Abstract
Hippocampal sharp wave ripples (SWRs) represent irregularly occurring synchronous neuronal population events that are observed during phases of rest and slow wave sleep. SWR activity that follows learning involves sequential replay of training-associated neuronal assemblies and is critical for systems level memory consolidation. SWRs are initiated by CA2 or CA3 pyramidal cells and require initial excitation of CA1 pyramidal cells as well as participation of parvalbumin (PV) expressing fast spiking (FS) inhibitory interneurons. These interneurons are relatively unique in that they represent the major neuronal cell type known to be surrounded by perineuronal nets (PNNs), lattice like structures composed of a hyaluronin backbone that surround the cell soma and proximal dendrites. Though the function of the PNN is not completely understood, previous studies suggest it may serve to localize glutamatergic input to synaptic contacts and thus influence the activity of ensheathed cells. Noting that FS PV interneurons impact the activity of pyramidal cells thought to initiate SWRs, and that their activity is critical to ripple expression, we examine the effects of PNN integrity on SWR activity in the hippocampus. Extracellular recordings from the stratum radiatum of horizontal murine hippocampal hemisections demonstrate SWRs that occur spontaneously in CA1. As compared to vehicle, pretreatment (120 min) of paired hemislices with hyaluronidase, which cleaves the hyaluronin backbone of the PNN, decreases PNN integrity and increases SWR frequency. Pretreatment with chondroitinase, which cleaves PNN side chains, also increases SWR frequency. Together, these data contribute to an emerging appreciation of extracellular matrix as a regulator of neuronal plasticity and suggest that one function of mature perineuronal nets could be to modulate the frequency of SWR events.
Keywords: Hyaluronidase, Chondrotinase, PV interneuron, protease, hippocampus
Introduction
Hippocampal sharp wave ripples (SWRs) represent synchronous neuronal population events characterized by a low frequency large amplitude deflection and an associated high frequency ripple oscillation (Buzsaki, 1986). SWRs have been implicated in the rapid sequential replay of neuronal assemblies that were initially activated during a learning experience (Alme et al., 2014; Colgin, 2016; O'Keefe and Dostrovsky, 1971; Wilson and McNaughton, 1994). During these events, replay occurs at an accelerated rate and is thought to be critical for memory consolidation and transfer (Buzsaki, 2015). Consistent with this are studies showing that disruption of SWR activity during sleep impairs memory for previously experienced information and that sustained increases in SWR activity are observed during sleep that follows learning (Ego-Stengel and Wilson, 2010; Eschenko et al., 2008; Girardeau et al., 2009). In addition, sequential replay reverberates between varied brain regions implicated in the acquisition and/or long-term storage including hippocampus and cortical projection areas (Ji and Wilson, 2007; Place et al., 2016; Rothschild et al., 2017). Intriguingly, replay of previously activated neuronal assemblies occurs in a bidirectional manner (Diba and Buzsaki, 2007; Foster and Wilson, 2006), and forward replay may facilitate the ability to plan for future events (Dragoi and Tonegawa, 2011; Sadowski et al., 2011).
Pyramidal cells (PCs) of CA3 have been implicated in the initiation of hippocampal SWRs both in vivo and in vitro (Buzsaki, 2015). The sharp wave is an excitatory event typically initiated in CA3 that represents depolarization of CA1 pyramidal cell dendrites, while the ripple is generated locally in CA1 by ripple –frequency activity of fast spiking interneurons (Schlingloff et al., 2014; Whittington et al., 1995).
PV expressing basket cells participate in ripple frequency oscillations and importantly, they can influence the excitability of the principal cells that initiate sharp wave ripples. In one study that examined pyramidal cell excitability in mice engineered so that AMPA mediated excitation of PV interneurons was selectively suppressed, it was observed that pyramidal cells were more likely to fire (Racz et al., 2009). Moreover, PV expressing interneurons represent the class of hippocampal interneurons that have been well implicated in ripple expression (Schlingloff et al., 2014). This was demonstrated by experiments with perisomatic injection of ω-agatoxin, which targets voltage dependent calcium channels on PV but not cholecystokinin expressing basket cell axon terminals. Ripples disappeared when ω-agatoxin was injected into the stratum pyramidale (Schlingloff et al., 2014).
PV interneurons represent the major population of neurons that is ensheathed in a perineuronal net, a specialized extracellular matrix comprised of a hyaluronin backbone that surrounds the soma and proximal dendritic area (reviewed in (Kwok et al., 2011; Pantazopoulos and Berretta, 2016; Sorg et al., 2016)). The presence of the PNN may enhance PV interneuron excitability (Balmer, 2016) as well as the frequency of inhibitory currents in pyramidal neurons (Slaker et al., 2015). Though the mechanism(s) by which this is achieved are not completely understood, PNNs may serve to prevent diffusion of glutamate released at PV post-synaptic contacts and/or restrict the lateral mobility of GluAs on this population (Balmer, 2016; Bikbaev et al., 2015; Frischknecht et al., 2009; Slaker et al., 2015). Consistent with the latter possibility, the diffusion constant of GluA1 drops as the subunit reaches hyaluronin-enriched area. Hyaluronidase digestion increased both the diffusion coefficient and the total surface area covered by individual subunits (Frischknecht et al., 2009).
PNN integrity varies as a function of normal physiology and pathology (Kwok et al., 2011; Pantazopoulos and Berretta, 2016). Developmental maturation of the PNN correlates with closure of critical periods of plasticity in which appropriate experience molds neuronal network organization (Pizzorusso et al., 2002). PNN formation is neuronal activity dependent (Dityatev et al., 2007), and region specific development is influenced by relevant neuronal activity or lack thereof. Moreover, PNN deposition and critical period closure in the visual cortex are both reduced by dark rearing (Gianfranceschi et al., 2003; Hockfield et al., 1990; Mower, 1991). In addition, in the mouse barrel cortex, whisker trimming during the first post-natal month is associated with a reduction in aggrecan rich PNNs (McRae et al., 2007). Of interest, the monoamine reuptake inhibitor fluoxetine has been shown to reduce PNN integrity and to reopen the critical window for visual plasticity (Guirado et al., 2014; Maya Vetencourt et al., 2008).
