Abstract
A novel robot-aided assist-as-needed gait training paradigm has been developed recently. This paradigm encourages subjects’ active participation during training. Previous pilot studies demonstrated that assist-as-needed robot-aided gait training (RAGT) improves treadmill walking performance post-stroke. However, it is not known if there is an over-ground transfer of the training effects from RAGT on treadmill or long-term retention of the effects. The purpose of the current study was to examine the effects of assist-as-needed RAGT on over-ground walking pattern post-stroke. Nine stroke subjects received RAGT with visual feedback of each subject’s instantaneous ankle malleolus position relative to a target template for fifteen 40-minute sessions. Clinical evaluations and gait analyses were performed before, immediately after and 6 months post-training. Stroke subjects demonstrated significant improvements and some long-term retention of the improvements in their self-selected over-ground walking speed, Dynamic Gait Index, Timed Up and Go, peak knee flexion angle during swing phase and total hip joint excursion over the whole gait cycle for their affected leg (p<0.05). These preliminary results demonstrate that subjects improved their over-ground walking pattern and some clinical gait measures post-training suggesting that assist-as-needed RAGT including visual feedback may be an effective approach to improve over-ground walking pattern post-stroke.
Keywords: Force field, gait training, locomotion, lower-limb exoskeleton, rehabilitation, robotics, stroke
I. INTRODUCTION
STROKE is a leading cause of serious long-term disability in the elderly. Each year approximately 795,000 people experience a new or a recurrent stroke [1]. Lower-extremity sensorimotor impairment is frequently seen in people following stroke [2]. Stroke survivors usually have reduced joint excursion, insufficient forward propulsion, and hyperactive reflex responses, which lead to a slow, asymmetrical, and unstable walking pattern [3-5]. In addition, stroke survivors tend to use inefficient compensatory strategies for the gait deficits such as a leg circumduction or abnormal elevation of the pelvis to compensate for insufficient foot clearance during swing. Overall, these altered walking patterns lead to high energy expenditure [6] and may further reduce their social participation and independence in activities of daily living [7].
Body weight supported treadmill training (BWSTT) is one of the approaches for gait retraining post-stroke [8, 9]. An advantage of BWSTT is that partial body weight support makes it easier for patients to control their lower limbs and trunk during training. BWSTT has shown significant therapeutic effects in improving patients’ gait speed, walking endurance, and functional walking capacity [8-10]. However, the major drawback of this training is that it is labor-intensive, requiring one or two therapists to manually assist patients’ leg motion and stabilize their trunk. In addition, the manual assistance provided during the training varies from one therapist to another and thus, the training may not be performed in a consistent manner [11]. Duncan et al (2011) suggested that BWSTT is not superior to a home-exercise program in promoting functional gait recovery. Thus, clear evidence for the effectiveness of BWSTT in the post-stroke population is still lacking [12].
Robotic lower-limb exoskeletons have been developed to overcome some of the aforementioned limitations of BWSTT. Robot-aided gait training (RAGT) with continuous assistance tends to move the patient’s leg passively through a prescribed target path, and has been shown to be less effective than conventional therapy [13]. This may be because the subjects’ physical effort is substantially reduced due to the continuous assistance. A novel compliant force field for RAGT was developed to provide assistance when needed, as an alternative to continuous assistance [14, 15]. Previous studies showed that both neurologically impaired and intact individuals increased their active participation by showing greater heart rate, muscle activation, and physical exertion during training when using an assist-as-needed paradigm [16-18]. In addition, a recent case study compared the two training strategies, assist-as-needed and continuous assistance paradigms, on a single stroke survivor [19]. Their preliminary results suggest that the assist-as-needed paradigm is more effective than the continuous assistance paradigm, showing more improvements in clinical measures and walking speed. The compliant force field used during the assist-as-needed paradigm preserves a basic feature of walking, that is, the presence of step-to-step variability [20]. It is possible that the movement variability encourages more active participation from the subjects, and leads to better gait recovery [21]. However, there is still limited evidence supporting the effectiveness of the assist-as-needed RAGT on individuals post-stroke. Furthermore, it has been suggested that restricting degrees of freedom of the trunk as a result of walking with the robotic exoskeleton may limit improvements in subjects' gait pattern [22]. An Active Leg Exoskeleton (ALEX) [23] developed in our laboratory provides additional degrees of freedom for trunk and hip movement in comparison to the commercially available Lokomat [24]. This may allow a more natural gait pattern and can be an advantage over the widely used Lokomat.
The purpose of the current study was to investigate the effectiveness of assist-as-needed RAGT with ALEX on the functional walking ability of individuals post-stroke. A previous pilot study from our lab demonstrated that two stroke survivors improved their treadmill walking patterns after 15 sessions of assist-as-needed RAGT that included visual feedback of their instantaneous and target ankle malleolus position [15]. In addition, a recent pilot study from Krishnan et al. showed that a single stroke survivor had substantially improved muscle coordination, propulsive ground reaction forces, and malleolus path after receiving the assist-as-needed RAGT with Lokomat [25]. Although those results are promising, the conclusions were based on the data from only one or two stroke survivors. Moreover, no information regarding long-term retention of the training effects from the assist-as-needed paradigm is available in the previous studies. In the current study, we investigated whether the RAGT using an assist-as-needed paradigm would facilitate changes in the walking patterns and functional ability of individuals post-stroke and whether these changes would be retained 6 months following training. We also investigated if the training effects could be transferred to over-ground walking. We hypothesized that subjects would show improvements in their walking patterns and sensorimotor function following gait training, and these changes would be retained to some extent even 6 months after the training.
