Abstract
While several systemic therapies are approved for cutaneous T cell lymphoma (CTCL), a non-Hodgkin lymphoma of skin-homing T cells that may involve lymph nodes and peripheral blood in advanced stages, relapses are common. Mutational analysis of CTCL cells has revealed frequent amplification of the MYC oncogene, and bromodomain and extraterminal (BET) protein inhibitors have been shown to repress MYC expression in various malignancies. Towards a potential novel therapy, we thus sought to examine the effect of BET inhibition on CTCL cells in vitro. Each of the four tested BET inhibitors (JQ1, ABBV-075, I-BET762, CPI-0610) consistently induced dose-dependent decreases in viability of isolated patient-derived CTCL cells and established CTCL cell lines (MyLa, Sez4, HH, Hut78). This effect was synergistically potentiated by combination of BET inhibition with BCL2 inhibition (e.g. venetoclax) or histone deacetylase (HDAC) inhibition (e.g. vorinostat or romidepsin). There was also a marked increase in caspase 3/7 activation when JQ1 was combined with either vorinostat or romidepsin, confirming that the observed synergies are due in major part to induction of apoptosis. Furthermore, MYC and BCL2 expression were each synergistically repressed when CTCL cells were treated with JQ1 plus HDAC inhibitors, suggesting cooperative activities at the level of epigenetic regulation. Taken together, these data indicate that targeting BET proteins in CTCL represents a promising novel therapeutic strategy that may be substantially potentiated by combination with BCL2 or HDAC inhibition.
Keywords: CTCL, apoptosis, BET inhibition, HDAC inhibition
INTRODUCTION
Cutaneous T cell lymphoma (CTCL), including the most common subtypes mycosis fungoides (MF) and Sézary syndrome (SS), represents a group of non-Hodgkin lymphomas of skin-homing, usually CD4+, malignant T cells [1, 2]. MF typically presents as cutaneous patches and plaques, but in more advanced disease, malignant T cells may disseminate to the blood, lymph nodes, and viscera [2, 3]. SS is a frank leukemic variant of CTCL that may progress from MF or develop de novo and is further characterized by erythroderma and bulky lymphadenopathy. Malignant T cells may comprise the majority of circulating T cells in patients with SS, with a median survival of 2 to 4 years [4–7]. The malignant T cells show constitutive activation and propensity for T-helper 2 cytokine production [8] that suppresses cell-mediated immunity and increases infection risk [1]. Unfortunately, CTCL remains generally incurable except in rare cases of allogeneic stem cell transplantation [9]. Overall response rates to single agent systemic therapies, including the retinoid bexarotene, and histone deacetylase (HDAC) inhibitors vorinostat and romidepsin, range between 20–45% and relapses are not uncommon [10, 11]. There is an unmet need for the treatment of advanced CTCL, and novel single or combination targeted therapies could be transformative.
Next-generation sequencing efforts have improved our understanding of the genetic alterations driving CTCL and may help shape novel approaches to therapeutic targeting of this malignancy [12–17]. CTCL is distinctive from the vast majority of other malignancies in that somatic copy number variants (SCNVs) comprise 92% of all driver mutations present within CTCL cells, and the resulting genetic derangements can be clustered into three pathways: T cell activation, cell cycle dysregulation/apoptosis, and DNA structural dysregulation affecting gene expression [12]. Within these pathways, prioritization of targeted therapies based on their specific mechanisms of action may be considered. Inhibition of the antiapoptotic protein B-cell lymphoma 2 (BCL2) was previously suggested as a targetable pathway based on common gene alterations that increase BCL2 activity and dependence, including STAT3 and STAT5B amplification, TP53 deletions and CTLA4 deletions [18–22]. We recently showed that venetoclax (ABT-199), a BCL2-selective inhibitor approved for relapsed or refractory chronic lymphocytic leukemia (CLL) with 17p deletion, efficiently induces apoptosis in patient-derived CTCL cells in vitro and this effect is synergistically potentiated by combination with HDAC inhibition [23, 24].
Mutational analysis in CTCL has also revealed 12 significant broad SCNVs [12]. The most common of these are amplifications on chromosome 8q that include the MYC oncogene in 42.5% of leukemic CTCLs [12]. MYC family genes play critical roles in cell growth and survival, and therefore the frequent amplification of MYC in CTCL lends itself to therapeutic intervention [25]. Findings showing that NF-κB is a potent transcriptional activator of the MYC promoter [26] and that the NF-κB pathway is constitutively active in CTCL [27] further suggest MYC as a viable therapeutic target. Bromodomain and extra-terminal (BET) proteins are important in initiating and enhancing transcription and, in particular, the BET-protein BRD4 regulates key genes for cell cycle progression, including MYC [25, 28, 29]. JQ1, a small-molecule BET inhibitor, prevents BRD4 binding and shows potent antiproliferative effects via downregulation of MYC gene expression in several other hematologic and non-hematologic malignancies [30–35]. JQ1 has also been shown to have antiproliferative effects on CTCL cell lines [36]. However, the effects of BET inhibition on patient-derived CTCL cells or in combination with other targeted agents have not been reported previously.
Herein, we show that BET targeting substantially decreases the viability of advanced patient-derived CTCL cells in vitro and that this effect can be synergistically potentiated by either BCL2 inhibition or HDAC inhibition. The effect is consistent across a spectrum of BET inhibitors: all four BET inhibitors tested (JQ1, ABBV-075, I-BET762, CPI-0610) demonstrate activity against CTCL cells, with ABBV-075 being the most potent. Combination of BET inhibition and HDAC inhibition, in particular, showed significant attenuation of MYC and BCL2 gene expression. Taken together, these data strongly suggest that BET inhibitors, alone and in combination with other agents, may allow for novel therapeutic strategies in the treatment of CTCL by cooperative repression of MYC and BCL2 expression.
RESULTS
BET inhibition via JQ1 reduces viability of patient-derived CTCL cells and CTCL cell lines in vitro
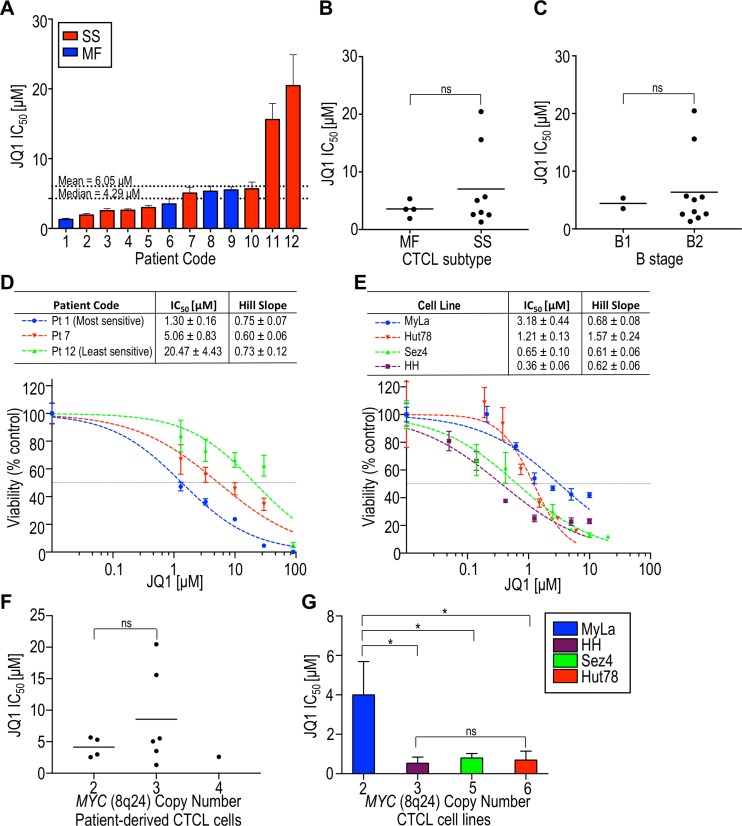
To study the effects of BET inhibition, malignant cells were purified from the peripheral blood of 12 CTCL patients (Table 1) and exposed to JQ1 in vitro. We consistently observed a dose-dependent decrease in CTCL cell viability following a 72 hr exposure (Figure 1A). Patient samples showed varying sensitivities to JQ1, with IC50s ranging from 1.30 to 20.47 µM (mean 6.05 ± 5.88 µM). Patients were categorized according to their initial diagnosis as either MF or SS, and as either B1 or B2 using the 2007 International Society for Cutaneous Lymphomas (ISCL) classification and the 2016 Gibson et al. criteria [37, 38]. While the two highest IC50s were observed with malignant cells from patients with SS, we also observed five SS patient-derived samples with IC50s less than the mean. We found no correlation of IC50 with MF vs SS or B1 vs B2 status but there was notable heterogeneity with more advanced disease, which may reflect further acquisition of mutations and chromosomal abnormalities (Figure 1B, 1C) [39].
