Abstract
In epidemiological studies on the toxic effects of prenatal exposure to hexachlorobenzene (HCB), researchers report HCB concentrations, either as wet-weight or per lipid weight basis, in matrices like breast milk, and maternal and cord blood. Conversion of exposures across matrices is needed for comparisons of concentrations and dose effect across cohorts. Using data from a birth cohort study in eastern Slovakia, we derived the maternal blood to cord blood HCB concentration ratio utilizing measured concentrations in 1027 paired maternal and cord blood samples, on a per-lipid basis. In addition to data from the Slovak study, the maternal milk to maternal serum ratio was summarized from 23 published studies on partitioning of HCB between human milk lipid and blood lipid. We identified two distinct groups of milk:blood ratios, those ≤0.45 and those ≥0.85. We assumed that using partition ratios ≤0.45 will underestimate HCB exposure estimates. Taking into account this precautionary measure, we suggest a conversion ratio of 1.21, which is the median of the 16 ratios identified in our literature review. We consider our estimate as conservative and providing appropriate safety in risk analysis.
Keywords: Concentration conversion, Hexachlorobenzene, Breast milk, Cord blood, Lipids, Partitioning
1. Introduction
Hexachlorobenzene (HCB) use as a pesticide was banned by the EU in 1981(EFSA, 2006) and its production and uses were phased out by the Stockholm Convention on Persistent Organic Pollutants in 2004 (Stockholm Convention, 2001). Before its ban, HCB was used widely as an agricultural pesticide. As a result of its prevalent use and its slow decay (EFSA, 2006), HCB can remain in the environment for a long time. Indeed, serum specimens from populations worldwide indicate that HCB is ubiquitous (Patayová et al., 2013; Woodruff et al., 2011; Fisher et al., 2016) despite being phased out several decades ago.
The primary targets of toxicity for HCB after occupational or accidental exposure include hepatic, reproductive, and developmental end points. Additionally, HCB exposures have been associated with cancer endpoints (ATSDR, 2015). Presently, investigators focus mainly on prenatal HCB exposures, i.e., on possible associations between levels of HCB in maternal blood or breast milk, cord blood, or children's blood, and developmental end points such as birth size (weight and/or length) or preterm birth, recurrent miscarriage, postnatal growth, postnatal neurodevelopment, maturation, cryptorchidism, hypospadias, and indicators of postnatal thyroid function (ATSDR, 2015). Since the 2015 ATSDR publication, additional studies have documented associations between higher HCB exposure and obesity (Vafeiadi et al., 2015), reduced cognitive development (Kyriklaki et al., 2016), airway obstruction (Hansen et al., 2016), decreased birth length (Lopez-Espinosa et al., 2016), increases in the proportion of optimal birth weight (Callan et al., 2016), small for gestational age (SGA) (Lauritzen et al., 2016), decreased follicle number (Kristensen et al., 2016) and development of metabolic diseases via insulin homeostasis (Tang-Péronard et al., 2015). According to ATSDR (2015), limitations of HCB studies include the lack of quantifiable exposure to HCB, small numbers of subjects, and/or the presence of measureable levels of other organochlorine compounds. The insufficient information on human toxicology of HCB characterizes the statement (ATSDR, 2015) that: “none of the human studies with HCB have provided reliable direct exposure data (dose and duration) and therefore, no evidence of an exposure-response relationship has been possible.” Such insufficient knowledge warrants further investigation of health outcomes of prenatal exposure to HCB. Moreover, the extremely variable elimination half-life of HCB in young adults (Gallo et al., 2015) seems to complicate the developmental health risk analysis of HCB. The assessment of perinatal exposures is based mainly on determination of the toxicant concentration in matrices derived from the body of either mother or infant. Matrices commonly used for this purpose are maternal blood, cord blood, or breast milk. Most relevant to the effect however, are toxicant concentrations in the central compartment represented by circulating blood either of the mother or the fetus. In order to compare exposures using different matrices as maternal serum/plasma, cord serum/plasma or mother milk, it is necessary to convert concentration data across matrices. The present paper summarizes what is known about HCB concentrations in paired matrices, and derives an HCB maternal serum/plasma vs. breast milk ratio based on 23 studies.