II. METHODS
A. Subject information
Nine stroke survivors (7 males, 2 females) who had sustained a stroke more than 3 months prior to the study gave written informed consent to participate in the study, approved by the University’s Review Board. Demographic details of the stroke survivors’ are listed in Table I. A physical therapist screened all subjects for the exclusion criteria. Subjects were excluded if they had evidence of multiple strokes, chronic white matter disease on magnetic resonance imaging, congestive heart failure, peripheral artery disease with intermittent claudication, cancer, pulmonary or renal failure, unstable angina, uncontrolled hypertension (>190/110 mmHg), dementia (Mini-Mental State Exam < 22) [26], severe aphasia, orthopedic conditions affecting the legs or the back, or cerebellar signs (e.g., ataxia).
TABLE I. DEMOGRAPHIC DETAILS.
| Subject ID |
AGE (yrs) |
Duration Post-stroke (mos) |
Side Affected (R/L) |
Gender (M/F) |
Diagnosis |
|---|---|---|---|---|---|
| S1 | 72 | 41 | R | M | ischemic |
| S2 | 47 | 38 | R | M | hemorrhagic |
| S3 | 78 | 29 | R | M | ischemic |
| S4 | 56 | 95 | L | M | ischemic |
| S5 | 80 | 53 | L | M | ischemic |
| S6 | 60 | 3 | R | F | hemorrhagic |
| S7 | 43 | 3 | R | M | ischemic |
| S8 | 67 | 20 | L | M | ischemic |
| S9 | 70 | 149 | L | F | hemorrhagic |
B. Device and assist-as-needed force field description
This study used ALEX developed at the University of Delaware (Figure. 1), details of which were described previously [15, 23]. ALEX was used to apply an assist-as-needed compliant guidance force on the affected leg of the subjects during training. Each subject was provided with visual feedback of the instantaneous malleolus position and a target template based on the spatial location of the lateral malleolus. The target template for the training was based on the normalized walking pattern of ten healthy elderly individuals that were recorded previously at 17 different speeds from 0.6 to 2.2 mph. Templates for the malleolus path were adjusted to each stroke survivor’s leg length at a given speed. The malleolus path of healthy individuals was considered to be 100%, and the stroke survivors’ baseline pattern was considered as 0%. The stroke survivors’ malleolus path was scaled at each data point to a certain percentage of the healthy data to generate the target template [15]. Scaling of the stroke survivor’s path was increased towards that of the healthy template across training sessions. A compliant force field with an assist-as-needed paradigm provided guidance in the form of a virtual (elastic) tunnel around the target template that works similar to an elastic band, tending to bring the subject’s ankle towards the target path. The force field included normal and tangential forces. The normal force was applied when the subject’s instantaneous malleolus position went beyond the virtual tunnel surrounding the target template. A minimal tangential force helped the subjects to move along the target malleolus path (Figure. 2).
Fig. 1.
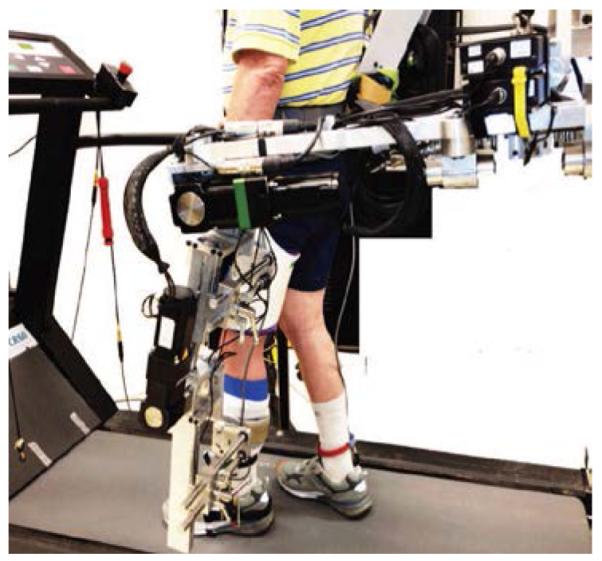
The active leg exoskeleton (ALEX) worn by the subjects on their affected extremity during training and treadmill evaluation.
Fig. 2.
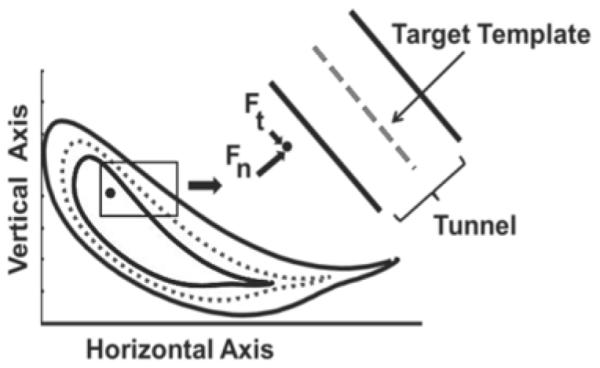
Target template based on ankle malleolus and application of the compliant force field. The dashed line represents the target template based on the malleolus path and the black solid lines represent the walls of the virtual tunnel on either sides of the template. The solid black dot represents instantaneous malleolus position of the subject with the normal (Fn) and tangential (Ft) forces represented by the solid black arrows moving the subject’s ankle closer to the template.
