Abstract
Background
In a phase II North American study (NP28761; NCT01871805), the anaplastic lymphoma kinase (ALK) inhibitor alectinib demonstrated both systemic and central nervous system (CNS) efficacy with good tolerability in patients with ALK-positive non-small cell lung cancer. We describe patient-reported outcomes (PROs) from the NP28761 study.
Patients and methods
PROs and health-related quality of life (HRQoL) benefits were assessed using two self-administered questionnaires (the European Organisation for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire-Core (EORTC QLQ-C30), and the 13-item EORTC QLQ-lung cancer-specific module) at enrolment and every 6 weeks until week 66, disease progression or death.
Results
Clinically meaningful mean improvements (≥10 point change from baseline) were observed in 10 domains, including global health status (GHS), role and social functioning, fatigue, pain, dyspnoea, and appetite loss. A clinically meaningful improvement was observed in GHS from the first assessment (6 weeks) until week 60. Alectinib demonstrated a rapid effect, with a median time to symptom improvement, using the composite endpoint of cough, dyspnoea and pain in the chest, of 1.4 months (6.1 weeks) (95% CI 1.4 to 1.6) and a median time to symptom deterioration of 5.1 months (22.1 weeks) (95% CI 2.8 to 6.8). Patients with CNS metastases at baseline experienced comparable HRQoL over the duration of the study as patients without CNS metastases. Exploratory analysis showed that the occurrence of an objective response may be associated with a better HRQoL.
Conclusions
Patients treated with alectinib in this phase II study achieved clinically meaningful improvements in HRQoL and symptoms and had delayed time to symptom deterioration.
Keywords: alectinib, health-related quality of life, non-small-cell lung cancer, patient-reported outcomes
Key questions.
What is already known about this subject?
Alectinib has shown promising systemic and central nervous system (CNS) efficacy in the treatment of patients with anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC) who were previously treated with crizotinib, and is well tolerated.
Additionally, alectinib has now demonstrated significantly superior efficacy compared with crizotinib in patients with advanced treatment-naïve ALK+ NSCLC.
What does this study add?
This current analysis is the first to demonstrate that alectinib can both improve patient-reported symptom burden and enhance health-related quality of life (HRQoL).
The median time to patient-reported outcome (PRO) symptom improvement with alectinib was 1.4 months (95% CI 1.4 to 1.6).
Baseline CNS metastases did not impact HRQoL over the duration of the study.
How might this impact on clinical practice?
Patients treated with alectinib in the North American NP28761 study reported clinically meaningful improvement in key lung cancer symptoms, patient-reported function and HRQoL while remaining a tolerable treatment option.
These PRO data are consistent with the safety data previously reported and confirm the tolerability of alectinib in patients with ALK+ NSCLC who were previously treated with crizotinib.
Introduction
Non-small cell lung cancer (NSCLC) is the most common subtype of lung cancer, accounting for around 80%–85% of all cases. In the majority of patients, the disease has reached an advanced stage by the time of diagnosis1 and often results in substantial symptom burden (eg, fatigue, dyspnoea, cough and pain).2 While more conventional anticancer therapies, such as chemotherapy, provide a marginal improvement in overall survival, the side effects are often significant, which may overshadow the treatment benefits. This can be detrimental to a patient’s health-related quality of life (HRQoL), impacting physical, emotional and social functioning. Hence, many clinical trials now include patients’ self-ratings on the impact of disease treatment on symptoms, functioning and HRQoL (collectively referred to as patient-reported outcomes (PRO)) as study endpoints to inform the assessment of clinical benefit.
Anaplastic lymphoma kinase-positive NSCLC (ALK-positive NSCLC) is a distinct subset of lung cancer which is characterised by rearrangements of the ALK gene. The current standard of care for ALK-positive NSCLC is the ALK tyrosine kinase inhibitor (TKI) crizotinib, which has demonstrated improved response rates and HRQoL compared with chemotherapy.3 4 However, progression usually occurs within 1 year of treatment on crizotinib, with the most common site of progression being the central nervous system (CNS).
