Abstract
Cytotoxic function and cytokine profile of natural killer cells are compromised in patients with systemic lupus erythematosus (SLE). CD3ζ, an important molecule for NK cell activation, is downregulated in SLE T cells and contributes to their altered function. However, little is known about the role of CD3ζ in SLE NK cells. We studied CD3ζ levels and its contribution to cytotoxic, degranulation, and cytokine production capacity of NK cells from patients with SLE. Furthermore, we studied the human NK cell line, NKL, where manipulation of CD3ζ levels was achieved using siRNA, and NK cells from Rag2 mice deficient in CD3ζ. We found reduced CD3ζ expression in NK cells from SLE patients independent of disease activity. Downregulation of CD3ζ expression in NK cells is mediated, at least in part, by Caspase 3, the activity of which is higher in NK cells from patients with SLE compared to NK cells from healthy donors. CD3ζ levels correlated inversely with natural cytotoxicity and the percentage of cells capable of producing the pro-inflammatory cytokines IFNγ and TNF. In contrast, CD3ζ levels showed a direct correlation with levels of antibody-dependent cellular cytotoxicity (ADCC). Experiments performed in CD3ζ-silenced NKL and CD3ζ-deficient NK cells from Rag2 mice confirmed the dependence of NK cell function on CD3ζ levels. Our results demonstrate a differential role for CD3ζ in natural cytotoxicity and ADCC. We conclude that downregulated CD3ζ confers a pro-inflammatory phenotype to SLE NK cells and contributes to their altered function in patients with SLE.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by loss of immune system tolerance that leads to multi-organ damage and tissue inflammation (1). Despite the improvement in the diagnosis and treatment of the disease, SLE patients continue to experience significant morbidity and mortality related to infections (2). Although immunosuppressive drug can account for the increased ratio of infections, the contribution of reduced natural killer and CD8 T cell cytotoxic activity cannot be ignored (3–5).
Natural killer (NK) cells are innate lymphoid cells with an important role in immune surveillance and immune response against infected and tumor cells through natural cytotoxicity or antibody-dependent cellular cytotoxicity (ADCC) (6). NK cells are also a major source of chemokines and cytokines such as IFNγ and TNF, which modulate adaptive immune responses upon activation (7).
Alteration of NK cell numbers and function leads to deregulation of the immune system and the development of SLE in humans and mice (3). Peripheral blood from SLE patients display a reduced number of NK cells with an activated phenotype and increased capacity to produce IFNγ, decreased ADCC, and altered natural cytotoxicity (8–12). NK cells in the kidney and lungs from MRL/lpr also display an activated phenotype with increased natural cytotoxicity and IFNγ production, but reduced ADCC (13, 14). Both are suggested contributors to tissue damage (3, 14). The molecular alterations responsible for the SLE NK cell deregulation are largely unknown.
Activation of NK cells occurs as a result of the integration of signals from inhibiting and activating receptors (15). As part of activating receptors, NKp30 and NKp46 are associated with natural cytotoxicity (15). CD16, however, is associated with antibody-dependent cellular cytotoxicity (15). These receptors share their association with the signaling molecules CD3ζ and FcεRIγ (15).
CD3ζ is a transmembrane molecule expressed in T and NKT cells where it associates with the TCR complex (16, 17), and in NK cells where associates with CD16, NKp30 and NKp46 (15). Decreased levels of CD3ζ in T cells have been reported in SLE patients attributed to decreased transcription rates and increased degradation (18, 19) and contributes to altered early signaling events and aberrant cytokine production (18). However, nothing is known about the role of CD3ζ in NK cells in patients with SLE.
We show that levels of CD3ζ in NK cells from patients with active or inactive SLE are decreased. Downregulation of CD3ζ expression does not depend on mRNA levels or serum factors but is in part controlled by Caspase 3, the activity of which is higher in NK cells from patients with SLE compared to control subjects. CD3ζ levels inversely correlate with natural cytotoxicity, as well as IFNγ and TNF production capacity and directly correlate with antibody-dependent cellular cytotoxicity from SLE NK cells. We confirmed the dependence of these observations on CD3ζ by modulating its expression level in the human NK cell line, NKL, using siRNA and by analyzing a Rag2 mouse lacking CD3ζ. Our results show that CD3ζ is not only downregulated in SLE T cells but also in NK cells and it contributes to the pro-inflammatory phenotype of SLE NK cells.
Patients and Methods
Human samples
Patients (n = 55, women) fulfilling the American College of Rheumatology criteria for lupus and age-similar and sex-matched healthy volunteers (n = 32, women) were recruited at the Division of Rheumatology at Beth Israel Deaconess Medical Center, and 5ml of blood was collected for this study. Disease activity of patients was measured using the SLE Disease Activity Index (SLEDAI). Patient and healthy donor demographic information are listed in Table I. The study was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center. Written informed consent was obtained from all subjects and investigation was conducted according to the principles expressed in the Declaration of Helsinki.
Table I.
Characteristics of the SLE and healthy donors included in the study.
| Parameter | SLE patients | Healthy controls |
|---|---|---|
| n=55 | n=32 | |
| Mean age, years ± SEM | 39.8 ± 1.5 | 43.1 ± 1.5 |
| Females, n (%) | 55 (100) | 32 (100) |
| SLEDAI ± SEM | 3.4 ± 0.5 | |
| Ethnicity | ||
| African-American, n (%) | 19 (34) | 8 (25) |
| Caucasian, n (%) | 25 (46) | 21 (66) |
| Others, n (%) | 11 (20) | 3 (9) |
| Treatment | ||
| Prednisone, n (%) | 24 (43.6) | |
| Hydroxychloroquine, n (%) | 39 (70.9) | |
| Mycophenolate, n (%) | 20 (36.4) | |
| Azathioprine, n (%) | 10 (15.6) | |
| Others, n (%) | 11 (20) | |
Cell lines and mouse strains
K562 and EL-4 cells were purchased from ATCC. NKL cell line was kindly provided by Dr. Jerome Ritz (Dana-Farber Cancer Institute, Boston, MA), RAJI cells by Dr. Frederick Wang (Brigham and Women’s Hospital Channing Labs, Boston, MA) and YAC-1 cells by Dr. Cox Terhost (Beth Israel Deaconess Medical Center, Boston, MA.) Rag2 knockout were purchased from Jackson Laboratories. Our group generated C57BL/6 deficient for CD3ζ which were then crossed with Rag2 knockout mice to produce a strain deficient for both CD3ζ and Rag2. All experiments were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Cells isolation, culture and electroporation
Peripheral blood mononuclear cells (PBMCs) were enriched by density gradient centrifugation using Lymphocyte Separation Medium (Corning Life Sciences.) Cells were maintained in RPMI 1640 (Corning Life Sciences) supplemented with 10% FBS (Gibco), 100 mg/ml streptomycin (Gibco), and 100U/ml penicillin (Gibco). For some experiments, NK cells were isolated from PBMCs using NK Cell Isolation Kit (Miltenyi). In the case of NKL cell line, the medium was supplemented with 50U/ml of recombinant interleukin-2 (IL-2, Peprotech). Mouse NK cells were obtained from splenocytes, purified using NK cell isolation kit II (Miltenyi) and cultured with additional 50μM of β-Mercaptoethanol, and 500U/ml of recombinant IL-2. Cells were maintained at 37°C and 5% CO2. NKL cells were electroporated with 100nM of CD3ζ siRNA or control (Dharmacon) using a nucleofector device (Lonza). Briefly, 3×106 cells were washed twice with PBS, resuspended in 100μl of Solution V (Lonza) and electroporated using the program O-017. After electroporation, cells were placed in prewarmed medium and cultured for 72h.
Cytokine and CD3ζ detection
PBMCs or 7 days cultured mouse NK cells were stimulated with PMA and Ionomycin (Sigma-Aldrich) in the presence of Brefeldin A (50ng/ml, 1μM, and 1μM respectively) at 37°C and 5% CO2 for 6h. Cells were washed twice with cold PBS and stained for extracellular markers for 30min at 4°C in PBS staining (PBS, 1% BSA and 2mM EDTA). After two washes with FACS-staining buffer, cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) and stained overnight for cytokines in Perm/Wash buffer at 4°C. After two washes with Perm/Wash buffer, cells were resuspended in FACS-staining buffer and analyzed on a Beckman Coulter Gallios cytometer. The same staining protocol was used to detect CD3ζ expression levels in ex vivo PBMCs but without any stimulation. The antibodies used are detailed in Supplemental Table 1. In some experiments, IFNγ was measured in supernatants using a commercial ELISA kit (Biolegend) following the manufacturer’s protocol. Supernatants were collected from 2.5 × 106 NKL cells, previously electroporated with CD3ζ siRNA or control siRNA, stimulated with PMA and Ionomycin.
