Abstract
Drosophila hemocytes are akin to mammalian myeloid blood cells that function in stress and innate immune-related responses. A multi-potent progenitor population responds to local signals and to systemic stress by expanding the number of functional blood cells. Here we show mechanisms that demonstrate an integration of environmental carbon dioxide (CO2) and oxygen (O2) inputs that initiate a cascade of signaling events, involving multiple organs, as a stress response when the levels of these two important respiratory gases fall below a threshold. The CO2 and hypoxia-sensing neurons interact at the synaptic level in the brain sending a systemic signal via the fat body to modulate differentiation of a specific class of immune cells. Our findings establish a link between environmental gas sensation and myeloid cell development in Drosophila. A similar relationship exists in humans, but the underlying mechanisms remain to be established.
In mammals, crosstalk between O2 and CO2 sensing central and peripheral chemoreceptors has been linked to functions of the hematopoietic system. Here, the authors show an evolutionarily relevant cascade involving multiple organs that links CO2- and O2-chemosensation to immune cell maturation in Drosophila.
Introduction
Carbon dioxide (CO2) is the first identified gaseous molecule that evokes innate avoidance behavior in Drosophila1, and is a critical sensory and respiratory cue that alters a variety of animal behaviors2. CO2 is detected by a heterodimeric receptor encoded by Gr21a and Gr63a that is expressed in the terminal organ of the larval head or in the antennal olfactory receptor neurons called ab1C in adult flies3,4. Even though CO2 was initially identified as a stress molecule, fruits, yeast, and animals emit CO2 as a respiration by-product that lead to complex combinatorial responses to odorants5.
Drosophila hemocytes are akin to mammalian myeloid cells and are sentinels for stress and innate immune-related responses6,7. Drosophila hemocytes arise from multi-potent blood progenitors and are comprised of three representative classes of myeloid-like cells: plasmatocytes, crystal cells (CCs), and lamellocytes8. The majority of mature hemocytes are macrophage-like plasmatocytes while a small fraction becomes CCs known to function in wound healing and innate immune responses7,9. Lamellocytes are seldom found in conventional culture conditions and are evident only upon immune challenge10.
The maintenance of hematopoietic stem- and progenitor populations and their interactions with the niche has been extensively studied in both humans and in model systems6,8,11,12. However, the importance of extrinsic cues that originate outside the stem- or progenitor compartment has not been carefully characterized and requires extensive future studies. Complex systemic responses often involve multiple organs and a combination of developmental and stress-related signals13. With the use of modern genetic techniques, the Drosophila hematopoietic system allows us to delineate mechanistic insights into intricate responses of the myeloid progenitor population to multiple systemic signals14–16. However, how sensory neurons that detect the level of ambient gases communicate with the myeloid blood system has not been elucidated although functional analogies have been identified in mammals.
In this study, we identify a genetic link between the respiratory gas-chemosensation and myeloid blood development in Drosophila. CO2-sensing and hypoxia-sensing neurons interact at the synaptic level. Low CO2 or O2 triggers the stabilization of Hypoxia inducible factor-α in a small set of neurons in the ventral nerve cord (VNC), promoting transcription of the cytokine unpaired3 in the brain. This secreted cytokine activates the JAK/STAT pathway in fat bodies (considered similar to the liver), resulting in the expression and secretion of an insulin-like protein, Dilp6. This secreted protein activates the insulin receptor in the hematopoietic organ and this leads to increased levels of the protein Serrate, a ligand for Notch. Increased Notch signaling raises the number of a specific class of immune cells. Notably, this phenotype is recapitulated by modulating atmospheric CO2 or O2, emphasizing that gas perception is directly associated with differentiation of the hematopoietic system in Drosophila.
Results
Respiratory chemosensation and CC differentiation
CO2 activates a transmembrane, heterodimeric gustatory receptor complex, called Gr21a/Gr63a3,4. It is specifically expressed in the terminal organ of the larval head (Supplementary Fig. 1a). Other tissues, including the hematopoietic organ called the lymph gland (Fig. 1a), do not express this receptor (Supplementary Fig. 1b). The CO2-sensing neuron (CO2SN for simplicity) sends its projection to the subesophageal ganglion (SEG), which in turn connects, through largely unmapped circuits, to the central brain and VNC3,4,17. Receptors capable of responding to oxygen levels (or monoxide gases and free radicals) are more widely expressed and belong to the intracellular soluble guanylyl cyclase class of proteins18,19. The receptor Gyc89da is activated by low molecular oxygen (O2) and the multiple neurons expressing it specifically sense hypoxia (HypSNs for simplicity). HypSNs are inhibited in normoxia and hyperoxia20,21.
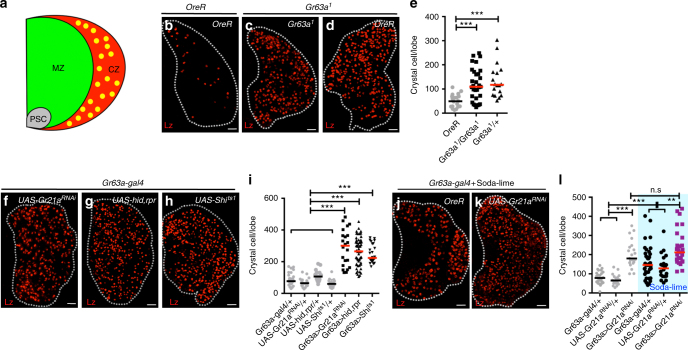
Fig. 1.
CO2 chemosensation controls the differentiation of crystal cells. Graphs indicate the number of crystal cells (CC) per lymph gland lobe. Bars in graphs: the median. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Scale Bar: 20μm. White dotted lines: lymph glands. Statistical results and genotypes are indicated in Supplementary Table 3. a A schematic representation of the primary lobe of the hematopoietic organ, the larval lymph gland. Undifferentiated blood progenitors reside in the inner core, termed the medullary zone (MZ), and give rise to mature blood cell lineages including crystal cells (CC, marked in yellow) that occupy a region termed the cortical zone (CZ). The posterior signaling center (PSC) secretes multiple factors to maintain the progenitors. Only the features relevant to this study are shown. b–l Loss of CO2SN activity causes increased CC number. Wild-type third-instar larvae express fewer than 100-CCs per primary lymph gland lobe (CCs are marked in red, Lz) (b) Both homozygous (Gr63a1/ Gr63a1) (c) and heterozygous (Gr63a1/+) mutants (d) of Gr63a, the gene encoding the chemoreceptor for CO2 sensing, exhibit increased numbers of CCs. Quantitation of CC numbers shown in (e). Gr21a encodes the second subunit of the CO2 chemoreceptor that functions with Gr63a. Knockdown of Gr21a function (Gr63a-gal4; UAS-Gr21aRNAi) (f), or genetic ablation of the CO2 receptor neuron by expression of pro-apoptotic genes, hid and rpr (Gr63a-gal4; UAS-hid,rpr) (g) or attenuation of synaptic transmission by expression of a temperature-sensitive form of the dynamin-like protein Shibire (Gr63a-gal4; UAS-Shits1) (h), each causes a significant increase in the number of CCs. This phenotype is not observed in Gal4- or UAS-controls alone. Quantitation is shown in i. Absorption of ambient CO2 in larval culture vial with the use of soda-lime, a mixture of bases that eliminates gaseous CO2 (see Methods for detail), mimics the CO2SN mutant phenotype (j). This phenotype is enhanced by a simultaneous knockdown of Gr21a in the CO2SN (Gr63a-gal4; UAS-Gr21aRNAi) (k). Quantitation shown in l. The blue shading in panels (l) represents soda-lime-induced low environmental CO2 condition
We modified the activities of the CO2SN or the HypSNs using a variety of genetic and environmental manipulations (Supplementary Fig. 1c). As a hematopoietic readout, we count CCs, which function in wound healing, clotting, innate immunity, and hypoxic stress response9,22,23. Compared with wild-type larvae raised under conventional environmental conditions, we find between 2 and 4-fold increase in the number of CCs upon reduced CO2SN activity (Fig. 1b-l and Supplementary Fig. 1d-l). This phenotype is specific to the CCs and does not alter the number of other cell types or the overall size of the lymph gland (Supplementary Fig. 1m-o). Also, the numbers of sessile and circulating CCs within the larvae are not affected (Supplementary Fig. 1p-s). Loss of HypSN activity has no effect on CC number (Fig. 2a, b, d) while elevated activity of HypSNs causes 2-fold increase in CCs (Fig. 2c, d) when specifically activated in neurons (Fig. 2e–g and Supplementary Fig. 1t). This phenotype is recapitulated upon inhibition of neuronal Gyc89da (Supplementary Fig. 1u). Thus, low CO2SN activity (low CO2 availability) or high HypSNs activity (low O2 availability) favors extra CC formation.
