Abstract
Dependence on drugs has enduring effects on drug intake and relapse. The role of choice in enhanced susceptibility to drug use in drug dependence has been little studied. Here we determine the effects of alcohol dependence on the choice between alcohol and a non-drug reward, saccharin, using the discrete choice model in food-restricted male rats. We trained rats to self-administer alcohol (12% w/v) and saccharin (0.05, 0.1%), tested their choice of alcohol vs. saccharin, and determined the effects of deprivation and intertrial interval (ITI) duration on choice. We then determined the effects of alcohol dependence, induced by repeated intermittent exposure to alcohol vapor on choice of alcohol vs. saccharin (0.1%) in discrete choice trials as well as on the effects of adulteration of alcohol with quinine on choice. We trained another group of rats to self-administer intravenous (i.v.) nicotine (0.03 mg/kg/infusion) and oral saccharin (0.1%), determined their choice, and examined the roles of ITI duration and concurrent access on choice. Rats chose equivalent amounts of 0.05% saccharin and 12% alcohol, showed a stronger choice for 0.1% saccharin, and alcohol and saccharin choice were modestly decreased and increased, respectively, by deprivation. Alcohol dependence led to profound increases in the choice of alcohol over saccharin while adulteration of alcohol with quinine did not affect choice in non-dependent or dependent rats. Rats showed marked choice for 0.1% saccharin over i.v. nicotine. The strong effect that dependence had on alcohol choice is an important validation of the discrete choice procedure.
Introduction
Dependence on alcohol and other drugs has profound and enduring effects on drug-related behaviors including intake, relapse, and impulsivity [1, 2]. The behavioral and neurobiological processes underlying the effects of dependence are poorly understood. Human drug abusers have access to drugs as well as to other reinforcers and often forgo these other reinforcers in order to take the drug, which is especially prevalent in drug-dependent individuals [3–5]. This underscores a limitation in most currently used animal models of drug addiction, the provision of drug access in the absence of other reinforcers. In these models, it may be argued that drug-seeking is driven by lack of an alternative, rather than increased motivation to obtain the drug [6]. A number of different preclinical models have been developed that incorporate choice of alternative reinforcers, such as concurrent access [7, 8], and the focus of the present work, the discrete choice procedure [9].
In the discrete choice procedure, rats are allowed to choose between a drug and non-drug reward (typically saccharin or palatable food pellets), such that selection of one precludes the availability of the other. Most of this work shows that rats choose the non-drug reward over the drug reward [9–12]. To date, this increased choice of the non-drug reinforcer over drug has been demonstrated primarily with intravenous (i.v.) self-administered drugs, including cocaine [9, 13], heroin [14, 15], nicotine [16], and methamphetamine [15, 17, 18].
The greater choice of the non-drug reward over i.v. drugs is a highly robust effect. It has been hypothesized that drug dependence could shift choice away from the non-drug reward toward the drug reward [19]. This has been explored using the approach of increased access to enhance drug intake. Choice of saccharin is not changed by extended (6–9 h) daily access to i.v. heroin, cocaine, or methamphetamine [9, 15, 18, 20, 21] or by prolonged training (25 days) with i.v. nicotine [16]. In the case of heroin, choice is shifted towards heroin if extended daily access is combined with a “within session” escalating dose regimen (dose quadrupled during last 5 h of 6 h session) [14]. However, the degree of heroin dependence induced in this study is unclear, since withdrawal symptoms were not reported.
The present study (1) determined choice behavior for orally self-administered solutions of alcohol and saccharin and examined the effects of reinforcer deprivation on such choice, (2) determined the effects of alcohol dependence on choice behavior between oral alcohol and saccharin, (3) determined the effects of adulteration of alcohol with quinine (a test of compulsive-like alcohol-taking) on choice in non-dependent and dependent rats [22, 23] and (4) determined choice of i.v. nicotine vs. oral saccharin in order to replicate the results of previous discrete choice studies with i.v. drugs and saccharin.
Our data comparing oral saccharin and i.v. nicotine-trained rats’ choice was in line with previous findings of enhanced saccharin vs. i.v. nicotine choice. In contrast, we observed that rats trained to self-administer oral alcohol and saccharin showed equivalent choice of the two reinforcers that was only slightly affected by deprivation. Based on these results, we then determined the effects of alcohol dependence, induced by repeated intermittent exposure to alcohol vapor, on the choice of oral alcohol vs. saccharin, and we also determined whether adulteration of alcohol with quinine would affect choice and whether this would interact with alcohol dependence.
Materials and methods
For subjects, alcohol, nicotine and saccharin self-administration, discrete choice tests, alcohol vapor chambers, assessment of alcohol withdrawal symptoms, i.v. catheterization, concurrent access tests, alcohol and saccharin deprivation, and tests of compulsive-like alcohol taking, see Supplementary Online Material.
Experiment 1: Choice between alcohol and saccharin
The goal of Exp. 1 was to determine choice behavior for orally self-administered solutions of alcohol and saccharin and to examine the effects of reinforcer deprivation on such choice. Forty male Long Evans rats were trained to self-administer alcohol (12% w/v) and saccharin (0.05%) as described previously [24, 25] to FR-3. They then received alternating daily sessions of alcohol and saccharin self-administration (8 sessions/reinforcer) followed by 5 days of discrete choice tests. Rats then received daily alternating self-administration sessions of alcohol and 0.1% saccharin (3 sessions/reinforcer) followed by 6 days of discrete choice tests. Rats then underwent 10 days of alcohol and saccharin deprivation, remaining in their home cages, after which they received daily discrete choice tests (2 days with a 2 min intertrial interval (ITI) and 2 days with a 5 min ITI).
