Abstract
The discovery of microbial communities in extreme conditions that would seem hostile to life leads to the question of how the molecules making up these microbes can maintain their structure and function. While microbes that live under extremes of temperature have been heavily studied, those that live under extremes of pressure, or “piezophiles”, are now increasingly being studied because of advances in sample collection and high-pressure cells for biochemical and biophysical measurements. Here, adaptations of enzymes in piezophiles against the effects of pressure are discussed in light of recent experimental and computational studies. However, while concepts from studies of enzymes from temperature extremophiles can provide frameworks for understanding adaptations by piezophile enzymes, the effects of temperature and pressure on proteins differ in significant ways. Thus, the state of the knowledge of adaptation in piezophile enzymes is still in its infancy and many more experiments and computational studies on different enzymes from a variety of piezophiles are needed.
Keywords: piezophilicity, pressure adaptation
1. Introduction
The discoveries of “extremophilic” organisms that thrive under extremes of many conditions such as temperature, pressure, salinity, pH, etc. raise many questions on how life can exist under such conditions [1, 2]. Protection against an extreme may involve the chemical composition of the biological macromolecules, the intracellular environment, and repair pathways. Determining adaptations to extreme conditions can lead to a greater fundamental understanding of life as well as guide the search for life in extreme environments such as beneath the continental and oceanic surface or even on other planetary bodies. Practically, determination of these adaptations is also important for developing methods for sterilization and food preservation as well as for biotechnology.
While adaptations to temperature have been heavily studied, studies of adaptations to pressure have lagged even though pressure has influenced the evolution and distribution of life [3–5]. High-pressure environments, including the deep sea, the sub-seafloor, and the continental subsurface, are the largest part of the biosphere [2, 6], with a large fraction of total organism numbers, biomass, and evolutionary history [7, 8]. Piezophiles, which grow best at greater than atmospheric pressure [9], are widespread in phylogenetic distribution, so that piezophilicity appears to have evolved multiple times. However, piezophiles are among the least understood extremophiles, in part because of the difficulty in collecting samples and performing experiments at high pressures. The upper limit of pressure at which life has been found so far is ~1.1 kbar (1 bar ≈ 1 atm ≈ 0.1 MPa) [10, 11]. Since the deepest human SCUBA dive [12] corresponds to a pressure of only ~33 bar and 1.4 kbar is close to the compressive strength of bone [13], life is found at pressures far beyond everyday experience where physical intuition fails.
When a microbe is under high pressure, the effects of pressure are transmitted into the intracellular domain so that proteins inside the cell are affected. Understanding adaptations to protect proteins against pressure is still in its infancy, but appears to involve more than adaptation by repair mechanisms. Thus, how proteins retain their structure and function under pressure is important for understanding piezophilicity. The main focus here is on enzymes, which are essential for growth and reproduction of an organism.
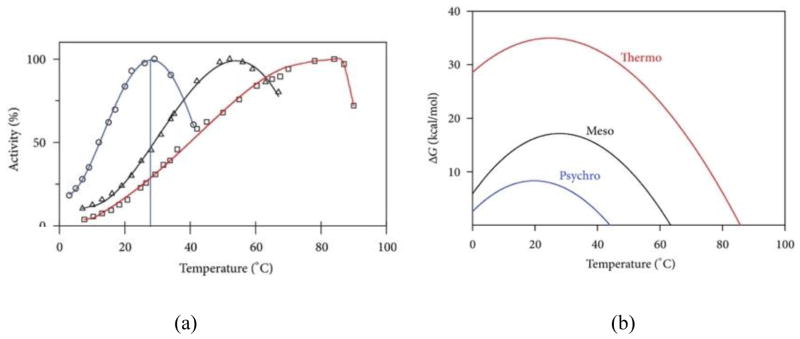
Comparisons of homologous proteins from extremophiles and mesophiles have long been used to identify adaptations for extremes, particularly of temperature. Since proteins need to function at the growth conditions of the parent organisms, the growth conditions can be considered to be “corresponding states” where proteins from extremophiles and mesophiles should have similar behavior [14, 15]. For instance, α-amylase from a psychrophile (preferring low temperatures), a mesophile (preferring moderate temperatures), and a thermophile (preferring high temperatures) [16] has maximum activity that correlate with the growth temperature, Tg, of the parent microbe (Fig. 1a). In addition, the stability of α-amylase to thermal denaturation increases as Tg increases; in other words, the temperature at which the free energy of unfolding, ΔGu, becomes negative increases as Tg increases (Fig. 1b). Of course, it is advantageous for a thermophile enzyme to be stable to thermal denaturation at high temperatures. However, it is apparently disadvantageous for a psychrophile enzyme to be stable at high temperatures presumably because it needs to be less stable to maintain flexibility at low temperatures. Thus, both the stability and flexibility of homologous enzymes are similar at their corresponding states so that good activity is maintained at their growth conditions [14, 15]. The importance of flexibility was also confirmed in studies such as a comparison of flexibilities as measured by hydrogen-deuterium exchange of 3-isopropylmalate dehydrogenase (IPMDH) from a thermophile and a mesophile [17]. In addition, differences in flexibility have been used to explain effects of adaptations on different stages of enzymatic reactions via the Michaelis constant KM, which is an inverse measure of substrate affinity, and turnover rate kcat, which measures the maximum catalytic rate. For instance, while the flexibility of psychrophile enzymes is needed for good catalytic activity at low temperatures, resulting in higher kcat at low temperatures, flexible active sites would tend to bind substrate weakly, resulting in higher KM. Thus, many enzymes have improved kcat at expense of KM, although some enzymes also optimize KM by specific mutations to optimize substrate binding, resulting good catalytic efficiency, or kcat/KM [18].
Figure 1.
Corresponding states and the role of stability and flexibility in enzyme activity: comparison of (a) percentage activity and (b) Gibbs free energy of unfolding (ΔG) as a function of temperature for α-amylase from a psychrophile (blue), a mesophile (black), and a thermophile (red). Figure from ref. [20].
An interesting consequence of the low stability of the psychrophile α-amylase is that it has the highest cold-unfolding temperature (i.e., where ΔGu becomes negative at low temperature) amongst the three α-amylases mentioned above (Fig. 1b). Apparently, this is because sufficient flexibility under cold conditions generally means weaker intramolecular interactions, but weaker interactions also lead to less stable proteins that are not only more readily heat denatured, but also cold denatured. This has led to an activity-stability-flexibility hypothesis in which flexibility is more important than stability for psychrophile enzyme activity, as long as the enzyme is stable enough to maintain its structure at the corresponding state conditions [19]. More generally, these studies suggest that an enzyme from an extremophile does not necessarily have less sensitivity to a negative consequence of that extreme (i.e., unfolding, rigidity, etc.) than a mesophile as long as that consequence does not interfere with sufficient activity at the corresponding state conditions.
These observations about the roles of stability and flexibility in enzyme activity have led to many studies to determine the structural origins of these adaptations. Since there are generally no obvious differences in crystal structures of homologous proteins from extremophiles versus mesophiles, a typical approach to identify the structural adaptations in proteins from extremophiles has been to look for at differences in interactions (i.e., hydrogen bonding, salt-bridges, hydrophobic interactions, etc.), differences in proportions of different amino acid residue types (i.e., nonpolar vs polar vs charged). Trends have been noticed, but can involve many small changes that are not necessarily consistent between proteins. A likely reason is that Nature has had a long time to make small changes to optimize protein function under extreme conditions, and the changes are not necessarily the same for each type of protein. However, an interesting observation is that since proteins from thermophiles relative to those from mesophiles either have generally many more hydrogen bonds if they come from archaea or a few more salt-bridges if they are from bacteria, this may be a reflection of archaea originally evolving in high temperature environments while bacteria originally evolving at lower temperatures but becoming adapted to some high temperature environments [21]. Thus, evolution of an enzyme from a high to low temperature environment may occur by random loss of many small stabilizing interactions to gain flexibility while evolution from a low to a high temperature environment may occur by adding a few larger stabilizing interactions to gain stability.