2. Material and methods
We performed a literature search in PubMed, Web of Science, and SCOPUS databases to identify studies that report on simultaneously measured HCB in serum and breast milk. We used the following keywords: “concentration,” “hexachlorobenzene,” “breast milk,” “blood,” “plasma,” “serum,” “lipids,” “partitioning.” We combined the keywords by Boolean operator AND. As the topic of the search was quite specific, the search results with given keywords did not optimally reflect our objective. The final selection of relevant publications was therefore done after a thorough inspection of each result. More papers were identified using literature references included in already identified publications. The literature sources were restricted to human studies and where paired sampling could reasonably be assumed. All studies reporting on HCB concentrations in maternal blood and milk were included, regardless of sample size and other potential exclusion criteria. In such way we obtained a set of 23 data points (item 1).
The following parameters were needed for conversion the lipid adjusted HCB concentration in mother milk to lipid adjusted/wet weight based HCB concentration in cord serum:
1) The ratio of HCB concentration in mother milk lipids to that in mother blood lipids; 2) The ratio of HCB concentration in cord blood plasma/serum to mother blood plasma/serum, using both lipid based and wet weight concentrations; and, 3) The concentration of mother and cord plasma/serum lipids. The ratio under 1) was derived from our literature search. The ratio under 2) was based on our data on partition of HCB between maternal and cord blood lipids (Patayová et al., 2013). Briefly, maternal and cord HCB concentrations were determined in 1027 mother-infant pairs taken part in a birth cohort study in eastern Slovakia (Hertz-Picciotto et al., 2003). Maternal serum was collected during the mother's hospital delivery stay, and cord blood was collected from the infant at birth. Concentrations of HCB were determined using gas chromatography with electron capture detection (GC-ECD). Cord/Maternal serum ratios were calculated for pairs in which data on concentration was available for both mother and her child. Percentage of samples with concentration of HCB ≥ LOD was 96.3%. For switching between wet weight and lipid based data we used our data on lipid concentration in 1065 paired maternal and cord blood serum (Lancz et al., 2015). Lipid concentration was measured using the enzymatic summation method (Akins et al., 1989) (item 3). Specifically, total serum cholesterol (TC) and triglyceride (TG) concentrations were determined at the Department of Clinical Biochemistry of TOP-MED General Hospital Bratislava using a DuPont Automatic Clinical Analyzer III (DuPont, Jonesboro, AR, USA), and cholesterol oxidase without cholesterol esterase was used to detect free cholesterol (FC). The method by Takayama and colleagues was used to determine serum choline-containing phospholipids (PL).45 Total serum lipids were calculated using the formula: TL = 1.677 × (TC − FC) + FC + TG + PL.
3. Results
3.1. The ratios of HCB concentration in maternal milk lipids to that in maternal blood lipids
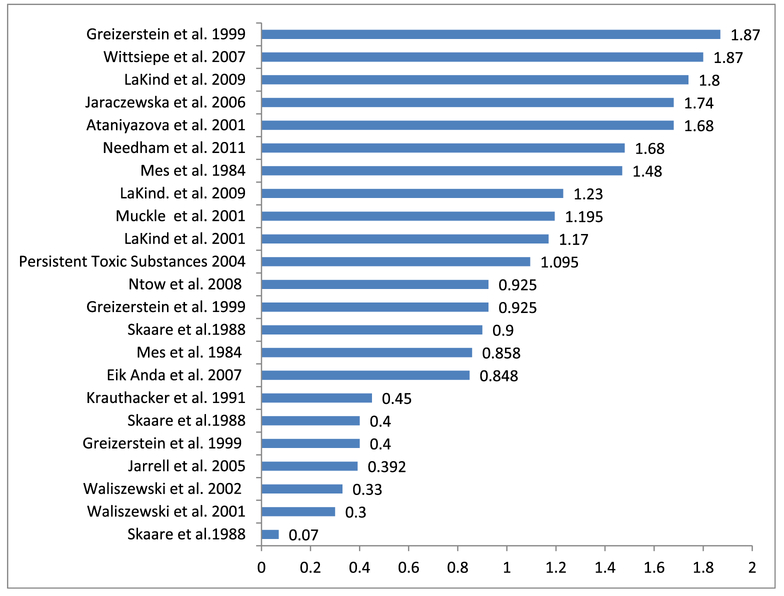
The 23 lipid-adjusted milk/serum HCB concentration ratios reported in the selected papers are shown in Fig. 1. The summarized information from the individual sources can be found in the Supplement.