C. Training and evaluation protocol
Subjects received a total of 15 training sessions by having 5 daily sessions per week, every other week for 3 weeks. Each training session included eight 5-minute training bouts with rest breaks after every bout. Subjects received visual feedback on their instantaneous malleolus position and the target template as well as functional electrical stimulation (FES) of their ankle plantarflexors and dorsiflexors during alternating minutes. The stimulation intensity for both muscle groups was set using 300-ms long, 30-Hz train with 150-Volt amplitude. Pulse duration for dorsiflexors was set with subjects seated, to achieve a neutral ankle joint position (0°) with minimal ankle eversion or inversion. For plantarflexors, the subjects stood in a position similar to terminal double support of the paretic leg. Pulse duration was set to achieve lifting of the paretic heel off the ground or until the subject’s maximal tolerance was reached, whichever occurred first. The FES stimulation pattern comprised a high-frequency (200-Hz) 3-pulse burst followed by a lower frequency (30-Hz) constant frequency train [27, 28]. FES was provided to S1 only for the third week of training, and S2 only for the plantarflexors for the entire period of training. The force field was applied continuously for entire training bout. The size of the target was not changed within a single session. However, the assistance was decreased gradually over the eight training bouts, allowing the subjects to control their malleolus position more independently [15]. The amount of normal force was proportional to the square of the deviation between subject’s instantaneous malleolus position and the tunnel around the desired target path (equation 1):
| (1) |
where KN or stiffness is a constant with force units per length units squared, d is the distance between subject’s instantaneous malleolus position and the desired position on the target path, and D0 is the width of virtual tunnel. We reduced the robotic assistance across training bouts by decreasing the stiffness and/or increasing the width of the virtual tunnel around the target template. Two levels of stiffness and two sizes of the virtual tunnel were used in the study: a high (HS = 0.760 N/mm2) and a low (LS = 0.125 N/mm2) stiffness coefficient (KN) as well as a narrow width (NW = 10mm) and a larger width (LW = 20mm) of the virtual tunnel (Table II).
TABLE II. TRAINING PROTOCOL FOR EACH BOUT IN A SINGLE SESSION.
| Bout | Bout | Bout | Bout | Bout | Bout | Bout | Bout |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| (NW- HS) |
(NW- HS) |
(NW- LS) |
(NW- LS) |
(LW- HS) |
(LW- HS) |
(LW- LS) |
(LW- LS) |
The training speeds and the size of the target template were increased gradually with the progression of the training, based on the subject’s performance on the previous training session. The details of the training protocol and computation of the target template have been documented previously [15].
All subjects underwent gait analyses on the treadmill using ALEX for the paretic leg and during over-ground walking using eight-camera motion capture system, as well as a clinical examination before training (baseline), immediately after training (post-training), and 6 months after training (follow-up). Each clinical evaluation session included lower-extremity Fugl-Meyer Assessment (FMA) [29], Dynamic Gait Index (DGI) [30], Six-Minute Walk Test (6MWT) [31], and Timed Up and Go test (TUG) [32]. A summary of the stroke survivors’ baseline characteristics is listed in Table III. The walking evaluation on the treadmill with ALEX was performed without the force field guidance and visual feedback at subjects’ baseline treadmill walking speeds. Subjects walked at their self-selected speed for the gait evaluation during over-ground walking.
TABLE III. PARTICIPANTS’ CHARACTERISTICS AT BASELINE.
| Subject ID |
Timed Up and Go (s) |
Dynamic Gait Index |
Fugl-Meyer Assessment |
Six- Minute Walk Test (m) |
Self- Selected Walking Speed (m/s) |
|---|---|---|---|---|---|
| S1 | 14.5 | 13 | NA | NA | 0.51 |
| S2 | 9.7 | 20 | NA | 474 | 1.06 |
| S3 | 18.6 | 13 | 19 | 311 | 0.74 |
| S4 | 7.6 | 16 | 24 | 476 | 1.04 |
| S5 | 15.6 | 12 | 25 | 268 | 0.75 |
| S6 | 29.3 | 9 | 11 | 72 | 0.15 |
| S7 | 16.2 | 12 | 21 | 232 | 0.53 |
| S8 | 29.3 | 8 | 12 | 150 | 0.29 |
| S9 | 13.2 | 15 | 28 | 332 | 0.78 |
D. Data acquisition and analysis
Lower-limb joint angles and foot pressure sensor data were collected when walking with ALEX on the treadmill. Interlink Electronics FSR 406 pressure sensors placed on the sole of the shoes were used to define the gait events of each leg. The area between the target and the actual malleolus path during the swing phase was computed to measure the effects of training on learning of the normalized target path [15]. If subject’s actual malleolus path matches the prescribed malleolus path more closely post-training, the area between the actual and the target paths would be smaller than the area computed at baseline. A smaller area after training would indicate a pattern closer to healthy individuals.
During over-ground walking, lower-body kinematic data sampled at 120 Hz were collected by using an eight-camera motion capture system. For the first three subjects, a VICON motion capture system (Oxford, UK) was used. Kinematic data for the remaining individuals were captured using an eight-camera Qualisys motion capture system (Gothenburg, Sweden) because of a switch in labs. Visual 3D (C-Motion Inc., Rockville, MD) was used to estimate over-ground walking speed, and compute hip, knee and ankle joint angles. Peak flexion angles during swing and total joint excursion during gait cycle from the paretic leg were computed for further analysis. Foot clearance during swing phase was computed as the maximal vertical position of the reflective marker attached on top of the 5th metatarsal.
E. Statistical analysis
Due to the small sample size, we used the non-parametric Friedman’s test to test for differences in the gait parameters and clinical outcome measures among the three evaluation sessions (i.e., baseline, post-training, 6-month follow-up). The significance level was set at p<0.05. If the main effect was significant, we used Wilcoxon signed rank test to compare each pair of the evaluation sessions with adjusted p<0.017. All statistics were performed in SPSS version 20 (IBM Co., Somers, NY).
III. RESULTS
A. Clinical measures
Stroke survivors exhibited significant changes in their self-selected over-ground walking speed (Friedman’s test, p=0.001), DGI (p=0.005), and TUG (p=0.016) following training (Figure. 3). The walking speed improved significantly immediately after the training (Wilcoxon signed rank tests, p=0.008), and the improvements were retained 6-months post- training (Wilcoxon signed rank tests, p=0.008). Changes equal to or greater than the minimum clinically important difference (MCID) in the self-selected speed (i.e., 0.16 m/s) [33] were observed in three out of the nine subjects after the training. In addition, at the 6-month follow-up, five out of the nine subjects achieved clinically meaningful changes greater than the MCID compared to the baseline. MCID is the reference value for the smallest change in an outcome measure that an individual will identify as beneficial [34].
