Abstract
Background and purpose
The role of preoperative short-course radiotherapy (SCRT) in rectal cancer treatment, when compared to long-course radiochemotherapy (LCRT), is still controversial. Thus the meta-analysis with trial sequential analysis (TSA) was performed to evaluate the long-term survival of SCRT and LCRT as therapeutic regimens for locally advanced rectal cancer.
Material and methods
PubMed, Embase, and the Cochrane Central Register of Controlled Trials were searched up to August 2017 for eligible studies. Hazard ratios (HRs) or odds ratios (ORs) of overall survival (OS), disease free survival (DFS) and local recurrence (LR) with the corresponding 95% confidence intervals (CIs) were calculated and TSA was applied.
Results
11 studies with 1984 patients were included. There was no significant difference in OS (HR = 0.92, 95% CI: 0.75–1.13, p = 0.44), DFS (HR = 0.94, 95% CI: 0.79–1.12, p = 0.50) and LR (OR = 0.73, 95% CI: 0.49–1.08, p = 0.11) between SCRT and LCRT groups. TSA suggested firm evidence for lacking on average a -10% relative risk reduction (RRR) in 4-year OS but no statistical significance in 4-year DFS.
Conclusions
Preoperative SCRT is as effective as LCRT for locally advanced colorectal cancer in long-term survival. SCRT could be preferential while facing long waiting lists or lacking medical resource.
Introduction
Preoperative radiotherapy has been shown conclusively to improve local control for rectal cancer [1–2]. For locally advanced stage II-III resectable rectal cancer (mostly cT3 without threatened or involved mesorectal fascia), either preoperative short-course radiotherapy (SCRT) of 25 Gy in 5 consecutive days or long-course chemoradiotherapy (LCRT) (45-50Gy, 1.8-2Gy/fr with concomitant 5-FU-based chemotherapy) followed by radical Total Mesorectal Excision is recommended [3–4]. The benefit of SCRT, as proposed by Swedish Rectal Cancer Trial [5], is a lower rate of early toxicity when compared to chemoradiation [6–8]. Short-course irradiation reduced the risk of local recurrence (LR) by half and showed evident overall survival (OS) improvement [9]. Short-course schedule is less expensive and more convenient as well, especially in centers with long waiting lists [10]. The superiority of LCRT, as proposed by Sauer [6], was demonstrated in comparison to postoperative chemoradiotherapy in terms of local control.
Although both SCRT and LCRT have been practiced in parallel for more than 20 years, it is not clear which form of preoperative radiotherapy provides better tumor control and long term outcomes. Two meta-analyses [11–12] showed that, in terms of sphincter preservation rate, LR rate, grade 3–4 acute toxicity, R0 resection rate and downstaging rate, SCRT is as effective as LCRT for management of rectal cancer. A randomized controlled trial (RCT) [13] reported that The 5-year disease free survival (DFS) and OS were significantly better in the LCRT group than the SCRT group. However, some other RCTs [10,14–15] showed that there was no significant difference in local control and OS between SCRT and LCRT groups.
Based on this situation, we performed meta-analyses to evaluate the long-term prognoses of preoperative SCRT and LCRT as the therapeutic regimens for locally advanced rectal cancer. However, meta-analysis may obtain false positive results (type I errors) or overestimate treatment effects due to systematic errors (bias) and random errors (play of chance). So we performed Trial sequential analysis (TSA) as well, which combines a priori information size calculation for a meta-analysis with the adaptation of monitoring boundaries to evaluate the accumulating data [16–17]. Therefore, we carried out this meta-analysis with TSA to investigate long-term outcomes of the SCRT and LCRT regimens for rectal cancer.
Methods
Literature search
A comprehensive literature search in Pubmed, Embase, and the Cochrane Library databases up to August 2017 was conducted to identify relevant literatures. The search was based on different combinations of the following terms: “rectal cancer”, “long-course chemoradiotherapy”, “preoperative chemoradiotherapy”, “conventional chemoradiotherapy”, “radiotherapy”, “survival” and “short-course radiotherapy”. In addition, references cited in the relevant review articles and meta-analyses were also checked for potentially eligible studies. There was no other limit imposed on this search. PRISMA statement and guidelines [18] were consulted during the stages of design, analysis, and reporting of this meta-analysis (PRISMA Checklist is available in S1 File).
Inclusion and exclusion criteria
(1) Studies that compared SCRT with LCRT in the treatment of locally advanced rectal cancer with follow-up of at least 2 years. (2) Studies that evaluated at least one of the three primary outcomes (OS, DFS or LR). (3) In cases of duplicates, the most recent study was included. (4) No language limitation was imposed. (5) Case reports, review articles and letters were excluded.
