Abstract
Histone H3 mutations are frequently found in diffuse midline gliomas (DMGs), which include diffuse intrinsic pontine gliomas and thalamic gliomas. These tumors have dismal prognoses. Recent evidence suggests that one reason for the poor prognoses is that O6-methylguanine-DNA methyltransferase (MGMT) promoter frequently lacks methylation in DMGs. This review compares the epigenetic changes brought about by histone mutations to those by isocitrate dehydrogenase-mutant gliomas, which frequently have methylated MGMT promoters and are known to be sensitive to temozolomide.
Keywords: MGMT, diffuse midline gliomas, Histone H3 mutation, resistance, epigenetics
Introduction
Diffuse midline gliomas (DMGs), including diffuse intrinsic pontine gliomas (DIPGs) and thalamic gliomas, have dismal prognoses: 8–11 months for DIPGs1,2) and about 25 and 12 months for World Health Organization (WHO) grades 3 and 4 thalamic gliomas, respectively.3,4) Possible explanations for the poor prognosis include difficulty of surgery5) and the ineffectiveness of temozolomide.6)
It is well known that malignant gliomas with isocitrate dehydrogenase (IDH) mutation have a good prognosis7,8) compared to IDH-wildtype gliomas. A majority of IDH-mutant gliomas are known to have O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and respond to temozolomide.9)
Recent genetic studies have shown that up to 90% of DMGs have mutations in histone H3.3 H3K27M encoding the gene H3F3A or H3.1 H3K27M encoding HIST1H3B.10–15) H3.3 H3K27M mutations are about 2.5-fold more frequent, present at an older age, have a gender predisposition toward boys, and carry a worse prognosis compared to DIPGs with H3.1 H3K27M mutations.12) Epigenetic studies have shown that histone mutations cause DNA hypomethylation,16,17) whereas IDH mutation causes DNA hypermethylation.17,18) We review the increasing evidence that this epigenetic modification renders IDH-mutant gliomas sensitive to temozolomide, but not DMGs.19)
IDH-mutant gliomas have frequent MGMT promoter methylation and Are sensitive to temozolomide
A seminal study in glioblastomas showed that recurrent mutations in IDH1 is seen in approximately 10% of glioblastomas.20) Subsequent studies have shown that IDH1 and IDH2 mutation are frequently seen in WHO grades 2 and 3 astrocytomas and oligodendrogliomas,8) and that IDH-mutation is a vital, early event in gliomagenesis.
IDH mutations are known to be gain-of-function mutations, which produce the oncometabolite R-2-hydroxyglutarate (2HG).21) The 2HG is structurally similar to alpha-ketoglutarate (α-KG), which is necessary to produce the DNA demethylase TET2 and histone demethylases (JMJs). 2HG competitively inhibits DNA and histone demethylases,22) causing diffuse deoxyribonucleic acid (DNA) hypermethylation [the so-called “glioma-CpG island methylator phenotype (G-CIMP) phenotype”]18) and histone hypermethylation.23)