Fig. 1.
The plot of the 23 lipid adjusted ratios identified in the literature of HCB concentration in maternal milk lipids to that in maternal blood lipids (Ataniyazova et al., 2001; EikAnda et al., 2007; Greizerstein et al., 1999; Jaraczewska et al., 2006; Jarrell et al., 2005; Krauthacker, 1991; LaKind et al., 2001; LaKind et al., 2009; Mes et al., 1984; Muckle et al., 2001; Ntow et al., 2008; Persistent Toxic Substances, 2004; Waliszewski et al., 2001; Waliszewski et al., 2002; Wittsiepe et al., 2007).
Two distinct groups of ratios can be seen in Fig. 1. Those ≤0.45 and those ≥0.848. We assume that using partition ratios ≤0.45 will underestimate HCB exposure estimates and consequently the results of exposure/outcome relationships. We show statistics on the ratios from all 23 values and after deleting values ≤ 0.45 in Table 1. Taking into account this precautionary measure, we suggest for conversion purposes the value of 1.213 which is a median of the 16 ratios ≥0.848.
Table 1.
Descriptive statistics on the lipid adjusted milk/serum HCB concentration ratio calculated from all 23 values and after omitting values of ratios ≤0.45.
| Values of ratios |
||
|---|---|---|
| Statistic |
All |
≥0.848 |
| Number of observations | 23 | 16 |
| Minimum | 0.070 | 0.848 |
| Maximum | 1.870 | 1.870 |
| 1st Quartile | 0.4 | 0.925 |
| Median | 0.925 | 1.213 |
| 3rd Quartile | 1.48 | 1.680 |
| Mean | 1.009 | 1.304 |
| Variance (n-1) | 0.305 | 0.135 |
| Standard deviation (n-1) | 0.552 | 0.368 |
3.2. The ratio of HCB concentration in maternal plasma/serum lipids to cord plasma/serum lipids and concentration of maternal and cord plasma/serum lipids
We previously reported a median wet weight HCB cord serum/maternal serum concentration ratio of 0.265 based on analyzing 931 samples. For lipid adjusted HCB concentrations, we obtained a median ratio of 1.097 (Patayová et al., 2013).
In some selected publications, the HCB concentrations are expressed as wet weight, in units of weight HCB/serum volume, and in others as lipid adjusted, in units of weight HCB/weight serum lipid. We have recalculated the wet weight based milk/serum concentration ratios and expressed them on a per-lipid basis. With HCB serum concentration expressed as wet weight, we calculated the concentration of total lipids in serum as 10 mg lipids/ml serum. We observed this value in 1065 subjects (Lancz et al., 2015) (Table 2). The descriptive statistical data on total lipid concentration in mother and cord serum samples are shown in Table 2 (Lancz et al., 2015).
Table 2.
Descriptive statistics of total lipids in maternal and cord blood serum samples (N = 1065).
| Total lipids mg/mL | Mean | Std. Deviation | Median | Minimum | Maximum | Geometric Mean |
|---|---|---|---|---|---|---|
| Mother | 10.23 | 1.98 | 10.17 | 1.75 | 20.17 | 10.03 |
| Cord | 2.52 | 0.52 | 2.46 | 0.13 | 5.34 | 2.47 |
3.3. Application of derived parameters on HCB conversion
We describe the maternal milk to cord plasma/serum conversion procedure for HCB as follows:
The first step is conversion between HCB lipid-adjusted concentrations in milk and maternal serum. We use the currently suggested conversion factor of 1.213. Therefore, we write:
| (1) |
We convert the maternal serum HCB level from ng/g lipid into ng/L serum as follows:
| (2) |
The coefficient of 10 reflects the average content of fat in serum (g/L) and is based on our data on lipid concentration in 1065 women from samples taken at delivery (Table 2).
The next step is the conversion between mother and cord lipid adjusted or wet-weight based HCB concentration. The respective equations, based on median HCB cord serum/maternal serum concentration ratios of 0.265 for wet weight and 1.097 for lipid adjusted HCB concentrations (Patayová et al., 2013), are as follows:
| (3) |
| (4) |
Combining the above equations we obtain equations (5) and (6) for direct conversion of maternal milk HCB level (ng/g lipid) to cord serum HCB levels.
| (5) |
or
| (6) |
4. Discussion
Maternal milk is the most commonly studied matrix for human biomonitoring of organochlorines (Fång et al., 2015). However the relationship between the concentration of the compounds in milk and the fetus is not straightforward. In our paper, we derive parameters necessary for conversion of breast milk HCB concentrations into cord serum levels, both, wet weighed and lipid adjusted, based on the extant published literature and our own data (Patayová et al., 2013; Lancz et al., 2015).