Selection and quality assessment
Two reviewers independently screened the titles, abstracts and full texts to determine whether the studies met the inclusion criteria, then assessed the qualities of the eligible studies and extracted data, discrepancies were resolved by consensus. The modified Jadad scale, developed by Greenhalgh [19] and Oremus [20], was used to assess the methodological quality of the included studies [21]. Specifically, the modified version of the Jadad scale consists of three additional questions for the 6-item Jadad scale: (1) was there a clear description of the inclusion/exclusion criteria? (2) was the method used to assess adverse effects described? (3) were the methods of statistical analysis described? One point would be awarded for each affirmative response, while no point would be awarded for a negative response. Scale scores ranged from 0 to 8 points, with higher scores indicating better quality [21]. In addition, to evaluate the pooled results, the Grading of Recommendations Assessment, Development, and Evaluation system (GRADE system) was employed to rate the quality of the evidence for each outcome [22].
Data extraction and synthesis
The extracted contents included: General study information (such as title, publication year, and first author), characteristics of participants and diseases, interventions (such as patients’ age and sex, type of study, sample size, interventions, tumor stage, length of follow-up) and outcomes (OS, DFS and LR).
Statistical analysis
For the time-to-event endpoints (OS and DFS), hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were combined as the effective value to assess the summary effects. The HRs and their 95% CIs were extracted explicitly from the included articles or calculated from the available numerical data using methods reported by Parmar [23], and calculated following the method developed by Tierney [24]. In addition, for LR, odds ratios (OR) with 95% CIs were calculated using the Mantel-Haenszel method.
Meta-analyses were performed using Review Manager Software version 5.2 (Cochrane Collaboration). The fixed-effect and random-effect models were used to calculate the outcomes, and the p-values less than 0.05 were considered statistically significant. In case of significant statistical heterogeneity, only the results from the random effect model were reported. Heterogeneity among the trials was determined by means of the Cochran Q value and quantified using the I2 inconsistency test. Subgroup analyses were performed to compare outcomes from RCT and non RCT respectively.
Trial sequential analysis
A required diversity (D2)-adjusted information size was calculated, with D2 being the relative variance reduction when the meta-analysis model was changed from a random-effect into a fixed-effect model [25]. D2 represents the percentage that the variability between trials, consists of the sum of the between-trial variability and a sampling error estimate considering the required information size. D2 differs from inconsistency (I2), which is the intuitively obvious adjusting factor based on the common quantification of heterogeneity, also which underestimates the required information size [25].
TSA was performed with a desire to maintain an overall 5% risk of type I error, being the standard in most meta-analyses and systematic reviews. In addition, the required information size was calculated (an alpha error of 5%, a beta error of 20%) [16,26–27]. Theoretically, If the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may be established. However, if the boundary is not surpassed, it is most probably necessary to continue doing trials. Trial sequential analysis version 0.9 beta was used for all these analyses.
Results
Literature search
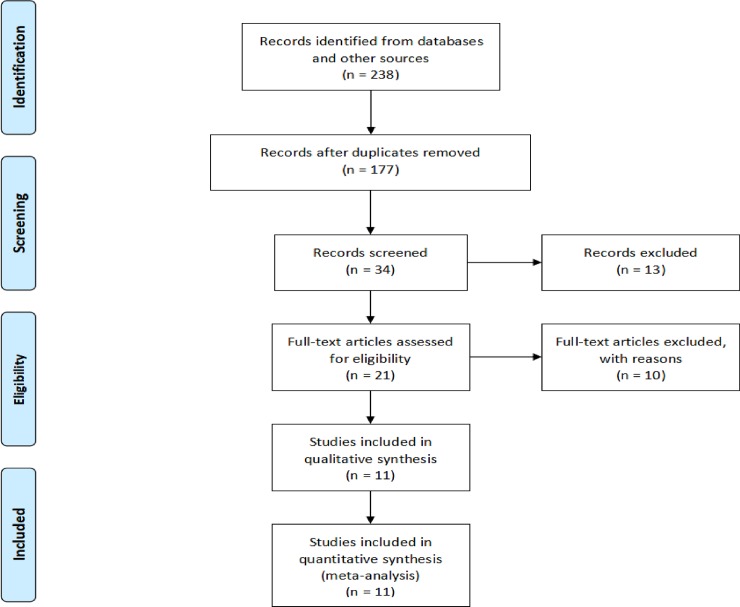
As shown in Fig 1, initially about 238 articles were searched from the databases up to August 2017. Based on the inclusion and exclusion criteria, 217 studies were excluded and 21 studies were subjected to a more detailed review. Finally, 11 studies (4 RCTs [10,13–15] and 7 non-RCTs [28–34]) with a total of 1984 patients were included in this meta-analysis.
Fig 1. Flowchart of eligible studies identification.
11 studies (4 RCTs and 7 non-RCTs) with a total of 1984 patients were included in this meta-analysis.
Study characteristics and methodological quality
Table 1 and S1 Table listed the main characteristics of the 11 studies. The sample sizes in the studies ranged from 29 to 427. 11 included studies all analyzed OS and DFS, and 10 of them analyzed LR. 4 RCTs [10,13–15] out of the 11 studies earned scores of 6 for quality assessment based on the modified Jadad scale, and the 7 non-RCTs [28–34] were scored 4.
