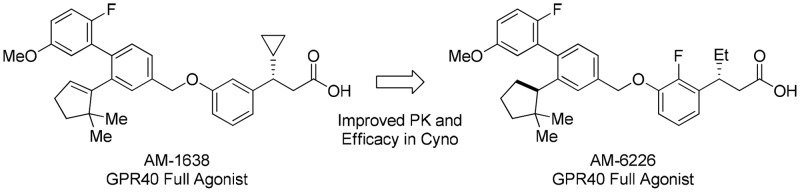
Abstract
GPR40 (FFA1) is a G-protein-coupled receptor, primarily expressed in pancreatic islets and enteroendocrine L-cells, and, when activated, elicits increased insulin secretion only in the presence of elevated glucose levels. We recently reported the discovery of AM-1638 (2), a full agonist of GPR40. Herein, we present further structure–activity relationships progressing from AM-1638 (2) to AM-6226 (14) that possesses a profile acceptable for dosing cynomolgus monkeys. The GPR40 full agonist AM-6226 (14) is the first molecule to display significant glucose lowering in cynomolgus monkeys providing additional evidence that GPR40 full agonists afford access to a powerful mechanism for maintaining glycemic control.
Keywords: GPR40, full agonist, AM-6226, AM-1638, AMG 837, insulin secretagogue, FFA1
Type II diabetics lose their ability to maintain glucose homeostasis due to multiple metabolic defects.1 GPR40 is a G-protein-coupled receptor, primarily expressed in pancreatic islets β-cells and enteroendocrine L-cells2 that possess the ability to modulate several metabolic defects when activated. Medium- to long-chain fatty acids bind to and elicit GPR40 to increase insulin secretion from β-cells and increased secretion of the gut hormones, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP).3−5
Agonists of GPR40 present low risk of hypoglycemia and have been considered by multiple groups leading to the discovery of multiple clinical candidates.4−9 These clinical candidates, such as AMG 837 (1), are all partial agonists of GPR40. We previously described the discovery of 1,10−12 which demonstrates antidiabetic activity in rodent models without exhibiting hypoglycemia. Favorable pharmacokinetic properties and efficacy of 1 led to its selection for clinical evaluation. We became interested in evaluating GPR40 full agonists in a nonhuman primate model of diabetes since partial agonist 1’s ability to maintain glycemic control was being tested in a clinical setting.
We recently described a series of GPR40 full agonists13−17 that were identified from compounds synthesized in the discovery of 1 by evaluating them in CHO cells transfected with lower levels of GPR40 expression plasmid (0.05 μg). This assay provides greater dynamic range with which to assess improvements in intrinsic efficacy (Figure 1). Optimization of the full agonist series afforded AM-1638 (2) that displays greater antidiabetic efficacy in several rodent diabetic models compared to partial agonist 1,13 providing compelling evidence that GPR40 full agonists engage a powerful mechanism for maintaining glycemic control.
Figure 1.
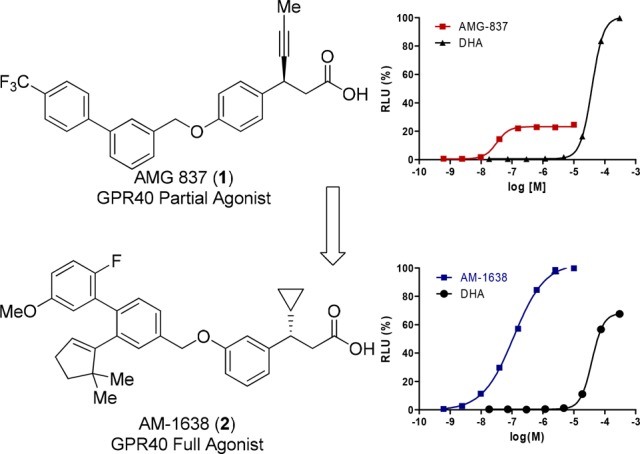
Effect of AMG 837 (1), AM-1638 (2), and docosahexaenoic acid (DHA) in CHO cells transfected with 0.05 μg of GPR40 expression plasmid.
