Abstract
Objective(s)
This study compares 1-year intrauterine device (IUD) continuation among women presenting for emergency contraception (EC) and initiating the copper (Cu T380A) IUD or the levonorgestrel (LNG) 52 mg IUD plus 1.5 mg oral LNG.
Study design
This cohort study enrolled 188 women who presented at a single family planning clinic in Utah between June 2013 and September 2014 and selected either the Cu T380A IUD or LNG 52 mg IUD plus oral LNG for EC. Trained personnel followed participants by phone, text or e-mail for 12 months or until discontinuation occurred. We assessed reasons for discontinuation and used Cox proportional hazard models, Kaplan–Meier estimates and log-rank tests to assess differences in continuation rates between IUDs.
Results
One hundred seventy-six women received IUDs; 66 (37%) chose the Cu T380A IUD and 110 (63%) chose the LNG 52 mg IUD plus oral LNG. At 1 year, we accounted for 147 (84%) participants, 33 (22%) had requested removals, 13 (9%) had an expulsion and declined reinsertion, 3 (2%) had a pregnancy with their IUD in place and 98 (67%) were still using their device. Continuation rates did not differ by IUD type; 60% of Cu T380A IUD users and 70% of LNG 52 mg IUD plus oral LNG users were still using their device at 12 months (adjusted hazard ratio 0.72, 95% confidence interval 0.40–1.3).
Conclusion(s)
Two-thirds of women who chose IUD placement at the EC clinical encounter continued use at 1 year. Women initiating Cu T380A IUD and LNG 52 mg IUD had similar 1-year continuation rates. These findings support same-day insertion of IUDs for women who are seeking EC and would like to use a highly effective reversible method going forward.
Implications
Providing IUD options for EC users presents an opportunity to increase availability of highly effective contraception.
Keywords: Emergency contraception, Copper IUD, Levonorgestrel IUD, Continuation
1. Introduction
Leaders in the field of family planning acknowledge the lack of impact oral emergency contraception (EC) has on reducing population rates of unplanned pregnancy [1–3]. Procedures to streamline the delivery of highly effective methods of contraception to women presenting for EC are needed [2]. Providers should ensure that women are able to select the EC method best suited to both their immediate and future needs.
The United States (U.S.) Food and Drug Administration (FDA) approved the 52 mg levonorgestrel intrauterine device (LNG 52 mg IUD) for up to 5 years of use and the copper T380A intrauterine device (Cu T380A IUD) for 10 years. Research on continuation demonstrates that most women discontinue device use well before FDA expiration [4]. One-year continuation rates for standard intrauterine device (IUD) initiation in previously published studies range from 82% to 88% for LNG 52 mg IUD and 84% to 90.5% for Cu T380A IUD [5–8]. Scant published data exist on 1-year continuation rates when an IUD is initiated at the time of EC. Rates range from 64% in a small U.S. sample to 94% in a large Chinese sample [9,10]. Available data are for Cu T380A IUD continuation and the generalizability of these data may be limited by cultural differences. The Cu T380A IUD is the most effective method of EC and a good option for many women [11]; however, U.S. women selecting IUDs outside the EC setting show a strong preference for the LNG 52 mg IUD [12]. Thus, many women may prefer the LNG 52 mg IUD as EC if it were demonstrated to be an effective option.
To address the gap in the literature on IUD continuation when initiated at the time of EC and explore the use of the LNG 52 mg IUD at the time of EC provision, we offered women a combination of the LNG 52 mg IUD along with oral LNG for EC or the Cu T380A IUD and followed participants for 1 year. This analysis estimates the 12-month continuation of Cu T380A IUDs and LNG 52 mg IUDs when initiated at the time of EC. In addition, we compared the 1-year continuation rates of women initiating the IUD for EC to previously documented IUD continuation rates in new contraceptive (standard, non-EC) users. We hypothesized that women initiating both Cu T380A and LNG 52 mg IUDs for EC would have lower continuation rates than women who initiate the IUD as a standard, non-EC start.