Table 1. Characteristics and Jadad scores of included studies.
| Study | Country | Study type | No. of patients | Sex, F/M | Age | DBTA, cm No. of patients |
Follow up, month | Jadad score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCRT | LCRT | SCRT | LCRT | SCRT | LCRT | SCRT | LCRT | |||||
| Bujko 2006[10] | Poland | RCT | 155 | 157 | 55/100 | 54/103 | 60(30–75)# | 59(34–73)# | 5.8(2–10)# | 5.7(2–9)# | 48(31–69)# | 6 |
| Kairevičė L 2017[13] | Lithuania | RCT | 68 | 72 | 25/43 | 22/50 | 66.5±9.5* | 63.14±10.1* | U:5 M:29 L:34 |
U:5 M:37 L:30 |
60.5(5–108)# | 6 |
| Ngan SY 2012[14] | Australia and New Zealand | RCT | 162 | 161 | 45/117 | 41/120 | 63(26–80)# | 64(29–82)# | U:26 M:88 L:48 |
U:42 M:88 L:31 |
70.8(36–93.6)# | 6 |
| Eitta MA 2010[15] | Egypt | RCT | 14 | 15 | 5/9 | 5/10 | 53(32–75)# | 45(25–65)# | U:0 M:3 L:11 |
U:0 M:2 L:13 |
18(6–28)# | 6 |
| Guckenberger M 2012[29] | Würzburg | PS | 108 | 107 | 32/76 | 27/80 | 64* | 66* | U:9 M:53 L:46 |
U:4 M:25 L:78 |
49(3–138)# | 4 |
| Beppu N 2015[28] | Japan | RS | 104 | 61 | 32/72 | 16/45 | 61(39–85)# | 63(34–79)# | U:0 M:49 L:55 |
U:0 M:28 L:33 |
44(12–85)# | 4 |
| Krajcovicova I 2012[30] | Slovak Republic | RS | 96 | 55 | 33/63 | 15/40 | F:63(36–84)# M:61(29–83)# |
F:58(42–72)# M:62(49–78)# |
NR | NR | 48(2–128)# | 4 |
| Yeh CH 2012[31] | Taiwan | RS | 28 | 37 | 11/17 | 13/24 | 67(42–87)# | 60(30–87)# | U:10 M:7 L:11 |
U:7 M:9 L:21 |
36(3.12–61.92)# | 4 |
| Inoue Y 2011[32] | Japan | RS | 51 | 22 | NR | NR | NR | NR | NR | NR | 49* | 4 |
| Klenova A 2007[33] | Bulgaria | RS | 51 | 33 | 21/30 | 13/20 | NR | NR | U:0 M:19 L:32 |
U:0 M:12 L:21 |
53(22–84)# | 4 |
| Abdel-Rahman O 2017[34] | Egypt and Canada | RS | 241 | 186 | 89/152 | 54/132 | 67* | 62* | NR | NR | NR | 4 |
* values are mean±standard deviation
# values are median (range)
SCRT: short-course radiotherapy, LCRT: long-course radiochemotherapy
DBTA: Distance between tumour and anal verge
RCT: randomized controlled trials, RS: retrospective study, PS: prospective study
U: high rectal cancer, M: middle rectal cancer, L: low rectal cancer
NR: not reported.
Overall survival
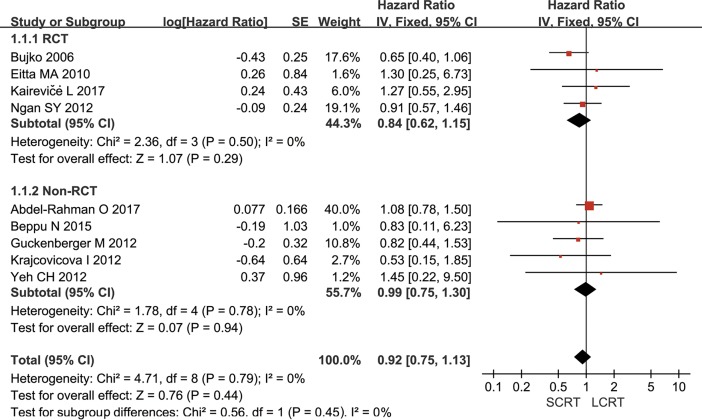
All included studies provided OS information, however, 2 [32–33] studies did not provide HR information. Klenova A [33] reported that the 4-year OS were 72% and 70% in SCRT and LCRT groups, respectively. Inoue Y [32] showed that the 5-year OS rate were 83.3% (LCRT) versus 83.4% (SCRT). Meta-analysis of the available data pooled by fixed-effect model showed that the result of the heterogeneity test was p = 0.79/I2 = 0%, and no significant difference was observed in OS between SCRT and LCRT groups (HR = 0.92, 95% CI: 0.75–1.13, p = 0.44) (Fig 2). The subgroup analysis of RCTs or non-RCTs showed similar results. The specific information of OS for the first 5 years was summarized in S2 Table.
Fig 2. Meta-analysis of cumulative overall survival.
