Abstract
Physically associated hydrogels based on strong hydrophobic interactions often have attractive mechanical properties that combine processability with elasticity. However, there is a need to study such interactions and understand their relation to the macroscopic hydrogel properties. Therefore, we use the surfactant sodium dodecyl sulfate (SDS) and urea as reagents that disrupt hydrophobic interactions. The model hydrogel is based on a segmented copolymer between poly(ethylene glycol) (PEG) and hydrophobic dimer fatty acid (DFA). We show that both agents influence viscoelastic properties, dynamics, and relaxation processes of the model hydrogel. In particular, the relaxation time is significantly reduced by urea, as compared to SDS, whereas the surfactant causes a decrease of the modulus of the hydrogel more efficiently. The reversibility of the effects of SDS and urea can be exploited, for instance, by using an injectable sol that solidifies when the SDS or urea diffuses out of the sample. Surfactant-induced processability may be advantageous in future applications of hydrophobically assembled physical hydrogels.
Introduction
Supramolecular hydrogels represent a group of hydrogel materials which are characterized by the transient nature of their networks. This is usually achieved through physical, noncovalent interactions, such as hydrogen bonding,1−3 ionic interactions,4 or hydrophobic associations.5,6 Recently, there has been an increasing interest in this class of soft materials as they can offer some unique advantages, the most important of which are their easy processability and shaping. This is due to their transient character and the reversible nature of the cross-links. As such, supramolecular hydrogels can be easily assembled or even disassociated, depending on the conditions applied. Consequently, they hold great potential as drug delivery, biomimetic, self-healing, shape-memory, or adaptive materials.5,7−13
A very important category of supramolecular hydrogels is entirely based on hydrophobic association. In such gels, the network is held together by the self-assembly of phase-separated hydrophobic blocks in water. Depending on the size of the hydrophobic units, the strength of the association will vary and therefore so will the resultant dynamics and material properties. However, in order to fully exploit the potential of such hydrogels, it is of crucial importance to understand the relation between the molecular interactions and the dynamics, structure, and macroscopic properties. One way to study hydrophobically assembled gels is to modify the strength of the interactions by the addition of surfactants or urea.
The effect of surfactants, in particular sodium dodecyl sulfate (SDS) on hydrophobically modified ethoxylated urethanes (HEURs), a class of associative polymers, has been studied.14 It was shown that at sufficiently high concentrations surfactant is able to dissolve the transient network completely. Amphiphilic surfactant molecules are able to interact with both hydrophilic PEG segments and hydrophobic flower-like micelles, resulting in micelle solubilization and network disintegration. Moreover, the effect of SDS has been seen in hydrophobically modified hydrogels as well. It was shown that SDS is able to facilitate the diffusion of polymer chains by interacting with hydrophobic micelles, thereby inducing self-healing.5,15 Urea, on the other hand, is known as a molecule able to disintegrate hydrogen bonds and induce protein denaturation.16−18 In addition, it has been reported that urea influences hydrophobic interactions, but the mechanisms by which this occurs are still under debate. It has been proposed that urea acts as a chaotrope, breaking the structure of water and increasing hydrocarbon solubility.19,20 However, recent results suggest that this indirect mechanism is not very likely to play an important role in the denaturation process.21,22 In particular, it was seen that in the systems based exclusively on hydrophobic interactions, it is most likely that urea actuates a direct way of interacting with the polymer structure. It appears that this mechanism is based on the preferential binding of urea to the hydrophobic structures and the formation of hydrogen bonds with water, resulting in a weakening of the hydrophobic associations.21
A study on the mechanisms by which SDS and urea alter the mechanics and properties of transient hydrogels thus may provide useful insights. The effects of SDS on some hydrogels containing hydrophobic modifications have been investigated,5,12,15,23 and a limited amount of research has been done on the interaction of urea with hydrogels.17,24−26 However, to the best of our knowledge there are no studies in which the effects of surfactants and urea on physical hydrogels are systematically compared. It is expected that since the network is transient, these molecules are able to significantly alter the strength of the associations and thereby change the macroscopic material properties.
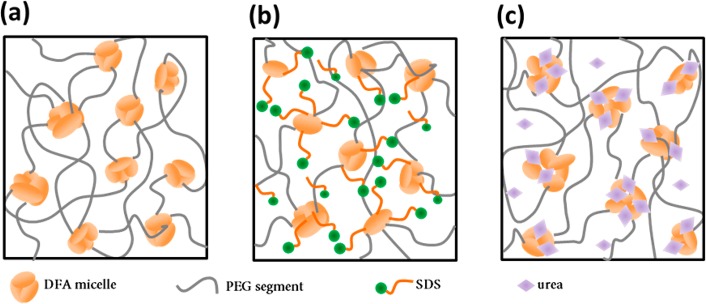
Recently, we have developed a supramolecular hydrogel entirely assembled via hydrophobic interactions.6 We used large hydrophobic dimer fatty acid (DFA) segments with 36 carbon atoms, copolymerized with poly(ethylene glycol) (PEG2000) in a one-step polycondensation reaction in the melt. The DFA units in the segmented block copolymer interact via hydrophobic interactions to form phase-separated nanodomains ∼3 nm in size and consisting of ∼200 DFA units that act as physical cross-linking points (micelles), giving rise to a supramolecular hydrogel (Figure 1a). As a result, these solid-like hydrogels displayed remarkable elasticity due to the strong hydrophobic association.
Figure 1.
Representation of the micellar structure of hydrogels. Network structure of the PEG-DFA based supramolecular hydrogels (a) in the absence of SDS and urea and in the presence of (b) SDS and (c) urea.
