Abstract
Background
Although the divergent male and female differentiation depends on key genes, many biological differences seen in men and women are driven by relative differences in estrogen and testosterone levels. Gender dysphoria denotes the distress that gender incongruence with the assigned sex at birth may cause. Gender-affirming treatment includes medical intervention such as inhibition of endogenous sex hormones and subsequent replacement with cross-sex hormones. The aim of this study is to investigate consequences of an altered sex hormone profile on different tissues and metabolic risk factors. By studying subjects undergoing gender-affirming medical intervention with sex hormones, we have the unique opportunity to distinguish between genetic and hormonal effects.
Methods
The study is a single center observational cohort study conducted in Stockholm, Sweden. The subjects are examined at four time points; before initiation of treatment, after endogenous sex hormone inhibition, and three and eleven months following sex hormone treatment. Examinations include blood samples, skeletal muscle-, adipose- and skin tissue biopsies, arteriography, echocardiography, carotid Doppler examination, whole body MRI, CT of muscle and measurements of muscle strength.
Results
The primary outcome measure is transcriptomic and epigenomic changes in skeletal muscle. Secondary outcome measures include transcriptomic and epigenomic changes associated with metabolism in adipose and skin, muscle strength, fat cell size and ability to release fatty acids from adipose tissue, cardiovascular function, and body composition.
Conclusions
This study will provide novel information on the role of sex hormone treatment in skeletal muscle, adipose and skin, and its relation to cardiovascular and metabolic disease.
Keywords: Transgender, Sex hormone, Adipose tissue, Skeletal muscle, Epigenetics, Sex change
Abbreviations: ANOVA, Andrology Sexual Medicine and Transgender Medicine at the Karolinska University Hospital; BSA, body surface area; CFR, coronary flow velocity reserve; GETS, GEnder Dysphoria Treatment in Sweden; GnRH, Gonadotropin releasing hormone; HOMA-IR, Homeostatic model assessment of insulin resistance; PBMC, peripheral blood mononuclear cells; TAPSE, right ventricular tricuspid annulus; TTE, transthoracic echocardiography
1. Introduction
The effects of sex on different biological mechanisms is a recurrent question in medical research and it is obvious that sex and gender have great impact on epidemiology, risk, clinical manifestations and course of disease [1]. Many of the biological differences seen in men and women in skeletal muscle, adipose, and cardiovascular risk factors are driven by relative differences in estrogen and testosterone levels, even though the primary divergent embryo and fetal development depend on the chromosome constitution and key genes [[2], [3], [4]]. Differences that are particularly significant but not well known are 1) regulation of skeletal muscle mass and adipose tissue, 2) metabolic changes in the regulation of glucose homeostasis and lipid metabolism, and 3) regulation of vascular function and structural effects on the heart and arteries. Traditionally, human studies investigating sex differences have compared men and women. However, it is very difficult to match subjects and avoid confounders in this comparison since individual differences are vast at genetic levels, as are environmental exposures during development in early and later life. Subjects exposed to both estrogen and testosterone at different time points would be ideal to compare the effects of sex-hormones in comparison to constitutional gene expression profiles.
Gender dysphoria denotes the distress that gender incongruence with the assigned sex at birth may cause. Gender-affirming treatment aims to align the body with the gender identity and includes treatment with designated sex hormones and may also include surgery to change primary and secondary sex characteristics, voice therapy, and hair removal, and could be either masculinizing or feminizing. The GETS study described here is designed to investigate the effects of altered sex hormone pattern on skeletal muscle, adipose, skin, heart, blood vessels and metabolic risk factors in subjects with gender dysphoria undergoing cross sex-hormone treatment. Both transgender men and women are studied. The primary outcome of the GETS study is transcriptomic and epigenomic changes in skeletal muscle. Secondary outcome measures include transcriptomic and epigenomic changes associated with metabolism in adipose and skin, muscle strength, fat cell size and ability to release fatty acids from adipose tissue, cardiovascular function, and body composition.
2. Methods
2.1. Study design
This study is designed as a single center observational cohort study.
2.2. Recruitment
The study population consists of individuals that have been referred to ANOVA, Andrology Sexual Medicine and Transgender Medicine at the Karolinska University Hospital, Stockholm, Sweden for evaluation of gender dysphoria and who have been accepted to start gender-affirming medical intervention. These individuals diagnosed with gender dysphoria are assessed for eligibility (see Table 1 for inclusion and exclusion criteria). If eligible, they are provided a brief oral and written presentation of the study background and practical implications. A total of 40 patients (20 transgender men and 20 transgender women) are planned to be recruited.
Table 1.
