Abstract
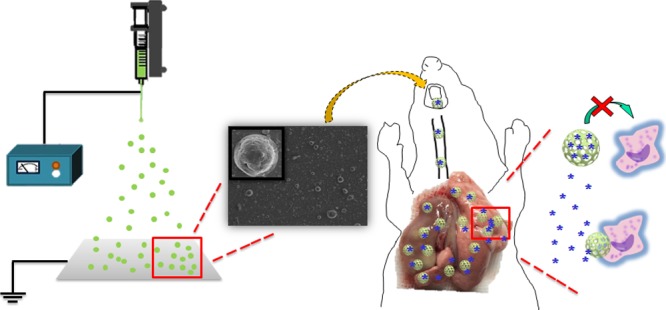
Nonsmall-cell lung cancer is a severe disease with high morbidity and mortality. However, the systemic administration of anticancer drugs generally leads to serious toxicity and low anti-lung cancer efficiency because of very limited drug distribution in the lung. In our previous research, we have confirmed the high anti-lung cancer effect of inhalable oridonin microparticles in spite of their long and complicated preparation process. Here, we develop a novel, simple, and quick method for preparing inhalable oridonin-loaded poly(d,l-lactic-co-glycolic)acid (PLGA) porous microspheres using the electrospraying technique. The formulation and preparation processes were screened. The electrospraying porous microspheres (EPMs) were rough, porous, and suitable for pulmonary delivery. Most of the oridonin was released from the EPMs within 20 h based on drug diffusion and via PLGA erosion. The EPMs exhibited efficient lung deposition in vitro and in vivo because of their ideal aerodynamic diameters. Chemical carcinogens were used to prepare primary lung cancer rat models by direct pulmonary delivery. The EPMs showed high anti-lung cancer effect after pulmonary delivery according to CT images and pathology. Inhibition of angiogenesis and enhancement of lung cancer cell apoptosis could be the major anticancer mechanism. Electrospraying is an efficient method for the preparation of inhalable drug-loaded porous microspheres. The oridonin-loaded EPMs are promising dry powder inhalers for the local therapy of primary lung cancer.
Introduction
Nonsmall-cell lung cancer is a severe disease, accounting for about 80–85% of all lung cancers, which readily metastasizes, and the prognosis is relatively poor (approximately 85% mortality within 5 years).1,2 Currently, the systematic administration of chemotherapeutic drugs is still the major treatment of lung cancer despite high side effects and insufficient drug exposure to the lungs. The direct pulmonary delivery of anticancer drugs could be an effective way for lung cancer therapy. It enables high doses of chemotherapeutics in the lung tumors, avoids the severe adverse side effects of systemic administration,3−5 and enhances patient compliance compared with intravenous injection6 because patients can self-medicate the anticancer agents. However, up to now, a local therapy for primary lung cancer is still absent. Therefore, it is necessary to find a highly effective local therapy. Oridonin, an active diterpenoid isolated from a traditional Chinese medicine Isodon rubescens (Hemsl) Hara, shows strong anticancer effect with little adverse reaction, attracting great attention from oncologists and pharmacologists.7
Inhalable porous microspheres, characterized by the particles of low mass density and large sizes, enable highly efficient delivery of inhaled drugs deep into the lungs and high respirable fractions of inhaled therapeutics. The key role of these large particles is to avoid being phagocytized by alveolar macrophages because of their large geometric sizes. The deposited particles have an opportunity to release entrapped drugs to the surroundings.8,9 Porous microspheres can be fabricated with the methods of solvent evaporation,10 spray drying,11 and supercritical fluid technology.12 However, these methods have disadvantages such as low encapsulation efficiency, difficulty in removing organic solvents, and of separation particles from the aqueous phase. In our previous research, we prepared oridonin-loaded porous microparticles for the treatment of lung cancer after pulmonary delivery, and the high anti-lung cancer effect of the treatment was confirmed.13 However, the preparation process was long and complicated. Moreover, residual organic solvents could be avoided in the preparation.
