Abstract
Insulin stimulates lipogenesis but insulin resistance is also associated with increased hepatic lipogenesis in obesity. However, the underlying mechanism remains poorly characterized. Here, we show a noncanonical insulin-Snail1 pathway that suppresses lipogenesis. Insulin robustly upregulates zinc-finger protein Snail1 in a PI 3-kinase-dependent manner. In obesity, the hepatic insulin-Snail1 cascade is impaired due to insulin resistance. Hepatocyte-specific deletion of Snail1 enhances insulin-stimulated lipogenesis in hepatocytes, exacerbates dietary NAFLD in mice, and attenuates NAFLD-associated insulin resistance. Liver-specific overexpression of Snail1 has the opposite effect. Mechanistically, Snail1 binds to the fatty acid synthase promoter and recruits HDAC1/2 to induce deacetylation of H3K9 and H3K27, thereby repressing fatty acid synthase promoter activity. Our data suggest that insulin pathways bifurcate into canonical (lipogenic) and noncanonical (anti-lipogenesis by Snail1) two arms. The noncanonical arm counterbalances the canonical arm through Snail1-elicited epigenetic suppression of lipogenic genes. Impairment in the insulin-Snail1 arm may contribute to NAFLD in obesity.
Insulin promotes lipogenesis but, on the other hand, insulin resistance is associated with increased lipogenesis in the liver. Here the authors show that Snail1 is upregulated by insulin and inhibits lipogenesis by repressing Fasn expression but insulin-mediated Snail1 upregulation is impaired during obesity and insulin resistance.
Introduction
Prevalence of nonalcoholic fatty liver disease (NAFLD) increases in an alarming pace due to the obesity epidemic1. The outcomes of NAFLD are ominous, including insulin resistance, type 2 diabetes, dyslipidemia, cardiovascular disease, liver fibrosis, cirrhosis, and/or hepatocellular carcinoma1–3. Liver lipid levels are determined by an interplay between de novo lipogenesis, lipid uptake, fatty acid β oxidation, and very low-density lipoprotein (VLDL) secretion. Notably, hepatic lipogenesis increases in NAFLD4,5, and genetic disruption of the hepatic lipogenic program prevents NAFLD6–8. Thus, inhibiting hepatic lipogenesis likely provides a therapeutic strategy for combatting NAFLD.
Liver lipogenesis is regulated predominantly by metabolic hormone insulin. Insulin stimulates the canonical lipogenic pathway, including activation of lipogenic transcription factors liver X receptor (Lxr), Srebp-1c, and upstream stimulatory factor-1 (Usf-1)5,9. These nuclear proteins activate expression of lipogenic enzymes ATP citrate lyase (Acl), acetyl coenzyme A carboxylase 1 (Acc1), and/or fatty acid synthase (Fasn)5. Paradoxically, insulin resistance is associated with increased hepatic lipogenesis in obesity, contributing to NAFLD10. However, the underlying mechanism remains poorly understood.
We recently reported that insulin upregulates adipose Snail1 that in turn suppresses expression of adipose triacylglycerol lipase (ATGL) and ATGL-mediated lipolysis11. Snail1 is a transcriptional repressor, and has been known to induce epithelial-to-mesenchymal transition (EMT) during development or in cancer metastasis12–14. Snail1 has been well documented to epigenetically suppress expression of E-cadherin and claudin, thus promoting EMT15–18. Mechanistically, Snail1 binds via its N-terminal SNAG domain to several epigenetic enzymes, including histone deacetylases (HDACs) and histone methyltransferases, and recruit them to target promoters where these enzymes catalyze repressive histone modifications14,15. Notably, we found two reports that describe the potential action of hepatic Snail1 in liver injury and regeneration19,20; however, the metabolic function of hepatic Snail1 has not been explored. In this study, we provide proof of concept evidence showing that hepatic Snail1 is an unrecognized suppressor of de novo lipogenesis. It epigenetically represses expression of lipogenic enzymes. We further demonstrate that insulin robustly upregulates Snail1 which defines the noncanonical anti-lipogenic pathway. Thus, this work unravels a bifurcation of insulin signaling into the canonical lipogenic and the noncanonical anti-lipogenic arms.
Results
Insulin upregulates hepatic Snail1 via PI 3-kinase pathway
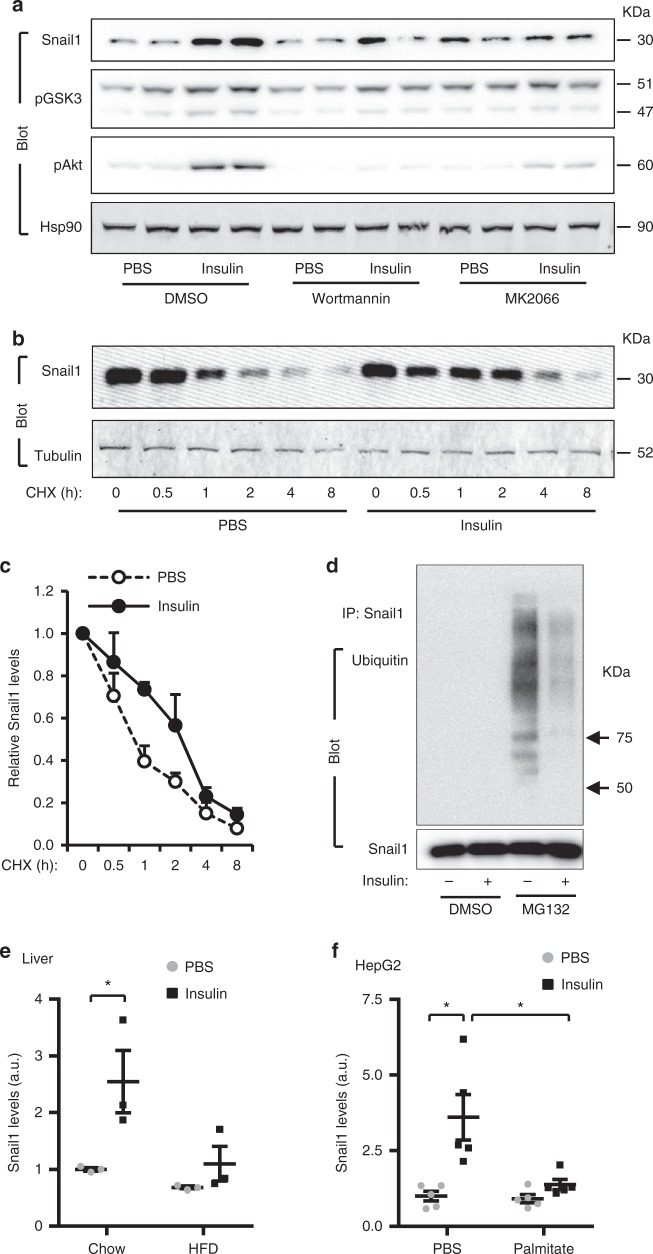
Given that insulin stimulates Snail1 expression in adipocytes11, we postulated that insulin might similarly upregulate Snail1 in hepatocytes. Indeed, insulin markedly increased Snail1 mRNA levels in both mouse primary hepatocytes and human HepG2 hepatocytes (Supplementary Fig. 1a, b), and substantially increased Snail1 protein levels in HepG2 hepatocytes (Fig. 1a). Likewise, insulin markedly increased the mRNA and protein levels of hepatic Snail1 in C57BL/6 mice (Supplementary Fig. 1c, d). Consistently, liver Snail1 levels were lower in the fasted state (low plasma insulin levels) than in the fed state (Supplementary Fig. 1e). To identify pathways responsible for upregulation of hepatic Snail1, we pretreated HepG2 hepatocytes with PI 3-kinase (wortmannin) or Akt (MK2066) inhibitors. Inhibition of either PI 3-kinase or Akt blocked upregulation of Snail1 by insulin (Fig. 1a and Supplementary Fig. 1f). Notably, Akt2 was reported to mediate TFGβ1-induced upregulation of Snail121. These data suggest that the PI 3-kinase/Akt pathway is required for insulin to upregulate hepatic Snail1.