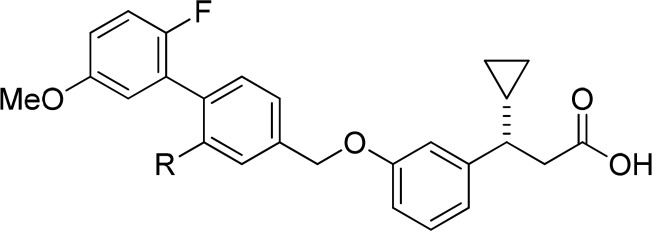
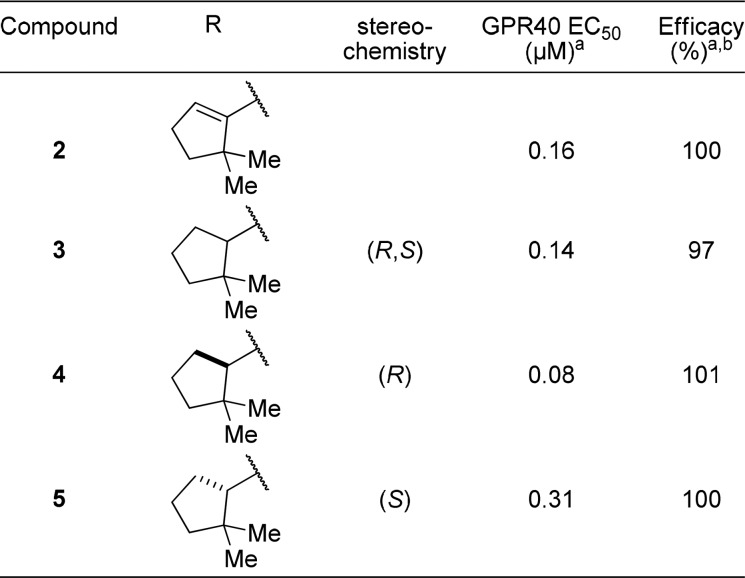
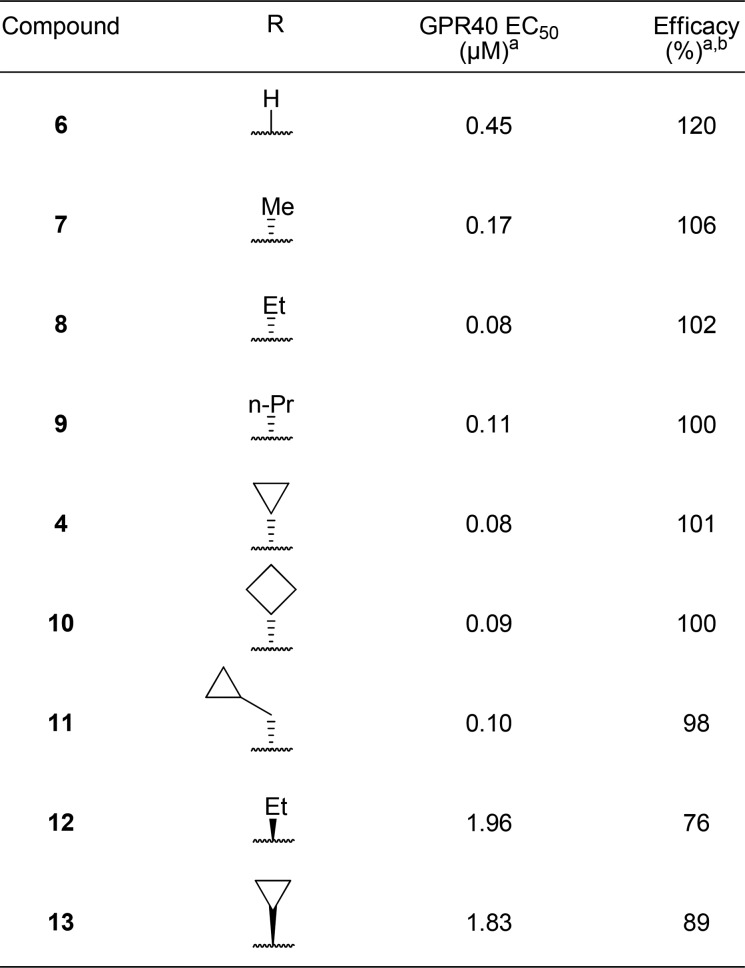
In an effort to improve the potency and pharmacokinetic properties of full agonist 2, we initiated further optimization of the full agonist series of compounds. Saturation of the cyclopentene ring led to an improvement in potency in the (R)-enantiomeric series (Table 1, 4, EC50 = 0.08 μM), while loss in potency was observed in the (S)-enantiomeric series (Table 1, 5, EC50 = 0.31 μM). While maintaining the 2,2-dimethyl-(R)-cyclopentane moiety, we next turned our attention to the β-substituent from the carboxylic acid (Table 2). Previous optimization had revealed that small alkyl substituents were preferred at this position. Reducing the size of the β-substituent to a hydrogen (6, EC50 = 0.45 μM) or methyl (7, EC50 = 0.17 μM) led to a loss in potency. β-Substituents with similar size to that of the cyclopropane of full agonist 4 displayed similar potency (8–11, EC50 = 0.08–0.11 μM). The two most potent substituents, the ethyl and cyclopropane, were selected for further optimization, while evaluation of their entipodes displayed a significant decrease in potency and efficacy (12, 13; EC50 = 1.96, 1.83 μM; 76%, 89%).
Table 1. Effect of Biphenyl Modification on GPR40 Activity in the Presence 100% Human Serum.