A caveat in comparing enzymes to determine adaptations of extremophiles is that while stability can be quantified via free energies of unfolding, the nature of the flexibility important for activity has not been clearly defined so that quantitative measures are difficult. For instance, it has been noted that the increase in flexibility may be limited only to a small but crucial part of the protein [19]. On the other hand, various functional states that can involve the entire protein have been shown to exist in equilibrium with the folded and unfolded states of various globular proteins [22]. In addition, many timescales may be involved. For instance, while ligand binding has little effect on backbone dynamics of Escherichia coli dihydrofolate reductase (DHFR) at the picosecond-nanosecond timescale, it has a marked effect at the microsecond-millisecond timescale, which can be detected by NMR relaxation dispersion [23]. More subtly, small atomic fluctuations may provide the “grease” that allows the conformational transitions between states. Thus, molecular dynamics simulations may play a role by quantitating the energy landscapes of different proteins [24, 25] so that they can be compared for underlying differences that affect conformational transitions.
2. Effects of pressure on proteins
To understand the adaptation of proteins to pressure, the effects of pressure on proteins must first be understood. Pressure has been used for a century to probe proteins much in the same way as temperature has been used, although much less readily given the need for high-pressure instrumentation. As noted by Bridgman [26], the major effects of pressure on protein structure are compression and unfolding. The effects of pressure on the structure and function of proteins have recently been reviewed thoroughly [1, 2, 22, 27], so only a few highlights are mentioned here. In this section, all of the proteins described come from mesophiles unless otherwise indicated.
2.1 Compaction
The compaction of proteins under pressure has been demonstrated using various structural methods [28, 29]. For instance, high-pressure NMR solution structures of GB1, a small (56 residue) folding domain of protein G, show that the domain compacts by ~1% between 30 bar and 2 kbar [30] and 2 microsecond molecular dynamics simulations of Clostridium acidurici ferredoxin (55 residue) also show a ~1% compaction between 1 bar and 2 kbar [31]. In addition, crystallographic studies of Shewanella oneidensis IPMDH show internal cavities are compressed monotonically up to 6.5 kbar [32].
Experimental measurements of the compressibility of proteins also show positive compressibility, indicating they are compacted by pressure. These measurements can be useful for new insights into protein dynamics and enzyme function [33]. For instance, partial specific adiabatic compressibility κS determined by precise sound velocity and density measurements for E. coli DHFR range from 6.6 to 9.8 Mbar−1 upon binding of different ligands at 30 °C [34]. Using results from molecular dynamics simulations of DHFR in complex with tetrahydrofolate (THF) at 37 °C [35], a computational estimate of κS is 8 Mbar−1, which in reasonable agreement with the experimental result of 8 Mbar−1 at 30 °C [34]. High-pressure crystallographic studies of monomeric and dimeric proteins have also been used to estimate their partial specific isothermal compressibilities κT, with values between 4 to 6 Mbar−1 [32, 36, 37].
2.2 Protein unfolding and conformational perturbations
Many studies have shown that pressures of above ~4 kbar can induce unfolding of globular proteins [2, 38, 39]. Using pressure as another perturbant in addition to temperature and chemical denaturants has led to new insights into protein folding and stability [40]. Although seemingly contrary to the reduction of volume due to pressure, pressure unfolding is driven by the reduction of volume of the entire system, which appears to be due to changes in interactions between the polypeptide chain and water. Although many specific effects have been proposed, the most compelling evidence is for the loss of internal void volume in the protein upon unfolding [41], or the “destruction of voids”. For instance, combined experimental and computational studies of mutants of staphylococcal nuclease show that larger internal cavities lead to lower unfolding pressures, apparently because larger changes in the system volume occur [42]. In addition, both X-ray [43] and NMR [44] studies of T4 lysozyme demonstrate that the equilibrium populations shift toward proteins with more water inside. Pressure unfolding is limited by the environment of the protein, since protein crystals do not show unfolding even at very high pressures, as pointed out by comparisons of pressure unfolding in solution versus reverse micelles by NMR [44]. Thermostable proteins are generally piezostable, indicating a relationship between heat resistance and pressure resistance [45]. However, two important differences between pressure and thermal or denaturant unfolding have been emerging: the pressure-induced unfolded state appears to be more compact than the thermally unfolded state [46] and pressure unfolding appears to involve extensive hydration in the interior of the protein rather than exposure of the inner hydrophobic core to the bulk solvent as in thermal unfolding [47].
However, care must be taken about interpreting the latter as a dynamic picture of water being pushed inside the protein by increasing pressure since the protein is also being compressed. Instead, a thermodynamic picture of shifting equilibrium of the protein state population towards states with greater numbers of water molecules inside the cavities with increasing pressure appears more consistent. In addition, the 2 microsecond molecular dynamics simulations of ferredoxin show less water penetrating as the pressure is increased up to 10 kbar because the atomic fluctuations that would allow water to penetrate become smaller, decreasing by about 10% between 1 bar and 2 kbar, with a slower decrease between 2 and 10 kbar [31]. These simulations are of a single protein with initially with no water inside, which is the most favored state at 1 bar, while the experiments are of an ensemble of proteins with varying amounts of water inside even at 1 bar.
Besides complete unfolding, pressure has other effects on the folded protein structure. One effect is that pressures as low as 1 to 2 bar can cause the dissociation of oligomers [48]. For instance, a recent study indicates that pressures of ~2 kbar will completely dissociate the dodecameric active complex between Aquifex aeolicus dihydroorotase (DHO) with aspartate transcarbamoylase (ATC) [49]. Water may also play a role in oligomer dissociation by pressure, as indicated by high-pressure crystallographic studies of the dimeric S. oneidensis IPMDH in which a cavity at the interface of the two monomers is empty at atmospheric pressure but has water inside as the pressure is increased to 4.1 kbar and above [32]. Another effect is that pressure also appears to induce conformational changes, generally to more open and thus more solvated structures, between 0.5 to 1.5 kbar [48]. The non-linear pressure dependence of NMR chemical shifts has been useful in identifying these changes [50]. For instance, high-pressure NMR studies show that while the Met20 loop of E. coli DHFR-THF is in the occluded state with respect to the nicotinamide ring-binding pocket at atmospheric pressure, increasing the pressure above 500 bar causes increasing populations of the open state [51, 52]. Also, high-pressure NMR studies of ubiquitin indicates pressure induces a transition from a closed to an open conformation suitable for enzyme recognition [53].
2.3 Pressure effects on the energy landscape
Of the two major effects of pressure on protein structure, compaction should tend to reduce atomic fluctuations since the local potential well for a given atom will become narrower as its neighboring atoms get closer. Studies of the protein energy landscape near the native state of staphylococcal nuclease and hen-egg white lysozyme [24] in pressure-temperature molecular dynamics simulations [54] are consistent because they show decreasing dynamics and increasing force constants of the underlying potential minima with pressure. In addition, a transition to slower increase in the force constants at 4 kbar was attributed to the loss of large amplitude, collective modes and restriction of large-scale solvent translational modes.
However, protein unfolding and conformational transitions by pressure implies that pressure can lead to larger motions, or at least a range of allowed structures. In particular, the “protein volume theorem” stipulates that increasing pressure can shift the equilibrium population from lower energy states to higher energy conformational states with smaller molar volumes [55, 56]. Recent re-examination of the hen-egg white simulations indicates that pressure may also lower barriers in the energy landscape to smaller amplitude collective motions [25], which might favor transitions to other conformational states.
