Abstract
Background
Viscosupplementation (VS) is a symptomatic treatment of knee osteoarthritis. Although systematic reviews of its repeat use showed favorable benefit/risk ratio, no study has focused on the indication of retreatment.
Methods
A task force was created to look at issues regarding retreatment with VS in knee osteoarthritis. An attempt was made to reach consensus on several issues: (1) to define treatment “success” and “failure,” (2) to determine when to retreat patients successfully treated by a previous VS, (3) to determine how to retreat patients in whom VS failed, (4) to define what to do in case of adverse reaction following previous VS, and (5) to examine the interests of soluble biomarkers to manage retreatment. After debate and review of literature the working group voted on 88 issues. Two “decision trees” were built based on the results of the votes.
Results
In case of failure, the authors draw attention to the need of a rigorous clinical and radiological analysis, and consider evidence-based medicine. When VS was previously successful, retreatment can be considered after recurrence or increase in pain. However, in subjects with high risk of disease progression, in young patients, and in professional sportsmen, retreatment could be considered systematically, because of the probability of hyaluronic acid to slow osteoarthritis progression. Evidence on soluble biomarkers was not considered as enough strong to support their use as decision tools for patient retreatment.
Conclusion
The decision algorithms are intended to facilitate consideration of the therapeutic options, in patients with knee osteoarthritis previously treated with VS.
Keywords: biomarkers, diagnostics, osteoarthritis, diagnosis, knee, joint involved, intraarticular delivery, therapeutic delivery, tissue lubrication, procedures
Current recommendations for the treatment of knee osteoarthritis (OA) include a combination of nonpharmacological and pharmacological modalities.1-4 Among the latter, viscosupplementation (VS) by intra-articular (IA) injection(s) of hyaluronic acid (HA)5 are aimed to reduce pain and to improve joint function, through complex mechanical and biological mechanisms that might restore joint homeostasis.6 VS is recommended by some scientific societies, in patients with knee OA where pain is not adequately relieved with conventional therapy.7-10 Recent meta-analysis and systematic reviews of randomized controlled trials (level of evidence 1) have ranked VS among the most effective treatments to relieve pain in patients with mild to moderate knee OA.11,12 Furthermore in some studies, patients perceived VS as the most effective treatment for knee OA13 whereas other level of evidence–1 studies showed controversial results regarding VS efficacy.14,15 Recently, 3 studies have demonstrated that HA decreased the serum or urine levels of soluble biomarkers reflecting cartilage metabolism.16-18 Nonetheless it is not known whether HA elicits an indirect reaction on the articular metabolism secondary to an increased use of the joint due to its analgesic effect or through a direct action on the cartilage metabolism.19 These finding suggested that some soluble biomarkers could be used as an indicator for HA reinjection.
In 2014, the EUROpean VIScosupplementation COnsensus working group (EUROVISCO) proposed a set of recommendations for the use of VS, based on both an extensive research of the literature and experts’ opinion.19 A consensus position was obtained for 16 of the 24 statements discussed, allowing to establish clear recommendations to help practitioners using VS.
In September 2015, the same group, expanded with 3 further experts, congregated to propose algorithms for the management knee OA patients, previously treated with VS.
The experts have examined 2 situations: (1) the reinjection in patients successfully treated with VS, 6 to 12 months ago and (2) the reinjection in patients where previous VS failed or caused adverse reactions. Finally, the experts discussed the interests and limits of using soluble biomarkers in the decision of VS retreatment.
Methods
Experts
Ten experts from Belgium, France, Germany, Italy, Spain, and the United Kingdom, congregated in a working group meeting held in Lyon, France, on September 17-18, 2015. This expert panel constituted of 7 rheumatologists, 2 orthopaedic surgeons, and 1 physiotherapist. All had expertise in clinical research methodology in the field of OA and VS and experience in academic medicine and/or private practice.
Issues
Three members of the task force (RR, TC, YH) were tasked to collate an exhaustive literature analysis on the topic. Eighteen statements were discussed during the meeting. After extensive debate, the expert panel had to give opinion on each of the 88 issues within the 18 statements. The first step was to define “success” and “failure” of the treatment. The second step was to determine when and how to retreat patients successfully treated by a previous VS. The third step was to determine when and how to retreat patients in whom VS previously failed. The fourth step was to propose management options where the patient experienced moderate adverse reaction following previous VS. Finally, the task force examined the role of serum and urine biomarkers in retreatment with HA.
Scoring and Voting Methods
For each statement, the experts had to score according to their degree of agreement, using a 4-point Likert-type scale (0-3), with 0 = “I don’t agree,” 1 = “I tend to disagree,” 2 = “I tend to agree,” and 3 = “I agree.” After debate and review of literature, each item was finally classified into 2 categories: “Agree” or “Disagree.” The statement was adopted and was consequently included into the decision algorithm only if 8 experts or more voted either to “Agree” or “Disagree.” At the end of the session, 2 “Decision Trees” regarding retreatment with VS were built according to the results of the votes: one after failure and the second after success of a previous VS.
Recommendations
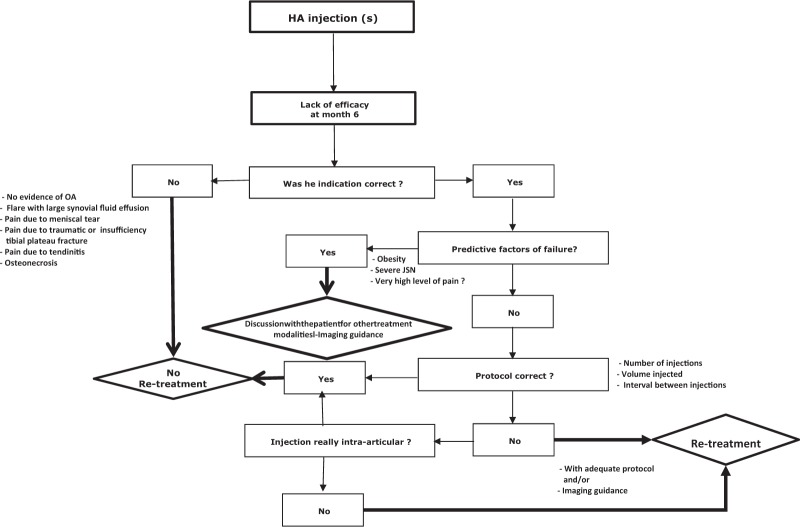
The algorithms of recommendations ( Figs. 1 and 2 ) were drafted by 1 expert (TC) after taking into account suggestions, comments, and approval of all the experts in the working group The decision trees presented in the present guidelines are intended to facilitate consideration of the therapeutic options in patients with knee OA previously treated with VS.
