Although dengue virus (DENV) elicits cross-neutralizing antibodies to Zika virus (ZIKV) that may be maintained for months to years, ZIKV lies antigenically outside the DENV serocomplex. Furthermore, traditional neutralization testing can be useful in certain contexts to distinguish between infecting flaviviruses.
Keywords: Dengue virus, Zika virus, neutralizing antibodies, cross-reactivity, Latin America, Asia, flavivirus, longitudinal, Nicaragua
Abstract
Background
The 4 dengue virus serotypes (DENV1–4) and Zika virus (ZIKV) are related mosquito-borne flaviviruses of major importance globally. While monoclonal antibodies and plasma from DENV-immune donors can neutralize or enhance ZIKV in vitro and in small-animal models, and vice versa, the extent, duration, and significance of cross-reactivity in humans remains unknown, particularly in flavivirus-endemic regions.
Methods
We studied neutralizing antibodies to ZIKV and DENV1–4 in longitudinal serologic specimens collected through 3 years after infection from people in Latin America and Asia with laboratory-confirmed DENV infections. We also evaluated neutralizing antibodies to ZIKV and DENV1–4 in patients with Zika through 6 months after infection.
Results
In patients with Zika, the highest neutralizing antibody titers were to ZIKV, with low-level cross-reactivity to DENV1–4 that was greater in DENV-immune individuals. We found that, in primary and secondary DENV infections, neutralizing antibody titers to ZIKV were markedly lower than to the infecting DENV and heterologous DENV serotypes. Cross-neutralization was greatest in early convalescence, then ZIKV neutralization decreased, remaining at low levels over time.
Conclusions
Patterns of antibody cross-neutralization suggest that ZIKV lies outside the DENV serocomplex. Neutralizing antibody titers can distinguish ZIKV from DENV infections when all viruses are analyzed simultaneously. These findings have implications for understanding natural immunity and vaccines.
(See the Editorial commentary by Anderson et al, on pages 516–8).
The 4 dengue virus (DENV) serotypes (DENV1–4) and Zika virus (ZIKV) are mosquito-borne flaviviruses of global health importance. Dengue is the most prevalent arboviral disease of humans, with up to 390 million infections annually [1]. ZIKV expanded dramatically throughout Latin America in 2015–2016 [2]. Although the majority of infections are mild or asymptomatic, ZIKV infection during pregnancy is linked with devastating birth defects, including microcephaly, and with Guillain-Barré syndrome in adults [3, 4]. Because of shared Aedes mosquito vectors, ZIKV spreads in geographic areas where DENV is endemic [5]. Since ZIKV also circulates in Africa and Asia [6, 7], billions of people are at risk of sequential or concurrent DENV and ZIKV infections.
The envelope protein (E) is a major target of the human antibody response to flaviviruses. The ectodomain of E comprises 3 domains (EDI, EDII, and EDIII), with distinct roles in virus attachment, entry, and membrane fusion. DENV and ZIKV are closely related (approximately 55% E amino acid identity) [8–10], giving rise to a high degree of structural and antigenic similarity [8, 9]. Therefore, it is not surprising that extensive antibody cross-reactivity is observed.
In fact, serologic cross-reactivity among flaviviruses has long been appreciated and used to categorize flaviviruses into serocomplexes and subcomplexes [11, 12]. With DENV, cross-reactive antibodies elicited by the infecting serotype bind and neutralize heterologous DENV serotypes, corresponding to a transient period of cross-protection or disease attenuation following primary DENV infection. In the absence of subsequent DENV infections, the neutralizing antibody (nAb) response typically narrows in regions of nonendemicity, conferring long-term immunity only to the infecting DENV serotype [13, 14]. However, cross-reactive nAb responses appear to be maintained over time in dengue-endemic regions [15]. Serologic cross-reactivity between DENV and ZIKV has been clearly demonstrated [8, 10, 16, 17]. Some monoclonal antibodies (mAbs) derived from DENV-immune patients cross-neutralize ZIKV [18] and protect against lethal ZIKV challenge in a mouse model [19]. Human plasma collected ≤100 days after reverse-transcription polymerase chain reaction (RT-PCR)–confirmed DENV infection also binds and cross-neutralizes ZIKV [8]. However, late-convalescent-phase plasma from DENV-immune travelers does not harbor durable, high levels of cross-neutralizing antibodies against ZIKV [20]. Thus, substantial deficiencies exist in our understanding of cross-neutralizing antibody responses among individuals with prior DENV exposure, particularly how these responses evolve over time and in various transmission contexts in flavivirus-endemic countries. Here, we characterized the extent of anti-DENV and anti-ZIKV neutralization in individuals from Latin America and Asia over months to years following molecularly confirmed DENV and ZIKV infections.
METHODS
Ethics Statement
All studies were approved by the relevant institutional review boards at the participating institutions (Supplementary Methods).