Inclusion/exclusion criteria.
| Inclusion criteria |
|---|
| Age 20 -<= 40 years |
| Individuals with Gender Dysphoria that have been accepted for cross-sex hormone treatment |
| Willingness to participate in the study |
|
Exclusion criteria |
| Already started with any hormone therapy |
| Ongoing infectious disease |
| Treatment with warfarin or other anticoagulants |
| History of cardiovascular disease |
| Type 1 diabetes |
| Other serious psychiatric or somatic morbidity |
| Alcohol or drug dependency |
| Language difficulties |
2.3. Informed consent
The subjects are informed that their participation in the study is completely voluntary and that they could withdraw their consent to participate at any time without the need of explanation. They are also informed that their decision to participate or not, or the withdrawal of consent to participate, would not in any way change their treatment. Oral and written informed consent is obtained from all subjects. The regional ethical review board in Stockholm, Sweden, approved the study (Dnr 2014/409-31/4).
2.4. Overview of study design
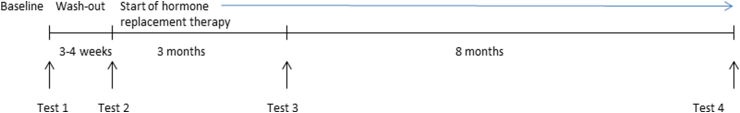
An overview of the study time-line is shown in Fig. 1. Examinations are conducted at four time points: (1) before treatment initiation, (2) four weeks after initiated gonadal hormonal down regulation but before hormone replacement, (3) three months after hormone replacement therapy, and (4) eleven months after hormone replacement therapy. Each time point is divided into two examination days (Mondays and Thursdays). On the first day, the participant comes to the laboratory in the morning after an overnight fast. After at least 5 min of rest, blood pressure is measured and blood samples are collected. After administration of local anesthesia, tissue samples from skeletal muscle, adipose and skin are collected. Subsequently, after a 15 min rest, arterial stiffness is measured with an arteriograph. On the second day, muscle strength is evaluated using isokinetic dynamometry. After a 15 min rest, a transthoracic echocardiography (TTE) is performed for estimation of chamber dimensions as well as ventricular and valvular function, and coronary flow velocity reserve (CFR) is assessed in the left anterior descending coronary artery by pulsed Doppler, followed by a carotid Doppler examination. On the first and last time points of the study, the participants undergo a CT muscle scan followed by whole body MRI. These investigations are performed on a separate day before the biopsies.
Fig. 1.
Study design. Examinations are conductged at four time points (1) at baseline before treatment initiation, (2) three to four weeks after initiated gonadal hormonal down regulation but before hormone replacement, (3) three months after the start of hormone replacement therapy and (4) eleven months after start of hormone replacement therapy.
2.5. Medical treatment
Endocrine therapy in order to reverse the endocrine environment from male to female and vice versa is initiated with injection of a Gonadotropin releasing hormone (GnRH) antagonist (Degarelix 240 mg sc). This results in immediate reduction in gonadotropin secretion and brings sex hormone levels (estradiol and testosterone) to castrate levels within 24 h and for the duration of the washout period of 4 weeks. Continued cross-hormone treatment is started after post castration assessments have been made. Transgender men (former called female-to-male) are treated with testosterone injections (testosterone undecanoate 1000 mg i.m.) with the two first injections given with a 6 week interval and thereafter one injection every tenth week, with dose adjustments in order to maintain androgen levels within the normal adult male reference range. Further gonadotropin suppression is maintained with GnRH-analogue administered i.m. every third months. Transgender women (former called male-to-female) are also given GnRH-analogue treatment to maintain suppression of the gonadal axis. Estradiol therapy is administered with either transdermal therapy or i.m. injections (estradiol polyphosphate) both aiming to produce serum estradiol levels within the mid-cycle normal range for fertile women.
2.6. Examinations
2.6.1. Blood and urine samples
Blood samples are collected for standard laboratory analyses, blood lipids, hematology and sex hormones. Granulocytes and peripheral blood mononuclear cells (PBMCs) are isolated from fresh whole blood, snap frozen in liquid nitrogen and subsequently stored at −80 °C until analyzed. In a subgroup of subjects, a baseline urine sample is collected to exclude previous cross sex hormone treatment.
2.6.2. Tissue samples
All tissue samples are collected under sterile conditions. A local anesthetic injection (10–20 ml Carbocain 10 mg/ml without adrenalin) is used for each biopsy.
