Abstract
Neuroendocrine neoplasms (NENs) arise from cells of the neuroendocrine system located in many sites amongst which most common are the gastrointestinal (GI) system and the lung. The efforts to assess the specific site of origin or predict the biological behavior of NENs is based upon a detailed study of neoplasm’s architectural pattern, immunohistochemical, genetic and molecular profile. Immunohistochemistry is used to characterize the aggressivity of NENs, by assessing the proliferation index Ki-67, as well as the neuroendocrine differentiation by assessing chromogranin A (CgA) and CD56. Basal panels of immunohistochemical markers such as CDX-2, Isl-1, TTF-1, PAX6/8 are currently being used to allocate the neoplasms, while in dubious cases new markers are investigating. Unraveling the genetic and molecular mechanisms of NENs pathogenesis along with shedding light on the molecular heterogeneity of neoplasms and the individual patterns of molecular lesions, underlining these neoplasms may provide new tools in terms of diagnostics and therapeutics. Molecular targeted therapies (MTTs) such as everolimus and sunitinib have been the first example of druggable molecular targets implicated in NENs that have been approved for NEN treatment. New investigational drugs are developing along with genetic tests that may allow the identification of the specific subset of patients that will respond to each individual MTT. Multiparametrical molecular and genetic analysis such as the NETest and the MASTER are already in trials shedding light in a step-by-step management of NENs that allow not only the selection of an appropriate therapeutic option but also the identification of response to treatment or early relapse allowing an early amendment of the strategy. Summarizing the combination of histopathological, immunohistochemical, genetic and molecular profile of a NEN opens new horizons in the efficient management of NENs.
Keywords: Neuroendocrine, neoplasm, immunohistochemistry, molecular, genetic
Introduction
Neuroendocrine neoplasms (NENs) arise from cells of the neuroendocrine system located in many sites amongst which most common are the gastrointestinal tract (GIT) and the lung. These cells may secrete bioactive substances, usually bioamines or peptide hormones in the blood stream causing distinct clinical syndromes such as the carcinoid syndrome. This group of NENs is denoted as functioning NENs; however, the majority of NENs is non-functioning. Although their course is usually indolent, a significant number of patients present with advanced disease. Their histological recognition is based on their characteristic cytologic features, distinct morphologic patterns and immunohistochemical profile. They share a common neuroendocrine origin, but there is great variability amongst them as they exhibit different organ-specific characteristics, biological behaviour, prognosis and treatment. Pathological, immunohistochemical, genetic and molecular markers are used to establish the diagnosis, distinguish between well-differentiated (WD) and poorly differentiated (PD) NENs, identify their proliferation rate, predict prognosis and select the most appropriate treatment (1-3).
The currently employed classification of NENs was initially proposed by the European Neuroendocrine Tumor Society (ENETS) and subsequently accepted and modified by the World Health Organization (WHO). The gastroenteropancreatic (GEP) group of NENs (GEP-NENs) follows a common classification/grading system based on the proliferation marker Ki-67, as opposed to lung NENs that mainly uses the number of mitoses. The recently published WHO Blue Book for Endocrine Organs in 2017 contains the modified grading scheme for pancreatic NENs (pNENs) and remains to be seen whether the same modifications regarding the proliferation index Ki-67 will also apply for the remaining gastrointestinal NENs (GI-NENs). The greatest difference in the new modified criteria is the distinction of G3 pNENs into two groups, namely the G3 WD-neuroendocrine tumors (WD-NETs) and the G3 PD-neuroendocrine carcinomas (PD-NECs). This new system separates two groups with histopathological and immunohistochemical heterogeneity reflecting their different biological behaviour and response to various therapeutic regimens (3-5).
In this review we will describe the histopathological, immunohistochemical genetic and molecular characteristics of NENs and delineate their role not only in establishing the diagnosis but also in predicting prognosis and directing the therapeutic choices.
Histopathological features of NENs
Microscopically WD-NENs are composed of cells possessing round or oval nuclei with “salt and pepper” chromatin and eosinophilic granular cytoplasm. NECs are classified as small-cell carcinomas (SC-NEC) or large-cell carcinomas (LC-NEC) especially because they differ in their cell size. The nuclear to cytoplasmic ratio of LC-NEC is lower than that of SC-NEC (6).
Since 1971, five different architectural patterns of GI-NENs (types A to D and mixed) were described for the first time while attempting to correlate each pattern with a specific tissue of origin. More specifically type A tumors are composed of solid nests (insular pattern) and this pattern is typical for ileal tumors; however, it can occasionally also be seen in appendiceal tumors. These tumors are mainly composed of enterochromaffin (EC)-cells. Type B tumors are trabecular or “ribbon-like” in morphology with anastomosing cords and this pattern is classically seen in rectal tumors composed of L-cells. Type C tumors are composed of tubular, acinar or rosette-like a structure usually accompanied by psammoma bodies and this histology is typically found in duodenal NENs including tumors of the ampulla. Type D tumors may be found in any anatomic site displaying a “diffuse” architecture. Apart from the jejunoileum (type A) and rectum (type B), NETs often exhibit mixed architectural pattern (7-9). NECs are characterized by organoid or nested pattern or exhibit diffuse growth completely lacking any particular pattern (6).
Immunohistochemical markers of NENs
Markers of neuroendocrine differentiation
Chromogranin A (CgA) and synaptophysin are currently considered the most specific immunohistochemical markers for NENs. CgA is an acidic glycoprotein of the granin family, being expressed in well to moderately differentiated NENs and tends to be only focally positive in PD-NECs/SC-NEC (10). PD-NECs do not react strongly with CgA antibodies (11). CgA may have limited sensitivity with some tumors, such as hindgut carcinoids (originating from left transverse colon to distal colon, rectum, and anus) found to stain in only 20–50% of cases (2,12,13).
Synaptophysin is a membrane glycoprotein, representing a good marker of neuroendocrine cells and NENs (14) with a diffuse cytoplasmic immunostaining (13). Immunolabeling for CgA and synaptophysin characterizes “pure” NENs but certain “non-pure”, i.e., consisting of cells of different origin, NENs like the solid pseudopapillary neoplasm of the pancreas or the mixed acinar NEC can also stain focally for both these markers (15). The WHO classification of tumors of endocrine organs and of the digestive system (4,6) specify that in mixed neuroendocrine non-NENs (MiNENs) either component represents at least 30% of the lesion. Thus, in cases that one of the two components represents only a minority of the tumor, such as adenocarcinomas or other non-NECs with interspersed expression of CgA and synaptophysin in less than 30% of the tumor cells, the diagnosis of neoplasms with a neuroendocrine component is made (16). The specific cut-offs have been suggested arbitrarily since a neoplastic component of less than 30% is not likely to affect the biological behaviour of the tumor (16).