We also examined differences between continuation and satisfaction rates of women who selected the Cu T380A IUD compared to those who selected the LNG 52 mg IUD plus oral LNG for EC. We hypothesized that women who selected the LNG 52 mg IUD plus oral LNG would have continuation rates and satisfaction levels at 1-year that were similar or no worse than those who initiated the Cu T380A IUD as part of an EC regimen.
2. Materials and methods
We conducted a prospective cohort study of women presenting for EC at a single family planning clinic in Utah. The details of the study design and report of the project’s primary outcomes including pregnancy rates and preference for the Cu T380A IUD or LNG 52 mg IUD in the EC setting have been previously published [13]. A brief description of the study design and the analysis for this report are described below.
We recruited women aged 18–35 years who reported unprotected intercourse in the previous 120 h, were interested in a same-day IUD, and spoke Spanish or English. The inclusion criteria also required a negative urine pregnancy test, desire to prevent pregnancy for at least 1 year, history of regular menstrual cycles (24–35 days) and knowledge of date of last menstrual period. Exclusion criteria included a positive urine pregnancy test, breastfeeding, vaginal bleeding of unknown etiology, current use of a highly effective method of contraception (sterilization, IUD or contraceptive implant), intrauterine infection within the past 3 months, untreated Neisseria gonorrhoeae or Chlamydia trachomatis infection, allergy to LNG or copper (respective to participants’ device choice), and known abnormalities of the uterus that distort the uterine cavity. Recruitment began in June 2013 and continued through September 2014. Trained clinic personnel approached and consented interested and eligible patients. After providing informed consent, participants selected either the Cu T380A IUD or the LNG 52 mg IUD plus 1.5 mg oral LNG. Participants received their desired EC regimen at no cost.
Participants completed self-administered surveys on the day of enrollment and at 1, 3, 6, 9 and 12 months or until device discontinuation using Research Electronic Data Capture (REDCap) [14]. REDCap is a secure Web application for data collection and management. Depending on participant contact method preference, we sent follow-up surveys via e-mail or a trained study staff member initiated a call and administered the survey over the phone. To aid follow-up, we tested cell phones at the enrollment visit and obtained two additional emergency contacts. We made attempts to contact participants up to three times per contact type at each follow-up point. An additional follow-up was attempted for all women still lost to follow-up at the time of last data collection point for the last enrolled participant. Participant demographic information including age, insurance status, ethnicity/race, relationship status, income, body mass index (BMI; kg/m2), obstetric history, reasons for needing EC (including exposure to unprotected intercourse, improper use of contraception or individual perceived risk), and method of EC selected were recorded at baseline. The study protocol was approved by the University of Utah Institutional Review Board (IRB 00050483).
We used Kaplan–Meier estimates and log-rank tests to assess differences in continuation rates between device types and estimate the proportion of patients continuing their selected IUD for 1 year (continuation proportion). We conducted univariate analyses for all categorical predictors of continuation, including IUD type, age, insurance, reasons for needing EC, BMI, ethnicity/race, relationship status, income and parity. We conducted the log-rank test of equality to explore variable inclusion in the final Cox model, and considered variables with log-rank test p values <.3 for inclusion. We then employed Cox regression models in an exploratory fashion to compare patient characteristics and time to discontinuation between the Cu T380A IUD and LNG 52 mg IUD groups. In the primary analysis, we censored those lost to follow-up per the standard non-informative assumption at the time of last contact. This method assumes that participants who drop out of the study are doing so for reasons unrelated to the study and that censored patients are considered to have survival prospects similar to participants who continued to be followed [15]. We also include a best- and worst-case sensitivity analysis to address concerns about the independence assumption. In addition, we explored interactions between potentially significant variables and used Cox–Snell residuals to confirm that the final model fit the data. We assessed differences in satisfaction between devices using chi-square tests. We conducted all analyses using Stata 14.0 statistical software (College Station, TX, USA).