The ratios of HCB concentration in maternal blood to breast milk observed in the selected literature sources show marked variability. This variability may be explained by the high lipophilicity of HCB {log KOW = 5.7 (Science Dossier, 2014)}, its affinity to triglycerides and cholesterol and changing levels of these main lipid components in maternal blood and milk linked to physiological processes. In the course of normal pregnancy, women show an increase in lipid levels, including levels of triglycerides and total cholesterol, as gestational age progresses (Sattar et al., 1997; Mazurkiewicz et al., 1994; Brizzi et al., 1999; Koletzko et al., 2001; van Stiphout et al., 1987). Moreover diurnal variations in the secretion of milk lipids (Jenness, 1979) and in concentrations of organochlorines in human milk (Pluim et al., 1992) have been described. Thus, heterogeneity in the ratios may reflect different time periods of HCB measurement during pregnancy. Additionally, it should be noted that the reviewed data on milk composition shows large geographic differences (Koletzko et al., 1992; Kumar et al., 2016). For instance, the ratios 0.9 and 0.07 (Skaare et al., 1988), while both derived from women in Norway, the 0.07 ratio was estimated based on concentrations from immigrants to Norway from developing countries. Moreover the value of 0.4 from the same author (Skaare et al., 1988) is for Norwegian mothers and their infants delivered by Caesarean operation. Furthermore, most studies indicate that HCB binds to blood lipoproteins (Mussalo-Rauhamaa, 1991; Gómez-Catalán et al., 1991; Norén et al., 1999) which by themselves, demonstrate variable levels (Mjøs et al., 1979). HCB vectors, triglycerides and cholesterol, being nonpolar lipid substances (insoluble in water), need to be transported in the plasma associated with various lipoprotein particles (Cox and García-Palmieri, 1990). Similarly, chlorinated hydrocarbon pesticides in milk are preferentially concentrated in the surface layers of the fat globule. Although many milk components were associated with pesticides, most of the pesticide on a total concentration basis was associated with triglycerides in the interior of the fat globule (Hugunin and Bradley, 1971). It was shown that because of the differences in these chlorinated hydrocarbon concentrations observed with different sample collection regimens, meaningful comparison of analytical results requires correction by total serum lipid levels (Phillips et al., 1989; Pluim et al., 1994) which has not been sufficiently documented in all literature sources reviewed in the present study.
We did not weigh the published values relative to their study size. Neither we used any QA/QC measures to select the studies from the literature (e.g., sample size). Thus, a mean ratio derived from a few paired samples was given equal weight as a study with a greater number of paired samples. When the concentration was expressed as wet weight, we had to impute lipid concentrations in serum with regard to comments on adjustment of concentration of lipophilic compounds on serum lipids (Schisterman et al., 2005; O'Brien et al., 2016). As already mentioned, the data on concentrations of serum lipids show great variability. For the conversion between maternal wet weight and lipid weight HCB serum concentrations, we used the coefficient of 10 reflecting the average content of fat in serum (g/L) based on our data from 1065 women (Table 2). Other sources give lower, however variable values (Dayton et al., 1966; The Serum Lipids and Lipoproteins, 1958; Cheek and Wease, 1969). Our value is close to mean maternal lipid concentration of 8.9 g/l reported by Needham et al. (2011). In secondary analyses using this value, we derived a ratio of 1.94, which was not meaningfully different from our ratio of 2.18 (data not shown). Here and in our study (Lancz et al., 2015), the lipid level might have been influenced by labor. Furthermore, in some of the reviewed papers the authors reported that blood and milk samples from the same individual were not collected at the same time. This also may contribute to variability in the data.