Despite the importance of the PNN to PV activity, and the role of PV interneurons in SWR expression, few if any studies have explicitly examined effects of PNN integrity on SWR events. The present study addresses the impact of hyaluronidase or chondroitinase processing of the PNN on SWR event frequency. We use acute hippocampal slices of thickness sufficient for the expression of spontaneous events (Schlingloff et al., 2014), and paired hemislices that are concomitantly pre-incubated in control, hyaluronidase or chondoitinase-containing ACSF.
Materials and Methods
Slice preparation
6–8 week old male and female C57/Bl6 mice were obtained from Jackson laboratories and used to prepare paired hippocampal hemi-slices in accordance with National Institutes of Health guidelines and a protocol that had been approved by the Institutional Animal Care and Use Committee at Georgetown University Medical Center. PVCre-tdTomato mice, used for immunostaining, were generated from crosses between PV-Cre (B6;129P2-Pvalbtm1(cre)Arbr/J; JAX#008069) driver and tdTomato (Ai14; JAX #07914) reporter animals and bred so as to avoid the confound of ectopic expression (Kobayashi and Hensch, 2013). Following deep isoflourane anaesthesia, animals were rapidly decapitated. The whole brain was subsequently removed and chilled in cold (0° C) sucrose-based cutting artificial cerebrospinal fluid (sACSF) containing (in mM) 252 sucrose; 3 KCL; 2 CaCl2; 2 MgSO4; 1.25 NaH2PO4; 26 NaHCO3; 10 dextrose and bubbled by 95% O2, 5% CO2. Hippocampal slices (490 um thick unless otherwise indicated) were cut in horizontal sections from dorsal to ventral brain with a vibratome (Leica, VT1000S). Horizontal sections were then bisected to obtain paired hemislices for subsequent incubation of one versus the other paired hemislice with control or enzyme containing ACSF. Right and left sided sections were randomly placed to reduce potential confounds due to hemispheric differences, and dorsal and ventral most slices were excluded due to potential PNN density differences along this axis. ACSF contained (in mM) NaCl, 132; KCl, 3; CaCl2, 2; MgSO4, 2; NaH2PO4, 1.25; NaHCO3, 26; dextrose 10; and saturated with 95% O2, 5% CO2 at 26° C. Slices were incubated for at least 120 minutes before being moved to the recording chamber.
Chemicals and reagents
Hyaluronidase and chondroitinase ABC were purchased from Sigma Chemical (St. Louis, MO; catalogue numbers H4272 and C3667). Lyophilized preparations were reconstituted in ACSF just prior to slice incubation. The final concentration of hyaluronidase in the incubating solution was .25 gm/ml and the final concentration of chondroitinaseABC was 0.1U/ml. Both concentrations were based on previously published in vitro studies (Filous et al., 2014) (Bikbaev et al., 2015).
PNN staining and microscopy
Following treatment, brain sections from PV-Cre/tdTomato mice were fixed overnight in 4% PFA/sucrose at 4° C. Sections were then washed briefly and placed in 20% sucrose solution and later subsectioned on a cryostat in order to obtain 30 µm-thick sections. Sections were then washed twice with 1X-PBS containing .1% Tween-20, blocked with 10% normal goat serum, and incubated with fluorescein-labeled Wisteria floribunda lectin (WFA) (1:1000, Vector Laboratories, FL-1351) for 2 hours at room temperature. Sections were then washed three times with 1X-PBS, mounted with Hydromount (National Diagnostics, HS-106) and allowed to dry several days at 4° C prior to confocal imaging. Images were acquired using a Leica SP8 laser scanning confocal microscope Leica Application Suite was used for image acquisition. Laser intensity, gain, and pinhole settings were kept constant for all samples.
PV cell counting and fluorescence
Z-stack images from fixed 250 µm-thick brain sections of PV-Cre/tdTomato mice were acquired using a Leica SP8 laser scanning confocal microscope with a dry, 10X objective with .40 numerical aperture. Images were taken through a Z plane (8.5 µm) containing 10 stacks (.94 µm/stack). Laser intensity, gain, and pinhole settings were kept constant for all samples. Images were transferred to ImageJ (NIH) and representative images were obtained using the ZProject mean intensity projection. Cell numbers and mean fluorescence of individual cells were acquired from regions of interest using 6 control fields and 5 hyaluronidase fields (1392 cells from the control fields and 927 cells from hyaluronidase fields).
Local field potential (LFP) recordings
Low resistance glass microelectrodes were used for LFP recordings of the SW/R signals. The electrodes were pulled with a Sutter P87 puller with 6 controlled pulls, resulting in an approximate 150K tip resistance. Electrodes were filled with 1M NaCl in 1% agar, which prevents leakage of the electrode solution that could potentially alter conditions at the recording site. The recordings were done in a submerged chamber, and slices were perfused on both sides at a high flow rate (20 ml /min). In our recording arrangement SWR signals have an adequate signal-to-noise ratio. Hippocampal slices from dorsal and ventral locations demonstrated SWR activity. Comparisons between control and treated slices were performed with matched left and right slices of specific dorsal to ventral level sections (980–2450 microns from the dorsal most section). Thus, recordings were performed using control and treated pairs prepared at the same time and with the same cutting or recording ACSF.