To put this analysis in context, we also compared the 1-year continuation rates for the EC IUD insertions to previously published 1-year IUD continuation rates of women who initiated their IUDs with a standard of care encounter using chi-square tests and binomial proportion confidence intervals (CIs) [5–8]. We chose data from several standard start IUD studies as the comparators, as they provide a broad and rigorous assessment of IUD continuation rates among a large and diverse population of standard start IUD users [5–8].
In addition, we evaluated continuers’ satisfaction level at each follow-up contact at 1, 3, 6, 9 and 12 months. Satisfaction was evaluated using a 5-point Likert scale with categories of “very unsatisfied,” “unsatisfied,” “neutral,” “satisfied” and “very satisfied.” For the analysis, we grouped the categories “very unsatisfied” and “unsatisfied” together and “very satisfied” and “satisfied” together.
3. Results
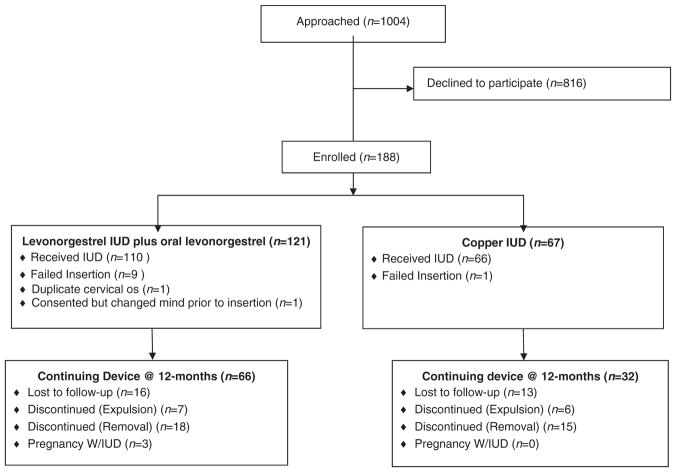
One hundred seventy-six women received IUDs at the time of their EC visit; 66 (38%) chose the Cu T380A IUD and 110 (63%) chose the LNG 52 mg IUD plus oral LNG. Participant study flow is outlined in Fig. 1. We examined differences in baseline characteristics by device type chosen on the day of enrollment (Table 1). The groups were similar except in BMI distribution.
Fig. 1.
Study flow diagram.
Table 1.
Characteristics of IUD for emergency contraception participants (n=176)
| Copper IUD (n=66) | LNG IUD+ (n=110) | p Value* | |||
|---|---|---|---|---|---|
|
|
|
||||
| n | % | n | % | ||
| Age category | |||||
| 18–19 | 9 | 14% | 12 | 11% | .67 |
| 20–24 | 31 | 48% | 55 | 50% | |
| 25–29 | 15 | 23% | 24 | 22% | |
| 30+ | 10 | 16% | 19 | 17% | |
| BMI category | |||||
| Underweight/Normal | 37 | 59% | 38 | 36% | .02 |
| Overweight | 15 | 24% | 43 | 41% | |
| Obese | 10 | 16% | 25 | 24% | |
| Race/Ethnicity | |||||
| White, non-Hispanic | 38 | 58% | 67 | 62% | .56 |
| Hispanic | 19 | 29% | 33 | 31% | |
| Other, non-white | 8 | 12% | 8 | 7% | |
| Relationship status | |||||
| Single/divorced/separated | 48 | 73% | 72 | 65% | .32 |
| Married/living with partner | 18 | 27% | 38 | 35% | |
| Previous pregnancy | |||||
| No | 34 | 52% | 49 | 45% | .37 |
| Yes | 32 | 48% | 61 | 55% | |
| Insurance status | |||||
| None | 36 | 56% | 61 | 56% | .97 |
| Public/private | 28 | 44% | 48 | 44% | |
| Income category | |||||
| <$12,000 | 29 | 45% | 41 | 37% | .75 |
| $12,000–$23,999 | 20 | 31% | 35 | 32% | |
| $24,000–$35,999 | 11 | 17% | 22 | 20% | |
| $36,000+ | 5 | 8% | 12 | 11% | |
| Reason needed emergency contraception | |||||
| No contraception | 33 | 52% | 51 | 47% | .54 |
| Contraceptive failure | 31 | 48% | 58 | 53% | |
| Total | 66 | 38% | 110 | 63% | |
Key: IUD, intrauterine device; LNG IUD+: same-day 52 mg levonorgestrel IUD plus 1.5 mg oral levonorgestrel; BMI, body mass index.