Study Site and Sample Selection
Nicaragua
Samples were from 2 prospective studies of pediatric dengue and Zika in Managua, Nicaragua. In the hospital study (1998–present), study enrollment occurs in the Nicaraguan Hospital Infantil Manual de Jesús Rivera. Children 6 months to 14 years of age suspected of having dengue or Zika (<7 days of illness) are eligible [21]. All suspected dengue and Zika cases are confirmed by DENV serotype–specific and ZIKV RT-PCR and serologic methods [22, 23]. Samples from dengue cases for this study (n = 180) were collected at the acute (days 1–6) and convalescent (days 14–28) phases, as well as 3, 6, 12, and 18 months following primary and secondary symptomatic DENV infections (DENV1, DENV2, and DENV3; Table 1). Primary and secondary dengue cases were defined by an Inhibition enzyme-linked immunosorbent assay (ELISA) [24, 25] titer of <2560 or ≥2560, respectively, in the convalescent-phase sample [23]. Samples from the 14-year ongoing community-based Pediatric Dengue Cohort Study (2004–present) included annual healthy blood samples collected 1–3 years following secondary DENV1, DENV2, DENV3, and DENV4 infections (n = 42). All samples from dengue cases were collected prior to the introduction of ZIKV into Nicaragua in 2016. Children with RT-PCR–confirmed Zika from the hospital-based study [22] included those with (n = 7) and those without (n = 10) prior DENV immunity who had samples collected during the acute and early convalescent phases and 3 and 6 months after ZIKV infection (Table 1). All blood samples were collected in 5 mM ethylenediaminetetraacetic acid (EDTA), and plasma was separated by centrifugation, aliquoted, and stored at −80°C.
Table 1.
Samples Included in the Study
| Study Site, Infecting Virus | Participants, No. | Immune Status | Collection Time Points | Samples, No. |
|---|---|---|---|---|
| Nicaragua | ||||
| Hospital study | ||||
| DENV1 | 10 | 5 had primary infection, and 5 had secondary infection | Acute phase, convalescent phase, and 3, 6, 12, and 18 mo | 60 |
| DENV2 | 10 | 5 had primary infection, and 5 had secondary infection | Acute phase, convalescent phase, and 3, 6, 12, and 18 mo | 60 |
| DENV3 | 10 | 5 had primary infection, and 5 had secondary infection | Acute phase, convalescent phase, and 3, 6, 12, and 18 mo | 60 |
| ZIKV | ||||
| Group 1 | 10 | DENV naive | Acute phase, convalescent phase, and 3 and 6 mo | 40 |
| Group 2 | 7 | DENV immune | Acute phase, convalescent phase, and 3 and 6 mo | 28 |
| Cohort study | ||||
| Nonea | 5 | 5 | ||
| DENV1 | 4 | Secondary infection | 1, 2, and 3 y after infection | 12 |
| DENV2 | 4 | Secondary infection | 1, 2, and 3 y after infection | 12 |
| DENV3 | 3 | Secondary infection | 1, 2, and 3 y after infection | 9 |
| DENV4 | 3 | Secondary infection | 1, 2, and 3 y after infection | 9 |
| Sri Lanka | ||||
| DENV1 | 18 | 10 had primary infection, and 8 had secondary infection | Variable: up to 217 d after infection | 41 |
| Other DENV serotypes | 7 | 3 had primary infection, and 4 had secondary infection | Variable: up to 202 d after infection | 16 |
| Thailand | ||||
| DENV1 | 6 | Secondary infection | Acute phase and convalescent phase (2–4 wk and 6 mo) | 18 |
| DENV2 | 11 | Secondary infection | Acute phase and convalescent phase (2–4 wk and 2 and 6 mo) | 51 |
| DENV3 | 1 | Secondary infection | Acute phase and convalescent phase (2–4 wk and 6 mo) | 3 |
| DENV4 | 2 | Secondary infection | Acute phase and convalescent phase (2–4 wk and 6 mo) | 6 |
Abbreviations: DENV, dengue virus; ZIKV, Zika virus.
aSpecimens were negative for ZIKV and DENV.
Sri Lanka
In a cohort study, archived dried blood spots (DBS) specimens stored at 4°C on protein saver cards from the Sri Lankan Pediatric Dengue Vaccine Initiative cohort [26] were processed with validated methods as previously described [26, 27]. From November 2008 to January 2010, a surveillance study of approximately 800 children ≤12 years of age was conducted by obtaining baseline, 1-year, and 2-year follow-up DBS specimens, which were tested for DENV-specific IgG. Children experiencing a febrile illness (temperature ≥38°C for ≤7 days) had blood specimens collected at presentation and during the convalescent phase, ≥10 days after symptom resolution. Diagnostic RT-PCR analysis was performed on acute specimens [26]. The baseline DENV serostatus of each child was used to classify subsequent RT-PCR–confirmed infection as primary or secondary [26].
In a hospital study, individuals presenting with acute febrile illness suspected of dengue, based on the clinical expertise of local clinicians, were enrolled. Serum was collected at presentation, and convalescent-phase serum specimens were obtained ≥10 days later. In hospital cases, the presence of DENV-specific IgG in the acute-phase samples (collected 1–5 days after onset of symptoms) was used to define primary or secondary cases [27]. Only RT-PCR–confirmed cases were used in both these studies.
Thailand
Suspected dengue cases were identified on presentation to Khon Kaen Hospital. Patients with acute febrile illness clinically suspected to be dengue were enrolled. Only cases that showed dengue symptoms confirmed by DENV RT-PCR were included in this study. Samples collected during hospitalization were considered acute-phase samples. Convalescent-phase samples were collected at 2–4 weeks and again at 5–7 months. The infecting DENV serotype was determined by RT-PCR analysis [28]. Secondary DENV infection was classified as a ratio of anti-DENV immunoglobulin M (IgM) to IgG of <1.8, as measured by IgM and IgG capture ELISA [29]. During the acute phase, the day of defervescence was defined as day 0, the day before as day −1, the day after as day 1, and so on. Blood samples were collected in 5 mM EDTA, and plasma was separated by centrifugation, aliquoted, and stored at −80°C.