We conclude, after acknowledging all these sources of uncertainty, that it is possible to derive a valid milk/cord serum conversion factor for HCB from the published data when adopting a certain measure. The median of 0.925 obtained as a result of consideration of all 23 papers reviewed (Table 1) is markedly influenced by the ratios ≤0.45. For precautionary reasons we have suggested a cut-off limit of 0.848. For the milk/serum ratios ≥0.848 the calculated median value of 1.213 (Table 1) seems to warrant safety in risk analysis. We are aware that such proceeding may raise some concern, however our choice of the value of 1.213 is close to the value of 1.48 (Needham et al., 2011) which was derived from a large number of observations. However the best way to eliminate all uncertainties would be to conduct a study under controlled conditions in which HCB will be determined in human serum and in milk at the same time, in many subjects, at multiple time points.
In the course of searching for relevant original data, we found a review article on partitioning of persistent organic pollutants, HCB included, between human serum and breast milk (Mannetje et al., 2012). The 11 literature sources considered in this review were limited to studies that were conducted in humans and those reporting on paired samples including at least five individuals. In the 11 studies resulting from that literature search, 4 were excluded because the serum and breast milk concentrations were not reported on a lipid weight basis. Another 2 publications were excluded because the serum samples were not fully paired with the breast milk samples. We consider using the suggested estimate of the serum/milk ratio of 0.633 (range 0.33−1.25), based on 5 publications, in risk assessment, problematic.
5. Conclusions
In epidemiological studies on toxic effects of prenatal exposure to HCB, researchers report assessment of HCB concentration, either wet weight or lipid adjusted, in matrices like breast milk, maternal blood and cord blood. Conversion of exposures across matrices is needed for comparison of health outcomes between cohorts. From three parameters needed for conversion between breast milk and cord blood, we derived two, maternal blood to cord blood HCB concentration ratio and the concentration of mother and cord serum lipids, from our previously published data. We assessed the value of the maternal milk to maternal serum ratio from 23 published data on partitioning of HCB between human milk lipid and blood lipid. We consider our estimate as conservative and as providing appropriate safety in risk analysis.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health, United States, Grants R01 CA096525, R03 TW007152, P30 ES001247, and K12 ES019852; Slovak Research and Development Agency, Slovakia, Grants APVT-21–016804, APVV-0571–12, APVV-0444–11; European Community’s Seventh Framework Programme (FP7/2007−2013) under grant agreements OBELIX n° 227391.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.envpol.2017.07.087.
This paper has been recommended for acceptance by David Carpenter.
References
- Akins JR, Waldrep K, Bernert JT Jr., 1989. The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin. Chim. Acta 184, 219–226. 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry), 2015. Toxicological Profile for Hexachlorobenzene (Georgia, Atlanta: ). [PubMed] [Google Scholar]
- Ataniyazova OA, Baumann RA, Liem AKD, Mukhopadhyay UA, Vogelaar EF, Boersma ER, 2001. Levels of certain metals, organochlorine pesticides and dioxins in cord blood, maternal blood, human milk and some commonly used nutrients in the surroundings of the Aral Sea (Karakalpakstan, Republic of Uzbekistan). Acta Paediatr 90, 801–808. 10.1111/j.1651-2227.2001.tb02808.x. [DOI] [PubMed] [Google Scholar]
- Brizzi P, Tonolo G, Esposito F, Puddu L, Dessole S, Maioli M, Milia S, 1999. Lipoprotein metabolism during normal pregnancy. Am. J. Obstet. Gynecol 181, 430–434. 10.1016/S0002-9378(99)70574-0. [DOI] [PubMed] [Google Scholar]
- Callan AC, Hinwood AL, Heyworth J, Phi DT, Odland J.è., 2016. Sex specific influence on the relationship between maternal exposures to persistent chemicals and birth outcomes. Int. J. Hyg. Environ. Health 219, 734–741. 10.1016/j.ijheh.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Cheek CS, Wease DF, 1969. A summation technic for serum total lipids. Comparison of methods. Clin. Chem 15, 102–107. [PubMed] [Google Scholar]
