Abstract
This review focuses on one family of the known cAMP receptors, the exchange proteins directly activated by cAMP (EPACs), also known as the cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs). Although EPAC proteins are fairly new additions to the growing list of cAMP effectors, and relatively “young” in the cAMP discovery timeline, the significance of an EPAC presence in different cell systems is extraordinary. The study of EPACs has considerably expanded the diversity and adaptive nature of cAMP signaling associated with numerous physiological and pathophysiological responses. This review comprehensively covers EPAC protein functions at the molecular, cellular, physiological, and pathophysiological levels; and in turn, the applications of employing EPAC-based biosensors as detection tools for dissecting cAMP signaling and the implications for targeting EPAC proteins for therapeutic development are also discussed.
I. INTRODUCTION: FUNDAMENTAL ASPECTS FOR cAMP SIGNAL TRANSDUCTION
A. cAMP, an Ancient and Prototypical Second Messenger
The discovery of cAMP, as the heat-stable factor mediating the intracellular function of hormones epinephrine and glucagon, by Sutherland and colleagues in 1957 led to the “second messenger” theory and ushered in the era of signal transduction research (851, 997). This theory has since revolutionized the understanding of cellular signaling cascades and opened the doors to a plethora of major discoveries centered on elucidating the regulation and physiological roles of cAMP-mediated signaling, including the discoveries of adenylyl cyclases (ACs), guanine nucleotide-binding proteins (G proteins), and G protein-coupled receptors (GPCRs). Over the years, many innovative technologies that exploit the cyclic nucleotide signaling cascade for the study of pathologies and the development of therapeutics have also been established.
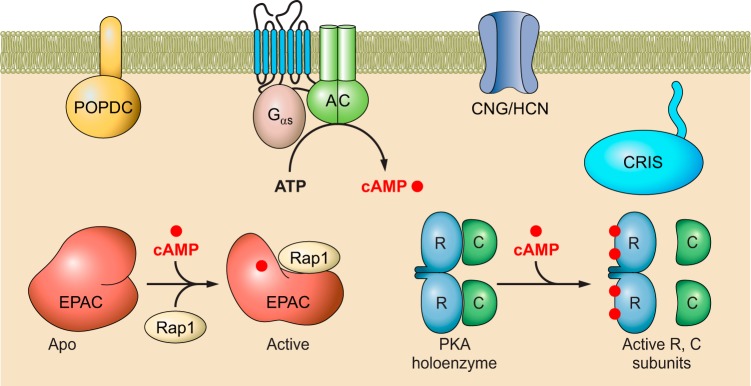
Intracellular cAMP is generated from ATP by the action of ACs in response to the activation of G proteins instigated by the binding of extracellular ligands to GPCRs. The signal transduction process mediated by cAMP second messengers is initiated by binding of the ligand to various cAMP sensors (FIGURE 1). In mammals, at least five families of cAMP effector proteins are known: the classic protein kinase A (PKA) (1020), the cyclic nucleotide regulated ion channels (CNG and HCN) (1219), the exchange proteins directly activated by cAMP (EPAC1 and EPAC2) (229, 510), the Popeye domain containing (POPDC) proteins (913), and the cyclic nucleotide receptor involved in sperm function (CRIS) (556). Despite their diverse functionalities, these cAMP effectors all share a common cyclic nucleotide binding (CNB) domain that is evolutionary conserved with an ancient ancestor: the bacterial cAMP receptor protein (CRP) (496). As a versatile regulatory module, the CNB domain, when coupled to different functional components, can act as a molecular switch for controlling various cellular activities (72).
FIGURE 1.
Introduction of mammalian second messenger cAMP signaling pathways. Generation of cAMP in response to the ligand induced activation of the G protein-coupled receptor (GPCR), G protein and adenylyl cyclase (AC) cascade at the cell membrane. Increase in intracellular levels of cAMP results in the activation of cAMP sensors, including the ubiquitously expressed cAMP-dependent protein kinase/protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC), as well as tissue-specific cyclic nucleotide-regulated ion channels (CNG and HCN), the Popeye domain containing (POPDC) proteins, and the cyclic nucleotide receptor involved in sperm function (CRIS). See text for additional details and abbreviations.
The CNB domain is small in size with roughly 120 amino acid residues that fold into a distinct three-dimensional structure consisting of an eight-stranded β-barrel core and an α-helical subdomain. Extensive structural analyses of CNB domain-containing proteins have led to the proposal of a general allosteric mechanism by which cyclic nucleotides activate their effectors. In this model, the binding of a cyclic nucleotide rearranges the phosphate binding cassette (PBC) within the β-barrel core that anchors the phosphate-sugar moiety of the nucleotide. This interaction relieves steric hindrance from the hinge allowing a COOH-terminal “lid” to move closer to the β-barrel core thus folding on top of the nucleotide base. As a consequence, these allosteric conformational changes activate the effector proteins by repositioning the autoinhibitory regulatory module away from the functional catalytic module (866).
B. Compartmentalization of cAMP Signaling
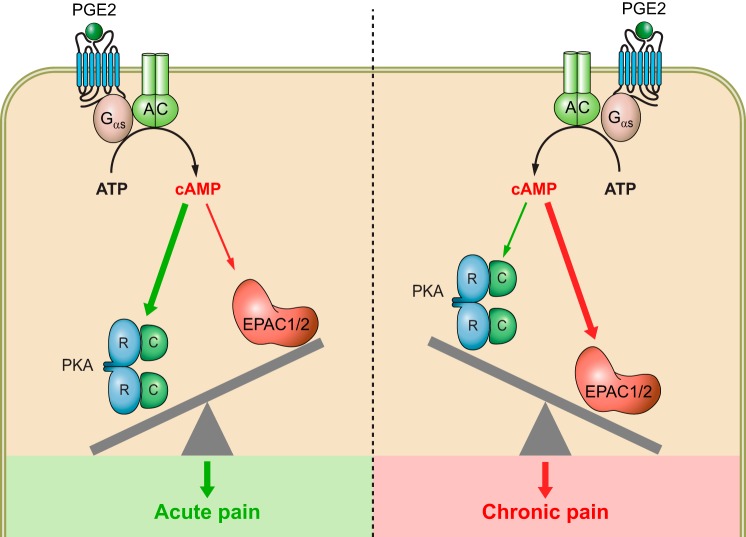
Initially, intracellular cAMP signaling in response to an external stimulus was believed to occur through free diffusion of the cAMP messenger from the site of generation to the intracellular effectors within the cytoplasm. However, as the complexity of the cAMP signaling cascade and associated physiological responses increased, this simple notion was no longer viable to explain how a single ubiquitous signaling molecule could effectively integrate the myriad of extracellular stimuli into such a diverse array of responses while also maintaining specificity and strength in the response. The accepted hypothesis for this question, still held today, was proposed in the early 80s by Brunton and colleagues (115, 127) while investigating cellular responses of prostaglandin E1 (PGE1) and isoproterenol in cardiomyocytes. They suggested the observed range of physiological responses created by a variety of stimuli that all produce cAMP must be implicative of compartmentalization of the cAMP molecule in the cell, as such only a specific pool of PKAs at distinct intracellular compartments are activated (115, 127).
Compartmentalization of cAMP signaling is an attractive notion for multiple reasons. By creating discrete microdomains of cAMP, cells could implement more effective and precise coordination of signaling events at various subcellular localizations. Spatiotemporal regulation of such a signaling system could then be readily modulated in different cellular settings to generate diverse readouts by also positioning various modulatory proteins at proper cellular locales. This realization led to the birth of an even more active field of study that investigates the mechanisms for precise spatial and temporal regulations of cAMP signaling. Studies throughout the past three and a half decades have helped us identify and understand a vast number of functions for these modulatory components. Three major types of proteins are known to assist in the compartmentalization of cAMP signaling in cells and generate the diverse array of physiological responses. These regulatory systems include ACs, cyclic nucleotide phosphodiesterases (PDEs), and A-kinase anchoring proteins (AKAPs).
1. Adenylyl cyclase
The first group of molecules responsible for regulation of the cAMP signal are cAMP manufacturing enzymes, the AC family. The biochemical properties of ACs have been known since the early 1960s when Sutherland et al. (998) described the isolation ACs, and in the 1980s, the activation of ACs was made apparent by Manning and Gilman (659) who recognized ACs as being downstream of G protein signaling, even before G proteins themselves were uncovered. Since these initial discoveries however, the family of mammalian ACs has grown to encompass nine closely related membrane-bound enzymes that convert ATP into cAMP and pyrophosphate upon activation by regulatory proteins including Gαs subunits (237). The 10th member of this family, soluble AC (sAC), is not directly regulated by G protein signaling, but rather by calcium and sodium bicarbonate where these enzymes are important for sperm functionality and neuronal outgrowth (172, 279, 461, 619, 1132, 1136). Mammalian sACs are not randomly distributed within the cell, but targeted to discrete subcellular loci such as centrioles, mitochondria, or nuclei (977, 1216). The general structure of all membrane-bound members of the AC family includes two highly conserved catalytic regions (C1 and C2) that dimerize to form the active catalytic core and two hydrophobic six-transmembrane segments (TM1 and TM2) that firmly anchor the enzyme to the lipid membrane (561, 1025, 1190). The dimerization of the two catalytic subunits is highly dependent on the presence of an activator of AC, such as Gαs, that binds to the two subunits to regulate the formation of the active catalytic core (1025, 1115, 1190).
The regulation, as well as the level of each AC isoform, differs in specific tissues (395). Most cells express multiple AC isoforms. For example, all AC isoforms are expressed in the brain, albeit different regions of the brain are richer in specific isoforms suggesting different regulatory mechanisms available for distinct signaling cascades of these regions. Although the most common regulators of ACs are Gαs and Gαi subunits being stimulatory and inhibitory (151, 163, 236, 996, 1014, 1016, 1025), respectively, other modulators of AC activity include, but are not limited to, Gβγ, calcium and calmodulin, protein kinase C (PKC), and PKA (69, 132, 181, 241, 459, 507, 574, 979, 1009, 1015, 1215). The differential control of the nine transmembrane ACs by various regulators further subdivides the family into four major groups. Group I ACs are calcium stimulated (AC1, 3, and 8), group II are stimulated by Gβγ subunits (AC2, 4, and 7), group III are inhibited by calcium and Gαi (AC5 and 6), and group IV are forskolin resistant (AC9). Thus cells have found effective means to regulate initiation of the cAMP signaling cascade by targeting second messenger generation. Importantly, the integration of extracellular stimuli perceived by the cell must be tightly regulated to promote the proper intracellular response, and the spatially predetermined presence of specific ACs that are differentially regulated by precisely utilized G proteins, intracellular ionic alterations, and kinases underscore the intricate nature of this regulation.
2. Phosphodiesterase
Opposing the action of ACs, another group of molecules that are also responsible for cAMP compartmentalization are PDEs. These enzymes degrade cAMP to 5′-AMP, restoring the basal cAMP state after activation of ACs (440, 870). The activation of PDEs is vital for regulating the strength and duration of the cAMP signal within a cell. The PDE superfamily has been studied quite extensively, resulting in the identification of 11 families (possibly 12), encompassing at least 40 members attributed to splice variants and altered NH2-terminal targeting sequences for various subcellular localizations (441, 641). The presence of this enzymatic family is highly conserved through evolution, further demonstrating the importance of PDEs for proper and efficient cellular signaling through degradation of cAMP and/or cGMP in cells (440). As seen in the AC family, cAMP compartmentalization is driven by differences existing between the kinetics of individual PDE members and regulation by various modulators. Eight of these families are found to hydrolyze cAMP, including PDE4 which has received much attention in the recent years for its diverse tissue distribution and potential therapeutic benefits in diseases of the immune system, where PDE4 is a major regulator (442, 477, 675, 935).
In support of PDEs modulating the cAMP signaling system, Zaccolo and Pozzan (1179) demonstrated quite eloquently that the cAMP signaling in cardiomyocytes was indeed compartmentalized and that free diffusion of the second messenger was limited by the presence of PDEs. This pivotal result depicts that PDEs are not just reset switches to restore basal cAMP, but rather required for the compartmentalization of the signal to specific subcellular regions. Further observations demonstrate that the activation of PDE4 is increased by phosphorylation at Ser54 by PKA, one of the downstream targets of cAMP signaling, thus creating a negative feedback loop in this signaling system (578, 650, 941). These conclusions illustrate the intricate signaling mechanism in place to avoid excessive stimulation of the cAMP second messenger.
3. Scaffolding proteins: A-kinase anchor proteins
Interestingly, even with the specific tissue distribution of ACs and PDEs, and variable regulatory mechanisms for each set of family members, the myriad of biological effect in response to cAMP warranted further investigation to fully explain compartmentalization and specificity achieved by this signaling cascade. For the aforementioned compartmentalization of cAMP by ACs and PDEs to function efficiently, relevant signaling components would need to colocalize to allow regulation that does not rely on random collision of pertinent signaling molecules (484). Intriguingly, investigators discovered a third set of molecules, the structurally diverse family of AKAPs that tether relevant signaling components to specific subcellular organelles or regions and form discrete multi-protein signalosomes for efficient biological responses (292, 309, 372, 497, 537). AKAPs are scaffolding proteins that act to assist in the spatiotemporal arrangement of pertinent components of cAMP signaling, including the regulatory subunit of PKA for where the family name originates (104, 907, 1026). To date, more than 50 AKAP family members have been identified, and this scaffold family coordinates the assembly of more than just PKA signaling molecules; instead, AKAPs are key regulators of cAMP signaling by assembling member-specific sets of cAMP effectors, ACs, PDEs, kinases, phosphatases, G proteins, and ion channels required to effectively optimize cAMP signaling at specific subcellular locales (195, 245–247, 280). The occurrence of these multifunctional signalosomes appears to be cell-type specific depending on the expression of AKAP members (246, 247, 713, 762, 856, 931). Furthermore, the close association of cyclic nucleotide producing and degrading enzymes as well as effector proteins, positions these AKAP scaffolding proteins as key modulators in temporal regulation of cyclic nucleotide signaling.
Taken together, these three protein families, along with the multitude of regulatory systems acting in concert, effectively modulate cAMP levels for intracellular signaling. Overall, this signal is commonly initiated by receptor stimulation leading to activation of cell-specifically expressed ACs to generate cAMP. Additionally, AKAPs assemble localized signalosomes positioning relevant downstream effectors near respective substrate proteins to propagate downstream signaling, while feedback mechanisms activate PDEs to halt signaling by degradation of the second messenger. With each of these mechanisms in place, cells can efficiently create an asymmetrical gradient of cAMP throughout the cytoplasm to induce local signaling events. Importantly, as cAMP is generated, the second messenger acts on downstream effectors such as PKA, EPAC, or ion-gated channels with exquisite spatiotemporal precision promoting the appropriate signal response.
II. DISCOVERY OF EPAC PROTEINS: A NOVEL FAMILY OF cAMP EFFECTORS
A. The Discovery of EPAC Family cAMP Sensors
A little over a decade after the discovery of cAMP, an intracellular receptor for the second messenger was identified as the cAMP-dependent protein kinase or PKA (1081). PKA was the second protein kinase to be discovered after phosphorylase kinase, a substrate of PKA (194). The tetrameric PKA holoenzyme is composed of two separate subunits, two catalytic (C) subunits that phosphorylate substrates and two regulatory (R) subunits that bind cAMP. As the most well-known and studied protein kinase, the PKA C subunit serves as the prototype for understanding structure and function of the eukaryotic protein kinase families (1019), while the R subunit exemplifies a general allosteric regulatory mechanism shared by diverse cyclic nucleotide receptor proteins (496). In the absence of cAMP, the R and C subunits form an inactive holoenzyme complex, in which a pseudo-substrate sequence in the type I R subunit or an actual substrate sequence in the type II R subunit docks into the active site of the C subunit. Binding of cAMP to the cAMP-binding domains of the R subunit induces conformational changes that allosterically dislodge the inhibitory sequence from the active cleft of the C subunit, consequently leading to the activation of PKA (1018). For more than three decades, common knowledge implied that cAMP exerted its action in eukaryotic cells largely through the ubiquitously expressed PKA, with the exception of tissue-specific cyclic nucleotide-regulated ion channels (CNG and HCN) in photoreceptor cells, olfactory sensory neurons, and cardiac sinoatrial node cells (1219). However, over the years, accumulating evidence suggested not all cellular effects of cAMP were mediated by PKA exclusively (23, 254, 622, 868), which led to speculation for the existence of additional cAMP receptors.