Fig. 1.
Insulin upregulates hepatic Snail1 via the PI 3-kinase pathway. a HepG2 cells were pretreated with wortmannin or MK2066, and then stimulated with insulin for 2 h. Cell extracts were immunoblotted with the indicated antibodies. Snail1 levels were normalized to Hsp90 levels (n = 4). b, c HepG2 cells were transfected with Snail1 plasmids and stimulated with insulin in the presence of cycloheximide. Cell extracts were immunoblotted with antibodies against Snail1 or α-tubulin. Snail1 levels (normalized to α-tubulin levels) were plotted against cycloheximide treatment durations (n = 3). d HepG2 cells were transduced with Snail1 adenoviral vectors and stimulated with insulin with or without MG132 (5 μM). Cell extracts were immunoprecipitated with antibody against Snail1 and immunoblotted with antibodies against ubiquitin or Snail1. e C57BL/6 males were fed a HFD or chow diet for 6 weeks, fasted overnight (14 h), and stimulated with insulin for 2 h. Liver nuclear extracts were immunoblotted with antibodies against Snail1 or lamin A/C. Snail1 levels were normalized to lamin A/C levels (n = 3). f HepG2 cells were pretreated with palmitate overnight and then stimulated with insulin. Cell extracts were immunoblotted with antibodies against Snail1 or Hsp90. Snail1 levels were quantified and normalized to Hsp90 levels (n = 4). Data are presented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
Given that GSK3β induces ubiquitination and degradation of Snail122,23, we speculated that insulin might suppress proteasome-mediated degradation of Snail1 via GSK3β. Insulin stimulated phosphorylation and inactivation of GSK3β (Fig. 1a), confirming that insulin inhibits GSK3β via the PI 3-kinase/Akt pathway24. First, we assessed Snail1 stability in HepG2 hepatocytes using protein synthesis inhibitor cycloheximide. Insulin profoundly inhibited degradation of Snail1 (Fig. 1b). Half-life of Snail1 was prolonged from 0.8 h in PBS-treated cells to 2.4 h in insulin-stimulated cells (Fig. 1c). Second, we measured ubiquitination of Snail1. We did not detect ubiquitinated Snail1 in DMSO-treated HepG2 cells (Fig. 1d), presumably due to rapid degradation. Hence, we blocked degradation by pretreating cells with proteasome inhibitor MG132. We detected robust ubiquitination of Snail1; importantly, insulin dramatically decreased the levels of ubiquitinated Snail1 (Fig. 1d).
We asked if the insulin/Snail1 axis is impaired in obesity, owing to insulin resistance. C57BL/6 mice were fed a high fat diet (HFD) for 6 weeks to induce obesity. Insulin treatment substantially increased hepatic Snail1 levels in chow-fed mice but not in HFD-fed mice (Fig. 1e and Supplementary Fig. 1g). To validate these findings in vitro, we pretreated HepG2 hepatocytes with palmitic acid to model metabolic environments in obesity. Palmitic acid pretreatment abrogated the ability of insulin to upregulate Snail1 (Fig. 1e and Supplementary Fig. 1h, i). Thus, the hepatic insulin/Snail1 axis is impaired in obesity.
Insulin/Snail1 axis suppresses hepatic lipogenesis
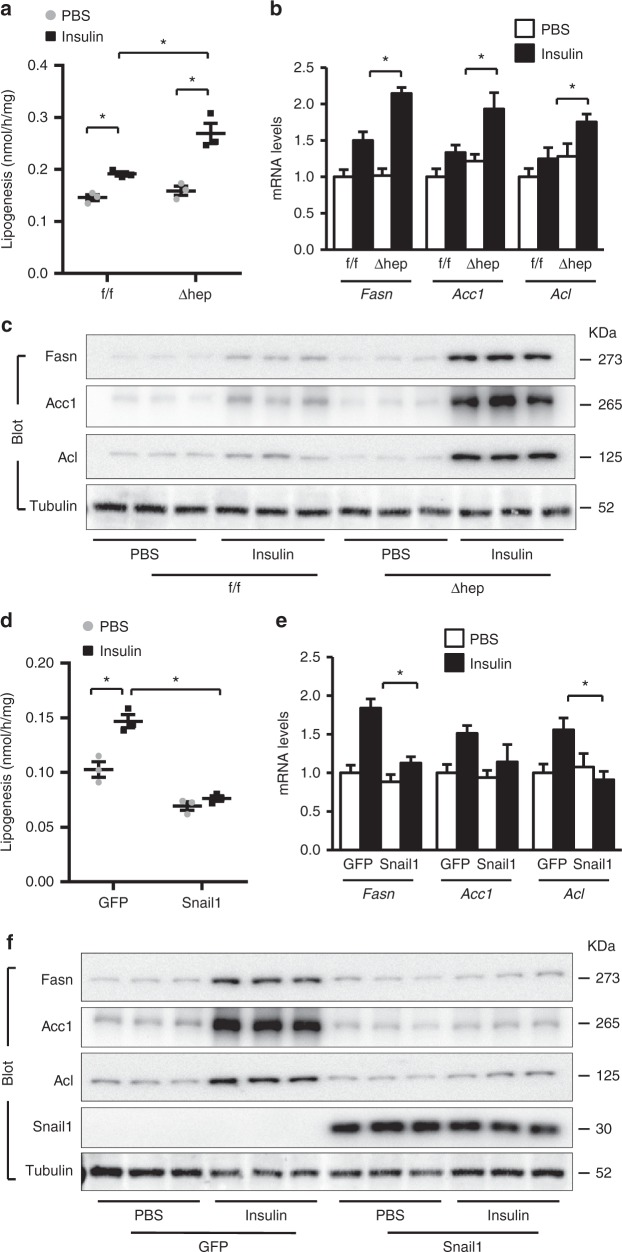
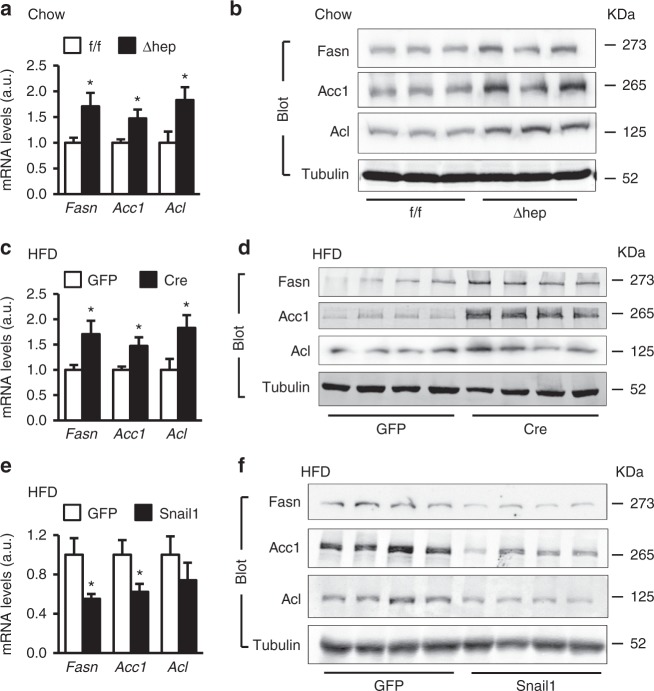
We sought to delineate the role of the insulin/Snail1 axis in hepatic lipogenesis. To disrupt this axis, we generated inducible, hepatocyte-specific Snail1 knockout (Snail1Δhep) mice by crossing Snail1flox/flox mice with albumin-CreERT2 drivers25,26. Snail1flox/flox;CreERT2 mice were treated with tamoxifen to obtain Snail1Δhep mice. Snail1flox/flox littermates were treated similarly with tamoxifen as control. Snail1 was disrupted in the livers but not white adipose tissue (WAT) of Snail1Δhep mice (Supplementary Fig. 2a). We assessed lipogenesis in primary hepatocytes isolated from Snail1Δhep and Snail1flox/flox mice. Deletion of Snail1 substantially increased the ability of insulin to stimulate lipogenesis (Fig. 2a), and caused an upward shift in insulin dose response curves (Supplementary Fig. 2b). To corroborate these findings, we measured expression of key lipogenic enzymes Fasn, Acc1, and Acl. Snail1 deficiency markedly enhanced the ability of insulin to increase both mRNA (Fig. 2b) and protein (Fig. 2c) levels of these enzymes. Given upregulating Snail1 by insulin, these results unveil that the insulin/Snail1 axis inhibits lipogenesis in a feedforward manner.
