Abstract
This study aims to investigate antioxidative and antibacterial properties of fresh garlic (non-aged, NG) and aged garlic (AG) by-products extracted with distilled water, ethanol, or chloroform. To determine their antioxidative and antibacterial capacities, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assay, and H2O2 radical scavenging activity, Fe2+ chelating activity, total ferric reducing antioxidant power (FRAP), and disc diffusion tests were performed. Total phenol and flavonoid contents from distilled water extract of AG were significantly higher than those of NG. DPPH, ABTS, FRAP, and H2O2 scavenging activities of distilled water extract of AG were higher than those of NG. However, Fe2+ chelating activities of ethanol and chloroform extracts were higher than those of distilled water extracts for both NG and AG. Antibacterial effects of AG were higher than those of NG. In conclusion, aged garlic showed more potent antioxidant and antibacterial effects than fresh garlic.
Keywords: Garlic, Aged garlic, Antioxidant, Antibacterial, Extract
Introduction
Garlic (Allium sativum L.) is originated from western Asia and Mediterranean coast. It has been used as spice and natural medicine. Garlic is known to contain natural antioxidants that can remove reactive oxygen species (ROS) and reduce lipid peroxides and low-density lipoprotein (LDL) oxidation [1, 2]. Because garlic contains allicin, diallyl sulfides, and other sulfur compounds, it shows many physiological effects and activities in various metabolic pathways [3].
However, the strong and tangy flavor of garlic is big problem when developing products using garlic compounds. This is related to alliin and its precursor, S-allyl-(l)-cysteine. When cell structure of garlic is broken, alliin is transformed to allicin by alliinase. Allicin is responsible for the strong flavor of garlic. Because allicin is unstable, it is decomposed to lipophilic organic sulfur compounds such as diallyl sulfide, diallyl disulfide, and diallyl trisulfide [4]. The most common and traditional method to reduce the strong flavor of garlic is by steaming or roasting. When garlic is heat treated, alliinase activity is greatly reduced and the flavor is softened [5]. Aged garlic or black garlic is attracting attention of customers these days. It is a type of processed garlic produced by aging of whole garlic under constant temperature and humidity [6]. During the aging process, volatile components of garlic are decreased due to high temperature. On the other hand, water soluble flavonoids and phenolic compounds are increased [7].
There have been studies about aged garlic, aged garlic juice, and products such as bread (flour dough) and Yakju (Korean rice-wine) that contain aged garlic [8, 9]. However, research about the by-product after the extraction process of garlic juice is insufficient. Therefore, the purpose of this study was to determine the antioxidative activity of garlic and aged garlic by-products after extracting them with different solvents (distilled water, ethanol, and chloroform).
Materials and methods
Samples
Samples of non-aged garlic (NG) and aged-garlic (AG) were obtained from OZL DNF Inc. (Dam Yang, Korea). Experiments were carried out using powdered garlic by-product samples. Briefly, after pressing out juice from whole garlic without removing the husk, the remaining was dried at 60 °C for 6 h (powdering). Then 50 g of NG or AG powder was mixed with 250 mL of solvent (distilled water, ethanol, or chloroform) and incubated at 25 °C in a shaking (250 rpm) incubator for 24 h. The extract was then filtered with Whatman filter paper (No. 2).
Chemicals
Folin–Ciocalteu’s phenol reagent, Na2CO3, MeOH, AlCl3, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate (K2S2O8), potassium ferricyanide, trichloroacetic acid, ferric chloride, FeCl2, ferrozine, Peroxidase from horseradish, hydrogen peroxide (H2O2) were obtained from sigma (Sigma-Aldrich GmbH, Sternheim, Germany).
Antioxidant activities
Determination of total phenol contents
Total phenol contents were determined using Folin–Ciocalteu’s phenol according to published method [10] with slight modification. Briefly, tenfold diluted sample (0.5 mL) and Folin–Ciocalteu’s phenol reagent (2.5 mL) were mixed and reacted at room temperature for 5 min. After that, 75 g/L Na2CO3 (2 mL) was added to the mixture and reacted at room temperature for 2 h. Absorbance value was then measured at 760 nm on a spectrophotometer. MeOH was used as blank. Garlic acid equivalents were used to construct standard curve.