- Cox RA, García-Palmieri MR, 1990. Cholesterol, triglycerides, and associated lipoproteins. Chapter 31 : Walker HK, Hall WD, Hurst JW (Eds.), Clinical Methods: the History, Physical, and Laboratory Examinations, third ed Butterworths, Boston. [Google Scholar]
- Dayton S, Hashimoto S, Dixon W, Pearce ML, 1966. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J. Lipid Res 7, 103–111. [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to hexachlorobenzene as undesirable substance in animal feed. Eur. Food Saf. Auth. J 402, 1–49. 10.2903/j.efsa.2007.434. [DOI] [Google Scholar]
- EikAnda E, Nieboer E, Dudarev AA, Sandanger TM, Odland JO, 2007. Intraand intercompartmental associations between levels of organochlorines in maternal plasma, cord plasma and breast milk, and lead and cadmium in whole blood, for indigenous peoples of Chukotka, Russia. J. Environ. Monit 9, 884–893. 10.1289/ehp.6616. [DOI] [PubMed] [Google Scholar]
- Fång J, Nyberg E, Winnberg U, Bignert A, Bergman Å, 2015. Spatial and temporal trends of the Stockholm Convention POPs in mothers' milk - a global review. Environ. Sci. Pollut. Res. Int 22, 8989–9041. 10.1007/s11356-015-4080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, Haines D, Davis K, Fraser WD, 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ. Health 15, 59 10.1186/s12940-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo MV, Deane GD, DeCaprio AP, Schell LM, 2015. Changes in persistent organic pollutant levels from adolescence to young adulthood. Environ. Res 140, 214–224. 10.1016/j.envres.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Catalán J, To-Figueras J, Rodamilans M, Corbella J, 1991. Transport of organochlorine residues in the rat and human blood. Arch. Environ. Contam. Toxicol 20, 61–66. 10.1007/BF01065329. [DOI] [PubMed] [Google Scholar]
- Greizerstein HB, Stinson C, Mendola P, Buck GM, Kostyniak PJ, Vena JE, 1999. Comparison of PCB congeners and pesticide levels between serum and milk from lactating women. Environ. Res 80, 280–286. 10.1006/enrs.1999.3956. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kocan A, Charles MJ, Ciznar P, Langer P, Sovcikova E, James R, 2003. PCBs and early childhood development in Slovakia: study design and background. Fresen Environ. Bull 12, 208–214. [Google Scholar]
- Hugunin AG, Bradley RL Jr., 1971. Distribution of organochlorine pesticides among some milk components. J. Dairy Sci 54, 355–359. 10.3168/jds.S0022-0302(71)85843-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Strøm M, Olsen SF, Dahl R, Hoffmann HJ, Granström C, Rytter D, Bech BH, Linneberg A, Maslova E, Kiviranta H, Rantakokko P, Halldorsson TI, 2016. Prenatal exposure to persistent organic pollutants and offspring allergic sensitization and lung function at 20 years of age. Clin. Exp. allergy 46, 329–336. 10.1111/cea.12631. [DOI] [PubMed] [Google Scholar]
- Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P, 2006. Distribution of poly-chlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region. Pol. Sci. Total Environ 372, 20–31. 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Jarrell J, Chan S, Hauser R, Hu H, 2005. Longitudinal assessment of PCBs and chlorinated pesticides in pregnant women from Western Canada. Environ. Health 4, 10 10.1186/1476-069X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness R, 1979. The composition of human milk. Semin. Perinatol 3, 225–239. [PubMed] [Google Scholar]
- Koletzko B, Rodriguez-Palmero M, Demmelmair H, Fidler N, Jensen R, Sauerwald T, 2001. Physiological aspects of human milk lipids. Early Hum. Dev 65, 3–18. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Thiel I, Abiodun PO, 1992. The fatty acid composition of human milk in Europe and Africa. J. Pediatr 120, 62–70. 10.1016/S0022-3476(05)81238-7. [DOI] [PubMed] [Google Scholar]
- Krauthacker B, 1991. Levels of organochlorine pesticides and polychlorinated biphenyls (PCBs) in human milk and serum collected from lactating mothers in the northern Adriatic area of Yugoslavia. Bull. Environ. Contam. Toxicol 46, 797–802. 10.1007/BF01689721. [DOI] [PubMed] [Google Scholar]
- Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, Halldorsson TI, Rantakokko P, Kiviranta H, Toft G, 2016. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ. Int 92–93, 366–372. 10.1016/j.envint.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, Nicol MP, Isolauri E, Yang B, Collado MC, Salminen S, 2016. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol 7,1619 10.3389/fmicb.2016.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriklaki A, Vafeiadi M, Kampouri M, Koutra K, Roumeliotaki T, Chalkiadaki G, Anousaki D, Rantakokko P, Kiviranta H, Fthenou E, Bitsios P, Kyrtopoulos SA, Kogevinas M, Chatzi L, 2016. Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4years of age: the Rhea mother-child cohort, Crete, Greece. Environ. Int 97, 204–211. 10.1016/j.envint.2016.09.012. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Naiman DQ, 2001. Infant exposure to chemicals in breast milk in the United States: what we need to learn from a breast milk monitoring program. Environ. Health Perspect 109, 75–88. 10.1289/ehp.0110975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM Jr., Sjödin A, Turner W, Wang RY, Needham LL, Paul IM, Stokes JL, Naiman DQ, Patterson DG Jr., 2009. Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ. Health Perspect 117, 1625–1631. 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancz K, Murǐnová Ľ, Patayová H, Drobná B, Wimmerová S, Sovčíková E, Kováč J, Farkašová D, Hertz-Picciotto I, Jusko TA, Trnovec T, 2015. Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int. J. Hyg. Environ. Health 218, 91–98. 10.1016/j.ijheh.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, èien T, Sandanger TM, Odland J.è., van de Bor M, Jacobsen GW, 2017. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatr. Res 81, 33–42. 10.1038/pr.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Murcia M, Iniguez C, Vizcaino E, Costa O, Fernández-Somoano A, Basterrechea M, Lertxundi A, Guxens M, Gascon M, Goñi-Irigoyen F, Grimalt JO, Tardóan A, Ballester F, 2016. Organochlorine compounds and ultrasound measurements of fetal growth in the INMA cohort (Spain). Environ. Health Perspect 124, 157–163. 10.1289/ehp.1408907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannetje A, Coakley J, Mueller JF, Harden F, Toms LM, Douwes J, 2012. Partitioning of persistent organic pollutants (POPs) between human serum and breast milk: a literature review. Chemosphere 89, 911–918. 10.1016/j.chemosphere.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz JC, Watts GF, Warburton FG, Slavin BM, Lowy C, Koukkou E, 1994. Serum lipids, lipoproteins and apolipoproteins in pregnant non-diabetic patients. J. Clin. Pathol 47, 728–731. 10.1136/jcp.47.8.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes J, Doyle JA, Adams BR, Davies DJ, Turton D, 1984. Polychlorinated biphenyls and organochlorine pesticides in milk and blood of Canadian women during lactation. Arch. Environ. Contam. Toxicol 13, 217–223. 10.1007/BF01055879. [DOI] [PubMed] [Google Scholar]
- Mjøs OD, Rao SN, Bjøru L, Henden T, Thelle DS, Førde OH, Miller NE, 1979. A longitudinal study of the biological variability of plasma lipoproteins in healthy young adults. Atherosclerosis 34, 75–81. 10.1016/0021-9150(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Muckle G, Ayotte P, Dewailly EE, Jacobson SW, Jacobson JL, 2001. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ. Health Perspect. 109, 1291–1299. 10.1289/ehp.011091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussalo-Rauhamaa H, 1991. Partitioning and levels of neutral organochlorine compounds in human serum, blood cells, and adipose and liver tissue. Sci. Total Environ 103,159–175. 10.1016/0048-9697(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG Jr., Sjödin A, Turner WE, Weihe P, 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol 45, 1121–1126. 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén K, Weistrand C, Karpe F, 1999. Distribution of PCB congeners, DDE, hexachlorobenzene, and methylsulfonyl metabolites of PCB and DDE among various fractions of human blood plasma. Arch. Environ. Contam. Toxicol 37, 408–414. 10.1007/s002449900532. [DOI] [PubMed] [Google Scholar]
- Ntow WJ, Tagoe LM, Drechsel P, Kelderman P, Gijzen HJ, Nyarko E, 2008. Accumulation of persistent organochlorine contaminants in milk and serum of farmers from Ghana. Environ. Res 106, 17–26. 10.1016/j.envres.2007.05.020. [DOI] [PubMed] [Google Scholar]