Cell Culture and Virus Production
Vero-81, C6/36, and U937 cells were cultured as described in the Supplementary Methods. At the University of California–Berkeley, all 4 DENV serotypes (DENV1 N1265-04, DENV2 N172-06, DENV3 N7236-98, and DENV4 N703-99) and ZIKV Nica 1–16 were isolated in Nicaragua and propagated (low passage: 2–3) in C6/36 cells [30]. All viral stocks were Mycoplasma free, and viral titers were determined by focus-forming assays on Vero cells. At the University of North Carolina–Chapel Hill, ZIKV strain H/PF/2013 was provided by the Centers for Disease Control and Prevention [31]; DENV World Health Organization reference strains (DENV1 West Pac 74, DENV2 S-16803, DENV3 CH54389, and DENV4 TVP-360) were obtained from Dr R. Putnak (Walter Reed Army Institute of Research). Virus stocks were prepared in C6/36 Aedes albopictus cells (ATCC CRL-1660). All DENV strains used were passage 4 and ZIKV was passage 3. At Imperial College London, ZIKV strain PF13/251013–18 (PF13) from French Polynesia in 2013 was provided by Dr V.-M. Cao-Lormeau (Institut Louis Malardé). DENV1 (Hawaii), DENV2 (16681), and DENV3 (H87) were gifts from the Armed Forces Research Institute of Medical Sciences. DENV-4 (1-0093) was isolated from a DENV4-infected patient. Virus-containing supernatants were collected and stored at −80°C. Viral titers were determined by focus-forming assays on Vero cells. All cell lines and virus stocks were free from Mycoplasma contamination.
Focus Reduction Neutralization Test (FRNT)
An FRNT was performed at the University of California–Berkeley, the University of North Carolina–Chapel Hill, and Imperial College London, using Vero cells and all 4 DENV serotypes and ZIKV in parallel (see the Supplementary Methods for experimental details, calculation of nAb titers, and quality control measures).
Antibody-Dependent Enhancement (ADE) Assay
Serially diluted plasma samples were incubated with virus at a multiplicity of infection of 2 for 1 hour at 37°C before addition to U937 cells. After incubation for 2 days (for ZIKV) or 3 days (for DENV), supernatants were harvested, and viral titers were determined by focus-forming assays as described in the Supplementary Methods. Fold enhancement was calculated by comparison to viral titers in the presence or absence of plasma.
RESULTS
Immune Plasma From ZIKV-Infected Individuals With and Those Without Prior DENV Immunity Have High ZIKV nAb Titers
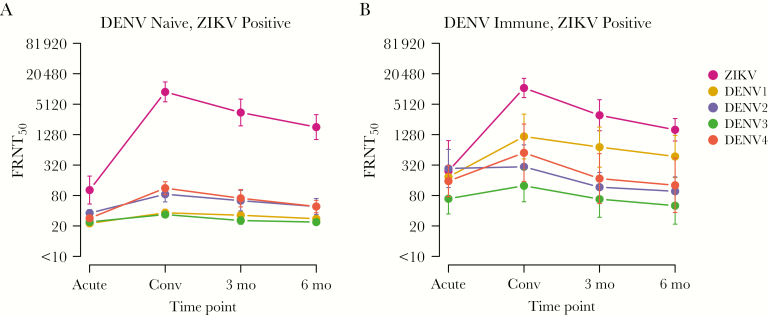
We selected Nicaraguan children from our hospital-based study who had RT-PCR–confirmed, symptomatic Zika with (n = 7) or without (n = 10) evidence of prior DENV immunity. All had nAb titers against DENV1–4 and ZIKV determined in samples collected during the acute and early convalescent phases and 3 and 6 months after ZIKV infection. First, the assay background value was confirmed as undetectable by testing specimens from DENV-naive, ZIKV-naive controls (Supplementary Figure 1). We found that peak nAb titers against ZIKV following ZIKV infection were higher than any 50% focus reduction neutralization test titer (FRNT50) to DENV1–4. Interestingly, the magnitude of the peak ZIKV nAb response (ie, the FRNT50 during the convalescent phase) and the responses in the 3- and 6-month follow-up specimens was similar regardless of preexisting DENV immunity (Figure 1A, B). ZIKV infection elicited detectable cross-neutralization to DENV1–4 at all time points after infection. As expected, samples from DENV-immune children with ZIKV infection exhibited greater neutralization to the 4 DENV serotypes than those who were DENV-naive (Figure 1A, B).
Figure 1.
Longitudinal neutralizing antibody responses against dengue virus serotypes 1–4 (DENV1–4) and Zika virus (ZIKV) in samples obtained after ZIKV infection. The 50% focus reduction neutralization test titer (FRNT50) for reverse-transcription polymerase chain reaction–confirmed ZIKV cases without (n = 10; A) or with (n = 7; B) evidence of prior DENV infection. Plasma samples obtained during the acute and convalescent (Conv) phases and 3 and 6 months after infection were evaluated by focus reduction neutralizing testing against all 4 DENV serotypes (Nicaraguan DENV1–4) and a Nicaraguan ZIKV isolate. All samples were processed in duplicate. Data for each sample set are geometric means ± standard deviations.