2.6.3. Skeletal muscle
Skeletal muscle biopsies are obtained at rest from the vastus lateralis muscle using the percutaneous needle technique [5]. The sample is divided into three parts for genomic, epigenetic and histology analysis including measuring of muscle fiber size. The histology sample is frozen in liquid nitrogen pre-cooled in isopentane, and the others are snap frozen directly in liquid nitrogen and then stored at −80 °C until analyzed. The endpoints that will be studied in skeletal muscle include 1) cross sectional area of the muscle fibers, fiber type composition, capillarization and number of myonuclei, 2) epigenetic changes in skeletal muscle including genome wide DNA methylation, as described [6]. 3) alterations in gene expression in skeletal muscle as well as protein expression of key targets.
2.6.4. Adipose
Adipose tissue biopsies are obtained approximately 10 cm laterally of the umbilicus after a subcutaneous local anesthetic injection as described above. A small incision is made to help the aspiration needle to penetrate the skin, and using a 10 ml syringe with generated negative pressure, subcutaneous fat is aspired. The sample is divided and one part is directly analyzed to determine fat cell size and lipolysis as described previously [7]. Both isolated fat cells and fat tissue are subsequently snap frozen in liquid nitrogen and then stored at −80 °C until subsequent genomic and epigenetic analyses. The endpoints that will be studied in adipose include 1) fat cell size (measured in picoliters) and fat cell number (total and in different compartments calculated from fat cell size and quantification of fat mass from MRI scans), and its potential impact on metabolic complications, as described [8], 2) lipolysis (quantified by spontaneous release of glycerol from isolated fat cells) and a ratio of basal/stimulated lipolysis (stimulated with isoprenaline or noradrenaline), and its potential impact on cardiovascular risk, as described [9], 3) epigenetic changes in adipose tissue including genome wide DNA methylation in fat cells, 4) gene expression analysis in adipose tissue as well as secretion of adipokines, as described [10].
2.6.5. Skin
Skin biopsies are obtained approximately 2 cm proximal of the muscle biopsy. After local intradermal and subcutaneous anesthetic injection, a 6 mm circular punch is used to obtain the skin biopsy. After biopsy collection, the subcutaneous fat is cut off and the sample is divided into three parts for subsequent genomic, epigenetic and histology analyses. The epigenetic and histology samples are frozen in liquid nitrogen pre-cooled in isopentane, while the genomic sample is snap frozen directly in liquid nitrogen and then stored at −80 °C until analyzed. The endpoints that will be studied in skin include epigenetic changes including genome wide DNA methylation and gene expression alterations, as described [11].
2.6.6. Computed tomography (CT)
The cross sectional area and radiological density of the thigh muscles are assessed by CT scans performed bilaterally at the midpoint of femur of each subject. To minimize the effect of fluid shifts on the cross sectional area, subjects rest in the supine position for 30 min before the scan. Preliminary scout images are obtained to ensure accurate positioning. All scans are obtained using a second-generation 64-slice dual-source CT system (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany) operating at 120 kV and a fixed flux of 100 mA. Areas of interest will be manually defined on 5 mm thick slices and measured using manual planimetry with associated imaging software (Image J, National Institutes of Health, Bethesda, MD). Cross sectional area and radiological density of the quadriceps muscle are measured at baseline and after 11 months of cross sex hormone treatment.
2.6.7. MRI
Body composition is determined by MR imaging of the whole body at baseline and after 11 months of cross sex hormone treatment. Each subject undergo whole body MR imaging in the supine position (arms by their sides) with a modified 2 point Dixon fat and water sequence on a 3 T MR platform (Siemens Prisma, Siemens Healthcare, Erlangen, Germany) with the following settings: slice thickness 4.5 mm, repetition time 6.69, echo time 2.39, number of averages 1, resolution 0.448 pixels per mm. The total acquisition time is < 10 min. After image processing, automated image analysis will be performed using segmentation software provided by AMRA™ (Advanced MR Analytics AB, Linköping, Sweden). This analysis will allow for fully automated quantification of whole body and compartmental muscle, liver and fat volumes.
2.6.8. Isokinetic and isometric peak torque
Isokinetic and isometric peak torque are determined for the knee flexors and extensors using isokinetic dynamometry (Biodex System 4 Pro, Medical Systems, Shirley, NY) at all four time points. The chest, hip and thigh are stabilized using straps, and the ankle is strapped to the lever arm, which is aligned with the axis of rotation of the knee joint. Maximal isometric (0°/s) and isokinetic strength (60°/s and 90°/s) are measured for both the right and left leg in newton meters with a sampling frequency of 2000 Hz. The subject performs 4 all-out repetitions for each leg, alternating between knee flexion and extension, at each angular velocity. A 30 s rest period is employed between the trials. For the isometric test (performed at the fixed knee angle of 120°), the subjects are instructed to apply as much force as possible for 5 s. Three trials are administered for each leg interspersed with 30 s recovery.
