Abstract
Myasthenia gravis (MG) is an autoimmune disorder caused by autoantibodies targeting proteins expressed at the neuromuscular junction (NMJ). In most cases the targets are acetylcholine receptor (AChR), muscle-specific tyrosine kinase (MuSK), or occasionally low-density lipoprotein receptor-related protein 4 (LRP4), but there is still a group of patients, often called seronegative MG (SNMG), with unknown antibody targets. One potential target is collagen Q (COLQ), which is restricted to the NMJ and is crucial for anchoring the NMJ-specific form of acetylcholinesterase (AChE). 415 serum samples with a clinical diagnosis of MG and 43 control samples were screened for the presence of COLQ autoantibodies using a cell-based assay (CBA) with HEK293 cells overexpressing COLQ at the cell surface. COLQ antibodies were detected in 12/415 MG sera and in one/43 control samples. Five of the COLQ-Ab + individuals were also positive for AChR-Abs and 2 for MuSK-Abs. Although the COLQ antibodies were only present at low frequency, and did not differ significantly from the small control cohort, further studies could address whether they modify the clinical presentation or the benefits of anti-cholinesterase therapy.
Keywords: Collagen Q (COLQ), Myasthenia gravis (MG), Neuromuscular junction (NMJ), Cell-based assay (CBA), Autoantibodies, Neuroimmunology
1. Introduction
Myasthenia gravis (MG) is a classic autoimmune disorder resulting from the presence of autoantibodies targeting neuromuscular junction (NMJ) proteins, and leading to defects in neuromuscular transmission with fatiguable muscle weakness. In most cases the antibodies recognise acetylcholine receptor (AChR) or muscle-specific tyrosine kinase (MuSK) [1], but there are still individuals, often referred to as seronegative MG (SNMG), in whom AChR and MuSK autoantibodies are not detected in present assays. Additional antibody targets include low-density lipoprotein receptor-related protein 4 (LRP4) [2–4], agrin (AGRN) [5] and acetylcholinesterase (AChE) [6]. Despite some evidence of antibodies to these proteins [2–6], there remain patients presenting with an autoimmune MG with no specific antibody identified.
Collagen Q (COLQ) is a protein crucial for anchoring and concentrating AChE at the NMJ, where its expression is restricted to the extracellular matrix and accessible to circulating antibodies. Indeed, mutations in COLQ can underlie one form of congenital myasthenic syndrome (CMS) [7,8]. Although COLQ possesses a number of features which make it a potential target for autoantibodies in MG, the presence of these have not been reported.
Traditionally autoantibodies have been detected by radioimmunoprecipitation assays (RIA) or in some cases by enzyme-linked immunosorbent assays (ELISA) or fluorescence immunoprecipitation assays (FIPA). These assays can be sensitive and highly specific, but do not necessarily detect the most pathogenic antibodies. Recently cell-based assays (CBAs) have been established in order to look for antibodies that bind to the extracellular domains of proteins that are naturally expressed on the cell surface. To apply this technique to proteins that are not membrane tethered, it is necessary to fuse them with a transmembrane protein or domain. Here we expressed COLQ fused with the transmembrane domain of contactin-associated protein-like 2 (CASPR2) and looked for antibodies in MG patients and controls.
2. Materials and methods
2.1. Ethics statement
The MG samples were archived from therapeutic plasmaphereses in the 1980s and 1990s when written consent was not required, but verbal was obtained. Ethical approval for use of pre 2006 stored patient samples without patient written consent was obtained from the Oxfordshire REC C 09/H0606/74. Samples from healthy individuals were obtained with written consent and ethical approval from the Oxfordshire REC Rf 07/Q1604/28.
2.2. Cloning of pcDNA-COLQ-CASPR2TM
Construct encoding COLQ in pcDNA™3.1/Hygro(+) (Invitrogen, V87020) was kindly provided by Dr Janet Kenyon. COLQ cDNA was engineered into pcDNA-Lgi1-CASPR2TM to replace the leucine-rich glioma inactivated 1 (Lgi1) cDNA. The C-terminus of COLQ was chosen for the fusion with the transmembrane domain (TM) of contactin-associated protein-like 2 (CASPR2TM). A construct with a Myc tag, introduced immediately downstream the N-terminal signal peptide, was also cloned to control for the cell surface expression of the protein.