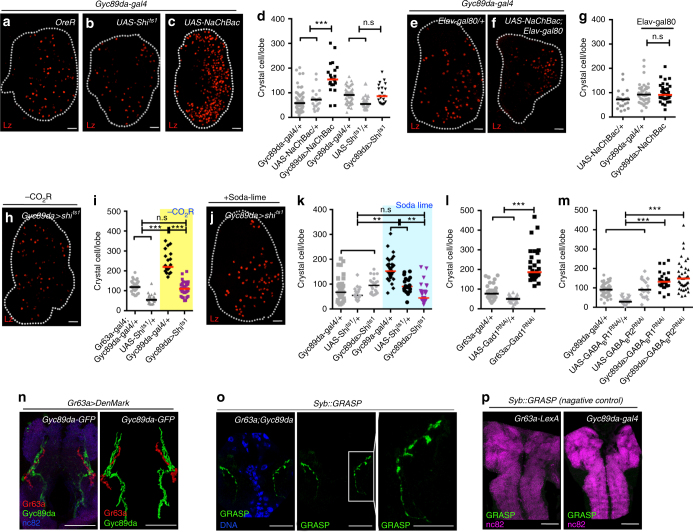
Fig. 2.
CO2SN forms inhibitory synapses with HypSN. Graphs indicate the number of crystal cells (CC) per lymph gland lobe. Bars in graphs: the median. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Scale Bar: 20μm, unless otherwise indicated. White dotted lines: lymph glands. CO2SN inhibited conditions: –CO2R with shaded yellow in i, and Soda-lime generated low CO2: shaded blue in k for clarity. a–g Constitutive activation of HypSNs induces CC differentiation. Control animals (Gyc89da-gal4/ + ) have an average of 70-90 CCs (a). Inactivation of HypSNs (Gyc89da-gal4; UAS-Shits1) does not alter CC number (b). Constitutive activation of HypSNs by expressing the bacterial sodium channel, NaChBac (Gyc89da-gal4; UAS-NaChBac) results in increased CC differentiation (c). Quantitation is shown in d. When the expression of NaChBac is specifically blocked in neurons (Gyc89da-gal4, Elav-gal80; UAS-NaChBac) the average CC number is unchanged (f) from that in control (e). Quantitation shown in g. h–k HypSNs function downstream of CO2SN. As shown in (Fig. 1h), CO2SN inhibition increases CC number (Gr63a-gal4; UAS-Shits1). This phenotype reverts to wild type when HypSNs are also inhibited (Gr63a-gal4; Gyc89da-gal4, UAS-Shits1) (h). Quantitation shown in i. Soda-lime-mediated CC phenotype is also alleviated by inhibition of HypSNs (low atmospheric CO2 + Gyc89da-gal4; UAS-Shits1) (j). Quantitation shown in k. l–m GABA-mediated inhibition of HypSNs by CO2SN. Knockdown of Gad1 in the CO2SN (Gr63a-gal4; UAS-Gad1RNAi) enhances differentiation of CCs (l). Similarly, loss of either GABABR1 or GABABR2 in HypSNs (Gyc89da-gal4; UAS-GABABR1RNAi or Gyc89da-gal4; UAS-GABABR2RNAi) also leads to increased CC differentiation (m). n–o Physical proximity and overlap of CO2SN and HypSNs in the SEG. Projections of the CO2SN adjoining HypSNs at the level of the SEG shows significant co-localization: CO2SN in red and HypSN in green (Gr63a-gal4; UAS-DenMark; Gyc89da-GFP). Rendered image of the co-localization data shown in right panel (n). Syb::GRASP expression of the CO2SN and HypSNs in the SEG (o). Syb::GRASP signal resulting from points of contact is shown in green (Magnified images in the following panels) (o). p Negative control experiments for the Syb::GRASP data shown in o. Gr63a-LexA alone (Gr63a-LexA; UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10) or Gyc89da-gal4 alone (Gyc89da-gal4; UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10) does not give rise to a GRASP signal. Scale bar: 50μm
The increased CC phenotype due to low CO2SN activity is fully suppressed to wild-type numbers by concurrent low HypSN activity (Fig. 2h–k), raising the possibility of a coupled response. An RNAi-based mini-screen for enzymes that synthesize neurotransmitters revealed that knockdown of Gad1 (encoding the GABA synthesis enzyme, glutamate decarboxylase) in the CO2SN gives increased CC numbers (Fig. 2l and Supplementary Fig. 1v). Remarkably, knockdown of the GABAB receptors, R1/R2 in the HypSNs similarly raises CC numbers (Fig. 2m and Supplementary Fig. 1w, x). This prompted us to investigate a possible direct interaction between these neurons at inhibitory synapses.
We expressed the dendritic marker, DenMark24, in the CO2SN and simultaneously marked HypSNs with GFP. Separate neurons with nuclei residing within the terminal ganglion express these two receptors. Although both send anterior projections to the terminal organ, at this location, they appear to be non-overlapping (Supplementary Fig. 1y). In contrast, the posterior projection from the single CO2SN approaches the SEG, where it comes into extremely close association with a projection from HypSNs (Fig. 2n).
We next utilized the GRASP technique25,26, in which a positive fluorescence signal indicates molecular level proximity between the two neurons. Using membrane-GRASP, we detect such close association between the CO2SN and the HypSNs at the level of the SEG (Supplementary Fig. 1z, aa). For even finer analysis, we used Synaptobrevin::GRASP that would only highlight points of active synaptic contacts (Full genotype: Gyc89da-gal4; Gr63a-LexA, UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10). Punctate signals are readily evident in the anterior SEG indicating synapse formation between these two classes of neurons (Fig. 2o, p). Taken together, the genetic data on the involvement of GABAergic neurons, the DenMark data on proximity of labeled branches and the GRASP analyses, we conclude that the CO2SN forms inhibitory synapses with HypSN branches at the level of the SEG. These data do not preclude additional parallel interactions elsewhere within the neuronal circuitry of the central brain.