Determination of total flavonoid contents
Total flavonoid contents were determined according to Dowd method [11] with slight modifications. Briefly, 2% AlCl3 (1.5 mL) and tenfold diluted sample (0.5 mL) were mixed and reacted at room temperature for 10 min. Absorbance of the mixture was then measured at 415 nm on a spectrophotometer. Quercetin was used to obtain standard curve.
DPPH free radical scavenging activity
Free radical scavenging activity of each sample was measured using the method of Blois [12], with some modifications. Briefly, 5 mL of 0.1 mM DPPH in 95% ethanol was added to 1 mL of each tenfold diluted sample. The mixture was shaken vigorously and incubated at room temperature for 30 min in the dark. After that, the mixture was filtered through a nylon syringe filter (0.45 μm). Its absorbance was then measured at 517 nm. DPPH radical scavenging activity was then calculated using the following equation:
ABTS radical cation scavenging activity
ABTS radical cation scavenging activity was determined using the method of Re et al. [13]. Briefly, 2 mM ABTS was dissolved in distilled water (DW) containing 2.45 mM potassium persulfate (K2S2O8) and kept at room temperature for 12 h in the dark. The ABTS·+ solution was adjusted with sodium phosphate buffer (0.1 M, pH 7.4) to an initial absorbance of about 0.75 ± 0.005 at 734 nm. Then 0.1 mL of each tenfold diluted sample was mixed 3 mL of ABTS·+ solution. After incubating at room temperature for 10 min, the absorbance was measured at 734 nm. ABTS·+ radical scavenging activity was then calculated using the following equation:
Ferric reducing antioxidant power (FRAP)
FRAP assay was performed following the modified Oyaizu method [14]. Briefly, 1 mL of each tenfold diluted sample was mixed with 2.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide (potassium hexacyanoferrate). The mixture was then incubated at 50 °C for 20 min. After adding 2.5 mL of 10% trichloroacetic acid, the mixture was then centrifuged at 4000 rpm for 10 min. The upper layer (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% FeCl3 (ferric chloride). After 5 min of reaction at room temperature, the absorbance was measured at 700 nm.
Ferrous ions (Fe2+) chelating activity
Fe2+ chelating activity of the extract was determined using published method [15]. Briefly, 0.5 mL of each tenfold diluted sample was mixed with 2 mL of 1 mM FeCl2 in 95% ethanol. The reaction was initiated by adding 2.5 mL of 2 mM ferrozine in 95% ethanol. The mixture was then vortexed and incubated at room temperature for 10 min. It was then filtered through a nylon syringe filter (0.45 μm). The absorbance of the filtrate was then measured at 562 nm. Ferrous ions chelating activity was then calculated using the following equation:
Hydrogen peroxide (H2O2) scavenging activity
Hydrogen peroxide scavenging activity was determined using previously described method [16]. Briefly, each extract sample (0.1 mL) was mixed with H2O2 (0.01 mL, 50 mM), peroxidase from horseradish (0.6 mL, 10 U/mL), ABTS (0.6 mL, 0.1%), and 1.8 mL of 0.1 M phosphate buffer (pH 6.0). The solution was then incubated at 37 °C for 15 min. Absorbance of the resulting solution was then measured spectrophotometrically at 414 nm. Garlic acid equivalents were used to construct standard curve.
Antimicrobial activity based on disc diffusion test
Antimicrobial activity of each extract (NG or AG) was tested against Salmonella enteritidis, Staphylococcus aureus, Escherichia coli, Bacillus cereus, and Listeria monocytogenes using agar diffusion technique [17]. Bacterial colonies were suspended in normal saline to obtain approximate concentrations (106–107 CFU/mL). Filter paper discs (Whatman, 6 mm in diameter) were dipped in each sample (50 μL) and dried for 3 h. All plates were incubated at 37 °C for 24 h. The diameter of the resulting zone of inhibition was measured in mm. Penicillin was used as positive control while distilled water was used as negative control.
Statistical analysis
All experiments were carried out in triplicates. Results are presented as mean ± standard error of means. Data were analyzed using IBM SPSS statistics 24.0. One-way analysis of variance (ANOVA) was performed. Significant differences between different solvents and treatments (NG and AG) were analyzed using Tukey test. A p value of less than 0.05 was considered statistically significant.