Table 1. Summary of CTCL patient characteristics.
| Pt ID | Sex | Age (yrs) |
CTCL subtype at diagnosis | Stage at diagnosis |
MYC copy number | TCR Vβ+ | Current therapy |
Previous therapy |
CD4+/ CD8+ ratio |
% Blood Involvement |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 78 | MF | B2 | 3 | Yes | Romidepsin, vorinostat | ECP, bexarotene, IFNγ-1b | 23.97 | 25–30 |
| 2 | F | 78 (dc) | SS | B2 | Unknown | Yes | Pralatrexate | Romidepsin, CHOEP, EPOCH, GND | 60.20 | 26 |
| 3 | M | 67 | SS | B2 | 2 | Yes | ECP, nbUVB | nbUVB, topical steroids | 3.89 | 17 |
| 4 | F | 65 | SS | B2 | 4 | Yes | ECP, bexarotene, intron-A | Topical steroids | 1.27 | 39 |
| 5 | M | 73 | SS | B2 | 2 | Yes | Gemcitabine | ECP with bexarotene, romidepsin, pralatrexate | 51.55 | 65 |
| 6 | M | 65 | MF | B1 | 3 | Yes | ECP, bexarotene, intron-A, IFNγ-1b | Topical steroids, nbUVB | 3.17 | 11 |
| 7 | M | 85 | SS | B2 | 3 | Yes | Pralatrexate | PUVA, bexarotene, vorinostat, methotrexate, intron, ECP, romidepsin, doxorubicin, gemcitabine, alemtuzumab, brentuximab | 322.00 | 53 |
| 8 | F | 63 | MF | B1 | 2 | Suspicion | ECP, topical nitrogen mustard, nbUVB, bexarotene | Topical steroids, topical nitrogen mustard | 1.84 | 19–20 |
| 9 | M | 53 | MF | B2 | 3 | Yes | Pentostatin, cyclophosphamide | ECP, IFNγ-1b, bexarotene, romidepsin, pralatrexate, gemcitabine | 161.33 | 75 |
| 10 | F | 72 | SS | B2 | 2 | Yes | Vorinostat | Romidepsin, belinostat, gemcitabine | 21.65 | 31 |
| 11 | F | 62 | SS | B2 | 3 | Yes | ECP, bexarotene, intron-A | Phototherapy, oral and topical steroids | 27.16 | 44–47 |
| 12 | F | 81 | SS | B2 | 3 | Yes | None | None | 44.80 | 40 |
CTCL subtypes are subtypes at the time of diagnosis. B stage based on ISCL classification [37] and the 2016 criteria proposed by Gibson et al. [38]. TCR-Vβ+ if >50% of the population of atypical cells express a single Vβ or if there is <20% expression of the entire 27 Vβ panel. Current therapy is defined as treatment at the time of experiment. CD4+/CD8+ ratio and % blood involvement of malignant cells are based on clinical flow cytometry of patients at the time of the experiment. CHOEP, cyclophosphamide, doxorubicin, etoposide, vincristine, prednisone; dc, deceased; ECP, extracorporeal photopheresis; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; F, female; GND, gemcitabine, navelbine, doxorubicin; IFN, interferon; M, male; MF, mycosis fungoides; nbUVB, narrow band UV-B; SS, Sézary syndrome; Pt ID, patient identifier.
Figure 1. The BET inhibitor JQ1 substantially decreases the viability of patient-derived CTCL cells and CTCL cell lines.
All samples were incubated with JQ1 for 72 hours and dose-response curves were generated, from which IC50 and hill slopes were calculated. (A) IC50 of patient-derived samples in increasing order. The median and mean IC50s were 4.29 µM and 6.05 µM, respectively. Mycosis fungoides (MF) patients are in blue and Sézary syndrome (SS) patients are in red. (B) Comparison of IC50 with CTCL subtype at the time of diagnosis as either MF or SS. (C) Comparison of IC50 with CTCL B stage based on ISCL classification. (D) Representative dose-response curves for patient-derived samples. (E) Dose-response curves for cell lines. (F) Comparison of IC50 with MYC amplification status in patient-derived samples. (G) Comparison of IC50 with MYC amplification status in cell lines. ns, p > 0.05; pt, patient; *p < 0.05.
The effect of BET inhibition by JQ1 on cell viability was also studied in four established CTCL cell lines: MyLa 2059, HH, Sez4, and Hut78. Three cell lines (HH, Sez4, and Hut78), were considerably more sensitive to JQ1 than any of the patient-derived samples (Figure 1D, 1E). Since JQ1 is known to reduce MYC expression in other hematologic and solid malignancies, and gene amplification may augment gene expression, we determined MYC copy number by fluorescence in situ hybridization [40] and compared this to JQ1 sensitivity. While we found no correlation between JQ1 IC50 values and MYC amplification status in our patient-derived samples (Figure 1F), MyLa (normal MYC copy number) showed a significantly higher IC50 than each of the cell lines harboring MYC amplifications. However, a greater degree of MYC amplification (i.e. 6 vs 3 copies) did not render cells more sensitive to BET inhibition (Figure 1G). The CTCL subtype represented by each cell line may be relevant; MyLa originated from the skin of a patient with MF and HH from the blood of a patient with leukemic MF, while Sez4 and Hut78 were derived from patients with frank SS [41]. Of note, Hut78 showed a hillslope of >1, which may indicate allostery [42]. Taken together, the CTCL patient-derived and established cell-line datasets strongly suggest that MYC amplification status is not predictive of sensitivity to BET inhibition.
A spectrum of BET inhibitors consistently reduces CTCL cell viability in vitro
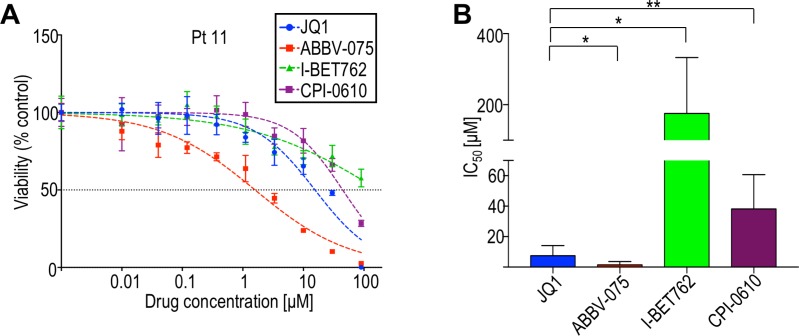
BET inhibitors with more favorable pharmacological characteristics than JQ1 are being developed for clinical use, including I-BET762, CPI-0610, and ABBV-075 (in order of discovery) [43–45]. To evaluate relative activities of these BET inhibitors, we compared their effects on cell viability using eight CTCL patient-derived samples and four CTCL cell lines. In all samples tested, patient-derived and cell lines, ABBV-075 was the most potent on a per molar basis (Figure 2; Supplementary Table 1). Two-way ANOVA of dose-response curves for ABBV-075 and JQ1 showed a statistically significant difference in each patient-derived sample. I-BET762, which has a benzotriazoloazepine core similar to JQ1 [44], was the least potent against patient-derived samples. Comparison of the potency of ABBV-075 to other BET inhibitors has not been previously described, so it is yet unclear whether the observed differences may be generalized, or are specific to CTCL. Nonetheless, the consistent sensitivity of CTCL cells to this panel of BET inhibitors further implicates the BET pathway as a viable therapeutic target.
