Abstract
Background
Resident memory T cells have emerged as key players in the immune response generated against a number of pathogens. Their ability to take residence in non-lymphoid peripheral tissues allows for the rapid deployment of secondary effector responses at the site of pathogen entry. This ability to provide enhanced regional immunity has gathered much attention, with the generation of resident memory T cells being the goal of many novel vaccines.
Objectives
This review aimed to systematically analyze published literature investigating the role of resident memory T cells in human infectious diseases. Known effector responses mounted by these cells are summarized and key strategies that are potentially influential in the rational design of resident memory T cell inducing vaccines have also been highlighted.
Methods
A Boolean search was applied to Medline, SCOPUS, and Web of Science. Studies that investigated the effector response generated by resident memory T cells and/or evaluated strategies for inducing these cells were included irrespective of published date. Studies must have utilized an established technique for identifying resident memory T cells such as T cell phenotyping.
Results
While over 600 publications were revealed by the search, 147 articles were eligible for inclusion. The reference lists of included articles were also screened for other eligible publications. This resulted in the inclusion of publications that studied resident memory T cells in the context of over 25 human pathogens. The vast majority of studies were conducted in mouse models and demonstrated that resident memory T cells mount protective immune responses.
Conclusion
Although the role resident memory T cells play in providing immunity varies depending on the pathogen and anatomical location they resided in, the evidence overall suggests that these cells are vital for the timely and optimal protection against a number of infectious diseases. The induction of resident memory T cells should be further investigated and seriously considered when designing new vaccines.
Keywords: resident memory T cells, infectious diseases, vaccine development, immunity, microbiology
Introduction
Traditionally, memory T cells have been subdivided into two broad categories: effector memory and central memory T cells (TEM and TCM, respectively). After the realization that some memory T cells fail to egress out of peripheral tissues back into the blood stream, it became clear that this dichotomous distinction of memory T cells did not account for the complete diversity of the memory T cell population. This led to the discovery of a third subset of memory T cell. Appropriately, dubbed “Tissue-resident memory T cells” (here after referred to as TRM), this newly defined population exhibits the unique feature of remaining localized in peripheral tissues (1). As such, these cells provide enhanced localized immunosurveillance and protection of peripheral tissues when compared to TEM and TCM. TRM have been characterized in many peripheral tissues, including skin, lungs, brain, liver, the female reproductive tract, and the gastrointestinal mucosa. Given the huge variance in their location of residence, this subset of memory cell is highly heterogeneous, phenotypically varying depending on their anatomic location and the inflammatory cues produced by their respective microenvironment. Although experimental techniques such as parabiosis can definitively distinguish TRM from circulating memory T cells, other less complex methods of identifying TRM are more frequently used. The co-expression of CD69 and CD103 is commonly used as a marker of tissue residence, although it appears not all bona fide TRM are defined by this particular phenotype. Regardless, TRM have been implicated in a wide range of physiological functions, such as providing protection against pathogens and cancerous cells, as well as in many pathological states such as autoimmune and other inflammatory diseases. The exploration of TRM biology and the role they play in maintaining homeostasis has broad implications for human health. Currently, our understanding of TRM function is largely constrained within the context of infectious diseases. As of now, it appears that TRM are better adapted to providing rapid protection against pathogen invasion when compared to their circulating counter parts (2–4). Thus, vaccines of the future would ideally establish a population of protective TRM at the portals of entry most at risk of pathogen invasion to provide immediate and effective immunity, rather than relying on the delayed recruitment of effector cells from the circulating pool of memory cells. Since parenterally administered vaccines induce minimal tissue-specific protection, current routes of administering vaccines may need to be revised (5, 6). The present review will primarily focus on the role of TRM in the immune response generated to a range of human pathogens and discuss future avenues for the development of TRM-based vaccines.
Methodology
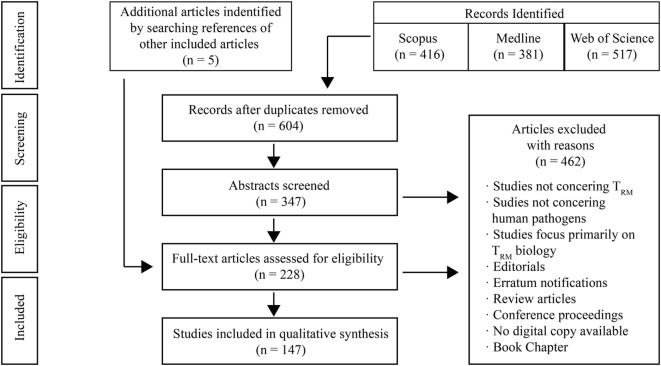
A systematic search of published literature was conducted. Literature was critically evaluated for evidence of the role TRM play during infections and in vaccinology. A flowchart summarizing our methodology has been included (Figure 1). The preparation of this review was guided by the PRISMA-P 2015 guideline (7).
Figure 1.
Literature search strategy. The search strategy used revealed 381 records in Medline (Ovid), 416 in SCOPUS, and 517 in Web of Science. This resulted in a total number of 1,314 records. After removing duplicates, there were 604 records. Screening of titles resulted in the exclusion of 257 records, as they did not address resident memory T cells, human infectious diseases, or neither. Others records were excluded as they were reviews, editorials, meeting abstracts, book chapters, poster presentations, or erratum notifications. The abstracts of the remaining 347 records were analyzed and a further 124 publications were excluded due to their focus on TRM biology. The full texts of the remaining studies were reviewed. 81 of these texts were excluded for aforementioned reasons. Co-authors were consulted when there was ambiguity regarding the relevance of a study. In total, 142 publications from the search were included. 5 additional studies were included by screening the references of studies from the search results and following external review.
Final searches of literature were performed on March 23, 2018 in Medline, SCOPUS and Web of Science by the first author. The Boolean search strategy used was as following (“resident memory t cell*” OR “t resident memory cell*” OR “tissue resident memory cell*” OR “resident memory” OR “tissue memory”). The references of included studies were also screened for other relevant publications.
Both human and animal studies that use surface markers of residence or other established techniques such as intravascular staining and parabiosis to illustrate localization of T cells to peripheral tissues, as well as T cell phenotyping were included. Studies were also screened for their relevance to human pathogens, and thus animal infection models that are analogous to human infectious diseases were included. Studies were included irrespective of published date. Only published and accepted manuscripts of original research were included. Publications that primarily focused on TRM biology (ontogeny, cellular metabolism, etc.) or non-infectious diseases were not included. Certain non-communicable diseases such as hepatocellular carcinoma and cervical cancer that can be caused by pathogens are briefly mentioned within the broader discussion of TRM-mediated immunity.
Results of Search
The results of the search strategy are summarized in Figure 1.
Data Synthesis and Analysis
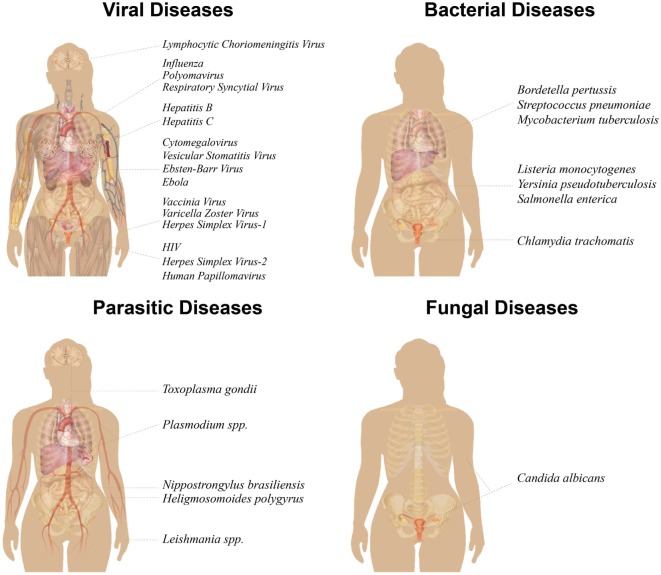
The first author conducted extraction of data from relevant studies. This review has been divided into sections based on pathogen type: viruses, bacteria, parasites/helminths, and fungi (Figure 2). The studies included in this review contain the most relevant findings related to immune responses generated by TRM against human pathogens, or make use of novel strategies for TRM generation. We apologize to authors whose work could not be included in this review.
Figure 2.
Illustration of human-relevant diseases for which a role of TRM has been reported. TRM have been studied in 16 viral diseases (top left), seven bacterial diseases (top right), five parasitic diseases (bottom left), and one fungal disease (bottom right). Pathogens have been grouped according to organs or organ systems that have been studied in the context of TRM, including the respiratory, gastrointestinal, and urogenital tracts, as well as brain, skin, liver, lymphatics, and circulation. Nippostrongylus brasiliensis and Heligmosomoides polygyrus are mouse pathogens for human Necator americanus and gastrointestinal helminth infections, respectively. Image modified from https://commons.wikimedia.org/wiki/File:Female_shadow_anatomy_without_labels.svg.
The Role of TRM in Viral Infections
As of present, TRM immune responses are by far mostly studied in the context of viral pathogens. The following section will present findings by specific viral pathogen/viral disease.
Herpes Simplex Virus (HSV)
Herpes simplex virus causes infections that present with a varying range of symptoms. The primary clinical manifestations of HSV infection are intraepithelial vesicles. There are two antigenically distinct HSV subtypes: HSV-1 and HSV-2, causing cold sores and genital warts, respectively (8). However, both sub-types can be the etiology of either clinical disease as sexual transmission allows for spread between the two sites (9). Both viruses establish a life-long latent infection within the surrounding nervous tissue, and control of HSV infection requires effective cell-mediated immune responses to prevent reactivation. However, co-morbid illnesses, immunosuppressive drugs, UV exposure, and psychological stress can hinder immune control. A number of studies suggest that TRM are implicated in controlling HSV-1 latency in the trigeminal ganglia (10). HSV-1 infection models primarily focus on infections of the skin and nervous tissue such as the trigeminal ganglia and the eyes (11–22). Following acute infection with HSV-1, CD8+ TRM remain localized to the skin initially infected and are also found surrounding latently infected sensory ganglia (11). However, evidence suggests that multiple exposures to cognate antigen can substantially increase the TRM population in not only the site of HSV infection, but also in distant skin (12). CD8+ skin TRM appear to resemble the antigen-presenting Langerhans cells of the skin, extending dendritic projections into the surrounding tissue, probably in an attempt to survey the local area for antigen (13). This is supported by evidence from confocal microscopy and intravital imaging (21) that suggests these TRM can travel between keratinocytes (13). However, unlike Langerhans cells, these TRM do not extend into the stratum corneum (13, 17). It also appears that skin TRM are not specifically attracted to virally infected cells, and thus migrate throughout the epidermis in a random manner (17, 21). By extension, it can be inferred that skin TRM may take a considerable period of time before identifying virally affected cells. As such, it may be safe to assume that a critical mass of skin TRM is needed in order to afford timely protection. This notion is supported by the observation that protection appears to be dependent upon the local density of TRM (17). Upon antigen recognition, skin TRM undergo a change in their morphological and motility pattern, decelerating their migratory rate and losing their dendricity (13, 17). This is probably indicative of a shift in role from immunosurveillance to effector function. Furthermore, the maintenance of HSV-1-specific TRM populations appears to be independent of circulating T cells in both skin and trigeminal ganglia (14, 17). Skin TRM appear to be able to sustain their numbers through local proliferation after secondary infection (17). However, after a combined corticosterone and stress-induced reduction of trigeminal ganglia CD8+ T cells (presumably TRM), there appeared to be no increased proliferation of remaining T cells when compared to the homeostatic proliferation rates as indicated by BrdU incorporation (14). Analysis of HSV-1-specific skin and dorsal root ganglia TRM during acute immunity and later time points revealed that transcription of cytolytic molecules decreases with time. As such, TRM-based immunity in the long term may not be reliant on enhanced cytolytic effector functions, but rather on the localization of these cells at sites susceptible to reinfection (15). The chronic inflammatory response induced by persistent viral gene expression during latency leads to TRM exhaustion in the brain ependymal region, rendering them unable to control HSV-1 infection (16). Perhaps the reason why TRM downregulate their cytolytic genes during times of homeostasis is because continuous expression may lead to exhaustion. Nevertheless, it appears that the generation of CD8+ TRM in skin and ganglia may be a viable option for protection against HSV-1 infection or reactivation. Local inflammation of the skin and mucosa alone can encourage the recruitment of TEM to these peripheral sites where differentiation into the TRM phenotype occurs. This was demonstrated using 2,4-dinitrofluorobenzene, a contact-sensitizing agent. Furthermore, the application of nonoxynol-9 (a spermicide agent) to the female genital tract enhanced protection against HSV challenge, correlating with higher numbers of CD103+ T cells localizing to the epithelium (19). Hence, agents that can be applied to specific tissue and that cause a localized, general inflammatory response may be a strategy worth exploring for the generation of TRM. More specifically, however, the CXCL10/CXCR3 chemokine pathway appears to be vital in generating TRM, as mice deficient in either CXCL10 or CXCR3 were unprotected against HSV-1 UV-B light-induced reactivation challenge. Furthermore, the administration of CXCL10 into deficient mice through the use of a neurotropic virus vector amplified TRM in the trigeminal ganglia, conferring better protections against reactivation challenge (20). CXCL10 administration through the use of a rAAV8-CamKIIa-GFP-CamKIIa-CXCL10 vector showed similar results (18). Samples from human patients that were asymptomatic but seropositive for HSV-1 infection were used to determine which 467 HLA-A*0201-restircted CD8+ T cell epitopes were immunodominant in the HSV-1-specific immune response. These asymptomatic individuals generated a high number of polyfunctional CD8+ TEM against three epitopes. HLA-A*0201 transgenic mice were primed with these epitopes and subsequently treated with an ocular topical preparation containing rAAV8-CamKIIa-GFP-CamKIIa-CXCL10 to deliver exogenous CXCL10 chemokine. Results from UV-B reactivation challenge demonstrated that this strategy was able to reduce viral shedding in tears and recurrent herpetic ocular disease (18). This strategy may be beneficial in rationally developing novel vaccines against other diseases.