In the present work, we characterize PEG-DFA hydrogel swollen in aqueous surfactant and urea solutions. We compare the effects of these agents on the dynamics, viscoelastic properties, and mechanics of this purely hydrophobically assembled supramolecular hydrogel. We rely on oscillatory rheology to study the viscoelasticity of the hydrogels. Both SDS and urea affect the viscoelastic properties by changing the number and the lifetime of cross-links in a concentration-dependent manner. We find that SDS has a stronger effect on the plateau modulus than does urea.
Experimental Section
Materials
The segmented copolymer PE PEG2000 was synthesized as described previously.6 Poly(ethylene glycol) 2000 (PEG2000) was purchased from Merck. Tin(II) chloride anhydrous was obtained from Alfa Aesar. Dimerized fatty acid (DFA), sodium dodecyl sulfate (SDS), and urea were all purchased from Sigma-Aldrich. Bulk solvents were obtained from Biosolve BV Chemicals. PEG was dried by azeotropic distillation with toluene before use; all other reagents were used without further purification. The segmented copolymer was synthesized by a polycondensation reaction in the melt under vacuum, and its characteristics are listed in Table S1.
Hydrogel Preparation
Dry PE PEG2000 was compression-molded at 95 °C, at 100 bar for 10 min. Teflon sheets were used to prevent the material from sticking. Upon cooling, the disks were removed from the mold and were used for hydrogel preparation. The size of the prepared polymer disks was 25 mm diameter, with a thickness of 0.5 mm. In general, hydrogels were prepared either by immersing the disks in solution until reaching 75 wt % water content or by adding the amount of solution to a polymer disk in order to form the gel at the same polymer fraction. Reference samples were prepared by using deionized water for swelling, while surfactant- and urea-containing hydrogels were prepared by swelling dry disks with SDS/urea aqueous solutions at designated concentrations, as described in the main text. Prior to all measurements, hydrogels were kept in a humid chamber for several hours to ensure complete equilibration.
Rheology
Oscillatory shear and stress relaxation measurements were performed on a stress-controlled rheometer (Anton Paar, Physica MCR 501), equipped with 25 mm parallel plates and an antievaporation accessory to maintain the samples hydrated and to minimize water evaporation. All measurements were conducted on hydrogels at 25 wt % polymer fraction and at a temperature of 25 °C. The linear viscoelastic regime was determined using a strain sweep measurement at an oscillation frequency of 1 rad/s, establishing a strain of 0.1% as safely within the linear viscoelastic regime. As a consequence, dynamic measurements in the frequency range between 0.1 and 100 rad/s were carried out at a strain amplitude of 0.1%. Finally, stress relaxation experiments were performed, applying a step strain of 0.1%.
Results and Discussion
Viscoelastic Behavior of PEG-DFA Hydrogel in the Presence of Surfactant and Urea
In order to study how the surfactant (SDS) and urea influence the viscoelastic properties of the PE PEG2000 hydrogel and the lifetime of the hydrophobic cross-links, oscillatory shear measurements were performed.
The hydrogels were prepared at a polymer concentration of 25 wt %, using aqueous solutions of SDS or urea at designated concentrations. The polymer fraction was kept constant throughout all the measurements, unless otherwise specified. A series of hydrogel samples were prepared, including one of PE PEG2000 swollen in water, which was used as control. Hydrogels were swollen in SDS solutions at six different concentrations from around its critical micelle concentration27 0.002–0.1 g/mL. Urea-containing hydrogels were swollen with solutions at four concentrations from 0.09 to 0.54 g/mL.
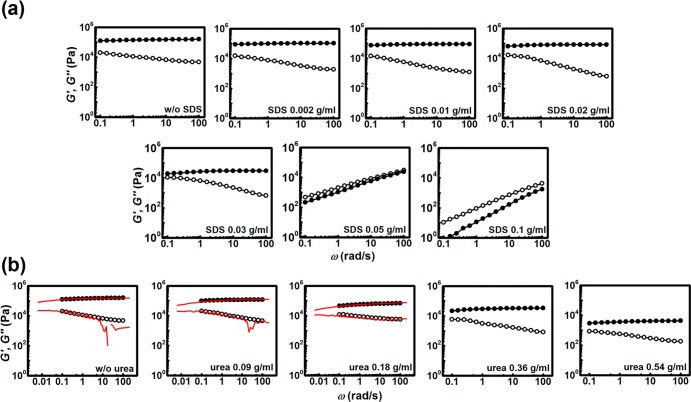
The most important information about the structure and dynamics can be obtained by measuring the frequency-dependent dynamic moduli G′(ω) and G″(ω) in the frequency range of ω = 0.1–100 rad/s and at a constant strain γ = 0.1%, where the material has a linear response (Figure S1). Figure 2 displays the viscoelastic response of the samples.
Figure 2.
Viscoelasticity of the hydrogels in the presence of SDS and urea. Frequency sweep at γ = 0.1% at 25 °C of the PE PEG2000 at (a) varying SDS concentrations and (b) varying urea concentrations, as indicated in the panels (closed symbols, G′; open symbols, G″). Red lines are data obtained from Fourier transforming stress-relaxation data from Figure 6b.
Figure 2a shows the frequency dependence of shear moduli of the SDS-containing hydrogels. As seen in our previous work,6 in the reference hydrogel sample we notice that at the plateau region the storage modulus G′(ω) is larger by an order of magnitude than the loss modulus G″(ω). This indicates the elastic, solid-like nature of the hydrogel. The large value of G′(ω) is also an indication of a high cross-link density of the network. Moreover, G′(ω) and G″(ω) show no significant variation upon changes in frequency, which showcases a typical viscoelastic response of strong physical hydrogels3,28,29 or permanently cross-linked chemical gels.