Experiment 2: Effect of alcohol dependence on alcohol vs. saccharin choice
The goal of Exp. 2 was to determine the effects of alcohol dependence on choice behavior between oral alcohol and saccharin and the effects of adulteration of alcohol with quinine (a test of compulsive-like alcohol-taking) on choice in non-dependent and dependent rats. Forty male Long Evans rats were trained to self-administer alcohol (12% w/v) and saccharin (0.1%) to FR-3 as described previously and then received 5 days of discrete choice tests. On the basis of their choice (alcohol/saccharin), rats were assigned to alcohol vapor (dependent) or control (non-dependent) groups and received 5 cycles of vapor exposure (5 days/cycle, with 6 days between cycles) or remained in their home cages. Briefly, after Cycles 1–3, rats received 4 alternating days of alcohol and saccharin self-administration (2 days of each) followed by 2 days of discrete choice tests. After Cycle 4, rats received 4 days of discrete choice tests followed by 2 days of concurrent access tests. Compulsive-like drinking was examined after Cycle 5; rats received 2 days self-administration of unadulterated alcohol and then 2 days with quinine-adulterated alcohol (first: 0.05 g/L, second: 0.1 g/L quinine). Rats then received 2 days of discrete choice tests with unadulterated alcohol and saccharin followed by 2 days of discrete choice tests with adulterated alcohol (0.05, 0.1 g/L quinine) and unadulterated saccharin. Somatic withdrawal symptoms were tabulated 24 h after termination of alcohol vapor exposure in Cycles 3–5.
Experiment 3: Choice between i.v. nicotine and saccharin
The goal of Exp. 3 was to determine choice of i.v. nicotine vs. oral saccharin in order to replicate the results of previous discrete choice studies with i.v. drugs and saccharin. Fourteen male Sprague Dawley rats were implanted with i.v. catheters and, after recovery, were trained to self-administer i.v. nicotine (0.03 mg/kg/infusion) and saccharin (0.1%) on alternating days for 20 days to a final ratio of FR-3 [25]. They then received 6 days of discrete choice tests, the first 4 with a 2 min ITI and the last 2 with a 5 min ITI and then 2 days of concurrent access sessions. While both Long Evans and Sprague Dawley rats are commonly used in i.v. nicotine self-administration studies, Sprague Dawley rats were used in this experiment because Long Evans rats from our current supplier are more susceptible to infections following surgical procedures.
Statistical analysis
We analyzed the data using SPSS (version 24). In Exp. 1 and 2, analyses were conducted using within-subjects analyses of variance (ANOVAs). In Exp. 2, mixed ANOVAs were used because of the additional between-subjects factor of Dependence condition. We followed up on significant main effects or interactions (p < 0.05) with post-hoc tests using the Bonferroni correction. The proportion of rats showing the somatic withdrawal signs of tail stiffness and hyper-reactivity observed after termination of alcohol vapor exposures 3–5 were analyzed with Χ2 tests.
Results
Experiment 1: Choice between alcohol and saccharin
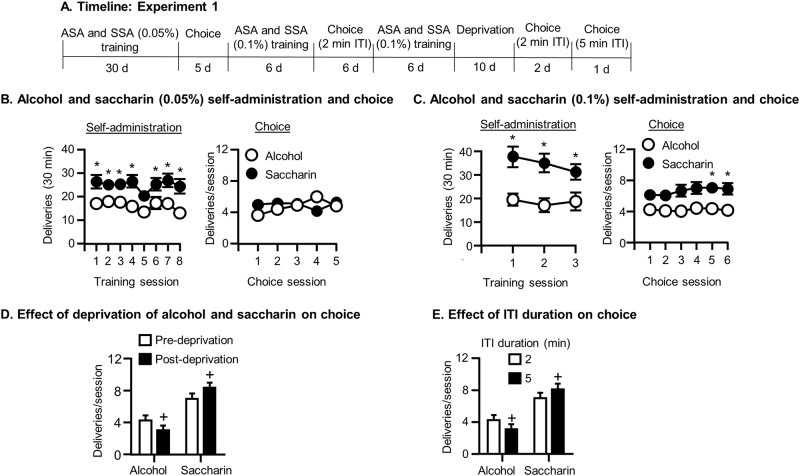
See Fig. 1a for experimental timeline.
Fig. 1.
