Abstract
Most general anesthetics enhance GABA type A (GABAA) receptor activity at clinically relevant concentrations. Sites of action of volatile anesthetics on the GABAA receptor remain unknown, whereas sites of action of many intravenous anesthetics have been identified in GABAA receptors by using photolabeling. Here, we used photoactivatable analogs of isoflurane (AziISO) and sevoflurane (AziSEVO) to locate their sites on α1β3γ2L and α1β3 GABAA receptors. As with isoflurane and sevoflurane, AziISO and AziSEVO enhanced the currents elicited by GABA. AziISO and AziSEVO each labeled 10 residues in α1β3 receptors and 9 and 8 residues, respectively, in α1β3γ2L receptors. Photolabeled residues were concentrated in transmembrane domains and located in either subunit interfaces or in the interface between the extracellular domain and the transmembrane domain. The majority of these transmembrane residues were protected from photolabeling with the addition of excess parent anesthetic, which indicated specificity. Binding sites were primarily located within α+/β− and β+/α− subunit interfaces, but residues in the α+/γ− interface were also identified, which provided a basis for differential receptor subtype sensitivity. Isoflurane and sevoflurane did not always share binding sites, which suggests an unexpected degree of selectivity.—Woll, K. A., Zhou, X., Bhanu, N. V., Garcia, B. A., Covarrubias, M., Miller, K. W., Eckenhoff, R. G. Identification of binding sites contributing to volatile anesthetic effects on GABA type A receptors.
Keywords: photoaffinity labeling, isoflurane, sevoflurane, crosslinking
Since the mid-20th century, mounting evidence has implicated GABA type A (GABAA) receptors (Fig. 1A) —the major inhibitory receptors in the adult mammalian brain (1, 2) —as significant targets that underlie general anesthesia (3–6). Within the past decade, studies have focused largely on identifying binding sites for i.v. anesthetics, such as propofol, etomidate, and barbiturates (7); however, binding sites for the lower-affinity volatile anesthetics remain enigmatic. Isoflurane (ISO) and sevoflurane (SEVO; Fig. 1B) are modern volatile anesthetics that are widely used in patients across the world. Both anesthetics have been demonstrated to affect synaptic transmission within the CNS, in part, by enhancing synaptic GABAA receptor activity (4, 8). ISO at clinical concentrations prolongs both fast and slow forms of phasic GABAA receptor–mediated inhibition in mouse hippocampal neurons (9), and knock-in mutations within either the α2- or β3-subunit have resulted in varying degrees of right-shifts of anesthesia end points for volatile anesthetics in mice (10, 11). The latter studies, although clearly providing evidence for the role of synaptic GABAA receptors in vivo, cannot definitively define the binding sites of ISO and SEVO, nor can in vitro site–directed mutagenesis studies as a result of the highly allosteric nature of GABAA receptors.
Figure 1.
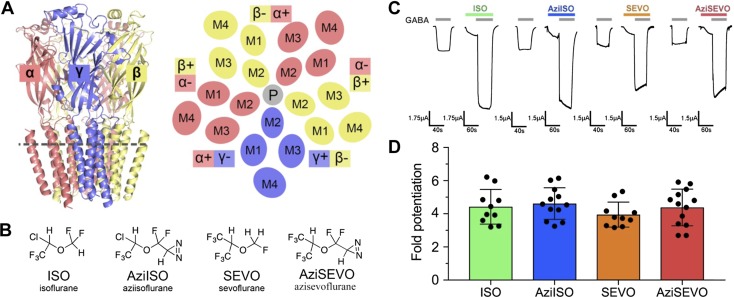
ISO, AziISO, SEVO, and AziSEVO α1β3γ2L GABAA receptor activity. A) Membrane view of the α1β3γ2 GABAA receptor homology model and schematic of the cross-section as indicated by the gray dashed line of the receptor transmembrane domain viewed from the synaptic cleft. Receptor is colored by subunit type (α1, salmon; β3, yellow; and γ2, slate), and membrane-spanning helices (M1–M4) are labeled accordingly for each subunit. The plus and minus subunit interfaces are indicated for each subunit, and the pore (P) is indicated as a gray circle. B) Chemical structures of volatile anesthetics and their photolabel analogs. C) Representative traces of evoked current by GABA EC10 control and after combined GABA EC10 and 0.3 mM ISO, AziISO, SEVO, and AziSEVO exposures within individual X. laevis oocytes expressing the α1β3γ2L GABAA receptor. D) Fold potentiation of GABA EC10 by 0.3 mM ISO, AziISO, SEVO, and AziSEVO within X. laevis oocytes expressing the α1β3γ2L GABAA receptor. Data were analyzed by Mann–Whitney U test that compared the evoked currents of the parent anesthetic with the corresponding photolabel analog. No significant differences were observed.
GABAA receptors are composed of 5 homologous subunits, each with an agonist-binding extracellular domain, an ion-conducting transmembrane domain that contains 4 transmembrane helices, and an intracellular domain (data not shown) (Fig. 1A). Subunits are organized in a pseudosymmetric manner around a central ion-conducting pore. In the brain, GABAA receptors are expressed with a diverse range of subunits that can be broadly classified by their relative distributions in either synaptic or extrasynaptic regions (12). Synaptic GABAA receptors are responsible for fast phasic inhibition and are formed by 2 α-, 2 β-, and 1 γ-subunit arranged around the central pore in the anticlockwise order, βαβαγ, as seen from the synaptic cleft (13–15). In the α1β3 receptors, one third of the β3-subunit takes the place of the γ-subunit (16), and evidence demonstrates that α1β3 receptors are present in vivo (2, 16, 17). Furthermore, it has been suggested that α1β3 receptors contribute to tonic inhibition (18), perhaps via an extrasynaptic location. The importance of subunit composition in general anesthetic pharmacology is emphasized by photolabeling studies that demonstrate, for example, that etomidate binds between the β3- and α1-subunits (β3+/α1−, for notation, see Fig. 1A), whereas mephobarbital has a preference for the γ2+/β3− interface and does not bind in the β3+/α1− interface (19, 20). These anesthetic sites are in the transmembrane domain, but the smaller, lower-affinity volatile anesthetics might well interact with cavities and pockets in the other domains and within subunits as well as between them.
MATERIALS AND METHODS
Heterologous expression of GABAA receptor subunits and electrophysiologic recordings
GABAA receptor expression in Xenopus laevis oocytes was completed in a manner similar to that described previously (21). Human cDNAs for GABAA receptor α1-, β3-, and γ2L-subunits were generously provided by Dr. Robert Pearce (University of Wisconsin, Madison, WI, USA). All animal care and experimental procedures that involved X. laevis frogs were carried out according to a protocol approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. GABAA receptor currents were recorded as previously reported (21, 22). In brief, GABAA receptor whole-oocyte currents were recorded at room temperature (21–23°C) under 2-electrode voltage clamp conditions (OC-725C; Warner Instruments, Hamden, CT, USA). Recordings were made at a holding voltage of −80 mV. Oocytes were perfused with ND96 (mM: 96 NaCl, 2 KCl, 1 MgCl2, 1 CaCl2, and 5 HEPES, pH 7.5) -based solutions by gravity-driven perfusion using a Six-Channel Valve VC-6 Control System (Warner Instruments, Hamden, CT, USA) with an approximate perfusion rate of 2–4 ml/min. The perfusion system was outfitted with Teflon tubing for drug exposure studies. ND96 and GABA solutions (Sigma-Aldrich, St. Louis, MO, USA) that were prepared daily in ND96 were delivered by using a gravity-driven perfusion system. Volatile anesthetics and photolabels were directly dissolved in ND96 to 0.3 mM by sonication in sealed glass vials and were delivered manually by using a Hamilton gastight syringe (Hamilton, Reno, NV, USA). Bath drainage was performed by gravity flow. Initially, each oocyte was exposed to 3–5 μM GABA for the effective concentration (EC) 7–13 of maximum GABAA receptor activation. Maximum GABA response was determined by a 10-mM GABA perfusion postdrug exposure and washout. To determine modulatory activity, oocytes were perfused for 20 s with the test compounds, which was immediately followed by 20 s of perfusion with the test compound and GABA at determined EC10. Data acquisition and initial analysis were performed by using pClamp 9.2/10.3 (Molecular Devices, Sunnyvale, CA, USA). Macroscopic currents were low-pass filtered at 1 kHz and digitized at 2 kHz. Data acquisition and initial analysis were performed by using pClamp 9.2/10.3.
Construction and stable cell line generation
Genes that encode the GABAA receptor α1-, β3-, and γ2L-subunits were cloned into expression vectors that contained independent antibiotic selections. We prepared the plasmids, FLAG–bGABAA receptor α1/pcDNA4/TO–Zeocin, hGABAA receptor β3/pcDNA3.1/TO–Hygro1, and hGABAA receptor γ2-(GGS)3GK-1D4/pACMV/TO–blasticidin as previously described (23, 24). Transfection using 293 fectin and colony selection and amplification were conducted as previously reported (23, 24). Optimal inducible cell lines were validated and chosen on the basis of quantitative RT-PCR, Western blot analysis, growth rate, and the number of [3H]muscimol and/or [3H]flunitrazepam sites (24).