However, a breakthrough would not occur until 1998 when two independent research groups identified a novel family of cAMP sensors challenging the long-accepted dogma for cAMP signaling, which was quite unexpected considering the initial discovery of PKA was established back in 1968. One group led by Dr. Ann Graybiel at MIT aimed to identify “a novel protein kinase A-like molecule” involved in the induction of Fos-like protein in striatal neurons responding to dopaminergic stimulation by screening novel brain-enriched genes containing cAMP-binding motifs in the striatum using a differential display protocol (622). Her group found a family of novel cAMP sensors, which they named cAMP-regulated guanine nucleotide exchange factor I and II (cAMP-GEFI and II), corresponding to EPAC1 and 2, respectively (510). These cAMP-GEFs harbor a similar structural arrangement of an NH2-terminal cAMP-binding domain and a COOH-terminal GEF domain for a Ras superfamily small GTPase, Rap1, which appears to be a critical link for the discovery of EPAC proteins by the Bos group at University of Utrecht in the Netherlands. Rap1, initially identified as an antagonist for the transforming activity of Ras (532), is known to be activated by cAMP in various cell types (20, 1078). While PKA phosphorylates Rap1 at position 180 (19, 598), this phosphorylation site is not required for cAMP-dependent activation of Rap1 (20, 229). This discrepancy motivated de Rooij and colleagues (229) to perform a sequence-homology search of an expressed sequence tag database using the cAMP-binding domain sequence of PKA R subunit, revealing EPAC1 as a novel cAMP sensor that mediates the PKA-independent Rap1 activation in response to cAMP. EPAC2 was also incidentally discovered as a cAMP sensor coupled to the sulfonylurea receptor (SUR1) in a yeast two-hybrid screen (796).
B. Origin and Phylogeny of EPAC
The origin of EPAC is evolutionally more contemporary than that of PKA. While PKA is present in unicellular eukaryotes like Saccharomyces cerevisiae, EPAC proteins have been only found in Metazoa within the evolutionary hierarchy. Unlike PKA proteins, which consist of separate R and C subunits encoded by distinct genes, EPAC proteins are single polypeptide molecules. The first EPAC gene was likely the product of a recombination event, which resulted in the fusion of two DNA fragments with coding sequences for a CNB-containing regulatory module and a GEF-containing catalytic module, respectively. The NH2-terminal regulatory half of EPAC is presumably originated from the R subunit of PKA while the COOH-terminal catalytic half of EPAC is most closely related to the Ras superfamily GEF proteins.
Only one EPAC gene, EPAC2, with both CNB domains is found in lower animals, such as Caenorhabditis elegans and Drosophila. On the other hand, vertebrates have two EPAC genes, EPAC1 and EPAC2. Most likely, a gene duplication event during evolution led to the formation of EPAC1, which lacks the NH2-terminal CNB domain (CNB-A). Interestingly, although EPAC2 retains the CNB-A site, its cAMP binding affinity is very low at 87 µM, significantly above the physiological concentrations of cAMP, and much weaker than that of the second CNB site (CNB-B) at 1.2 µM, which is similar to that of EPAC1 (4 µM) (228). Therefore, the CNB-A site in both EPAC1 and EPAC2 has degenerated in its original functionality for cAMP binding.
C. EPAC Genes and Transcripts
To date, the majority of our knowledge on the functional role of EPAC proteins has been derived from studies in mouse and human. The mouse Epac1 gene Rapgef3 is located on chromosome 15 (15qF1) and has three validated transcript variants in the NCBI database (55). Transcript variant 1 encodes the longest Epac1 isoform 1 with 926 amino acids. Transcript variant 2 and 3, using two alternate in-frame exons or lacking an alternate in-frame exon both in the central coding region, produce shorter isoforms 2 (918 amino acids) and 3 (909 amino acids). While no information is available for the regulation and expression of these individual transcript variants, promoter analysis of a 1.6 kb DNA fragment upstream of the 5′ flanking region for mouse Epac1 cDNA reveals two glucose responsive element (GRE) (CACGTG) sites corresponding to nucleotides −1112 to −1106 and −479 to −473, as well as two E-box motifs (CAGCTG) known to be important for glucose responsiveness. Furthermore, hyperglycemia stimulates transcription and translation of Epac1 leading to cellular hypertrophy of the renal tubules by increasing protein kinase B (PKB/Akt) phosphorylation along with P21 and P27 activities (992). The human EPAC1 promoter also contains a hypoxia responsive element (HRE) (ACGTG) site located at −1232 to −1228. Importantly, hypoxia-induce recruitment of HIF-1α to the Epac1 promoter enhances Epac1 expression in mouse primary cortical cells (575).
Mouse Rapgef4, located on chromosome 2 (2qc3), also has three NCBI validated transcript variants 1, 2, and 3, translating into protein with 1011, 993, and 867 amino acids in length, respectively. While the translation product of variant 1 corresponds to the full-length mouse Epac2 isoform Epac2A1, variant 2 of the gene encodes a putative Epac2A2, differing by omitting exon 7 and the associated 18 amino acid sequence sandwiched between the CNB-A and DEP domain (424). The transcript variant 3 produces the Epac2B isoform, which is specifically expressed in the mouse adrenal glands (766). A liver-specific mouse Epac2 isoform, Epac2C, is also identified. Northern blot and sequence analyses suggest Epac2C mRNA is initiated from exon 10 using an alternative promoter in intron 9 of mouse Epac2. Epac2C is composed of 696 amino acids and lacks the first CNB-A (1049). The expression of Epac2A, 2B, and 2C isoforms appears to be regulated epigenetically at the level of promoter methylation in different tissues (424).
In humans, EPAC1 is encoded by the RAPGEF3 gene encompassing 28 exons located on chromosome 12q13.11, while the EPAC2 gene, RAPGEF4, is located on chromosome 2 (2q31.1) and contains 31 exons. EPAC1 has up to 20 potential predicted transcripts or splice variants (55), among which three have been validated. Transcript variant 1 (6,239 bp) encodes EPAC1A with 923 amino acids while transcript variants 2 (5,773 bp) and 3 (6,003 bp) encode the same EPAC1B isoform consisting of a shorter polypeptide chain of 881 amino acids. On the other hand, EPAC2 has five validated transcript variants (EPAC2A-EPAC2E), produced by alternative promoter usage and/or alternative splicing, in the NCBI database. EPAC2A represents the longest transcript and encodes a polypeptide chain of 1011 amino acids while EPAC2B-EPAC2E all lack several exons in the 5′ coding region and encode shorter polypeptide chains (867–791 amino acids) without the CNB-A at the NH2 terminus, compared with EPAC2A. Besides the aforementioned HRE motif in the human EPAC1 promoter, little is known about the transcriptional regulation of human EPAC1 and 2 variants.
D. Tissue Distribution of EPAC Proteins
While isolated EPAC1 and 2 proteins exert similar biochemical activities in vitro, their physiological functions are largely different owing to distinct tissue distributions. Initial reports suggest that the expression of Rapgef3 is relatively ubiquitous in mice, while expression of Rapgef4 is more restricted. Particularly, elevated levels of Epac1 mRNA were detected in kidney, ovary, skeletal muscle, thyroid, and brain, while Epac2 mRNA is primarily associated with the CNS and adrenal gland with limited levels in heart, small intestine, and testis (510). Subsequent studies based on Northern-blot analyses of Epac2 also reveal high levels of full-length transcript in the pituitary gland and a truncated transcript corresponding to Epac2C in liver, as well as low levels of Epac2 expression in lung, kidney, and pancreatic islets (796, 1049). Studies in rodents suggest that the expressions of Epac1 and Epac2 are regulated developmentally. Real-time PCR analysis of Epac1 and Epac2 mRNA in brain, heart, kidney, and lung at embryonic, neonatal, and adult stages demonstrate more drastic developmental changes for Epac2 expression among the two isoforms in mice. Epac1 mRNA expression increases moderately after birth, reaching maximal expression levels at 3 wk of age in all described tissues. Although Epac2 mRNA follows a similar expression pattern in brain, heart, and lung, Epac2 levels decline dramatically after birth in kidney (1050). Analyses of Epac1 and Epac2 protein levels in rat brain, spinal cord, and dorsal root ganglion (DRG) neurons at different stages of development reveal a developmental regulation of Epac in the rat nervous system and indicate high Epac1 expression at embryonic and neonatal stages that decreases in adult cells, whilst Epac2 exhibits an inverted expression pattern to Epac1 (746).
In humans, EPAC1 mRNA is detected in all tissues with high levels in hippocampus, thyroid, kidney, breast and adipose tissues, while EPAC1 protein is detected at medium or low expression levels in all 45 tissue types examined, with the exception of ovarian tissue (http://www.proteinatlas.org/ENSG00000079337-RAPGEF3/tissue). On the other hand, high levels of EPAC2 mRNA are detected in the central nervous system and adrenal glands, whereas high EPAC2 protein levels are also observed. EPAC2 protein expression is also detected in 44 tissues and only missing in oral mucosa (http://www.proteinatlas.org/ENSG00000091428-RAPGEF4/tissue).
E. Structure and Mechanism of Activation
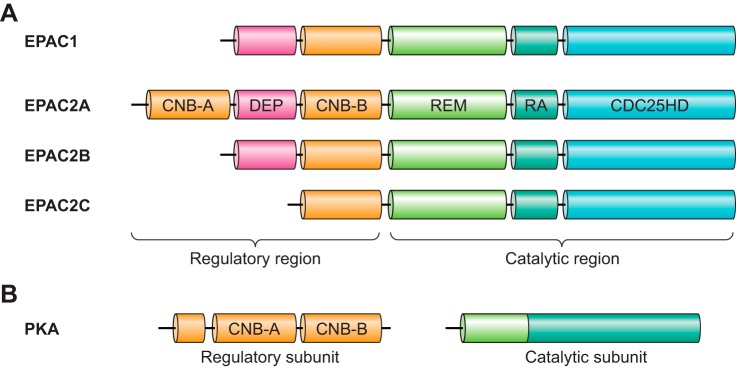
Structural similarities between EPAC1 and EPAC2 are obvious and considerable based on protein sequence analysis. Both proteins contain an NH2-terminal regulatory region and a COOH-terminal catalytic region (510). The catalytic region of EPAC is characterized by a RAS exchange motif (REM), a RAS-association (RA) domain, and successive CDC25 homology domain (also known as the guanine nucleotide exchange factor for Ras-like small GTPases [RasGEF] domain) responsible for nucleotide exchange activity. The regulatory regions of EPAC1 and EPAC2 share a Dishevelled/Egl-10/pleckstrin (DEP) domain and the cAMP-binding domain CNB, whereas an additional CNB domain expressed NH2 terminal to the DEP domain is recognized in full-length EPAC2 (FIGURE 2). Early studies focused on examining communication between the regulatory and catalytic halves considered important for ligand-mediated EPAC activation. Biochemical analyses using isolated recombinant proteins suggest that apo-EPAC exists in an autoinhibitory state, in which the regulatory region sterically blocks the catalytic site. This is confirmed by the observation that an EPAC1 deletion mutant lacking the regulatory region is constitutively active in vitro (228). Moreover, individually isolated NH2-terminal regulatory (EPAC-NR) and COOH-terminal catalytic (EPAC-CC) halves of EPAC are able to form stable inactive complexes sensitive to activation by cAMP similar to the intact EPAC protein (228).
FIGURE 2.
Domain architecture of EPAC isoforms and PKA. Individual domains indicated: CNB, cyclic nucleotide-binding domain; DEP, disheveled, EGL-10 and pleckstrin homology domain; REM, Ras exchange motif; RA, Ras association domain; CDC25HD, CDC25 homology domain. See text for additional details and abbreviations.
Ligand-binding studies with isolated cAMP binding domains from EPAC1 and EPAC2 further reveal that CNB-A of EPAC2 exerts much weaker affinity towards cAMP (87 μM) than that of CNB-B (1.2 μM), which is similar to the only CNB in EPAC1 (4 μM). This extra CNB-A of EPAC2, as well as the DEP domain, is not required for keeping EPAC2 in an autoinhibitory state as isolated EPAC2 CNB-B domain is sufficient to block GEF activity of the EPAC2 catalytic half (228). Remarkably, while an isolated EPAC1 CNB (EPAC1-NR149–328) domain is capable of inhibiting the EPAC1 catalytic half (EPAC1-CC324–881), a construct shorter by a mere 11 COOH-terminal amino acids (EPAC1-NR149–317) is not. This observation led to the identification of a conserved sequence motif 321VLVLE325 required for the autoinhibition of EPAC1. Mutation of the VLVLE motif into AAAAA results in a constitutively active EPAC1 protein in the absence of cAMP (864). The mechanistic importance of this sequence motif is revealed by crystallographic determination of the full-length EPAC2 structure showing the VLVLE motif as a part of a five-strand, β-sheet-like “switchboard” structure, critical for maintaining the proper orientation between the regulatory and catalytic halves and retention of the EPAC autoinhibitory state (862).
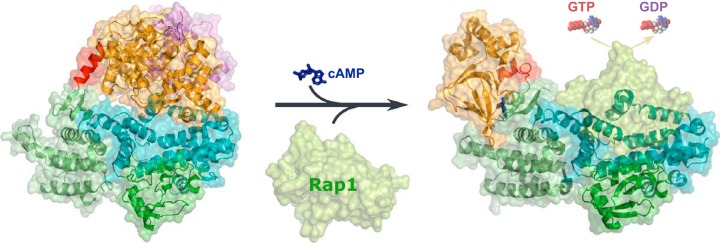
Subsequent determination of the three-dimensional structure complex (EPAC2Δ305:Sp-cAMPS:Rap1B), consisting of an NH2-terminal deletion of the first CNB and DEP domains in EPAC2 bound with its ligands Sp-cAMPS and Rap1B, reveals important conformational changes induced by cAMP and Rap1 binding during EPAC activation (861). Comparison of the inactive (apo-EPAC2) and active (EPAC2Δ305:Sp-cAMPS:Rap1B) structures delineates that cAMP-mediated EPAC activation is centered around a localized hinge motion, during which the hinge helix swings toward the core of the CNB domain bringing the switchboard closer to the cAMP binding pocket to form the lid enclosing the cAMP binding site (861, 862). As a consequence, the last two turns of the hinge helix dissolve to form a loop, which allows the catalytic core to rotate ~90° sideways away from the regulatory lobe and lead to the exposure of the effector binding site and eventual activation of EPAC (FIGURE 3). Unlike PKA, conformational changes associated with binding of cAMP to EPAC mainly involve rigid body movements without major changes in overall secondary structure. This notion is consistent with results based on solution biophysical studies using amide hydrogen-deuterium (H-D) exchange coupled with Fourier transform infrared spectroscopy (1176). Nonetheless, EPAC activation shares a similar mechanism, namely hinge motion, with other cyclic nucleotide binding proteins (866). Indeed, disrupting the hinge helix by mutating the conserved residue Phe435 with Gly leads to a constitutive active EPAC2 (1040).
FIGURE 3.
cAMP induced EPAC activation. EPAC in its ligand-free form remains in an autoinhibitory mode wherein the regulatory region sterically blocks the catalytic site. Binding of cAMP induces a hinge (colored red) motion and causes the regulatory lobe to move away from the catalytic lobe, thereby exposing the GEF domain to allow the binding of Rap GTPases and, consequently, the activation of Rap through the exchange of the G protein-bound GDP for GTP. Structures of the apo-EPAC2 and EPAC2 in complex with cAMP and Rap1 shown are based on PDB files 2BYV and 3CF6, respectively.
While the X-ray structures for inactive and active EPAC provided “before and after” snapshots of the cAMP-induced activation process in atomic detail, important questions remained. For example, cAMP-induced membrane translocation of EPAC1 is essential for efficient cAMP-mediated activation of Rap1 at the plasma membrane and requires the DEP domain (834, 845). However, no active EPAC X-ray structure containing the DEP domain is available. Therefore, how cAMP binding primes the DEP domain for membrane association is still unclear. Moreover, the two CNBs of EPAC2 in their ligand-free state directly face each other in close contact to form a seemingly continuous structural entity and mutually occlude the binding of cAMP (862, 863). How then does cAMP gain access to the ligand binding sites to activate EPAC2? In addition, extensive studies have revealed that in solution EPAC proteins exist as a dynamic ensemble of multiple conformations (864, 1040), which are difficult for conventional X-ray crystallographic techniques to discern, since usually only one of the many possible low-energy conformations that is compatible with the crystal lattice is captured. Thus different approaches are still required to address these challenges.
Solution biophysical analyses, such as enhanced deuterium exchange-mass spectrometry (DXMS), NMR, and small-angle X-ray scattering (SAXS), as well as molecular simulation studies, have provided valuable insights and suggest a critical role for protein dynamics in EPAC activation (107, 607, 1040, 1064, 1117). A detailed study using DXMS, a powerful technique for examining protein dynamics and conformational changes, depicts the region undergoing the largest changes (increases) in solvent accessibility in response to cAMP binding is located at the hinge helix (607), which exactly overlaps with the structural observation of a partial melting of the hinge helix in the EPAC2Δ305:Sp-cAMPS:Rap1B crystal structure (861). Importantly, in addition to the conformational change at the hinge, perturbations of protein dynamics induced by cAMP are also observed at other regions, which are either missing or not detected in the X-ray structure. In particular, cAMP binding leads to a reduction in the dynamics of a helical hairpin directly involved in the interaction with Rap1. This observation suggests that cAMP binding not only relieves steric hindrance imposed by the regulatory lobe to the catalytic lobe, but also may contribute directly to modulate EPAC-Rap1 interaction. Such a notion is consistent with a previous report showing that binding of cAMP and Rap1 is coupled as cAMP binds to EPAC1 more tightly in the presence of Rap1 (554). Furthermore, increases in amide hydrogen exchange in response to cAMP binding are detected within the DEP domain and also at the interface between the DEP and CNB-B domains. These results suggest that cAMP induces conformational changes within the DEP domain and/or a domain rearrangement between the DEP and CNB-B domains (607). These conformational changes may be responsible for the membrane translocation of EPAC1 by allowing the DEP domain to bind phosphatidic acid (PA) in the plasma membrane (198). Another interesting finding from the DXMS analyses demonstrates the amide hydrogens are exchanged rapidly at the interface between CNB-A and CNB-B, indicating this region is highly dynamic and flexible in solution, which may allow cAMP to gain access to the ligand binding pockets that are seemingly inaccessible in the crystal structure.