A large proportion of G-CIMP cases are known to have MGMT promoter methylation. Data from the NOA-04 trial found that 96% of G-CIMP cases had methylated MGMT promoters.24) Also, 88% of oligodendrogliomas, which are known to harbor IDH mutations, were found to have MGMT promoter methylation.25)
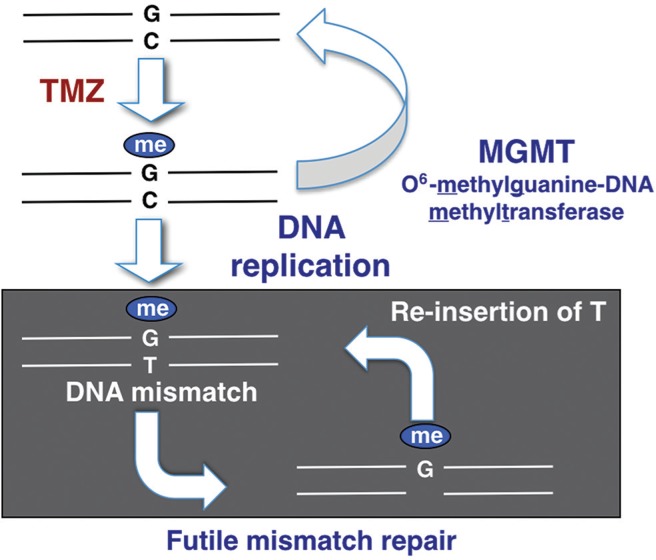
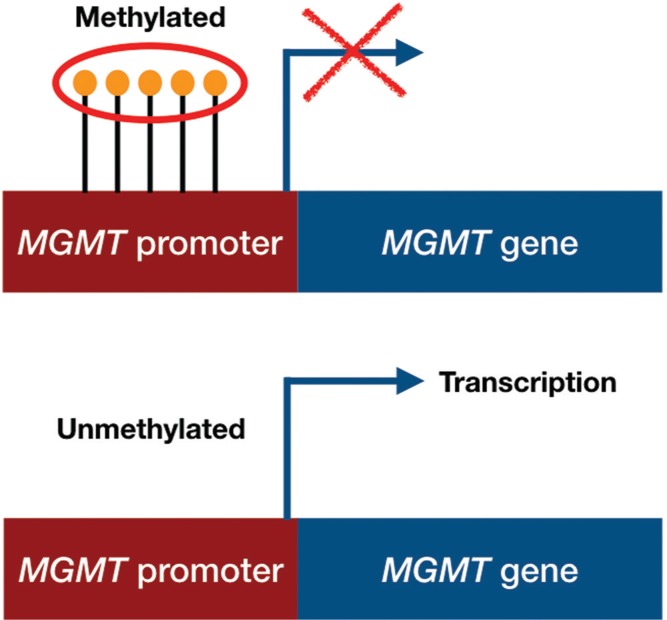
It is well known that MGMT promoter methylation is a predictive factor of response to temozolomide.9,26) The main mechanism of action of temozolomide is to add a methyl-group at the O6 position of guanine (G) in the DNA of glioma cells, causing a methyl-guanine (meG)-to-thymine (T) mismatch at DNA replication, instead of cytosine (C) (Fig. 1). Mismatch repair genes locate the meG-T mismatch and remove the T, only to have a T re-inserted. This insertion and removal of T, called the “futile mismatch repair”, contributes to the vulnerability of tumor DNA and ultimately leads to death of the tumor cell. MGMT, which is expressed in normal cells but lost in a percentage of brain tumors, removes the methyl group at the O6 position of guanine added by temozolomide, neutralizing its effect (Fig. 1). MGMT expression is epigenetically regulated.9) Thus, MGMT promoter methylation inhibits the transcription of MGMT leading to MGMT silencing (Fig. 2).
Fig. 1.
A schematic drawing showing the relationship between MGMT promoter methylation and MGMT protein expression. When the MGMT promoter is methylated, transcription is repressed and thus MGMT protein is not produced.
Fig. 2.
The main mechanism of action of temozolomide is to add a methyl-group at the O6 position of guanine (G) in the DNA of glioma cells, causing a methyl-guanine (meG)-to-thymine (T) mismatch at DNA replication, instead of cytosine (C). Mismatch repair genes locate the meG-T mismatch and remove the T, only to have a T re-inserted. This insertion and removal of T, called the “futile mismatch repair”, contributes to the vulnerability of tumor DNA and ultimately leads to apoptosis. MGMT, which is expressed in normal cells but lost in a percentage of brain tumors, removes the methyl group at the O6 position of guanine added by temozolomide, neutralizing its effect.
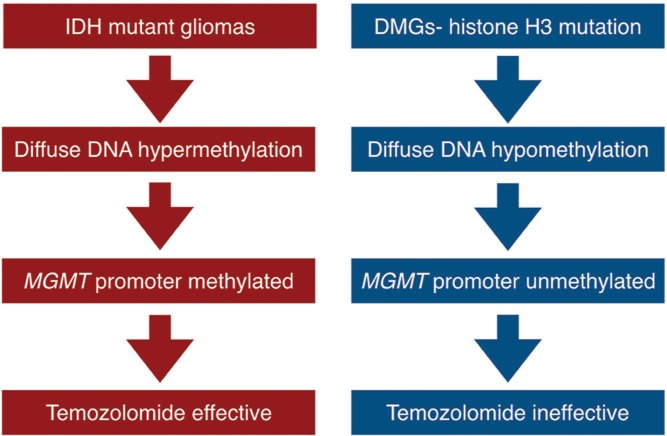
Taken together, we can conclude that IDH-mutant gliomas express the G-CIMP phenotype, frequently have MGMT promoter methylation and are sensitive to temozolomide (Fig. 3, left side). Secondary glioblastomas harboring IDH-mutations are known to be sensitive to temozolomide therapy.27)
Fig. 3.
A flow chart showing the relationship between epigenetic changes in DNA, MGMT promoter methylation and response to temozolomide in IDH-mutant gliomas (left side) and diffuse midline gliomas with histone H3K27 mutations (right side).