Figure 1.
Algorithm of recommendations for retreatment with viscosupplementation of patients after failure of a previous viscosupplementation.
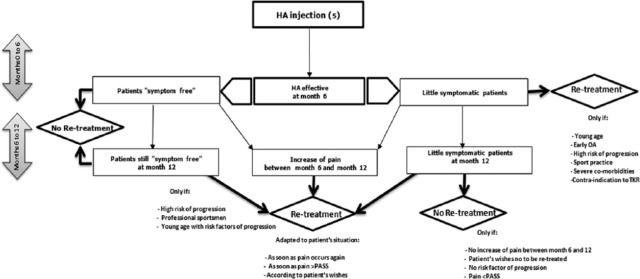
Figure 2.
Algorithm of recommendations for retreatment with viscosupplementation of patients improved by a previous viscosupplementation.
Issues
Definition of Treatment Failure
Viscosupplementation is aimed to alleviate pain and decrease disability in patients suffering from knee OA “not sufficiently improved” by the first-line treatments, including analgesics and nonpharmacological modalities.1,4 However, the definition of “sufficiently” improved remains unclear. The practitioner is faced with this several times a day, before a treatment decision is made. The optimal duration of efficacy is yet to be defined. Compared with IA corticosteroids, HA injections have a delayed onset of action. They are more effective in the long term, up to 26 weeks.20 Some studies have demonstrated that VS might be effective up to 1 year.21,22 So the task force agreed that duration of efficacy should not be less than 6 months.
To define the concept of treatment failure, the panel of experts had to answer the following issues:
Do you agree or disagree with the following definition of treatment failure six months after VS? Pain decrease on 100 mm visual analogue scale (VAS) <20 mm; Pain on VAS or Western Ontario and McMaster Universities Osteoarthritis (WOMAC)23 score decrease <50%; Pain on VAS or WOMAC score decrease <Minimal Clinically Important Improvement (MCII)24; Residual pain >Patient Acceptable Symptom State (PASS)24; Pain decrease <MCII and residual pain >PASS; Pain decrease >MCII and residual pain<PASS but patient dissatisfied; Pain decrease <MCII and residual pain >PASS but patient satisfied.
To demonstrate the “treatment failure”, what are the most useful tools, in daily practice and clinical trials: WOMAC pain score variation, Knee injury and Osteoarthritis Outcome Score (KOOS)25 variation, MCII, PASS, OMERACT-OARSI response criteria,26 Patient’s overall opinion?
Retreatment of Patients Successfully Treated by Previous VS
The following issues were discussed when re-treating patients who improved with previous VS:
Retreatment with VS must be considered in the following scenarios. Systematically every 6 to 12 months, even if patients remain asymptomatic, only if pain returns to pretreatment levels, only from a certain level of pain (i.e., PASS) or as soon as pain occurs again, as per patient’s wishes?
Must we systematically retreat patients with little symptoms? If so: every 3 months, every 6 months, every year, systematically, but the time interval between 2 treatments must be adapted to the patient’s situation (i.e., age, anatomical severity, activities, etc.).
Which of these clinical situations may push you to retreating patients: early stage of OA, advanced stage of OA, young age, elderly, risk factors of rapid progression? Sports practice (leisure), sports practice (professional), contra-indication to arthroplasty, severe comorbidities.
Do the potential chondroprotective properties of HA influence your decision to retreat asymptomatic or mildly symptomatic patients with HA?
Retreatment of Patients after Failure of Previous VS
After the review of literature and based on their clinical experience, the working group members had to answer the following questions:
Choose among the following, the items you consider as predictive factors of VS failure: advanced stage of OA (Kellgren-Lawrence grade [KL]27 III and IV), advanced stage of OA (KL grade IV only), overweight (body mass index [BMI] between 25 and 30 kg/m2), obesity (BMI >30 kg/m2), clinical severity assessed by pain on VAS >6 and ≤8. Clinical severity assessed by pain on VAS ≥8. Severe patellofemoral involvement, isolated patellofemoral OA, synovial fluid effusion <10 mL, synovial fluid effusion >10 mL, pain due to meniscus tear. OA flare as defined by the Knee Osteoarthritis Flare-up Score (KOFUS), which is a 6-item questionnaire, ranging from 0 to 14. A score ≥7 points corresponds to a diagnosis of flare-up.28
In your opinion may the following statements influence the results of VS? Choice of the viscosupplement (i.e. are particular viscosupplements better than others)? Inappropriate protocol (inadequate number of injections, time interval not respected between 2 injections, inaccurate clinical analysis of origin of pain (i.e., meniscus lesion, neuropathic pain, osteonecrosis, tendonitis, etc.), wrong analysis of anatomical severity (i.e, wrong analysis of X-rays, inadequate radiological evidence), extra-articular injection.
Do you think there are significant differences between marketed viscosupplements that could influence the clinical results? If yes, which among these characteristics are important to consider: origin (animal or bacterial), injected volume, HA concentration, total amount of HA, molecular weight of HA, HA structure (linear, cross-linked), rheological properties of the gel (viscosity, elasticity, crossover frequency), mannitol or sorbitol addition.
Do you agree with these assertions: Only cross-linking allows a “single injection” regimen, Repeated injections (minimum 3) are always necessary for viscosupplements made with linear HA?
What imaging modality(s) do you consider before any course of VS: standard X-rays, magnetic resonance imaging (MRI), ultrasonography.
Among the following responses, which are those that ensure the intra-articular placement of the needle: imaging guidance, aspiration of synovial fluid, physician experience lateral mid-patellar route of injection, absence of pain at injection.