DENV Infection Elicits Low but Sustained Cross-neutralizing Antibody Responses to ZIKV in Nicaraguan Children
To determine the extent and duration of cross-neutralization of ZIKV by DENV-immune plasma, longitudinal samples collected at the acute and convalescent phases and 3, 6, 12, and 18 months after infection from primary and secondary DENV1, DENV2, and DENV3 cases (before the introduction of ZIKV into Nicaragua) in our hospital study were tested for nAb activity against all 4 DENV serotypes (DENV1–4) and ZIKV by the FRNT. As expected, plasma from primary dengue cases showed the highest nAb titers to the infecting serotype and lower FRNT50 values to heterologous DENV serotypes (Figure 2A). However, nAb titers to heterologous DENV strains were consistently higher than the FRNT50 to ZIKV (Figure 2A). A peak in nAb titers for DENV and ZIKV was observed at the convalescent phase, and FRNT50 titers were relatively stable from 3 to 18 months after infection. Similarly, individuals with secondary DENV infections showed the highest nAb titers to the DENV serotypes, although not necessarily the secondary infecting serotype, and the lowest titers to ZIKV (Figure 2B). The kinetics of nAb responses in secondary dengue cases were comparable to those for primary dengue cases, with a peak in FRNT50 titers during the convalescent phase and stabler titers from 3 to 18 months after infection, although there was more-gradual antibody waning than in primary cases between 3 and 18 months. Primary and secondary dengue cases had similar levels of nAb titers to ZIKV over time.
Figure 2.
Longitudinal neutralizing antibody responses against dengue virus serotypes 1–4 (DENV1–4) and Zika virus (ZIKV) after primary and secondary DENV infections among participants in the Nicaraguan hospital and cohort studies. A and B, Longitudinal plasma samples from primary (n = 5/serotype; A) and secondary (n = 5/serotype; B) dengue cases from the Nicaraguan hospital study, collected during the acute and convalescent phases and 3, 6, 12, and 18 months after infection. C, Longitudinal samples from the Nicaraguan cohort study, collected 1, 2 and 3 years after secondary infection with DENV1 (n = 4), DENV2 (n = 4), DENV3 (n = 3), and DENV4 (n = 3). Focus reduction neutralizing testing was performed against Nicaraguan DENV1–4 and ZIKV strains, and the 50% focus reduction neutralization test titer (FRNT50) was calculated and plotted. All samples were processed in duplicate. Data are geometric means ± standard deviations.
To examine the kinetics of the nAb response along a longer time frame, we evaluated longitudinal annual samples from children with secondary DENV infections in our community-based cohort study. Overall, these revealed the same trend, in that the highest titers were against the first infecting DENV serotype, which in most cases was DENV2, and the next highest titers were against the infecting serotype, followed by the other heterologous DENV serotypes and ZIKV (Figure 2C). Anti-DENV nAb titers, as well as low levels of cross-reactivity against ZIKV, were maintained 1–3 years after infection.
Antigenic Cartography Places ZIKV Outside the DENV Serocomplex
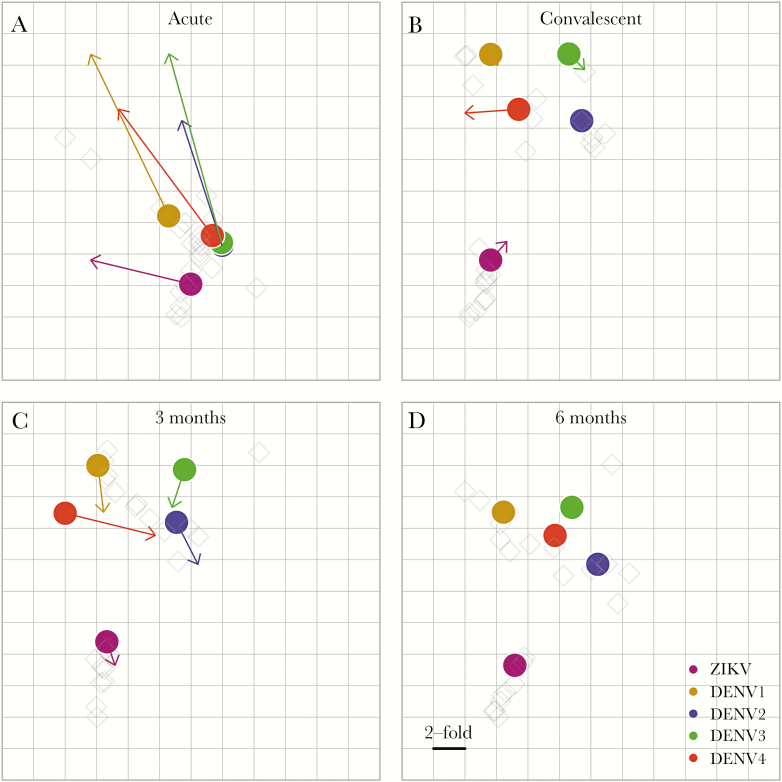
To estimate the antigenic relationships among DENV1–4 and ZIKV, we used antigenic cartography [32] to analyze neutralization titers in plasma collected after primary DENV1–3 and ZIKV infection from participants in the Nicaraguan hospital-based study. Each neutralization titer was treated as a measure of distance between a plasma sample and virus, and simultaneous fitting of these distances produced an antigenic map. In the acute phase, DENV1–4 and ZIKV formed a single cluster (Figure 3A), but ZIKV separated from the DENV1–4 cluster by the convalescent phase, and this is maintained out to 6 months after infection (Figure 3B).