Histone H3-mutant diffuse midline gliomas Have frequent unmethylated MGMT promoter and Are resistant to temozolomide
In contrast to IDH-mutation, in which diffuse DNA hypermethylation occurs, epigenetic studies have shown that histone mutations, including H3K27M and H3G34R/V (seen in pediatric glioblastoma of the cerebrum), cause DNA hypomethylation.16,17,28) Recent studies suggest that MGMT is almost always expressed in DMGs. None of the 46 DMGs with confirmed H3F3A mutation showed MGMT promoter methylation in a report by Banan et al.29) Similarly, Korshunov et al.30) reported that MGMT promoter was methylated in only 3% of DIPGs with H3K27M mutations.
Furthermore, Oka et al.31) showed that MGMT was expressed in 9 out of 11 (82%) brainstem gliomas in which immunohistochemical analysis of MGMT was feasible. From these reports, we can postulate that epigenetic changes driven by histone H3K27M mutation cause frequent lack of MGMT promoter methylation, thus expression of MGMT and resistance to temozolomide therapy (Fig. 3, right side).
Future directions and therapeutic implications
Temozolomide is a key drug used in the treatment of glioblastomas, and is often used in the treatment of WHO grade 3 malignant gliomas as well. However, increasing evidence suggests that temozolomide is not effective in DMGs. Despite some effort for aggressive surgical intervention,4) the clinical outlook for DMGs remain dismal. Here, we outline just some of the new preclinical and clinical efforts to eradicate this disease.
Epigenetic modification
Since global reduction of H3K27 methylation is a key epigenetic event in H3K27M mutant DMGs, pharmacologic restoration of H3K27 methylation either by enhancing H3K27 methyltransferase (PRC2) activity or by inhibiting H3K27 demethylase activity for the lysine 27 residue is a rational method to treat DMGs.32) The latter can be achieved by using the H3K27 demethylase inhibitor GSKJ4. Decreased histone methylation at H3K27 causes increased histone acetylation in DIPG, which can also be targeted. The HDAC inhibitor panobinostat was found to be effective in DIPG cell lines through a screening of 83 drugs. Panobinostat was found to increase H3 acetylation and restore H3K27 trimethylation.19) This data has led to the commencement of clinical trials looking at the efficacy of panobinostat in DIPGs. Two recent high-profile papers show the efficacy of BET bromodomain inhibitors, which prevent the interaction of BRD4 with acetylated histone, leading to the repression of BRD4 transcriptional targets and proliferation.33,34)
Targeting of associated mutations
Epigenetic modification can be very toxic, as drugs will affect the epigenetic status of normal cells as well as tumor. Less toxic treatments including localized delivery and targeted treatments need to be explored. One potential avenue of treatment is targeting of mutations associated with H3K27M mutations. Mutations in activin receptor type 1 (ACVR1) are frequently seen in H3.1 H3K27M mutant, but not H3.3 H3K27M mutant DMGs.12,35) ACVR1 encodes for type I bone morphogenic protein (BMP) receptor ALK2, and mutation of this receptor leads to constitutive activation of BMP signaling pathway.35) Targeted treatment using the ALK2 inhibitor LDN-193189 showed moderate response in vitro.15)
FGFR1 mutations are seen in 4–27% of thalamic high-grade gliomas, but not DIPGs,35) and is a potential target for thalamic gliomas.
Targeting of PARP
As stated above, the main mechanism of action for temozolomide is to add a methyl-group at the O6-position of guanine, which is removed by MGMT (Fig. 1). However, temozolomide is also known to methylate adenine at N3-position and guanine at the N7-position. These do not generally induce cytotoxicity, as poly(ADP-ribose) polymerase (PARP) activation allows for base excision repair of damaged DNA. Evidence suggests that inhibition of PARP or depletion of NAD+ which is a co-enzyme of PARP, can lead to cytotoxicity.36) Interestingly, a study by Chornenkyy et al.37) shows PARP1 expression in DIPG cell lines and sensitivity to the PARP inhibitor niraparib.
Inhibition of PTEN/Akt/mTOR signaling pathway
Approximately, 70% of DIPGs have either AKT gain or phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss,38,39) suggesting that targeting of the PTEN/AKT/mechanistic target of rapamycin (mTOR) signaling pathway is a potential therapeutic strategy for DIPGs. Miyahara et al.40) and others41) reported the efficacy of dual mTOR inhibition in vitro and in vivo.