Attenuation of CO2SN stabilizes Hifα in HypSNs in the VNC
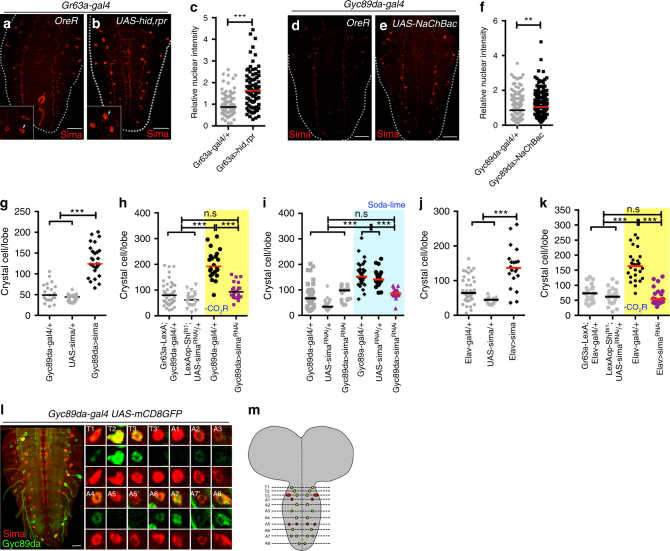
Hypoxic conditions allow stabilization of Hypoxia inducible factor-α (Hifα, called Sima in Drosophila) and favor CC differentiation through non-canonical activation of Notch27. A small number of cells in the VNC express very low levels of Sima protein even in wild-type larvae grown under normoxic conditions (Fig. 3a) and this Sima protein expression is upregulated if CO2SN activity is attenuated (Fig. 3b, c), or if HypSN activity is increased (Fig. 3d–f). Additionally, loss of Gad1 in the CO2SN raises Sima levels in the VNC neurons (Supplementary Fig. 2a-c), highlighting the interaction between CO2SN and HypSNs in this process. Overexpression of sima in HypSNs is sufficient to induce increased CC differentiation under normal gaseous ligand sensation (Fig. 3g and Supplementary Fig. 2d). Also, knockdown of sima transcript in HypSNs rescues the CC phenotype seen under low CO2SN activity (Fig. 3h, i and Supplementary Fig. 2e-g). These two results are also seen if sima levels are manipulated specifically in all neurons (Fig. 3j, k and Supplementary Fig. 2g-i). Thus, no non-neuronal participant is essential and Sima increase in HypSNs is both necessary and sufficient for linking the sensory signals to CC formation. The high VNC Sima expression is seen in 14 pairs of neurons of which 10 pairs are HypSNs (Fig. 3l, m). These results establish that HypSNs that are also Sima+ are important for the CC phenotype.
Fig. 3.
Sima (Hifα) stabilization in HypSNs affects crystal cell number. c and f indicate the relative intensities of nuclear Sima, and Graphs in g–k indicate the number of CCs in a single lymph gland lobe. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Scale Bar: 50μm. Bars in graphs: the median. CO2SN inhibited conditions: –CO2R with shaded yellow in h, k, and Soda-lime generated low CO2: shaded blue in i for clarity. a–c Inhibition of the CO2SN elevates Sima expression in the VNC. Control brains (Gr63a-gal4/ + ) exhibit very low Sima expression in the VNC (Sima is in red) (a). Genetic ablation of CO2SN (Gr63a-gal4; UAS-hid,rpr) leads to 2-fold increase in accumulation of nuclear Sima in specific VNC cells (white arrows in inset) (b). Quantitation of data shown in c. d–i Activation of HypSNs elevates Sima expression in the VNC. Control brains (Gyc89da-gal4/ + ) show very low Sima expression (d). Constitutive activation of HypSNs significantly increases accumulation of nuclear Sima in specific VNC cells (Gyc89da-gal4; UAS-NaChBac) (e). Quantitation of relative nuclear Sima intensity shown in f. CC differentiation is significantly enhanced when sima is overexpressed in HypSN cells (Gyc89da-gal4; UAS-sima) (g). simaRNAi expressed in HypSNs suppresses the extra CC differentiation phenotype caused by loss of CO2SN function (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-simaRNAi) (h). This rescue is also seen upon loss of sima in HypSNs when animals are grown in low CO2 (soda-lime) condition (i). j-k sima expression in neurons is linked to CC differentiation. Larvae expressing sima in the brain (Elav-gal4; UAS-sima) facilitates CC differentiation (j). Also, increased CC differentiation caused by CO2SN inhibition is rescued by concurrent inhibition of sima in the brain (Gr63a-LexA, LexAop-Shits1; Elav-gal4, UAS-simaRNAi) (k). l, m Sima co-localizes with a subset of HypSNs. Within the VNC, neurons express Sima in total 14 pairs per hemineuromere from T1 to A8. Amongst these, 10 pairs co-localize with Gyc89da in the T1-T3, A2-A4 and A6-A8 (Gyc89da-gal4; UAS-mCD8GFP). A magnified view of Sima and Gyc89da-expressing neurons in the thoracic and abdominal ganglia (Sima, red; Gyc89da, green) (l). In the reconstructed image of VNC, Sima+ HypSNs are shown in yellow and Sima+ cells that are not HypSNs are marked in red (m)
Upd3 from the brain signals to the fat body
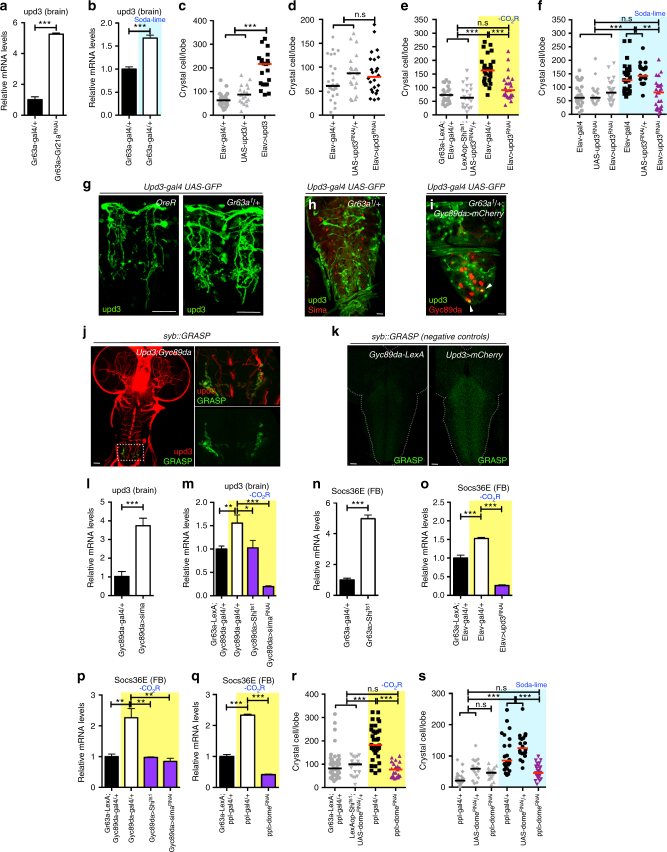
VNC neurons are known to secrete hormones and cytokines into the hemolymph28. We conducted a targeted mini-screen of known hormone and secreted factor-encoding genes (Supplementary Fig. 3a) to determine if any of these is differentially expressed at the mRNA level in the Gr63a mutant brain compared with controls. Three of these genes are upregulated in the mutant at the transcriptional level, of which only one, that encodes the cytokine unpaired3 (upd3) gives increased CC numbers upon pan-neuronal overexpression (Fig. 4a–c and Supplementary Fig. 3b). Loss of upd3 in neurons does not cause any hematopoietic defect (Fig. 4d) but upd3-RNAi or upd3 mutants suppress the extra CC phenotype of CO2SN inhibition (Fig. 4e, f and Supplementary Fig. 3c-f). This indicates a role for Upd3 in transmitting the gaseous ligand-generated stress signal to regions outside the brain.