Retreatment of Patients with Moderate Adverse Reaction after Previous VS
The task force agreed on a management plan in patients who experienced a mild to moderate adverse reaction, such as increase of pain, swelling, and synovial effusion after a previous VS. The questions analyzed were:
In case of moderate local adverse reaction at index course do you use the same VS/protocol for repeat injections?
If not, what do you do: You use another viscosupplement. You use another protocol (i.e., 3 vs. 1 injection). You use the same protocol with addition of IA steroid. You use the same protocol and advise a NSAIDs treatment for several days. You substitute it with biofermentative HA if the first VS was of animal origin. You don’t perform any new VS injection.
Interests and Limits of Soluble Biomarkers for Managing Retreatment with HA
Four issues were debated on the value of serum and urine biomarkers for the decision of retreatment with VS:
The effect of viscosupplementation on cartilage metabolism is a valuable outcome measure in the follow-up of OA patients.
Soluble biomarkers are good tools in monitoring the effects of viscosupplementation on cartilage metabolism.
Soluble biomarkers are predictive of the response to viscosupplementation.
Soluble biomarkers can be used as an indicator of HA reinjection.
Results
Definition of Treatment Failure
Agreement and level of consensus are summarized in Table 1 .
Table 1.
Level of Consensus on Definition of Treatment Failure in Knee Osteoarthritis.
| Issues on Definition of Treatment Failure in Knee OA | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| Do you agree or disagree with the following definition of “treatment failure six months after VS”? | |||
| Pain decrease on VAS <20 mm | Moderately in favor | 7 | 3 |
| Pain decrease on VAS or WOMAC score <50% | Strongly against | 2 | 8 |
| Pain decrease on VAS or WOMAC score <MCII | Strongly in favor | 8 | 2 |
| Remaining pain >PASS | Strongly in favor | 9 | 1 |
| Pain decrease <MCII and pain >PASS | Moderately in favor | 7 | 3 |
| Pain decrease >MCII and pain <PASS but patient dissatisfied | Strongly in favor | 8 | 2 |
| Pain decrease <MCII and pain >PASS but patient satisfied | Strongly against | 2 | 8 |
| To demonstrate the “treatment failure,” what are the most useful tools, in daily practice | |||
| WOMAC pain score variation | No consensus | 5 | 5 |
| KOOS variation | Strongly against | 2 | 8 |
| MCII | Weakly in favor | 6 | 4 |
| PASS | Strongly in favor | 8 | 2 |
| OMERACT-OARSI response criteria | Moderately against | 3 | 7 |
| Patient’s overall opinion | Unanimously in favor | 10 | 0 |
| To demonstrate the “treatment failure,” what are the most useful tools, in clinical trials | |||
| WOMAC pain score variation | Strongly in favor | 9 | 1 |
| KOOS variation | Moderately in favor | 7 | 3 |
| MCII | Strongly in favor | 8 | 2 |
| PASS | Strongly in favor | 8 | 2 |
| OMERACT-OARSI response criteria | Moderately in favor | 7 | 3 |
| Patient’s overall opinion | Strongly in favor | 9 | 1 |
OA = osteoarthritis; VAS = 100 mm visual analogue scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; PASS = Patient’s Acceptable Symptom State; MCII = Minimal Clinically Important Improvement; KOOS = Knee injury and Osteoarthritis Outcome Score; OMERACT-OARSI = Outcome Measures in Clinical Trials–Osteoarthritis Research Society International.
To define the failure of treatment, the working group overwhelmingly endorsed the concept of PASS.24 It stressed that PASS, defined as the value beyond which patients consider themselves well (i.e., 4 on a 10-point rating scale),24 is a clinically relevant outcome for the patient. Asking the question “Are you feeling good” is much more relevant to assess the treatment efficacy in daily practice than the question “Are you feeling better,”24,29 that can be assessed using MCII, defined as the smallest change in measurement that signifies an important improvement in a patient’s symptom.24 The patient’s opinion being the primary concern, the experts, emphasized the concept of “patient’s satisfaction” with respect to the treatment regardless of the results of PASS and MCII. However a decrease of pain inferior to MCII threshold (−2 points and 20% on a 10-point rating scale)24 was also rated as treatment failure by 8 out of the 10 experts. Among the main tools for assessing treatment’s success or failure, only the patient’s overall opinion and PASS were rated as useful in clinical practice, whereas all others were considered useful in clinical trials.
Issues on Retreatment after Success of Previous Viscosupplementation
Agreement and level of consensus are summarized in Table 2 .
Table 2.
Level of Consensus on Retreatment after Success of Viscosupplementation.
| Issues on Retreatment after Success of Viscosupplementation | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| Retreatment with VS must be considered | |||
| Systematically every 6-12 months, even if patients remain asymptomatic | Strongly against | 2 | 8 |
| Only if pain returns to pretreatment levels | Strong against | 2 | 8 |
| Only from a certain level of pain (i.e., PASS) | Strongly in favor | 8 | 2 |
| As soon as pain occurs again | Strongly in favor | 9 | 1 |
| According to the patient’s wishes | Moderately in favor | 7 | 3 |
| Must we retreat systematically little symptomatic patients? | |||
| Yes | Weakly in favor | 6 | 4 |
| Every 3 months | Strongly against | 0 | 6 |
| Every 6 months | Strongly against | 1 | 5 |
| Every year | Strongly against | 1 | 5 |
| Yes but the time interval between 2 treatments must be adapted to the patient’s situation (i.e., age, anatomical severity, activities, etc.) | Unanimously in favor | 6 | 0 |
| Which of these clinical situations may push you into retreating patients? | |||
| Early stage of OA? | Strongly in favor | 9 | 1 |
| Advanced stage of OA? | Strongly against | 2 | 8 |
| Young age? | Strongly in favor | 9 | 1 |
| Elderly | Moderately against | 3 | 7 |
| Risk factors of rapid progression? | Strongly in favor | 9 | 1 |
| Sports practice (leisure)? | No consensus | 5 | 5 |
| Sports practice (professional)? | Strongly in favor | 9 | 1 |
| Contraindication to arthroplasty? | Moderately in favor | 7 | 3 |
| Severe comorbidities? | Strongly in favor | 8 | 2 |
| Does the chondroprotective properties of HA influence your decision to retreat asymptomatic or little symptomatic patients with HA? | Strongly in favor | 8 | 2 |
VS = viscosupplementation; OA = osteoarthritis; PASS = Patient’s Acceptable Symptom State; HA = hyaluronic acid.
