Abstract
Background
Traditionally, milk proteins have been recommended for skeletal health; recently, soy proteins have emerged as popular alternatives. Excess adiposity appears detrimental to skeletal health, as obese adolescents have increased fracture rates compared with healthy controls. However, soy protein effects on skeletal health during excess adiposity remain unknown.
Objective
The study objective was to examine the effects of isocaloric diets containing milk protein isolate (MPI), soy protein isolate (SPI), or a 50/50 combination (MIX) as the sole protein source on metabolic health indicators and bone outcomes in rapidly growing, hyperphagic, male Otsuka Long Evans Tokushima Fatty (OLETF) rats.
Methods
OLETF rats, aged 4 wk, were randomly assigned to 3 treatment groups (MPI, SPI, or MIX, n = 20 per group) and provided with access to experimental diets ad libitum for 16 wk.
Results
Body mass did not differ between the groups, but SPI had lower percentage body fat than MPI (P = 0.026). Insulin was lower in MPI than in MIX (P = 0.033) or SPI (P = 0.044), but fasting blood glucose was not different between the groups. SPI significantly reduced serum cholesterol compared with MPI (P = 0.001) and MIX (P = 0.002). N-terminal propeptide of type I collagen (P1NP) was higher in MIX than MPI (P = 0.05); C-terminal telopeptide of type 1 collagen (CTx) was higher in MPI than SPI (P < 0.001) and MIX (P < 0.001); the P1NP to CTx ratio was significantly higher in SPI and MIX than in MPI (P < 0.001). Trabecular separation was reduced in SPI compared with MPI (P = 0.030) and MIX (P = 0.008); trabecular number was increased in SPI compared with MIX (P = 0.038). No differences were seen in cortical geometry and biomechanical properties.
Conclusions
In the context of excess adiposity, soy- and milk-based proteins have comparable effects on cortical bone geometry and biomechanical properties, whereas soy-based proteins favorably affect the trabecular microarchitecture, and the combination of both proteins may offer additional benefits to bone remodeling in rapidly growing male OLETF rats.
Keywords: soy protein, milk protein, bone, OLETF rats, trabecular microarchitecture, bone turnover markers
Introduction
Individuals achieve peak bone mass early in the second decade of life, which makes adolescence a critical time for skeletal growth and bone accrual (1). Peak bone mass is influenced by a variety of lifestyle factors, such as diet, physical activity, and body mass (1). Higher body mass is generally correlated with higher bone mineral density (BMD) owing to increased mechanical loading (2). However, obesity, especially excess adiposity, is associated with increased systemic inflammation, insulin resistance, and PPAR-γ signaling, which negatively impact the skeleton (3, 4) by increasing osteoclast activity (5) and decreasing osteoblast activity (6), leading to an imbalance in bone remodeling. When adjusted for body mass, obese children tend to have lower whole-body BMD and bone mineral content, as well as higher fracture rates in the lower limbs, compared with lean controls (3, 7). Epidemiologic evidence points to a positive association between dairy intake and bone mass in childhood and adolescence (8–10). In addition, animal studies indicate that soy protein might counteract the detrimental effects of obesity on bone, by reducing PPAR-γ signaling and insulin resistance, and thus correct the imbalance of remodeling (11, 12). However, whether soy- or dairy-based proteins confer greater skeletal benefits in the context of obesity has yet to be determined.
Traditionally, the consumption of cow's milk-based proteins is recommended for optimal skeletal development because dairy milk and dairy products are excellent sources of dietary calcium and vitamin D (13). However, recent evidence suggests that the skeletal benefits of whole dairy products are greater than those derived from calcium and vitamin D supplementation (14). These data support the conclusion that the skeletal benefits of dairy products are due in part to milk protein, in addition to the benefits of calcium and vitamin D. In adolescents, 12–18 mo of milk supplementation resulted in an increase in spine (15) and whole-body BMD (16) compared with those without milk supplementation. A meta-analysis showed that this increase in BMD after an increase in dairy consumption is especially significant in individuals with a history of low intakes (17). Milk consumption during adolescence is also associated with a lower risk of osteoporotic fracture as an adult (18). In healthy men, short-term (i.e., 16 d) milk-protein supplementation increased urinary markers of bone formation and decreased urinary markers of bone resorption compared with baseline (19). Epidemiologic evidence in adolescents (20) supports a strong positive correlation between milk consumption and circulating insulin-like growth factor (IGF-1), which is essential for osteoblast differentiation and bone formation (21, 22). Additionally, short-term supplementation with casein (23) and milk protein increased circulating IGF-1 in prepubescent boys (24). Together, this evidence supports milk-based proteins being a benefit to bone health outside of their micronutrient content.
While milk-based proteins remain popular among consumers, soy-based proteins have emerged as a popular vegetarian, plant-based dairy alternative (25). Unique among plant-based proteins, soy is a high-quality protein equivalent to egg protein (26), which is used as the reference protein in determination of biological value. Not only does soy protein as the sole dietary protein source support positive nitrogen balance in growing humans; data from experimental animal models suggest that soy protein has equivalent or even superior skeletal effects compared with casein in adolescent male animals (27, 28). Soy protein significantly improved femoral BMD, as well as cancellous bone properties, such as trabecular number and bone volume, in male C57BL/6 mice (27). Following soy protein consumption, expression of intestinal calcium transporters, specifically TRPV6, were increased in rats compared with casein controls (28). In young and old men, soy protein intake has been shown to increase circulating IGF-1 concentrations (29). Therefore, a diet containing soy protein isolate should also positively affect skeletal development, although whether animal- or plant-based proteins confer the most benefit is not clear (28).
Given the increasing prevalence of excess adiposity and insulin resistance in adolescents and their adverse effects on bone health, the effects of protein source are clinically relevant. To our knowledge, no other studies have looked at protein source and the effect it can have on bone turnover, trabecular microarchitecture, or cortical geometry and biomechanical strength in the context of obesity and insulin resistance. Thus, the current study was performed to compare the effects of milk- and soy-based protein on bone outcomes in the Otsuka Long Evans Fatty (OLETF) rat model of obesity and insulin resistance. We chose the OLETF model because the progression of obesity and insulin resistance relative to skeletal maturity is similar to that of humans (30). We hypothesized that soy-based protein could have an equivalent effect on serum markers of bone turnover, trabecular microarchitecture, and cortical geometry and biomechanical strength in rapidly growing male OLETF rats. The OLETF rat is selectively bred for null expression of cholecystokinin-1 (CCK-1) receptor in the hypothalamus. Because CCK is a gut hormone that signals satiety through interaction with the receptor in the hypothalamus, null expression of the CCK-1 receptor results in hyperphagia (31) and subsequent obesity and insulin resistance with a standard rodent chow diet. Excess adiposity is evident starting at 5 wk of age, followed by the onset of insulin resistance at 12 wk of age, hyperglycemia at 20 wk of age, and frank type 2 diabetes by 40 wk of age (31–33). This progression of disease coincides with skeletal growth, which peaks at around 16 wk of age in the rat, consistent with the development of insulin resistance and obesity relative to skeletal maturity in humans (30).