2.4 Pressure effects on enzyme activity
Considerable research has been done on the effects of pressure on enzyme activity [57, 58]. Obviously, pressure will destroy enzyme activity if the protein becomes unfolded or if complexes needed for activity are dissociated. For instance, E. coli DHFR shows large conformational changes between 1.3 to 2.5 kbar in fluorescence studies [59], a pressure range where enzyme activity decreases by more than half [52]. However, at lower pressures, enzyme activity may be modulated. This has allowed pressure to be used as another perturbant in addition to temperature to understand the molecular mechanisms of enzyme-catalyzed reactions. The pressure dependence of enzyme catalytic parameters (kcat, KM, kcat/KM) allows determination of volume changes so that pressure effects can be understood in terms of the volume change upon reaction ΔV or in the activation volume ΔV* [58, 60]. In other words, increased pressure would tend to disfavor the reaction if ΔV is positive and slow the reaction if ΔV* is positive, or vice versa. However, pressure effects on binding specificity can be even harder to predict [45].
Compaction can have affect enzyme activity in several ways. For instance, it can lead to deformation of the active site as indicated by crystallographic studies of yellow fluorescent protein (citrine) at pressures up to 5 kbar in which a shift in the fluorescence spectra between 1 and 2.8 kbar was attributed to the progressive deformation of its chromophore by up to 0.8 Å [61]. More generally, compaction can disfavor the reaction if ΔV is positive and slow the reaction if ΔV* is positive by 15% to 45% [15, 52, 62].
Changes in conformational populations can also affect enzyme activity. For instance, in the recently observed pressure activation of the isolated DHO, which is inactive when not in the complex with ATC at normal pressures, a conformational change induced by the complex formation is presumably mimicked by pressure. In particular, a flexible loop occludes the active site in the isolated protein, but opens upon complex formation or presumably by pressure [49].
3. Adaptation of enzymes to pressure
So far, the most extensive studies of piezophiles have been mainly on microbes from the deep-sea [4, 63–65], which have been found at large ranges of pressures and temperatures. Since piezophilic microbes appear to belong mainly to genera with members found at atmospheric pressures, they have proteins with homologs from mesophiles so that comparisons can be made of proteins with a high degree of sequence identity that are and are not adapted for high pressure. The deepest ocean environment where that life has been found is at bottom of Challenger Deep in the Mariana Trench, where temperatures are 1 to 4 °C and pressures are 1.1 kbar. Microbes have also been found near deep-sea hydrothermal vents, which have pressures near ~0.5 kbar and temperatures near ~100 °C. The optimum and maximum growth pressures and temperatures of microbes varies over an even larger range than from where they were isolated; in particular, their maximum growth pressures and temperatures can range up to 1.2 kbar and from −12 to 121 °C [66]. In addition, more studies on the combined effects of temperature, pressure, and salinity on microbes are warranted, such has been done for four species of Halomonas [67].
Interestingly, microbes from the cold, deep sea appear to be mostly bacteria while those found near hydrothermal vents appear to be mostly archaea [3]. This implies different evolutionary driving forces have been present when considering comparisons to mesophiles; for instance, evolving from high to low pressure environments for archaea versus the reverse for bacteria since life in the primordial world may have been high temperature and high pressure. Thus, bacteria and archaea may have different adaptations to high pressures, as may microbes from cold versus hot high-pressure environments. In addition, a few piezophiles have been shown to preferentially accumulate certain osmolytes in response to pressure, indicating they are “piezolytes” that protect against hydrostatic pressure [68, 69]. This suggests that genetic modification of enzymes may not be the only means of protecting enzymes in high-pressure environments so the effects of different solutes in the solvent environment are also beginning to be studied [70, 71].
Since protein adaptations in archaeal piezophiles have been reviewed recently [72], this review focuses on enzymes with crystal structures from bacterial piezophiles, which are mostly from cold, deep-sea environments and therefore are psychrophilic as well as piezophilic. As with enzymes from most extremophiles, crystal structures of piezophile enzymes show no obvious differences with those of their mesophile homologs [73]. Since pressure unfolding occurs at much higher pressures than piezophiles have been found at so far, the most important adaptation of proteins from piezophiles appeared to be in their flexibility. In fact, an interesting correlation has been made between cold and pressure unfolding of proteins [74]. Thus, the activity-stability-flexibility hypothesis at the corresponding states of growth temperature Tg and growth pressure Pg, which are collectively referred to as GTP, for piezophiles and mesophiles should be examined. A caveat is that adaptations to temperature have been pointed out to be potentially more important than adaptations to pressure for enzymes from microbes found in cold deep-sea environments, since a temperature drop of 20 °C may decelerate a reaction by a factor of four to ten depending on the activation energy while a pressure increase of 1 kbar may decelerate it by only 15% [15]. Newer studies of mesophiles show rates that are decelerated by pressure by 35 to 45% [52, 62] but this is still far from the reduction expected by temperature. However, it is not clear what aspects of flexibility are influenced by pressure and whether it needs to be increased or decreased. Moreover, the contributions of low temperature and high pressure may be difficult to separate so that combined studies of enzymes from psychropiezophiles and from thermopiezophiles would be of great interest.
Key target enzymes for understanding adaptations to extremes should demonstrate sensitivity to the extreme of interest and be found in organisms from a variety of growth conditions. Henceforth, microbes will be referred by a two-letter code after the first mention, with mesophilic microbes in plain text and piezophilic microbes in italics. DHFR is a prime target because it is essential to all organisms in its role in the biosynthesis of purine nucleotides and some amino acids [75]. Over 400 crystal structures of DHFR can be found in the Protein Data Bank (PDB) [76], including studies of E. coli (Ec) DHFR with different ligands bound to understand its mechanism [77] as well as DHFR from a variety of mesophiles and extremophiles. In particular, crystal structures can be found for DHFR from Moritella profunda (Mp) [78], a facultative psychropiezophile with an optimal Pg of 220 bar at 6 °C [79]. EcDHFR is piezosensitive, losing 35% of its atmospheric activity by 1 kbar while MpDHFR retains activity out to 1 kbar [79]. IPMDH is another essential enzyme, which is involved in the biosynthesis of leucine. While there are fewer crystal structures of IPMDH in the PDB, they come from a variety of mesophiles and extremophiles. In particular, structures of IPMDH from the mesophile S. oneidensis (So) [80] and the obligate hyperpiezophile S. benthica (Sb) from the bottom of the Mariana Trench [81] allow comparison of the largest difference in Pg between currently available microbes with relative few differences in sequence. However, S. oneidensis MR1 is not a typical mesophile because it grows over a wide range of temperatures including near 0 °C [82]. SoIPMDH is piezosensitive, with a 50% drop in kcat above 0.5 kbar, while SbIPMDH has less than a 15% drop in kcat even at 2 kbar [62]. In addition, adenylate kinase (AK) catalyzes the interconversion of adenine nucleotides (ATP, ADP, and AMP). While it is well represented in the PDB with over 500 structures, only one appears to be from a piezophile, Photobacterium profundum (Pp). While this microbe is especially interesting because it has a wide Pg range and appears to have a piezolyte [68], there do not seem to be published biochemical characterizations of PpAK or pressure sensitivity studies of a mesophile AK. However, crystal structures of enzymes from other piezophiles are appearing more frequently in the PDB.