Electrospraying is an attractive technique for generating particles on the nano- and microscales and has recently gained interest for pharmaceutical purposes. It works on the principle of applied electric fields. The high applied voltage generates an electrostatic force inside of the liquid droplet, which counteracts with the surface tension of the liquid droplet. A Taylor cone of the liquid is formed at the tip of the needle. According to Coulomb’s repulsion of charges, the highly charged liquid droplets are sprayed and evaporated or coagulated in the air. The formed particles are collected on the collector plate. The droplet size can be precisely controlled by regulating both the flow rate and the applied voltage. In practice, the electrospraying technique enables better control over the structure, size, and composition of the particles compared with other traditional fabrication methods.14−16
Here, we develop inhalable drug-loaded electrospraying porous microspheres (EPMs) for the local therapy of primary lung cancer. The technique is simple and fast without organic residual solvents. The formulation and preparation method of EPMs are optimized. Drug release is investigated. The high anticancer effect of EPMs on the primary lung cancer rat models is confirmed.
Results and Discussion
Formulation and Preparation Process of EPMs
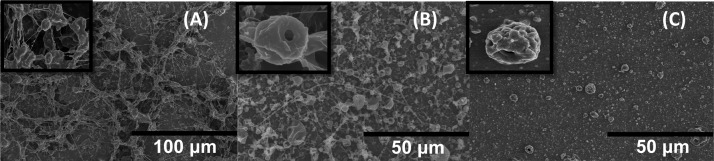
According to preliminary studies, we found that the preparation of EPMs needed low voltage and long collection distance of the electrospinning instrument. These two conditions may provide enough time for the components in the electrosprayed solution to aggregate to microscale droplets based on the interface tension. After the solvents in the droplets evaporated in the air, the microspheres immediately formed. High applied voltage and short collection distance resulted in strings of beads (Figure 1A,B). We primarily selected 15 kV as the applied voltage and 20 cm as the collection distance for the preparation of PLGA [poly(d,l-lactic-co-glycolic)acid] EPMs after electrospraying the W/O emulsions consisting of 1.5% NH4HCO3 (pore-forming agent) solution/2% PLGA-in-dichloromethylene (DCM) solution (containing 0.5% Span 80 to stabilize emulsions) (5:1, v/v). Independent microspheres were obtained on the collector aluminum foil (Figure 1C).
Figure 1.
SEM images of PLGA EPMs prepared with different applied voltages and collection distances, including 20 kV/10 cm (A), 15 kV/15 cm (B), and 15 kV/20 cm (C).
To obtain porous microspheres, pore-forming agents, such as NH4HCO3, need to be added to the electrosprayed polymer solutions in advance. The surface morphologies of porous microspheres mainly depend on the concentrations of PLGA solutions, NH4HCO3 aqueous solutions, and W/O ratios (Figure 2). When no pore-forming agents were added, only smooth microspheres were obtained [Figure 2B(d),C(a)]. Too much of PLGA (e.g., 15% PLGA in the solution) resulted in strings containing beads [Figure 2A(d)]. Finally, the optimal formulation of blank EPMs included 6% or 8% PLGA solution, 1.5% NH4HCO3 solution, and 1:10 W/O ratio.
Figure 2.
SEM images of the EPMs prepared with different formulations and processes. (A) 1.5% NH4HCO3 solution, 1:3 W/O ratio, and 2%, 6%, 8%, or 15% PLGA solution from (a) to (d). (B) 1.5% NH4HCO3 solution, 6% PLGA solution, and the W/O ratios of 1:3, 1:5, 1:10, and no water phase from (a) to (d). (C) 8% PLGA solution, 1:10 W/O ratio, and 0%, 1.5%, 3%, or 4.5% NH4HCO3 solution from (a) to (d). Finally, the formulation and process of C(b) is optimal.
Oridonin-loaded EPMs were also prepared by referring to the above optimal blank formulation and process. A different aspect was that some fibers appeared and the size distribution became very wide when 8% PLGA solution was used (Figure 3). However, 6% PLGA solution resulted in uniform EPMs. Hydrophobic oridonin may change the W/O ratio. In addition, some oridonin nanocrystals could appear on the EPM surface. In summary, oridonin-loaded EPMs were prepared with 15 kV electrospraying applied voltage, 20 cm collection distance, and 1:10 W/O emulsion of 1.5% NH4HCO3 aqueous solution/6% PLGA/0.6% oridonin/0.5% Span 80 solution in DCM. High oridonin encapsulation efficiency of 58.59 ± 2.0% and drug loading efficiency of 5.86 ± 0.2% were achieved.