Results and discussion
Determination of total phenol and flavonoid contents
Results of total phenol and flavonoid contents of NG and AG extracts using different solvents are shown in Table 1. Distilled water extract from AG had significantly (p < 0.001) higher total phenol content (147.58 mg GAE/g) than other extracts. Mean values of total phenol contents were in the following order: AG-E (43.01 mg GAE/g) > NG-D (5.68 mg GAE/g) > NG-E (2.86 mg GAE/g) > NG-C = AG-C (0.27 and 0.69 mg GAE/g, respectively). In general, antioxidant activities of plant extracts are mostly interactive with phenolic-type compounds [18]. Regardless of solvents, flavonoid contents of AG groups were significantly higher compared to those of NG groups. In particular, flavonoid content of water extract from AG was approximately 4.5-fold higher than that of NG (AG-W, 338.04 mg GAE/g; NG-W, 70.82 mg QE/g). Consequently, water extract from AG showed the highest total phenols and flavonoid contents. The significant increase in total phenol and flavonoids in AG might be due to the conversion of some components in garlic into these highly hydrophilic compounds. According to Bozin et al. [19], 80% methanol extract from aged garlic had higher total phenol and flavonoids contents (0.98 mg GAE/g and 6.99 μg QE/g, respectivley) than that from fresh garlic (0.05 mg GAE/g and 4.16 μg QE/g, respectively). Nencini et al. [20] have found that aged garlic extract with 15% ethanol contains 0.73 mg GAE/g (bulbs) and 1.23 mg GAE/g (leaves) of total phenol. Flavonoids and phenolic compounds have antioxidant abilities such as regeneration of α-tocopherol, free radical scavengers, and metal chelation [13].
Table 1.
Total phenol and flavonoid contents in fresh garlic and aged garlic by-products extracted with different solvents
| NG | AG | |||||
|---|---|---|---|---|---|---|
| DW | Ethanol | Chloroform | DW | Ethanol | Chloroform | |
| Total phenol | 5.68 ± 0.51c | 2.86 ± 0.14c | 0.27 ± 0.06c | 147.58 ± 5.27a | 43.01 ± 2.85b | 0.69 ± 0.11c |
| Total flavonoid | 70.82 ± 0.22c | 35.07 ± 0.78d | 25.44 ± 1.08e | 338.04 ± 1.60a | 124.08 ± 3.22b | 28.07 ± 1.97de |
Total phenolic contents: mg GAE/g, total flavonoid content: μg QE/g
NG non-aged garlic powder, AG aged garlic powder, DW distilled water
Values with different superscripts are significantly different among samples (p < 0.05)
DPPH and ABTS radical scavenging activity
Results of DPPH radical scavenging activity showed a decreasing tendency according to the extraction solvent (DW > ethanol > chloroform) as shown in Table 2. DPPH radical scavenging activities of the AG group extracted with DW, ethanol, and chloroform were significantly (p < 0.05) higher than those of the NG group: AG-D (79.21%) > AG-E (51.16%) > NG-D (20.86%) > AG-C (13.44%) > NG-E (11.22%) > NG-C (5.28%). ABTS radical cation scavenging activity results were similar to results of DPPH radial scavenging test. That is, AG-D had the highest activity and AG group had higher activity than the NG group. Radical cation scavenging activities of extracts were in the following order: AG-D (99.56%) > AG-E (83.49%) > NG-D (35.92%) > AG-C (19.23%) = NG-E (19.78%) > NG-C (17.43%). Accordingly, AG group showed greater radical scavenging activities for DPPH and ABTS than NG group, with AG-D showing the greatest activity. Previous studies have suggested that aged garlic contains plentiful phenol, flavonoid, and various sulfur compounds such as S-ally-(l)-cysteine (SAC, hydrophilic) and disulfide (hydrophobic) compared to fresh garlic. In addition, SAC has high radical scavenging activities [21, 22]. The number of phenolic compounds and flavonoids has positive correlation with DPPH and ABTS radical scavenging activities due to hydrogen and electron donation from hydroxyl groups of these compounds [23, 24]. In particular, ABTS method is regarded as potentially more efficient than DPPH method since ABTS can measure both hydrophilic and hydrophobic substances [25].