Mean of at least two runs.
% Compared to reference agonist 2.
Table 2. Exploration of β-Substituent SAR.
Mean of at least two runs.
% Compared to reference agonist 2.
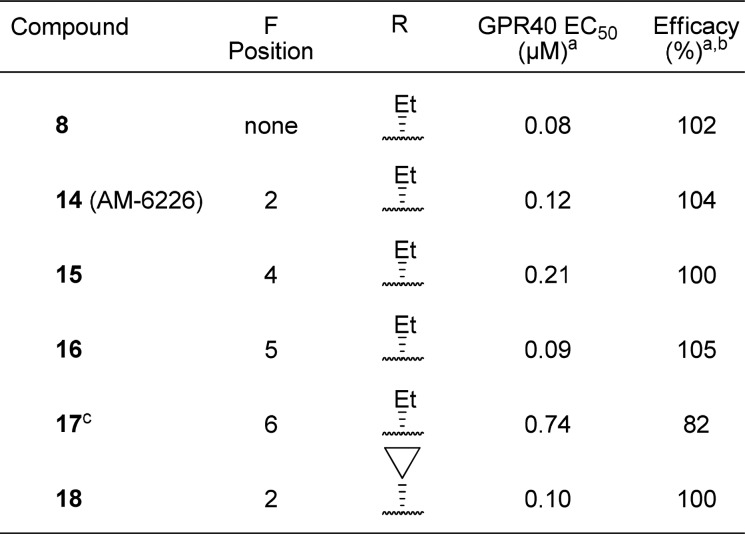
To decrease the electron density of the oxy-aryl ring, we evaluated fluorination of the four possible positions. Installment of a fluorine at the 2- and 5-positions provided full agonists with a minimal loss in potency (Table 3; 14, 0.12 μM; 16, 0.09 μM), while fluorination at the 4- and 6-positions led to more significant losses in potency (Table 3; 15, 0.21 μM; 17, 0.74 μM). Because they maintained potency, the pharmacokinetic properties of the fluorinated full agonist 14 and 16 were evaluated (Table 4). Full agonist 14 displayed low iv clearance and high oral bioavailability. Because of this favorable profile the β-ethyl substituent was exchanged for a cyclopropane that displayed a similar in vitro (Table 3, 18, EC50 = 0.10 μM) and pharmacokinetic profile (Table 4, 18), albeit a lower oral bioavailability.
Table 3. Evaluation of Aryl-fluorine Substitutions.
Mean of at least two runs.
% Compared to reference agonist 2.
Compound 17 contains a 5,5-dimethyl-1-cyclopenten-1-yl moiety instead of the (1R)-2,2-dimethylcyclopentyl moiety.