2.6.9. Arterial stiffness
Arterial stiffness is measured by pulse wave velocity and aortic augmentation index using the Arteriograph system (Tensiomed Kft, Budapest, Hungary), a non-invasive oscillometric method using an occlusion technique. The pulse wave velocity is measured in the brachial artery by calculating the return time of the first and second systolic pressure waves and divides it with the aortic length. The wave reflection, measured as augmentation index, corresponds to the pressure difference between the first and second wave. Measurements are made with a brachial cuff in supine position after 15 min rest. The recommended procedures for standardization of arterial stiffness measurements are followed, as described [12].
2.6.10. Transthoracic echocardiography and ECG
A 12-lead ECG is recorded before the echocardiographic investigation. The echocardiographic examinations are performed on a high-end ultrasound scanner (Vivid E9, GE Healthcare, Horten, Norway) with the subjects in a lateral supine position. Two different transducers are used: the M5S transducer for the two-dimensional images and Doppler, and the 4V-D transducer for the 3-dimensional (3D) volumes. Data are stored on a local server and analyzed off-line on a dedicated workstation (EchoPac, GE Healthcare, Horten, Norway) by one experienced investigator.
The echocardiographic examinations are made in agreement with the ASE/EACVI guidelines [13]. Parasternal and apical four- and two-chamber views are recorded and left ventricular volume, stroke volume and ejection fraction are calculated using the modified biplane Simpson formula. Also, the left ventricular and left atrial three-dimensional full volumes are recorded. Left ventricular end-diastolic and end-systolic volumes, stroke volume and ejection fraction are calculated as well as the end systolic atrial volume after delineation of the cavities in accordance to the dedicated software. Deformation analysis is performed by peak left ventricular strain, averaging 18 segments in the three standard apical views.
Right ventricular end-diastolic dimension is measured in a slightly modified four-chamber view at the base of the right ventricle and fractional area change is calculated after delineation of the cavity in end-diastole and end-systole. Systolic displacement of the right ventricular tricuspid annulus (TAPSE) is measured using the M-mode placed in the lateral annulus. The right atrial area is measured in the apical view.
A pulse-wave Doppler with a sample volume of 5 mm is placed at the tip of the mitral leaflets in apical four-chamber view and the early (E) and late (A) diastolic blood flow velocities as well as the deceleration time (DT) and intraventricular relaxation time (IVRT) are registered and measured, and the E/A-ratio calculated. Tissue Doppler early diastolic velocity is recorded in the base of the left ventricular septum and lateral wall in apical four-chamber view, and a mean of the two velocities is calculated (e'). In accordance to Nagueh et al., the E/e'-ratio is calculated as part of the evaluation of the left diastolic function [14].
Body surface area (BSA) is calculated using the Dubois & Dubois formula and dimensions and volumes are indexed when needed [15].
2.6.11. Coronary flow velocity reserve
Adenosine is a potent vasodilatator producing maximal coronary vasodilatation within 40–50 s. The plasma half-life is less than 10 s and side-effects (mainly hyperpnea and flush) and hyperaemia thus sub-side quickly after termination of infusion. Adenosine is administered intravenously (0.140 mg/kg/min) for up to 5 min. Blood pressure is determined at rest and at 1–2 min intervals during the infusion. Preset criteria for reducing or stopping the infusion are acute bronchospasm, advanced AV block, decrease in systolic blood pressure >20 mmHg or patient refusal. Patients are instructed to refrain from caffeine use for 24 h before the investigation.
The mid to distal part of the left anterior descending coronary artery is identified using color Doppler as a guide, and the flow velocity is measured by pulsed wave Doppler, baseline velocity at rest and subsequently during the adenosine infusion. Gate size is set at 4–5 mm. Angle correction is performed if the angle between the color Doppler flow and the Doppler beam exceeded 20° and is maintained during both rest and stress studies. The spectral trace of the coronary velocity flow is characteristically biphasic with a dominating diastolic component. Stop frames and clips are digitally recorded for off-line analysis.
2.6.12. Carotid artery ultrasound
The right and left carotid arteries are examined with a duplex scanner (Siemens Helix S3000, Munchen Germany) using a −13 MHz linear array transducer, as described by Ajeganova et al. [16]. The carotid intima-media thickness (cIMT) is measured at the far wall of the common carotid artery (CCA), 0.5–1.0 cm proximal to the beginning of the carotid bulb. The cIMT are defined as the distance between the leading edge of the lumen-intima echo and the leading edge of the media-adventitia echo. All examinations are digitally stored for subsequent analyses and will be done with an automated tracing of echo interfaces and measurements of distances between the wall echoes within a 10-mm-long section of CCA in late diastole, defined by a simultaneous electrocardiographic recording, as described [17]. The mean values of the cIMT within the 10-mm-long section will be calculated of the CCA.