Alectinib is an oral ALK TKI which has demonstrated efficacy for patients with ALK-positive NSCLC, both systemically and in the CNS.5–9 In the single-arm, phase II, North American study of alectinib in ALK-positive NSCLC in patients who were previously treated with crizotinib (NP28761; NCT01871805), at the April 2015 data cut-off, an objective response rate (ORR) of 52.2% (n=35/67) was reported in patients with measurable disease at baseline according to the independent review committee (IRC), with a median progression-free survival of 8.1 months (95% CI 6.2 to 12.6) and a median duration of response (DOR) of 13.5 months (95% CI 6.7 to not estimable). In patients with measurable CNS metastases at baseline, alectinib demonstrated a CNS ORR of 75.0% (95% CI 47.6 to 92.7) and a CNS DOR of 11.1 months (95% CI 5.8 to 11.1). Here, we summarise the PRO data from this study.
Patients and methods
Study design and patients
The design of the NP28761 study has been published previously.7 Briefly, this was a single-arm, multicentre study. Eligible patients were aged ≥18 years, with locally advanced or metastatic (stage IIIB–IV) ALK-positive NSCLC, which was confirmed by a US Food and Drug Administration-approved fluorescent in situ hybridisation test, and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2. Patients had progressed on crizotinib. Baseline CNS metastases were permitted, providing they were asymptomatic.
Patients received 600 mg of oral alectinib twice daily. The primary endpoint of the study was ORR according to the Response Evaluation Criteria in Solid Tumours V.1.1 as assessed by an IRC. HRQoL, as measured by the global health status (GHS) scale of the European Organisation for Research and Treatment of Cancer 30-Item Quality of Life Questionnaire-Core (EORTC QLQ-C30), was defined as the secondary endpoint.
The study was undertaken in accordance with the principles of the Declaration of Helsinki, and written informed consent was obtained from all patients.
Patient-completed questionnaires
Patient-reported symptoms and HRQoL were assessed using two self-administered questionnaires that have been routinely used to describe PROs in lung cancer studies.4 10 The first questionnaire is the EORTC QLQ-C30,11 which comprises 30 questions across five functional scales (physical, role, social, cognitive and emotional), a GHS scale, three symptom scales (fatigue, pain and nausea/vomiting) and various single-item symptom scales. The second questionnaire is the EORTC QLQ-lung cancer-specific module (EORTC QLQ-LC13),12 which comprises 13 questions assessing lung cancer-specific symptoms, including cough, dyspnoea, pain in the chest and others. Data were collected at randomisation and every 6 weeks until week 66, disease progression or death.
Statistical analyses
Questionnaire items were scored according to the EORTC algorithms,13 which standardise the raw score to a range of 0–100. For the functional scales and GHS items, a higher score represents a better level of functioning. For each of the symptom scales, a lower score represents a lower level of that particular symptom (ie, lower symptom burden). The mean change from baseline analyses was documented for all subscales, with a 10-point change considered to be clinically meaningful.14 15 The time to symptom deterioration or improvement was defined as the time from randomisation to the first appearance of a score ≥10 points worse or better than baseline, respectively, and was assessed for the symptoms of cough, dyspnoea or pain in the chest using the QLQ-LC13 scale.14 The time to symptom deterioration or improvement was summarised using the Kaplan-Meier methodology, without imputation of missing baseline values. Symptom deterioration was also examined for patients with and without baseline CNS metastases. Multiple exploratory analyses were conducted to understand the patient-reported benefit of alectinib’s CNS activity, including the relationship between best overall response (complete or partial) and emotional functioning and cognitive functioning. In addition, a comparison of baseline ECOG PS and PRO scores was done to evaluate differences in these subgroups.
Results
Patients
In total, 87 patients were enrolled between 4 September 2013 and 4 August 2014. The median age of patients was 54 years (range 29–79), 45% of patients were male, 90% had an ECOG PS of 0 or 1, and 84% of patients were white. At baseline, 60% (52/87) of patients had measurable and/or non-measurable CNS metastases.