As discussed above, major advances have been accomplished in recent years expanding our structural and mechanistic understanding of EPAC functions. However, most structural information of EPAC proteins are based on studies with EPAC2. To date, full-length or deletion fragments of EPAC1 are refractory to crystallographic analysis. Our current knowledge of EPAC1 protein structure is largely derived from homology modeling employing EPAC2 structures as templates, as well as NMR analyses of the deletion EPAC1 CNB fragments, which have provided additional insight into the roles of allostery and dynamics during EPAC1 activation (221, 222, 400, 671, 936–938). When the apo- and cAMP-bound EPAC1 CNB fragments were analyzed using NMR spectroscopy, only minor chemical shift changes for residues at the hinge helix were observed upon cAMP binding, indicating no significant changes in local structure. However, comparative analysis of the 15N relaxation rates in apo- and cAMP-bound states revealed that cAMP binding was associated with increased dynamics for the region. These results suggest that cAMP promotes the transition from a “closed” inactive conformation to an “open” active conformation by increasing the entropic penalty of the hinge, and thereby cAMP contributes to weakening of the inhibitory interactions between the regulatory and the catalytic regions (222). Moreover, while binding of cAMP to EPAC1 CNB is coupled with an anti- to syn-conformational transition, cGMP is present in a syn conformation while free in solution and switches to an anti-conformation in the EPAC1-bound state. Surprisingly, the structures of ligand-bound EPAC1 CNB and the ligand binding affinities between cAMP and cGMP are not significantly different. A key difference observed between cAMP and cGMP are the opposing effects on the dynamic profile of the hinge helix. These observations further support a dynamically driven allosteric mechanism underlying the selective response of EPAC to cAMP over cGMP (221).
F. Development of EPAC-Based Optical Sensors
The broad implication of cAMP’s ability in regulating diverse cellular signaling events and physiological functions provoked extensive efforts to develop optical sensors capable of visually imaging cAMP in live cells. Pioneering work by Roger Tsien and colleagues (2) in the early 1990s led to the first successful applications of an optic sensor for monitoring intracellular cAMP in real-time. The first intracellular cAMP sensor developed was a PKA-based indicator in which the R and C subunits were labeled with a pair of fluorescence resonance energy transfer (FRET)-compatible dyes, which were in close proximity in the PKA holoenzyme to allow resonance energy transfer from the donor to the acceptor fluorophores. Binding of cAMP promoted dissociation of the holoenzyme complex and abolishment of FRET signal (2). While this new approach was a major breakthrough and permitted imaging of cAMP dynamics in single living cells for the first time, the procedure is riddled with technical challenges and is not suitable for all cell types with requirements of in vitro labeling, purification, and microinjection of the PKA holo-complex. With the discovery and development of green fluorescent proteins (GFPs) (1045), an improved PKA-based biosensor was developed replacing chemical labeling of fluorescence probes with genetic tagging of GFPs, leading to generation of genetically encoded cAMP sensors that can be applied in a wide variety of cells conveniently via transfection (1178).
1. First-generation EPAC-based FRET cAMP biosensors
EPAC-based FRET biosensors were first reported independently by three different research groups in 2004 (244, 768, 835). These EPAC-based cAMP sensors are comprised of a full-length or the CNB fragment of EPAC, sandwiched between a pair of suitable donor and acceptor GFPs. While these sensors differ slightly in design, they all explore the same conceptual basis: a large cAMP-mediated conformational change associated with EPAC proteins. The development of such single-chain, genetically encoded biosensors have made it possible for real-time monitoring of spatial and temporal aspects of intracellular cAMP gradients.
An indicator for cAMP using Epac1 (ICUE1) was designed by sandwiching the full-length EPAC1 between enhanced yellow fluorescent protein (eYFP) and enhanced cyan fluorescent protein (eCFP). When expressed in HEK293, HeLa, and pheochromocytoma (PC12) cells, ICUE1 showed a typical EPAC1 cellular expression pattern with cytosolic, perinuclear, and mitochondrial distribution. Increase of intracellular cAMP in response to isoproterenol or forskolin treatment led to a reversible decrease in FRET signal. Fusions of ICUE1 to various specific cellular targeting motifs allowed the direct imaging of cAMP production at the plasma membrane, mitochondria, and inside the nucleus. Simultaneous imaging of cAMP dynamics and PKA phosphorylation, using EPAC- and PKA-based sensors, revealed a much delayed PKA response despite a rapid accumulation of cAMP in the nucleus. These results suggest that intracellular cAMP signaling is precisely controlled in a spatiotemporal manner (244). A similar CFP-EPAC1-YFP biosensor was constructed and shown to be functionally active in eliciting robust FRET decreases in response to various cAMP-elevating agents when expressed in mammalian cells. Further optimization of the sensor was conducted by deleting the membrane associating DEP domain (amino acids 1–148) and introducing mutations (T781A, F782A), which disrupt the binding of Rap1, to prevent elevation of basal Rap1 activity with overexpression of the sensor. These modifications led to the generation of an improved cAMP indicator, FP-EPAC1(DEP-CD)-YFP, boasting an extended dynamic range and improved signal-to-noise ratio (835). On the other hand, Nikolaev et al. (768) tested FRET efficiency between eYFP and eCFP fused directly to the NH2 and COOH terminal of the CNB of EPAC1, EPAC2, or PKA RIIβ, respectively. They showed that a single CNB was sufficient to generate detectable FRET signal and a significantly larger signal amplitude and activation speed could be achieved using constructs based on the EPAC1 CNB. These sensors, EPAC1-camps, permitted rapid imaging of β-adrenergic receptor-induced cAMP signals with high speed ~40 µm/s throughout the entire cell body of hippocampal neurons and peritoneal macrophages (768).
2. Applications of EPAC-based cAMP sensors
The high-resolution imaging of cAMP dynamics in single living cells offers researchers the opportunity to not only determine the absolute free cAMP concentrations in living cells with high temporal and spatial resolution (95, 900), but to also address several important unanswered questions involving cAMP signaling, in particular potential cross-talk mechanisms with other signaling pathways, as well as the roles of various GPCRs (3, 85, 1067, 1129), G proteins (553, 672), ACs (1113), and PDEs (789, 1023) in controlling intracellular cAMP dynamics in response to distinct stimuli (383, 408, 698, 1077, 1185). For example, cAMP and Ca2+ signaling pathways are known to be interconnected spatially and temporally in eukaryote cells (202, 357). However, the underlying mechanisms as how these dynamic signals are integrated in single cells remain unresolved. By concurrent imaging of Ca2+ and cAMP dynamics with fura 2 and EPAC1-camps, respectively, a close temporal and causal interrelationship between the enhancement of cytoplasmic Ca2+ and cAMP levels following membrane depolarization were observed in the insulin-secreting MIN6 cells. These results suggest that periodic activation and inactivation of Ca2+-sensitive ACs and PDEs may be responsible for Ca2+-dependent cAMP oscillations in electrically excitable insulin-secreting MIN6 β-cells (397, 579). Using HEK293 cells expressing the EPAC1-camps indicator, Willoughby and Cooper (1121) explored the role of the Ca2+-sensitive AC, AC8, in mediating Ca2+-stimulated periodic cAMP oscillations, and determined AC8 acts as a low-pass filter for high-frequency Ca2+ oscillations. These results demonstrate that Ca2+, through its effects on AC8 and subsequent activation of PDE4, can induce concurrent changes in intracellular cAMP dynamics (1121).
The crosstalk between Ca2+ and cAMP signaling also plays an important role during the regulation of endothelial functions. The endothelial monolayer of blood vessels functions as a semi-permeable barrier between blood and interstitial tissues. The permeability of this barrier is differentially regulated by Ca2+ and cAMP (685). Thrombin, a blood coagulation factor, has been known to enhance endothelial permeability by activation of the Gq-mediated Ca2+ signaling cascade (642), as well as by sustained suppression of cAMP levels (190). Real-time imaging of thrombin-mediated regulation of Ca2+ and cAMP signals using a calcium indicator and cAMP FRET sensor, EPAC1-camps, illustrated that thrombin treatment prompted a transient, Ca2+-dependent but Gαi-independent decrease of cAMP levels in isoproterenol-primed human umbilical vein endothelial cells (HUVEC) cells (1113) or human dermal microvascular endothelial cells (HDMEC) (64). Mechanistic analysis suggests the apparent thrombin-evoked decrease in cAMP is mediated by Ca2+-dependent inhibition of AC6 (1113). Furthermore, EPAC-Rap1 signaling is likely to play a direct role in thrombin-mediated barrier interruption as an EPAC-selective agonist can block barrier-destabilizing effects of thrombin in HDMEC (64). Subsequent studies also show that thrombin induces a delayed increase in cAMP in HUVECs via a Ca2+-dependent activation of cytosolic phospholipase A2 (PLA2) and synthesis of prostacyclin, which stimulates Gαs-coupled prostacyclin receptors (1112).
Assessing the hypothesis that cAMP production mediated by different receptors and AC isoforms is not uniformly distributed between lipid raft and non-lipid raft domains of the plasma membrane, a freely diffusible EPAC2-camps sensor and the membrane tethered versions, EPAC2-MyrPalm and EPAC2-CAAX, which target the sensor to the lipid raft or non-raft membrane domains, respectively, were applied to monitor global and local cAMP production near the plasma membrane. Isoproterenol-induced activation of the β-AR, which is enriched in lipid raft domains, produced similar FRET signal changes for EPAC2-MyrPalm and EPAC2-camps, while the response reported by EPAC2-CAAX was significantly smaller. Surprisingly, PGE1-mediated activation of E-type PG receptors (EPRs), which are believed to be absent from lipid raft domains, resulted in similar responses in all three probes as those induced by β-AR activation. Disruption of lipid rafts by cholesterol depletion decreased cAMP responses associated with β-AR stimulation in all three subcellular locations but had no effects on the responses to EPR activation. Moreover, basal cAMP levels appear significantly higher in non-raft domains. Consistent with this notion, pharmacological inhibition of AC activity reduced basal cAMP level detected by EPAC2-CAAX, but not EPAC2-MyrPalm and EPAC2-camps. On the other hand, FRET signals reported by EPAC2-CAAX were also more sensitive to direct stimulation of AC activity, but less sensitive to pharmacological PDE inhibition. Taken together, these results confirm regulated compartmentalization of cAMP signaling associated with lipid raft, and non-lipid raft, membrane domains, thus suggesting contributions of both AC and PDE activities in intracellular cAMP dynamics (4).
The EPAC-based cAMP sensors may have potentially exciting applications in clinical diagnosis. For example, activating autoantibodies against β1-adrenergic receptors (anti-β1-Abs) are implicated in a pathophysiological role during heart failure. The contemporary assays for anti-β1-Abs detection are complex and highly divergent. Bridging this gap, Nikolaev et al. (767) devised a robust assay for selective identification of anti-β1-Abs by measuring β1-adrenergic receptor-mediated intracellular cAMP production in HEK293 cells stably expressing human β1-adrenergic receptor and the EPAC-based cAMP sensor (EPAC1-camps). Analysis of anti-β1-Abs prevalence in a cohort of previously antibody-typed patients with ischemic cardiomyopathy (ICM) or dilated cardiomyopathy (DCM) revealed IgG from 22 patients (5 ICM/17 DCM) previously tested positive with anti-β1-Abs targeting the second extracellular β1-receptor loop (anti-β1-ECII) induced significant cAMP productions (~50% of maximal isoproterenol-induced signal, “high-activator” IgG); furthermore, IgG from 50 control patients and 32 anti-β1-ECII-negative patients (17 ICM/15 DCM) had no effect on cAMP generation. Unexpectedly, IgG from 23 DCM patients, previously considered anti-β1-Abs negative, were now positively identified to induce lower but noticeable cAMP signals (~30% of maximal isoproterenol-induced signal, “low-activator” IgG). The effect of “high-activator” IgG could be blocked specifically with synthetic peptides corresponding to the second extracellular β1-receptor loop (β1-ECII), but not by peptides corresponding to the first extracellular β1-receptor loop (β1-ECI) and β-blockers. Contrarily, FRET signals generated by “low-activator” IgG are inhibited by β1-ECI peptides and β-blockers, but not by β1-ECII peptides. These results suggest the EPAC cAMP sensor-based assay is highly sensitive and capable of distinguishing high or low activator anti-β1-Abs that recognize different epitopes within fluctuating active β1-receptor conformations (767).
In addition to imaging cAMP dynamics in live cell culture, EPAC-based FRET sensors can be adapted to measure intracellular distribution of cAMP in tissues via viral gene transfer or transgenic techniques. Neuronal expression of the FRET cAMP sensor EPAC1-camps in organotypic brain stem slices using the strictly neuron-restricted synapsin 1 gene promoter and adeno-associated viruses (AAV)-driven gene delivery system allows real-time examinations of the crosstalk between Ca2+ and cAMP signaling in living tissue (706). Transgenic mice ubiquitously expressing the EPAC1-camps cAMP sensor under the control of a hybrid CMV enhancer/chicken β-actin (CAG) promoter (CAG-EPAC1-camps) were generated and utilized to study thyroid-stimulating hormone (TSH)-mediated cAMP signaling. Rapid internalization of TSH receptors was ascertained after stimulation where these receptors continually activate Gαs and AC to sustain cAMP production intracellularly in living thyroid follicles isolated from CAG-EPAC1-camps mice. Functionally, internalized TSH receptors produce distinct downstream cellular responses in comparison to signal transductions triggered by cell surface receptors. These results revealed a new paradigm for GPCR signaling where spatiotemporal regulation of cAMP production by TSH receptors occurs both at the cell surface and intracellular sites (131).
Following these studies and using the same transgenic mice, cAMP imaging in living pituitary slices and primary pituitary cells was conducted to evaluate the contribution of Gαi-coupled somatostatin receptors (SSTRs) in the regulation of cAMP levels under physiological conditions (460). In somatotropic cells, somatostatin and dopamine receptors are the predominant Gαi-coupled receptors, but whose actions on cAMP signaling have been challenging for real-time live cell imaging studies. The transgenetically expressed EPAC1-camps sensor adequately exposed decreases in basal cAMP levels from ~0.4 μM to <0.1 μM in response to somatostatin-14 stimulation in pituitary cells. Furthermore, by crossing the CAG-EPAC1-camps mice with SSTR2−/− mice, the authors successfully established a major role of SSTR2 in mediating the effects of octreotide, a clinically used somatostatin analog. This study demonstrates that transgenic EPAC sensor mice can be crossbred with other genetic murine models for dissecting functions of various GPCRs under physiological conditions (460).
More recently, a transgenic mouse model expressing a targeted cAMP sensor by fusing EPAC1-camps with phospholamban (PLN, PLB), a small integral membrane protein regulator of the sarcoplasmic/endoplasmic reticulum (SR) calcium ATPase 2a (SERCA2a) in cardiac muscle and skeletal muscle cells, was generated as a relevant model to directly monitor subcellular cAMP dynamics in the context of cardiac disease. Live-imaging analyses of adult ventricular cardiomyocytes isolated from healthy and hypertrophic hearts expressing the EPAC1-PLN sensor led to the discovery that SERCA2a-associated and cytosolic PDE4 effects are both reduced while local PDE2-dependent effects are increased, leading to disrupted receptor-microdomain communication in hypertrophic cardiomyocytes (973). Similarly, transgenic fly models expressing the EPAC1-camps senor have also been generated and applied to monitor intracellular cAMP dynamics in neurons of isolated living fly brains responding to stimulation of neuropeptide pigment-dispersing factor (PDF), a critical hormone for maintaining normal circadian rhythmicity in flies. These studies not only experimentally demonstrate that PDF directly modulates most neurons in the Drosophila circadian clock network (943), but also reveal a novel role for PDF in ellipsoid body control of locomotor activity, suggesting a potential link between the central clock and the locomotor circuit (830).
3. New generations EPAC-based FRET cAMP biosensors
Directly comparing to PKA-based cAMP indicators, EPAC-based cAMP sensors offer several major advantages including being genetically encoded as single-chain proteins capable of being conveniently introduced into cells, tissues, and whole organisms where superior spatial and temporal resolution is provided for real-time imaging in live cells. However, the CFP/YFP FRET pair commonly incorporated in the first-generation of EPAC-based cAMP sensors is susceptible to photobleaching, as well as pH and ionic strength variations (468, 715). These properties limit the cellular applications of these sensors considering that it is often a common necessity to observe physiological responses within a considerable time window and potentially significant changes in pH or ion concentrations associated with many cellular activities (51, 257). Therefore, development of cAMP sensors with increased photostability and reduced ion sensitivity are crucial in obtaining FRET signals which reliably mirror the actual cellular cAMP levels and do not artificially report unrelated secondary effects.
