Abstract
Blood glucose levels influence brain regulation of food intake. This study assessed the effect of mild physiological hyperglycemia on brain response to food cues in individuals with obesity (OB) versus normal weight individuals (NW). Brain responses in 10 OB and 10 NW nondiabetic healthy adults [body mass index: 34 (3) vs. 23 (2) kg/m2, means (SD), P < 0.0001] were measured with functional MRI (blood oxygen level-dependent contrast) in combination with a two-step normoglycemic-hyperglycemic clamp. Participants were shown food and nonfood images during normoglycemia (~95 mg/dl) and hyperglycemia (~130 mg/dl). Plasma glucose levels were comparable in both groups during the two-step clamp (P = not significant). Insulin and leptin levels were higher in the OB group compared with NW, whereas ghrelin levels were lower (all P < 0.05). During hyperglycemia, insula activity showed a group-by-glucose level effect. When compared with normoglycemia, hyperglycemia resulted in decreased activity in the hypothalamus and putamen in response to food images (P < 0.001) in the NW group, whereas the OB group exhibited increased activity in insula, putamen, and anterior and dorsolateral prefrontal cortex (aPFC/dlPFC; P < 0.001). These data suggest that OB, compared with NW, appears to have disruption of brain responses to food cues during hyperglycemia, with reduced insula response in NW but increased insula response in OB, an area involved in food perception and interoception. In a post hoc analysis, brain activity in obesity appears to be associated with dysregulated motivation (striatum) and inappropriate self-control (aPFC/dlPFC) to food cues during hyperglycemia. Hyperstimulation for food and insensitivity to internal homeostatic signals may favor food consumption to possibly play a role in the pathogenesis of obesity.
Keywords: brain activity, fMRI, food cues, hyperglycemia, obesity
INTRODUCTION
Obesity, a major health problem in Western societies (7), is caused by an excess in energy intake relative to the amount of energy expended. Body weight, generally, tends to remain constant throughout the years (23), suggesting the existence of well-developed mechanisms regulating energy balance (17). However, the significant increased prevalence of obesity in the past few decades indicates that environmental factors may be influencing the body’s ability to regulate energy balance, although the precise mechanisms driving this adverse change are not fully defined.
The interplay between behavioral, environmental, and homeostatic factors modulates energy intake (food consumption). Hormones and nutrients, such as blood glucose, provide key homeostatic signals indicative of short- and long-term energy status influencing food consumption (39). In 1953, Jean Mayer proposed the glucostatic theory (29), suggesting that the lateral hypothalamus senses acute changes in glucose levels, processes this information, and sends it to other brain regions involved in the regulation of hunger and satiety, which in turn translates into eating behavior and food consumption. Indeed, studies show that blood glucose levels influence eating behavior in animals (14, 25) as well as humans (5, 19, 33) and that both acute blood glucose decrements (31, 33) and increments (19, 28, 32, 40) can alter brain activity in humans. However, most of these studies evaluated the effect of blood glucose levels after oral ingestion of glucose. Thus, it is unclear whether an increase in blood glucose levels per se, independent of ingestion-associated signals, could affect brain control of eating behavior in humans.
Within the brain, specific areas play a major role in regulating eating behavior, such as the hypothalamus (homeostatic sensing), the insula and limbic-striatal system (reward-motivation), and the prefrontal cortex (PFC; executive-control) (6). In addition, hormones (ghrelin, leptin, and insulin), nutrients (glucose and fat), and body weight status have also been shown to influence brain perception of food cues (11, 34). Hunger appears to be a main driver promoting food consumption, but emotional and situational factors also influence eating behavior (8). Thus, the brain processes hedonic, environmental, and homeostatic signals, which act in concert to modulate food intake. Studies have shown that hypoglycemia stimulates hunger and influences food-seeking behavior (22). Mild plasma glucose decrements (from ~90 to 65 mg/dl) in nonobese healthy human subjects elicit enhanced brain activity in the reward-motivation areas in conjunction with decreased activity in executive control brain regions (33), suggesting that hypoglycemia stimulates desire for food, while reducing capacity to exert control over food stimuli. In contrast, individuals with obesity demonstrate distinct brain activity patterns during mild hypoglycemia, namely, augmented activity in reward-motivation brain regions and no change in cortical regions that promote a heightened response to food stimuli and less control over eating behavior (33). These inappropriate brain responses to food cues in obesity during mild hypoglycemia, as could occur in the postprandial state, may promote desire for food and food intake, thereby serving to play a role in the pathogenesis of obesity (26). Whether brain responses to food cues in individuals with obesity compared with normal weight individuals are also similarly affected by physiological increases in blood glucose per se that are comparable to those observed in the postprandial state is, however, not well established.
To investigate the impact of mild hyperglycemia (~130 mg/dl) on brain responses to food motivation, we used the hyperglycemic clamp technique to raise glucose levels in a standardized fashion while obtaining blood oxygen level-dependent (BOLD) measurements with functional MRI (fMRI) to compare brain responses to visual food and nonfood (control) cues in individuals with obesity and normal weight individuals. Although visual food stimuli have been shown to have a significant effect on food motivation and intake (3), we anticipated that the human brain could detect and respond to mild hyperglycemia as it does to mild hypoglycemia. Thus, we hypothesized that in normal weight individuals, a small increase in blood glucose (without oral intake) would diminish responses in the reward-motivation while increasing responses in the executive control brain areas involved in modulating food motivation and intake, whereas individuals with obesity would exhibit deregulated brain responses that could be playing a major role in stimulating desire for food during the postprandial period, a time when increased control over food intake behavior is important for regulating energy balance and influencing body weight.