Neural cell-adhesion molecule (NCAM) also called CD56 is a membrane-bound glycoprotein, involved in neuron-neuron and nerve-muscle interactions. CD56 is sensitive, but not highly specific marker for NENs because it is often expressed in several non-NENs. In addition, small cell lung cancer (SCLC) frequently stains with NCAM antibodies (11).
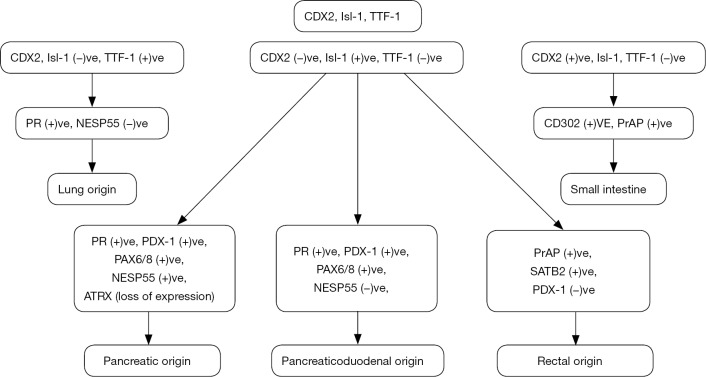
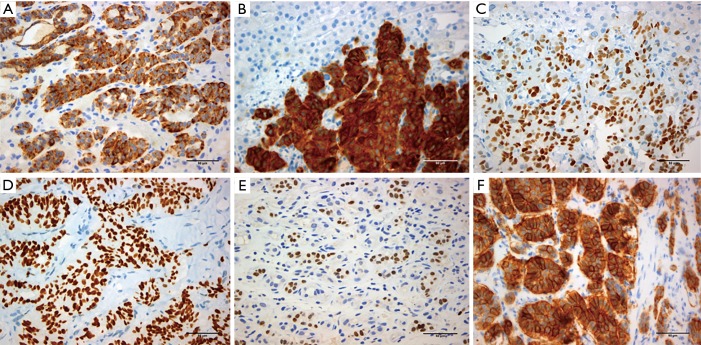
A step by step approach of immunohistochemical markers is used currently in pathology departments in order to confirm the site of origin of NENs, or to identify the origin of a metastatic site with not obvious primary neoplasm (17) (Figures 1,2).
Figure 1.
Algorithmic histopathologic approach to neuroendocrine neoplasms. CDX2, caudal type homeobox 2; Isl-1, islet 1; TTF-1, thyroid transcription factor-1; PR, progesterone receptor; NESP55, neuroendocrine secretory protein 55; PrAP, prostatic acid phosphatase; PDX-1, pancreatic and duodenal homeobox 1; PAX6/8, paired box genes 6/8; ATRX, alpha thalassemia/mental retardation syndrome X-linked; SATB1, special AT-rich sequence binding protein 1.
Figure 2.
Expression of immunohistochemical markers related to neuroendocrine neoplasms. (A,B) Granular cytoplasmic immunoexpression of chromogranin (A) and synaptophysin (B); (C,D,E) nuclear immunoexpression of Isl-1 (C), CDX-2 (D) and TTF-1 (E); (F) membranous immunoexpression of SSTR2a. Scale bar: 50 µm. Isl-1, islet 1; TTF-1, thyroid transcription factor-1; CDX2, caudal type homeobox 2; SSTR2a, somatostatin receptor 2a.
Proliferation index Ki-67
Ki-67 labeling index (LI) is accepted by both WHO and ENETS as a proliferative marker for NENs being independently correlated with survival and representing the most reliable prognostic factor of GEP-NENs. Common problems in evaluating the Ki-67 index include intertumoral and intratumoral staining heterogeneity in the primary site of the tumor or among the metastatic sites early or in during the course of the disease and the counting methods even when the improved automated staining machines are used (18,19). Intratumoral heterogeneity is another issue affecting the reliability of Ki-67 index in liver metastases from pNETs when diagnosed by a core needle biopsy despite its values as a prognostic factor of metastatic NETs to the liver (20). Other factors affecting its reproducibility are represented by differences in tissue processing and especially fixation methods, the loss of antigenicity over time, particularly in paraffin-embedded formalin-fixed blocks, and the assessment of pale stained nuclei (21). If all nuclei that have been taken in consideration in a Ki-67 slide display sharp borders and stain intensely or less intensely, but homogeneous or speckled pattern, the counts have an excellent reproducibility (5).
Immunohistochemical markers of site of origin
Caudal type homeobox 2 (CDX2)
CDX2 is a homeodomain-containing transcription factor critical for the intestinal differentiation (22). Although CDX2 is a highly sensitive immunohistochemical marker for GI adenocarcinomas, it can also be found in tumors with intestinal differentiation (i.e., pancreas, bile ducts, bladder, lung, uterine cervix, endometrium, ovary) (23,24).
The expression of CDX2 was documented in a large amount of WD-GI-NENs with a high sensitivity and specificity for NENs of the midgut (jejunoileum and appendix) origin (25). Immunohistochemical detection of CDX2 has been demonstrated in 90% of the primary and 91% of the metastatic jejunoileal WD-NENs (25) and in 93% of the primary appendiceal NENs (ANENs) and in 83% of the few cases of metastatic appendiceal tumors (26,27). CDX2 has also been detected in tumors originating from other parts of the GIT but in significantly less frequency (31% of duodenal, 14% of gastric, 16% of pancreatic and 29% of rectal). Rare positivity in lung NENs (9), as well as high positivity in WD-NENs of the ovary along with an insular pattern has also been reported (28). Additionally, the expression of CDX2 in midgut tumors shows strong and diffuse nuclear staining, whereas in foregut and hindgut tumors the staining is weak and patchy (9).
Thyroid transcription factor-1 (TTF-1)
TTF1 is a homeodomain-containing nuclear transcription protein of the NK2 homeobox (NKX2) gene family being involved in the organogenesis of the thyroid gland and lung as well as the development of the neurohypophysis and the ventral brain (29).
In the setting of WD-NENs, TTF-1 seems to have a variable sensitivity, but a very high specificity for tumors of lung origin. Expression of TTF1 has been documented in typical and atypical lung NENs, lung LC-NECs, and SC-NECs in approximately 35%, 50%, 47%, and 90%, respectively (29). On the contrary, the expression of TTF1 in WD-NENs of GIT is rare (9). It must be noted that although it is unlikely for a medullary thyroid carcinoma (MTC) to present as metastasis of unknown primary site, its differential diagnosis from lung NEN that expresses calcitonin and carcinoembryonic antigen (CEA) can be very difficult (30,31). TTF-1 expression in PD-NECs has been reported in many extra-pulmonary SC-NECs (prostate, urinary bladder, breast) but not in Merkel cell carcinoma (32).