Upon introduction of SDS in the system, it is expected that the hydrophobic DFA micelles become weaker due to interactions with amphiphilic surfactant molecules. This would result in local solubilization of the micelles (Figure 1b) and a decrease of the energy barrier for escape of DFA units from the hydrophobic cores, thus speeding up the dynamics of the network.23 However, at SDS concentrations of 0.002 and 0.01 g/mL (corresponding to DFA to SDS weight ratio of 7:1) (Figure 2a), the hydrogels exhibit properties quite similar to those of the reference sample, suggesting that the corresponding surfactant concentrations are not sufficient to induce drastic changes in material properties at the probed time scale. When SDS concentration was raised to 0.02 and 0.03 g/mL, different features started to appear. The hydrogel with 0.02 g/mL SDS has a slightly narrower gap between G′(ω) and G″(ω) at low frequencies, indicating that the viscous response is becoming more significant compared to the three previously tested samples. At 0.03 g/mL SDS the change is even more clear; first, at very low frequency, G′(ω) and G″(ω) are very close, indicating that the modulus crossover takes place at slightly longer time scale than probed by this experiment, and second, G′(ω) is reduced nearly 5-fold compared to the samples tested at lower SDS concentrations. According to rubber network theory, the plateau value of G′(ω) is directly proportional to the cross-link density,30 and therefore a lowering of this modulus indicates that the cross-link density of the network has decreased. Since reduction of the size of the micelles would preserve the number of active cross-links (being the number of chains that connect different micelles), a reduction in cross-link density implies that free, nonassociated DFA units are formed by the addition of SDS.
The response of the samples containing 0.02 or 0.03 g/mL SDS, is still predominantly elastic (G′ > G″), but the elastic modulus is reduced by SDS. At even higher SDS concentrations (SDS 0.05 and 0.1 g/mL) the response was predominantly viscous in the entire frequency range probed, as G″(ω) was larger than G′(ω). While some DFA units are completely dissociated, reducing the modulus, the dynamics of DFA exchange from micelles is also strongly affected by the presence of surfactant molecules, giving rise to a liquid-like sample. The time scale of exchange is shorter than the fastest time scale probed in this experiment (10 ms). This is also in accordance with our previous observation that the PE PEG2000 gel kept in large volume of SDS 0.1 g/mL solution dissolved completely after 5 days.6
In addition to surfactant, we also examine the effect of another agent influencing hydrophobic associations—urea. Urea is known as a molecule able to disrupt hydrogen bonds and cause protein denaturation, but it has been seen that it also affects pure hydrophobic interactions. Even though there are different plausible mechanisms described by which urea affects hydrophobic interactions, it is most probably due to urea’s binding to hydrophobic species. In fact, by strong dispersion interactions it interacts with hydrophobic cores, making hydrogen bonding with water and urea from the next shell (Figure 1c). That way, it penetrates in the hydrophobic core causing the weakening of the interactions.21 Since our system is entirely based on nondirectional hydrophobic interactions, with no hydrogen bonds present, we aim to investigate in more detail whether urea is able to induce changes in material properties and correlate them to those caused by SDS. To do so, we prepared a series of four hydrogels swollen with aqueous solutions of urea at different concentrations: 0.09, 0.18, 0.36, and 0.54 g/mL. The reference sample is the same used in the study with SDS, swollen with pure water. The composition of all hydrogels was kept at 25 wt % of polymer.
When urea is introduced in the system, the general shape of the response does not change significantly between the gels containing different amounts of urea, with G′(ω) larger than G″(ω) over the whole range of frequency, indicating that all samples are elastic and solid-like. The most prominent change, however, is observed in the value of the plateau modulus. The trend is the same as observed with SDS: there is a remarkable decrease in Gplateau with increasing urea concentration, as shown in Figure 2b.
From the frequency-dependent measurements we observe that urea indeed appears to influence the hydrophobic associations, as it exerts a notable effect on both the plateau modulus and the association energy of DFA, similar to SDS. Therefore, we believe that urea is indeed able to significantly weaken hydrophobic interactions, probably by having strong dispersion interactions with DFA segments, which allows for urea’s binding to DFA micelles and eventually penetrating into them, as proposed by Zangi et al.21 There could also be the effect of increased solubility of DFA, since large hydrocarbons have better solubility in concentrated urea solutions than in water.31
Additionally, we performed Fourier transformation of the stress relaxation data to obtain G′(ω) and G″(ω) of the urea-containing hydrogels, and the results are displayed in Figure 2b as solid red lines (also shown in Figure S2). The data agree remarkably well with the experimental frequency sweep, and we are confident that the same holds true for the remaining urea- and SDS-containing hydrogels. This proves the reliability of the stress relaxation experiments reported further in the text. The procedure of the mentioned Fourier transformation is described in the Supporting Information.