MATERIALS AND METHODS
Subjects
Twenty subjects (13 women and 7 men) participated in this study. The participants were divided in two groups: normal weight [NW; 6 women/4 men, body mass index (BMI) 23 (2) kg/m2, age 28 (8) yr, hemoglobin A1c (HbA1c) 5.2% (0.2%), means (SD)] and obese [OB; 7 women/3 men, BMI 34 (3) kg/m2, age 31 (12) yr, HbA1c 5.2% (0.3%)]. Other than BMI (P < 0.0001), the two groups were comparable for age (P = 0.52), HbA1c (P = 0.531), and education status [NW: 14 (3) yr and OB: 15 (2) yr, P = 0.562]. Participants were screened at the Yale New Haven Hospital Research Unit. Subjects who were vegetarian, had any major medical or psychological disorders, had elevated HbA1c (all <5.7%, except 1 OB participant who was 5.8%), or had contraindications for MRI scans were excluded. Participants enrolled in the study were required to have stable body weight at least 3 mo before the study. Written informed consent was obtained from each study participant. The study was approved by the Human Investigation Committee from the Yale Human Research Protection Program and was registered with ClinicalTrials.gov, no. NCT00580710.
Study Design
On the day of the study, participants were asked to come to the Yale Magnetic Resonance Research Center, where they ate a standardized meal (turkey sandwich, diet ginger ale, and an apple, consisting of 360 cal: 44% carbohydrate, 31% fat, and 25% protein) ~2 h before the MRI scan. Catheters were then inserted into the antecubital vein on each arm. One catheter was used for intravenous glucose infusion, while the other was used for blood sampling. Subjects were then brought to the scan room. Once in the scanner, they underwent a two-step glucose infusion: normoglycemia (~95–100 mg/dl) and mild hyperglycemia (~125–130 mg/dl; Fig. 1). The MRI scan and intravenous glucose infusion were started concomitantly at time 0. During normoglycemia, a slow intravenous glucose infusion rate was used to keep blood glucose levels at the desired target range levels and well matched between the NW and OB groups. After completion of the normoglycemic session, a weight-based bolus dose of intravenous glucose was calculated as previously described (12); however, to avoid severe hyperglycemia, only a third of the calculated dose was given. Blood glucose levels were then kept at a mild hyperglycemic range until the end of the scan. The target glucose levels during the hyperglycemic phase of the clamp were chosen on the basis of the normal glucose levels observed after a 2-h oral glucose tolerance test (<140 mg/dl), per American Diabetes Association diagnostic criteria (1). Glucose levels were kept at the target range by adjusting the intravenous glucose infusion based on the plasma glucose levels measured every 5 min. At baseline before the scan and during the MRI-infusion procedure, blood samples were drawn for hormone level analysis. Anatomical scans were acquired at the beginning of the scan. At each step of the infusion, subjects were presented with a visual task while BOLD measurements were acquired.
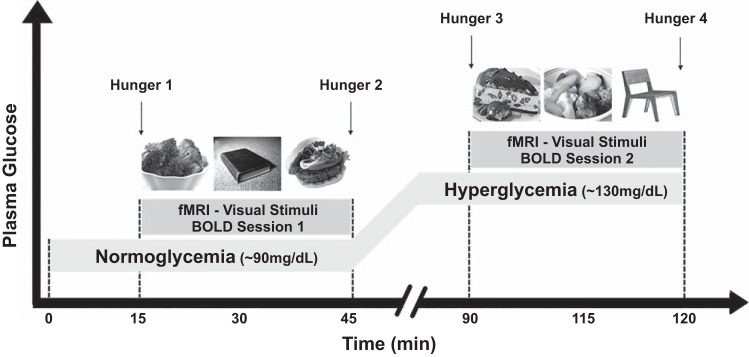
Fig. 1.
Study procedure [event-related functional MRI (fMRI) design]. An fMRI scan was obtained while blood glucose levels were manipulated with a two-step normoglycemic-hyperglycemic clamp. Two sessions of blood oxygen level-dependent (BOLD) acquisitions were obtained during each step of the clamp. With the use of a well-validated fast event-related design, each session consisted of 4 runs of 21 pictures (7 high-calorie food, 7 low-calorie food, and 7 nonfood) in random order that were projected onto a screen in the scanner. Wanting and liking ratings were recorded for each picture (not shown). Hunger ratings were obtained with a visual analog scale of 1 (“not at all hungry”) to 9 (“very hungry”) immediately before and immediately after each session (time points 1, 2, 3, and 4).