Symptoms and HRQoL
The completion rate for patients who undertook PRO assessments at baseline was 90% (n=79) for both questionnaires. At the next subsequent assessment (week 6), the completion rates were 78% (n=68) for the EORTC QLQ-C30 and 72% (n=63) for the EORTC QLQ-LC13.
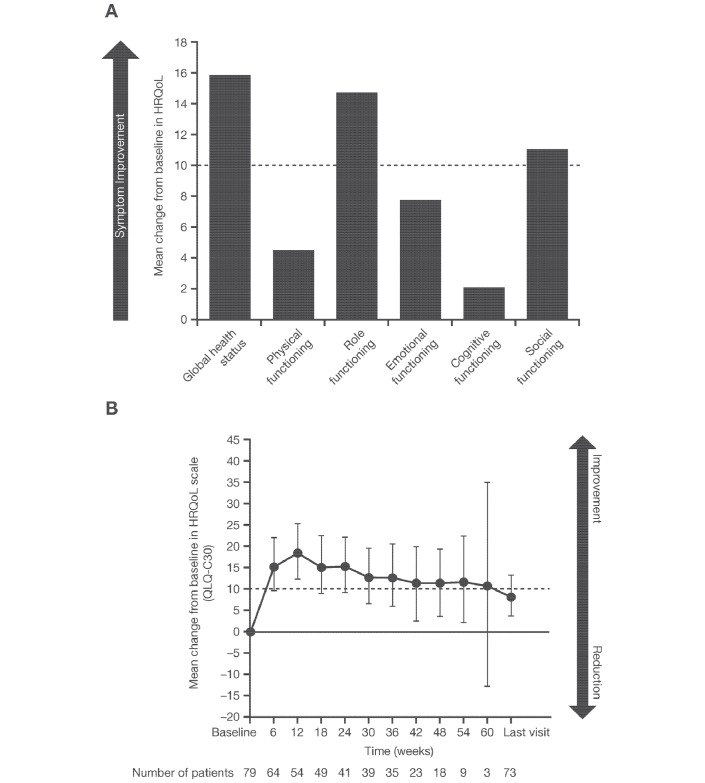
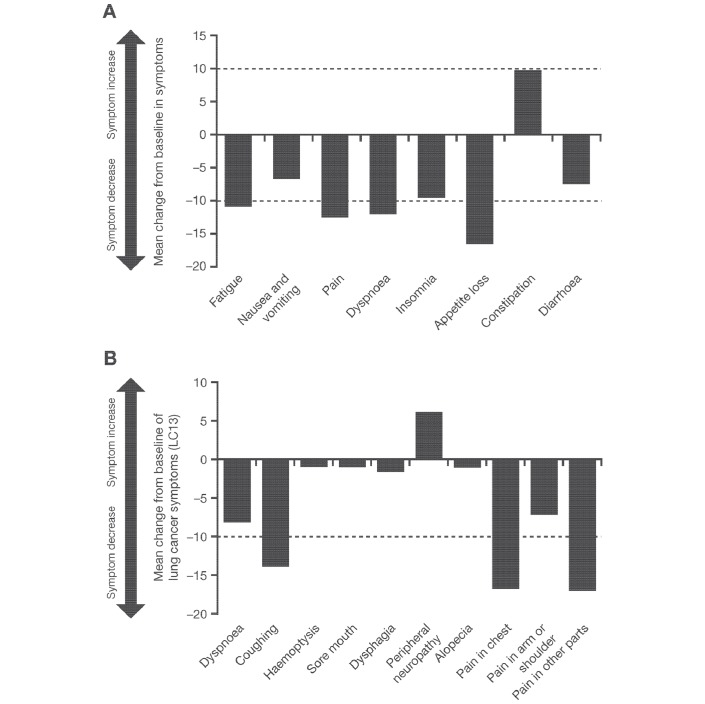
Changes from baseline values in symptoms, functioning and HRQoL scales are shown in table 1. According to the EORTC QLQ-C30, on average, patients at the first postbaseline visit (week 6) showed improvements in their GHS and all five functioning scales compared with baseline, with clinically meaningful mean changes in GHS (mean change±SD: 15.89±25.66), social functioning (11.20±23.20) and role functioning (14.84±32.00) (table 1; figure 1A). At week 12, the change from baseline in emotional functioning subscale had reached the clinically meaningful threshold at a mean value of 11.01±17.04 (n=54). The initial clinically meaningful improvement in GHS from the first assessment date was maintained during study treatment, as the mean value remained ≥10 points above baseline until just before the last study visit (figure 1B). Immediate improvements postbaseline were noted in all but one of the symptom subscale assessments using the EORTC QLQ-C30 (figure 2A). Clinically meaningful mean symptom reductions were seen for fatigue (10.94±25.70), pain (–12.50±33.47), dyspnoea (–12.17±32.96) and appetite loss (−16.67±30.28).