Alcohol and saccharin choice (Exp. 1). a Timeline of the experiment. b Self-administration and choice of alcohol and saccharin (0.05%): Mean ± SEM number of alcohol and saccharin (0.05%) deliveries during the last 8 days of self-administration (left) and the 5 daily choice tests (right). c Self-administration and choice of alcohol and saccharin (0.1%): Mean ± SEM number of alcohol and saccharin (0.1%) deliveries during self-administration (left) and subsequent choice tests (right). d Effect of deprivation of alcohol and saccharin on choice between alcohol and saccharin: Mean ± SEM number of alcohol and saccharin deliveries during choice tests before and after 10 days of alcohol and saccharin deprivation. e Effect of ITI duration on choice between alcohol and saccharin: Mean ± SEM number of alcohol and saccharin deliveries during choice tests with 2 and 5 min ITIs. *Different from alcohol, +different from pre-deprivation baseline (d) or from 2 min ITI (e) (ps < 0.05). N = 31. ASA alcohol self-administration, SSA saccharin self-administration, ITI intertrial interval
Alcohol and saccharin self-administration
During training with 12% alcohol and 0.05% saccharin, rats self-administered more saccharin than alcohol (Reinforcer: F(1,25) = 13.71, p = 0.001; Fig. 1b, left). During training with 12% alcohol and 0.1% saccharin rats also self-administered more saccharin (Reinforcer: F(1,30) = 14.46, p = 0.001; Fig. 1c, left).
Discrete choice tests with alcohol and saccharin
Rats chose equivalent amounts of alcohol and saccharin (0.05%) in discrete choice tests (Reinforcer: p > 0.05; Fig. 1b, right). There was a significant Reinforcer×Day interaction (F(4,27) = 3.38, p = 0.021), because alcohol choice increased across days while 0.05% saccharin choice remained the same. Analysis conducted on the 6 days of choice between alcohol and 0.1% saccharin showed a strong trend toward increased saccharin choice (Reinforcer: F(1,30) = 4.41, p = 0.053; Fig. 1c, right) and post hoc tests showed that more 0.1% saccharin was chosen on days 5 and 6 (p < 0.05). Rats chose significantly more 0.1% than 0.05% saccharin, but alcohol choice was not affected by saccharin concentration (Reinforcer×Concentration interaction: F(1,30) = 16.05, p = 0.00) in an analysis of the mean of the last 2 days of the choice tests with each saccharin concentration (not shown).
Effects of deprivation of alcohol and saccharin on choice
Rats chose more saccharin (0.1%) and less alcohol after 10 days of deprivation of both reinforcers (Fig. 1d; Reinforcer×Deprivation interaction: F(1,30) = 14.92, p = 0.001).
Effects of increasing the ITI in discrete choice tests for alcohol and 0.1% saccharin
Rats chose more saccharin (0.1%) and less alcohol when tested with a 5 min ITI compared to a 2 min ITI (Reinforcer×ITI length interaction: F(1,30) = 4.91, p = 0.034; Reinforcer: F(1,30) = 10.91, p = 0.002; Fig. 1e).
Experiment 2: Effect of alcohol dependence on choice between alcohol and saccharin
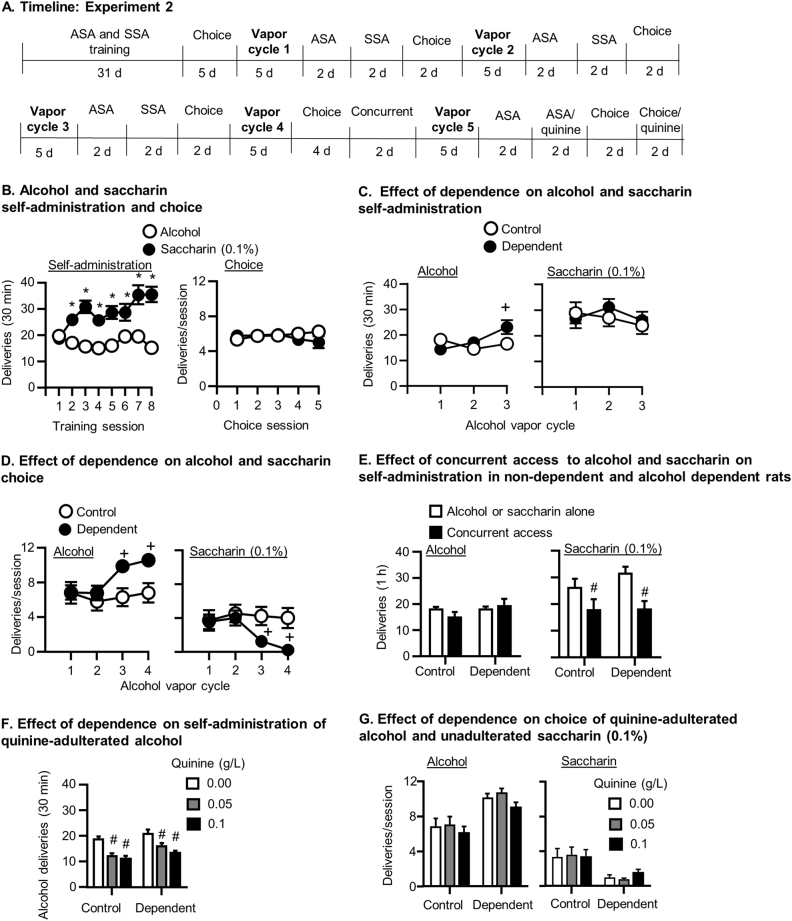
See Fig. 2a for experimental timeline.
Fig. 2.