Fig. 3.
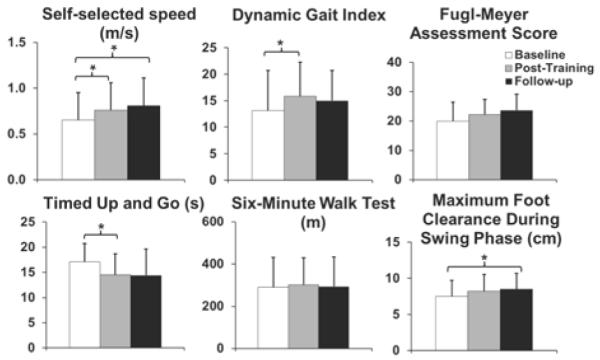
Clinical outcome measures and maximum foot clearance averaged across subjects at baseline, after training (post-training), and 6 months after training (follow-up). Error bars represent the standard deviation across subjects. * p<0.017
DGI and TUG scores of the subjects improved significantly after training (Wilcoxon signed rank test, DGI: p=0.007, TUG: p=0.008) but the changes were not retained 6-months post-training. Five out of nine subjects showed immediate improvements greater than minimal detectable change (i.e., 2.9) [35] in their DGI scores. Minimal detectable change (MDC) is the smallest change that exceeds changes due to measurement error [36]. Only one subject achieved a change greater than the reported MDC in TUG (i.e., 7.84 seconds) [37]. The FMA was not performed for the first two subjects. Three out of the remaining seven subjects showed changes greater than the MDC (i.e., 3.57) [37]. There was a significant main effect in FMA (Friedman’s test, p=0.022); however, post-hoc tests revealed no statistically significant difference in the FMA scores after training or at 6-month follow-up compared to the baseline (Wilcoxon signed rank tests, p>0.017) (Figure. 3). The 6MWT was not performed on the first subject. Furthermore, there were no statistically significant differences in the 6MWT following training (Friedman’s test, p=0.3) (Figure. 3). However, one out of the eight subjects did show changes in the 6MWT greater than the MDC (i.e., 54.1 m) [38].
B. Over-ground kinematic data
Subjects showed significant changes in peak knee flexion during the swing phase (Friedman’s test, p=0.013), total hip joint excursion of the whole gait cycle (p=0.003), and maximum foot clearance (p=0.013) of their affected legs after the training. Peak knee flexion increased significantly (baseline=45.9°±15.4°, post-training=50.6°±14.9°) after training (Wilcoxon signed rank test, p=0.008). This increase was towards the average value of neurologically intact healthy elderly (i.e., 61.9°±6.4°) [39]. However, the increase was not retained at the 6-month follow-up. Only one subject showed a clinically meaningful change in peak knee flexion (i.e., 5.7°) [40]. There were no significant changes for the peak hip flexion or peak ankle dorsiflexion during swing.
Subjects had significantly greater total hip joint excursion (baseline=30.4°±10.6°, post-training=34.4°±10.7°, follow-up=37.4°±12.2°) both after training (Wilcoxon signed rank tests, p=0.011) and at the 6-month follow-up compared to the baseline (Wilcoxon signed rank tests, p=0.008). The total hip joint excursion was greater than normal at baseline and following training. The normal or the average value of neurologically intact young adults is 24°±4° [41]. No significant changes were seen for total knee (Friedman’s test, p=0.05) or ankle joint excursion (p=0.64), among the three evaluation sessions (Figure. 4). Subjects did not show any significant change in maximum foot clearance after the training, but a significantly greater foot clearance was found at the 6-month follow-up (Wilcoxon signed rank test, p=0.011) (Figure. 3).
Fig. 4.
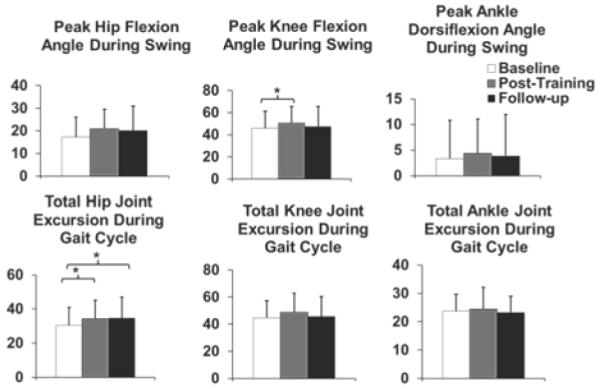
Peak hip and knee flexion angles and peak ankle dorsiflexion angle (degrees) during the swing phase and total hip, knee, and ankle joint excursion (degrees) during the gait cycle averaged across subjects for the paretic leg. Error bars represent the standard deviation across subjects. * p<0.017
C. Ankle lateral malleolus path area
There were no significant differences in the area between subjects’ malleolus path and the target path derived from the healthy subjects recorded previously (Friedman’s test, p>0.05). However, five out of the nine subjects demonstrated a smaller area following training. This indicates that these stroke survivors walked with their malleolus paths closer to the target path even without the visual display. On the contrary, three subjects showed an increase in the area. This increase resulted from an exaggerated high stepping pattern while walking in the robotic exoskeleton without the compliant force field. The single remaining subject did not show changes following training in the area relative to the target path (Figure. 5).
Fig. 5.
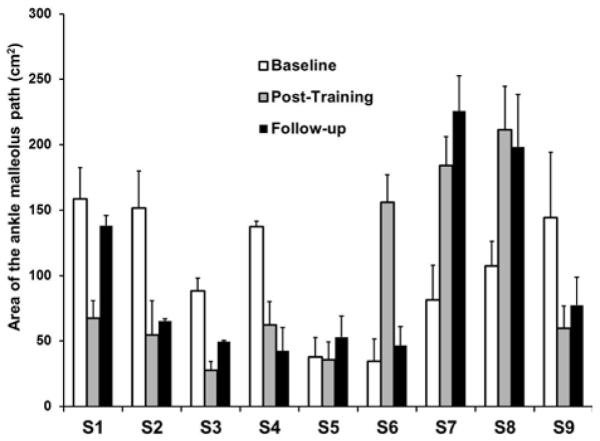
Each subject’s area between the ankle malleolus path at their self-selected walking speed and the target template averaged across swing phase. Error bars represent standard deviation across gait cycles.