Table 1.
Baseline, week 6 and mean change from baseline values for the QLQ-C30 and QLQ-LC13
| Baseline mean±SD | Baseline median | Week 6 mean±SD | Week 6 median | Mean change from baseline±SD | Median change from baseline | |
| Global health status (QLQ-C30) | 53.59±24.27 | 58.33 | 71.94±20.05 | 75.00 | 15.89±25.66 | 16.67 |
| Functioning (QLQ-C30) | ||||||
| Physical | 69.96±24.43 | 73.33 | 76.18±20.19 | 80.00 | 4.69±17.44 | 0.00 |
| Social | 60.55±35.32 | 66.67 | 75.98±25.85 | 83.33 | 11.20±23.20 | 0.00 |
| Role | 58.86±34.06 | 66.67 | 76.72±27.34 | 83.33 | 14.84±32.00 | 0.00 |
| Cognitive | 77.85±24.13 | 83.33 | 81.62±24.63 | 83.33 | 2.34±20.76 | 0.00 |
| Emotional | 73.49±21.40 | 75.00 | 81.13±19.03 | 83.33 | 7.99±18.75 | 8.33 |
| Symptoms (QLQ-C30) | ||||||
| Fatigue | 45.57±27.02 | 44.44 | 33.33±24.13 | 33.33 | –10.94±25.70 | –11.11 |
| Nausea and vomiting | 15.61±20.73 | 0.00 | 7.84±17.15 | 0.00 | –6.77±19.63 | 0.00 |
| Pain | 36.29±33.63 | 33.33 | 22.30±26.95 | 16.67 | –12.50±33.47 | 0.00 |
| Dyspnoea | 33.33±30.86 | 33.33 | 22.55±24.73 | 33.33 | –12.17±32.96 | 0.00 |
| Insomnia | 31.22±30.82 | 33.33 | 22.06±25.50 | 33.33 | –9.38±29.38 | 0.00 |
| Appetite loss | 29.96±33.16 | 33.33 | 11.76±23.58 | 0.00 | –16.67±30.28 | 0.00 |
| Constipation | 20.25±26.38 | 0.00 | 27.45±29.89 | 33.33 | 9.90±30.68 | 0.00 |
| Diarrhoea | 13.25±23.01 | 0.00 | 4.90±15.52 | 0.00 | –7.41±25.00 | 0.00 |
| Symptoms (QLQ-LC13) | ||||||
| Dyspnoea | 30.85±27.13 | 22.22 | 23.28±21.56 | 22.22 | –8.24±22.57 | 0.00 |
| Cough | 33.76±27.47 | 33.33 | 19.58±21.28 | 33.33 | –13.89±31.47 | 0.00 |
| Haemoptysis | 1.27±6.41 | 0.00 | 0.53±4.20 | 0.00 | –1.11±6.03 | 0.00 |
| Sore mouth | 5.06±16.09 | 0.00 | 2.12±8.19 | 0.00 | –1.11±13.68 | 0.00 |
| Dysphagia | 4.64±11.61 | 0.00 | 1.59±7.16 | 0.00 | –1.67±11.36 | 0.00 |
| Peripheral neuropathy | 13.08±22.28 | 0.00 | 15.87±25.30 | 0.00 | 6.11±17.88 | 0.00 |
| Alopecia | 11.69±25.80 | 0.00 | 9.52±22.74 | 0.00 | –1.13±22.29 | 0.00 |
| Pain in the chest | 21.52±28.6 | 0.00 | 5.82±17.50 | 0.00 | –16.67±29.75 | 0.00 |
| Pain in the arm or shoulder | 16.88±28.18 | 0.00 | 9.52±19.33 | 0.00 | –7.22±25.37 | 0.00 |
| Pain in other parts | 44.00±36.42 | 33.33 | 25.27±33.43 | 0.00 | –16.96±42.78 | 0.00 |
Values in bold represent clinically meaningful changes from baseline (ie, ≥10 point change from baseline).