Methods
Experimental design and animal protocol
In this 16-wk longitudinal study, sixty 4-wk-old, hyperphagic male OLETF rats (Tokushima Research Institute) were randomly assigned to 1 of 3 experimental dietary treatments: milk protein isolate (MPI; Idaho Milk Products), soy protein isolate (SPI; DuPont Nutrition and Health), or a 50/50 combination of MPI and SPI (MIX; Research Diets, Inc.) as the sole protein source (n = 20 per group). All rats were housed individually in a temperature-controlled environment (21°C) with a 0600–1800 light, 1800–0600 dark cycle maintained throughout the experimental period. Rats were allowed ad libitum consumption of the diets from 4 to 20 wk of age. Body weight, food intake, and body composition via EchoMRI were recorded weekly. The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri and the Harry S Truman VA Subcommittee for Animal Studies. Not all rats were used for all analyses, based on availability of samples.
Experimental diets
Animals were randomly assigned to experimental diets containing MPI, SPI, or MIX as the sole protein source for 16 wks. The MIX group was included to compare the effects of a mixed protein source diet on bone outcomes to the effects of the diets containing only soy- or only milk-based proteins. Each diet was formulated to be isonitrogenous and isocaloric on the basis of the guaranteed analysis provided by the manufacturer, and to meet or exceed the AIN-93G micronutrient requirements for the growing rat, as previously reported (34) (Table 1). Diets differed from AIN-93G in macronutrient composition, in that the diets were higher in fat and contained sucrose. This was done so that the diets would mimic a Western-style diet (35), each providing 19% of energy from protein, 45% from carbohydrate, and 36% from fat. The isoflavone content of the SPI protein (μg aglycone/g protein) was as follows: 453 μg daidzein/g protein, 731 μg genistein/g protein, and 62 μg glycitein/g protein. Isoflavone content of the SPI diet was 90.6 mg of daidzein, 146.2 mg of genistein, and 12.4 mg of glycitein; isoflavone content of the MIX diet was 45.3 mg of diadzein, 73.1 mg of genistein, and 6.2 mg of glycitein. The calcium content (mass %) of the SPI, MIX, and MPI diets was 0.74%, 0.96%, and 1.18%, respectively; the phosphorus content was 0.56%, 0.58%, and 0.60%, respectively. The MPI and MIX diets had higher calcium and phosphorus contents because of the calcium and phosphorus associated with the MPI protein.
TABLE 1.
| Diet, g/kg | |||
|---|---|---|---|
| Ingredient | SPI | MIX2 | MPI |
| Cornstarch | 240 | 240 | 240 |
| Sucrose | 100 | 100 | 100 |
| Maltodextrin | 75 | 75 | 75 |
| Cellulose | 50 | 50 | 50 |
| MPI | 0 | 108.8 | 217.5 |
| SPI | 200 | 100 | 0 |
| DL-methionine | 3 | 3 | 3 |
| Palm oil, bleached, deodorized | 52.5 | 52.5 | 52.5 |
| Cocoa butter, deodorized | 37.5 | 37.5 | 37.5 |
| Safflower oil, USP | 28.5 | 28.5 | 28.5 |
| Sunflower oil | 27 | 27 | 27 |
| Linseed oil | 4.5 | 4.5 | 4.5 |
| t-Butylhydroquinone | 0.03 | 0.03 | 0.03 |
| Mineral mix3 | 10 | 10 | 10 |
| Potassium citrate | 16.5 | 16.5 | 16.5 |
| Dicalcium phosphate | 13 | 13 | 13 |
| Calcium carbonate | 5.5 | 5.5 | 5.5 |
| Vitamin mix4 | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 |
| Protein, % energy | 19 | 19 | 19 |
| Carbohydrate, % energy | 45 | 45 | 45 |
| Fat, % energy | 36 | 36 | 36 |
| Caloric density (kcal/g) | 4.41 | 4.41 | 4.41 |
MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; SPI, soy protein isolate.
MIX, 50/50 combination of MPI (MPI-85, Idaho Milk Products) and SPI (SUPRO 670, DuPont Nutrition & Health).
Mineral Mix S10026 (Research Diets, Inc) contains (in g/kg of mineral mix): NaCl, 259; MgO, heavy, 41.9; MgSO4⋅7H2O, 257.6; (NH4)6Mo7O24 4H2O, 0.3; KCrS2O8, 1.925; CuCO3, 1.05; C6H5FeO7, 21; CO3MnH2O, 12.25; KIO3, 0.035; NaF, 0.2; Na2SeO3, 0.035; ZnCO3, 5.6; sucrose, 399.105.
Vitamin Mix V13401(Research Diets, Inc) contains (in g/kg of vitamin mix): vitamin A palmitate (500,000 IU/g), 0.8; vitamin D3 (100,000 IU/g) 1.0; menadione sodium bisulfate (62.5% menadione), 0.08; biotin (1%), 2.0; cyanocobalmin (0.1%), 1.0; folic acid, 0.2; nicotinic acid, 3.0; calcium pantothenate, 1.6; pyridoxine-HCl, 0.7; riboflavin, 0.6; thiamin-HCl, 0.6; sucrose 988.42.
Animal sacrifice and tissue collection
At 20 wk of age, the final body mass was measured, then rats were anesthetized via intraperitoneal injection of pentobarbital (80 mg/kg) and exsanguinated via removal of the heart, as previously described (34). Blood was collected via cardiac puncture, allowed to clot for 20 min at room temperature, then spun at 1500 × g for 10 min at 4°C for serum collection. Serum was aliquoted and stored at –80°C for subsequent analysis of metabolic markers and serum markers of bone formation (N-terminal propeptide of type I collagen, P1NP) and resorption (C-terminal telopeptide of type I collagen, CTx). Right tibias and femurs were collected, cleaned of soft tissue, wrapped in 1× PBS-soaked gauze, and frozen at –80°C for subsequent analysis.
Metabolic outcomes
Fasting serum glucose, insulin, free fatty acids, triglycerides, and total cholesterol were measured using commercially available kits as previously described (34).
Serum markers of bone formation and resorption
The concentrations of the bone formation marker P1NP and the resorption marker CTx were measured in serum using commercially available, rodent-specific ELISA kits (ImmunoDiagnostic Systems). The intra-assay CVs were <4% for CTx and <6% for P1NP. All assays were run on the same day to avoid inter-assay variation; all samples were run in duplicate.
Femur calcium and phosphorous contents
Right femurs were cleaned of all soft tissue, weighed, and then defatted in hexane and diethyl ether each for 24 h. Following lipid extraction, femurs were dried to a constant weight at 60°C. Femurs were then placed in a muffle furnace (800°C) overnight to collect ash. The final weight of the ash content was recorded and ashed femurs were dissolved in 12 N HCl for subsequent analysis of calcium and phosphorus contents via inductive coupled plasma-optical emission spectroscopy (University of Missouri, Agricultural Experiment Station Chemical Laboratories). Results are expressed as milligrams of calcium or phosphorus per gram of dry bone (mg/g).