Human studies have also been conducted in HSV-2 infection. Samples from the genital tract of HSV-2-infected women contained populations of HSV-2-specific T cells with a TRM phenotype (23). More interestingly, a population of CD8αα+ TRM that reside at the dermal–epidermal junction have also been described in biopsies of HSV-2-infected humans. This unique positioning suggests that these cells may be able to survey the neural tissue from which virus travels to the skin during reactivation (24). Thus, TRM play a role in the natural immune response against HSV-2 infection. Although it was already demonstrated that T cells could be recruited to peripheral tissues using inflammatory agents (19), the “prime and pull” vaccine strategy was first described in a HSV-2 infection model (25). In this study, the investigators explored the novel idea of parenterally immunizing mice and subsequently topically administering CXCL10 into the vagina before challenging with HSV-2. Mice that underwent the prime and pull protocol showed minimal signs of clinical disease and had a survival rate of 100%. Naive and parenterally immunized mice that did not receive the pull treatment developed clinical disease and exhibited high mortality rates. This strategy also demonstrated the capacity to prevent infection of sensory neurons (25). Further investigation of this protocol revealed that immunity was largely dependent on INF-γ produced by CD8+ TRM (26). Re-stimulation of this CD8+ TRM was dependent on a population of CD301b+ dendritic cells that resided in the lamina propria. In fact, depletion of CD301b+ dendritic cells using a diphtheria toxin model rendered the prime and pull strategy ineffective and mice suffered high morbidity and mortality rates (26). Although a non-specific inflammatory stimulus such as nonoxynol-9 may be sufficient to pull CD8+ T cells in to the female reproductive tract and subsequently convert them to TRM, it appears that antigen presentation by CD301b+ dendritic cells is needed for CD8+ TRM-mediated immunity at this site. A different study that made use of a topical vaccine containing a human papillomavirus (HPV) vector expressing gB and gD ectodomains of HSV-2 has shown the capacity to generate TRM in the reproductive tract, and reduce viral shedding and clinical disease (27). This study highlights the capacity of HPV vectors to induce TRM in the genital tract, a vaccine strategy that may be applicable to other sexually transmitted infections. HSV-2-specific CD8+ TRM can also be generated using a “chemical-free and biological-free” laser adjuvant, a protocol that could be explored in other infectious models (28). While the vast majority of studies have assessed the protective capabilities of CD8+ TRM during HSV infection, very few studies have analyzed the role of CD4+ TRM in HSV infections (21, 29). Intravaginal vaccination of mice with thymidine kinase negative HSV-2 (an attenuated form of the virus) provided full protection against challenge with wild-type HSV-2, independent of CD8+ T cells and B cells. Instead, parabiosis studies demonstrated that CD4+ TRM are required within the genital tract mucosa for immunity in this model. These CD4+ TRM are polyfunctional, secreting IFN-γ, TNF-α, and IL-2, and resided in organized, non-tertiary immune structures called memory lymphocyte clusters (MLCs). These MLCs appear to assemble under the influence of macrophage-secreted CCL5. Upon antigen stimulation, the CD4+ TRM within the MLCs expand and secrete high levels of IFN-γ. The investigators of this study also report that circulating memory T cells were “barely recruited” when MLCs were present in the mucosa. This suggests that CD4+ TRM may be capable of clearing or controlling infection even before recruiting signals are generated in a magnitude large enough to attract circulating T cells to the site of infection (29). It still remains necessary to explore whether a critical mass of CD4+ TRM-containing MLCs are needed within the genital tract to provide protection. It is likely that this profound role of CD4+ TRM in mediating immunity during HSV-2 infection is due to the location of the infection (genital tract) rather than the viral factors alone. This is supported by the fact that MLCs have also been found in the genital tract of both human and mice secondary to infections caused by a bacterial pathogen [refer to Chlamydia trachomatis (Ct) section of this review]. Despite the difficulties in generating CD4+ TRM following prime and pull vaccination, an ideal vaccine against HSV-2 should generate CD8+ and CD4+ TRM (25).
Influenza
Influenza viruses are a major cause of respiratory infections. Although influenza vaccines have been in use for many years, antigenic drift of surface hemagglutinin and neuraminidase proteins require annual immunizations. Antigenic shift can result in highly virulent strains of influenza that cause devastating pandemics (30). The ideal influenza vaccine would provide heterotypic immunity that prevents the escape of newly mutated viruses. Current influenza vaccines rely on generating high neutralizing antibody titers to protect against infection. Although this strategy has demonstrated efficacy in mediating protection, the inability of antibodies to neutralize new variants of the virus has sparked research into alternate strategies (31). Growing evidence suggests that efforts should be focused on developing vaccines that generate TRM-mediated immunity (32). Analysis of human samples has revealed that influenza-specific TRM can be found in substantial numbers in lung tissue, highlighting their role in natural infection (33, 34). Despite expressing low levels of granzyme B and CD107a, these CD8+ TRM had a diverse T cell receptor (TCR) repertoire, high proliferative capacities, and were polyfunctional (34). Influenza infection history suggests a greater level of protection against re-infections likely due to the accumulation of CD8+ TRM in the lungs (35). Furthermore, the natural immune response to influenza A virus infection in a rhesus monkey model demonstrated that a large portion of influenza-specific CD8+ T cells generated in the lungs were phenotypically confirmed as CD69+CD103+ TRM (36). Unlike lung parenchymal TRM, airway CD8+ TRM are poorly cytolytic and participate in early viral replication control by producing a rapid and robust IFN-γ response (37, 38). Bystander CD8+ TRM may also take part in the early immune response to infection through antigen non-specific, NKG2D-mediated immunity (39). The generation of functional TRM that protect against heterosubtypic influenza infection appear to be dependent on signals from CD4+ T cells (40). A role for CD4+ TRM has also been reported (41). Much like their CD8+ counterparts, CD4+ TRM also produce a significant IFN-γ response during early infection (42, 43). Aside from the CD8+ and CD4+ subsets of TRM, a subset of NK1.1+ double negative T memory cells which reside in the lungs also play a role in influenza infection (44). Taken together, these studies and others (45–47) demonstrate that TRM are required for optimal protection. However, unlike TRM in other locations, such as the skin, lung TRM are not maintained for extended periods of time. This gradual loss of lung TRM appears to be the reason for the loss in heterotypic immunity against influenza infection (45, 46, 48). Lung TRM exhibit a transcriptional profile that renders them susceptible to apoptosis (48). Despite conflicting evidence (49), it appears that maintenance of the lung CD8+ TRM populations relies on the continual seeding from circulating CD8+ T cells. However, with time, circulating CD8+ T cells adopt a transcriptional profile that reduces their capacity to differentiate into TRM. Expanding the CD8+ TEM compartment through booster vaccination may circumvent the problem of these time-sensitive transcriptional changes (48). There is also conflicting evidence regarding the requirement of local antigen for the generation of TRM within the lung (48, 50). Continuing to find ways to generate and maintain lung TRM is of great importance for vaccines against pulmonary infections. Intranasal administration of vaccines seems to encourage the development of a strong mucosal immune response (51, 52). Intranasal administration of Live Attenuated Influenza Vaccine (FluMist) in a mouse model induced both CD4+ and CD8+ TRM that provided a degree of cross-strain protection independent of TCM and antibodies (53). The intranasal administration of a PamCys2 or Adjuplex has demonstrated capacity for producing protective influenza-specific lung CD8+ TRM in similar numbers and IFN-γ secreting potential when compared to the natural response to influenza infection (54, 55). Furthermore, a vaccine containing virus-like particles with tandem repeat M2e epitopes generated heterotypic immunity through the induction of antibodies, and protection correlated with IFN-γ-secreting CD8+ TRM (56). A Modified Vaccinia Ankara-vectored virus expressing conserved influenza nucleoprotein and matrix protein 1 elicited an IFN-γ secreting CD4+ T cell and CD8+ TRM response (57). Co-administration of 4-1BBL (CD137 signal) along with an influenza nucleoprotein expressing replication defective adenovirus vector via the intranasal route stimulated and boosted a lung CD8+ TRM response through the recruitment of circulating T cells (58). Intranasal administration of 4-1BBL may serve as a promising “pull” strategy in systemically primed individuals. Another potential “pull” strategy is the intranasal administration of Fc-fused IL-7. This protocol was used as a pre-treatment before influenza A infection, and demonstrated protective capacities in mice against lethal challenge. It appears that Fc-fused IL-7 recruits polyclonal circulating T cells into the lungs, which subsequently reside in the lung tissue as “TRM-like cells” (59). Intranasal administration of Fc-fused IL-7 after systemic priming may be able to recruit influenza-specific T cells into the lungs, and may be a strategy for inducing lung TRM. An antibody targeted vaccination strategy in which antigens are coupled to monoclonal antibodies against CD103+ or DNGR-1+ dendritic cells has also been shown to elicit a protective CD8+ TRM response (47, 60).
Human Immunodeficiency Virus (HIV)
Human immunodeficiency virus is a retrovirus that is transmitted via contact with infected blood and other fluids such as semen and vaginal secretions. The virus specifically targets the surface proteins CD4, CXCR4, and CCR5, with the natural progression of disease resulting in the depletion of CD4+ T cells. As a consequence, infected individuals are left in an immunocompromised state referred to as aquired immunodeficieny syndrome (AIDS), which is characterized by fatal opportunistic infections and malignancies. Although the development of therapeutics such as anti-retroviral therapy has reduced the incidence of AIDS, HIV/AIDS continues to contribute significantly to global morbidity and mortality. Evidence shows that CD8+ T cells are vital in controlling early infection (61). Studies of human tissue samples have revealed that TRM are generated in response to HIV infection in multiple locations including the gastrointestinal tract and the female reproductive tract (62–65). Furthermore, individuals who appeared to naturally control infection had TRM that were capable of producing the highest polyfunctional immune responses when compared to individuals who did not. However, the TRM population within the HIV-specific CD8+ T cell compartment in individuals who controlled infection was under-represented when compared to individuals who were viremic (62). Although not confirmed, this may be due to the higher ability of polyfunctional TRM in these individuals to recruit circulating T cells, thereby only altering the TRM proportion. Similar to other infections in various sites, CD8+ TRM in the context of HIV can be sub-divided into two subsets based on the expression of CD103 (62, 63). Analysis of the ectocervial epithelium and menstrual blood revealed that HIV-infected women were more likely to have CD103− TRM when compared to healthy individuals (63, 64). This reduced expression of CD103 may be explained by the HIV-induced depletion of CD4+ T cells which appear to be vital in providing help to CD8+ T cells for up-regulating CD103 (64). The CD103− populations of the ectocervix resided closer to the basement membrane of the epithelium when compared to their CD103+ counterparts. Interestingly, the CD103+ population from infected individuals appears to express higher levels of PD-1 (63). In a separate study, adipose PD-1+ CD4+ TRM, appeared to remain relatively inactive during HIV infection and may serve as a reservoir for HIV (65). As such chronically activated TRM and TRM exposed to immunomodulated environments (such as the adipose tissue) may be unable to elicit a full effector response, favoring the progression of HIV infection. It also appears that HIV has the ability to disrupt CCR5-mediated CD8+ T cell migration into the cervical mucosa, thereby impairing the development of TRM populations (66). Regardless, human studies suggest that TRM, especially CD8+ TRM, play an important role in combating HIV infection and thus may be valuable targets for vaccine development. Since the most common mode of transmission of HIV is through sexual intercourse, it may be desirable to explore strategies that induce anti-HIV CD8+ TRM in the female and male reproductive tract and rectosigmoid epithelium. In a Simian Immunodeficiency Virus model of rhesus macaques, intravenous administration of SIVmac239Δnef generated a population of CD8+ TRM in the vaginal tissue and the gut that participated in protection (67). In a murine model, a mucosal vaccination strategy in which intranasal administration of an influenza-vector expressing the HIV-1 Gag protein p24 followed by an intravaginal booster induced CD8+ TRM in the vagina. Antigen stimulation of these CD8+ TRM resulted in the recruitment of B cells, natural killer cells, and CD4+ T cells (68). While the recruitment of innate and adaptive immune cells may be beneficial in early viral clearance, the recruitment of CD4+ T cells may be detrimental in the context of HIV as they are the target for HIV. Hence, incidental recruitment of CD4+ T cells to sites of HIV entry (female reproductive tract and rectum) by prime and pull vaccination strategies may unintentionally increase susceptibility to infection. A micro-needle array delivery system that utilizes a recombinant adenovirus vector containing the HIV-1 protein Gag, has also produced promising results in generating TRM. These HIV-specific TRM were found in the female reproductive tract and respiratory tract of immunized mice and responded to local antigenic stimulation through expansion and production of IFN-γ and granzyme B (69). Using this micro-needle array delivery system as a priming strategy followed by intravaginal delivery of a booster concoction serving as a pull strategy may be an interesting protocol worth exploring.