Figure 3.
SEM images and size distribution of the oridonin-loaded EPMs prepared with 8% PLGA (A) or 6% PLGA (B) solution. Enlarged images of oridonin-loaded EPMs prepared with 6% PLGA solution (C) and blank EPMs (D).
Characteristics of Oridonin-Loaded EPMs
Oridonin-loaded EPMs were rough spheres with many nanopores on the surfaces according to the scanning electron microscopy (SEM) (Figure 3C). The average geometric size of EPMs was 5.23 μm (D50) according to the laser light scattering measurement. The tapped density of EPMs was very small, only 0.15 ± 0.02 g/mL, which can be attributed to the porous structures that were further evidenced in the next investigation of EPM erosion. The porous structures of EPMs led to the small mass mean aerodynamic diameter of 2.1 ± 0.1 μm. The flowability of EPMs was good, with a small repose angle of 29.37 ± 6.6°. On the basis of the appropriate aerodynamic diameter and good flowability, EPMs had a high emitted dose of 67.9%, that is, a large proportion of inhaled EPMs would deposit into the lung. Additionally, the X-ray diffraction (XRD) pattern, differential scanning calorimetry (DSC), and Fourier transform infrared (FT-IR) spectra showed that oridonin adopted an amorphous form and high dispersion in the PLGA microsphere matrix (Figures S1–S3).
High Lung Deposition of EPMs
In vitro EPM distribution in the lung was investigated using the Next Generation Impactor (NGI). The fine particle fraction (FPF) of the oridonin-loaded EPMs was 19.1%, higher than that (12%) of the traditional nonporous solid PLGA microspheres (Figure S4). The 2D CT imaging further showed the in vivo lung deposition of Cy7-loaded microspheres, wherein the lung deposition of EPMs was much more than that of the solid microspheres (Figure 4). More importantly, most of the EPMs deposited in the deep sites of the lung according to the images. By contrast, almost all of the solid microspheres deposited on the upper trachea and bronchi (Figure 4), indicating low inhalation efficacy. High lung deposition of EPMs should result from the porous structure and appropriate aerodynamic diameter. Therefore, EPMs are good carriers for the pulmonary delivery of drugs.
Figure 4.
Lung deposition of microspheres shown using CT 2D (A), 3D (B), and lung tissue section images (C, 100×) after 2 h postrat pulmonary delivery. The arrows in the images (C) show the deposited microspheres in the lung tissues.
Rapid Release of Oridonin from EPMs
Oridonin rapidly released in the early stage of the dissolution investigation. One hour later, 46% of oridonin had been released, possibly resulting from surface-adhered oridonin nanocrystals and rapid release of the embedded oridonin through the exposed nanopores in EPMs (Figure 5). About 65% of oridonin had been released after 24 h. The nanopores were formed after electrospraying evaporation of NH4HCO3. A lot of inner pores were exposed when the microspheres were eroded over 1 h (Figure 5A). By contrast, the solid PLGA microspheres had no pores, so oridonin released slowly (Figure 5). Therefore, EPMs not only improve the deposition of microspheres in the lung but also supply the nanopores as the drug-releasing routes.
Figure 5.
Oridonin release profiles from the EPMs and the related SEM images of microspheres (A) and the release profiles from the solid PLGA microspheres (B).
Large microspheres can avoid the uptake of alveolar macrophages that phagocytize nanoparticles but not microspheres.17 SEM images showed the sizes of EPMs decreasing to 1 μm or less after 24 h of erosion (Figure 4), and most of the oridonin had released into the surroundings and would go into lung cancer cells. Therefore, EPMs are excellent pulmonary delivery carriers to transport drugs to the deep sites of the lungs and rapidly release drugs into the surroundings. Furthermore, the safety of PLGA EPMs may be ensured because of erosion, and the final degradation product of PLGA is CO2.