Tibia cortical geometry and trabecular microarchitecture
Microcomputed tomography (μCT) imaging of the tibia was performed using a high-resolution (32-µm slice increment) imaging system (Siemens INVEON Micro SPECT/CT, Siemens Medical). The methods used were in accordance with guidelines for the use of μCT in rodents (36). Scans were acquired using an isotropic voxel size of 0.0316 mm, a peak X-ray tube potential of 80 kVp with a tube current of 500 µA, and a 600-ms exposure at a medium-high magnification using a bin of 2. In a single rotation, 360 projections were collected at 1° increments and calibration images were collected prior to data acquisition. Images were reconstructed in real time using a Feldkamp cone beam filtered back projection algorithm (2D-FDP). Trabecular bone microarchitecture was evaluated in a 1-mm region of interest that started 1 mm below the growth plate of the proximal tibia. Cortical bone cross-sectional geometry was evaluated in the tibia mid-diaphysis, between the crest of the tibia and the distal edge of the tibiofibular joint in a 0.5-mm region of interest 0.25 mm proximal and 0.25 mm distal to the midslice. The optimize threshold function was used to delineate mineralized bone from soft tissue. Segmentation thresholds of 214 mg/cm3 and 570 mg/cm3 were used for evaluation of trabecular and cortical bone, respectively. Scans were analyzed using BoneJ software (37), a subset of ImageJ (ver. 1.50d) (National Institutes of Health public domain), and measures of cortical geometry and trabecular microarchitecture were collected. Cortical morphometric outcomes included: tibia length, total cross-sectional area inside the periosteal envelope (Tt.Ar), marrow area (Ma.Ar), cortical bone area (Ct.Ar), cortical area fraction (Ct.Ar/Tt.Ar, %), average cortical thickness (Ct.Th), and robustness (R, total bone area over tibia length, calculated as R = Tt.Ar/Le). Outcomes for trabecular microarchitecture included: total volume (TV, volume of region of interest), bone volume (BV, volume of region segmented as bone), bone volume fraction (BV/TV), connectivity density (Conn.D, degree of trabeculae connectivity normalized to TV), trabecular number (Tb.N, mean number of trabeculae per unit length, calculated as 1/(Tb.Th + Tb.Sp) (38)), trabecular thickness (Tb.Th, mean trabecular thickness), trabecular separation (Tb.Sp, distance between trabeculae), structural model index (SMI), and degree of anisotropy.
Tibial biomechanical properties
Torsional loading to failure was used to assess the biomechanical properties of the tibia. The distal and proximal ends of the right tibia were embedded in a cylindrical steel holder that was placed in a test fixture. A machined cross bar was used to prevent the proximal end of the holder/tibia from rotating about its long axis while the distal end was rotated about its long axis at a speed of 10 mm/s with a load cell of 5 kg. The machine's control software (Stable Micro Systems) measured the cable force (F, in grams) and the applied torque (T). The load-displacement curve from this analysis is analogous to the torque-twist curve, which was used along with geometrical properties determined from μCT (i.e., length of specimen and polar moment of inertia) to calculate: maximal torque at fracture (Tmax), torsional stiffness (Ks), shear modulus of elasticity (G), ultimate tensile strength or maximal shear stress (Su), and energy absorbed to failure (U) as previously described (30).
Statistics
Four-wk-old, hyperphagic male OLETF rats were randomly assigned to 1 of 3 experimental diet groups (MPI, MIX, or SPI). Pearson correlations between metabolic (body weight, body fat percentage, and serum insulin, glucose, triglycerides, and cholesterol) and bone (bone turnover markers, trabecular microarchitecture, and cortical geometry and biomechanical strength) outcomes were performed to determine direct effects of diet compared with indirect effects of metabolic health on bone outcomes. One-way ANOVA was used to test for significant differences between groups for metabolic outcomes, serum bone turnover markers, and trabecular outcomes. Body weight is a strong predictor of cortical bone growth and biomechanical strength, so cortical and biomechanical outcomes were assessed by one-way ANCOVA, with final body weight included as a covariate. When there was a significant difference among groups, post hoc pairwise comparisons were made using the least significant difference technique. Data are means ± SEMs; statistical significance was set at P < 0.05. All analyses were performed using SPSS software (SPSS/23.0).
Results
Rat characteristics
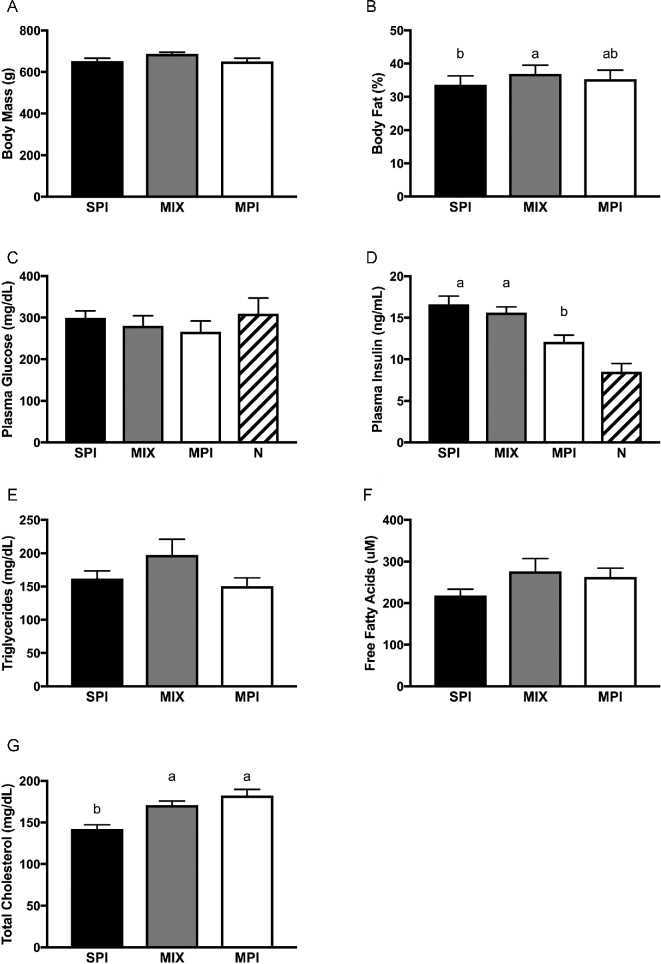
Metabolic characteristics, including liver function and microbiome composition, of a subset of OLETF rats have been reported previously (34). Initial body mass (P = 0.808), final body mass (P = 0.259), serum glucose (P = 0.519), triglycerides (P = 0.118), and free fatty acids (P = 0.119) were not significantly different among groups (34). However, percentage body fat was lower in SPI-fed rats (P = 0.026) compared with MIX-fed rats, but not MPI-fed rats. Rats consuming the MIX diet had the highest average weekly food intake (181.0 ± 2.3 g) compared with rats consuming the SPI (174.0 ± 2.7 g) and MPI (170.4 ± 2.9 g) diets (34). At time of death, all rats were insulin resistant, as they had elevated fasting insulin but normal glucose concentrations (31). Fasting insulin was significantly different among groups (P = 0.002): it was significantly lower in MPI-fed rats than in those fed MIX (P = 0.006) and SPI (P = 0.001). Rats consuming the SPI diet had significantly reduced serum cholesterol compared with those consuming the MPI and MIX diets (P = 0.001 and P = 0.002, respectively) (Figure 1).
FIGURE 1.
Final body weights and fasting metabolic characteristics in OLETF rats fed a Western-style diet with SPI, MPI, or MIX. Body mass (A), body fat percentage (B), plasma glucose (C), plasma insulin (D), triglycerides (E), free fatty acids (F), and serum total cholesterol (G). Data are means ± SEMs; n = 9–10 rats/group. Different letters denote significance, P < 0.05. For reference, normoglycemic and normoinsulinemic values have been provided. MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; N, normoglycemic and normoinsulinemic; OLETF, Otsuka Long Evans Tokushima Fatty; SPI, soy protein isolate.