Figure 2. BET inhibitors in clinical development (ABBV-075, I-BET762, CPI-0610) are variably effective in limiting CTCL cell viability.
(A) Representative dose-response curves of CTCL cells derived from patient 11 to different BET inhibitors. (B) Comparison of average IC50s of BET inhibitors from patient-derived samples. ABBV-075 and JQ1 were tested against eight patient-derived samples; I-BET-762 and CPI-0610 were tested against five patient-derived samples. Patient codes and individual IC50s are provided in Supplementary Table 1. *p < 0.05; **p < 0.01.
Decreased CTCL cell viability by BET inhibition is synergistically potentiated by BCL2 or HDAC inhibition
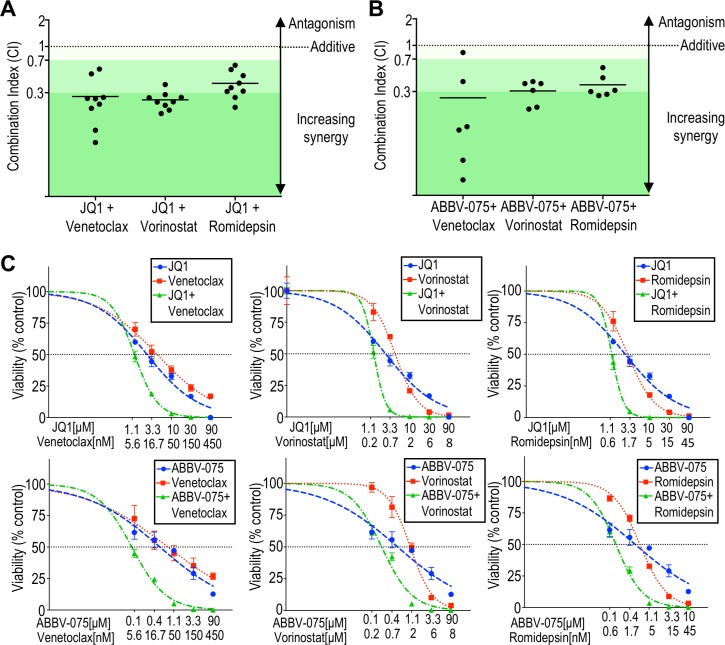
We next sought to explore the potential potentiation of BET inhibition against CTCL. Malignant cells purified from nine CTCL patients were incubated with BET inhibitor JQ1 alone or in combination with the BCL2 inhibitor venetoclax, or one of two HDAC inhibitors, vorinostat or romidepsin, to assess for synergy by the cell viability assay, and hill slopes and IC50 values calculated. We observed a marked shift in the dose response curves when JQ1 was combined with a BCL2 inhibitor or HDAC inhibitor. The degree of synergy was quantified as combination index (CI) using the Chou-Talalay method, from dose-response curves with constant ratios of agents tested (CI = 1 indicates a purely additive effect, while CI < 1 reveals synergy) [46, 47]. All (9/9) patient-derived samples demonstrated synergy when JQ1 was combined with either a BCL2 inhibitor or HDAC inhibitor (Figure 3A, 3C; Supplementary Table 2) and the degree of synergy was either moderate (CI < 0.7) or strong (CI < 0.3). This result is striking given the heterogeneity of genetic aberrations in CTCL. Even in the case of patient 9, who was previously treated with romidepsin and relapsed, there still was moderate synergy (CI = 0.31). The degree of synergy did not correlate with sensitivity to JQ1 or MYC amplification status (data not shown).
Figure 3. BCL2 inhibitors or HDAC inhibitors synergistically potentiate BET inhibition against patient-derived CTCL cells.
(A) The combination index (CI) at 10% viability was calculated for nine patient-derived samples (listed in Supplementary Table 2) exposed to JQ1 combined with venetoclax, vorinostat, or romidepsin, using the Chou-Talalay method. Strong synergy is defined as CI < 0.3, moderate synergy is CI < 0.7, and weak synergy is CI < 0.9 (adapted from Chou). (B) Calculated CI at 10% viability for six patient-derived samples exposed to ABBV-075 combined with venetoclax, vorinostat, or romidepsin. Synergy was note in all patient-derived samples. (C) Representative viability curves for BET inhibitors (JQ1 or ABBV-075) and their combinations with venetoclax, vorinostat, or romidepsin (patient 10).
We also assayed for synergy with ABBV-075, the most potent of the BET inhibitors assessed against CTCL cells in vitro. As for JQ1, synergy against CTCL was observed with ABBV-075 in combination with HDAC or BCL inhibition in the vast majority of patient-derived samples at the moderate or strong level (Figure 3B). Cell lines showed more varied results (Supplementary Table 2). While MyLa and Sez4 demonstrated synergy for all combinations, HH showed an antagonistic effect with JQ1 plus venetoclax, an additive effect with JQ1 plus vorinostat, and synergy with JQ1 plus romidepsin. Hut78 also showed an antagonistic effect with JQ1 plus venetoclax, and an additive effect when JQ1 was combined with either HDAC inhibitor. While cell lines serve as in vitro models for translational investigation and show similar gene expression patterns as advanced MF/SS patients, molecular changes inherent in cell culture may result in lines that do not reflect all aspects of freshly isolated samples [48]. It had been previously shown that JQ1 induces apoptosis in HH and Hut78, two cell lines that showed antagonism with JQ1 plus venetoclax, while JQ1 induces senescence in MyLa cells [36]. Despite that JQ1 and venetoclax each target components of the apoptosis pathway in HH and Hut78, these data suggest that for certain genetic or epigenetic alterations, this combination of agents may result in antagonism. Such antagonism was not observed in any of the CTCL patient samples tested.
Combination BET inhibition and HDAC inhibition leads to marked increases in apoptosis induction
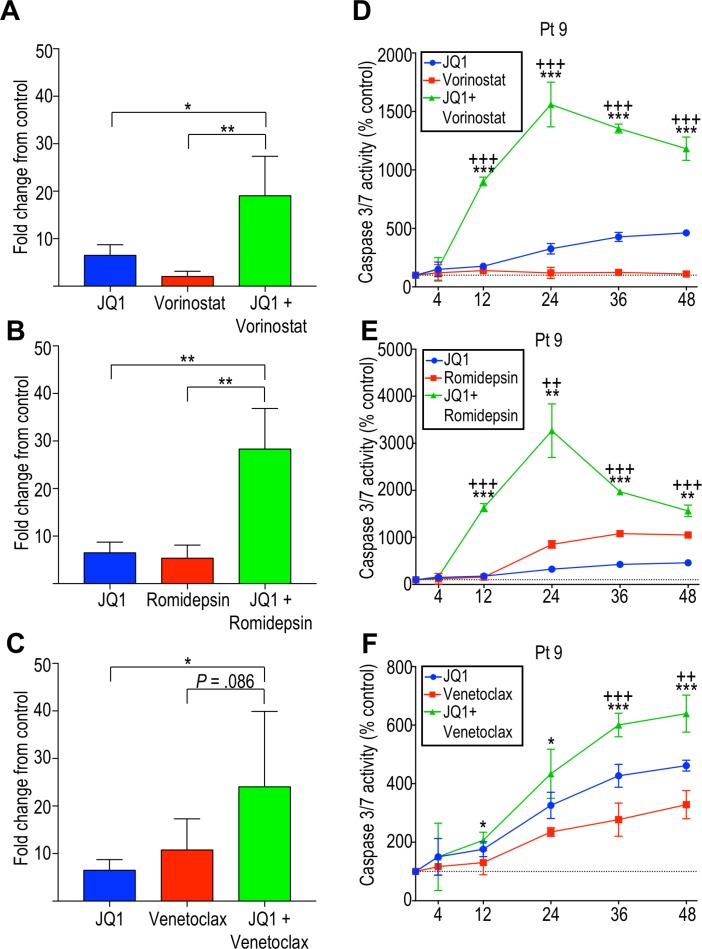
To determine whether the observed dose-dependent decrease in cell viability was due in part to apoptosis induction, caspase 3/7 activation was measured. Patient-derived cells were incubated with single agents or combinations of agents, as described above. JQ1, venetoclax, and HDAC inhibitors independently induced caspase-dependent apoptosis (Figure 4). However, the combination of JQ1 with BCL2 inhibitor or HDAC inhibitors showed a higher rate of apoptosis than the rate observed with individual agents. In particular, JQ1 in combination with either HDAC inhibitor showed a striking increase in caspase-dependent apoptosis in all patient-derived samples tested (Figure 4A, 4B, 4D, 4E). While JQ1 plus venetoclax resulted in significantly increased caspase activity in some patients (Figure 4F), there was great variability and on average, this combination only approached statistical significance (Figure 4C). BET inhibition efficiently induces apoptosis in CTCL cells in vitro, and this is potentiated by HDAC inhibition, strongly suggesting this combinatorial therapy might benefit advanced CTCL patients, including those refractory to single agent therapy.