High Anti-Lung Cancer Effect of Oridonin-Loaded EPMs
In the lung cancer rat models, a great amount of tumor nodes appeared in the left lungs (Figure 6). The oridonin powder group had less tumor nodes than the saline group. The gemcitabine group had few tumor nodes. Surprisingly, the oridonin EPM group showed nearly no nodes. Therefore, the therapeutic efficiency of oridonin-loaded EPMs could be better than gemcitabine, which was the clinical first-line anticancer agent. The high anti-lung cancer effect of oridonin-loaded EPMs should be attributed to the high lung deposition of EPMs, rapid release of oridonin, and avoidance of the uptake of macrophages. In the following study, the anti-lung cancer mechanism of oridonin-loaded EPMs is explored and some advantages are found.
Figure 6.
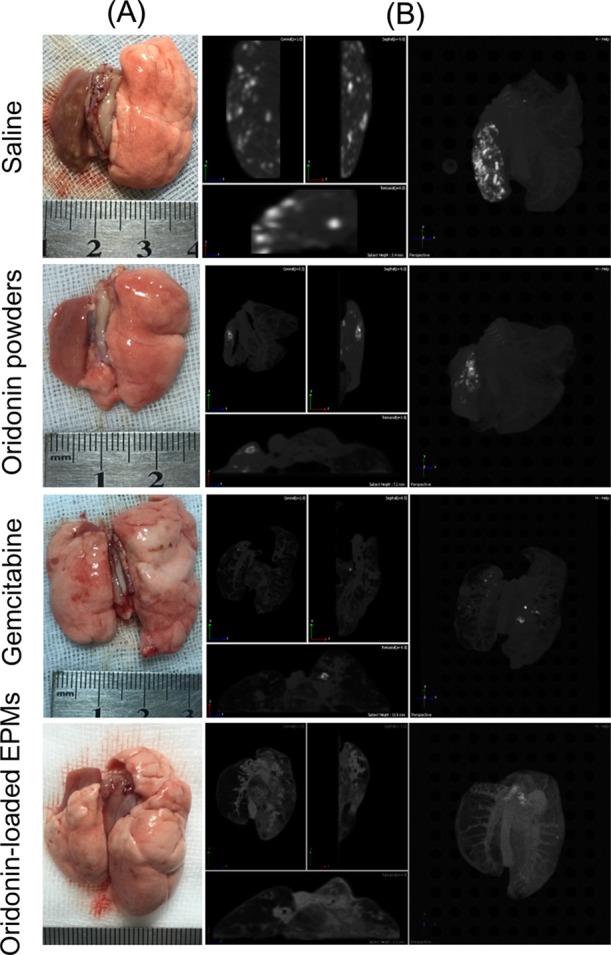
Lung appearances (A) and CT images (B) of the primary lung cancer rats treated with saline, oridonin powders, gemcitabine, and oridonin-loaded EPMs via pulmonary delivery. The light points in the CT images indicate tumor nodes.
Inhibition of Angiogenesis Induced by Oridonin
Tumor growth and metastasis require sufficient nutrients and oxygen via angiogenesis.18 CD31, a membrane protein on the surface of the endothelial cells of blood vessels, indicates the growth prosperity of cancer. The saline group showed a great number of CD31 expressions (Figure 7A). The oridonin powder and gemcitabine groups showed some CD31 expressions (Figure 7B,C). However, very little CD31 was expressed in the oridonin-loaded EPM group (Figure 7D). Therefore, the antiangiogenesis effect of oridonin may be one of its anti-lung cancer mechanisms.
Figure 7.
CD31 expressions in the primary lung cancer tissues in rats after treatment via pulmonary delivery. The arrows indicate CD31 expressions shown as brown spots.