- O'Brien KM, Upson K, Cook N, Weinberg CR, 2016. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ. Health Perspect 124, 220–227. 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patayovaá H, Wimmerovaá S, Lancz K, Palkovičová L, Drobná B, Fabišiková A, Kováč J, Hertz-Picciotto I, Jusko TA, Trnovec T, 2013. Anthropometric, socioeconomic, and maternal health determinants of placental transfer of organochlorine compounds. Environ. Sci. Pollut. Res. Int 20, 8557–8566. 10.1007/s11356-013-1786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persistent Toxic Substances, 2004. Food Security and Indigenous Peoples of the Russian North - Final Report. AMAP, Oslo: http://www.amap.no/documents/doc/persistent-toxic-substances-food-security-and-indigenous-peoples-of-the-russian-north.-final-report/795. [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr., Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 18,495–500. 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Pluim HJ, Slot PC, Olie K, Koppe JG, 1992. Diurnal variations in concentrations of PCDDs and PCDFs in human milk. Chemopsphere 25, 307–311. 10.1016/0045-6535(92)90547-5. [DOI] [Google Scholar]
- Pluim HJ, Boersma ER, Kramer I, Olie K, van der Slikke JW, Koppe JG, 1994. Influence of short-term dietary measures on dioxin concentrations in human milk. Environ. Health Perspect 102, 968–971. 10.1289/ehp.94102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, Greer IA, Louden J, Lindsay G, McConnell M, Shepherd J, Packard CJ, 1997. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. J. Clin. Endocrinol. Metabol 82, 2483–2491. 10.1210/jc.82.8.2483. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA, 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect 113, 853–857. 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Science Dossier, 2014. Aquatic Environmental Risk Assessment of Hexachlorobenzene. Eurochlor 17, Brussels. http://www.eurochlor.org/media/90477/sd16-hcbaquaticra-final.pdf. [Google Scholar]
- Skaare JU, Tuveng JM, Sande HA, 1988. Organochlorine pesticides and polychlorinated biphenyls in maternal adipose tissue, blood, milk, and cord blood from mothers and their infants living in Norway. Arch. Environ. Contam. Toxicol 17, 55–63. 10.1007/BF01055154. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention on Persistent Organic Pollutants, 2001, 2256 UNTS 119, 40 ILM 532, Stockholm. [Google Scholar]
- Tang-Péronard JL, Heitmann BL, Jensen TK, Vinggaard AM, Madsbad S, Steuerwald U, Grandjean P, Weihe P, Nielsen F, Andersen HR, 2015. Prenatal exposure to persistent organochlorine pollutants is associated with high insulin levels in 5-year-old girls. Environ. Res 142, 407–413. 10.1016/j.envres.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Serum Lipids and Lipoproteins, 1958. Review of Earlier Work Acta Medica Scandinavica, vol. 161, pp.9–28. 10.1111/j.0954-6820.1958.tb04619.x. http://onlinelibrary.wiley.com/doi/10.1111/j.0954-6820.1958.tb04619.x/epdf. [DOI] [Google Scholar]
- Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, Fthenou E, Venihaki M, Sarri K, Vassilaki M, Kyrtopoulos SA, Oken E, Kogevinas M, Chatzi L, 2015. Association of prenatal exposure to persistent organic pollutants with obesity and car-diometabolic traits in early childhood: the rhea mother-child cohort (crete, Greece). Environ. Health Perspect 123, 1015–1021. 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stiphout WA, Hofman A, de Bruijn AM, 1987. Serum lipids in young women before, during, and after pregnancy. Am. J. Epidemiol 126, 922–928. 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzon RM, Silva CS, Siliceo J, 2001. Organochlorine pesticide levels in maternal adipose tissue, maternal blood serum, umbilical blood serum, and milk from inhabitants of Veracruz, Mexico. Arch. Environ. Contam. Toxicol 40, 432–438. 10.1007/s002440010194. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Aguirre AA, Infanzon RM, Siliceo J, 2002. Persistent organochlorine pesticide levels in maternal blood serum, colostrum, and mature milk. Bull. Environ. Contam. Toxicol 68, 324–331. 10.1007/s002440010194. [DOI] [PubMed] [Google Scholar]
- Wittsiepe J, Fürst P, Schrey P, Lemm F, Kraft M, Eberwein G, Winneke G, Wilhelm M, 2007. PCDD/F and dioxin-like PCB in human blood and milk from German mothers. Chemosphere 67, 286–294. 10.1016/j.chemosphere.2006.05.118. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect 119, 878–885. 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Takayama M, Itoh S, Nagasaki T, Tanimizu I, 1977. A new enzymatic method for determination of serum choline-containing phospholipids. Clin. chimica acta; Int. J. Clin. Chem 79, 93–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.