Vaccinia
Vaccinia is a poxvirus that usually causes a very mild or asymptomatic infection in immunocompetent individuals. Immunity to vaccinia virus also provides sufficient protection against smallpox, which allowed for its eradication following administration of the live vaccinia virus (70). Despite elimination, smallpox remains a priority on the global agenda given the potential for the virus to be used as a biological weapon (71). For this reason and its ability to serve as a vector, vaccinia virus continues to be used in research. Murine models demonstrate that TRM are generated in response to vaccinia and play a significant role in mediating protection against infection (72–76). Dermal-resident γδ T cells have also been implicated in the immune response against cutaneous vaccinia infection (77). Following skin infection CD8+ T cells are recruited independently of CD4 T+ cells and IFN-γ (72), many of which subsequently assume the TRM phenotype (72, 73, 75, 78, 79) and are capable of initiating potent inflammatory responses upon re-stimulation (79). Of particular interest is the capacity of local vaccinia skin inoculation to globally seed skin tissue even at remote sites with long lasting TRM (72) as well as generating TRM responses in non-related non-lymphoid organs such as the lungs and liver (76). Multiple exposures to cognate viral antigens have also shown to selectively expand TRM (72, 73, 78, 79). In a lung infection model of vaccinia, higher numbers of lung TRM correlated with better protection against subsequent infection as indicated by a rapid reduction in viral loads. TRM seem to expand more rapidly and localize to the infection site as indicated by a 5-ethynyl-2′-deoxyuridine proliferation assay when compared to their circulating counter parts. Depletion of lung CD8+ T cells by intranasal administration of αCD8 antibody, resulted in previously protected mice becoming susceptible to infection, indicating that CD8+ TRM play a vital role in mediating immunity (74). In another study, parabiosis experiments demonstrated that TRM were exceedingly better at clearing vaccinia virus skin infection than TCM within a shorter timeframe. In fact, it appears that skin TRM can clear vaccinia skin infection even in the absence of neutralizing antibodies and TCM (72). However, vaccinia-specific CD8+ skin TRM appear to have an impaired ability to recruit circulating effector cells during polymicrobial sepsis infection (80). Whether there are other physiologically challenging conditions that impair skin TRM functionality remains largely unexplored. Surprisingly, vaccinia lung infection revealed that not all TRM are equally capable of conferring protection. TRM that resided in the lung interstitium were better positioned to rapidly kill infected lung cells in a contact-dependent manner when compared to TRM situated in association with the tissue vasculature. Furthermore, TRM found within the interstitium, unlike vascular-associated TRM, were able to up-regulate CD69 expression, potentially indicating an enhanced ability to respond during early infection (74). Investigations of vaccinia infection has also reinforced that epithelial immunization routes, such as skin scarification and intranasal exposure, demonstrate significant efficacy for generating protective TRM responses (72–76, 78). In fact, vaccination via skin scarification is capable of protecting against clinical disease (pock lesions of the skin) whereas not all mice vaccinated via systemic routes such as intramuscular and intraperitoneal were protected from pock lesions. More astonishingly, mice immunized via skin scarification demonstrated greater resistance to disease when challenged via a heterologous route (intranasal), compared to mice immunized subcutaneously or intraperitoneally, in spite of generating reduced antibody titers (75). These observations may be attributed to TRM-mediated immunity given the evidence that TRM can be generated in distant tissues after skin scarification (76). Overall, studies that use vaccinia infection models have shed light on the ability of skin scarification to elicit a robust and somewhat unique immune response.
Respiratory Syncytial Virus (RSV)
Respiratory syncytial virus is a common cause of lower respiratory tract infections in children and the elderly. Common reinfection with RSV suggests absence of protective immunity (81). A number of studies have shown the importance of TRM in providing protection against RSV (82–86). An experimental human infection study showed that adults with higher frequencies of RSV-specific CD8+ T cells, many of which displayed a TRM phenotype, developed less severe lower respiratory tract symptoms and reduced viral loads. This increase in protection was not correlated with higher numbers of circulating CD8+ T cells, suggesting the localization of TRM was vital for mediating immediate protection (82). TRM induction in lung tissue and airway fluid was also demonstrated following intranasal RSV infection in mice. Adoptive transfer of airway lymphocytes from RSV-infected mice into naïve recipients reduced disease burden upon infection challenge, compared to adoptive transfer of airway lymphocytes from sham-infected mice. It was concluded that both airway CD8+ and CD4+ T cells play a role in protecting against RSV infection and reducing disease severity, respectively (83). However, given that only bulk CD4+ or CD8+ T cells were transferred, it remains to be investigated if the protective capacity is mediated by airway TEM or TRM cells. In support of the latter, an African green monkey model of RSV infection illustrated that antibody and CD4+ T cell responses are unlikely to protect against reinfection. On the contrary, it appears that lung CD8+ T cells, of which up to half displayed a TRM phenotype, were more capable of protecting against secondary infection (84). From the available evidence (82–86), it appears that an ideal RSV vaccine should elicit a CD8+ TRM response in the lung. Of note, some experimental RSV vaccines have already shown promising results with regards to TRM generation: intranasal administration of an RSV antigen-expressing murine cytomegalovirus generated an IFNγ- and MIP-1β-secreting population of TRM (85); co-administration of the TLR9 agonist CpG and an inhibitor of notch signaling (L-685,458) with formalin-inactivated RSV elicited a strong protective TRM response (86); intranasal administration of virus-like particles containing RSV M and M2 proteins as antigen delivery systems has also shown propensity to induce the production of TRM (87); and a dendritic cell-Listeria monocytogenes immunization strategy, when administered locally, was able to avoid circulating T cell-induced immunopathology and protect against RSV infection challenge through the generation of TRM (88).
Cytomegalovirus (CMV)
Cytomegalovirus establishes life-long latency in many organs including mucosal tissues. It has long been known that CMV infection induces a sustained clonal expansion of specific CD8+ T cells, a phenomenon referred to as memory inflation (89). However, only recently has it been explicated that CMV infection promotes the formation of TRM in various mucosal tissues, especially the salivary glands (90–92). Although the CD8+ T cell response is vital for the control of CMV infection, the virus-induced downregulation of MHC I on acinar glandular cells of the salivary glands (long-term target tissue of CMV) resulting in the reliance on CD4+ T cells for control of lytic replication at this site (93). Surprisingly, salivary gland CD8+ TRM were capable of controlling viral replication. It appears that murine CMV is unable to completely inhibit the expression of MHC I on CD8+ TRM of the salivary glands, thereby providing an opportunity for these T cells to mediate localized immunity (90). Although it remains unclear whether these TRM inhibit viral replication through effector cytokines or direct cytotoxicity, it certainly appears that salivary gland TRM may inhibit the shedding of CMV, hence reducing the chances of transmission. These mucosal TRM typically form early after infection. However, mucosal seeding continuously occurs through the recruitment and differentiation of circulating populations. As such, the immunodominance of mucosal TRM against CMV changes with time, favoring the TCR repertoire that remains high in circulation (91). TRM have also been found in brain tissue after murine CMV infection (94–96). In the brain, CMV-specific TRM formation seems to be dependent on regulatory T cell (Treg) activity. Furthermore, Treg cells seem to have a suppressive effect on brain TRM’s capacity to produce granzyme B, potentially a precautionary measure to prevent detrimental neuroinflammation (94). From the studies that have dissected the role of TRM in protecting against CMV infection and inhibiting reactivation there seems to be a clear role for these tissue tropic T cells in limiting CMV replication. A number of studies also demonstrate the capacity of CMV to be used as a viral vector in novel vaccines that generate TRM-mediated immunity (85, 91, 97). Manipulating CMV’s capacity to induce a robust CD8+ T cell response within mucosal tissues may be a promising avenue for the generation of new vaccines.
Lymphocytic Choriomeningitis Virus (LCMV)
Lymphocytic choriomeningitis virus, a rodent-borne disease can cause meningoencephalitis in humans (98). While LCMV infection models have been used to study TRM in multiple tissues (99), the protective role of TRM has only been clearly investigated in the brain, thymus, and female reproductive tract. Depletion of circulating T cells or NK cells demonstrated that TRM have the capacity to protect against infection independently of NK cells, TCM, and TEM populations (100, 101). Upon MHC-I-antigen stimulation, LCMV brain TRM displayed effector functions and mediated virus control through IFN-γ release and perforin-mediated cytotoxicity (100). Thymic TRM, when stimulated with gp33, released both IFN-γ and TNF-α, suggesting that TRM at this location may be polyfunctional. It also appears that T cells that took residence in the thymus were more likely to respond to antigen stimulation when compared to their splenic counterparts, further exemplifying the protective nature of these cells (101). Since infection of the thymus can significantly reduce T cell generation due to increased thymocyte deletion and reduced proliferation, it is vital to have protective mechanisms in place that act rapidly to minimize pathogen-induced damage in the thymus. From the available evidence, thymic TRM seem to be capable of adequately fulfilling this task. LCMV infection also induces the production of TRM in various peripheral tissues, such as the lungs, intestines, and female reproductive tract (102–104). While the role of TRM in the lung and intestines following LMCV infection is not well established, it was found that re-activation of CD8+ TRM in the female reproductive tract was able to produce a general anti-viral immune response that is almost able to confer sterilizing immunity when challenged with an non-cognate virus (105). This TRM induced antiviral state may be of great interest in the aim to generate vaccines that create heterotypic protection.
Varicella Zoster Virus
Varicella zoster virus, the cause of chicken pox, is an alpha-herpes virus that can establish latency within the dorsal root ganglia. Reactivation of the virus results in a painful disease called shingles. Although vaccines are available against both chicken pox and shingles (106), recent evidence suggests that TRM may be key players in controlling latent infection, a phenomenon that could be exploited to improve current vaccines. One study analyzed skin samples from human donors of varying ages who were serologically confirmed VZV positive. 80–90% of T cells from the sampled tissue expressed CD69, suggesting that the majority of T cells in skin were TRM. IL-2 responses from stimulated VZV-specific T cells demonstrated that host age did not influence the numbers of responsive cells. However, it was found that skin from older donors demonstrated a lesser capacity to mount a clinical response and decreased CD4+ T cell infiltration when challenged with VZV antigen. This correlated with higher proportions of Foxp3+ cells. Furthermore, TRM of older skin expressed PD-1 in higher amounts (107). Together, this data suggest that VZV-specific TRM may be suppressed with age. This may be a reason for the high incidence of reactivation of VZV in older individuals. Results from a different study that utilized samples of human trigeminal ganglia suggests that TRM do not seem to play a role in controlling latent infection in the trigeminal ganglia (10). Regardless, further investigation into the role of TRM in controlling latent VZV infection may help to develop therapeutics or vaccines that prevent shingles.
Human Papillomavirus
Human papillomavirus is a sexually transmitted pathogen that generally causes an asymptomatic, self-limiting infection. However, certain subtypes of HPV can cause cancer of the cervix, anus, and oropharynx (108). The routine administration of HPV preventative vaccines has led to a significant reduction in the incidence of infection in many parts of the world. However, immunization of individuals with an established HPV infection has not shown to protect against the progression of HPV-induced lesions into carcinoma. As such, a therapeutic vaccine that is administered by post infection may subvert this problem. Current HPV vaccines rely on the induction of antibodies to neutralize viral particles (109). The potential for generating anti-HPV TRM as a strategy for eliminating previously established HPV infection is yet to be fully explored. One study evaluated the capacity of two adenoviruses (Ad26 and Ad35) that express a fusion of the HPV16 oncoproteins E6 and E7 to elicit a protective response in the cervicovaginal mucosa. Intra-vaginal administration of either vector was able to elicit the generation of CD8+ TRM within the cervicovaginal mucosa. Furthermore, systemic priming with Ad35 followed by an intra-vaginal booster immunization of Ad26 induced polyfunctional, E6/E7-specific, cytokine-secreting CD8+ T cells within the cervicovaginal mucosa (110, 111). Although it remains to be resolved if protection against established HPV infection causally relies on TRM, this and other studies (111) provide impetus to further explore the intra-vaginal route of administration and the use of viral vectors as strategies for the induction of cervicovaginal TRM.
Viral Hepatitis
Viral hepatitis is an inflammatory disease of the liver that is caused by a range of viruses (112). Two studies, both of which utilized human donor liver tissue and paired blood samples, analyzed the role of TRM in the context of viral hepatitis. One study focused on patients with hepatitis B viral infections (HBV), while the other study included patients with HBV or hepatitis C viral infections. A higher proportion of liver T cells from patients who demonstrated partial control of HBV infection had a TRM phenotype, when compared to healthy controls. Given that the overall numbers of T cells in the liver of healthy and HBV-infected individuals were similar, this threefold increase in TRM numbers appear to be due to an increased predisposition of T cells to adopt the TRM phenotype in virally infected liver tissue, rather than expansion of pre-existing TRM (113). The numbers of T cells co-expressing CD69 and CD103 increased by fourfold in chronic hepatitis C patients (114). Furthermore, the reciprocal relationship between viral loads and liver TRM numbers indicates that TRM play a vital role in infection control (113). Ex vivo stimulation of TRM showed heterogeneous antigen specificity, with a number of HBV antigens being able to initiate effector responses. However, viral envelope peptides seemed to generate the greatest capacity to induce production of IFNγ, TNFα, and IL-2. Analysis of TRM from healthy liver tissue revealed a noticeably reduced expression of granzyme B, when compared to non-resident counter parts. This suggests that hepatic TRM have less cytolytic capacity than circulating T cells (113, 114). However, liver TRM of patients with chronic hepatitis B expressed markedly higher amounts of granzyme B when compared to healthy controls (114). Liver TRM also showed increased expression of the inhibitory molecule PD-1 compared to non-resident T memory cells (113, 114). The downregulation of granzyme B and upregulation of PD-1 in healthy liver tissue may be a precautionary measure intended to prevent immunopathology, given the liver’s role in filtering high amounts of antigen draining from the mesenteric circulation. This is of great importance in viral hepatitis infections as immunopathology is largely involved in the progression of viral hepatitis that leads to cirrhosis and hepatocellular cancer. The increased production of granzyme by TRM in CHB patients may be part of the pathogenesis of fulminant hepatitis. Further exploring the role of TRM in protection against viral hepatitis (including hepatitis A, D, and E) and the immunopathology implicated in the progression of the disease may aid in the development of immunomodulatory therapeutics to prevent viral cirrhosis and hepatocellular cancer.