Fig. 2.
Insulin/Snail1 axis suppresses de novo lipogenesis in hepatocytes. a–c Primary hepatocytes were isolated from Snail1Δhep and Snail1flox/flox littermates and stimulated with insulin (50 nM) or PBS for 12 h (a, c) or 4 h (b). a De novo lipogenesis assays (normalized to protein levels; n = 3). b Fasn, Acc1, and Acl mRNA levels (normalized to 36B4 levels; n = 4). c Cell extracts were immunoblotted with the indicated antibodies. d–f Primary hepatocytes were transduced with Snail1 or GFP adenoviral vectors and stimulated with insulin (50 nM) for 12 h (d, f) or 4 h (e). d De novo lipogenesis (normalized to protein levels; n = 3). e Fasn, Acc1, and Acl mRNA levels (normalized to 36B4 levels; n = 4). f Cell extracts were immunoblotted with the indicated antibodies. Data are presented as mean ± SEM. *p < 0.05 by two-tailed unpaired Student’s t test
To further confirm the antagonistic effect of the insulin/Snail1 axis on lipogenesis, we overexpressed Snail1 in primary hepatocytes using Snail1 adenoviral vectors. Green fluorescent protein (GFP) vectors were used as control. Overexpression of Snail1 completely blocked insulin-stimulated lipogenesis; notably, it also decreased baseline lipogenesis (Fig. 2d). In agreement with these results, Snail1 blocked insulin-stimulated expression of Fasn, Acc1, and Acl (Fig. 2e, f). Notably, Snail1 did not inhibit insulin-stimulated phosphorylation of Akt (Supplementary Fig. 2c), further supporting the notion that the insulin/Snail1 axis suppresses lipogenesis via a feedforward circuit. Snail1 did not alter the ability of insulin to suppress gluconeogenesis in primary hepatocytes (Supplementary Fig. 2d), suggesting that the insulin/Snail1 axis specifically regulates lipid but not glucose metabolism in hepatocytes. In light of these findings, we propose that insulin signaling bifurcates into the canonical (lipogenic) and the noncanonical Snail1 (anti-lipogenic) arms.
Hepatocyte-specific deletion of Snail1 promotes NAFLD
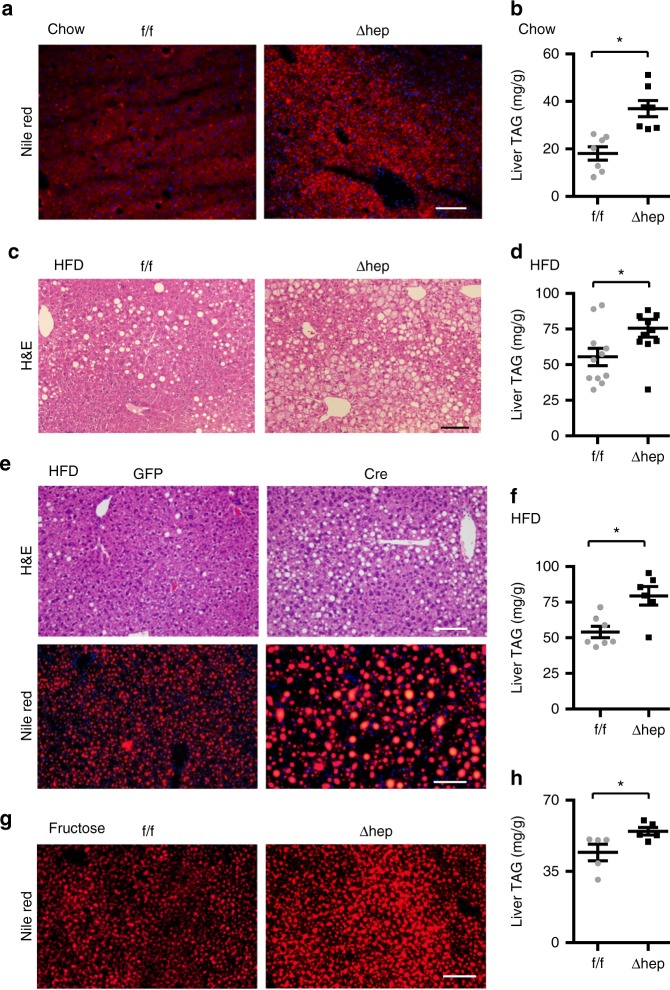
We set out to explore the function of the noncanonical Snail1 arm in vivo by characterizing Snail1Δhep mice. Snail1Δhep mice were grossly normal. Body weight and plasma triacylglycerol (TAG) and nonesterified fatty acids (NEFA) were comparable between Snail1Δhep and Snail1flox/flox littermates (Supplementary Fig. 3a, b). Hepatocyte lipid droplets were larger and more abundant in Snail1Δhep mice relative to Snail1flox/flox littermates, as assessed by staining liver sections with neutral lipid dye Nile red (Fig. 3a). Consistently, liver TAG levels were significantly higher in Snail1Δhep mice (Fig. 3b). To extend these findings in obesity, we placed Snail1Δhep and Snail1flox/flox mice on a HFD for 10 weeks. Hepatocyte lipid droplets were substantially more abundant in Snail1Δhep mice relative to Snail1flox/flox littermates (Fig. 3c). Liver TAG levels were significantly higher in Snail1Δhep mice (Fig. 3d), although body weight and plasma TAG and NEFA levels were comparable between Snail1Δhep and Snail1flox/flox mice (Supplementary Fig. 3c, d).
Fig. 3.
Hepatocyte-specific deletion of Snail1 promotes NAFLD. a, b Snail1flox/flox (n = 7) and Snail1Δhep (n = 7) males (18 weeks) were fed a normal chow diet. Livers were isolated under non-fasted conditions. a Representative Nile red staining of liver sections. b Liver TAG levels (normalized to liver weight). c, d Snail1flox/flox (n = 11) and Snail1Δhep (n = 11) male littermates were fed a HFD for 10 weeks. c Representative H&E staining of liver sections. d Liver TAG levels (normalized to liver weight). e, f Snail1flox/flox males were fed a HFD for 6 weeks and transduced with AAV-TBG-GFP (n = 7) or AAV-TBG-Cre (n = 6) vectors for 4 weeks. e Representative H&E or Nile red staining of liver sections. f Liver TAG levels (normalized to liver weight). g, h Snail1flox/flox (n = 5) and Snail1Δhep (n = 5) males were fed a fructose diet for 10 weeks. Scale bars: 100 µm. Data are represented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
To address concerns about the potential effect of tamoxifen on the observed phenotypes, we generated an independent line of Snail1Δhep mice by crossing Snail1flox/flox mice with albumin-Cre drivers. These mice were fed a HFD for 10 wks, and body weight was comparable between sex-matched Snail1Δhep and Snail1flox/flox mice (Supplementary Fig. 3e). Liver TAG levels were significantly higher in Snail1Δhep males and females relative to sex-matched Snail1flox/flox littermates (Supplementary Fig. 3f). Additionally, we deleted hepatocyte Snail1 in adult Snail1flox/flox mice using adeno-associated virus (AAV) Cre vectors27. Snail1flox/flox mice were fed a HFD for 6 weeks and then transduced with AAV-TBG-Cre or AAV-TBG-GFP (control) vectors. Snail1 was deleted in the livers but not the WAT of AAV-TBG-Cre-transduced mice (Supplementary Fig. 3g). Hepatocyte lipid droplets were more abundant and larger (Fig. 3e), and liver TAG levels were significantly higher in AAV-TBG-Cre mice relative to AAV-TBG-GFP mice (Fig. 3f). Body weight was similar between these two groups (Supplementary Fig. 3h). Furthermore, deletion of hepatocyte Snail1 similarly exacerbated fructose-induced liver steatosis in Snail1Δhep mice relative to Snail1flox/flox mice (Fig. 3g, h). Taken together, these data suggest that suppression of the noncanonical Snail1 arm exacerbates NAFLD independently of changes in body weight.