Real-time quantitative reverse transcriptase-polymerase chain reaction (Q-RT-PCR)
Total RNA was isolated from purified NK cells (Purity higher than 90%) by RNeasy Mini Kit (Qiagen). Reverse transcription was performed using RNA to cDNA EcoDry (Takara). Quantitative real-time PCRs for CD3ζ were performed (Light Cycler 480, Roche) with 40 cycles at 95 °C for 10 sec and 60 °C for 60 sec using TaqMan assays (Applied Biosystems). All PCR reactions were run in triplicates with a control reaction containing no RT enzyme. The comparative Ct method was used to quantify transcripts relative to GAPDH.
Confocal microscopy
PBMCs (105) or purified NK cells (5 × 104) were stained extracellularly for CD56 and intracellularly for CD3ζ as indicated above. Cells were resuspended in 200μl of PBS, plated on poly-l-lysine–coated glass slides using a cytospin, and mounted in 33% glycerol-PBS. Confocal microscopy and double-fluorescence analysis were performed with a Nikon Eclipse Ti. Images were analyzed using FIJI.
Cytotoxicity and degranulation assays
Antibody-Dependent Cellular Cytotoxicity (ADCC) and Natural cytotoxicity of NK cells were detected using GranToxiLux (OncoImmunin) according to manufacturer instructions in 96-well U-bottom plates. K562 or YAC-1 cells were used as targets to detect natural cytotoxic activity in human or mouse respectively, while for ADCC the target cells were RAJI cells stained with human-antiCD20 (InvivoGen) or EL-4 cells stained with rat-anti-CD90 (Biolegend). Different ratios of effector to target cells were incubated for 2.5h at 37°C and 5% CO2. After two washes with Wash Buffer, cells were resuspended in Wash Buffer and analyzed using an LSR II cytometer (Becton Dickinson). Degranulation was also performed in a 96 well U-bottom plate with 5μl of anti-CD107a APC (Biolegend) in the presence of K562 or anti-CD16 (Biolegend) coated plates at different concentrations as stimuli. After 30min of incubation at 37°C and 5% CO2, Brefeldin A (1μM) (BD Bioscience) was added, and incubation continued for 5h. Cells were washed twice with cold PBS and stained for extracellular markers for 30min at 4°C in FACS-staining buffer (PBS 1% BSA 2mM EDTA). After two washes with FACS-staining buffer cells were analyzed using a Gallios or a Cytoflex cytometer (Beckman Coulter). Data were analyzed using Kaluza software (Beckman Coulter).
Co-Immunoprecipitation
Total protein was extracted from NKL cells electroporated with CD3ζ siRNA or control siRNA using 1% Triton X-100 lysis buffer plus proteinase and phosphatase inhibitor. 250μg of lysate was used for CD16 immunoprecipitation using an anti-CD16 antibody (3G8, BioLegend). FcεRIγ was detected by Western Blot using anti-FcεRIγ (Upstate). Loading control was performed in immunoprecipitation-supernatants using anti-β-actin (Abcam). Images were acquired with a FUJI LAS-4000 imager (GE Healthcare Life Sciences) and analyzed using FIJI.
Phospho-Tyrosine detection
NKL cells (5×106 cells/condition) electroporated with CD3ζ siRNA or control siRNA were stimulated with 5μg/ml of anti-CD16 (3G8) and 2.5μg/ml of goat anti-mouse crosslinker (EMD Millipore) or 5 ×104 K562 cells for one minute. Stimulation was stopped by washing cells twice with cold PBS. In the case of murine NK cells, 3×106 IL-2 stimulated NK cells were stimulated using 3×104 YAC-1 cells. Total protein was extracted using RIPA buffer plus proteinase and phosphatase inhibitors. For Western blot anti-p-Tyr (PY20, Santa Cruz Biotechnology), anti-CD3ζ (6B10.2, Santa Cruz Biotechnology) and anti-β actin (Abcam) were used. Images were acquired with a FUJI LAS-4000 imager (GE Healthcare Life Sciences) and analyzed using FIJI. Lanes from Western blot of lysates from murine NK cells were cut and pasted to place together with their control.
Statistical Analysis
Data were analyzed using GraphPad Prism v6. The specific tests used are indicated in the legend of each figure.
Results
CD3ζ is decreased in NK and T cells from SLE patients
Although important both for NK cell signaling and activation (15) and despite reduced levels having been reported in SLE T cells related to their altered function (18, 19), CD3ζ levels have not been studied in SLE NK cells. We evaluated CD3ζ expression in NK (CD3−CD56+), T cell (CD3+CD56-) and different T cell subsets from SLE patients and healthy donors (Supplemental Figure 1) using intracellular flow cytometry. We found reduced CD3ζ expression in SLE NK cells (mean of CD3ζ MFI ± SEM = 30.7 ± 1.4 n=23 healthy donor vs. 21.7 ± 1.3 n=45 SLE patients, p < 0.001; Figure 1A) and T cells (20.2 ± 1.3 vs. 17.2 ± 1.4, p = 0.02; Figure 1B and Supplemental Figure 1B). CD3ζ downregulation was independent of treatment as we did not find any correlation between treatment and CD3ζ levels (mean of CD3ζ MFI ± SEM in patients treated with: Prednisone = 23.0 ± 2.5; Hydroxychloroquine = 22.4 ± 1.9; Azathioprine = 20.0 ± 5.1; Mycophenolate 20.1 ± 2.2). Becuase CD3ζ is also expressed in NKT cells, we assessed its expression in the NKT type I subpopulation which have been implicated in SLE pathogenesis (20). We did not find significant differences in the expression levels of this molecule compared with those from healthy donors (22.7 ± 2.8 vs. 20.1 ± 1.8, p = 0.42; Supplemental Figure 1C.)
Figure 1. Downregulation of CD3ζ in SLE NK and NK T cells is independent of disease activity.
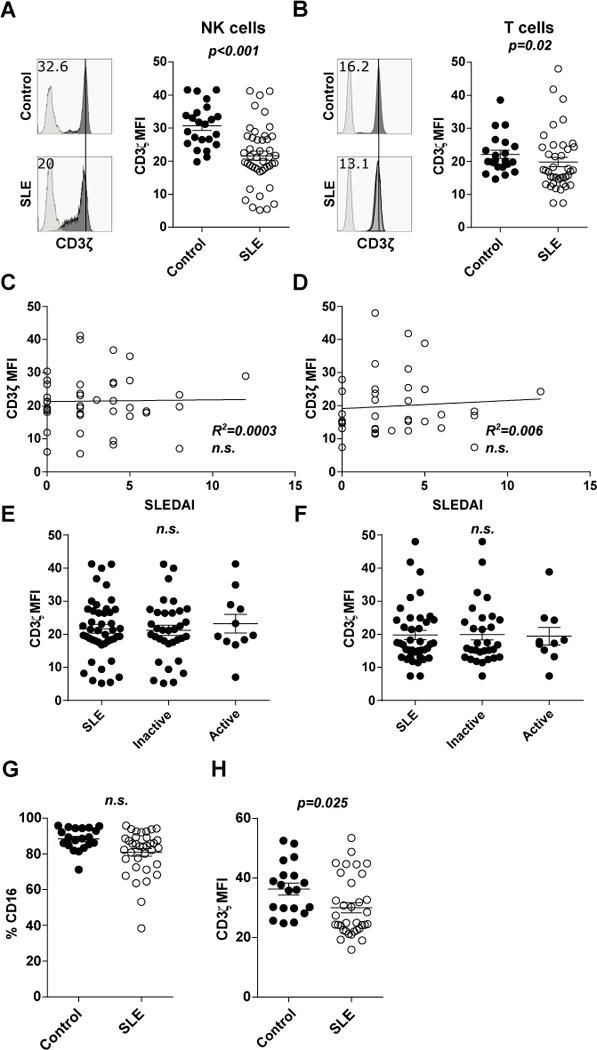
PBMCs from SLE patients were stained to identify NK- (CD3−CD56+) or T-cells (CD3+CD56−), as well as intracellular CD3ζ. Individual MFI values, indicating the mean ± SEM for CD3ζ gated in NK- (A) or T-cells (B) from healthy donors (Control, n=23) and SLE patients (SLE, n=45). Representative histograms are shown with isotype control in lighter grey. Correlation between SLEDAI scores from each patient and the CD3ζ MFI values in NK cells (C) and T cells (D). Individual MFI values, indicating the mean ± SEM for CD3ζ gated in NK- (E) or T-cells (F) from total SLE patients, those with the disease in an inactive status (SLEDAI ≤4), and those with the disease in an active status (SLEDAI >4). Scatter diagram showing cumulative data of (G) % of CD16+CD56+CD3− NK cells and (H) CD3ζ MFI values in CD16+CD56+CD3− NK cells from healthy donors and SLE patients indicating mean ± SEM.
Differences between CD3ζ levels expression in cells from controls or SLE patients were evaluated by unpaired T-test in (A) and (B), and ANOVA test in (E), (F), (G) and (H). Pearson’s correlation was applied to the relationship between CD3ζ levels and the SLEDAI score from each patient.