Figure 3.
Antigenic relationships among dengue virus serotypes 1–4 (DENV1–4) and Zika virus (ZIKV), based on neutralizing antibody titers in plasma from primary DENV and ZIKV infections. Antigenic maps were generated by fitting 50% focus reduction neutralization test titers (FRNT50) for primary DENV and ZIKV infection plasma (light gray diamonds) from the Nicaraguan hospital-based study against DENV1–4 and ZIKV (colored circles) as distances in Euclidean space. Each grid-square corresponds to a two-fold dilution in the FRNT50. Arrows show shifts in antigenic relationships between DENV1–4 and ZIKV Nicaraguan viruses, based on how they were recognized by plasma specimens collected at each time point after infection in relation to the next time point: (A) acute; (B) convalescent; (C) 3 months, and (D) 6 months after infection.
ZIKV Cross-neutralization Is Variable and Inferior to Heterologous DENV Neutralization in Plasma From Sri Lankan Subjects
To investigate the magnitude and kinetics of cross-neutralizing antibody responses to ZIKV following DENV infection in another geographical region, we examined archived serologic specimens from 2 sources in Sri Lanka (Table 1, Supplementary Table 1). From a community-based study of dengue conducted in 2008–2010 [26], we selected 12 subjects (6 with primary and 6 with secondary DENV infections) who experienced a laboratory-confirmed, symptomatic DENV infection and had at least 1 additional specimen available after incident DENV infection; the total number of samples varied depending on the basis of how many febrile episodes a child reported. From a Sri Lankan hospital-based study conducted in 2014, we selected 13 subjects (7 with primary and 6 with secondary DENV infections), from whom convalescent-phase blood samples collected between 11 and 77 days after infection were available.
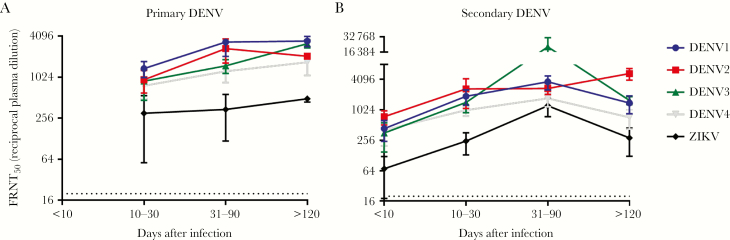
We grouped specimens tested by primary versus secondary DENV infection and time after symptom onset. Neutralization profiles (ie, FRNT50 values) to each virus at each time point are shown in Supplementary Table 1. In primary cases (Figure 4A), DENV1 was the most common infecting serotype, reflected in the higher neutralization titers to DENV1. The FRNT50 for DENV2–4 clustered at averages below those for DENV1 at each time point, while the ZIKV FRNT50 was approximately 10-fold lower than the DENV1 FRNT50. ZIKV neutralization persisted beyond 120 days after infection (range, 128–217 days) at substantial levels but were subordinate to DENV titers.
Figure 4.
Cross-neutralization of Zika virus (ZIKV) following dengue virus (DENV) infection in Sri Lanka. 50% focus reduction neutralization test titers (FRNT50) were determined for DENV1–4 and ZIKV. Samples were grouped by time after laboratory-confirmed DENV infection and by primary (A) and secondary (B) DENV infection. Points are group means ± standard errors of the mean.
In secondary DENV infections (Figure 4B), ZIKV FRNT50 titers were again the lowest of all groups. ZIKV cross-neutralizing titers rose later during the convalescent phase (range, 31–90 days after infection) among secondary cases to levels approximating the mean FRNT50 of several DENV serotypes. At time points >120 days after infection (range, 123–202 days) in secondary cases, the mean FRNT50 for ZIKV declined markedly and began to further separate from that of the DENV serotypes. Thus, ZIKV cross-neutralization is detected after primary and secondary DENV cases, but typically with an FRNT50 at least 4-fold lower than to the infecting DENV serotype. Notably, most Sri Lankan hospital samples were not tested beyond a dilution of 1:1280, and many DENV1–4 FRNT50 values are >1:1280; thus, the difference in the magnitude of ZIKV versus DENV FRNT50 values is underestimated.
Transient Cross-neutralization of ZIKV Following DENV Infections Observed in a Thai Cohort
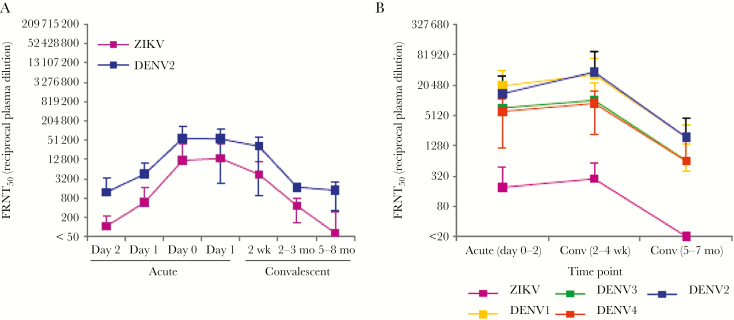
In a third country, we studied samples collected from Thai children (ages 6–14 years) admitted to the hospital with acute DENV infection during 2001–2003, when ZIKV was not reported in the area. Samples were collected on each day after hospital admission and at convalescent-phase time points of 2 weeks, 2 months, and 6 months following discharge (Table 1). Neutralization of DENV2 and ZIKV was measured in plasma samples from 5 children with secondary DENV2 infection (Figure 5A, Supplementary Figure 2 and Table 2). In general, neutralization titers for both DENV and ZIKV peaked between day 0 and day 14 and declined thereafter. Five of 5 cases showed DENV neutralization (FRNT50 > 50% at a plasma dilution of 1:50), which was maintained until the 6-month time point. Five of 6 cases also showed neutralization of ZIKV at early time points, but this decayed more rapidly than the titers to DENV at 6 months. To validate these findings in a larger sample set representing infection by all 4 DENV serotypes, 15 additional secondary DENV cases were tested (Supplementary Table 3). These again showed strong neutralization of all DENV serotypes at all time points, whereas nAb titers against ZIKV were lower during early time points and at 6 months (Figure 5B).