FRET signals are commonly recorded by donor quenching and/or acceptor sensitization using either ratiometric detection or fluorescent lifetime imaging (FLIM) techniques. Optimal FRET imaging requires specific matching properties for the donor and acceptor fluorophores and different FRET readout methods entail corresponding design requirements (464). To further improve the EPAC-based FRET cAMP biosensors, a panel of constructs consisting of different donor and acceptor fluorescent proteins were systematically tested. For radiometric and sensitized emission FRET detection, replacing YFP by Venus, a variant with significantly lower pKa and pH sensitivity (751), and varying the orientation of Venus to CFP by substituting the YFP acceptor with a circularly permuted (cp) mutants (43, 752, 1034), such as cp173Venus, resulted in a significantly improved sensor, CFP-EPAC-cp173Venus. This FRET sensor exhibits reduced photosensitivity and enhanced FRET efficiency. In an independent study to optimize the cAMP sensor, pairing a nondimerizing GFP mutant (GFPnd) (1180) with a monomeric red fluorescent protein mCherry (946) led to the identification of a new GFPnd-EPAC-mCherry for optimal detection by fluorescent lifetime imaging (1058, 1200). Further development of this sensor combining the GFP/mCherry FRET pair with a cAMP-affinity enhancement mutation (K405E) in the cAMP binding domain of EPAC2 effectively increased cAMP affinity threefold (217, 775). Similarly, a GFP-EPAC2-camps-mCherry sensor with greater photostability, lower noise level, and higher sensitivity to sparse concentrations of cAMP was also developed (434).
To develop ion-insensitive cAMP sensors, the eCFP/eYFP FRET pair of the established EPAC1-based biosensor was replaced by Cerulean/Citrine fluorophores, which are engineered eCFP/eYFP variants with improved fluorescent properties. Citrine improves stability by exhibiting less sensitivity to pH changes, indifference to halide interference, and increased photostability compared with eYFP (369), while Cerulean boasts a significantly improved quantum yield, higher extinction coefficient, and more desirable fluorescence lifetime profile that is best fit by a single exponential (875). When compared with the conventional eCFP/eYFP EPAC sensor side-by-side, the new Cerulean/Citrine-based sensor demonstrates a twofold enhanced dynamic range, is less sensitivity to fluctuations in ion concentration, and provides more reliable live cAMP concentration measurements in metabolically active cells under various physiological conditions (899). Similarly, an improved cAMP sensor based on the full-length EPAC2 sandwiched between Cerulean and Venus was developed by Zhang and colleagues (412).
While the second generations of EPAC sensors overcome many disadvantages associated with the first generation, these tools are still often optimized only for either ratiometric or FLIM FRET detection. A “third generation” cAMP sensor, mTurquoiseΔ-EPAC(CD, ΔDEP)-cp173Venus-Venus, has been constructed by replacing the eCFP donor with mTurquoise, a high quantum yield and single-exponentially decaying CFP variant (352) and using a double acceptor consisting of a tandem cp173Venus and Venus fluorophores. This new sensor has an increased FRET span optimal for both FLIM and ratiometric detections, demonstrating ~35% increased change in lifetime as detected by frequency-domain FLIM measurements and ~22% improved ratio change (535, 536). Building on the sensors based on mTurquoise, which is currently the brightest and most photobleaching-resistant GFP donor, a fourth generation of cAMP sensors were designed by incorporating a new tandem repeat of two cp173Venus acceptor fluorophores, as well as a single point EPAC mutation, Q270E, which increases the affinity for cAMP by ~2.5-fold. The combination of modifications has produced improved cAMP sensors with outstanding photostability, dynamic range, and signal-to-noise ratios that outperform all previously reported EPAC sensors. The improvements are remarkable with maximal FRET ratio changes around 100% while also performing quite well in FLIM experiments. Furthermore, replacement of the fluorescent acceptors with non-emitting (dark) dedicated variants provides additional improvements for dedicated single-wavelength FLIM acquisition that displays near-doubling of fluorescence lifetime under saturating cAMP levels (534).
4. EPAC-based BRET cAMP biosensors
An EPAC-based bioluminescence resonance energy transfer (BRET) sensor for cAMP was developed using CFP-EPAC1-YFP (835) as a template by replacing the donor ECFP used in the FRET sensor with a Renilla luciferase (476). An additional optimization was introduced by replacing the eYFP (citrine) with a circularly permuted version (citrine-cp229) to maximize the BRET ratio upon binding cAMP by twofold. This BRET sensor, CAMYEL (cAMP sensor using YFP-EPAC-RLuc), overcomes many disadvantages associated with the requirement for photo-excitation of the donor molecule when using FRET probes; therefore, CAMYEL provides more quantifiable measurements with improved dynamic ranges and signal-to-noise ratios (100). Using this novel BRET sensor, the authors characterized cAMP production in response to dual ligand stimulation in mouse macrophage-like RAW 264.7 cells. Specifically, the sensor determined that although sphingosine 1-phosphate (S1P) produced a minimal effect on cAMP production alone, intracellular cAMP induced by isoproterenol or PGE2 was dramatically enhanced by S1P costimulation. The enhancement of cAMP by S1P is mediated by the S1P2 receptor and heterotrimeric G13 protein, acting independent of Ca2+ and exhibiting resistance to pertussis toxin (476). A similar EPAC BRET cAMP sensor based on ICUEs (244, 1072) was also developed and used to characterize the activity of putative ligands of the human trace amine-associated receptor 1 (TAAR1), a poorly expressing GPCR that has been challenging to investigate in the past (58, 278).
With improved signal-to-noise ratios and dynamic range, BRET-based cAMP sensors are suitable for low to medium throughput screening for modulators of various components involved in the cAMP signaling pathway, including GPCRs and EPAC proteins. Insel and colleagues (111, 112) found that the EPAC-based BRET sensor, CAMYEL, is capable of distinguishing between EPAC agonists, partial agonists, and super agonists, as well as identifying competitive and noncompetitive EPAC inhibitors. Through a combination of a CAMYEL-based screening assay and computational molecular modeling, a thiobarbituric acid derivative was identified that allosterically inhibits EPAC1 activity through binding the hinge region of EPAC1 (111, 112).
III. EPAC SIGNALOSOMES
Although EPAC1 and EPAC2 act on the same immediate downstream effectors, the Ras superfamily small GTPases Rap1 and Rap2, their physiological and cellular functions are mostly nonredundant due to distinct tissue and cellular distributions, as well as their abilities to form discrete signalosomes through interaction with specific cellular partners. In this section, the subcellular localizations and binding partners of EPAC1 and EPAC2 will be discussed.
A. EPAC1 Cellular Distribution and Signalosomes
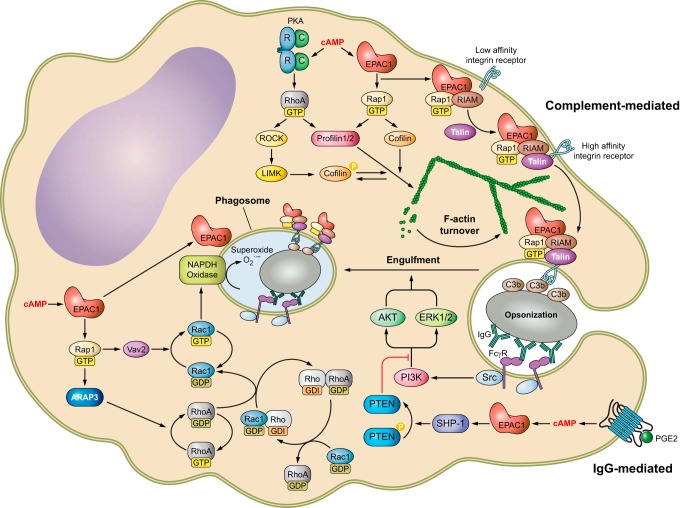
EPAC1 has been observed at various subcellular locations and its cellular distribution appears to be cell-cycle dependent. EPAC1 is mainly found in the cytosol around the nuclear membrane (NM) and in mitochondria during the interphase, but EPAC1 is translocated to the mitotic spindle, centrosome, and the contractile ring during mitosis in COS7 cells (845). The dynamic cellular distribution of EPAC1 indicates that its cellular localization is highly regulated and has important functional implications. Not surprisingly, cAMP, in addition to directly activating EPAC1, is crucial in controlling EPAC1’s subcellular targeting (108, 834, 845). For example, in primary rat alveolar macrophage (AM), EPAC1 is detected on punctate and tubular membranes throughout the cell body, predominantly at the perinuclear region with low levels present on the plasma membrane. In addition, EPAC1 is also colocalized with microtubule organizing centers (MTOCs). Following treatment with PGE2, EPAC1 redistributes from MTOC to nuclear envelope and accumulates on late phagosomes (108). This cAMP-regulated subcellular EPAC1 targeting may play a role in the apparent cell-cycle-dependent distribution of EPAC1 as intracellular cAMP concentration not only fluctuates in response to external stimuli, but also oscillates at different stages of the cell cycle (124, 368).
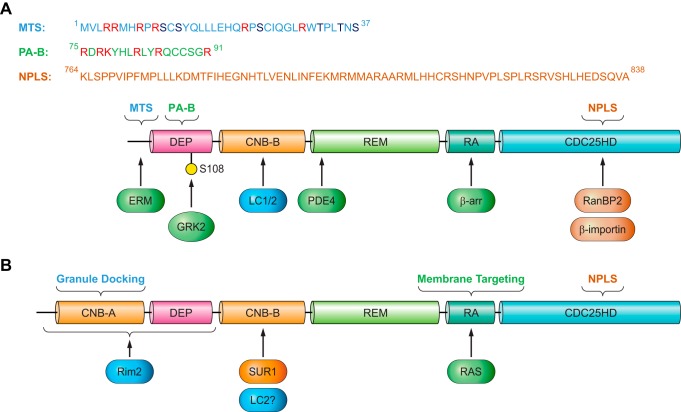
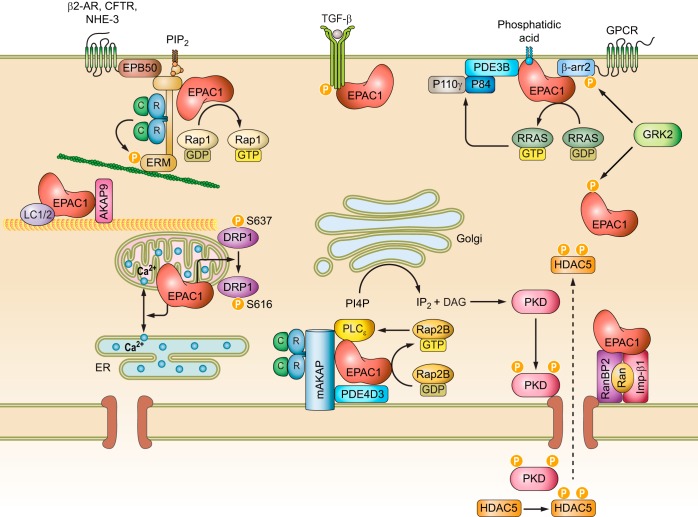
In general, it appears that high intracellular cAMP concentrations enhance membrane association of EPAC1 (834, 845, 962). Although involvement of the DEP domain in EPAC1’s membrane association and functions was recognized quite early on (228, 687, 845), the mechanism of cAMP-induced, DEP domain dependent EPAC1 plasma membrane (PM) translocation was not elucidated until several years later. In addition to directly activating EPAC GEF activity, binding of cAMP also induces a conformational change in the DEP domain (607), which results in the exposure of a 17-amino acid polybasic PA binding motif within the DEP domain (FIGURE 4A) and enable EPAC1 binding to PA on the PM (198). In contrast, low intracellular cAMP levels favor distribution of EPAC1 towards the microtubule (MT) cytoskeleton (108, 845). This is likely attributable to EPAC1’s ability to directly interact with MT and to promote tubulin polymerization independent of Rap1 (686, 930). This dynamic cellular distribution of EPAC1 allows signal induction in distinct intracellular compartments via interaction with subcellular-specific binding partners to form functional signalosomes (FIGURE 5) as described below.
FIGURE 4.
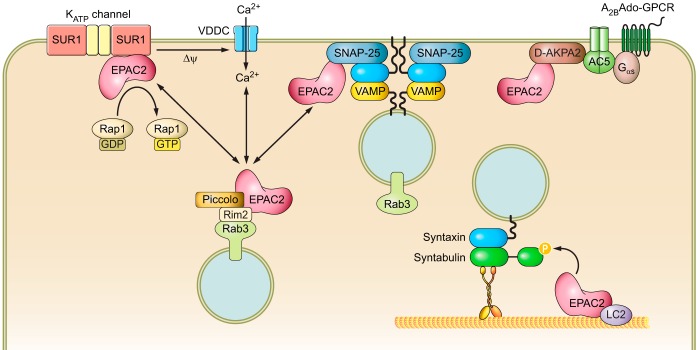
Cellular targeting sequences and binding partners of EPAC1 (A) and EPAC2 (B). The mitochondrial targeting sequence (MTS), phosphatidic acid binding motif (PA-B), and nuclear pore localization sequence (NPLS) of EPAC1 are shown. In addition, protein binding partners of EPAC1 and EPAC2 and their interacting domains are depicted by arrows. See text for additional details and abbreviations.
FIGURE 5.
Intracellular signalosomes of EPAC1. See text for additional details and abbreviations.
1. EPAC1 signalosomes at the plasma membrane
While the PA-binding ability of the DEP domain is important for EPAC1’s general interaction with the PM, other interactions are necessary for a more clustered localization of EPAC1 at the PM permitting more precise and compartmentalized signaling. One additional mechanism for membrane targeting involves the ezrin-radixin-moesin (ERM) family of scaffolding proteins that connect the PM and actin cytoskeleton by virtue of their NH2-terminal FERM (4.1 protein, ezrin, radixin, moesin) lipid binding domain, middle helical domain and COOH-terminal actin binding domain (ABD). ERMs exist in a dormant, autoinhibitory “closed” conformation with both the F-actin and membrane binding sites concealed while in the cytosol. PIP2 binding to FERM and threonine phosphorylation of the ABD induces the open conformation, releasing the autoinhibition and subsequently activating ERM proteins. Activated ERMs then translocate from the cytoplasm to the PM and can serve as PM anchors for other proteins (676). Through a yeast two-hybrid screen and coimmunoprecipitation experiments, the 49-amino acid NH2 terminal of EPAC1 was shown to directly bind with activated ERM (FIGURE 4A). Unlike the interaction between PM and EPAC1 via its DEP domain, EPAC1-ERM binding does not require cAMP (i.e., an active EPAC1 conformation). Functionally, GPCR-induced ERM activation leads to the recruitment and clustering of EPAC1 at the PM, where efficient cell adhesion to the extracellular matrix is promoted (349). Subsequent studies further reveal that knockdown of ezrin, but not radixin or moesin, inhibits cell spreading induced by EPAC activation in A549 cells and HUVECs. Surprisingly, the effect of ezrin on cell spreading is independent from the EPAC1 anchoring function, but downstream of Rap1 (886). These studies reveal the complexity of EPAC1 and ERM signalosomes and the nonredundant functions among ezrin, radixin, and moesin: where all three proteins are important for the EPAC-induced cell adhesion, but only ezrin is required for EPAC-induced cell spreading.
In an independent study, Altschuler and colleagues (421) performed a yeast two-hybrid screen using the first 200 residues of EPAC1 as the bait with the intent of classifying potential DEP domain binding partners, which led to the identification of radixin, an ERM family member, as an additional EPAC1 cellular partner. Unexpectedly, the DEP domain of EPAC1 is not required for radixin interaction, whereas an ERM binding motif spanning residues 1–52 in EPAC1 is responsible for direct binding of EPAC1 to the NH2-terminal FERM domain of radixin (421). In addition to interacting with EPAC1, ERM proteins are bona fide AKAPs, capable of binding both type I and II PKA with their helical domain (252, 892). Indeed, colocalization and functional analyses further define radixin acting as an integrated scaffold for EPAC1 and PKA through FERM and helical domains (FIGURE 5), respectively, and generating functional signalosomes at specific cellular loci to mediate TSH-induced cell proliferation in thyroid cells (421).