Table 2.
DPPH and ABTS radical scavenging activity (%) of fresh garlic and aged garlic by-products extracted with different solvents
| NG | AG | |||||
|---|---|---|---|---|---|---|
| DW | Ethanol | Chloroform | DW | Ethanol | Chloroform | |
| DPPH radical scavenging activity | 20.86 ± 0.29c | 11.22 ± 0.14e | 5.28 ± 0.33f | 79.21 ± 0.74a | 51.16 ± 0.13b | 13.44 ± 5.14d |
| ABTS radical scavenging activity | 35.92 ± 0.43c | 19.78 ± 0.15d | 17.43 ± 0.09e | 99.56 ± 0.06a | 83.49 ± 0.28b | 19.23 ± 0.20d |
NG non-aged garlic powder, AG aged garlic powder, DW distilled water
Values with different superscripts are significantly different among samples (p < 0.05)
Ferric reducing antioxidation power (FRAP)
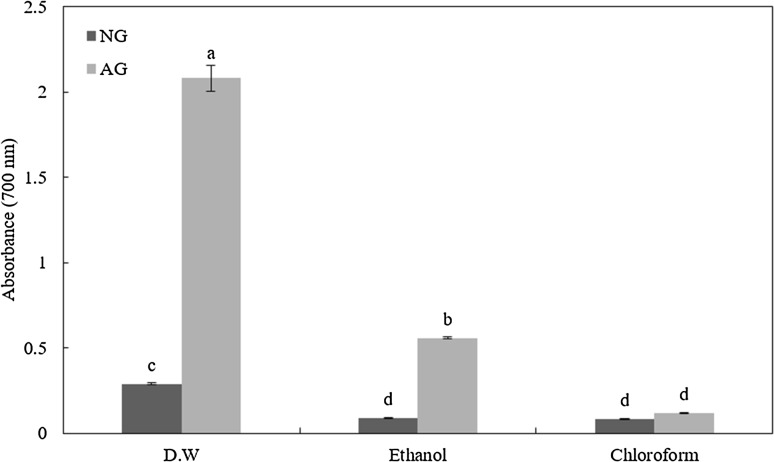
FRAP is a method of verifying antioxidant activity through electron donating ability. In FRAP, ferric tripyridyltriazine (Fe3+-TPTZ) complex is reduced to ferrous tripyridyltriazine (Fe2+-TPTZ) by the sample. The higher the absorbance, the higher the antioxidant activity. Results of total ferric reducing power showed that the AG group had a decreasing tendency of reducing power according to extraction solvent (DW > ethanol > chloroform) based on absorbance at 700 nm (Fig. 1). Among these sample extracts, AG-D had significantly (p < 0.001) higher reducing power than other groups. The reducing power of these samples was in the following order: AG-E > NG-D > NG-E = NG-C = AG-C. This result might be due to high phenol and flavonoid contents in distilled water and ethanol extracts of aged garlic compared to those of non-aged garlic extracts. Positive correlation between polyphenol content of Allium species and FRAP value (r 2 = 0.669) has been observed previously [20].
Fig. 1.