Liver-specific expression of Snail1 protects against NAFLD
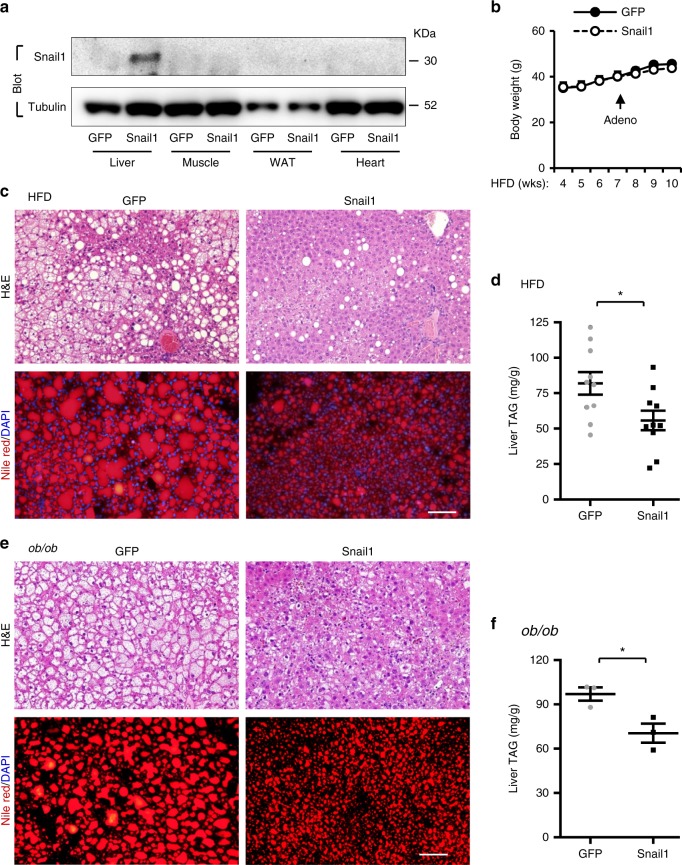
We reasoned that enhancing the hepatic insulin/Snail1 arm might inhibit liver lipogenesis and NAFLD progression. C57BL/6 mice were fed a HFD for 7 weeks and transduced with Snail1 (GFP as control) adenoviral vectors. Recombinant Snail1 was detected in the liver but no other tissues as expected (Fig. 4a). Although body weight was comparable between Snail1 and GFP adenoviral-transduced mice (Fig. 4b), hepatocyte lipid droplets were considerably smaller and less abundant in Snail1 adenoviral-transduced mice (Fig. 4c). Liver TAG levels were significantly lower in Snail1 than in GFP mice (Fig. 4d). Likewise, liver-specific overexpression of Snail1 attenuated liver steatosis in ob/ob mice with genetic obesity (Fig. 4e, f). These data suggest that the insulin/Snail1 arm puts a brake on lipogenesis, thereby protecting against liver steatosis and lipotoxicity.
Fig. 4.
Liver-specific overexpression of Snail1 protects against NAFLD. a–d C57BL/6 male mice (8–9 weeks) were fed a HFD for 7 weeks and transduced with Snail1 (n = 10) or GFP (n = 10) adenoviral vectors. Livers were harvested 3 weeks after transduction. a Tissue extracts were immunoblotted with the indicated antibodies. b Growth curves. c Representative H&E or Nile red staining of liver sections. Scale bar: 100 µm. d Liver TAG levels (normalized to liver weight). e, f ob/ob male mice (9 weeks) were transduced with GFP (n = 3) or Snail1 (n = 3) adenoviral vectors for 3 weeks. e Representative H&E or Nile red staining of liver sections. f Liver TAG levels (normalized to liver weight). Scale bars: 100 µm. Data are presented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
Hepatic Snail1 suppresses liver lipogenesis in vivo
To confirm inhibition of lipogenic programs by the Snail1 arm in vivo, we measured hepatic levels of key lipogenic enzymes Fasn, Acc1, and Acl in Snail1Δhep mice fed a chow diet. Deletion of hepatic Snail1 substantially increased both mRNA (Fig. 5a) and protein (Fig. 5b) levels of these enzymes in Snail1Δhep mice compared to Snail1flox/flox littermates. To extend these findings to NAFLD, we placed Snail1flox/flox mice on a HFD for 6 weeks, followed by transduction with AAV-TBG-Cre (delete hepatic Snail1) or AAV-TBG-GFP (control) vectors. Deletion of hepatocyte Snail1 markedly increased both mRNA and protein levels of liver Fasn, Acc1, and Acl (Fig. 5c, d). We also performed an unbiased gene expression profiling analysis, and found that expression of lipogenic genes as well as genes encoding lipid droplet proteins was higher in mice with hepatocyte-specific ablation of Snail1 (Supplementary Fig. 4a). In contrast, expression of the genes that control lipid uptake, fatty acid β oxidation, and VLDL secretion was relatively normal (Supplementary Fig. 4a). Consistently, liver-specific overexpression of Snail1 did not alter expression of the genes controlling β oxidation and VLDL secretion (Supplementary Fig. 4b). Thus, protection against NAFLD by hepatic Snail1 can be explained, at least in part, by reduction in liver de novo lipogenesis. To further test this notion, C57BL/6 mice were fed a HFD for 6 weeks (increasing baseline lipogenesis) and transduced with Snail1 (overexpressing Snail1 in the liver) or GFP (control) adenoviral vectors. Overexpression of Snail1 profoundly decreased both mRNA and protein levels of Fasn, Acc1, and Acl in the liver (Fig. 5e, f). In contrast, expression of the genes that control liver inflammation (TNFα, IL6, MCP1, F4/80), fibrosis (Fn1, vimentin, αSMA, Colla 1a1), and EMT (Ecad, Cldh1) were similar between these two groups (Supplementary Fig. 4b).
Fig. 5.
Snail1 directly suppresses liver lipogenic programs in vivo. a, b Snail1∆hep (n = 6) and Snail1flox/flox (n = 6) males fed a normal chow diet for 18 weeks. c, d Snail1flox/flox male mice (8–9 weeks) were fed a HFD for 6 weeks and transduced with AAV-TBG-GFP (n = 6) or AAV-TBG-Cre (n = 6) vectors. Livers were harvested 4 weeks after AAV transduction. e, f C57BL/6 male mice (8–9 weeks) were fed a HFD for 7 weeks and transduced with Snail1 (n = 5) or GFP (n = 5) adenoviral vectors. e Liver Fasn, Acc1, and Acl expression was assessed by qPCR (normalized to 36B4 expression). f Liver extracts were immunoblotted with the indicated antibodies. Fasn, Acc1, and Acl levels were quantified and normalized to α-tubulin levels. Data are presented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
Given Srebp-1c mediating insulin stimulation of lipogenesis, we tested if Snail1 counteracts Srebp-1c action. Deletion of hepatic Snail1 upregulated Srebp-1c in the liver; conversely, liver-specific overexpression of Snail1 downregulated hepatic Srebp-1c (Supplementary Fig. 5a, b). Srebp-1c potently stimulated Fasn promoter activity, as assessed by Fasn luciferase reporter assays; in contrast, Snail1 suppressed Fasn promoter activity (Supplementary Fig. 5c). Deletion of the serum response element (SRE), referred to as Fasn(ΔSRE), completely abolished Srebp-1c-stimulated activation of Fasn(ΔSRE); in contrast, Snail1 still suppressed Fasn(ΔSRE) activity (Supplementary Fig. 5d). Furthermore, Snail1 decreased Srebp-1c-stimulated activation of the Fasn promoter (Supplementary Fig. 5e). These data further support the concept that the noncanonical Snail1 arm counterbalances the canonical Srebp-1c lipogenic arm in response to insulin.