Fig. 4.
Upd3 secreted from the brain triggers a systemic signal. c–f, r–s indicate CC numbers in a single lymph gland. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Error bars in a–b, l–q: standard deviation. Bars in c–f, r–s: the median. Scale Bar: 50μm. CO2SN inhibited conditions: –CO2R with shaded yellow in e, m, o–r. Soda-lime generated low CO2 conditions: shaded blue in b, f, s. a-b upd3 upregulation in the brain is linked to CC differentiation. Loss of Gr21a (Gr63a-gal4; UAS-Gr21aRNAi) in the CO2SN (a) or scavenging of CO2 (b) results in increased upd3 mRNA in the brain. c-m. Upd3 functions downstream of Sima. upd3 in the brain causes increased CC formation (Elav-gal4; UAS-upd3) (c). Loss of neuronal upd3 (Elav-gal4; UAS-upd3RNAi) alone does not alter CC number (d). Silencing neuronal upd3 suppresses the CC phenotype caused by inhibition of CO2SN (Gr63a-LexA, LexAop-Shits1; Elav-gal4, UAS-upd3RNAi) (e) or by soda-lime treatment (Elav-gal4; UAS-upd3RNAi) (f). Compared with control, upd3 is elevated in the posterior VNC of Gr63a1/ + mutants (Upd3-gal4, UAS-GFP; Gr63a1/ + ) (g). No overlap is observed between Sima+ and upd3+ neurons (h). HypSN seen in the posterior VNC are occasionally upd3-positive (white arrow heads), but the majority of upd3+ cells are not HypSNs (Upd3-gal4, UAS-GFP; Gyc89da-LexA; LexAop-mCherry) (i). Syb::GRASP expression of the HypSNs and Upd3+ neurons in the VNC (Magnified images in the following panels) (Upd3, red; GRASP, green) (j). Gyc89da-LexA alone (Gyc89da-LexA; UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10) or Upd3-gal4 alone (Upd3-gal4 UAS-mCherry; UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10) does not give rise to a GRASP signal (k). Overexpression of sima in HypSNs increases neuronal upd3 mRNA (Gyc89da-gal4; UAS-sima) (l). sima RNAi or inhibition of HypSNs suppresses the elevated upd3 levels (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-simaRNAi or Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-Shits1) (m). n–s Brain-secreted Upd3 functions in the fat body. Socs36e is induced in the fat body upon loss of CO2SN (Gr63a-LexA; LexAop-Shits1) (n). This expression is suppressed upon: loss of neuronal upd3 (Gr63a-LexA, LexAop-Shits1; Elav-gal4, UAS-upd3RNAi) (o), by simultaneous inhibition of HypSNs (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-Shits1) (p), by sima RNAi in the HypSNs (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-simaRNAi) (p), or upon loss of dome in the fat body (Gr63a-LexA, LexAop-Shits1; ppl-gal4, UAS-domeRNAi) (q). dome RNAi in the fat body reverts the CC numbers in the CO2SN mutant (Gr63a-LexA, LexAop-Shits1; ppl-gal4, UAS-domeRNAi) (r) or in soda-lime conditions (ppl-gal4; UAS-domeRNAi) (s) to wild-type
Under conditions of reduced CO2SN activity, upd3 is detected in a large number of cells in the brain that are not obligatorily HypSNs or Sima+ (Fig. 4g, h). Yet, upd3 and HypSNs co-localize and synapse onto each other at the posterior region of VNC (Full genotype: Upd3-gal4, UAS-mCherry; Gyc89da-LexA, UAS-CD4-spGFP11, LexAop-nSyb-spGFP1-10) (Fig. 4i–k). Consistently, experimental evidence presented below suggests that the observed increase in upd3 is critically dependent on Sima+ HypSNs. Overexpression of sima in HypSNs is sufficient to induce a 4-fold increase in upd3 transcription in the brain (Fig. 4l). Also, loss of sima in HypSNs rescues the high upd3 transcription in a reduced CO2SN activity background (Fig. 4m). Simultaneous loss of CO2SN and HypSN activities gives wild-type levels of upd3 transcription (Fig. 4m).
Socs36e is a direct downstream transcriptional target of the JAK/STAT signaling pathway initiated by Upd3 upon binding its receptor, Domeless (Dome)29. qPCR analysis of dissected tissues from larvae lacking CO2SN activity shows upregulation of Socs36e specifically in the fat body (considered similar to the liver) (Fig. 4n), and importantly, not in the lymph gland (Supplementary Fig. 3g). Fat body Socs36e expression is suppressed when either: upd3 is down-regulated in the brain, or when sima expression is decreased within HypSNs, or upon simultaneous inhibition of CO2SN and HypSNs (Fig. 4o, p). Additionally, overexpression of either sima or upd3 in the brain is sufficient to induce Socs36e in the fat body (Supplementary Fig. 3h, i). Finally, dome-RNAi autonomously suppresses Socs36e in the fat body and non-autonomously affects CC number in CO2SN activity-depleted larvae (Fig. 4q–s and Supplementary Fig. 3j, k).
Dilp6 induces Serrate via InR in blood progenitors
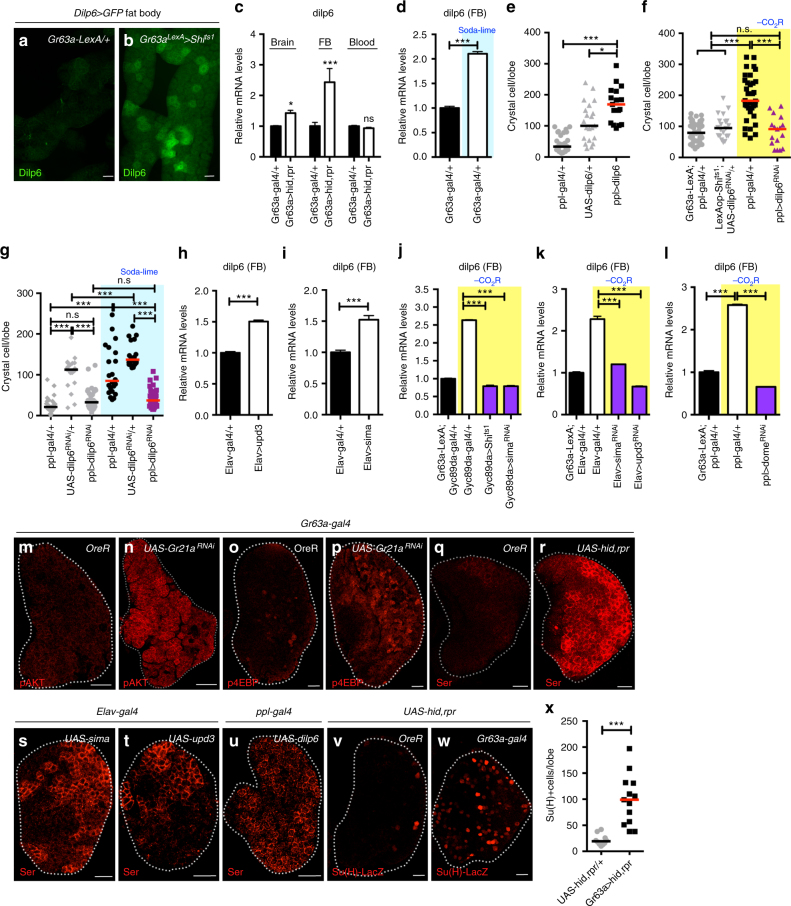
We screened for increased expression of RNAs encoding neurosecretory peptides upon loss of CO2SN activity. Only one positive candidate, the Drosophila Insulin-like peptide-6 (dilp6), is normally low in the fat body during larval stages30 and is specifically upregulated in this organ when the CO2SN is mutated (Fig. 5a–d and Supplementary Fig. 4a-d). While dilp6 is additionally expressed in glia31, we found that overexpression in the fat body, but not in glia, has an effect on CC number (Fig. 5e and Supplementary Fig. 4e-g). Similarly, the CC phenotype due to reduced CO2SN activity is efficiently rescued when dilp6 is specifically blocked in the fat body or in the dilp641 mutant, but not when dilp6-RNAi is expressed in glial cells (Fig. 5f, g and Supplementary Fig. 4h-k).