IV. DISCUSSION
There were significant improvements in walking speed, some of the over-ground kinematic measures, and some of the clinical measures after training. The improvement in walking speed and total hip joint excursion were retained 6 months after training. However, little change was seen in the ankle path area following training. Therefore, the current findings partially support our hypothesis that assist-as-needed RAGT would improve walking patterns and functional capacity post-stroke.
Current literature lacks information regarding changes in over-ground kinematics of the paretic leg following RAGT. This information is critical because the ultimate goal of rehabilitation is to transfer the training effects to over-ground walking. Our results showed that stroke survivors improved joint kinematics during over-ground walking, such as significant increases in the peak knee flexion angle during the swing phase and the total hip joint excursion over the whole gait cycle. However, there were no significant improvements in the ankle joint angles, suggesting limited training effects of FES of ankle plantarflexors and dorsiflexors. Further investigation is required to determine whether the improvements in the walking pattern post-training were due to the effects of the compliant force field, or FES, or a combination of both. There were significant changes in some of the clinical measures and gait speed after training. The measures that improved significantly post-training such as DGI, TUG, and gait speed also showed changes greater than the MDC for some of the subjects. However, not all the subjects demonstrated an improvement greater than the MDC. Although some of the changes in the clinical measures might result from the measurement error, all subjects demonstrated a trend towards improvement following training. In addition, five out of the nine stroke survivors walked with smaller area following training suggesting a malleolus path that was closer to the target template post-training. Three subjects demonstrated an increase in their malleolus path area post-training. This increase in the area was due to a higher stepping during treadmill walking post-training and suggests greater compensations for foot clearance used by these subjects. Among the three subjects who exhibited high stepping post-training, subject S8 also demonstrated an increase in maximum foot clearance during over-ground walking, whereas before training, S8 tended to drag his foot during swing phase due to spasticity in the plantarflexors of the affected leg. The increase in the foot clearance during the swing phase might help this subject reduce the risks of trips or falls [42-44]. However, the other two subjects did not show a transfer of higher foot clearance from their treadmill to over-ground walking pattern. These results suggest that there is relatively modest training effect from the robot-aided treadmill training that could be transferred to the over-ground walking pattern and functional walking ability.
There have been a limited number of studies investigating the effectiveness of RAGT post-stroke. Previous studies compared the effects of RAGT with BWSTT or conventional physical therapy [13, 22, 45]. However, there is still a lack of strong evidence supporting the effectiveness of any gait training intervention on the functional recovery post-stroke [8, 9, 12, 13, 22]. In these previous studies, the RAGT employed a control algorithm that provided continuous assistance to the subjects regardless of their online performance [22]. Conversely, a compliant force field as tested in the current study allows variability of stepping movements and requires greater active participation from the subjects. The compliant force field may result in larger improvements of functional walking ability than the continuous assistance that forces the limb through a fixed movement path because, in the latter case, participants may rely on the robotic assistance and thus, reduce their effort [19, 46].
Further investigation is needed to determine whether assist-as-needed RAGT is superior to training with continuous assistance or BWSTT. Although the assist-as-needed paradigm theoretically provides advantages over continuous assistance, the changes in gait in the current study e.g., self-selected walking speed (0.65±0.3 to 0.76±0.3 m/s) and the 6MWT (289.4±142.2 to 301.7±128.1 meters) were similar to changes in these parameters (self-selected speed: 0.45±0.19 to 0.52±0.21 m/s; 6MWT: 170±86 to 186±88 meters) in a previous study that employed a Lokomat to provide continuous assistance [22]. In addition, larger improvements have been reported after BWSTT (self-selected walking speed: 0.43±0.22 to 0.56±0.28 m/s; 6MWT: 170±86 meters to 204±96 meters). However, subjects’ baseline walking speeds and endurance in the current study were generally higher than the subjects in Hornby et al. [22], and it is possible that some of our subjects may have shown a ceiling effect. A recent study in our lab with healthy individuals suggests that an error-augmentation training paradigm, where the robotic force field exaggerates the subjects’ error in matching the target can result in better short-term learning effects than the assist-as-needed paradigm [47]. Therefore, it may be possible that stroke survivors with higher functional walking ability may benefit from a more challenging training protocol such as an error augmentation paradigm.
The current study provided intermittent visual feedback of the instantaneous ankle malleolus positions and target template to the subjects as compared to the previous studies where feedback was based on hip or knee joint information [14]. Previous studies have suggested that the central nervous system controls important variables for task success [48], for example center of pressure to maintain standing posture using multiple degrees of freedom of the joints or muscles [49]. In addition, to regain walking ability, it has been shown that people with neurological disorders might benefit more from task-based training that encourages developing new muscle activity pattern for task success instead of a training that imposes reconstruction of normal muscle activity pattern [50]. Thus, it might be more effective to provide feedback on the performance of a critical task-relevant variable (e.g., malleolus position) than feedback on the performance of an individual joint (e.g., hip).