There was no significant difference in OS between SCRT and LCRT groups (HR = 0.92, 95% CI: 0.75–1.13, p = 0.44). The subgroup analysis of RCTs or non-RCTs found similar results.
Disease free survival
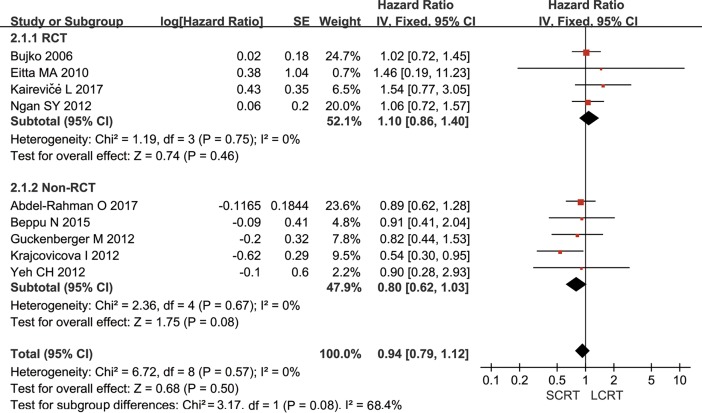
10 out of 11 studies reported DFS. Inoue Y [32] provided that the 4-year DFS were 66% and 68% in SCRT and LCRT groups, respectively. However, no information for obtaining HR was provided. Meta-analysis of 4 RCTs and 5 non-RCTs using the fixed-effect model demonstrated that the heterogeneity between studies was p = 0.57/I2 = 0% and no significant difference was found (HR = 0.94, 95% CI: 0.79–1.12, p = 0.50). Subgroup analysis showed that the difference remained insignificant when RCTs and non-RCTs were analyzed separately (Fig 3). The specific information of DFS for the first 5 years was summarized in S3 Table.
Fig 3. Meta-analysis of cumulative disease free survival.
No significant difference was found (HR = 0.94, 95% CI: 0.79–1.12, p = 0.50) in disease free survival. Subgroup analysis showed that the difference remained insignificant when RCTs and non-RCTs were analyzed separately.
Local recurrence and distant recurrence
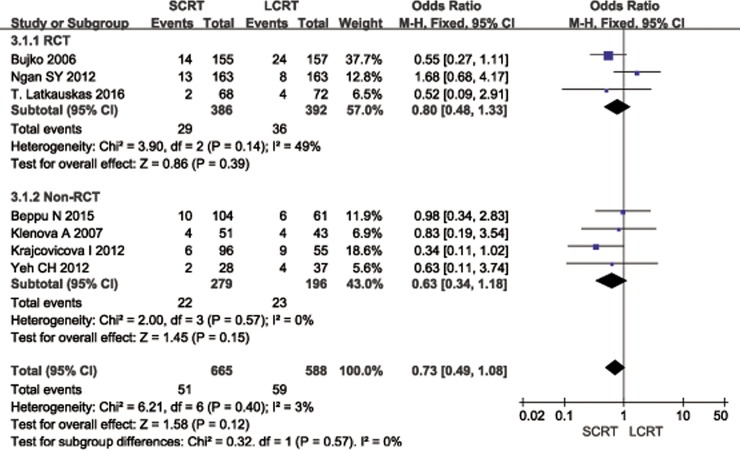
The data of 3-year LR was reported in 3 RCTs [10,13–14] and 4 non-RCTs [28,30–31,33]. Meta-analysis of the data reported no difference between SCRT and LCRT in terms of LR (OR = 0.73, 95% CI: 0.49–1.08, p = 0.11). Subgroup analyses of either RCTs or non-RCTs both showed similar results (Fig 4). The specific information of LR for the first 5 years was summarized in S4 Table.
Fig 4. Meta-analysis of 3-year local recurrence.
There was no difference between CRT and LCRT (OR = 0.73, 95% CI: 0.49–1.08, p = 0.11) in 3-year local recurrence. Subgroup analysis found no significant difference in either RCTs or non-RCTs as well.
Bujko K [10] showed that the crude incidence of distant metastasis was 31.4% in the short-course group and 34.6% in the chemoradiation group (p = 0.54). Ngan SY [14] reported that 5-year distant recurrence rates were 27% for SCRT and 30% for LCRT (HR (LCRT:SCRT) = 1.04, 95% CI: 0.69–1.56, p = 0.92). A RCT [13] reported that distant metastases developed in 14 (21.9%) cases after SCRT and in 9 (12.7%) cases after LCRT (p > 0.05) during the follow-up of 39.7 months, and the HR of distant metastasis for SCRT patients compared to LCRT patients was 2.2 (95% CI: 0.95–5.10). Yeh CH [31] showed that the distant metastasis rates of patients received SCRT and LCRT were 31.5% and 31.1% (p = 0.21), respectively.