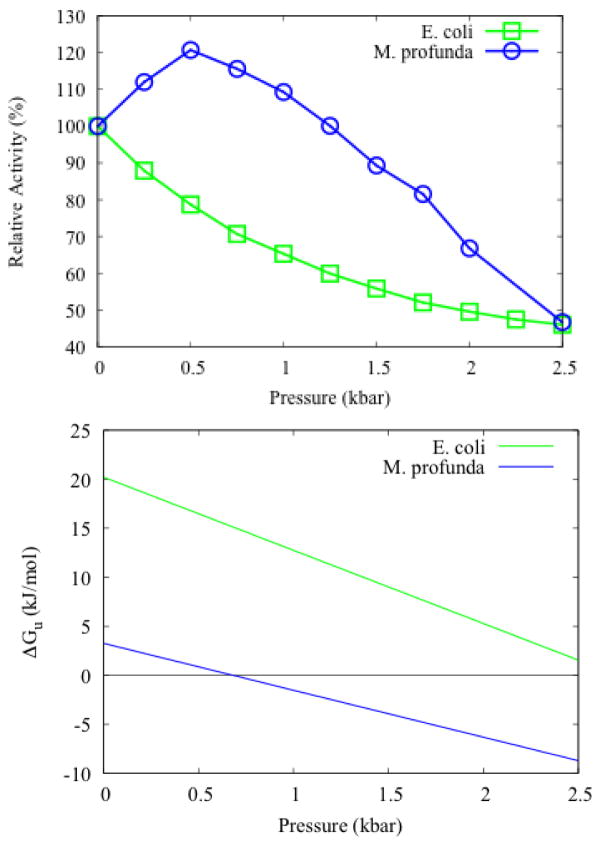
Perhaps the most extensive comparison between a piezophile enzyme with its mesophile homolog so far is between MpDHFR and EcDHFR, which have 55% sequence identity. Interestingly, EcDHFR shows continuously decreasing relative activity (i.e., relative to its atmospheric pressure activity) with pressure, while MpDHFR has relative activity that increases with pressure up to 0.5 kbar followed by a decrease (Fig. 2A). In addition, the unfolding pressure for MpDHFR (0.66 to 0.73 kbar between 15.6 and 28.8 °C) is much lower than for EcDHFR (2.58 to 2.72 kbar between 15.2 and 27.0 °C) [59] (Fig. 2B). This indicates that the piezophile enzyme is more sensitive to pressure unfolding than the mesophile homolog, similar to psychrophile enzymes being more sensitive to cold unfolding than mesophilic homologs. Thus, further studies of piezophile enzymes in light of the activity-stability-flexibility hypothesis [19] are indicated.
Figure 2.
Pressure dependence of (A) the relative enzyme activity and (B) free energy of unfolding of E. coli DHFR (filled circle) and M. profunda DHFR (open circle) at 25 °C and pH 7. Figures redrawn from [59]; more information given in paper.
However, the initial increase in activity with pressure found for MpDHFR, while intriguing, is not found in DHFR from all of the piezophiles examined so far [83]. In addition, when the pressure dependence of the activity of MpDHFR is measured at the Tg of the microbe, there is no initial increase but rather relatively constant activity up to the Pg [78]. Table 1 summarizes results that have been obtained for DHFR from piezophiles, as well as some mesophiles and thermophiles. Thus, other factors appear to be needed to maintain activity under pressure than the simple picture, which is discussed in the following sections.
Table 1.
Experimental data for DHFR, including microbial source, growth conditions, stability, and factors influencing activity
| Microbe* | Tg† (°C) | Pg† (bar) | ΔGu† (kJ/mol) | kcat† (s−1) | Increase† | Res 27† |
Ref‡ |
|---|---|---|---|---|---|---|---|
| S. benthica (Sb) | 10 | 700 | 8.7±1.2 | 68.3±1.2 | Yes | D | [84] |
| S. benthica (Sb′) | 15 | 600 | 4.7±0.9 | 70.2±1.1 | No | D | [84] |
| S. violacea (Sv) | 10 | 300 | 8.0±0.5 | 86.8±2.0 | Yes | D | [84] |
| M. yayanosii (My) | 10 | 800 | NA | 80.3±2.0 | Yes | E | [83] |
| M. japonica (Mj) | 10 | 500 | NA | 109.2±1.1 | No | E | [83] |
| M. profunda (Mp) | 6 | 220 | 13.4 | 18.7±0.8 | Yes | E | [85]/[86] |
| P. profundum (Pp) | 15 | 280 | NA | 150.5±2.3 | No | D | [83] |
| S. frigidimarina (Sf) | 0 | 1 | 8.3±0.9 | 93.3±1.3 | No | D | [84] |
| S. oneidensis (So) | 30 | 1 | 6.7±1.0 | 45.1±1.1 | No | D | [84] |
| S. putrefaciens (Sp) | 37 | 1 | 8.3±1.4 | 98.7±1.7 | No | D | [84] |
| E. coli (Ec) | 37 | 1 | 26.9±3.7 | 19.2±0.5 | No | D | [84] |
| E. coli (Ec) | 37 | 1 | 24.7 | 16.4±3.6 | No | D | [85]/[86] |
| G. stearothermophilus (Gs) | 55 | 1 | NA | 14.6±1.3 | No | D | [85] |
| T. maritima (Tm) dimer | 80 | 1 | 144.4 | 0.10±0.01 | No | D | [85]/[86] |
Sb was isolated at 10,898 m; Sb′ was isolated at 6,356 m; G. is Geobacillus; T. is Thermatoga
Tg and Pg are from Ref. [63] for piezophiles; Res 27 is amino acid residue at position 27; Increase refers to relative activity with pressure; kcat of NADPH at 25 °C; ΔGu using urea at 15°C.
Reference is for kcat at 25 °C and ΔGu at 15 °C unless two given, in which case, first is for kcat and second is for ΔGu
3.1. Low stability
The low stability observed in MpDHFR may be a common theme in deep-sea enzymes, since low stability is associated with high flexibility. The deep-sea DHFR characterized so far are relatively less stable, as indicated by low ΔGu (Table 1). At atmospheric pressures, MpDHFR is more flexible than EcDHFR as measured by hydrogen-deuterium exchange NMR studies, which may be important in generating the reaction ready conformation but apparently does not influence the chemical step [87]. The piezophile SbIPMDH is also less stable than its mesophilic homolog SoIPMDH (T1/2 are 56.6 °C versus 60.7 °C, respectively) [88]. Analogous to psychrophile enzymes and cold unfolding, stability does not appear to be a very strong evolutionary driving force for piezophile enzymes as long as they are stable at the Pg of the parent microbe. However, some DHFR have Pg that are higher than Pu of MpDHFR, which may indicate other stabilizing factors such as strong interactions at key points or piezolytes. In addition, not all mesophile DHFR have higher stability, including SoDHFR, which is from the same organism as SoIPMDH (Table 1). This implies that low stability and by inference high flexibility is not necessarily detrimental to life at normal pressure. Overall, low stability and inferred greater flexibility appear to be favorable for enzymes in both cold and high-pressure environments, but do not disallow mesophilic environments.