Fig. 5.
CO2SN/HypSNs systemically control Dilp6 and Serrate. e–g indicate the number of CCs in a single lymph gland lobe. Scale bar: 20μm; except in a, b: 50μm. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Error bars in c–d and h–l: standard deviation. Bars in graph e–g, x: the median. CO2SN inhibited conditions: –CO2R with shaded yellow in f, j–l. Soda-lime generated low CO2 conditions: shaded blue in d, g. a–d Dilp6 secreted from the fat body is the second systemic signal. dilp6 is expressed upon loss of CO2SN activity (Gr63a-LexA, LexAop-Shits1; Dilp6-gal4, UAS-GFP) in the fat body (Dilp6, green) (a, b, d), but not the brain or blood (c). e–l dilp6 is necessary and sufficient in extra CC formation. Overexpression of dilp6 in the fat body is sufficient to induce CC differentiation (ppl-gal4; UAS-dilp6) (e). Loss of dilp6 in the fat body reverts CCs in the CO2SN mutant background to wild-type numbers (Gr63a-LexA, LexAop-Shits1; ppl-gal4, UAS-dilp6RNAi) (f). This is also seen under soda-lime treatment conditions (ppl-gal4; UAS-dilp6RNAi) (g). Expression of either upd3 (h) or sima (i) in the brain is sufficient to enhance dilp6 expression in the fat body (Elav-gal4; UAS-upd3 or Elav-gal4; UAS-sima). Increased expression of dilp6 is disrupted by: silencing HypSNs (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-Shits1) (j), by sima RNAi in HypSNs (Gr63a-LexA, LexAop-Shits1; Gyc89da-gal4, UAS-simaRNAi) (j), upon inhibition of neuronal upd3 (Gr63a-LexA, LexAop-Shits1; Elav-gal4, UAS-upd3RNAi) (k), or when domeRNAi is expressed in the fat body (Gr63a-LexA, LexAop-Shits1; ppl-gal4, UAS-domeRNAi) (l). m–x Insulin receptor (InR) activation by Dilp6 increases Serrate expression. pAKT (pAKT, red) (m–n) and p4EBP (p4EBP, red) (o–p) are upregulated in the lymph gland upon CO2SN inhibition (Gr63a-gal4; UAS-Gr21aRNAi). Compared with wild type (q), Serrate expression is substantially enhanced when CO2SN activity is lost (Gr63a-gal4; UAS-hid,rpr) (Serrate, red) (r). Neuronal expression of either sima (Elav-gal4; UAS-sima) (s) or upd3 (Elav-gal4; UAS-upd3) (t), is sufficient to enhance Serrate protein expression. This phenotype is also seen when dilp6 is overexpressed in the fat body (ppl-gal4; UAS-dilp6) (u). Su(H)-LacZ is activated in cells that receive active Notch signal. The number of such cells increases when CO2SN activity is attenuated (Gr63a-gal4, Su(H)-LacZ; UAS-hid,rpr) (Su(H)-LacZ, red) (v–w). Quantitation is shown in x
Overexpression of sima or upd3 in the brain raises fat body levels of dilp6 (Fig. 5h, i). Increased dilp6 in a CO2SN activity-depleted background is suppressed: upon concurrent inhibition of HypSNs, with attenuation of brain sima or upd3, or when dome-RNAi is expressed in the fat body (Fig. 5j-l). A different insulin related peptide, Dilp2, functions in hematopoiesis, but not directly in CC formation14,32 and no previously known role in hematopoiesis was identified for Dilp6. Both Dilps function by binding to the insulin receptor (InR), which is known to promote differentiation of CCs16,32. In CO2SN activity-depleted larvae, we detect high pAKT and p4EBP, direct phosphorylation targets of the InR pathway in the lymph gland (Fig. 5m–p and Supplementary Fig. 4l-q). This is also seen when dilp6 is overexpressed in the fat body (Supplementary Fig. 4r, s). We hypothesize that altered gaseous signaling leads to systemic secretion of Dilp6 from the fat body that activates InR pathway30,31,33.
A hallmark of CC fate specification is the interaction between the ligand Serrate, expressed in internal signaling centers within the lymph gland, with its receptor Notch, present in neighboring cells27,34,35. Wild-type lymph glands from mid-second instar larvae exhibit low Serrate protein expression at the edge where differentiation is initiated (Fig. 5q). Reduced CO2SN activity has a pronounced effect on membrane Serrate expression as this protein is detected in more cells at a much higher level when compared with wild type (Fig. 5r and Supplementary Fig. 4t-v). Increased Serrate is also seen upon sima or upd3 overexpression in the brain, or when dilp6 is driven in the fat body (Fig. 5s–u). We also detect an increase in number of cells in which Notch is active, but there is no change in the activation level per cell (Fig. 5v–x). We conclude that the cascade leading up to InR increases Serrate expression, which in turn activates the Notch pathway in an increased number of cells causing them to take on CC fates.
CC differentiation controlled by altered environmental gases
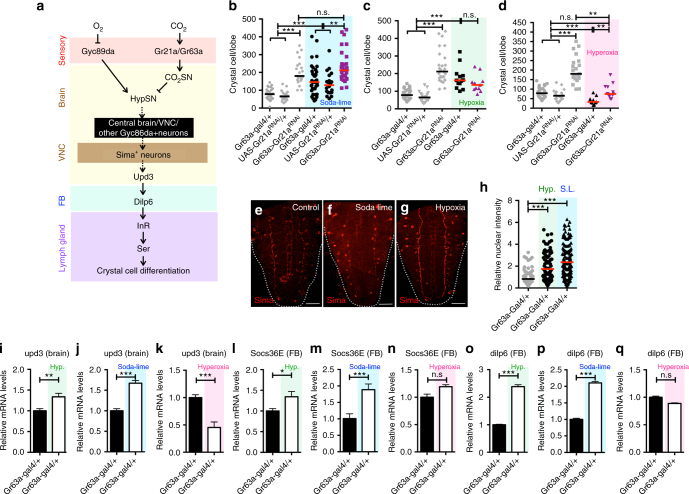
A model summarizing the results presented in this study is shown in Fig. 6a. Environmental CO2 activates its receptor Gr21a/Gr63a expressed on the CO2SN, while environmental levels of O2 repress HypSNs. This occurs at the level of sensory neurons, and the information is relayed to higher brain centers. Genetic data support communication between these sensory neurons in the suboesophageal ganglion (SEG) and accumulation of Sima in a different set of HypSNs within a small number of VNC neurons. The neuronal circuits between the SEG, VNC and higher brain centers have not been mapped yet. However, it is clear that Sima enhances upd3 expression in the brain and that secretion of this cytokine activates the JAK/STAT pathway in the fat body and this results in Dilp6 expression and secretion into the hemolymph. The resulting Dilp6/InR signal within the lymph gland causes an increase in the level and number of cells that express Serrate. As is well known from the published literature, the Serrate–Notch interaction is critical in determining CC fate and number within the lymph gland19,26. The interorgan communication system identified in this study allows the monitoring of ambient gas levels in the environment and through their integration, allows any chronic imbalance of respiratory gases for the purpose of proper stress response and the maintenance of immune homeostasis.