Paired box genes 6/8 (PAX 6/8)
PAX8 is a transcription factor involved in thyroid and kidney development, it is also highly expressed in the surface of ovarian carcinomas, implying its use to identify carcinomas of the thyroid, of the Müllerian system and the kidney (33,34). The polyclonal PAX8 antibody is also expressed in the islets of Langerhans and pNENs suggesting the pancreatic origin of WD-NENs (35,36). Additionally, polyclonal PAX8 is often expressed in WD-NENs of duodenal and rectal origin (36) while it is not expressed in WD-NENs of ileal origin and only rarely in the lung, stomach and appendix (9).
However, more recent studies have reported that monoclonal PAX8 antibodies are universally non-reactive with either islets of Langerhans or pNENs (26,37). The likely reason that the polyclonal PAX8 antibody is expressed in pNENs is because it cross-reacts and detects the PAX6 transcription factor which seems to be the main transcription factor expressed in islets of Langerhans and pNENs (37,38). PAX6 expression has been found mostly in pNENs as well as in most duodenal and rectal WD-NENs (26).
Insulin gene enhancer binding protein islet 1 (Isl-1)
Isl-1 is a homeobox-gene transcriptional factor expressed in all endocrine but not exocrine, pancreatic cells (9), implying its role in the development of endocrine pancreas and in the diagnosis of pNENs. However, pancreatic gastrinomas display lower rate of expression of this factor. Isl-1 is not expressed in 90% of WD-GI-NENs and in 84% of pulmonary endocrine tumors (26). Isl-1 positivity is also typical of duodenal and rectal WD-NENs (39,40). Twelve percent (12%) of 94 pulmonary and 17% of 42 primaries ANENs have also shown positivity (41,42), and it is also expressed in MTC (43).
Other markers
pNENs may also express the progesterone receptor (PR), pancreatic and duodenal homeobox 1 (PDX-1) as well as the neuroendocrine secretory protein 55 (NESP55) (9). PR was positive in 40–75% of pNENs (44) whereas in other GI-NENs is usually negative (45). PDX-1 is mostly positive in pNENs and the majority of duodenal NENs, whereas its expression is less frequent in rectal but not jejunoileal NENs (27,40,46). NESP55 is a member of the granin family found in secretory granules in the adrenal medulla, pituitary gland and brain. In normal pancreas is most often expressed in B cells while a significant percentage of pNENs (40–50%) also express NESP55 at least focally (47). Ileal, duodenal, gastric and ANENs are negative whereas only rarely it is expressed in rectal tumors (27).
The loss of ATRX (alpha thalassemia/mental retardation syndrome X-linked)/DAXX (death-domain associated protein) expression has been found in pNENs, and predicts aggressive behavior since its loss is found in larger tumors, tumors with vascular and lymphatic invasion, infiltration of adjacent organs and with infiltrative borders (48). Prostatic acid phosphatase (PrAP) was found to be expressed in rectal pNENs (9). Finally, special AT-rich sequence binding protein-2 (SATB2) is selectively expressed in the colorectal GI-NENs (9).
Somatostatin receptors 2a and 5 (SSTR2a and SSTR5)
NETs overexpress SSTRs, mostly SSTR2a and SSTR5 (49). For the evaluation of SSTR2a and SSTR5 immunoexpression, a standardized scoring system is used, that also display a good correlation with the findings of octreoscan (50). The immunohistochemical expression of SSTR2a and SSTR5 in GEP-NENs range within 60–93% and 33–83%, respectively (49,51,52).
Patients with pNENs show increased SSTR2a expression compared to GI-NENs whereas G3 WD-NETs express SSTR2a in 78% of cases. On the contrary, G3 PD-NECs and mixed adenoneuroendocrine carcinomas (MANECs: since the terminology of MiNENs is currently in use only for pancreatic neoplasms) show lower SSTR2a expression compared to well- and moderately-differentiated tumors G1, G2 NENs (53).
Melatonin
Melatonin is an indoleamine, a small lipophilic molecule, two enzymatic steps away from serotonin, which easily passes through cell membranes exhibiting autocrine, paracrine and endocrine actions (54). In humans there are three different membrane receptors and one nuclear receptor of melatonin: melatonin receptor type 1a (Mel 1a, ML1a, MT1, MTNR1A), Melatonin receptor type 1b (Mel 1b, ML1b, MT2, MTNR1B), Melatonin receptor type 3 (MT3, ML2, NQO2, QR2) and retinoid-related orphan nuclear hormone receptor (RZR/RORα) (55).
Melatonin and receptors MT1 and MT2 are expressed in the normal human GIT and pancreas (56). Melatonin and MT2, but not MT1 are expressed in EC-cells (57). Melatonin and MT2 expression has been detected in the majority of the small intestine NETs (SI-NETs) but the expression of MT1 receptors was not frequent (15). The expression of melatonin in SI-NETs correlates with a lower Ki-67 index implying its anti-neoplastic effect (15). On the contrary, in neural, blood cell and other tissues melatonin promotes the cell survival by reducing apoptosis (58). Melatonin can also reduce the toxicity of chemotherapeutic agents while it exerts oncostatic effects of certain forms of cancer (59).
Programmed death ligand 1 (PD-L1)
PD-L1, is expressed on the cell surface of many tumor cells. The binding of PD-L1 to programmed cell death protein 1 (PD-1) has as a result the initiation of intracellular signals which inhibit CD8+ T cells proliferation and T cell activation, thus blocking the antitumor immune response, favoring tumor growth and metastasis (60). PD-1 has been detected in tumor-infiltrating lymphocytes (TILs) present in the tumor microenvironment, and PD-L1 aberrant expression by tumor cells is associated with a poor prognosis in several human tumors (61). Recently, it has been shown that PD-L1 blockade improves antitumor immunity and offers a promising cancer immunotherapy approach. The blockade of PD-L1 in non-SC-NEC is already being pursued as antitumor immunotherapy (62).
The expression of PD-L1 in GEP-NENs has been shown to correlate positively with increased grade of these tumors, and PD-L1 positivity was much stronger in PD-GEP-NECs than in low-grade NENs (63). G3 tumors exhibited strong PD-L1 expression in both the tumor and infiltrating immune cells, reflecting an unfavorable environment for T-cell-mediated tumor surveillance by T cells. An increase in membrane expression of PD-L1 in tumors was observed in neoplasms with more aggressive behavior (62). Thus, target therapy against PD-L1 may be an alternative efficient therapy and immunohistochemically documented PD-L1 expression may represent a predictive biomarker for the selection of GEP-NENs patients who will benefit from the immunotherapy (63).
Genetic markers of NENs
Molecular analysis has been recently under investigation, particularly in cases of unknown primary site (17). A number of techniques have been used such as copy number variance (CNV), DNA methylation and RNA expression.