Figure 4. Effects of BET inhibition and its potentiation by HDAC inhibition are mediated in part by induction of apoptosis by caspase 3/7.
Average caspase 3/7 activity at 24 hours, shown as fold change from the control, for four patient-derived samples (patient 4, 9, 11, and 12) incubated with (A) JQ1 and vorinostat, (B) JQ1 and romidepsin, and (C) JQ1 and venetoclax. Caspase 3/7 activity over 48 hours shown for patient 9 following incubation with (D) JQ1 and vorinostat, (E) JQ1 and romidepsin and (F) JQ1 and venetoclax. *p < 0.05; **p < 0.01; ***p < 0.001. For (D–F), +indicates p-value against JQ1 and *indicates p-value against vorinostat, romidepsin or venetoclax.
MYC and BCL2 gene expression are greatly attenuated by combination BET inhibition and HDAC inhibition
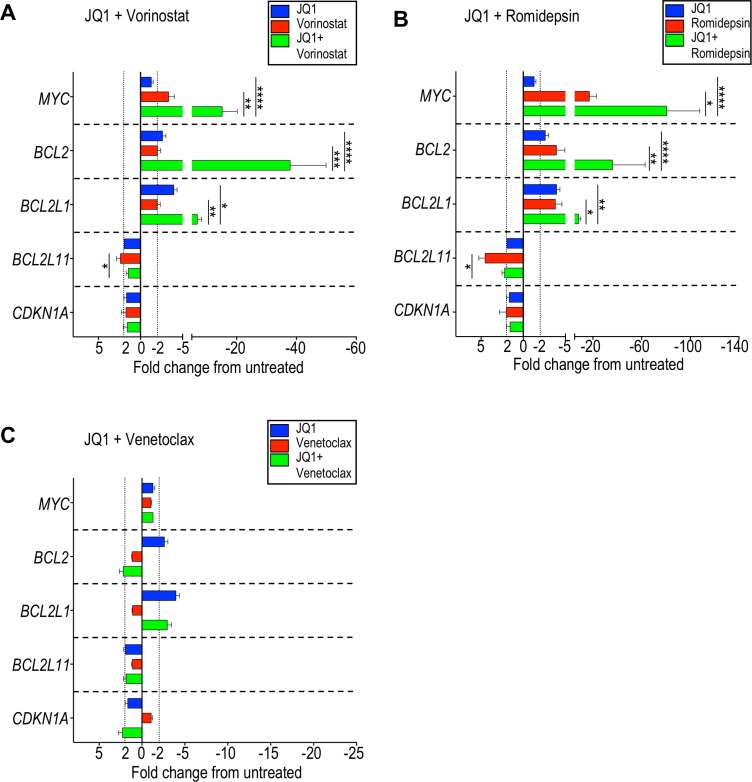
MYC, BCL2, BCL2L1, BCL2L11, and CDKN1A are genes that were previously reported to show altered expression under BET inhibition in several hematologic and solid cancer cell lines [32–34, 49–54]. In CTCL cell lines, specifically, MyLa, SeAx, Hut78, and HH, MYC expression was reported to decrease after exposure to JQ1 [36]. We were interested in examining changes in gene expression that may be important for the mechanisms of synergy we observed in patient-derived CTCL cells. Patient-derived CTCL samples were therefore incubated for 24 hours with 10 µM JQ1, 50 nM venetoclax, 2 µM vorinostat, or 5 nM romidepsin, as well as combinations of JQ1 with each of the other agents, and the relative expression of 5 genes were compared with a vehicle control. Notably, JQ1 alone did not affect MYC expression while vorinostat and romidepsin induced an average 3-fold and 17-fold decrease, respectively (Figure 5A, 5B; Supplementary Table 3). However, when JQ1 was combined with vorinostat, a 15-fold decrease in MYC expression was seen, and an 80-fold decrease was seen with JQ1 plus romidepsin, revealing synergistic repression of gene expression. This trend was also seen when ABBV-075 was combined with an HDAC inhibitor (Supplementary Table 4). This was also true for BCL2 expression and, to a lesser degree, for BCL2L1. The BCL2L11 gene encodes proapoptotic BIM, which binds to BCL2 and is suggested to play a key role in the mechanism of synergy of BET inhibition and BCL2 inhibition. While we did observe an increase in BCL2L11 expression following HDAC inhibition, we did not see significant changes following treatment with JQ1, except in two patient samples. As expected, no significant changes in expression of BCL2, BCL2L1, and BCL2L11 were observed with venetoclax (Figure 5C), consistent with its known mechanism of action of inhibiting BCL2 protein binding, thereby sequestering proapoptotic proteins BAX or BAK [55].
Figure 5. Combination of BET inhibition and HDAC inhibition markedly represses MYC and BCL2 expression in CTCL cells.
Seven patient-derived samples (listed in Supplementary Table 3) were incubated with the indicated agents or vehicle control for 24 hours and changes in gene expression evaluated by qRT-PCR. Change in gene expression as represented by fold change from untreated for (A) JQ1 and vorinostat, (B) JQ1 and romidepsin, and (C) JQ1 and venetoclax. There was a striking decrease in MYC and BCL2 expression when JQ1 was combined with either vorinostat or romidepsin. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
DISCUSSION
The presented pre-clinical data provides substantial evidence for the potential of BET inhibitors in the treatment of advanced CTCL. BET protein BRD4 regulates transcription of key genes for cell cycle progression, such as the MYC oncogene that is often amplified in CTCL, by recruiting the positive transcription elongation factor and phosphorylating the C terminal domain serine 2 on RNA polymerase II [25, 28, 29]. Anti-tumor activity and repression of MYC by BET inhibitors have been shown in various malignancies including multiple myeloma (MM), Burkitt’s lymphoma, and acute myelogenous leukemia (AML) [32, 33, 56, 57]. Decreased MYC expression occurs due to BRD4 depletion in enhancer regions that drive MYC expression [57, 58]. This effect may be intensified in MYC-amplified tumors; in MYC-amplified medulloblastoma cell lines, JQ1 had a greater effect on limiting cell proliferation [59].