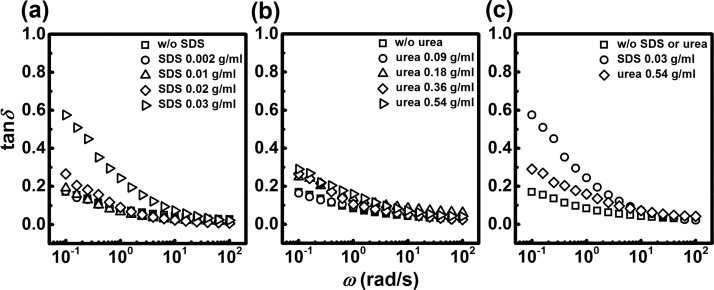
Furthermore, the loss tangent (tan δ = G″/G′) is plotted against the angular frequency ω (Figure 3). In Figure 3a, it is evident that tan δ < 0.1 for the largest part of the frequency range, confirming the strong, elastic nature of the material (reference gel, SDS 0.002 and 0.01 g/mL gels). However, tan δ > 0.1 at lower frequencies (ω = 0.3 rad/s), indicating a weak dynamic, nonpermanent character of the cross-links for these samples at longer time scales. At SDS 0.02 g/mL, tan δ > 0.1 at frequency of ω = 0.7 rad/s, whereas at SDS 0.03 g/mL it is even more evident, as tan δ > 0.1 at much higher frequencies (ω = 6.3 rad/s). This shows that surfactant indeed increases the viscous contribution of the present PE PEG2000 hydrogel and thereby its dynamics. These results thus show that surfactant is able to influence and disturb the strong DFA hydrophobic interactions in the material, thereby speeding up the hydrogel’s dynamics, as previously observed for other similar systems.23
Figure 3.
Viscous contribution in the hydrogels with SDS and urea. Loss factor tan δ as a function of angular frequency, determined from the data in the Figure 2. (a) SDS-containing hydrogels; (b) urea-containing hydrogels; (c) comparison between the reference hydrogel and samples at the highest investigated SDS and urea concentrations.
For the samples with urea, a less pronounced concentration dependence is observed (Figure 3b). This suggests that the dynamics of the network is not as strongly affected by urea as it is by the SDS surfactant. We attribute this lack of a larger increase of viscous character to a different mechanism by which urea interacts with hydrophobic micelles. In Figure 3c, we show the comparison between the effects of SDS and urea, relative to the pure PE PEG2000. The increase of tan δ at low frequencies is much more pronounced for SDS (0.03 g/mL) compared to both the reference and urea-containing hydrogel (0.54 g/mL). Therefore, we can conclude that the dynamics of the PE PEG2000 hydrogel is greatly increased by SDS at 0.03 g/mL, whereas the effect by urea is weaker.
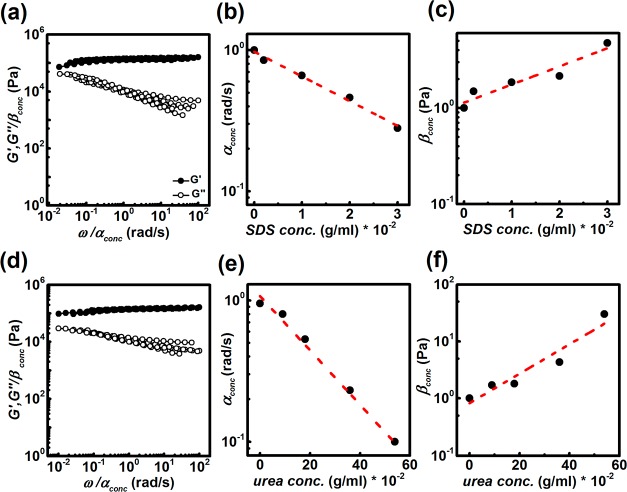
Interestingly, we tried to construct the corresponding master curves by shifting the obtained frequency-dependent responses. We do so because the general shape of the response remains remarkably similar among different samples. By applying both, horizontal and vertical shift factors, it was possible to obtain the master curve, as shown in Figure 4a,d. It can be noticed from Figure 4b,e that the horizontal shift factors scale exponentially with SDS and urea concentrations, indicating that the activation energy (Ea), corresponding to the association energy of hydrophobic blocks, is inversely proportional to the amount of the reagent used.
Figure 4.
Master curves of the frequency-dependent responses. (a) Master curve obtained after shifting the frequency sweep measurements when different amounts of SDS were added; (b) horizontal shift factor; (c) vertical shift factor, at varying SDS concentrations; (d) master curve obtained after shifting the responses when urea was used; (e) horizontal shift factor; (f) vertical shift factor, at varying urea concentrations. The dashed red lines in (b), (c), (e), and (f) represent the fitting.
By comparing the changes in the characteristic relaxation time (the horizontal shift factor α) to those of the modulus (corresponding to the vertical shift factor β), it is possible to highlight the effect of SDS and urea on these parameters. In particular, from Figures 4b,c,e,f it can be concluded that the changes in the relaxation time of a factor of 2 in the presence of SDS and urea correspond to the changes in the modulus of a factor of 2.7 and 1.7, respectively. Therefore, we notice that the effect of SDS on G′(ω) is larger than that of urea, whereas urea exerts stronger effect on the relaxation time compared to SDS.
In order to assess the nonlinear mechanical properties of these gels, the samples were subjected to large amplitude oscillatory shear measurements (LAOS), performed at varying strain amplitudes (0.01–100%), at a constant frequency ω = 1 rad/s. Figure S1 displays the results of these measurements.
As expected from the frequency-dependent measurements, the linear elastic modulus decreases upon addition of SDS. At low strains, G′(ω) and G″(ω) are independent of strain, indicating linear response. In this region, the gels deform elastically, with G′(ω) larger than G″(ω). However, beyond a certain strain value, the gels start to display increasingly viscous behavior. This implies that the cross-links are broken by mechanical force more quickly than they are able to re-form, leading to a breakdown of the network structure. This is obviously a reversible process, as we tested in our previous work.6 However, here we are interested in the critical strain value and how it is affected by SDS. In the nonlinear regime, the sample without SDS exhibited a yield strain of γc= 2%, which is in accordance with previously observed results.6 However, as SDS is added to the system, the critical yield strain shifts to larger values. For the gel with 0.002 g/mL SDS it goes up to ∼7%, whereas for 0.01, 0.02, and 0.03 g/mL SDS gels γc increases to ∼15%. The explanation for this is most likely related to the solubilization of DFA micelles by SDS. Some of the DFA micelles are becoming elastically inactive, causing the PEG segments between the next two active cross-links to be longer than in the reference sample (PEG 2000).