Immunoaffinity purification of GABAA receptor
We performed protein purification and reconstitution as previously described (23, 24). In brief, stably transfected HEK293-TetR cells were grown, induced with tetracycline and 5 mM sodium butyrate, harvested, and lysed, and membrane suspensions were collected. Membrane pellets were solubilized by the dropwise addition of (mM) 50 Tris-HCl (pH 7.4), 150 NaCl, 2 CaCl2, 5 KCl, 5 MgCl2, and 4 EDTA that was supplemented with 10% (v/v%) glycol, protease inhibitors (leupeptin, chymostatin, pepstatin A, aprotinin, and PMSF), and dodecyl-d-maltoside (final concentration, 1.5% m/v%) to a final protein concentration of 1 mg/ml. Insoluble material was removed by ultracentrifugation, and the supernatant was loaded onto prepared anti-FLAG or anti-1D4 affinity columns. Dodecyl-d-maltoside was replaced by dimethyl (3-stearamidepropyl) (3-sulfonatopropyl) ammonium (CHAPS)/asolectin by repeated washes and 1 h of equilibration. Columns were then washed with asolectin (0.025–0.86 mM as required) and CHAPS (5 mM) before elution by 90 min of equilibration in 1 column volume of the same solution that was supplemented with 0.1 mM FLAG or 0.15 mM 1D4 peptide. Eluate was collected and the elution process was performed a total of 3 to 4 times. Eluted protein fractions were frozen in liquid nitrogen and stored at −80°C.
Photoaffinity labeling of FLAG-α1β3 or FLAG-α1β3γ2L-L3-1D4 GABAA receptor for protein microsequencing
Aziisoflurane (AziISO) and azisevoflurane (AziSEVO) at a final concentration 30 μM was added to 4 μg FLAG-α1β3γ2L-L3-1D4 in 200 μM azolectin and 5 mM CHAPS, and to 6.3 μg FLAG-α1β3 GABAA receptor in 200 μM asolectin:cholesterol (2:1), 5 mM CHAPS, and 1 μM GABA, respectively, using DMSO vehicle [<0.01% (v/v%)]. Sample was equilibrated on ice in the dark for 5 min before being exposed to 300 nm RPR-3000 Rayonet lamp in 1-mm path-length quartz cuvettes through a WG295 295-nm glass filter (Newport Corp., Irvine, CA, USA) for 25 min.
In-solution protein digestion
Photolabeled samples were concentrated to ∼15–20 μl by using 10-kDa MW cutoff Amicon Ultra Centrifugal Filters (EMD Millipore, Billerica, MA, USA). ProteaseMax Surfactant (Promega, Madison, WI, USA) was added to 0.2%, and samples were vortexed vigorously for 30 s. Samples were diluted to 93.5 μl to a final concentration of 50 mM NH4HCO3. One microliter of 0.5 M DTT was added, and samples were incubated at 56°C for 20 min. Iodoacetamide (2.7 μl of 0.55 M) was then added, and protein samples were incubated at room temperature in the dark for 20 min. One microliter of 1% (w/v%) ProteaseMax Surfactant was added, followed by CaCl2 to a 1-mM final concentration. Sequencing grade–modified trypsin (Promega) was added to a final ratio of 1:20 protease:protein (w/w). Proteins were digested overnight at 37°C. Trypsin-digested peptides were diluted to 200 μl, with final concentration of 100 mM NH4HCO3 and 0.2% ProteaseMax Surfactant before the addition of sequencing-grade chymotrypsin (Promega) to a final ratio of 1:20 protease:protein (w/w). Proteins were digested overnight at 37°C. TFA was added to 0.5% (v/v%), and peptide digests were incubated at room temperature for 10 min before being centrifuged at 16,000 g for 20 min before desalting using C18 stage tips that were prepared in-house. Samples were dried by Speedvac (Thermo Fisher Scientific, Waltham, MA, USA) and resuspended in 0.1% formic acid immediately before mass spectrometry analysis.
In-gel protein digestion
Photolabeled receptor samples were concentrated to ∼20 μl by using 10-kDa MW cutoff Amicon Ultra Centrifugal Filters (EMD Millipore). SDS loading buffer was added to the sample that contained a final concentration 80 mM DTT, and samples were vortexed vigorously, then incubated at room temperature for 45 min before the entire sample was separated by SDS-PAGE. Resulting gels were stained with Coomassie Blue G250 (Bio-Rad, Hercules, CA, USA). Gels were destained and washed with double-distilled H2O (ddH2O). Identified protein bands between ∼50 and 60 kDa—corresponding to FLAG-α1, β3, or γ2L-(L3)-1D4 GABAA receptor subunits—were excised. Excised bands were destained, dehydrated, and dried by Speedvac before proteins were reduced by incubation at 56°C for 20 min in 5 mM DTT and 50 mM NH4HCO3. DTT solution was removed and proteins were then alkylated by adding 55 mM iodoacetamide in 50 mM NH4HCO3 and incubating at room temperature in the dark. Bands were dehydrated and dried by Speedvac before being resuspended in 100 μl 0.2% ProteaseMax surfactant, 1 mM CaCl2, and 50 mM NH4HCO3 solution that contained trypsin at a ratio of 1:20 protease:protein (w/w). Proteins were digested overnight at 37°C. Samples were diluted to 200 μl to a final concentration of 100 mM NH4HCO3 and 0.2% ProteaseMax Surfactant before addition of sequencing-grade chymotrypsin (Promega) to a final ratio of 1:20 protease:protein (w/w). Proteins were digested overnight at 37°C. To increase hydrophobic peptide retrieval from the gel, we performed multiple peptide extractions. First, the initial peptide digest solution was removed and 100 μl 30% acetyl nitrile and 5% formic acid in ddH2O (v/v%) was added. Samples were sonicated for 20 min. The second peptide extraction was removed before addition of 100 μl 70% acetyl nitrile and 5% formic acid in ddH2O (v/v%). Samples were sonicated for 20 min. All peptide digests were pooled and dried by Speedvac before being resuspended in 0.5% TFA and desalted using C18 stage tips that were prepared in-house. Samples were dried by Speedvac and resuspended in 0.1% formic acid immediately before mass spectrometry analysis.
Mass spectrometry
Desalted peptides were analyzed on an Orbitrap Elite Hybrid Ion Trap-Orbitrap Mass Spectrometer (Thermo Fisher Scientific) that was coupled to an Easy-nanoLC 1000 system with a flow rate of 300 nl/min. Peptides were eluted for100 min with linear gradients from 2 to 40% acetyl nitrile (85 min), from 40 to 85% ACN (5 min), and finally 85% (10 min) acetonitrile in 0.1% formic acid (v/v%). Data-dependent acquisition mode was applied with a dynamic exclusion of 45 s. In every 3-s cycle, 1 full mass spectrometer scan was collected with a scan range of 350–1500 m/z, a resolution of 60 K. Maximum injection time was 50 ms and automatic gain control was 500,000. MS2 scans then followed using the most intense MS1 parent ions with filtered charges of 2–5. An isolation window of 1.5 m/z was used with quadruple isolation mode. Ions were fragmented by using collision-induced dissociation (CID) with collision energy of 35%. Iontrap detection was used with normal scan range mode and rapid iontrap scan rate. Automatic gain control was set at 10,000 with a maximal injection time of 100 ms.
Mass spectrometry analysis
We performed analysis as previously reported (25) with the addition of predicted photolabel modifications (26, 27). In brief, we conducted spectral analysis by using Thermo Proteome Discoverer 2.0 (Thermo Fisher Scientific) and the Mascot Daemon search engine using a customized database that contained GABAA receptor protein sequences that were supplied for heterologous expression or the human proteome UniProt database (UniProtKB: P28472; http://www.uniprot.org/uniprot/P28472). All analyses included dynamic oxidation of methionine (+15.9949 m/z) and N(Q) deamidation [+0.98402 Da, when PNGase F-specific, defined as N(Q) deglycosylation], as well as static alkylation of cysteine (+57.0215 m/z; iodoacetamide alkylation). Photolabeled samples were run with the additional dynamic AziSEVO (+230.0559 m/z) or AziISO (+195.97143 m/z) modifications. A mass variation tolerance of 10 ppm for mass spectrometry and 0.8 Da for tandem mass spectrometry were used. Both the in-solution and in-gel sequential trypsin/chymotrypsin digests were searched without enzyme specification, with a false discovery rate of 0.01%. Sample experiments were conducted in triplicate, and samples that contained no photoaffinity ligand were treated in a manner similar to control for false-positive detection of photoaffinity ligand modifications.
Docking
A homology model of the α1β3γ2 GABAA receptor was built by mutating 31 residues in the β-subunits from a α1β1γ2 GABAA model 3 reported in Hénin et al. (28) and on the basis of the Gloeobacter ligand-gated ion channel GluCl with ivermectin-bound structure (PDB ID: 3RHW). Mutations were made by using the Mutator plugin of Visual Molecular Dynamics (29). Ions and crystallographic ligands were removed by using PyMOL (30). We used AutodockTools4 (31) to add hydrogens and Kollman charges, merge nonpolar hydrogen, and define flexible residues. Molecular coordinates for ISO and SEVO were downloaded from the ZINC small-molecule library (32) using provided physical representations. Molecular coordinates for AziISO and AziSEVO were generated by using MarvinSketch (v.16.3.28.0; ChemAxon, Budapest, Hungary) and AutodockTools4 to generate Geisteiger charges and merge nonpolar hydrogens. Maximum torsions were allowed for all investigated ligands—that is, ligands were fully flexible. We performed separate docking simulations using AutoDock Vina (33), as previously reported (26, 34), of the entire accessible photolabeled cavities formed by subunit dimers. Grid box dimensions were set at 1 Å resolution for ISO and AziISO sites α+/γ−/γ-intrasubunit (22 × 24 × 22 Å), α+/β− (22 × 22 × 22 Å), and β+/α− (22 × 24 × 42 Å) sites. Grid box dimensions were sited at 1 Å resolution for SEVO and AziSEVO sites α+/γ− (22 × 20 × 20 Å), α+/β− (20 × 18 × 22 Å), β+/α− (22 × 24 × 42 Å), and γ+/β− (18 × 18 × 22 Å) sites. Images and distance measurements were prepared by using PyMOL (30).
Statistics
We used Prism 7.0 (GraphPad Software, La Jolla, CA, USA), unless otherwise noted, for preparation and statistical data analysis. Details of statistical analyses are provided in Materials and Methods and figure legends.
