Abstract
From childhood to adolescence, strengthened coupling in frontal, striatal and parieto‐temporal regions associated with cognitive control, and increased anticorrelation between task‐positive and task‐negative circuits, subserve the reshaping of behavior. ADHD is a common condition peaking in adolescence and regressing in adulthood, with a wide variety of cognitive control deficits. Alternate hypotheses of ADHD emphasize lagging circuitry refinement versus categorical differences in network function. However, quantifying the individual circuit contributions to behavioral findings, and relative roles of maturational versus categorical effects, is challenging in vivo or in meta‐analyses using task‐based paradigms within the same pipeline, given the multiplicity of neurobehavioral functions implicated. To address this, we analyzed 46 positively‐correlated and anticorrelated circuits in a multivariate model in resting‐state data from 504 age‐ and gender‐matched youth, and created a novel in silico method to map individual quantified effects to reverse inference maps of 8 neurocognitive functions consistently implicated in ADHD, as well as dopamine and hyperactivity. We identified only age‐ and gender‐related effects in intrinsic connectivity, and found that maturational refinement of circuits in youth with ADHD occupied 3‐10x more brain locations than in typical development, with the footprint, effect size and contribution of individual circuits varying substantially. Our analysis supports the maturational hypothesis of ADHD, suggesting lagging connectivity reorganization within specific subnetworks of fronto‐parietal control, ventral attention, cingulo‐opercular, temporo‐limbic and cerebellar sub‐networks contribute across neurocognitive findings present in this complex condition. We present the first analysis of anti‐correlated connectivity in ADHD and suggest new directions for exploring residual and non‐responsive symptoms.
Keywords: ADDH, adolescence, attention, cerebellum, child, development, frontal lobe, parietal lobe
1. INTRODUCTION
ADHD is the most prevalent developmental neurobehavioral condition, affecting ∼7% of youth (Thomas, Sanders, Doust, Beller, & Glasziou, 2015) and over 3% of adults (Fayyad et al., 2007; Uchida, Spencer, Faraone, & Biederman, 2015) worldwide. ADHD is clinically diagnosed by soliciting subjective observations from parents, teachers and/or affected individuals using rating scales with questions oriented toward the cardinal DSM5 criteria of inattentiveness or hyperactivity‐impulsivity, and individuals may qualify with symptoms that are wholly inattentive, hyperactive‐impulsive, or a mixture. Unfortunately, these categorical DSM subtypes have limited utility in predicting treatment response. Beyond the categorical symptom axes, impairment in 8 domains of neurocognitive function have been consistently associated with ADHD in targeted and meta‐analytic studies: cognitive flexibility, goal selection, response inhibition, response precision, selective attention, sustained attention, temporal information processing and working memory (Mueller, Hong, Shepard, & Moore, 2017). However, the categorical DSM nosology does not fully capture this multi‐system neurocognitive spectrum, and there is no clear way to map these 8 neurocognitive domains onto the categorical symptom axes of inattention and hyperactivity‐impulsivity. However, this neurocognitive spectrum is of great interest since it may more easily be related to models of brain‐behavior correspondence and neurodevelopment, and potentially explain residual and treatment‐nonresponsive symptoms.
The reported prevalence of ADHD increases throughout childhood to peak in adolescence at ∼12% (Thomas et al., 2015), reducing to a quarter of this in adulthood. This pattern strongly suggests a relationship between ADHD symptoms and brain functional maturation. Many studies have probed the association between behavioral maturation and concomitant changes in the organization of intrinsic networks (IN). INs are large‐scale functional neural networks observable while the brain is in the resting (task‐free) state, and during the performance of specific tasks (Erhardt, Allen, Damaraju, & Calhoun, 2011; Smith et al., 2009). During the period from latency to young adulthood, intrinsic functional networks undergo maturational changes concomitant with ongoing development of core behavioral processes associated with adolescence, such as self‐concept, risk‐taking, response inhibition, working memory, goal‐directed behavior, and executive function more broadly (Blakemore, 2012). An increasing evidence base suggests these changes represent functionally‐continuous—though not necessarily smoothly‐incrementing—intermediate stages between childhood and adulthood, with IN reorganization and refinement at the macroscale serving as a substrate for behavioral and cognitive development (Ernst, Torrisi, Balderston, Grillon, & Hale, 2015). Perhaps the best‐described phenomenon is increasing long‐ versus short‐range connectivity (Dosenbach et al., 2010; Fair et al., 2009; Supekar, Musen, & Menon, 2009), particularly between task‐positive lateral/medial frontal, striatal and parieto‐temporal regions associated with cognitive control. These developments in youth are a segment of a longer arc extending from infancy to adulthood where network organization shifts from local to distributed, with increased efficiency and specialization associated with stronger connectivity within individual networks to support the behavioral shifts entrained in youth (Casey, Jones, & Somerville, 2011; Ernst, 2014; Rubia, 2013).
Intrinsic functional networks are known to exhibit positively‐correlated and anticorrelated relationships (Fox et al., 2005). A growing body of evidence suggests that increasing anticorrelation between networks during childhood and adolescence contributes to the refinement of discrete networks in adulthood. For example, robustly increasing negative correlations during maturation in this age period have been demonstrated between default mode network nodes and task‐positive network nodes associated with attention and working memory (Chai, Ofen, Gabrieli, & Whitfield‐Gabrieli, 2014). Since superior cognitive performance and executive function in adults is associated with greater anticorrelation between task‐positive and default mode networks (Keller et al., 2015), it has been proposed that anticorrelative strengthening is related to observed attention and executive function development in this period. Taken together, the evidence from fMRI research suggests a shift over this period of late childhood to adolescence towards more mature, top‐down controlled cognition effected via the reshaping of task‐directed fronto‐striatal/fronto‐parietal‐temporal circuits, and strengthening dissociation between these circuits and task‐negative anticorrelations (Rubia, 2013).
Collectively, these observations have contributed to the developmental delay hypothesis of ADHD, where symptoms are theorized to arise from lagging development of cognitive control processes and their underlying circuits, and which many volumetric studies support. Recent high‐profile findings from the ENIGMA study in >3,000 subjects ages 4–63 years old (yo) identified a right‐shifted brain maturational trajectory in ADHD, with delays in development, and later degeneration, of the accumbens, amygdala, caudate, hippocampus and intracranial volume versus controls (Hoogman et al., 2017). Overall, the most consistent findings in whole‐brain cross‐sectional studies in ADHD are of reduced gray matter volume in the basal ganglia, and reductions in frontal, temporal and cerebellar volume and cortical thickness (Rubia, Alegria, & Brinson, 2014). These converge with an earlier generation of fMRI studies examining task‐activation effects, that consistently demonstrated fronto‐striatal differences and more recently, fronto‐parietal and fronto‐cerebellar differences. Recent meta‐analytic studies have suggested that effects in these oft‐implicated regions may be mapped to separable intrinsic networks and neurocognitive tasks and deficits. A meta‐analysis of 55 task fMRI studies by Cortese et al highlighted hypoactivation in children in ventral attention and fronto‐parietal networks with hyperactivation in default mode, ventral attention and somatomotor networks. In adults, hypoactivation was almost completely associated with the fronto‐parietal network, illustrating the maturationally path‐dependent natural history of ADHD, though this analysis was limited to cortical areas in a 7‐network model (Cortese et al., 2012). Meta‐analysis of activation differences in ADHD versus TD in individual neurocognitive functions has been limited by insufficient numbers of studies, but a series of informative studies by Hart et al found hypoactivation in response inhibition tasks in right inferior frontal cortex‐anterior cingulate‐striato‐thalamic areas versus reduced activation for attention tasks in right dorsolateral prefrontal‐parietal‐striato‐thalamic areas (Hart, Radua, Nakao, Mataix‐Cols, & Rubia, 2013) and in left inferior frontal‐parietal‐insula‐cerebellar regions in timing tasks (Hart, Radua, Mataix‐Cols, & Rubia, 2012). An important contribution to our emerging understanding of ADHD also comes from studies describing a “DMN interference” hypothesis. Multiple studies have demonstrated that problems in appropriately inactivating the DMN during cognitively‐effortful tasks occur as part of normal development, since this ability improves with age. Attenuated deactivation of the DMN has been seen in ADHD during sustained attention (Christakou et al., 2013), timing tasks (Hart et al., 2012) and more generally (Cortese et al., 2012). DMN interference is likely part of the larger theme of brain maturational delay in ADHD with respect to the strengthening in anticorrelated brain function observed in normal development. Abnormally strong coherence between the dorsolateral prefrontal cortex and DMN, and reduced anticorrelation between these networks, correlates with poor attentional performance in the resting‐state (Hoekzema et al., 2014) and sustained attentional task performance (Christakou et al., 2013).
To help resolve the relative roles of developmental lag versus categorical diagnosis differences in connectivity in individual intrinsic networks and their anticorrelated circuits, direct comparison in the same subject group of these two variables and their relative effect sizes is desirable. However, there is a paucity of studies directly examining the relative explanatory power of maturational (age) effects versus categorical diagnosis in high‐order models of brain function in ADHD, particularly with respect to anticorrelated circuits, and how these differences may map onto the neurocognitive functions associated with ADHD. Even fewer studies have addressed the question of relative effect sizes between circuits. The question of model order is also important, since connectivity findings within networks in a 7‐network model may be conceptually similar to findings between (sub)networks in a 30‐ or 40‐network model. Further complications arise from the large number of neurocognitive functions implicated in ADHD and differences in methodology. Our evidence comes from many smaller studies in individual task‐paradigms and meta‐analyses of these studies, and is still limited by insufficient power and methodologic differences. This is understandable since constructing an in vivo task‐based fMRI experiment to examine multiple tasks in the same subjects is highly challenging given the number of paradigms required and the ambiguity of task performance with age, since younger individuals may use different strategies to perform the same task as an older individual.