Figure 5.
Longitudinal neutralizing antibody responses to dengue virus (DENV) and Zika virus (ZIKV) from a Thai hospital study. A, Mean 50% focus reduction neutralization titers (FRNT50) to infecting DENV serotype (DENV2) and ZIKV strain PF13 of plasma specimens from subjects with secondary DENV infection (n = 5), during the course of acute infection, at the convalescent phase (2 weeks after infection), and at 2 and 6 months after infection. Data are presented as FRNT50 and representative of 1 experiment with 5 samples. B, Mean FRNT50 against DENV1–4 and ZIKV strain PF13 among longitudinal specimens from 15 Thai subjects with secondary infection by any DENV serotype. Data are means ± standard deviations.
DENV-Immune Plasma Transiently Enhances DENV and ZIKV Infection In Vitro
Finally, we found that DENV2-immune plasma promoted ADE of both DENV and ZIKV infection in vitro at a similar peak enhancement titer, although the magnitude of enhancement was greater for DENV2 (Supplementary Figure 3).
DISCUSSION
In this study, we characterized the nAb response against DENV and ZIKV over time following primary and secondary DENV infections and drew 2 main conclusions: (1) cross-neutralizing antibody responses to ZIKV are consistently detected after DENV infection, but they occur at magnitudes notably less than to the infecting DENV serotype and generally less than to heterologous DENV serotypes, and (2) the magnitude and kinetics of ZIKV nAb responses are unaffected by previous DENV infection. Both findings are consistent with ZIKV and DENV belonging to a distinct serocomplex.
Across multiple well-characterized DENV infection cohorts representing populations in Latin America and Asia, we consistently observed cross-neutralizing activity against ZIKV, but ZIKV FRNT50 values tended to be 4–10-fold lower than DENV FRNT50 values. Cross-neutralization to ZIKV variably declines over time and is maintained at low levels in children for months to years after infection. We have previously seen that high levels of ZIKV cross-neutralizing antibodies are not maintained into the late convalescent phase (ie, ≥6 months after infection) in DENV-infected travelers [20], raising the possibility that ongoing DENV exposure in regions of endemicity may drive maintenance of cross-neutralization of ZIKV following DENV infection [15]. Most studies reporting strong cross-neutralization between ZIKV and DENV have used early convalescent-phase sera [8] or mAbs derived from plasma blasts obtained at very early time points after DENV infection [18, 19]. Using antigenic maps, we show that DENV1–4 and ZIKV group closely when plasma specimens from the acute phase of primary DENV and ZIKV infection are analyzed; however, by the convalescent phase and out to 6 months after infection, DENV1–4 group as a serocomplex, with ZIKV clearly outside of the DENV antigenic cluster.
Overall, it appears that ZIKV–cross-neutralizing antibodies are frequently induced by DENV infection at a fraction of the magnitude of DENV-neutralizing antibodies, with anti-ZIKV titers reaching a steady-state level by 6–12 months. Furthermore, neutralization antibody assays can distinguish ZIKV from DENV infections when all viruses are analyzed simultaneously, particularly if relative titers of ZIKV versus DENV serotypes, rather than specific cutoffs for positivity, are considered [33], and thus may be useful in surveillance, research, and potentially some clinical settings outside of the acute phase of illness [20].
One assumption is that none of the subjects with dengue included in the study were infected by ZIKV. While it is plausible that Zika in Asia is grossly underreported owing to a high rate of asymptomatic infections or attribution of Zika cases to other causes of acute febrile illness, such as dengue [6, 34, 35], this is highly unlikely in our study. Samples from Asian subjects did not exhibit high FRNT50 values at the earliest time points interrogated after DENV infection, nor did they display maintenance of high ZIKV FRNT50 titers into the convalescent phase, which would be expected after a true prior ZIKV infection [12, 13]. The samples from Nicaraguan patients with dengue were collected years before ZIKV was reported or projected to be circulating in Central America [36].
The neutralization assay has been an important tool in flavivirology, although variations in titer between laboratories have been recognized [37]. Here we observed some differences in overall titer magnitude but reached similar conclusions across the 3 sites. This variation may be due to unique features of study populations or to details such as the virus strain used in the assay, DENV infection order, intensity of local transmission, and strain of DENV exposure, which were not systematically analyzed here.