Serum markers of bone formation and resorption
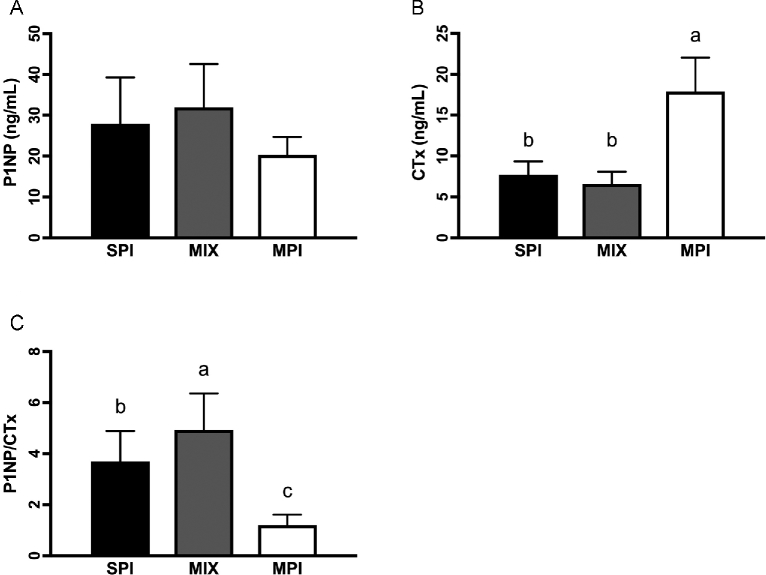
Rats fed the MIX diet had significantly greater P1NP than those on the MPI diet (P = 0.017). SPI-fed and MIX-fed rats had significantly lower CTx than MPI-fed rats (P < 0.001). Consequently, the ratio of P1NP/CTx was significantly different between groups (P < 0.001), with the ratio being significantly greater in rats consuming the MIX diet than on either the SPI (P = 0.026) or MPI (P < 0.001) diet and the ratio in the SPI group being greater than that in the MPI group (P < 0.001) (Figure 2).
FIGURE 2.
Serum markers of bone turnover in OLETF rats fed a Western-style diet with SPI, MPI, or MIX. P1NP (A), CTx (B), and P1NP/CTx (C). Data are means ± SEMs; n = 9–10 rats/group. Different letters denote significance, P < 0.05. CTx, C-terminal telopeptide of type I collagen; MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; OLETF, Otsuka Long Evans Tokushima Fatty; P1NP, N-terminal propeptide of type I collagen; SPI, soy protein isolate.
Femur calcium and phosphorous content
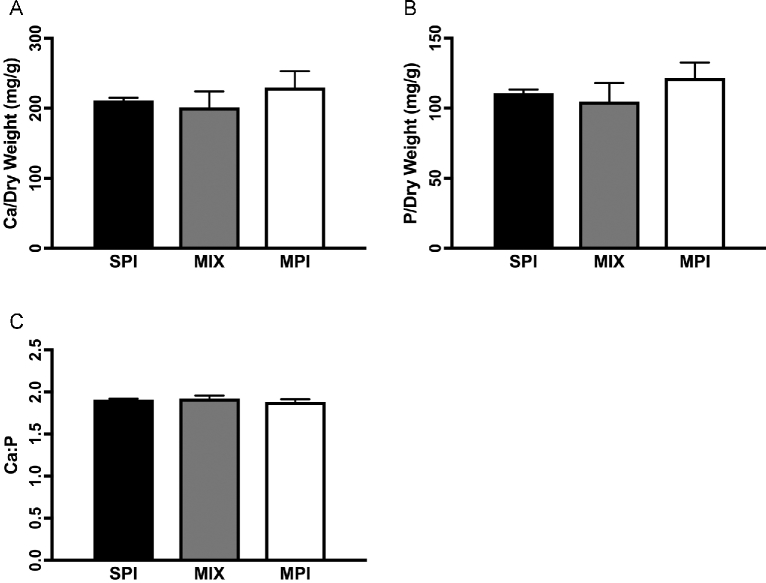
There were no differences in calcium content (milligrams of mineral per gram of dry bone) among groups (P = 0.105). However, phosphorus content (milligrams of mineral per gram of dry bone) trended toward group differences (P = 0.059), with the MPI group having the highest phosphorus content and the MIX group the lowest (Figure 3).
FIGURE 3.
Mineral content of the femur in OLETF rats fed a Western-style diet with SPI, MPI, or MIX. Ca/dry weight (A), P/dry weight (B), and Ca-to-P ratio (C). Data are means ± SEMs; n = 5 rats/group. Different letters denote significance, P < 0.05. MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; OLETF, Otsuka Long Evans Tokushima Fatty; SPI, soy protein isolate.
Tibial cortical geometry
Tibia length was not different among groups, suggesting no differences in longitudinal growth. Tt.Ar, Ma.Ar, Ct.Ar, Ct.Th, R, and Ct.Ar/Tt.Ar of the tibia mid-diaphysis were not different among groups, indicating similar bone mass accumulation (36). There were no differences in maximum or minimum moment of inertia (Imax, Imin) among groups; the Imax/Imin ratio, which is a measure of the circularity of the diaphysis (39), also showed no difference, indicating similarly shaped tibias among groups (Table 2).
TABLE 2.
Cortical geometry of the tibia mid-diaphysis in OLETF rats fed a Western-style diet with SPI, MPI, or MIX1
| SPI | MIX | MPI | P-value (Diet)2 | |
|---|---|---|---|---|
| Tibia length, mm | 44.60 ± 0.30 | 45.04 ± 0.36 | 44.46 ± 0.32 | 0.43 |
| Tibia diameter, mm | 3.93 ± 0.08 | 3.99 ± 0.08 | 3.92 ± 0.08 | 0.78 |
| Tt.Ar, mm2 | 9.75 ± 0.11 | 9.89 ± 0.11 | 9.91 ± 0.10 | 0.43 |
| Ma.Ar, mm2 | 2.52 ± 0.06 | 2.69 ± 0.06 | 2.59 ± 0.06 | 0.11 |
| Ct.Ar, mm2 | 7.22 ± 0.12 | 7.20 ± 0.11 | 7.32 ± 0.12 | 0.74 |
| Ct.Ar/Tt.Ar, mm2 | 0.74 ± 0.01 | 0.73 ± 0.01 | 0.74 ± 0.01 | 0.30 |
| Ct.Th, mm2 | 1.02 ± 0.01 | 1.02 ± 0.01 | 1.01 ± 0.01 | 0.69 |
| Imax, mm4 | 7.61 ± 0.09 | 7.55 ± 0.10 | 7.50 ± 0.10 | 0.72 |
| Imin, mm4 | 4.70 ± 0.10 | 4.78 ± 0.11 | 4.74 ± 0.11 | 0.86 |
| Imax/Imin | 1.63 ± 0.03 | 1.58 ± 0.03 | 1.60 ± 0.03 | 0.40 |
| K, mm4 | 13.8 ± 0.55 | 12.9 ± 0.56 | 13.2 ± 0.57 | 0.53 |
| R, mm | .219 ± 0.003 | .220 ± 0.003 | .223 ± 0.003 | 0.48 |
Data are means ± SEMs adjusted with final body weight as a covariate; n = 18–20 rats/group. Ct.Ar, cortical area; Ct.Ar/Tt.Ar, cortical volume fraction; Ct.Th, cortical thickness; Imax, maximum moment of inertia; Imin, minimum moment of inertia; Imax/Imin, ratio of maximum to minimum moment of inertia; K, polar moment of area; Ma.Ar, marrow area; MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; R, robustness (Tt.Ar/length); SPI, soy protein isolate; Tt.Ar, total area.
P-values are a one-way ANCOVA with body weight as a covariate.