2.6.13. Insulin sensitivity
Insulin sensitivity is assessed with Homeostatic model assessment of insulin resistance (HOMA-IR) using fasting glucose and insulin in the formula HOMA-IR = glucose (mmol/l) x insulin (mU/l)/22.5, as described by Matthews et al. [18].
2.6.14. Blinding
The staff doing quantitative measurements of fat cell size and skeletal muscle fiber size is blinded to group allocation. The staff performing quantitative measurements on MRI or CT is also blinded to allocation.
2.7. Statistical analysis
The results from the examinations of transgender men and transgender women will be analyzed separately using paired t-test, linear and multiple regression analysis or repeated ANCOVA. We will do a complete case analysis that excludes subjects without a complete data set. We assume that this missing data are not related to any observed variables and therefore the complete case analysis can be applied. We expect 15% missing data based on previous investigations and this has been taken into account in the study design. Each variable will be analyzed separately and not as composite variables.
The statistical approach to studies involving the primary outcome transcriptomic or epigenomic methodology will generally be parametric statistics in combination with empirically estimated confidence intervals. Multiple hypothesis tests will be compensated for utilizing false discovery rate where an adjusted p-value of 0.05 will be considered significant. Measures to reduce the number of false negatives include Bayesian-moderated t-statistics (Linear Models for Microarray Data (LIMMA)) or boot-strapped empirical distributions. For transcriptomic data, generally an effect size of ≥2SD is considered biologically relevant. Based on previous data on inter- and intra-individual variance in skeletal muscle gene expression the number of observations needed to detect an effect size of 2SD with a power of 80% and a type I error cut-off of 5% after compensation for multiple hypothesis testing is 12 for paired longitudinal data and 28 for case-control studies. In addition to the aforementioned conservative univariate analytical approach, explorative multivariate approaches aiming to identify clusters of genes with a similar pattern of expression (or methylation) in relation to phenotypical characteristics such as changes in muscle mass or isokinetic force production. The methods used for such explorative and data-driven analysis involves Principal Component Analysis (PCA), Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) and T-Distributed Stochastic Neighbor Embedding (t-SNE). As these strategies are data-driven and for the most part based on empirical distribution of data, a formal power-analysis is not applicable. Based on work by us and others we estimate that a study size of 16–18 independent observations is sufficient to retrieve biologically relevant information from the data. To address issues with over-fitting we will utilize the fact that we have access to several independent datasets (albeit not involving hormone treatment) with muscle transcriptomic data in relation to relevant clinical characteristics in all the proposed study arms of the project, which can be used for validation and thus minimize the risk for over-fitted models and false discoveries.
3. Discussion
Treatment with gender-affirming hormones was described already in the 1950's by Hamburger and colleagues [19], and since then several studies have been performed on individuals undergoing cross-sex hormone treatment [20,21]. However, this is the first study focusing on individuals undergoing cross-sex hormone treatment with extensive mapping of the effects on transcriptomic and epigenomic changes in skeletal muscle, adipose and skin, and also cardiovascular function which together presents a unique opportunity to discern the hormonal effects from constitutional genome-driven programming.
A few studies have been performed to study changes in body composition and body fat distribution in transgender individuals, both feminizing treatment in transgender women (former called male-to-female) [22] and masculinizing treatment in transgender men (former called female-to-male) [[23], [24], [25], [26]] using dual X-ray absorptiometry (DEXA) [[22], [23], [24]] or abdominal and thigh MRI [25,26]. Some studies have been cross-sectional [23,24], comparing transgender individuals with cisgender females and or males (not transgender) controls [23,24]. In general, cross-sex hormone treated transgender women have a decrease in lean body mass and an increased fat mass while cross-sex hormone treated transgender men had an increased lean body mass and a decrease in fat mass, as reviewed [27]. One previous prospective study has shown an increase in muscle cross-sectional area of the forearm in transgender men after one year of testosterone undecanoate therapy [28]. In contrast to previous studies, the present study includes a whole body MRI, which enables the analysis of fat mass and lean body mass in several different specific compartments. We will also be able to measure changes in cross sectional area of the muscles in the thigh. In addition, we have the opportunity to evaluate the presence of ectopic fat distribution in different tissues. Observational studies have shown that strength is reduced in men with low testosterone levels [29], and an increase in handgrip strength in transgender men after one year of testosterone therapy [28]. The present study will investigate the mechanisms behind these changes in skeletal muscle and is, to our knowledge, the first to evaluate changes in strength in transgender women. Furthermore, this study will investigate how an altered sex-hormone profile affects the transcriptome, epigenome and metabolism in multiple tissues. The epigenetic analyses will include longitudinal genome wide interrogation of DNA methylation in the various phases of the gender-affirming treatments in the collected tissues. This will be of particular interest from a mechanistic point of view since they may highlight specific effects on the genome that is not immediately visible by gene expression and phenotype changes, but may poise for future expression alterations. One conceptual example might be breast malignancy where gene expression profiles were shown to be associated with estrogen treatment in transgender women [30]. Similarly, the hypothesis that autoimmune disease is directly or indirectly related to estrogen levels might put transgender women at higher risk, as was suggested in a case of systemic lupus erythematosus (SLE) [31]. Interestingly, immune responsiveness was actually shown to correlate with sex steroid treatment in transgender men and women [32].