In fact, in our previous study, the same hydrogel, containing PEG8000, showed the same critical strain of 15%, confirming the network is more flexible.6 It seems that the disruption of DFA micelles has a twofold effect on these gels: first, the chain segment between active cross-links appears significantly longer than PEG2000, resulting in a higher γc, and second, it results in decreased cross-link density and therefore stiffness.
The trend of increasing γc as a function of SDS concentration is displayed in Figure 5.
Figure 5.
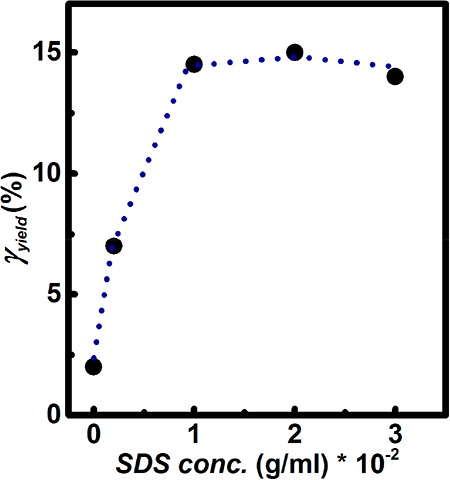
Yielding strain against SDS concentration. Values are determined from the strain sweep experiment (Figure S1a).
As opposed to SDS, in urea-containing hydrogels, we failed to observe a clear increasing trend of the critical strain γc as a function of urea concentration. The critical strain for all measured samples is in the range of 1–3%. The lack of γc increasing with urea might be due to the fact that urea, when interacting with the micelles, is not dissolving them significantly in order to result in apparent longer PEG intermicellar segments which give rise to a more flexible network and increased γc.
Influence of SDS and Urea on Stress Relaxation
Stress relaxation of the hydrogels was studied to get more insight into the lifetime of the cross-links at varying SDS and urea concentrations.
Here, we discuss how the stress relaxes in these physical hydrogels, when a step strain γ = 0.1% is applied. Several samples were tested, and the results are displayed in Figure 6. It is evident that as SDS and urea concentrations increase, there is also a faster stress relaxation. This is due to a more dynamic network, which is able to rearrange at both structural and conformational levels in order to release the stress. The DFA micelles lifetime is reduced, allowing them to break and re-form faster. Also, this partial disassociation of the micelles leads to detachment of PEG chains from the micellar core, which results in a more flexible system and allows for relaxation of the stress through the network more quickly compared to the reference hydrogel.
Figure 6.
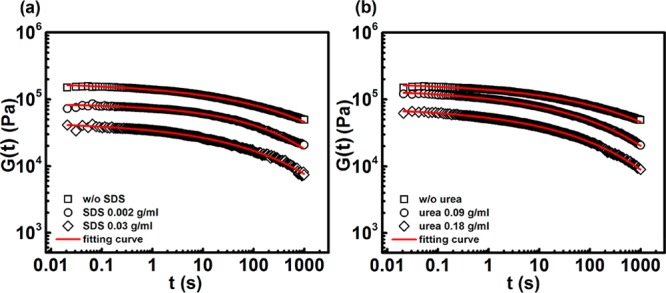
Stress relaxation responses in the presence of SDS and urea. Stress relaxation of PE PEG2000 hydrogel at varying (a) SDS and (b) urea contents, plotted as relaxation modulus G(t) versus time, at step strain of 0.1%. The specific samples are listed in the panels.
Since the network is transient and there are distributions of chain lengths, topology, and DFA micelle sizes, the systems in question are most likely characterized by a distribution of relaxation times and not by a single relaxation process. Moreover, there is also the relaxation process related to DFA associations. The same has been seen in other transient networks.28,32 Therefore, as has been often successfully applied to viscoelastic materials with transient cross-links,33,34 we fit the obtained data using a stretched exponential, as
| 1 |
where τ is the mean relaxation time, corresponding to the average residence time of a hydrophobic unit in the micelle, β is the exponent associated with the moments, as described previously,35 and A is the initial value of the relaxation modulus G(t). The resulting fits are shown as solid red curves in Figure 6, while derived fitting parameters are listed in Table 1.
Table 1. Fitted Parameters Based on Stretched Exponential for PE PEG2000 Hydrogels at Various SDS and Urea Amounts.
| sample (g/mL) | A [Pa] | τ [s] | β | r2 | sample (g/mL) | A [Pa] | τ [s] | β | r2 |
|---|---|---|---|---|---|---|---|---|---|
| no SDS | 180000 | 270 | 0.239 | 0.99 | no urea | 180000 | 270 | 0.239 | 0.99 |
| 0.002 SDS | 87000 | 243 | 0.319 | 0.99 | 0.09 urea | 143000 | 70 | 0.251 | 0.99 |
| 0.03 SDS | 46000 | 102 | 0.257 | 0.99 | 0.18 urea | 77000 | 47 | 0.251 | 0.99 |
It has been shown that stress relaxation requires dissociation of mechanically active chains bound to micelles.36 Since SDS and urea cause solubilization of single DFA units and weakening of the DFA domains, we expect this to result in a faster stress relaxation. Indeed, it is clear from the figure that the relaxation time τ decreases as the amount of SDS present is raised, meaning that the lifetime of the hydrophobic associations in the sample swollen with SDS 0.03 g/mL is much shorter compared to the reference hydrogel. The mean relaxation time for the sample without SDS is 270 s, whereas it drops to 102 s when SDS 0.03 g/mL is used. This implies that the stress is dissipated more quickly, which is in line with previous rheological experiments. When urea is present, because of the urea-dependent weakening of hydrophobic interactions and the increased solubility of hydrocarbons, we obtain the same effect on the stress relaxation as seen for the surfactant system. In fact, the urea-free hydrogel is characterized by a mean relaxation time of 270 s, whereas 0.18 g/mL urea hydrogel has a mean relaxation time of 47 s.