Fig. 6.
Environmental alterations support results of genetic manipulations. b–d indicate the number of CCs in a single lymph gland lobe. Scale bar: 20μm. n.s: not significant (p > 0.01). *p < 0.01; **p < 0.001; ***p < 0.0001. Error bars in i–q: standard deviation. Bars in graph b–d, h: the median. Soda-lime generated low CO2 conditions: blue; hypoxic (10% O2) conditions: green; hyperoxic (40% O2) conditions: pink. a A model summarizing the systemic response events. b–d Physiological relevance probed by altering environmental gases. Environmental CO2 is scavenged by soda-lime. Hypoxic (<10% O2) or hyperoxic (>40% O2) conditions are achieved in hypoxia chambers. Elimination of CO2 using soda-lime significantly increases the number of CCs in the lymph gland, and this response is further aggravated by the concurrent loss of CO2SN (Gr63a-gal4; UAS-Gr21aRNAi) (b). Hypoxia increases CC number, but this phenotype cannot be further enhanced by low CO2SN activity (Gr63a-gal4; UAS-Gr21aRNAi) (c). Hyperoxia on its own significantly suppresses CC development, and also efficiently rescues the low CO2SN-induced CC differentiation to normal levels (d). e–q Phenotypic parallels between decreased levels of ambient CO2 and O2. In addition to low CO2SN activity induced by genetic manipulation (Fig. 3a–c), soda-lime treatment accumulates Sima protein expression in a specific subset of VNC neurons (e, f). Similar accumulation of Sima is observed by rearing larvae in hypoxic conditions (g). Quantitation shown in h. Both hypoxia and soda-lime treatments enhance upd3 in the brain (i–j), Socs36e and dilp6 transcript levels in the fat body (l–m, o–p). This expression is not observed in hyperoxia treatment (k, n, q)
A unique feature of this model is the integration of CO2 and O2 sensation to achieve a common blood phenotype. For the results to be physiologically relevant, alteration of environmental gaseous ligands should phenocopy the effects of genetic manipulations. The normal atmosphere contains approximately 0.04% of CO2 with additional CO2 released from fermenting food sources. Environmental CO2 is scavenged in larval culture vials with the use of a mixture of bases, “soda-lime” (see Supplementary Fig. 1c and Methods). The soda-lime method scavenges atmospheric CO2 to very low levels without altering development, while hypoxia chambers can create controlled hypoxia, hyperoxia, or hypercapnia environments. Most importantly, exposure of wild-type larvae to either soda-lime or to hypoxia increases CC numbers (Fig. 6b, c and Supplementary Fig. 5a, b compare with the genetic manipulations in Fig. 1i). Lowering CO2SN activity does not further enhance the hypoxia phenotype (Fig. 6c and Supplementary Fig. 5c). On its own, hyperoxia decreases CC numbers compared to wild type and also suppresses the low CO2SN-activity phenotype (Fig. 6d and Supplementary Fig. 5d, e compare Fig. 2d, i), whereas hypercapnia does not alter the CC formation (Supplementary Fig. 5f). Finally, soda-lime and hypoxia both: increase nuclear Sima in neurons (Fig. 6e-h compare Fig. 3a-f), and increase upd3 in the brain (Fig. 6i–k compare Fig. 4a, b), and Socs36e (Fig. 6l–n compare Fig. 4n, p) and dilp6 in the fat body (Fig. 6o–q compare Fig. 5c, j). These results demonstrate that the CC phenotypes can be triggered by imbalances in respiratory gases in wild-type animals, establishing a physiological relevance for our observations.
Discussion
A wild-type number of CCs is generated through local developmental signals independent of sensory input27,34,35. The multi-organ and multi-pathway cascade described here represents a stress signal activated upon alteration in respiratory gases over the developmental time period. Such sustained variations in gaseous components are likely to be experienced fairly often during larval development. During the time period over which hematopoiesis is at its peak within the lymph gland, larvae experience hypoxic conditions buried into the food that they forage through. Decomposition and yeast (a primary food source) cause variations in CO2 levels. Toward the end of the hematopoietic developmental period, larvae spend extended periods of time in a very different environment awaiting pupariation. Finally, molting is associated with the shedding of the cuticular intima and degeneration of tracheoles that lack these inner linings36. As the tracheal tube is filled with fluid and devoid of gases at this stage, we speculate that this process is also likely to alter oxygen tension in the hemolymph. In past studies, CCs have been associated with hypoxia and hypoxic stress as well as innate immune response23,27,37. How increased numbers of these cells will mitigate the effects of gaseous imbalance will require detailed analysis in the future. However, in order to determine whether the presence of CCs provides a benefit to the whole animal, we generated flies in which the final step in the cascade, Serrate, is eliminated in cells from which CCs are derived during larval development. We then tested the emerging adults for sensitivity to hypoxia and found that these flies fully paralyze in a hypoxia chamber much more readily than genetically matched control flies (Supplementary Fig. 5g, h; the p-value is <0.0001). Thus, in addition to their other functions, CCs provide general protection against hypoxia to the animal. For myeloid progenitors, which are sentinels for stress and infection, we consistently find that stress signals feed into developmental pathways, in this case Serrate–Notch signaling, to enhance the homeostatic response to a level more appropriate for rapid immune and stress response9,38.
To determine if loss of gaseous sensation is linked to innate immunity, we tested levels of antimicrobial peptides in animals lacking CO2SN. Indeed, loss of CO2 sensation is associated with a four to six-fold increase in the transcription of Drosomycin and a four-fold increase for Drosocin (Supplementary Fig. 5i). However, the significance of this response by the blood cells in the absence of any microbial infection is not clear. We attribute this global and preemptive augmentation of the innate immune system to the increased concentrations of cytokines such as Upd3 and Dilp6 that result from long-term loss of gaseous sensation. Additional physiological effects such as altered lifespan in Drosophila39 and innate immune response in C. elegans40,41 have been associated with CO2 and O2 sensation. Together, these studies allow us to infer that O2 and CO2 chemosensation has a conserved role in animal physiology and immunity.
Although the mechanistic details are not yet deciphered, it seems clear that this conservation extends to mammalian species including humans. Several studies suggest a crosstalk between CO2 and O2 in mammals42 and establish an influence of gaseous sensation on the hematopoietic system43,44. The ventral surface of the mammalian medulla oblongata senses CO245 and responds to O2 sensing by the carotid chemoreceptor neurons46,47. Chemosensation and immunity are closely linked and each is evolutionarily conserved at a mechanistic level. Whether a multi-organ cascade involving multiple cytokines similar to that described in this study links gaseous signaling to myeloid cell function and development in humans will be attractive to investigate.