Data cut-off: 27 April 2015.
QLQ-C30, 30-Item Quality of Life Questionnaire-Core; QLQ-LC13, 13-Item Quality of Life Questionnaire-lung cancer-specific module.
Figure 1.
(A) Mean change from baseline in global health status and functional scales of the QLQ-C30 after 6 weeks of alectinib treatment. (B) Maintenance of clinically meaningful improvement in global health status throughout the study. HRQoL, health-related quality of life; QLQ-C30, 30-Item Quality of Life Questionnaire-Core.
Figure 2.
Mean change from baseline in symptom scales and single-item assessments after 6 weeks of alectinib treatment according to (A) the QLQ-C30 and (B) the QLQ-LC13. QLQ-C30, 30-Item Quality of Life Questionnaire-Core; QLQ-LC13, 13-Item Quality of Life Questionnaire-lung cancer-specific module.
An improvement from baseline was also seen in common treatment-related symptoms such as nausea and vomiting, insomnia and diarrhoea. The only treatment-related symptom to worsen during alectinib treatment was constipation (mean change: 9.90±30.68); however, this did not reach the clinically meaningful threshold.
Analysis of the EORTC QLQ-LC13 also showed mean improvement in all but one of the symptom and subscale assessments (figure 2B). By the first postbaseline assessment (6 weeks), clinically meaningful improvement was seen in patient-reported cough (–13.89±31.47), pain in the chest (–16.67±29.75) and pain in other parts (–16.96±42.78) (table 1, figure 2B).
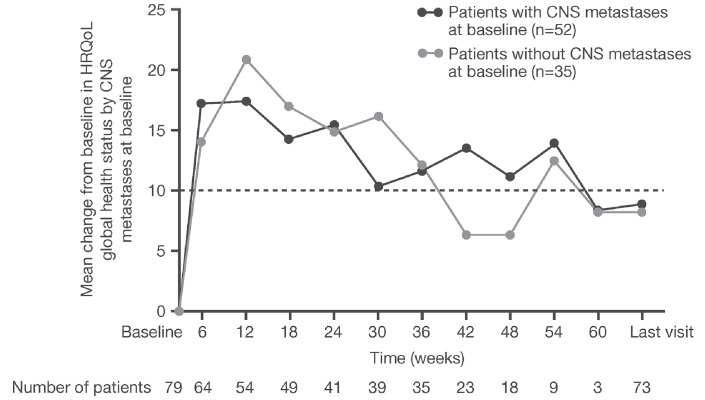
Clinically meaningful improvement of similar magnitude was seen in the mean GHS over time in both patients with or without CNS metastases at baseline (figure 3).
Figure 3.
Mean change from baseline in HRQoL scores for patients with and without baseline CNS metastases. Data cut-off: 27 April 2015. CNS, central nervous system; HRQoL, health-related quality of life.
Treatment with alectinib resulted in a median time to symptom improvement, using the composite endpoint of cough, dyspnoea and pain in the chest, of 1.4 months (95% CI 1.4 to 1.6; 6.1 weeks (95% CI 6.1 to 7.0 weeks)), with more than 50% of patients showing improvement within the first few weeks (figure 4A). The median time to symptom deterioration, defined as being free of a clinically meaningful deterioration in cough, dyspnoea or chest pain, was 5.1 months (95% CI 2.8 to 6.8; 22.1 weeks) using the EORTC QLQ-LC13 (figure 4B).