The phenomenon of ADE, whereby cross-reactive non neutralizing antibodies facilitate infection of FcγR-bearing cells and lead to higher levels of viremia and more-severe disease manifestations, is a well-established concept in the dengue field [13, 38, 39] and has recently been established in human populations [40]. Although clear evidence of enhancement of ZIKV infection in DENV-immune humans is still lacking [41], it remains a major concern. While DENV and other flaviviruses are readily enhanced by antibodies in cell culture ADE assays with FcγR-bearing cell lines, such assays have not been useful for predicting severe DENV disease in people [42]. Here, we observed that plasma from Thai individuals who had recovered from DENV2 infections enhanced DENV2 to a greater degree than ZIKV in cell culture assays, further highlighting the challenges of extrapolating the human flavivirus disease risk from findings of in vitro ADE assays. The enhancing activity peaked during the acute and convalescent phases and declined thereafter, mirroring the curve of neutralizing antibody profiles.
Alternatively, ZIKV cross-neutralizing antibodies elicited by DENV may mitigate ZIKV infection, in line with claims that a single vaccine against DENV1–4 and ZIKV could be developed [8, 12, 18]. Preinfection nAb titers have been associated with a decreased probability of symptomatic secondary DENV infection [15, 27], and nAb correlates of protection exist for other flavivirus infections [43]. However, no specific correlate of protection has been identified for DENV or ZIKV, and many individuals who seroconverted, based on neutralization testing, remained susceptible to DENV infection in vaccine studies [44, 45]. Interestingly, DENV-immune nonhuman primates are readily infected with ZIKV, with no observation of enhancement or protection [46, 47]. Ongoing cohort studies relating the known sequence and timing of DENV infections or prevalent DENV serostatus to ZIKV infection outcome will help address these critical questions.
Since 2015, many individuals in Latin America have experienced ZIKV as a primary or secondary flavivirus infection, raising the question of how ZIKV infection will affect immunity to DENV. A dramatic reduction in dengue cases throughout Latin America was observed in 2017 [48], suggesting that ZIKV infection may induce at least temporary immune cross-protection from DENV. Our data demonstrate that ZIKV infection clearly elicits cross-neutralizing antibodies to DENV and may boost DENV FRNT50 values in a DENV-immune host. Whether higher or lower rates of DENV infection occur among ZIKV-immune individuals will be determined in longitudinal cohort studies. Interestingly, we found that the magnitude of the anti-ZIKV nAb titer was similar regardless of prior DENV infection, which may be inconsistent with recent reports [49, 50] that prior DENV infection (at least by certain serotypes) may potentiate the nAb response to ZIKV. We also noted that nAb titers to ZIKV after ZIKV infection were higher than nAb titers to the infecting DENV serotype after DENV infection.
Overall, ZIKV appears to be antigenically related but distinct from the DENV serotypes. Our study provides a framework for comprehensive assessment of the serologic immune responses to multiple flaviviruses that is needed to inform vaccine development and epidemiologic studies aimed at reducing the burden of disease caused by these important pathogens in areas of endemicity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Daniela Weiskopf and Dr Alessandro Sette, for assistance procuring Sri Lankan hospital samples, and Nattaya Tangthawornchaikul, for collecting Thai specimens.
Financial support. This work was supported by the National Institutes of Health (grants P01AI106695 [to E. H. and A. D. d. S.], U19AI118610 [to E. H.], R01AI099631 [to A. B.], and R21/R33AI100186 [to A. B.]), the Centers for Disease Control and Prevention (contract 00HVCLJB-2017-04191 to A. D. d. S.), the Wellcome Trust (to G. S.), and the Newton Medical Research Council (J. M.).
Potential conflicts of interest. A. D. d. S. has consulted for Takeda, Glaxo Smith Kline, and Merck on dengue vaccines and is an inventor on patent applications involving flavivirus vaccines and diagnostic assays. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: Regional Consultation Toward Southeast Asia Framework for Zika Virus Preparedness and Response, Bangkok, Thailand, 12 July 2017; Reunión Técnica Sobre Análisis de Factores Virológicos, Epidemiológicos, y Entomológicos Vinculados a la Disminución de los Casos de Dengue en los Países de las Américas, Washington, D. C., 3–5 October 2017; Immunology2017, Washington, D. C., 12–16 May 2017; 15th International Dengue Course Challenges of Zika and Chikungunya Transmission, Havana, Cuba, 7–11 August 2017; 66th Annual Meeting of the American Society of Tropical Medicine and Hygiene, Baltimore, Maryland, 5–9 November 2017.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. PAHO, WHO. Zika cumulative cases http://www.paho.org/hq/index.php?option=com_content&view=article&id=12390&Itemid=42090&lang=en. Accessed 30 October 2017.