RESULTS
α1β3γ2L GABAA receptor electrophysiology with AziISO and AziSEVO
AziISO and AziSEVO were functionally active on α1β3γ2L receptors that were heterologously expressed in X. laevis oocytes. Both photolabel analogs demonstrated positive modulation that was similar to their respective parent drugs, ISO and SEVO. In our system, the ED50 (35) (300 μM) of the volatile anesthetics or photolabel analogs resulted in a 4- to 4.5-fold potentiation of the current induced by an EC10 concentration (3–5 μM) of GABA (Fig. 1C, D). ISO, SEVO, AziISO, and AziSEVO also demonstrated minimal direct activation (Fig. 1C). The similar functional activity that was displayed by photolabels and their parent drugs is most consistent with shared α1β3γ2L receptor binding sites and molecular mechanisms.
Photoaffinity labeling and mass spectrometry analysis of α1β3 and α1β3γ2L GABAA receptors
We used purified α1β3 and α1β3γ2L receptors from our previously validated, tetracycline-inducible HEK293 cell line expression systems (23, 24). For expression and purification, tags were added to the N or C termini of subunits—the addition of these tags do not significantly alter the activity or response of the channel to positive modulators compared with wild-type α1β3γ2 receptors (23, 24). Supplemental Tables 1 and 2 shows photolabeled residues aligned between the tagged and nontagged receptor subunits. Affinity-purified α1β3 and α1β3γ2L receptors were suspended in a mixture of phospholipid and detergent. AziISO or AziSEVO was added to a final concentration of 30 μM, and samples were irradiated at 300 nm using a 295-nm low-pass filter for 25 min.
To determine volatile anesthetic specific sites—that is, displaceable binding sites—within the α1β3γ2L receptor, separate samples that contained the same amount of receptor and photolabel were irradiated in the presence of the parent drug. A high concentration (≥100-fold) of parent drug relative to photolabel analog is generally required to inhibit photoincorporation of saturable sites (36). This is a result of the nonequilibrium nature of photoaffinity labeling with the irreversible (photolabel analog) vs. reversible (parent drug) binding events (37, 38). Indeed, a 100-fold excess concentration of the high-affinity intravenous anesthetic, Propofol, was required to observe displaceable binding sites within the α1β3 receptor (34). We therefore introduced 3 mM ISO or SEVO—100-fold excess to AziISO and AziSEVO—to determine binding specificity.
Photoaffinity labeling experiments were performed in 6 replicates with one half of replicates subjected to in-solution and the other subjected to in-gel sequential trypsin/chymotrypsin digestion. The application of replicates and both forms of digestion were used to enhance mass spectrometry coverage of the GABAA receptor subunit sequences. Liquid chromatography–tandem mass spectrometry (LC-MS/MS) with nanoelectrospray ionization was used to analyze all peptides that were generated by the digestion of photolabeled receptors. In our MS/MS analysis, the most intense peptide ions by m/z were selected and randomly fragmented along the peptide backbone. This resulted in smaller ion fragments that contained the N terminus of the peptide (a, b, and c ions) or the C terminus (x, y, and z ions). By convention, the subscript numbers that follow the ion fragment letters denote the remaining residues that are present within the fragment.
LC-MS/MS analysis of photolabeled α1β3 receptors resulted in high coverage of the α1- and β3-subunit sequences. In both AziISO and AziSEVO photolabeling experiments, the α1-subunit displayed the highest coverage—92.24 and 91.16%, respectively—with near complete coverage of the transmembrane domain helices with both photolabels (Fig. 2). The β3-subunit displayed less, but still considerable, coverage after AziISO (89.43%) and AziSEVO (80.76%) photolabeling experiments. In both photolabeling experiments, the M4 helix had the lowest coverage (30–55%), whereas M1–M3 helices displayed >78% coverage (Fig. 2). The significant coverage of the α1- and β3-subunits was similarly reflected in the LC-MS/MS analysis of photolabeled α1β3γ2L GABAA receptors. The α1-subunit displayed 95.04 and 95.04% coverage after AziISO and AziSEVO experiments, respectively (Figs. 3 and 4). There was slightly lower coverage of the β3-subunits with AziISO (89.01%) and AziSEVO (84.57%), again with the lowest coverage being that of the M4 helix (58%; Figs. 3 and 4). Perhaps because there is only 1 copy per pentamer, the γ2L-subunit had the lowest sequence coverage of the 3 subunits after AziISO (85.66%) and AziSEVO (78.59%) with and without ISO (77.58%) or SEVO (81.62%; Figs. 3 and 4). Of the transmembrane domain, M3 and M4 helices displayed the least coverage, with the M3 helix showing 50–80% coverage and the M4 helix 34–65% coverage. No correlation was observed in our experiments between GABAA receptor subunit coverage, exposure to UV light, or the addition of ISO or SEVO.
Figure 2.
Coverage map for FLAG-α1β3GABAA receptor mass spectrometry analysis for photolabeled samples with ISO (A) and SEVO (B). Sequence of the purified FLAG-α1β3 GABAA receptor with high-confidence coverage in the mass spectrometry analysis denoted as bold residue codes. Transmembrane domain helices are underlined.
Figure 3.
Coverage map for FLAG-α1β3γ2L-L3-1D4 GABAA receptor mass spectrometry analysis for AziISO photolabeled samples without (A) and with (B) ISO. Sequence of the purified FLAG-α1β3γ2L-L3-1D4 GABAA receptor with high-confidence coverage in the mass spectrometry analysis denoted as bold residue codes. Transmembrane domain helices are underlined.
Figure 4.
Coverage map for FLAG-α1β3γ2L-L3-1D4 GABAA receptor mass spectrometry analysis for AziSEVO photolabeled samples without (A) and with (B) SEVO. Sequence of the purified FLAG-α1β3γ2L-L3-1D4 GABAA receptor with high-confidence coverage in the mass spectrometry analysis denoted as bold residue codes. Transmembrane domain helices are underlined.
Residues photolabeled by AziISO in α1β3 and α1β3γ2L GABAA receptors
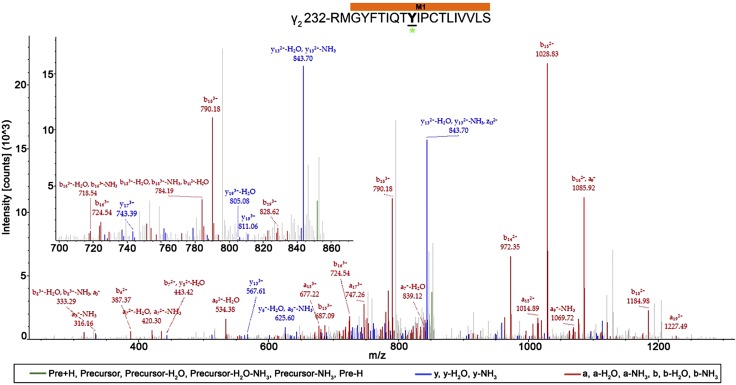
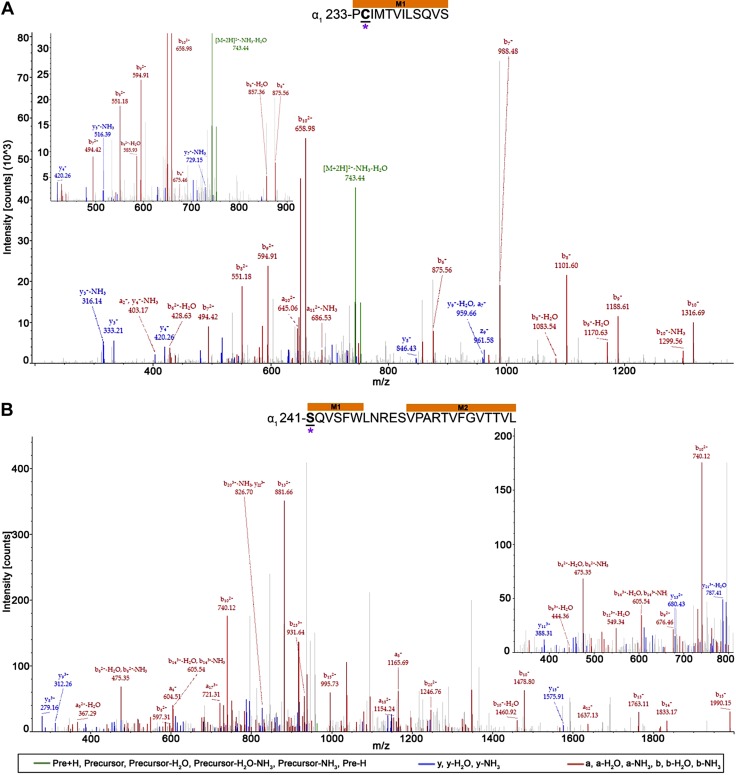
For the remainder of the work, we use the prime numbering approach for the notation of modified residues that are located within transmembrane helices, where the M1 prime system counts from the conserved pre-M1 arginine (M1 0′), thus placing the conserved M1 proline at M1 12′. Residues with AziISO adducts and their location within the α1β3 and α1β3γ2L receptors are shown in Table 1, and all spectra are provided in Supplemental Figs. 1–15. A total of 10 residues were photolabeled by AziISO within the α1β3 receptor. Eight residues had AziISO adducts in α1β3γ2L receptor, including one in the γ2L-subunit M1 helix. The 20-aa sequence of the peptide (Fig. 5) and the amino acid modified with AziISO were deduced from this spectrum. The AziISO mass shift in a10 (m/z 723.83), b10 (m/z 729.48; −NH3), and y11 (m/z 736.98) indicated that the modification was present within these fragments. The lack of AziISO-associated shift in b9 (m/z 1113.75) further narrowed the modification to γ2-M1 10′ Y241.
TABLE 1.