The ability of EPAC1 to interact with ERMs provides potential mechanisms for precise targeting of EPAC1 with upstream effectors at the PM, in accordance with ERMs being known to associate with a multitude of membrane receptors, channels, and adaptors (676), including the β2-adrenergic receptor (β2-AR). This ERM/β2-AR association is attributed to the ERM-binding phosphoprotein-50 (EBP50), also known as the Na+/H+ exchanger regulatory factor isoform 1 (NHERF1) (FIGURE 5) (143). EPAC1 also associates with both β1-AR and β2-AR when they are coexpressed in HEK293 cells (76). β-ARs are spatially and temporally regulated by various modulators and scaffolding proteins, such as β-arrestin (β-arr) and PDE. Through direct interaction with β-arr2, EPAC1 participates in the coordination of β1-AR- and β2-AR-mediated cardiac hypertrophic signaling (FIGURE 4A). Specifically, β1-AR activation recruits EPAC1:β-arr2 to the PM, where EPAC1 activates H-Ras via Rap2B-PLC signaling and induces pro-hypertrophic gene expression during cardiac myocyte remodeling (FIGURE 5). On the other hand, activation of β2-AR results in the recruitment of PDE4D5:β-arr2 complex allowing the EPAC1:β-arr2 to activate Rap1-dependent nonhypertrophic signaling in the cytosol (76). In addition to interactions with β-arr2, a direct interaction between EPAC1 and GPCR kinase 2 (GRK2), an important regulator of GPCR signaling, has been demonstrated (268). GRK2 modulates cAMP signaling by phosphorylating β-ARs, which leads to the recruitment of arrestins to block reassociation of G proteins and reactivation of GPCRs (283). Subsequent studies demonstrate that GRK2 directly phosphorylates EPAC1 at serine 108 adjacent to the PA binding motif in the DEP domain (FIGURE 4A). Furthermore, phosphorylation of EPAC1 by GRK2 inhibits agonist-induced translocation of EPAC1 to the PM (FIGURE 5) (962).
While cAMP, as a small molecule second messenger, is capable of diffusing freely in solution, cellular signaling mediated by cAMP is highly compartmentalized with exquisite spatiotemporal precision. As previously mentioned, this regulation is achieved in part by a highly coordinated network of two multi-membered families of enzymes asserting opposing functions, namely, the ACs and PDEs. The regulation of cAMP signaling is further coordinated by a family of structurally diverse scaffolding proteins, AKAPs, which tether PKA and related signaling molecules to form signalosomes at discrete subcellular locales (926). Accumulating evidence suggests that EPAC1 directly contributes to the compartmentalization of cAMP signaling by interacting with various PDEs and AKAPs (246, 713, 762, 853, 856, 931). Using immunoprecipitation and peptide array approaches, Maurice and colleagues (1123) show that PDE3B interacts directly with EPAC1 and PI3Kγ regulatory subunit, P84. These interactions are mediated by two separate regions of PDE3B: while the NH2 terminal of PDE3B (residues 1–25) interacts with EPAC1, a hydrophobic section (residues 436–460) of PDE3B is responsible for binding to P84 (FIGURE 5). This PDE3B-based signalosome integrates EPAC1 and PI3Kγ signaling permitting dynamic cAMP-dependent regulation of cell adhesion, spreading, and tubule formation in human arterial endothelial cells (HAECs) (1123).
EPAC1 is identified as one of the 16 interacting partners of the type I transforming growth factor (TGF)-β receptor (TGF-βRI), a key player in propagating TGF-β signaling, in an affinity pulldown-based proteomic study (FIGURE 5). Interaction between EPAC1 and TGF-βRI requires the intact kinase activity of TGF-βRI and is independent of the cAMP-binding domain of EPAC1. Functionally, ectopic overexpression of EPAC1 inhibits TGF-β1-induced Smad2 phosphorylation and antagonizes TGF-β1/TGF-βRI-mediated transcriptional activation, inhibition of cell adhesion and stimulation of cell migration in mink lung epithelial Mv1Lu cells (197). These observations are consistent with the report that overexpression of EPAC1 inhibits TGF-β1-induced collagen synthesis in fibroblasts (790, 1171) and activation of EPAC reverses microtubule-dependent increases in endothelial permeability induced by TGF-β (930). Conversely, TGF-β1 decreases the expression of EPAC1 (61, 790, 1171). Therefore, it appears that TGF-β1 and EPAC1 signaling forms a reciprocal negative-feedback loop. However, the general application of this opposing relationship between EPAC and TGF-β signaling in other cell types, particularly in vivo, is not clear. A recent study using EPAC1 knockout mice suggests that EPAC1 positively modulates TGF-β signaling in boosting Treg-mediated immunosuppression. Specifically, deletion of EPAC1 leads to increased SMAD7 expression, while reducing SMAD4 expression and SMAD2 phosphorylation in response to TGF-β treatment in T cells (16).
In addition to participating in GPCR- and receptor kinase-mediated signaling, EPAC1 has also been implicated in interacting and regulating channel functions at the PM. For example, EPAC1 is reported to associate with the cystic fibrosis transmembrane conductance regulator (CFTR) via direct interaction with NHERF1/EBP50 (FIGURE 5). Activation of EPAC1 promotes translocation to the plasma membrane, where EPAC1 colocalizes with CFTR and promotes membrane stability of the receptor by suppressing endocytosis pathways (632).
2. EPAC1 signalosomes at the nuclear envelope
The identification of a putative cAMP-responsive signaling complex containing EPAC1, PDE4D3, PKA, and extracellular signal-regulated kinase 5 (ERK5), held together by mAKAP (also called AKAP6), at the perinuclear membranes of rat neonatal ventriculocytes provided one potential avenue for EPAC1 targeting to its most prominent subcellular locus: the nuclear envelope (FIGURE 5) (246). This macromolecular complex not only targets selective signaling molecules to a distinct subcellular locus, but also brings together two cAMP effectors, as well as PDE4D3, a cAMP attenuator, for self-modulation of the degree and duration of cAMP signal activation. A more recent study identified another mAKAP/EPAC1 macromolecular signaling complex, along with phospholipase C-ε (PLC-ε), PKC-ε and protein kinase D (PKD) at the nuclear envelope of cardiomyocytes (1192). This perinuclear EPAC1-containing signalosome positions PLC-ε in close proximity to a specific pool of phospholipid substrates, namely, phosphatidylinositol 4-phosphate (PI4P) in the Golgi apparatus. Localization of this complex functions to regulate the activation of nuclear PKD and hypertrophic signaling pathways in response to hypertrophic stimuli by generating diacylglycerol (DAG) (FIGURE 5) (1192). A previous study by Schmidt et al. (916) showed that cAMP/EPAC1 selectively stimulated PLC-ε signaling, but not PLC-β or PLC-γ, through activation of a specific Rap GTPase isoform, Rap2B. Taken together, these studies implicate EPAC1 interaction with mAKAP scaffolding proteins in the formation of signalosomes to integrate specific signaling components at the NM and coordinate discrete physiological responses in a spatiotemporally regulated fashion.
In addition to mAKAP-mediated EPAC1 NM localization, a general NM targeting mechanism for EPAC1 exists, especially for cells other than myocytes and neurons that do not express mAKAP. Affinity purification and mass spectrometry analyses identified five proteins as potential EPAC1 interaction partners in a HEK293 cell line stably transfected with Flag-EPAC1 (620). These proteins include the small G protein Ran, Ran binding protein 2 (RanBP2), importin β1, and nucleoporins 98 and 205, which are components of the nuclear pore complex (NPC) (FIGURE 5). This association of EPAC1 with the NPC appears to be stable under both basal and cAMP-stimulated conditions. Studies based on EPAC1 deletion mutations suggest that the RA domain of EPAC1 was critical for mediating the interaction with Ran-GTP and RanBP2 proteins and for targeting EPAC1 to the NM. This RA-dependent EPAC1-Ran interaction was also determined to be required for efficient EPAC1-induced Rap1 activation, suggesting that nuclear localization of EPAC1 may facilitate Rap1 activation in response to elevated levels of cAMP (620).
The interaction between EPAC1 and RanBP2 has been independently validated by a yeast two-hybrid study, which demonstrates that the zinc finger (ZNF) domain of RanBP2 directly interacts with EPAC1 and tethers it to the NPC (350). However, in contrast to the earlier report, Ran was determined to be expendable for targeting EPAC1 to NPC, for which interaction between EPAC1 and RanBP2 is necessary and sufficient. Additionally, the EPAC1 domain required for RanBP2 binding was identified to be the CDC25-HD domain (FIGURE 4A), not the RA domain as previously hypothesized (620). Furthermore, although RanBP2 binding suppresses EPAC1-induced Rap activation, disruption of EPAC1 and RanBP2 interactions via siRNA mediated knockdown of RanBP2 or phosphorylation of the ZNF sites reverse the negative regulation by restoring Rap1 activation (350). These results challenge the notion that nuclear localization of EPAC1 enhances Rap1 activation (620), instead suggesting the interaction between EPAC1 and RanBP2 antagonizes EPAC1 signaling by sequestration of the inactive pool of EPAC1 at the NPC. A subsequent study further narrows down the region responsible for EPAC1 nuclear pore localization to amino acids 764–838 within the CDC25-HD domain of EPAC1 (FIGURE 4A). This region is in fact conserved between EPAC1 and EPAC2. However, structural modeling reveals that the nuclear pore localization motif is blocked by the CNB-A domain in EPAC2A, countering nuclear envelope association of EPAC2. This notion is supported by the observation that a naturally occurring EPAC2 variant, EPAC2B, that lacks the CNB-A domain is targeted to the nuclear fraction in a similar fashion as EPAC1 (36, 811).
A more recent study further supports that interaction between EPAC1 and importin β1 may contribute to dynamic partitioning of EPAC1 between the PM and NM (38). Similar to RanBP2, association between importin β1 and EPAC1 sequesters EPAC at the NM (FIGURE 5), while silencing importin β1 promotes targeting of EPAC1 to the PM where neurite outgrowth is strongly inhibited by a cAMP-independent EPAC1 mechanism (38).
3. EPAC1 signalosomes at the cytoskeleton and other cellular loci
Several accounts implicate cAMP-mediated EPAC signaling participation in important roles for cytoskeleton organization and dynamics. EPAC1 has been shown to associate with the MT cytoskeleton network (FIGURE 5) (845). In fact, EPAC1 can interact directly with tubulin, copurifies with cellular MTs, and colocalizes with the mitotic spindle assembly. The binding of EPAC1 promotes microtubule formation (686). In addition to directly interacting with tubulin/MT, EPAC1 is also known to associate with microtubule binding proteins. Using EPAC1 as a bait, the light chain 2 (LC2) of microtubule-associated protein 1A (MAP1A) was identified as a protein capable of interaction with EPAC1 in a yeast two-hybrid screen. This interaction is mediated by the CNB domain of EPAC1 (FIGURE 4A) (653). EPAC1, again through the CNB domain, has also been shown to interact with the light chain 1 (LC1) of MAP1B (94). Interestingly, while interaction between EPAC1 and MT suppresses EPAC1-mediated Rap1 activity (686), association of LC1 or LC2 enhances EPAC1-mediated Rap1 activation (94, 381). These results reveal a dynamic linkage between cAMP-mediated EPAC signaling and cytoskeleton dynamics. Studies in HUVECs confirm that EPAC1 colocalizes with MTs. Activation of EPAC1 by 007 initiates a Rap-independent increase in MT growth rate and elongation of the MTs toward the cell periphery (930), but also induces a Rap-dependent increase in cortical actin, consequently enhancing endothelial barrier function (548, 930). A subsequent study illustrated an AKAP9 association with EPAC1 and MTs which is necessary for the EPAC1-induced increase in MT growth rate (FIGURE 5). While AKAP9 is required for integrin-mediated adhesion and barrier function downstream of EPAC1, the scaffolding protein is not essential for EPAC-induced Rap activation, reorganization of cortical actin, or basal barrier properties (931). In relation to the function of EPAC1 in MT dynamics, a recent study shows that although EPAC1 activity is not required for maintaining the relative positioning of the centrosome and nucleus under normal conditions, EPAC1/Rap1B signaling is essential for hypoxia/ATP-induced centrosome-nuclear separation and cell migration downstream of the purinergic A2b receptor in human retinal epithelial pigment (RPE) cells (794). The centrosome is the main MT-organizing center and promotes de novo assembly of MTs (77). Thus the finding that EPAC1 signaling is correlated and important for centrosome positioning under stress conditions is intriguing and exciting. Further studies are required to elucidate the physiological and pathophysiological functions of this EPAC1-mediated regulation for centrosome positioning. Additionally, the composition of the EPAC1 signalosome at the centrosome is not resolved, but considering the centrosome attachment to the NPC is well-accepted (5), the involvement of previously identified partners associated with the EPAC1 signalosome at the nuclear envelope will be of great interest.
The MT and actin cytoskeleton network is critical for mitochondrial dynamics and positioning (833). EPAC1-mediated signaling is likely to participate in MT-associated mitochondrial functions as EPAC1 is also known to localize to the mitochondria (FIGURE 5). While an NH2-terminal mitochondrial-targeting sequence within the first 37 amino acid residues of EPAC1b has been identified to be important for mitochondrial localization (FIGURE 4A) (845), currently the mechanism governing distribution of EPAC1 between mitochondria and other cellular compartments or potential mitochondrial partners for EPAC1 remain to be defined. Nevertheless, several recent papers have demonstrated that mitochondrial EPAC1 plays important roles in various physiological and pathophysiological conditions. For instance, EPAC1 is implicated in regulation of mitochondrial fission/fusion dynamics and in promoting VSMC proliferation during neointima formation in response to vascular injury (1088). Another study by Brenner and colleagues (1100) suggests that EPAC1 could act as a key effector for mitochondrial cAMP by reducing mitochondrial Ca2+ entry, stabilizing mitochondrial membrane potential, and inhibiting apoptosis in cardiomyocytes. However, a more recent study contradicts these conclusions by demonstrating activation of EPAC1 in mitochondria increases Ca2+ overload and induces cardiomyocyte death. Furthermore, deletion of EPAC1 is cardioprotective against ischemia/reperfusion injury in vivo (289). Mechanistically, activation of EPAC1 by 007-AM stimulates Ca2+ exchange between the endoplasmic reticulum (ER) and the mitochondrion by promoting the formation of a Ca2+-handling macromolecular complex composed of voltage-dependent anion channel 1 (VDAC1), the chaperone glucose-regulated protein 75 (GRP75), and the inositol-1,4,5-triphosphate (IP3) receptor 1 (IP3R1) between the ER and the mitochondrion interface (FIGURE 5) (289).
B. EPAC2 Cellular Distribution and Signalosomes
Compared with EPAC1, the cellular distribution and interacting partners of EPAC2 are mostly distinct. This disparity in cellular dynamics between EPAC1 and EPAC2 is largely determined by their sequence and structural variations. The extra NH2-terminal CNB domain, while very poor at binding cAMP, appears to be critical for EPAC2’s cellular localization and function (FIGURE 4B). While the full-length EPAC2A is primarily targeted to the proximity of PM, the adrenal gland specific splice variant EPAC2B, lacking the first CNB domain, is localized mainly to the cytoplasm (766). Later reports further affirm that the CNB-A of EPAC2A interferes sterically with the nuclear pore localization sequence conserved between EPAC1 and EPAC2, blocking association with the nuclear envelope. Accordingly, EPAC2B is enriched in the perinuclear fractions with a localization profile similar to EPAC1 (811). In addition to the CNB-A, sequence disparities of the RA domain between EPAC1 and EPAC2 also contribute to EPAC2’s distinctive ability to interact with activated Ras proteins, which regulate EPAC2’s recruitment to the PM (FIGURE 4B) (608). Although binding of EPAC2 to Ras-GTP is independent of cAMP, Ras-binding appears to be required for the cAMP-dependent activation of Rap1 via EPAC2 (621). A latest report by Tengholm and colleagues (9) shows that the RA domain is required for cAMP-induced PM translocation of EPAC2A. On the other hand, while CNB-A domain is expendable for cAMP-mediated translocation to PM, this domain is essential for the clustering of EPAC2A to the granule docking sites in β-cells. Intriguingly, the typical membrane association DEP domain is dispensable for cAMP-induced PM translocation and granule docking of EPAC2A. In fact, deletion of the DEP domain in EPAC2A leads to increased basal membrane and granule localizations regardless of cAMP levels (9).
1. EPAC2 signalosomes at insulin-secreting granules and synaptic vesicles
Early studies identify Rab3-interacting molecule 2 (Rim2) and Piccolo (a cytoskeletal matrix associated with the active zone protein) as EPAC2 interacting partners in mouse insulin-secreting MIN6 cell line employing an EPAC2-baited yeast two-hybrid screening (318, 796). Piccolo is structurally related to Rim2. While the PDZ domain of Rim2 and Piccolo is responsible for binding of EPAC2 (318, 796), the C2A domain of Piccolo is involved with the C2A domain of Rim2 in a Ca2+-dependent manner (318). In relation to EPAC2, the NH2-terminal 290 residues of EPAC2 is responsible for Rim2 binding (FIGURE 4B) (955); however, it is not clear if this same region of EPAC2 is involved in the interaction with Piccolo. Binding of EPAC2 to Rim2 and Piccolo, as well as Rim1, is confirmed by affinity pull-down approaches. Furthermore, functional analyses suggest EPAC2, Piccolo, Rim2, and Rab3 form a signaling complex to mediate cAMP-induced, PKA-independent exocytosis (FIGURE 6) (318, 796). Overexpression of a Rim2 deletion mutant containing the EPAC2-binding domain, but lacking the NH2-terminal zinc-finger and COOH-terminal C2 domains, disrupts endogenous interactions between EPAC2 and Rim2 and inhibits cAMP/EPAC2-mediated exocytosis. This supports the notion that the effects of EPAC2 on exocytosis are mediated by Rim2 (499, 796). Additional mapping reveals that the NH2-terminal zinc-finger of Rim1 and Rim2, known to bind Rab3, is important for both subcellular localization and cAMP-mediated exocytosis. These results also illustrate that the interaction between Rim2 and Rab3 is required in cAMP-dependent insulin secretion (955). Similarly, overexpression of the PDZ domain of Piccolo in MIN6 cells significantly suppressed cAMP-induced insulin secretion, suggesting that the interaction between EPAC2 and Piccolo is also important for cAMP-induced exocytosis (318).