Visual Stimuli
The present study followed a validated fMRI-metabolic study protocol, the details of which have been previously described (3, 33). Brain anatomical measurements were performed in the initial first 15 min followed immediately by BOLD measurements. E-Prime software (Psychological Software Tools) was used to present the visual stimuli (total: 168 pictures across 8 runs) to subjects. Each BOLD session (normoglycemia and hyperglycemia) lasted ~30 min, and sessions were performed at the end of each step of the clamp. They consisted of 4 runs of 21 images each: 7 high-calorie (HC) foods, 7 low-calorie (LC) foods, and 7 nonfood (NF) items per run. Affective valence of the food pictures was previously evaluated, and it was not statistically different between HC and LC food pictures (33). With the use of an event-related design, images were shown for 6 s each and were counterbalanced and randomized across participants. Subsequently after images, two visual-analog scales (VAS) appeared for 3 s each to ask the subject to rate their liking and wanting of the item in the picture on a scale of 1 (not at all) to 9 (very much). Then a fixation cross was shown with a jittered intertrial interval (mean of 6 s) to differentiate events of interest within a trial (10). This process was then repeated for each of the 21 images in the run. Before and after each four-run session, a VAS was displayed, and the subject was asked to rate their hunger from 1 (not at all hungry) to 9 (very hungry). Images for the tasks were projected onto a screen mounted at the back of the scanner bore and viewed by the subjects on a mirror mounted onto the head coil. The entire duration of the fMRI scan and infusion was ~2 h.
Biochemical Analysis
Plasma glucose concentration was determined by the glucose oxidase method (YSI, Yellow Springs, OH). Plasma insulin, ghrelin, leptin (Millipore, St. Charles, MO), and cortisol (Diagnostic Products, Los Angeles, CA) were measured by double-antibody radioimmunoassay.
Statistical Analysis
For behavioral ratings and hormone data, statistical analyses were performed using SPSS 22.0 software (IBM). All values represent means (SD). A two-way repeated-measures ANOVA was performed to identify the interaction effect of glucose level over time within each group. Comparisons within subjects were determined by the two-tailed Student’s t-test for paired samples and between subjects by the unpaired Student’s t-test for equality of means. A value of P < 0.05 was considered statistically significant.
Functional MRI Analysis
The digital data [Digital Imaging and Communication in Medicine (DICOM)] were converted to analyze data by XMedCon, where the first 3 images were discarded from the run to enable the signal to achieve steady-state equilibrium between radio frequency pulsing and relaxation leaving 195 images per slice per run for analysis. The data were motion corrected using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5/), and they were discarded if linear motion was >1.5 mm or rotation was >2°. For individual subject data analysis, general linear model was used to determine the regions with changes in signal in response to the visual task (HC, LC, or NF image) in each session. The functional images were spatially smoothed with a 6-mm Gaussian kernel to account for variations in the location of activation across subjects. The output maps were normalized beta-maps, which were in the acquired space (3.44 × 3.44 × 4 mm). To adjust for anatomical differences in each individual, the Yale BioImage Suite software package (https://medicine.yale.edu/bioimaging/suite/) was used to calculate two linear and one nonlinear registration. These three registrations were concatenated and applied as one registration to bring the data into a common reference brain space. The Colin27 brain in the Montreal Neurological Institute (MNI) space was used as the reference brain. For group level data analysis, analysis of variance using Analysis of Functional Neuroimages (AFNI) ANOVA (https://afni.nimh.nih.gov) was implemented with a 2 (group: NW, OB) × 2 (session: normoglycemia, hyperglycemia) × 3 (task: HC, LC, NF) design. In this design, task and session were treated as the within-subjects fixed-effect factors, group as the between-subjects factor, and subject as the random effect factor. To correct for multiple comparisons, we used family-wise errors (FWE) correction determined by Monte Carlo simulation using the AFNI 3dClustSim version (16.3.05, October 2016) program. A value of P < 0.001 was considered statistically significant for whole brain FWE correction (15).
RESULTS
Glucose and Hormones
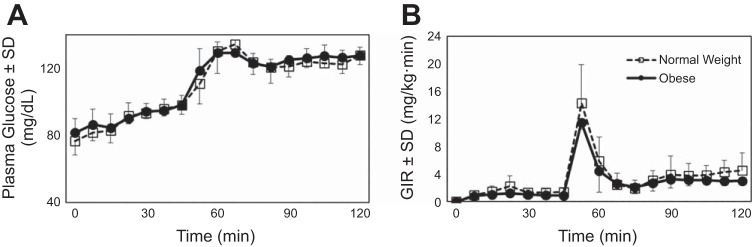
The NW and OB groups were studied in a neutral hunger/satiety state, 2 h after eating a standard meal. Baseline nonfasting plasma glucose levels were comparable between the two groups [NW: 80 (9) mg/dl and OB: 82 (9) mg/dl; P = 0.753]. Insulin and leptin levels were significantly higher in the OB than in the NW group, whereas ghrelin levels were lower (Table 1). Glucose levels during the two-step normoglycemic-hyperglycemic clamp were comparable in both groups (Fig. 2A), as was the glucose infusion rate required to keep glucose levels at the desired target range during the study (Fig. 2B). The hormonal differences observed at baseline for insulin, leptin, and ghrelin between the two groups persisted throughout the glucose clamp studies.
Table 1.