Tibia trabecular microarchitecture
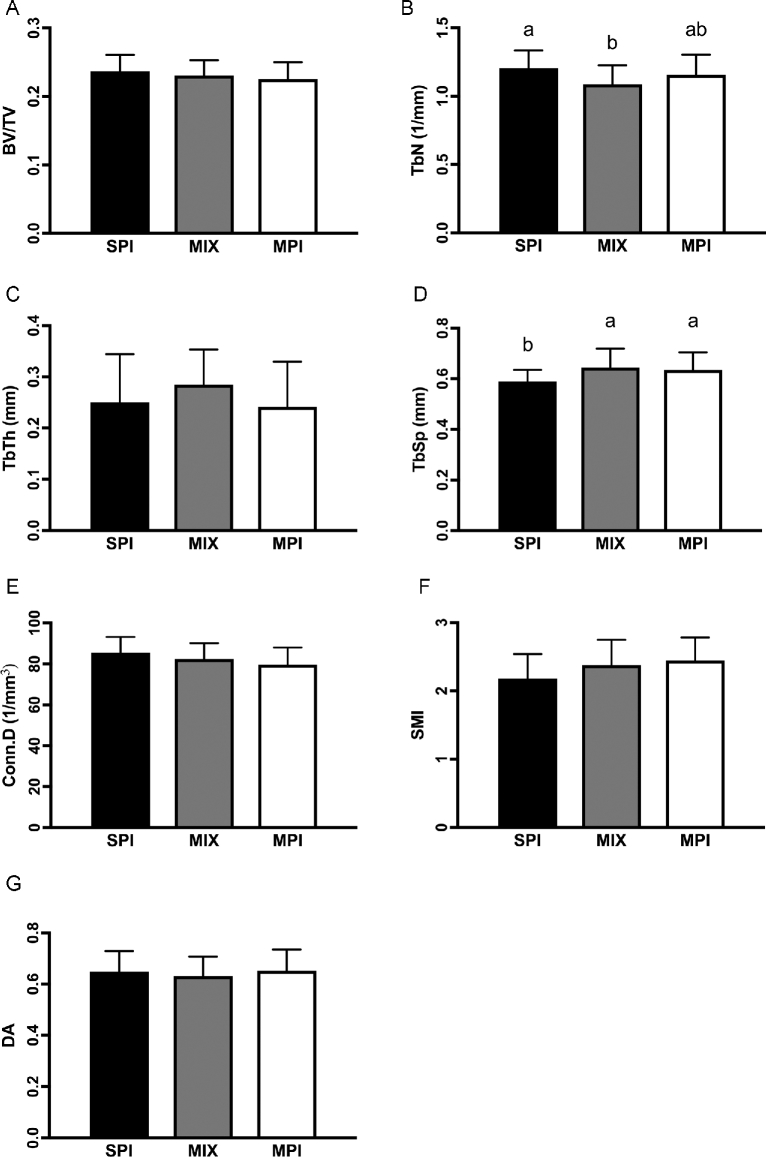
BV/TV (P = 0.364), Tb.Th (P = 0.242), and degree of anisotropy (P = 0.251) of the proximal tibia were not different among groups. However, Tb.Sp was significantly lower in rats fed the SPI diet compared with those fed the MIX (P = 0.014) and MPI (P = 0.025) diets. Tb.N was also significantly increased in the SPI group relative to the MIX group (P = 0.011), but not compared with the MPI group (P = 0.297). There was also a trend for Conn.D (P = 0.077) and SMI (P = 0.060) to be different among groups, with the SPI group having the highest Conn.D but the lowest SMI compared with the MIX and MPI groups (Figure 4).
FIGURE 4.
Trabecular microarchitecture of the proximal tibia in OLETF rats fed a Western-style diet with SPI, MPI, or MIX. BV/TV (A), TbN (B), TbTh (C), TbSp (D), Conn.D (E), SMI (F), and DA (G). Data are means ± SEMs; n = 19–20 rats/group. Different letters denote significance, P < 0.05. BV/TV, trabecular bone volume fraction; Conn.D, connectivity density; DA, degree of anisotropy; MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; OLETF, Otsuka Long Evans Tokushima Fatty; SMI, structural mode index; SPI, soy protein isolate; TbN, trabecular number; TbSp, trabecular separation; TbTh, trabecular thickness.
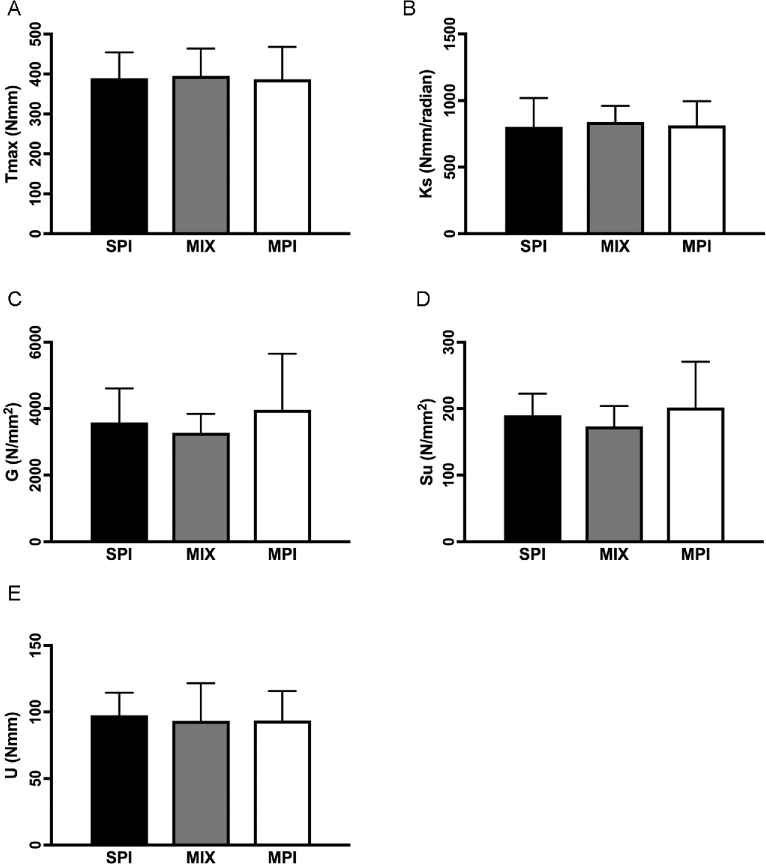
Tibial biomechanical properties
Whole-bone (Tmax, P = 0.929; Ks, P = 0.753; U, P = 0.836) and tissue-level (G, P = 0.152; Su, P = 0.151) biomechanical properties of the tibia mid-diaphysis were not different among groups (Figure 5).
FIGURE 5.
Biomechanical strength measures of the tibia mid-diaphysis in OLETF rats fed a Western-style diet with SPI, MPI, or MIX. Tmax (A), Ks (B), G (C), Su (D), and U (E). Data are means ± SEMs adjusted with final body weight as a covariate; n = 19–20 rats/group. Different letters denote significance, P < 0.05. G, shear modulus of elasticity; Ks, torsional stiffness; MIX, a 50/50 mixture of MPI and SPI; MPI, milk protein isolate; OLETF, Otsuka Long Evans Tokushima Fatty; SPI, soy protein isolate; Su, ultimate tensile strength or maximal shear stress; Tmax, maximal torque at fracture; U, energy absorbed to failure.