Combination approaches using BET inhibitors and other targeted therapies also have been described in multiple hematologic and solid tumors, but not previously in CTCL [49–52, 60]. For example, synergy between JQ1 and the BCL2 inhibitor navitoclax (ABT-263) against MYCN-amplified SCLC has been reported [49] and preclinical studies combining BET inhibitors and HDAC inhibitors showed synergistic activity against urothelial carcinoma cell lines, melanoma cells, and murine models of pancreatic ductal adenocarcinoma [50, 51, 60]. Based on genetic alterations in CTCL that may affect BCL2, we identified BCL2 as promising target in CTCL, and revealed that the BCL2 inhibitor venetoclax exhibits marked activity against CTCL viability [24], an effect synergistically potentiated by HDAC inhibitors, vorinostat and romidepsin (both agents have been previously approved by the U.S. FDA for CTCL) [10]. In the current studies, we show that the cytotoxic effect of BET inhibition in CTCL cells is synergistically potentiated by either BCL2 inhibition or HDAC inhibition in the vast majority of both patient-derived samples and CTCL cell lines. No correlation was observed between MYC copy number and IC50 in our CTCL patient-isolated cells; MYC amplification may not necessarily translate to increased MYC expression. However, even samples derived from patients who have tried and failed multiple single therapies showed a marked decrease in MYC expression when exposed to drug combinations. One prior case-based study reported 2 patients with NUT midline carcinoma treated with OTX015, a BET inhibitor, with rapid clinical response in less than 2 weeks. However, both later experienced disease progression and biopsies revealed high MYC levels, suggesting resistance to MYC suppression [61]. Combination therapy may be a promising approach to overcome resistance in such cases.
To study more clinically applicable BET inhibitors in CTCL, we selected and assessed those currently in clinical trials for other cancers. I-BET762 is undergoing phase I/II studies for cancers including relapsed refractory AML and MM as well as ER+ breast cancer and prostate cancer (NCT01943851, NCT02964507, NCT03150056). A phase I study using CPI-0610 in diffuse large B-cell lymphoma and follicular lymphoma, found doses of 170 mg and 230 mg once daily were associated with plasma concentrations of ≥3 µM and were generally well tolerated [62]. ABBV-075 is undergoing phase I study to evaluate the safety profile in cancers including AML, prostate cancer, and SCLC (NCT02391480). While I-BET762 has a very similar core structure to JQ1 [44], it was not as potent in CTCL patient-derived cells and cell lines in this study. CPI-0610 has a 3,4-dimethylisozazole moiety added to its core, allowing for additional hydrogen bonding, but it was not more potent than JQ1 [45]. Multiple BRD4 binding sites may allow for more potency and selectivity. ABBV-075 has a more distinct pyrrolopyridone core that additionally binds the conserved Asn433 residue of BET proteins [43, 63], which may account for the higher potency seen in this study.
We found that ABBV-075 also shows synergy with BCL2- or HDAC- inhibition, as for JQ1. This further supports the hypothesis that synergy observed with BET plus BCL2- or HDAC- inhibition is due to targeting of specific pathways that are affected by BRD4. In our study, we observed synergistic effects on expression of MYC and BCL2, and to a lesser degree BCL2L1, following exposure to combined BET inhibitors and HDAC inhibitors. A prior study of gene expression in lymphoma cells indicated a ∼25% overlap of genes induced by either BET inhibitors or HDAC inhibitors and suggested that the mechanism of synergy of BET plus HDAC inhibition in MYC-overexpressing cells is partly due to induction of HDAC-silenced genes [64]. While differential effects on gene expression may be responsible for synergy, BET and HDAC inhibition may also work in concert through BRD4. BRD4 binds to target gene promoters or super-enhancers, including those of oncogenes such as MYC and BCL2 [57, 58, 65]. BET inhibitors prevent BRD4 from binding to acetyl-lysine and recruiting transcriptional machinery by occupying the binding pocket. In fact, treatment with JQ1 preferentially reduced BRD4 at super-enhancers for MYC in MM cells [57] and reduced BRD4 occupancy at promoters of MYC, BCL2, and CDK6 in AML cell lines [66] while HDAC inhibitors caused a substantial increase in global acetylation of genes, resulting in translocation and redistribution of BRD4 as it binds to newly acetylated sites [67]. In another study, while there was an overall increase in marks bound by BRD4 following treatment with HDAC inhibitors, there was a loss of proper localization of BRD4 for specific gene transcription [68]. Direct blocking of BRD4 binding by BET inhibitors, as previously described [69, 70], and translocation of BRD4 due to global acetylation by HDAC inhibitors are two independent mechanisms that converge on BRD4 and may be responsible for the marked attenuation of MYC and BCL2 transcription observed in CTCL cells.
Other genes of interest included BCL2L11, encoding proapoptotic BIM, and CDKN1A, encoding cell cycle regulator p21. BIM expression has been shown to be upregulated 2-fold in MYC-amplified SCLC following treatment with ABBV-075 [71]. Other BET inhibitors have been shown to upregulate BIM in AML cells and melanoma [51, 66]. BIM has been suggested to play a key role in the mechanism of synergy between BET inhibition and BCL2 inhibition in primary double-hit lymphoma cells, and SCLC [71, 72]. BIM binds BCL2, altering the balance between pro-apoptotic and anti-apoptotic signals. Although there was an increase in BIM expression, it was on average less than 2-fold in our patient-derived CTCL cells following culture with JQ1. Nonetheless, synergy was observed with JQ1 and venetoclax. HDAC inhibition has previously been shown to upregulate BIM [73]. In all patient samples, while HDAC inhibition led to increased BCL2L11 expression, this was diminished when combined with JQ1. This suggests that while there is an antagonistic effect on BCL2L11 expression, the net decrease in expression of MYC and BCL2 predominates and cooperates, leading to the observed synergistic effects.
In summary, BET inhibition effectively limits the viability of leukemic CTCL cells, in part via induction of apoptosis. There was a clear synergistic effect when a BET inhibitor was combined with either a BCL2 inhibitor or an HDAC inhibitor, and expression data further suggested synergy at the epigenetic level with HDAC inhibitors. Our pre-clinical data strongly suggests that therapeutic targeting of CTCL using BET inhibition, alone or in combination with BCL2 inhibition or HDAC inhibition, represents a promising strategy in the treatment of CTCL that warrants clinical testing.
MATERIALS AND METHODS
CTCL patient samples
Peripheral blood was obtained from CTCL patients at the Yale Cancer Center. All procedures were approved by the Yale Human Investigational Review Board, and informed consent was obtained. Blood was collected in lithium heparin tubes and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient. Malignant T cells were purified with a CD4+ negative selection kit (Miltenyi Biotec, Bergisch Gladbach, Germany) supplemented with antibodies to remove CD26+ and/or CD7+ cells, depending on the known malignant cell phenotype. Purity was assessed by flow cytometry using phenotypic markers of individual patient’s malignant cells (previously clinically identified), including Vβ in 10 of 12 patients, and was consistently >97%. For assays, cells were cultured in RPMI 1640 containing 10% heat-inactivated FBS (HI-FBS), 2 mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin (L-glutamine/Pen/Strep), and the following interleukins (IL): IL2 (10 ng/mL), IL7 (5 ng/mL), IL15 (10 ng/mL), and IL13 (10 ng/mL; all from R&D Systems, Minneapolis) at 37° C, 5% CO2, and 95% humidity. Clinical fluorescence in situ hybridization (FISH) testing was used to determine the patients’ MYC copy number status.
CTCL cell lines
MyLa, HH, Hut78, and Sez4 CTCL cell lines were described previously [74–78]. MyLa (Myla2059) was provided by Dr. E. Contassot (University Hospital, Zurich, Switzerland), HH and Hut 78 were purchased from American Type Culture Collection and Sez4 was provided by Dr. A. Rook (University of Pennsylvania, Philadelphia, PA). We have previously characterized genetic alterations in HH, Hut78, and Sez4 [78]. MyLa and HH were cultured in RPMI 1640 plus 10% HI-FBS and L-glutamine/Pen/Strep. Sez4 used the same medium supplemented with IL2 (20 ng/mL). Hut78 required Iscove’s Modified Dulbecco’s Medium plus 20% HI-FBS and L-glutamine/Penicillin/Strep. All cells were cultured at 37° C, 5% CO2, and 95% humidity. All media and supplements were obtained from Invitrogen (Carlsbad, CA). FISH testing to determine MYC copy number status was performed by the Molecular Cytogenetics Laboratory at Yale University School of Medicine.