Table 4. Rat Pharmacokinetic Properties of Full Agonistsa.
| Compound | Fu | oral Cmax (μM) | oral AUC (μM·h) | Cl (L/h/kg) | Vdss (L/kg) | iv t1/2 (h) | % F |
|---|---|---|---|---|---|---|---|
| 8 | 0.006 | 0.37 | 1.74 | 0.67 | 1.12 | 2.1 | 29 |
| 4 | 0.006 | 0.25 | 1.83 | 0.72 | 1.68 | 2.0 | 34 |
| 14 | 0.003 | 1.18 | 8.22 | 0.33 | 0.81 | 2.0 | 72 |
| 18 | 0.003 | 0.36 | 2.43 | 0.49 | 1.9 | 3.3 | 32 |
Administered at a dose of 0.5 mg/kg, iv; 2 mg/kg, po, in rats. Data are expressed as mean values (n = 3).
Due to the similar in vitro profiles of the fluorinated compounds 14 and 18, 14 was selected for further pharmacokinetic evaluation in higher species because of the greater exposure achieved in rats (AUC = 8.22 μM·h). Full agonist 14 displayed both low clearance and good oral bioavailability in dog and cynomolgus monkeys (Table 5; Cl = 0.21, 0.22 L/h/kg; %F = 62, 91), respectively. This profile is a significant improvement over compound 2 with a >4-fold improvement in AUC, while the fraction unbound in plasma is similar between the two compounds (Table 5). We deemed the profile acceptable to evaluate the antidiabetic activity of full agonist 14, which we have named AM-6226, in a nonhuman primate model of diabetes.
Table 5. Pharmacokinetic Properties of Full Agonist 14a.
| Compound | Species | Fu | oral dose (mg/kg) | oral AUC (μM·h) | Cl (L/h/kg) | Vdss (L/kg) | iv t1/2 (h) | % F |
|---|---|---|---|---|---|---|---|---|
| 2 | rat | 0.010 | 2 | 15.1 | 0.91 | 1.1 | 1.8 | 72 |
| 14 | rat | 0.003 | 2 | 8.22 | 0.33 | 0.81 | 2.0 | 72 |
| 14 | dog | 0.034 | 2 | 11.6 | 0.21 | 0.68 | 4.1 | 62 |
| 2 | cyno | 0.012 | 10 | 18.8 | 0.81 | 2.0 | 2.1 | 71 |
| 14 | cyno | 0.017 | 10 | 80.4 | 0.22 | 0.5 | 3.3 | 91 |
Administered at a dose of 0.5 mg/kg, iv. Data are expressed as mean values (n = 3).
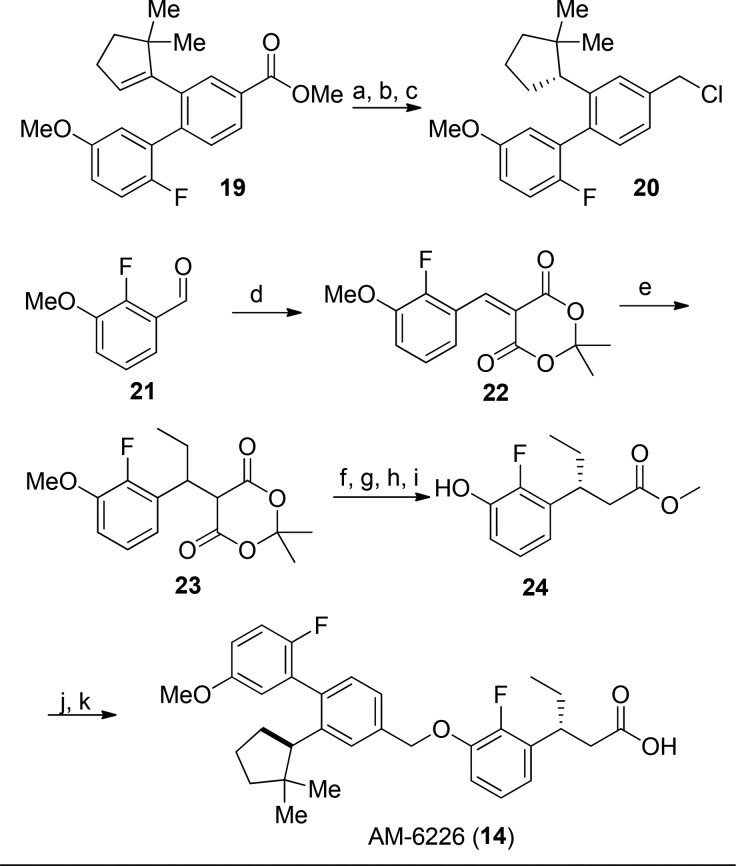
The synthesis of full agonist 14 begins with hydrogenation of the trisubstituted olefin 19 (Scheme 1), followed by ester reduction, which yielded racemic alcohols in excellent yield. Separation of the enantiomers and subsequent chlorination yielded the desired benzylic chloride 20. Condensation of aldehyde 21 with Meldrum’s acid yielded olefin 22, and subsequent conjugate addition of ethyl magenesium bromide yielded 23. Hydrolysis of the diester, removal of methyl ether using dodecylthiol, subsequent esterification, and chiral separation delivered 24 in good overall yield. Alkylation of 24 with 20, followed by hydrolysis yielded AM-6226.