To understand whether downregulation of CD3ζ is related to disease status, we examined the relationship between CD3ζ levels from patients with their SLEDAI, but we did not find any correlation in either NK (R2<0.001, Figure 1C) or T cells (R2 = 0.006, Figure 1D). Additionally, no difference was found in CD3ζ levels between patients segregated by their disease active or inactive status (Figure 1E for NK cells and 1F for T cells).
CD16 receptor (also called low-affinity immunoglobulin gamma Fc region receptor III-A) is one of the receptors associated with CD3ζ in NK cells (15). CD16 is expressed in most peripheral blood NK cells (6). Both, SLE patients and healthy donors, express greater levels of CD3ζ in CD16+NK compared to CD16− (Supplemental Figure S1A). To exclude the possibility that the differences found in CD3ζ levels were merely due to an increased proportion of CD16− NK cells in SLE, we evaluated the expression of CD16 in NK cells. We found a slight but not significant reduction of CD16+ NK cells in our cohort of SLE patients compared with healthy donors (mean of %NK CD16+ ± SEM = 88.5 ± 1.4 vs. 80.9 ± 2.1, n.s.; Figure 1G). Additionally, we found decreased CD3ζ expression in CD16+ NK cells from SLE patient samples compared with those from healthy donors (mean of CD3ζ MFI ± SEM = 36.28 ± 2.0 vs. 30.03 ± 1.7, p = 0.025; Figure 1H), confirming that downregulation of CD3ζ is a defect intrinsic to SLE NK cells.
To demonstrate how CD3ζ expression is affected, we performed confocal microscopy experiments in purified NK cells from patients with SLE or matched healthy donors. We stained the cells for intracellular CD3ζ and cell surface CD56 to determine localization or not on the surface membrane. Quantification of cells from 5 patients and 4 healthy donors showed that NK cells from patients with SLE display higher cytoplasmic localization and lower levels of CD3ζ on the cell surface compared to those from healthy donors (p=0.0001, Fisher’s exact test), although overall lower staining levels (Figure 2A).
Figure 2. Caspase 3 inhibition preserves CD3ζ levels in NK cells from patients with SLE.
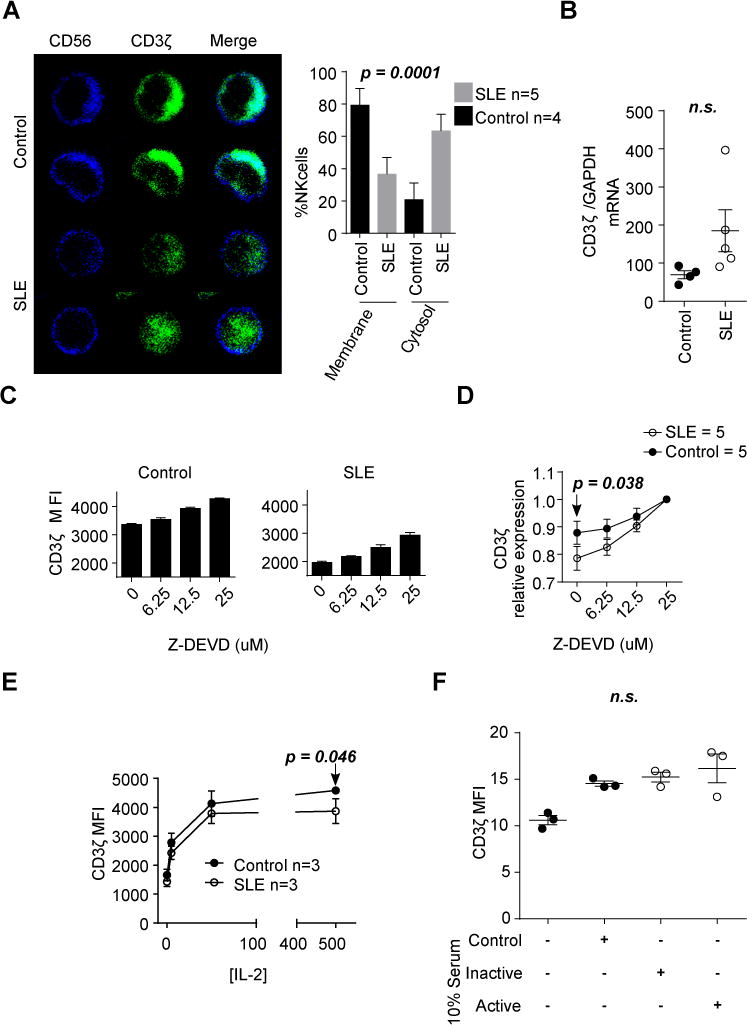
(A) NK cells from healthy donors and patients with SLE were stained with anti-CD56 (Blue) and anti-CD3ζ (Green) and visualized by confocal microscopy. Representative images are shown. The graph shows the percentage of cells with CD3ζ staining in the membrane or cytosol (mean ± SEM; n = 25 cells/patient). (B) CD3ζ gene expression was evaluated by qPCR. (C) CD3ζ proteins levels were evaluated in duplicates by flow cytometry in NK cells from healthy donors and patients with SLE after culture for five hours in the presence of 20μg/ml of cycloheximide and increasing concentrations of z-DEVD. A representative experiment including results from a control donor and a patient with SLE are shown. (D) Values of CD3ζ expression were normalized to those obtained in NK cells treated with 25μM of z-DEVD to show the different slopes. Data are shown as mean ± SEM. (E) NK cells from patients with SLE and healthy donors were treated with increasing concentrations of IL-2 for seven days. CD3ζ values are presented as mean ± SEM. (F) Individual MFI values are indicating the mean ± SEM for CD3ζ gated in NK cells from a healthy donor treated for 48h with heat-inactivated serum from healthy donors (Control), patients with SLEDAI≤4 (Inactive) or patients with SLEDAI >4 (Active). Each dot represents the value of cells treated with different sera. Statistics were performed using Fisher’s exact test in A, Student’s T-test to compare values from control and patients with SLE in B, D (0 μM z-DEVD dose) and E (500IU dose), and ANOVA was used in F.
Caspase 3 degrades CD3ζ in SLE NK cells
Several mechanisms including transcriptional regulation and protein degradation have been described to control CD3ζ expression in T cells from patients with SLE (21). We first addressed whether CD3ζ mRNA is decreased in NK cells from patients with SLE. From a small number of patients from whom we were able to purify sufficient numbers of NK cells we measured CD3ζ mRNA. We found no differences in the expression levels of CD3ζ mRNA in NK cells between healthy donors and patients with SLE (mean of CD3ζ/GAPDH ± SEM = 69.46 ± 10.47 vs. 185.3 ± 55.34, n.s.; Figure 2B), even though the protein levels were reduced (Figure 1A).
Next, we asked whether CD3ζ protein is stable in NK cells from patients with SLE. It is known that Caspase 3 can degrade CD3ζ (22, 23), and it has been reported that Caspase 3 activity is increased both in T cells and NK cells from patients with SLE (22). Therefore, we asked whether Caspase 3 is responsible for CD3ζ downregulation in NK cells from patients with SLE. We cultured PBMCs from healthy donors or patients with SLE in the presence of cycloheximide, to inhibit de novo synthesis of proteins, and increasing concentrations of Z-DEVD-FMK, a Caspase 3 specific inhibitor, for 5 hours and we found that CD3ζ degradation was more prominent and susceptible to Caspase 3 inhibition in NK cell from patients with SLE than in NK cells from healthy donors (Figure 2C and D).
One well-established defect in SLE is the deficiency of IL-2 (1). Because IL-2 is an important cytokine for NK cell function(24), and because some reports have shown that IL-2 can restore CD3ζ expression in tumor infiltrating lymphocytes (TILs, 25), we asked whether IL-2 can restore CD3ζ levels in NK cells from patients with SLE. Accordingly, we cultured NK cells from patients with SLE and healthy donors for seven days in the presence of increasing concentrations of IL-2 and measured CD3ζ levels. As shown in Figure 2E, exposure of NK cells from patients with SLE to IL-2 results in increased expression of CD3ζ but to a lesser extent compared to NK cells from healthy controls. Additionally, to understand whether other serum factors present in SLE sera can affect CD3ζ levels in NK cells, we cultured NK cells from healthy donors with serum from control, inactive or active SLE patients for 48h but we failed to observe any downregulation of CD3ζ (Figure 2F.)
Downregulation of CD3ζ impairs ADCC in SLE NK cells without affecting CD16 levels
CD3ζ is associated with several receptors linked to the NK cell cytotoxic mechanism ADCC and natural cytotoxicity (15)both of which are altered in SLE patients (3). Given the fact that CD3ζ is downregulated in SLE NK cells, we asked whether altered cytotoxic functions were linked to CD3ζ levels.