The molecular profile of 14 patients with metastatic GI-NENs was studied by quantitative RT-PCR for candidate gene expression. In approximately 50% of them one or more aberrations were detected [2 had SMARCB1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1)], 2 TP53 (tumor protein p53) mutations, 1 had each STK11 (serine/threonine kinase 11), RET (ret proto-oncogene), and BRAF mutations (B-Raf proto-oncogene, serine/threonine kinase), while CCNE1 (cyclin E1) amplification was found in one patient with an additional TP53 mutation) (64). Moreover, TP53, mitogen activated protein kinase (MAPK3), RET and fibronectin genes up-regulation has been found in pNENs (65,66). Chromosomal imbalances were shown but not in parallel with a repeatable DNA-aberration pattern. Small DNA-gains were found more frequently than DNA losses; DNA-gains involved chromosome 16p, 7p, 14q, 15q, 17q, while 5p, 8q, 9q, and the X-chromosome were amplified as opposed to DNA-losses involving chromosome 1, 10q, 11, 13q, 14q, 15q, 17p and 21q and chromosome 18 loss (66). In 10q11.2 continuous gains or losses were found implying a specific function of the RET proto-oncogene in GI-NET (67). Three genes clusters were suggested as predictors of the primary site of origin defining a decision tree (CD302 up-regulation implying ileum, PPWD1 (peptidylprolyl isomerase domain and WD repeat containing 1) down-regulation implying pancreas and ABHD14B (abhydrolase domain containing 14B) up-regulation implying stomach (66). Other genetic abnormalities included CDKN1B gene mutation (encoding p27, a cell cycle regulator) in SI-NETs and the amplification of chromosome 17q in SI-NETs and pNEN metastatic foci (68).
Circulating tumour cells (CTCs)
CTCs have been documented in patients with breast, prostate and colorectal cancer and are related to the progression-free survival (PFS) and overall survival (OS). NENs express epithelial cellular adhesion molecule (EpCAM) heterogeneously, and CTCs can be isolated from patients’ plasma, making them a possible prognostic factor. In a study with 176 patients with metastatic NENs the value of ≥ one CTC, detected in the blood, was correlated with worse PFS and OS; moreover, within grades of differentiation, presence of CTCs was able to define a poor prognostic subgroup of patients (69).
miRNAs
Small RNA molecules (miRNAs) control gene expression post-transcriptionally. Circulating miRNAs may represent a promising prognostic marker or even a marker associated with resistance to treatment. In patients with GI-NENs, miR-21 levels were strongly related to the proliferation marker Ki-67 and the presence of liver metastases. In 37 patients with pNENs, miR-642 and miR-210 were positively associated with Ki-67 index and metastatic potential, respectively (70,71). Furthermore, in this group of patients, increased expression of miR-196a was related with higher number of mitoses and Ki-67 labelling, increased metastatic potential and decreased OS (71).
Comparative genomic hybridization (CGH)
The array-CGH displayed an enormous number of DNA aberrations [epidermal growth factor receptor (EGFR), erb-B2, E-cadherin, BIRC5 (survivin) MEN1, p53, H19, IGFII (insulin-like growth factor II), c-Myc, c-Met, cyclin D, wnt] that is known to affect tumor suppressor genes as well as proto-oncogenes, but in GEP-NENs no common aberration pattern for primaries and metastases was isolated (66).
NETest
The NETest is a multianalyte algorithm analysis PCR-based test recently proposed using 51 “finger-print” genes characterizing a NET (72). Its clinical value is under investigation and the early reports are promising to predict treatment response as well as early identification of recurrence (73).
Genetic heterogeneity in primary and metastatic sites
Tο assess mutational heterogeneity in primary tumor and metastases, tissue from five patients with SI-NENs and synchronous hepatic metastases, was submitted to whole exome sequencing. The number of mutations that was detected per sample was variable (9–32, mean 22 mutations), while common and private (i.e., gene mutation found in a few members of a family without passing to next generations) mutations were also variable (74).
Molecularly Aided Stratification for Tumor Eradication Research (MASTER)
Molecular diversity of NENs along with individual patterns of molecular aberrations led to a new approach, in terms of diagnostics and therapeutics, employing fast track exome and RNA sequencing, in young adults with advanced stage cancer and in patients with rare tumors (75).
Epigenetics
Epigenetic drugs for cancer therapy constitute the new target of the pharmaceutical industry. Histone deacetylase (HDAC) inhibitors have already been used in the treatment of hematopoietic malignancies, while in neuroendocrine cells studies they induce tumor growth suppression through activation of the Notch1 signalling pathway. Panobinostat, a HDAC, administered in patients with low-grade (grade 1/2) SI-NENs and pNENs, in the context of a phase II trial, resulted in stable disease (100% rate of stability, i.e., with no disease progression), with had a PFS 9.9 months but low response rate (76). Combination therapy of belinostat, another HDAC, and cisplatin-etoposide, in 28 patients with advanced solid tumors, including lung SC-NEC and other NENs of a phase I study, was effective, although further studies are needed (77).
BRAF mutations
Whole exome sequencing was performed on tissue from nine patients with colonic NECs and lead to the detection of BRAF exon 15 mutations in four patients (78).
Small intestinal NEN genetic profiling
Mutation in the cyclin-dependent kinase inhibitor 1B gene (CDKN1B) is the most common mutation found in SI-NENs. Results from genome-wide profiling of tissues from the primary tumor showed that SI-NENs patients can be further categorized into groups with different PFS intervals (79).
Bronchial NEN genomic identity
Integrated genome analyses of tissues from pulmonary NENs showed that the genes involved in the chromatin remodelling pathway are the most commonly affected (80).
Molecular targets of NENs
It has become clear that decrypting the molecular profile of a patient may help to a better therapeutic decision since specific drugs have been discovered that target a specific molecular pathway. Current treatment modalities include somatostatin receptor analogs (SRAs), peptide receptor targeted therapy, molecular targeted therapies (MTTs) interferon-α, conventional chemotherapy, surgery and locoregional therapy. Particularly, MTTs such as the mTOR inhibitor, everolimus and the multiple tyrosine kinase inhibitor (MKI) sunitinib have been approved with promising results (1,2), making the need, for the discovery of newer molecular targeted agents, imperative (81).
Vascular endothelial growth factor receptor (VEGFR) pathway
NENs are highly vascular tumors and increased expression of VEGF, along with VEGFR subtypes 1 and 2 have been discovered in midgut and pNENs (82,83).
Bevacizumab is a monoclonal antibody against VEGF, which reduced tumor angiogenesis in xenograft animal models but without growth inhibition in human carcinoid cell lines (1,84). The comparison of depot octreotide plus interferon alfa-2b versus depot octreotide plus bevacizumab in patients with advanced carcinoid tumors was studied in a phase III prospective randomized trial, but no difference in PFS was seen, implying similar antitumor action of interferon and bevacizumab in this type of tumor (85).
Further, in a multicenter phase II trial, that assessed the combination of bevacizumab with mTOR inhibitor, temsirolimus, in patients with pNENs and disease progression, response rate was found 41% and 6-month PFS was 79%, implying synergistic action although long-term follow-up is needed (86).