p Value of the chi-square test comparing EC regimen.
At 1-year following the EC clinical encounter, we accounted for a total of 147 (84%) participants, of which 33 (22%) had their device removed, 13 (9%) had a device expulsion and declined reinsertion, 3 (2%) had an unintended pregnancy and 98 (67%) were still using their device. Continuation rates did not differ by device type chosen, with 32 (60%; 95% CI 0.46–0.74) Cu T380A IUD users and 66 (70%; 95% CI 0.60–0.79) LNG 52 mg IUD users still using their device at 12 months (p=.58) (Table 2). When assessing expulsion and patient requested removals separately, there were no differences using the log-rank test between the device types for either outcome.
Table 2.
Continuation and satisfaction at 12 months by emergency contraception choice
| EC chosen | Enrolled (n) | Followed up at 12 months (n) | Continuing use, n (%) | Very satisfied or satisfied, n (%)a | Neutral, n (%)a | Very unsatisfied or unsatisfied, n (%)a |
|---|---|---|---|---|---|---|
| LNG IUD+ | 110 | 94 | 66 (70) | 41 (71) | 12 (21) | 5 (8) |
| Copper IUD | 66 | 53 | 32 (60) | 17 (65) | 5 (19) | 4 (15) |
Key: IUD, intrauterine device; LNG IUD+: same-day 52 mg levonorgestrel IUD plus 1.5 mg oral levonorgestrel; CU IUD: copper IUD.
Women continuing device use completed satisfaction questioning [completion of satisfaction question 84/98 (86%)].
The three unintended pregnancies occurred in women receiving the LNG 52 mg IUD plus oral LNG, yielding a 12-month pregnancy rate of 1.7% (95% CI 0.3%–4.9%). The first unintended pregnancy was a luteal phase pregnancy which occurred in the index EC cycle; this pregnancy is described in detail in a prior publication [13]. The second unintended pregnancy occurred without an IUD in place following an unrecognized IUD expulsion at 10 weeks post-insertion, and the third unintended pregnancy occurred with an IUD in place at 7 months post-insertion.
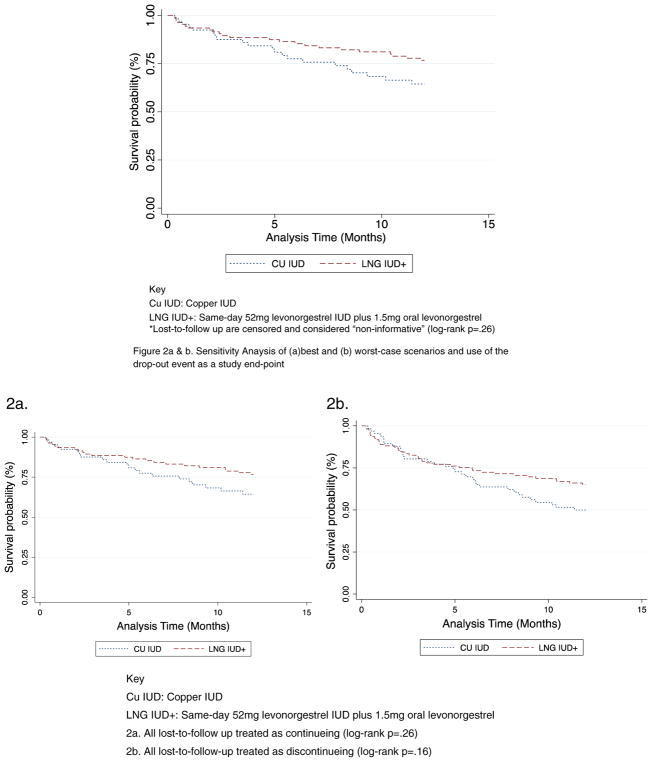
In the univariate analysis, using the log-rank test for equality of survivor functions, there were no differences in discontinuation (expulsion and requested removal) between the two types of devices (p=.26) (Fig. 2). In the best-case sensitivity analysis (Fig. 2a), we assume that all individuals who are lost to follow-up are continuing use; similar to the primary analysis, there is no difference in the log-rank test (p=.26). In the worst-case sensitivity analysis (Fig. 2b), we assume that all individuals who are lost to follow-up have discontinued use. In this worst-case analysis, the log-rank test is also nonsignificant (p=.16). These sensitivity analyses affirm the robustness of the primary analysis.