Residues photolabeled by AziISO within α1β3 and α1β3γ2L GABAA receptors within the ECD and/or TMD as well as associated TMD helices M1–M4
| Receptor/subunit | ECD | TMD |
|||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | ||
| α1β3 | |||||
| α | N103 | I239, T230a, L240 | I271, V252, S272 | — | — |
| β | A45 | — | T266 | V290 | — |
| α1β3γ2L | |||||
| α | E250 | P278a, S276a | — | — | |
| β | — | I222, Q224, Y226 | I255, I264 | — | — |
| γ | — | Y241 | — | — | |
ECD, extracellular domain; TMD, transmembrane domain. aResidues near or linking TMD helices.
Figure 5.
AziISO photolabeled residue within the γ2L-subunit M1 helix within the α1β3γ2L GABAA receptor. Mass spectrum of γ2L-M1′ Y241 AziISO photolabeled peptide. Focused view of the spectrum within ∼700–900 m/z range, with b (red), y (blue), and precursor ions (green) shown (inset). Above the spectrum is the subunit peptide sequence that contains the γ2L-subunit M1 transmembrane helix. The predicted photolabeled residue is shown in bold, underline, and is indicated by a green asterisk. See the Supplemental Material for associated peptide fragment table.
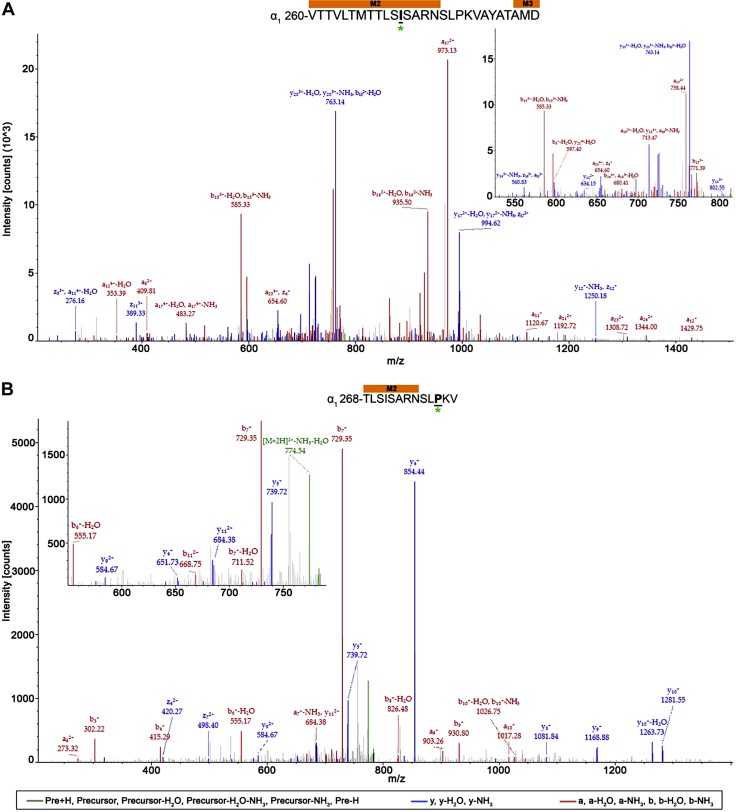
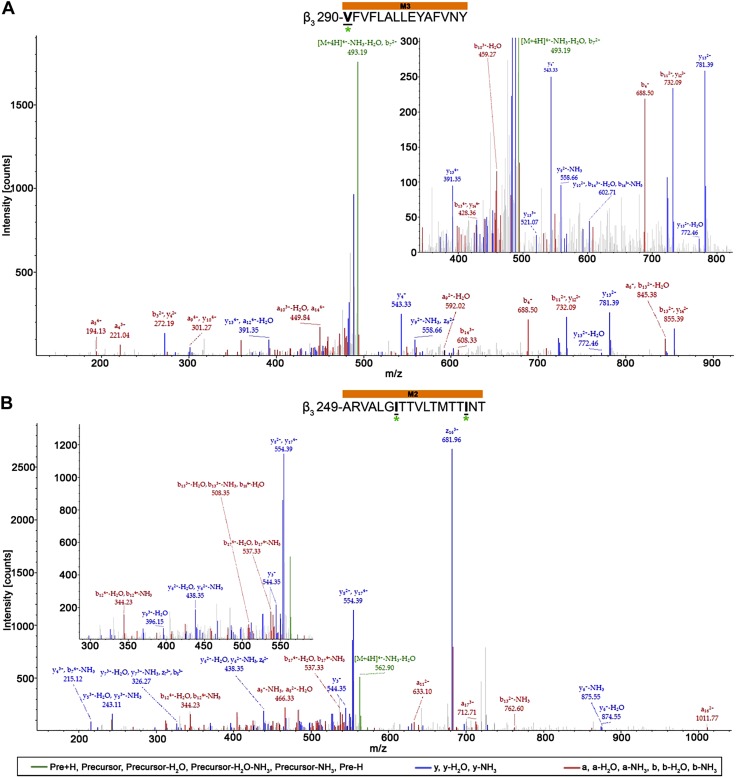
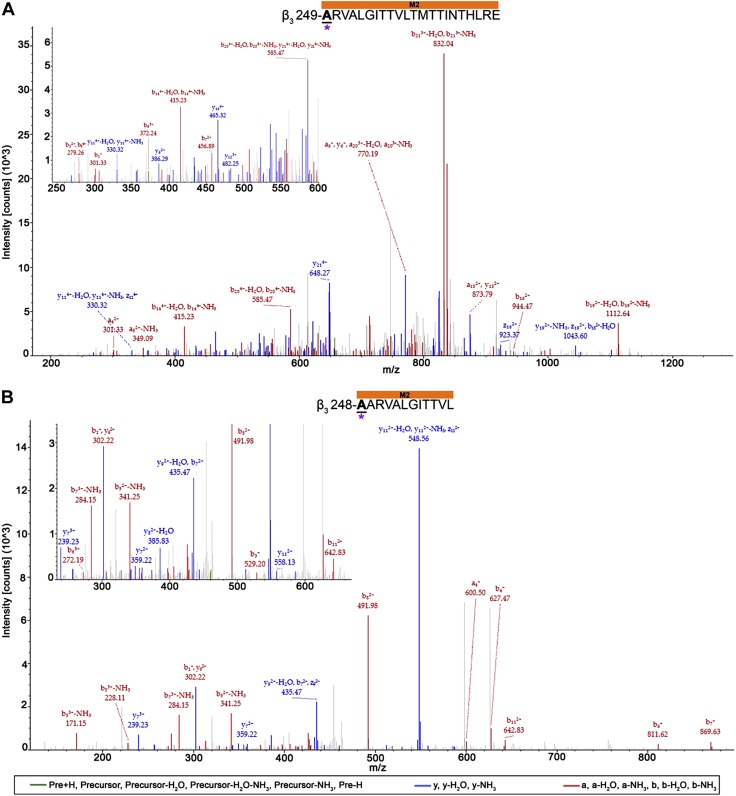
The majority of photolabeled residues within the α1- and β3-subunits in both receptors were located within the receptors’ transmembrane domain, with exception of 2 residues (α1-N103 and β3-A45) within the α1β3 receptor. Whereas no direct overlap of photolabeled residues was observed for the α1β3 and α1β3γ2L receptor, similar regions within some helices were photolabeled. These include the end of the α1-subunit M2 helix within the α1β3 receptor (Fig. 6A) and the linker between the α1-subunit M2 and M3 transmembrane domain helices within the α1β3γ2L receptor (Fig. 6B). The 28- and 13-aa long α1-subunit peptides for α1β3 and α1β3γ2L receptors, respectively, demonstrate the associated AziISO mass shift. The longer α1β3 receptor peptide displayed a12 (m/z 353.39; −H2O) and y17 (m/z 994.62; −NH3) ion fragments with the AziISO modification, whereas the neighboring a11 (m/z 276.16; −H2O) fragment did not, which indicates that the α1-M2 16′ I271 is the adducted residue (the prime numbering system used in M2 places the conserved leucine at M2 9′). The mass gain in the b11 (m/z 668.75) and a12 (m/z 711.52; −NH3) ion fragments, as well as the gain in the following y3–y12 ion fragments that were detected in the α1β3γ2L receptor α1-subunit peptide provide evidence for the α1-M2 23′ P278 modification (in the M2–M3 loop). In both α1β3 and α1β3γ2L receptors, the middle of the β3-subunit M3 and M2 helices displayed AziISO modifications (Fig. 7). Within the α1β3 receptor, a 15-aa long peptide displayed the associated AziISO mass shifts; however, the ion fragment that directly assigned β3-M3′ V290 as containing the modification was not detected (the prime numbering system used in M3 is the based on the structures of glutamate-gated chloride channel and the β3-GABAA receptor homopentamer (39) that indicate that the conserved alanine is at the start of the M3 helix, thus, β3-M3 0′ A280). The following b2 (m/z 442.36) fragment displayed the gain in m/z, whereas y14 (m/z 846.61; −H2O) did not, which, together, strongly indicates that β3-M3 10′ V290 contains the AziISO modification. Two isoleucine residues within the α1β3γ2L receptor β3-subunit peptide were modified with AziISO. In both instances, modifications were detected in ion fragments that were unique to each AziISO modification, including a7 (m/z 424.56) and neighboring a8 (m/z 466.33; −H2O) for β3-M2 5′ I255 and b16 (m/z 513.30) and y3 (m/z 544.35) for β3-M2 14′ I264.
Figure 6.
AziISO photolabeled residues within the α1-subunits of α1β3 and α1β3γ2L GABAA receptors. Mass spectra of α1-subunit AziISO photolabeled residues α1-M2′ I271 (A) and α1-M2′ P278 (B) within α1β3 and α1β3γ2L GABAA receptors, respectively. Above the spectra are the subunit peptide sequences that contain the α1-subunit transmembrane helices. Focused view of the spectrum within ∼525–825 (A) and 550–800 m/z (B), with a and/or b (red), y and/or z (blue), and precursor ions (green) shown (inset). Predicted photolabeled residue is shown in bold, underline, and is indicated by a green asterisk. See the Supplemental Material for associated peptide fragment tables.