Methods
Drosophila stocks and genetics
The following Drosophila stocks were used in this study: Gr63a-gal4 (BL9942), Gr21a-gal4 (BL24147), Gyc89da-gal4 and Gyc89da-GFP (D. Morton), Elav-gal4 (BL8765), ppl-gal4 (BL58768), HHLT-gal4 (C. Evans), Upd3-gal4 (H. Agaisse), Repo-gal4 (BL7415), Dilp6-gal4 (A. Brand), HmlΔ-gal4 (S. Sinenko), Hml-dsRed; Dome-Meso-GFP (U. Banerjee), Gr21a RNAi (BL31281 and VDRC104122), dilp6 RNAi (BL33684 and VDRC102465), upd3 RNAi (VDRC106869), sima RNAi (VDRC106187), dome RNAi (VDRC19717), Gad1 RNAi (BL51794), GBR1 RNAi (VDRC101440), GBR2 RNAi (BL50608), Serrate RNAi (VDRC27172), UAS-sima (BL9582), UAS-dilp6 (E. Hafen), UAS-upd3 (B. Lemaitre), UAS-hid, rpr (Nambu JR), UAS-Shits1 (T. Kitamoto), UAS-syb::GRASP (BL64315), UAS-CD4::GRASP (BL58755), UAS-GTrace (C. Evans), UAS-NaChBac (BL9469), UAS-mCD8GFP (BL5137), UAS-DenMark (BL33063), Elav-gal80 (Y.N. Jan), LexAop-Shits1 (G. Rubin), 13XLexAop2-6XmCherry-HA (BL52271), 12xSu(H)-LacZ (S. Artavanis-Tsakonas), Gr63a1 (BL9941), Df[Gr21a] (DGRC150003), dilp641(BL30885), upd2Δupd3Δ (BL55729), upd3Δ(BL55728).
Generation of Gr63a-LexA, Gyc89da-LexA and Lz-LexA flies: Gr63a enhancer4, Gyc89da enhancer20 or Lz enhancer (Forward primer sequence:GGGATTAGGCAGTGTTCCC, Reverse primer sequence:GTACCAATCGCTCCATCCAC) was amplified from fly genomic DNA and ligated into the TOPO-TA vector (Invitrogen) for Gateway cloning. Each entry vector was ligated into the pBPnlsLexA::p65Uw (Addgene 26230) destination vector using the LR ligase (Invitrogen). Transgenic flies were generated by BestGene Inc.
All fly stocks were maintained at 18 °C. Unless indicated, crossed flies were maintained at 29 °C with dextrose-cornmeal based conventional food for maximum Gal4-UAS/LexA-LexAoP expression. Experiments with soda-lime/hypoxia/hyperoxia/hypercapnia and synchronization15 of larvae were performed at 25 °C. Gyc89da-gal4 crossed with UAS-sima flies were maintained at 18 °C until reaching the mid-second instar (approximately 5 days) and shifted to 25 °C. Elav-gal4 crossed with UAS-sima or UAS-upd3, or Gyc89da-gal4 crossed with UAS-NaChBac was maintained at 25°C. These above four genotypes show a drowning or lethal phenotype at 29 °C. Gr63a1 mutants were back-crossed more than 50 generations. Gyc89da-gal4 or Dilp6-gal4 was recombined with UAS-mCD8GFP; Gyc89da-LexA, or Lz-LexA was recombined with 13xLexAoP2-6XmCherry-HA. Efficiencies of RNAi lines used in this study are indicated in Supplementary Table 1.
Soda-lime, CO2, and O2 control experiments
For the soda-lime treatment: the soda-lime (Sigma 72073) experiment was designed based on the previous study48. To avoid crowding, eight females and six male flies were crossed for all experiments and vials were shifted to new vials every day. Twenty soda-lime particles were wrapped and sealed with gauze (referred to as a soda-lime pocket). This soda-lime pocket was attached 5 mm above the food to diminish metabolic CO2 emitted from larvae. To eliminate atmospheric CO2, a 1000 μL pipette tip containing fifteen loosely-packed particles of soda-lime was inserted into a vial sealed with parafilm (Supplementary Fig. 1c). Putting more than twenty soda-lime particles in the pipette tip inhibits air flow and putting the pocket inside the food negatively affected larval growth. With this number of soda-lime particles, there was no developmental influence on larvae. Experiments were independently repeated at least three times.
For O2 and CO2 modulation experiments: hypoxia, hyperoxia and hypercapnia experiments were done in a hypoxia chamber (Modular Incubator Chamber MIC-101, Billups-Rothenberg.Inc or ProOX C21, BioSpherix). 10% (±0.5%) O2 was used for hypoxia experiments, 40% (±0.5%) O2 for hyperoxia, 13% (±0.5%) CO2 for hypercapnia. Drosophila larvae were synchronized and cultured in normoxic conditions until 72 h after egg laying, and shifted to either hypoxic or hyperoxic condition. After rearing animals for 48 h in the chamber, wandering third-instar larvae were dissected immediately. For hypercapnia, the first-instar larvae were synchronized and shifted to the chamber, and dissected when they reached the wandering third-instar. Hypoxia/hyperoxia/hypercapnia and soda-lime experiments were done at 25 °C.
Immunohistochemistry
Lymph glands were dissected and stained as previously described8. Following primary antibodies were used in this study: αLz (DSHB, 1:10), αSima49 (1:100), αβgal (Promega, 1:1000), αnc82 (DSHB, 1:10), αAntp (DSHB, 1:10), αp4EBP (Cell signaling, 2855 S, 1:100), αpAKT (Cell signaling, 4060 S, 1:100) and αSerrate (K. Irvine, 1:1000). Cy3-, FITC- or Alexa Fluor 647-conjugated secondary antibody (Jackson Laboratory) was used for staining. Alexa Flour 594 Phalloidin (Thermo Fisher, A12381) was used for F-actin staining. All samples were mounted in VectaShield (Vector Laboratory) and imaged by Zeiss Axiocam 503, Nikon C2 Si-plus or Zeiss LSM880 Airyscan confocal microscopy.
For αSerrate staining, a pre-absorption step was essential for clear lymph gland staining. To do so, a 1:100 concentration (2% sodium azide) of antibody was incubated together with nine fixed larval cuticles overnight at 4 °C. Lymph glands were dissected at 72 h AEL and fixed in 3.7% formaldehyde for 25 min at room temperature. After fixation, lymph glands were washed 3 times (10 min each) nutating in 0.1% Tween20 in 1 × PBS and blocked in 1% BSA/0.1% Tween20 in 1xPBS for 30 min on a table-top shaker. Lymph glands were incubated overnight in αSerrate primary antibody (used at a final concentration of 1:1000) at 4 °C. Lymph glands were washed 3 times (10 min each) nutating in 0.1% Triton-X in 1xPBS and then incubated in Rat secondary antibody with 1% BSA/0.1% Triton-X in 1xPBS for 3 h at room temperature. After washing 3 times (10 min each) with 0.1% Triton-X in 1xPBS, samples were mounted in Vectashield with DAPI and imaged as described above.
Quantitative real-time PCR analysis
At least 20 larval organs (at least 100 for the lymph gland) were dissected to extract RNA. cDNA was synthesized with qPCR-RT kit (TOYOBO). qRT-PCR was performed by comparative CT method using SYBR Green Realtime PCR Master Mix (TOYOBO) and a StepOne-Plus Real-Time PCR detection thermal cycler (Applied Biosystems). Specific primers used for qRT-PCR are described in Supplementary Table 2.