There was a strong level of consensus to re-treat patients as soon as pain occurs again and not to retreat asymptomatic or minimally symptomatic patients systematically. In the latter case, there was a consensus to adapt the frequency of treatment to patients’ individual situation. PASS threshold24 was also rated to be a useful tool in the decision of retreatment. A large majority of the experts identified 4 clinical situations that can potentially persuade physicians to retreat patients sooner: early stage of OA, young age, risks factors of progression and professional sportsperson. Severe comorbidities that contra indicate NSAIDs and surgery were also considered as arguments in favor of an earlier retreatment. Most experts acknowledge being influenced by the results of studies intended to demonstrate the chondroprotective properties of HA.
Issues on Retreatment after Failure of Previous Viscosupplementation
Agreement and level of consensus are summarized in Table 3 .
Table 3.
Level of Consensus on Retreatment after Failure of Viscosupplementation.
| Issues on Retreatment after Failure of Viscosupplementation | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| Among the following items which are those you consider as predictive factors of viscosupplementation failure? | |||
| Kellgren-Lawrence grade III and IV | Moderately against | 3 | 7 |
| Kellgren-Lawrence grade IV only | Unanimously in favor | 10 | 0 |
| Overweight (BMI between 25 and 30 kg/m2) | No consensus | 5 | 5 |
| Obesity (BMI >30 kg/m2) | Unanimously in favor | 10 | 0 |
| Clinical severity: pain on VAS >6 and ≤8 | Strongly against | 2 | 8 |
| Clinical severity: pain on VAS ≥8 | Weakly in favor | 6 | 4 |
| Severe patellofemoral involvement | Strongly in favor | 9 | 1 |
| Isolated patellofemoral OA | Strongly in favor | 8 | 2 |
| Synovial fluid effusion<10 mL | Strongly against | 2 | 8 |
| Synovial fluid effusion >10 mL | Moderately in favor | 7 | 3 |
| Pain due to meniscus tear | Strongly in favor | 9 | 1 |
| OA flare | Strongly in favor | 8 | 2 |
| In your opinion, which of the following statements influence the results of VS? | |||
| Choice of the viscosupplement | Strongly in favor | 8 | 2 |
| Inappropriate protocol (inadequate number of injections, time interval not respected between 2 injections?) | Strongly in favor | 8 | 2 |
| Wrong clinical analysis of pain origin | Unanimously in favor | 10 | 0 |
| Wrong analysis of anatomical severity | Strongly in favor | 8 | 2 |
| Extra-articular injection | Unanimously in favor | 10 | 0 |
BMI = body mass index; VS = viscosupplementation; OA = osteoarthritis; VAS = 100 mm visual analogue scale.
Among the main reasons for VS failure, 4 have obtained a unanimous vote: wrong clinical analysis of the pain origin, extra-articular injection(s), obesity and radiographic KL grade IV. Severe femoropatellar involvement, pain due to meniscus tear, OA flare, isolated patellofemoral involvement, inappropriate protocol, inaccurate analysis of anatomical severity were also considered as predictive factors of VS failure by most of the experts.
Issues on Retreatment after Adverse Reaction with Previous Viscosupplementation
Agreement and level of consensus are summarized in Table 4 .
Table 4.
Level of Consensus on Retreatment in Case of Adverse Reaction with Viscosupplementation.
| Issues on Retreatment in Case of Adverse Reaction with Viscosupplementation | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| In case of moderate local adverse reaction at index course do you use the same VS and/or protocol for repeat injections? | Strongly against | 2 | 8 |
| If not, what do you do? | |||
| You use another viscosupplement | Moderately in favor | 6 | 2 |
| You use another protocol (i.e., 3 vs. 1 injection or vice versa) | Weakly against | 3 | 5 |
| You use the same protocol with addition of IA steroid | Weakly in favor | 5 | 3 |
| You use the same protocol and advise treatment with NSAIDs for several days. | No consensus | 4 | 4 |
| You substitute it with biofermentative HA if the first VS was of animal origin | Weakly in favor | 5 | 3 |
| You do not perform any new VS injection | Weakly against | 3 | 5 |
VS = viscosupplementation; IA = intra-articular; NSAIDs = nonsteroidal anti-inflammatory drugs; HA = hyaluronic acid.
Eight out of the 10 experts advised to change the viscosupplement and/or the injection protocol in case of a nonserious local adverse reaction at index course of VS. Adding an IA corticosteroid or choosing another viscosupplement (i.e., biofermentative instead of animal origin) were the 2 main proposals but opinions were divided and no consensus was obtained.
Issues on Viscosupplementation and Techniques of Injection
Agreement and level of consensus are summarized in Table 5 .
Table 5.
Level of Consensus on Retreatment in Case of Adverse Reaction with Viscosupplementation.
| Issues on Viscosupplement and Techniques of Injection | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| Do you think the following differences between marketed viscosupplements could influence the clinical results? | Strongly in favor | 9 | 1 |
| Origin (animal or bacterial) | Weakly against | 5 | 5 |
| Injected volume | Strongly in favor | 8 | 2 |
| HA concentration | Moderately in favor | 7 | 3 |
| Total amount of HA | Moderately in favor | 8 | 2 |
| Molecular weight of HA | Strongly in favor | 8 | 2 |
| HA structure (linear, cross-linked) | Strongly in favor | 8 | 2 |
| Rheological properties of the gel (viscosity, elasticity, crossover frequency) | Moderately in favor | 7 | 3 |
| Mannitol or sorbitol addition | Moderately in favor | 8 | 2 |
| Do you think the dosing regimen must be supported by evidence-based-medicine? | Unanimously in favor | 10 | 0 |
| Do you agree with these assertions? | |||
| Only cross-linking allows a “single injection” regimen? | Unanimously in favor | 10 | 0 |
| Repeated injections (minimum 3) are always necessary for viscosupplements made of linear HA? | Strongly in favor | 8 | 2 |
| What imaging technique(s) do you consider before any new course of injection? | |||
| Standard X-rays | Moderately against | 4 | 6 |
| MRI | Strongly against | 1 | 9 |
| Ultrasonography | Unanimously against | 0 | 10 |
| How to ensure the intra-articular administration of the viscosupplement? | |||
| Imaging guidance | Moderately in favor | 7 | 3 |
| Synovial fluid aspiration | Strongly in favor | 9 | 1 |
| Absence of pain at injection | Weakly against | 4 | 6 |
| Lateral mid-patellar route of injection | Strongly in favor | 9 | 1 |
| Physician experience | Strongly in favor | 9 | 1 |
HA = hyaluronic acid; VS = viscosupplementation; OA = osteoarthritis; MRI = magnetic resonance imaging.