3.2 High compressibility
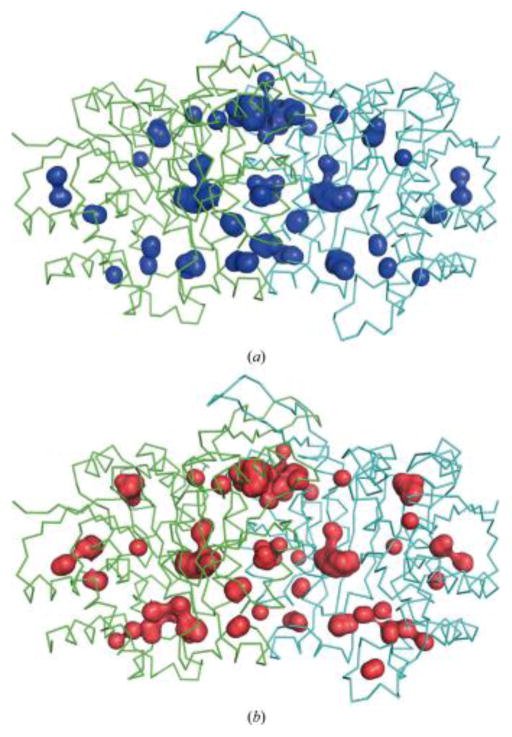
A theme that may be common to all enzymes from piezophiles is high compressibility. While a larger internal cavity volume would seemingly make a protein more susceptible to pressure unfolding, it has been suggested to also make the protein more compressible and less subject to pressure-induced distortion, thus allowing it to remain active at higher pressures [81]. Studies of crystal structures indicate that MpDHFR is more loosely packed and more highly hydrated than Ec DHFR with a larger estimated total internal cavity volume in MpDHFR than in EcDHFR (340 vs. 270 Å) [59]. In addition, crystallographic studies indicated that SbIPMDH [81] has a more open structure than SoIPMDH [80], with a larger estimated total cavity volume in SbIPMDH than in SoIPMDH (655 vs. 592 Å) (Fig. 3). However, since high-pressure unfolding becomes a concern if the protein has low stability, piezophile enzymes may have larger compressibility by having more small cavities rather than a few large cavities.
Figure 3.
The internal cavities of (a) S. oneidensis IPMDH (blue) and (b) S. benthica IPMDH (red) dimers. The wire representation shows the overall structure of the IPMDHs (each subunit is drawn in green and cyan). Figures from [80].
Comparisons of experimental compressibility measurements of piezophile and mesophile homologs apparently have yet to be made. Recent 50 nanosecond molecular dynamics simulations of MpDHFR and EcDHFR bound with THF at 1 bar and 220 bar and different temperatures, which indicate that nanosecond scale atomic fluctuations are slightly larger in MpDHFR-THF at a given temperature and pressure [35]. In addition, intrinsic isothermal compressibilities from quasiharmonic analyses [25] of these simulations show greater compressibility for MpDHFR-THF than EcDHFR-THF (64 vs. 58 Mbar−1) [89]; the intrinsic compressibility only includes cavity contributions and not from changes in solvation.
In addition, questions still remain over how the increased compressibility seen in the molecular dynamics simulations of MpDHFR over EcDHFR is connected with the increased flexibility of the activity-stability-flexibility hypothesis. In particular, while larger atomic fluctuations are seen in MpDHFR than EcDHFR, consistent with the increased compressibility, larger atomic fluctuations are also seen at the higher pressure on the nanoseconds timescale [35]. Since 2 microsecond simulations of ferredoxin show decreasing atomic fluctuations as pressure increases [31], this indicates that pressure may increases collective motions of loops and domains to more solvated states on the nanosecond timescale, while overall decreasing motion on a longer timescale.
3.3 High absolute activity
One mechanism for maintaining catalytic activity in deep-sea enzymes may be high absolute catalytic activity, regardless of temperature or pressure. For instance, the kcat of most deep-sea DHFR tends to be about four to five times higher than that of EcDHFR (Table 1). This implies that even if activity is reduced by high pressure or cold temperature, the activity is apparently sufficient at the microbe to survive at its GTP. This might to due at least in part to greater flexibility, since deep-sea microbes have low ΔGu (Table 1), or to specific modifications to enhance catalytic activity. However, the converse is not necessarily true because the three non-piezophilic Shewanella DHFR also have relatively high kcat. This implies that high catalytic activity is favorable for cold-temperature, high-pressure environments, but it is not necessarily unfavorable for mesophilic environments.
3.4 High relative activity at high pressures
Another mechanism for maintaining catalytic activity for piezophile enzymes may be adaptations that maintain or increase activity at higher pressures. MpDHFR is the only DHFR in Table 1 that apparently uses maintaining relative activity to higher pressures without also having high absolute kcat to maintain activity at its GTP, since the other microbes that show a plateau or increase in relative activity with increasing pressure also have high absolute kcat. A clue about the adaptation is that the D27E mutant of EcDHFR actually shows increasing relative activity out to 2.5 bar [70], with a 50% increase in kcat and slight 2 kJ/mol increase in ΔGu. Asp27 in EcDHFR plays a critical role in the hydride transfer step [90], and the results indicated that the D27E mutant has a slightly opened substrate-binding cleft. Although it is not clear why the D27E mutant had increased activity, interestingly all of the Moritella DHFR in Table 1 have Glu27. Thus, an explanation for the behavior of MpDHFR compared to EcDHFR in Fig. 2 is that the Glu27 in MpDHFR leads to the increase in activity with pressure (Fig. 2A), while the lower stability of MpDHFR leads to the subsequent decrease in activity above 500 bar (Fig. 2B).
SbIPMDH maintains relative activity to higher pressures without also having a high absolute kcat. Both SbIPMDH and SoIPMDH have kcat of ~16 to 19 s−1 for either IPM or NAD at atmospheric pressure but the activity of SoIPMDH decreases monotonically with pressure while SbIPMDH is pressure tolerant out to 10 kbar [88]. A recent study attributes the change in pressure sensitivity to a single amino acid substitution at residue 266, which is Ala in SbIPMDH and Ser in SoIPMDH, since mutations switching amino acids at this residue appear to switch the pressure tolerance [88]. Since three water molecules were observed to penetrate in a cleft around Ser266 in SoIPMDH under high-pressure [32] while no water is observed in the mutant, it was proposed that the water penetration reduced enzyme activity by preventing hinge motion in the wild-type SoIPMDH and SbIPMDH Ala266Ser. However, the motion was allowed in SoIPMDH Ser266Ala and wild-type SbIPMDH.
Although far from generalizable, these two cases appear to be specific sequence determinants that can extend activity to higher pressures.
4. Outlook
While studies of the structural origins of piezophilicity in enzymes are still in their infancy, several ideas are suggested by the studies described here. First, protection against a negative effect of pressure is only needed if that effect occurs at the growth pressure of the organism, as indicated in the activity-stability-flexibility hypothesis. Second, at least some types of adaptations to cold and high pressure can be complimentary and thus difficult to separate. Third, not all types of adaptations that increase tolerance to high pressure are necessarily deleterious at atmospheric pressure. Finally, not all types of adaptations are universal to homologous enzymes from different piezophiles.
In addition, at least three types of adaptations for pressure are indicated so far. First, low stability and high compressibility is a general adaptation that could maintain flexibility at high pressure, although whether nonpiezophilic psychrophile enzymes also have high compressibility as a consequence of maintaining flexibility at cold temperatures has not been investigated. Second, high catalytic activity can lead to better adaptations for both cold and high pressure, but is an adaptation that is not necessarily deleterious at atmospheric pressure. High catalytic activity could potentially be achieved by generally more flexible proteins or by specific sequence differences that enhance specific parts of the mechanism. Finally, changes in size of cavities at critical regions may be important in sequence determinants of piezophilicity for specific proteins. In fact, the need for large total cavity volume associated with high compressibility needs to be balanced by the need that no individual cavities are large enough to cause disruptive water penetration. Thus, both general properties of the protein such as high compressibility, which can protect against the effects of compaction, and specific sequence determinants, which can govern water penetration at key points in a specific protein or otherwise enhance activity, may play roles in adaptation to high pressure.
Overall, further progress towards understanding piezophilicity requires examining more types of proteins from different organisms, especially piezophiles that live under other extremes. An important aspect of this is defining better measures of flexibility.