Flow cytometry
Unfractionated PBMC and purified malignant T-cells were analyzed by flow cytometry using the Stratedigm-13 (Stratedigm Inc, San Jose CA). Cells were blocked with human TruStain FcX (BioLegend, San Jose, CA) for 10 minutes, incubated with monoclonal antibodies directed against CD3 (BD Biosciences, San Jose, CA), CD4, CD7, CD26 (eBioscience, San Jose, CA), TCR-Vβ (Beckman Coulter, Brea, CA) or matched isotype controls for 20 minutes at 4° C, washed three times and fixed in 1% paraformaldehyde. FlowJo Software (v10; FlowJo, LLC) was used for data analysis.
Cell viability assay
Cells were plated at 10,000/well in 96-well black optical plates and cultured for 72 hours in vehicle control (0.2% dimethyl sulfoxide (DMSO) or the following range of drug concentrations, alone or in combination: 0.01 to 90 µM BET inhibitor (JQ1, ABBV-075, I-BET762, CPI-0610), 5.6 to 450 nm venetoclax, 0.2 to 18 µM vorinostat, and 0.6 to 45 nM romidepsin. All drugs were obtained from ApexBio (Houston, TX) except ABBV-075 (Cayman Chemicals, Ann Arbor, MI). Drug concentrations were applied in approximate half-log10 increments to patient samples and two-fold increments for cell lines. Viability was measured using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, WI) as per the manufacturer’s protocol and plates were read with the Victor X Light Luminescence Counter (Perkin Elmer, Waltham, MA). Cell luminescence was normalized to vehicle control and corrected for media.
Apoptosis assay
Patient derived cells were incubated for 24 hr as described for the cell viability assay. Following incubation, the Promega Caspase-Glo 3/7 assay (Madison, WI) was used to quantitate caspase activity, as per the manufacturer’s protocol. Plates were read using the Victor X Light Luminescence Counter.
Gene expression profiling
RNA was isolated using the Qiagen RNeasy Micro (patient-derived cells) or Mini kit (cell lines), per the manufacturer’s protocol (Hilden, Germany). The High Capacity cDNA Reverse Transcription Kit and TaqMan PreAmp Master Mix (Applied Biosystems Inc., Foster City, CA) were used for cDNA synthesis and preamplification, respectively. Quantitative real time PCR (qRT-PCR) was performed (ABI 7500, SDS 2.0 software) using TaqMan Gene Expression Master Mix and TaqMan primers. Hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT1) was used as the reference gene to normalize cycle threshold (Ct) values and expression differences relative to controls calculated using RQ = 2−ΔΔCt. Statistical analysis was done using RQ values.
Calculation of IC50 values and drug synergy
For the cell viability and apoptosis assays, each drug concentration was performed in quadruplicate, and the mean values were plotted with their respective standard deviation. The mean inhibitor concentration (IC50) was determined using GraphPad Prism (version 7.01). Drug combinations were done in fixed dose ratios, which were determined based on the IC50 of the individual drugs. At least five different concentrations were performed and combination index (CI) values were calculated using the Chou-Talalay method in Microsoft Excel [46, 47].
Statistical analysis
Graphpad Prism (version 7.01) was used for all statistical calculations. P values were calculated by parametric, unpaired two-tailed t tests.
SUPPLEMENTARY MATERIALS TABLES
Acknowledgments
The authors are grateful for the nurses of the Yale Smilow Cancer Center and Yale Photopheresis Unit for providing phlebotomy support for these studies.
Abbreviations
- AML
acute myelogenous leukemia
- BCL2
B-cell lymphoma 2
- BET
bromodomain and extraterminal proteins
- CTCL
cutaneous T cell lymphoma
- HDAC
histone deacetylase
- MF
mycosis fungoides
- MM
multiple myeloma
- SCLC
small cell lung cancer
- SS
Sézary syndrome
Author contributions
S.K., J.M.L., B.M.C., and M.G. designed experiments; S.K., J.M.L., and M.G. analyzed the data, prepared figures, and wrote the manuscript. S.K., B.M.C., P.F.M., and F.N.M. performed experiments. M.G., F.M.F., and K.R.C. provided samples; S.K., J.M.L., and M.G. participated in discussion of the data; and all authors reviewed and edited the manuscript.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
FUNDING
This work was supported by the Drs Martin & Dorothy Spatz Charitable Foundation (M.G.), and the James G. Hirsch, M.D. Endowed Medical Student Research Fellowship (S.K).
REFERENCES
- 1.Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, Ubriani R, Vittorio CC, Junkins-Hopkins JM, Wysocka M, Rook AH. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- 3.Kazakov DV, Burg G, Kempf W. Clinicopathological spectrum of mycosis fungoides. J Eur Acad Dermatol Venereol. 2004;18:397–415. doi: 10.1111/j.1468-3083.2004.00937.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox RA. Cutaneous T-cell lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:837–851. doi: 10.1002/ajh.23756. [DOI] [PubMed] [Google Scholar]
- 5.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, et al. WHO-EORTC classification for cutaneous T cell lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 6.Scarisbrick JJ, Whittaker S, Evans AV, Fraser-Andrews EA, Child FJ, Dean A, Russell-Jones R. Prognostic significance of tumor burden in the blood of patients with erythrodermic primary cutaneous T-cell lymphoma. Blood. 2001;97:624–630. doi: 10.1182/blood.v97.3.624. [DOI] [PubMed] [Google Scholar]
- 7.Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM, Wilkins B, Fields PA, Dean A, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 8.Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, Schlapbach C, Schaekel K, Rook AH, Tawa M, Fisher DC, Kupper TS, Clark RA. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T cell lymphoma. Clin Cancer Res. 2013;19:3755–3763. doi: 10.1158/1078-0432.CCR-12-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atilla E, Atilla PA, Bozdag SC, Yuksel MK, Toprak SK, Topcuoglu P, Akay BN, Sanli H, Gurman G, Ozcan M. Allogeneic hematopoietic stem cell transplantation for refractory mycosis fungoides (MF) and Sezary syndrome (SS) Int J Hematol. 2017;106:426–430. doi: 10.1007/s12185-017-2245-x. [DOI] [PubMed] [Google Scholar]
- 10.Janiga J, Kentley J, Nabhan C, Abdulla F. Current systemic therapeutic options for advanced mycosis fungoides and Sézary syndrome. Leuk Lymphoma. 2018;59:562–577. doi: 10.1080/10428194.2017.1347650. [DOI] [PubMed] [Google Scholar]
- 11.Rozati S, Cheng PF, Widmer DS, Fujii K, Levesque MP, Dummer R. Romidepsin and azacitidine synergize in their epigenetic modulatory effects to induce apoptosis in CTCL. Clin Cancer Res. 2016;22:2020–2031. doi: 10.1158/1078-0432.CCR-15-1435. [DOI] [PubMed] [Google Scholar]