Scheme 1.
Reagents and conditions: (a) Pd/C, H2O, MeOH, 90%; (b) LiAlH4, THF, 0 °C, 96%; chiral separation of enantiomers; analytical column (Chiracel-OD (2% IPA in hexane, 45 min run), Peak 1–15.5 min, Peak 2–38.0 min); (c) SOCl2, CH2Cl2, 87%; (d) C6H8O4, H2O, 90 °C, 59%; (e) EtMgBr, THF, 0 °C, 93%; (f) DMF/H2O (10/1), 91%; (g) C12H10S, NaOH, NMP, 125 °C, 88%; (h) H2SO4, MeOH, 80 °C, 90%; (i) chiral HPLC (Chiralcel OD column, 3% IPA/hexane, detection at 220 nm) to afford two separable peaks, 40% for each peak; (j) 20, H2O, Cs2CO3, DMF; (k) LiOH, EtOH, H2O, 95% over 2 steps.
Sitagliptin and 14 were orally dosed to prediabetic cynomolgus monkeys selected from a high-fat feed colony. The GPR40 full agonist 14 displays significant glucose lowering during an oral glucose tolerance test (OGTT) at 1 and 10 mg/kg (Figure 2). Moreover, full agonist 14 and the antidiabetic drug sitagliptin afforded similar reduction in plasma glucose AUC (18% vs 13%) when 10 mg/kg of either compound was administered orally.
Figure 2.
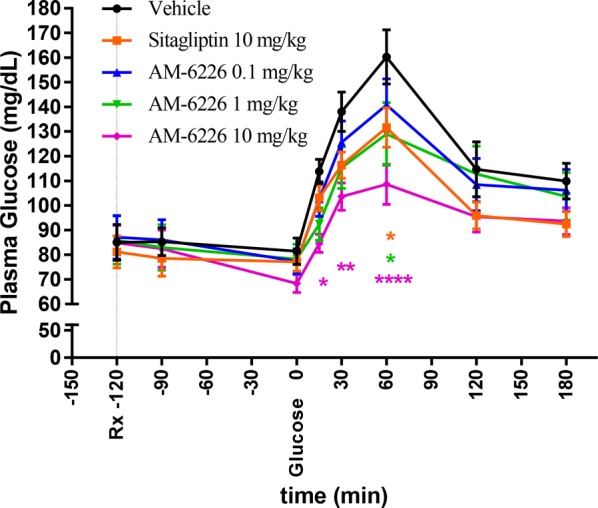
Effect of full agonist 14 (AM-6226) and Sitagliptin during an OGTT in cynomolgus monkeys. Time-dependent changes in plasma glucose after oral administration of either full agonist 14 or Sitagliptin followed by 4 g/kg oral glucose challenge. Values are means ± SE (n = 16). *p ≤ 0.05, ****p ≤ 0.001 as compared with control by two-way ANOVA with Dunnett’s test.