2.3. HEK293 cell culture and transfection
Human embryonic kidney cells (HEK293) were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Sigma, D6429) supplemented with 10% foetal calf serum (Sigma, F2442) and antibiotics (Invitrogen, 15240-062). Transfections were performed using polyethylenamine (PEI). Transfection mixes used for one well of a 6-well plate contained 3 μg DNA, 1.25 μl 20% glucose, 1.5 μl PEI, nuclease-free water up to 6.5 μl and 1 ml growth medium.
2.4. A cell-based assay for the detection of COLQ antibodies
Cells plated on poly-L-leucine-coated coverslips were transfected with pcDNA-COLQ-CASPR2TM, pcDNA-Myc-COLQ-CASPR2TM or pcDNA™3.1(+) vectors, respectively. 2 days after the transfection, the cells were incubated for 1 hour with sera diluted 1:20 in blocking solution (DMEM, 1% BSA, 20 mM HEPES) or a mouse monoclonal anti-Myc antibody (Cell Signalling, 2276), fixed with 3% paraformaldehyde and then incubated for one hour with Alexa Fluor 568 secondary antibodies (1:500) against human or mouse IgG, respectively. The immunostaining of the cells was analysed by widefield or confocal fluorescence microscopy using an Olympus X71 Fluorescence Microscope and SimplePCI software, or a Zeiss 780 Inverted Microscope and ZEN lite software, respectively.
2.5. Serum samples
Serum samples that were tested were either archived SNMG who were negative on a standard radioimmunoprecipitation assays for detection of autoantibodies to either AChR and MuSK or from patients with a clinical diagnoses of MG sent to Oxford to be analysed by CBAs for clustered AChR or MuSK antibodies (n = 415). Control serum samples were either healthy laboratory workers (n = 22) or disease control serum samples obtained from patients with established epilepsy identified either on admission or attending specialist epilepsy clinics at a large teaching hospitals, approximately 60% of whom had focal seizures and the remaining had generalized epilepsy (n = 21).
3. Results
3.1. A cell-based assay for the detection of anti-COLQ antibodies
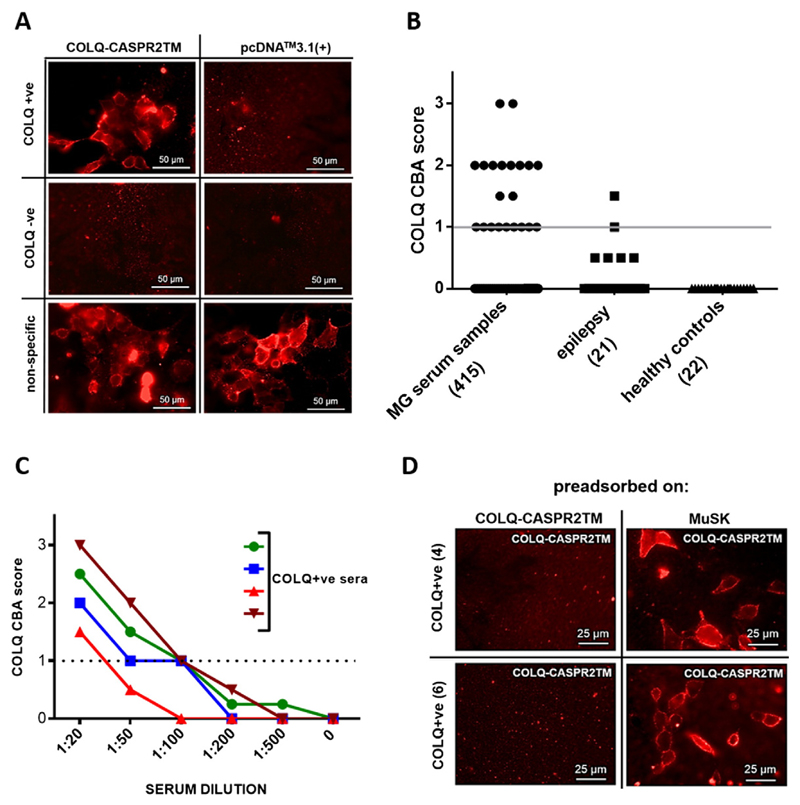
To detect anti-COLQ antibodies, HEK293 cells transfected with the construct encoding COLQ-CASPR2TM were incubated with serum samples and Alexa Fluor 568 anti-human IgG secondary antibodies. HEK293 cells transfected with pcDNA-Myc-COLQ-CASPR2TM labelled with an anti-Myc antibody were used to control for the cell surface expression of the protein. The assay was used to screen 415 MG sera for the presence of antibodies to COLQ. The results of those assays were not known at the time of testing for COLQ antibodies. Red fluorescence at the surface of COLQ-CASPR2TM expressing cells, but not mock-transfected cells, indicated the presence of COLQ autoantibodies in the samples (e.g. Fig. 1A). The scores (0–3), corresponding to the intensity of the fluorescence at the cell surface of COLQ-CASPR2TM transfected cells, are shown in Fig. 1B. 12 out of 415 samples were found to be COLQ + ve, based on the mean ± 3SDs of the scores assigned to the healthy and epilepsy control samples (2.9%). In the control group a relatively weak (score = 1.5) COLQ binding was detected in only one out of 43 individuals (0 out of 22 in the healthy control, 1 out of 21 in the epilepsy patients). In addition 5 of the 12 MG samples were also positive on CBA or for binding to AChR and two in the RIA to MuSK. For patients confirmed as SNMG by assay (negative on CBA or RIA for antibodies to the AChR, MUSK, LRP4 or AGRN) 5 out of 149 were COLQ + ve (3.4%). To control against the possibility of a non-specific binding to CASPR2-TM (though this was considered unlikely since the CASPR2-TM domain would be hidden within the membrane) the COLQ + ve samples were tested using assays in which the NMJ proteins AGRN and LRP4 were similarly expressed at the cell surface by fusion with CASPR2-TM. The COLQ + ve samples were negative in all these assays.
Fig. 1. CBA for the presence of anti-COLQ autoantibodies in patient sera.
A. HEK293 cells were transfected with pcDNA-COLQ-CASPR2TM or pcDNA™3.1(+) vectors, respectively, and stained with patient sera, and an Alexa Fluor 568 anti-human IgG secondary antibody. The panel shows example staining of COLQ + ve, COLQ-ve serum samples or the ones exhibiting unspecific cell surface binding. B. COLQ CBA scores in the cohort of serum samples. The dot plot presents the binding scores, reflecting the intensity of the fluorescence obtained with the patient and control sera. The numbers in brackets indicate a number of individuals in a particular subgroup. The grey line indicates the cut-off of the CBA (score = 1), based on mean ± 3SDs of all controls. C. Titration of COLQ + ve sera. HEK293 cells were transfected with the pcDNA-COLQ-CASPR2TM plasmid, stained with anti-COLQ sera diluted 1:20, 1:50, 1:100, 1:200, and 1:500, and binding detected with Alexa Fluor 568 anti-human IgG secondary antibody. The intensity of the fluorescence was scored (0–3). Different symbols represent serum samples from different individuals. The dotted line indicates the cut-off of the CBA (score = 1), based on mean± 3SDs of all controls. D. Preadsorption of anti-COLQ antibodies from COLQ + ve sera. HEK293 cells were transfected with COLQ-CASPR2TM-encoding plasmid and incubated with two different COLQ + ve serum samples (from patients #4 and #6), preadsorbed against either COLQ-CASPR2TM or MuSK expressing cells, respectively. Binding of the patients' antibodies to the COLQ-CASPR2TM expressing cells was not found after adsorption against COLQ-CASPR2TM-expressing cells, but adsorption against MuSK-expressing cells did not reduce binding of the patients' antibodies.
Four COLQ positive samples, with sufficient volume available, were titrated. The titres of the analysed samples were 1:100, 1:100, 1:100 and 1:20, respectively (Fig. 1C).
To confirm the specificity of the COLQ antibodies, the samples were preadsorbed against COLQ on COLQ-CASPR2TM-expressing HEK293 cells. As a control, the sera were incubated with HEK293 cells overexpressing MuSK at their surface. The binding of the serum antibodies to COLQ-HEK293 cells was abrogated by adsorption against COLQ-HEK293 cells but not against MuSK-HEK293 cells. (Fig. 1D).