- 12.Choi J, Goh G, Walradt T, Hong BS, Bunick CG, Chen K, Bjornson RD, Maman Y, Wang T, Tordoff J, Carlson K, Overton JD, Liu KJ, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011–1019. doi: 10.1038/ng.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ungewickell A, Bhaduri A, Rios E, Reuter J, Lee CS, Mah A, Zehnder A, Ohgami R, Kulkarni S, Armstrong R, Weng WK, Gratzinger D, Tavallaee M, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056–1060. doi: 10.1038/ng.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva Almeida AC, Abate F, Khiabanian H, Martinez-Escala E, Guitart J, Tensen CP, Vermeer MH, Rabadan R, Ferrando A, Palomero T. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465–1470. doi: 10.1038/ng.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Ni X, Covington KR, Yang BY, Shiu J, Zhang X, Xi L, Meng Q, Langridge T, Drummond J, Donehower LA, Doddapaneni H, Muzny DM, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGirt LY, Jia P, Baerenwald DA, Duszynski RJ, Dahlman KB, Zic JA, Zwerner JP, Hucks D, Dave U, Zhao Z, Eischen CM. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508–519. doi: 10.1182/blood-2014-11-611194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litvinov IV, Netchiporouk E, Cordeiro B, Doré MA, Moreau L, Pehr K, Gilbert M, Zhou Y, Sasseville D, Kupper TS. The use of transcriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL) Clin Cancer Res. 2015;21:2820–2829. doi: 10.1158/1078-0432.CCR-14-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya S, Ray RM, Johnson LR. STAT3-mediated transcription of Bcl-2, Mcl-1, and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem J. 2005;392:335–344. doi: 10.1042/BJ20050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekulic A, Liang WS, Tembe W, Izatt T, Kruglyak S, Kiefer JA, Cuyugan L, Zismann V, Legendre C, Pittelkow MR, Gohmann JJ, De Castro FR, Trent J, et al. Personalized treatment of Sézary syndrome by targeting a novel CTLA4:CD28 fusion. Mol Genet Genomic Med. 2015;3:130–136. doi: 10.1002/mgg3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung JT, Kim DH, Kwak EK, Kim JG, Park TI, Sohn SK, Do YR, Kwon KY, Song HS, Park EH, Lee KB. Clinical role of Bcl-2, Bax, or p53 overexpression in peripheral T-cell lymphomas. Ann Hematol. 2006;85:575–581. doi: 10.1007/s00277-006-0127-z. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen M, Kaestel CG, Eriksen KW, Woetmann A, Stokkedal T, Kaltoft K, Geisler C, Röpke C, Odum N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia. 1999;13:735–738. doi: 10.1038/sj.leu.2401415. [DOI] [PubMed] [Google Scholar]
- 22.Lindahl LM, Fredholm S, Joseph C, Nielsen BS, Jønson L, Willerslev-Olsen A, Gluud M, Blümel E, Petersen DL, Sibbesen N, Hu T, Nastasi C, Krejsgaard T, et al. STAT5 induces miR-21 expression in cutaneous T cell lymphoma. Oncotarget. 2016;7:45730–45744. doi: 10.18632/oncotarget.10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 24.Cyrenne BM, Lewis JM, Weed JG, Carlson KR, Mirza FN, Foss FM, Girardi M. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T cell-lymphoma patients. Blood. 2017;130:2073–2083. doi: 10.1182/blood-2017-06-792150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doroshow DB, Eder JP, LoRusso PM. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28:1776–1787. doi: 10.1093/annonc/mdx157. [DOI] [PubMed] [Google Scholar]
- 26.La Rosa FA, Pierce JW, Sonenshein GE. Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol Cell Biol. 1994;14:1039–1044. doi: 10.1128/mcb.14.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damsky WE, Choi J. Genetics of Cutaneous T Cell Lymphoma: From Bench to Bedside. Curr Treat Options Oncol. 2016;17:33. doi: 10.1007/s11864-016-0410-8. [DOI] [PubMed] [Google Scholar]
- 28.Devaiah BN, Singer DS. Two faces of brd4: mitotic bookmark and transcriptional lynchpin. Transcription. 2013;4:13–17. doi: 10.4161/trns.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, Zhou Q. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19:535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott CJ, Kopp N, Bird L, Paranal RM, Qi J, Bowman T, Rodig SJ, Kung AL, Bradner JE, Weinstock DM. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–2852. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faivre EJ, Wilcox D, Lin X, Hessler P, Torrent M, He W, Uziel T, Albert DH, McDaniel K, Kati W, Shen Y. Exploitation of castration-resistant prostate cancer transcription factor dependencies by the novel BET inhibitor ABBV-075. Mol Cancer Res. 2017;15:35–44. doi: 10.1158/1541-7786.MCR-16-0221. [DOI] [PubMed] [Google Scholar]
- 36.Kamijo H, Sugaya M, Takahashi N, Oka T, Miyagaki T, Asano Y, Sato S. BET bromodomain inhibitor JQ1 decreases CD30 and CCR4 expression and proliferation of cutaneous T-cell lymphoma cell lines. Arch Dermatol Res. 2017;309:491–497. doi: 10.1007/s00403-017-1749-9. [DOI] [PubMed] [Google Scholar]
- 37.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S, Wood G, Dummer R, Ranki A, et al. Revisions to the staining and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 38.Gibson JF, Huang J, Liu KJ, Carlson KR, Foss F, Choi J, Edelson R, Hussong JW, Mohl R, Hill S, Girardi M. Cutaneous T-cell lymphoma (CTCL): current practices in blood assessment and the utility of T-cell receptor (TCR)-Vβ chain restriction. J Am Acad Dermatol. 2016;74:870–877. doi: 10.1016/j.jaad.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 40.Weed J, Gibson J, Lewis J, Carlson K, Foss F, Choi J, Li P, Girardi M. FISH panel for leukemic CTCl. J Invest Dermatol. 2017;137:751–753. doi: 10.1016/j.jid.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Netchiporouk E, Gantchev J, Tsang M, Thibault P, Watters AK, Hughes JM, Ghazawi FM, Woetmann A, Ødum N, Sasseville D, Litvinov IV. Analysis of CTCL cell lines reveals important differences between mycosis fungoides/Sézary syndrome vs. HTLV-1+ leukemic cell lines. Oncotarget. 2017;8:95981–95998. doi: 10.18632/oncotarget.21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prinz H. Hill coefficients, dose-response curves and allosteric mechanisms. J Chem Biol. 2010;3:37–44. doi: 10.1007/s12154-009-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel KF, Wang L, Soltwedel T, Fidanze SD, Hasvold LA, Liu D, Mantei RA, Pratt JK, Sheppard GS, Bui MH, Faivre EJ, Huang X, Li L, et al. Discovery of N-(4-(2,4-Difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl)ethanesulfonamide (ABBV-075/Mivebresib), a Potent and Orally Available Bromodomain and Extraterminal Domain (BET) Family Bromodomain Inhibitor. J Med Chem. 2017;60:8369–8384. doi: 10.1021/acs.jmedchem.7b00746. [DOI] [PubMed] [Google Scholar]
- 44.Mirguet O, Gosmini R, Toum J, Clément CA, Barnathan M, Brusq JM, Mordaunt JE, Grimes RM, Crowe M, Pineau O, Ajakane M, Daugan A, Jeffrey P, et al. Discovery of epigenetic regulator I-BET762: lead optimization to afford a clinical candidate inhibitor of BET bromodomains. J Med Chem. 2013;56:7501–7515. doi: 10.1021/jm401088k. [DOI] [PubMed] [Google Scholar]
- 45.Albrecht BK, Gehling VS, Hewitt MC, Vaswani RG, Côté A, Leblanc Y, Nasveschuk CG, Bellon S, Bergeron L, Campbell R, Cantone N, Cooper MR, Cummings RT, et al. Identification of a Benzoisoxazoloazepine Inhibitor (CPI-0610) of the Bromodomain and Extra-Terminal (BET) Family as a Candidate for Human Clinical Trials. J Med Chem. 2016;59:1330–1339. doi: 10.1021/acs.jmedchem.5b01882. [DOI] [PubMed] [Google Scholar]