Glucose and hormonal levels during the two-step normoglycemic-hyperglycemic clamp
| Normal Weight |
Obese |
P Value Baseline† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | Normoglycemia | Hyperglycemia | P Value Normoglycemia × Hyperglycemia* | Basal | Normoglycemia | Hyperglycemia | P Value Normoglycemia × Hyperglycemia* | ||
| Glucose, mg/dl | 80 (9) | 98 (5) | 128 (5) | <0.001 | 82 (9) | 98 (6) | 128 (5) | <0.001 | 0.753 |
| Insulin, µU/ml | 18 (11) | 17 (11) | 24 (16) | 0.013 | 31 (15) | 32 (23) | 45 (28) | 0.005 | 0.037 |
| Leptin, ng/ml | 8 (8) | 9 (9) | 10 (9) | 0.100 | 27 (15) | 33 (23) | 35 (22) | 0.522 | 0.002 |
| Ghrelin, ng/ml | 841 (251) | 911 (259) | 831 (277) | 0.012 | 630 (168) | 658 (219) | 590 (153) | 0.120 | 0.041 |
| Cortisol, µg/dl | 17 (10) | 15 (9) | 16 (4) | 0.552 | 12 (4) | 10 (4) | 13 (6) | 0.199 | 0.180 |
Values are means (SD).
P value indicates comparison within groups for glucose and hormone levels at the end of the hyperglycemia vs. the normoglycemia clamp step.
P value indicates comparison between groups for glucose and hormone levels at baseline.
Fig. 2.
Plasma glucose levels (A) and glucose infusion rate (GIR; B). Data are means (SD), during the two-step normoglycemic-hyperglycemic clamp in normal weight study participants (□, n = 10) and study participants with obesity (●, n = 10).
Food Motivation
During the fMRI scans, the participants rated how hungry they were before and after each BOLD session. There were no statistical differences in hunger ratings between NW and OB groups. Similar to our previous findings (3), hunger ratings progressively increased in response to food pictures over time and thus were significantly higher during hyperglycemia, a later event, than during normoglycemia (Fig. 3A). Wanting and liking ratings were obtained for each food (HC and LC) and NF picture. Both groups showed a preference for images of food compared with NF [average ratings of the 2 sessions for both groups combined: wanting, food = 5.2 (1.4) vs. NF = 2.7 (1.0), P < 0.001; liking, food = 6.5 (1.0) vs. NF = 3.4 (1.1), P < 0.001; all statistical analysis corrected for multiple comparisons (Bonferroni adjusted)]. When hyperglycemia and normoglycemia were compared, there was a significant decrease in liking NF (P < 0.01) and wanting food pictures (P < 0.05; Fig. 3B). However, there was no group by task interaction effect for food preference when comparing the NW and OB groups [F(2,36) = 0.330, P = 0.721].
Fig. 3.
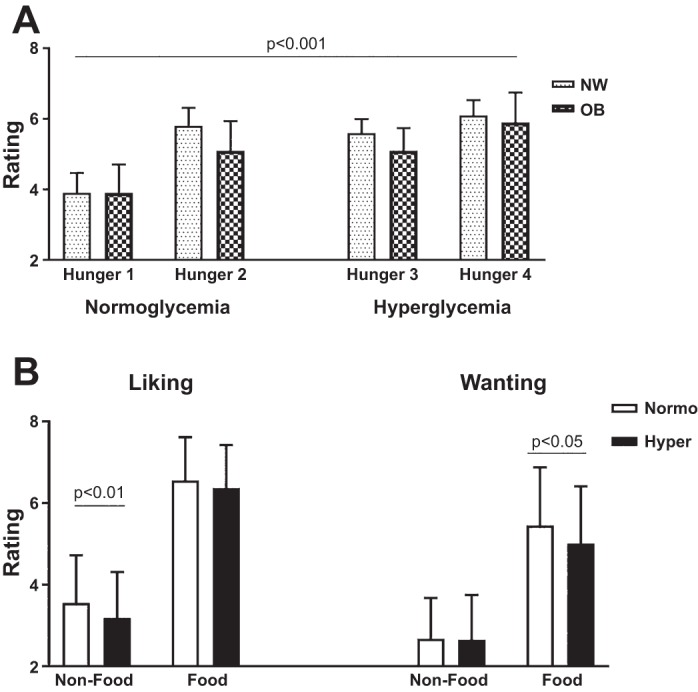
Hunger, liking, and wanting for food. Average hunger (A) and liking and wanting ratings (B) while viewing nonfood and food (high-calorie and low-calorie food) pictures during normoglycemia (Normo) and hyperglycemia (Hyper) for both groups [normal weight (NW) and obese (OB)] combined (n = 20). Data are means (SD). Statistically significant P values (P < 0.05) comparing normoglycemia and hyperglycemia are indicated.
Functional MRI: Whole Brain Response
Group × session × task interaction.