Ferric reducing antioxidation power (FRAP) of non-aged garlic powder (NG) and aged garlic powder (AG) by-products extracted with distilled water (DW), ethanol, and chloroform. Values with different superscripts are significantly different among samples (p < 0.05)
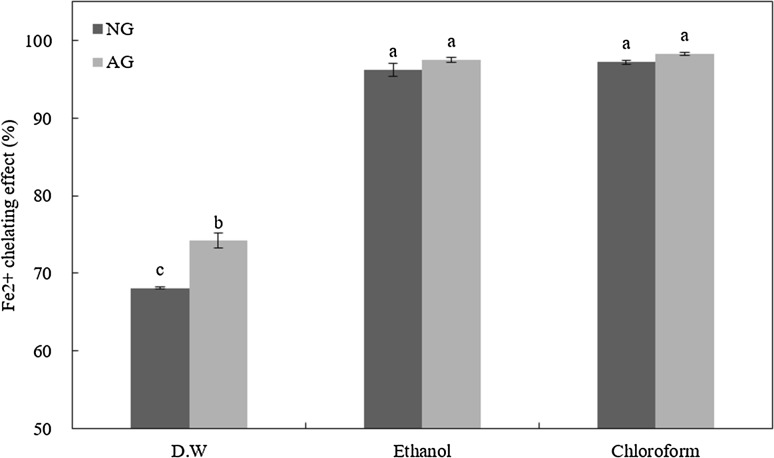
Ferrous ions (Fe2+) chelating activity
Ferrozine reacts with Fe2+ to form a complex and become purple. The chelating activity of a sample can inhibit the formation of Fe2+-ferrozine complex, resulting in degradation of color. Results of ferrous ion chelating activity of aged and fresh garlic extracts using different solvents are shown in Fig. 2. Ferrous ions chelating activity was detected in all groups. Especially, extracts using ethanol and chloroform (organic solvent) had significantly (p < 0.05) higher ferrous ion chelating activity than extracts using DW. Distilled water extract from aged garlic showed significant (p < 0.001) higher ferrous ion chelating activity than all groups. However, ethanol and chloroform extracts did not show significant difference in ferrous ion chelating activity for aged or non-aged garlic. Similarly, it has been reported that aqueous extract from garlic has higher reducing power and lower metal chelating ability than methanol extracts [26]. The inhibition effect of diallyl sulfide and diallyl disulfide derived from garlic on Fe2+ lipid oxidation has been proved previously [27]. The high metal chelating effect of ethanol and chloroform extracts could be due to thiol groups such as hydrophobic disulfides with metal chelating ability [28].
Fig. 2.
Fe2+ chelating activity (%) of non-aged garlic powder (NG) and aged garlic powder (AG) by-products extracted with distilled water (DW), ethanol, and chloroform. Values with different superscripts are significantly different among samples (p < 0.05)
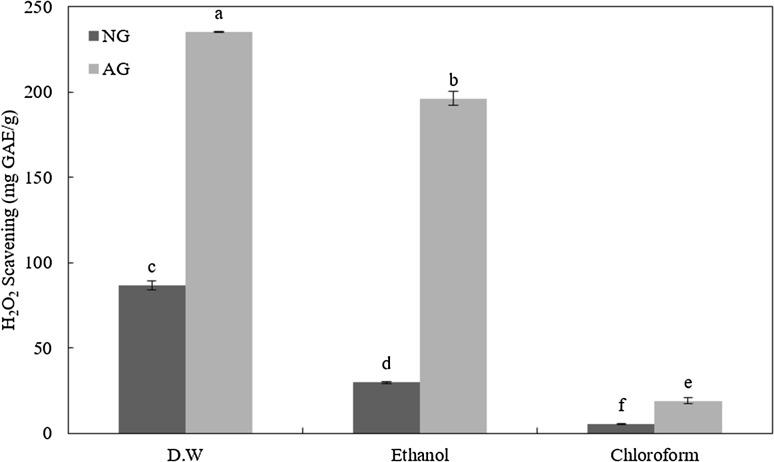
Hydrogen peroxide (H2O2) scavenging activity
Results of hydrogen peroxide scavenging activities of various extracts are show in Fig. 3. AG-D extract had the highest hydrogen peroxide scavenging activity (235.02 mg GAE/g), followed by AG-E (196.11 mg GAE/g), NG-D (86.48 mg GAE/g), NG-E (29.68 mg GAE/g), AG-C (19.00 mg GAE/g), and NG-C (5.32 mg GAE/g). NG and AG groups showed a decreasing tendency in hydrogen peroxide scavenging activity according to extraction solvent (DW > ethanol > chloroform). Hydrogen peroxide is considered a non-free radical species. It has high toxicity in presence of some metal ions such as Fe2+ and Cu2+. Lipid oxidation generated by Fenton reaction (Fe2+ and hydrogen peroxide) can be inhibited by garlic extracts [19]. Ide et al. [29] have reported that sulfur compounds such as SAC and alliin of aged garlic can scavenge hydrogen peroxide. Furthermore, fructosyl arginine generated from Maillard reaction during aging of garlic has shown hydrogen peroxide scavenging ability [30]. Therefore, the high hydrogen peroxide scavenging activity of aged garlic extract using distilled water might be useful for protection against oxidative toxicity.
Fig. 3.