FIGURE 6.
Intracellular signalosomes of EPAC2. See text for additional details and abbreviations.
In addition to Rim2 and Piccolo, EPAC2 also interacts specifically with the nucleotide-binding fold-1 (NBF-1) of sulfonylurea receptor SUR1, a subunit of the ATP-sensitive potassium channel (KATP) (FIGURE 6) (796). Truncation mapping and affinity pull-down analyses further determine the direct interaction sites (FIGURE 4B): residues 351–458 within CNBD-B of EPAC2 and residues 859–881 within NBF-1 of SUR1 (1199). This direct interaction between EPAC2 and SUR1 may be responsible for EPAC-mediated inhibition of the KATP (495) and for PKA-independent cAMP-induced granule priming in pancreatic β-cells (271), as well as in dentate granule cells (1199). Interestingly, interaction between EPAC2 and SUR1 is inhibited by cAMP but not affected by ATP. Alternatively, interaction between EPAC2 and Rim2 or Piccolo is cAMP-independent (955, 956). Moreover, while Piccolo binds to the SUR1-EPAC2 complex (956), both Rim2 and Piccolo interact with L-type voltage-dependent Ca2+ channel (VDCC) via their respective C2 domain (955). Thus these results suggest that EPAC2 is integrally implicated in coordinating ATP, cAMP, and Ca2+ signals to regulate exocytosis of insulin granules in β-cells (955, 956).
As an approach to explore the dynamics of insulin granule exocytosis in primary cultured pancreatic beta cells, total internal reflection fluorescence microscopy was conducted using an insulin fusion construct tagged with enhanced yellow fluorescent protein (Venus), insulin-Venus. The authors were then able to characterize the fusion events of insulin granules to the PM, segregating these events into three modes based on the dynamics of the granules in response to various stimuli. These modes include predocked granules that fused to the PM (old face), newly recruited granules that immediately fused to the PM without docking (restless newcomer), and newly recruited granules which docked and then fused to the PM (resting newcomer) (957). While cAMP alone did not induce fusion of the granules to the PM, it potentiated both the first phase and the second phase of glucose-induced fusion events. Interestingly, cAMP promoted the fusion events only by increasing the number of restless newcomers. Moreover, cAMP-potentiated fusion events in the first phase of glucose-induced exocytosis were markedly abridged in EPAC2−/− mice (957). Membrane fusion events during exocytosis require the assembly of a ternary SNARE (Soluble NSF Attachment Protein REceptor) core complex incorporating the vesicle-associated membrane protein (VAMP) localized at the secretory vesicle, syntaxin-1 and synaptosome-associated protein of 25 kDa (SNAP-25) on the PM (173). A direct interaction between EPAC2 and the NH2-terminal domain of SNAP-25 was demonstrated in an affinity pulldown study (FIGURE 6). While full-length SNAP-25 protein enhanced cAMP-stimulated rapid component of exocytosis, overexpression of a COOH-terminal truncated SNAP-25 renders INS-1 cells insensitive to cAMP potentiation. Taken together, these studies suggest that EPAC2 is important in regulating cAMP-stimulated insulin granule dynamics by interacting with SNAP-25 and controlling granule density at the PM (1070).
A similar signalosome is also observed for EPAC2 in the CNS during neurotransmitter release, which is but another form of exocytosis employed by neurons to rapidly communicate throughout the body. EPAC is observed to be a major regulator in presynaptic terminal release probability of synaptic vesicles (490, 852, 897). This is accomplished through a signalosome where EPAC2 binds Rim1α, increasing association with Rab3 to maneuver synaptic vesicles in closer proximity to the plasma membrane (300, 301). Furthermore, EPAC2-mediated generation of DAG through the PLC pathway may be involved in the activation and translocation of Munc13–1 promoting binding to Rim1α and subsequent SNARE-mediated synaptic vesicle release (301). EPAC2’s binding of SUR1 is also observed to affect exocytosis in dentate granule presynaptic terminals, where this interaction attenuates action of the neuronal-type KATP while promoting voltage-dependent Ca2+ channel activity (1199). Observed attenuation of excitatory postsynaptic current (EPSC) generation in animals where EPAC is deleted (1155) further confirms the action of this EPAC-dependent analogous exocytic signalosome in neurotransmission and insulin secretion.
2. EPAC2 signalosomes at other cellular loci
Similar to EPAC1, EPAC2 has also been shown to interact and colocalize with LC2 or MAP1A in PC12 cells (653). Activation of EPAC2 stimulated the phosphorylation of syntabulin, a microtubule-associated and syntaxin-1-binding protein that attaches syntaxin-containing vesicles to microtubules and kinesin I and mediates anterograde transport of syntaxin-1 to neuronal processes in INS-1E cells (988). Silencing of syntabulin diminished EPAC-potentiated, glucose-stimulated insulin secretion (1166). These studies suggest a potential role of EPAC2’s association with the MT cytoskeleton in vesicle trafficking (FIGURE 6).
A recent study also suggests the existence of two putative cAMP-responsive signalosomes acting as functionally unique signaling units to produce sequestered cAMP pools near hepatocyte plasma membranes. Through interactions with D-AKAP2, AC5, and PDE3B, EPAC2 is selectively regulated by a compartmentalized cAMP pool activated by adenosine GPCR (Ado-GPCR) A2B (FIGURE 6). In contrast, Ado-GPCR A2A, AKAP79/150, PDE3A, and AC6 appear to form a separate functional macromolecular complex that generates an unconnected cAMP pool to selectively activate PKA (379). A note of caution should be executed for the proposed signalosomes, which are mainly based on functional analyses, as well as affinity pull-down and colocalization studies, and have not yet been validated by rigorous biophysical and biochemical approaches.
IV. PHYSIOLOGICAL FUNCTIONS OF EPAC
EPAC proteins have been found to be involved or responsible for regulating a myriad of physiological functions throughout multiple biological systems (FIGURE 7). The following sections will begin to identify and categorize these biological effects in each system, including some functions initially attributed to PKA effects. Some of the physiological functions of EPAC tested in various animal model systems are listed in TABLE 1.
FIGURE 7.
Overview of key biological functions regulated by EPAC proteins in various physiological systems.
Table 1.
Physiological functions of EPAC proteins in murine models
| Genotype | System | Decrease | Increase | Unaltered | |
|---|---|---|---|---|---|
| EPAC activation/overexpression | Neurology | C57 | Reinstated cocaine seeking (1083) | LTP (343), memory (516, 647, 793) | |
| Sprague-Dawley rat | LT memory (370), learning and memory (1096) | ||||
| Cardiovascular | Neointima formation (1168), ductus arteriosus closure (1169), spontaneous VT (439) | ||||
| Endocrinology | Plasma leptin (1150) | Hypothalamic leptin sensitivity (1150), GSIS (176, 515, 922) | |||
| Pain | Hyperalgesia (268), chronic postoperative pain (142) | ||||
| Renal | IRI renal failure (813, 986) | ARPKD hepatic cystogenesis (54), tubular epithelial adhesion (813) | |||
| Pulmonary inflammation | Vascular barrier recovery (81) | ||||
| EPAC1 null | Cardiovascular | Basal contractility (785), stress-induced myopathies and arrhythmias (785), ANP-mediated vascular permeability (549) | Baseline vascular permeability (549) | Hypertrophy (785), basal cardiac function (819), Ca2+ handling (819) | |
| β-AR induced hypertrophy (586), neointima formation (502, 1088) | |||||
| Endocrinology | Global null | Food intake/HFD-induced obesity (1150), WAT/plasma leptin (1150), GSIS (487) | Hypothalamic leptin sensitivity (1150) | ||
| WAT null | WAT leptin secretion (444) | Food intake/HFD-induced obesity (444) | |||
| Pain | KO | Inflammatory hyperalgesia (962), SNT-induced mechanical allodynia (267) | |||
| KO | Chronic inflammatory pain (1087) | ||||
| Infection | Rickettsiosis infection (353) | ||||
| Pulmonary inflammation | Matrix secretion and remodeling (787) | ||||
| Cancer | Ovarian tumor growth (334) | ||||
| EPAC2 null | Neurology | C57 | LTD (590), spatial learning/memory (590), fear memory (793, 1207), socialization and vocalization (974), anxiety and depression (1207) | Spatial learning (974, 1207), olfaction and anxiety (974) | |
| Neurology | 129Sv | Cocaine CPP (627) | |||
| Cardiovascular | β-AR-induced arrhythmias (819), Ca2+ sparks (819), ANP secretion (523) | Hypertension (523) | Hypertrophy (785), basal cardiac function (819), Ca2+ handling (819), baseline and ANP-mediated vascular permeability (549) | ||
| Endocrinology | Hypothalamic leptin signaling (454), plasma adiponectin (454), GSIS (957, 968), HFD-induced glucose tolerance (968) | Plasma leptin (454), food intake/HFD-induced obesity (454) | |||
| Pulmonary inflammation | BALF total inflammatory cells (787), BALF cytokine secretion (787) | ||||
| EPAC1/2 null | Neurology | 129Sv | LTP (1155), spatial learning and socialization (1155), glutamate release (1155) | ||
| Cardiovascular | β-AR induced arrhythmias (819) | Basal cardiac function (819), Ca2+ handling (819) |
ANP, atrial natriuretic peptide; ARPKD, autosomal recessive polycystic kidney disease; BALF, bronchoalveolar lavage fluid; CPP, conditioned place preference; GSIS, glucose-sensitive insulin secretion; HFD, high-fat diet; IRI, ischemia-reperfusion injury; LT, long term; LTD, long-term depression; LTP, long-term potentiation; SNT, spinal nerve transection; VT, ventricular tachycardia; WAT, white adipose tissue. Reference numbers are in parentheses.
A. Central Nervous System
The major transfer of information in the CNS is carried out by the temporal release of neurotransmitters into the synapse between two proximal neurons. This transmission can be triggered by nerve stimulation, depolarization, or developmental cues, but must be tightly regulated to ensure adequate stimulation or inhibition of the subsequent postsynaptic neuron. The collection of excitatory and inhibitory signals that a neuron receives determines whether the signal propagates further.
1. Synaptic neurotransmission
From the time Greengard’s group observed increased cAMP in rabbit superior cervical ganglia after stimulation, research groups focused tremendous effort in investigating the role of cAMP in the CNS (367, 673). G proteins and calcium signaling are now known to be major modulators for the release of neurotransmitters, along with the ligand-gated ion channels. During neuronal stimulation, GPCRs are activated and the Gαs-protein heterotrimer dissociates into Gαs subunits to effectively activate ACs raising intracellular cAMP concentrations, while Gβγ subunits assist in the calcium influx. Increased levels of cAMP induce enhanced neurotransmission from presynaptic terminals (599, 1111). Until recently, this cAMP-mediated enhancement was attributed to the action of PKA, as there were few other alternatives (162, 1111). But interestingly, loss of different PKA subunits in neurons did not drastically affect the basal level of transmission (101). Thus the field began to search for alternative signaling pathways that cAMP could possibly use to alter neurotransmission.
a) neurotransmitter release.
The role of EPAC versus PKA in vesicle release remains controversial and may be inevitably determined to be dependent on cell type. The development of the EPAC-selective activator 007 has proven to be instrumental in the determination of EPAC’s function in neurotransmitter release. In initial studies considering the mechanism of cAMP on neurotransmitter release, independent research groups treated the presynaptic neuron of the calyx of Held with either forskolin, an EPAC-specific agonist, or cAMP alone. The first of these studies was conducted in the presence of a nonhydrolyzable analog of GDP, GDPβS, to disable G protein signaling. Cessation of G protein activation decreased the EPSC. Attempting to recover the signal by targeting downstream effectors of G protein activation, the universal second messenger cAMP, was found less effective than a selective EPAC activator (897). A later study focused on the mechanism in which increased cAMP enhanced neurotransmitter release. To directly increase the EPSC, cAMP was postulated to enhance neurotransmitter release through potentiating the probability of neurotransmitter vesicle release or increasing the number of vesicles available for release. Treatment with forskolin demonstrated the involvement of cAMP in increasing both parameters. Further inquiry into the precise mechanism revealed that PKA inhibitors had no effect on EPSC generation, while the EPAC-specific activator increased EPSC levels, supporting the importance of EPAC as a major regulator in synaptic vesicle release (490). Additionally, EPAC activation could prevent presynaptic silencing, as well as accelerate awakening, of axonal terminals after prolonged cannabinoid type 1 receptor stimulation, while PKA modulation was ineffective (852). Furthermore, EPAC-null animals display decreased EPSC generation and spontaneous activation in neurons of the hippocampus (1155). Long-term potentiation (LTP) in CA1 pyramidal neurons and granule cells from EPAC-null mice rapidly decayed to basal levels after only 30 min of tetanus in comparison with wild-type littermates that sustained elevated EPSCs through 90 min (1155).
A potential mechanism for EPAC to increase neurotransmitter release involves the known association between the NH2 terminus of EPAC2 and RIM proteins (796). RIM proteins associating with Munc13–1 have been implicated in vesicular release time and time again (24, 78, 233, 920, 1098). RIM proteins have also been observed to increase the size of the active zone by recruiting calcium channels, which scales linearly to the probability of vesicle release (425, 485). Furthermore, a direct correlation between RIM1α, but not Munc13–1, and release capacity of vesicles was determined using a FM1–43 membrane probe to detect a range of vesicle release mechanisms at the nerve terminal (852). With a common association partner, investigation into a potential relationship between EPAC and Munc-13–1 in vesicle release revealed an additive effect on EPSC potentiation with phorbol ester and forskolin cotreatment (490). This would suggest EPAC and Munc13–1 pathways are distinct from one another, but both serve to enhance neurotransmission. However, a more recent account supports the role of EPAC in enhancing glutamate release in direct correlation to Munc13–1 translocation and binding with RIM1α in synaptosomes from the cerebral cortex. This EPAC-modulated translocation involves the activation of PLC to hydrolyze PIP2 and generate DAG which activates and redistributes Munc13–1 to the active zone (301). Furthermore, EPAC activation increased RIM1α association with Rab3A to shift vesicles closer to the presynaptic membrane as shown by electron microscopy (300). Taken together, there is suitable evidence for the involvement of EPAC in vesicle release (FIGURE 8); however, the complete function of EPAC in neurotransmitter release may be more complex than a single mechanism of action and may even be dependent on neuronal type.
FIGURE 8.
Functions of EPAC2 in neurons of the CNS. Schematic depicting the roles and regulation of EPAC2 during depolarization (top), neurotransmission (middle), and neurite outgrowth and spine plasticity (bottom). See section IVA for additional details and abbreviations.
As seen in a later study, only 38% of forskolin-mediated neurotransmitter release was attributed to the action of EPAC signaling in autaptic neurons from the dentate gyrus of the hippocampus. Treatment of these neurons with an EPAC-specific agonist successfully increased the number of EPSCs. But upon addition of hypertonic sucrose solution to release vesicles in close proximity to the active zone, there was no observable change in the number of vesicles released after activation of EPAC (342, 885). The authors concluded that EPAC increases the probability of vesicle release by a general mechanism or through modulation of vesicle population shifts. More studies in the hippocampal regions of RIM1α knockout mice show that forskolin-induced EPSC are unaffected by the loss of RIM, creating a point of controversy in the field (152). However, one possibility is that Rim2 can act as a surrogate in the absence of RIM1 to propagate the signal through a redundant mechanism.