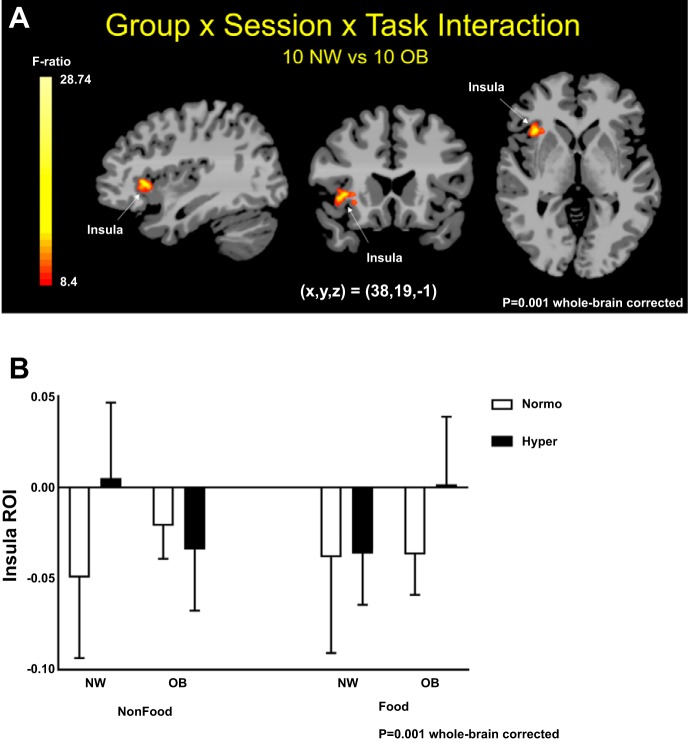
An overall significant interaction effect of group (NW, OB) × session (normoglycemia, hyperglycemia) × task (HC, LC, NF) was found (P < 0.001, whole brain FWE correction). This significant interaction resulted from a significant group difference during both the normoglycemia and hyperglycemia sessions in response to visual cues in the right insula, after whole brain FWE correction at P < 0.001 (Fig. 4A). Both groups demonstrated comparable brain activity in response to HC and LC in contrast to NF pictures, at P < 0.001. In addition, there were no effects of HC relative to LC food pictures, and therefore the results for whole brain contrast maps were grouped by food (HC and LC combined) in contrast to NF pictures. NW displayed increased right insula activity during NF, whereas OB showed increased right insula activity while viewing food pictures, when comparing hyperglycemia with normoglycemia (Fig. 4B).
Fig. 4.
Whole brain maps of group, session, and task interaction. A: whole brain maps showing in red/yellow statistically significant interaction of group [normal weight (NW), obese (OB)], session (normoglycemia, hyperglycemia), and task [high-calorie (HC) food, low-calorie (LC) food, nonfood (NF)] on right insula activity. B: right insula blood oxygen level-dependent contrast beta-values during normoglycemia (Normo) and hyperglycemia (Hyper) in response to NF and food (HC and LC) pictures in NW and OB individuals. Montreal Neurological Institute (MNI) coordinates were used. ROI, region of interest. F ratio, family-wise errors correction at P < 0.001, cluster correction at P < 0.05.
Group, task, and session effect.
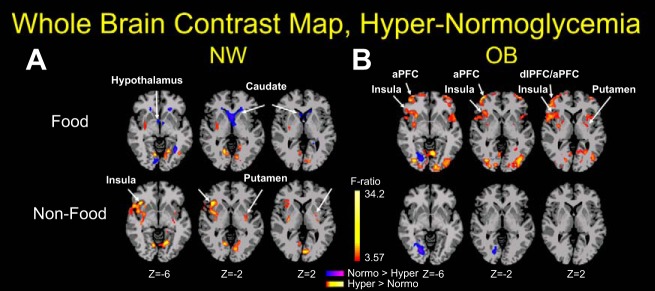
The group main effect map showed that there were no brain regions that survived whole brain FWE correction at P < 0.001. The task main effect was found in ventrolateral PFC, amygdala, insula, putamen, and visual occipital areas at P < 0.001, with food conditions showing increased activity in these regions compared with nonfood condition. To further understand the effect of the food and NF images on brain activity during each step of the glucose clamp, we observed that in NW, hyperglycemia increased brain activity (yellow-red) to visual food stimuli in the putamen (striatum) and visual occipital cortical areas and decreased activity (blue) in the hypothalamus and caudate (Fig. 5A, top; P < 0.001). OB individuals, in contrast to the NW group, demonstrated a significantly different brain activity pattern in response to food pictures, i.e., enhanced responses in the insula (taste perception/interoception) and striatum (reward-motivation) and no changes in the caudate and hypothalamus (Fig. 5B, top; P < 0.001). In the presence of hyperglycemia, viewing NF pictures by NW individuals resulted in increased brain responses in insula and striatum, anterior and dorsolateral PFC (aPFC and dlPFC, respectively), and occipital brain regions (visual cortex; Fig. 5A, bottom), whereas this response was absent (or even diminished in the occipital cortex) in the OB group (Fig. 5B, bottom). These results suggest that the brain of OB subjects is diversely activated by food cues and relatively insensitive to the changes in blood glucose levels per se (homeostatic milieu).
Fig. 5.
Differences between hyperglycemia and normoglycemia by groups. Whole brain contrast maps during hyperglycemia relative to normoglycemia while viewing food (high-calorie and low-calorie food) and nonfood pictures. Yellow and red depict increased activity, and blue indicates decreased activity during hyperglycemia in contrast to normoglycemia. A: during hyperglycemia, normal weight (NW) participants showed decreased activity in the hypothalamus and caudate while viewing food images and increased activity in the insula and putamen while viewing nonfood images. B: in contrast, participants with obesity (OB) showed increased activity in the insula, anterior prefrontal cortex (aPFC), and dorsolateral prefrontal cortex (dlPFC) while viewing food images but no activity changes while viewing nonfood images. Montreal Neurological Institute (MNI) coordinates were used. Student’s t-test, family-wise errors correction at P < 0.001, cluster correction at P < 0.05. Red/yellow, hyperglycemia (Hyper) > normoglycemia (Normo); blue/purple, hyperglycemia < normoglycemia.