Mortality rates after cross-sex hormone treatment have been evaluated at an epidemiological level in a large study by Asscheman et al., [33] with no increase in mortality rates for transgender men treated with testosterone while there was an increased mortality in transgender women (due to suicide, acquired immunodeficiency syndrome, cardiovascular disease, drug abuse, or unknown cause). The increased cardiovascular disease was associated with etinyl oestradiol and this type of treatment is nowadays less common than before. A nation-wide population cohort study found a three times elevated mortality rate for transgender men and women combined after cross-sex hormone treatment compared with cisgender controls of both sexes. Causes of death were suicide and cardiovascular disease [34]. There is a limited number of studies regarding the effects of cross-sex hormone treatment and the results are inconclusive on the direct effects of cross-sex hormone treatment on risk factors for cardiovascular disease [35]. Higher carotid arterial stiffness in testosterone treated transgender men has previously been shown [36]. Estrogen improve flow-mediated vasodilation [37,38], while androgens seem to impair vascular reactivity in transgender persons [39]. No studies measuring pulse wave velocity and coronary artery endothelial function in transgender subjects have been performed.
Finally, the GETS study will also be the first to investigate skin alterations caused by cross sex hormone treatment with focus on genomic, epigenetic and histology analyses. The only previous studies in a transgender population on skin effects are non-invasive and focused on the hormonal effects on acne and body hair [40,41].
To conclude, the long term effects of cross-sex hormone treatment is not fully understood and have not been studied at all in some areas such as adipose and skin. Thus, the GETS study will provide novel and deeper insight into the effects of cross-sex hormone treatment on skeletal muscle, adipose, skin, heart, immune system and endothelial function. This is important in order to improve gender-affirming treatment and future care and will further define the role of sex-hormone treatment and its relation to development of metabolic complications and cardiovascular disease.
Funding
This work was supported by the Stockholm County Council grant numbers 20160337 and K0138-2015, the Thuring foundation and the 1.6 Million Club.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.04.005.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Federman D.D. The biology of human sex differences. N. Engl. J. Med. 2006;354(14):1507–1514. doi: 10.1056/NEJMra052529. [DOI] [PubMed] [Google Scholar]
- 2.Vitale C., Fini M., Speziale G., Chierchia S. Gender differences in the cardiovascular effects of sex hormones. Fund. Clin. Pharmacol. 2010;24(6):675–685. doi: 10.1111/j.1472-8206.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- 3.Lundsgaard A.M., Kiens B. Gender differences in skeletal muscle substrate metabolism - molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014;5:195. doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattsson C., Olsson T. Estrogens and glucocorticoid hormones in adipose tissue metabolism. Curr. Med. Chem. 2007;14(27):2918–2924. doi: 10.2174/092986707782359972. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand. J. Clin. Lab. Invest. 1975;35(7):609–616. [PubMed] [Google Scholar]
- 6.Pidsley R., Zotenko E., Peters T.J., Lawrence M.G., Risbridger G.P., Molloy P., Van Djik S., Muhlhausler B., Stirzaker C., Clark S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016;17(1):208. doi: 10.1186/s13059-016-1066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson D.P., Lofgren P., Thorell A., Arner P., Hoffstedt J. Visceral fat cell lipolysis and cardiovascular risk factors in obesity. Horm. Metab. Res. 2011;43(11):809–815. doi: 10.1055/s-0031-1287767. [DOI] [PubMed] [Google Scholar]
- 8.Ryden M., Andersson D.P., Bergstrom I.B., Arner P. Adipose tissue and metabolic alterations: regional differences in fat cell size and number matter, but differently: a cross-sectional study. J. Clin. Endocrinol. Metab. 2014;99(10):E1870–E1876. doi: 10.1210/jc.2014-1526. [DOI] [PubMed] [Google Scholar]