Data analysis
SWR frequency was quantified through complementary approaches. 1) Raw LFP traces were filtered between 0.5–30 Hz, and the SWR peaks were counted manually by a hypothesis-blind investigator. 2) SWR events were also characterized via a custom MATLAB algorithm based on previously published techniques (Csicsvari et al., 1999; Eschenko et al., 2008; Siapas and Wilson, 1998). A Gaussian FIR band-pass filter with corrected phase delay was applied to the LFP in two frequency bands: 2–30Hz and 80–250Hz to extract the low-frequency (LF) SW and high-frequency (HF) ripple components, respectively. The 80Hz lower cutoff for the ripple band was chosen to better match the room temperature recordings (Papatheodoropoulos et al., 2007). For both the LF and HF filtered signals, the root mean square (RMS) was computed every 5ms in a 10ms moving window. Based on published studies (Ccedil;aliskan et al., 2015; Maier et al., 2003), the threshold for peak detection was 3–4 SDs of the RMS signal as indicated. Peak duration was defined as the contiguous period around a peak where the RMS signal remained 2 SDs above baseline. In this way, both SW and ripple events could be individually detected. SWR events were defined as a subset of these, requiring both a simultaneous SW and ripple event. The duration of SWR events was defined as the greater of the overlap of simultaneous SW and ripple events. Event power was calculated by integrating either the LF, HF, or unfiltered LFP for the SW, ripple and SWR events, respectively.
Statistical analysis
Data was entered into a Graph Pad Prism program and statistical analysis performed using Student’s t test for 2 group comparisons or ANOVA for comparisons of more than 2 groups. Significance was set at p < 0.05
Results
I. Spontaneous SWR activity in 490 µm hippocampal slices
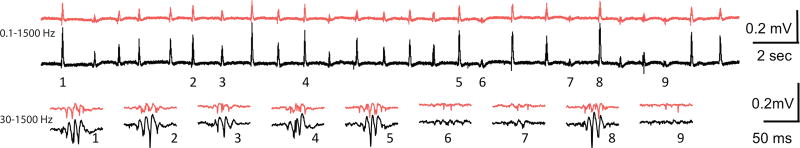
Spontaneous SWR activity has previously been reported in ex vivo hippocampal slices of thickness thought sufficient for adequate network complexity in work by Schlingloff and colleagues (Schlingloff et al., 2014). As shown in figure 1, we also observe spontaneous SWR activity in 490 µm sections. Raw SWR signals from two electrodes that were placed within the proximal section of the CA1 stratum radiatum are shown. As can be appreciated from this 15 second epoch shown, events with varied inter-event intervals and morphology are apparent at both recording sites. Twenty six events are displayed with nine expanded to show the associated ripple component.
Figure 1.
Spontaneous SWRs in 490 mM horizontal hippocampal slices. Top: raw LFP traces (red and black) showing sharp waves from two recording locations in CA1 (filtered; 0.1 to 1500 Hz). Bottom: examples of ripples from the top trace are displayed on an expanded time scale (filtered; 30 to 1500 Hz). Note that in this 15 second section of recordings there are 26 SWRs, some large events (note 1,4,8) and some small events (note 6,7,9).
II. PNN staining in hippocampal slices
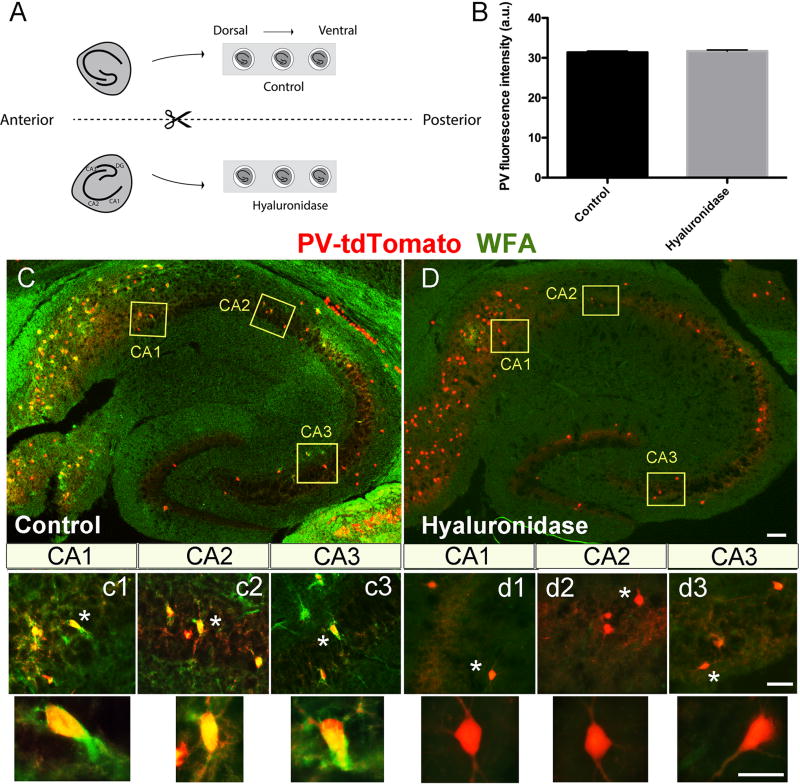
The ability of hyaluronidase to disrupt PNN integrity has been demonstrated in varied settings. Due to the potential for reduced enzyme activity in the presence of carbogenated ACSF, however, we examined PNN integrity in slices that were incubated for 2h in control or hyaluronidase containing carbogenated ACSF. The experimental set up (2A) involved two separate chambers containing multiple wells so that matched horizontal hemisection pairs could recover in one of the two solutions. This set up would be maintained for future studies of SWR activity following the recovery period. In these experiments, slices were incubated for 2h with (0.25 grams/ml) hyaluronidase, a concentration that is consistent with that recently used in the treatment of hippocampal cultures (Bikbaev et al., 2015), then formalin fixed, cryostat sectioned, and stained to detect PNN ensheathed PV neurons. Shown in 2B is average PV fluorescence intensity. Neither PV fluorescence intensity nor PV cell number per field (232 +/− 18.8; 185 +/1 39.87; p= 0.29; data not shown), which are surrogate measures of PV viability (Marx et al., 2013), were significantly affected by treatment. Shown in 2C–D are representative WFA immunostaining results from control and hyaluronidase pretreated conditions. Overall WFA positive PNN signal is not appreciably above background following 2h treatment with hyaluronidase (C versus D). Individual PV positive neurons (red) which are surrounded by WFA positive PNNs (green) can be observed in control but not hyaluronidase treated slices (c1–c3 versus d1–d3).