Snail1 suppresses lipogenesis by an epigenetic mechanism
We next sought to interrogate the genomic mechanism of Snail1 action, using the Fasn gene as a prototype of Snail1 targets. In mouse liver, Snail1 directly bound to the Fasn promoter as detected by chromatin immunoprecipitation (ChIP) (Fig. 6a). Snail1 directly inhibited Fasn promoter activity in HepG2 cells (Fig. 6b). These data further confirm that Snail1 is a transcriptional repressor of lipogenic genes.
Fig. 6.
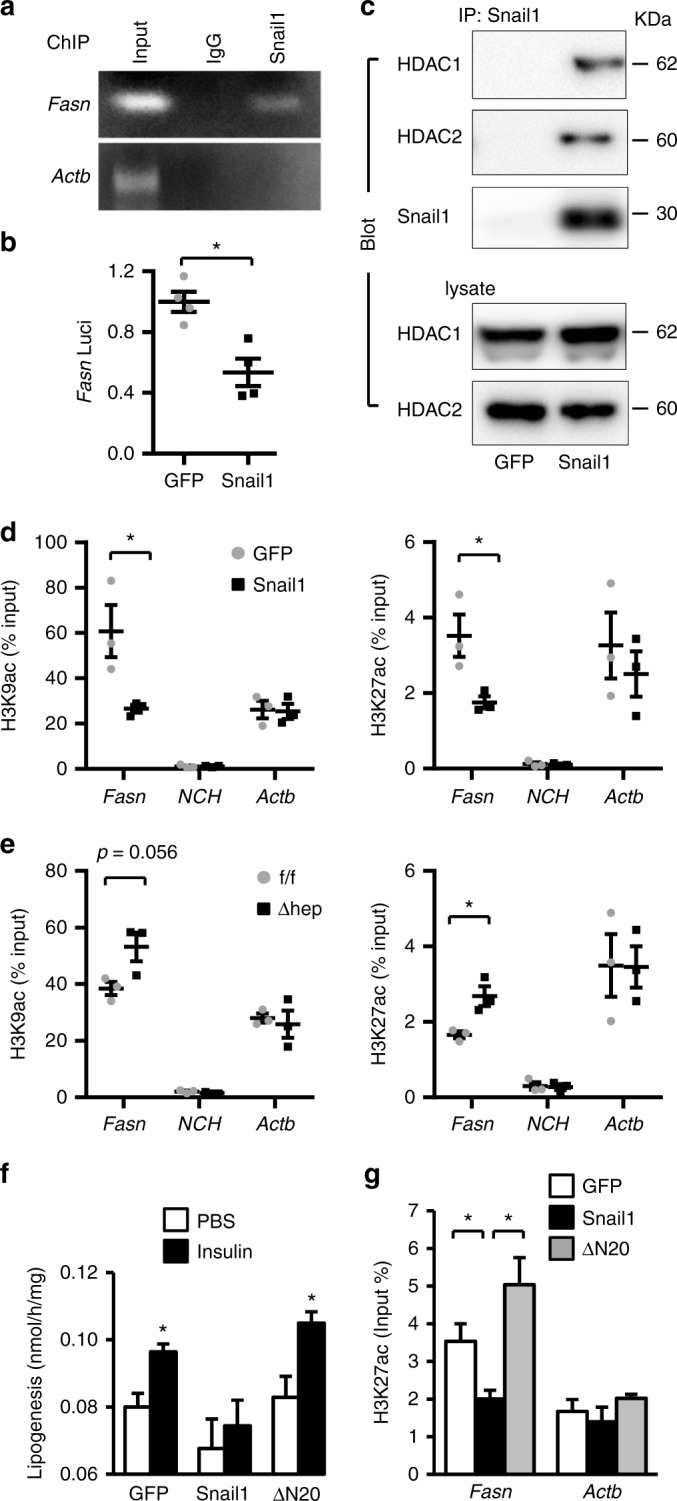
Snail1 epigenetically represses the Fasn promoter. a Male mice were transduced with Snail1 adenoviral vectors for 3 weeks. Binding of Snail1 to the Fasn promoter was assessed in the liver by ChIP. b Fasn luciferase activity (normalized to β-gal internal controls). c Male mice were transduced with Snail1 adenoviral vectors for 3 weeks. Liver extracts were immunoprecipitated with antibody against Snail1 and immunoblotted with antibodies against HDAC1 or HDAC2. d C57BL/6 male mice (8–9 weeks) were fed a HFD for 7 weeks and then transduced with Snail1 (n = 3) or GFP (n = 3) adenoviral vectors. Livers were harvested 3 weeks after transduction. The levels of liver H3K9ac and H3K27ac on the Fasn, NCH, or Actb promoter were measured by ChIP-qPCR and normalized to inputs. e Snail1∆hep (n = 3) and Snail1flox/flox (n = 3) males were fed a HFD for 10 weeks. The levels of liver H3K9ac or H3K27ac on the Fasn, NCH, or Actb promoter were measured by ChIP. f, g Primary hepatocytes were transduced with Snail1, ΔN20, or GFP adenoviral vectors and stimulated with or without insulin (50 nM) for 12 h. f Lipogenesis. g H3K27ac levels on the Fasn promoter. Data are presented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
HDAC1 and HDAC2 have been known to repress expression of target genes by deacetylating histone H3 lysine-9 (H3K9) and H3K27, critical epigenetic modifications. We found that Snail1 coimmunoprecipitated with both HDAC1 and HDAC2 in mouse liver (Fig. 6c). Hence, we measured acetylation of H3K9 (H3K9ac) and H3K27 (H3K27ac) on the Fasn promoter. Liver-specific overexpression of Snail1 significantly reduced both H3K9ac and H3K27ac levels in the liver (Fig. 6d). In contast, Snail1 did not alter the levels of H3K9ac and H3K27ac on unrelated NCH and Actb promoters (Fig. 6d). To extend these findings to endogenous Snail1, we assessed H3K9ac and H3K27ac levels in the livers of Snail1Δhep mice. Ablation of hepatic Snail1 markedly increased both H3K9ac and H3K27ac levels on the Fasn promoter in the livers of Snail1Δhep mice relative to Snail1flox/flox littermates (Fig. 6e). Notably, other forms of histone modifications on the Fasn promoter, including trimethylation of H3K4, H3K9, and H3K27, were not altered by either overexpression or ablation of Snail1 in the liver (Supplementary Fig. 6a, b).
To confirm inhibition of lipolysis by Snail1-elicited histone deacetylation, primary hepatocytes were transduced with Snail1 and treated with trichostatin A (TSA), a selective HDAC inhibitor. TSA blocked the ability of Snail1 to inhibit lipogenesis (Supplementary Fig. 6c). TSA also reversed Snail1-induced suppression of Fasn expression (Supplementary Fig. 6d, e). To corroborate these findings, we deleted the SNAG domain of Snail1 (amino acids 1–20) responsible for binding to HDAC1/2, referred to as ΔN20. Primary hepatocytes were transduced with Snail1 or ΔN20 adenoviral vectors, followed by stimulation with insulin. ΔN20, unlike Snail1, was unable to suppress insulin stimulation of Fasn expression (Supplementary Fig. 6f) and lipogenesis (Fig. 6f). Importantly, ΔN20 also lost the ability to elicit deacetylation of H3K9 (Supplementary Fig. 6g) and H3K27 (Fig. 6g). Collectively, these data suggest that the insulin/Snail1 axis suppresses de novo lipogenesis through epigenetic reprogramming of lipogenic genes, particularly via deacetylating H3K9 and H3K27.