A consensus was reached on the assertion that viscosupplements must be used in accordance with recommendations based on scientific evidence. Thus, all the group members agreed that any single injection regimen should only use cross-linked HA. A large majority of them agreed with the fact that only repeat injections can be effective when cross-linked HA is used. A strong agreement was obtained on the differences between viscosupplements that can have an impact on the clinical result: injected volume, molecular structure, and molecular weight were the items that obtained the highest level of agreement. To ensure IA delivery of HA, the group stressed the importance of synovial fluid aspiration, lateral mid-patellar route approach and physician experience. Surprisingly, only a moderate consensus was obtained for the use of imaging guidance.
Finally, the experts have considered it not necessary to perform X-rays before any new course of injection. There was overall agreement on the uselessness to perform routine MRI or ultrasonography before any new treatment.
Issues on Interests and Limits of Soluble Biomarkers for Managing Retreatment with HA
Agreement and level of consensus are summarized in Table 6 .
Table 6.
Level of Consensus on Interests and Limits of Soluble Biomarkers for Managing Retreatment with Viscosupplementation.
| Issues on Interests and Limits of Soluble Biomarkers for Managing Retreatment with Viscosupplementation | Level of Consensus | Agreement |
|
|---|---|---|---|
| Agree | Disagree | ||
| The effect of viscosupplementation on cartilage metabolism is a valuable outcome in the follow-up of OA patient | Strongly in favor | 8 | 2 |
| Soluble biomarkers are predictive of the response to viscosupplementation | Moderately against | 3 | 7 |
| Soluble biomarkers can be used as indicator of HA reinjection | Strongly against | 2 | 8 |
| Soluble biomarkers are good tools, useful for monitoring the effects of VS on cartilage metabolism in daily practice | Strongly against | 2 | 8 |
| Soluble biomarkers are good tools, useful for monitoring the effects of VS on cartilage metabolism in clinical trials | Strongly in favor | 9 | 1 |
VS = viscosupplementation; OA = osteoarthritis; HA = hyaluronic acid.
The experts were almost unanimous to consider that effect of viscosupplementation on cartilage metabolism is a valuable outcome in the follow-up of OA patient. However, analyzing the current evidence in the literature, it was agreed that there is not enough evidence to support the use of soluble biomarkers as indicator of HA re-injection or for monitoring the effects treatment in the daily practice. However, they recommend the use of biomarkers in clinical trials to monitor the effect of VS on joint tissue metabolism.
Discussion
Several studies have been conducted to investigate safety and efficacy of retreatment with IA hyaluronans.30-33 Repeat courses of the hyaluronans have been shown to be safe and effective in the treatment of pain associated with OA of the knee.30 A multicenter, randomized, controlled study in 306 patients with knee who received 4 cycles of 5 IA HA or placebo injections suggested that HA repeat injections not only improve knee OA symptoms during the in-between cycle period, but also exert a marked carry-over effect for at least 1 year after the last injections.22 Altman et al.31 showed that repeat IA injections of BioHA (2 cycles of 3 injections at 6 month interval) were effective, well tolerated, and not associated with an increase in adverse events, such as synovial effusions. Patients not only maintained their improvement from baseline but also experienced an additional pain score decrease after the second cycle of injections. Comparable favorable results were obtained after repeat injections of a single injection of a biofermentative cross-linked HA.32 On the opposite, a retrospective study of patients who received multiple courses of 3 weekly Hylan G-F 20 injections showed that the incidence of local adverse reactions tended to increase with subsequent courses of therapy.33
However, so far, no work has been published about indications of retreatment with HA therapy. The purpose of the meeting was to consider all the factors that can influence, positively or negatively, a doctor’s decision to retreat patients with HA and also to build a decisional algorithm. The first part of the work emphasizes the importance of the patient’s opinion in the therapeutic decision, particularly in the definition of treatment failure or success. The task force highlighted the importance of PASS and patient’s satisfaction in the assessment of VS efficacy. On the contrary, composite indices (WOMAC, KOOS, and OMERACT-OARSI criteria) were not considered as useful in daily practice, but remained as valuable tools in clinical trials. Although useful for assessing the overall impact of the disease on daily activities, these indices do not necessarily reflect the real perception of the patients. Pain on nominated activity has been shown to be at least as, and in some cases more, sensitive to change than the KOOS/WOMAC questionnaire.34 In patients not satisfied with previous VS, several predictors of treatment failure have been identified. The unanimously identified factors were a wrong clinical analysis of pain and the extra-articular delivery of the HA. Indeed, the high disparity between the radiographic structural damage and the severity of symptoms in knee OA patients implies the prominent role of other factors than the joint pathology itself.35 Among the mechanisms of pain in OA, peripheral and central sensitizations, meniscus lesions, subchondral bone microcracks and inflammatory mechanisms, have been suggested to be of great importance.35,36,37 Despite the diversity, HA exhibits various and complex mechanisms that can explain its efficacy to alleviate OA pain (mild anti-inflammatory effect, analgesic actions, effect on subchondral bone, increase of glycosaminoglycan synthesis, inhibition of several matrix metalloproteinases).37 It is likely that HA cannot act, with the same efficiency, on all the elements resulting in OA pain. Other studies have shown that obesity and very severe joint space narrowing were predictors of VS failure.38 Interestingly, based on their own experience, most of the experts considered patellofemoral OA (isolated or severe) as a predictor of VS failure, despite the lack of evidence in the literature.39,40