Immunotherapy
Okada and colleagues established T cell receptor-transduced T cells recognizing a peptide sequence encompassing the H3.3K27M mutation. Preclinical data shows significant suppression of glioma xenografts in mice.42) Development of a peptide vaccine recognizing IDH1 R132H mutant glioma43) has led to exploration of a similar peptide vaccine recognizing H3K27M.44) Major histocompatibility complex (MHC) class 2 response, which allows proteins to be degraded into peptides and sent to the surface of the cell,45) enables intracellular mutant proteins to be expressed at the surface of tumor cells. A Phase I clinical trial (NCT02960230) testing the safety of an H3.3K27M peptide vaccine is currently underway.
Convection-enhanced delivery and other methods of delivery
Convection-enhanced delivery (CED) of drugs to the brainstem remains a promising candidate for treatment of DIPGs. Preclinical brainstem tumor models have been treated with CED of various drugs including temozolomide.46) In Japan, Saito et al.47) have published a case report showing radiographical response after CED of nimustine hydrochloride (ACNU) in a patient with recurrent glioblastoma infiltrating into the brainstem. Intranasal delivery (IND) is also a promising method of delivery, as it is far less invasive than CED.48) IND was shown to be effective in a brainstem tumor model when combined with nanoliposomal chemotherapy.49)
Conclusion
This paper focused on what we currently known about the reason H3 mutant DMGs are not sensitive to temozolomide. Epigenetic changes brought about by H3 mutation cause DMGs to frequently lack MGMT promoter methylation thus express MGMT. Since radical surgery is difficult in almost all cases of DMGs, there is an urgent need for new, more effective therapies targeting DMGs. Safe, local delivery, as well as more targeted therapies are rapidly being developed and tested, but a real breakthrough remains elusive. Worldwide collaboration in research as well as clinical treatment is critical to overcome this uncommon but deadly disease.
Acknowledgment
The authors would like to acknowledge Drs. Rintaro Hashizume and Hiroaki Miyahara for helpful insights regarding this paper.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflicts of interest to declare.
References
- 1).Hargrave D, Bartels U, Bouffet E: Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7: 241–248, 2006 [DOI] [PubMed] [Google Scholar]
- 2).Buczkowicz P, Bartels U, Bouffet E, et al. : Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128: 573–581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Esquenazi Y, Moussazadeh N, Link TW, et al. : Thalamic glioblastoma: clinical presentation, management strategies, and outcomes. Neurosurgery 83: 76–85, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Saito R, Kumabe T, Kanamori M, Sonoda Y, Tominaga T: Distant recurrences limit the survival of patients with thalamic high-grade gliomas after successful resection. Neurosurg Rev 40: 469–477, 2017 [DOI] [PubMed] [Google Scholar]
- 5).Kelly PJ: Stereotactic biopsy and resection of thalamic astrocytomas. Neurosurgery 25: 185–195, 1989 [PubMed] [Google Scholar]
- 6).Chassot A, Canale S, Varlet P, et al. : Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol 106: 399–407, 2012 [DOI] [PubMed] [Google Scholar]
- 7).Ogura R, Tsukamoto Y, Natsumeda M, et al. : Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology 35: 324–335, 2015 [DOI] [PubMed] [Google Scholar]
- 8).Yan H, Parsons DW, Jin G, et al. : IDH 1 and IDH 2 mutations in gliomas. N Engl J Med 360: 765–773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Hegi ME, Diserens AC, Gorlia T, et al. : MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352: 997–1003, 2005 [DOI] [PubMed] [Google Scholar]
- 10).Wu G, Diaz AK, Paugh BS, et al. : The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46: 444–450, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Aihara K, Mukasa A, Gotoh K, et al. : H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro-oncology 16: 140–146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Castel D, Philippe C, Calmon R, et al. : Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130: 815–827, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. : K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124: 439–447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Mackay A, Burford A, Carvalho D, et al. : Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32: 520–537.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Taylor KR, Mackay A, Truffaux N, et al. : Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46: 457–461, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Bender S, Tang Y, Lindroth AM, et al. : Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24: 660–672, 2013 [DOI] [PubMed] [Google Scholar]
- 17).Sturm D, Witt H, Hovestadt V, et al. : Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22: 425–437, 2012 [DOI] [PubMed] [Google Scholar]