Ablation of hepatic Snail1 exacerbates insulin resistance
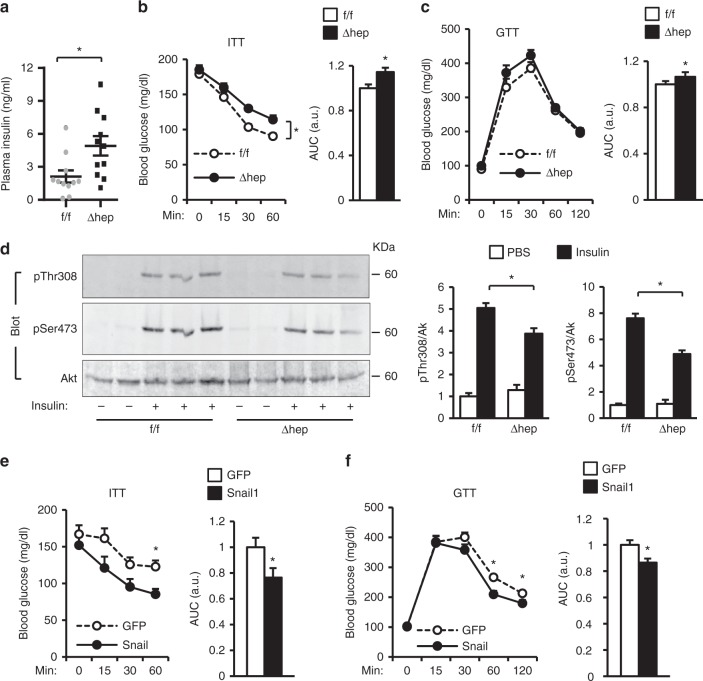
Liver steatosis has been thought to induce insulin resistance, prompting us to examine glucose metabolism in Snail1Δhep mice. Plasma insulin levels was normal in Snail1Δhep mice fed a normal chow diet (Snail1Δhep: 0.70 ± 0.07 ng/ml, n = 5; Snail1flox/flox: 0.61 ± 0.09 ng/ml, n = 5; p = 0.42). We placed Snail1Δhep and Snail1flox/flox littermates on a HFD for 10 weeks. Body weight was similar between these two groups (Supplementary Fig. 3c). Snail1Δhep mice developed more severe hyperinsulinemia, a hallmark of systemic insulin resistance, relative to Snail1flox/flox littermates (Fig. 7a). To confirm insulin resistance, we performed insulin (ITT) and glucose (GTT) tolerance tests. Insulin had reduced ability to decrease blood glucose in Snail1Δhep mice compared to Snail1flox/flox mice (Fig. 7b). The areas under the curves (AUC) of both ITT and GTT were significantly higher in Snail1Δhep mice (Fig. 7b, c). Furthermore, the levels of insulin-stimulated phosphorylation of Akt (pSer473 and pThr308) in the liver was significantly lower in Snail1Δhep than in Snail1flox/flox littermates (Fig. 7d). Thus, Snail1Δhep mice are prone to both liver steatosis and insulin resistance.
Fig. 7.
Hepatic Snail1 ameliorates insulin resistance in obesity. a–d Snail1∆hep (n = 11) and Snail1flox/flox (n = 11) male mice (8–9 weeks) were fed a HFD for 10 weeks. a Overnight fasting plasma insulin levels. b ITT. AUC areas under curves. c GTT. d Mice were fasted overnight (14 h) and injected with insulin (0.5 units/kg). Liver extracts were prepared 5 min later and immunoblotted with anti-phospho-Akt (pSer473 or pThr308) or anti-Akt antibodies. Akt phosphorylation was normalized to total Akt levels. e, f C57BL/6 male mice (8–9 weeks) were fed a HFD for 7 weeks and transduced with Snail1 (n = 10) or GFP (n = 10) adenoviral vectors for 2 weeks after transduction. e ITT. f GTT. Data are presented as mean ± SEM. *p < 0.05, two-tailed unpaired Student’s t test
To determine whether liver-specific overexpression of Snail1 has the opposite effect, C57BL/6 mice were fed a HFD for 7 weeks and transduced with Snail1 or GFP (control) adenoviral vectors. Overexpression of Snail1 significantly attenuated HFD-induced insulin resistance and glucose intolerance (Fig. 7e, f), although body weight was similar between Snail1 and GFP adenoviral-transduced mice (Fig. 4b). Concurrently, hepatic Snail1 substantially decreased liver steatosis in obese mice (Fig. 4c). Together, these data suggest that the hepatic insulin/Snail1 axis ameliorates insulin resistance in obesity, presumably through decreasing liver steatosis.
Discussion
In this study, we have identified hepatic Snail1 as an unrecognized suppressor of lipogenesis. Snail1 bound to the promoters of lipogenic genes where it recruited HDAC1/2 to catalyze repressive deacetylation of H3K9 and H3K27. Insulin robustly upregulated hepatic Snail1 in both primary hepatocytes and livers, defining an unrecognized noncanonical insulin/Snail1 pathway that epigenetically suppresses lipogenesis.
In hepatocytes, insulin substantially increased both expression and stability of Snail1 in a PI 3-kinase-dependent manner, defining an insulin-PI 3-kinase-Snail1 cascade. Snail1 overexpression blocked insulin-stimulated expression of lipogenic enzymes and lipogenesis in primary hepatocytes. Liver-specific overexpression of Snail1 suppressed the hepatic lipogenic program and protected against dietary NAFLD. Conversely, ablation of hepatic Snail1, using three distinct approaches (tamoxifen, AAV-TBG-Cre, and albumin-Cre), had the opposite effect. These findings demonstrate that insulin concomitantly stimulates both the canonical lipogenic (e.g., Srebp-1c) and the noncanonical Snail1 (anti-lipogenesis) pathways. The noncanonical Snail1 arm likely puts a brake on the canonical lipogenic arm, combating excessive lipogenesis and lipotoxicity. In obesity, the insulin/Snail1 brake was impaired, likely contributing to increased hepatic lipogenesis and NAFLD. Aside from insulin, Wnts, TGFβ1, and additional factors also upregulate Snail118,28,29. Both Wnt and TGFβ1 suppress lipogenesis30,31. Therefore, Snail1 may serve as a common node upon which these factors converge to regulate lipogenesis and intracellular lipid content. It is worth mentioning that obesity is associated with compensatory hyperinsulinemia in part due to adipose insulin resistance. Hyperinsulinemia further drives hepatic lipogenesis, particular when liver is still sensitive to insulin and/or the Snail1 arm is dysfunctional, thereby exacerbating NAFLD.
Mechanistically, we found that Snail1 directly bound to the Fasn promoter and repressed Fasn promoter activity. Snail1 has been known to bind, via its SNAG domain, to many epigenetic enzymes, including HDAC1/2, LSD1, Ezh2, and G9a15,16,32. We confirmed that Snail1 bound to HDAC1/2 in the liver. Overexpression of Snail1 decreased, whereas ablation of Snail1 increased, the levels of H3K9ac and H3K27ac, active epigenetic marks, on the Fasn promoter. Remarkably, inhibition of HDAC1/2 by TSA blocked Snail1-induced suppression of lipogenesis. Deleting the SNAG domain also abrogated the ability of Snail1 to inhibit insulin stimulation of lipogenesis. These findings support the notion that Snail1 suppresses lipogenesis at least in part by recruiting HDAC1/2 to lipogenic gene promoters where HDAC1/2 catalyze repressive deacetylation of H3K9 and H3/K27.
Given that liver steatosis is linked to insulin resistance, it is not unexpected that ablation of hepatic Snail1 exacerbated, whereas liver-specific overexpression of Snail1 ameliorated, HFD-induced insulin resistance and glucose intolerance. It is worth mentioning that simple liver steatosis has been reported to be dissociated from insulin resistance33. Diacylglycerol and several lipid species, rather than inert TAG, are able to induce insulin resistance34–37. In light of these observations, we propose that hepatic Snail1 may selectively inhibit production of toxic lipid species that induce insulin resistance. Additional studies are needed to test this hypothesis in the future.