DISCUSSION
In this study, we investigated whether modest increments in blood glucose levels (~130 mg/dl), comparable to those observed in the normal postprandial state in nondiabetic lean individuals and individuals with obesity (4, 24), affect brain food motivation responses when presented with visual food cues. We observed that mild hyperglycemia per se influences brain activity in response to visual food cues and that NW and OB individuals have very distinct brain activity patterns when exposed to identical increases in blood glucose levels (Fig. 4B). NW participants demonstrated no change in insula activity [a brain region involved in taste perception, decision making, and interoception (6, 11)] in response to hyperglycemia while seeing food pictures but increased insula activity in the presence of NF pictures. This shift in insula activity in the presence of mild hyperglycemia was not observed in the OB group, which showed no change in the insula response to NF pictures but increased activity while viewing food images. In addition, while viewing food pictures (Fig. 5), hyperglycemia decreased brain activity in the hypothalamus (homeostasis) and caudate (reward-motivation) in the NW group, whereas the OB group in contrast displayed more robust responses in the insula (taste perception/interoception), the striatum (reward-motivation), and the aPFC and dlPFC (self-control). These findings suggest that food motivation and intake-associated brain regions in OB individuals may be less responsive to changes in peripheral energy status signals compared with NW individuals (i.e., by absent effect in hypothalamic activity during hyperglycemia). Thus, obesity appears to be a state of neural insensitivity to the homeostatic milieu, such as during increases in desire and motivation for food cue stimuli in individuals with obesity during hyperglycemia.
Similar to the reported effects of oral feeding on brain responses to visual food stimuli (9, 13, 27), we observed that hyperglycemia induced by glucose infusion also influences brain activity, findings consistent with the glucostatic theory (29), which posits that blood glucose levels per se have a direct effect on feeding behavior. OB individuals, compared with NW individuals, demonstrated absent (or in some cases opposite) responses to hyperglycemia in brain regions associated with desire and motivation for food (insula), in line with previous studies showing that in OB individuals, brain responses to food cues are not minimized by food intake (13, 27). It is noteworthy that the dlPFC, a brain region involved with self-monitoring and restraint, showed increased activity in individuals with obesity but not lean individuals when asked to reappraise the reward value of food during hyperglycemic states (36). The increased activity in this region seen in our OB group could indicate an increased effort to restrain the effect of the food images in the reward-associated brain regions during hyperglycemia. In addition, in NW, hyperglycemia decreased activity in the caudate in response to food cues, a brain region involved with reward anticipation (18), and increased in the putamen in response to NF cues, an area related to stimulus-action (18). These results suggest that in NW, hyperglycemia decreased food-related reward anticipation and increased readiness and motivation toward NF-related stimuli. In contrast, increased putamen activity during hyperglycemia in OB individuals suggests greater action-oriented readiness toward food-related reward stimuli.
In NW individuals, the shift in brain responses to food and NF visual cues induced by hyperglycemia may help determine eating behavior, in which increased desire for food before eating at normoglycemia is minimized or redirected to neutral (NF) stimuli after meal-induced hyperglycemia. Thus, postprandial glucose elevations per se appear to be one of the mechanisms modulating satiety in NW individuals. In marked contrast, brain reward-motivation regions of OB individuals are activated by food cues and relatively insensitive to increases in blood glucose levels. In addition, hyperglycemia failed to suppress hypothalamic activity in individuals with obesity. Taken together, these data suggest that abnormal brain response patterns to external food cues in OB compared with NW individuals in the postprandial period may play a role in the pathogenesis of obesity, where a persistent desire for food and diminished interest toward NF-related stimuli would promote food intake as well as weight gain.
OB compared with NW individuals had a distinct hormonal profile, namely, higher insulin and leptin levels and lower ghrelin levels. These hormones have been shown to influence feeding behavior; that is, leptin and insulin increase satiety whereas ghrelin is orexigenic (30). Although in our previous study we did not observe a direct effect of insulin on brain responses to food cues in lean individuals (3), it is very likely that hormonal status differences influenced our results. In OB individuals, insulin and leptin resistance may impair hormonal regulation of food intake (2, 38), which could interfere with the effect of glucose on modulating brain control of eating behavior. However, we cannot exclude the possibility that obesity may affect brain glucose directly: In humans, decreased glucose entry in the brain was observed in individuals with obesity (20), which could be explained by a decrease in blood-brain barrier glucose transporters, as is seen in mice fed a high-fat diet (21). Furthermore, rodent studies have shown that high-fat diets cause hypothalamic abnormalities, such as inflammation and neuronal injury, which could impair hypothalamic glucose-sensing mechanisms (30). It remains to be determined whether improving insulin and/or leptin resistance could help restore brain responses to hyperglycemia in obesity.
The standardized meal 2-h before the scan was effective in maintaining the participants in a neither-too-hungry-nor-too-full state; however, because the number of calories in the meal was not calculated by the individual’s weight, we cannot exclude that it could have affected some of our results. In addition, it is conceivable that different metabolic and hormonal responses to the meal in OB and NW individuals could have affected our results. Nevertheless, hyperglycemia appears to further accentuate these intrinsic differences. Although we observed differences in brain responses between the two groups, ratings of food motivation (hunger, wanting, and liking) were not significantly different at baseline (before viewing pictures) or throughout the study (Fig. 2). The small sample size may have limited our ability to detect small subjective changes in these measurements. In addition, social desirability bias may have influenced VAS responses when the participants were selecting their answers. A limitation of this study was that the order of the sessions (normoglycemia and hyperglycemia) was not randomized, which resulted in a progressive increase in hunger ratings. This study design was chosen to limit the participants’ exposure to the visual tasks, but it resulted in a mismatch between hunger and brain responses to hyperglycemia. However, these results are consistent with previous studies showing that intravenous glucose infusion fails to elicit changes in subjective measurements of food motivation, such as hunger and satiety (35, 37), but does modulate the brain response in food motivation regions (40).