III. SWR ripple event frequency is increased following exposure to hyaluronidase
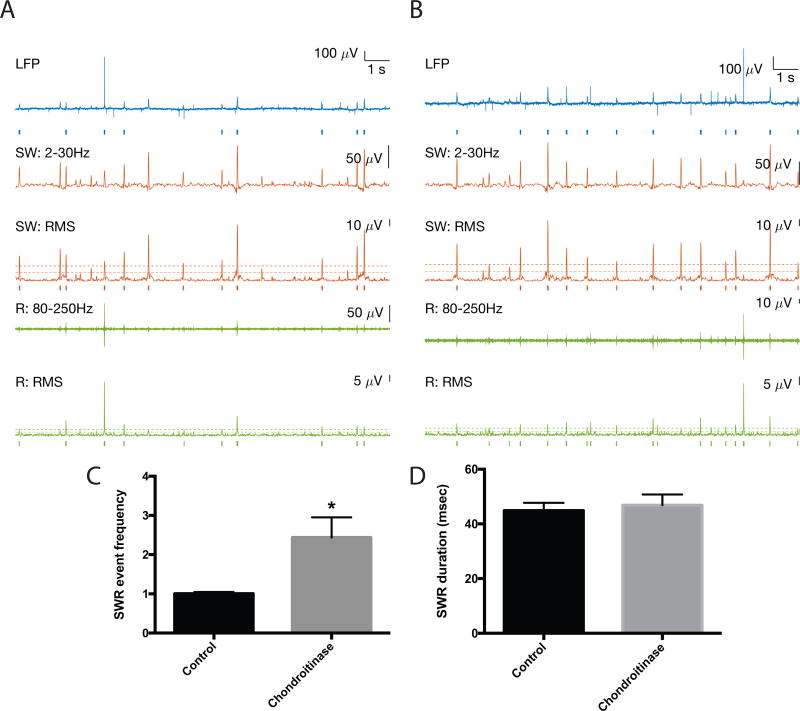
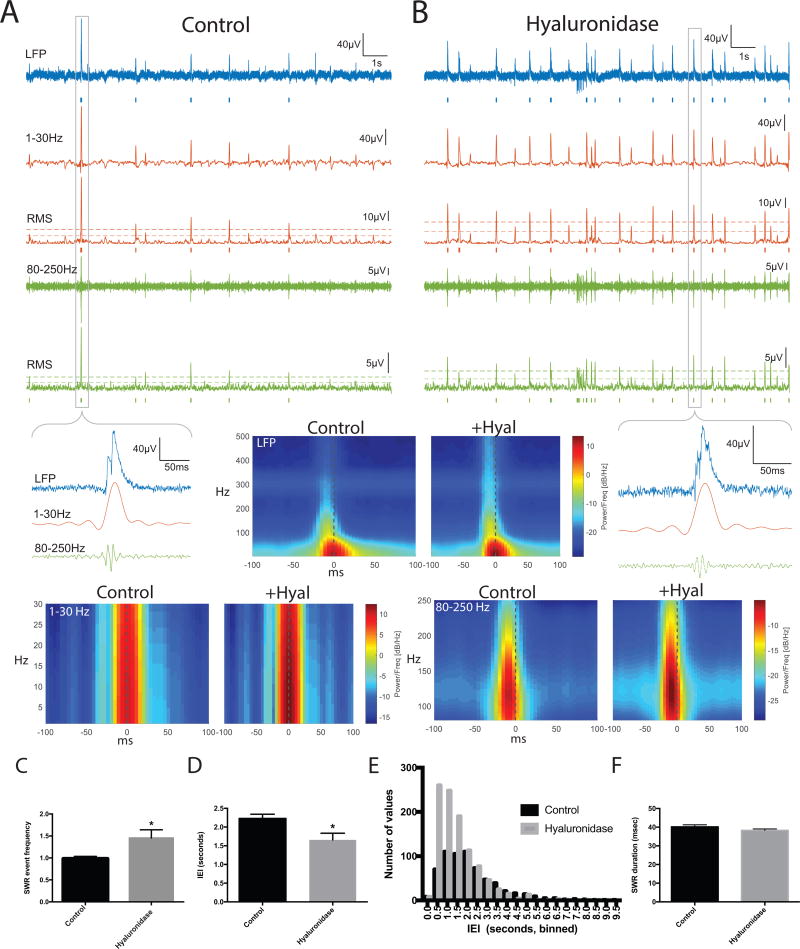
We next examined the frequency of SWR events in paired control and hyaluronidase treated hemislices. Representative traces are shown in 3A and B, with SW events in red and ripples in green. Events are underlined by tics for SWs and ripples that were detected with a 4 SD threshold. Tic length reflects event duration. A zoomed in view is shown for one event, and a spectrographic representation corresponding to the average of all detected events across 15 15s epochs for 8 control and 8 treated slices (2–3 slice pairs for each of 3 animals) is also shown as indicated (Control or +Hyal). In 3C, we show average SWR event frequency results from the 8 control and 8 hyaluronidase pretreated slices. This endpoint is significantly increased in the hyaluronidase group (p < 0.05). In 3D we show average inter-event interval (IEI) data as an additional form of this measure and in 3E we show a frequency distribution histogram comparing IEIs in control and hyaluronidase treatments. An increase in relatively short IEIs can be appreciated in the hyaluronidase pretreated slices. In contrast to SWR event frequency, SWR and ripple duration were unchanged by hyaluronidase treatment [40.2 +/− 1.1; 38.3 +/− 0.73; p=0.18 (3F) and 23.63 +/−0.95; 26.3 +/− 1.3; p=0.11, data not shown)].
