. Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dayyat EA, Spruyt K, Molfese DL, Gozal D. Sleep estimates in children: parental versus actigraphic assessments. Nat Sci Sleep. 2011;3:115–123. doi: 10.2147/NSS.S25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Bruin EJ, Bogels SM, Oort FJ, Meijer AM. Efficacy of cognitive behavioral therapy for insomnia in adolescents: a randomized controlled trial with internet therapy, group therapy and a waiting list condition. Sleep. 2015;38(12):1913–1926. doi: 10.5665/sleep.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palermo TM, Beals-Erickson S, Bromberg M, Law E, Chen M. A single arm pilot trial of brief cognitive behavioral therapy for insomnia in adolescents with physical and psychiatric comorbidities. J Clin Sleep Med. 2017;13(3):401–410. doi: 10.5664/jcsm.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conroy DA, Czopp AM, Dore-Stites DM, et al. Modified cognitive behavioral therapy for insomnia in depressed adolescents: a pilot study. Behav Sleep Med. 2017 Mar 23; doi: 10.1080/15402002.2017.1299737. . [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 70.Gringras P, Green D, Wright B, et al. Weighted blankets and sleep in autistic children--a randomized controlled trial. Pediatrics. 2014;134(2):298–306. doi: 10.1542/peds.2013-4285. [DOI] [PubMed] [Google Scholar]
- 71.Paine S, Gradisar M. A randomised controlled trial of cognitive-behaviour therapy for behavioural insomnia of childhood in school-aged children. Behav Res Ther. 2011;49(6-7):379–388. doi: 10.1016/j.brat.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Badin E, Haddad C, Shatkin JP. Insomnia: the sleeping giant of pediatric public health. Curr Psychiatry Rep. 2016;18(5):47. doi: 10.1007/s11920-016-0687-0. [DOI] [PubMed] [Google Scholar]
- 73.Owens J. Classification and epidemiology of childhood sleep disorders. Prim Care. 2008;35(3):533–546. vii. doi: 10.1016/j.pop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Owens JA, Mindell JA. Pediatric insomnia. Pediatr Clin North Am. 2011;58(3):555–569. doi: 10.1016/j.pcl.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 75.Tham EK, Schneider N, Broekman BF. Infant sleep and its relation with cognition and growth: a narrative review. Nat Sci Sleep. 2017;9:135–149. doi: 10.2147/NSS.S125992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am. 2011;58(3):649–665. doi: 10.1016/j.pcl.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coogan AN, McGowan NM. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Atten Defic Hyperact Disord. 2017;9(3):129–147. doi: 10.1007/s12402-016-0214-5. [DOI] [PubMed] [Google Scholar]
- 78.Lunsford-Avery JR, Krystal AD, Kollins SH. Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clin Psychol Rev. 2016;50:159–174. doi: 10.1016/j.cpr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meltzer LJ, Mindell JA. Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. J Pediatr Psychol. 2014;39(8):932–948. doi: 10.1093/jpepsy/jsu041. [DOI] [PubMed] [Google Scholar]
- 80.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 81.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87(4):494–499. [PubMed] [Google Scholar]
- 82.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hyde M, O'Driscoll DM, Binette S, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16(2):213–216. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 84.Pickering L, Jennum P, Gammeltoft S, Poulsgaard L, Feldt-Rasmussen U, Klose M. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur J Endocrinol. 2014;170(6):873–884. doi: 10.1530/EJE-13-1025. [DOI] [PubMed] [Google Scholar]
- 85.De Rui M, Middleton B, Sticca A, et al. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: effect of light therapy. Neurochem Res. 2015;40(2):284–292. doi: 10.1007/s11064-014-1414-z. [DOI] [PubMed] [Google Scholar]
- 86.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17(1):89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 88.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 89.Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17(12):1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 90.Palmer CR, Kripke DF, Savage HC, Jr, Cindrich LA, Loving RT, Elliott JA. Efficacy of enhanced evening light for advanced sleep phase syndrome. Behav Sleep Med. 2003;1(4):213–226. doi: 10.1207/S15402010BSM0104_4. [DOI] [PubMed] [Google Scholar]