Trial sequential analyses
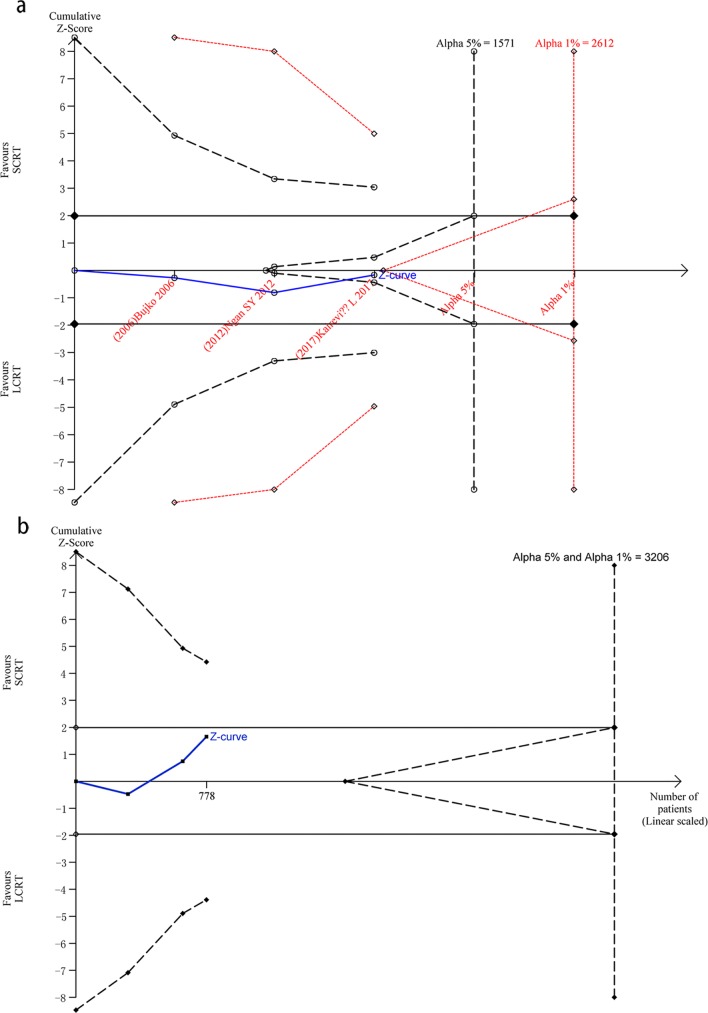
We conducted trial sequential analyses using the information size adjusting for the presence of heterogeneity based on three RCTs [10,13–14] with modified Jadad scale score of at least 6. The required heterogeneity-adjusted information size with 5% risk of type I error (risk of obtaining a false ‘positive’ result), 20% risk of type II error (risk of obtaining false ‘negative’ result) and an anticipated RR = 1.1 (i.e. a relative risk reduction in 4-year OS by SCRT of -10%) was calculated to 1571 patients. The cumulative z curve crossed the futility boundary, suggesting firm evidence for lack of on average a -10% RRR in 4-year OS. The required information size using 1% risk of type I error instead was 2612 patients. The analyses did not yield any sign of statistical significance whatsoever. The cumulative z curve crossed neither the traditional boundary nor the trial sequential monitoring boundary but was very close to the futility boundaries, suggesting a lack of firm evidence for a -10% RRR in the SCRT group compared to the LCRT group regarding to 4-year OS (Fig 5A).
Fig 5. Trial sequential analysis of 4-year overall survival.
5a, Trial sequential analysis of 4-year overall survival. The required heterogeneity-adjusted information size using 5% risk of type I error and 20% risk of type II error. The cumulative z curve crossed the futility boundary, suggesting firm evidence for lack of on average a -10% relative risk reduction in 4-year OS. 5b, Trial sequential analysis of 4-year disease free survival. When compared with LCRT treatment in 4-year DFS, neither the traditional boundary nor the trial sequential monitoring boundary was crossed for a -10% relative risk reduction with SCRT. In addition, the futility boundary was not crossed.
In addition, we performed trial sequential analysis for 4-year DFS with a type I error of 5% and 1%, type II error of 20% (80% power), and adjusted for heterogeneity among included trials [10,13–14]. When compared with LCRT treatment, neither the traditional boundary nor the trial sequential monitoring boundary was crossed for a -10% RRR with SCRT. In addition, the futility boundary was not crossed (Fig 5B), suggesting the need for more evidence to establish additional benefits of SCRT over LCRT treatment.
Evidence rating of the critical outcomes
The GRADE system was used to synthesize and rate the evidence for each outcome, and the quality of evidence was summarized in Table 2. The overall qualities of evidence for those outcomes were of high quality. Hence, further research is unlikely to change our confidence in the estimate of effect.