Effects of alcohol dependence on choice of alcohol over saccharin (Exp. 2). a Timeline of the experiment. b Self-administration and choice of alcohol and saccharin (0.1%) prior to dependence induction: Mean ± SEM number of alcohol and saccharin (0.1%) deliveries during the last 8 days of alcohol and saccharin self-administration (FR-3) (left) and during the 5 daily choice tests (right). c Effects of alcohol dependence on self-administration of alcohol and saccharin: Mean ± SEM number of alcohol (left) and saccharin (right) deliveries during self-administration sessions conducted after the first 3 cycles of alcohol vapor exposure. d Effects of alcohol dependence on choice between alcohol and saccharin: Mean ± SEM number of alcohol (left) and saccharin (right) deliveries during the choice tests conducted after the first 4 cycles of alcohol vapor exposure. e Effects of concurrent access to alcohol and saccharin on self-administration in non-dependent and alcohol dependent rats: Mean ± SEM number of alcohol (left) and saccharin (right) deliveries during sessions with access to alcohol or saccharin alone or with concurrent access to both reinforcers. f Effects of alcohol dependence on decreases in alcohol self-administration produced by adulteration of alcohol with quinine: Mean ± SEM number of alcohol deliveries during self-administration tests with unadulterated alcohol (0 concentration) or alcohol adulterated with 0.05 and 0.1 g/L quinine in control (left) and alcohol dependent (right) rats. g Effects of alcohol dependence and quinine adulteration of alcohol on choice between alcohol and saccharin: Mean ± SEM number of alcohol (left) and saccharin (right) deliveries during choice tests with unadulterated alcohol (0 concentration) or alcohol adulterated with 0.05 and 0.1 g/L quinine in control and alcohol dependent rats. *Different from alcohol, +different from non-dependent controls, #different from saccharin alone (e) or from 0 quinine concentration condition (f) (ps < 0.05). N = 15–16/group. ASA alcohol self-administration, SSA saccharin self-administration
Alcohol and saccharin self-administration prior to alcohol vapor exposure
Rats self-administered more saccharin (0.1%) than alcohol (Reinforcer: (F(1,29) = 25.93, p = 0.000; Fig. 2b, left). Saccharin self-administration increased, while alcohol self-administration remained steady across days (Reinforcer×Day interaction: F(7,23) = 11.85, p = 0.000).
Discrete choice tests of alcohol and saccharin prior to alcohol vapor exposure
The choice of 12% alcohol and 0.1% saccharin was similar in the discrete choice tests conducted prior to alcohol vapor exposure. (Reinforcer p > 0.05) (Fig. 2b, right).
Effects of alcohol vapor exposure on alcohol and saccharin self-administration
Dependence increased alcohol self-administration as a function of the number of alcohol vapor cycles experienced (Fig. 2c, left) but did not affect saccharin self-administration (Fig. 2c, right). There was a Dependence condition×Cycle interaction (F(2,28) = 8.02, p = 0.001). Alcohol self-administration of dependent rats was significantly higher than the control rats after the third vapor cycle (p < 0.05).
Discrete choice tests after alcohol vapor exposure
Compared to controls, alcohol choice increased in dependent rats as a function of vapor cycle (Fig. 2d, left)(Dependence condition×Cycle interaction: F(3,80) = 4.61, p = 0.005) and was significantly higher than control rats after vapor cycles 3 and 4 (p < 0.05). Compared to control rats, saccharin choice decreased across alcohol vapor cycles in dependent rats (Fig. 2d, right) (Dependence group×Cycle: F(3,80) = 4.43, p = 0.006) and was significantly lower than the control group after vapor cycles 3 and 4 (p < 0.05).
Effects of alcohol dependence on concurrent access to alcohol and saccharin
Concurrent access to alcohol and saccharin did not affect alcohol choice (Fig. 2e, left), but reduced saccharin choice (2e, right) (Dependence condition×Reinforcer interaction: F(1,29) = 8.69, p = 0.006). There was no effect of Dependence condition nor interaction (ps > 0.05).
Effects of alcohol adulteration with quinine on alcohol self-administration and choice between alcohol and saccharin
Self-administration
Alcohol-dependent rats self-administered more alcohol than non-dependent rats (Dependence condition: F(1,29) = 6.20, p = 0.019), and quinine reduced alcohol self-administration (Quinine concentration: F(2,28) = 24.76, p = 0.000), but there was no interaction (p > 0.05) (Fig. 2f ).
Discrete choice
Alcohol-dependent rats chose more alcohol than control rats (Dependence condition: F(2,25) = 8.79, p = 0.006; Fig. 2g). Quinine reduced alcohol choice across the dependent and control groups (Quinine concentration: F(2,25) = 5.13, p = 0.009), but the post hoc tests done between the concentrations within each dependence condition were not significant (ps > 0.05). Alcohol-dependent rats chose saccharin less than non-dependent rats (Dependence condition: F(1,26) = 5.05, p = 0.033), but there was no effect of Quinine concentration or interaction (ps > 0.05).
Effects of withdrawal from alcohol vapor on somatic withdrawal symptoms
Twenty-four hours after termination of alcohol vapor exposure in cycles 3–5, rats exhibited tail stiffness and hyper-reactivity (Table 1). The proportion of rats showing tail stiffness was significant after vapor cycles 4 and 5 (Cycle 4: Χ2 = 2, p = 0.045; Cycle 5: Χ2 = 21.16, p = 0.000)
Table 1.