Hydrogen peroxide (H2O2) scavenging activity of non-aged garlic powder (NG) and aged garlic powder (AG) by-products extracted with distilled water (DW), ethanol, and chloroform. Values with different superscripts are significantly different among samples (p < 0.05)
Antimicrobial activity
Results of antimicrobial activities of aged and non-aged garlic extracts using different solvents are shown in Table 3. Non-aged garlic extracts except chloroform extract did not show significant antimicrobial activity against S. aureus, S. enteritidis, E. coli, B. cereus, or L. monocytogens. Similar results have been reported by Dziri et al. [31] showing that antibacterial activity of rosy garlic (Allium roseum var. odoratissimum) against pathogenic bacteria was mostly not detected. In contrast, Benkeblia [32] has demonstrated that essential oil extract from garlic has high antimicrobial activity against or S. aureus and S. Enteritidis. However, chloroform extracts of both non-aged and aged garlic inhibited (p < 0.05) the growth of B. cereus. Yin and Cheng [27] have also observed that the addition of lipophilic sulfur compounds such as diallyl sulfide and diallyl disulfide into meat products can reduce the growth of pathogenic bacteria whereas hydrophilic sulfur compounds such as s-ethyl cysteine and n-acetyl cysteine have low antibacterial activities. Inhibition of B. cereus growth is sensitively affected by solvent used to extract plants [33]. The growth of E. coli was significantly (p < 0.05) reduced by distilled water and ethanol extracts of aged garlic. This result might be due to the fact that hydrophilic sulfur compounds and phenol/flavonoids contents were increased in aged-garlic.
Table 3.
Disc diffusion test results of fresh garlic and aged garlic by-products extracted with different solvents
| unit: mm | NG | AG | SEM | ||||
|---|---|---|---|---|---|---|---|
| Extract solvent | DW | Ethanol | Chloroform | DW | Ethanol | Chloroform | |
| S. aureus | nd | nd | nd | 9.67a | nd | nd | 0.11 |
| S. enteritidis | nd | nd | nd | nd | nd | nd | – |
| E. coli | nd | nd | nd | 10.67a | 10.00a | nd | 0.34 |
| B. cereus | nd | nd | 9.67b | nd | nd | 13.3a | 0.29 |
| L. monocytogenes | nd | nd | nd | nd | nd | nd | – |
NG non-aged garlic powder, AG aged garlic powder, DW distilled water, nd not detected, SEM standard error of means
Values with different superscripts are significantly different among samples (p < 0.05)
Acknowledgements
This work was supported by the Brain Korea 21 Plus Project of Department of Food Science and Biotechnology of Animal Resources, Konkuk University in 2017.
References
- 1.Saravanan G, Prakash J. Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol. 2004;94(1):155–158. doi: 10.1016/j.jep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Ide N, Lau BH. Garlic Compounds Protect Vascular Endothelial Cells from Oxidized Low Density Lipoprotein-induced Injury. J. Pharm. Pharmacol. 1997;49(9):908–911. doi: 10.1111/j.2042-7158.1997.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 3.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Recent advances on the nutritional effects associated with the use of garlic as a supplement: intake of garlic and its bioactive components. J. Nutr. 2001;131(3):955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 4.Rose P, Whiteman M, Moore PK, Zhu YZ. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep. 2005;22(3):351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 5.Song K, Milner JA. Recent Advances on the Nutritional Effects Associated with the Use of Garlic as a Supplement: The Influence of Heating on the Anticancer Properties of Garlic. J. Nutr. 2001;131(3):1054S–1057S. doi: 10.1093/jn/131.3.1054S. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Kang OJ, Gweon OC. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J Funct Foods. 2013;5(1):80–86. doi: 10.1016/j.jff.2012.08.006. [DOI] [Google Scholar]
- 7.Shin JH, Choi DJ, Chung MJ, Kang MJ, Sung NJ. Changes of Physicochemical Components and Antioxidant Activity of Aged Garlic at Different Temperatures. J Korean Soc Food Sci Nutr. 2008;37(9):1174–1181. doi: 10.3746/jkfn.2008.37.9.1174. [DOI] [Google Scholar]
- 8.Wang SJ, Lee JH, Choi MJ, Lee SK. Effects of aged black garlic extracts on the rheology of flour dough. J Korean Soc Food Sci Nutr. 2012;41(3):430–435. doi: 10.3746/jkfn.2012.41.3.430. [DOI] [Google Scholar]