We observed that our visual food cue paradigm was a strong enough stimulus to induce brain responses detectable by fMRI. Although the small sample could have affected our fMRI results, we would not expect that it would lead to enhanced brain responses to food in contrast to NF stimuli in the presence of hyperglycemia. Furthermore, we used a very stringent statistical threshold of P < 0.001, as recommended for fMRI data analysis (15). This threshold cutoff has been shown to be reliable and replicable as well as to give high power to detect brain regions involved in food processing in studies with a relatively small number of subjects.
In this study, we chose an increment in blood glucose levels from 95 to 130 mg/dl based on the levels found in normal subjects after a meal (16). It is possible that greater increments in glucose levels would be necessary to cause effects in brain responses to food cues in the OB group that are similar to those in the NW group. The two groups were comparable with regard to age, gender, education, HbA1c levels, and nonfasting baseline glucose levels. Therefore, for this healthy nondiabetic OB group, we would expect that this experimentally induced increase in blood glucose levels would reflect their usual postprandial blood glucose pattern. However, in the OB group, this mild hyperglycemia not only had no suppressive effect on brain activity in the hypothalamus and the caudate but also led to an increase in brain activity in the insula, putamen, and aPFC/dlPFC. Indeed, similar findings were observed by other investigators when participants were instructed to eat until sated (13).
In summary, we observed that modest physiological increments in blood glucose from normoglycemia to hyperglycemia modulate brain responses to food cues. In NW individuals, hyperglycemia promotes a brain activity pattern toward a decrease in motivation for food (hypothalamus and caudate) and an increase in reward-motivation (insula and putamen) to neutral (NF-related) objects. OB individuals have a distinct brain activity pattern in response to hyperglycemia, namely, enhanced activity in brain regions regulating reward-motivation (insula and putamen) and self-control (aPFC/dlPFC). These findings indicate that obesity may induce a state of augmented desire for food that is insensitive to the peripheral signals concerning the body’s energy storage. This hyperstimulated state of the brain may play a role in the pathogenesis of obesity, which, in our current environment overwhelmed by highly palatable calorie-dense food, may be fueling the epidemic of obesity.
GRANTS
We acknowledge support for the BioImage Suite software used in the fMRI analysis from National Institutes of Health (NIH) National Institute of Biomedical Imaging and Bioengineering (NIBIB) Grant 1R01-EB-006494-01. This work was funded in part by NIH Grants K23-DK-098286-02 to R. Belfort-DeAguiar, K08-AA-023545 to D. Seo, DK-20495, P30-DK-45735, and T32-DK-07058 to R. S. Sherwin, and R01-DK099039 and UL1-DE-19586 to R. Sinha. This publication was made possible by Clinical and Translational Science Awards Grant UL1-TR-00142 from the National Center for Advancing Translational Science, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
DISCLOSURES
R. Belfort-DeAguiar has received research support from GlaxoSmithKline. J. Hwang has received research support from Pfizer and Regeneron. R. S. Sherwin is an adjudication committee member for studies sponsored by Novo Nordisk Pharmaceuticals Corporation via ICON Clinical Research Limited (ICON) and Quintiles and a member of the Data Safety Monitoring Board for MannKind Corporation via PPD Development, L.P. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
R.B.-D., D.S., S.N., T.C., R.S., and R.S.S. conceived and designed research; R.B.-D., D.S., S.N., and J.H. performed experiments; R.B.-D., D.S., C.L., S.N., C.S., W.L., and R.S. analyzed data; R.B.-D., D.S., C.L., C.S., W.L., J.H., T.C., R.S., and R.S.S. interpreted results of experiments; R.B.-D., D.S., C.L., C.S., W.L., and R.S.S. prepared figures; R.B.-D. drafted manuscript; R.B.-D., D.S., C.L., S.N., C.S., W.L., J.H., T.C., R.S., and R.S.S. edited and revised manuscript; R.B.-D., D.S., C.L., S.N., C.S., W.L., J.H., T.C., R.S., and R.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen Allen, Anne O’Connor, Gina Solomon, Catherine Parmelee, Hedy Sarofin, Terry Hickey, Kristen A. Tsou, Edward Gaiser, Ralph Jacob, Mikhail Smolgovsky, Irene Chernyak, and Codruta Todeasa for assistance as well as the subjects who participated in this study.
Present addresses: S. Naik, University College London Hospitals NHS Foundation Trust, 235 Euston Rd., London NW1 2BU, UK; C. Schmidt, University of Illinois College of Medicine at Rockford, 1601 Parkview Ave., Rockford, IL 61107.