A major limitation of the current experiment is the limited sample size. The conclusions of this study were based upon data from nine stroke survivors. Additionally, in the current study, most of the subjects had mild to moderate impairment based on Perry’s [51] classification. Long-term training of stroke survivors with more diverse levels of motor impairments may better determine which levels of subjects will benefit most from the assist-as-needed RAGT. Additionally, there is an absence of a control group in the current study. Comparisons with BWSTT or the conventional therapy would help to determine whether the assist-as-needed training paradigm is a better training strategy to improve gait post-stroke. Furthermore, it is not possible to determine whether the therapeutic effects shown in the current study were a result of a single component of the training paradigm or a combined effect of all the components. However, the purpose of the current study was to evaluate the potential of a comprehensive, robotic training paradigm that includes RAGT with visual feedback of the malleolus path and FES of the ankle plantarflexors and dorsiflexors as a training alternative for improving functional walking ability in stroke survivors.
V. CONCLUSION
This preliminary study suggests that RAGT including a compliant force field with an assist-as-needed paradigm and visual feedback of malleolus path is a potential alternative for gait rehabilitation in people post-stroke. Subjects demonstrated improvements in their walking pattern on the treadmill. The effects of training were also modestly transferred to over-ground walking with changes in the functional walking ability, sensorimotor function, and walking speed, although the changes were not superior to previously reported results from RAGT using a continuous assistance or BWSTT.
ACKNOWLEDGMENT
The authors would like to thank the research core team in helping with the recruitment, scheduling, and clinical evaluations of the stroke survivors. The authors are also thankful to the functional electrical stimulation team for helping with training sessions.
This work was supported by grant R01HD038582 from the National Institutes of Health.
Biographies

Shraddha Srivastava received the Bachelors and Masters degrees in physical therapy from Guru Gobind Singh Indraprasth University, Delhi, India. She received the Ph.D. degree in Biomechanics and Movement Sciences from University of Delaware, Newark in 2014. Her research interests include gait-training, movement coordination in healthy and neurologically impaired populations, and robotic rehabilitation.

Pei-Chun Kao received the physical therapy degree from the National Taiwan University, Taipei, Taiwan, and the M.S. and Ph.D. degrees from the University of Michigan, Ann Arbor. She received her postdoctoral training from the University of Delaware, Newark. Her research interests are in gait biomechanics & rehabilitation, human neuromechanical control, and robotic exoskeletons.

Seok Hun Kim received the B.S. degree in physical therapy from Daegu University, Daegu, Korea, and the M.S. degree in physical therapy and the Ph.D. degree in rehabilitation science from the University of Kansas Medical Center, Kansas City. He is currently an Assistant Professor in the School of Physical Therapy and Rehabilitation Sciences, University of South Florida, Tampa. His research interests include robot-assisted rehabilitation, neuromuscular control, and motor learning in individuals with neurological disorders.

Paul Stegall (S'10) received the B.S. degree in mechanical engineering from Johns Hopkins University, Baltimore, MD, USA, in 2009. He is currently working towards the Ph.D. degree in mechanical engineering at Columbia University, New York, NY, USA. He is with the Robotics and Rehabilitation Laboratory, Columbia University. His research interests include robotic rehabilitation, human learning, and gait training.

Damiano Zanotto (M’12) received the B.S. degree (with honors), in 2005, the M.S. degree (with honors), in 2007, both in mechanical engineering, and the Ph.D. degree in industrial engineering (curriculum in mechatronics) in 2011, all from the University of Padua, Padua, Italy. Between 2011 and 2013, he was a Postdoctoral Researcher with the Mechanical Systems Laboratory, University of Delaware, Newark, DE, US. Since 2013 he has been working with the ROAR Laboratory (Columbia University, New York, NY, US) as an Associate Research Scientist. His research interests include assistive and rehabilitation robotics, wearable robotics, and cable-driven robotic devices.

Jill. S. Higginson is an Associate Professor in the Departments of Mechanical Engineering and Biomedical Engineering at the University of Delaware. She has also served as the Director of the Center for Biomechanical Engineering Research and Associate Director of Biomedical Engineering at UD. She trained at Cornell University (BS Mechanical Engineering ‘96), Penn State University (MS Bioengineering ‘98), and Stanford University (PhD Mechanical Engineering ‘05). Her research applies experimental and computational techniques to study muscle coordination during walking in healthy and impaired populations. Ongoing projects target abnormal muscle control strategies in stroke and osteoarthritis.

Sunil K. Agrawal (M’92) received the Ph.D. degree in mechanical engineering from Stanford University, CA, USA, in 1990. He is currently a Professor with the Department of Mechanical Engineering, Columbia University, NY, USA. He has authored more than 350 journal and conference papers and two books in the areas of controlled mechanical systems, dynamic optimization, and robotics. He is a Fellow of the American Society of Mechanical Engineers (ASME). He received a Presidential Faculty Fellowship from the White House, a Bessel Prize in Germany, and a Humboldt U.S. Senior Scientist Award. He has been an editorial board member of ASME and the IEEE journals.

John P. Scholz (deceased) received the physical therapy degree from the University of Pennsylvania, Philadelphia, the M.S. degree from the University of North Carolina, Chapel Hill, and the Ph.D. degree from the University of Connecticut, Storrs. He was a Professor of physical therapy at the University of Delaware, Newark. His primary research interests included study of basic processes underlying movement coordination and characterizing movement coordination in patients with motor dysfunction. Dr. Scholz was a founding member of the International Society for Motor Control.
Contributor Information
Shraddha Srivastava, Department of Physical Therapy, University of Delaware, Newark, DE 19713 USA (shraddha@udel.edu)..
Pei-Chun Kao, Department of Physical Therapy, University of Delaware, Newark, DE 19713 USA.
Seok Hun Kim, School of Physical Therapy and Rehabilitation Sciences, University of South Florida, Tampa, FL 33612..
Paul Stegall, Department of Mechanical Engineering, Columbia University, New York, NY 10027 USA..
Damiano Zanotto, Department of Mechanical Engineering, Columbia University, New York, NY 10027 USA..
Jill S. Higginson, Department of Mechanical Engineering, University of Delaware, Newark, DE 19713.
Sunil K. Agrawal, Department of Mechanical Engineering, Columbia University, New York, NY 10027 USA..