Acknowledgments
The authors are grateful for support from the National Institutes of Health through Grant No. R01-GM122441 and from the McGowan Trust. This work used computer time on the Extreme Science and Engineering Discovery Environment (XSEDE) granted via MCB990010, which is supported by National Science Foundation Grant No. OCI-1053575; the Medusa cluster, which is maintained by University Information Services at Georgetown University; and the LoBos cluster at the Laboratory of Computational Biology in the National Heart, Lung, & Blood Institute of the National Institutes of Health, which was generously provided by Bernard R. Brooks.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winter R. High pressure effects in molecular bioscience. In: Manaa MR, editor. Chemistry at Extreme Conditions. Elsevier B. V; Amsterdam, The Netherlands: 2005. pp. 29–82. [Google Scholar]
- 2.Meersman F, Daniel I, Bartlett DH, Winter R, Hazael R, McMillan PF. High-pressure biochemistry and biophysics. In: Hazen RM, Jones AP, Baross JA, editors. Carbon in Earth. Mineralogical Society of America; Washington, DC: 2013. pp. 607–648. [Google Scholar]
- 3.Fang JS, Zhang L, Bazylinski DA. Deep-sea piezosphere and piezophiles: geomicrobiology and biogeochemistry. Trends Microbiol. 2010;18:413–422. doi: 10.1016/j.tim.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Prieur D, Jebbar M, Bartlett D, Kato C, Oger P. Piezophilic prokaryotes. In: Sebert P, editor. Comparative High Pressure Biology. Science Publishers; Enfield, New Hampshire: 2009. pp. 281–318. [Google Scholar]
- 5.Yayanos AA. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci USA. 1986;83:9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picard A, Daniel I. Pressure as an environmental parameter for microbial life - A review. Biophys Chem. 2013;183:30–41. doi: 10.1016/j.bpc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Kallmeyer J, Pockalny R, Adhikari RR, Smith DC, D’Hondt S. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc Natl Acad Sci USA. 2012;109:16213–16216. doi: 10.1073/pnas.1203849109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95:6578–6583. doi: 10.1073/pnas.95.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yayanos AA. Deep-sea piezophilic bacteria. Methods Microbiol. 2001;30:615–637. [Google Scholar]
- 10.Kato C. Microbiology of piezophiles in deep-sea environments. In: Anitori RP, editor. Extremophiles: Microbiology and Biotechnology. 2012. pp. 233–263. [Google Scholar]
- 11.Yayanos AA. Microbiology to 10,500 meters in the deep sea. Ann Rev Microbiol. 1995;49:777–805. doi: 10.1146/annurev.mi.49.100195.004021. [DOI] [PubMed] [Google Scholar]
- 12.Guinness World Records. 2017 Available from: http://www.guinnessworldrecords.com.
- 13.Pal S. Design of Arificial Human Joints & Organs. Springer; New York: 2014. p. 419. [Google Scholar]
- 14.Somero GN. Proteins and temperature. Ann Rev Physiol. 1995;57:453–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- 15.Jaenicke R, Závodszky P. Proteins under extreme physical conditions. FEBS Lett. 1990;268:344–349. doi: 10.1016/0014-5793(90)81283-t. [DOI] [PubMed] [Google Scholar]
- 16.D’Amico S, Marx JC, Gerday C, Feller G. Activity-stability relationships in extremophilic enzymes. J Biol Chem. 2003;278:7891–7896. doi: 10.1074/jbc.M212508200. [DOI] [PubMed] [Google Scholar]
- 17.Závodszky P, Kardos J, Svingor Á, Petsko GA. Adjustment of conflexibility is a key event in the thermal adaptation of proteins. Proc Natl Acad Sci USA. 1998;95:7406–7411. doi: 10.1073/pnas.95.13.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feller G, Gerday C. Psychrophilic enzymes: Hot topics in cold adaptation. Nature Rev Microbiol. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 19.Georlette D, Blaise V, Collins T, D’Amico S, Gratia E, Hoyoux A, Marx J-C, Sonan G, Feller G, Gerday C. Some like it cold: biocatalysis at low temperatures. FEMS Microbiol Rev. 2004;28:25–42. doi: 10.1016/j.femsre.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Feller G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica. 2013:512840. doi: 10.1155/2013/512840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berezovsky IN, Shakhnovich EI. Physics and evolution of thermophilic adaptation. Proc Natl Acad Sci USA. 2005;102:12742–12747. doi: 10.1073/pnas.0503890102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen LM, Roche J. High-pressure NMR techniques for the study of protein dynamics, folding and aggregation. J Magn Reson. 2017;277:179–185. doi: 10.1016/j.jmr.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Boehr DD, McElheny D, Dyson HJ, Wright PE. Millisecond timescale fluctuations in dihydrofolate reductase are equisitely sensitive to the bound ligands. Proc Natl Acad Sci USA. 2010;107:1373–1378. doi: 10.1073/pnas.0914163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinhold L, Smith JC, Kitao A, Zewail AH. Picosecond fluctuating protein energy landscape mapped by pressure-temperature molecular dynamics simulation. Proc Natl Acad Sci USA. 2007;104:17261–17265. doi: 10.1073/pnas.0708199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodgers JM, Hemley RJ, Ichiye T. Quasiharmonic analysis of protein energy landscapes from pressure-temperature molecular dynamics simulations. J Chem Phys. 2017;147:125103. doi: 10.1063/1.5003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bridgman PW. The coagulation of albumen by pressure. J Biol Chem. 1914;19:511–512. [Google Scholar]
- 27.Balny C. Review: What lies in the future of high-pressure bioscience? Biochim Biophys Acta. 2006;1764:632–639. doi: 10.1016/j.bbapap.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Gross M, Jaenicke R. Review: Proteins under pressure: The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur J Biochem. 1994;221:617–630. doi: 10.1111/j.1432-1033.1994.tb18774.x. [DOI] [PubMed] [Google Scholar]
- 29.Kharakoz DP. Protein compressibility, dynamics, and pressure. Biophys J. 2000;79:511–525. doi: 10.1016/S0006-3495(00)76313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilton DJ, Tunnicliffe RB, Kamatari YO, Akasaka K, Williamson MP. Pressure-induced changes in the solution structure of the GB1 domain of protein G. Proteins Struc Func Bioinf. 2008;71:1432. doi: 10.1002/prot.21832. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Tran KN, Rodgers JM, Bartlett DH, Hemley RJ, Ichiye T. A molecular perspective on the limits of life: Enzymes under pressure. Cond Matter Phys. 2016;19:1–16. [Google Scholar]; Anthony DJ. Haymet Festschrift. doi: 10.54488/CMP.19.20101. [DOI] [Google Scholar]
- 32.Nagae T, Kawamura T, Chavas LMG, Niwa K, Hasegawa M, Kato C, Watanabe N. High-pressure-induced water penetration into 3-isopropylmalate dehydrogenase. Acta Crysta. 2012;D68:300–309. doi: 10.1107/S0907444912001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gekko K. Review: Compressibility gives new insight into protein dynamics and enzyme function. Biochim Biophys Acta. 2002;1595:382–386. doi: 10.1016/s0167-4838(01)00358-2. [DOI] [PubMed] [Google Scholar]