Fig. 2.
Kaplan–Meier survival curve estimating IUD continuation rates by chosen method of emergency contraception. Key: Cu IUD, copper IUD;LNG IUD+, same-day 52 mg levonorgestrel IUD plus 1.5 mg oral levonorgestrel. Sensitivity analysis of (a) best and (b) worst-case scenarios and use of the drop-out event as a study end-point.
Univariate analysis of other potential predictors of removals indicated that age [hazard ratio (HR) 1.4, 95% CI 0.8–2.5), IUD type chosen (HR 0.7, 95% CI 0.4–1.3), insurance used at the visit (HR 1.3, 95% CI 0.7–3.1) and reason for needing EC (HR 0.9, 95% CI 0.5–1.6) were potentially associated with discontinuations. However, when looking at the adjusted HRs, the model indicates that there are no statistically significant predictors of discontinuation in this cohort of women initiating IUDs at the time of EC.
Using chi-square analysis and binomial proportion CIs to assess IUD continuation, this study’s IUD continuation rates were significantly lower than in the studies with a standard IUD start (p<.001). However, this study’s IUD continuation rate did not differ from those reported in another IUD for EC study (p=.60) [9]. Of note, continuation rates did not differ by device type chosen in either our study or any of the comparator studies [5–8]. Fig. 3 compares the continuation rate and 95% CIs seen in this study to the continuation rates reported in previous studies. In addition, these continuation rates are higher than 1-year continuation of less effective methods.
Fig. 3.
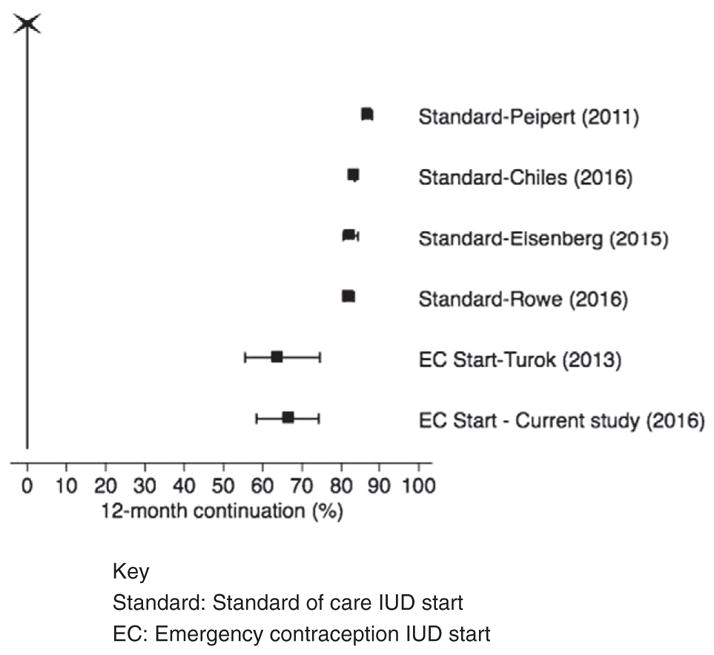
Forest plot demonstrating IUD continuation rates and 95% confidence intervals for current study and comparator studies. Key: Standard, standard of care IUD start;EC, emergency contraception IUD start.