John P. Scholz, Department of Physical Therapy and the Program of Biomechanics and Movement Science, University of Delaware, Newark, DE 19713 USA.
REFERENCES
- [1]. Go AS Mozaffarian D Roger VL Benjamin EJ Berry JD Borden WB Bravata DM Dai S Ford ES Fox CS Franco S Fullerton HJ Gillespie C Hailpern SM Heit JA Howard VJ Huffman MD Kissela BM Kittner SJ Lackland DT Lichtman JH Lisabeth LD Magid D Marcus GM Marelli A Matchar DB McGuire DK Mohler ER Moy CS Mussolino ME Nichol G Paynter NP Schreiner PJ Sorlie PD Stein J Turan TN Virani SS Wong ND Woo D Turner MB C. American Heart Association Statistics S. Stroke Statistics Heart disease and stroke statistics--2013 update: a report from the American Heart Association Circulation 2013. 127 1 e6–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Thom T HN Rosamond W et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Circulation 2006. 113 e85–e151 [DOI] [PubMed] [Google Scholar]
- [3]. Woolley SM Characteristics of gait in hemiplegia Top Stroke Rehabil 2001. 7 4 1–18 [DOI] [PubMed] [Google Scholar]
- [4]. Neptune RR Kautz SA Zajac FE Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking J Biomech 2001. 34 11 1387–98 [DOI] [PubMed] [Google Scholar]
- [5]. Olney SJ Richards C Hemiparetic gait following stroke. Part I: Characteristics Gait & Posture 1996. 4 2 136–148 [Google Scholar]
- [6]. Bard G Energy expenditure of hemiplegic subjects during walking Arch Phys Med Rehabil 1963. 44 368–70 [PubMed] [Google Scholar]
- [7]. Muren MA Hutler M Hooper J Functional capacity and health-related quality of life in individuals post stroke Top Stroke Rehabil 2008. 15 1 51–8 [DOI] [PubMed] [Google Scholar]
- [8]. Visintin M Barbeau H Korner-Bitensky N Mayo NE A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation Stroke 1998. 29 6 1122–1128 [DOI] [PubMed] [Google Scholar]
- [9]. Hesse S Bertelt C Jahnke MT Schaffrin A Baake P Malezic M Mauritz KH Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients Stroke 1995. 26 6 976–81 [DOI] [PubMed] [Google Scholar]
- [10]. Barbeau H Visintin M Optimal outcomes obtained with bodyweight support combined with treadmill training in stroke subjects Arch Phys Med Rehabil 2003. 84 10 1458–65 [DOI] [PubMed] [Google Scholar]
- [11]. Galvez JA Budovitch A Harkema SJ Reinkensmeyer DJ Trainer variability during step training after spinal cord injury: Implications for robotic gait-training device design J Rehabil Res Dev 2011. 48 2 147–60 [DOI] [PubMed] [Google Scholar]
- [12]. Duncan PW Sullivan KJ Behrman AL Azen SP Wu SS Nadeau SE Dobkin BH Rose DK Tilson JK Cen S Hayden SK Team LI Body-weight-supported treadmill rehabilitation after stroke N Engl J Med 2011. 364 21 2026–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Hidler J Nichols D Pelliccio M Brady K Campbell DD Kahn JH Hornby TG Multicenter Randomized Clinical Trial Evaluating the Effectiveness of the Lokomat in Subacute Stroke Neurorehabilitation and Neural Repair 2009. 23 1 5–13 [DOI] [PubMed] [Google Scholar]
- [14]. Riener R Lunenburger L Jezernik S Anderschitz M Colombo G Dietz V Patient-cooperative strategies for robot-aided treadmill training: first experimental results IEEE Trans Neural Syst Rehabil Eng 2005. 13 3 380–94 [DOI] [PubMed] [Google Scholar]
- [15]. Banala SK Kim SH Agrawal SK Scholz JP Robot Assisted Gait Training With Active Leg Exoskeleton (ALEX) IEEE Trans Neural Syst Rehabil Eng 2009. 17 1 2–8 [DOI] [PubMed] [Google Scholar]
- [16]. Duschau-Wicke A von Zitzewitz J Caprez A Lunenburger L Riener R Path Control: A Method for Patient-Cooperative Robot-Aided Gait Rehabilitation Transactions on Neural Systems and Rehabilitation Engineering, IEEE 2010. 18 1 38–48 [DOI] [PubMed] [Google Scholar]
- [17].Duschau-Wicke A, Caprez A, Riener R. Patient-cooperative control increases active participation of individuals with SCI during robot-aided gait training. J Neuroeng Rehabil. 2010;7(1):43. doi: 10.1186/1743-0003-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Koenig A, Omlin X, Bergmann J, Zimmerli L, Bolliger M, Muller F, Riener R. Controlling patient participation during robot-assisted gait training. J Neuroeng Rehabil. 2011;8:14. doi: 10.1186/1743-0003-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Krishnan C Kotsapouikis D Dhaher YY Rymer WZ Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor Arch Phys Med Rehabil 2013. 94 6 1202–6 [DOI] [PubMed] [Google Scholar]
- [20]. Hausdorff JM Yogev G Springer S Simon ES Giladi N Walking is more like catching than tapping: gait in the elderly as a complex cognitive task Exp Brain Res 2005. 164 4 541–8 [DOI] [PubMed] [Google Scholar]
- [21]. Cai LL Fong AJ Otoshi CK Liang YQ Burdick JW Roy RR Edgerton VR Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning Journal of Neuroscience 2006. 26 41 10564–10568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Hornby Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: A randomized controlled study (vol 39, pg 1786, 2008) Stroke 2008. 39 8 E143–E143 [DOI] [PubMed] [Google Scholar]
- [23].Winfree KN, Stegall P, Agrawal SK. Design of a minimally constraining, passively supported gait training exoskeleton: ALEX II. IEEE Int Conf Rehabil Robot. 2011;2011:5975499. doi: 10.1109/ICORR.2011.5975499. [DOI] [PubMed] [Google Scholar]