Blood alcohol concentrations and somatic withdrawal signs in alcohol vapor-exposed rats (Exp. 2)
| Vapor cycle | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| BAL (mg/dL) | ND | 165.7±15.9 | 338.3±21.7 | 187.0±23.8 | 248.8±35.1 |
| Withdrawal | |||||
| Tail stiffness (%) | ND | ND | 7 (47%) | 9 (60%)a | 11 (73%)a |
| Hyper-reactivity (%) | ND | ND | 2 (13%) | 5 (33%) | 5 (33%) |
Blood alcohol concentrations during alcohol vapor exposure cycles 2–5 (top). Numbers and proportion of rats showing withdrawal signs of tail stiffness and hyper-reactivity, 24 h after termination of vapor cycles 3–5 (bottom). N = 15
BAL blood alcohol level (mg/dL), ND not determined
aSignificant proportion of rats showing tail stiffness (ps < 0.05)
Experiment 3: Choice between i.v. nicotine and oral saccharin
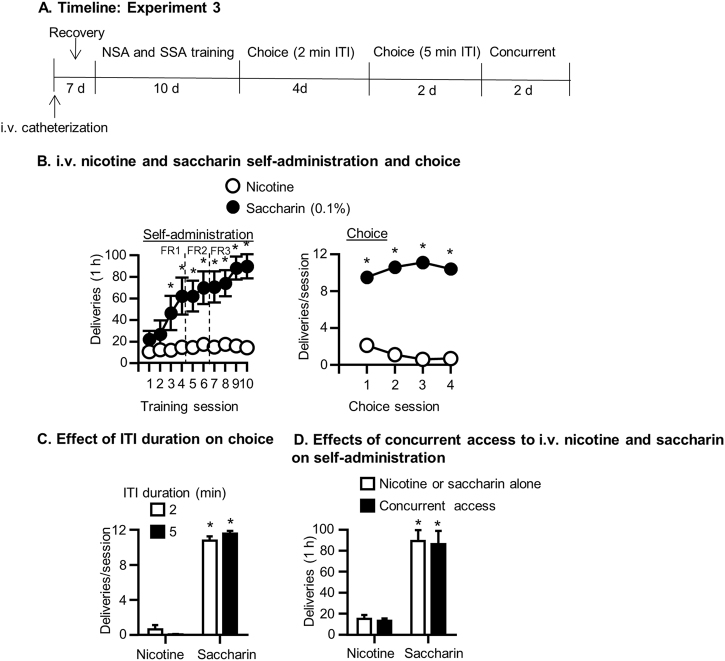
See Fig. 3a for the experimental timeline.
Fig. 3.
i.v. nicotine and saccharin choice (Exp. 3). a Timeline of the experiment. b Nicotine and saccharin self-administration and choice: Mean ± SEM number of nicotine and saccharin deliveries during the 8 days of i.v. nicotine and saccharin self-administration (left) and subsequent 4 daily choice tests (right). c Effect of ITI duration on choice between i.v. nicotine and saccharin: Mean ± SEM number of nicotine and saccharin deliveries during choice tests with 2 and 5 min ITIs. d Effects of concurrent access on self-administration of i.v. nicotine and saccharin: Mean ± SEM number of nicotine and saccharin deliveries during sessions with access to nicotine or saccharin alone or with concurrent access to both. *Different from nicotine (ps < 0.05). N = 10. NSA nicotine self-administration, SSA saccharin self-administration, ITI intertrial interval, FR fixed ratio
Nicotine and saccharin self-administration
Rats self-administered more saccharin than nicotine (Reinforcer: F(1,9) = 23.61, p = 0.001; Fig. 3b, left). Across days, saccharin self-administration increased while nicotine self-administration remained the same (Reinforcer×Day: F(1,9) = 6.42, p = 0.003). Self-administration of saccharin was higher than nicotine beginning on training day 3 and this persisted to the end of training (p < 0.05).
Discrete choice tests with nicotine and saccharin
Rats chose saccharin more than nicotine in discrete choice tests (Reinforcer (F(1,9) = 177.75, p = 0.000; Fig. 3b, right).
Effect of increasing ITI on choice of nicotine and saccharin
Changing the ITI from 2 min to 5 min did not significantly affect choice of nicotine or saccharin (p > 0.05; Fig. 3c). Rats chose significantly more saccharin than nicotine (Reinforcer: F(1,9) = 921.95, p = 0.000), but there was no effect of ITI duration or interaction (ps > 0.05).
Concurrent access to nicotine and saccharin
During concurrent access tests, rats self-administered more saccharin than nicotine (Reinforcer: F(1,9) = 41.81, p = 0.000; Fig. 3d). There was no effect of Access condition or interaction (p > 0.05).
Discussion
Consistent with previous research using the discrete choice procedure with i.v. drugs, we found that rats strongly preferred oral saccharin over i.v. nicotine. We found, however, that rats chose equivalent amounts of oral alcohol and saccharin. In alcohol-dependent rats, the choice of alcohol over saccharin was profound and this increased choice was directly related to the number of alcohol vapor cycles experienced, with exclusive choice of alcohol seen after the fourth vapor exposure. Our data show that drug dependence can enhance choice of a drug of abuse over a natural reward using the discrete choice model. In contrast to the strong effects of dependence on choice, two other manipulations that affect the intake of alcohol, deprivation, and adulteration with the bitter tastant, quinine, did not affect choice. These findings are relevant to how we understand data from discrete choice studies as well as other preclinical models designed to measure the addictive properties of drugs.