- 18).Turcan S, Rohle D, Goenka A, et al. : IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483: 479–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Grasso CS, Tang Y, Truffaux N, et al. : Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 21: 555–559, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Parsons DW, Jones S, Zhang X, et al. : An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807–1812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Dang L, White DW, Gross S, et al. : Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462: 739–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Xu W, Yang H, Liu Y, et al. : Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19: 17–30, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Lu C, Ward PS, Kapoor GS, et al. : IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483: 474–478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Wiestler B, Capper D, Hovestadt V, et al. : Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro-oncology 16: 1630–1638, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Möllemann M, Wolter M, Felsberg J, Collins VP, Reifenberger G: Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer 113: 379–385, 2005 [DOI] [PubMed] [Google Scholar]
- 26).Jacinto FV, Esteller M: MGMT hypermethylation: a prognostic foe, a predictive friend. DNA Repair (Amst) 6: 1155–1160, 2007 [DOI] [PubMed] [Google Scholar]
- 27).SongTao Q, Lei Y, Si G, et al. : IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103: 269–273, 2012 [DOI] [PubMed] [Google Scholar]
- 28).Ahsan S, Raabe EH, Haffner MC, et al. : Increased 5-hydroxymethylcytosine and decreased 5-methylcytosine are indicators of global epigenetic dysregulation in diffuse pontine glioma. Acta Neuropathol Commun 2: 59, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Banan R, Christians A, Bartels S, Lehmann U, Hartmann C: Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun 5: 98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Korshunov A, Ryzhova M, Hovestadt V, et al. : Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129: 669–678, 2015 [DOI] [PubMed] [Google Scholar]
- 31).Oka H, Utsuki S, Tanizaki Y, et al. : Clinicopathological features of human brainstem gliomas. Brain Tumor Pathol 30: 1–7, 2013 [DOI] [PubMed] [Google Scholar]
- 32).Hashizume R: Epigenetic targeted therapy for diffuse intrinsic pontine glioma. Neurol Med Chir (Tokyo) 57: 331–342, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Piunti A, Hashizume R, Morgan MA, et al. : Therapeutic targeting of polycomb and BET bromodomain proteins in diffuse intrinsic pontine gliomas. Nat Med 23: 493–500, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Nagaraja S, Vitanza NA, Woo PJ, et al. : Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell 31: 635–652.e6, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. : Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46: 462–466, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Tateishi K, Higuchi F, Miller JJ, et al. : The alkylating chemotherapeutic temozolomide induces metabolic stress in IDH1-mutant cancers and potentiates NAD+ depletion–mediated cytotoxicity. Cancer Res 77: 4102–4115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Chornenkyy Y, Agnihotri S, Yu M, et al. : Poly-ADP-ribose polymerase as a therapeutic target in pediatric diffuse intrinsic pontine glioma and pediatric high-grade astrocytoma. Mol Can Ther 14: 2560–2568, 2015 [DOI] [PubMed] [Google Scholar]
- 38).Zarghooni M, Bartels U, Lee E, et al. : Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28: 1337–1344, 2010 [DOI] [PubMed] [Google Scholar]
- 39).Warren KE, Killian K, Suuriniemi M, Wang Y, Quezado M, Meltzer PS: Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro-oncology 14: 326–332, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Miyahara H, Yadavilli S, Natsumeda M, et al. : The dual mTOR kinase inhibitor TAK228 inhibits tumorigenicity and enhances radiosensitization in diffuse intrinsic pontine glioma. Cancer Lett 400: 110–116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Flannery PC, DeSisto JA, Amani V, et al. : Preclinical analysis of MTOR complex 1/2 inhibition in diffuse intrinsic pontine glioma. Oncol Rep 39: 455–464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Chheda ZS, Kohanbash G, Okada K, et al. : Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med 215: 141–157, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Schumacher T, Bunse L, Pusch S, et al. : A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512: 324–327, 2014 [DOI] [PubMed] [Google Scholar]
- 44).Ochs K, Ott M, Bunse T, et al. : K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology 6: e1328340, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Coulie PG, van den Eynde BJ, van der Bruggen P, Boon T: Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14: 135–146, 2014 [DOI] [PubMed] [Google Scholar]
- 46).Yoshimura J, Siu IM, Thomale UW, Jallo GI: The effects of temozolomide delivered by prolonged intracerebral microinfusion against the rat brainstem GBM allograft model. Childs Nerv Syst 28: 707–713, 2012 [DOI] [PubMed] [Google Scholar]
- 47).Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T: Regression of recurrent glioblastoma infiltrating the brainstem after convection-enhanced delivery of nimustine hydrochloride. J Neurosurg Pediatr 7: 522–526, 2011 [DOI] [PubMed] [Google Scholar]
- 48).Hashizume R, Ozawa T, Gryaznov SM, et al. : New therapeutic approach for brain tumors: intranasal delivery of telomerase inhibitor GRN163. Neuro-oncology 10: 112–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Louis N, Liu S, He X, et al. : New therapeutic approaches for brainstem tumors: a comparison of delivery routes using nanoliposomal irinotecan in an animal model. J Neurooncol 136: 475–484, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]