Sunitinib blocks VEGFR-1, VEGFR-2, VEGFR-3, KIT [stem-cell factor (SCF) receptor], platelet derived growth factor receptor (PDGFR) alpha (PDGFR-α) and beta (PDGFR-β), involved in the angiogenesis of the WD-pNENs (1,87). In advanced pNEN patients, sunitinib increased PFS, OS, and the objective response rate and therefore sunitinib was added to the pharmaceutical armamentarium (88).
Another oral, potent, and selective inhibitor of VEGFR 1, 2, and 3, axitinib along with SRAs is under investigation in G1–G2 NETs of advanced non-pNETs (NCT01744249).
Phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway
The PI3K/AKT/mTOR pathway is implicated in cell metabolism, growth and survival. A serine threonine kinase, the mTOR protein, has central role in the formation of two distinct multi-protein complexes mTOR complex 1 (mTORC1) and 2 (mTORC2). mTORC1 merges signals from growth factors, energy status, oxygen and amino acids and fine-tunes processes involved in cell growth while the exact role of mTORC2 is under investigation (89). Monotherapy with mTOR inhibitors, such as everolimus, with or without the addition of molecules such as a recent trial of everolimus and a VEGF pathway inhibitor, bevacizumab seems promising treatments (90). In a two-stage phase II trial, that evaluated the efficacy of the dual PI3K/mTOR inhibitor BEZ235 in patients with everolimus-resistant pancreatic NENs, the results were promising, although intolerable toxicity did not allow for the trial to proceed to stage 2 (91).
In cancer cells, PI3K/AKT/mTOR pathway is implicated in proliferation and angiogenesis. In NENs the pathway is activated through growth factor receptors, activating mutations/amplification of kinases, or loss of the tumor suppressor gene PTEN. In NENs, the overexpression of insulin growth factor receptor-1 (IGF-1R) and fibroblast growth factor receptor-3 (FGFR-3) activates the mTOR pathway (83,92). The design of the MetNET-1 trial was based on the effects of metformin on IGF-1 and mTOR complex through AMPK, in combination with the mTOR inhibitor everolimus, in patients with advance pancreatic NENs (93). Genomic profiling of patients with sporadic WD-pNENs, identified mutations in genes of the mTOR pathway in 14% of tumors (94). Apoptotic regulator DAXX and the chromatin modifier ATRX, were associated with better clinical outcome (94). Moreover, biallelic inactivation of ATRX or DAXX through loss of heterozygosity (LOH) was strongly associated with an increase in telomere length as quantified by whole-genome sequencing data (95) while their mutations promoted alternative lengthening of telomeres (ALT) and chromosomal instability (96,97).
The genetic modifications involved in PD-pNENs are different and are associated with the cell cycle, including TP53 and RB1 (98). In familial forms of pNENs such as the syndromes: multiple endocrine neoplasia 1 (MEN-1), von Hippel-Lindau (VHL), neurofibromatosis type 1 (NF-1), tuberous sclerosis complex (TSC), the mTOR pathway is activated (92). In patients with VHL, the mutated VHL gene cause hypoxia-inducible factor (HIF) pathway alteration (99).
IGF system
The IGF system has an important pathophysiological role in several human cancers (100). The IGF system is composed of IGF-I and IGF-II, type 1 (IGF-IR) IGF receptor and type 2 (IGF-IIR) IGF receptor, insulin receptor (IR) isoform A (IR-A) and isoform B (IR-B), and at least six IGF-binding proteins (IGFBP1–6) (101). Expression of IGFs and their receptors correlate with increased angiogenesis, increased metastatic potential, reduced survival and tumor dedifferentiation in several cancer types including NENs. IGF-I, in tissue cell model of pNENs (BON cells), regulates cell-cycle proteins and cellular secretion of CgA. IGF-II and IGF-IR are also expressed in 30% and 70% of NENs, respectively, whereas high IGF-II levels stimulate BON cell growth (102-105). Recently, we have demonstrated that the immunohistochemical expression of IGF-1Ec, a product from alternative splicing of the igf1 gene, was more prevalent in specimens derived from metastatic sites compared to primary sites and in higher Ki-67 specimens compared to specimens with low Ki-67 expression (105). This finding will need to be studied further since a specific pharmaceutical agent for this molecule is under way.
Conclusions
The introduction of the histopathological, immunohistochemical, genetic and molecular markers in NENs has been proven critical in the diagnosis and management of such patients so far. Since NENs are still considered rare diseases and their natural history is variable we have to expand our knowledge on their pathogenesis by molecular and pathological analysis. The better understanding onto their molecular profile will shed light in different immunohistochemical subtypes that may benefit from similar treatments or may identify genetic alterations that may reverse their oncogenic dynamic by targeting specific molecular pathways. Hence, the identification of an immunohistochemical pattern may direct towards a specific organ as metastatic foci origin, implying the therapeutic interventions that have been licenced for this site of NENs origin. Moreover, the molecular and/or genetic profile may also reveal a molecular pathway that is more altered than another one supporting one therapeutic option over another one. Hence, the future of NENs management seems to include an integrated profile of a patient with NEN, providing data on all the features of the neoplasm, from the Ki-67 to the specific site of origin and with specific genetic and molecular alterations resulting in the most efficient therapeutic alteration to achieve the best therapeutic response. Test and programs such as the NETest and the MASTER have been already validated from scientific groups and they have been proved effective to select patients who will benefit from specific medications and to predict the response to the treatment. The collaboration in intra-national level by creating Centers of Excellence on NETs and in inter-national level by creating collaborations between Centers of Excellence of different countries will increase the value of the clinical studies by increasing the number of the participants making the findings more valid and reproducible.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pavel M. Translation of molecular pathways into clinical trials of neuroendocrine tumors. Neuroendocrinology 2013;97:99-112. 10.1159/000336089 [DOI] [PubMed] [Google Scholar]
- 2.Alexandraki KI, Kaltsas G. Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine 2012;41:40-52. 10.1007/s12020-011-9562-2 [DOI] [PubMed] [Google Scholar]