Apoptosis of Lung Cancer Cells Induced by Oridonin
Apoptosis is a key mechanism by which chemotherapeutic agents induce cytotoxic effects in cancer cells.19 In this study, the apoptosis proportions of lung cancer cells are shown after merging the images of DAPI (4′,6-diamidino-2-phenylindole) and TUNEL (terminal deoxynucleotidyl transferase Biotin-dUTP nick end labeling) staining (Figure 8). The saline group showed no apoptosis. The oridonin powder group showed little apoptosis. The oridonin-loaded EPM group and the gemcitabine group showed much apoptosis. Therefore, oridonin-loaded EPMs showed a high lung cancer cellular apoptosis effect. The pathological sections also exhibited histological results similar to the apoptosis (Figure 8), wherein the oridonin-loaded EPMs could attenuate the cell proliferation in the pulmonary alveoli and small bronchus compared with the other groups.
Figure 8.

Apoptosis of lung cancer cells and pathological sections of the lung tissues of rats with primary lung cancer after treatment via pulmonary delivery. Apoptosis was indicated using TUNEL staining (100×). The nuclei were shown using DAPI staining (100×). The merged images of TUNEL and DAPI staining showed the proportions of apoptosis in the lung cancer cells. H&E staining (100×) showed the states of lung cancer cells.
Conclusions
Drug-loaded efficient lung inhalers are very beneficial to the local therapy of primary lung cancers. We designed a novel, simple, and rapid electrospraying method to prepare drug-loaded porous microspheres, that is, inhaled oridonin-loaded EPMs, for the local therapy of primary lung cancer. The EPMs had a nanoporous structure and low tapping density, resulting in appropriate aerodynamic diameters and high lung deposition, that is, ideal lung inhalable particles. The outstanding advantage of oridonin-loaded EPMs may be that oridonin had released to the surroundings through nanoporous routes before alveolar macrophages could phagocytize the eroded nanoporous PLGA microspheres. Therefore, oridonin had an opportunity to enter the lung cancer cells. Our pharmacodynamic study demonstrated that the oridonin-loaded PLGA EPMs had a high anti-lung cancer effect following pulmonary delivery. An improvement in the lung cancer cell apoptosis may be the major mechanism. Electrospraying is an effective method for the preparation of inhalable drug-loaded nanoporous microspheres. Oridonin-loaded PLGA EPMs are promising dry powder inhalers for the local therapy of primary lung cancer.
Experimental Section
Materials
Oridonin was obtained from Shaanxi Huike Botanical Development Co., Ltd. (Shaanxi, China). PLGA [poly(d,l-lactic-co-glycolic)acid, lactide/glycolide, 75:25, mol/mol, MW, 10 kDa] was obtained from Jinan Daigang Biomaterial Co., Ltd. (Shandong, China). Gemcitabine was used as a positive drug and purchased from Hansoh Pharmaceutical Co., Ltd. (Jiangsu, China). NH4HCO3 was purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Cy7 was purchased from Fanbo Biochemicals Co., Ltd. (Beijing, China). All other chemicals and solvents were of analytical grade or high-performance liquid chromatographic (HPLC) grade.
Animals
Male Wistar rats (4–6 weeks old, 180–220 g) were from Beijing Vital River Experimental Animal Technology Co., Ltd., China. The handling and surgical procedures of animals were conducted strictly according to the Guiding Principles for the Use of Laboratory Animals. All of the studies were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals. The animal experiments were approved by the Animal Care Committee of Beijing Institute of Radiation Medicine. Lung tissues were excised after sacrifice. Humane care of the animals was always done.
Formulation Screening of EPMs
EPMs were prepared using an emulsion/electrospraying method. A PLGA solution (2–15%) in dichloromethane (DCM) containing 0.5% Span 80 was mixed with an NH4HCO3 solution (1.5–4.5%). A water-in-oil (W/O) emulsion was prepared following sonication with a probe-type sonicator (HUP-100, Hengao Technology Development Co., Ltd., China) at 70 W for 60 s in an ice bath. Oridonin-loaded EPMs were prepared after oridonin was dissolved in the PLGA solution. The W/O emulsions were sprayed from the nozzle (inner diameter 0.88 mm, outer diameter 1.27 mm) of the electrospraying equipment (SS-2535H, Beijing Ucalery Technology Development Co., Ltd., China). An aluminum foil straightly faced the nozzle as the collector. PLGA microspheres were collected from the aluminum foil using a brush. Other conditions included room temperature, a relative humidity of 30%, an applied voltage of +15 kV, a spray solution flow rate of 0.8 mL/h, and a distance of 20 cm between the nozzle and the aluminum foil.