The role of EPAC in neuropeptide release in other neurons was also investigated with mixed conclusions. In Drosophila motor neurons and at the neuromuscular junction, forskolin, PKA, and EPAC activators enhanced neuropeptide release in accordance with the initial studies; however, the use of a genetically altered fly line expressing an EPACKG00434 allele harboring a P-element insertion in the CNB domain to disrupt EPAC activation did not exhibit diminished forskolin-induced neurotransmission in motor neurons (945). In contrast at the neuromuscular junction, the cAMP-induced increase in neurotransmission is inhibited by hyperpolarization-activated, cyclic nucleotide-regulated channel (HCN) blockers, but a selective EPAC agonist only partially rescues the diminished synaptic transmission (70, 178). A similar phenomenon is found in the neuromuscular junction of crayfish, when HCNs activity switches to cAMP and actin-independent states the level of neurotransmitter release continues to rise in the absence of calcium with elevation of cAMP through an EPAC-dependent pathway (1203, 1205). Back in mammalian pyramidal neurons from the prefrontal cortex, EPAC was found to be dispensable in forskolin-induced EPSCs, while the combined inhibition of PKA and MAPK could abolish the action of forskolin in these cells, brefeldin A treatment and HCN blockers were ineffective (445). These discrepancies in the function of EPAC in neurotransmitter release and potentiation could represent the use of brefeldin A as an EPAC inhibitor (1204). Brefeldin A, a lactone metabolite produced by Eupenicillium brefeldianum, inhibits a high-molecular-weight Golgi-associated ARF guanine nucleotide exchange factor, GBF1 (772). However, brefeldin A is not an EPAC inhibitor as intended (860). New chemical inhibitors that are selective for EPAC have become more readily available and could assist in clarifying functional roles for EPAC in neurons, whether that function is more consistent across different neurons and species or, as previously reported studies suggest, diversified across neuronal subtypes.
b) long-term potentiation.
Excitatory transmission at synapses in the hippocampus primarily function through glutamate release; the efficacy of this transmission is controlled by LTP and long-term depression. The development of knockout mice harboring deletions of EPAC1, EPAC2 or both isoforms in the forebrain supported investigation of the activity of EPACs in this process.
In the absence of EPAC2, mossy fiber CA3 synapses in the hippocampus have unaffected basal activity and short trains of synaptic transmission, but long-term activity and forskolin-dependent potentiation were impaired. The number of available vesicles to release after extended synaptic activity were found to be diminished in the absence of EPAC2. These data suggest that EPAC2 is essential in the regulation of the reserve pool of vesicles as well as vesicle release in response to increased cAMP levels (294). In slightly different synapses of the hippocampus, Schaffer collateral-CA1, the presynaptic transmission was found to be unaltered; however, it would be interesting to see the effect of forskolin on these synapses to observe if transmission in the presence of elevated cAMP is affected as was the case in the mossy fiber CA3 synapses. EPAC2 knockout synapses also demonstrate impairment of NMDA receptor dependent long-term depression (590). The EPAC null, EPAC1/2 knockout, mouse exhibits impaired LTP paired with spatial learning and social interaction abnormalities that are absent in the single knockout animals, suggesting a possible functional redundancy in brain between the two isoforms (1155).
c) action potential generation.
The induction of the action potential is a key characteristic of neuronal transmission. Through voltage-gated ion channels, neurons can sufficiently provoke a full depolarization as the membrane potential rises above the threshold level. The induction and maintenance of these membrane potentials are accomplished through cation channels that are activated by a variety of mechanisms.
Investigating the role of EPACs in this process illustrates involvement of EPACs in neuronal communication from the external environment through sensory neuron generation of action potentials. In the presence of nerve growth factor (NGF), sensory neurons demonstrate increased expression of EPAC2, while EPAC1 levels remain constant. Activation of these neurons results in increased number of action potentials. Interestingly in the absence of NGF, PKA inhibition is sufficient to block the increase of action potentials, but in the presence of NGF only loss of EPAC2 can accomplish this inhibition. This suggests crucial involvement of the external cellular environment in establishing the signaling cascade responsible for action potential generation in sensory neurons (1068). Investigation of the mechanism of action for EPAC in action potential generation demonstrated that inhibition of Ras signaling, but not Rap1, can block these effects (949). This suggests the presence of NGF implicates an EPAC/Ras pathway during action potential generation in sensory neurons.
Effects of EPACs on ion channels to depolarize or hyperpolarize the neuron may also have functional consequence. In cerebellar granule cells, EPAC2 activation is found to enhance Rap and p38 activation to mobilize intracellular calcium, which in turn activates calcium-sensitive big potassium channels, an ion channel commonly associated with PKA activation. Stimulation of these ion channels produces slight membrane hyperpolarization diminishing neuron firing (982). Although EPAC is implicated in promotion of the action potential and synaptic excitation by numerous reports, EPAC attenuation of membrane depolarization could be representative of a negative-feedback mechanism following extended neural activity to dampen depolarizations, or differential signaling of EPAC in postsynaptic neurons compared with functions in the presynaptic terminal.
Another ion channel regulated by both cAMP sensors is the fast-delayed rectifier potassium channel Kv3. This channel is activated by vasoactive intestinal peptide (VIP) treatment of neurons comprising the suprachiasmatic nucleus of the hypothalamus increasing neuronal electrical activity, but is potentially blocked by inhibition of EPAC or PKA (562). However, use of brefeldin A to inhibit EPAC impedes proper interpretation of these results; thus repeating these studies with a specific inhibitor of EPAC is warranted to evaluate EPAC’s role on Kv3 channels. Similarly, supporting the function of EPAC in enhancing action potential generation, activation of EPAC in the pre-Botzinger complex region of the brainstem enhances activity of the transient receptor potential cation channel, TRPM4, through calcium from IP3 stores. The function of EPAC extends further to inhibit ATP-sensitive potassium channels leading to greater neuronal excitability (FIGURE 8) (707). Therefore, the function of EPAC in neuronal excitability is still under development, but currently, the majority of data lends towards EPACs functioning to propagate neurotransmission by increasing neuropeptide release, neuronal potentiation, and enhancing firing rate of action potentials.
2. Neurite development and differentiation
The formation of the primary axon and neurite projections is often associated with functional differentiation in neurons. The function of elevated cAMP to induce neuritogenesis is no secret. Although axonal regeneration in DRG neurons (129, 746) and neurite extension in PC12 (170, 185, 519) have been attributed to both the effects of PKA and EPAC, the mechanism of action for each of these modulators is still controversial. While the complete mechanics of how cells finely integrate both cAMP receptors to reach an accurate physiological outcome are not completely understood, different investigative groups are pursuing this question with much sustained effort. Studies selectively activating the two cAMP effectors have begun to shed light on the individual functions of these proteins, and the collective results of these studies in terms of development and differentiation will be reviewed below.
In support of EPAC’s role in neurite development and differentiation, expression of EPAC1 and EPAC2 isoforms is developmentally regulated in the rat nervous system. EPAC1 is more abundantly expressed in early development, but as neurons mature, EPAC1 expression decreases and EPAC2 becomes upregulated (746). Additionally, expression of EPAC2 was found to be highly localized in growth cones and neurites (1208).
During development, the axonal growth cones switch from an attraction to repulsion corresponding to a decrease in cAMP levels. Considering the role of EPAC in the embryonic growth cone, attraction properties are linked to the direct action of netrin-1 on EPAC. Balancing this EPAC-mediated attraction, diminishing levels of cAMP promote netrin-1 to target PKA inducing repulsion in postnatal growth cones (747). This switch between EPAC to PKA is important to distinct control of growth cone dynamics during development. Another interesting study considering neuronal polarity showed EPAC1 was expressed in the nascent axon, while EPAC2 had more universal expression in all neurites. Further pharmacological probing determined that the inhibition of both isoforms of EPAC, but not EPAC2 alone could reduce the essential Rap1B activity and formation of axons in cultured hippocampal neurons. This would suggest that during axon specification, EPAC1 may be the main activator of Rap1B (744). Intriguingly other downstream effectors of EPAC that are activated by Rap1- and Rap2-independent mechanisms are also important to axonal growth including Rit, c-Jun NH2-terminal kinase (JNK), and microtubule growth (423, 930, 954). This suggests that the functions of EPAC in axonal elongation may not only be isolated to EPAC’s activation of small GTPases.
Additionally, the spatiotemporal activation of cAMP sensors may also be involved in proper signaling for dendrite extension. Elevation of cAMP activates ERK1/2 and is known to induce neuritogenesis. Interestingly, PKA induces transient ERK1/2 activation to promote proliferation in these neurons, while simultaneous activation of both EPAC and PKA extends ERK1/2 activation and is accompanied by neurite outgrowth and differentiation (FIGURE 8) (519, 805). Further considering the spatiotemporal regulation of cAMP signaling required for development of axonal elongation and branching, a recent investigation by Zhou et al. (1208) utilizes an optogenetic AC construct to generate cAMP in dentate granule cells at various lengths of time. Short-term elevation of cAMP exhibited branching of the main axon through PKA signaling, but long-term activation of the AC resulted in EPAC-dependent branching and elongation of the axon (1208). Although axonal growth is important to the development of mature neurons, growth arrest is also required to confer complete differentiation. In these regards, a coordinated action between the cAMP-independent NGF activation of ERK and EPAC2 induction of p38 MAPK provokes growth arrest and neuritogenic effects, respectively, in neuroscreen-1 (NS-1) and PC12 cells (273).
With EPAC2 expression increasing and subsequent depletion of EPAC1, as well as decreased cAMP levels in mature neurons, one could predict that the function of the EPAC isoforms may differ slightly during neuron development. Indeed, enhanced EPAC1 expression in neonatal neurons facilitated 007-mediated attraction of growth cone extensions, and inhibition of PKA was ineffective in blocking this process. Additionally, a distinct role for EPAC2 on the promotion of DRG neurite outgrowth is supported as 007 activation of EPAC could effectively induce neurite outgrowth which was abolished with loss of the predominant EPAC2 expression in adult neurons (746). These results support the distinction of EPAC isoform functions: expression of EPAC1 in early neurons contributes to the development of the primary axon, whereas mature neurons elevate the levels of EPAC2 to modulate dendrite stability and outgrowth. Since adult axon regeneration is naturally inhibited, an interesting notion develops as to whether use of EPAC activators could be an effective therapy to assist new axonal growth after axonal injury.
The differentiation of the initial axon and subsequent dendrites is quite an important process to connect the circuitry of the nervous system in early development, but the neuroplasticity of the nervous system is paramount for learning and recovering from injuries in an organism, while disruption can lead to neurological disorders. The development and maintenance of dendrites are important to the processes of synaptic remodeling and plasticity of the brain. In synaptic remodeling, EPAC2 activation of Rap promotes synapse destabilization of the dendritic spines. To destabilize the synapse while avoiding synaptic elimination, EPAC2 affects spine reduction and internalization of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) to reduce excitatory synaptic transmission. This action may be coordinated by interaction of EPAC2 with neuroligin, which recruits EPAC2 to the membrane and enhances the GEF activity (FIGURE 8) (1127). Furthermore, the expression of a rare EPAC2 mutation in the RA domain found in several autistic patients established the maintenance of basal dendrites to be a function of EPAC2. In pyramidal neurons, this mutation effectively decreased the number and length of basal dendrites that was linked to the action of EPAC2 through a Ras-mediated pathway (975).
However, controversy still exists between the effects of PKA versus EPAC in neurite development. The promotion of neurite outgrowth by PKA has been extensively investigated (393, 703, 966, 967, 1147). In other neuronal populations, such as spiral ganglion neurons, high levels of cAMP and constitutively active PKA both strongly inhibit neurite length, while direct activation of EPAC was unable to initiate elongation. Even though PKA inhibition did not rescue the neurite length, constant activation of phosphatidylinositol 3-kinase (PI3K) in the presence of active PKA was effective in reversing the effect (1139). Others have demonstrated that PKA is required for neurite outgrowth through a neurturin-mediated biphasic activation of ERK (1082). With these various conclusions, one could conjecture that the two cAMP receptors work dynamically to propagate the outgrowth of neurites (185). One recent study by Wei et al. (1104) shows that PKA inhibition alone enhances neurite outgrowth in DRG neurons, but co-inhibition of PKA and EPAC effectively reduces neurite outgrowth. Furthermore, in a spinal cord injury (SCI) model, the authors determined that PKA inhibition could effectively enhance recovery and sprouting of injured axons, where they propose EPAC “takes over” cAMP signaling and promotes neurite axonal outgrowth (1104). Thus more work is required to dissect the independent and synergistic roles of EPAC and PKA in neuritogenesis.
Pituitary adenylate cyclase activating polypeptide 38 (PACAP-38) is the ligand of a GPCR, PAC1, which activates AC and is implicated in the differentiation process of neurons (153, 238, 714). The potent PACAP-38 stimulation of neurite outgrowth was found to be mimicked by forskolin treatment; however, PKA inhibition was unable to affect the differentiation, while EPAC activation partially recapitulated the effect of forskolin (720). Further implication of a potential role for EPAC in neuronal differentiation was suggested by the action of PACAP-38 in other cells. The downstream mechanism of differentiation in other cell types, such as PACAP-38 activated PC12 cells, involves the activation of a Ras-like small GTPase, Rit. Shi and co-workers’ investigation (953, 954) into the potential activation pathway of Rit in neuronal differentiation by EPAC demonstrated that although EPAC does not directly bind Rit, EPAC-dependent Rit activation occurs through an intricate signaling cascade where EPAC activates Src kinase to promote TrkA receptor transactivation of tyrosine residue 499, which subsequently activates Rit. Additionally, in neurons Rit was found to be required to sustain p38 activation, while ERK1/2 activity was unaltered (954). Although these results begin to depict the necessity for EPAC in the differentiation of neurons by PACAP-38, they do not rule out the potential of additional pathways also being involved in this intricate process. During the development of astrocytes from neuronal precursor cells, PACAP induces cAMP and glial fibrillary acid protein (GFAP) gene expression. Neither EPAC-Rap1 nor Ras activation alone was sufficient to induce astrocytogenesis, but concurrent activation of both pathways was a viable option to induce differentiation and GFAP expression (585). Additional support for the importance of EPAC2 to astrocyte differentiation is observed in EPAC2 knockout mice, where EPAC2 was found to be required for PACAP to regulate external influx of calcium essential to GFAP expression and differentiation (939). Remarkably, another Ras-like small GTP binding protein, Rin, was also implicated in the PACAP-38-mediated differentiation process through a PKA-dependent mechanism (952). These studies suggest that neuronal differentiation is tightly controlled by independent actions of EPAC and PKA through activation of various small GTPases at precise times.
The differentiation of other populations of cells in the nervous system is also affected by EPAC signaling. Differentiation of oligodendrocytes into myelin forming cells is hindered by GPR17 and GPR37 that act to suppress PKA activation of cAMP response element (CRE) binding protein (CREB) as well as EPAC-mediated activation of MAPK and subsequent nuclear translocation of ERK1/2, respectively (960, 1153). Additionally, in Schwann cells, elevated cAMP was involved in enhanced differentiation and myelin formation, in part, through an EPAC-dependent mechanism (39).
Considering the actions of EPAC on neuronal differentiation, the functions of specific activators and inhibitors of EPACs are becoming an attractive idea for therapies targeting neuronal injuries. For example, plasmalemmal repair is essential for neurons to maintain circuit connections post-injury (764, 971). This repair process is accomplished by merging undamaged membranes (263, 970) through myelin delamination (52) and calcium-dependent accumulation of vesicles and undamaged membrane (262, 558, 858, 1031). PKA and EPAC activators are found to increase the rate of repair of this process through redundant and parallel pathways (970).
3. Behavior
As the functional roles of EPAC in the CNS continue to accumulate, the loss of such an important regulator should manifest abnormalities in behavior of EPAC null animals. The full extent of EPAC functions in neurological behaviors is only beginning to be appreciated and is still the focus of many ongoing investigations. Below the results of these initial studies defining the physiological function of EPACs in behavior will be discussed.
a) memory.
For some time now, the role of cAMP in learning and memory in mammals has been reported (114, 313). Supporting the requirement for cAMP, forskolin treatment can rescue long-term memory in mice lacking AC1 and AC8 (1124). Blocking PDE4 to enhance cAMP levels also augments LTP and improves long-term memory (57). PKA was initially presumed to be the major component driving the formation of memories (73, 99, 910); however, recent studies have begun to implicate EPAC signaling as a parallel regulator in these processes (343, 795). Some studies have even begun to forge the hypothesis that EPAC signaling, in some instances, is the major contributor to long-term memory formation and learning.
Generation of memories is thought to rely on the plasticity of the neurons in the hippocampus and regulated by increases and decreases in synaptic strength mediating LTP and long-term depression (LTD), respectively (426). Interestingly, EPAC signaling is implicated in both LTP and LTD in hippocampal cells supporting a potential role for EPACs in the cognitive process (343, 981, 1116). Further implication of EPAC in memory formation involves EPAC’s role in neurite outgrowth and plasticity, as described in section IVA2 (1127). Synaptic plasticity in the hippocampus has been attributed to the action of ERK on protein translation to form subsequent long-term memories (514). Stimulation of hippocampal slices with an EPAC agonist leads to increased LTP stability and activation of ERK (343, 514). A similar formation of preferential long-term odor memory in rats was observed to be enhanced by the activation of an EPAC/ERK pathway (370). Another study also concluded that loss of EPAC2 could impair LTD in the hippocampus and illustrated a moderate reduction in learning behaviors in these animals (590). In support of this mechanism, activation of EPAC was determined to be responsible for enhanced memory retrieval in C57BL/6J mice (516, 647, 793). Furthermore, impairment of fear memory by PKA inhibition is rescued by cotreatment of the EPAC activator, suggesting EPAC can function independent of PKA in this signaling system (647).