From the obtained parameters it is possible to correlate the change in relaxation time to the change in modulus between different samples. When the relaxation times are changed by a factor of 2 in SDS and urea-containing hydrogels, the moduli change by a factor of 2.9 and 0.8, respectively. Therefore, the stress relaxation data suggest that SDS has a very significant effect on the modulus. This is due to the amphiphilic structure and detergent-like properties of SDS, hence its ability to solubilize and isolate single DFA units more effectively than urea. On the one hand, urea displays a weaker effect on the modulus, but, on the other hand, it exhibits a more pronounced effect on the relaxation time than does SDS. The effect of urea on the stress relaxation can be explained by the fact that it is able not only to increase the solubility of large hydrocarbons31 but also to interact with DFA micelles and weaken the hydrophobic interactions.21 The trends in the variation of relaxation time and modulus caused by SDS and urea are comparable to those observed from the superimposed frequency measurements discussed above, thus indicating consistency between the frequency-dependent and the stress relaxation measurements.
Qualitative Assessment of Reversibility and Application
When PE PEG2000 hydrogel was treated with SDS, it became more viscoelastic, and it was liquid-like if swollen with 0.05 or 0.10 g/mL SDS. The liquid state is expected to go back to its original, elastic and solid-like state upon removal of surfactant. Gelation by removal of SDS was tested on a solution in 0.05 g/mL SDS. The liquid-like nature of this system was demonstrated by casting the solution directly onto a glass plate. Figure 7a shows that the hydrogel flows and has no fixed shape. However, if the same hydrogel was injected directly into a large volume of water, the material maintained the elongated shape assumed during injection. Because of diffusion of SDS into the water, a gel with fixed shape was formed (Figure 7b), of which the shape persistence after approximately 5 min is shown in contrast with the freely flowing behavior of the parent solution in Figure 7c. The use of SDS can thus be a useful way to increase processability of the material for specific applications and to restore gel-like properties afterward. Similar observations regarding mechanical properties were described by Okay and co-workers.23
Figure 7.

Surfactant-induced processability. (a) Hydrogel at SDS 0.05 g/mL being injected onto a glass plate; (b) the same hydrogel being injected in water; (c) comparison between the two samples. Red dye was used for easier visualization of samples.
The most striking differences between the SDS- and urea-containing hydrogels are related to the degree to which these agents influence the plateau modulus and the relaxation time. This is evident from frequency-dependent measurements as well as from stress relaxation experiments. In Figure 8, a direct comparison between the effects of SDS and urea on the modulus and relaxation time is shown. Both relaxation time and plateau modulus decrease when the concentration of interacting agents is increased. However, the effect on the plateau modulus caused by SDS is much stronger than that of urea (Figure 8b).
Figure 8.
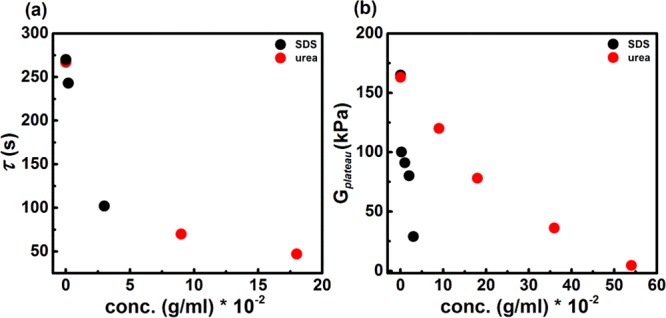
Main effects on viscoelastic behavior of hydrogels caused by SDS and urea. (a) Relaxation time derived from data in Figure 6; (b) plateau modulus, taken at ω = 100 rad/s, plotted as a function of SDS/urea concentration.
These differences between surfactant and urea can be explained by a different way in which they interact with the network, as depicted in Figure 1. SDS micelles solubilize isolated DFA units from the aggregates, leading to a decrease in network density. Urea does not form micelles and has a weaker capacity to solubilize isolated DFA units, but it does weaken hydrophobic interactions and in that way lowers the activation energy for removal of DFA units from DFA aggregates, which results in shorter relaxation times.
Conclusions
We have studied in detail the viscoelastic properties and changes in dynamics of a purely hydrophobic supramolecular hydrogel when external chemical stimuli are applied, such as the presence of surfactant molecules or urea in the background liquid. Worth noticing is the fact that the surfactant SDS in particular was able to drastically decrease the plateau modulus of the PEG-DFA hydrogel. Our results strongly indicate that these significant changes caused by SDS are due to its ability to interact with and isolate DFA segments. Moreover, our work shows that urea indeed alters the hydrophobic interactions and, surprisingly, while affecting the material’s stiffness with the same trend as SDS exhibits a significantly more pronounced effect on the relaxation time. These findings could potentially be very significant also for other hydrogel systems based either fully or partially on hydrophobic associations, as the addition of either surfactant or urea might help tune their mechanical and viscoelastic properties. These reversible changes in dynamics and viscoelasticity caused by SDS and urea suggest that under optimized and suitable conditions properties of supramolecular hydrogels could be tailored to fit a variety of needs. This might widen the range of useful applications of supramolecular hydrogels by helping to induce or improve self-healing, processability, or even injectability of hydrogels with hydrophobic cross-links.1,37−40
Acknowledgments
This project was supported by the Marie Curie ITN project SASSYPOL (grant no. 607602, EU-FP7-PEOPLE-2013-ITN) and by the Ministry of Education, Culture and Science of The Netherlands (Gravity program 024.001.035).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.macromol.8b00892.