The most common reason participants reported for IUD discontinuation in the first year of use was pain and/or bleeding; 4/66 (6%) of Cu T380A IUD users and 8/110 (7%) of LNG 52 mg IUD users reported this reason for discontinuation. Other reasons reported for removal in the LNG 52 mg IUD group were changes in mood, accidental self-removal and desired pregnancy [one each; 1/110 (0.9%)]. Additional reasons for removal among Cu T380A IUD users included infection and desire for pregnancy [one each; 1/66 (1.5%)].
At 12 months, 84 participants provided satisfaction data and over two-thirds (69%) of the continuing users reported being “very satisfied” or “satisfied” and 11% of continuers reported being “dissatisfied” or “very dissatisfied.” For women who responded to the satisfaction question at 12 months, reported satisfaction levels did not differ by device type (p=.65) (Table 2).
4. Discussion
Two-thirds of women participating in this study continued their chosen IUD for 1 year. The IUD at time of EC continuation rate is lower than rates reported in clinical studies on standard IUD insertion; however, women presenting for EC are at an increased risk of unintended pregnancy and may benefit from even a short time using a highly effective contraception method [9]. Initiation of an IUD at the time of an EC clinic visit provides an opportunity to transition from no method or a less effective method to a highly effective method of contraception. The 1-year pregnancy rate for the LNG 52 mg IUD plus oral LNG for EC users of 2% is substantially lower than oral EC users initiating routine contraceptive care, who have pregnancy rates up to 12% [17–20].
The 1-year continuation rates demonstrated in this study are lower than IUD continuation rates reported for women choosing a standard IUD start in multiple studies [5–8]. However, the study continuation rates are also higher than the 1-year continuation rates reported for women initiating less effective methods of contraception, which range from 55% for oral contraceptive users in the CHOICE project [5] to 12%–33% in adolescents initiating other hormonal methods [16]. It is reasonable to consider that women deciding to use an IUD at the time of an EC visit are different from standard start IUD users. While it is possible that women willing to initiate an IUD at the time of EC may have unique motivations and contraceptive goals that explain the differences in observed continuation rates, we do not have data to suggest that these women make impulsive contraceptive decisions or that they are more likely to discontinue an IUD abruptly. In this dataset, we are unable to assess the impact of no cost contraception on the continuation rates. These topics are worthy of future research efforts.
The 8% expulsion rate over 1 year in this group is higher than previously published IUD expulsion rates [6,21,22]. It is possible that the continuation rate may have been higher at 1 year if expelled devices were replaced at no cost to the study participants, but this was not provided as part of the study. The trend toward higher continuation rates among LNG 52 mg IUD rather than Cu T380A IUD users seen here may become statistically significant in a larger sample. This warrants further study.
The main strength of this study lies in the novelty of assessing IUD continuation for EC users with a sample of both Cu T380A IUD and LNG 52 mg IUD users. The few exclusion criteria were also reflective of real-world practice in family planning clinics. A loss to follow-up of 16% at 1 year is acceptable in this population that may be difficult to follow over a long period of time.
The most pressing limitation is that the study was not initially powered to detect differences in 1-year continuation rates between women initiating two different types of IUD. We based the sample size for this study on creating a point estimate and CI for pregnancy among women initiating the LNG 52 mg IUD at an EC visit; thus a priori power calculations are not available. However, in a post hoc analysis a sample of 176, with 66 Cu T380A IUD and 110 LNG 52 mg IUD individuals, we have 70% power to determine a two-sample comparison of survivor functions of method continuation with an HR of 0.72. In addition, this study relies on a comparison of historical controls [23,24]; however, this method of analysis is regularly used by other investigators in noninferiority trials. Finally, only 18% of eligible EC users approached agreed to participate in the study which limits generalizability, but it is within the range of previous reports of 12% of EC users desiring an IUD [25,26]. The increased availability of over the counter oral EC limits the study’s external validity, as the number of women presenting to clinics for EC and potentially receiving an IUD in that setting may be low.