Pearson correlations
Body weight was significantly positively correlated with Ct.Th (r = 0.260, P = 0.047), Tmax (r = 0.436, P = 0.003), and Ks (r = .0481, P = 0.001), and trended toward significance with G (r = .0257, P = 0.098) and Su (r = 0.250, P = 0.102). Body fat percentage was significantly positively correlated with Tmax (r = 0.496, P = 0.019), Ks (r = .0568, P = 0.006), G (r = 0.460, P = 0.031), and Tb.Sp (r = 0.392, P = 0.036), and trended toward significance with Su (r = 0.411, P = 0.058). There was a significant negative correlation between body fat percentage and Conn.D (r = −0.398, P = 0.033). There was a significant positive correlation between serum insulin and serum P1NP (r = 0.437; P = 0.020). No other correlations were significant.
Discussion
In this study, we examined the effects of 3 experimental diets (MPI, MIX, and SPI) on cancellous and cortical bone outcomes in obese, insulin-resistant rapidly growing male OLETF rats. We chose the OLETF rat model because the progression of obesity and insulin resistance relative to skeletal maturity in that model is similar to that of humans (30). We hypothesized that soy- and milk-based proteins would have an equivalent effect on bone outcomes during a time of rapid growth. In support of our hypothesis, the diets had a similar effect on cortical geometry and biomechanical properties of the tibia. However, rats fed the SPI diet showed significant improvements in trabecular microarchitecture, specifically trabecular spacing and trabecular number, and rats fed the MIX diet showed an increased ratio of bone formation to bone resorption, as measured by serum markers. Taken together, these results suggest that, in a male rodent model of young obesity, soy- and dairy-based proteins are comparable for cortical bone geometry and biomechanical strength, soy protein might favorably affect cancellous bone microarchitecture, and a mix of both proteins might benefit bone remodeling.
Rats fed the MPI diet had significantly greater serum CTx than rats fed the SPI and MPI diets (104% and 172% higher, respectively), suggesting a suppression of bone resorption with the presence of soy protein. This supports previous studies where soy protein suppresses bone resorption through decreased levels of receptor activator of nuclear factor-κB ligand (RANKL), a known promoter of osteoclastogenesis (40). Additionally, this significant increase of CTx in the MPI diet group is clinically relevant, as evidence points to a fracture relative risk of 2.1 owing to a 25% increase in serum CTx (41). While soy protein independently improved bone remodeling, the effect of combining soy- and milk-based proteins resulted in a significant increase in bone formation relative to resorption based on serum markers, which is consistent with a previous report on the combination of milk protein and soy isoflavones in protection against the loss of BMD in hind-limb unloading through an increase in osteogenic genes in the bone marrow (42). This implies that the combination of soy- and milk-based proteins might be most beneficial to bone remodeling in rapidly growing rats. This is significant, considering that an imbalance in bone remodeling in favor of resorption could be one of the primary mechanisms behind the impaired bone health seen in childhood obesity (43).
A proposed mechanism behind the effects of soy on bone are the estrogen-like actions of soy isoflavones on the estrogen receptor (ER), specifically ERβ (44), and the effects of soy protein intake on bone health in women have been studied extensively (45–47). Fewer studies have examined the skeletal effects of soy in men, partly because of concerns surrounding increased levels of estrogen-like molecules in males and the possibility that bioactive phytoestrogens could have an effect on reproductive hormones in men (48). However, most studies have shown that soy intake has no effect on reproductive hormone levels in either adults or children (49, 50). Additionally, 1 study in older rats showed that soy protein could attenuate orchidectomy-induced bone loss (51), and estrogen levels are generally a stronger predictor of BMD in males than testosterone (52). In addition to its estrogen-like actions (53), other studies show that soy protein may decrease levels of calveolin-1 (54), which could lead to a decrease in osteoblast senescence through a decrease in PPAR-γ (11). SPI could also prevent bone deterioration induced by a high-fat diet by preventing loss of insulin signaling in the bone (12). Additional studies in this area are warranted.
Cancellous bone and cortical bone responded differently to 16 wk of a soy-protein diet. Cortical geometry and biomechanical properties were not significantly different among the dietary treatments, but the trabecular microarchitecture was affected. Specifically, Tb.Sp was significantly decreased and Tb.N was significantly increased in rats fed the SPI diet. One explanation for the effects of soy protein on cancellous, but not cortical, bone is the distribution of the ER isoforms α and β. Soy isoflavones preferentially bind to the ER-β subspecies (44), and cancellous bone has greater expression of ER-β compared with cortical bone (55). Additionally, the rate of turnover in trabecular bone is considerably higher than that of cortical bone (56), owing to an increase in active surface area (57). Other researchers have also shown that soy preferentially protects trabecular bone through increases in bone formation (58), which would be consistent with our findings. However, the bone markers that we measured represent the whole body, and cannot distinguish between cancellous and cortical bone. Loss of individual trabeculae and thinning of existing trabeculae (59), as well as a lower trabecular bone volume (60), significantly contribute to loss of bone strength. Thus, data from the present study suggest that soy protein isolate might increase cancellous bone strength. However, we did not have the capacity to test the compression strength of cancellous bone and further study is warranted.
Finally, we measured the calcium and phosphorus contents of the femur, as mineral content is an important determinant of BMD and bone strength in growing rats (61). While there were small differences in the calcium and phosphorous contents of the MPI and SPI proteins and, therefore, the respective diets, all of the diets met or exceeded the calcium and phosphorous recommendations for growing rodents (62). Thus, it was not surprising that there were no differences in calcium content of the femur. While there was a trend toward a difference in total femoral phosphorus content, with the MPI group being the highest, the ratio of calcium to phosphorus was not different among groups, indicating that th eprotein source did not significantly affect the relative composition of the 2 primary minerals in bone during skeletal growth.
Because overweight and obesity are now linked to poor bone heath and increased fracture risk (3, 7), there is potential for dietary protein intake to indirectly improve bone health in overweight adolescents through improvements in metabolic health. Epidemiologic evidence suggests that a dietary pattern incorporating more low-fat dairy products might lower the risk of type 2 diabetes (63) and hypertension (64). In adolescents, dairy consumption is inversely associated with central adiposity (65). Soy protein has also been widely studied for its reported metabolic health benefits (66, 67). In adults, soy consumption results in a clinically significant decrease in circulating total and LDL cholesterol (68, 69) and triglycerides (53). In this study, there were few metabolic differences among the dietary treatments. However, the rats fed the SPI diet had a lower body fat percentage compared with the rats fed the MIX diet, and had decreased circulating cholesterol compared with those fed MIX or MPI. Additionally, the rats fed SPI had significant improvements in liver function (34), which is often used as a surrogate measure of metabolic health, indicating improved metabolic health following a soy-based diet. This improvement in metabolic health and decrease in circulating cholesterol have significance for skeletal health, as both excess adiposity (4) and hypercholesterolemia (70) have detrimental effects on bone, and mouse models show that adiposity induced by a high-fat diet especially affects cancellous bone (71).
All groups showed insulin resistance, but rats in the SPI group had higher serum insulin than those in the MPI group. We observed a significant positive correlation between insulin and P1NP. This result was contrary to our hypothesis that insulin resistance would be detrimental to bone health. However, insulin is a major anabolic hormone that can have significant effects on bone growth through osteoblast activity (72), and previous studies have shown that insulin resistance can be beneficial to trabecular microarchitecture (73, 74). Additionally, we showed positive correlations between body weight and body fat percentage and all biomechanical strength outcomes, as well as a significant positive association between body weight and cortical thickness. This was unsurprising, considering body weight is a strong determinant of cortical bone growth and strength (75). Finally, we showed a positive correlation between body fat percentage and Tb.Sp and a negative correlation between body fat percentage and Conn.D. These results support our hypothesis that dietary protein intake could indirectly affect bone health through actions on metabolic health.