Table 2. Quality of evidence for each outcome using GRADE system.
| Outcome | Study design | Studies (participants) |
Quality assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | HR or OR (95%CI) | Heterogeneity | ||||
| I2 (%) | p value | ||||||||||
| OS | 9(1895) | 0.92 (0.75–1.13) | 0 | 0.79 | |||||||
| RCT | 3(778) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊕⊕ high | 0.84 (0.62–1.15) | 0 | 0.50 | |
| Non-RCT | 6(1117) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊝⊝low | 0.99 (0.75–1.30) | 0 | 0.78 | |
| DFS | 9(1895) | 0.94 (0.79–1.12) | 0 | 0.57 | |||||||
| RCT | 3(778) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊕⊕ high | 1.10 (0.86–1.40) | 0 | 0.75 | |
| Non-RCT | 6(1117) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊝⊝low | 0.80 (0.62–1.03) | 0 | 0.87 | |
| LR | 7(1250) | 0.73 (0.49–1.08) | 3 | 0.41 | |||||||
| RCT | 3(775) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊕⊕ high | 0.80 (0.48–1.33) | 48 | 0.15 | |
| Non-RCT | 4(475) | No serious | No serious | No serious | No serious | Undetected | ⊕⊕⊝⊝low | 0.63 (0.34–1.18) | 0 | 0.57 | |
Discussion
According to the updated ESMO clinical practice guidelines of 2017, both LCRT and SCRT for resectable locally advanced rectal cancer are recommended [3]. However, due to the various routine deliveries of neoadjuvant treatment regimens, different treatment strategies were adopted among countries or even in the same country.
Several meta-analyses [11–12,35–37] compared these two different preoperative treatment regimens and found no difference in DFS and OS. Among them, 3 meta-analyses reported that LCRT resulted in significantly lower LR rate, but the recent 2 meta-analyses [11–12] showed that no significant difference in LR rate between the two regimens. All systematic reviews and meta-analyses made a consensus that LCRT resulted in significantly higher pathological complete response rate and higher acute toxicity.
The present meta-analysis investigated long-term outcomes of the SCRT versus LCRT for advanced rectal cancer. OS, DFS and LR were not significantly different between the patients treated using the SCRT and the LCRT regimens. However, the prevalence of meta-analysis at risk of random error due to repetitive testing seemed too high to be ignored. TSA might eliminate early false positive findings due to imprecision and repeated significance testing in meta-analyses. And TSA could also provide a required diversity adjusted information size, a threshold for a statistically significant treatment effect, and the threshold for futility [26,38]. We therefore undertook a trial sequential analysis to consolidate the available literature.
Presented by a systematic overview of radiotherapy in rectal cancer, statistically lower LR rates were observed in most trials comparing preoperative radiotherapy (followed by surgery) versus surgery alone [39]. In the Australia and New Zealand RCT [14], there was no statistically significant difference in LR rate between preoperative SCRT and LCRT radiotherapy, although the trend favored LC (3-year LR rates: 10% in SC group and 2% in LC group; inferred 95% CI for SCRT-LCRT approximately -2.1% to 8.3%). However, our present study demonstrated a small difference in 3-year LR rate (8.8%), favoring SCRT. The 95% CI (SCRT:LCRT) was 0.49 to 1.08, including differences of 3.2% or more, in favor of SCRT (i.e. 6.8% vs 10%). But the trial did not exclude the potential important clinical difference in 3-year LR rates.
Kapiteijn E [39] considered that LR was significantly related to the distance from the anal verge. This may be due to that 82.8% of patients had a tumor in the lower third. However, no significant difference was found in terms of LR in the present meta-analysis. The data was consistent with either no difference or an important clinical difference in favor of SCRT, and it’s unlikely that there was an important difference favoring LCRT.
Latkauskas T [40] considered that the death of rectal cancer was mainly correlated with distant metastases, but not LR. This could be the explaination for the trivial survival benefit reported by most trials, while comparing short-term with long course neoadjuvant treatment for rectal cancer. However, 3 RCTs [10,13–14] reported that no significant difference was found in incidence of distant metastases between SCRT and LCRT groups. The France FFCD 9203 did not certify any superiority for the addition of 5-Fu to RT in terms of either DFS or OS, when comparing preoperative radiotherapy with chemoradiotherapy [41]. According to the results of the Lithuania RCT [40], 3-year DFS was better in LCRT group compared to SCRT group without significant difference in OS. Surgical recovery and perioperative morbidity were similar between the groups. Kairevičė L [13] reported that the 5-year DFS and OS were significantly better in the LCRT group than that in the SCRT group. However, the study was based on a small number of patients, and this could be one of its biggest limitations. Data on positive pathological lymph nodes in Lithuania RCT [40] -25 (36.8%) cases in the SCRT group and 18 (25%) cases in CRT group (p > 0.05)—could tell that there was an imbalance in the original nodal status between both arms.
While the current study is focused on comparing LCRT and SCRT, the use of sequential chemotherapy in SCRT has a role in the long-term survival. A population-based cohort study recruited 123 patients who were with stage II rectal cancer and received preoperative SCRT plus chemotherapy, and its subgroup analysis suggested that adjuvant chemotherapy improved DFS (HR:0.24; 95% CI:0.07–0.85; p = 0.027) and OS (HR = 0.22; 95% CI: 0.069–0.70; p = 0.011) in patients with ≥ 2 risk features [42]. A phase II trial, which included 50 patients with stage VI rectal cancer, demonstrated a clear OS advantage with SCRT plus chemotherapy (p = 0.004) [43]. Multicenter RCTs are needed to confirm this advantage.