- 3. Coyne CB, Lazear HM. Zika virus—reigniting the TORCH. Nat Rev Microbiol 2016; 14:707–15. [DOI] [PubMed] [Google Scholar]
- 4. dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the Guillain–Barré syndrome—case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 5. Weaver SC, Costa F, Garcia-Blanco MA, et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res 2016; 130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salehuddin AR, Haslan H, Mamikutty N, et al. Zika virus infection and its emerging trends in Southeast Asia. Asian Pac J Trop Med 2017; 10:211–9. [DOI] [PubMed] [Google Scholar]
- 8. Priyamvada L, Quicke KM, Hudson WH, et al. Human antibody responses after dengue virus infection are highly cross- reactive to Zika virus. Proc Natl Acad Sci U S A 2016; 113:7852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kostyuchenko VA, Lim EX, Zhang S, et al. Structure of the thermally stable Zika virus. Nature 2016; 533:425–8. [DOI] [PubMed] [Google Scholar]
- 10. Dejnirattisai W, Supasa P, Wongwiwat W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 2016; 17:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mansfield KL, Horton DL, Johnson N, et al. Flavivirus- induced antibody cross-reactivity. J Gen Virol 2011; 92:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinz FX, Stiasny K. The antigenic structure of Zika virus and its relation to other flaviviruses: implications for infection and immunoprophylaxis. Microbiol Mol Biol Rev 2017; 81:doi: 10.1128/MMBR.00055-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses 2011; 3:2374–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Alwis R, Beltramello M, Messer WB, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 2011; 5:e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci U S A 2016; 113:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stettler K, Beltramello M, Espinosa DA, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016; 353:823–6. [DOI] [PubMed] [Google Scholar]
- 17. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barba-Spaeth G, Dejnirattisai W, Rouvinski A, et al. Structural basis of potent Zika-Dengue virus antibody cross-neutralization. Nature 2016; 539:314. [DOI] [PubMed] [Google Scholar]
- 19. Swanstrom JA, Plante JA, Plante KS, et al. Dengue virus envelope dimer epitope monoclonal antibodies isolated from Dengue patients are protective against Zika virus. MBio 2016; 7:pii:e01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins MH, McGowan E, Jadi R, et al. Lack of durable cross-neutralizing antibodies against Zika virus from Dengue virus infection. Emerg Infect Dis 2017; 23:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Narvaez F, Gutierrez G, Pérez MA, et al. Evaluation of the traditional and revised WHO classifications of dengue disease severity. PLoS Negl Trop Dis 2011; 5:e1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waggoner JJ, Gresh L, Mohamed-Hadley A, et al. Single-reaction multiplex PCR for detection of Zika, chikungunya, and dengue viruses. Emerg Infect Dis 2016; 22:1295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gutiérrez G, Gresh L, Pérez MÁ, et al. Evaluation of the diagnostic utility of the traditional and revised WHO dengue case definitions. PLoS Negl Trop Dis 2013; 7:e2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernández RJ, Vázquez S. Serological diagnosis of dengue by an ELISA inhibition method (EIM). Mem Inst Oswaldo Cruz 1990; 85:347–51. [DOI] [PubMed] [Google Scholar]
- 25. Balmaseda A, Hammond SN, Tellez Y, et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health 2006; 11:935–42. [DOI] [PubMed] [Google Scholar]
- 26. Tissera H, Amarasinghe A, De Silva AD, et al. Burden of dengue infection and disease in a pediatric cohort in urban Sri Lanka. Am J Trop Med Hyg 2014; 91:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, de Silva AM. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 2015; 211:590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yenchitsomanus PT, Sricharoen P, Jaruthasana I, et al. Rapid detection and identification of dengue viruses by polymerase chain reaction (PCR). Southeast Asian J Trop Med Public Health 1996; 27:228–36. [PubMed] [Google Scholar]
- 29. Innis BL, Nisalak A, Nimmannitya S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 1989; 40:418–27. [DOI] [PubMed] [Google Scholar]
- 30. Diamond MS, Edgil D, Roberts TG, Lu B, Harris E. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J Virol 2000; 74:7814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc 2014; 2:pii: e00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katzelnick LC, Fonville JM, Gromowski GD, et al. Dengue viruses cluster antigenically but not as discrete serotypes. Science 2015; 349:1338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rabe IB, Staples JE, Villanueva J, et al. ; MTS Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 2016; 65:543–6. [DOI] [PubMed] [Google Scholar]
- 34. Dyer O. Zika virus is set to spread through Asia, WHO says. BMJ 2016; 355:i5577. [DOI] [PubMed] [Google Scholar]
- 35. Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metsky HC, Matranga CB, Wohl S, et al. Zika virus evolution and spread in the Americas. Nature 2017; 546:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salje H, Rodríguez-Barraquer I, Rainwater-Lovett K, et al. Variability in dengue titer estimates from plaque reduction neutralization tests poses a challenge to epidemiological studies and vaccine development. PLoS Negl Trop Dis 2014; 8:e2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halstead SB. Pathogenesis of dengue: dawn of a new era. F1000Res 2015; 4:doi: 10.12688/f1000research.7024.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010; 328:745–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358:929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halstead SB. Biologic evidence required for Zika disease enhancement by dengue antibodies. Emerg Infect Dis 2017; 23:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Libraty DH, Acosta LP, Tallo V, et al. A prospective nested case-control study of dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med 2009; 6:e1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. SAGE Working Group on Japanese encephalitis vaccines. Background paper on Japanese encephalitis vaccines. 2014. http://www.who.int/immunization/sage/meetings/2014/october/1_JE_Vaccine_Background_Paper.pdf. [Google Scholar]
- 44. Simmons CP. A candidate dengue vaccine walks a tightrope. N Engl J Med 2015; 373:1263–4. [DOI] [PubMed] [Google Scholar]
- 45. Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011; 29:7229–41. [DOI] [PubMed] [Google Scholar]
- 46. McCracken MK, Gromowski GD, Friberg HL, et al. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLOS Pathog 2017; 13:e1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pantoja P, Pérez-Guzmán EX, Rodríguez IV, et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 2017; 8:15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. PAHO, WHO. Dengue. http://www.paho.org/hq/index.php? option=com_topics&view=article&id=1&Itemid=40734. Accessed 31 October 2017.
- 49. Robbiani DF, Bozzacco L, Keeffe JR, et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017; 169:597–609.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogers TF, Goodwin EC, Briney B, et al. Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2017; 2:eaan6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.