Liver steatosis is considered, historically, as the first hit during NAFLD development38. In the presence of the secondary and/or multiple other hits (e.g., oxidative stress), relatively benign fatty liver progresses to pathogenic NAFLD/NASH manifested by liver inflammation, injury, and fibrosis38,39. The severity of liver steatosis is influenced by multiple metabolic pathways, which are controlled largely at the transcriptional level. Transcription factors Srebp-1c, ChREBP, and Lxr have been extensively characterized for their ability to stimulate de novo lipogenesis and liver steatosis6,40–43. Hepatic PPARγ, which is upregulated in obesity, stimulates expression of lipid droplet proteins as well as fatty acid transporters, thereby promoting liver steatosis8,44–48. In contrast, hepatic PPARα, PPARβ/δ, Foxa2, and circadian clock BMAL1 exert anti-steatosis action by promoting fatty acid β oxidation49–54. Recent studies have highlighted the impact of negative regulators of lipogenesis on NAFLD. Fxr, nuclear receptors for bile acids, suppresses lipogenesis and liver steatosis55–57. Circadian clock Rev-erbα/β also suppresses lipogenesis, and liver-specific ablation of rev-erbα/β results in NAFLD58,59. Notably, in contrast to genetic lipogenic programs that have been extensively investigated, epigenetic regulation of lipid metabolism remains largely unclear. In this regard, Snail1-elicited epigenetic reprogramming of hepatic lipogenesis, identified in this study, likely points to a new direction in the NAFLD field.
In conclusion, we have unveiled the noncanonical insulin-Snail1 pathway that epigenetically suppresses hepatic lipogenesis. Impairment in this pathway increases hepatic de novo lipogenesis, contributing to NAFLD in obesity. Our data suggest that the insulin/Snail1/epigenetic axis may serve as a potential therapeutic target for the treatment of NAFLD and metabolic disease.
Methods
Animal treatments
Animal experiments were conducted following the protocols approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC). Snail1flox/flox and albumin-CreERT2 mice (C57BL/6 background) have been characterized previously25,26. Mice were housed on a 12-h light-dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan (ULAM), and fed ad libitum either a normal chow diet (9% fat in calories; TestDiet, St. Louis, MO), a HFD (60% fat in calories; Research Diets, New Brunswick, NJ), or a fructose diet (60% Fructose; in calories; Research Diets, New Brunswick, NJ).
To generate mice hepatocyte-specific ablation of Snail1, Snail1flox/flox mice were crossed with albumin-CreERT2 or albumin-Cre drivers. Snail1flox/flox;albumin-CreERT2 mice were intraperitoneally injected with tamoxifen (Cayman) (0.5 mg/mouse, twice 2 days apart) to obtain Snail1Δhep mice. Snail1flox/flox mice were similarly treated with tamoxifen as control. Alternatively, adult Snail1flox/flox mice were fed a HFD for 6–7 wks and injected with AAV-TBG-Cre or AAV-TBG-GFP vectors via tail veins (1011 viral particles/mouse). To generate mice with liver-specific overexpression of Snail1, C57BL/6 male mice (12 wks) were fed a HFD for 6 weeks and then transduced with Snail1 or GFP (control) adenoviral vectors via tail vein injection (1011 viral particles/mouse). ob/ob mice (9 wks) were similarly transduced with Snail1 or GFP adenoviral vectors.
To measure liver Snail1 levels, C57BL/6 males (8–10 wks) were fed a HFD or chow diet for 6 weeks, fasted overnight (14 h), and stimulated with insulin (0.75 units/kg body weight, i.p.) for 2 h. Liver nuclear extracts were prepared and immunoblotted with antibodies against Snail1 or lamin A/C (loading control).
Glucose (GTT) and insulin (ITT) tolerance tests
Mice were fasted overnight (GTT) or for 6 h (ITT), and intraperitoneally injected with glucose (1 g/kg body weight) for GTT or human insulin (1 unit/kg body weight) for ITT. Blood glucose was measured in tail veins 0, 15, 30, 60, and 120 min after injection. Plasma insulin levels were measured using mouse insulin ELISA kits (CRYSTAL CHEM, Downers Grove, IL).
Nile red staining and liver TAG levels
Liver frozen sections were fixed with 4% paraformaldehyde for 20 min, washed twice with PBS, stained with Nile red (1 µg/ml in PBS) for ~30 min, washed twice with PBS, and visualized using fluorescent microscope. Liver samples were homogenized in 1% acetic acid, and lipids were extracted using 80% chloroform/Methanol (2:1). Organic fractions were dried in a chemical hood, resuspended in a KOH (3 M)/ethanol solution, incubated at 70 °C for 1 h, mixed with MgCl2 (0.75 M), and centrifuged. Aqueous fractions were used to measure TAG levels using Free Glycerol Reagent (Sigma).
Immunoblotting and immunoprecipitation
Tissues or cells were homogenized in a lysis buffer (50 mM Tris HCl, pH 7.5, 1.0% NP-40, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 1 mM PMSF, 10 mg/ml aprotinin, and 10 mg/ml leupeptin). Tissue or cell extracts were immunoprecipitated and/or immunoblotted with the indicated antibodies (Supplementary table 1). Uncropped scans of important blots were provided in the Supplementary Figs. 7–9.
Cell cultures and treatments
HepG2 cells (ATCC) were grown in DMEM containing 5 mM glucose, 10% calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 5% CO2 and 37 °C. Primary hepatocytes were isolated using liver perfusion with type II collagenase (Worthington Biochem, Lakewood, NJ), and were grown in William E Medium (Sigma, St. Louis, MO) supplemented with 2% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin.
HepG2 cells were deprived of serum overnight, pretreated with wortmannin (100 nM) or MK2066 (100 nM) for 30 min, and then stimulated with insulin (50 nM) for 2 h. Additionally, HepG2 cells were pretreated with palmitate (100 µM) overnight and then stimulated with insulin (100 nM) for 2 h. Cell extracts were prepared for various assays.
In separate cohorts, HepG2 cells were transfected with Snail1 plasmids. Forty-eight hour later, the cells were derived of serum overnight, and were then treated with cycloheximide (5 μg/ml) in the presence of either insulin (100 nM) or PBS (control) for 0–8 h. Cell extracts were immunoblotted with antibodies against Snail1 or α-tubulin. To calculate half-life, Snail1 protein was quantified and normalized to α-tubulin levels. Snail1 abundance was presented as a ratio to its baseline levels prior to cycloheximide treatment, and was plotted against durations of cycloheximide treatment. In separate experiments, HepG2 cells were transduced with Snail1 adenoviral vectors for 24 h, and then stimulated with insulin (100 nM) with or without MG132 (5 μM) for 2 h. Cell extracts were immunoprecipitated with antibody against Snail1 and immunoblotted with antibodies against ubiquitin or Snail1.
Primary hepatocytes were transduced with Snail1 or GFP adenoviral vectors (1000 viral particles per cell) for 24 h. Hepatocytes were deprived of serum overnight in DMEM supplemented with 5 mM glucose, and then grown in DMEM with 22.5 mM glucose in the presence or absence of insulin (50 nM) for additional 4 h or 12 h. Cell extracts were prepared for various assays.
Fasn luciferase reporter assays
The rattus Fasn promoter (from −225 to + 45) was prepared by PCR (forward primer: 5′-AGTGCCTCTCATGTATGCTTAA-3′, and reverse primer: 5′-TCCCGCAGTCTCGATACCTTGG-3′) and inserted into pGL3 vectors. HepG2 cells were co-transfected with Fasn, or Fasn (ΔSRE), luciferase reporter plasmids and the indicated vectors60. Luciferase activities were measured 72 h after transfection using a kit (Progema, Madison, WI), and were normalized to β-gal levels (internal control).