- 91.Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89(1):1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 92.Dorrian J, Darwent D, Dawson D, Roach GD. Predicting pilot's sleep during layovers using their own behaviour or data from colleagues: implications for biomathematical models. Accid Anal Prev. 2012;45(Suppl):17–21. doi: 10.1016/j.aap.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Arendt J, Middleton B, Williams P, Francis G, Luke C. Sleep and circadian phase in a ship's crew. J Biol Rhythms. 2006;21(3):214–221. doi: 10.1177/0748730405285278. [DOI] [PubMed] [Google Scholar]
- 94.Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, Vedaa O, Nordhus IH, Bjorvatn B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: effects on subjective and objective sleep. Chronobiol Int. 2014;31(1):72–86. doi: 10.3109/07420528.2013.823200. [DOI] [PubMed] [Google Scholar]
- 95.Wasdell MB, Jan JE, Bomben MM, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res. 2008;44(1):57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 96.van Maanen A, Meijer AM, van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: A systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Appleton RE, Jones AP, Gamble C, et al. The use of melatonin in children with neurodevelopmental disorders and impaired sleep: a randomised, double-blind, placebo-controlled, parallel study (MENDS) Health Technol Assess. 2012;16(40):i–239. doi: 10.3310/hta16400. [DOI] [PubMed] [Google Scholar]
- 98.Kim MJ, Lee GH, Kim CS, et al. Comparison of three actigraphic algorithms used to evaluate sleep in patients with obstructive sleep apnea. Sleep Breath. 2013;17(1):297–304. doi: 10.1007/s11325-012-0689-z. [DOI] [PubMed] [Google Scholar]
- 99.Elbaz M, Roue GM, Lofaso F, Quera Salva MA. Utility of actigraphy in the diagnosis of obstructive sleep apnea. Sleep. 2002;25(5):527–531. [PubMed] [Google Scholar]
- 100.Pallin M, O'Hare E, Zaffaroni A, et al. Comparison of a novel non-contact biomotion sensor with wrist actigraphy in estimating sleep quality in patients with obstructive sleep apnoea. J Sleep Res. 2014;23(4):475–484. doi: 10.1111/jsr.12126. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Diaz E, Quintana-Gallego E, Ruiz A, et al. Respiratory polygraphy with actigraphy in the diagnosis of sleep apnea-hypopnea syndrome. Chest. 2007;131(3):725–732. doi: 10.1378/chest.06-1604. [DOI] [PubMed] [Google Scholar]
- 102.Sharif MM, Bahammam AS. Sleep estimation using BodyMedia's SenseWear armband in patients with obstructive sleep apnea. Ann Thorac Med. 2013;8(1):53–57. doi: 10.4103/1817-1737.105720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 104.Gagnadoux F, Nguyen XL, Rakotonanahary D, Vidal S, Fleury B. Wrist-actigraphic estimation of sleep time under nCPAP treatment in sleep apnoea patients. Eur Respir J. 2004;23(6):891–895. doi: 10.1183/09031936.04.00089604. [DOI] [PubMed] [Google Scholar]
- 105.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27(8):1560–1566. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 106.Ting H, Huang RJ, Lai CH, et al. Evaluation of candidate measures for home-based screening of sleep disordered breathing in Taiwanese bus drivers. Sensors. 2014;14(5):8126–8149. doi: 10.3390/s140508126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 108.Bradshaw DA, Yanagi MA, Pak ES, Peery TS, Ruff GA. Nightly sleep duration in the 2-week period preceding multiple sleep latency testing. J Clin Sleep Med. 2007;3(6):613–619. [PMC free article] [PubMed] [Google Scholar]
- 109.Simons R, Wilschut ES, Valk PJ. Sleep and alertness in North Sea helicopter operations. Aviat Space Environ Med. 2011;82(7):704–710. doi: 10.3357/asem.2960.2011. [DOI] [PubMed] [Google Scholar]
- 110.Fietze I, Strauch J, Holzhausen M, et al. Sleep quality in professional ballet dancers. Chronobiol Int. 2009;26(6):1249–1262. doi: 10.3109/07420520903221319. [DOI] [PubMed] [Google Scholar]
- 111.Kim HJ, Kim JH, Park KD, Choi KG, Lee HW. A survey of sleep deprivation patterns and their effects on cognitive functions of residents and interns in Korea. Sleep Med. 2011;12(4):390–396. doi: 10.1016/j.sleep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Thorne H, Hampton S, Morgan L, Skene DJ, Arendt J. Differences in sleep, light, and circadian phase in offshore 18:00-06:00 h and 19:00-07:00 h shift workers. Chronobiol Int. 2008;25(2):225–235. doi: 10.1080/07420520802106850. [DOI] [PubMed] [Google Scholar]