Quantification of samples
CCs were quantified and analyzed by ImageJ (plug in: 3D object counter) or Imaris (Bitplane). CCs in individual primary lobes were counted for this study. Whole Z-stacks were compressed and analyzed for the quantification and figure presentation. Other stainings including pAKT, p4EBP or Serrate are shown in a single middle Z-stack slice. For CCs in circulation, Lz-LexA LexAop-mCherry positive blood cells in a larva were counted after bleeding. Prior to bleeding, animals were vortexed for 2 min to detach sessile population. Statistical significance of the CC phenotype was analyzed by Wilcoxon rank sum test after determining normality with the use of SPSS. Given natural variability of the number of CCs, we considered samples are significantly different only when *p < 0.01. Statistical results and genotypes are indicated in Supplementary Table 3.
Relative nuclear Sima intensity was analyzed with the use of IMARIS software. Amongst Sima immunoreactivity shown in the brain, we only selected high Sima+ cells to avoid background expressions, and of which nuclear intensity was measured and calculated. Relative intensity of mutants compared to wild type was presented in figures.
Hypoxia tolerance experiment
Three-day-old male flies were used for hypoxia tolerance experiments. 15 flies were placed in one empty vial and conditioned for 2 h before transferring to 1% oxygen-containing hypoxia chamber. Fly movement was recorded for 1 h. 1 point was given when any fly from one vial shows a movement in 5 s; therefore, 12 point per 1 min for maximum. Flies were never placed in hypoxic condition before this experiment. Behavior assay was repeated more than three times with biologically independent samples.
Data availability
All data generated during and/or analyzed during the current study are included in this published article and its supplementary information files.
Electronic supplementary material
Acknowledgements
The authors thank members of the Shim lab and the Banerjee lab for helpful discussions. The authors acknowledge the Bloomington, VDRC, DGRC, and KDRC Drosophila stock centers and the DSHB hybridoma bank. The authors thank the following individuals for stocks and reagents: C. Evans, H. Agaisse, A. Brand, E. Hafen, B. Lemaitre, T. Kitamoto, Y.N. Jan, G. Rubin, S. Artavanis-Tsakonas, D. Morton and K. Irvine. This work was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (NRF-2014S1A2A2028388) and by the Ministry of Science, ICT and Future planning (NRF-2014R1A1A1002685) to J.S.; The UCLA part of this collaboration was supported by a Training Grant in Developmental Hematology (T32 HL086345) to C.M.S.; and the NHLBI grant R01 HL067395 and the Broad Stem Cell Research Center at UCLA to U.B.
Author contributions
B.C., C.M.S., S.Y., N.C., and J.S. performed experiments; B.C., C.M.S., and J.S. analyzed data; B.C., C.M.S., U.B., and J.S. contributed to writing the manuscript; U.B. and J.S. conceived the idea and supervised the project.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04990-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Utpal Banerjee, Email: banerjee@mbi.ucla.edu.
Jiwon Shim, Email: jshim@hanyang.ac.kr.
References
- 1.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 2.Guerenstein PG, Hildebrand JG. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008;53:161–178. doi: 10.1146/annurev.ento.53.103106.093402. [DOI] [PubMed] [Google Scholar]
- 3.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 4.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc. Natl Acad. Sci. USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 6.Gold KS, Bruckner K. Drosophila as a model for the two myeloid blood cell systems in vertebrates. Exp. Hematol. 2014;42:717–727. doi: 10.1016/j.exphem.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MJ. Drosophila hemopoiesis and cellular immunity. J. Immunol. 2007;178:4711–4716. doi: 10.4049/jimmunol.178.8.4711. [DOI] [PubMed] [Google Scholar]
- 8.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 9.Bidla G, Dushay MS, Theopold U. Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci. 2007;120:1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 10.Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–580. doi: 10.1046/j.1462-5822.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 11.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2011;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Droujinine IA, Perrimon N. Interorgan communication pathways in physiology: focus on Drosophila. Annu. Rev. Genet. 2016;50:539–570. doi: 10.1146/annurev-genet-121415-122024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 2012;14:394–400. doi: 10.1038/ncb2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim J, et al. Olfactory control of blood progenitor maintenance. Cell. 2013;155:1141–1153. doi: 10.1016/j.cell.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 2011;31:15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evgenov OV, et al. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat. Rev. Drug. Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton DB. Atypical soluble guanylyl cyclases in Drosophila can function as molecular oxygen sensors. J. Biol. Chem. 2004;279:50651–50653. doi: 10.1074/jbc.C400461200. [DOI] [PubMed] [Google Scholar]
- 20.Morton DB, Stewart JA, Langlais KK, Clemens-Grisham RA, Vermehren A. Synaptic transmission in neurons that express the Drosophila atypical soluble guanylyl cyclases, Gyc-89Da and Gyc-89Db, is necessary for the successful completion of larval and adult ecdysis. J. Exp. Biol. 2008;211:1645–1656. doi: 10.1242/jeb.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermehren-Schmaedick A, Ainsley JA, Johnson WA, Davies SA, Morton DB. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186:183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann JA. Innate immunity of insects. Curr. Opin. Immunol. 1995;7:4–10. doi: 10.1016/0952-7915(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 23.Binggeli O, Neyen C, Poidevin M, Lemaitre B. Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS. Pathog. 2014;10:e1004067. doi: 10.1371/journal.ppat.1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolai LJ, et al. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc. Natl Acad. Sci. USA. 2010;107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feinberg EH, et al. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Macpherson LJ, et al. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 2015;6:10024. doi: 10.1038/ncomms10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332:1210–1213. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits. PLoS. One. 2008;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karsten P, Hader S, Zeidler MP. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech. Dev. 2002;117:343–346. doi: 10.1016/S0925-4773(02)00216-2. [DOI] [PubMed] [Google Scholar]
- 30.Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P. A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chell JM, Brand AH. Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- 33.Chatterjee D, et al. Control of metabolic adaptation to fasting by dILP6-induced insulin signaling in Drosophila oenocytes. Proc. Natl Acad. Sci. USA. 2014;111:17959–17964. doi: 10.1073/pnas.1409241111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr. Biol. 2002;12:1923–1927. doi: 10.1016/S0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 35.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell. Dev. Biol. 2003;19:623–647. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- 37.Neyen C, et al. The Black cells phenotype is caused by a point mutation in the Drosophila pro-phenoloxidase 1 gene that triggers melanization and hematopoietic defects. Dev. Comp. Immunol. 2015;50:166–174. doi: 10.1016/j.dci.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Meister M. Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 2004;16:10–15. doi: 10.1016/j.coi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8:e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323:382–384. doi: 10.1126/science.1166527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Styer KL, et al. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science. 2008;322:460–464. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott K. Out of thin air: sensory detection of oxygen and carbon dioxide. Neuron. 2011;69:194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soliz J. Erythropoietin and respiratory control at adulthood and during early postnatal life. Respir. Physiol. Neurobiol. 2013;185:87–93. doi: 10.1016/j.resp.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 44.Tramezzani JH, Morita E, Chiocchio SR. The carotid body as a neuroendocrine organ involved in control of erythropoiesis. Proc. Natl Acad. Sci. USA. 1971;68:52–55. doi: 10.1073/pnas.68.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulkey DK, et al. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 46.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat. Rev. Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 47.Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J. Appl. Physiol. (1985) 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 48.Hallem EA, et al. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C. W., Purkayastha A., Jones K. T., Thaker S. K., Banerjee U. In vivo genetic dissection of tumor growth and the Warburg effect. eLife5, e18126 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during and/or analyzed during the current study are included in this published article and its supplementary information files.