Epstein–Barr Virus (EBV)
Epstein–Barr virus is one of the most prominent causes of infectious mononucleosis. After exposure to infected saliva, the virus infects and replicates in B cells and epithelial cells of the new host. Although the clinical disease of glandular fever is usually self-limiting, EBV remains latent in circulating B cells and episodes of reactivation are known to occur. It appears that reactivation of EBV occurs in the lymphoid tissue of the oropharynx, where the virus switches from a latent form into a lytic cycle. Control of infection is mediated by a T cell response against infected B cells (115). EBV-specific CD8+ memory T cells localize to the epithelium of the oropharynx (116), where they up-regulate CD69 and CD103 in an IL-15- and TGF-β-dependent fashion (117). CD103+ EBV-specific T memory cells found in tonsillar tissue are more sensitive to antigen stimulation and produce a greater effector response when compared to circulating EBV-specific T cells (116). Furthermore, a substantial CD103+ T cell population only seems to appear as viral replication and disease tapers (118). Taken together, it appears as though TRM play a crucial role in rapidly controlling viral replication of EBV within the oropharyngeal tissue upon reactivation to prevent full clinical relapse.
Vesicular Stomatitis Infection (VSV)
Vesicular stomatitis infection is a zoonotic disease that can cause a mild febrile illness in humans (119). Intranasal infection of mice with VSV has shown to produce CD103+ CD8 TRM population in the brain (120, 121), as the virus travels along the olfactory bulb to the brain where it causes infection. These brain TRM were found to be functional in situ, responding to cognate antigen (120, 121). Staining for effector molecules revealed that many of these TRM cells were positive for granzyme B, suggesting cytolytic abilities. Once removed from the brain parenchyma, these cells appear dysfunctional, suggesting they are highly adapted to the brain microenvironment. Maintenance of this population of TRM appears to be independent of circulating T cells, and BrdU incorporation indicates a slow homeostatic rate of proliferation to sustain the population (120). Interestingly, brain TRM appear to form clusters within specific sites of the brain parenchyma that contain CD4+ T cells, perhaps indicating a role for CD4+ T cells in the generation and/or maintenance of brain CD8+ TRM. These clusters may have formed around sites of previous VSV replication sites, where persisting antigen may be drawing the TRM to these locations. Although, viral RNA could not be detected at these sites (120), this does not exclude the possibility that undetectable levels of antigen may be present at these sites. TRM may also form clusters around local dendritic cells that are still presenting antigen from a previous infection. This hypothesis is supported by the observation that antigen presentation by bone marrow-derived-APCs was able to support CD103 expression by TRM (120).
Other Viruses
Polyomaviruses are opportunistic pathogens that usually remain latent following infection. However, in immunocompromised individuals, infection can cause multifocal leukoencephalopathy (122). TRM are generated in the context of polyomarvirus infection (123–126), and polyomavirus-specific brain CD8+ TRM in mice maintain a high TCR affinity for pathogen epitopes. In fact, TRM TCR affinity appears to be higher than the TCR affinity of T cells from the spleen. This observation supports a role of TRM in mediating rapid control of viral replication during reactivation, as high TCR affinity allows for the early detection of low amounts of virus (123). In contradiction to this finding, evidence from another study suggests that lower TCR stimulation increases the generation of brain TRM (125). One way of interpreting these seemingly contradicting observations is that brain TRM initially differentiate from circulating effector T cells with low TCR stimulation capacity, but after taking residence in the brain, undergo functional avidity maturation (127) increasing their ability to respond to antigen. A renal transplant clinical study suggests that renal BK Polyomavirus-specific TRM were rendered incapable of protecting against infection leading to interstitial nephritis, likening these TRM to dysfunctional tumor-infiltrating lymphocytes (126).
Ebola virus causes a form of hemorrhagic fever characterized by intravascular coagulation and maculopapular rash. Although the natural reservoirs for the virus are thought to be fruit bats, human-to-human transmission can occur when contaminated body fluids breach mucosal barriers or skin. Absence of specific treatment and epidemic potential of the virus highlights the need for a vaccine (128). Aerosol administration of a human parainfluenza virus type 3-vectored vaccine expressing an Ebola envelope glycoprotein was capable of not only eliciting neutralizing antibodies but also a CD103+ T cell response in the lungs of macaques. A large proportion of these TRM were polyfunctional, demonstrating positivity for two or more activation markers. Furthermore, a single dose of this vaccine conferred 100% protection against infection challenge (129). Since a large proportion of transmission in the recent Ebola epidemic was through skin contact, vaccination via scarification is worth exploring.
Norovirus is a highly infectious virus, and is a common cause of gastroenteritis. Although infection is generally self-limiting, chronic forms have been reported in immunocompromised patients. A clinical study has implicated CD8+ T cells resembling TRM in the immune response against norovirus (130). However, a genetically manipulated strain of murine norovirus causing chronic infection revealed that despite a robust and functional TRM response being generated, clearance of the virus was not achieved, likely due to inadequate antigen sensing (131).
The Role of TRM in Bacterial Infections
Although there is significantly less literature about TRM in the context of bacterial infections, the evidence largely implies that TRM have a noteworthy role in protecting against pathogenic bacteria. The following section groups bacterial pathogens together depending on their location of primary infection.
Bacterial Infections of the Lungs and Airways
Pertussis, also known as whooping cough, is caused by Bordetella pertussis, a Gram-negative coccobacillus. Despite high vaccination coverage, whooping cough remains a serious public health concern. T cell responses are critical for immunity against B. pertussis (132). While the existing whole-cell pertussis (wP) vaccine is generally associated with a strong Th1 response, immunization with the widely used acellular pertussis (aP) vaccine induces a Th2-dominated humoral response (133). Immunity to the aP vaccine wanes over time compared to wP vaccines (134). This diminished immunity allows for the transmission of B. pertussis to susceptible individuals. A recent study reported that following B. pertussis infection, IL-17- and IFN-γ-secreting CD4+ TRM congregate in the lungs of infected mice where they persisted for 120 days, and expanded up to sixfold upon reinfection. Egress inhibitor FTY720 did not affect the control of bacterial burden during secondary infection, suggesting that TRM were capable of providing immunity irrespective of peripheral T cell recruitment. Bacterial clearance in reinfected mice also correlated with CD4+ TRM expansion, with a large portion of cells displaying a Th17 phenotype (135). Adoptive transfer of lung CD4+ TRM from infected mice into naïve hosts conferred protection against B. pertussis challenge (135), suggesting that Th17-like CD4+ TRM seemed to play a crucial role in long-term immunity. Interestingly, γδ T cells that express CD69 and CD103, classically known to provide innate-like protection during primary infection, also provided a significant early-release IL-17 response during secondary infection in convalescent mice. However, γδ TRM, especially Vγ4+ γδ T cells persisted in the lungs of convalescent mice and produced a greater IL-17 response on re-exposure to B. pertussis in an antigen-specific manner (136). Therefore, a long-lasting B. pertussis vaccine should not only promote the generation of B. pertussis-specific CD4+ TRM but also γδ TRM.
Pneumonia is one of the largest infectious causes of mortality in children worldwide (137). The most common cause of community-acquired pneumonia is Streptococcus pneumoniae, a Gram-positive polysaccharide-encapsulated bacterium (138). Modern pneumococcal vaccines are polysaccharide based and are thus poorly immunogenic, providing serotype-specific immunity that wanes over time. Although CD4+ Th17 responses are considered vital in providing protection against pneumococcal infections, the role of TRM is yet to be fully characterized. Experimental S. pneumoniae infection was found to promote the production of heterotypic CD4+ TRM of both Th17 and Th1 phenotypes in niches located within pneumonia-affected lobes of the lung. It was also observed that immunity was restricted to pathogen-experienced tissue, suggesting that TRM reside in primary infection sites, rather than providing immunosurveillance throughout the entire respiratory mucosa. Despite spatial restriction, TRM provided superior protection to the local tissue when compared to systemic immune responses elicited by antigen-specific CD4+ TCM. Neither adoptive transfer of splenic CD4+ T cells from infected mice into naïve recipients, nor inhibiting lung translocation of circulating CD4+ T cells with FTY720 in pathogen-experienced mice, had a significant effect on protection against pneumococcal infection challenge. Therefore, protective immunity against bacterial pneumonia is likely due to the aggregation of CD4+ TRM in susceptible tissues (139). Interestingly, combining whole virion influenza and whole cell pneumococcal vaccine also promoted the generation of lung CD4+ TRM. It is likely that these TRM, in combination with the accompanying high antibody titers elicited by the combined vaccine, played a role in providing protection against pneumococcal-influenza co-infection (140). Overall CD4+ TRM may play a role in the generation of naturally acquired immunity against pneumococcal infections, and should be considered in the development of heterotypic pneumococcal vaccines.
Mycobacterium tuberculosis (Mtb), an acid-fast staining intracellular bacterium, is the causative agent of tuberculosis (TB). The deadly infection can present as pulmonary, as well as extra pulmonary disease (141). Currently, Bacillus Calmette–Guérin (BCG) is the only licensed vaccine against TB, and prevents dissemination in children. However, BCG does not provide strong enough immunity against pulmonary TB in adults, therefore, allowing transmission (142). Immune control of Mtb infection largely relies on the production of IFNγ by CD4+ T cells, which enhances macrophage killing of persisting intracellular Mtb and leads to the formation of granulomas around sites of bacterial replication (141). A clinical study revealed that individuals previously exposed to tuberculosis were likely to have a population of lung-resident Th1 effector memory cells that released IFN-γ in response to Mtb antigen re-exposure (143). However, the delay in activation and recruitment of TB-specific T cells to the lungs during primary infection allowed for Mtb to proliferate, resulting in a high bacterial burden. The importance of airway-residing memory T cells (then called airway luminal cells) in mediating protection against TB has been described well before the dawn of TRM (144–146). However, these cells most likely represent the same cell type. Lung TRM induced by mucosal vaccination have shown to be effective in limiting the early control of bacterial replication (147). Despite the defined role of CD4+ T cells in controlling TB, recent evidence from vaccine studies suggest that CD8+ lung TRM also play an important role in protection against Mtb (148, 149). Only mucosal administration of BCG led to the generation of airway TRM that produce higher levels of pro-inflammatory cytokines, including IFN-γ than CD8+ TEM. Furthermore, adoptive transfer of sorted airway CD8+ TRM from BCG-vaccinated mice demonstrated enhanced protection against Mtb challenge in recipient mice. Transfer of CD8+ TRM decreased the numbers of alveolar macrophages, while increasing the number of CD4+ T cells and B cells in the infected lung tissues (148). It was hypothesized that CD8+ TRM kill Mtb-infected alveolar macrophages, thereby depleting intracellular reservoirs of the bacteria and limiting the entry into the lung parenchyma (148). Likewise, the viral-vectored vaccines SeV85AB and AdAg85A, administered via the intranasal route have also shown to elicit an immune response that favors the production of CD8+ rather than CD4+ TRM (149, 150). In a rhesus monkey model, a cytomegalovirus vector delivering a range of Mtb antigens (RhCMV/TB) provided significant protection against tuberculosis, presumably through it its ability to generate and maintain pathogen-specific CD4+ and CD8+ circulating and more importantly resident memory T cells that selectively express VLA-1 (151). Finally, aerosol vaccination with an attenuated Mtb strain lacking sigH not only led to an enormous influx of T cells expressing CD69 into the lung airways (likely to include TRM), but also to a significant long-term protection against virulent Mtb challenge (152). Collectively, these studies indicate that rationally designed TB vaccines should generate immune responses that prevent the establishment of infection and/or provide sterilizing immunity by inducing both lung CD4+ TRM and CD8+ TRM in the lungs.
Bacterial Infections of the Urogenital Tract
A number of bacteria cause disease of the reproductive tract and urinary system. One such example is Ct, an obligate intracellular bacterium that causes infections of the genitals and eyes. It is the leading cause of infectious blindness worldwide, and can cause infertility when sexually transmitted (153). According to clinical evidence, it appears that spontaneous clearance of clinical infection correlates with at least partial protection against C. trachomatis through the production of INF-γ-secreting cells such as CD4+ Th1 cells. However, IFN-γ responses alone do not seem to provide complete protection. It has also been documented that B cell-antibody responses are involved in immunity, especially against secondary infection (154–156). Importantly, intraepithelial CD8+ lymphocytes and MLCs composed of B cells and CD4+ T cells border the vaginal and uterine tract, respectively in pathogen-experienced tissue. These immunocyte structures hinder C. trachomatis from replicating and establishing a clinical infection (157). Optimal protection against Chlamydia requires both the recruitment of TCM and the presence of TRM within the urogenital tract (158, 159). It is likely that protective immunity occurs in response to chronic or repeated infection, which leads to the seeding of TRM throughout the epithelial surface (160). A vaccine composed of Chlamydia major outer membrane protein and ISOCMATRIX adjuvant was able to provide enough protection to prevent the sexual transmission of C. trachomatis, however, was not capable of providing complete immunity. This may be attributed to the inability of the vaccine to generate a large enough TRM population, underscoring the essential role of TRM in Chlamydia infection (159). In a separate study, mice were either inoculated with infectious C. trachomatis or UV-inactivated C. trachomatis (UV-Ct). Mice infected with the infectious form demonstrated capacity to control future infections better than naïve controls, which may be attributable to the production of both Chlamydia-specific TCM and TRM populations. However, the group of mice inoculated with UV-Ct suffered higher bacterial burdens when compared to naïve controls. This data in conjunction with the generation of de novo Ct-specific Treg suggest that a tolerogenic immune response occurred in these mice. On the contrary, intra-uterine administration of UV-Ct conjugated with charge-switching synthetic adjuvant peptides (UV-Ct-cSAP) conferred a superior protection to Ct in both conventional and humanized mice. The rapid clearance of Ct in UV-Ct-cSAP-vaccinated mice has been attributed to the immediate release of IFN-γ by mucosal TRM (158). Taken together, the ideal vaccine against Chlamydia should promote the generation of local MLC, TCM, and TRM in the epithelium, even though partial protection appears to be sufficient to prevent disease transmission.