Before taking the decision to retreat with HA in patients where VS failed, the first step in the decision tree ( Fig. 1 ), is to confirm the appropriate indication: evidence of OA, no other cause of knee pain (osteonecrosis, tendonitis, inflammatory or septic arthritis, microcrystal deposition disease, pain due to meniscus extrusion, complex regional pain syndrome), no OA flare or severe neuropathic pain. If indication is incorrect do not repeat HA injections and the instead change to a more appropriate treatment by choosing one more adapted to the clinical situation (i.e., IA corticosteroids in case of flare-up with synovial fluid effusion, injection of corticosteroids into the meniscus wall or arthroscopic meniscus repair if pain due to meniscus lesion, knee arthroplasty in case of advanced OA, pregabalin or duloxetine in case of neuropathic pain, weight loss program or bariatric surgery in case of obesity, etc., to name a few). If the indication was correct but with concomitant one or several predictive factors of failure, the working group recommends to discuss other treatment options with the patient after explaining that the chances of success of a new or repeat VS is low.38 If indication is correct and there are no major predictive factors of failure, one should confirm, “Whether the treatment was properly administered?” (i.e., number of injections, volume injected, interval between injections, in agreement with the product recommendations as described on the products’ SmPC [Summary of Product Characteristics]). Indeed, any new dosing regimen should be supported by controlled versus comparator or placebo trials.19 In this way, a randomized prospective trial comparing 2 different dosages of an intermediate-molecular-weight linear HA (3 × 2 mL weekly injections vs. one 6 mL injection), has demonstrated that a 3-weekly injection regimen was more effective than a single 6 mL injection.41 The choice of viscosupplement is also subject to debate and controversies regarding possible differences of efficacy between marketed viscosupplements, related to their molecular weight, origin, or extraction mode. There was only a moderate level of agreement between the experts of the task force while considering the choice of the viscosupplement as a predictor of clinical result despite a recent review reporting that high-molecular-weight HA was superior to lower molecular weight HA products and that HA obtained through biofermentation process had a better safety profile than avian-derived HA products.37 After ensuring the HA is administered as protocol, we must guarantee that viscosupplement has been administered intra-articularly. It is often difficult to be sure, except in case of synovial fluid aspiration during the procedure and where imaging guidance was used. The working group felt that ultrasound or fluoroscopy guidance is indispensable in IA injections to deep or small joints, such as the hip and trapeziometacarpal joints. However, a good knowledge of the injection techniques and anatomy allows for IA injection without imaging guidance in the knee. The lack of pain during injection does not guarantee the correct placement of the needle into the IA space, but a painful injection is usually a sign of an extra-articular injection. Debate continues on the “best” approach portal for knee injection.42,43 On this topic, the working group had a consensual point of view, admitting that no approach was 100% accurate, but lateral mid-patellar approach had to be preferred to the anterior approaches16 due to improved accuracy.42-44 The task force also stressed the importance of the physician experience in IA injection techniques. If all precautions have been taken to ensure optimal IA injection and the treatment failed, it must be concluded not to try new or repeat VS and change therapeutic option. The algorithm details are given in Figure 1 .
Another very frequent clinical scenario is a patient, who significantly improved with VS, and returns to follow-up, 6 to 12 months later. Four different case scenarios can be identified—(1) the patient remains “symptom free,” (2) the patient remains “minimally symptomatic” but with no increasing pain, (3) the patient is “minimally symptomatic” but with increasing pain, or (4) the patient is symptomatic again. In the first situation, the task force did not recommend to retreat systematically. Consequently, there was a strong agreement to advise retreatment as soon as the pain recurs and if pain exceed the PASS threshold. In “minimally symptomatic” patients with no increase of pain, the EUROVISCO group members proposed to retreat young patients, early stages of OA, patients with risk factors of progression,45-49 professional sportsmen and patients with severe comorbidities. One expect to benefit of potential protective effect of viscosupplement, and then prevent OA onset or progression. Those patients at high risk of disease progression can be re-treated 12 months after the first injection even if they are asymptomatic. This statement is supported by increasing evidence of chondroprotective properties of HA16,17,50-55 and hence can possibly postpone prosthetic surgery.56 Of course, the management of risk factors is recommended during the remission period. The algorithm for retreatment decision in patients who were satisfied with previous VS is given in Figure 2 .
Benefits and limitations of the use of soluble biomarkers in patients treated with HA injections have also been discussed. The experts concluded that, with current knowledge, there is not enough evidence to use soluble biomarkers as decision tools for reinjection or for monitoring the effects treatment in the daily practice. However, the experts encourage the use of soluble biomarkers to investigate the effect of VS on joint tissue metabolism and to assess their role as surrogate biomarkers of clinical and/or imaging outcomes.
In conclusion, the EUROVISCO working group drew up a set of suggestions aimed to help practitioners in the decision of retreatment with VS in patients with knee OA who were previously treated with IA HA injections. The task force built 2 separate decision algorithms based on the clinical result (failure or success) of the previous treatment. In case of failure, the authors draw attention to the necessity of a rigorous clinical and radiological analysis, and to the use of VS in concordance with data from the evidence-based medicine. A lateral mid-patellar approach is to be preferred to anterior approach and imaging guidance must be used in difficult cases such as obese patients. In patients who previously improved with VS, retreatment can be considered as soon as pain recurs or increases again. However, in subjects with a high risk of progression, in young patients, early OA, professional sportsmen, VS retreatment can be considered systematically even in asymptomatic patients as there is compelling new evidence on HA to retard OA progression.37,57
Footnotes
Acknowledgments and Funding: The authors acknowledge LABRHA SAS (Lyon, France) who organized and funded the meeting. LABRHA SAS, 19 place Tolozan, Lyon, France, provided financial support for the conduct of the meeting.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yves Henrotin received honorarium from Menarini, Flexion therapeutics, IBSA, BioIberica, Expansciences, Royal canin, MagPharm and LABRHA and Tilman SA, for consultant services; and is the founder and shareholder of KIOmed pharma and Artialis SA. Raghu Raman received honorarium from Sanofi and LABRA for consultant services. Pascal Richette received fees from BioIbérica, Fidia, IBSA, Expanscience, Genévrier, Sanofi, Rottapharm, Servier, Flexion Therapics, and Ménarini. Hervé Bard received speaker and expert fees from Sanofi, Rottapharm-Madaus, Pfizer, and LABRHA. Jörg Jerosch received honorarium from Sanofi for speaker services. Thierry Conrozier received honorarium from Genevrier, Aptissen Bioventus, and LABRHA for expert and consultant services. Xavier Chevalier received fees as a Genevrier Board member, Sanofi-Aventis expert, member of the IBSA fundation, speaker in IBSA meetings, Moebius and Flexion therapics consultant. Alberto Migliore received consulting fees from Abbvie, BMS, MSD, Fidia, Sanofi, IBSA, Pfizer, and LABRHA, for national and international studies and courses. Jordy Monfort received consulting fees from Sanofi and BioIberica. Dominique Baron received speaker fees from LCA and Expansciences.