The resting‐state condition offers an attractive complement and removes the methodologic challenges of task performance. A few whole‐brain studies have identified lagging connectivity within networks in ADHD versus typically‐developing (TD) individuals during childhood and adolescence (Sripada, Kessler, & Angstadt, 2014). These have generally used grid‐based ROI methods in low model orders. While these methods have the benefits of sparsity and low dimensionality, nodes are exclusively allocated to hypothesized network boundaries such as the Yeo‐Krienen networks (Yeo et al., 2011), not allowing for nodes functioning in multiple networks. Multifunctional brain nodes are not only biologically likely, but are of special interest in ADHD since networks involved in higher‐order cognition and cognitive control tend to contain large proportions of association cortex. As well, accumulating evidence suggests that functional parcellations are more useful for network identification (Craddock, James, Holtzheimer, Hu, & Mayberg, 2012; Sepulcre, Sabuncu, & Johnson, 2012; Smith et al., 2011). By contrast, data‐driven methods such as independent component analysis blindly decompose the whole‐brain fMRI signal into multiple, temporally‐coherent individual signals corresponding to brain networks (Allen et al., 2011). These techniques are well‐suited to data discovery, can allow for multifunctional nodes and are robust to noise and motion, though expertise is required to separate intrinsic networks from noise components, and identify the networks extracted (Calhoun & de Lacy, 2017). Surprisingly few ICA‐based studies have been performed in ADHD in the large samples offered by multi‐site public data repositories and none to our knowledge have focused equal attention on anti‐correlations or used a high model order capable of delineating (sub)networks of particular interest such as the right versus left fronto‐parietal control network (Smith et al., 2009), or subnetworks of the DMN (Andrews‐Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010).
We wanted to bring together several major strands of evidence in ADHD, and aimed to compare the relative explanatory power of categorical diagnosis versus maturation in both intrinsic networks and their anticorrelated circuits in a large sample of TD and ADHD youth in a multivariate model capable of quantifying the relative effect sizes in each circuit of interest. Though reassuring similarities between resting‐state intrinsic networks and the networks activated during tasks have been identified (Smith et al., 2009), a significant disadvantage of the resting‐state condition is the “loss” of close connection to individual neurocognitive functions that task performance enables. An additional exploratory aim we formulated was to examine the relative contribution of identified effects to each of the 8 major neurocognitive functions implicated in ADHD and TD subjects within a consistent processing pipeline and analytic methodology. To accomplish this, we developed a novel in silico approach to map resting‐state effects to neurocognitive functional maps, integrating newer resources available in the Neurosynth (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011) computational neuroscience tools. We hypothesized that more effects differentiating youth with ADHD versus TD would be associated with age versus categorical diagnosis, that anti‐correlated circuits would exhibit more differences in ADHD versus TD youth, and that affected subjects would have higher loading of circuit effects onto the neurocognitive functions implicated in ADHD.
2. METHODS
2.1. Functional MRI processing
Unprocessed resting‐state fMRI data for subjects with ADHD and TD ages 7.0–17.9 were obtained from the ADHD‐200 repository and matched for age and gender. The ADHD‐200 repository is a public data resource containing resting‐state fMRI and some phenotypic information from 776 subjects collected by the ADHD‐200 Consortium from 8 independent sites, comprising 491 TD individuals and 285 youth with ADHD (http://fcon_1000.projects.nitrc.org/indi/adhd200/) (Consortium, 2012). The study was declared exempt from human subjects research considerations by the University of Washington Institutional Review Board. The first five timepoints were discarded from the beginning of each scan to account for possible MRI equilibration effects. Scans were slice‐time corrected to the middle scan volume, realigned to the first image in the series and coregistered and normalized to the functional template using standard algorithms in SPM12. After processing, data were submitted to quality control to assess the quality of the normalization and degree of subject motion by computing (a) spatial regression between each normalized functional image and a group mask constructed from all subjects and (b) root mean square difference of volume N to volume N + 1, also known as DVARS (Christodoulou et al., 2013; Power et al., 2014). Subjects with <95% correspondence between their normalized image and the mask, and subjects with motion >2 SDs from the mean DVARS were eliminated from consideration.
2.2. Construction of subject sample
Our goal was to create an age‐matched subject sample that was approximately representative of the youth population with ADHD in terms of age distribution, handedness, co‐morbidities, gender and head motion while preserving statistical power given the effect size in resting‐state imaging. Subjects passing imaging quality control criteria were selected to create an approximately age‐ and gender‐matched sample of 252 youth with ADHD and 252 TD youth, ages 7.0–17.9 with demographic characteristics summarized in Table 1.
Table 1.
Demographic characteristics of subjects
| Typically‐developing | ADHD | |||
|---|---|---|---|---|
| Age group (years) | Number | Percentage of male | Number | Percentage of male |
| 7–8.99 | 38 | 52.6 | 40 | 65.0 |
| 9–10.99 | 72 | 62.5 | 73 | 71.2 |
| 11–12.99 | 65 | 78.5 | 60 | 80.0 |
| 13.0–14.99 | 51 | 94.1 | 54 | 94.4 |
| 15.0–17.99 | 26 | 80.8 | 25 | 80.0 |
| Total | 252 | 73.4 | 252 | 78.1 |
Age and IQ for this sample exhibited skew and kurtosis in the range of ±3.0, considered acceptable for a normal distribution (Kline, 2005). Subjects had full scale IQ (FSIQ) ranging from 73 to 153 with a mean of 110. There was no significant difference in FSIQ between groups. Our sample contains 3:1 male to female ratio and is weighted toward children over 11 yo, directionally similar to the overall population distribution in ADHD. Within the younger age bands we included a slightly greater proportion of females given our desire to include more younger subjects and the relative paucity of male controls of this age.
The clinically‐diagnosed ADHD population is enriched for left‐handedness, though its significance is disputed (Ghanizadeh, 2013). We retained this characteristic and there was a significant difference in handedness scores between groups (p = .003) with more left‐handedness in the ADHD group. Similarly, in the ADHD group 96 subjects (38%) reported past or present co‐morbidities including specific learning disorders, oppositional defiant disorder, depression, anxiety disorders and Tourette's Syndrome, approximately equivalent to the general population with ADHD. 59 subjects with ADHD were reported to not be medication‐naive. Subjects with ADHD are also known to have higher rates of head motion during MRI scanning and this has been proposed as a genuine trait—and perhaps genetic—difference (Couvy‐Duchesne et al., 2016). Group differences in DVARS, the frame‐to‐frame measure of head motion, were significant at p < .05, with ADHD subjects having more head motion.
2.3. Group independent component analysis
After processing the fMRI data, we performed ICA using the Group ICA of fMRI Toolbox (GIFT) developed in our group, and widely used in ICA of fMRI (Calhoun & Adali, 2012; Calhoun, Adali, Pearlson, & Pekar, 2001). Resting‐state scans were first pre‐whitened followed by a subject‐specific data reduction principal components analysis retaining 105 principle components (PCs) with the objective of stabilizing back reconstruction and retaining maximum variance at the individual level. A high‐order 100‐component group ICA was then performed using the Infomax algorithm run 10 times with random initialization using ICASSO (Himberg, Hyvarinen, & Esposito, 2004; Li, Adali, & Calhoun, 2007). Aggregate spatial maps were estimated as the centrotypes of component clusters to reduce sensitivity to initial algorithm parameters. Single‐subject images were concatenated in time to perform the single group ICA estimation and subject specific spatial maps estimated using back reconstruction (Erhardt et al., 2011) with the group information guided ICA (GIG‐ICA) algorithm (Du et al., 2016), an approach which we have shown well‐captures individual subject variability (Allen, Erhardt, Wei, Eichele, & Calhoun, 2012). GIG‐ICA estimates single‐subject images and timecourses from the single group ICA estimation, thereby allowing individual variation in spatial maps. The resulting independent components were scaled by converting each subject component image and the time course to z‐scores.
2.4. Functional brain network identification and grouping
Independent components were sorted into gray‐matter networks versus artifactual noise components using a combination of expert visual inspection and quantitative metrics. For each of the 100 components we computed the spectral metrics of (a) Fractional amplitude of low frequency fluctuations (fALFF) and (b) Dynamic range (Allen et al., 2011). fALFF is the ratio of the integral of spectral power below 0.10 Hz to the integral of power between 0.15 and 0.25 Hz. Dynamic range is the difference between the peak power and minimum power at frequencies to the right of the peak. Generally, components representing brain networks have higher values in these spectral metrics, while noise components (such as signals accruing from cerebrospinal fluid, vascular pulsations, white matter or head motion) have lower values. Components were inspected and those with poor overlap with cerebral gray matter or low spectral metrics were discarded.
The primary neurocognitive function of each network was attributed by visual inspection and quantitative comparisons using two methods. Firstly, maxima of coordinates in Montreal Neurologic Space (MNI) associated with each of the INs were calculated and these compared with the literature. Multiple literature‐based confirmatory sources with specific Talairach or MNI coordinates were available for all members of the task‐positive network group, the DMN and primary sensorimotor and visual networks (Dosenbach et al., 2007; Dosenbach et al., 2006; Fox et al., 2005; Laird et al., 2011; Power et al., 2011; Seeley et al., 2007; Smith et al., 2009; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Vernet, Quentin, Chanes, Mitsumasu, & Valero‐Cabre, 2014). Secondly, the neurocognitive function of the top 5 spatial locations in each IN were examined using the using the Brodmann Interactive Atlas (http://www.fmriconsulting.com/brodmann/Interact.html).
2.5. Statistical analysis
We constructed network spatial maps by selecting voxels that represented the strongest and most consistent coactivations for each component of interest by performing a voxelwise one‐sample t test on the individual subject component imaging and thresholding individual voxels at (mean + 4 SDs). Nuisance regressors composed of individual sites, DVARS measure and the six realignment parameters and their six first derivatives were regressed from the analysis. Thus, these spatial maps represent the brain regions most associated with each component's timecourse, instantiated in thresholded t maps. This procedure enabled us to construct a group spatial map for each of the networks assembled from the relevant individual subject timecourses, and perform statistical tests on a voxelwise basis
We performed a multivariate analysis of covariance (MANCOVA) using the MANCOVAN toolbox in GIFT, to compare the effects of age with other possible predictors of variance in the same set of network maps for (a) all 504 subjects, (b) the 252 TD subjects, and (c) the 252 subjects with ADHD. To optimize for the large dimensions of the data but enable statistical testing at each voxel, predictors were submitted to the MANCOVA with an F test at each iteration to produce a final reduced model for each outcome measure and network, before univariate testing of significant predictors was performed on the original model with correction for multiple comparisons (among the 46 networks analyzed) and false discovery rate (FDR) at α = 0.01. We used age, gender, FSIQ‐level, scan site and DVARS measure as predictors for all three analyses. For the first analysis of the combined group of 504 subjects, categorical diagnosis (group) was also added as a predictor. We retained DVARS measure and site to test for any residual effects of motion or site on the statistical testing. Significant effects were computed for both positively‐correlated voxels in each network and for voxels with corresponding anti‐correlated timecourses.