ADCC is an important mechanism in the elimination of virus-infected and tumor cells (26–28). This mechanism is mediated through the recognition of antibodies decorating the infected or tumoral cell surface by Fc receptors on cells including NK cells, monocytes or activated CD8 T cells (29). Due to limitations of our patient blood draw volumes (5ml) did not allow purification of sufficient numbers of NK cells to perform in vitro ADCC assays and because ADCC can be carried out by other cells in the PBMCs expressing Fc receptors, we used an indirect method to test this function. We evaluated degranulation by measuring levels of CD107a (lysosomal-associated membrane protein 1, LAMP1) in NK cells from SLE PBMCs stimulated with plate-bound anti-CD16 antibody using multicolor flow cytometry, which has been shown to correlate with cellular killing capacity (30).
We found a direct correlation between the levels of CD3ζ and the increase of CD107a positive cells stimulated with anti-CD16 (R2 = 0.46, p = 0.04; Figure 3A). To confirm the dependence of ADCC on CD3ζ levels, we tested the ability of NKL to kill RAJI cells labeled with an anti-CD20 antibody (31). NKL cells electroporated with CD3ζ siRNA exhibited reduced killing capacity compared to those transfected with a control siRNA (mean of % RAJI GranToxiLux+ ± SEM = 23.9 ± 0.1 vs. 29.5 ± 1.5, p < 0.05 at 10:1 effector: target ratio, n = 3; Figure 3B). Additionally, NK cells from CD3ζ-deficient Rag2 knockout mice present lower ADCC levels than those from Rag2 CD3ζ-sufficient mice (mean of % EL4 GranToxiLux+ ± SEM 36.1 ± 3.4 vs. 54.6 ± 4.1, p = 0.03; Figure 3C).
Figure 3. Antibody-dependent cellular cytotoxicity, mediated through CD16 signaling, depends on CD3ζ expression.
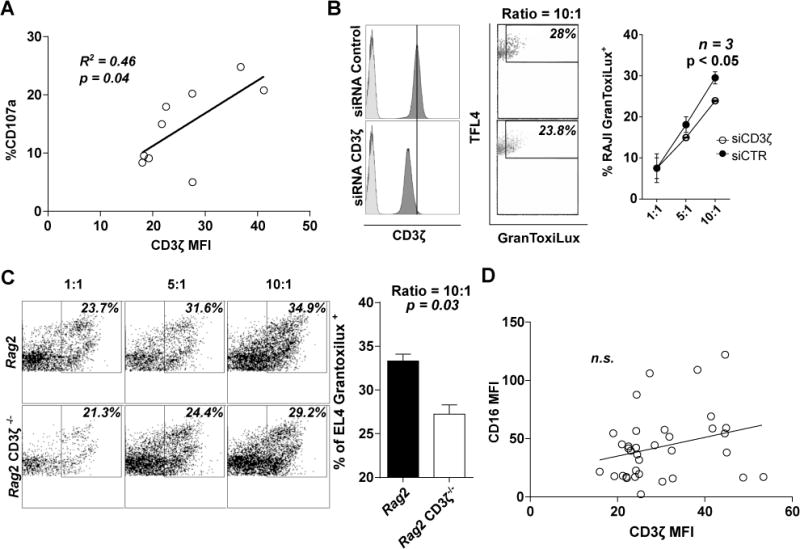
(A) Relationship between % of CD107a+CD56+CD3− NK cells and CD3ζ levels from SLE patients are shown. (B) Representative histograms of CD3ζ levels showing isotype control in lighter grey (left) and dot plot of ADCC (center) in siRNA control (upper) or CD3ζ (lower) treated NKL cells. Dot plot of the mean ± SEM of the percentage of RAJI dead cells in ADCC (right) with NKL cells treated with siRNA control or against CD3ζ (n=3 individual experiments). (C, left) Representative dot plot of ADCC in NK cells from Rag2 knockout or Rag2 knockout CD3ζ−/−, and (C, right) column bar graph representing the mean ± SEM of the percentage of EL4 dead cells in ADCC at ratio 10:1 (right) from two different experiments with at least three mouse per group. (D) Relationship between CD16 and CD3ζ MFI levels in CD16+CD56+CD3− NK cells. Differences in (A), (B) and (C) were evaluated by unpaired T-test. Pearson’s correlation was applied to determine the relationship in (D).
CD16 is the principal receptor mediating ADCC in NK cells (32). As CD16 associates with CD3ζ, we addressed whether CD16 levels are affected by those of CD3ζ. There was no correlation between CD3ζ and CD16 levels in CD16+NK cells (R2=0.07, n.s.; Figure 3D). Silencing of CD3ζ in the human NK cell line NKL (33), confirmed that CD16 levels do not depend on CD3ζ expression levels (Supplemental Figure 2A). Additionally, NK cells from CD3ζ-deficient Rag2 mice do not show alteration in CD16 levels (Supplemental Figure 2B).
Downregulation of CD3ζ increases natural cytotoxic activity of NK cells
Natural cytotoxicity is the other NK cell cytotoxic mechanism which has also been described as decreased in SLE NK cells (9, 11), although some reports have indicated no alteration (10, 12). To determine whether natural cytotoxicity is altered in SLE NK cells and if CD3ζ has a role in this mechanism, we studied the natural cytotoxic activity of SLE NK cells against K562 cells by flow cytometry. Natural cytotoxicity is reduced in SLE PBMCs compared to healthy donor cells (mean of %K562 GranToxiLux+ ± SEM = 20.0 ± 2.5 n = 5 vs. 13.13 ± 2.4 n=7 at ratio 25:1 effector:targets; Figure 4A). However, this difference did not persist when cytotoxicity was adjusted by the percentage of NK cells present in the assay (mean of killing capacity (%K562 GranToxiLux+/%NK cells ratio) ± SEM = 2.3 ± 0.7 vs. 4.6 ± 1.4 in healthy donors and SLE, respectively; Figure 4B). Surprisingly, we observed an inverse correlation between the levels of CD3ζ and the killing capacity in NK cells from both SLE and healthy donors (R2 = 0.69, n = 7, p = 0.02 and R2 = 0.49, n = 5, n.s., respectively; Figure 4C). A similar correlation, although not significant, was found for NK cell degranulation, determined by expression of CD107a following stimulation with K562 cells in a different group of patients (R2 = 0.61, n = 6, p = 0.051; Figure 4D).
Figure 4. Downregulation of CD3ζ leads to increased natural cytotoxicity.
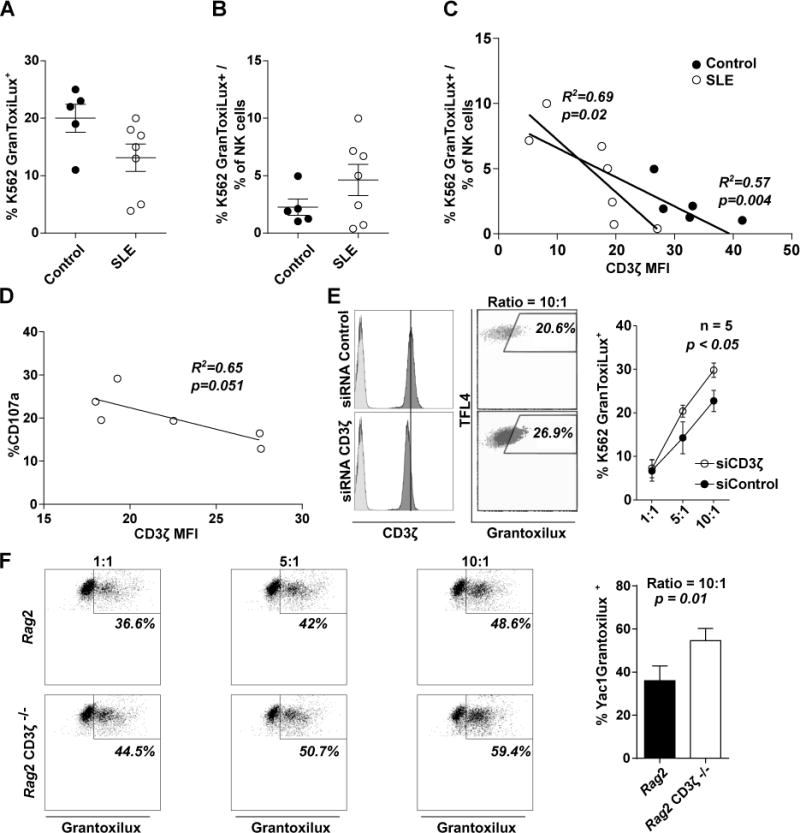
(A) % of K562 dead cells or (B) following correction by the percentage of NK cells in the PBMCs, after coculture with PBMCs from healthy donors (n=3) or SLE patients (n=6) at a ratio 25:1 (effector:target cells). (C) Correlation between % of dead K562 cells and levels of CD3ζ from patients or controls are shown. (D) Correlation between % of CD107a+ NK cells and levels of CD3ζ after coculture with K562, assayed in a different set of patients than (A), (B) and (C). (E) Representative histograms of CD3ζ levels showing isotype control in lighter grey (E, left) and dot plot showing Natural Cytotoxicity measurement (E center), in siRNA control (upper) or CD3ζ (lower) treated NKL cells are shown. (E, right) Dot plot is showing the mean ± SEM of the percentage of K562 death cells under natural cytotoxicity assay (n=5 individual experiments). (F, left) Representative dot plot showing natural cytotoxicity in NK cells from Rag2 knockout or Rag2 knockout CD3ζ−/−, and (F, right) column bar graph representing the mean ± SEM of the percentage of Yac1 dead cells in natural cytotoxicity assay at ratio 10:1 (right) from two different experiments with at least three mouse per group. Differences in (A), (B) and (F) were evaluated by unpaired T-test. Pearson’s correlation was applied to determine the relationship in (C) and (D). A Two-way ANOVA was performed to detect differences in (E) and (F).