Choice of oral alcohol vs. oral saccharin in non-dependent rats
The key finding from Experiment 1 was that food-restricted rats trained to self-administer solutions of alcohol and saccharin chose both reinforcers equally in discrete choice tests. This was surprising for three reasons: (1) rats responded for saccharin more than alcohol during self-administration; (2) previous work on alcohol self-administration with progressive ratio schedules shows alcohol is a relatively weak reinforcer; and (3) the majority of previous discrete choice studies report robust saccharin choice over drug. We then investigated this finding parametrically and found that increasing the saccharin concentration or the ITI enhanced saccharin choice without affecting alcohol choice. A recent study examining choice between 0.2% saccharin and 20% alcohol using a 1 min ITI reported that during the first 5 choice tests, the degree of choice of alcohol vs. saccharin was comparable to what we found [26].
We also investigated the effects of deprivation, a procedure shown to increase consumption of alcohol following a period of abstinence [27–29]. We found that in non-dependent rats, concurrent deprivation of alcohol and saccharin increased saccharin choice and decreased alcohol choice. While this pattern is surprising, saccharin deprivation has been shown to increase its subsequent intake [30]. It is possible that deprivation might have a more pronounced effect on saccharin than alcohol, and therefore, under conditions of concurrent deprivation of alcohol and saccharin, choice may be shifted toward saccharin.
Therefore, increasing saccharin concentration, the ITI, or employing deprivation can shift the preference toward saccharin; the effects of these manipulations are, however, modest because rats still choose significant amounts of alcohol compared to i.v. drugs, for which a much higher degree of non-drug vs. drug choice is observed.
The reasons for the greater relative choice of oral alcohol vs. saccharin compared to i.v. drugs vs. saccharin are not known. One potential explanation is that alcohol and saccharin are delivered and consumed via the same means and route, compared to different routes (i.v. vs. oral) in most previous studies employing discrete choice. Oral alcohol and saccharin may recruit the same sensory pathways thereby reducing the bias toward saccharin. Another reason may be that perception of orally self-administered reinforcers such as alcohol or saccharin is immediate, while, in the case of i.v. drugs, there is a significant time lag (6 s for i.v. cocaine) between infusion and the perception of the infusion, as indexed by behavioral change [9]. Arguing against this is that, while oral alcohol is perceived immediately, its pharmacological effect is delayed, potentially leading to decreases in its value in a choice situation due to delay discounting, although this might be countered by their learning of the contingency between alcohol taste and its pharmacological effects. Another argument is that quinine adulteration of alcohol had no effect on choice of alcohol over saccharin; had the relatively enhanced choice of alcohol been based solely on its immediate taste, its adulteration by quinine would be predicted to inhibit the choice of alcohol.
Effects of alcohol dependence on choice between alcohol and saccharin
The most important finding from our study is that, in food-restricted rats, choice of alcohol over saccharin increased dramatically in alcohol vapor-exposed rats as a function of the number of alcohol vapor cycles to a near-exclusive choice of alcohol after the fourth vapor cycle; at this time, 13 out of the 15 alcohol vapor-exposed rats chose alcohol exclusively. This provides a critical validation of the discrete choice model as a tool to measure the choice of alcohol, because it shows that the procedure is sensitive to a key feature of alcohol addiction, dependence.
The possible role of drug dependence in choice has been examined using the approaches of extended access (6–9 h daily sessions) to heroin or methamphetamine [15, 17, 18, 21], extended training with nicotine (25 days) [16], or extended heroin [14] or cocaine [9] access combined with a within-session dose escalation procedure. Only extended i.v. heroin access with dose escalation significantly shifted choice toward drug [14]. While withdrawal symptoms in this study were not reported, it should be noted that naloxone-precipitated withdrawal symptoms have been reported following extended access to heroin [31, 32]. Our present results show that an alcohol exposure regimen that produces physical dependence leads to exclusive alcohol choice. These data suggest there may be a difference between stimulants (methamphetamine, nicotine) and depressants/opioids (alcohol, heroin) on the effects of chronic, high-level drug exposure on choice. The reasons for this are not known, but it could be speculated to be related to their propensities to cause physical withdrawal symptoms upon cessation of exposure, with alcohol and heroin causing such symptoms, whereas physical withdrawal symptoms with nicotine or methamphetamine are equivocal.
Alcohol dependence also induces states of negative affect, such as anxiety [33], that persist beyond the physical withdrawal period and are associated with increased motivation to take alcohol. This altered motivational state produced by dependence could also contribute to the increases in alcohol choice that we observed.