IV. SWR frequency is also increased following exposure to chondroitinase
Multiple in vitro and in vivo studies have also utilized chondrotinase, which targets chondroitin sulfate glycosaminoglycans, to reduce or eliminate PNNs (Lin et al., 2008). Additional studies were therefore performed with chondroitinase to further support a connection between PNN integrity and SWR event frequency. Results are shown in figure 4. Representative traces are shown in 4A–B, and in 4C the average SWR event frequency in 4 control and 4 chondroitinase slices is shown. Based on visual inspection (Koniaris et al., 2011) and consistency with hand count results, the detection threshold for data shown from these experiments was based on SD=3. For both SD3 and SD4, the difference between the groups is significant at p < 0.05. As was observed with hyaluronidase, chondroitinase did not have a significant effect on SWR or ripple duration [44.9 +/− 2.8; 46.8 +/− 4.0; p=0.7 (4D), and 20.44 =/− 0.7; 23.1 +/− 0.9; p= 0.05 (data not shown)].
Figure 4.
SWR event frequency in chondroitinase treated slices. Representative traces are shown in 4A and B, with SW events again in red and ripples in green. The fold change in average SWR event frequency results for 4 control and 4 treated slices is shown in 4C. The difference between control and treatment SWR frequency in 4C is significant (p < 0.05). In 4D, we show that in contrast to SWR event frequency, SWR duration is unchanged by chondroitinase treatment.
V. Hypothetical schematic
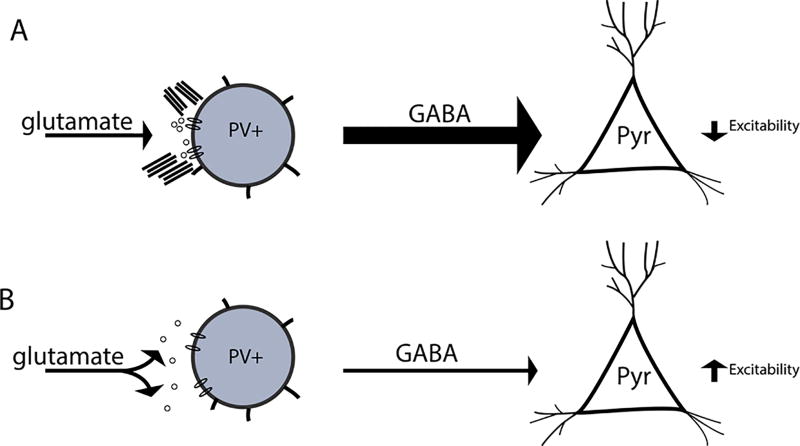
In figure 5 we show a schematic representing a potential mechanism by which removal of perineuronal nets may increase SWR event frequency. In this model, an intact PNN can spatially restrict glutamatergic input to the PV neuron. This allows the PV neuron to inhibit activity of principal pyramidal cell. When PNN integrity is disrupted, however, the diffusion of glutamate and lateral mobility of post-synaptic receptors is likely increased, leading to reduced decreased excitatory input to PV neurons. This in turn leads to reduced inhibition, and thus increased excitability, of pyramidal cells.
Figure 5.
Hypothetical model by which PNN disruption influences the frequency of SWR events. In figure 6 we show a hypothetical model. A PV neuron with an intact PNN is shown at the top left. This neuron receives spatially localized glutamatergic input, allowing it to in turn inhibit pyramidal cell excitability. In contrast, a PV neuron with a disrupted PNN is shown at bottom left. In this scenario, glutamate may more easily diffuse from the synaptic cleft and/or glutamate receptors may demonstrate enhanced lateral mobility. A potential result is that this PV is less activated and thus less able to inhibit pyramidal cell excitability.
Discussion
In the present study we observe that the frequency of SWR events is increased in hippocampal slices that are pretreated with proteases known to disrupt integrity of the PNN (Bikbaev et al., 2015). This suggests that PNN disruption is sufficient not only to reduce glutamatergic input to individual PV cells as previously described (Favuzzi et al., 2017), but to affect the dynamics of large-scale population events initiated by hippocampal pyramidal cells. Since PV FSIs represent the predominant cell type known to be ensheathed by a PNN (Pantazopoulos and Berretta, 2016), PNN digestion likely affects pyramidal cell excitability through modulation of inhibitory input from the PV population. This possibility is supported by studies showing reduced excitability of PV cells, and a reduction in the frequency of inhibitory currents in pyramidal cells, following enzyme-mediated cleavage of PNNs (Slaker et al., 2015). A recent study also suggests loss of the PNN can decrease inhibition in the visual cortex (Lensjo et al., 2017b), an area with an abundance of PNN enwrapped PV expressing neurons (Lensjo et al., 2017a). Of particular interest, this was associated with an increase in gamma frequency activity (Lensjo et al., 2017b). Additionally, in a study of post-traumatic injury in rats, a loss of the PNN was associated with reduced inhibitory tone, leading the authors to suggest that PNN disruption might contribute to post traumatic epilepsy (Hsieh et al., 2016). In related modeling work, it was demonstrated that reduced AMPA input to interneurons can lead to induction of SW bursts followed by high frequency gamma tails (Traub et al., 1996). Disinhibition induced hyperexcitability has also been described in cultured hippocampal neurons following treatment with hyaluronidase (Bikbaev et al., 2015; Vedunova et al., 2013).
In terms of why disrupted PNN integrity would substantially reduce the ability of PV interneurons to inhibit pyramidal cell excitability, hypothetical mechanisms include the possibility that the PNN serves to properly localize glutamatergic input to this cell type (Bikbaev et al., 2015; Frischknecht et al., 2009). Glutamate receptors on PV interneurons are localized to soma and proximal dendrites, both of which are typically ensheathed by PNN (Favuzzi et al., 2017). Of interest, the subpopulation of PV cells that is rich in PNN, as determined by staining with the CSPG brevican, receives relatively robust glutamatergic input (Favuzzi et al., 2017). With PNN disruption released glutamate may more easily diffuse. In the absence of a robust PNN, receptor subunits might also be more motile and less precisely localized (Frischknecht et al., 2009). The negatively charged PNN may also buffer ions such as potassium (Bruckner et al., 1993; Hartig et al., 1999), and it has been proposed that removal of negative charge at the cell membrane could potentially affect the local electric field sensed by gating subunits of ion channels (Balmer, 2016).