The motivation to pursue this study was to test a method of broadening access to highly effective methods of contraception in a high-risk population. This approach was successfully demonstrated in a variety of clinical settings, including at the time of abortion [27,28], childbirth [29–31] and EC administration [9]. This study demonstrates that although women initiating an IUD at the time of EC have lower 1-year continuation rates than women initiating the IUD as a standard start, the majority continue IUD use at 1 year, and these women have high satisfaction rates. In addition, these continuation rates are substantially higher than 1-year continuation rates of less effective methods. The EC clinical encounter affords providers the opportunity to offer women with a high risk of unintended pregnancy options for more effective fertility control. Offering IUDs at the time of EC provides another opportunity to reduce unintended pregnancies by offering immediate access to highly effective contraception, an important principal in improving contraceptive care.
Acknowledgments
The authors wish to thank the study participants and the staff at Planned Parenthood Association of Utah, who made this study possible, especially administrators Kathy Burke, Penny Davies and Marci Fjelstad.
Footnotes
Funding: This study was independently funded by the University of Utah Department of Obstetrics and Gynecology Division of Family Planning. Use of REDCap was provided by a Eunice Kennedy Shriver National Institute of Child Health and Development grant [8UL1TR000105 (formerly UL1RR025764) NCATS/NIH].
Conflict of interest: D.K.T. receives speaking honoraria from Allergan, Medicines360, Merck and Teva. He serves on advisory boards for Actavis, Bayer, Pharmanest and Teva. The Department of Obstetrics and Gynecology, University of Utah, receives contraceptive clinical trials research funding from Bayer, Bioceptive, Contramed, Medicines360, Merck and Teva. L.M.G. reports personal fees from Evofem Medical Advisory Meeting, outside the submitted work.
ClinicalTrials.gov Identifier: NCT01963962.
References
- 1.Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181–8. doi: 10.1097/01.AOG.0000250904.06923.4a. [DOI] [PubMed] [Google Scholar]
- 2.Glasier A, Fairhurst K, Wyke S, Ziebland S, Seaman P, Walker J, et al. Advanced provision of emergency contraception does not reduce abortion rates. Contraception. 2004;69:361–6. doi: 10.1016/j.contraception.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Polis CB, Schaffer K, Blanchard K, Glasier A, Harper CC, Grimes DA. Advance provision of emergency contraception for pregnancy prevention: a meta-analysis. Obstet Gynecol. 2007;110:1379–88. doi: 10.1097/01.AOG.0000295603.84568.f6. [DOI] [PubMed] [Google Scholar]
- 4.Sanders JN, Turok DK, Gawron LM, Law A, Wen L, Lynen R. Two-year continuation of intrauterine devices and contraceptive implants in a mixed-payer setting: a retrospective review. Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peipert JF, Zhao Q, Allsworth JE, Petrosky E, Madden T, Eisenberg D, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105–13. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92:10–6. doi: 10.1016/j.contraception.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Chiles DP, Roberts TA, Klein DA. Initiation and continuation of long-acting reversible contraception in the United States military healthcare system. Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Rowe P, Farley T, Peregoudov A, Piaggio G, Boccard S, Landoulsi S, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498–506. doi: 10.1016/j.contraception.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turok DK, Jacobson JC, Dermish AI, Simonsen SE, Gurtcheff S, McFadden M, et al. Emergency contraception with a copper IUD or oral levonorgestrel: an observational study of 1-year pregnancy rates. Contraception. 2014;89:222–8. doi: 10.1016/j.contraception.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Godfrey EM, Wojdyla D, Dong J, Cong J, Wang C, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. BJOG. 2010;117:1205–10. doi: 10.1111/j.1471-0528.