CD3ζ knockdown in NKL cells by siRNA electroporation results in increased natural cytotoxicity against K562 silencing compared with cells electroporated with control siRNA (Mean of %K562 GranToxiLux+ 29.8 ± 1.7 vs. 22.8 ± 2.4, p <0.05 at 10:1 effector:target cell ratio, n = 5; Figure 4E). Furthermore, NK cells from our CD3ζ-deficient Rag2 knockout mouse show increased cytotoxicity compared with those from CD3ζ wild-type (50.2 ± 2.5 vs. 36.4 ± 3.6, p = 0.01; Figure 4F).
CD3ζ downregulation confers a proinflammatory phenotype to NK cells
In addition to their cytotoxic function, NK cells produce multiple cytokines such as IFNγ and TNFα which amplify the inflammatory response and cause tissue damage (13, 34, 35). Reduced CD3ζ levels are linked to a proinflammatory phenotype in SLE T cells (36, 37), and SLE NK cells produce more proinflammatory cytokines than those from healthy donors (38, 39), so we examined the relationship between CD3ζ levels and the proinflammatory phenotype of SLE NK cells. We found a higher proportion of NK cells producing both IFNγ and TNFα after PMA/Ionomycin stimulation in SLE patients compared to healthy donors (mean of %NK cell IFNγ+TNFα+ ± SEM = 10.4 ± 1.3, n = 6 healthy donors vs. 18.67 ± 2.2, n = 11 SLE patients, p = 0.02; Figure 5A). Interestingly, CD3ζ levels in NK cells correlated inversely with the percentage of IFNγ and TNFα double positive NK cells (R2 = 0.66, p = 0.002, n=11; Figure 5B). We also found an inverse correlation between CD3ζ levels and the proportion of IFNγ positive (R2 = 0.47, n = 11, p = 0.02; Figure 5C) and TNF–positive NK cells (R2 = 0.37, n = 11, p = 0.04; Figure 5D).
Figure 5. Downregulation of CD3ζ leads to a proinflammatory phenotype in NK cells from SLE patients.
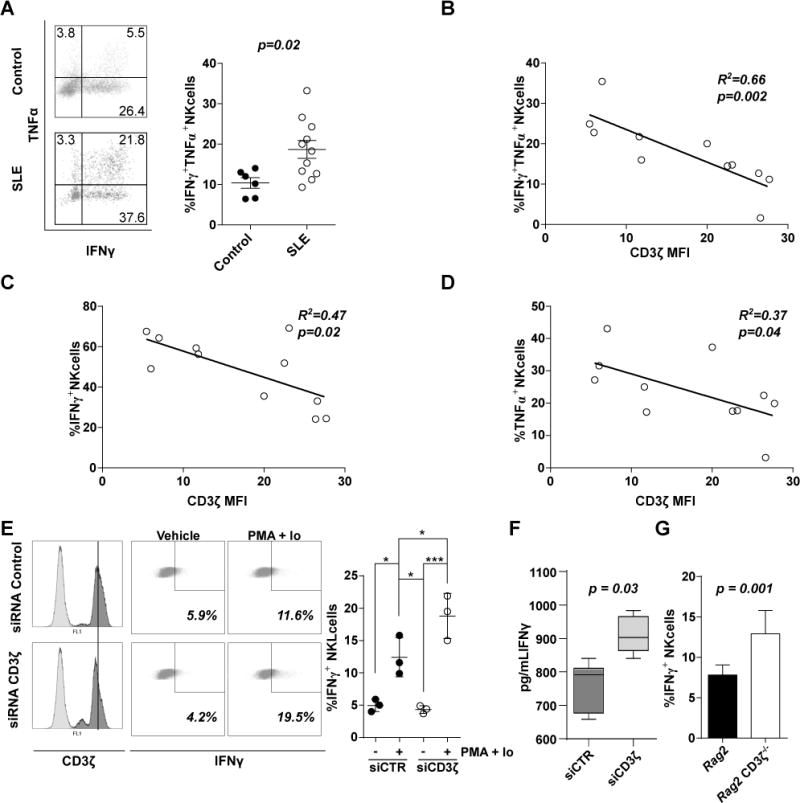
(A, left) Representative dot plot showing percentage of NK cells from healthy donors (Control, upper) or SLE patients (SLE, lower) producing IFNγ, TNFα or both. (A, right) Scatter diagram of cumulative values of IFNγ+TNFα+ NK cells from healthy donors and SLE patients. Correlations between (B) IFNγ+TNFα+, (C) IFNγ+, and (D) TNFα+ with CD3ζ values in NK cells from SLE patients are shown. (E) Representative histograms of CD3ζ basal levels (E, left) and % of IFNγ+ (E center) in siRNA control (upper) or CD3ζ (lower) treated NKL cells are shown. (E, right) Scatter diagram showing cumulative data of % of IFNγ+ NKL cells under different conditions as indicated in the graph (n=3 independent experiments). (F) IFNγ was also measured in supernatants from NKL cells electroporated with CD3ζ siRNA or control siRNA using an ELISA kit, values are represented with a box and whiskers graph. (G) Column bar graph representing the mean ± SEM of % of IFNγ+ NK cells from Rag2 knockout or Rag2 knockout CD3ζ−/− mice from two experiments with at least three mice per experiment. Differences in (A), (F) and (G) were evaluated by unpaired T-test. Pearson’s correlation analysis was conducted to determine the relationship in (B), (C) and (D). A One-way ANOVA was performed to detect differences in (E; * = p < 0.05, ** = p < 0.001, *** = p < 0.0001).
To confirm the dependence of these findings on CD3ζ levels we examined the behavior of NKL cells and NK cells from CD3ζ deficient mice. Silencing CD3ζ in NKL cells resulted in increased proportion of cells producing IFNγ compared with control cells (mean of %NKL cell IFNγ+ ± SEM = 18.8 ± 2.0 vs. 12.4 ± 1.8, n=3; Figure 4E), while TNFα was not detected. Similarly, IFNγ was increased in the supernatant of NKL cells in which CD3ζ was downregulated by siRNA (mean of IFNγ concentration in pg/ml ± SEM = 910 ± 22.13 vs. 761 ± 29.68, p = 0.03; Figure 5F). Additionally, we observed that mouse NK cells deficient in CD3ζ express higher levels of IFNγ under PMA/Ionomycin stimulation (mean of %NK cell IFNγ+ = 7.82 ± 0.5 in Rag2 knockout vs. 12.92 ± 1.3 in Rag2 knockout CD3−/−, p = 0.001; Figure 5G). Again, we did not find higher levels of TNFα, as observed with NKL cells.
Downregulation of CD3ζ altered early NK cell signaling
Downregulation of CD3ζ in T cells from patients with SLE is associated with a rewiring of the T cell receptor and altered early TCR signaling (18). CD3ζ is also important for NK cell function and especially for CD16 expression and signaling (15, 30). Because our data indicate that downregulation of CD3ζ does not alter CD16 levels, we asked whether CD3ζ deficiency is compensated by other molecules such as FcεRIγ chain, as it is the case in T cells from patients with SLE (40). We immunoprecipitated CD16 from NKL cells previously electroporated with CD3ζ siRNA or control siRNA. Our results show that when CD3ζ is downregulated CD16 associates more with FcεRIγ which supports its expression (Figure 6A, B and C) confirming previous reports (41, 42). Next we asked whether this recruitment of FcεRIγ alters NK cell signaling. We stimulated NKL cells previously electroporated with CD3ζ siRNA or control siRNA with crosslinked CD16 antibodies or K562 cells for one minute to determine if early signaling events are altered. We found that NKL cells in which CD3ζ was downregulated showed a defect in signaling induction after CD16 stimulation but presented a stronger pattern of phosphorylation when exposed to K562 (Figure 6D). Similarly, we noted a stronger pattern of phosphorylation in IL-2 activated NK cells from Rag2 CD3ζ-deficient mice when exposed to YAC-1 cells (Supplemental Figure 3).
Figure 6. Downregulation of CD3ζ alters early NK cell signaling.