For the TSA of 4-year OS, the calculated diversity-adjusted required information size (DARIS) was 1571 participants, considerating the patient proportion in the control group with the outcome of 5.28%, a RRR of 20%, an alpha of 5%, a beta of 20% (the required information size using 1% risk of type I error is 2612 patients instead). 35.9% of the DARIS has been reached after accruing the patients to altogether 564 from 3 RCTs. The cumulative z curve crossed the futility boundary. We also performed TSA for 4-year DFS with a type I error of 5% and 1%, type II error of 20% (80% power). The required information size using 1% or 5% risk of type I error was 3206 patients. Accordingly, with 483 accrued participants in 3 RCTs, only 15.1% of the DARIS had been reached.
Considering medical expenses, Beppu N [28] reported that the SCRT regimen cost about $2,650 while the LCRT regimen cost about $7,050. Therefore, convenience and a lower cost were the major benefits of short term treatment. In addition, when considering patient convenience, the waiting period was about half a month for patients who received SCRT, while it was > 3 months for LCRT regimen recipients. SCRT could be the preferential treatment for locally advanced colorectal cancer while facing long waiting lists or lack of medical resource.
Limitation
First of all, though all included studies earned more than scores of 4 for quality, all RCT exist some potential risks of bias. Only 3 out of all included RCTS reported the method of adequate randomized sequence generation [10,13–14], the other RCT showed unclear randomization [15]. This could have an influence on diagnostic procedures, RT and surgical techniques, as well as survival results. Moreover, the trial sequential analyses demonstrated that we have never had convincing evidence in favor of SCRT over LCRT for rectal cancer and we are still far from having it.
In conclusion, the present study demonstrated that preoperative SCRT is as effective as LCRT for the treatment of rectal cancer in terms of OS, DFS and LR. Further adequately powered trials with lower risk of bias are necessary to provide more robust evidence.
Supporting information
(PDF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for respectable rectal cancer: A meta-analysis. JAMA 2000;284(8):1008–1015. [DOI] [PubMed] [Google Scholar]
- 2.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358(9290):1291–1304. 10.1016/S0140-6736(01)06409-1 [DOI] [PubMed] [Google Scholar]
- 3.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017. July 1;28(suppl_4):iv22–iv40. 10.1093/annonc/mdx224 [DOI] [PubMed] [Google Scholar]
- 4.Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, et al. Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol 2009;92(2):148–163. 10.1016/j.radonc.2009.06.027 [DOI] [PubMed] [Google Scholar]
- 5.Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336(14):980–987. 10.1056/NEJM199704033361402 [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351(17):1731–1740. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 7.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72(1): 15–24. 10.1016/j.radonc.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Calais G, Daban A, Berger C, Radosevic-Jelic L, Maingon P, et al. Preoperative chemoradiotherapy versus preoperative radiotherapy in rectal cancer patients: assessment of acute toxicity and treatment compliance. Eur J Cancer 2004;40(2):219–224. [DOI] [PubMed] [Google Scholar]
- 9.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005,23(24):5644–5650. 10.1200/JCO.2005.08.144 [DOI] [PubMed] [Google Scholar]
- 10.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93(10):1215–1223. 10.1002/bjs.5506 [DOI] [PubMed] [Google Scholar]
- 11.Zhou ZR, Liu SX, Zhang TS, Chen LX, Xia J, Hu ZD, et al. Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol 2014;23(4):211–221. 10.1016/j.suronc.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Liu SX, Zhou ZR, Chen LX, Yang YJ, Hu ZD, Zhang TS. Short-course Versus Long-course Preoperative Radiotherapy plus Delayed Surgery in the Treatment of Rectal Cancer: a Meta-analysis. Asian Pac J Cancer Prev 2015;16(14):5755–5762. [DOI] [PubMed] [Google Scholar]
- 13.Kairevičė L, Latkauskas T, Tamelis A, Petrauskas A, Paužas H, Žvirblis T, et al. Preoperative long-course chemoradiotherapy plus adjuvant chemotherapy versus short-course radiotherapy without adjuvant chemotherapy both with delayed surgery for stage II-III resectable rectal cancer: 5-year survival data of a randomized controlled trial. Medicina (Kaunas) 2017;53(3):150–158. [DOI] [PubMed] [Google Scholar]
- 14.Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30(31):3827–3833. 10.1200/JCO.2012.42.9597 [DOI] [PubMed] [Google Scholar]
- 15.Eitta MA, El-Wahidi GF, Fouda MA, El-Hak NG, Abo El-Naga EM. Preoperative radiotherapy in resectable rectal cancer: a prospective randomized study of two different approaches. J Egypt Natl Canc Inst 2010;22(3):155–164. [PubMed] [Google Scholar]