Characterization of Oridonin-Loaded EPMs
The XRD method was used to differentiate raw oridonin, PLGA, the physical mixture of oridonin/PLGA(1:10, w/w), and the oridonin-loaded PLGA EPMs on an X-ray diffractometer (Bruker D8-advance, Germany) with the θ range from 5° to 50°. The thermal behavior of microparticles was detected using a DSC Q2000 (TA instruments, USA) instrument at a speed of 10 °C/min in the 30–300 °C range. The volume diameters of powders were measured on the BT2001 particle size analyzer (Bettersize Instruments Ltd., Dandong, China) based on the laser light diffraction method. The surface morphologies of oridonin-loaded PLGA EPMs were observed on a scanning electron microscope (SEM, S-4800, Hitachi, Japan). The microparticles were mounted on metal stubs with an adhesive carbon tape, sputter-coated with gold, and examined under the microscope at an acceleration voltage of 10 kV. The repose angle (deg) of microparticles was measured using the funnel method. The bulk density was calculated with the graduated flask method.
Measurements of Drug Loading and Encapsulation Efficiencies
Oridonin-loaded PLGA EPMs (10 mg) were dissolved in dimethyl sulfoxide (DMSO, 1 mL) and then diluted 10-fold with the mobile phase of methanol/water (60:40, v/v). The sample was filtered through a 0.45 μm filter and analyzed on an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, USA) to determine oridonin. The determination was performed on a Diamonsil C18 column (250 mm × 4.6 mm, 5 μm, Dikma Technologies, China) with the column temperature at 25 °C. The mobile phase was delivered at a flow rate of 1 mL/min. The detection wavelength was set at 239 nm, and the injection volume was 20 μL. All measurements were performed in triplicates. Drug loading and entrapment efficiencies were calculated with eqs 1 and 2, respectively.
| 1 |
| 2 |
Lung Deposition Study
The in vitro deposition was determined using an NGI (Copley Scientific Limited, UK) at an airflow rate of 60 L/min. The aerosolization performance of the ∼15 mg microparticles that had been filled into a size 3# hard capsule was investigated with a linker to the NGI. The FPF, emitted dose, and total recovery were assessed. The powders collected in each stage were dissolved in the mobile phase of methanol/water (60:40, v/v), and the solutions were analyzed with HPLC.
In Vitro Release of Oridonin
Oridonin-loaded EPMs (10 mg) were suspended in the simulated lung fluid (SLF, 10 mL) containing 0.02% Tween 80. The suspension was shaken at 160 rpm and 37 °C. At the predetermined time points, an aliquot (1 mL) of suspensions was withdrawn and centrifuged at 5000 rpm for 10 min. The supernatant was filtered through 0.45 μm filters and analyzed with HPLC (see the Supporting Information). Fresh SLF of equal volume was supplemented to the suspension after pipetting. The experiments were performed in triplicates. Additionally, the surface morphologies of the microspheres at different time points were investigated with SEM (S-4800, 10 kV, Hitachi, Japan).
Lung Deposition Measurement of Microspheres
Cy7-loaded EPMs were prepared as above and administered to the rat lung using an insufflator (DP-4M, Penn-Century Inc., PA, USA) through the trachea without anesthesia. To confirm the lung deposition of EPMs, the tissue sections and the whole lungs were observed with a fluorescence microscopy and an imaging station (IVIS Spectrum CT, PerkinElmer, USA), respectively. Nonporous solid PLGA microspheres were prepared with the above-mentioned electrospraying method without aqueous phases. Their lung deposition efficiency was also investigated as the control.