- 9.Lee HH, Kim IJ, Kang ST, Kim YH, Lee JO, Ryu CH. Development of black garlic Yakju and its antioxidant activity. Korean J. Food Sci. Technol. 2010;42(1):69–74. [Google Scholar]
- 10.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 11.Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardization of propolis extract and identification of principal constituents. J Pharm Belg. 1994;49(6):462–468. [PubMed] [Google Scholar]
- 12.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 13.Re P, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 14.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japanese Journal of Nutrition. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 15.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate, and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophy. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto G, Hayase F, Kato H. Scavenging of active oxygen species by glycated proteins. Biosci. Biotechnol. Biochem. 1992;56(6):928–931. doi: 10.1271/bbb.56.928. [DOI] [PubMed] [Google Scholar]
- 17.Meena MR, Sethi VIJAY. Antimicrobial activity of essential oils from spices. J Food Sci. Technol. 1994;31(1):68–70. [Google Scholar]
- 18.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozin B, Mimica-Dukic N, Samojlik I, Goran A, Igic R. Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae) Food Chem. 2008;111(4):925–929. doi: 10.1016/j.foodchem.2008.04.071. [DOI] [Google Scholar]
- 20.Nencini C, Menchiari A, Franchi GG, Micheli L. In vitro antioxidant activity of aged extracts of some Italian Aliium species. Plant Foods Hum Nutr. 2011;66(1):11–16. doi: 10.1007/s11130-010-0204-2. [DOI] [PubMed] [Google Scholar]
- 21.Colin-Gonzalez AL, Santana RA, Silva-Islas CA, Chanez-Cardenas ME, Santamaria A, Maldonado PD. The antioxidant mechanisms underlying the aged garlic extract- and S-allyl cysteine-induced protection. Oxid Med Cell Longev. 2012;2012:16. doi: 10.1155/2012/907162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bae SE, Cho SY, Won YD, Lee SH, Park HJ. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. Lebenson Wiss Technol. 2014;55(1):397–402. doi: 10.1016/j.lwt.2013.05.006. [DOI] [Google Scholar]
- 23.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 24.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 25.Martysiak-Żurowska D, Weronika W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci Pol Technol Aliment. 2012;11(1):83–89. [PubMed] [Google Scholar]
- 26.Liu C, Yang X, Yao Y, Huang W, Sun W, Ma Y. Determination of antioxidant activity in garlic (Allium sativum) extracts subjected to boiling process in vitro. Journal of Food and Nutrition Research. 2014;2(7):383–387. doi: 10.12691/jfnr-2-7-9. [DOI] [Google Scholar]
- 27.Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organosulfur compounds in ground beef. Meat Sci. 2003;63(1):23–28. doi: 10.1016/S0309-1740(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 28.Saha B, Iglesias M, Dimming IW, Streat M. Sorption of trace heavy metals by thiol containing chelating resins. Solvent Extraction and Ion Exchange. 2000;18(1):133–167. doi: 10.1080/07366290008934676. [DOI] [Google Scholar]
- 29.Ide N, Matsuura H, Itakura Y. Scavenging effect of aged garlic extract and its constituents on active oxygen species. Phytother Res. 1996;10(4):340–341. doi: 10.1002/(SICI)1099-1573(199606)10:4<340::AID-PTR831>3.0.CO;2-4. [DOI] [Google Scholar]
- 30.Ide N, Lau BHS, Ryu K, Matsuura H, Itakura Y. Antioxidant effects of fructosyl arginine, a Maillard reaction product in aged garlic extract. J. Nutr. Biochem. 1999;10(6):372–376. doi: 10.1016/S0955-2863(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 31.Dziri S, Hassen I, Fatnassi S, Mrabet Y, Casabianca H, Hanchi B, Hosni K. Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). J Funct Foods. 4(2):423–432 (2012)
- 32.Benkeblia N. Antimicrobial activityof essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum) Lebenson Wiss Technol. 2004;37(2):263–268. doi: 10.1016/j.lwt.2003.09.001. [DOI] [Google Scholar]
- 33.Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol. 2003;80(3):223–230. doi: 10.1016/S0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]