For each predictor that proved significant in the univariate analysis, the effect size (beta) was determined by computing connected voxel clusters (similarly to the bwlabeln function in MATLAB) and then calculating an average beta over the cluster of voxels. Of note, it is to be expected that voxels closer to cluster boundaries may have smaller betas than those obtained in more central voxels. This is true in general of any ROI‐based approach which assumes a homogeneous behavior without a fixed region. Larger clusters will thus have more voxels with lower values compared to smaller clusters (in addition to more voxels with larger values). However, compared to an external ROI the homogeneity within the ROIs in the present study is expected to be higher as this is a data‐driven cluster. The fraction of the network map accounted for by each effect was determined by calculating the percentage of the total voxels in each network map represented by these voxels with significant effects. Effect sizes were computed for both positively correlated and anticorrelated signals. To assess for possible effects of handedness, presence of co‐morbid diagnosis and medication usage, we constructed an additional multivariate analysis in the ADHD group only, where sensitivity to these effects would be larger than if diluted by the addition of TD controls. We used age, gender, FSIQ‐level, scan site, DVARS, handedness, presence of co‐morbid diagnosis and medication usage as predictors and otherwise duplicated our multivariate methodology. Comprehensive meta‐analysis of fMRI studies suggests that ADHD‐related dysfunction is present regardless of comorbid psychiatric conditions or history of stimulant treatment (Cortese et al., 2012).
2.6. Mapping significant effects to neurocognitive functional maps
Effect spatial maps were created to map voxels with significant effects in the univariate analysis at α < 0.01, corrected for FDR and multiple comparisons, for each of the networks and their anti‐correlated signals. For example, a map of the effects of age in voxels with timecourses anti‐correlated to the right fronto‐parietal network. Reverse inference maps were created using custom code written in Python to access the Neurosynth (Yarkoni et al., 2011) database and analytic engine for each of the following terms: cognitive flexibility; goal selection; response inhibition; response precision; selective attention; sustained attention; duration discrimination; working memory; dopamine and hyperactivity. Of note, we substituted duration discrimination, the best‐studied task used in humans to test time discrimination, for temporal information processing, since empirically we found the latter compound term did not derive a sufficiently specific reverse inference map. In addition, we selected the terms “dopamine” and “hyperactivity” for analysis, given the former's longstanding association with ADHD and ADHD treatment, and the latter's status as a cardinal categorical symptom.
Neurosynth reverse inference maps are z‐score fMRI activation maps derived from a database of >11,000 studies in the neuroscience literature in task‐based fMRI. Neurosynth (http://neurosynth.org/) uses text mining to identify terms of interest (e.g. “attention”) within neuroscience articles occurring at a frequency of >1/10,000 words, and extracts fMRI activation coordinates from tables in the corresponding article text. These term to activation mappings are used to construct the database. Automated meta‐analysis is performed for a psychological term of interest (e.g., “cognitive flexibility”) to construct a whole‐brain reverse inference map of the posterior probability of a term of interest occurring given activation at each voxel. This contrasts with forward inference maps such that are commonly obtained in task‐based fMRI, or conventional meta‐analyses, which often display the probability of brain activation given a task, or term. Therefore, reverse inference maps may be conceptualized as meta‐analytic maps identifying brain location activations, that are relatively more selective for the neurocognitive function of interest than forward inference maps. This procedure controls for the fact that many brain locations are implicated in multiple functions and are non‐specifically activated in experiments. The process by which maps are generated by Neurosynth is wholly automated, and multiple validation techniques were applied by the original authors to compare results with manual techniques. These included comparing lexically‐defined regions of interest (ROI) with known anatomically‐defined ROIs, replicating previous research in the well‐studied domain of visual category‐specific activations, and comparing results from Neurosynth meta‐analyses with conventional manual meta‐analyses. These validation studies may be reviewed in detail in Yarkoni et al (Yarkoni et al., 2011). Overall, for broad domains of cognition, such as are considered in the present study, the composite Neurosynth algorithm extracts the majority of coordinates accurately to form the underlying database, and produces results comparable in sensitivity and specificity to manual meta‐analytic approaches. In the present study, we used custom Python code to access the Neurosynth database and generate reverse inference maps corresponding to terms of interest in ADHD, but otherwise all computational procedures were similar. We selected terms of interest based on the prior neurocognitive literature pertaining to ADHD (e.g., the excellent review by Mueller et al. (2017) and accessed the entire database of studies that is currently available in Neurosynth. Limitations remain, that are reviewed below (see Section 2.3).
Custom code was written in MATLAB to identify locations in the brain where individual effect maps were spatially coincident with activations in reverse inference maps for each neurocognitive function. Every combination of significant effects and reverse inference maps was computed for (a) intrinsic networks (b) their anticorrelated signals to determine voxels that were present in both maps for each combination. To create aggregated maps of maturational effects in each subject across each neurocognitive function, overlapping voxels from a) all networks and b) their anticorrelated signals were collected, and redundancies eliminated to determine only unique voxels. We calculated the relative numbers of brain locations implicated in each neurocognitive functional map for TD and ADHD youth by summing the unique voxels for each neurocognitive functional map and dividing into the relative proportions for each subject group. Finally, to delineate which brain structures were implicated in age‐dependent effects in each subject group for each neurocognitive function, overlapping voxels were individually mapped onto specific brain structures using the Talairach Daemon.
3. RESULTS
3.1. A high order model of 46 functional brain networks was constructed for youth with TD and ADHD
We identified 46 intrinsic functional networks in whole‐brain resting‐state fMRI data from 504 subjects. These were grouped into 6 primary domains: task positive cognitive control, DMN, subcortical, visual, sensorimotor and cognitive process network groups. The cognitive process group includes networks with functional specializations other than visual and sensorimotor networks, for example speech and language networks.
3.2. Categorical ADHD diagnosis did not explain differences in intrinsic networks or their anticorrelated circuits between TD and ADHD youth
In the pooled group of 504 TD and ADHD youth, we found no significant effect (α < .01, corrected) of ADHD categorical diagnosis. Categorical diagnosis was significant in nine networks in multivariate testing, but did not survive univariate testing. Significant effects of age (α < .01, corrected) were present in the combined 504‐subject group in the same networks and their anticorrelated circuits as seen when ADHD and TD youth were considered separately (see below). Interestingly, in this larger group of 504 subjects with higher power, there were three additional significant (α < .01, corrected) effects of female gender including the ventral frontal portion of the ventral attention network (VAN:VFC) and IN17, a DMN subnetwork anchored in the posterior cingulate cortex (PCC). There was no age × gender interaction in univariate testing, suggesting these were independent effects. There were no significant (α < .01, corrected) effects of FSIQ‐level or scan site, or their interaction with age or gender. Similarly, DVARS, DVARS × age and DVARS x gender were not significant, indicating residual head motion effects did not influence our results. In the analysis of ADHD youth only, where we examined the effects of handedness, comorbid diagnosis and medication usage, age was a significant predictor but otherwise the only significant effect was in handedness in circuits anticorrelated to the right fronto‐parietal network. This effect did not overlap others in our analysis.
3.3. TD and ADHD youth displayed differentiated maturational effect patterns in functional brain networks and their anticorrelated regions
When effects were analyzed separately in TD and ADHD youth across the same 46 intrinsic networks, we found significant (α < .01, corrected for FDR) maturational effects. Some were shared by both groups, but others were found only in either TD or ADHD youth. TD and ADHD youth shared significant maturational effects in the temporo‐limbic, basal ganglia‐thalamic, and cortico‐cerebellar networks and their anticorrelated circuits (Figure 2). The only cortical network where we found a significant maturational effect shared by both subject groups was in the precuneus, though its anticorrelated circuits displayed a significant maturational effect only in TD youth. TD youth demonstrated further age‐dependent effects only in the left fronto‐parietal control network, and in circuits anticorrelated to the frontal portion of the cingulo‐opercular network. In contrast, many additional maturational effects were present in youth with ADHD. These were identified in two posterior DMN subnetworks and the VAN:VFC, and included their anticorrelated regions. As well, significant maturational effects were seen in ADHD youth in a frontopolar network (anchored in Brodmann Area 10), the right fronto‐parietal control network, and anticorrelated circuits to the insular subnetwork of the cingulo‐opercular network, visual attention, cerebellar and speech production networks.
Figure 2.
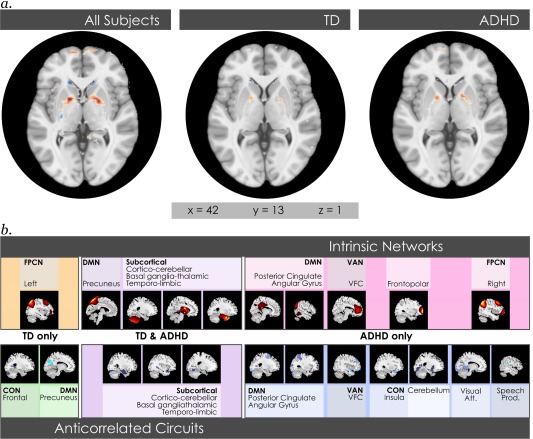
Shared and divergent maturational effects between youth with TD and ADHD. (a) Representative slice of significant (α < 0.01, corrected for FDR and multiple comparisons) maturational effects is shown across the whole brain for all intrinsic networks and their anticorrelated regions in the same two planes for maturational effects in all subjects, TD youth, and ADHD youth. Intensities are converted to z‐scores and thresholded at z = 1. These may be viewed interactively in 3D in Neurovault at https://www.neurovault.org/collections/HNHJFKBI/. (b) The individual intrinsic networks and their anticorrelated regions in which significant maturational effects (α < 0.01, corrected) were detected are shown for those common to TD and ADHD youth, and those found only in either TD or ADHD youth [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 1.