Bacterial Infections of the Gastrointestinal Tract
Gastrointestinal infections are generally acquired through ingestion of contaminated food or water. These bacteria may remain in the gut, or may disseminate to other parts of the body causing systemic disease (161). Targeted induction of TRM along the gastrointestinal epithelium could enhance protection against these pathogens. Listeria monocytogenes, a food-borne Gram-positive coccobacillus, is of particular concern in immunocompromised and pregnant individuals, and can cause meningitis and stillbirth (162). Its capacity to replicate within host cells facilitates immune evasion. Thus, protection against L. monocytogenes is largely dependent on cell-mediated immunity (163). First observed in 1981 as a distinct population of long-lived T memory cells “positioned” in tissue following listeriosis (164), a more recent study highlighted the role of intestinal CD8+ TRM in mediating immunity against L. monocytogenes following oral infection. In fact, blockage of integrin α4β7 prevented the formation of these intestinal TGF-β-dependent TRM resulting in diminished protection upon re-challenge (165). Revealed by multi-photon dynamic microscopy, a population of Vγ4+ γδ TRM was found within the mesenteric lymph nodes in response to L. monocytogenes infection, which remained largely stationary under homeostatic conditions. However, upon re-challenge, activation of these cells resulted in organized clusters around bacterial replication foci where they released IL-17 and subsequently, the recruitment of neutrophils to facilitate bacterial elimination. Similarly to γδ TRM function seen in B. pertussis infection (136), neutralization of IL-17 hindered bacterial clearance, highlighting the importance of early IL-17 release by γδ TRM (166).
Yersinia pseudotuberculosis (Yptb), a food-borne pathogen causing of Far East scarlet-like fever, is a Gram-negative bacterium responsible for gastroenteritis, mesenteric lymphadenitis, and can clinically mimic acute appendicitis (167). A Yptb oral infection mouse model showed a robust CD8+ T cell response in the intestines including a population of Yptb-specific CD103+ CD8+ TRM uniformly distributed throughout the intestine, while CD103− TRM formed around sites of primary infection where they carried out effector functions (168). Although found in antigen-rich areas, their development is independent of local antigen stimulation (168). The development of TRM populations in the intestine seems to rely on inflammatory signals from the site of infection rather than antigens (169). Production of IFN-β and IL-12 from intestinal macrophages effectively suppresses TGF-β-mediated CD103 expression thereby leading to the development of CD69+ CD103- TRM population in mice during Yptb infection. This data suggest a central role for inflammatory monocytes in the differentiation and maintenance of different CD8+ TRM populations to achieve optimal protection against intestinal infections. Additionally, this study raises the question as to whether CD103 is necessary for residence within the intestinal tissue, or whether it negatively regulates TRM capacity to migrate within their residential tissues.
Salmonella spp. is a group of Gram-negative bacilli that is a common cause of gastrointestinal infections responsible for “food poisoning.” Transmitted orally, this heterogeneous group of bacteria contains typhoid or enteric-fever causing serovars that can be potentially fatal to humans (170). Current vaccines against Salmonella are poorly immunogenic and risk disease in immunocompromised individuals (171). An effective vaccine that prevents gastrointestinal infection is much needed to prevent outbreaks of salmonellosis. Subcutaneous co-administration of Salmonella SseB and flagellin has shown to provide protection against systemic disease in mice. However, parabiosis studies suggest that this protection can be transferred via the circulation, diminishing the role of TRM in the observed immunity (172). Nevertheless, it may be beneficial to assess the capacity of this vaccine and others to protect against gastrointestinal infection by oral administration. However, the barriers of oral tolerance and destruction of vaccine components by digestive enzymes and chemicals must be overcome in order to develop oral vaccines.
In summary, TRM responses in bacterial infections appear to be more diverse compared to viral infections, an observation that may be attributed to the varying locations of bacterial replication (intracellular versus extracellular), the more complex lifestyles and the presence or more sophisticated immune evasion mechanisms. Future studies should assess the role of TRM in the natural immune response to other bacterial infections.
The Role of TRM in Parasitic (Protozoa and Helminths) Infections
Protozoa are unicellular organisms that are of great importance to human health. Most prevalent in tropical regions of the world, protozoan infections are difficult to treat due to their complex life cycles and their ability to evade host immune responses through antigenic variation, residence within various intracellular compartments, and their capacity to assume protective forms such as cysts (173, 174). At this juncture, our collective understanding of TRM responses to protozoan infections remains relatively deficient. However, studies have shown that TRM play a significant role in protecting against a few protozoan species.
Malaria, the most prevalent protozoan infection of humans, is caused by five species of Plasmodium. Transmitted by the bite of an infected female Anopheles mosquito, Plasmodium parasites enter the circulation and take residence inside erythrocytes during part of their complex life cycle. Natural immunity to Plasmodium infection involves a mixture of humoral, CD4+, and CD8+ T cell responses (175). However, liver TRM have emerged as a promising target for protecting against malaria (176). Unlike TRM of the epithelium, such as the lungs, intestines, and skin, liver-TRM appear to reside in sinusoids (the blood vessels of the liver), rather than the parenchymal tissue (177–179). The heavily fenestrated architecture of these blood vessels and the distinct slow flow rate of blood allows for TRM to traverse through the organ without being dispatched into circulation. Furthermore, liver sinusoids provide a prime niche for close interaction of TRM and antigen presenting cells, such as Kupffer cells and dendritic cells. This allows for the rapid detection of antigen. Each hepatocyte is also in close association with a sinusoid, thereby providing easy access to liver TRM for assessment of surface antigen presentation (177). Intravital imaging revealed that these TRM traversed around 10 µm per minute and, as reported in HSV-1 infection of skin, TRM assume an amoeboid form, extending dendrites to survey the liver for antigens (2). Rather than relying on CD103-αE integrin interactions for maintaining tissue residence, liver TRM appear to utilize the adhesion molecule LFA-1 (3). A rhesus monkey P. knowlesi infection model that assessed sporozoite immunization demonstrated capacity for generating liver TRM. These TRM appear to be protective as their depletion resulted in the loss of immunity (1). Experiments with radiation-attenuated sporozoites also support the notion that inducing high numbers of liver TRM can afford protection against malaria. In this study, a “prime-and-trap” strategy was used in which primed T cells from the spleen were drawn to the liver using a recombinant adeno-associated virus that infected hepatocytes and subsequently caused them to express Plasmodium antigen. The immunity generated by this strategy was attributed to the increased numbers of CD8+ liver TRM (2). The emergence of liver TRM as a potential target for pre-erythrocytic malaria vaccines warrants further research.
Leishmaniasis is a heterogeneous vector-borne disease that is caused by an intracellular protozoa parasite. There are over 20 known leishmania species, all of which are transmitted by bites from infected female Phlebotomine sandflies. Clinical disease presents in three main forms: cutaneous, mucocutanous, and visceral. Subjugation of Leishmania parasites relies on the establishment of IFN-γ-producing CD4+ Th1 cells (180). While it is widely known that clearance of primary infection can lead to protective immunity, the effector response that leads to protection remains unclear. It has, however, become apparent that TRM play a crucial role in providing such protection (181–183). L. major infection models illustrated that following infection, long-lived TRM are rapidly fabricated and seeded universally throughout the skin, as they can be detected in tissue far away from the primary site of infection (181). It was previously believed that these TRM provided immunity by rapidly recruiting circulating effector T cells. However, more recent studies suggest that the recruitment of circulating T cells may not be as important as previously thought, as FTY720 and αCXCR3-treated mice re-challenged with L. major showed minimal difference in early parasite control (182, 183). Furthermore, parabiosis studies demonstrated that Leishmania-specific circulating T cells alone provide little or no protection during early infection. Data exemplifies that CD4+ TRM are rather likely to provide immunity by eliciting a delayed-type hypersensitivity response. Early immunity is attributable to the capacity of TRM to rapidly recruit reactive oxygen species/nitric oxide producing inflammatory monocytes to control parasite burden (182). Liver TRM have also been implicated in the immune response against Leishmania. The recombinant proteins LirCyp1 and LirSOD of L. infantum appear to be good candidates for promoting the expansion of liver memory T cells (184). No current vaccine exists for this potentially fatal disease. Together, these data suggest that Leishmaniasis vaccines should be tailored to generate TRM to provide heterotypic protection against the many species that cause disease.
Toxoplasma gondii, the causative agent of toxoplasmosis, is an intracellular protozoan that is generally acquired through contaminated food. In humans, T. gondii can form persistent cysts in multiple tissues (185). In both acute and chronic infection, cell-mediated immunity and effector cytokines play vital roles in limiting the progression of disease (186). Mice deficient in TNF-α suffer from increased pathology, and IFN-γ production in the brain stimulates microglia and astrocytes to inhibit protozoan proliferation. In a chronic infection model of T. gondii, CD103+ CD8+ TRM established in the brain produced a more robust IFN-γ and TNF-α response when compared to CD103− T cell subsets (187). It appears that brain TRM provide superior protection against T. gondii infection of the central nervous system when compared to CD8+ TEM and TCM. During T. gondii and Y. pseudotuberculosis infection TRM also seem to accumulate in white adipose tissue in what appears to be a depot of protective memory cells (188).
Helminthic infections are highly prevalent around the world. Although most infections are not fatal, they account for a large proportion of disease burden, causing secondary conditions, such as anemia and malnutrition. Immunity against helminthic infection is largely mediated by the Th2 effector arm of the adaptive immune system (189). The role of TRM in protecting against helminthic infections, however, has only been explored recently in two species: Heligmosomoides polygyrus and Nippostrongylus brasiliensis (190, 191). While neither of these species are human pathogens, they provide analogous models to gastrointestinal helminthic infections and Necator americanus infection in humans, respectively (192, 193). Adoptive transfer of peritoneal-cavity CD4+ TRM from convalescent mice into naïve mice prior to H. polygrus infection challenge, has demonstrated that peritoneal-cavity derived CD4+ TRM are capable of hindering the reproductive capacity of female worms without reducing worm burden (190). This phenomenon provides new insight into what appears to be a unique interaction between TRM and pathogen. A different study that used a N. brasiliensis model demonstrated that even a small number of lung-interstitial TRM were capable of providing protective immunity. This was confirmed as cognate mice treated with FTY720 and lymphotoxin beta-receptor fusion protein (which causes lymphopenia) were able to clear secondary infection, suggesting that circulating T cells are not necessary to mount a protective secondary response (191). In spite of the lack of knowledge surrounding the interaction between TRM and helminths, there is a clear role for this subset of T cells in worm infections that needs to be explored further.
The Role of TRM in Fungal Infections
Typically, fungal infections are less frequent compared to viral and bacterial diseases. However, due to the increasing use of immunomodulatory drugs for cancer and organ transplant patients, the increasing incidence of mycosis is of clinical importance (194). TRM responses are least studied in the context of fungal infections. In fact, only one fungus appears to have been used in TRM studies.
Candida albicans is a dimorphic yeast and opportunistic pathogen. Although it forms part of the normal commensal biome of humans, it can cause infections known as candidiasis especially in immunocompromised individuals (195). Skin and tongue-resident CD4+ IL-17-producing TRM can provide effective protection against C. albicans (196, 197). Murine skin and oral infection models demonstrated that during early infection, γδ T cells release IL-17 in response to C. albicans invasion (196, 197). In skin, by day 7 post-infection, the vast majority of IL-17-producing cells are of the Th17 phenotype. Eventually, the T cells at the site of initial infection upregulate CD103 and CD69 suggesting they assume the CD4+ TRM phenotype between 30 and 90 days post infection. During this time, the cells first become less motile, eventually “sessile” and localize to the papillary dermis. However, upon reinfection, these TRM were capable of rapidly clearing infection and appear to be superior at doing so than circulating TEM. It was also reported that C. albicans-specific Th17 cells were found in high numbers in normal human skin (196), and that low doses of C. albicans antigen exposure stimulates the production of regulatory skin TRM that substantially suppress the activity of skin TEM (198). This may be attributed to the widespread presence of C. albicans in human tissue. Resident memory Treg cells may play a protective role in preventing a hyper-inflammatory response to benign C. albicans antigen exposure. Although the development of a vaccine against C. albicans is not of vital importance, these studies provide an initial insight into understanding TRM responses to fungal infections. Other fungal infections that would be of interest include tinea, cryptococcosis, and aspergillosis.
Discussion
The discovery of TRM has enhanced the possibility to develop new and improved vaccines. From the available literature, it appears that the most important factor for generating TRM is to match the route of vaccination to the route of pathogen entry. In general, these are the mucosal and epithelial barriers that provide the first line of defense against pathogens: the respiratory, gastrointestinal, urogenital mucosa, and integumentary epithelium (Figure 3). Thus, the long-standing method of administering vaccines parenterally may be less effective at conferring optimal protection when compared to the novel mucosal and epithelial vaccine strategies highlighted throughout this review. The “prime and pull” method in which a parenteral vaccine is administered (prime) and an inflammatory agent is applied at a later time point to the desired peripheral tissue (pull) has also proven itself to be an effective vaccine strategy for generating TRM. The combination of mucosal vaccination and “pull” strategies may be an avenue worth exploring in future experiments. Another promising vaccine strategy is the use of viral-vectored vaccines. Regardless, it is also imperative to keep in mind the balance that these tissues must constantly strike in inducing a tolerogenic versus effector immune response to antigens, given their dual function in both their physiological roles (respiration, digestion, reproduction, etc.) and in serving as barriers against infection. Additionally, it will be important to consider the local cytokine milieu that influences TRM generation (199). Generating memory T cells in peripheral tissues with cytolytic effector functions and the capacity to recruit inflammatory cells may pose the risk of inducing a hyper-inflammatory state leading to immunopathology. However, the natural persistence of TRM in most peripheral tissues for extended periods of time under homeostatic conditions implies that mechanisms are in place to regulate TRM responses. Developing a greater understanding of these mechanisms is vital in creating safe TRM-based vaccines.
Figure 3.