In conclusion, we have described the further optimization of GPR40 full agonists resulting in the identification of AM-6226 (14) that displays an improved pharmacokinetic profile and was suitable for evaluation in cynomolgus monkeys. The antidiabetic activity that full agonist 14 exhibits in cynomolgus monkeys along with the previously disclosed efficacy of GPR40 full agonists in rodent models of diabetes provides compelling evidence that GPR40 full agonists afford access to a mechanism for maintaining glycemic control and potential for the treatment of type II diabetic patients.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00213.
Synthesis and spectroscopic characterization of compound 14; 1H NMR and LRMS characterization of final compounds; cell-based and in vivo methods (PDF)
The authors declare the following competing financial interest(s): We own stock in Amgen Inc.
Dedication
In memory of Dr. XianYun Jiao.
Supplementary Material
References
- Defronzo R. A. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes 2009, 58, 773–795. 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y.; Kawamata Y.; Harada M.; Kobayashi M.; Fujii R.; Fukusumi S.; Ogi K.; Hosoya M.; Tanaka Y.; Uejima H.; Tanaka H.; Maruyama M.; Satoh R.; Okubo S.; Kizawa H.; Komatsu H.; Matsumura F.; Noguchi Y.; Shinohara T.; Hinuma S.; Fujisawa Y.; Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Stoddart L. A.; Smith N. J.; Milligan G. International union of pharmacology. LXXI. Free fatty acid receptors FFA1, −2, and −3: pharmacology and pathophysiological functions. Pharmacol. Rev. 2008, 60, 405–417. 10.1124/pr.108.00802. [DOI] [PubMed] [Google Scholar]
- For a review, see:Brown S. P.; Taygerly J. P. Small-Molecule Modulators of GPR40 (FFA1). Annu. Rep. Med. Chem. 2014, 49, 77–86. 10.1016/B978-0-12-800167-7.00006-7. [DOI] [Google Scholar]
- For a review, see:Bharate S. B.; Nemmani K. VS.; Vishwakarma R. A. Progress in the discovery and development of small-molecule modulators of G-protein-coupled receptor 40 (GPR40/FFA1/FFAR1): An emerging target for type 2 diabetes. Expert Opin. Ther. Pat. 2009, 19, 237–264. 10.1517/13543770802665717. [DOI] [PubMed] [Google Scholar]
- Negoro N.; Sasaki S.; Mikami S.; Ito M.; Suzuki M.; Tsujihata Y.; Ito R.; Harada A.; Takeuchi K.; Suzuki N.; Miyazaki J.; Santou T.; Odani T.; Kanzaki N.; Funami M.; Tanaka T.; Kogame A.; Matsunaga S.; Yasuma T.; Momose Y. Discovery of TAK-875: A potent, selective, and orally bioavailable GPR40 agonist. ACS Med. Chem. Lett. 2010, 1, 290–294. 10.1021/ml1000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen E.; Due-Hansen M. E.; Urban C.; Merten N.; Pfleiderer M.; Karlsen K. K.; Rasmussen S. S.; Steensgaard M.; Hamacher A.; Schmidt J.; Drewke C.; Petersen R. K.; Kristiansen K.; Ullrich S.; Kostenis E.; Kassack M. U.; Ulven T. Structure-activity study of dihydrocinnamic acids and discovery of the potent FFA1 (GPR40) agonist TUG-469. ACS Med. Chem. Lett. 2010, 1, 345–349. 10.1021/ml100106c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.; Yano J.; Hirozane Y.; Kefala G.; Gruswitz F.; Snell G.; Lane W.; Ivetac A.; Aertgeerts K.; Nguyen J.; Jennings A.; Okada K. High-resolution structure of the human GPR40 receptor bound to allosteric agonist TAK-875. Nature 2014, 513, 124–127. 10.1038/nature13494. [DOI] [PubMed] [Google Scholar]