REFERENCES
- 1.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 40, Suppl 1: S11–S24, 2017. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 2.Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 55: 2986–2992, 2006. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 3.Belfort-DeAguiar R, Seo D, Naik S, Hwang J, Lacadie C, Schmidt C, Constable RT, Sinha R, Sherwin R. Food image-induced brain activation is not diminished by insulin infusion. Int J Obes 40: 1679–1686, 2016. doi: 10.1038/ijo.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Mag Reson Med 34: 537–541, 1995. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 5.Campfield LA, Smith FJ, Rosenbaum M, Hirsch J. Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev 20: 133–137, 1996. doi: 10.1016/0149-7634(95)00043-E. [DOI] [PubMed] [Google Scholar]
- 6.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 13: 43–56, 2012. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention Adult Obesity Causes & Consequences (Online) https://www.cdc.gov/obesity/adult/causes.html.
- 8.Cohen DA. Obesity and the built environment: changes in environmental cues cause energy imbalances. Int J Obes 32, Suppl 7: S137–S142, 2008. doi: 10.1038/ijo.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly L, Coveleskie K, Kilpatrick LA, Labus JS, Ebrat B, Stains J, Jiang Z, Tillisch K, Raybould HE, Mayer EA. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol Motil 25: 579–e460, 2013. doi: 10.1111/nmo.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp 8: 109–114, 1999. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 11.De Silva A, Salem V, Matthews PM, Dhillo WS. The use of functional MRI to study appetite control in the CNS. Exp Diabetes Res 2012: 764017, 2012. doi: 10.1155/2012/764017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 58: 303–312, 2012. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci 29: 7015–7022, 2009. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 113: 7900–7905, 2016. [Erratum in Proc Natl Acad Sci USA 113: E4929, 2016. doi: 10.1073/pnas.1612033113.] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freckmann G, Hagenlocher S, Baumstark A, Jendrike N, Gillen RC, Rössner K, Haug C. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol 1: 695–703, 2007. doi: 10.1177/193229680700100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30: 367–398, 2007. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- 18.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol 95: 948–959, 2006. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 19.Heni M, Kullmann S, Ketterer C, Guthoff M, Bayer M, Staiger H, Machicao F, Häring HU, Preissl H, Veit R, Fritsche A. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp 35: 918–928, 2014. doi: 10.1002/hbm.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang JJ, Jiang L, Hamza M, Sanchez Rangel E, Dai F, Belfort-DeAguiar R, Parikh L, Koo BB, Rothman DL, Mason G, Sherwin RS. . Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2: 95913, 2017. doi: 10.1172/jci.insight.95913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jais A, Solas M, Backes H, Chaurasia B, Kleinridders A, Theurich S, Mauer J, Steculorum SM, Hampel B, Goldau J, Alber J, Förster CY, Eming SA, Schwaninger M, Ferrara N, Karsenty G, Brüning JC. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 165: 882–895, 2016. [Erratum in Cell 166: 1338–1340, 2016. doi: 10.1016/j.cell.2016.08.010.] doi:. [DOI] [PubMed] [Google Scholar]
- 22.Janowitz HD, Ivy AC. Role of blood sugar levels in spontaneous and insulin-induced hunger in man. J Appl Physiol 1: 643–645, 1949. doi: 10.1152/jappl.1949.1.9.643. [DOI] [PubMed] [Google Scholar]
- 23.Kyle UG, Zhang FF, Morabia A, Pichard C. Longitudinal study of body composition changes associated with weight change and physical activity. Nutrition 22: 1103–1111, 2006. doi: 10.1016/j.nut.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Lennerz BS, Alsop DC, Holsen LM, Stern E, Rojas R, Ebbeling CB, Goldstein JM, Ludwig DS. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr 98: 641–647, 2013. doi: 10.3945/ajcn.113.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis-Sylvestre J, Le Magnen J. Fall in blood glucose level precedes meal onset in free-feeding rats. Neurosci Biobehav Rev 4, Suppl 1: 13–15, 1980. doi: 10.1016/0149-7634(80)90041-X. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics 103: E26, 1999. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 27.Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 18: 254–260, 2010. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48: 1801–1806, 1999. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- 29.Mayer J. Glucostatic mechanism of regulation of food intake. N Engl J Med 249: 13–16, 1953. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 30.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci 15: 367–378, 2014. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musen G, Simonson DC, Bolo NR, Driscoll A, Weinger K, Raji A, Théberge J, Renshaw PF, Jacobson AM. Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab 93: 1450–1457, 2008. doi: 10.1210/jc.2007-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, Sherwin RS. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 309: 63–70, 2013. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest 121: 4161–4169, 2011. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 1: 7, 2014. doi: 10.3389/fnut.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism 34: 826–831, 1985. doi: 10.1016/0026-0495(85)90106-4. [DOI] [PubMed] [Google Scholar]
- 36.Scharmüller W, Übel S, Ebner F, Schienle A. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett 518: 106–110, 2012. doi: 10.1016/j.neulet.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 37.Schultes B, Panknin AK, Hallschmid M, Jauch-Chara K, Wilms B, de Courbière F, Lehnert H, Schmid SM. Glycemic increase induced by intravenous glucose infusion fails to affect hunger, appetite, or satiety following breakfast in healthy men. Appetite 105: 562–566, 2016. doi: 10.1016/j.appet.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science 307: 375–379, 2005. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 40.Smeets PA, Vidarsdottir S, de Graaf C, Stafleu A, van Osch MJ, Viergever MA, Pijl H, van der Grond J. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am J Physiol Endocrinol Metab 293: E754–E758, 2007. doi: 10.1152/ajpendo.00231.2007. [DOI] [PubMed] [Google Scholar]