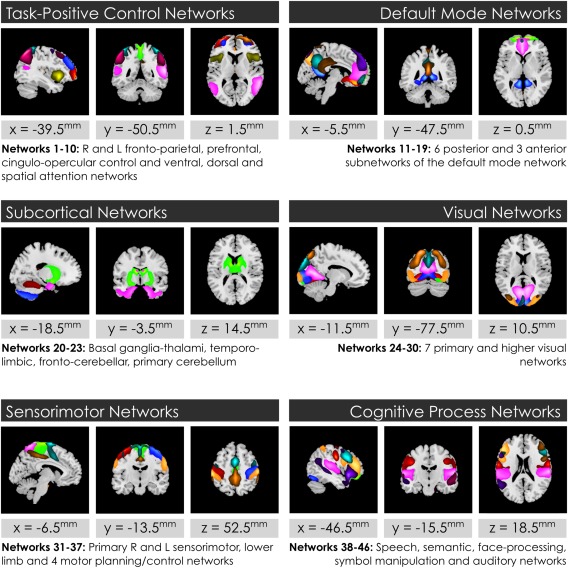
Intrinsic networks analyzed in the study grouped by primary neurocognitive function. Forty‐six functional networks were identified and analyzed from whole‐brain resting‐state functional MRI data in 504 age‐ and gender‐matched subjects 7–17 yo: 252 typically‐developing youth, and 252 with ADHD. Networks are shown grouped into domains. Top constituent anatomic regions for each network and its anticorrelated circuits, and an attribution of its function, may be inspected in Supporting Information Table S1 [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Maturational effects were larger in fronto‐striatal and default mode networks, but more network territory was involved in cerebellar circuitry
In youth with ADHD, age‐dependent effects were generally larger across most networks than those seen in TD youth (Figure 3). In some cases, the discrepancy was quite large. For example, in the temporo‐limbic network the size of the maturational effect for youth with ADHD was over twice that seen in TD subjects. The exception to this was in the basal ganglia‐thalamic network, where similarly calibrated maturational effects were obtained in both groups. In anticorrelated circuits, we observed negative age‐dependent effects, indicating these circuits become more anticorrelated over the developmental arc. In anticorrelated circuits, the general pattern of larger effects in youth with ADHD was not present, and effect sizes were roughly equivalent in TD versus ADHD youth. However, in basal ganglia‐thalamic and precuneus anticorrelated circuits where maturational effect sizes in TD youth were the largest we found in our entire analysis, this pattern was broken. Here, the maturational effect was considerably smaller or absent, respectively.
Figure 3.
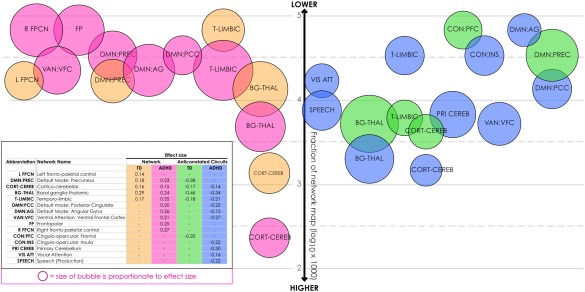
Maturational effect size and proportion of intrinsic networks and their anticorrelated circuits implicated in maturational effects. The relative size of maturation effects found in individual intrinsic networks and their anticorrelated circuits is displayed, calculated by averaging the beta over clustered voxels with significant effects in each network. The position of each effect relative to the vertical axis represents the fraction of voxels in the entire network displaying significant maturational effects. This proportion is shown in a log scale, where 2 = larger fraction and 5 = smaller fraction [Color figure can be viewed at http://wileyonlinelibrary.com]
We also analyzed the relative proportion of each network that exhibited significant maturational effects. This was similar across TD and ADHD youth with the notable exception of the cortico‐cerebellar network, where the maturational footprint was over an order of magnitude larger in TD youth versus most other networks, and two orders of magnitude larger in youth with ADHD.
3.5. The maturational footprint across neurocognitive functions is consistently larger in youth with ADHD versus TD
We computed the spatial overlap between unique locations with age‐dependent effects (concatenated across all networks) and reverse inference maps for individual neurocognitive functions associated with ADHD. The overlap was consistently greater in ADHD versus TD youth (Figure 4). The ADHD developmental footprint ranged from 3 to 10× more brain locations versus TD youth, and was greatest in response inhibition, selective and sustained attention and dopamine and hyperactivity maps, and lowest in duration discrimination.
Figure 4.
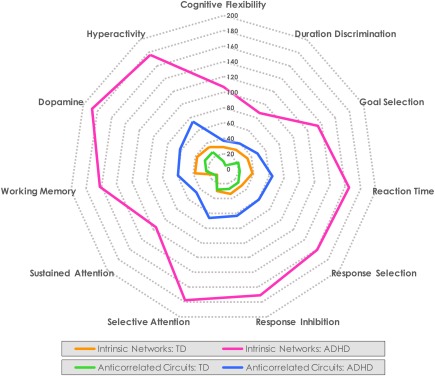
Unique brain locations with maturational effects and their overlap with neurocognitive functions implicated in ADHD. The number of unique brain locations with significant (α < 0.01, corrected) maturational effects in all intrinsic networks (orange = TD, magenta = ADHD) and all anticorrelated circuits (green = TD, blue = ADHD) map to locations in reverse inference maps associated with each neurocognitive function. Numbers on the concentric rings reflect the number of unique locations [Color figure can be viewed at http://wileyonlinelibrary.com]
A similar pattern was evident in anticorrelated circuits of a larger maturational footprint across neurocognitive function maps in ADHD versus TD youth. However, the disparity was generally toward the lower end of that seen in the networks themselves, trending more consistently at ∼3×. The exception was again in duration discrimination. These exceptions in duration discrimination may be associated with our use of a specific task versus a meta‐analytic term to calibrate effects in temporal information processing.
3.6. Differential maturation of DMNs and subcortical INs are most consistently associated with neurocognitive functions implicated in ADHD
We examined how these maturationally‐dependent locations mapped onto individual intrinsic networks and neurocognitive functions by subject group (Figure 5). Each of the neurocognitive functions implicated in ADHD displayed a slightly different combination of maturational network effects across the two subject groups. Of note, each neurocognitive map had network maturational effects appearing only in TD subjects. Conversely, each neurocognitive function also had multiple maturational effects only in ADHD subjects. The cortico‐cerebellar network was the only network consistently involved across all neurocognitive functions in both groups. Basal ganglia‐thalamic and precuneus networks were also consistently involved across functions, but with some individual functions where only TD or ADHD subjects showed maturational effects.
Figure 5.
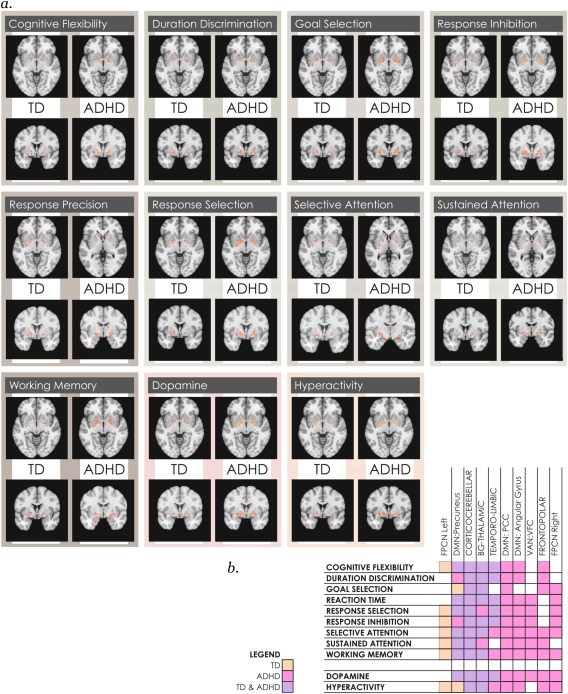
Maturationally‐dependent locations in intrinsic networks in each neurocognitive function map in youth with TD and ADHD. (a) Representative slice is shown of images capturing overlap in age‐effects in networks (positively‐correlated circuits) with individual neurocognitive maps of functions associated with ADHD, for each group of subjects. Full 3D images may be viewed in Neurovault at https://www.neurovault.org/collections/REEXTAWB/ (TD subjects) and/collections/BEMOJGJNL/(ADHD subjects). (b) Summarizes where maturational effects are present in individual intrinsic networks mapping onto each neurocognitive functional reverse inference map [Color figure can be viewed at http://wileyonlinelibrary.com]
3.7. Maturational differences in anticorrelated circuits across neurocognitive functions highlighted the ventral attention network
We performed a similar mapping of maturational effects to individual neurocognitive reverse inference maps for anticorrelated circuits (Figure 6a,b). As may be particularly appreciated in the 3D maps, fewer unique brain locations with maturational effects mapped onto neurocognitive functional maps in anticorrelated circuits, though these were well‐distributed across individual functions. In terms of maturationally‐dependent effects shared by both subject groups, in anticorrelated circuits the basal ganglia‐thalamic was the most consistently represented across all neurocognitive functions. Though, as with the intrinsic networks themselves, anticorrelated circuits where age effects were present in both subject groups were well‐represented in all functions. We continued to observe a pattern where maturational effects that mapped onto certain functions were only present in TD youth, in anticorrelated circuits to the precuneus (DMN) and temporo‐limbic networks and frontal cingulo‐opercular subnetwork. Of particular note, anticorrelated circuits to the VAN:VFC were associated with every neurocognitive function map. Where maturational effects in networks appear only in their anticorrelated circuits (insula cingulo‐opercular subnetwork, cerebellar, visual attention and speech production networks), these were pervasively involved across all neurocognitive functions. Interestingly, while the angular gyrus subnetwork of the DMN was well‐involved in neurocognitive functional maps, its anticorrelated circuits were almost uninvolved.