Given that PV FSI activity is critical to ripple expression, it is of interest that despite the potential of PNN degradation to reduce PV FSI activity (Slaker et al., 2015), SWRs are well expressed in hyaluronidase and chondroitinase pre-treated slices. One possibility is that while PV cells are less excitable between SW events, allowing SW events to occur more frequently, the PV population remains sufficiently excitable to express ripple activity when a SW initiating event occurs. Consistent with this, a prior study focused on PNN surrounded fast spiking neurons in the medial nucleus of the trapezoid body demonstrated that while PNN digestion reduced the gain of spike output in response to current steps in the background of Gaussian white noise currents, the ability of the neurons to fire up to 1000 Hz in response to current pulses was not affected (Balmer, 2016). In addition, work by Caputi et al. showed that reduced expression of GluA4 on FSIs was associated with increased firing of pyramidal cells but no alteration in the firing of FSIs during SWRs (Caputi et al., 2012). In contrast to SWR event frequency, we did not observe a change in ripple duration. A different combination of factors may influence SWR density as compared to ripple duration (Malerba et al., 2016). Though both may be affected by select perturbations, others including age and select neurochemicals can affect SWR density in the absence of an effect on SWR or ripple duration (Decker et al., 2009; Wiegand et al., 2016).
Because PV interneurons represent the main cell type known to be ensheathed by a PNN, and because this cell type may be critical to SWR initiation and expression, our hypothetical mechanism (figure 5) is focused on changes in PV excitability. We acknowledge, however, that future studies will be necessary to examine the effect of PNN degradation on both PV cell and pyramidal cell activity during SWs and SWRs. It will also be of interest to determine where SWR events are initiated in control and hyaluronidase conditions. These experiments will be important in that within CA2 hippocampus, a subpopulation of pyramidal cells is ensheathed by a PNN, and degradation of CA2 PNNs has been linked to recovery of the potential for long-term potentiation in this normally resistant region (Carstens et al., 2016). As is the case for CA3, pyramidal cell activation within CA2 can initiate SWRs (Oliva et al., 2016) and thus PNN degradation in this area might directly increase pyramidal cell activation. It should be noted, however, that basal synaptic transmission in CA2 pyramidal cells was not affected by hyaluronidase treatment of slices (Carstens et al., 2016).
In terms of the significance of PNN net integrity as an effector of SWR event frequency, expression and/or integrity of PNN components can be influenced by age, enrichment or disease (Berretta, 2012; Matuszko et al., 2017; Quattromani et al., 2017; Slaker et al., 2016; Wiegand et al., 2016). Glial cells express critical components of the PNN, and expression of components is regulated by factors including neuronal activity (Howell and Gottschall, 2012; Matthews et al., 2002; McRae et al., 2007; Song and Dityatev, 2017). In addition, several PNN cleaving enzymes are expressed by neurons and glia (Brzdak et al., 2017; Deb and Gottschall, 1996; Dubey et al., 2017; Janusz et al., 2013; Pollock et al., 2014; Szklarczyk et al., 2002; Wiera et al., 2017; Yong et al., 1998). Soluble and transmembrane matrix metalloproteinases have been particularly well studied for their potential to disrupt PNN integrity, and their expression and/or activity is can be dramatically altered by physiological or pathological neuronal activity (Conant et al., 2010; Meighan et al., 2006; Nagy et al., 2006), disease states and select therapeutics (Pollock et al., 2014; Szklarczyk et al., 2002).
Of additional relevance are studies focused on psychiatric diseases including schizophrenia and major depression. These conditions have been associated with altered PNN integrity in combination with altered neuronal population activity (Berretta et al., 2015; Khalid et al., 2016; Korotkova et al., 2010). Major depression has been associated with both increased and decreased levels of PNN degrading enzymes, which may reflect the opposing influence of stress or inflammation in the former as opposed to reduced monoamine-stimulated protease expression in the latter. The antidepressant fluoxetine, which enhances monoaminergic tone, has been shown to increase MMP-9 levels and to reduce PNN integrity (Guirado et al., 2014). While multiple mechanisms likely contribute to fluoxetine’s antidepressant efficacy (Lee et al., 2015; Nestler et al., 1990; Nibuya et al., 1996), given that reduced excitatory/inhibitory balance has been implicated in the condition (Thompson et al., 2015), fluoxetine’s potential to reduce PNN integrity could contribute.
In summary, we demonstrate that the PNN disrupting enzymes hyaluronidase and chondroitinase can significantly increase the frequency of SWR events in horizontal hippocampal slices. We suggest that further study may be warranted to test the importance of PNNs on in vivo SWR dynamics and memory consolidation, in both normal physiology and disease states.
Figure 2.
Horizontal slice preparation and staining. Horizontal slices were prepared and bisected, with matched hemisections placed in one of two recovery solutions (ACSF or ACSF with hyaluronidase). Following a 2h recovery period, hemislices were used for additional studies. A schematic of the set up is shown in 2A, and representative PV fluorescence in cells from control (n=1392; 6 fields) and hyaluronidase (n=927; 5 fields) treated slices, prepared from PVtd-Tomato mice, is shown in 2B. No significant difference was detected. In 2C and D, we show representative immunostaining of a hippocampal slice from a PVtd-Tomato mouse with WFA. Layers of CA1, CA2, and CA3 areas indicated by yellow rectangles are shown magnified in c1–c3 for control slices, and d1–d3 for hyaluronidase treated slices, respectively. WFA positive PV expressing cells can be appreciated in control but not hyaluronidase treatment conditions. Scale bars: 100 µm (C, D), 50 µm (c1–c3, d1–d3), and 20 µm for high magnification single neurons.
Figure 3.