Ethics Approval: Ethical approval was not sought for the present study because the work did not involve patients, since it was an experts meeting.
Informed Consent: Informed consent was not sought for the present study because the work did not involve patients.
References
- 1. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. ; Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan, et al. ; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465-74. [DOI] [PubMed] [Google Scholar]
- 3. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra S, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363-88. [DOI] [PubMed] [Google Scholar]
- 4. Bruyère Cooper C, Pelletier JP, Branco J, Luisa Brandi M, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin Arthritis Rheum. 2014;44:253-63. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 5. Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol. 1993;39(Suppl):3-9. [PubMed] [Google Scholar]
- 6. Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int. 2004;12:278-89. [PubMed] [Google Scholar]
- 7. Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28-33. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 8. Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, Jones D, et al. ; British Geriatric Society. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1-57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 9. Trojian TH, Concoff AL, Joy SM, Hatzenbuehler JR, Saulsberry WJ, Coleman CI. AMSSM scientific statement concerning viscosupplementation injections for knee osteoarthritis: importance for individual patient outcomes. Br J Sports Med. 2016;50:84-92. doi: 10.1136/bjsports-2015-095683. [DOI] [PubMed] [Google Scholar]
- 10. Bruyère O, Cooper C, Pelletier JP, Maheu E, Rannou F, Branco J, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis—from evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3-11. doi: 10.1016/j.semarthrit.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 11. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 12. Campbell KA, Erickson BJ, Saltzman BM, Mascarenhas R, Bach BR, Jr, Cole BJ, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2036-45.e14. doi: 10.1016/j.arthro.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 13. Posnett J, Dixit S, Oppenheimer B, Kili S, Mehin N. Patient preference and willingness to pay for knee osteoarthritis treatments. Patient Prefer Adherence. 2015;9:733-44. doi: 10.2147/PPA.S84251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthritis Cartilage. 2007;15:1424-36. [DOI] [PubMed] [Google Scholar]
- 15. Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97:2047-60. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 16. Herrero-Beaumont G, Guerrero R, Sánchez-Pernaute O, Acebes C, Palacios I, Mas S, et al. Cartilage and bone biological markers in the synovial fluid of osteoarthritic patients after hyaluronan injections in the knee. Clin Chim Acta. 2001;308:107-15. [DOI] [PubMed] [Google Scholar]
- 17. Conrozier T, Balblanc JC, Richette P, Mulleman D, Maillet B, Henrotin Y, et al. Early effect of hyaluronic acid intra-articular injections on serum and urine biomarkers in patients with knee osteoarthritis: an open-label observational prospective study. J Orthop Res. 2012;30:679-85. doi: 10.1002/jor.21580. [DOI] [PubMed] [Google Scholar]
- 18. Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, et al. Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO2) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: a research study part of the Biovisco study. J Orthop Res. 2013;31:901-7. doi: 10.1002/jor.22297. [DOI] [PubMed] [Google Scholar]
- 19. Henrotin Y, Raman R, Richette P, Bard H, Jerosch J, Conrozier T, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45:140-9. doi: 10.1016/j.semarthrit.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 20. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704-11. doi: 10.1002/art. [DOI] [PubMed] [Google Scholar]
- 21. Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of Hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee—a prospective randomized clinical trial. Knee. 2008;15:318-24. doi: 10.1016/j.knee.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 22. Navarro-Sarabia F, Coronel P, Collantes E, Navarro FJ, de la, Serna AR, Naranjo A, et al. ; Amelia Study Group. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70:1957-62. doi: 10.1136/ard.2011.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-40. [PubMed] [Google Scholar]
- 24. Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res. 2012;64:1699-707. doi: 10.1002/acr.21747. [DOI] [PubMed] [Google Scholar]
- 25. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88-96. [DOI] [PubMed] [Google Scholar]
- 26. Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389-99. [DOI] [PubMed] [Google Scholar]
- 27. Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marty M, Hilliquin P, Rozenberg S, Valat JP, Vignon E, Coste P, et al. Validation of the KOFUS (Knee Osteoarthritis Flare-Ups Score). Joint Bone Spine. 2009;76:268-72. doi: 10.1016/j.jbspin.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 29. Bellamy N, Hochberg M, Tubach F, Martin-Mola E, Awada H, Bombardier C, et al. Development of multinational definitions of minimum clinically important improvement and patient acceptable symptom state in osteoarthritis. Arthritis Care Res (Hoboken). 2015;67:972-80. [DOI] [PubMed] [Google Scholar]
- 30. Pagnano M, Westrich G. Successful nonoperative management of chronic osteoarthritis pain of the knee: safety and efficacy of retreatment with intra-articular hyaluronans. Osteoarthritis Cartilage. 2005;13:751-61. [DOI] [PubMed] [Google Scholar]