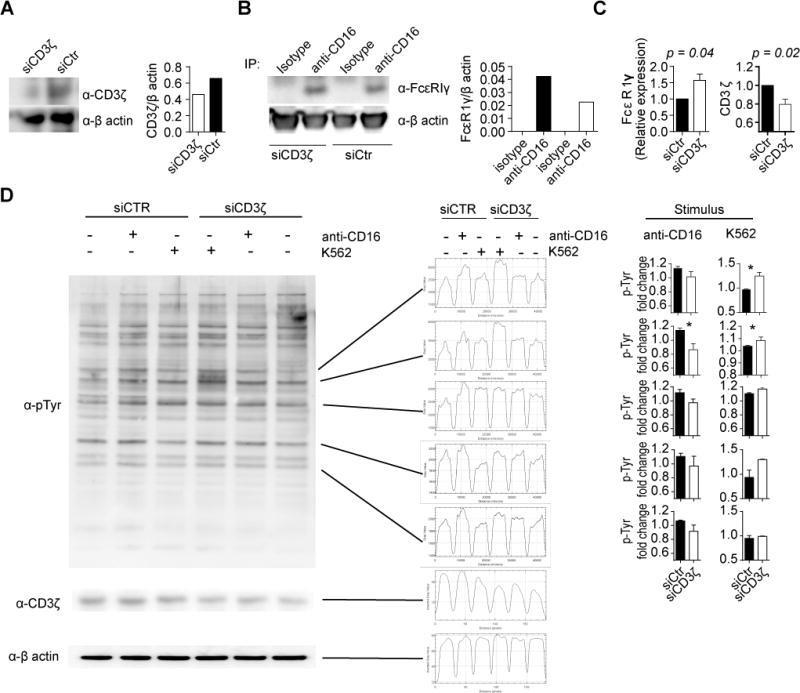
(A) NKL cells were electroporated with CD3ζ siRNA or control siRNA, and cell lysates immunoblotted for CD3ζ and β-actin. Densitometric quantitation is shown in the graph (right panel). (B) CD16 was immunoprecipitated from lysates from NKL cells electroporated with CD3ζ siRNA or control siRNA. Coimmunoprecipitated FcεRIγ was determined by Western blotting (representative experiment out of 3). Densitometric quantitation is shown in the graph (right panel). (C) Expression levels of FcεRIγ and CD3ζ were normalized to levels obtained from cells electroporated with control siRNA. Cumulative data from the 3 independent experiments are shown. D) NKL cells electroporated with CD3ζ siRNA or control siRNA were stimulated with a crosslinking CD16 antibody or with K562 cells for one minute. A representative Western blot (out of 3) is shown for phospho-tyrosine, CD3ζ and β-actin, and band density profile are shown on the center. Data were normalized (fold change over the unstimulated condition) and cumulative data for each quantified band are shown on the left. T test was used to evaluate differences. *p < 0.05
DISCUSSION
CD3ζ is decreased in T cells from SLE patients and it is known that this downregulation is linked to the hyperactivation and the proinflammatory phenotype of these cells (18, 36, 37). Although SLE NK cells also present a proinflammatory phenotype and increased levels of activation markers (12, 38, 39), CD3ζ levels have not been studied in SLE NK cells. We, therefore, asked whether CD3ζ levels are downregulated and whether this downregulation can account for NK cell dysfunction in SLE patients.
In this study, we found decreased CD3ζ levels in NK cells and T cells from SLE patients but not in NKT type I cells. This can occur as a consequence of common mechanisms affecting NK and T cells but not NKT type I, however, additional factors can contribute to this discrepancy including cohort bias and treatment. Nonetheless, we did not find any relation between patients’ therapy and CD3 ζ levels.
Several mechanisms have been proposed to account for the downregulation of CD3ζ in SLE T cells including genetic polymorphisms, epigenetic changes, altered transcription or increased degradation, some of which can be induced by serum factors (18). After we noted that CD3ζ mRNA levels in NK cells from patients with SLE were comparable to those from normal subjects we considered that CD3ζ protein is degraded by Caspase 3, a mechanism that has been previously reported to occur in T cells from patients with SLE (23). Indeed, Caspase 3 activity is increased in NK cells from patients with SLE (22) and exposure of NK cells from patients with SLE to a Caspase 3 inhibitor preserves CD3ζ expression. Because IL-2 production is decreased in patients with SLE (43) and IL-2 can restore CD3ζ levels in TIL (25), we decided to test whether IL-2 could similarly restore CD3ζ expression in NK cells from patients with SLE. Indeed, exposure of NK cells from patients with SLE to IL-2 led to increased expression of CD3ζ albeit to a lesser extent when compared to NK cells from healthy controls. To determine if CD3ζ levels are affected by other factors in the serum, we treated NK cells from healthy donors with serum from SLE patients with active or inactive disease but we did not record any changes, indicating that downregulation of CD3ζ in NK cells does not depend only on inflammation (19). Also, we did not find a correlation between SLEDAI and CD3ζ levels from SLE NK cells.
As CD3ζ is critical for proper signaling in NK cells (15), and NK cell dysfunction has been described in SLE patients (3), we have studied the relationship between CD3ζ levels in NK cells and their function. We found a direct correlation between the levels of CD3ζ and antibody-dependent cellular cytotoxicity (ADCC), a mechanism which depends on CD16 (32), a molecule associated with CD3ζ and FcεRIγ (15), and an inverse correlation with natural cytotoxicity.
In SLE patients we noted a direct correlation between CD3ζ levels in NK cells and their ADCC capacity. After silencing of CD3ζ in the human NK cell line NKL and by analyzing ADCC in NK cells from CD3ζ-deficient mice, we confirmed the dependence of ADCC on CD3ζ and further that this does not necessarily accompany changes in CD16 expression levels. While the interaction between CD16 and CD3ζ is well established, the dependence of CD16 surface expression levels on CD3ζ is controversial. Two patients who lacked CD3ζ had a profound defect in both CD16 expression and cytotoxic capacity of NK cells (44). We found that CD16 is neither downregulated in NK cells from patients with SLE, even though CD3ζ expression is decreased, nor in our NKL model. We found that CD16 associated with FcεRIγ in NKL cells in which CD3ζ was silenced, and accordingly FcεRIγ has been reported to associate and support the expression of CD16 (41, 42). On the other hand, a study in mice reported a negative role for CD3ζ in CD16 expression and ADCC (45), because CD3ζ deficient mice showed increased CD16 expression in NK cells and increased ADCC. However, a previous report using the same mouse model showed no alteration in the expression of CD16 and a reduced ADCC activity in CD3ζ-deficient NK cells (46). To eliminate the influence of the autoinflammatory T cells observed in the CD3ζ-deficient C57BL/6 mice (37), we generated the CD3ζ–deficient Rag2 mouse which does not show sign of autoinflammation even in advanced age (data not shown). Our results, both from human and mouse NK cells is consistent with the findings of Liu et al. (46) as we observed no differences in CD16 expression levels but decreased ADCC in CD3ζ-reduced or -deficient NK cells.
Natural cytotoxicity from NK cells involves a wide number of receptors, including NKp30 and NKp46 in humans (15, 47), which also signal via CD3ζ or the FcRγ chain (15). While it has been described that NKp30 levels are affected by CD3ζ downregulation, those of NKp46 remains unchanged (44). However, NKp30 and NKp46 levels have been described as unaltered or increased in SLE NK cells in several publications (48, 49). Even though we did not study these receptors in NK cells from patients with SLE, we confirmed in NKL cells that the levels of NKp30 and NKp46 as well as CD16, are not affected by the downregulation of CD3ζ (Supplemental Figure 2).
Although we find a decreased cytotoxicity against K562 in SLE NK cells, this appears to be the result of a decreased proportion of NK cells in PBCMs from SLE patients. Upon calculation of the killing index (% of K562 cell death/% of NK cells in the sample), we found no difference between patients and controls as noted by others previously (10). Interestingly, we found an inverse correlation between the killing index and the levels of CD3ζ in NK cell from SLE patients which we confirmed by downregulating CD3ζ in the NKL cell line and by analyzing natural cytotoxicity in mouse NK cells deficient for CD3ζ. These data confirm a previous study in mice which showed that CD3ζ-deficient NK cells display increased natural cytotoxicity (46). Altogether, these results indicate that although CD3ζ in NK cells has a negative effect on natural cytotoxicity, it is required for ADCC.
Our results show that FcεRIγ replaces CD3ζ in NK cells from SLE patients as it has been shown to be the case in T cells (40). FcεRIγ can support CD16 expression (41, 42), which can explain why we do not observe downregulation of CD16 in patients with SLE. While FcRγ can support CD16 expression, it has been previously demonstrated that CD16 signals preferentially through CD3ζ. The presence of three ITAMs in the intracellular tail of CD3ζ facilitates signaling by CD16 (50). In contrast, NKp30 and NKp46, two receptors with significant roles in natural cytotoxicity, are more dependent on FcRγ than CD3ζ (51, 52). A possible explanation for this could be the reliance of natural cytotoxicity on spleen tyrosine kinase (Syk) function (53). This protein is mainly activated by FcRγ (54) and can mediate interactions with other receptors such as 2B4 and CD59, both critical for natural cytotoxicity (52, 55). Rewiring of the TCR in T cells from patients with SLE, where CD3ζ is partially replaced by FcεRIγ and its signaling partner Zap70 is replaced by Syk, results in increased early signaling events (18). Here we showed that downregulation of CD3ζ in the human NKL cells led to decreased signaling induced by engagement of CD16 but increased in response to K562 cells. Similar results were observed when NK cells from a Rag2 CD3ζ-deficient mice were exposed to Yac-1 cells compared to those from Rag2 mice. These results confirm the importance of CD3ζ in CD16 signaling and the reliance of natural cytotoxicity on FcεRIγ.