Figure 6.
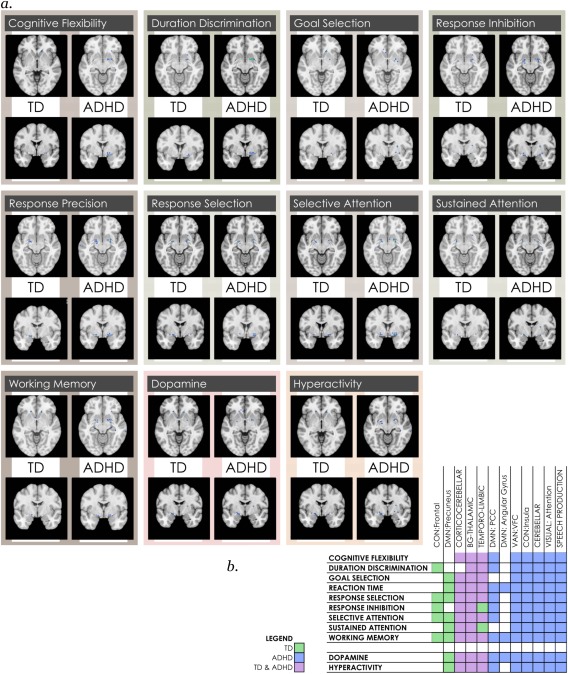
Maturationally‐dependent locations in anticorrelated circuits in each neurocognitive function map in youth with TD and ADHD. (a) Representative slice is shown of images capturing overlap in age‐effects in anticorrelated circuits with individual neurocognitive maps of functions associated with ADHD, for each group of subjects. Full 3D images may be viewed at https://www.neurovault.org/collections/CMOOMNPW/ (TD subjects) and/collections/RUHPKRPO/(ADHD subjects). (b) Summarizes where maturational effects are present in anticorrelated circuits to individual intrinsic networks mapping onto each neurocognitive functional reverse inference map [Color figure can be viewed at http://wileyonlinelibrary.com]
3.8. Reorganization of DMN and ventral attention subnetworks is lagging or altered in ADHD and maps to neurocognitive functions
When significant maturational effects are present in both the correlated and anticorrelated areas of a brain network, it suggests a network undergoing reorganization. We isolated these effects to examine networks and brain regions that displayed this pattern (Figure 7).
Figure 7.
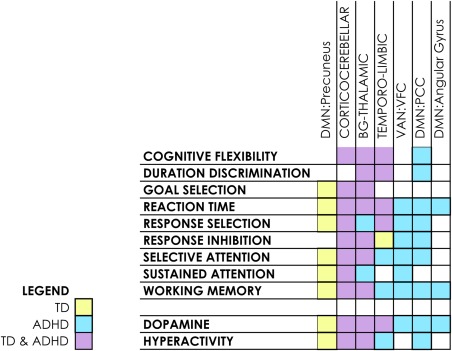
Intrinsic networks with maturational effects present in both networks and their anticorrelated circuits in the same subject group. Mapping of individual circuits to neurocognitive functional maps is shown where maturational effects are present in both positively‐correlated and anticorrelated circuits in the same function in the same subject group [Color figure can be viewed at http://wileyonlinelibrary.com]
In TD youth, the precuneus displays this pattern in the 7–17‐year age group mapping to most neurocognitive functions other than cognitive flexibility, response inhibition and time discrimination. In contrast, two posterior DMN subnetworks and the ventral attention network only display this pattern in ADHD, with the VAN:VFC well represented in attentional and inhibitory functions. In both groups at this age, the frontocerebellar and two limbic networks were highlighted, mapping to response selection/inhibition and sustained attention in ADHD.
3.9. Outside the striatal and limbic systems, different brain structures are involved in neurocognitive maturational effects in TD versus ADHD youth
While we mapped age‐dependent effects onto individual networks, individual voxels with maturational effects may be present anywhere in these large, distributed networks and we wished to see if there were regional anatomic patterns which varied from typical development in ADHD and promote comparability with the extant literature. We therefore mapped the same maturational effects detected in individual neurocognitive function maps onto the anatomic brain structures in which they were located (Figure 8).
Figure 8.
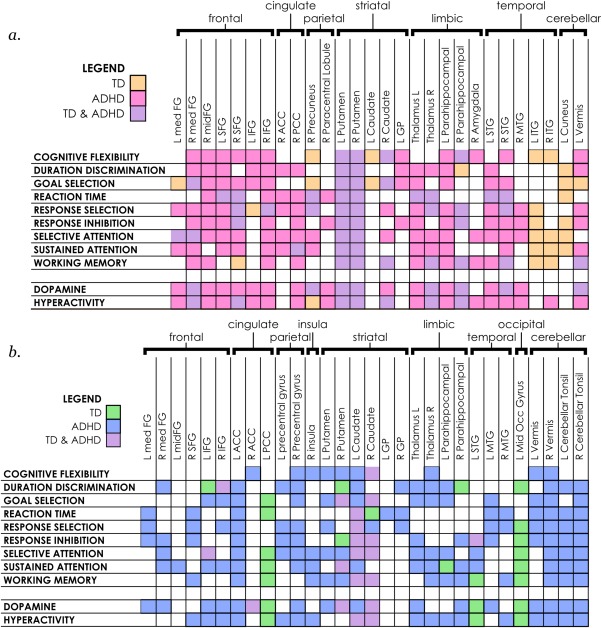
Regions implicated in maturational effects in individual neurocognitive functions in TD and ADHD youth. Anatomic locations where maturational effects are present in neurocognitive reverse inference maps in (a), intrinsic networks and (b), in anticorrelated circuits, with color coding for locations where both TD and ADHD youth exhibit maturational effects, and those where effects occur only for one group. Anatomic locations were identified using the Talairach Daemon [Color figure can be viewed at http://wileyonlinelibrary.com]
Many more individual brain regions were implicated in maturational effects in youth with ADHD than in TD youth (Figure 3). We found that TD and ADHD youth shared developmental effects mostly in limbic regions: the caudate and putamen bilaterally, and to some extent in the right parahippocampus and thalamus. Scattered commonality also existed in some fronto‐temporal areas. However, outside the limbic system, the two groups displayed different regional patterns of maturational effects. In TD youth, age‐dependent effects in brain network function were concentrated in more caudal areas: bilateral inferior temporal gyrus and the left cuneus, and in the left posterior cingulate cortex and middle occipital gyrus in anticorrelated signals. These are areas associated with the ventral stream of visual processing and complex visual processing, with the posterior cingulate cortex considered the posterior anchor of the default mode network. In youth with ADHD, many additional areas were significant including multiple regions of the middle, medial, inferior and superior frontal cortex and more extensive involvement of the limbic system and temporal lobe. Of note, the cerebellum was much more prominent in ADHD, including both vermis and tonsillar areas, particularly in anticorrelated circuits.
3.10. A small number of networks are prominent contributors to maturational effects in the neurocognitive functions associated with ADHD
We computed the relative proportions of maturational effects attributable to individual networks within each neurocognitive function of interest for TD youth (Figure 9a) and youth with ADHD (Figure 9b). We found that across all neurocognitive functions associated with ADHD, the cortico‐cerebellar and basal‐ganglia networks were the largest contributors to maturational effects. This bias was more prominent in ADHD. In TD youth, the left fronto‐parietal network, basal ganglia‐thalamic precuneus assumed a larger share, especially in cognitive flexibility, response selection/inhibition and working memory.
Figure 9.
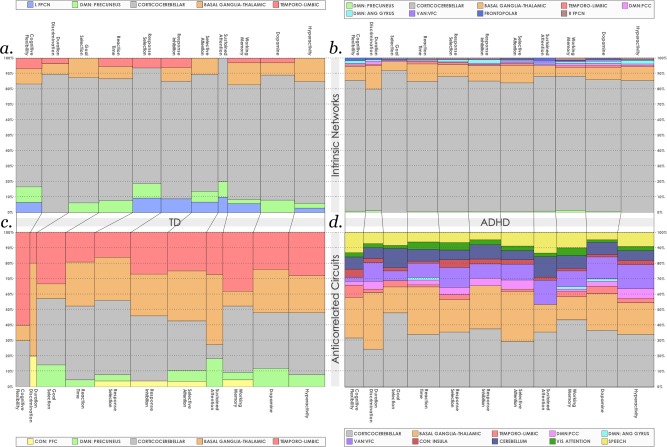
Relative contributions of each intrinsic network and their respective anticorrelated regions to maturational effects in each neurocognitive function. Unique voxels with significant maturational effects in each intrinsic network were summed and the relative percentage associated with each neurocognitive function of interested was computed for TD youth (a) and youth with ADHD (b). Similar calculations for anticorrelated circuits were performed for TD youth (c) and youth with ADHD (d). Width of columns reflects relative numbers of locations with maturational effects in each neurocognitive function [Color figure can be viewed at http://wileyonlinelibrary.com]
In anticorrelated circuits, this skew towards the cortico‐cerebellar network was reduced. Here, areas anticorrelated to the basal ganglia‐thalamic and temporo‐limbic networks were much more prominent in TD youth (Figure 9c) across all functions, though the cortico‐cerebellar network was still influential. Strikingly, a different pattern was seen in youth with ADHD, where regions anticorrelated to the VAN:VFC, DMN: PCC, primary cerebellum and speech network came to the foreground. Inspection of Figure 9 also illustrates that during typical development, anticorrelated circuits associated with response selection/inhibition and selective attention have larger shares of maturational effects relative to other functions.