- 9.Ryden M., Arner P. Cardiovascular risk score is linked to subcutaneous adipocyte size and lipid metabolism. J. Intern. Med. 2017;282(3):220–228. doi: 10.1111/joim.12641. [DOI] [PubMed] [Google Scholar]
- 10.Dahlman I., Sinha I., Gao H., Brodin D., Thorell A., Ryden M., Andersson D.P., Henriksson J., Perfilyev A., Ling C., Dahlman-Wright K., Arner P. The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes. Int. J. Obes. 2015;39(6):910–919. doi: 10.1038/ijo.2015.31. [DOI] [PubMed] [Google Scholar]
- 11.Tervaniemi M.H., Katayama S., Skoog T., Siitonen H.A., Vuola J., Nuutila K., Sormunen R., Johnsson A., Linnarsson S., Suomela S., Kankuri E., Kere J., Elomaa O. NOD-like receptor signaling and inflammasome-related pathways are highlighted in psoriatic epidermis. Sci. Rep. 2016;6:22745. doi: 10.1038/srep22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson L.A. Methods for assessing arterial stiffness: technical considerations. Curr. Opin. Nephrol. Hypertens. 2012;21(6):655–660. doi: 10.1097/MNH.0b013e32835856e3. [DOI] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. : Official Publ. J. Am. Soc. Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. e14. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., 3rd, Dokainish H., Edvardsen T., Flachskampf F.A., Gillebert T.C., Klein A.L., Lancellotti P., Marino P., Oh J.K., Popescu B.A., Waggoner A.D. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. : Official Publ. J. Am. Soc. Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Du Bois D., Du Bois E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. discussion 312–313. [PubMed] [Google Scholar]
- 16.Ajeganova S., Gustafsson T., Jogestrand T., Frostegard J., Hafstrom I. Bone mineral density and carotid atherosclerosis in systemic lupus erythematosus: a controlled cross-sectional study. Arthritis Res. Ther. 2015;17:84. doi: 10.1186/s13075-015-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendelhag I., Liang Q., Gustavsson T., Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28(11):2195–2200. doi: 10.1161/01.str.28.11.2195. [DOI] [PubMed] [Google Scholar]
- 18.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Hamburger C., Sturup G.K., Dahl-Iversen E. Transvestism; hormonal, psychiatric, and surgical treatment. J. Am. Med. Assoc. 1953;152(5):391–396. doi: 10.1001/jama.1953.03690050015006. [DOI] [PubMed] [Google Scholar]
- 20.Tangpricha V., den Heijer M. Oestrogen and anti-androgen therapy for transgender women, the lancet. Diabetes Endocrinol. 2017;5(4):291–300. doi: 10.1016/S2213-8587(16)30319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisner S.L., Deutsch M.B., Bhasin S., Bockting W., Brown G.R., Feldman J., Garofalo R., Kreukels B., Radix A., Safer J.D., Tangpricha V., T'Sjoen G., Goodman M. Advancing methods for US transgender health research. Curr. Opin. Endocrinol. Diabetes Obes. 2016;23(2):198–207. doi: 10.1097/MED.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller A., Zollver H., Kronawitter D., Oppelt P.G., Claassen T., Hoffmann I., Beckmann M.W., Dittrich R. Body composition and bone mineral density in male-to-female transsexuals during cross-sex hormone therapy using gonadotrophin-releasing hormone agonist. Exp. Clin. Endocrinol. Diabetes. 2011;119(2):95–100. doi: 10.1055/s-0030-1255074. [DOI] [PubMed] [Google Scholar]
- 23.Pelusi C., Costantino A., Martelli V., Lambertini M., Bazzocchi A., Ponti F., Battista G., Venturoli S., Meriggiola M.C. Effects of three different testosterone formulations in female-to-male transsexual persons. J. Sex. Med. 2014;11(12):3002–3011. doi: 10.1111/jsm.12698. [DOI] [PubMed] [Google Scholar]
- 24.Van Caenegem E., Wierckx K., Taes Y., Dedecker D., Van de Peer F., Toye K., Kaufman J.M., T'Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J. Clin. Endocrinol. Metab. 2012;97(7):2503–2511. doi: 10.1210/jc.2012-1187. [DOI] [PubMed] [Google Scholar]
- 25.Giltay E.J., Elbers J.M., Gooren L.J., Emeis J.J., Kooistra T., Asscheman H., Stehouwer C.D. Visceral fat accumulation is an important determinant of PAI-1 levels in young, nonobese men and women: modulation by cross-sex hormone administration. Arterioscler. Thromb. Vasc. Biol. 1998;18(11):1716–1722. doi: 10.1161/01.atv.18.11.1716. [DOI] [PubMed] [Google Scholar]