Properties of the segmented copolymer determined by GPC, strain-dependent measurements, description of the procedure for the Fourier transform of the stress relaxation data, Fourier transform of the stress-relaxation responses in urea-containing samples (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Guo M.; Pitet L. M.; Wyss H. M.; Vos M.; Dankers P. Y. W.; Meijer E. W. Tough Stimuli-Responsive Supramolecular Hydrogels with Hydrogen-Bonding Network Junctions. J. Am. Chem. Soc. 2014, 136 (19), 6969–6977. 10.1021/ja500205v. [DOI] [PubMed] [Google Scholar]
- Pape A. C. H.; Bastings M. M. C.; Kieltyka R. E.; Wyss H. M.; Voets I. K.; Meijer E. W.; Dankers P. Y. W. Mesoscale Characterization of Supramolecular Transient Networks Using SAXS and Rheology. Int. J. Mol. Sci. 2014, 15 (1), 1096–1111. 10.3390/ijms15011096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar G. M.; Koenigs M.; Fahimi Z.; Cox M.; Voets I. K.; Wyss H. M.; Sijbesma R. P. Injectable Hydrogels from Segmented PEG-Bisurea Copolymers. Biomacromolecules 2012, 13 (12), 3966–3976. 10.1021/bm301242v. [DOI] [PubMed] [Google Scholar]
- Sun J.-Y.; Zhao X.; Illeperuma W. R. K.; Chaudhuri O.; Oh K. H.; Mooney D. J.; Vlassak J. J.; Suo Z. Highly Stretchable and Tough Hydrogels. Nature 2012, 489 (7414), 133–136. 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncaboylu D. C.; Sari M.; Oppermann W.; Okay O. Tough and Self-Healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44 (12), 4997–5005. 10.1021/ma200579v. [DOI] [Google Scholar]
- Mihajlovic M.; Staropoli M.; Appavou M.-S.; Wyss H. M.; Pyckhout-Hintzen W.; Sijbesma R. P. Tough Supramolecular Hydrogel Based on Strong Hydrophobic Interactions in a Multiblock Segmented Copolymer. Macromolecules 2017, 50 (8), 3333–3346. 10.1021/acs.macromol.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangeetha N. M.; Maitra U. Supramolecular Gels: Functions and Uses. Chem. Soc. Rev. 2005, 34 (10), 821–836. 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe S.; Dubruel P.; Schacht E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12 (5), 1387–1408. 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- Drury J. L.; Mooney D. J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24 (24), 4337–4351. 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101 (7), 1869–1880. 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Akay G.; Hassan-Raeisi A.; Tuncaboylu D. C.; Orakdogen N.; Abdurrahmanoglu S.; Oppermann W.; Okay O. Self-Healing Hydrogels Formed in Catanionic Surfactant Solutions. Soft Matter 2013, 9 (7), 2254–2261. 10.1039/c2sm27515e. [DOI] [Google Scholar]
- Gulyuz U.; Okay O. Self-Healing Poly(Acrylic Acid) Hydrogels with Shape Memory Behavior of High Mechanical Strength. Macromolecules 2014, 47 (19), 6889–6899. 10.1021/ma5015116. [DOI] [Google Scholar]
- Li G.; Yan Q.; Xia H.; Zhao Y. Therapeutic-Ultrasound-Triggered Shape Memory of a Melamine-Enhanced Poly(Vinyl Alcohol) Physical Hydrogel. ACS Appl. Mater. Interfaces 2015, 7 (22), 12067–12073. 10.1021/acsami.5b02234. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Xu B.; Winnik M. A.; Macdonald P. M. Surfactant Interactions with HEUR Associating Polymers. J. Phys. Chem. 1996, 100 (23), 9834–9841. 10.1021/jp953558f. [DOI] [Google Scholar]
- Argun A.; Algi M. P.; Tuncaboylu D. C.; Okay O. Surfactant-Induced Healing of Tough Hydrogels Formed via Hydrophobic Interactions. Colloid Polym. Sci. 2014, 292 (2), 511–517. 10.1007/s00396-013-3121-8. [DOI] [Google Scholar]
- Hopkins F. G. Denaturation of Proteins by Urea and Related Substances. Nature 1930, 126, 328–330. 10.1038/126328a0. [DOI] [Google Scholar]
- Ratner B. D.; Miller I. F. Interaction of Urea with Poly(2-Hydroxyethyl Methacrylate) Hydrogels. J. Polym. Sci., Part A-1: Polym. Chem. 1972, 10 (8), 2425–2445. 10.1002/pol.1972.150100818. [DOI] [Google Scholar]
- Tanford C. Isothermal Unfolding of Globular Proteins in Aqueous Urea Solutions. J. Am. Chem. Soc. 1964, 86 (10), 2050–2059. 10.1021/ja01064a028. [DOI] [Google Scholar]
- Rupley J. A. The Effect of Urea and Amides upon Water Structure1. J. Phys. Chem. 1964, 68 (7), 2002–2003. 10.1021/j100789a503. [DOI] [Google Scholar]
- Finer E. G.; Franks F.; Tait M. J. Nuclear Magnetic Resonance Studies of Aqueous Urea Solutions. J. Am. Chem. Soc. 1972, 94 (13), 4424–4429. 10.1021/ja00768a004. [DOI] [Google Scholar]