3.2. Clinical presentation of COLQ antibody positive cases
All the positive patients were female, and all but two (out of the ten with known age of onset) had disease onset below 40 years (defined as early-onset MG). Three had purely ocular disease but the others had generalised (n = 7) or ocular/bulbar involvement (n = 2). The patients were treated with steroids or AChE inhibitors, and some data on the response to these therapies were available. Seven individuals responded well to steroids and in four out of nine patients, for whom the data were available, the response to AChE inhibitors was considered poor (Table 1).
Table 1.
Clinical information for the COLQ antibody + ve cases.
| Gender | Age of onset | Form of MG | EMG (decrement or jitter) | Reported response to AChE inhibitors | Response to steroids | Anti AChR or anti-MuSK antibodies | COLQ CBA score | |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 15 | Oculobulbar | Abn | Good | Yes | – | 3 |
| 2 | F | 23 | Generalised | Abn | Poor | ND | – | 3 |
| 3 | F | 23 | Ocular | Abn | Poor | ND | Clustered AChR | 2 |
| 4 | F | 33 | Generalised | Abn | Poor | ND | Clustered AChR | 2 |
| 5 | F | 31 | Ocular | Abn | Good | ND | Clustered AChR | 2 |
| 6 | F | 20 | Generalised | Abn | Good | Yes | AChR | 2 |
| 7 | F | 39 | Ocular | Abn | Good | Yes | Clustered AChR | 2 |
| 8 | F | NA | Generalised | ND | ND | Yes | MuSK RIA | 2 |
| 9 | F | NA | Oropharyngeal Respiratory | ND | ND | ND | MuSK RIA | 2 |
| 10 | F | 65 | Generalised | ND | ND | Yes | – | 2 |
| 11 | F | 6 | Generalised | Abn | Good | Yes | – | 1.5 |
| 12 | F | 45 | Generalised | Abn | Poor | Yes | – | 1.5 |
Abn – abnormal, ND – not done, NA – not available, RIA - radioimmunoprecipitation assay.
4. Discussion
A CBA was developed for the detection of COLQ binding in patient sera and detected antibodies to COLQ in ~ 3% of the sera. This was at a similar frequency as found in our control samples (1/43), though the small number makes this result difficult to assess. The antibodies were at relatively low titres (1:20–1:100) but preadsorption indicated that they were specific for COLQ.
Although their frequency in patients without AChR or MuSK antibodies is low, these antibodies could still play a role in the disease. COLQ anchors AChE in the extracellular matrix and it is possible that the COLQ antibodies disrupt the concentration of asymmetric AChE complexes [9,10], and reduce the amounts of AChE. In CMS patients with genetic defects in the gene for COLQ [7,8,11,12], there is thought to be impaired formation of AChE-COLQ complexes, or lack of attachment to the NMJ [10]. The AChE-COLQ complexes are anchored by two mechanisms. First, two heparan-binding domains of COLQ interact with the heparan sulphate proteoglycan–perlecan [13,14] in the matrix. Second, the C-terminal fragment of COLQ interacts with MuSK [15]. These interactions have been shown to be affected by COLQ mutations in CMS and by MuSK antibodies in MG, and hence the mechanism could be analogous [16–18]. The impaired COLQ function could lead to a prolonged lifetime of acetylcholine (ACh) in the synaptic cleft, an increased duration of endplate currents and an increased ion flux, resulting in an endplate myopathy and compromised neuromuscular transmission or a desensitisation of the endplate receptors [19].
In the CMS patients with mutations in COLQ, there is a loss of asymmetric AChE at the NMJ and many patients do not show any benefit from AChE inhibitors [11]. Indeed four of the patients had poor responses to these drugs, but five had responded. Nevertheless, even though these antibodies may not be directly pathogenic, they could contribute to the highly varied clinical presentations in MG patients, and/or modify the response to AChE inhibitors and warrant further investigation.
Acknowledgements
We would like to thank Muscular Dystrophy Campaign for the funding.