- 34.Kamiyama T, Gekko K. Effect of ligand binding on the flexibility of dihydrofolate reductase as revealed by compressibility. Biochim Biophys Acta. 2000;1478:257–266. doi: 10.1016/s0167-4838(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Rodgers JM, Hemley RJ, Ichiye T. Extreme biophysics: Enzymes under pressure. J Comput Chem. 2017;38:1174–1182. doi: 10.1002/jcc.24737. [DOI] [PMC free article] [PubMed] [Google Scholar]; Charles L. Brooks Festschrift. doi: 10.1002/jcc.24737. [DOI] [Google Scholar]
- 36.Kundrot CE, Richards FM. J Mol Biol. 1987;193:157–170. doi: 10.1016/0022-2836(87)90634-6. [DOI] [PubMed] [Google Scholar]
- 37.Ascone I, Savino C, Kahn R, Fourme R. Acta Crysta. 2010;D66:654–663. doi: 10.1107/S0907444910012321. [DOI] [PubMed] [Google Scholar]
- 38.Heremans K. High pressure effects on proteins and other biomolecules. Ann Rev Biophys, Bioeng. 1982;11:1–21. doi: 10.1146/annurev.bb.11.060182.000245. [DOI] [PubMed] [Google Scholar]
- 39.Royer CA. Review: Revisiting volume changes in pressure-induced protein unfolding. Biochim Biophys Acta. 2002;1595:201–209. doi: 10.1016/s0167-4838(01)00344-2. [DOI] [PubMed] [Google Scholar]
- 40.Silva JL, Foguel D, Royer CA. New insights into protein folding, dynamics and structure from high pressure studies. Trends Biochem Sci. 2001;26:612–618. doi: 10.1016/s0968-0004(01)01949-1. [DOI] [PubMed] [Google Scholar]
- 41.Frye KJ, Royer CA. Probing the contribution of internal cavities to the volume change of protein unfolding under pressure. Protein Sci. 1998;7:2217–2222. doi: 10.1002/pro.5560071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roche J, Caro JA, Noberto DR, Barthe P, Roumestand C, Schlessman JL, Garcia AE, Garcia-Moreno BE, Royer CA. Cavities determine the pressure unfolding of proteins. Proc Natl Acad Sci USA. 2012;109:6945–6950. doi: 10.1073/pnas.1200915109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins MD, Hummer G, Quillin ML, Matthews BW, Gruner SM. Cooperative water filling of a nonpolar protein cavity observed by high-pressure crystallography and simulation. Proc Natl Acad Sci USA. 2005;46:16668–16671. doi: 10.1073/pnas.0508224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nucci NV, Fuglestad B, Athanasoula EA, Wand JA. Role of cavities and hydration in the pressure unfolding of T4 lysozyme. Proc Natl Acad Sci USA. 2014;111:13846–13851. doi: 10.1073/pnas.1410655111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mombelli E, Shehi E, Fusi P, Tortora P. Exploring hyperthermophilic proteins under pressure: theoretical aspects and experimental findings. Biochim Biophys Acta. 2002;1595:392–396. doi: 10.1016/s0167-4838(01)00361-2. [DOI] [PubMed] [Google Scholar]
- 46.Panick G, Malessa R, Winter R, Rapp G, Frye KJ, Royer CA. Structural characterization of the pressure-denatured state and unfolding/refolding kinetics of staphylococcal nuclease by synchrotron small-angle X0-ray scattering and Fourier-transform infrared spectroscopy. J Mol Biol. 1998;275:389–402. doi: 10.1006/jmbi.1997.1454. [DOI] [PubMed] [Google Scholar]
- 47.Hummer G, Garde S, Garcia AE, Paulaitis ME, Pratt LR. The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proc Natl Acad Sci USA. 1998;95:1552–1555. doi: 10.1073/pnas.95.4.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva JL, Weber G. Pressure stability of proteins. Ann Rev Phys Chem. 1993;44:89–113. doi: 10.1146/annurev.pc.44.100193.000513. [DOI] [PubMed] [Google Scholar]
- 49.Hervé G, Evans HG, Fernado R, Patel C, Hachem F, Evans DR. Activation of latent dihydroorotase from Aquifex aeolicus by pressure. J Biol Chem. 2017;292:629–637. doi: 10.1074/jbc.M116.739862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akasaka K, Li H. Low-lying excited states of proteins revealed from nonlinear pressure shifts in 1H and 15N NMR. Biochem. 2001;40:8665–8671. doi: 10.1021/bi010312u. [DOI] [PubMed] [Google Scholar]
- 51.Kitahara R, Sareth S, Yamada H, Ohmae E, Gekko K, Akasaka K. High pressure NMR reveals active-site hinge motion of folate-bound Escherichia coli dihydrofolate reductase. Biochem. 2000;39:12789–12795. doi: 10.1021/bi0009993. [DOI] [PubMed] [Google Scholar]
- 52.Ohmae E, Tatsuka M, Abe F, Kato C, Tanaka N, Kunugi S, Gekko K. Effects of pressure on enzyme functiion of Escherichia coli dihydrofolate reductase. Biochim Biophys Acta. 2008;1784:1115–1121. doi: 10.1016/j.bbapap.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Kitahara R, Yokoyamaa S, Akasaka K. NMR snapshots of a fluctuating protein structure: Ubiquitin at 30 bar–3 kbar. J Mol Biol. 2005;347:277–285. doi: 10.1016/j.jmb.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 54.Meinhold L, Smith JC. Pressure-dependent transition in protein dynamics at about 4 kbar revealed by molecular dynamics simulations. Phys Rev E. 2005;72:061908. doi: 10.1103/PhysRevE.72.061908. [DOI] [PubMed] [Google Scholar]
- 55.Kitahara R, Akasaka K. Close identity of a pressure-stabilized intermediate with a kinetic intermediate in protein folding. Proc Natl Acad Sci USA. 2003;100:3167–3172. doi: 10.1073/pnas.0630309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Akasaka K. Conformational fluctuations of proteins revealed by variable pressure NMR. Biochim Biophys Acta. 2006;1764:331–345. doi: 10.1016/j.bbapap.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Boonyaratanakornkit BB, Park CB, Clark DS. Review: Pressure effects on intra-and intermolecular interactions within proteins. Biochim Biophys Acta. 2002;1595:235–249. doi: 10.1016/s0167-4838(01)00347-8. [DOI] [PubMed] [Google Scholar]
- 58.Ohmae E, Murakami C, Gekko K, Kato C. Review: Pressure effects on enzyme functions. J Biol Macromolec. 2007;7:23–29. [Google Scholar]
- 59.Ohmae E, Murakami C, Tate S-i, Gekko K, Hata K, Akasaka K, Kato C. Pressure dependence of activity and stability of dihydrofolate reductases of the deep-sea bacterium Moritella profunda and Escherichia coli. Biochim Biophys Acta. 2012;1824:511–512. doi: 10.1016/j.bbapap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Northrop DB. Review: Effects of high pressure on enzymatic activity. Biochim Biophys Acta. 2002;1595:71–79. doi: 10.1016/s0167-4838(01)00335-1. [DOI] [PubMed] [Google Scholar]
- 61.Barstow B, Ando N, Kim CU, Gruner SM. Alteration of citrine structure by hydrostatic pressure explains the accompanying spectral shift. Proc Natl Acad Sci USA. 2008;105:13362–13366. doi: 10.1073/pnas.0802252105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasahara Ryota, Sato T, Tamegai H, Kato C. Piezo-adapted 3-isopropylmalate dehydrogenase of the obligate piezophile Shewanella benthica DB21MT-2 isolated from the 11,000-m depth of the Mariana Trench. Biosci Biotechnol Biochem. 2009;73:2541–2543. doi: 10.1271/bbb.90448. [DOI] [PubMed] [Google Scholar]
- 63.Bartlett DH. Review: Pressure effects on in vivo microbial processes. Biochim Biophys Acta. 2002;1595:367–381. doi: 10.1016/s0167-4838(01)00357-0. [DOI] [PubMed] [Google Scholar]