Downregulation of CD3ζ in SLE T cells has been linked to increased production of IFNγ (38, 39). Here we observed that levels of CD3ζ in NK cells inversely correlated with the percentage of cells producing IFNγ and TNFα. We confirmed this observation by silencing CD3ζ in the NKL line which resulted in increased IFNγ production. Our failure to confirm a similar correlation with TNFα indicates that other factors are involved in the expression of this cytokine in addition to signaling mediated by CD3ζ. The mechanism whereby CD3ζ regulates IFNγ expression is not completely understood. However, it is known that in T cells, CD3ζ facilitates tonic signaling in the absence of TCR stimulation through Zap-70 (56). Activation of Zap70 leads to the phosphorylation and activation of ERK, even without TCR stimulation (57). Decreased ERK activity is a well-known SLE T cell abnormality and leads to decreased DNA methyltransferase 1 (DNMT1) activity (58). This causes widespread DNA hypomethylation and overexpression of some genes such as IFNγ (59), which is one possible explanation for the increment in IFNγ expression in lupus NK cells.
A population of human NK cells deficient in FcRγ was reported to have increased ADCC, reduced natural cytotoxicity and reduced cytokine production (51). Our results are in agreement with these indicating a negative role for CD3ζ in natural cytotoxicity and cytokine production.
As CD16 is involved in the response against herpes simplex virus (26), cytomegalovirus (27), and tumor cells (28), the decreased CD16 function that accompanies CD3ζ loss in SLE NK cells could contribute to the increased risk of viral infections in SLE patients (60–62). In addition, these proinflammatory NK cells may infiltrate the kidneys in patients with SLE (13), produce proinflammatory cytokines and contribute to tissue damage. Our results shed light into the mechanisms which may account for NK cell dysfunction and increased susceptibility to infections and widespread organ inflammation in patients with SLE.
In conclusion, we found CD3ζ downregulation in SLE NK cells which is independent of the disease status or treatment. CD3ζ levels account for the proinflammatory phenotype observed in SLE NK cells and for their defect in ADCC.
Supplementary Material
Acknowledgments
We would like to acknowledge all patients who consented to participate in the study. We thank Dr. Jerome Ritz (Dana-Farber Cancer Institute, Boston, MA), Dr. Frederick Wang (Brigham and Women’s Hospital Channing Labs, Boston, MA) and Dr. Cox Terhost for sharing cell lines.
FUNDING STATEMENT
This work was funded by the United States National Institutes of Health with grant number R01 AI042269 to GCT, R01 AR060849 to VCK, NIH NIAMS K01 AR060781 and R01 AR068974 to VRM, and ASF and SJB were funded by training grant T32 AI074549 to GCT. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
REFERENCE LIST
- 1.Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Fors Nieves CE, Izmirly PM. Mortality in Systemic Lupus Erythematosus: an Updated Review. Curr Rheumatol Rep. 2016;18:21. doi: 10.1007/s11926-016-0571-2. [DOI] [PubMed] [Google Scholar]
- 3.Spada R, Rojas JM, Barber DF. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol. 2015;98:479–487. doi: 10.1189/jlb.4RU0315-081RR. [DOI] [PubMed] [Google Scholar]
- 4.Kis-Toth K, Comte D, Karampetsou MP, Kyttaris VC, Kannan L, Terhorst C, Tsokos GC. Selective Loss of Signaling Lymphocytic Activation Molecule Family Member 4–Positive CD8+ T Cells Contributes to the Decreased Cytotoxic Cell Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016;68:164–173. doi: 10.1002/art.39410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comte D, Karampetsou MP, Yoshida N, Kis-Toth K, Kyttaris VC, Tsokos GC. SLAMF7 engagement restores defective effector CD8+ T cells activity in response to foreign antigens in systemic lupus erythematosus. Arthritis Rheumatol Hoboken NJ. 2017 doi: 10.1002/art.40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2007;15:226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JK, Hughes P, Gelsthorpe K, Ward AM, Rowell NR. Antibody-dependent and phytohaemagglutinin-induced lymphocyte cytotoxicity in systemic lupus erythematosus. Ann Rheum Dis. 1981;40:11–17. doi: 10.1136/ard.40.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlowski SH, Hirsen DJ, Jackson EJ. Comparison of natural killing with antibody dependent cell mediated cytotoxicity in patients with systemic lupus erythematosus. J Rheumatol. 1982;9:59–62. [PubMed] [Google Scholar]
- 10.Green MRJ, Kennell ASM, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol. 2005;141:165–173. doi: 10.1111/j.1365-2249.2005.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, Shin MH, Park JJ, Kim TJ, Lee SS, Yoo DH, Kang HS. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–1763. doi: 10.1002/art.24556. [DOI] [PubMed] [Google Scholar]
- 12.Ye Z, Ma N, Zhao L, Jiang ZY, Jiang YF. Differential expression of natural killer activating and inhibitory receptors in patients with newly diagnosed systemic lupus erythematosus. Int J Rheum Dis. 2014 doi: 10.1111/1756-185X.12289. [DOI] [PubMed] [Google Scholar]
- 13.Spada R, Rojas JM, Pérez-Yagüe S, Mulens V, Cannata-Ortiz P, Bragado R, Barber DF. NKG2D ligand overexpression in lupus nephritis correlates with increased NK cell activity and differentiation in kidneys but not in the periphery. J Leukoc Biol. 2015;97:583–598. doi: 10.1189/jlb.4A0714-326R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson N, Carlsten H. Enhanced natural but diminished antibody-mediated cytotoxicity in the lungs of MRLlpr/lpr mice. Clin Exp Immunol. 1996;105:480–485. doi: 10.1046/j.1365-2249.1996.d01-787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher LA, van Oers NSC. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Becker AM, Blevins JS, Tomson FL, Eitson JL, Medeiros JJ, Yarovinsky F, Norgard MV, van Oers NSC. Invariant NKT Cell Development Requires a Full Complement of Functional CD3 Immunoreceptor Tyrosine-Based Activation Motifs. J Immunol. 2010;184:6822–6832. doi: 10.4049/jimmunol.0902058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest. 2015;125:2220–2227. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciszak L, Pawlak E, Kosmaczewska A, Potoczek S, Frydecka I. Alterations in the expression of signal-transducing CD3ζ chain in T cells from patients with chronic inflammatory/autoimmune diseases. Arch Immunol Ther Exp (Warsz) 2007;55:373–386. doi: 10.1007/s00005-007-0042-6. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Zhang H, Caimol M, Benike CJ, Chakravarty EF, Strober S, Engleman EG. Invariant natural killer T cells in lupus patients promote IgG and IgG autoantibody production. Eur J Immunol. 2015;45:612–623. doi: 10.1002/eji.201444760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nambiar MP, Juang YT, Krishnan S, Tsokos GC. DISSECTING THE MOLECULAR MECHANISMS OF TCR ζ CHAIN DOWNREGULATION AND T Cell SIGNALING ABNORMALITIES IN HUMAN SYSTEMIC LUPUS ERYTHEMATOSUS. Int Rev Immunol. 2004;23:245–263. doi: 10.1080/08830180490452602. [DOI] [PubMed] [Google Scholar]
- 22.Hanaoka N, Jabri B, Dai Z, Ciszewski C, Stevens AM, Yee C, Nakakuma H, Spies T, Groh V. NKG2D initiates caspase-mediated CD3ζ degradation and lymphocyte receptor impairments associated with human cancer and autoimmune disease. J Immunol Baltim Md 1950. 2010;185:5732–5742. doi: 10.4049/jimmunol.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan S, Kiang JG, Fisher CU, Nambiar MP, Nguyen HT, Kyttaris VC, Chowdhury B, Rus V, Tsokos GC. Increased Caspase-3 Expression and Activity Contribute to Reduced CD3ζ Expression in Systemic Lupus Erythematosus T Cells. J Immunol. 2005;175:3417–3423. doi: 10.4049/jimmunol.175.5.3417. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Tian Z, Wei H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front Immunol. 2017;8:930. doi: 10.3389/fimmu.2017.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Paola F, Ridolfi R, Riccobon A, Flamini E, Barzanti F, Granato AM, Mordenti GL, Medri L, Vitali P, Amadori D. Restored T-cell activation mechanisms in human tumour-infiltrating lymphocytes from melanomas and colorectal carcinomas after exposure to interleukin-2. Br J Cancer. 2003;88:320–326. doi: 10.1038/sj.bjc.6600679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moraru M, Black LE, Muntasell A, Portero F, López-Botet M, Reyburn HT, Pandey JP, Vilches C. NK Cell and Ig Interplay in Defense against Herpes Simplex Virus Type 1: Epistatic Interaction of CD16A and IgG1 Allotypes of Variable Affinities Modulates Antibody-Dependent Cellular Cytotoxicity and Susceptibility to Clinical Reactivation. J Immunol Baltim Md 1950. 2015;195:1676–1684. doi: 10.4049/jimmunol.1500872. [DOI] [PubMed] [Google Scholar]