Visual summary of key TRM effector responses and vaccine strategies at epithelial surfaces. (A) Represents the female reproductive tract. Topical application of both specific chemokines and general inflammatory agents such as nonoxynol-9 can be used to “pull” systemically primed TEM into the mucosal tissue. CD103+ TRM reside closer toward the apical surface of the mucosa. Both CD4+ and CD8+ TRM play a role in controlling viral infections. Memory lymphocyte clusters have been shown to be important in controlling infections at this site. CD8+ TRM re-stimulation appears to be dependent on CD301b+ dendritic cells that reside in the lamina propria. (B) Represent the integumentary epithelium. Topical application of both specific chemokines and general inflammatory agents such as 2,4-dinitroflourobenzene can be used to “pull” systemically primed TEM into the epidermal tissue. Skin scarification as a route of vaccination encourages the development of skin TRM. Upon antigen recognition, skin TRM lose their dendricity and become less motile. γδ TRM can mediate early immune responses. CD8+ αα+ TRM have been found in the dermal–epidermal junction where they may be able to survey local neural tissue for reactivation of latent viral infections. (C) Represents the respiratory epithelium. While different chemokines have shown the ability to “pull” TRM into the respiratory epithelium and airways, the presence of antigen appears to be important at this site. TRM-mediated control of Streptococcus pneumoniae is largely dependent on the CD4+ subset. Control of viral and Mycobacterium tuberculosis infection requires both CD4+ and CD8+ TRM. γδ TRM, in conjunction with CD4+ TRM have been shown to mediate immunity against Bordetella pertussis.
A very appealing aspect of TRM-based vaccines is that it may be possible to generate heterotypic protection, as shown by studies using influenza infection models. This is vital for protecting against a range of rapidly mutating pathogens, such as HIV, as well as infectious diseases such as leishmaniasis that are caused by heterogeneous pathogens. Furthermore, the promptitude with which TRM mediate immune responses is also of great interest when generating protection against infections such as tuberculosis that are capable of establishing latent infections. In order to achieve immediate TRM protection, it appears that there must be a minimal density of TRM within peripheral tissues to ensure pathogens are identified and eliminated in a timely manner. This may pose a challenge in developing TRM-based vaccines as sufficient numbers of TRM need to be generated and maintained evenly throughout infection-susceptible tissues. The spatial capacity in non-lymphoid tissue to accommodate for TRM may be limited, and thus establishing the capacity of different tissues has as well as determining the minimum threshold of TRM needed to provide protection is much needed.
Conclusion
In summary, the findings of this review largely accentuate the importance of TRM in protecting against a range of pathogens. Their localization to sites prone to infection appears to give TRM an enhanced capacity to mount swifter immune responses when compared to circulating memory T cells. Previous vaccine development has been largely centered on the generation of systemic memory response, which at times has shown to be ineffective. The capacity to form tissue-specific immunity through TRM may shape vaccines of the future. Continuing to foster the growing pool of knowledge about TRM will help to guide the field of TRM vaccinology and may lead to the generation of new and more effective vaccines which may help to reduce the incidence of many infectious diseases.
Author Contributions
VM performed the literature search and conducted extraction of data from relevant studies. AK, HS, and SN critically reviewed the literature search. VM and AK wrote the manuscript. All coauthors read and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor is currently co-organizing a Research Topic with one of the reviewers ET, and confirms the absence of any other collaboration.
Acknowledgments
This work was supported by NHMRC grant APP1120808 and NHMRC Career Development Fellowship APP1140709 to AK. SN was supported by a Children’s Hospital Foundation Early Career Fellowship (50197). We thank Saparna Pai for critically reviewing the manuscript.
References
- 1.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol (2015) 16:79. 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]
- 2.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med (2015) 7(269):269rv1. 10.1126/scitranslmed.3010641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8+ memory T cells. Front Immunol (2012) 3:340. 10.3389/fimmu.2012.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenkel Jason M, Masopust D. Tissue-resident memory T cells. Immunity (2014) 41(6):886–97. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S-H, Jang Y-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res (2017) 6(1):15–21. 10.7774/cevr.2017.6.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azegami T, Yuki Y, Kiyono H. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol (2014) 26(9):517–28. 10.1093/intimm/dxu063 [DOI] [PubMed] [Google Scholar]
- 7.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 349:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 8.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med (1987) 316(23):1444–9. 10.1056/NEJM198706043162304 [DOI] [PubMed] [Google Scholar]
- 9.Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis (2000) 181(4):1454–7. 10.1086/315395 [DOI] [PubMed] [Google Scholar]
- 10.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A (2007) 104(9):3496–501. 10.1073/pnas.0610847104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol (2009) 10(5):524–30. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 12.Davies B, Prier JE, Jones CM, Gebhardt T, Carbone FR, Mackay LK. Cutting edge: tissue-resident memory T cells generated by multiple immunizations or localized deposition provide enhanced immunity. J Immunol (2017) 198(6):2233–7. 10.4049/jimmunol.1601367 [DOI] [PubMed] [Google Scholar]
- 13.Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, Van Beek AE, Gomez-Eerland R, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A (2012) 109(48):19739–44. 10.1073/pnas.1208927109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae (2011) 2(1):5. 10.1186/2042-4280-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintern JD, Guillonneau C, Carbone FR, Doherty PC, Turner SJ. Cutting edge: tissue-resident memory CTL down-regulate cytolytic molecule expression following virus clearance. J Immunol (2007) 179(11):7220–4. 10.4049/jimmunol.179.11.7220 [DOI] [PubMed] [Google Scholar]
- 16.Menendez CM, Jinkins JK, Carr DJ. Resident T cells are unable to control herpes simplex virus-1 activity in the brain ependymal region during latency. J Immunol (2016) 197(4):1262–75. 10.4049/jimmunol.1600207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat Immunol (2018) 19:183–91. 10.1038/s41590-017-0027-5 [DOI] [PubMed] [Google Scholar]
- 18.Khan AA, Srivastava R, Chentoufi AA, Kritzer E, Chilukuri S, Garg S, et al. Bolstering the number and function of HSV-1-specific CD8+ effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J Immunol (2017) 199(1):186–203. 10.4049/jimmunol.1700145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A (2012) 109(18):7037–42. 10.1073/pnas.1202288109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava R, Khan AA, Chilukuri S, Syed SA, Tran TT, Furness J, et al. CXCL10/CXCR3- dependent mobilization of herpes simplex virus-specific CD8+ TEM and CD8+ TRM cells within infected tissues allows efficient protection against recurrent herpesvirus infection and disease. J Virol (2017) 91(14):e00278–17. 10.1128/JVI.00278-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature (2011) 477(7363):216–9. 10.1038/nature10339 [DOI] [PubMed] [Google Scholar]
- 22.Menendez CM, Carr DJJ. Herpes simplex virus-1 infects the olfactory bulb shortly following ocular infection and exhibits a long-term inflammatory profile in the form of effector and HSV-1-specific T cells. J Neuroinflammation (2017) 14:124. 10.1186/s12974-017-0903-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posavad CM, Zhao L, Dong L, Jin L, Stevens CE, Magaret AS, et al. Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol (2017) 10(5):1259–69. 10.1038/mi.2016.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature (2013) 497(7450):494–7. 10.1038/nature12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature (2012) 491(7424):463–7. 10.1038/nature11522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin H, Kumamoto Y, Gopinath S, Iwasaki A. CD301b+ dendritic cells stimulate tissue-resident memory CD8+ T cells to protect against genital HSV-2. Nat Commun (2016) 7:13346. 10.1038/ncomms13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuburu N, Wang KN, Goodman KN, Pang YY, Thompson CD, Lowy DR, et al. Topical Herpes Simplex Virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J Virol (2015) 89(1):83–96. 10.1128/JVI.02380-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patricia PL, Todorov G, Pham TT, Nesburn A, Bahraoui E, BenMohamed L. Laser adjuvant-assisted peptide vaccine promotes skin mobilization of dendritic cells and enhances protective CD8 + T EM and T RM cell responses against herpes infection and disease. J Virol (2018) 92:JVI.2156–2117. 10.1128/JVI.02156-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science (2014) 346(6205):93–8. 10.1126/science.1257530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine (2008) 26(Suppl 4):D49–53. 10.1016/j.vaccine.2008.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: formulation and production strategies. Eur J Pharm Biopharm (2015) 94:251–63. 10.1016/j.ejpb.2015.05.023 [DOI] [PubMed] [Google Scholar]
- 32.Pizzolla A, Nguyen THO, Smith JM, Brooks AG, Kedzieska K, Heath WR, et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol (2017) 2(12):02. 10.1126/sciimmunol.aam6970 [DOI] [PubMed] [Google Scholar]
- 33.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS One (2011) 6(1):e16245. 10.1371/journal.pone.0016245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizzolla A, Nguyen TH, Sant S, Jaffar J, Loudovaris T, Mannering SI, et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest (2018) 128(2):721–33. 10.1172/JCI96957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinones-Parra SM, Clemens EB, Wang ZF, Croom HA, Kedzierski L, McVernon J, et al. A role of influenza virus exposure history in determining pandemic susceptibility and CD8(+) T cell responses. J Virol (2016) 90(15):6936–47. 10.1128/JVI.00349-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichyangkul S, Yongvanitchit K, Limsalakpetch A, Kum-Arb U, Im-Erbsin R, Boonnak K, et al. Tissue distribution of memory T and B cells in rhesus monkeys following influenza A infection. J Immunol (2015) 195(9):4378–86. 10.4049/jimmunol.1501702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity (2010) 33(1):96–105. 10.1016/j.immuni.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMaster SR, Wilson JJ, Wang H, Kohlmeier JE. Airway-resident memory CD8 T cells provide antigen-specific protection against respiratory virus challenge through rapid IFN-γ production. J Immunol (2015) 195(1):203–9. 10.4049/jimmunol.1402975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sckisel GD, Tietze JK, Zamora AE, Hsiao HH, Priest SO, Wilkins DE, et al. Influenza infection results in local expansion of memory CD8(+) T cells with antigen non-specific phenotype and function. Clin Exp Immunol (2014) 175(1):79–91. 10.1111/cei.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laidlaw BJ, Zhang N, Marshall HD, Staron M, Guan T, Hu Y, et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity (2014) 41(4):633–45. 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman TJ, Lambert K, Topham DJ. Rapid reactivation of extralymphoid CD4 T cells during secondary infection. PLoS One (2011) 6(5):e20493. 10.1371/journal.pone.0020493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J Immunol (2010) 184(7):3841–9. 10.4049/jimmunol.0902281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol (2011) 187(11):5510–4. 10.4049/jimmunol.1102243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neyt K, GeurtsvanKessel CH, Lambrecht BN. Double-negative T resident memory cells of the lung react to influenza virus infection via CD11c(hi) dendritic cells. Mucosal Immunol (2016) 9(4):999–1014. 10.1038/mi.2015.91 [DOI] [PubMed] [Google Scholar]
- 45.Wu T, Hu YH, Lee YT, Bouchard KR, Benechet A, Khanna K, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal crossprotection against pulmonary virus infection. J Leukoc Biol (2014) 95(2):215–24. 10.1189/jlb.0313180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarnitsyna VI, Handel A, McMaster SR, Hayward SL, Kohlmeier JE, Antia R. Mathematical model reveals the role of memory CD8 T cell populations in recall responses to influenza. Front Immunol (2016) 7:165. 10.3389/fimmu.2016.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1+ dendritic cells. Immunity (2016) 45(4):847–60. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol (2017) 2(7):06. 10.1126/sciimmunol.aag2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med (2016) 213(13):3057–73. 10.1084/jem.20160938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, Denning TL, et al. Pulmonary antigen encounter regulates the establishment of tissue-resident CD8 memory T cells in the lung airways and parenchyma. Mucosal Immunol (2018). 10.1038/s41385-018-0003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pizzolla A, Wang Z, Groom JR, Kedzierska K, Brooks AG, Reading PC, et al. Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc Natl Acad Sci U S A (2017) 114(20):5225–30. 10.1073/pnas.1620194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J Virol (2014) 88(16):9010–6. 10.1128/JVI.00329-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory provides heterosubtypic protection to influenza infection. JCI Insight (2016) 1(10):e85832. 10.1172/jci.insight.85832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasper DJ, Neldner B, Plisch EH, Rustom H, Carrow E, Imai H, et al. Effective respiratory CD8 T-cell immunity to influenza virus induced by intranasal carbomer-lecithin-adjuvanted non-replicating vaccines. PLoS Pathog (2016) 12(12):e1006064. 10.1371/journal.ppat.1006064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deliyannis G, Kedzierska K, Lau YF, Zeng WG, Turner SJ, Jackson DC, et al. Intranasal lipopeptide primes lung-resident memory CD8+ T cells for long-term pulmonary protection against influenza. Eur J Immunol (2006) 36(3):770–8. 10.1002/eji.200535217 [DOI] [PubMed] [Google Scholar]
- 56.Lee YN, Lee YT, Kim MC, Gewirtz AT, Kang SMA. Novel vaccination strategy mediating the induction of lung-resident memory CD8 T cells confers heterosubtypic immunity against future pandemic influenza virus. J Immunol (2016) 196(6):2637–45. 10.4049/jimmunol.1501637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullin J, Ahmed MS, Sharma R, Upile N, Beer H, Achar P, et al. Activation of cross-reactive mucosal T and B cell responses in human nasopharynx-associated lymphoid tissue in vitro by modified vaccinia Ankara-vectored influenza vaccines. Vaccine (2016) 34(14):1688–95. 10.1016/j.vaccine.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 58.Zhou AC, Wagar LE, Wortzman ME, Watts TH. Intrinsic 4-1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol (2017) 10(5):1294–309. 10.1038/mi.2016.124 [DOI] [PubMed] [Google Scholar]