Pharmacodynamic Study
We prepared the primary lung cancer rat models with the chemical induction method where 3-methylcholanthrene (MCA) and diethylnitrosamine (DEN) were pulmonary-administered.20 Thirty days were allowed for the models to mature. Twenty-four rats were equally divided into four groups. The rats with lung cancer were administered saline (0.2 mL per rat) through the airway using an intratracheal aerosolizer (IA-1B, Penn-Century Inc., PA, USA) once a week for 4 weeks. Oridonin powders (1 mg each rat) and oridonin-loaded EPMs (20 mg each rat, containing 1 mg of oridonin) were pulmonary administered to the lungs of the rats using the DP-4M insufflator through the trachea without anesthesia once a week for 4 weeks. A gemcitabine (10 mg/mL) solution in saline was also sprayed into the lungs of the rats using an intratracheal aerosolizer with a dose of 0.1 mL each rat once a week for 4 weeks. The rats were sacrificed after treatment for 31 days, that is, after 3 days following four times of administration. The whole lung was observed with the imaging station as above. The left lung was split into two parts. One was frozen with liquid nitrogen followed by maintaining at −80 °C for biological measurement. The other was fixed in the 4% paraformaldehyde solution followed by histopathological evaluation.
Immunohistochemistry
The sections of the left lung, initially embedded in paraffin, were deparaffinized, rehydrated, and microwave-heated for 15 min in the EDTA antigen retrieval solution (pH 8.0). A 3% H2O2 solution was used to block the endogenous peroxidase activity. The tissues were further blocked with bovine serum albumin (BSA). The primary antibody of CD31 (Good Bio, China) diluted with a 3% BSA solution was added to the above tissues and incubated overnight at 4 °C. The sections were washed with PBS three times. The secondary antibody of the primary antibody was added and incubated for 30 min at room temperature followed by interval PBS washing. The sections were immersed in the coloring substrate 3,3′-diaminobenzidine (DAB, 0.4 mg/mL, DAKO, USA) containing 0.003% H2O2 for 5 min, rinsed with water, counterstained with hematoxylin, dehydrated, and cover slipped. The sections were observed under a microscope.
Apoptosis Assay
The left lung was fixed in a 4% paraformaldehyde solution and embedded in paraffin. TUNEL (Roche, Switzerland) was performed and incubated for 1 h at 37 °C. After being washed with PBS, the sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature to detect nucleoli. Images of TUNEL and DAPI fluorescence were recorded using a fluorescent microscope.
Statistical Analysis
Student t tests were used to determine significance. All error bars represent standard deviations. The statistical difference was determined when p < 0.05, and the significant difference was defined as p < 0.01.
Acknowledgments
The work was partly supported by the Beijing Natural Science Foundation of China (no. 7154230).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00456.
X-ray diffraction graphs, differential scanning calorimetric graphs of PLGA, infrared spectra of raw oridonin powders, the physical mixture of them, and the oridonin-loaded PLGA EPMs (PDF)
Author Contributions
L.Z. and M.L. contributed equally to this article.
The authors declare no competing financial interest.
Supplementary Material
References
- Rafei H.; El-Bahesh E.; Finianos A.; Nassereddine S.; Tabbara I. Immune-based therapies for non-small cell lung cancer. Anticancer Res. 2017, 37, 377–387. 10.21873/anticanres.11330. [DOI] [PubMed] [Google Scholar]
- Xu C.; Wang P.; Zhang J.; Tian H.; Park K.; Chen X. Pulmonary codelivery of doxorubicin and sirna by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small 2015, 11, 4321–4333. 10.1002/smll.201501034. [DOI] [PubMed] [Google Scholar]
- Levet V.; Rosière R.; Merlos R.; Fusaro L.; Berger G.; Amighi K.; Wauthoz N. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 2016, 515, 209–220. 10.1016/j.ijpharm.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Luo T.; Loira-Pastoriza C.; Patil H. P.; Ucakar B.; Muccioli G. G.; Bosquillon C.; Vanbever R. Pegylation of paclitaxel largely improves its safety and anti-tumor efficacy following pulmonary delivery in a mouse model of lung carcinoma. J. Controlled Release 2016, 239, 62–71. 10.1016/j.jconrel.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Tagami T.; Ando Y.; Ozeki T. Fabrication of liposomal doxorubicin exhibiting ultrasensitivity against phospholipase A2 for efficient pulmonary drug delivery to lung cancers. Int. J. Pharm. 2017, 517, 35–41. 10.1016/j.ijpharm.2016.11.039. [DOI] [PubMed] [Google Scholar]