- 3.Klimstra DS. Pathologic Classification of Neuroendocrine Neoplasms. Hematol Oncol Clin North Am 2016;30:1-19. 10.1016/j.hoc.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 4.Lloyd RV, Osamura RY, Klöppel G, et al. WHO classification of tumours of endocrine organs. 4th edition. World Health Organization; International Agency for Research on Cancer, 2017. [Google Scholar]
- 5.Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch 2018;472:341-9. 10.1007/s00428-017-2258-0 [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System. 4th edition. WHO/IARC, Placed Published, 2010. [Google Scholar]
- 7.Soga J, Tazawa K. Pathologic analysis of carcinoids. Histologic reevaluation of 62 cases. Cancer 1971;28:990-8. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Thomas RM, Elsayed AM, et al. Carcinoids of the jejunum and ileum: an immunohistochemical and clinicopathologic study of 167 cases. Cancer 1997;79:1086-93. [DOI] [PubMed] [Google Scholar]
- 9.Bellizzi AM. Assigning site of origin in metastatic neuroendocrine neoplasms: a clinically significant application of diagnostic immunohistochemistry. Adv Anat Pathol 2013;20:285-314. 10.1097/PAP.0b013e3182a2dc67 [DOI] [PubMed] [Google Scholar]
- 10.Lin F, Liu H. Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. Arch Pathol Lab Med 2014;138:1583-610. 10.5858/arpa.2014-0061-RA [DOI] [PubMed] [Google Scholar]
- 11.Lloyd R. Immunohistochemical markers of endocrine/neuroendocrine tumors. J Histotechnol 1999;22:231-7. 10.1179/his.1999.22.3.231 [DOI] [Google Scholar]
- 12.Al-Khafaji B, Noffsinger AE, Miller MA, et al. Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Hum Pathol 1998;29:992-9. 10.1016/S0046-8177(98)90206-4 [DOI] [PubMed] [Google Scholar]
- 13.Erickson LA, Lloyd RV. Practical markers used in the diagnosis of endocrine tumors. Adv Anat Pathol 2004;11:175-89. 10.1097/01.pap.0000131824.77317.a7 [DOI] [PubMed] [Google Scholar]
- 14.Gould VE, Lee I, Wiedenmann B, et al. Synaptophysin: a novel marker for neurons, certain neuroendocrine cells, and their neoplasms. Hum Pathol 1986;17:979-83. 10.1016/S0046-8177(86)80080-6 [DOI] [PubMed] [Google Scholar]
- 15.Klimstra DS, Pitman MB, Hruban RH. An algorithmic approach to the diagnosis of pancreatic neoplasms. Arch Pathol Lab Med 2009;133:454-64. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol 2016;27:284-311. 10.1007/s12022-016-9432-9 [DOI] [PubMed] [Google Scholar]
- 17.Alexandraki K, Angelousi A, Boutzios G, et al. Management of neuroendocrine tumors of unknown primary. Rev Endocr Metab Disord 2017;18:423-31. 10.1007/s11154-017-9437-9 [DOI] [PubMed] [Google Scholar]
- 18.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182:311-22. [DOI] [PubMed] [Google Scholar]
- 19.Nadler A, Cukier M, Rowsell C, et al. Ki-67 is a reliable pathological grading marker for neuroendocrine tumors. Virchows Arch 2013;462:501-5. 10.1007/s00428-013-1410-8 [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 2011;35:853-60. 10.1097/PAS.0b013e31821a0696 [DOI] [PubMed] [Google Scholar]
- 21.Combs SE, Han G, Mani N, et al. Loss of antigenicity with tissue age in breast cancer. Lab Invest 2016;96:264-9. 10.1038/labinvest.2015.138 [DOI] [PubMed] [Google Scholar]
- 22.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther 2004;3:593-601. 10.4161/cbt.3.7.913 [DOI] [PubMed] [Google Scholar]
- 23.Moskaluk CA, Zhang H, Powell SM, et al. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16:913-9. 10.1097/01.MP.0000086073.92773.55 [DOI] [PubMed] [Google Scholar]
- 24.Barbareschi M, Murer B, Colby TV, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol 2003;27:141-9. 10.1097/00000478-200302000-00001 [DOI] [PubMed] [Google Scholar]
- 25.Erickson LA, Papouchado B, Dimashkieh H, et al. Cdx2 as a marker for neuroendocrine tumors of unknown primary sites. Endocr Pathol 2004;15:247-52. 10.1385/EP:15:3:247 [DOI] [PubMed] [Google Scholar]
- 26.Schmitt AM, Riniker F, Anlauf M, et al. Islet 1 (Isl1) expression is a reliable marker for pancreatic endocrine tumors and their metastases. Am J Surg Pathol 2008;32:420-5. 10.1097/PAS.0b013e318158a397 [DOI] [PubMed] [Google Scholar]
- 27.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol 2009;33:626-32. 10.1097/PAS.0b013e31818d7d8b [DOI] [PubMed] [Google Scholar]
- 28.Rabban JT, Lerwill MF, McCluggage WG, et al. Primary ovarian carcinoid tumors may express CDX-2: a potential pitfall in distinction from metastatic intestinal carcinoid tumors involving the ovary. Int J Gynecol Pathol 2009;28:41-8. 10.1097/PGP.0b013e31817a8f51 [DOI] [PubMed] [Google Scholar]
- 29.Ordóñez NG. Value of thyroid transcription factor-1 immunostaining in tumor diagnosis: a review and update. Appl Immunohistochem Mol Morphol 2012;20:429-44. 10.1097/PAI.0b013e31825439bc [DOI] [PubMed] [Google Scholar]
- 30.La Rosa S, Imperatori A, Giovanella L, et al. Thyroid metastases from typical carcinoid of the lung differentiating between medullary thyroid carcinoma and neuroendocrine tumor metastasis to the thyroid. Thyroid 2009;19:521-6. 10.1089/thy.2008.0424 [DOI] [PubMed] [Google Scholar]
- 31.Matias-Guiu X, LaGuette J, Puras-Gil AM, et al. Metastatic neuroendocrine tumors to the thyroid gland mimicking medullary carcinoma: a pathologic and immunohistochemical study of six cases. Am J Surg Pathol 1997;21:754-62. 10.1097/00000478-199707000-00003 [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology 2000;36:415-20. 10.1046/j.1365-2559.2000.00890.x [DOI] [PubMed] [Google Scholar]
- 33.Nonaka D, Tang Y, Chiriboga L, et al. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol 2008;21:192-200. 10.1038/modpathol.3801002 [DOI] [PubMed] [Google Scholar]
- 34.Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol 2008;32:1566-71. 10.1097/PAS.0b013e31816d71ad [DOI] [PubMed] [Google Scholar]
- 35.Tong GX, Yu WM, Beaubier NT, et al. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 2009;22:1218-27. 10.1038/modpathol.2009.88 [DOI] [PubMed] [Google Scholar]
- 36.Long KB, Srivastava A, Hirsch MS, et al. PAX8 Expression in well-differentiated pancreatic endocrine tumors: correlation with clinicopathologic features and comparison with gastrointestinal and pulmonary carcinoid tumors. Am J Surg Pathol 2010;34:723-9. [DOI] [PubMed] [Google Scholar]
- 37.Lorenzo PI, Jimenez Moreno CM, Delgado I, et al. Immunohistochemical assessment of Pax8 expression during pancreatic islet development and in human neuroendocrine tumors. Histochem Cell Biol 2011;136:595-607. 10.1007/s00418-011-0866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai JP, Mertens RB, Mirocha J, et al. Comparison of PAX6 and PAX8 as immunohistochemical markers for pancreatic neuroendocrine tumors. Endocr Pathol 2015;26:54-62 10.1007/s12022-014-9346-3 [DOI] [PubMed] [Google Scholar]