- 59.Kang MC, Choi DH, Choi YW, Park SJ, Namkoong H, Park KS, et al. Intranasal introduction of Fc-fused interleukin-7 provides long-lasting prophylaxis against lethal influenza virus infection. J Virol (2016) 90(5):2273–84. 10.1128/JVI.02768-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol (2015) 8(5):1060–71. 10.1038/mi.2014.133 [DOI] [PubMed] [Google Scholar]
- 61.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet (2014) 384(9939):258–71. 10.1016/S0140-6736(14)60164-1 [DOI] [PubMed] [Google Scholar]
- 62.Kiniry BE, Li S, Ganesh A, Hunt PW, Somsouk M, Skinner PJ, et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8+ T-cells in chronic infection. Mucosal Immunol (2017) 15:15. 10.1038/mi.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibbs A, Buggert M, Edfeldt G, Ranefall P, Introini A, Cheuk S, et al. HIV-infected women have high numbers of CD103-CD8+ T cells residing close to the basal membrane of the ectocervical epithelium. J Infect Dis (2017) 20:20. 10.1093/infdis/jix661 [DOI] [PubMed] [Google Scholar]
- 64.Moylan DC, Goepfert PA, Kempf MC, Saag MS, Richter HE, Mestecky J, et al. Diminished CD103 (αEβ7) expression on resident T cells from the female genital tract of HIV-positive women. Pathog Immun (2017) 1(2):371–87. 10.20411/pai.v1i2.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Damouche A, Pourcher G, Pourcher V, Benoist S, Busson E, Lataillade JJ, et al. High proportion of PD-1-expressing CD4+ T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur J Immunol (2017) 47(12):2113–23. 10.1002/eji.201747060 [DOI] [PubMed] [Google Scholar]
- 66.Pattacini L, Baeten J, Lingappa J, Lund J. HIV infection impairs CD8+resident memory T cell frequency in cervical mucosae. J Immunol (2016) 196:208–22. [Google Scholar]
- 67.Adnan S, Reeves RK, Gillis J, Wong FE, Yu Y, Camp JV, et al. Persistent low-level replication of SIVΔnef drives maturation of antibody and CD8 T cell responses to induce protective immunity against vaginal SIV infection. PLoS Pathog (2016) 12(12):e1006104. 10.1371/journal.ppat.1006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan HX, Wheatley AK, Esterbauer R, Jegaskanda S, Glass JJ, Masopust D, et al. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol (2017) 25:25. 10.1038/mi.2017.89 [DOI] [PubMed] [Google Scholar]
- 69.Zaric M, Becker PD, Hervouet C, Kalcheva P, Yus BI, Cocita C, et al. Long-lived tissue resident HIV-1 specific memory CD8(+) T cells are generated by skin immunization with live virus vectored microneedle arrays. J Control Release (2017) 268:166–75. 10.1016/j.jconrel.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roberts KL, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol (2008) 16(10):472–9. 10.1016/j.tim.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 71.Lofquist JM, Weimert NA, Hayney MS. Smallpox: a review of clinical disease and vaccination. Am J Health Syst Pharm (2003) 60(8):749–56; quiz 57–8. [PubMed] [Google Scholar]
- 72.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature (2012) 483(7388):227–31. 10.1038/nature10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaide O, Emerson RO, Jiang XD, Gulati N, Nizza S, Desmarais C, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med (2015) 21(6):647–53. 10.1038/nm.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilchuk P, Hill TM, Guy C, McMaster SR, Boyd KL, Rabacal WA, et al. A distinct lung-interstitium-resident memory CD8(+) T cell subset confers enhanced protection to lower respiratory tract infection. Cell Rep (2016) 16(7):1800–9. 10.1016/j.celrep.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hersperger AR, Siciliano NA, DeHaven BC, Snook AE, Eisenlohr LC. Epithelial immunization induces polyfunctional CD8(+) T cells and optimal mousepox protection. J Virol (2014) 88(16):9472–5. 10.1128/JVI.01464-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kadoki M, Patil A, Thaiss CC, Brooks DJ, Pandey S, Deep D, et al. Organism-level analysis of vaccination reveals networks of protection across tissues. Cell (2017) 171(2):398–413.e21. 10.1016/j.cell.2017.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woodward Davis AS, Bergsbaken T, Delaney MA, Bevan MJ. Dermal resident versus recruited γδ T cell response to cutaneous Vaccinia virus infection. J Immunol (2015) 194(5):2260–7. 10.4049/jimmunol.1402438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muschaweckh A, Buchholz VR, Fellenzer A, Hessel C, König PA, Tao S, et al. Antigen-dependent competition shapes the local repertoire of tissue-resident memory CD8+ T cells. J Exp Med (2016) 213(13):3075–86. 10.1084/jem.20160888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med (2016) 213(6):951–66. 10.1084/jem.20151855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue HH, et al. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog (2017) 13(9):e1006569. 10.1371/journal.ppat.1006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus – a comprehensive review. Clin Rev Allergy Immunol (2013) 45(3):331–79. 10.1007/s12016-013-8368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun (2015) 6:10224. 10.1038/ncomms10224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, Tregoning JS. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol (2018) 11(1):249–56. 10.1038/mi.2017.79 [DOI] [PubMed] [Google Scholar]
- 84.Li HL, Callahan C, Citron M, Wen ZY, Touch S, Monslow MA, et al. Respiratory syncytial virus elicits enriched CD8+ T lymphocyte responses in lung compared with blood in African green monkeys. PLoS One (2017) 12(11):e0187642. 10.1371/journal.pone.0187642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, Graham BS. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol (2017) 10(2):545–54. 10.1038/mi.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang L, Li HY, Hai Y, Yin W, Li WJ, Zheng BY, et al. CpG in combination with an inhibitor of Notch signaling suppresses formalin-inactivated respiratory syncytial virus-enhanced airway hyperresponsiveness and inflammation by inhibiting Th17 memory responses and promoting tissue-resident memory cells in lungs. J Virol (2017) 91(10):e02111–16. 10.1128/JVI.02111-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarz B, Morabito KM, Ruckwardt TJ, Patterson DP, Avera J, Miettinen HM, et al. Viruslike particles encapsidating respiratory syncytial virus M and M2 proteins induce robust T cell responses. ACS Biomater Sci Eng (2016) 2(12):2324–32. 10.1021/acsbiomaterials.6b00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, et al. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog (2018) 14(1):e1006810. 10.1371/journal.ppat.1006810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol (2016) 16(6):367–77. 10.1038/nri.2016.38 [DOI] [PubMed] [Google Scholar]
- 90.Thom JT, Weber TC, Walton SM, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8(+) T cells, facilitating protection from local cytomegalovirus infection. Cell Rep (2015) 13(6):1125–36. 10.1016/j.celrep.2015.09.082 [DOI] [PubMed] [Google Scholar]
- 91.Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep (2015) 13(6):1137–48. 10.1016/j.celrep.2015.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caldeira-Dantas S, Furmanak T, Smith C, Quinn M, Teos LY, Ertel A, et al. The chemokine receptor CXCR3 promotes CD8+ T cell accumulation in uninfected salivary glands but is not necessary after murine cytomegalovirus infection. J Immunol (2018) 200(3):1133–45. 10.4049/jimmunol.1701272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A. Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4 T cells. PLoS Pathog (2011) 7(8):e1002214. 10.1371/journal.ppat.1002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prasad S, Hu S, Sheng WS, Singh A, Lokensgard JR. Tregs modulate lymphocyte proliferation, activation, and resident-memory T-cell accumulation within the brain during mcmv infection. PLoS One (2015) 10(12):e0145457. 10.1371/journal.pone.0145457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prasad S, Hu S, Sheng WS, Chauhan P, Singh A, Lokensgard JR. The PD-1: PD-L1 pathway promotes development of brain-resident memory T cells following acute viral encephalitis. J Neuroinflammation (2017) 14:82. 10.1186/s12974-017-0860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Venturi V, Nzingha K, Amos TG, Charles WC, Dekhtiarenko I, Cicin-Sain L, et al. The neonatal CD8+ T cell repertoire rapidly diversifies during persistent viral infection. J Immunol (2016) 196(4):1604–16. 10.4049/jimmunol.1501867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barouch DH, Picker LJ. Novel vaccine vectors for HIV-1. Nat Rev Microbiol (2014) 12(11):765–71. 10.1038/nrmicro3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knust B, Holman RC, Redd J, Mehal JM, Grube SM, MacNeil A, et al. Lymphocytic choriomeningitis virus infections among American Indians. Emerg Infect Dis (2013) 19(2):328–9. 10.3201/eid1902.120888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell (2015) 161(4):737–49. 10.1016/j.cell.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steinbach K, Vincenti I, Kreutzfeldt M, Page N, Muschaweckh A, Wagner I, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med (2016) 213(8):1571–87. 10.1084/jem.20151916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hofmann M, Oschowitzer A, Kurzhals SR, Krüger CC, Pircher H. Thymus-resident memory CD8+ T cells mediate local immunity. Eur J Immunol (2013) 43(9):2295–304. 10.1002/eji.201343519 [DOI] [PubMed] [Google Scholar]
- 102.Beura LK, Anderson KG, Schenkel JM, Locquiao JJ, Fraser KA, Vezys V, et al. Lymphocytic choriomeningitis virus persistence promotes effector-like memory differentiation and enhances mucosal T cell distribution. J Leukoc Biol (2015) 97(2):217–25. 10.1189/jlb.1HI0314-154R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol (2012) 188(10):4866–75. 10.4049/jimmunol.1200402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hondowicz BD, Kim KS, Ruterbusch MJ, Keitany GJ, Pepper M. IL-2 is required for the generation of viral-specific CD4+ Th1 tissue-resident memory cells and B cells are essential for maintenance in the lung. Eur J Immunol (2018) 48(1):80–6. 10.1002/eji.201746928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science (2014) 346:98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nat Rev Dis Primers (2015) 1:15016. 10.1038/nrdp.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, et al. The characterization of varicella zoster virus-specific T cells in skin and blood during aging. J Invest Dermatol (2015) 135(7):1752–62. 10.1038/jid.2015.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cutts FT, Franceschi S, Goldie S, Castellsague X, de Sanjose S, Garnett G, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ (2007) 85(9):719–26. 10.2471/BLT.06.038414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol (2017) 146(1):196–204. 10.1016/j.ygyno.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 110.Cuburu N, Khan S, Thompson CD, Kim R, Vellinga J, Zahn R, et al. Adenovirus vector-based prime-boost vaccination via heterologous routes induces cervicovaginal CD8+ T cell responses against HPV16 oncoproteins. Int J Cancer (2018) 142(7):1467–79. 10.1002/ijc.31166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest (2012) 122(12):4606–20. 10.1172/JCI63287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koff RS. Review article: vaccination and viral hepatitis – current status and future prospects. Aliment Pharmacol Ther (2007) 26(10):1285–92. 10.1111/j.1365-2036.2007.03517.x [DOI] [PubMed] [Google Scholar]
- 113.Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, et al. IL-2high tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J Exp Med (2017) 214(6):1567–80. 10.1084/jem.20162115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stelma F, De Niet A, Sinnige MJ, Van Dort KA, Van Gisbergen KPJM, Verheij J, et al. Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep (2017) 7(1). 10.1038/s41598-017-06352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol (2015) 33:787–821. 10.1146/annurev-immunol-032414-112326 [DOI] [PubMed] [Google Scholar]
- 116.Woodberry T, Suscovich TJ, Henry LM, August M, Waring MT, Kaur A, et al. Alpha E beta 7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J Immunol (2005) 175(7):4355–62. 10.4049/jimmunol.175.7.4355 [DOI] [PubMed] [Google Scholar]
- 117.Woon HG, Braun A, Li J, Smith C, Edwards J, Sierro F, et al. Compartmentalization of total and virus-specific tissue-resident memory CD8+ T cells in human lymphoid organs. PLoS Pathog (2016) 12(8):e1005799. 10.1371/journal.ppat.1005799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, et al. Tonsillar homing of Epstein-Barr virus–specific CD8+ T cells and the virus-host balance. J Clin Invest (2005) 115(9):2546–55. 10.1172/JCI24810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Timoney P. Vesicular stomatitis. Vet Rec (2016) 179(5):119–20. 10.1136/vr.i4075 [DOI] [PubMed] [Google Scholar]
- 120.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A (2010) 107(42):17872–9. 10.1073/pnas.1010201107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wakim LM, Woodward-Davis A, Liu R, Hu YF, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol (2012) 189(7):3462–71. 10.4049/jimmunol.1201305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang M, Abend JR, Johnson SF, Imperiale MJ. The role of polyomaviruses in human disease. Virology (2009) 384(2):266–73. 10.1016/j.virol.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frost EL, Kersh AE, Evavold BD, Lukacher AE. Cutting edge: resident memory CD8 T cells express high-affinity TCRs. J Immunol (2015) 195(8):3520–4. 10.4049/jimmunol.1501521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shwetank, Abdelsamed HA, Frost EL, Schmitz HM, Mockus TE, Youngblood BA, et al. Maintenance of PD-1 on brain-resident memory CD8 T cells is antigen independent. Immunol Cell Biol (2017) 95(10):953–9. 10.1038/icb.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maru S, Jin G, Schell TD, Lukacher AE. TCR stimulation strength is inversely associated with establishment of functional brain-resident memory CD8 T cells during persistent viral infection. PLoS Pathog (2017) 13(4):e1006318. 10.1371/journal.ppat.1006318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Aalderen MC, Remmerswaal EBM, Heutinck KM, ten Brinke A, Feltkamp MCW, van der Weerd NC, et al. Clinically relevant reactivation of polyomavirus BK (BKPyV) in HLA-A02-positive renal transplant recipients is associated with impaired effector-memory differentiation of BKPyV-specific CD8+ T cells. PLoS Pathog (2016) 12(10):e1005903. 10.1371/journal.ppat.1005903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.von Essen MR, Kongsbak M, Geisler C. Mechanisms behind functional avidity maturation in T cells. Clin Dev Immunol (2012) 2012:163453. 10.1155/2012/163453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moghadam SRJ, Omidi N, Bayrami S, Moghadam SJ, SeyedAlinaghi S. Ebola viral disease: a review literature. Asian Pac J Trop Biomed (2015) 5(4):260–7. 10.1016/S2221-1691(15)30341-5 [DOI] [Google Scholar]