De novo lipogenesis
Primary hepatocytes were transduced with the indicated adenoviral vectors as described previously61, and concomitantly treated with or without TSA (2 μM). De novo lipogenesis was assessed and normalized to total protein levels as described previously62. To assess insulin stimulation of lipogenesis, hepatocytes were deprived of serum overnight in the presence of 5 mM glucose, and stimulated with 50 nM insulin for 12 h prior to lipogenesis assays.
Quantitative real time RT-PCR (qPCR)
Total RNAs were extracted using TRIzol reagent (Invitrogen life technologies, Carlsbad, CA). The first-strand cDNAs were synthesized using random primers and M-MLV reverse transcriptase (Promega, Madison, WI). qPCR was performed using Absolute QPCR SYBR Mix (Thermo Fisher Scientific, UK) and Mx3000P real time PCR system (Stratagene, LA Jolla, CA). qPCR primers were listed in supplementary table 2.
Chromatin immunoprecipitation (ChIP)
ChIP assays were described previously11. Briefly, liver samples were treated with 1% formaldehyde for 10 min to crosslink DNA-protein complexes. Genomic DNA was extracted, and sheared to 200–500 bp fragments using a sonicator (Model Q800R, QSONICA). DNA-protein complexes were immunoprecipitated with the indicated antibodies (Supplementary table 1). Crosslink was reversed by heating at 65 °C for 4 h. DNA was purified and used for PCR or qPCR analysis. Primers flanking the putative Snail1-binding motifs of the Fasn promoter were listed in supplementary table 2.
Statistical analysis
Differences between two groups were analyzed by two-tailed Student’s t test. Longitudinal data (growth curves, GTT and ITT) were further analyzed by ANOVA and Bonferroni posttest using Prism 7. P < 0.05 was considered statistically significant.
Data availability
All relevant data are available upon request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
We thank Drs. Zheng Chen, Mark J. Canet, Hong Shen, Gong Cheng, Deqiang Zhang, Yin Lei, Xin Tong, and Bishr M. Omary for assistance and discussions. We thank Dr. Stephen J. Weiss (University of Michigan) for providing Snail1flox/flox mice. This study was supported by grants DK094014, DK114220, and DK115646 (to L.R.) and DK095201 (to Y.S.) from the National Institutes of Health, American Heart Association Postdoctoral Fellowship 14POST20230007 (to Yan Liu), and National Natural Science Foundation of China Grant 81420108006 (to Yong Liu). This work utilized the cores supported by the Michigan Diabetes Research and Training Center (NIH DK20572), the University of Michigan Nathan Shock Center (NIH P30AG013283), and the University of Michigan Gut Peptide Research Center (NIH DK34933).
Author contributions
Yan Liu, L.J., C.S., and N.I. conducted the experiments, Yan Liu and L.R. designed the experiments and wrote the paper, and Yan Liu, L.J., C.S., N.I., Y.M.S., Yong Liu, and L.R. performed data analysis and edited the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-05309-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allen AM, et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig. Dis. Sci. 2010;55:560–578. doi: 10.1007/s10620-009-1081-0. [DOI] [PubMed] [Google Scholar]
- 4.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaven SW, et al. Reciprocal regulation of hepatic and adipose lipogenesis by liver x receptors in obesity and insulin resistance. Cell. Metab. 2013;18:106–117. doi: 10.1016/j.cmet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon YA, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell. Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran-Salvador E, et al. Role for PPARgamma in obesity-induced hepatic steatosis as determined by hepatocyte- and macrophage-specific conditional knockouts. FASEB J. 2011;25:2538–2550. doi: 10.1096/fj.10-173716. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl Acad. Sci. USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun C, et al. Adipose Snail1 regulates lipolysis and lipid partitioning by suppressing adipose triacylglycerol lipase expression. Cell Rep. 2016;17:2015–2027. doi: 10.1016/j.celrep.2016.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batlle E, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 13.Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: the Snail story. Curr. Pharm. Des. 2014;20:1698–1705. doi: 10.2174/13816128113199990512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol. Cell. Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herranz N, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol. Cell. Biol. 2008;28:4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong C, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J. Clin. Invest. 2012;122:1469–1486. doi: 10.1172/JCI57349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BJ, et al. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J. Clin. Invest. 2015;125:2473–2483. doi: 10.1172/JCI74159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojima T, et al. Transforming growth factor-beta induces epithelial to mesenchymal transition by down-regulation of claudin-1 expression and the fence function in adult rat hepatocytes. Liver Int. 2008;28:534–545. doi: 10.1111/j.1478-3231.2007.01631.x. [DOI] [PubMed] [Google Scholar]
- 19.Sekiya S, Suzuki A. Glycogen synthase kinase 3 beta-dependent Snail degradation directs hepatocyte proliferation in normal liver regeneration. Proc. Natl Acad. Sci. USA. 2011;108:11175–11180. doi: 10.1073/pnas.1016122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe RG, et al. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol. Cell. Biol. 2011;31:2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan A, Qi Y, Du J. Akt2 mediates TGF-beta1-induced epithelial to mesenchymal transition by deactivating GSK3beta/snail signaling pathway in renal tubular epithelial cells. Cell. Physiol. Biochem. 2014;34:368–382. doi: 10.1159/000363006. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BP, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell. Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 23.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 24.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 25.Rowe RG, et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 2009;184:399–408. doi: 10.1083/jcb.200810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler M, Dierich A, Chambon P, Metzger D. Efficient temporally controlled targeted somatic mutagenesis in hepatocytes of the mouse. Genesis. 2004;39:167–172. doi: 10.1002/gene.20039. [DOI] [PubMed] [Google Scholar]
- 27.Bell P, et al. Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs. Hum. Gene Ther. 2011;22:985–997. doi: 10.1089/hum.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yook JI, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat. Cell. Biol. 2006;8:1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 29.Kaimori A, et al. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J. Biol. Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 30.Abiola M, et al. Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS ONE. 2009;4:e8509. doi: 10.1371/journal.pone.0008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNairn AJ, et al. TGFbeta signaling regulates lipogenesis in human sebaceous glands cells. BMC Dermatol. 2013;13:2. doi: 10.1186/1471-5945-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong C, et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene. 2013;32:1351–1362. doi: 10.1038/onc.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monetti M, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spruss A, et al. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 36.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012;15:574–584. doi: 10.1016/j.cmet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J. Gastroenterol. Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 39.Mendez-Sanchez N, Arrese M, Zamora-Valdes D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423–433. doi: 10.1111/j.1478-3231.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 40.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl Acad. Sci. USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dentin R, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 42.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 43.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi: 10.1172/JCI0215593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gavrilova O, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J. Biol. Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 45.Matsusue K, et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 2003;111:737–747. doi: 10.1172/JCI200317223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YJ, et al. Nuclear receptor PPARgamma-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl Acad. Sci. USA. 2012;109:13656–13661. doi: 10.1073/pnas.1203218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsusue K, et al. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 2008;7:302–311. doi: 10.1016/j.cmet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steneberg P, et al. Hyperinsulinemia enhances hepatic expression of the fatty acid transporter Cd36 and provokes hepatosteatosis and hepatic insulin resistance. J. Biol. Chem. 2015;290:19034–19043. doi: 10.1074/jbc.M115.640292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kersten S, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montagner A, et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanderson LM, et al. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 2009;29:6257–6267. doi: 10.1128/MCB.00370-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 53.Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Jacobi D, et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015;22:709–720. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caron S., et al. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell. Biol. 33, 2202-2211 (2013). [DOI] [PMC free article] [PubMed]
- 56.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe M, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bugge A, et al. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang B, et al. Carboxyl terminus of HSC70-interacting protein (CHIP) down-regulates NF-kappaB-inducing kinase (NIK) and suppresses NIK-induced liver injury. J. Biol. Chem. 2015;290:11704–11714. doi: 10.1074/jbc.M114.635086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen H, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60:2065–2076. doi: 10.1002/hep.27348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheng L, Cho KW, Zhou Y, Shen H, Rui L. Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid beta-oxidation. J. Biol. Chem. 2011;286:38128–38135. doi: 10.1074/jbc.M111.256677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant data are available upon request.