Figure 7.
AziISO photolabeled residues within the β3-subunits of α1β3 and α1β3γ2L GABAA receptors. Mass spectra of β3-subunit AziISO photolabeled residues β3-M3′ V290 (A) and β3-M2′ I255/β3-M2′ I264 (B) within α1β3 and α1β3γ2L GABAA receptors, respectively. Focused view the spectrum within ∼350–825 (A) and 280–590 m/z (B), with b (red), y (blue), and precursor ions (green) shown. Predicted photolabeled residues is shown in bold, underlined, and is indicated by a green asterisk. Above the spectra are the subunit peptide sequences that contain the β3-subunit transmembrane helices. Predicted photolabeled residue is shown in bold, underlined, and is indicated by a green asterisk. See the Supplemental Material for associated peptide fragment tables.
Residues photolabeled by AziSEVO in α1β3 and α1β3γ2L GABAA receptors
A total of 9 residues were photolabeled by AziSEVO in the α1β3 receptor, and 8 residues were photolabeled in the α1β3γ2L receptor. All spectra are provided in Supplemental Figs. 16–31. Photolabeled residues and their locations within either receptor are displayed in Table 2. Three residues within the α1β3 receptor, 1 in the α1-subunit (α1-G104), and 2 in the β3-subunit (β3-E179 and β3-P185) were located in the extracellular domain. No extracellular domain residues were photolabeled by AziSEVO within the α1β3γ2L receptor. There were no identical residues that were photolabeled by AziSEVO between the 2 ion channels; however, there was overlap (clustering) in the photolabeled regions within the transmembrane domain helices. Photolabeled residues were identified toward the middle of the α1-subunit M1 helices (Fig. 5A, B) within the α1β3 and α1β3γ2L receptors (Fig. 8). A 12-aa residue long peptide within the α1β3 receptor α1-subunit included the mass shift as a result of an AziSEVO modification. Gain in mass was observed within the a2 (m/z 403.17) and downstream b4 (m/z 675.06) ion fragments, whereas the majority of y ions detected (y3–y10) did not display the mass increase, which suggests that α1-M1′ C234 was the modified residue. Evidence for photolabeling of α1-M1′ S241 was also detected with a 24-aa-long peptide that contained the AziSEVO mass shift. The ion fragment that directly associated with the modified site, b1, was not detected; however, a low-intensity y23 (m/z 870.56) ion fragment was detected, which supports the serine assignment. Comparatively higher-intensity a4 (m/z 604.51) and y21 (m/z 787.41; −H2O) ion fragments better support the AziSEVO modification at the beginning of the peptide sequence and within the α1-subunit M1 helix.
TABLE 2.
Residues photolabeled by AziSEVO within α1β3 and α1β3γ2L GABAA receptors within the ECD and/or TMD as well as associated TMD helices M1–M4
| Receptor/subunit | ECD | TMD |
|||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | ||
| α1β3 | |||||
| α | G104 | C234 | P253, V257 | — | — |
| β | E179, P184 | W241 | T255, A249 | — | L417a |
| α1β3γ2L | |||||
| α | — | S241 | R255, V260, T261, T265 | — | — |
| β | — | — | A248 | — | — |
| γ | — | — | L268, G269 | — | — |
ECD, extracellular domain; TMD, transmembrane domain. aResidues near or linking TMD helices.
Figure 8.
AziSEVO photolabeled residues within the α1-subunits of α1β3 and α1β3γ2L GABAA receptors. Mass spectra of α1-subunit AziSEVO photolabeled residues α1-M1′ C234 (A) and α1-M1′ S241 (B) within α1β3 and α1β3γ2L GABAA receptors, respectively. Above the spectra are the subunit peptide sequences that contain the α1-subunit transmembrane helices. Focused view of the spectrum within ∼425–900 (A) and 325–800 m/z (B), with b (red), y (blue), and precursor ions (green) shown. Predicted photolabeled residue is shown in bold, underlined, and is indicated by a magenta asterisk. See the Supplemental Material for associated peptide fragment tables.
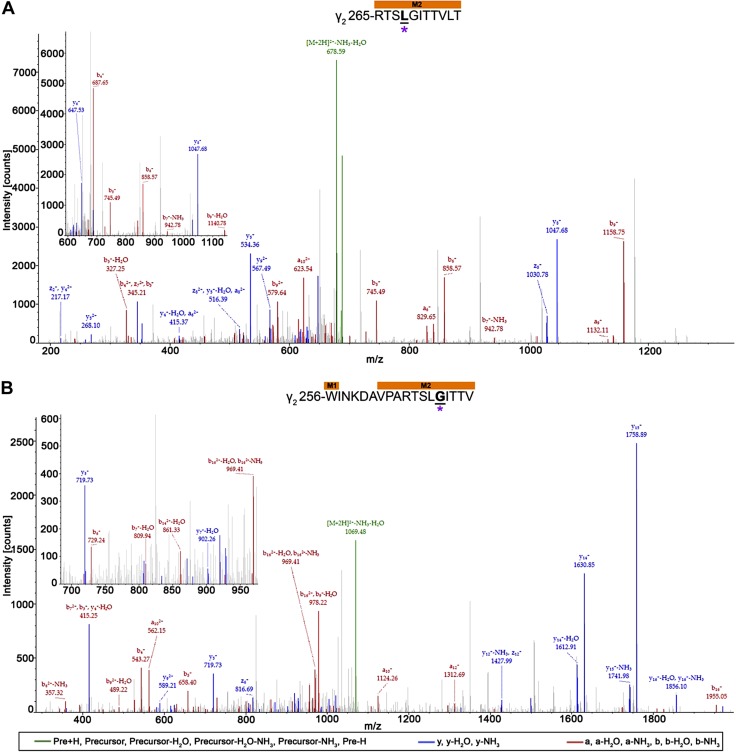
In addition, AziSEVO-photolabeled residues were identified in the beginning of the β3-subunit M2 helix of both the α1β3 and α1β3γ2L receptors (Fig. 9) on adjacent residues. Within the α1β3 receptor, β3-M2′ A249 was identified with an AziSEVO modification within a 22-aa-long peptide. The ion fragment that directly assigned the modification, b1 (m/z 301.33), was detected, as well as the corresponding y21 (m/z 585.47; −H2O) ion fragment without the modification. Similarly, within the 12-aa-long peptide of the α1β3γ2L receptor β3-subunit, the ion fragment, b1 (m/z 302.22), that contained the β3-M2 2′ A248 modified residue was detected, as well as a corresponding y11 (m/z 558.13) ion fragment. Finally, 2 neighboring residues within the γ2L-subunit of the α1β3γ2L receptor M2 helix were photolabeled by AziSEVO within 2 unique peptides (Fig. 10). An 11-aa-long peptide was detected with an AziSEVO modification that was identified within the contained leucine residue. The higher-intensity y8 (m/z 1047.68) ion fragment that contained the modification, as well as the b3 (m/z 327.25; −H2O) and b4 (m/z 687.65) fragments without and with the gain in mass, respectively, support γ2L-M2′ L268 as an adducted residue. The adjacent residue within the γ2L-subunit M2 helix, γ2L-M2 4′ G269, was also identified as adducted within an 18-aa-long peptide. Gain in mass from the AziSEVO modification was observed within both the b14 (m/z 1741.03) and y5 (m/z 719.73) ion fragments; however, the lower-intensity a13 (m/z 1425.83) fragment and no detection of the y1–y5 ion fragments lends some ambiguity to the γ2L-M2 4′ G269 assignment vs. a γ2L-M2 3′ L268 assignment.
Figure 9.
AziSEVO photolabeled residues within the β3-subunits of α1β3 and α1β3γ2L GABAA receptors. Mass spectra of β3-subunit AziSEVO photolabeled residues β3-M2′ A249 (A) and β3-M2′ A248 (B) within α1β3 and α1β3γ2L GABAA receptors, respectively. Above the spectra are the subunit peptide sequences that contain the β3-subunit transmembrane helices. Focused view of the spectrum within ∼250–600 (A) and 250–600 m/z (B), with b (red), y (blue), and precursor ions (green) shown. Predicted photolabeled residue is shown in bold, underlined, and is indicated by a magenta asterisk. See the Supplemental Material for associated peptide fragment tables.
Figure 10.
AziSEVO photolabeled residue within the γ2L-subunit M1 helix within α1β3γ2L GABAA receptor. Mass spectra of γ2L-M2′L268 (A) and γ2L-M2′G269 (B) AziSEVO photolabeled peptides. Above the spectra is the subunit peptide sequence that contains the γ2L-subunit M1 transmembrane helix. Focused view of the spectrum within ∼600–1150 (A) and 690–975 m/z (B), with b (red), y (blue), and precursor ions (green) shown. Predicted photolabeled residue is shown in bold, underlined, and is indicated by a magenta asterisk. See the Supplemental Material for associated peptide fragment table.
Inhibition of AziISO and AziSEVO photolabeling by ISO or SEVO in α1β3γ2L GABAA receptors
We used spectral counting as a semiquantitative means to determine the inhibition (or protection) of α1β3γ2L GABAA receptor photoincorporation by the parent drugs, ISO and SEVO. We accomplished this by normalizing the number of spectra counts for photolabeled peptides by the total number of spectral counts for the receptor. By using this approach, in the presence of a 100-fold concentration (3 mM) of ISO and SEVO, there was a ∼65 and 69% decrease in total AziISO and AziSEVO photoincorporation, respectively. The total number of identified photolabeled residues within the α1β3γ2L GABAA receptor for both photolabel analogs in the presence of their corresponding parent anesthetic was also reduced, despite similar sequence coverages (Figs. 3 and 4). All spectra are provided in Supplemental Figs. 32–36. The majority of residues that were photolabeled by AziISO or AziSEVO within the transmembrane helices were not photolabeled with the addition of excess parent anesthetics. Two residues within the α-subunit were photolabeled by AziISO in the presence of ISO: the extracellular domain residue α1-P175 and α1-M2 13′ T268 (see Supplemental Fig. 9). A total of 3 modifications were detected for AziSEVO in the presence of SEVO: an extracellular domain residue (β3-N197) and 2 transmembrane domain residues, α1-M4 ′R394 and γ2L-M2 17′ I282. Only the homologous α1-M2 17′ T268 that was photolabeled by AziISO in the presence of ISO displayed a degree of overlap with residues without the presence of the parent drug in the α1β3γ2L GABAA receptor.