We also assessed the effects of adulteration of alcohol with the bitter tastant, quinine, on alcohol self-administration and choice under non-dependent and dependent conditions to determine whether dependence would result in compulsive-like alcohol self-administration and choice. We found that quinine concentration-dependently reduced alcohol self-administration, but although dependent rats self-administered more alcohol, the quinine-induced decreases were only slightly and non-significantly affected by alcohol dependence. Although two studies showed clear effects of alcohol vapor exposure on quinine-induced reductions in alcohol self-administration [23, 34], another showed less robust effects [35]. The two studies showing that dependence blunted the effect of quinine reported that lower concentrations of quinine (0.005–0.025 g/L) reduced alcohol self-administration in controls but not in dependent rats, while a dose of 0.05 g/L reduced self-administration in both control and dependent rats to a similar degree [23, 34]. We used concentrations of 0.05 and 0.1 g/L and did not see an effect of dependence. From this, it could be suggested that, had we used a lower quinine dose range, we may have detected an effect of alcohol dependence on the effects of quinine on self-administration.
Adulteration of alcohol with quinine had no effect on choice of alcohol over saccharin in either control or alcohol-dependent rats, despite the greatly increased alcohol choice in dependent rats. The reasons for the insensitivity of the choice procedure to quinine adulteration of alcohol are not known, but there are a number of possible explanations. We used quinine concentrations in the range shown to reduce alcohol self-administration, but it is possible that higher concentrations of quinine are required to shift choice away from alcohol, a hypothesis we will explore in future studies. Another is that the previous experience the rats had with quinine-adulterated alcohol during the self-administration tests reduced its effects on choice. Another possibility is that the large number of self-administration and choice sessions over ~3 months experienced by the rats may have impaired the expression of inhibitory effects of quinine on choice of alcohol. Finally, shorter periods between reinforcements (i.e., short ITI) may increase the likelihood of seeing quinine effects on choice.
Choice of i.v. nicotine vs. oral saccharin
The results of Experiment 3 on the choice of i.v. nicotine and oral saccharin in food-restricted rats replicate previous findings with the discrete choice trials' procedure showing a pronounced choice of oral saccharin over i.v. nicotine [16] or other i.v. administered drugs. They also contrast with our findings of equivalent alcohol and saccharin choice from Exp. 1. This pronounced degree of choice of saccharin over i.v. nicotine was still noted despite our use of a lower saccharin concentration (0.1%) than used in previous studies (0.2%) with i.v. drugs and at 2 different ITIs. This suggests that, even at this lower concentration, saccharin has a greater reinforcing value relative to i.v. nicotine. During sessions when rats had concurrent access to i.v. nicotine and saccharin, rats also self-administered more saccharin than nicotine, which is consistent with this and with the results of previous studies [7].
Methodological considerations
Effectiveness of alcohol-dependence induction
During alcohol vapor exposure, rats in the dependent group had blood alcohol levels (BALs) in the range shown to result in physical dependence (Table 1) [36], exhibited behavioral signs of intoxication during vapor exposure, and showed physical withdrawal symptoms 24 h after termination of alcohol vapor exposure (Table 1). These observations, coupled with increased alcohol self-administration in alcohol vapor-exposed rats, indicate that they were alcohol dependent. In addition, increased alcohol self-administration by the dependent rats in our study occurred after the third vapor exposure cycle (a total of 15 days of intermittent alcohol vapor), consistent with previous work showing that the increased motivation for alcohol associated with alcohol vapor exposure is dependent on the numbers of vapor exposures experienced [36]. Variations in the extent of increases in alcohol self-administration by alcohol vapor exposure have been observed in different laboratories. Some of the variation may be related to the schedule of exposure, the use of self-administration during the withdrawal phase, and strain differences [37, 38].
Saccharin concentration
Different concentrations of saccharin were used to initiate saccharin self-administration (0.5 and 0.1% in Exp. 1 and 0.1% in Exp. 2), but the levels of choice of saccharin vs. alcohol were similar. These concentrations are lower than the concentration (0.2%) used by the majority of studies on choice of saccharin vs. other drugs of abuse. It should be noted that, in another study that used 0.2% saccharin, the choice of alcohol vs. saccharin during the first 5 days of choice testing was comparable to what we observed [26].
We used non-caloric saccharin as the alternative reinforcer in these initial studies because it is standard in discrete choice studies. While sucrose may be a better non-drug reinforcer for comparison with alcohol, it has been reported that oral administration of alcohol with sucrose results in lower BALs compared to alcohol and saccharin (Roberts et al., 1999), potentially impacting alcohol choice. In future work, we will investigate the potential role of the caloric value of alcohol in choice by testing different alternative reinforcers.
Another factor related to the caloric issue is that we fed the rats 23–25 g chow/day rather than ad lib. Under this feeding schedule, rats were healthy and gained weight steadily with an average body weight of about 420 g by the end of the experiment, but the possibility that this restriction altered choice behavior cannot be ruled out.
The discrete choice model and the reinforcing effects of alcohol
We examined the effects of three different treatments used to study the reinforcing effects of alcohol on the choice of alcohol vs. saccharin in the discrete choice procedure. The first two, deprivation and dependence induction, have been shown to enhance alcohol intake. The third, the adulteration of alcohol with the bitter tastant, quinine, reduces alcohol intake, and alcohol-dependent rats are more resistant to its effects. We expected that deprivation and alcohol dependence would enhance choice, while quinine adulteration would reduce choice and that quinine-induced reductions in choice would possibly be reduced by dependence. We found that choice of alcohol was markedly increased by alcohol dependence, but it was resilient to both deprivation and quinine adulteration of alcohol, the latter in both non-dependent and dependent rats. Further studies of this pattern of findings may help to better understand which aspects of the reinforcing effects of alcohol are measured by the discrete choice procedure.