- [24]. Stegall P Winfree KN Agrawal SK Degrees-of-freedom of a robotic exoskeleton and human adaptation to new gait templates IEEE International Conference on Robotics and Automation (ICRA) 2012. 4986–4991 [Google Scholar]
- [25].Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Active robotic training improves locomotor function in a stroke survivor. J Neuroeng Rehabil. 2012;9:57. doi: 10.1186/1743-0003-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cockrell JR, Folstein MF. Mini-mental state examination. Geriatric Psychiatry. 2002:140. [Google Scholar]
- [27]. Kesar TM Perumal R Jancosko A Reisman DS Rudolph KS Higginson JS Binder-Macleod SA Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke Physical Therapy 2010. 90 1 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Binder-Macleod S Kesar T Catchlike property of skeletal muscle: recent findings and clinical implications Muscle & nerve 2005. 31 6 681–693 [DOI] [PubMed] [Google Scholar]
- [29]. Gladstone DJ Danells CJ Black SE The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties Neurorehabilitation and Neural Repair 2002. 16 3 232–240 [DOI] [PubMed] [Google Scholar]
- [30].Shumway-Cook A. Motor Control: Theory and Practical Implications. Williams and Wilkins; Baltimore: 1995. Dynamic Gait Index. [Google Scholar]
- [31].Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919. [PMC free article] [PubMed] [Google Scholar]
- [32].Podsiadlo D, Richardson S. The timed" Up & Go": a test of basic functional mobility for frail elderly persons. Journal of the American geriatrics Society. 1991;39(2):142. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- [33]. Tilson JK Sullivan KJ Cen SY Rose DK Koradia CH Azen SP Duncan PW Locomotor T Experience Applied Post Stroke Investigative Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference Phys Ther 2010. 90 2 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Jaeschke R Singer J Guyatt GH Measurement of health status: ascertaining the minimal clinically important difference Controlled clinical trials 1989. 10 4 407–415 [DOI] [PubMed] [Google Scholar]
- [35]. Romero S Bishop MD Velozo CA Light K Minimum detectable change of the Berg Balance Scale and Dynamic Gait Index in older persons at risk for falling J Geriatr Phys Ther 2011. 34 3 131–7 [DOI] [PubMed] [Google Scholar]
- [36]. Beckerman H Roebroeck M Lankhorst G Becher J Bezemer P Verbeek A Smallest real difference, a link between reproducibility and responsiveness Quality of Life Research 2001. 10 7 571–578 [DOI] [PubMed] [Google Scholar]
- [37]. Hiengkaew V Jitaree K Chaiyawat P Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed "Up & Go" Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone Arch Phys Med Rehabil 2012. 93 7 1201–8 [DOI] [PubMed] [Google Scholar]
- [38]. Fulk GD Echternach JL Nof L O'Sullivan S Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke Physiotherapy theory and practice 2007. 24 3 195–204 [DOI] [PubMed] [Google Scholar]
- [39]. Chen G Patten C Kothari DH Zajac FE Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds Gait Posture 2005. 22 1 51–6 [DOI] [PubMed] [Google Scholar]
- [40]. Kesar TM Binder-Macleod SA Hicks GE Reisman DS Minimal detectable change for gait variables collected during treadmill walking in individuals post-stroke Gait Posture 2011. 33 2 314–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Alton F Baldey L Caplan S Morrissey M A kinematic comparison of overground and treadmill walking Clinical Biomechanics 1998. 13 6 434–440 [DOI] [PubMed] [Google Scholar]
- [42]. Berg WP Alessio HM Mills EM Tong C Circumstances and consequences of falls in independent community-dwelling older adults Age Ageing 1997. 26 4 261–8 [DOI] [PubMed] [Google Scholar]
- [43]. Blake AJ Morgan K Bendall MJ Dallosso H Ebrahim SB Arie TH Fentem PH Bassey EJ Falls by elderly people at home: prevalence and associated factors Age Ageing 1988. 17 6 365–72 [DOI] [PubMed] [Google Scholar]
- [44]. Begg R Best R Dell'Oro L Taylor S Minimum foot clearance during walking: strategies for the minimisation of trip-related falls Gait Posture 2007. 25 2 191–8 [DOI] [PubMed] [Google Scholar]
- [45]. Husemann B Muller F Krewer C Heller S Koenig E Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke - A randomized controlled pilot study Stroke 2007. 38 2 349–354 [DOI] [PubMed] [Google Scholar]
- [46]. Reinkensmeyer DJ Akoner O Ferris DP Gordon KE Slacking by the human motor system: computational models and implications for robotic orthoses Conf Proc IEEE Eng Med Biol Soc 2009. 2009 2129–32 [DOI] [PubMed] [Google Scholar]
- [47]. Kao PC Srivastava S Agrawal SK Scholz JP Effect of robotic performance-based error-augmentation versus error-reduction training on the gait of healthy individuals Gait Posture 2013. 37 1 113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gelfand I, Tsetlin M. On mathematical modeling of the mechanisms of the central nervous system. 1966 [Google Scholar]
- [49]. Krishnamoorthy V Latash ML Scholz JP Zatsiorsky VM Muscle synergies during shifts of the center of pressure by standing persons Exp Brain Res 2003. 152 3 281–292 [DOI] [PubMed] [Google Scholar]
- [50]. Ivanenko Y Poppele R Lacquaniti F Distributed neural networks for controlling human locomotion: lessons from normal and SCI subjects Brain Res Bull 2009. 78 1 13–21 [DOI] [PubMed] [Google Scholar]
- [51]. Perry J The mechanics of walking in hemiplegia Clin Orthop Relat Res 1969. 63 23–31 [PubMed] [Google Scholar]