4. DISCUSSION
4.1. High‐order multivariate modeling of both intrinsic networks and anticorrelated circuits supports the maturational hypothesis of ADHD in children and adolescents
We found the effect of ADHD diagnosis was uniformly weaker than age, and did not detect significant group x age interactions, suggesting that during this developmental period, maturation is a more important determinant of variation in brain network function than categorical diagnosis of ADHD. In youth with ADHD the expected strengthening of connectivity in the left fronto‐parietal network is missing, but there is ongoing maturation that may reflect lagging development in DMN subnetworks, VAN:VFC, frontopolar and right fronto‐parietal networks, or an altered developmental trajectory. While both groups have ongoing functional development in subcortical and precuneus networks, here too there is divergence, with a much more pronounced effect size of temporo‐limbic network in ADHD and a larger spatial footprint in the cortico‐cerebellar network. Overall, almost an order of magnitude more brain locations are still maturing in 7–17 yo youth with ADHD than in TD youth of the same age, and maturational effects in affected youth extend across considerably more of the neurocognitive “footprint” associated with ADHD (Figure 4). Our analysis therefore supports the developmental hypothesis of ADHD, and is consistent with volumetric evidence suggesting more of the brain is “younger” relative to chronologic age in ADHD, and may continue to be so through adulthood in symptomatic individuals (Sato, Hoexter, Castellanos, & Rohde, 2012). In this respect our whole‐brain network analysis using ICA in a high‐order model is at odds with studies where categorical effects have been detected used grid‐based ROI methods in low‐order models, including studies performed in the same ADHD‐200 dataset (Sripada et al., 2014), though broadly consistent with other low‐order studies in the same data (Sripada et al., 2014), highlighting the import of methodologic differences in mapping the “analytic topology” (Castellanos & Aoki, 2016) of ADHD.
Our study is novel in focusing equal attention to the examination of multivariate effects in anticorrelated circuits. Generally, we identified increasing anticorrelation with age in many INs, consistent with the growing evidence that increasing anticorrelation is a developmental process reflecting circuit maturation. Our analysis further supported the developmental hypothesis of ADHD and fleshes out conceptualized links between increasing anticorrelation and maturation. We identified alteration to the expected developmental trajectory of anticorrelated circuits in multiple specific networks in ADHD. The expected increasing anticorrelation we see in TD youth in frontal cingulo‐opercular and precuneus networks is absent in ADHD, and others with lagging or altered development included anticorrelated circuits to DMN subnetworks, the VAN:VFC and insula component of the cingulo‐opercular networks. Of note, our findings suggested analysis of anticorrelated circuits is of particular import to understanding cerebellar involvement in ADHD, since regions anticorrelated to the primary cerebellum were significant in addition to the cortico‐cerebellar network. Somewhat unexpectedly, we identified significant maturational effects in anticorrelated circuits to the speech production network, since expressive language deficits do not form part of the diagnostic criteria in ADHD. However, >40% of affected children score below the 15th percentile in expressive language testing. Moreover, testing across developmental domains indicates expressive language deficits are interrelated with social and attentional deficits in the disorder (Dyck & Piek, 2014), consistent with our exploratory finding (Figure 9) that anticorrelated maturational effects in this network made important contributions across neurocognitive functions. Generally, while anticorrelated circuits had a smaller spatial maturational footprint on the brain during this age period than primary networks, 3x as many anticorrelated brain locations were still maturing in ADHD > TD, suggesting more widespread ongoing refinement of networks as well as more individually reorganizing networks. Moreover, these additional maturational differences in anticorrelated circuits in subjects with ADHD fan out much more evenly over neurocognitive functions (Figure 9) than in TD and are of equal effect size, underlining the potential value of a more detailed exploration of the role of anticorrelated circuit maturation in ADHD.
4.2. Altered development of posterior > anterior DMN circuits exists in ADHD where female gender may be a protective factor
The preponderance of connectivity evidence for DMN involvement in ADHD comes from low‐order models, where it is approached as a single network. The DMN is now considered to have multiple subnetworks with differentiated functions (Andrews‐Hanna et al., 2010) and separable anticorrelated circuit relationships (Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). To our knowledge, no extant studies in ADHD have previously fractionated the DMN and examined effects in subnetworks or their anticorrelated circuits in ADHD. In the current sample, our high order model yielded six posterior and three anterior DMN components with significant maturational effects in three of these. Our findings were concentrated in posterior‐anchored DMN INs. Two of these appeared only in youth with ADHD, and were in IN17, a PCC‐anchored network with connectivity to parietal and frontopolar areas, and IN18, anchored in the angular gyrus with rich temporal and cingulate connectivity. In both cases, anticorrelated circuits were also implicated, suggesting these posterior DMN components may manifest lagging reorganization in ADHD. Of note, in the larger combined analysis of 504 subjects with higher power, we detected an additional effect of gender in IN17 that may be a protective effect of female gender worthy of exploration in more well‐powered studies.
A precuneus network (IN14) was the final DMN component with maturational effects in both groups of youths, but only in TD youth in anticorrelated circuits, suggesting lagging or different reorganization in ADHD youth. Other studies have noted differences specific to the precuneus in ADHD, with structural voxel‐based meta‐analysis indicating that the precuneus is one of the only brain regions that is larger in ADHD than controls (Nakao, Radua, Rubia, & Mataix‐Cols, 2011), perhaps relating to different plasticity. Recent intriguing work from Yang et al (Yang et al., 2014) in subjects aged 7–85 yo identified a functional developmental trajectory for the precuneus that differentiated it from other DMN constituents. This study proposed that the precuneus subnetwork, while sharing nodes with other DMN components, has a distinguishable and stronger age dependence than the rest of the DMN that weakens over the life course as it integrates with the rest of the DMN. Our analysis suggests that in the 7–17 yo age group, the individual precuneus component undergoing refinement has connectivity to PCC and middle frontal gyrus (BA8, containing the frontal eye fields) areas versus other precuneus DMN components differentiated by connections to limbic, cingulate and frontopolar cortex, respectively.
4.3. Lagging or altered reorganization of the ventral‐frontal > temporo‐parietal portion of the VAN is implicated in ADHD
The VAN has been frequently associated with ADHD symptoms, particularly in meta‐analyses (Cortese et al., 2012), an intuitive connection given its theorized role in “bottom‐up” stimulus‐driven attention to unexpected or unattended events, in contrast to a dorsal attention network thought to implement “top‐down” goal‐driven attention (Vossel, Geng, & Fink, 2014). The ventral system is anchored in ventral frontal areas and the temporo‐parietal junction, and our high‐order model separated these into subnetworks, allowing us to examine effects in each and supporting a more prominent role for the former. Our analysis suggests that VAN circuitry is not undergoing significant maturation in youth ages 7–17 yo with TD, but reorganization in the ventral frontal circuits may lag or possess a differentiated developmental trajectory in children with ADHD. Of note, exploratory mapping suggests these effects extend beyond sustained/selective attention to response functions and working memory. These ventral frontal regions of the VAN are activated in reward paradigms, with the ventro‐medial frontal cortex frequently implicated in ADHD (Castellanos & Aoki, 2016). While the maturational effect of VAN:VFC anticorrelated circuits was slightly smaller relative to the network itself, its spatial footprint was larger and affected more neurocognitive functions. We did not identify significant maturational effects in the subnetwork of the VAN anchored in the temporo‐parietal junction (IN4) or the dorsal attention system (IN6). In our anatomic mapping we found significant maturational effects in medial frontal gyrus (consistent with the literature) but also in inferior and middle frontal gyrus, which are areas which may control the interplay between dorsal and ventral attention networks.
4.4. Differential development in right and left lateralized fronto‐parietal control networks distinguishes ADHD from TD youth
Fronto‐parietal control circuits have been well‐linked with ADHD (Lin et al., 2015). Using a grid‐based model, Sripada et al found lagging maturation within fronto‐parietal ROIs and between DMN and right fronto‐parietal ROIs (Sripada et al., 2014). We pursued a high‐order decomposition partly to fractionate these key control networks into their right and left lateralized components using data‐driven methods. We found striking differences, with maturational effects present only in TD youth in the left FPCN, and only in ADHD youth in the right FPCN, with the latter effect size being quite large. In neither case was there involvement of anticorrelated circuits. The fronto‐parietal control networks are among the few large‐scale INs that fractionate laterally, and studies link aberrant lateralization to ADHD, though this is nonspecific and also seen in e.g. autism and schizophrenia. Previous work has suggested fronto‐parietal networks play important roles in task start cues (Dosenbach et al., 2007; Petersen & Posner, 2012), alerting, and executive control of attention (Markett et al., 2014), though further research is required to disambiguate individual roles of the right versus left networks. In our exploratory mapping, we found the right and left networks were both associated with selective attention, response selection/inhibition and working memory, but that otherwise their functions appeared to diverge in youth. These findings echo the differential neurocognitive functional involvement of right versus left frontal cortical areas identified in task meta‐analyses in ADHD, and may serve as useful hypotheses to explore in task‐based studies using high‐order models capable of fractionating these networks. In combination with an additional ADHD‐specific maturational effect in a network anchored in frontopolar cortex (IN1) without involvement of anticorrelated circuits, these three frontal network effects are consistent with findings suggesting remission of ADHD symptoms arises from prefrontal maturation (Halperin & Schulz, 2006) and the dominance of fronto‐parietal involvement in adult ADHD (Cortese et al., 2012).
4.5. Differentiated control network maturation is also present in cingulo‐opercular circuits
In a parallel observation, we also detected differentiated maturation in the cingulo‐opercular network (CON), which we fractionated into frontal, cingulate and insula subnetworks. The CON is thought to be involved in the integration of interoceptive and cognitive information, acting as a control network in the maintenance of a task‐general state and in error response (Dosenbach et al., 2007; Dosenbach et al., 2006). Some studies have identified specific deficits in cingulo‐opercular function in subjects with ADHD, but comparative analysis of the involvement of this network is challenged by the somewhat indistinct differentiation in the current literature regarding the cingulo‐opercular versus “salience” networks (Power et al., 2011), that may spatially overlap or in fact be the same network, and many studies also exist linking the salience network to ADHD. Interestingly, we saw maturational effects in the frontal portion of the network only in TD youth, and the insula portion only in youth with ADHD. In contrast to our findings in the frontoparietal control networks, these were both only in anticorrelated circuits.