- Hauge M.; Vestmar M. A.; Husted A. S.; Ekberg J. P.; Wright M. J.; Di Salvo J.; Weinglass A. B.; Engelstoft M. S.; Madsen A. N.; Luckmann M.; Miller M. W.; Trujillo M. E.; Frimurer T. M.; Holst B.; Howard A. D.; Schwartz T. W. GPR40 (FFAR1) - Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. 10.1016/j.molmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J. C. S.; Cui S.; Walker S. D.; Faul M. M. Asymmetric syntheses of a GPR40 receptor agonist via diastereoselective and enantioselective conjugate alkynylation. Tetrahedron 2010, 66, 4730–4737. 10.1016/j.tet.2010.04.019. [DOI] [Google Scholar]
- Luo J.; Zhang J.; Zhuang R.; Li F.; Nguyen K.; Chen M.; Tran T.; Lopez E.; Lin Y.-J.; Li N.; Swaminath G.; Reagan J.; Chen J.-L.; Houze J.; Lin D. AMG 837: A novel GPR40/FFA1 agonist that enhances insulin secretion and lowers glucose levels in rodents. PLoS One 2011, 6, e27270. 10.1371/journal.pone.0027270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houze J. B.; Zhu L.; Sun Y.; Akerman M.; Qiu W.; Zhang A.; Sharma R.; Schmitt M.; Wang Y.; Liu J.; Liu J.; Medina J. C.; Reagan J. D.; Luo J.; Tonn G.; Zhang J.; Lu J. Y.; Chen M.; Lopez E.; Nguyen K.; Yang L.; Tang L.; Tian H.; Shuttleworth S.; Lin D. AMG 837: A potent, orally bioavailable GPR40 agonist. Bioorg. Med. Chem. Lett. 2012, 22, 1267–1270. 10.1016/j.bmcl.2011.10.118. [DOI] [PubMed] [Google Scholar]
- Brown S. P.; Dransfield P. J.; Vimolratana M.; Jiao X.; Zhu L.; Pattaropong V.; Sun Y.; Liu J.; Luo J.; Zhang J.; Wong S.; Zhuang R.; Guo Q.; Li F.; Medina J. C.; Swaminath G.; Lin D. C.-H.; Houze J. B. Discovery of AM-1638: A potent and orally bioavailable GPR40/FFA1 full agonist. ACS Med. Chem. Lett. 2012, 3, 726–730. 10.1021/ml300133f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Liu J.; Dransfield P. J.; Zhu L.; Wang Z.; Du X.; Jiao X.; Su Y.; Li A.; Brown S. P.; Kasparian A.; Vimolratana M.; Yu M.; Pattaropong V.; Houze J. B.; Swaminath G.; Tran T.; Nguyen K.; Guo Q.; Zhang J.; Zhuang R.; Li F.; Miao L.; Bartberger M. D.; Correll T. L.; Chow D.; Wong S.; Luo J.; Lin D. C.-H.; Medina J. C. Discovery and optimization of potent GPR40 full agonists containing tricyclic spirocycles. ACS Med. Chem. Lett. 2013, 4, 551–555. 10.1021/ml300427u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.; Dransfield P. J.; Lin D. C. H.; Wong S.; Wang Y.; Wang Z.; Kohn T.; Yu M.; Brown S. P.; Vimolratana M.; Zhu L.; Li A.-R.; Su Y.; Jiao X.; Liu J.; Swaminath G.; Tran T.; Luo J.; Zhuang R.; Zhang J.; Guo Q.; Li F.; Connors R.; Medina J. C.; Houze J. B. Improving the pharmacokinetics of GPR40/FFA1 full agonists. ACS Med. Chem. Lett. 2014, 5, 384–389. 10.1021/ml4005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Swaminath G.; Brown S. P.; Zhang J.; Guo Q.; Chen M.; Nguyen K.; Tran T.; Miao L.; Dransfield P. J.; Vimolratana M.; Houze J. B.; Wong S.; Toteva M.; Shan B.; Li F.; Zhuang R.; Lin D. C.-H. A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS One 2012, 7, e46300. 10.1371/journal.pone.0046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C.-H.; Guo Q.; Luo J.; Zhang J.; Nguyen K.; Chen M.; Tran T.; Dransfield P. J.; Brown S. P.; Houze J.; Vimolratana M.; Jiao X. Y.; Wang Y.; Birdsall N. J. M.; Swaminath G. Identification and pharmacological characterization of multiple allosteric binding sites on the free fatty acid 1 receptor. Mol. Pharmacol. 2012, 82, 843–859. 10.1124/mol.112.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.