Figure 4.
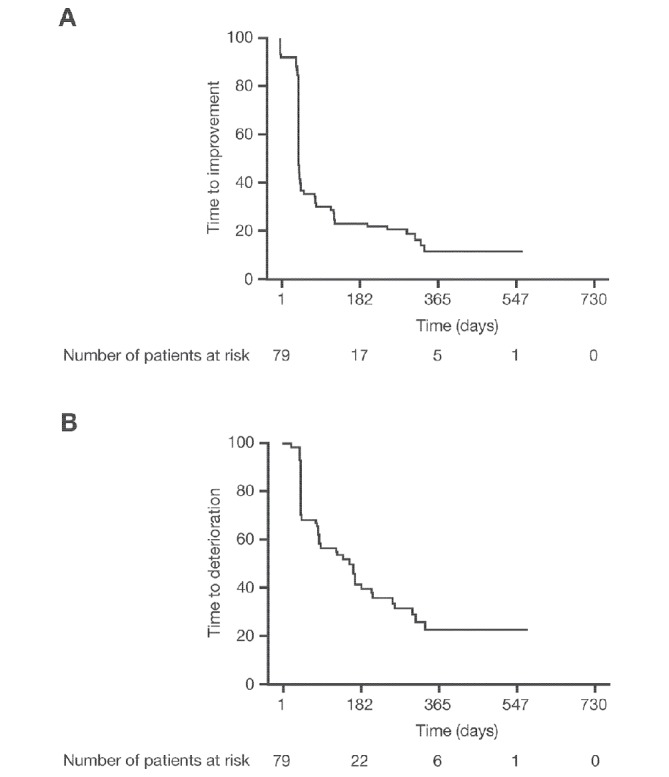
Kaplan-Meier plots of (A) time to first improvement and (B) time to first deterioration in pain in the chest, cough and dyspnoea, according to the 13-Item Quality of Life Questionnaire-lung cancer-specific module.
Relationship between best overall response, emotional functioning and cognitive functioning
Online supplementary figure 1 shows the mean changes from baseline in patient-reported emotional and cognitive functioning for both responders (complete or partial response) and non-responders over the 66-week study period. On average, patients who were responders generally maintained or improved their level of emotional functioning and cognitive functioning while on treatment through week 66. Patients who were non-responders reported either maintained or modestly decreased scores for both functioning scales while on treatment.
esmoopen-2018-000364supp001.pdf (136.7KB, pdf)
Relationship between ECOG PS and PROs
An exploratory analysis to show the relationship between ECOG PS and baseline PRO scores was undertaken and, according to the respective items and scales of the EORTC QLQ-C30, patients with a higher ECOG PS (1 or 2) had worse mean symptom values at baseline for appetite loss, constipation, fatigue, nausea and vomiting, and pain, compared with patients with an ECOG PS of 0 (online supplementary table 1), as would be expected (patients with an ECOG PS of 0 are by definition asymptomatic). Similarly, patients with a higher ECOG PS had lower mean scores across all five functioning scales. With respect to the items and scales of the EORTC QLQ-LC13, a higher ECOG PS was associated with worse mean baseline symptom scores in dysphagia, alopecia, pain in other parts and sore mouth, compared with an ECOG PS of 0 (online supplementary table 1).
Discussion
Alectinib has shown promising efficacy in the treatment of ALK-positive NSCLC, both systemically and in the CNS,5–9 and is well tolerated. Additionally, alectinib has now demonstrated significantly improved progression-free survival over crizotinib in patients with advanced treatment-naïve ALK+ NSCLC.9 This current analysis is the first to demonstrate that alectinib can both improve patient-reported symptom burden and enhance HRQoL.