- 16.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61(1):64–75. 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38(1):276–286. 10.1093/ije/dyn179 [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhalgh T. Assessing the methodological quality of published papers. BMJ 1997; 315(7103):305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord 2001;12(3):232–236. 10.1159/000051263 [DOI] [PubMed] [Google Scholar]
- 21.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther 2008;88(2):156–175. 10.2522/ptj.20070147 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009;9:86 10.1186/1471-2288-9-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol 2008;61(8):763–769. 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 27.Wang Quan, Zheng Bobo, Ma Bin, Yang KeHu. Anterior approach versus conventional liver resection for hepatocellular carcinoma. Cochrane Hepato-Biliary Group. First published: 31 May 2013. [Google Scholar]
- 28.Beppu N, Matsubara N, Noda M, Yamano T2, Kakuno A3, Doi H, et al. Short-course radiotherapy with delayed surgery versus conventional chemoradiotherapy: A comparison of the short- and long-term outcomes in patients with T3 rectal cancer. Surgery 2015;158(1):225–235. 10.1016/j.surg.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 29.Guckenberger M, Saur G, Wehner D, Sweeney RA, Thalheimer A, Germer CT, et al. Comparison of preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Strahlenther Onkol 2012;188(7):551–557. 10.1007/s00066-012-0131-2 [DOI] [PubMed] [Google Scholar]
- 30.Krajcovicova I, Bolješíková E, Sandorova M, Zavodska A, Zemanová M, Chorváth M, et al. Preoperative radiotherapy of locally advanced rectal cancer: clinical outcome of short-course and long-course treatment with or without concomitant chemotherapy. Klin Onkol 2012;25(5):364–369. [PubMed] [Google Scholar]
- 31.Yeh CH, Chen MF, Lai CH, Huang WS, Lee SP, Chen WC. Comparison of treatment results between surgery alone, preoperative short-course radiotherapy, or long-course concurrent chemoradiotherapy in locally advanced rectal cancer. Int J Clin Oncol 2012;17(5):482–490. 10.1007/s10147-011-0317-0 [DOI] [PubMed] [Google Scholar]
- 32.Inoue Y, Saigusa S, Hiro J, Toiyama Y, Tanaka K, Mohri Y, et al. Clinical and molecular comparison between short-and long-course preoperative radiotherapy for rectal cancer. J Clin Oncol 2011;29(Suppl. 1):e14093. [Google Scholar]
- 33.Klenova A, Georgiev R, Kurtev P, Kurteva G. Short versus conventional preoperative radiotherapy of rectal cancer: indications. J BUON 2007;12(2):227–232. [PubMed] [Google Scholar]
- 34.Abdel-Rahman O, Kumar A, Kennecke HF, Speers CH, Cheung WY. Impact of Duration of Neoadjuvant Radiation on Rectal Cancer Survival: A Real World Multi-center Retrospective Cohort Study. Clin Colorectal Cancer 2017;pii: S1533-0028(17)30095-6. [DOI] [PubMed] [Google Scholar]
- 35.Ceelen W, Fierens K, Van Nieuwenhove Y, Pattyn P. Preoperative chemoradiation versus radiation alone forstage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer 2009;124(12):2966–2972. 10.1002/ijc.24247 [DOI] [PubMed] [Google Scholar]
- 36.Latkauskas T, Paskauskas S, Dambrauskas Z, Gudaityte J, Saladzinskas S, Tamelis A, et al. Preoperative chemoradiation vs radiation alone for stage II and III resectable rectal cancer: a meta-analysis. Colorectal Dis 2010;12(11):1075–1083. 10.1111/j.1463-1318.2009.02015.x [DOI] [PubMed] [Google Scholar]
- 37.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2013;(2):CD006041 10.1002/14651858.CD006041.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive—Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38(1):287–298. 10.1093/ije/dyn188 [DOI] [PubMed] [Google Scholar]
- 39.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345(9):638–646. 10.1056/NEJMoa010580 [DOI] [PubMed] [Google Scholar]
- 40.Latkauskas T, Pauzas H, Kairevice L, Petrauskas A, Saladzinskas Z, Janciauskiene R, et al. Preoperative conventional chemoradiotherapy versus short-course radiotherapy with delayed surgery for rectal cancer: results of a randomized controlled trial. BMC Cancer 2016;16(1):927 10.1186/s12885-016-2959-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24(28):4620–4625. 10.1200/JCO.2006.06.7629 [DOI] [PubMed] [Google Scholar]
- 42.Loree JM, Kennecke HF, Renouf DJ, Lim HJ, Vickers MM, Speers CH, et al. Effect of Adjuvant Chemotherapy on Stage II Rectal Cancer Outcomes After Preoperative Short-Course Radiotherapy. Clin Colorectal Cancer. 2016;15(4):352–359.e1. 10.1016/j.clcc.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 43.Bisschop C, van Dijk TH, Beukema JC, Jansen RLH, Gelderblom H, de Jong KP, et al. Short-Course Radiotherapy Followed by Neoadjuvant Bevacizumab, Capecitabine, and Oxaliplatin and Subsequent Radical Treatment in Primary Stage IV Rectal Cancer: Long-Term Results of a Phase II Study. Ann Surg Oncol. 2017;24(9):2632–2638. 10.1245/s10434-017-5897-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.