- 113.Rosekind MR, Gregory KB, Mallis MM. Alertness management in aviation operations: enhancing performance and sleep. Aviat Space Environ Med. 2006;77(12):1256–1265. doi: 10.3357/asem.1879.2006. [DOI] [PubMed] [Google Scholar]
- 114.Gohar A, Adams A, Gertner E, et al. Working memory capacity is decreased in sleep-deprived internal medicine residents. J Clin Sleep Med. 2009;5(3):191–197. [PMC free article] [PubMed] [Google Scholar]
- 115.Forberg K, Waage S, Moen B, Bjorvatn B. Subjective and objective sleep and sleepiness among tunnel workers in an extreme and isolated environment: 10-h shifts, 21-day working period, at 78 degrees north. Sleep Med. 2010;11(2):185–190. doi: 10.1016/j.sleep.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 116.Barger LK, Flynn-Evans EE, Kubey A, et al. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol. 2014;13(9):904–912. doi: 10.1016/S1474-4422(14)70122-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76(11):1058–1063. [PubMed] [Google Scholar]
- 118.Rose M, Manser T, Ware JC. Effects of call on sleep and mood in internal medicine residents. Behav Sleep Med. 2008;6(2):75–88. doi: 10.1080/15402000801952914. [DOI] [PubMed] [Google Scholar]
- 119.Bjorvatn B, Stangenes K, Oyane N, et al. Randomized placebo-controlled field study of the effects of bright light and melatonin in adaptation to night work. Scand J Work Environ Health. 2007;33(3):204–214. doi: 10.5271/sjweh.1129. [DOI] [PubMed] [Google Scholar]
- 120.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 121.Centers for Disease Control and Prevention (CDC) Short sleep duration among workers--United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(16):281–285. [PubMed] [Google Scholar]
- 122.Morita Y, Sasai-Sakuma T, Asaoka S, Inoue Y. Prevalence and correlates of insufficient sleep syndrome in Japanese young adults: a web-based cross-sectional study. J Clin Sleep Med. 2015;11(10):1163–1169. doi: 10.5664/jcsm.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 124.Centers for Disease Control and Prevention (CDC) Drowsy driving - 19 states and the District of Columbia, 2009-2010. MMWR Morb Mortal Wkly Rep. 2013;61(51-52):1033–1037. [PubMed] [Google Scholar]
- 125.Geiger-Brown J, Rogers VE, Trinkoff AM, Kane RL, Bausell RB, Scharf SM. Sleep, sleepiness, fatigue, and performance of 12-hour-shift nurses. Chronobiol Int. 2012;29(2):211–219. doi: 10.3109/07420528.2011.645752. [DOI] [PubMed] [Google Scholar]
- 126.Hartzler BM. Fatigue on the flight deck: the consequences of sleep loss and the benefits of napping. Accid Anal Prev. 2014;62:309–318. doi: 10.1016/j.aap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovas JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–659. doi: 10.3945/an.115.008623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gschliesser V, Frauscher B, Brandauer E, et al. PLM detection by actigraphy compared to polysomnography: a validation and comparison of two actigraphs. Sleep Med. 2009;10(3):306–311. doi: 10.1016/j.sleep.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 129.Kobayashi M, Namba K, Ito E, et al. The validity of the PAM-RL device for evaluating periodic limb movements in sleep and an investigation on night-to-night variability of periodic limb movements during sleep in patients with restless legs syndrome or periodic limb movement disorder using this system. Sleep Med. 2014;15(1):138–143. doi: 10.1016/j.sleep.2013.08.790. [DOI] [PubMed] [Google Scholar]
- 130.Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study. Sleep Med. 2005;6(5):407–413. doi: 10.1016/j.sleep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 131.Montgomery-Downs HE, Crabtree VM, Gozal D. Actigraphic recordings in quantification of periodic leg movements during sleep in children. Sleep Med. 2005;6(4):325–332. doi: 10.1016/j.sleep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 132.Rogers VE, Gallagher PR, Marcus CL, Ohene-Frempong K, Traylor JT, Mason TB. Capturing PLMS and their variability in children with sickle cell disease: does ankle activity monitoring measure up to polysomnography? Sleep Med. 2012;13(8):1013–1020. doi: 10.1016/j.sleep.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.