In summary, our findings suggest that, in the context of excess adiposity, soy-based and milk-based proteins have comparable effects on cortical bone geometry and biomechanical properties, while soy-based proteins favorably affect trabecular microarchitecture, and the combination of both proteins may offer additional benefits to bone remodeling in young, rapidly growing male OLETF rats. These findings are of key significance as many people consume protein from a variety of animal- and plant-based sources.
Acknowledgements
The authors’ responsibilities were as follows—JPT, RSR, and PSH: designed the research; RKD, MWR, and GMM: conducted the research; DNB and ESK: provided the diets; RKD, MWR, and PSH: analyzed the data; RKD and PSH: wrote the paper; RKD: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Partially supported by grants from DuPont Nutrition and Health (JPT and RSR), National Institutes of Health Grant DK-088940 (JPT), VA Merit Review I01 RX000123 (JPT), and VA-Merit Grant I01BX003271-01 (RSR). Also supported with resources and the use of facilities at the Harry S. Truman Memorial VA Hospital in Columbia, MO.
Author disclosures: RKD, MWR, and GMM, no conflicts of interest; DNB, Employee of DuPont Nutrition and Health during the time of this study; ESK, Employee of DuPont Nutrition and Health during the time of this study; JPT, RSR, and PSH, no conflicts of interest.
Abbreviations used:
- BMD
bone mineral density
- BV
bone volume
- CCK
cholecystokinin
- Conn.D
connectivity density
- Ct.Th
cortical thickness
- CTx
C-terminal peptide of type I collagen
- ER
estrogen receptor
- G
shear modulus of elasticity
- IGF-1
insulin-like growth factor 1
- Ks
torsional stiffness
- MIX
50/50 mixture of soy and milk protein isolate
- MPI
milk protein isolate
- OLETF
Otsuka Long Evans Tokushima Fatty
- P1NP
amino-terminal propeptide of type I collagen
- R
robustness
- SMI
structural mode index
- SPI
soy protein isolate
- Su
ultimate tensile strength or maximal shear stress
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- Tb.Th
trabecular thickness
- Tmax
maximal torque at fracture
- Tt.Ar
total area
- TV
total volume; μCT, microcomputed tomography
References
- 1. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 2016;27(4):1281–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao L-J, Liu Y-J, Liu P-Y, Hamilton J, Recker RR, Deng H-W. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92(5):1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol Cell Endocrinol 2015;410:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farr JN, Dimitri P. The impact of fat and obesity on bone microarchitecture and strength in children. Calcif Tissue Int 2017;100:500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halade GV, El Jamali A, Williams PJ, Fajardo RJ, Fernandes G. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol 2011;46(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC et al. . Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 2010;142(2):309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res 2013;471(4):1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore LL, Bradlee ML, Gao D, Singer MR. Effects of average childhood dairy intake on adolescent bone health. J Pediatr 2008;153(5):667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heaney RP. Dairy and bone health. J Am Coll Nutr 2009;28(Suppl 1):82S–90S. [DOI] [PubMed] [Google Scholar]
- 10. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res 2016;60(1):32527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J-R, Lazarenko OP, Blackburn ML, Badger TM, Ronis MJJ. Soy protein isolate inhibits high-fat diet-induced senescence pathways in osteoblasts to maintain bone acquisition in male rats. Endocrinology 2015;156(2):475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JR, Zhang J, Lazarenko OP, Cao JJ, Blackburn ML, Badger TM, Ronis MJ. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J 2013;27(9):3514–23. [DOI] [PubMed] [Google Scholar]
- 13. Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr 2002;76(3):675–80. [DOI] [PubMed] [Google Scholar]
- 14. Thorning TK, Bertram HC, Bonjour J-P, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC, et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr 2017;105(5):1033–45. [DOI] [PubMed] [Google Scholar]
- 15. Chan GM, Hoffman K, McMurry M. Effects of dairy products on bone and body composition in pubertal girls. J Pediatr 1995;126(4):551–6. [DOI] [PubMed] [Google Scholar]
- 16. Cadogan J, Eastell R, Jones N, Barker ME. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 1997;315(7118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone 2008;43(2):312–21. [DOI] [PubMed] [Google Scholar]
- 18. Kalkwarf HJ, Khoury JC, Lanphear BP. Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr 2003;77(1):257–65. [DOI] [PubMed] [Google Scholar]
- 19. Toba Y, Takada Y, Matsuoka Y, Morita Y, Motouri M, Hirai T, Suguri T, Aoe S, Kawakami H, Kumegawa M, et al. Milk basic protein promotes bone formation and suppresses bone resorption in healthy adult men. Biosci Biotechnol Biochem 2001;65(6):1353–7. [DOI] [PubMed] [Google Scholar]
- 20. Esterle L, Sabatier J-P, Guillon-Metz F, Walrant-Debray O, Guaydier-Souquières G, Jehan F, Garabédian M. Milk, rather than other foods, is associated with vertebral bone mass and circulating IGF-1 in female adolescents. Osteoporos Int 2009;20(4):567–75. [DOI] [PubMed] [Google Scholar]
- 21. Yakar S, Courtland H-W, Clemmons D. IGF-1 and bone: New discoveries from mouse models. J Bone Miner Res 2010;25(12):2543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guntur AR, Rosen CJ. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep 2013;2(437). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen K. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr 2009;63:1076–83. [DOI] [PubMed] [Google Scholar]
- 24. Hoppe C, Mølgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr 2004;58(9):1211–6. [DOI] [PubMed] [Google Scholar]
- 25. Messina M. Insights gained from 20 years of soy research. J Nutr 2010;140(12):2289S–95S. [DOI] [PubMed] [Google Scholar]
- 26. Young V, Puig M, Queiroz E, Scrimshaw N, Rand W. Evaluation of the protein quality of an isolated soy protein in young men: relative nitrogen requirements and effect of methionine supplementation. Am J Clin Nutr 1984;39(1):16–24. [DOI] [PubMed] [Google Scholar]
- 27. Yan L, Graef GL, Nielsen FH, Johnson LK, Cao J. Soy protein is beneficial but high-fat diet and voluntary running are detrimental to bone structure in mice. Nutr Res 2015;35(6):523–31. [DOI] [PubMed] [Google Scholar]
- 28. Gaffney-Stomberg E, Cao JJ, Lin GG, Wulff CR, Murphy NE, Young AJ, McClung JP, Pasiakos SM. Dietary protein level and source differentially affect bone metabolism, strength, and intestinal calcium transporter expression during ad libitum and food-restricted conditions in male rats. J Nutr 2014;144(6):821–9. [DOI] [PubMed] [Google Scholar]
- 29. Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr 2002;132(9):2605–8. [DOI] [PubMed] [Google Scholar]
- 30. Hinton PS, Shankar K, Eaton LM, Rector RS. Obesity-related changes in bone structural and material properties in hyperphagic OLETF rats and protection by voluntary wheel running. Metabolism 2015;64(8):905–16. [DOI] [PubMed] [Google Scholar]
- 31. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 1992;41(11):1422–8. [DOI] [PubMed] [Google Scholar]