- 39.Koo J, Mertens RB, Mirocha JM, et al. Value of Islet 1 and PAX8 in identifying metastatic neuroendocrine tumors of pancreatic origin. Mod Pathol 2012;25:893-901. 10.1038/modpathol.2012.34 [DOI] [PubMed] [Google Scholar]
- 40.Hermann G, Konukiewitz B, Schmitt A, et al. Hormonally defined pancreatic and duodenal neuroendocrine tumors differ in their transcription factor signatures: expression of ISL1, PDX1, NGN3, and CDX2. Virchows Arch 2011;459:147-54. 10.1007/s00428-011-1118-6 [DOI] [PubMed] [Google Scholar]
- 41.Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol 2007;15:407-14. 10.1097/01.pai.0000210416.53493.0f [DOI] [PubMed] [Google Scholar]
- 42.Denby KS, Briones AJ, Bourne PA, et al. IMP3, NESP55, TTF-1 and CDX2 serve as an immunohistochemical panel in the distinction among small-cell carcinoma, gastrointestinal carcinoid, and pancreatic endocrine tumor metastasized to the liver. Appl Immunohistochem Mol Morphol 2012;20:573-9. 10.1097/PAI.0b013e3182494009 [DOI] [PubMed] [Google Scholar]
- 43.Agaimy A, Erlenbach-Wünsch K, Konukiewitz B, et al. ISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic origin. Mod Pathol 2013;26:995-1003. 10.1038/modpathol.2013.40 [DOI] [PubMed] [Google Scholar]
- 44.Doglioni C, Gambacorta M, Zamboni G, et al. Immunocytochemical localization of progesterone receptors in endocrine cells of the human pancreas. Am J Pathol 1990;137:999-1005. [PMC free article] [PubMed] [Google Scholar]
- 45.Viale G, Doglioni C, Gambacorta M, et al. Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 1992;70:2268-77. [DOI] [PubMed] [Google Scholar]
- 46.Chan ES, Alexander J, Swanson PE, et al. PDX-1, CDX-2, TTF-1, and CK7: a reliable immunohistochemical panel for pancreatic neuroendocrine neoplasms. Am J Surg Pathol 2012;36:737-43. 10.1097/PAS.0b013e31824aba59 [DOI] [PubMed] [Google Scholar]
- 47.Jakobsen AM, Ahlman H, Kölby L, et al. NESP55, a novel chromogranin-like peptide, is expressed in endocrine tumours of the pancreas and adrenal medulla but not in ileal carcinoids. Br J Cancer 2003;88:1746-54. 10.1038/sj.bjc.6600924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav R, Kakkar A, Sharma A, et al. Study of clinicopathological features, hormone immunoexpression, and loss of ATRX and DAXX expression in pancreatic neuroendocrine tumors. Scand J Gastroenterol 2016;51:994-9. 10.3109/00365521.2016.1170195 [DOI] [PubMed] [Google Scholar]
- 49.Papotti M, Bongiovanni M, Volante M, et al. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch 2002;440:461-75. 10.1007/s00428-002-0609-x [DOI] [PubMed] [Google Scholar]
- 50.Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007;20:1172-82. 10.1038/modpathol.3800954 [DOI] [PubMed] [Google Scholar]
- 51.Zamora V, Cabanne A, Salanova R, et al. Immunohistochemical expression of somatostatin receptors in digestive endocrine tumours. Dig Liver Dis 2010;42:220-5. 10.1016/j.dld.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 52.Diakatou E, Alexandraki KI, Tsolakis AV, et al. Somatostatin and dopamine receptor expression in neuroendocrine neoplasms: correlation of immunohistochemical findings with somatostatin receptor scintigraphy visual scores. Clin Endocrinol (Oxf) 2015;83:420-8. 10.1111/cen.12775 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Wang W, Jin K, et al. Somatostatin receptor expression indicates improved prognosis in gastroenteropancreatic neuroendocrine neoplasm, and octreotide long-acting release is effective and safe in Chinese patients with advanced gastroenteropancreatic neuroendocrine tumors. Oncol Lett 2017;13:1165-74. 10.3892/ol.2017.5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerner AB, Case JD, Takahashi Y, et al. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes. J Am Chem Soc 1958;80:2587 10.1021/ja01543a060 [DOI] [Google Scholar]
- 55.Emet M, Ozcan H, Ozel L, et al. A Review of Melatonin, Its Receptors and Drugs. Eurasian J Med 2016;48:135-41. 10.5152/eurasianjmed.2015.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Söderquist F, Hellström PM, Cunningham JL. Human gastroenteropancreatic expression of melatonin and its receptors MT1 and MT2. PLoS One 2015;10:e0120195. 10.1371/journal.pone.0120195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Söderquist F, Janson ET, Rasmusson AJ, et al. Melatonin Immunoreactivity in Malignant Small Intestinal Neuroendocrine Tumours. PLoS One 2016;11:e0164354. 10.1371/journal.pone.0164354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das A, McDowell M, Pava MJ, et al. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-alpha toxicity involves membrane melatonin receptors. J Pineal Res 2010;48:157-69. 10.1111/j.1600-079X.2009.00739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xin Z, Jiang S, Jiang P, et al. Melatonin as a treatment for gastrointestinal cancer: a review. J Pineal Res 2015;58:375-87. 10.1111/jpi.12227 [DOI] [PubMed] [Google Scholar]
- 60.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012;1:1223-25. 10.4161/onci.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology 2014;3:e29288. 10.4161/onci.29288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jing W, Li M, Zhang Y, et al. PD-1/PD-L1 blockades in non-small-cell lung cancer therapy. Onco Targets Ther 2016;9:489-502. 10.2147/OTT.S94993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cavalcanti E, Armentano R, Valentini AM, et al. Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading. Cell Death Dis 2017;8:e3004. 10.1038/cddis.2017.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim ST, Lee SJ, Park SH, et al. Genomic Profiling of Metastatic Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET) Patients in the Personalized-Medicine Era. J Cancer 2016;7:1044-8. 10.7150/jca.14815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capurso G, Lattimore S, Crnogorac-Jurcevic T, et al. Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer 2006;13:541-58. 10.1677/erc.1.01153 [DOI] [PubMed] [Google Scholar]
- 66.Posorski N, Kaemmerer D, Ernst G, et al. Localization of sporadic neuroendocrine tumors by gene expression analysis of their metastases. Clin Exp Metastasis 2011;28:637-47. 10.1007/s10585-011-9397-5 [DOI] [PubMed] [Google Scholar]
- 67.Kaemmerer D, Posorski N, von Eggeling F, et al. The search for the primary tumor in metastasized gastroenteropancreatic neuroendocrine neoplasm. Clin Exp Metastasis 2014;31:817-27. 10.1007/s10585-014-9672-3 [DOI] [PubMed] [Google Scholar]
- 68.Karpathakis A, Dibra H, Pipinikas C, et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr Relat Cancer 2017;24:L21-5. 10.1530/ERC-16-0419 [DOI] [PubMed] [Google Scholar]
- 69.Khan MS, Kirkwood A, Tsigani T, et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol 2013;31:365-72. 10.1200/JCO.2012.44.2905 [DOI] [PubMed] [Google Scholar]