- 31. Altman RD, Rosen JE, Bloch DA, Hatoum HT. Safety and efficacy of retreatment with a bioengineered hyaluronate for painful osteoarthritis of the knee: results of the open-label Extension Study of the FLEXX Trial. Osteoarthritis Cartilage. 2011;19:1169-75. doi: 10.1016/j.joca.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32. Strand V, Lim S, Takamura J. Evidence for safety of retreatment with a single intra-articular injection of Gel-200 for treatment of osteoarthritis of the knee from the double-blind pivotal and open-label retreatment clinical trials. BMC Musculoskelet Disord. 2016;17:240. doi: 10.1186/s12891-016-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waddell DD, Bricker DC. Hylan G-F 20 tolerability with repeat treatment in a large orthopedic practice: a retrospective review. J Surg Orthop Adv. 2006;15:53-9. [PubMed] [Google Scholar]
- 34. Parkes MJ, Callaghan MJ, O’Neill TW, Forsythe LM, Lunt M, Felson DT. Sensitivity to change of patient-preference measures for pain in trials of patients with knee osteoarthritis: a secondary analysis from the BRACE and TASK trials. Arthritis Care Res (Hoboken). 2016;68:1224-31. doi: 10.1002/acr.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roubille C, Raynauld JP, Abram F, Paiement P, Dorais M, Delorme P, et al. The presence of meniscal lesions is a strong predictor of neuropathic pain in symptomatic knee osteoarthritis: a cross-sectional pilot study. Arthritis Res Ther. 2014;16:507. doi: 10.1186/s13075-014-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Havelin J, Imbert I, Cormier J, Allen J, Porreca F, King T. Central sensitization and neuropathic features of ongoing pain in a rat model of advanced osteoarthritis. J Pain. 2016;17:374-82. doi: 10.1016/j.jpain.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eymard F, Chevalier X, Conrozier T. Obesity and severity of joint space narrowing are associated with a lower rate of success of viscosupplementation in patients with knee osteoarthritis. Post-hoc analysis of a double-blind, controlled, multicenter, randomized trial. Osteoarthritis Cartilage. 2016;24:S528. [Google Scholar]
- 39. Clarke S, Lock V, Duddy J, Sharif M, Newman JH, Kirwan JR. Intra-articular Hylan G-F 20 (Synvisc) in the management of patellofemoral osteoarthritis of the knee (POAK). Knee. 2005;12:57-62. [DOI] [PubMed] [Google Scholar]
- 40. Conrozier T, Mathieu P, Schott AM, Laurent I, Hajri T, Crozes P, et al. Factors predicting long-term efficacy of Hylan GF-20 viscosupplementation in knee osteoarthritis. Joint Bone Spine. 2003;70:128-33. [DOI] [PubMed] [Google Scholar]
- 41. Zóboli AA, de Rezende MU, de Campos GC, Pasqualin T, Frucchi R, de Camargo OP. Prospective randomized clinical trial: single and weekly viscosupplementation. Acta Ortop Bras. 2013;21:271-5. doi: 10.1590/S1413-78522013000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Douglas RJ. Aspiration and injection of the knee joint: approach portal. Knee Surg Relat Res. 2014;26:1-6. doi: 10.5792/ksrr.2014.26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cardone DA, Tallia AF. Diagnostic and therapeutic injection of the hip and knee. Am Fam Physician. 2003;67:2147-52. [PubMed] [Google Scholar]
- 44. Telikicherla M, Kamath SU. Accuracy of needle placement into the intra-articular space of the knee in osteoarthritis patients for viscosupplementation. J Clin Diagn Res. 2016;10:RC15-7. doi: 10.7860/JCDR/2016/17127.7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paradowski PT, Lohmander LS, Englund M. Osteoarthritis of the knee after meniscal resection: long term radiographic evaluation of disease progression. Osteoarthritis Cartilage. 2016;24:794-800. doi: 10.1016/j.joca.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 46. Martel-Pelletier J, Raynauld JP, Dorais M, Abram F, Pelletier JP. The levels of the adipokines adipsin and leptin are associated with knee osteoarthritis progression as assessed by MRI and incidence of total knee replacement in symptomatic osteoarthritis patients: a post hoc analysis. Rheumatology (Oxford). 2016;55:680-8. doi: 10.1093/rheumatology/kev408. [DOI] [PubMed] [Google Scholar]
- 47. Simon D, Mascarenhas R, Saltzman BM, Rollins M, Bach BR, Jr, MacDonald P. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. doi: 10.1155/2015/928301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complications. 2016;30:944-50. doi: 10.1016/j.jdiacomp.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 49. Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16:6093-112. doi: 10.3390/ijms16036093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237-47. [DOI] [PubMed] [Google Scholar]
- 51. Li P, Raitcheva D, Hawes M, Moran N, Yu X, Wang F, et al. Hylan G-F 20 maintains cartilage integrity and decreases osteophyte formation in osteoarthritis through both anabolic and anti-catabolic mechanisms. Osteoarthritis Cartilage. 2012;20:1336-46. [DOI] [PubMed] [Google Scholar]
- 52. Li J, Gorski DJ, Anemaet W, Velasco J, Takeuchi J, Sandy JD, et al. Hyaluronan injection in murine osteoarthritis prevents TGFbeta 1-induced synovial neovascularization and fibrosis and maintains articular cartilage integrity by a CD44-dependent mechanism. Arthritis Res Ther. 2012;14:R151. doi: 10.1186/ar38871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Y, Hall S, Hanna F, Wluka AE, Grant G, Marks P, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yanagisawa K, Muneta T, Ozeki N, Nakagawa Y, Udo M, Saito R, et al. Weekly injections of Hylan G-F 20 delay cartilage degeneration in partial meniscectomized rat knees. BMC Musculoskelet Disord. 2016;17:188. doi: 10.1186/s12891-016-1051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu CC, Su LJ, Tsai WY, Sun HL, Lee HC, Wong CS. Hylan G-F 20 attenuates posttraumatic osteoarthritis progression: association with upregulated expression of the circadian gene NPAS2. Life Sci. 2015;141:20-4. doi: 10.1016/j.lfs.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 56. Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: evidence from a large U.S. health claims database. PLoS One. 2015;10:e0145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu CJ, Ko CJ, Hsieh CH, Chien CT, Huang LH, Lee CW, et al. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid–regulated proteins involved in chondroprotective effect under oxidative stress. J Proteomics. 2014;99:40-53. doi: 10.1016/j.jprot.2014.01.016. [DOI] [PubMed] [Google Scholar]