By the first postbaseline PRO assessment (week 6), patients reported a clinically meaningful improvement in many lung cancer symptoms (fatigue, dyspnoea, cough, pain, chest pain, pain in other parts), suggesting an immediate symptom benefit with treatment. Furthermore, time-to-event analyses concerning core lung cancer symptoms of dyspnoea, cough and chest pain demonstrated a median time to symptom improvement of 1.4 months, which corresponds to the first postbaseline PRO assessment. The immediate symptom benefit with alectinib was sustained for patients on treatment; additional time-to-event analyses indicated that the median time to symptom deterioration was approximately 5.1 months from baseline. Correspondingly, a clinically meaningful improvement in GHS was seen at the earliest postbaseline PRO assessment and maintained until before the last study visit, demonstrating an early and sustained improvement in HRQoL for alectinib-treated patients in this study. This pattern of lung cancer symptom and HRQoL improvement was observed in patients irrespective of the presence of CNS metastases at baseline. A clinically meaningful improvement in loss of appetite was also seen; a recent study by Patel et al16 demonstrated that weight gain, due to overall improvement of appetite loss and improvement in nausea/vomiting, can be used as an indicator of clinical benefit. This PRO finding was supported via observations of weight gain in 18.6% of patients in the North American study (NP28761; NCT01871805) and in 12.3% of patients in the phase II global study (NP28673; NCT018011) at the updated 2016 data cut-offs; however, this gain could also be related to possible increases in fluid retention.
The baseline values observed in this study were similar to historical values reported in patients with stage III/IV lung cancer using the EORTC QLQ-C30 questionnaire.17 The large SDs observed throughout these analyses are common for skewed populations, which is a limitation of the mean change score estimates; meaningful median improvements, however, were also seen in the GHS and fatigue domains of the EORTC QLQ-C30.
In conclusion, patients treated with alectinib in the North American NP28761 study reported clinically meaningful improvement in key lung cancer symptoms, patient-reported function and HRQoL while remaining a tolerable treatment option. In the ALUR study (NCT02604342), alectinib improved HRQoL, functioning and symptom burden versus chemotherapy in patients with ALK+ NSCLC after crizotinib failure (ALUR, NCT02604342).18 Quality of life endpoints are being evaluated in two first-line phase III studies investigating alectinib (ALEX, NCT020758409 and J-ALEX, JapicCTI-132316).19
Acknowledgments
The authors would like to thank all of the NP28761 investigators, their patients and their families. Third-party medical writing support was provided by Louise Clarke, PhD, of Gardiner-Caldwell Communications, and was funded by F Hoffmann-La Roche.
Footnotes
Contributors: Study concept: ATS, VS, GJR, WB, KD, SG, S-HIO and AZ. Study design: ATS, VS, GJR, WB, KD and SG. Data acquisition: ATS, VC, GJR, SG, HW, AAC, MAS, S-HIO and LG. Quality control of data and algorithms: EW and WB. Data analysis and interpretation: ATS, EW, VC, GJR, WB, KD, SG, HW, AAC, MAS, S-HIO, AZ and VS. Statistical analysis: EW. Manuscript preparation: EW, WB, KD, HW, AAC, MAS, S-HIO, AZ and VS. Manuscript editing: ATS, EW, GJR, KD, SG, HW, AAC, MAS, S-HIO and LG. Manuscript review: S-HIO, ATS, EW, VC, GJR, WB, KD, SG, HW, AAC, MAS, AZ and VS.
Funding: This work, including data analyses and submission of the article, was supported by F Hoffmann-La Roche. No grant number is applicable.