- 129.Meyer M, Garron T, Lubaki NM, Mire CE, Fenton KA, Klages C, et al. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J Clin Invest (2015) 125(8):3241–55. 10.1172/JCI81532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cutler AJ, Oliveira J, Ferreira RC, Challis B, Walker NM, Caddy S, et al. Capturing the systemic immune signature of a norovirus infection: an n-of-1 case study within a clinical trial. Wellcome Open Res (2017) 2:28. 10.12688/wellcomeopenres.11300.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomov VT, Palko O, Lau CW, Pattekar A, Sun YH, Tacheva R, et al. Differentiation and protective capacity of virus-specific CD8(+) T cells suggest murine Norovirus persistence in an immune-privileged enteric niche. Immunity (2017) 47(4):723–38. 10.1016/j.immuni.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Melvin JA, Scheller EV, Miller JF, Cotter PA. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol (2014) 12(4):274–88. 10.1038/nrmicro3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rowe J, Yerkovich ST, Richmond P, Suriyaarachchi D, Fisher E, Feddema L, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun (2005) 73(12):8130–5. 10.1128/IAI.73.12.8130-8135.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smits K, Pottier G, Smet J, Dirix V, Vermeulen F, De Schutter I, et al. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine (2013) 32(1):111–8. 10.1016/j.vaccine.2013.10.056 [DOI] [PubMed] [Google Scholar]
- 135.Wilk MM, Misiak A, McManus RM, Allen AC, Lynch MA, Mills KHG. Lung CD4 tissue-resident memory T cells mediate adaptive immunity induced by previous infection of mice with Bordetella pertussis. J Immunol (2017) 199(1):233–43. 10.4049/jimmunol.1602051 [DOI] [PubMed] [Google Scholar]
- 136.Misiak A, Wilk MM, Raverdeau M, Mills KHG. IL-17-producing innate and pathogen-specific tissue resident memory γδ T cells expand in the lungs of Bordetella pertussis-infected mice. J Immunol (2017) 198(1):363–74. 10.4049/jimmunol.1601024 [DOI] [PubMed] [Google Scholar]
- 137.Haq IJ, Battersby AC, Eastham K, McKean M. Community acquired pneumonia in children. BMJ (2017) 356:616–9. 10.1136/bmj.j686 [DOI] [PubMed] [Google Scholar]
- 138.Rozenbaum MH, Pechlivanoglou P, van der Werf TS, Lo-Ten-Foe JR, Postma MJ, Hak E. The role of Streptococcus pneumoniae in community-acquired pneumonia among adults in Europe: a meta-analysis. Eur J Clin Microbiol Infect Dis (2013) 32(3):305–16. 10.1007/s10096-012-1778-4 [DOI] [PubMed] [Google Scholar]
- 139.Smith NM, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, Lipsitz E, et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol (2018) 11(1):220–35. 10.1038/mi.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Babb R, Chen A, Ogunniyi AD, Hirst TR, Kara EE, McColl SR, et al. Enhanced protective responses to a serotype-independent pneumococcal vaccine when combined with an inactivated influenza vaccine. Clin Sci (2017) 131(2):169–80. 10.1042/CS20160475 [DOI] [PubMed] [Google Scholar]
- 141.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MPR. The immune response in tuberculosis. Annu Rev Immunol (2013) 31(1):475–527. 10.1146/annurev-immunol-032712-095939 [DOI] [PubMed] [Google Scholar]
- 142.Moliva JI, Turner J, Torrelles JB. Immune responses to Bacillus Calmette–Guérin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol (2017) 8:407. 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walrath J, Zukowski L, Krywiak A, Silver RF. Resident Th1-like effector memory cells in pulmonary recall responses to Mycobacterium tuberculosis. Am J Respir Cell Mol Biol (2005) 33(1):48–55. 10.1165/rcmb.2005-0060OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jeyanathan M, Heriazon A, Xing Z. Airway luminal T cells: a newcomer on the stage of TB vaccination strategies. Trends Immunol (2010) 31(7):247–52. 10.1016/j.it.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 145.Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, et al. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol (2004) 173(10):6357–65. 10.4049/jimmunol.173.10.6357 [DOI] [PubMed] [Google Scholar]
- 146.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol (2005) 174(12):7986–94. 10.4049/jimmunol.174.12.7986 [DOI] [PubMed] [Google Scholar]
- 147.Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Le Gros G, et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol (2010) 40(9):2482–92. 10.1002/eji.200940279 [DOI] [PubMed] [Google Scholar]
- 148.Perdomo C, Zedler U, Kühl AA, Lozza L, Saikali P, Sander LE, et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio (2016) 7(6):e01686–16. 10.1128/mBio.01686-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu ZD, Wong KW, Zhao HM, Wen HL, Ji P, Ma H, et al. Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol Ther (2017) 25(5):1222–33. 10.1016/j.ymthe.2017.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haddadi S, Thanthrige-Don N, Afkhami S, Khera A, Jeyanathan M, Xing Z. Expression and role of VLA-1 in resident memory CD8 T cell responses to respiratory mucosal viral-vectored immunization against tuberculosis. Sci Rep (2017) 7(1):9525. 10.1038/s41598-017-09909-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med (2018) 24(2):130–43. 10.1038/nm.4473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun (2015) 6:8533. 10.1038/ncomms9533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol (2016) 14(6):385–400. 10.1038/nrmicro.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brunham RC. Immunity to Chlamydia trachomatis. J Infect Dis (2013) 207(12):1796–7. 10.1093/infdis/jit095 [DOI] [PubMed] [Google Scholar]
- 155.Batteiger BE, Xu F, Johnson RE, Rekart ML. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis (2010) 201(Suppl 2):S178–89. 10.1086/652400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Geisler WM, Lensing SY, Press CG, Hook EW, III. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis (2013) 207(12):1850–6. 10.1093/infdis/jit094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnson RM, Yu H, Strank NO, Karunakaran K, Zhu Y, Brunham RC. B cell presentation of Chlamydia antigen selects out protective CD4gamma13 T cells: implications for genital tract tissue-resident memory lymphocyte clusters. Infect Immun (2018) 86(2):e00614–17. 10.1128/iai.00614-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science (2015) 348(6241):aaa8205. 10.1126/science.aaa8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Meara CP, Armitage CW, Kollipara A, Andrew DW, Trim L, Plenderleith MB, et al. Induction of partial immunity in both males and females is sufficient to protect females against sexual transmission of Chlamydia. Mucosal Immunol (2016) 9(4):1076–88. 10.1038/mi.2015.125 [DOI] [PubMed] [Google Scholar]
- 160.Johnson RM, Brunham RC. Tissue-resident T cells as the central paradigm of Chlamydia immunity. Infect Immun (2016) 84(4):868–73. 10.1128/IAI.01378-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fletcher SM, McLaws M-L, Ellis JT. Prevalence of gastrointestinal pathogens in developed and developing countries: systematic review and meta-analysis. J Public Health Res (2013) 2(1):42–53. 10.4081/jphr.2013.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.de Noordhout CM, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis (2014) 14(11):1073–82. 10.1016/S1473-3099(14)70870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zenewicz LA, Shen H. Innate and adaptive immune responses to Listeria monocytogenes: a short overview. Microbes Infect (2007) 9(10):1208–15. 10.1016/j.micinf.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jungi TW, Jungi R. Immunological memory to Listeria monocytogenes in rodents. IV. Studies on origin and fate of tissue-positioned T memory cells. Immunology (1981) 44(4):789–98. [PMC free article] [PubMed] [Google Scholar]
- 165.Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity (2014) 40(5):747–57. 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Romagnoli PA, Sheridan BS, Pham QM, Lefrançois L, Khanna KM. IL-17A-producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection. Proc Natl Acad Sci U S A (2016) 113(30):8502–7. 10.1073/pnas.1600713113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amphlett A. Far east scarlet-like fever: a review of the epidemiology, symptomatology, and role of superantigenic toxin: Yersinia pseudotuberculosis-derived mitogen A. Open Forum Infect Dis (2016) 3(1):ofv202. 10.1093/ofid/ofv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol (2015) 16(4):406–14. 10.1038/ni.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bergsbaken T, Bevan MJ, Fink PJ. Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep (2017) 19(1):114–24. 10.1016/j.celrep.2017.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eng S-K, Pusparajah P, Ab Mutalib N-S, Ser H-L, Chan K-G, Lee L-H. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci (2015) 8(3):284–93. 10.1080/21553769.2015.1051243 [DOI] [Google Scholar]
- 171.Gayet R, Bioley G, Rochereau N, Paul S, Corthesy B. Vaccination against Salmonella infection: the mucosal way. Microbiol Mol Biol Rev (2017) 81(3):e00007–17. 10.1128/MMBR.00007-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee SJ, Benoun J, Sheridan BS, Fogassy Z, Pham O, Pham QM, et al. Dual immunization with SseB/Flagellin provides enhanced protection against Salmonella infection mediated by circulating memory cells. J Immunol (2017) 199(4):1353–61. 10.4049/jimmunol.1601357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nourollahpour Shiadeh M, Niyyati M, Fallahi S, Rostami A. Human parasitic protozoan infection to infertility: a systematic review. Parasitol Res (2016) 115(2):469–77. 10.1007/s00436-015-4827-y [DOI] [PubMed] [Google Scholar]
- 174.Gurung P, Kanneganti T-D. Immune responses against protozoan parasites: a focus on the emerging role of Nod-like receptors. Cell Mol Life Sci (2016) 73(16):3035–51. 10.1007/s00018-016-2212-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rénia L, Goh YS. Malaria parasites: the great escape. Front Immunol (2016) 7:463. 10.3389/fimmu.2016.00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tse SW, Cockburn IA, Zhang H, Scott AL, Zavala F. Unique transcriptional profile of liver-resident memory CD8+ T cells induced by immunization with malaria sporozoites. Genes Immun (2013) 14(5):302–9. 10.1038/gene.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pichyangkul S, Spring MD, Yongvanitchit K, Kum-Arb U, Limsalakpetch A, Im-Erbsin R, et al. Chemoprophylaxis with sporozoite immunization in P. knowlesi rhesus monkeys confers protection and elicits sporozoitespecific memory T cells in the liver. PLoS One (2017) 12(2):e0171826. 10.1371/journal.pone.0171826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fernandez-Ruiz D, Ng WY, Holz L, Ma J, Zaid A, Wong YC, et al. Liver-resident memory T cells form a front-line defence against malaria liver-stage infection. Immunity (2016) 45(4):889–902. 10.1016/j.immuni.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 179.McNamara HA, Cai Y, Wagle MV, Sontani Y, Roots CM, Miosge LA, et al. Up-regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol (2017) 2(9):eaaj1996. 10.1126/sciimmunol.aaj1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res (2017) 6:750. 10.12688/f1000research.11120.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Glennie ND, Scott P. Memory T cells in cutaneous leishmaniasis. Cell Immunol (2016) 309:50–4. 10.1016/j.cellimm.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Glennie ND, Volk SW, Scott P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog (2017) 13(4):e1006349. 10.1371/journal.ppat.1006349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med (2015) 212(9):1405–14. 10.1084/jem.20142101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rodrigues A, Claro M, Alexandre-Pires G, Santos-Mateus D, Martins C, Valério-Bolas A, et al. Leishmania infantum antigens modulate memory cell subsets of liver resident T lymphocyte. Immunobiology (2017) 222(2):409–22. 10.1016/j.imbio.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 185.Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis (2012) 44(11):805–14. 10.3109/00365548.2012.693197 [DOI] [PubMed] [Google Scholar]
- 186.Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol (2012) 34(6):793–813. 10.1007/s00281-012-0339-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Landrith TA, Sureshchandra S, Rivera A, Jang JC, Rais M, Nair MG, et al. CD103+ CD8 T cells in the toxoplasma-infected brain exhibit a tissue-resident memory transcriptional profile. Front Immunol (2017) 8:335. 10.3389/fimmu.2017.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han SJ, Glatman Zaretsky A, Andrade-Oliveira V, Collins N, Dzutsev A, Shaik J, et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity (2017) 47(6):1154–1168.e6. 10.1016/j.immuni.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jackson JA, Friberg IM, Little S, Bradley JE. Review series on helminths, immune modulation and the hygiene hypothesis: Immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology (2009) 126(1):18–27. 10.1111/j.1365-2567.2008.03010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Steinfelder S, Rausch S, Michael D, Kühl AA, Hartmann S. Intestinal helminth infection induces highly functional resident memory CD4+ T cells in mice. Eur J Immunol (2017) 47(2):353–63. 10.1002/eji.201646575 [DOI] [PubMed] [Google Scholar]
- 191.Thawer SG, Horsnell WG, Darby M, Hoving JC, Dewals B, Cutler AJ, et al. Lung-resident CD4(+) T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol (2014) 7(2):239–48. 10.1038/mi.2013.40 [DOI] [PubMed] [Google Scholar]
- 192.Reynolds LA, Filbey KJ, Maizels RM. Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus. Semin Immunopathol (2012) 34(6):829–46. 10.1007/s00281-012-0347-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Brombacher TM, De Gouveia KS, Cruywagen L, Makena N, Booley F, Tamgue O, et al. Nippostrongylus brasiliensis infection leads to impaired reference memory and myeloid cell interference. Sci Rep (2018) 8(1):2958. 10.1038/s41598-018-20770-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res (2014) 139(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- 195.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol (2011) 49(2):171–7. 10.1007/s12275-011-1064-7 [DOI] [PubMed] [Google Scholar]
- 196.Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, et al. Staged development of long-lived T-cell receptor αβ TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol (2017) (17). 10.1016/j.jaci.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Conti HR, Peterson AC, Brane L, Huppler AR, Hernández-Santos N, Whibley N, et al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med (2014) 211(10):2075–84. 10.1084/jem.20130877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity (2012) 36(5):873–84. 10.1016/j.immuni.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Strutt TM, Dhume K, Finn CM, Hwang JH, Castonguay C, Swain SL, et al. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol (2018) 11(3):668–80. 10.1038/mi.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]