SWR event frequency in hyaluronidase treated slices. A: 1st trace: Control slice 15s recording of LFP (filtered 0.5–1500Hz). Blue bars indicate detected SWR event, defined as a coincident SW and ripple event. 2nd trace: lower frequency SW component filtered 1–30Hz. 3rd trace: Root mean square of SW filtered from 1–30Hz. Dashed lines indicate 2 and 4 SD above baseline for event detection. Red bars below indicate detected SW event. 4th trace: high frequency ripple component filtered from 80–250Hz. 5th trace: Root mean square of ripple filtered 80–250Hz. Dashed lines indicate 2 and 4 SD above baseline for event detection. Green bars below indicate detected ripple event. B shows the same features for Hyaluronidase treatment. Zoomed in views of indicated SWR events are shown, as are spectrogram averages for the indicated frequency ranges in all detected SWR events from 8 control and 8 hyaluronidase pretreated slices (time-locked to the SW peak; n= 743 control events and 1114 hyaluronidase events). C shows the fold change in average SWR event frequency for two to three paired slices from each of 3 different animals (n=8 slices per group), and overall SWR inter-event interval results from the 8 control and 8 treated slices is shown in D. The difference between control and treatment SWR frequency in 3C and D is significant (p < 0.05). A frequency histogram of inter-event intervals is shown in E. In 3F, we show that in contrast to SWR event frequency, SWR duration is unchanged by hyaluronidase treatment.
Acknowledgments
We would like to acknowledge funding from the Von Matsch professorship for neurological diseases, the Dean’s Toulmin award, and the National Institutes of Health (NS083410). AC and PLB received support from T32 NS041231. ZS received support from a Chinese State Scholarship (201408220100).
Grant Sponsor: National Institutes of Health (United States)
Grant numbers: NS083410; NS 041231
Footnotes
The authors report no conflicts of interest.
References
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: eleven maps for eleven rooms. Proc Natl Acad Sci U S A. 2014;111(52):18428–35. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer TS. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro. 2016;3(4) doi: 10.1523/ENEURO.0112-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012;62(3):1584–1597. doi: 10.1016/j.neuropharm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Markota M, Brown C, Batzianouli ET. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr Res. 2015;167(1–3):18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikbaev A, Frischknecht R, Heine M. Brain extracellular matrix retains connectivity in neuronal networks. Sci Rep. 2015;5:14527. doi: 10.1038/srep14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner G, Brauer K, Hartig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8(3):183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- Brzdak P, Wlodarczyk J, Mozrzymas JW, Wojtowicz T. Matrix Metalloprotease 3 Activity Supports Hippocampal EPSP-to-Spike Plasticity Following Patterned Neuronal Activity via the Regulation of NMDAR Function and Calcium Flux. Mol Neurobiol. 2017;54(1):804–816. doi: 10.1007/s12035-016-9970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398(2):242–52. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi A, Fuchs EC, Allen K, Le Magueresse C, Monyer H. Selective Reduction of AMPA Currents onto Hippocampal Interneurons Impairs Network Oscillatory Activity. Plos One. 2012;7(6) doi: 10.1371/journal.pone.0037318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens KE, Phillips ML, Pozzo-Miller L, Weinberg RJ, Dudek SM. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J Neurosci. 2016;36(23):6312–20. doi: 10.1523/JNEUROSCI.0245-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ccedil;aliskan G, Schulz SB, Gruber D, Behr J, Heinemann U, Gerevich Z. Corticosterone and corticotropin-releasing factor acutely facilitate gamma oscillations in the hippocampus in vitro. European Journal of Neuroscience. 2015;41(1):31–44. doi: 10.1111/ejn.12750. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17(4):239–49. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166(2):508–21. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19(16):RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S, Gottschall PE. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem. 1996;66(4):1641–7. doi: 10.1046/j.1471-4159.1996.66041641.x. [DOI] [PubMed] [Google Scholar]
- Decker JM, Wojtowicz AM, Ul Haq R, Braunewell KH, Heinemann U, Behrens CJ. C-type natriuretic peptide decreases hippocampal network oscillations in adult rats in vitro. Neuroscience. 2009;164(4):1764–75. doi: 10.1016/j.neuroscience.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10(10):1241–2. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67(5):570–88. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469(7330):397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey D, McRae PA, Rankin-Gee EK, Baranov E, Wandrey L, Rogers S, Porter BE. Increased metalloproteinase activity in the hippocampus following status epilepticus. Epilepsy Res. 2017;132:50–58. doi: 10.1016/j.eplepsyres.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20(1):1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15(4):222–8. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, Maeso P, Fernandes C, Ewers H, Rico B. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron. 2017 doi: 10.1016/j.neuron.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, Kang SH, Bergles DE, Lee SI, Levine JM, et al. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci. 2014;34(49):16369–84. doi: 10.1523/JNEUROSCI.1309-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440(7084):680–3. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12(7):897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100(21):12486–91. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–3. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castren E, Nacher J. Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Int J Neuropsychopharmacol. 2014;17(10):1635–46. doi: 10.1017/S1461145714000406. [DOI] [PubMed] [Google Scholar]
- Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, Reichenbach A, Bruckner G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842(1):15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Kalb RG, Zaremba S, Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–14. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Howell MD, Gottschall PE. Lectican proteoglycans, their cleaving metalloproteinases, and plasticity in the central nervous system extracellular microenvironment. Neuroscience. 2012;217:6–18. doi: 10.1016/j.neuroscience.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TH, Lee HH, Hameed MQ, Pascual-Leone A, Hensch TK, Rotenberg A. Trajectory of Parvalbumin Cell Impairment and Loss of Cortical Inhibition in Traumatic Brain Injury. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L, Dziembowska M. The Fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci. 2013;33(46):18234–41. doi: 10.1523/JNEUROSCI.2207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10(1):100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Khalid A, Kim BS, Seo BA, Lee ST, Jung KH, Chu K, Lee SK, Jeon D. Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neurosci. 2016;17:4. doi: 10.1186/s12868-016-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Hensch TK. Germline recombination by conditional gene targeting with Parvalbumin-Cre lines. Front Neural Circuits. 2013;7:168. doi: 10.3389/fncir.2013.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniaris E, Drimala P, Sotiriou E, Papatheodoropoulos C. Different effects of zolpidem and diazepam on hippocampal sharp wave-ripple activity in vitro. Neuroscience. 2011;175:224–34. doi: 10.1016/j.neuroscience.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68(3):557–69. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71(11):1073–89. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, Wang CL, Wang LJ, Lee IH, Wang TY, et al. Correlation of plasma brain-derived neurotrophic factor and metabolic profiles in drug-naive patients with bipolar II disorder after a twelve-week pharmacological intervention. Acta Psychiatr Scand. 2015;131(2):120–8. doi: 10.1111/acps.12324. [DOI] [PubMed] [Google Scholar]
- Lensjo KK, Christensen AC, Tennoe S, Fyhn M, Hafting T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro. 2017a;4(3) doi: 10.1523/ENEURO.0379-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M. Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J Neurosci. 2017b;37(5):1269–1283. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Kwok JC, Crespo D, Fawcett JW. Chondroitinase ABC has a long-lasting effect on chondroitin sulphate glycosaminoglycan content in the injured rat brain. J Neurochem. 2008;104(2):400–8. doi: 10.1111/j.1471-4159.2007.05066.x. [DOI] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. Journal of Physiology-London. 2003;550(3):873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba P, Krishnan GP, Fellous JM, Bazhenov M. Hippocampal CA1 Ripples as Inhibitory Transients. PLoS Comput Biol. 2016;12(4):e1004880. doi: 10.1371/journal.pcbi.1004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx M, Haas CA, Haussler U. Differential vulnerability of interneurons in the epileptic hippocampus. Front Cell Neurosci. 2013;7:167. doi: 10.3389/fncel.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22(17):7536–47. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszko G, Curreli S, Kaushik R, Becker A, Dityatev A. Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience. 2017 doi: 10.1016/j.neuroscience.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–8. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27(20):5405–13. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96(5):1227–41. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991;58(2):151–8. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26(7):1923–34. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, McMahon A, Sabban EL, Tallman JF, Duman RS. Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci U S A. 1990;87(19):7522–6. doi: 10.1073/pnas.87.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–72. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Oliva A, Fernandez-Ruiz A, Buzsaki G, Berenyi A. Role of Hippocampal CA2 Region in Triggering Sharp-Wave Ripples. Neuron. 2016;91(6):1342–55. doi: 10.1016/j.neuron.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Berretta S. In Sickness and in Health: Perineuronal Nets and Synaptic Plasticity in Psychiatric Disorders. Neural Plast. 2016;2016:9847696. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Sotiriou E, Kotzadimitriou D, Drimala P. At clinically relevant concentrations the anaesthetic/amnesic thiopental but not the anticonvulsant phenobarbital interferes with hippocampal sharp wave-ripple complexes. BMC Neurosci. 2007;8:60. doi: 10.1186/1471-2202-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, Eichenbaum H. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci. 2016;19(8):992–4. doi: 10.1038/nn.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock E, Everest M, Brown A, Poulter MO. Metalloproteinase inhibition prevents inhibitory synapse reorganization and seizure genesis. Neurobiol Dis. 2014;70:21–31. doi: 10.1016/j.nbd.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Quattromani MJ, Pruvost M, Guerreiro C, Backlund F, Englund E, Aspberg A, Jaworski T, Hakon J, Ruscher K, Kaczmarek L, et al. Extracellular Matrix Modulation Is Driven by Experience-Dependent Plasticity During Stroke Recovery. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz A, Ponomarenko AA, Fuchs EC, Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J Neurosci. 2009;29(8):2563–8. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G, Eban E, Frank LM. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat Neurosci. 2017;20(2):251–259. doi: 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski JH, Jones MW, Mellor JR. Ripples make waves: binding structured activity and plasticity in hippocampal networks. Neural Plast. 2011;2011:960389. doi: 10.1155/2011/960389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingloff D, Kali S, Freund TF, Hajos N, Gulyas AI. Mechanisms of sharp wave initiation and ripple generation. J Neurosci. 2014;34(34):11385–98. doi: 10.1523/JNEUROSCI.0867-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21(5):1123–8. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Slaker M, Barnes J, Sorg BA, Grimm JW. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. Plos One. 2016;11(12) doi: 10.1371/journal.pone.0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35(10):4190–202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2017 doi: 10.1016/j.brainresbull.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Berretta S, Blacktop JM, Fawcett JW, Kitagawa H, Kwok JC, Miquel M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J Neurosci. 2016;36(45):11459–11468. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22(3):920–30. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015;38(5):279–94. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493(Pt 2):471–84. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedunova M, Sakharnova T, Mitroshina E, Perminova M, Pimashkin A, Zakharov Y, Dityatev A, Mukhina I. Seizure-like activity in hyaluronidase-treated dissociated hippocampal cultures. Front Cell Neurosci. 2013;7:149. doi: 10.3389/fncel.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373(6515):612–5. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wiegand JPL, Gray DT, Schimanski LA, Lipa P, Barnes CA, Cowen SL. Age Is Associated with Reduced Sharp-Wave Ripple Frequency and Altered Patterns of Neuronal Variability. Journal of Neuroscience. 2016;36(20):5650–5660. doi: 10.1523/JNEUROSCI.3069-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiera G, Nowak D, van Hove I, Dziegiel P, Moons L, Mozrzymas JW. Mechanisms of NMDA Receptor- and Voltage-Gated L-Type Calcium Channel-Dependent Hippocampal LTP Critically Rely on Proteolysis That Is Mediated by Distinct Metalloproteinases. J Neurosci. 2017;37(5):1240–1256. doi: 10.1523/JNEUROSCI.2170-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21(2):75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]