Docking of ISO, AziISO, SEVO, and AziSEVO within α1β3γ2 GABAA receptor homology model
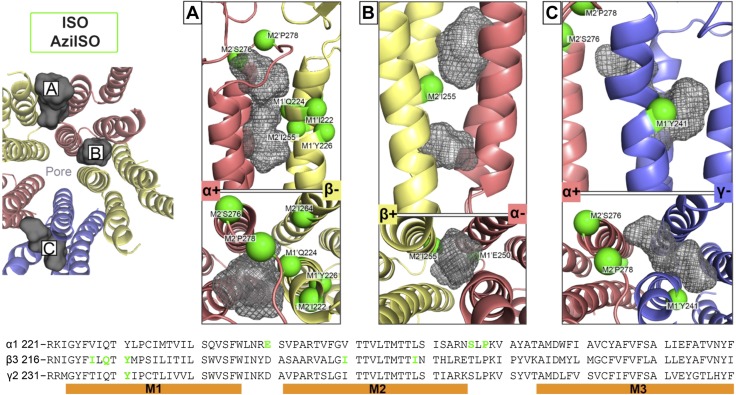
A homology α1β3γ2 GABAA receptor model that was based on the glutamate-gated chloride channel with ivermectin bound (PDB ID: 3RHW), used previously for propofol molecular dynamic simulations (28, 40), was used to map the location of photolabeled residues (Materials and Methods). Photolabeled residues indicated binding within the pore, as well as 3 and 3–4 unique cavities for AziISO (Fig. 11) and AziSEVO (Fig. 12), respectively, within the transmembrane domain of the heteropentameric receptor. We conducted docking simulations within these sites by using AutoDock Vina (33) with both the photolabel and the corresponding parent drug to determine the ability of the ligands to stably occupy binding sites. When comparing AziISO with ISO, both ligands shared similar highest-scored poses within candidate sites, including intersubunit cavities, α+/β− and β+/α−, as well as an intersubunit and/or intrasubunit cavity associated with the γ2-subunit (Fig. 11). AziSEVO and SEVO shared poses compared with one another within all 4 unique intersubunit cavities, α+/β−, β+/α−, α+/γ −, and γ+/β−, within the α1β3γ2 GABAA receptor (Fig. 12).
Figure 11.
Docking experiments within predicted ISO binding cavities as indicated by AziISO photolabeled residues in the α1β3γ2L GABAA receptor. The 3 suggested transmembrane domain binding cavities within the α+/β− (A) and β+/α− (B) interfaces and the α+/γ− intrasubunit/intersubunit cavity (C). All residues that face cavities were made flexible, whereas the backbone structure remained rigid during docking experiments. The gray Connolly surface/mesh dotted representations are of 5 ISO and 5 AziISO in the highest scored poses predicted by AutoDockVina (33). Green spheres indicate the Cα atoms of residues photolabeled by AziISO in the α1β3γ2L GABAA receptors and are labeled accordingly. The left panel includes a transmembrane domain view of the docking experiments for each unique interface from the synaptic cleft [only 1 of 2 β+/α− interfaces (B) shown]. The bottom panel includes the aligned α1-, β3-, and γ2L-subunit sequences that span the M1–M3 helices, with photolabeled residues colored in green.
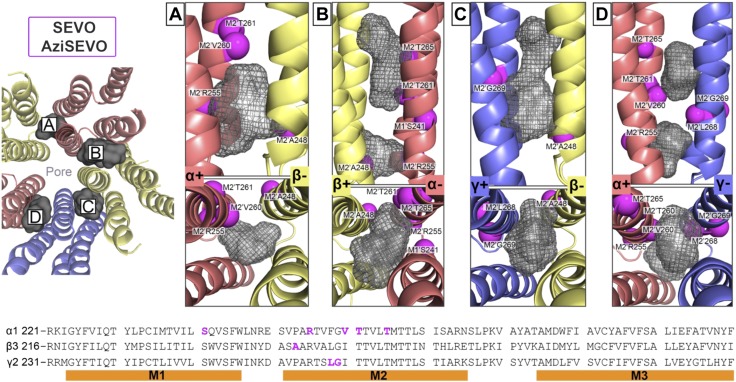
Figure 12.
Docking experiments within predicted ISO binding cavities as indicated by AziSEVO photolabeled residues in the α1β3γ2L GABAA receptor. The 4 suggested transmembrane domain binding cavities within the α+/β− (A), β+/α− (B), γ+/β− (C), and α+/γ− (D) interfaces. All residues that face cavities were made flexible, whereas the backbone structure remained rigid during docking experiments. The gray Connolly surface/mesh dotted representations are of 5 ISO and 5 AziSEVO in the highest scored poses predicted by AutoDockVina (33). Magenta spheres indicate Cα atoms of residues photolabeled by AziSEVO in the α1β3γ2L GABAA receptors and are labeled accordingly. The left panel includes a transmembrane domain view of the docking experiments for each unique interface from the synaptic cleft [only 1 of 2 β+/α− interfaces (B) shown]. The bottom panel includes the aligned α1-, β3-, and γ2L-subunit sequences that span the M1–M3 helices, with photolabeled residues colored in magenta.
DISCUSSION
In this study, we identified the binding sites for the commonly used volatile anesthetics, ISO and SEVO, within GABAA receptors. To accomplish this, we used the previously validated photolabel analogs, AziISO and AziSEVO, for the direct detection of photolabeled residues within the GABAA receptor by mass spectrometry. Our study has succeeded for the first time, to our knowledge, in directly identifying haloether binding sites within α1β3 and α1β3γ2L GABAA receptors. Previous evidence has suggested that the α1β3 receptor contributes to the tonic inhibition mediated by extrasynaptic GABAA receptors (2, 41, 42), whereas γ-subunit–containing receptor subtypes are largely associated with synaptic localization (1). As might be expected from the size differences between volatile and intravenous anesthetics (43), there were more binding sites compared with that available for the bulkier intravenous anesthetics. Although the location of some of these sites might have been expected, others are novel. Furthermore, we were surprised to find that AziISO and AziSEVO did not always occupy the same binding pocket.
The GABAA receptor is considered to be a major protein target for many general anesthetics (7, 44). A significant decrease in potency that was observed in the β3-M2′ N265M knock-in mouse has provided strong evidence for the role of the channel in propofol and etomidate hypnosis mechanisms (45). Application of photolabel analogs further identified the GABAA receptor as a protein target for these intravenous anesthetics, with the identification of at least 2 binding sites (19, 34, 46). In contrast, GABAA receptor molecular mechanisms for the volatile anesthetics, ISO and SEVO, have remained uncertain. Our experiments (Fig. 1B, C), as well as numerous previous in vitro and ex vivo studies, have demonstrated that the volatile anesthetics, ISO and SEVO, act as positive modulators of the channel at clinically relevant concentrations (8, 9). The α2-M2 15′ S270H/α2-M2 22′ L277A knock-in mouse displayed a significant decrease in potency for ISO, further implicating the receptor as a target for volatile anesthetics (10); however, mutagenesis cannot definitively identify a binding site or, like the β3-M2 15′ N265M knock-in mouse for intraveous anesthetics, all contributing binding sites and/or molecular mechanisms. In fact, despite the identification of the halothane binding sites on the homologous nicotinic acetylcholine receptor (47), direct evidence for the binding of the volatile anesthetics to the GABAA receptor has not been reported.
Two initial observations were apparent from our photolabeling results using AziISO and AziSEVO. First was the greater number of residues that were photolabeled by these photoactive analogs compared with that associated with the photoactive analogs of the intravenous anesthetics (34, 46). Of interest, some peptides contained >1 modification, likely indicating the dynamic nature of these small molecules in their binding sites, but also potentially indicating greater than single occupancy of the receptor. The large number of photolabeled residues in both the α1β3 and α1β3γ2L receptors is likely to represent clinically relevant sites as photolabeling concentrations were 10-fold lower (30 μM) than that usually associated with the ED50 (300 μM). In addition, photolabeling of the majority of the transmembrane helices was prevented by adding the corresponding parent anesthetic. Complete inhibition, or displacement, is not expected because progressive covalent incorporation will continue even with low occupancy as a result of the competitor. Previously, this degree of inhibition (50–60%) of photoincorporation with 100-fold molar excess parent anesthetic was interpreted to indicate specific, or saturable, sites (34), an interpretation that was additionally supported the results of by subsequent mutagenesis studies (7); therefore, we interpret the multiple photolabeled residues in the GABAA receptors as being indicative of both a high degree of mobility within a given binding site, as well as >1 volatile anesthetic binding site within the GABAA receptors. Second, there seemed to be selective photolabeling of the GABAA receptor transmembrane domain by AziISO and AziSEVO. Previous evidence has suggested that the majority of pharmacologically relevant binding sites for intravenous anesthetics was located in the transmembrane domain of GABAA receptors (7), and the selective photolabeling of transmembrane domain residues by AziISO and AziSEVO extends this observation to volatile anesthetics. The extracellular domain was only photolabeled within the α1β3 receptor, or in the presence of excess ISO or SEVO; therefore, the pharmacologic significance of these sites remains unclear. However, the location of these residues seems to align with the inhibitory ketamine site within the homologous bacterial Gloeobacter ligand-gated ion channel (48), which might transduce GABAA receptor inhibition at high volatile anesthetic concentrations.