- 32. Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 2010;52:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran TH. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans Biol Sci 2006;361(1471):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panasevich MR, Schuster CM, Phillips KE, Meers GM, Chintapalli S V, Wankhade UD, Shankar K, Butteiger DN, Krul ES, Thyfault JP, et al. Soy compared with milk protein in a Western diet changes fecal microbiota and decreases hepatic steatosis in obese OLETF rats. J Nutr Biochem 2017;46:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffé-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and AJ mice. Metabolism. W.B. Saunders 1995;44(5):645–51. [DOI] [PubMed] [Google Scholar]
- 36. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 37. Doube M, Kłosowski MM, Arganda-Carreras I, Cordelières FP, Dougherty RP, Jackson JS, Schmid B, Hutchinson JR, Shefelbine SJ. BoneJ: free and extensible bone image analysis in ImageJ. Bone 2010;47(6):1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruker-microCT Morphometric parameters measured by SkyscanTM CT – analyser software [Reference Manual on the Internet]. 2012. p. 1–49. [Google Scholar]
- 39. Shaw CN, Stock JT. Intensity, repetitiveness, and directionality of habitual adolescent mobility patterns influence the tibial diaphysis morphology of athletes. Am J Phys Anthropol 2009;140(1):149–59. [DOI] [PubMed] [Google Scholar]
- 40. Chen J-R, Singhal R, Lazarenko OP, Liu X, Hogue WR, Badger TM, et al. Short term effects on bone quality associated with consumption of soy protein isolate and other dietary protein sources in rapidly growing female rats. Exp Biol Med 2008;233(11):1348–58. [DOI] [PubMed] [Google Scholar]
- 41. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 2000;15(8):1526–36. [DOI] [PubMed] [Google Scholar]
- 42. Matsumoto Y, Tousen Y, Nishide Y, Tadaishi M, Kato K, Ishimi Y. Combined effects of soy isoflavones and milk basic protein on bone mineral density in hinddlimb unloaded mice. J Clin Biochem Nutr 2016;58(2):141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dimitri P, Wales JK, Bishop N. Adipokines, bone-derived factors and bone turnover in obese children; evidence for altered fat-bone signalling resulting in reduced bone mass. Bone 2011;48(2):189–96. [DOI] [PubMed] [Google Scholar]
- 44. Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull 2001;24(4):351–6. [DOI] [PubMed] [Google Scholar]
- 45. Kerstetter JE, Wall DE, O'Brien KO, Caseria DM, Insogna KL. Meat and soy protein affect calcium homeostasis in healthy women. J Nutr 2006;136(7):1890–5. [DOI] [PubMed] [Google Scholar]
- 46. Spence LA, Lipscomb ER, Cadogan J, Martin B, Wastney ME, Peacock M, Weaver CM. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr 2005;81(4):916–22. [DOI] [PubMed] [Google Scholar]
- 47. Ho SC, Woo J, Lam S, Chen Y, Sham A, Lau J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos Int 2003;14(10):835–42. [DOI] [PubMed] [Google Scholar]
- 48. D'Adamo CR, Sahin A. Soy foods and supplementation : a review of commonly perceived health benefits and risks. Altern Ther Health Med 2014;20(Supp 1):39–52. [PubMed] [Google Scholar]
- 49. Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ. Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertil Steril 2010;94(3):997–1007. [DOI] [PubMed] [Google Scholar]
- 50. Messina M, Rogero MM, Fisberg M, Waitzberg D. Health impact of childhood and adolescent soy consumption. Nutr Rev 2017;75(7):500–15. [DOI] [PubMed] [Google Scholar]
- 51. Khalil DA, Lucas EA, Smith BJ, Soung DY, Devareddy L, Juma S, Akhter MP, Recker R, Arjmandi BH. Soy isoflavones may protect against orchidectomy-induced bone loss in aged male rats. Calcif Tissue Int 2005;76(1):56–62. [DOI] [PubMed] [Google Scholar]
- 52. Khosla S, Melton LJ, Riggs BL. Estrogens and bone health in men. Calcif Tissue Int 2001;69:189–92. [DOI] [PubMed] [Google Scholar]
- 53. Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr 2008;138(6):1244S–9S. [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Lazarenko OP, Blackburn ML, Badger TM, Ronis MJJ, Chen J-R. Soy protein isolate down-regulates caveolin-1 expression to suppress osteoblastic cell senescence pathways. FASEB J 2014;28(7):3134–45. [DOI] [PubMed] [Google Scholar]
- 55. Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Expression of estrogen receptor β in rat bone. Endocrinology 1997;138(10):4509–12. [DOI] [PubMed] [Google Scholar]
- 56. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 2008;3(Suppl 3):S131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci 2006;1092:385–96. [DOI] [PubMed] [Google Scholar]
- 58. Soung DY, Devareddy L, Khalil DA, Hooshmand S, Patade A, Lucas EA, Arjmandi BH. Soy affects trabecular microarchitecture and favorably alters select bone-specific gene expressions in a male rat model of osteoporosis. Calcif Tissue Int 2006;78(6):385–91. [DOI] [PubMed] [Google Scholar]
- 59. Felsenberg D, Boonen S. The bone quality framework: Determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 2005;27(1):1–11. [DOI] [PubMed] [Google Scholar]
- 60. Karim L, Vashishth D. Role of trabecular microarchitecture in the formation, accumulation, and morphology of microdamage in human cancellous bone. J Orthop Res 2011;29(11):1739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hunt JR, Hunt CD, Zito CA, Idso JP, Johnson LK. Calcium requirements of growing rats based on bone mass, structure, or biomechanical strength are similar. J Nutr 2008;138(8):1462–8. [DOI] [PubMed] [Google Scholar]
- 62. Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc writing committee. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 63. Liu S, Choi HK, Ford E, Song Y, Klevak A, Buring JE, Manson JE. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006;29(7):1579–84. [DOI] [PubMed] [Google Scholar]
- 64. Mccarron DA, Morris CD, Henry HJ, Stanton JL. Blood pressure and nutrient intake in the united states. Source Sci New Ser 1984;224(4656):1392–8. [DOI] [PubMed] [Google Scholar]
- 65. Bradlee ML, Singer MR, Qureshi MM, Moore LL. Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public Health Nutr 2009;13(6):797–805. [DOI] [PubMed] [Google Scholar]
- 66. Messina M. A brief historical overview of the past two decades of soy and isoflavone research. J Nutr 2010;140(7):1350S–4S. [DOI] [PubMed] [Google Scholar]
- 67. Noriega-López L, Tovar AR, Gonzalez-Granillo M, Hernández-Pando R, Escalante B, Santillán-Doherty P, Torres N. Pancreatic insulin secretion in rats fed a soy protein high fat diet depends on the interaction between the amino acid pattern and isoflavones. J Biol Chem 2007;282(28):20657–66. [DOI] [PubMed] [Google Scholar]
- 68. Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr 2007;85(4):1148–56. [DOI] [PubMed] [Google Scholar]
- 69. Zhan S, Ho S. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr 2005;81(2):397–408. [DOI] [PubMed] [Google Scholar]
- 70. Majima T, Shimatsu A, Komatsu Y, Satoh N, Fukao A, Ninomiya K, Matsumura T, Nakao K. Increased bone turnover in patients with hypercholesterolemia. Endocr J 2008;55(1):143–51. [DOI] [PubMed] [Google Scholar]
- 71. Cao JJ, Gregoire BR, Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 2009;44(6):1097–104. [DOI] [PubMed] [Google Scholar]
- 72. Pramojanee SN, Phimphilai M, Chattipakorn N, Chattipakorn SC. Possible roles of insulin signaling in osteoblasts. Endocr Res 2014;39(4):144–51. [DOI] [PubMed] [Google Scholar]
- 73. Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association between insulin resistance and bone structure in nondiabetic postmenopausal women. J Clin Endocrinol Metab 2016;101(8):3114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010;95(11):5045–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The framingham study. J Bone Miner Res 2009;8(5):567–73. [DOI] [PubMed] [Google Scholar]