- 64.Eloe EA, Fadrosh DW, Novotny M, Zeigler Allen L, Kim M, Lombardo MJ, Yee-Greenbaum J, Yooseph S, Allen EE, Lasken R, Williamson SJ, Bartlett DH. Going deeper: metagenome of a hadopelagic microbial community. Plos One. 2011;6:e20388. doi: 10.1371/journal.pone.0020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Li X, Bartlett DH, Xiao X. Current developments in marine microbiology: High-pressure biotechnology and the genetic engineering of piezophiles. Curr Opin Biotechnol. 2015;33:157–164. doi: 10.1016/j.copbio.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Harrison JP, Gheeraet N, Tsigelnitskiy D, Cockell CS. The limits for life under multiple extremes. Trends Microbiol. 2013;21:204–212. doi: 10.1016/j.tim.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Kaye JZ, Baross JA. Synchronous effects of temperature, hydrostatic pressure, and salinity on growth, phospholipid profiles, and protein patterns of four Halomonas species from deep-sea hydrothermal vent and sea surface environments. Appl Environ Microbiol. 2004;70:6220–6229. doi: 10.1128/AEM.70.10.6220-6229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin DD, Bartlett DH, Roberts MF. Solute accumulation in the deep-sea bacterium Photobacterium profundum. Extremophiles. 2002;6:507–509. doi: 10.1007/s00792-002-0288-1. [DOI] [PubMed] [Google Scholar]
- 69.Amrani A, Bergon A, Holota H, Tamburini C, Garel M, Ollivier B, Imbert J, Dolla A, Pradel N. Transcriptomics reveal several gene expression patterns in the piezophile Desulfovibrio hydrothermalis in response to hydrostatic pressure. Plos One. 2014;9:e106831. doi: 10.1371/journal.pone.0106831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohmae E, Miyashita Y, Tate S-i, Gekko K, Kitazawa S, Kitahara R, Kuwajima K. Solvent environments significantly affect the enzymatic function of Escherichia coli dihydrofolate reductase: Comparison of wild-type protein and the active-site mutant D27E. Biochim Biophys Acta. 2013;1834:2782–2794. doi: 10.1016/j.bbapap.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 71.Herberhold H, Royer CA, Winter R. Effects of chaotropic and kosmotropic cosolvents on the pressure-induced unfolding and denaturation of proteins: An FT-IR study on staphylococcal nuclease. Biochem. 2004;43:3336–3345. doi: 10.1021/bi036106z. [DOI] [PubMed] [Google Scholar]
- 72.Reed CJ, Lewis H, Trejo E, Winston V, Evilia C. Review: Protein adaptations in archaeal extremophiles. Archaea. 2013;2013:373275. doi: 10.1155/2013/373275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohmae E, Miyashita Y, Kato C. Thermodynamic and functional characteristics of deep-sea enzymes revealed by pressure effects. Extremophiles. 2013;17:701–709. doi: 10.1007/s00792-013-0556-2. [DOI] [PubMed] [Google Scholar]
- 74.Meersman F, Smeller L, Heremans K. Comparative Fourier transform infrared spectroscopy study of cold-, pressure-, and heat-induced unfolding and aggregation of myoglobin. Biophys J. 2002;82:2635–2644. doi: 10.1016/S0006-3495(02)75605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammes GG, Benkovic SJ, Hammes-Schiffer S. Flexibility, diversity, and cooperativity: pillars of enzyme catalysis. Biochem. 2011;50:10422–10430. doi: 10.1021/bi201486f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sawaya MR, Kraut J. Loop and subdomain movements in the mechanism of Escherichia coli dihydrofolate reductase: Crystallographic evidence. Biochem. 1997;36:586–603. doi: 10.1021/bi962337c. [DOI] [PubMed] [Google Scholar]
- 78.Hay S, Evans RM, Levy C, Loveridge EJ, Wang X, Leys D, Allemann RK, Scrutton NS. Are the catalytic properties of enzymes from piezophilic organisms pressure adapted? ChemBioChem. 2009;10:2348–2353. doi: 10.1002/cbic.200900367. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y, Nogi Y, Kato C, Liang Z, Rüger HJ, De Kegel D, Glansdorff N. Moritella profunda sp. nov. and Moritella abyssi sp. nov., two psychropiezophilic organisms isolated from deep Atlantic sediments. Int J Sys Evol Microbiol. 2003;53:533–538. doi: 10.1099/ijs.0.02228-0. [DOI] [PubMed] [Google Scholar]
- 80.Nagae T, Kato C, Watanabe N. Structural analysis of 3-isopropylmalate dehydrogenase from the obligate piezophile Shewanella benthica DB21MT-2 and the nonpiezophile Shewanella oneidensis MR-1. Acta Crysta. 2012;F68:265–268. doi: 10.1107/S1744309112001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kato C, Li L, Nogi Y, Nakamura Y, Tamaoka J, Horikoshi K. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl Environ Microbiol. 1998;64:1510–1513. doi: 10.1128/aem.64.4.1510-1513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abboud R, Popa R, Souza-Egipsy V, Giometti CS, Tollaksen S, Mosher JJ, Findlay RH, Nealson KH. Low-temperature growth of Shewanella oneidensis MR-1. Appl Environ Microbiol. 2005;71:811–816. doi: 10.1128/AEM.71.2.811-816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murakami C, Ohmae E, Tate S-i, Gekko K, Nakasone K, Kato C. Cloning and characterization of dihydrofolate reductases from deep-sea bacteria. J Biochem-Tokyo. 2010;147:591–599. doi: 10.1093/jb/mvp206. [DOI] [PubMed] [Google Scholar]
- 84.Murakami C, Ohmae E, Tate S-i, Gekko K, Nakasone K, Kato C. Comparative study on dihydrofolate reductases from Shewanella species living in deep-sea and ambient atmospheric-pressure environments. Extremophiles. 2011;15:165–175. doi: 10.1007/s00792-010-0345-0. [DOI] [PubMed] [Google Scholar]
- 85.Evans RM, Behiry EM, Tey L-H, Guo J, Loveridge EJ, Allemann RK. Catalysis by dihydrofolate reductase from the psychropiezophile Moritella profunda. ChemBioChem. 2010;11:2010–2017. doi: 10.1002/cbic.201000341. [DOI] [PubMed] [Google Scholar]
- 86.Xu Y, Feller G, Gerday C, Glansdorff N. Moritella cold-active dihydrofolate reductase: Are there natural limits to optimization of catalytic efficiency at low temperature? J Bacteriol. 2003;185:5519–5526. doi: 10.1128/JB.185.18.5519-5526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loveridge EJ, Tey LH, Behiry EM, Dawson WH, Evans RM, Whittaker SBM, Günther UL, Williams C, Crump MP, Allemann RK. The role of large-scale motions in catalysis by dihydrofolate reductase. J Am Chem Soc. 2011;113:20561–20570. doi: 10.1021/ja208844j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamajima Y, Nagae T, Watanabe N, Ohmae E, Kato-Yamada Y, Kato C. Pressure adaptation of 3-isopropylmalate dehydrogenase from an extemely piezophilic bacterium is attributed to a single amino acid substitution. Extremophiles. 2016;20:177–186. doi: 10.1007/s00792-016-0811-4. [DOI] [PubMed] [Google Scholar]
- 89.Huang Q, Do QA, Rodgers JM, Hemley RJ, Ichiye T. Quasi-harmonic analysis of the energy landscapes of dihydrofolate reductase from piezophiles and mesophiles. doi: 10.1002/jcc.24737. unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schnell J, et al. Structure, Dynamics, and Catalytic Function of Dihydrofolate Reductase. Ann Rev Biophys Biomolecular Struc. 2004;33:119–140. doi: 10.1146/annurev.biophys.33.110502.133613. [DOI] [PubMed] [Google Scholar]