Competing interests: S-HIO has acted in a consulting or advisory role to ARIAD, AstraZeneca, Boehringer Ingelheim, Novartis and Roche, and participated in the speaker bureaus for AstraZeneca, Boehringer Ingelheim and Roche/Genentech. MAS has provided corporate-sponsored research support for Genentech and participated in the speaker bureaus for Genentech. SG has participated in the advisory boards for Genentech/Roche, ARIAD, Novartis and Pfizer. LG received consultancy fees from Genentech/Roche, Pfizer, Merck, AbbVie and AstraZeneca, and personal fees from Merck and BMS IION Foundation. HW has participated in the advisory boards for ARIAD, Genentech/Roche and Takeda, and received honoraria for non-branded presentations for ARIAD and Genentech/Roche. AAC has participated in the advisory boards for Novartis, Takeda, BMS and Lilly, provided corporate-sponsored research for Novartis and BMS, and has relationships with Genentech, Merck, Takeda, Boehringer Ingelheim, Pfizer, Celgene and Novartis. VC has participated in the advisory boards for Pfizer Canada. GJR has acted in a consulting role to Novartis, and provided corporate-sponsored research support to Chugai, Roche, Pfizer, Novartis and Millennium. VS, WB, EW, KD and AZ are employees of F Hoffmann-La Roche and hold shares in the company. ATS has participated in the advisory boards for Pfizer, Novartis, Roche/Genentech, ARIAD, Blueprint Medicines, Loxo and EMD Serono, and received honoraria for non-branded presentations for Pfizer, Novartis, Genentech/Roche, Ignyta, Taiho and Foundation Medicine.
Patient consent: Not required.
Ethics approval: The institution boards of each study site approved this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ferlay J, Shin HR, Bray F, et al. . Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2. Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer 2013;81:288–93. 10.1016/j.lungcan.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 3. Shaw AT, Kim DW, Nakagawa K, et al. . Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 4. Blackhall F, Kim DW, Besse B, et al. . Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol 2014;9:1625–33. 10.1097/JTO.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 5. Seto T, Kiura K, Nishio M, et al. . CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590–8. 10.1016/S1470-2045(13)70142-6 [DOI] [PubMed] [Google Scholar]
- 6. Ou SH, Ahn JS, De Petris L, et al. . Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase ii global study. J Clin Oncol 2016;34:661–8. 10.1200/jco.2015.63.9443 [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Gandhi L, Gadgeel S, et al. . Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–42. 10.1016/S1470-2045(15)00488-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gadgeel SM, Shaw AT, Govindan R, et al. . Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol 2016;34:4079–85. 10.1200/JCO.2016.68.4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peters S, Camidge DR, Shaw AT, et al. . Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377:829–38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 10. Geater SL, Xu CR, Zhou C, et al. . Symptom and quality of life improvement in LUX-Lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J Thorac Oncol 2015;10:883–9. 10.1097/JTO.0000000000000517 [DOI] [PubMed] [Google Scholar]
- 11. Aaronson NK, Ahmedzai S, Bergman B, et al. . The european organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 12. Bergman B, Aaronson NK, Ahmedzai S, et al. . The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer 1994;30A:635–42. [DOI] [PubMed] [Google Scholar]
- 13. Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer 2001;37:1331–4. [DOI] [PubMed] [Google Scholar]
- 14. Osoba D, Rodrigues G, Myles J, et al. . Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44. 10.1200/JCO.1998.16.1.139 [DOI] [PubMed] [Google Scholar]
- 15. Osoba D, Bezjak A, Brundage M, et al. . Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 2005;41:280–7. 10.1016/j.ejca.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 16. Patel JD, Pereira JR, Chen J, et al. . Relationship between efficacy outcomes and weight gain during treatment of advanced, non-squamous, non-small-cell lung cancer patients. Ann Oncol 2016;27:1612–9. 10.1093/annonc/mdw211 [DOI] [PubMed] [Google Scholar]
- 17. Scott NW, Fayers PM, Aaronson NK, et al. . EORTC QLQ-C30 reference values. 2008. http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf; (accessed 9 Feb 2018).
- 18. Mazières J, Novello S, Castro J, et al. . Patient-reported outcomes and safety from the phase III ALUR study of alectinib vs chemotherapy in pre-treated ALK+ NSCLC: WCLC, 2017. [Google Scholar]
- 19. Hida T, Nokihara H, Kondo M, et al. . Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29–39. 10.1016/S0140-6736(17)30565-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2018-000364supp001.pdf (136.7KB, pdf)