Summary and conclusions
We found that rats chose equivalent amounts of orally self-administered alcohol and saccharin in the choice tests. Alcohol dependence resulted in a shift to exclusive choice of alcohol over saccharin, and this occurred as a function of the number of alcohol vapor exposure cycles experienced. These data extend the validity of the discrete choice procedure as a model of drug abuse as it shows it can detect the heightened drive to self-administer drug over other reinforcers noted in drug-dependent humans.
Although choice was shifted markedly by alcohol dependence, it was unaffected by reward deprivation or adulteration of alcohol with a bitter tastant, quinine. This pattern of findings suggests that the choice procedure may measure different aspects of increased motivation to take drug compared to other animal models of addiction.
Our data are highly relevant to the development of medications for treatment of alcoholism. This is because the rationale behind treating drug addiction is not only to reduce drug-directed behavior but also to reallocate behavior toward alternative non-drug reinforcers. Therefore, the use of the discrete choice procedure in alcohol-dependent rats could be a potentially powerful model, as medications can be developed with the goal of shifting this exclusive choice of alcohol in dependent rats back toward an alternative reinforcer.
Electronic supplementary material
Acknowledgements
The study was supported by NIAAA-NIH funds to ADL (R01-AA024341).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0101-1).
References
- 1.Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41:1432–43. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camchong J, Endres M, Fein G. Decision making, risky behavior, and alcoholism. Handb Clin Neurol. 2014;125:227–36. doi: 10.1016/B978-0-444-62619-6.00014-8. [DOI] [PubMed] [Google Scholar]
- 3.Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/S0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- 4.Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: a brief review. Pharmacol Biochem Behav. 1997;57:419–27. doi: 10.1016/S0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- 5.Carroll ME. Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr. 1998;169:6–25. [PubMed] [Google Scholar]
- 6.Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–84. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Panlilio LV, Hogarth L, Shoaib M. Concurrent access to nicotine and sucrose in rats. Psychopharmacol (Berl) 2015;232:1451–60. doi: 10.1007/s00213-014-3787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks ML. Utility of preclinical drug versus food choice procedures to evaluate candidate medications for methamphetamine use disorder. Ann NY Acad Sci. 2017;1394:92–105. doi: 10.1111/nyas.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed SH. Trying to make sense of rodents’ drug choice behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2017. 10.1016/j.pnpbp.2017.09.027 [DOI] [PubMed]
- 11.Ahmed SH. Individual decision-making in the causal pathway to addiction: contributions and limitations of rodent models. Pharmacol Biochem Behav. 2018;164:22–31. doi: 10.1016/j.pbb.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Banks ML, Negus SS. Insights from preclinical choice models on treating drug addiction. Trends Pharmacol Sci. 2017;38:181–94. doi: 10.1016/j.tips.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhille N, Belin-Rauscent A, Mar AC, Ducret E, Belin D. High locomotor reactivity to novelty is associated with an increased propensity to choose saccharin over cocaine: new insights into the vulnerability to addiction. Neuropsychopharmacology. 2015;40:577–89. doi: 10.1038/npp.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology. 2017;42:1126–35. doi: 10.1038/npp.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh C, Fam J, Ahmed SH, Clemens KJ. Rats quit nicotine for a sweet reward following an extensive history of nicotine use. Addict Biol. 2017;22:142–51. doi: 10.1111/adb.12306. [DOI] [PubMed] [Google Scholar]
- 17.Caprioli D, Zeric T, Thorndike EB, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addict Biol. 2015;20:913–26. doi: 10.1111/adb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–73. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- 20.Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, et al. The anterior insular cortex-->central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron. 2017;96:e418. doi: 10.1016/j.neuron.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–73. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le AD, Funk D, Coen K, Tamadon S, Shaham Y. Role of kappa-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology. 2018;43:838–50. doi: 10.1038/npp.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–48. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- 26.Pelloux Y, Baunez C. Targeting the subthalamic nucleus in a preclinical model of alcohol use disorder. Psychopharmacology (Berl) 2017;234:2127–37. doi: 10.1007/s00213-017-4618-5. [DOI] [PubMed] [Google Scholar]
- 27.Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–7. [PubMed] [Google Scholar]
- 28.Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–43. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- 29.Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27:1048–54. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–9. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 31.Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- 32.Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–4. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL. Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology. 2015;89:157–67. doi: 10.1016/j.neuropharm.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimbrough A, Kim S, Cole M, Brennan M, George O. Intermittent access to ethanol drinking facilitates the transition to excessive drinking after chronic intermittent ethanol vapor exposure. Alcohol Clin Exp Res. 2017;41:1502–9. doi: 10.1111/acer.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leao RM, Cruz FC, Vendruscolo LF, de Guglielmo G, Logrip ML, Planeta CS, et al. Chronic nicotine activates stress/reward-related brain regions and facilitates the transition to compulsive alcohol drinking. J Neurosci. 2015;35:6241–53. doi: 10.1523/JNEUROSCI.3302-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–82. doi: 10.1097/01.ALC.0000145781.11923.4E. [DOI] [PubMed] [Google Scholar]
- 37.Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav. 2017;152:61–7. doi: 10.1016/j.pbb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–94. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.