- 70.Thorns C, Schurmann C, Gebauer N, et al. Global microRNA profiling of pancreatic neuroendocrine neoplasias. Anticancer Res 2014;34:2249-54. [PubMed] [Google Scholar]
- 71.Zatelli MC, Grossrubatscher EM, Guadagno E, et al. Circulating tumor cells and miRNAs as prognostic markers in neuroendocrine neoplasms. Endocr Relat Cancer 2017;24:R223-37. 10.1530/ERC-17-0091 [DOI] [PubMed] [Google Scholar]
- 72.Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat Cancer 2015;22:561-75. 10.1530/ERC-15-0092 [DOI] [PubMed] [Google Scholar]
- 73.Ćwikła JB, Bodei L, Kolasinska-Ćwikła A, et al. Circulating Transcript Analysis (NETest) in GEP-NETs Treated With Somatostatin Analogs Defines Therapy. J Clin Endocrinol Metab 2015;100:E1437-45. 10.1210/jc.2015-2792 [DOI] [PubMed] [Google Scholar]
- 74.Walter D, Harter PN, Battke F, et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci Rep 2018;8:3811. 10.1038/s41598-018-22115-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horak P, Klink B, Heining C, et al. Precision oncology based on omics data: The NCT Heidelberg experience. Int J Cancer 2017;141:877-86. 10.1002/ijc.30828 [DOI] [PubMed] [Google Scholar]
- 76.Jin N, Lubner SJ, Mulkerin DL, et al. A Phase II Trial of a Histone Deacetylase Inhibitor Panobinostat in Patients With Low-Grade Neuroendocrine Tumors. Oncologist 2016;21:785-6. 10.1634/theoncologist.2016-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balasubramaniam S, Redon CE, Peer CJ, et al. Phase I trial of belinostat with cisplatin and etoposide in advanced solid tumors, with a focus on neuroendocrine and small cell cancers of the lung. Anticancer Drugs 2018;29:457-65. 10.1097/CAD.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Idrees K, Padmanabhan C, Liu E, et al. Frequent BRAF mutations suggest a novel oncogenic driver in colonic neuroendocrine carcinoma. J Surg Oncol 2018;117:284-9. 10.1002/jso.24834 [DOI] [PubMed] [Google Scholar]
- 79.Karpathakis A, Dibra H, Pipinikas C, et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin Cancer Res 2016;22:250-8. 10.1158/1078-0432.CCR-15-0373 [DOI] [PubMed] [Google Scholar]
- 80.Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. 10.1038/ncomms4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alexandraki KI, Karapanagioti A, Karoumpalis I, et al. Advances and Current Concepts in the Medical Management of Gastroenteropancreatic Neuroendocrine Neoplasms. Biomed Res Int 2017;2017:9856140 10.1155/2017/9856140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology 1998;32:133-8. 10.1046/j.1365-2559.1998.00321.x [DOI] [PubMed] [Google Scholar]
- 83.Chan JA, Kulke MH. Progress in the treatment of neuroendocrine tumors. Curr Oncol Rep 2009;11:193-9. 10.1007/s11912-009-0028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Jia Z, Li Q, et al. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007;109:1478-86. 10.1002/cncr.22554 [DOI] [PubMed] [Google Scholar]
- 85.Yao JC, Guthrie KA, Moran C, et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518. J Clin Oncol 2017;35:1695-703. 10.1200/JCO.2016.70.4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hobday TJ, Qin R, Reidy-Lagunes D, et al. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J Clin Oncol 2015;33:1551-6. 10.1200/JCO.2014.56.2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delbaldo C, Faivre S, Dreyer C, et al. Sunitinib in advanced pancreatic neuroendocrine tumors: latest evidence and clinical potential. Ther Adv Med Oncol 2012;4:9-18. 10.1177/1758834011428147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 89.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 2009;122:3589-94. 10.1242/jcs.051011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yao JC, Phan AT, Hess K, et al. Perfusion computed tomography as functional biomarker in randomized run-in study of bevacizumab and everolimus in well-differentiated neuroendocrine tumors. Pancreas 2015;44:190-7. 10.1097/MPA.0000000000000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fazio N, Buzzoni R, Baudin E, et al. A Phase II Study of BEZ235 in Patients with Everolimus-resistant, Advanced Pancreatic Neuroendocrine Tumours. Anticancer Res 2016;36:713-9. [PMC free article] [PubMed] [Google Scholar]
- 92.Gajate P, Alonso-Gordoa T, Martínez-Sáez O, et al. Prognostic and predictive role of the PI3K-AKT-mTOR pathway in neuroendocrine neoplasms. Clin Transl Oncol 2018;20:561-9. 10.1007/s12094-017-1758-3 [DOI] [PubMed] [Google Scholar]
- 93.Pusceddu S, de Braud F, Concas L, et al. Rationale and protocol of the MetNET-1 trial, a prospective, single center, phase II study to evaluate the activity and safety of everolimus in combination with octreotide LAR and metformin in patients with advanced pancreatic neuroendocrine tumors. Tumori 2014;100:e286-9. [DOI] [PubMed] [Google Scholar]
- 94.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199-203. 10.1126/science.1200609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017;543:65-71. 10.1038/nature21063 [DOI] [PubMed] [Google Scholar]
- 96.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011;333:425. 10.1126/science.1207313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014;146:453-60.e5. 10.1053/j.gastro.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 98.Klöppel G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc Med 2017;33:324-30. 10.1159/000481390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 2004;18:2893-904. 10.1101/gad.1256804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LeRoith D, Roberts CT. The insulin-like growth factor system and cancer. Cancer Lett 2003;195:127-37. 10.1016/S0304-3835(03)00159-9 [DOI] [PubMed] [Google Scholar]
- 101.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995;16:3-34. [DOI] [PubMed] [Google Scholar]
- 102.Höpfner M, Baradari V, Huether A, et al. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer 2006;13:135-49. 10.1677/erc.1.01090 [DOI] [PubMed] [Google Scholar]
- 103.Wulbrand U, Remmert G, Zöfel P, et al. mRNA expression patterns of insulin-like growth factor system components in human neuroendocrine tumours. Eur J Clin Invest 2000;30:729-39. 10.1046/j.1365-2362.2000.00700.x [DOI] [PubMed] [Google Scholar]
- 104.Evers BM, Ishizuka J, Townsend CM, et al. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci 1994;733:393-406. 10.1111/j.1749-6632.1994.tb17289.x [DOI] [PubMed] [Google Scholar]
- 105.Alexandraki KI, Philippou A, Boutzios G, et al. IGF-IEc expression is increased in secondary compared to primary foci in neuroendocrine neoplasms. Oncotarget 2017;8:79003-11. 10.18632/oncotarget.20743 [DOI] [PMC free article] [PubMed] [Google Scholar]