- Zangi R.; Zhou R.; Berne B. J. Urea’s Action on Hydrophobic Interactions. J. Am. Chem. Soc. 2009, 131 (4), 1535–1541. 10.1021/ja807887g. [DOI] [PubMed] [Google Scholar]
- Rezus Y.; Bakker H. J. Effect of Urea on the Structural Dynamics of Water. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (49), 18417–18420. 10.1073/pnas.0606538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncaboylu D. C.; Sahin M.; Argun A.; Oppermann W.; Okay O. Dynamics and Large Strain Behavior of Self-Healing Hydrogels with and without Surfactants. Macromolecules 2012, 45 (4), 1991–2000. 10.1021/ma202672y. [DOI] [Google Scholar]
- Sagle L. B.; Zhang Y.; Litosh V. A.; Chen X.; Cho Y.; Cremer P. S. Investigating the Hydrogen-Bonding Model of Urea Denaturation. J. Am. Chem. Soc. 2009, 131 (26), 9304–9310. 10.1021/ja9016057. [DOI] [PubMed] [Google Scholar]
- Wang J.; Liu B.; Ru G.; Bai J.; Feng J. Effect of Urea on Phase Transition of Poly(N-Isopropylacrylamide) and Poly(N,N-Diethylacrylamide) Hydrogels: A Clue for Urea-Induced Denaturation. Macromolecules 2016, 49 (1), 234–243. 10.1021/acs.macromol.5b01949. [DOI] [Google Scholar]
- Fahimi Z.Structure and Mechanics of Physically Cross-Linked Hydrogels; Technische Universiteit Eindhoven: 2014. [Google Scholar]
- Mukerjee P.; Mysels K. J.. Critical Micelle Concentrations of Aqueous Surfactant Systems; U.S. National Bureau of Standards; for sale by the Supt. of Docs., U.S. Govt. Print. Off., 1971.
- Hao J.; Weiss R. A. Viscoelastic and Mechanical Behavior of Hydrophobically Modified Hydrogels. Macromolecules 2011, 44 (23), 9390–9398. 10.1021/ma202130u. [DOI] [Google Scholar]
- Wu J.; Ge Q.; Mather P. T. PEG-POSS Multiblock Polyurethanes: Synthesis, Characterization, and Hydrogel Formation. Macromolecules 2010, 43 (18), 7637–7649. 10.1021/ma101336c. [DOI] [Google Scholar]
- Mark J. E.; Erman B.. Rubberlike Elasticity: A Molecular Primer; Cambridge University Press: 2007. [Google Scholar]
- Graziano G. On the Solubility of Aliphatic Hydrocarbons in 7 M Aqueous Urea. J. Phys. Chem. B 2001, 105 (13), 2632–2637. 10.1021/jp004335e. [DOI] [Google Scholar]
- Meng F.; Pritchard R. H.; Terentjev E. M. Stress Relaxation, Dynamics, and Plasticity of Transient Polymer Networks. Macromolecules 2016, 49 (7), 2843–2852. 10.1021/acs.macromol.5b02667. [DOI] [Google Scholar]
- Gurtovenko A. A.; Blumen A. Relaxation of Disordered Polymer Networks: Regular Lattice Made up of Small-World Rouse Networks. J. Chem. Phys. 2001, 115 (10), 4924–4929. 10.1063/1.1395562. [DOI] [Google Scholar]
- Baeurle S. A.; Hotta A.; Gusev A. A. A New Semi-Phenomenological Approach to Predict the Stress Relaxation Behavior of Thermoplastic Elastomers. Polymer 2005, 46 (12), 4344–4354. 10.1016/j.polymer.2004.07.034. [DOI] [Google Scholar]
- Williams G.; Watts D. C. Non-Symmetrical Dielectric Relaxation Behaviour Arising from a Simple Empirical Decay Function. Trans. Faraday Soc. 1970, 66 (0), 80–85. 10.1039/tf9706600080. [DOI] [Google Scholar]
- Li Q.; Barrett D. G.; Messersmith P. B.; Holten-Andersen N. Controlling Hydrogel Mechanics via Bio-Inspired Polymer–Nanoparticle Bond Dynamics. ACS Nano 2016, 10 (1), 1317–1324. 10.1021/acsnano.5b06692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncaboylu D. C.; Argun A.; Algi M. P.; Okay O. Autonomic Self-Healing in Covalently Crosslinked Hydrogels Containing Hydrophobic Domains. Polymer 2013, 54 (23), 6381–6388. 10.1016/j.polymer.2013.09.051. [DOI] [Google Scholar]
- Thomas B. H.; Craig Fryman J.; Liu K.; Mason J. Hydrophilic–hydrophobic Hydrogels for Cartilage Replacement. J. Mech. Behav. Biomed. Mater. 2009, 2 (6), 588–595. 10.1016/j.jmbbm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Tedeschi A.; Auriemma F.; Ricciardi R.; Mangiapia G.; Trifuoggi M.; Franco L.; De Rosa C.; Heenan R. K.; Paduano L.; D’Errico G. A Study of the Microstructural and Diffusion Properties of Poly(Vinyl Alcohol) Cryogels Containing Surfactant Supramolecular Aggregates. J. Phys. Chem. B 2006, 110 (46), 23031–23040. 10.1021/jp061941m. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li L. SDS-Aided Immobilization and Controlled Release of Camptothecin from Agarose Hydrogel. Eur. J. Pharm. Sci. 2005, 25 (2–3), 237–244. 10.1016/j.ejps.2005.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.