- 27.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, Kaplan J. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180:1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 28.Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci U S A. 1999;96:5640–5644. doi: 10.1073/pnas.96.10.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimmerjahn F, Gordan S, Lux A. FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015;36:325–336. doi: 10.1016/j.it.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafiq S, Butchar JP, Cheney C, Mo X, Trotta R, Caligiuri M, Jarjoura D, Tridandapani S, Muthusamy N, Byrd JC. Comparative Assessment of Clinically Utilized CD20-directed Antibodies in Chronic Lymphocytic Leukemia Cells Reveals Divergent NK cell, Monocyte and Macrophage Properties. J Immunol Baltim Md 1950. 2013;190:2702–2711. doi: 10.4049/jimmunol.1202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vivier E, Rochet N, Ackerly M, Petrini J, Levine H, Daley J, Anderson P. Signaling function of reconstituted CD16: ζ:γ receptor complex isoforms. Int Immunol. 1992;4:1313–1323. doi: 10.1093/intimm/4.11.1313. [DOI] [PubMed] [Google Scholar]
- 33.Robertson MJ, Cochran KJ, Cameron C, Le JM, Tantravahi R, Ritz J. Characterization of a cell line, NKL, derived from an aggressive human natural killer cell leukemia. Exp Hematol. 1996;24:406–415. [PubMed] [Google Scholar]
- 34.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Jaw JJ, Stutzman NC, Zou Z, Sun PD. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J Leukoc Biol. 2012;91:299–309. doi: 10.1189/jlb.0611308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto K, Setoyama Y, Tsuzaka K, Abe T, Takeuchi T. Reduced expression of TCR zeta is involved in the abnormal production of cytokines by peripheral T cells of patients with systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/509021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng GM, Beltran J, Chen C, Terhorst C, Tsokos GC. T cell CD3ζ deficiency enables multiorgan tissue inflammation. J Immunol Baltim Md 1950. 2013;191:3563–3567. doi: 10.4049/jimmunol.1300634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P, Musset L, Debré P, Amoura Z, Vieillard V. Phenotype and function of natural killer cells in systemic lupus erythematosus: Excess interferon-γ production in patients with active disease. Arthritis Rheum. 2011;63:1698–1706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 39.Henriques A, Teixeira L, Inês L, Carvalheiro T, Gonçalves A, Martinho A, Pais ML, da Silva JAP, Paiva A. NK cells dysfunction in systemic lupus erythematosus: relation to disease activity. Clin Rheumatol. 2013;32:805–813. doi: 10.1007/s10067-013-2176-8. [DOI] [PubMed] [Google Scholar]
- 40.Enyedy EJ, Nambiar MP, Liossis SNC, Dennis G, Kammer GM, Tsokos GC. Fcε receptor type I γ chain replaces the deficient T cell receptor ζ chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 41.Lanier LL, Yu G, Phillips JH. Analysis of Fc gamma RIII (CD16) membrane expression and association with CD3 zeta and Fc epsilon RI-gamma by site-directed mutation. J Immunol. 1991;146:1571–1576. [PubMed] [Google Scholar]
- 42.Blázquez-Moreno A, Park S, Im W, Call MJ, Call ME, Reyburn HT. Transmembrane features governing Fc receptor CD16A assembly with CD16A signaling adaptor molecules. Proc Natl Acad Sci U S A. 2017;114:E5645–E5654. doi: 10.1073/pnas.1706483114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apostolidis SA, Lieberman LA, Kis-Toth K, Crispín JC, Tsokos GC. The Dysregulation of Cytokine Networks in Systemic Lupus Erythematosus. J Interferon Cytokine Res. 2011;31:769–779. doi: 10.1089/jir.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valés-Gómez M, Esteso G, Aydogmus C, Blázquez-Moreno A, Marín AV, Briones AC, Garcillán B, García-Cuesta E-M, López Cobo S, Haskologlu S, Moraru M, Cipe F, Dobbs K, Dogu F, Parolini S, Notarangelo LD, Vilches C, Recio MJ, Regueiro JR, Ikinciogullari A, Reyburn HT. Natural killer cell hyporesponsiveness and impaired development in a CD247-deficient patient. J Allergy Clin Immunol. 2016;137:942–945.e4. doi: 10.1016/j.jaci.2015.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Arase H, Suenaga T, Arase N, Kimura Y, Ito K, Shiina R, Ohno H, Saito T. Negative regulation of expression and function of Fc gamma RIII by CD3 zeta in murine NK cells. J Immunol Baltim Md 1950. 2001;166:21–25. doi: 10.4049/jimmunol.166.1.21. [DOI] [PubMed] [Google Scholar]
- 46.Liu CP, Ueda R, She J, Sancho J, Wang B, Weddell G, Loring J, Kurahara C, Dudley EC, Hayday A, Al E. Abnormal T cell development in CD3-zeta−/− mutant mice and identification of a novel T cell population in the intestine. EMBO J. 1993;12:4863. doi: 10.1002/j.1460-2075.1993.tb06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2014;92:221–229. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- 48.Puxeddu I, Bongiorni F, Chimenti D, Bombardieri S, Moretta A, Bottino C, Migliorini P. Cell surface expression of activating receptors and co-receptors on peripheral blood NK cells in systemic autoimmune diseases. Scand J Rheumatol. 2012;41:298–304. doi: 10.3109/03009742.2011.648657. [DOI] [PubMed] [Google Scholar]
- 49.Schepis D, Gunnarsson I, Eloranta M-L, Lampa J, Jacobson SH, Kärre K, Berg L. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–146. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang I, Zhang T, Scott JM, Kim AR, Lee T, Kakarla T, Kim A, Sunwoo JB, Kim S. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int Immunol. 2012;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bida AT, Upshaw Neff JL, Dick CJ, Schoon RA, Brickshawana A, Chini CC, Billadeau DD. 2B4 utilizes ITAM-containing receptor complexes to initiate intracellular signaling and cytolysis. Mol Immunol. 2011;48:1149–1159. doi: 10.1016/j.molimm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandey R, DeStephan CM, Madge LA, May MJ, Orange JS. NKp30 ligation induces rapid activation of the canonical NF-kappaB pathway in NK cells. J Immunol Baltim Md 1950. 2007;179:7385–7396. doi: 10.4049/jimmunol.179.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ottinger EA, Botfield MC, Shoelson SE. Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem. 1998;273:729–735. doi: 10.1074/jbc.273.2.729. [DOI] [PubMed] [Google Scholar]
- 55.Marcenaro E, Augugliaro R, Falco M, Castriconi R, Parolini S, Sivori S, Romeo E, Millo R, Moretta L, Bottino C, Moretta A. CD59 is physically and functionally associated with natural cytotoxicity receptors and activates human NK cell-mediated cytotoxicity. Eur J Immunol. 2003;33:3367–3376. doi: 10.1002/eji.200324425. [DOI] [PubMed] [Google Scholar]
- 56.Qian D, Mollenauer MN, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sjölin-Goodfellow H, Frushicheva MP, Ji Q, Cheng DA, Kadlecek TA, Cantor AJ, Kuriyan J, Chakraborty AK, Salomon AR, Weiss A. The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway. Sci Signal. 2015;8:ra49. doi: 10.1126/scisignal.2005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorelik G, Richardson B. Aberrant T cell ERK pathway signaling and chromatin structure in lupus. Autoimmun Rev. 2009;8:196–198. doi: 10.1016/j.autrev.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Araújo-Souza PS, Hanschke SCH, Viola JPB. Epigenetic control of interferon-gamma expression in CD8 T cells. J Immunol Res. 2015;2015:849573. doi: 10.1155/2015/849573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramos-Casals M, Cuadrado MJ, Alba P, Sanna G, Brito-Zerón P, Bertolaccini L, Babini A, Moreno A, D’Cruz D, Khamashta MA. Acute viral infections in patients with systemic lupus erythematosus: description of 23 cases and review of the literature. Medicine (Baltimore) 2008;87:311–318. doi: 10.1097/MD.0b013e31818ec711. [DOI] [PubMed] [Google Scholar]
- 61.Björnådal L, Löfström B, Yin L, Lundberg IE, Ekbom A. Increased cancer incidence in a Swedish cohort of patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31:66–71. doi: 10.1080/03009740252937568. [DOI] [PubMed] [Google Scholar]
- 62.Chen YJ, Chang YT, Wang CB, Wu CY. Malignancy in systemic lupus erythematosus: a nationwide cohort study in Taiwan. Am J Med. 2010;123:1150.e1–6. doi: 10.1016/j.amjmed.2010.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