4.6. A cognitive‐emotive cerebellar subnetwork has a prominent footprint in ADHD
Though traditionally conceptualized as a motor structure, the cerebellum is now understood to have a regionally dissociable topology and important role in cognitive and affective processes. We extended the growing recognition of an important role for the cerebellum in ADHD, previously weighted toward volumetric studies, examining maturational effects in two separable cerebellar networks. The first (IN 20) contains connections to primary motor cortex (BA 4), limbic, occipital and thalamic areas. The second is richly connected to fronto‐parietal somatosensory/premotor (BA 3, 6) and dorsolateral prefrontal cortex (BA 9), inferior ventral temporal areas (BA 20), globus pallidus, limbic areas and angular gyrus. These correspond closely to distinct cerebellar circuitry described using meta‐analytic mapping in task fMRI by Balsters, Laird, Fox, and Eickhoff (2014), who identified the former with action execution and the latter with cognition‐emotion behaviors. Our study foregrounds a role for reorganization in both TD and ADHD youth during this period in the cognitive‐emotion cerebellar network. While similar maturational effect sizes were present in both groups, and in the network and its anticorrelated circuits, the developmental spatial “footprint” of this network across the entire range of neurocognitive functions associated with ADHD was striking. Our findings suggest that while a large absolute number of brain locations involved in cerebellar circuitry possess significant maturational effects relevant to ADHD (Figure 9), and a much larger spatial proportion of this IN exhibited age‐dependent effects than other networks, the relative impact (effect size) of these differences is smaller than that of striatal, limbic, DMN and ventral attention networks (Figure 3).
4.7. Exploratory mapping of maturational effects to dopamine‐responsive and hyperactivity maps suggests hypotheses for further investigation
The most exploratory part of our analysis lay in mapping circuit effects to maps associated with “dopamine” and “hyperactivity.” In terms of the contribution of maturational effects in individual networks and anticorrelated circuits, both dopamine and hyperactivity (Figure 9) were essentially concatenations of overall trends among the more specific neurocognitive functions, though we saw some overweighting of striatal and limbic network contributions to hyperactivity. We found nearly all maturational effects in INs and anticorrelated circuits mapped onto dopamine‐responsive regions, consistent with the proposed pathophysiology of ADHD (Del Campo, Chamberlain, Sahakian, & Robbins, 2011). Notable exceptions were the left fronto‐parietal network, and in anticorrelated circuits the frontal component of the cingulo‐opercular network. These two circuits shared the features of being maturationally‐sensitive only in TD youth, and being associated with response selection/inhibition, selective attention and working memory. Additional information was given by mapping maturational effects to dopamine‐responsive anatomic areas. Here, we identified lacunae in the left caudate and globus pallidus and bilateral inferior temporal gyrus in networks, and in anticorrelated circuits, the left middle and right superior frontal gyri, left precentral gyrus, right insula, globus pallidus and some limbic areas. Collectively, these observations may represent a useful hypothesis regarding symptoms and patients that are not responsive to dopaminergically‐oriented treatments such as stimulants and amantadine.
Similarly, all networks and anticorrelated circuits with maturational effects were represented in hyperactivity, with the exceptions of the VAN:VFC and circuits anticorrelated to the DMN: angular gyrus. Our analysis supported a conceptualization of hyperactivity as a convergent phenomenon associated with maturational reorganization during this age period in corticocerebellar and striatal circuits, with additional lagging or differential reorganization in ADHD in temporo‐limbic and default mode circuits. Of note, we did not detect effects in more purely sensorimotor networks (INs 31–37). Almost all circuits that mapped onto hyperactivity were also present in the dopaminergic map, consistent with this symptom generally being one of the more treatment‐responsive. The small number of exceptions—the left fronto‐parietal network, right amygdala, right inferior temporal gyrus and circuits anticorrelated to a handful of fronto‐parietal and limbic areas—might be explored for associations with residual symptoms or selective response to noradrenergic regions. Unfortunately, the lack of a noradrenergic reverse inference map precluded us from exploring this hypothesis in the current analysis.
5. LIMITATIONS
Our analysis uses reverse inference maps generated by the Neurosynth database and analytic engine. While multiple validation studies were performed by the originators of Neurosynth to demonstrate that this resource produces results comparable to manual meta‐analytic approaches, a number of limitations remain. Firstly, the database is constructed from a sub‐set of neuroscience journals, and does not include all possible journals and therefore studies that might contribute to the body of research for each term (neurocognitive construct) of interest. Secondly, not all activation coordinates may be correctly extracted. Thirdly, while this fully‐automated approach has produced results comparable in sensitivity and specificity to manual meta‐analytic approaches, sensitivity and specificity of 100% is not guaranteed for each neurocognitive term examined in the present study.
6. CONCLUSION
Using a data‐driven ICA approach to define networks and subnetworks, we were not able to duplicate the results of other researchers in the same data who have used ROI and seed‐based approaches to identify effects arising from categorical diagnosis alone, albeit in lower‐order models. Our analysis identifies differences in children and adolescents with ADHD in networks and regions that are consistent with the extant literature in ADHD (Chabernaud et al., 2012; Elton, Alcauter, & Gao, 2014), but locates age‐ and a smaller number of gender effects in these areas, suggesting further examination of maturational and gender effects and their relationship to neurocognitive functions will continue to offer a rich explanatory model of the macroscale mechanisms of ADHD. We add considerable granularity to the extant literature, particularly with respect to the role of maturation in circuit anticorrelation in ADHD. Thus, of the two major strands of connectivity theory regarding brain functional maturation from childhood to adolescence, our analysis confirms the importance of both strengthening in fronto‐striato‐parietal‐cerebellar control circuits and increased refinement of circuit anticorrelation, to an understanding of the maturational lag or altered developmental trajectory in ADHD. We note that our study examined age effects in a transverse sample, and detailed analysis in true longitudinal samples extending through adulthood will be informative, as will very large samples such as the forthcoming ABCD and Healthy Brain Biobank collections with the potential to compare children in well‐powered, quite narrow age bands. Further studies in large, longitudinal datasets extending through young adulthood will help disambiguate a core remaining question of whether findings such as ours represent lagging circuit development, or an alternate developmental trajectory pertaining to ADHD diagnosis.
While ADHD is a widely‐researched disorder using neuroimaging, our current understanding has been shaped by a composite of many smaller individual studies in single task conditions and meta‐analyses that have highlighted the heterogeneity of this condition and implicated many neurocognitive functions. Our novel approach extends multivariate connectivity modeling by quantifying predictive effects in the resting‐state and mapping them onto a full range of neurocognitive functions within the same analytic protocol. While this exploratory in silico technique is not a replacement for in vivo studies, it offers the ability to map effects and circuits against a wide range of neurocognitive functions with the specificity offered by reverse, rather than forward, inference maps. Correspondence between our findings and current evidence in ADHD supports the results obtained by this approach, and may offer utility in generating further hypotheses and informing the specificity of this analysis in ADHD.
ACKNOWLEDGMENTS
The authors would like to thank B. Ernesto Johnson for his assistance with figure preparation. This study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000421 to NdL, and NIH 2R01EB005846, P20GM103472, and R01EB020407 and NSF 153906 to VDC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
de Lacy N, Kodish I, Rachakonda S, Calhoun VD. Novel in silico multivariate mapping of intrinsic and anticorrelated connectivity to neurocognitive functional maps supports the maturational hypothesis of ADHD. Hum Brain Mapp. 2018;39:3449–3467. 10.1002/hbm.24187
Funding information National Center for Advancing Translational Sciences of the National Institutes of Health, Grant/Award Number: KL2TR000421; NIH, Grant/Award Numbers: 2R01EB005846, P20GM103472, and R01EB020407; NSF, Grant/Award Number: 153906
REFERENCES
- Allen, E. A. , Erhardt, E. B. , Damaraju, E. , Gruner, W. , Segall, J. M. , Silva, R. F. , … Kalyanam, R. (2011). A baseline for the multivariate comparison of resting‐state networks. Frontiers in Systems Neuroscience, 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, E. A. , Erhardt, E. B. , Wei, Y. , Eichele, T. , & Calhoun, V. D. (2012). Capturing inter‐subject variability with group independent component analysis of fMRI data: A simulation study. Neuroimage, 59(4), 4141–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna, J. R. , Reidler, J. S. , Sepulcre, J. , Poulin, R. , & Buckner, R. L. (2010). Functional‐anatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters, J. H. , Laird, A. R. , Fox, P. T. , & Eickhoff, S. B. (2014). Bridging the gap between functional and anatomical features of cortico‐cerebellar circuits using meta‐analytic connectivity modeling. Human Brain Mapping, 35(7), 3152–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore, S. J. (2012). Imaging brain development: The adolescent brain. NeuroImage, 61(2), 397–406. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , & Adali, T. (2012). Multisubject independent component analysis of fMRI: A decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Reviews in Biomedical Engineering, 5, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , & de Lacy, N. (2017). Ten key observations on the analysis of resting‐state functional MR imaging data using independent component analysis. Neuroimaging Clinics of North America, 27(4), 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. , Jones, R. M. , & Somerville, L. H. (2011). Braking and accelerating of the adolescent brain. Journal of Research on Adolescence, 21(1), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos, F. X. , & Aoki, Y. (2016). Intrinsic functional connectivity in attention‐deficit/hyperactivity disorder: A science in development. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(3), 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabernaud, C. , Mennes, M. , Kelly, C. , Nooner, K. , Di Martino, A. , Castellanos, F. X. , & Milham, M. P. (2012). Dimensional brain‐behavior relationships in children with attention‐deficit/hyperactivity disorder. Biological Psychiatry, 71(5), 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, X. J. , Ofen, N. , Gabrieli, J. D. , & Whitfield‐Gabrieli, S. (2014). Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. Journal of Cognitive Neuroscience, 26(3), 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou, A. , Murphy, C. M. , Chantiluke, K. , Cubillo, A. I. , Smith, A. B. , Giampietro, V. , … Consortium, M. A. (2013). Disorder‐specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Molecular Psychiatry, 18(2), 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou, A. G. , Bauer, T. E. , Kiehl, K. A. , Feldstein Ewing, S. W. , Bryan, A. D. , & Calhoun, V. D. (2013). A quality control method for detecting and suppressing uncorrected residual motion in fMRI studies. Magnetic Resonance Imaging, 31(5), 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, H. D. (2012). The ADHD‐200 Consortium: A model to advance the translational potential of neuroimaging in clinical neuroscience. Frontiers in Systems Neuroscience, 6, 62 10.3389/fnsys.2012.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S. , Kelly, C. , Chabernaud, C. , Proal, E. , Di Martino, A. , Milham, M. P. , & Castellanos, F. X. (2012). Toward systems neuroscience of ADHD: A meta‐analysis of 55 fMRI studies. The American Journal of Psychiatry, 169(10), 1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvy‐Duchesne, B. , Ebejer, J. L. , Gillespie, N. A. , Duffy, D. L. , Hickie, I. B. , Thompson, P. M. , … Medland, S. E. (2016). Head motion and inattention/hyperactivity share common genetic influences: Implications for fMRI studies of ADHD. PLoS One, 11(1), e0146271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock, R. C. , James, G. A. , Holtzheimer, P. E., 3rd , Hu, X. P. , & Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo, N. , Chamberlain, S. R. , Sahakian, B. J. , & Robbins, T. W. (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention‐deficit/hyperactivity disorder. Biological Psychiatry, 69(12), e145–e157. [DOI] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. , … Raichle, M. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Nardos, B. , Cohen, A. L. , Fair, D. A. , Power, J. D. , Church, J. A. , … Lessov‐Schlaggar, C. N. (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach, N. U. , Visscher, K. M. , Palmer, E. D. , Miezin, F. M. , Wenger, K. K. , Kang, H. C. , … Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y. , Allen, E. A. , He, H. , Sui, J. , Wu, L. , & Calhoun, V. D. (2016). Artifact removal in the context of group ICA: A comparison of single‐subject and group approaches. Human Brain Mapping, 37(3), 1005–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck, M. J. , & Piek, J. P. (2014). Developmental delays in children with ADHD. Journal of Attention Disorders, 18(5), 466–478. [DOI] [PubMed] [Google Scholar]
- Elton, A. , Alcauter, S. , & Gao, W. (2014). Network connectivity abnormality profile supports a categorical‐dimensional hybrid model of ADHD. Human Brain Mapping, 35(9), 4531–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt, E. B. , Allen, E. A. , Damaraju, E. , & Calhoun, V. D. (2011). On network derivation, classification, and visualization: A response to Habeck and Moeller. Brain Connect, 1(2), 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt, E. B. , Rachakonda, S. , Bedrick, E. J. , Allen, E. A. , Adali, T. , & Calhoun, V. D. (2011). Comparison of multi‐subject ICA methods for analysis of fMRI data. Human Brain Mapping, 32(12), 2075–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M. (2014). The triadic model perspective for the study of adolescent motivated behavior. Brain Cognition, 89, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M. , Torrisi, S. , Balderston, N. , Grillon, C. , & Hale, E. A. (2015). fMRI functional connectivity applied to adolescent neurodevelopment. Annual Review of Clinical Psychology, 11, 361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair, D. A. , Cohen, A. L. , Power, J. D. , Dosenbach, N. U. , Church, J. A. , Miezin, F. M. , … Petersen, S. E. (2009). Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology, 5(5), e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyad, J. , De Graaf, R. , Kessler, R. , Alonso, J. , Angermeyer, M. , Demyttenaere, K. , … Lara, C. (2007). Cross‐national prevalence and correlates of adult attention‐deficit hyperactivity disorder. British Journal of Psychiatry, 190(05), 402–409. [DOI] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh, A. (2013). Lack of association of handedness with inattention and hyperactivity symptoms in ADHD. Journal of Attention Disorders, 17(4), 302–307. [DOI] [PubMed] [Google Scholar]
- Halperin, J. M. , & Schulz, K. P. (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention‐deficit/hyperactivity disorder. Psychology Bulletin, 132(4), 560–581. [DOI] [PubMed] [Google Scholar]
- Hart, H. , Radua, J. , Mataix‐Cols, D. , & Rubia, K. (2012). Meta‐analysis of fMRI studies of timing in attention‐deficit hyperactivity disorder (ADHD). Neuroscience and Biobehavioral Reviews, 36(10), 2248–2256. [DOI] [PubMed] [Google Scholar]
- Hart, H. , Radua, J. , Nakao, T. , Mataix‐Cols, D. , & Rubia, K. (2013). Meta‐analysis of functional magnetic resonance imaging studies of inhibition and attention in attention‐deficit/hyperactivity disorder: Exploring task‐specific, stimulant medication, and age effects. JAMA Psychiatry, 70(2), 185–198. [DOI] [PubMed] [Google Scholar]
- Himberg, J. , Hyvarinen, A. , & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22(3), 1214–1222. [DOI] [PubMed] [Google Scholar]
- Hoekzema, E. , Carmona, S. , Ramos‐Quiroga, J. A. , Richarte Fernandez, V. , Bosch, R. , Soliva, J. C. , … Casas, M. (2014). An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Human Brain Mapping, 35(4), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , … Jahanshad, N. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. Lancet Psychiatry, 4(4), 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, J. B. , Hedden, T. , Thompson, T. W. , Anteraper, S. A. , Gabrieli, J. D. , & Whitfield‐Gabrieli, S. (2015). Resting‐state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex, 64, 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline, R. B. (2005). Principles and practice of structural equation modeling (2nd ed.). New York: Guilford; p 50. ISBN 978‐1‐57230‐690‐5. [Google Scholar]
- Laird, A. R. , Fox, P. M. , Eickhoff, S. B. , Turner, J. A. , Ray, K. L. , McKay, D. R. , … Fox, P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience, 23(12), 4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. O. , Adali, T. , & Calhoun, V. D. (2007). Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28(11), 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. Y. , Tseng, W. Y. , Lai, M. C. , Matsuo, K. , & Gau, S. S. (2015). Altered resting‐state frontoparietal control network in children with attention‐deficit/hyperactivity disorder. Journal of the International Neuropsychological Society, 21(04), 271–284. [DOI] [PubMed] [Google Scholar]
- Markett, S. , Reuter, M. , Montag, C. , Voigt, G. , Lachmann, B. , Rudorf, S. , … Weber, B. (2014). Assessing the function of the fronto‐parietal attention network: Insights from resting‐state fMRI and the attentional network test. Human Brain Mapping, 35(4), 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, A. , Hong, D. S. , Shepard, S. , & Moore, T. (2017). Linking ADHD to the neural circuitry of attention. Trends in Cognitive Sciences, 21(6), 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, T. , Radua, J. , Rubia, K. , & Mataix‐Cols, D. (2011). Gray matter volume abnormalities in ADHD: Voxel‐based meta‐analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry, 168(11), 1154–1163. [DOI] [PubMed] [Google Scholar]
- Petersen, S. E. , & Posner, M. I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Schlaggar, B. L. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power, J. D. , Mitra, A. , Laumann, T. O. , Snyder, A. Z. , Schlaggar, B. L. , & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. (2013). Functional brain imaging across development. European Child & Adolescent Psychiatry, 22(12), 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Alegria, A. A. , & Brinson, H. (2014). Brain abnormalities in attention‐deficit hyperactivity disorder: A review. Revista De Neurologia, 58 Suppl 1, S3–16. [PubMed] [Google Scholar]
- Sato, J. R. , Hoexter, M. Q. , Castellanos, X. F. , & Rohde, L. A. (2012). Abnormal brain connectivity patterns in adults with ADHD: A coherence study. PLoS One, 7(9), e45671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , … Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre, J. , Sabuncu, M. R. , & Johnson, K. A. (2012). Network assemblies in the functional brain. Current Opinion in Neurology, 25(4), 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Laird, A. R. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Miller, K. L. , Salimi‐Khorshidi, G. , Webster, M. , Beckmann, C. F. , Nichols, T. E. , … Woolrich, M. W. (2011). Network modelling methods for FMRI. NeuroImage, 54(2), 875–891. [DOI] [PubMed] [Google Scholar]
- Spreng, R. N. , Sepulcre, J. , Turner, G. R. , Stevens, W. D. , & Schacter, D. L. (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience, 25(1), 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada, C. S. , Kessler, D. , & Angstadt, M. (2014). Lag in maturation of the brain's intrinsic functional architecture in attention‐deficit/hyperactivity disorder. Proceedings of the National Academy of Sciences of the United States of America, 111(39), 14259–14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar, K. , Musen, M. , & Menon, V. (2009). Development of large‐scale functional brain networks in children. PLoS Biology, 7(7), e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R. , Sanders, S. , Doust, J. , Beller, E. , & Glasziou, P. (2015). Prevalence of attention‐deficit/hyperactivity disorder: A systematic review and meta‐analysis. Pediatrics, 135(4), e994–1001. [DOI] [PubMed] [Google Scholar]
- Uchida, M. , Spencer, T. J. , Faraone, S. V. , & Biederman, J. (2015). Adult outcome of ADHD: An overview of results from the MGH longitudinal family studies of pediatrically and psychiatrically referred youth with and without ADHD of both sexes. Journal of Attention Disorders, 22(6), 523–534. [DOI] [PubMed] [Google Scholar]
- Uddin, L. Q. , Kelly, A. M. , Biswal, B. B. , Castellanos, F. X. , & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet, M. , Quentin, R. , Chanes, L. , Mitsumasu, A. , & Valero‐Cabre, A. (2014). Frontal eye field, where art thou? Anatomy, function, and non‐invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Frontiers in Integrative Neuroscience, 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel, S. , Geng, J. J. , & Fink, G. R. (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. The Neuroscientist, 20(2), 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Chang, C. , Xu, T. , Jiang, L. , Handwerker, D. A. , Castellanos, F. X. , … Zuo, X. N. (2014). Connectivity trajectory across lifespan differentiates the precuneus from the default network. Neuroimage, 89, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. A. , Nichols, T. E. , Van Essen, D. C. , & Wager, T. D. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo, B. T. , Krienen, F. M. , Sepulcre, J. , Sabuncu, M. R. , Lashkari, D. , Hollinshead, M. , … Polimeni, J. R. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]