- Tseng C.-L.; Wu S. Y.-H.; Wang W.-H.; Peng C.-L.; Lin F.-H.; Lin C.-C.; Young T.-H.; Shieh M.-J. Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials 2008, 29, 3014–3022. 10.1016/j.biomaterials.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Zhou G.-B.; Kang H.; Wang L.; Gao L.; Liu P.; Xie J.; Zhang F.-X.; Weng X.-Q.; Shen Z.-X.; Chen J.; Gu L.-J.; Yan M.; Zhang D.-E.; Chen S.-J.; Wang Z.-Y.; Chen Z. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 2006, 109, 3441–3450. 10.1182/blood-2006-06-032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. A.; Hanes J.; Caponetti G.; Hrkach J.; Ben-Jebria A.; Eskew M. L.; Mintzes J.; Deaver D.; Lotan N.; Langer R. Large porous particles for pulmonary drug delivery. Science 1997, 276, 1868–1871. 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- Fan J.-B.; Huang C.; Jiang L.; Wang S. Nanoporous microspheres: From controllable synthesis to healthcare applications. J. Mater. Chem. B 2013, 1, 2222–2235. 10.1039/c3tb00021d. [DOI] [PubMed] [Google Scholar]
- Lee J.; Oh Y. J.; Lee S. K.; Lee K. Y. Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J. Controlled Release 2010, 146, 61–67. 10.1016/j.jconrel.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Kundawala A.; Patel V.; Patel H.; Choudhary D. Preparation, in vitro characterization, and in vivo pharmacokinetic evaluation of respirable porous microparticles containing rifampicin. Sci. Pharm. 2014, 82, 665–681. 10.3797/scipharm.1307-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanda D. S.; Tyagi P.; Mirvish S. S.; Kompella U. B. Supercritical fluid technology based large porous celecoxib–PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single dose. J. Controlled Release 2013, 168, 239–250. 10.1016/j.jconrel.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Li M.; Liu X.; Du L.; Jin Y. Inhalable oridonin-loaded poly(lactic-co-glycolic)acid large porous microparticles for in situ treatment of primary non-small cell lung cancer. Acta Pharm. Sin. B 2017, 7, 80–90. 10.1016/j.apsb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P.; Gandhimathi C.; Venugopal J. R.; Becker D. L.; Ramakrishna S.; Srinivasan D. K. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv. Drug Delivery Rev. 2015, 94, 77–95. 10.1016/j.addr.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Guo X.; Xia T.; Wang H.; Chen F.; Cheng R.; Luo X.; Li X. Electrosprayed microparticles with loaded pDNA-calcium phosphate nanoparticles to promote the regeneration of mature blood vessels. Pharm. Res. 2013, 31, 874–886. 10.1007/s11095-013-1209-y. [DOI] [PubMed] [Google Scholar]
- Bohr A.; Boetker J. P.; Rades T.; Rantanen J.; Yang M. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: A particle engineering approach. Curr. Pharm. Des. 2014, 20, 325–348. 10.2174/13816128113199990399. [DOI] [PubMed] [Google Scholar]
- Lee W.-H.; Loo C.-Y.; Traini D.; Young P. M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Delivery 2015, 12, 1009–1026. 10.1517/17425247.2015.1039509. [DOI] [PubMed] [Google Scholar]
- Papetti M.; Herman I. M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol.: Cell Physiol. 2002, 282, C947–C970. 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- Li Y.; Ahmed F.; Ali S.; Philip P. A.; Kucuk O.; Sarkar F. H. Inactivation of nuclear factor κb by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005, 65, 6934–6942. 10.1158/0008-5472.can-04-4604. [DOI] [PubMed] [Google Scholar]
- Liu W.-b.; Ao L.; Zhou Z.-y.; Cui Z.-h.; Zhou Y.-h.; Yuan X.-y.; Xiang Y.-l.; Cao J.; Liu J.-y. CpG island hypermethylation of multiple tumor suppressor genes associated with loss of their protein expression during rat lung carcinogenesis induced by 3-methylcholanthrene and diethylnitrosamine. Biochem. Biophys. Res. Commun. 2010, 402, 507–514. 10.1016/j.bbrc.2010.10.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.