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 11.Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27:1994–2000. doi: 10.1093/humrep/des140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The contraceptive CHOICE project: reducing barriers to long-acting reversible contraception. Obstet Gynecol. 2010;203:115e1–7. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93:526–32. doi: 10.1016/j.contraception.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Survival probabilities (the Kaplan–Meier method) BMJ. 1998;317:1572. doi: 10.1136/bmj.317.7172.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raine TR, Foster-Rosales A, Upadhyay UD, Boyer CB, Brown BA, Sokoloff A, et al. One-year contraceptive continuation and pregnancy in adolescent girls and women initiating hormonal contraceptives. Obstet Gynecol. 2011;117:363–71. doi: 10.1097/AOG.0b013e31820563d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turok DK, Jacobson J, Simonsen SE, Gurtcheff SE, Trauscht-Van Horn J, Murphy PA. The copper T380A IUD vs. oral levonorgestrel for emergency contraception: a prospective observational study. Contraception. 2011;84:321–2. [Google Scholar]
- 18.Raine TR, Harper CC, Rocca CH, Fischer R, Padian N, Klausner JD, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62. doi: 10.1001/jama.293.1.54. [DOI] [PubMed] [Google Scholar]
- 19.Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Obstet Gynecol. 2009;201:146e1–6. doi: 10.1016/j.ajog.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh TL, Frezieres RG. Patterns of emergency contraception use by age and ethnicity from a randomized trial comparing advance provision and information only. Contraception. 2006;74:110–7. doi: 10.1016/j.contraception.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Shimoni N. Intrauterine contraceptives: a review of uses, side effects, and candidates. Semin Reprod Med. 2010;28:118–25. doi: 10.1055/s-0030-1248136. [DOI] [PubMed] [Google Scholar]
- 22.Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718–26. doi: 10.1097/AOG.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials. 2011;12:1–12. doi: 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States Food and Drug Administration. Guidance for industry: non-inferiority clinical trials. 2010. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz EB, Kavanaugh M, Douglas E, Dubowitz T, Creinin MD. Interest in intrauterine contraception among seekers of emergency contraception and pregnancy testing. Obstet Gynecol. 2009;113:833–9. doi: 10.1097/AOG.0b013e31819c856c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turok DK, Gurtcheff SE, Handley E, Simonsen SE, Sok C, North R, et al. A survey of women obtaining emergency contraception: are they interested in using the copper IUD? Contraception. 2011;83:441–6. doi: 10.1016/j.contraception.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Bednarek PH, Creinin MD, Reeves MF, Cwiak C, Espey E, Jensen JT. Immediate versus delayed IUD insertion after uterine aspiration. Med. 2011;364:2208–17. doi: 10.1056/NEJMoa1011600. [DOI] [PubMed] [Google Scholar]
- 28.Okusanya BO, Oduwole O, Effa EE. Immediate postabortal insertion of intrauterine devices. Cochrane Database Syst Rev. 2014;7:Cd001777. doi: 10.1002/14651858.CD001777.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? Obstet Gynecol. 2012;206:481e1–7. doi: 10.1016/j.ajog.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Cohen R, Sheeder J, Arango N, Teal SB, Tocce K. Twelve-month contraceptive continuation and repeat pregnancy among young mothers choosing postdelivery contraceptive implants or postplacental intrauterine devices. Contraception. 2016;93:178–83. doi: 10.1016/j.contraception.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Levi EE, Stuart GS, Zerden ML, Garrett JM, Bryant AG. Intrauterine device placement during cesarean delivery and continued use 6 months postpartum: a randomized controlled trial. Obstet Gynecol. 2015;126:5–11. doi: 10.1097/AOG.0000000000000882. [DOI] [PMC free article] [PubMed] [Google Scholar]