- 26.Elbers J.M., Asscheman H., Seidell J.C., Gooren L.J. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am. J. Physiol. 1999;276(2 Pt 1):E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- 27.Klaver M., Dekker M., de Mutsert R., Twisk J.W.R., den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017;49(5) doi: 10.1111/and.12660. [DOI] [PubMed] [Google Scholar]
- 28.Van Caenegem E., Wierckx K., Tae Y., Schreiner T., Vandewalle S., Toye K., Lapauw B., Kaufman J.M., T'Sjoen G. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI) Eur. J. Endocrinol. 2015;172(2):163–171. doi: 10.1530/EJE-14-0586. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein J.S., Lee H., Burnett-Bowie S.A., Pallais J.C., Yu E.W., Borges L.F., Jones B.F., Barry C.V., Wulczyn K.E., Thomas B.J., Leder B.Z. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013;369(11):1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentz E.K., Pils D., Bilban M., Kaufmann U., Hefler L.A., Reinthaller A., Singer C.F., Huber J.C., Horvat R., Tempfer C.B. Gene expression signatures of breast tissue before and after cross-sex hormone therapy in female-to-male transsexuals. Fertil. Steril. 2010;94(7):2688–2696. doi: 10.1016/j.fertnstert.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Chan K.L., Mok C.C. Development of systemic lupus erythematosus in a male-to-female transsexual: the role of sex hormones revisited. Lupus. 2013;22(13):1399–1402. doi: 10.1177/0961203313500550. [DOI] [PubMed] [Google Scholar]
- 32.Giltay E.J., Fonk J.C., von Blomberg B.M., Drexhage H.A., Schalkwijk C., Gooren L.J. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J. Clin. Endocrinol. Metab. 2000;85(4):1648–1657. doi: 10.1210/jcem.85.4.6562. [DOI] [PubMed] [Google Scholar]
- 33.Asscheman H., Giltay E.J., Megens J.A., de Ronde W.P., van Trotsenburg M.A., Gooren L.J. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur. J. Endocrinol. 2011;164(4):635–642. doi: 10.1530/EJE-10-1038. [DOI] [PubMed] [Google Scholar]
- 34.Dhejne C., Lichtenstein P., Boman M., Johansson A.L., Langstrom N., Landen M. Long-term follow-up of transsexual persons undergoing sex reassignment surgery: cohort study in Sweden. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016885. e16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streed C.G., Jr., Harfouch O., Marvel F., Blumenthal R.S., Martin S.S., Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann. Intern. Med. 2017;167(4):256–267. doi: 10.7326/M17-0577. [DOI] [PubMed] [Google Scholar]
- 36.Vermeersch S.J., Rietzschel E.R., De Buyzere M.L., De Bacquer D., De Backer G., Van Bortel L.M., Gillebert T.C., Verdonck P.R., Segers P. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J. Hypertens. 2008;26(7):1411–1419. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 37.New G., Timmins K.L., Duffy S.J., Tran B.T., O'Brien R.C., Harper R.W., Meredith I.T. Long-term estrogen therapy improves vascular function in male to female transsexuals. J. Am. Coll. Cardiol. 1997;29(7):1437–1444. doi: 10.1016/s0735-1097(97)00080-6. [DOI] [PubMed] [Google Scholar]
- 38.McCrohon J.A., Walters W.A., Robinson J.T., McCredie R.J., Turner L., Adams M.R., Handelsman D.J., Celermajer D.S. Arterial reactivity is enhanced in genetic males taking high dose estrogens. J. Am. Coll. Cardiol. 1997;29(7):1432–1436. doi: 10.1016/s0735-1097(97)00063-6. [DOI] [PubMed] [Google Scholar]
- 39.McCredie R.J., McCrohon J.A., Turner L., Griffiths K.A., Handelsman D.J., Celermajer D.S. Vascular reactivity is impaired in genetic females taking high-dose androgens. J. Am. Coll. Cardiol. 1998;32(5):1331–1335. doi: 10.1016/s0735-1097(98)00416-1. [DOI] [PubMed] [Google Scholar]
- 40.Wierckx K., Van de Peer F., Verhaeghe E., Dedecker D., Van Caenegem E., Toye K., Kaufman J.M., T'Sjoen G. Short- and long-term clinical skin effects of testosterone treatment in trans men. J. Sex. Med. 2014;11(1):222–229. doi: 10.1111/jsm.12366. [DOI] [PubMed] [Google Scholar]
- 41.Giltay E.J., Gooren L.J. Effects of sex steroid deprivation/administration on hair growth and skin sebum production in transsexual males and females. J. Clin. Endocrinol. Metab. 2000;85(8):2913–2921. doi: 10.1210/jcem.85.8.6710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.