- 46.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 47.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 48.Kaur G, Dufour JM. Cell lines. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Hong B, Li X, Deng K, Li H, Yan Lui VW, Lin W. JQ1 synergizes with the Bcl-2 inhibitor ABT-263 against MYCN-amplified small cell lung cancer. Oncotarget. 2017;8:86312–86324. doi: 10.18632/oncotarget.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hölscher AS, Schulz WA, Pinkerneil M, Niegisch G, Hoffmann MJ. Combined inhibition of BET proteins and class I HDACs synergistically induces apoptosis in urothelial carcinoma cell lines. Clin Epigenetics. 2018;10:1. doi: 10.1186/s13148-017-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinemann A, Cullinane C, De Paoli-Iseppi R, Wilmott JS, Gunatilake D, Madore J, Strbenac D, Yang JY, Gowrishankar K, Tiffen JC, Prinjha RK, Smithers N, McArthur GA, et al. Combining BET and HDAC inhibitors synergistically induces apoptosis of melanoma and suppresses AKT and YAP signaling. Oncotarget. 2015;6:21507–21. doi: 10.18632/oncotarget.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee DH, Qi J, Bradner JE, Said JW, Doan NB, Forscher C, Yang H, Koeffler HP. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int J Cancer. 2015;136:2055–2064. doi: 10.1002/ijc.29269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun B, Shah B, Fiskus W, Qi J, Rajapakshe K, Coarfa C, Li L, Devaraj SG, Sharma S, Zhang L, Wang ML, Saenz DT, Krieger S, et al. Synergistic activity of BET protein antagonist-based combination in mantle cell lymphoma cells sensitive or resistant ibrutinib. Blood. 2015;126:1565–1574. doi: 10.1182/blood-2015-04-639542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogg SJ, Newbold A, Vervoort SJ, Cluse LA, Martin BP, Gregory GP, Lefebure M, Vidacs E, Tothill RW, Bradner JE, Shortt J, Johnstone RW. BET inhibition induces apoptosis in aggressive B-cell lymphoma via epigenetic regulation of BCL-2 family members. Mol Cancer Ther. 2016;15:2030–2041. doi: 10.1158/1535-7163.MCT-15-0924. [DOI] [PubMed] [Google Scholar]
- 55.Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ., 3rd Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henssen A, Thor T, Odersky A, Heukamp L, El-Hindy N, Beckers A, Speleman F, Althoff K, Schäfers S, Schramm A, Sure U, Fleischhack G, Eggert A, et al. BET bromodomain protein inhibition is a therapeutic option for medulloblastoma. Oncotarget. 2013;4:2080–2095. doi: 10.18632/oncotarget.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazur PK, Herner A, Mello SS, Wirth M, Hausmann S, Sánchez-Rivera FJ, Lofgren SM, Kuschma T, Hahn SA, Vangala D, Trajkovic-Arsic M, Gupta A, Heid I, et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015;21:1163–1171. doi: 10.1038/nm.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T, Uro-Coste E, de Braud F, Pelosi G, French CA. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abramson JS, Blum KA, Flinn IW, Gutierrez M, Goy A, Maris M, Cooper M, O’Meara M, Borger D, Mertz J, Sims RJ, Jeffrey S, Younes A. BET inhibitor CPI-0610 is well tolerated and induces responses in diffuse large B-cell lymphoma and follicular lymphoma: preliminary analysis of an ongoing phase 1 study. Blood. 2015;126:1491. [Google Scholar]
- 63.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, Gingras AC, Arrowsmith CH, Knapp S. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhadury J, Nilsson LM, Muralidharan SV, Green LC, Li Z, Gesner EM, Hansen HC, Keller UB, McLure KG, Nilsson JA. BET and HDAC inhibitors induce similar genes and biological effects and synergize to skill in Myc-induced murine lymphoma. Proc Natl Acad Sci U S A. 2014;111:E2721–2730. doi: 10.1073/pnas.1406722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Da Costa D, Agathanggelou A, Perry T, Weston V, Petermann E, Zlatanou A, Oldreive C, Wei W, Stewart G, Longman J, Smith E, Kearns P, Knapp S, et al. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J. 2013;3:e126. doi: 10.1038/bcj.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiskus W, Sharma S, Qi J. Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol Cancer Ther. 2014;13:1142–1154. doi: 10.1158/1535-7163.MCT-13-0770. [DOI] [PubMed] [Google Scholar]
- 67.Greer CB, Tanaka Y, Kim YJ, Xie P, Zhang MQ, Park IH, Kim TH. Histone deacetylases positively regulate transcription through the elongation machinery. Cell Rep. 2015;13:1444–1455. doi: 10.1016/j.celrep.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acharya P, Sampathi S, Zhao Y, Wang J, Liu Q, Stengel K, Hiebert S. Histone deacetylase inhibitors mimic BET inhibitors and cause RNA polymerase pausing at MYC, KIT, and other oncogenes in t(8;21) AML. Blood. 2017;130:2470. [Google Scholar]
- 69.Stratikopoulos EE, Parsons RE. Molecular Pathways: Targeting the PI3K pathway in cancer-BET inhibitors to the rescue. Clin Cancer Res. 2016;22:2605–2010. doi: 10.1158/1078-0432.CCR-15-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mottok A, Gascoyne RD. Bromodomain inhibition in diffuse large B-cell lymphoma—giving MYC a brake. Clin Cancer Res. 2016;21:4–6. doi: 10.1158/1078-0432.CCR-14-1651. [DOI] [PubMed] [Google Scholar]
- 71.Lam LT, Lin X, Faivre EJ, Yang Z, Huang X, Wilcox DM, Bellin RJ, Jin S, Tahir SK, Mitten M, Magoc T, Bhathena A, Kati WM, et al. Vulnerability of small-cell lung cancer to apoptosis induced by the combination of BET bromodomain proteins and BCL2 inhibitor. Mol Cancer Ther. 2017;16:1511–1520. doi: 10.1158/1535-7163.MCT-16-0459. [DOI] [PubMed] [Google Scholar]
- 72.Esteve-Arenys A, Valero JG, Chamorro-Jorganes A, Gonzalez D, Rodriguez V, Dlouhy I, Salaverria I, Campo E, Colomer D, Martinez A, Rymkiewicz G, Pérez-Galán P, Lopez-Guillermo A, et al. The BET bromodomain inhibitor CPI203 overcomes resistance to ABT-199 (venetoclax) by downregulation of BFL-1/A1 in in vitro and in vivo models of MYC+/BCL2+ double hit lymphoma. Oncogene. 2018;37:1830–1844. doi: 10.1038/s41388-017-0111-1. [DOI] [PubMed] [Google Scholar]
- 73.Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaltoft K, Bisballe S, Dyrberg T, Boel E, Rasmussen PB, Thestrup-Pedersen K. Establishment of two continuous T-cell strains from a single plaque of a patient with mycosis fungoides. In Vitro Cell Dev Biol. 1992;28A:161–167. doi: 10.1007/BF02631086. [DOI] [PubMed] [Google Scholar]
- 75.Starkebaum G, Loughran TP, Jr, Waters CA, Ruscetti FW. Establishment of an IL-2 independent, human T-cell line possessing only the p70 IL-2 receptor. Int J Cancer. 1991;49:246–253. doi: 10.1002/ijc.2910490218. [DOI] [PubMed] [Google Scholar]
- 76.Gazdar AF, Carney DN, Bunn PA, Russell EK, Jaffe ES, Schechter GP, Guccion JG. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980;55:409–417. [PubMed] [Google Scholar]
- 77.Abrams JT, Lessin S, Ghosh SK, Ju W, Vonderheid EC, Nowell P, Murphy G, Elfenbein B, DeFreitas E. A clonal CD4-positive T-cell line established from the blood of a patient with Sezary syndrome. J Invest Dermatol. 1991;96:31–37. doi: 10.1111/1523-1747.ep12514693. [DOI] [PubMed] [Google Scholar]
- 78.Lin WM, Lewis JM, Filler RB, Modi BG, Carlson KR, Reddy S, Thornberg A, Saksena G, Umlauf S, Oberholzer PA, Karpova M, Getz G, Mane S, et al. Characterization of the DNA copy-number genome in the blood of cutaneous T-cell lymphoma patients. J Invest Dermatol. 2012;132:188–197. doi: 10.1038/jid.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.