Funding
Supported by the Muscular Dystrophy Campaign Grant RA3/792.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–8. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- [2].Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipo-protein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69:418–22. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- [3].Pevzner A, Schoser B, Peters K, Cosma NC, Karakatsani A, Schalke B, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259:427–35. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- [4].Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69:445–51. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- [5].Cossins J, Belaya K, Zoltowska K, Koneczny I, Maxwell S, Jacobson L, et al. The search for new antigenic targets in myasthenia gravis. Ann N Y Acad Sci. 2012;1275:123–8. doi: 10.1111/j.1749-6632.2012.06833.x. [DOI] [PubMed] [Google Scholar]
- [6].Wang WW, Hao HJ, Gao F. Detection of multiple antibodies in myasthenia gravis and its clinical significance. Chin Med J (Engl) 2010;123:2555–8. [PubMed] [Google Scholar]
- [7].Donger C, Krejci E, Serradell AP, Eymard B, Bon S, Nicole S, et al. Mutation in the human acetylcholinesterase-associated collagen gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency (Type Ic) Am J Hum Genet. 1998;63:967–75. doi: 10.1086/302059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci U S A. 1998;95:9654–9. doi: 10.1073/pnas.95.16.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rotundo RL, Rossi SG, Kimbell LM, Ruiz C, Marrero E. Targeting acetylcholinesterase to the neuromuscular synapse. Chem Biol Interact. 2005;157–158:15–21. doi: 10.1016/j.cbi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [10].Ohno K, Engel AG, Brengman JM, Shen XM, Heidenreich F, Vincent A, et al. The spectrum of mutations causing end-plate acetylcholinesterase deficiency. Ann Neurol. 2000;47:162–70. [PubMed] [Google Scholar]
- [11].Mihaylova V, Muller JS, Vilchez JJ, Salih MA, Kabiraj MM, D'Amico A, et al. Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain. 2008;131:747–59. doi: 10.1093/brain/awm325. [DOI] [PubMed] [Google Scholar]
- [12].Wargon I, Richard P, Kuntzer T, Sternberg D, Nafissi S, Gaudon K, et al. Long-term follow-up of patients with congenital myasthenic syndrome caused by COLQ mutations. Neuromuscul Disord. 2012;22:318–24. doi: 10.1016/j.nmd.2011.09.002. [DOI] [PubMed] [Google Scholar]
- [13].Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuro-muscular junction involves perlecan and dystroglycan. J Cell Biol. 1999;145:911–21. doi: 10.1083/jcb.145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kimbell LM, Ohno K, Engel AG, Rotundo RL. C-terminal and heparin-binding domains of collagenic tail subunit are both essential for anchoring acetylcholinesterase at the synapse. J Biol Chem. 2004;279:10997–1005. doi: 10.1074/jbc.M305462200. [DOI] [PubMed] [Google Scholar]
- [15].Cartaud A, Strochlic L, Guerra M, Blanchard B, Lambergeon M, Krejci E, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol. 2004;165:505–15. doi: 10.1083/jcb.200307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakata T, Ito M, Azuma Y, Otsuka K, Noguchi Y, Komaki H, et al. Mutations in the C-terminal domain of ColQ in endplate acetylcholinesterase deficiency compromise ColQ–MuSK interaction. Hum Mutat. 2013;34:997–1004. doi: 10.1002/humu.22325. [DOI] [PubMed] [Google Scholar]
- [17].Arredondo J, Lara M, Ng F, Gochez DA, Lee DC, Logia SP, et al. COOH-terminal collagen Q (COLQ) mutants causing human deficiency of endplate acetylcholinesterase impair the interaction of ColQ with proteins of the basal lamina. Hum Genet. 2014;133:599–616. doi: 10.1007/s00439-013-1391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawakami Y, Ito M, Hirayama M, Sahashi K, Ohkawara B, Masuda A, et al. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology. 2011;77:1819–26. doi: 10.1212/WNL.0b013e318237f660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Muller JS, Mihaylova V, Abicht A, Lochmuller H. Congenital myasthenic syndromes: spotlight on genetic defects of neuromuscular transmission. Expert Rev Mol Med. 2007;9:1–20. doi: 10.1017/S1462399407000427. [DOI] [PubMed] [Google Scholar]