Our investigation using volatile anesthetic photolabels confirmed the functional relevance of the transmembrane domain binding of volatile anesthetics, specifically the ISO binding site associated with the α+ interface as suggested by the α2-M2 15′ S270H/α2-M2 22′ L277A knock-in mouse (10). AziISO modifications located within the α1-subunit, α1-M2 21′ S276, and α1-M2 23′ P278, and the β3-subunit M1 helix, β3-M1 8′ Q224, also supports the presence of this binding site within the α1β3γ2L GABAA receptor α+/β− cavity (Fig. 6A). The photolabeled residue, α1-M2 16′ I271, identified within the α1β3 GABAA receptor further supports binding within not only the α+/γ− interface, but in the α+/β− interface. AziSEVO modifications of the α+/β− cavity were largely localized within a separate region of the α1-subunit M2 and M3 helices (Fig. 7A). AziSEVO photolabeling of the β3-subunit, β3-M1 25′ W241 and β3-M2 2′ A249, again supports this α+/β− interfacial binding cavity. By using mutagenesis and electrophysiology, other investigators have suggested that SEVO and ISO iso have different binding sites in a variety of potassium channels (22, 26, 49, 50), and our results confirm that this is also true in GABAA receptors. Although comparatively less than ISO, a decrease in SEVO-positive modulation was observed within in vitro studies using α1-M2 15′ S270W or S270I GABAA receptor mutants (8). These results suggest that the α1-M2 15′ S270 mutation has direct and well as long-range effects on volatile anesthetic binding within the α+/β− interface and/or the mutation may alter accessibility to interfacial binding sites (51).
Our study also demonstrated a shared ISO and SEVO binding site that is located within the β+/α− interfaces of the α1β3γ2L GABAA receptor (Figs. 6B and 7B). Intravenous anesthetics have also been associated with binding within this interface by using both mutagenesis and photolabeling (19, 34, 52). Indeed, a modest decrease in ISO potency for immobility was observed in β3-M2 15′ N265M GABAA receptor mutants, which suggests affinity for the β+/α− interface (11). Furthermore, ISO inhibited the photoincorporation of an etomidate photolabel analog within GABAA receptors, which is also consistent with a shared binding cavity (53). Taken together, the evidence suggests a shared, enhancing anesthetic binding site within the synaptic GABAA receptor β+/α− interface. Previously, mutation of a phenylalanine (α1-F380) located within the main α1 intracellular loop near the M4 transmembrane domain helix had been demonstrated to modulate GABAA receptor sensitivity to propofol but not ISO (54). This is consistent with our inability to detect neither an AziISO modification, nor an AziSEVO modification on this residue.
Extrasynaptic GABAA receptor subtypes, including α6β3δ and α1β3δ GABAA receptors, lack the γ-subunit and demonstrate an increased sensitivity to ISO and SEVO (55–57). Furthermore, the available evidence suggests that the more potent intravenous general anesthetics do not interact with the α+/γ− interface, although they do with the γ+/β− interface (7) Therefore, it is of interest that ISO and SEVO binding sites were associated with the γ2L subunit, potentially including both the α+/γ− interface and an intrasubunit location (Figs. 11C and 12C, D). Previously, anesthetic binding in the pentameric ligand-gated receptor intrasubunit cavities has been associated with channel inhibition; therefore, it is plausible that an ISO γ2L-subunit intrasubunit site would functionally oppose the effects resulting from the occupancy of αβ sites, thereby decreasing synaptic GABAA receptor sensitivity (58). In addition, molecular dynamics simulations have suggested that asymmetric occupancy of pentameric receptor interfaces results in greater effects on the pore radius of the ion channel compared with full, symmetric occupancy (59); therefore, the additional ISO and/or SEVO binding siteswithin the γ-subunit may provide an antagonistic element within synaptic GABAA receptors by promoting symmetric occupancy and whatever allosteric effect is transduced by intrasubunit occupancy. Additional studies that use extrasynaptic GABAA receptor subtypes will provide more information on these questions, as well as a basis for differences in GABAA receptor subtype sensitivity to volatile anesthetics.
There are recognized limitations present within the current study. Endogenous lipids are involved in the conformation and dynamics of ion channels, and previous work has indicated that the membrane microdomain might be involved in anesthetic molecular mechanisms (28, 60). GABAA receptors in our study were photolabeled within an artificial lipid/detergent micelle. Although allosteric interactions are maintained in this micelle, and the locations of the binding sites for etomidate, propofol, and barbiturates in the micelle have been confirmed in physiologic systems, it remains possible that it may not fully represent the native conformation of the receptor, especially with the smaller volatile agents studied herein. In addition, our study cannot explicitly determine the functional relevance—that is, positive, negative, or null—of individual photolabeled sites within the receptor. Nevertheless, the identification of a potential overlapping site with the intravenous anesthetics within the β+/α− interface suggests this interface acts as a positive modulation site (7, 34). We included 1 μM GABA within the photolabeling experiments to simulate potentiation, the likely clinically relevant volatile anesthetic activity; however, within the sample, various states would be present, including a desensitized state. Given that no crystal structure of heteromeric GABAA receptors is available, we were restricted to using a homology model that was based on symmetric homopentamers to interpret the location of photolabeled amino acid side chains or backbone atoms; therefore, we limited our computational studies to docking experiments to estimate candidate binding sites. It is thus possible that positions—and the associated mechanisms—might be different in actual structures of the heteropentameric receptor. Future investigations that determine the structure of the heteropentameric GABAA receptor, GABAA receptor-anesthetic binding states, and/or GABAA receptor-lipid interactions will help to individually distinguish the photolabeled sites with more confidence. Finally, despite high sequence coverage, some peptides were not detected, so we cannot exclude additional sites in some regions.
In summary, the GABAA receptor is considered to be a major target for general anesthetics; however, the binding sites for volatile anesthetics, until now, have not been clearly defined. In this study, we have directly identified through photolabeling and mass spectrometry residues that line the binding cavities for ISO and SEVO within synaptic and extrasynaptic GABAA receptors. Our study demonstrates that the 2 volatile anesthetics bind within the transmembrane domain α1- and β3-subunit interfaces as well as a γ-subunit–associated cavity within the α1β3γ2L GABAA receptor. In addition, this work indicates both shared and unique binding sites for the otherwise physiochemically similar volatile anesthetics, ISO and SEVO. Future investigations that use different GABAA receptor subtypes will provide additional insight into the basis for differential anesthetic action on GABAA receptors of differing subunit composition and regional distribution.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants P01-GM55876, GM107117, GM110174, P01–GM58448 (National Institute of General Medical Sciences), and AI118891 (Department of Defense); and by the National Science Foundation Graduate Research Fellowship Program (DGE-1321851). The authors declare no conflicts of interest.
Glossary
- AziISO
aziisoflurane
- AziSEVO
azisevoflurane
- ddH2O
double-distilled H2O
- EC
effective concentration
- EC10
effective concentration to elicit response 10% of experimental group
- GABAA
GABA type A
- ISO
isoflurane
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- SEVO
sevoflurane
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. A. Woll, N. V. Bhanu, B. A. Garcia, K. W. Miller, and R. G. Eckenhoff designed the research; K. A. Woll, X. Zhou, and N. V. Bhanu conducted the experiments; K. A. Woll and X. Zhou analyzed the data; and K. A. Woll, B. A. Garcia, M. Covarrubias, K. W. Miller, and R. G. Eckenhoff wrote the manuscript.
REFERENCES
- 1.Olsen R. W., Sieghart W. (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen R. W., Sieghart W. (2009) GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56, 141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis D. R., Duggan A. W., Felix D., Johnston G. A. (1971) Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 32, 69–96 [DOI] [PubMed] [Google Scholar]
- 4.Mihic S. J., McQuilkin S. J., Eger E. I., II, Ionescu P., Harris R. A. (1994) Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol. Pharmacol. 46, 851–857 [PubMed] [Google Scholar]
- 5.Brown C. L., Martin I. L. (1983) Photoaffinity labelling of the benzodiazepine receptor cannot be used to predict ligand efficacy. Neurosci. Lett. 35, 37–40 [DOI] [PubMed] [Google Scholar]
- 6.Brown C. L., Martin I. L. (1984) Photoaffinity labelling of the benzodiazepine receptor compromises the recognition site but not its effector mechanism. J. Neurochem. 43, 272–273 [DOI] [PubMed] [Google Scholar]
- 7.Forman S. A., Miller K. W. (2016) Mapping general anesthetic sites in heteromeric γ-aminobutyric acid type A receptors reveals a potential for targeting receptor subtypes. Anesth. Analg. 123, 1263–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishikawa K., Harrison N. L. (2003) The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology 99, 678–684 [DOI] [PubMed] [Google Scholar]
- 9.Dai S., Perouansky M., Pearce R. A. (2012) Isoflurane enhances both fast and slow synaptic inhibition in the hippocampus at amnestic concentrations. Anesthesiology 116, 816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner D. F., Swihart A., Rau V., Jia F., Borghese C. M., McCracken M. L., Iyer S., Fanselow M. S., Oh I., Sonner J. M., Eger E. I., II, Harrison N. L., Harris R. A., Homanics G. E. (2011) Inhaled anesthetic responses of recombinant receptors and knockin mice harboring α2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J. Pharmacol. Exp. Ther. 336, 134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert S., Arras M., Vogt K. E., Rudolph U. (2005) Isoflurane-induced surgical tolerance mediated only in part by beta3-containing GABA(A) receptors. Eur. J. Pharmacol. 516, 23–27 [DOI] [PubMed] [Google Scholar]
- 12.Olsen R. W. (2015) Allosteric ligands and their binding sites define γ-aminobutyric acid (GABA) type A receptor subtypes. Adv. Pharmacol. 73, 167–202 [DOI] [PubMed] [Google Scholar]
- 13.Baumann S. W., Baur R., Sigel E. (2001) Subunit arrangement of gamma-aminobutyric acid type A receptors. J. Biol. Chem. 276, 36275–36280 [DOI] [PubMed] [Google Scholar]
- 14.Baumann S. W., Baur R., Sigel E. (2002) Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 277, 46020–46025 [DOI] [PubMed] [Google Scholar]
- 15.Baur R., Minier F., Sigel E. (2006) A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett. 580, 1616–1620 [DOI] [PubMed] [Google Scholar]
- 16.Sieghart W., Sperk G. (2002) Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr. Top. Med. Chem. 2, 795–816 [DOI] [PubMed] [Google Scholar]
- 17.Sieghart W., Ramerstorfer J., Sarto-Jackson I., Varagic Z., Ernst M. (2012) A novel GABA(A) receptor pharmacology: drugs interacting with the α(+) β(-) interface. Br. J. Pharmacol. 166, 476–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tretter V., Ehya N., Fuchs K., Sieghart W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiara D. C., Dostalova Z., Jayakar S. S., Zhou X., Miller K. W., Cohen J. B. (2012) Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry 51, 836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiara D. C., Jayakar S. S., Zhou X., Zhang X., Savechenkov P. Y., Bruzik K. S., Miller K. W., Cohen J. B. (2013) Specificity of intersubunit general anesthetic binding sites in the transmembrane domain of the human α1β3γ2 GABAA receptor. J. Biol. Chem. 288, 19343–19357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woll K. A., Weiser B. P., Liang Q., Meng T., McKinstry-Wu A., Pinch B., Dailey W. P., Gao W. D., Covarrubias M., Eckenhoff R. G. (2015) Role for the propofol hydroxyl in anesthetic protein target molecular recognition. ACS Chem. Neurosci. 6, 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber A. F., Liang Q., Covarrubias M. (2012) Novel activation of voltage-gated K+ hannels by sevoflurane. J. Biol. Chem. 287, 40425–40432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dostalova Z., Liu A., Zhou X., Farmer S. L., Krenzel E. S., Arevalo E., Desai R., Feinberg-Zadek P. L., Davies P. A., Yamodo I. H., Forman S. A., Miller K. W. (2010) High-level expression and purification of Cys-loop ligand-gated ion channels in a tetracycline-inducible stable mammalian cell line: GABAA and serotonin receptors. Protein Sci. 19, 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dostalova Z., Zhou X., Liu A., Zhang X., Zhang Y., Desai R., Forman S. A., Miller K. W. (2014) Human α1β3γ2L gamma-aminobutyric acid type A receptors: high-level production and purification in a functional state. Protein Sci. 23, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Miller K. W. (2015) Dodecyl maltopyranoside enabled purification of active human GABA type A receptors for deep and direct proteomic sequencing. Mol. Cell. Proteomics 14, 724–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woll K. A., Peng W., Liang Q., Zhi L., Jacobs J. A., Maciunas L., Bhanu N., Garcia B. A., Covarrubias M., Loll P. J., Dailey W. P., Eckenhoff R. G. (2017) Photoaffinity ligand for the inhalational anesthetic sevoflurane allows mechanistic insight into potassium channel modulation. ACS Chem. Biol. 12, 1353–1362 [DOI] [PubMed] [Google Scholar]
- 27.Eckenhoff R. G., Xi J., Shimaoka M., Bhattacharji A., Covarrubias M., Dailey W. P. (2010) Azi-isoflurane, a photolabel analog of the commonly used inhaled general anesthetic isoflurane. ACS Chem. Neurosci. 1, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hénin J., Salari R., Murlidaran S., Brannigan G. (2014) A predicted binding site for cholesterol on the GABAA receptor. Biophys. J. 106, 1938–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphrey W., Dalke A., Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 30.Schrödinger (2015) The {PyMOL} molecular graphics system, version1.8, Schrödinger, New York: [Google Scholar]
- 31.Morris G. M., Huey R., Lindstrom W., Sanner M. F., Belew R. K., Goodsell D. S., Olson A. J. (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin J. J., Sterling T., Mysinger M. M., Bolstad E. S., Coleman R. G. (2012) ZINC: a free tool to discover chemistry for biology. J. Chem. Inf. Model. 52, 1757–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trott O., Olson A. J. (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 31, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayakar S. S., Zhou X., Chiara D. C., Dostalova Z., Savechenkov P. Y., Bruzik K. S., Dailey W. P., Miller K. W., Eckenhoff R. G., Cohen J. B. (2014) Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog. J. Biol. Chem. 289, 27456–27468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franks N. P., Lieb W. R. (1996) Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology 84, 716–720 [DOI] [PubMed] [Google Scholar]
- 36.Woll K. A., Dailey W. P., Brannigan G., Eckenhoff R. G. (2016) Shedding light on anesthetic mechanisms: application of photoaffinity ligands. Anesth. Analg. 123, 1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayley H. (1983) Photogenerated reagents in biochemistry and molecular biology. In Laboratory Techniques in Biochemistry and Molecular Biology, Vol. 12, Elsevier, Amsterdam [Google Scholar]
- 38.Weiser B. P., Woll K. A., Dailey W. P., Eckenhoff R. G. (2014) Mechanisms revealed through general anesthetic photolabeling. Curr. Anesthesiol. Rep. 4, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbs R. E., Gouaux E. (2011) Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woll K. A., Murlidaran S., Pinch B. J., Hénin J., Wang X., Salari R., Covarrubias M., Dailey W. P., Brannigan G., Garcia B. A., Eckenhoff R. G. (2016) A novel bifunctional alkylphenol anesthetic allows characterization of γ-aminobutyric acid, type A (GABAA), receptor subunit binding selectivity in synaptosomes. J. Biol. Chem. 291, 20473–20486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brickley S. G., Cull-Candy S. G., Farrant M. (1999) Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J. Neurosci. 19, 2960–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortensen M., Smart T. G. (2006) Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. 577, 841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lea W. A., Xi J., Jadhav A., Lu L., Austin C. P., Simeonov A., Eckenhoff R. G. (2009) A high-throughput approach for identification of novel general anesthetics. PLoS One 4, e7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen R. W., Li G. D. (2011) GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation. Can. J. Anaesth. 58, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 17, 250–252 [DOI] [PubMed] [Google Scholar]
- 46.Yip G. M., Chen Z. W., Edge C. J., Smith E. H., Dickinson R., Hohenester E., Townsend R. R., Fuchs K., Sieghart W., Evers A. S., Franks N. P. (2013) A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat. Chem. Biol. 9, 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiara D. C., Dangott L. J., Eckenhoff R. G., Cohen J. B. (2003) Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry 42, 13457–13467 [DOI] [PubMed] [Google Scholar]
- 48.Pan J., Chen Q., Willenbring D., Mowrey D., Kong X.-P., Cohen A., Divito C. B., Xu Y., Tang P. (2012) Structure of the pentameric ligand-gated ion channel GLIC bound with anesthetic ketamine. Structure 20, 1463–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lioudyno M. I., Birch A. M., Tanaka B. S., Sokolov Y., Goldin A. L., Chandy K. G., Hall J. E., Alkire M. T. (2013) Shaker-related potassium channels in the central medial nucleus of the thalamus are important molecular targets for arousal suppression by volatile general anesthetics. J. Neurosci. 33, 16310–16322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Q., Anderson W. D., Jones S. T., Souza C. S., Hosoume J. M., Treptow W., Covarrubias M. (2015) Positive allosteric modulation of Kv channels by sevoflurane: insights into the structural basis of inhaled anesthetic action. PLoS One 10, e0143363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stewart D. S., Pierce D. W., Hotta M., Stern A. T., Forman S. A. (2014) Mutations at beta N265 in γ-aminobutyric acid type A receptors alter both binding affinity and efficacy of potent anesthetics. PLoS One 9, e111470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchis-Segura C., Cline B., Jurd R., Rudolph U., Spanagel R. (2007) Etomidate and propofol-hyposensitive GABAA receptor beta3(N265M) mice show little changes in acute alcohol sensitivity but enhanced tolerance and withdrawal. Neurosci. Lett. 416, 275–278 [DOI] [PubMed] [Google Scholar]
- 53.Li G. D., Chiara D. C., Cohen J. B., Olsen R. W. (2010) Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J. Biol. Chem. 285, 8615–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moraga-Cid G., Yevenes G. E., Schmalzing G., Peoples R. W., Aguayo L. G. (2011) A single phenylalanine residue in the main intracellular loop of α1 γ-aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology 115, 464–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogawa S. K., Tanaka E., Shin M. C., Kotani N., Akaike N. (2011) Volatile anesthetic effects on isolated GABA synapses and extrasynaptic receptors. Neuropharmacology 60, 701–710 [DOI] [PubMed] [Google Scholar]
- 56.Lees G., Edwards M. D. (1998) Modulation of recombination human gamma-aminobutyric acidA receptors by isoflurane: influence of the delta subunit. Anesthesiology 88, 206–217 [DOI] [PubMed] [Google Scholar]
- 57.Yamashita M., Marszalec W., Yeh J. Z., Narahashi T. (2006) Effects of ethanol on tonic GABA currents in cerebellar granule cells and mammalian cells recombinantly expressing GABA(A) receptors. J. Pharmacol. Exp. Ther. 319, 431–438 [DOI] [PubMed] [Google Scholar]
- 58.Kinde M. N., Bu W., Chen Q., Xu Y., Eckenhoff R. G., Tang P. (2016) Common anesthetic-binding site for inhibition of pentameric ligand-gated ion channels. Anesthesiology 124, 664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mowrey D., Cheng M. H., Liu L. T., Willenbring D., Lu X., Wymore T., Xu Y., Tang P. (2013) Asymmetric ligand binding facilitates conformational transitions in pentameric ligand-gated ion channels. J. Am. Chem. Soc. 135, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arcario M. J., Mayne C. G., Tajkhorshid E. (2017) A membrane-embedded pathway delivers general anesthetics to two interacting binding sites in the Gloeobacter violaceus ion channel. J. Biol. Chem. 292, 9480–9492 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.