Two independent studies investigating mechanisms utilized by EPAC to affect learning and social interactions demonstrated activation of Rap1 by EPAC specifically reduces miR-124 gene expression protecting translation of Zif-268 (Egr1) levels. Scopolamine-mediated suppression of EPAC levels in Sprague-Dawley rats phenocopied the effect of simultaneously knocking out both EPAC isoforms (EPAC-null) in a 129Sv murine strain demonstrating enhanced miRNA-124 and coinciding with decreased Zif-268 translation in these animals. The genetic deletion of Zif-268 revealed severe defects in LTP maintenance and higher brain functions including learning and socialization. Genetic suppression of miR-124 or pharmacological treatment of scopolamine-treated rats with melatonin both successfully reversed apparent neurological disorders by rescuing the inhibition of Zif-268 translation in the EPAC-null animals or restoring EPAC levels in the scopolamine-induced model to inhibit miR-124, respectively (1096, 1155). Furthermore, overinduction of miRNA-124 was found to mimic the EPAC-null mice defects. Interestingly, the single knockout of EPAC1 or EPAC2 was not sufficient to induce the defects in learning and socialization, suggesting a redundancy between the two isoforms in these processes (1155).
There is slight controversy within the animal models however. Whereas the EPAC-null mice are the only ones to display neurological disorders in the above study, EPAC2-null mice on a C57BL/6 background were found to have intact learning and olfaction, but abnormalities in socialization and ultrasonic vocalizations disrupting proper communication (974). Two-photon imaging displayed defective spine development in cortical neurons presenting an issue at the synaptic level, like that mentioned previously (1127). Conversely, evidence supporting the abnormal socialization and communication of EPAC2-null mice is depicted in a study where deficiency in neuroligin-3 (NL-3) leads to autism-spectrum disorders (462). With NL3 directly acting on EPAC2 to enhance the GEF activity, EPAC2 is well-positioned as a potential modulator of this signaling cascade (1127). Some reasons for such discrepancies could be linked to the mouse genetic backgrounds, investigated brain region, or redundant mechanisms of EPAC1 that may conceivably account for some of the reported differences in learning (974).
b) feeding.
Interestingly, EPAC1 knockout mice have reduced food intake resulting in decreased body weight (1150). The combination of genetic and pharmacological manipulations of EPAC1 demonstrate the importance of this cAMP-signal modulator in energy homeostasis and leptin signaling. Molecularly these animals have heightened leptin sensitivity measured by an increase of signal transducer and activators of transcription 3 (STAT3) phosphorylation in the hypothalamus (1150). Conversely, activation of EPAC was found to reduce leptin signaling by attenuating STAT3 phosphorylation and subsequently inducing suppressor of cytokine signaling 3 (SOCS3) (322). Further support of these results is demonstrated in nodose ganglia neurons where ghrelin inhibits leptin-induced STAT3 phosphorylation through induction of SOCS3, which was reversible by silencing of EPAC1 (409). Taken together, these results depict EPAC1 as a potent modulator of leptin sensitivity governing the balance of energy in the hypothalamus. Almahariq et al. (14) have assembled an in-depth review of the actions of EPAC in energy homeostasis, and the major components of this system will be explored further in section IVC1a).
Thus the actions of EPAC in the CNS are being established at a molecular level and being linked to physiological phenotypes observed in genetically altered animals. The vast array of functions of EPACs in neurons including neurotransmission, modulation of action potentials, and neurite outgrowth have begun to depict a systematic cAMP-mediated modulation of signaling outcomes through activation of EPAC1, EPAC2, or PKA with extraordinary spatiotemporal precision. Therefore, specific targeting of these cAMP effectors may serve as an effective platform to combat neuronal pathologies, and will be discussed later in this review.
B. Cardiovascular
1. Cardiac
Compared with many other organ systems, functional roles for EPAC in the cardiovascular system are well-studied. For example, in human cardiac tissue from heart failure patients, EPAC1 expression is markedly upregulated, defining a role for the cAMP effector in cardiac function (692). Investigation into the role of EPAC in the heart revealed that EPAC activation increases Ca2+ transients in cardiomyocytes and employs a Rap/PLC/CaMKII-dependent ryanodine receptor (RyR) phosphorylation to enhance spontaneous Ca2+ leak from the SR (439, 729, 778, 779, 820, 821, 891). Unexpectedly, and in somewhat contrast to the function of EPAC in cardiomyocytes, the initial report exploring the consequence of genetic deletion of EPAC isoforms in mice demonstrated similar cardiac function and Ca2+ handling to wild-type mice (819). This may represent a more modulatory role for EPAC, where baseline function is not perturbed by loss of EPAC, but the functional consequence of this deletion is observed with aberrant insult to the system. Indeed, EPAC2-null animals were protected from β1-AR induced arrhythmias in this model, suggesting a role of EPAC2 in this pathology (819). However, a concurrent, independent investigation of the function of the two EPAC isoforms in the intact animal demonstrated that EPAC1-null mice have reduced basal cardiac contractility associated with attenuated Ca2+ transient and reduced Ca2+ reuptake into the SR. These effects in the absence of EPAC1 are attributed to reduced phosphorylation of RyR2 and PLN Ser16, independent of PKA. Furthermore, EPAC1-null mice exhibit resistance to several induced cardiac stresses, while EPAC2 deletion did not display cardioprotective features (785). The discrepancy in these results is currently attributed to the genetic background of the mice used in the studies; however, such an inverted phenotypic difference warrants further investigation to determine the relation of such observations to the human condition. These studies are in agreement, however, that inhibition of an EPAC isoform is beneficial to protect from cardiac stresses. As mentioned, EPAC’s function in the heart is well documented and has been extensively detailed in current reviews (84, 320, 601); thus we will focus this section of the review on recent advances for EPAC function in vascular tissues.
2. Vascular
The second messenger cAMP plays a crucial role in regulating endothelial permeability and barrier functions as well as modulating vascular contraction/relaxation. Among the known cAMP effectors, PKA and EPAC are abundantly expressed in the vasculature. In this section, the functions of EPAC proteins in vascular pressure and tone, vascular permeability and barrier integrity, and vascular proliferation and angiogenesis will be examined within the context of overall cAMP signaling in vascular regulation.
a) vascular pressure and tone.
One important aspect in vascular regulation is the dynamic of contracted and relaxed states of vascular smooth muscle cells (VSMCs) surrounding the arteries thus controlling blood pressure and vascular tone. Generally, activation of RhoA is implicated in the Rho kinase (ROCK) induction responsible for phosphorylation and inhibition of myosin light-chain phosphatase targeting regulatory subunit (MYPT1). Loss of phosphatase activity increases phosphorylation of myosin regulatory light chain (RLC20) and sequential force generation leading to contraction (533, 1000, 1048). With RhoA being a major regulator of cytoskeletal dynamics, intracellular cAMP signaling is commonly observed to invoke vasorelaxation in large vessels through inhibition of RhoA signaling (1213). Thus inhibition of RhoA is accomplished by downstream actions of cAMP sensors, PKA and EPAC/Rap1, decreasing contractile force generated by RhoA and inducing arterial relaxation. Major mechanisms utilized by cAMP signaling to attenuate contraction include direct inhibition of RhoA through Ser188 phosphorylation (272, 749), reduction of intracellular calcium influx from the SR (208), or Ca2+ desensitization of the contractile machinery through telokin phosphorylation or exclusion of MYPT1 Ser696 inhibitory phosphorylation to induce activation of the myosin light-chain phosphatase (MLCP) (1125, 1133).
Along these lines, increased Rap1 activity, demonstrated after EPAC activation, provides another mechanism to decrease RhoA activity concurrent with reduction in RLC20 and MLCP phosphorylation independent of PKA. Supporting this role of EPAC/Rap1, Rap1B-null animals are unable to reduce RhoA activity through EPAC activation (1213). Additionally, the regulation of cytosolic Ca2+ levels and consequent modulation of potassium current by EPAC are also pertinent to the relaxation of VSMCs. During vasorelaxation, EPAC activation is observed to increase cytosolic Ca2+ and induce Ca2+-activated large-conductance K+ (BKCa) channels. EPAC activates these channels by enhancing frequency of plasma membrane proximal Ca2+ release through sensitization of ryanodine receptors (RyRs) on the SR (878). Intriguingly, KATP is also found to be differentially regulated by cAMP attributing tonic and agonist-mediated KATP activation to PKA phosphorylation of channel subunits (404, 849, 901). Opposing the function of EPAC in BKCa channels, the transient increase in Ca2+ generated by direct EPAC activation with 007 is observed to attenuate pinacidil-mediated activation of KATP by 42% in a Ca2+-sensitive phosphatase 2B (calcineurin)-dependent mechanism. Supporting this effect, EPAC1 was found to immunoprecipitate SUR2B, a regulatory subunit of the KATP, suggesting a signaling complex, although interaction with other subunits of the receptor requires further investigation (844). The suppressive action of EPAC on KATP is only observed in the absence of PKA activation and therefore may only be relevant under pathological conditions where PKA signaling is perturbed in vascular cells. Taken together, these results suggest that the physiological function of EPAC on potassium current is more likely related to BKCa channel activation, additive with the action of PKA on KATP, promoting hyperpolarized cells and a relaxed vascular state. In support of the relaxant function of EPAC and PKA, in a Ca2+-free extracellular environment, both EPAC and PKA activation are also observed to slowly leak cytosolic Ca2+ from the SR reducing the SR load and attenuating contraction (208).
Interestingly, mice absent for the EPAC effector Rap1B present with elevated blood pressure leading to cardiac hypertrophy, as the hypertrophy can be suppressed with angiotensin II receptor antagonists. Further, cAMP/EPAC-mediated vascular relaxation was observed to be functionally deficient in the absence of Rap1B. Additionally, enhanced levels of MYPT1 and myosin RLC20 phosphorylation both basally and in response to the thromboxane analog U46619 signify increased RhoA signaling consequential to attenuated Rap1B in these animals. Together these observations support the relaxant role of EPAC/Rap1B in vascular smooth muscles and suggest that EPAC/Rap1B downregulates RhoA signaling to induce relaxation in these vessels, contributing, in part, to hypertension in Rap1B-null mice (577).
Although these mechanisms within the smooth muscle cells are important to the relaxation state, the presence of an intact endothelium is also found to be required in achieving maximal relaxation by EPAC activation. In support of this relaxation state, both PKA and EPAC were determined to activate endothelial nitric oxide synthase (eNOS) increasing nitric oxide (NO) production by 14 and 15%, respectively, and leading to enhanced relaxation of the overlying muscle (336, 878). Supporting this function, Rap1B-null mice exhibit reduced vascular endothelial growth factor (VEGF) stimulation of eNOS and subsequent NO generation promoting the development of hypertension in the model (577). These results demonstrate an independent pathway for EPAC/Rap1 that cooperates with PKA to relax smooth muscle cells exposed to increasing cAMP levels (878, 1213).
Curiously, glucagon-like peptide-1 receptor (GLP-1R) agonists, which are administered for diabetes mellitus type 2, also have a favorable effect in reducing hypertension (963). Investigation into the mechanism of action revealed that GLP-1R agonists were ineffective in inducing vasodilation of the aorta alone, but rather GLP-1R agonist-mediated enhanced secretion of atrial natriuretic peptide (ANP) from atrial cardiomyocytes resulting in the relaxant effect. Moreover, GLP-1R activation stimulates AC and with the aid of pharmacological agents, EPAC activation alone was observed to augment GLP-1 agonist induction of ANP secretion and inhibition of PLC blocked this process. In the presence of GLP-1R, agonist stimulation promotes EPAC2 translocation to the membrane fraction, and in the absence of GLP-1Rs direct activation of EPAC stimulates ANP secretion. Together, this suggests that EPAC2 functions downstream of GLP-1R to enhance ANP secretion potentially through membrane association. Indeed, correlating with these results EPAC2-null mice exhibit deficient ANP secretion in response to GLP-1R agonists, and the GLP-1R agonist is unable to reduce hypertension in these animals (523). These results suggest that GLP-1R agonists induce EPAC2 and potentially cross-talk with PLC pathways to promote ANP secretion and subsequent vasodilation to reduce blood pressure. Although these results begin to describe the action of GLP-1R agonist’s antihypertensive effect, the role of EPAC1 and the extent of PLC function in this secretory pathway are still not clear. Furthermore, the mechanism by which EPAC mediates the release of ANP from the large dense-core vesicle remains to be determined.
External stimuli that induce strain on the vascular system, such as extreme temperatures, may also affect the outcome of cAMP signaling in the modulation of vascular dilation; therefore, the involvement of EPAC in stress-induced vasoconstriction of human dermal arterioles (microvascular smooth muscle) was investigated. Although the typical resolution of elevated cAMP is relaxation, during stress conditions, such as cold stimuli, α2c-adrenoreceptors are translocated from the trans-Golgi to the cell surface where they induce vasoconstriction in response to norepinephrine (182, 183, 471). Inquiry into the mechanism of this trafficking demonstrated that EPAC/Rap1 increased α2c-adrenoreceptor expression through JNK/AP-1 activation (266, 472), while PKA was also implicated as disruption of the PKA-AKAP interaction and compartmentalization attenuated receptor expression (736). Furthermore, translocation of the receptors to the cell surface was observed in the presence of elevated cAMP levels, direct EPAC activation, or expression of constitutively active Rap1A, suggesting a predominant role for EPAC/Rap1 in the regulation of this receptor trafficking pathway (266, 472). The EPAC/Rap1-dependent translocation of α2c-adrenoreceptors to the cell surface was accomplished by a rearrangement of the actin cytoskeleton and increased F-actin, which are lost with treatment of actin destabilizing agents or expression of a dominant-negative Rap1A construct. As an additional implication of the inverted role of EPAC/Rap1 in microvascular smooth muscles during stress, the activation of EPAC and cAMP elevation are observed to surprisingly activate RhoA signaling, a central regulator of actin cytoskeleton organization, in a Rap1A-dependent manner. Supporting this functional activation of RhoA by EPAC/Rap1A, inhibition of RhoA or downstream Rho-associated protein kinase, ROCK, arrested the EPAC/Rap1A-dependent F-actin enhancement (472). A followup study searched for the relation between α2c-adrenoreceptors translocation and F-actin uncovering a specific interaction between the COOH terminus of α2c-adrenoreceptors and filamin-2, an actin cross-linker, using yeast-two hybrid screening (737). Indeed, forskolin, EPAC activation, and constitutively active Rap1A led to phosphorylation of filamin-2 at Ser2113 in a RhoA/ROCK-dependent manner, and consequently translocation of sequestered α2c-adrenoreceptors (737). Therefore, EPAC/Rap1A appears to be implicated in vasoconstriction to initiate heat conservation after exposure to cold stimuli in the microvasculature.
b) vascular barrier integrity and permeability.
The retention of barrier integrity is of utmost importance to the vasculature to maintain proper pressure control, transport of macromolecules and solutes, and allow for extravasation of cells for immunological surveillance throughout the body. Control of vascular permeability is ascribed to cAMP signaling with multiple studies attributing endothelial barrier integrity to EPAC and PKA functions which maintain the endothelial adhesion junctions (83, 210, 323, 548, 762, 853, 930). Importantly, the functional role of EPAC in regulating the tightness of the endothelial barrier is receiving greater attention as tools to probe EPAC and PKA functions have become available. In vascular endothelial cells, Rap1 is found to be associated with in cell-cell contacts, and loss of Rap1 activity results in attenuation of endothelial adhesion with gap formation. Treatment of these cells with 007 rapidly restores the endothelial junctions implicating EPAC in this pathway. Barrier enhancement is accomplished through EPAC/Rap1 reorganization of cortical actin, associated VE-cadherin, and other junction related macromolecules to enhance cell-cell contacts (210, 323). These results depict the critical function of EPAC/Rap1 in mediating vascular endothelial barriers.
Pro-inflammatory mediators such as thrombin and histamine are responsible for increasing barrier permeability in living animals. For example, hyperpermeability induced by thrombin occurs by activating RhoA and inducing contraction of the myosin-actin cytoskeleton. Elevation of cAMP and activation of EPAC are both observed to effectively attenuate this effect by restoring barrier function (210, 323). Barrier dysregulation in response to inflammatory mediators will be more readily discussed in section VE2b.
Two very recent accounts describe the function of EPAC isoforms in microvascular permeability. The first study demonstrated that loss of EPAC1 increased baseline permeability observed as increased transvascular transport of radiolabeled albumin. Further, vascular permeability induced by ANP was attenuated in EPAC1-null mice, while wild-type and EPAC2-null animals remained sensitive to ANP stimulation. To visualize the junctions between endothelial cells, dynamic contrast-enhanced magnetic resonance imaging was employed and illustrated heavily reduced density of junctions in EPAC1-knockout endothelial cells, suggesting reduced adhesion. Thus EPAC1, and not EPAC2, appears to be the responsible isoform for basal microvascular integrity and may be a target of ANP to increase permeability of the microvasculature (549). Following this notion, another investigation focused on hyperpermeability induced by ischemia-reperfusion. Korayem et al. (550) demonstrate that elevation of cAMP by forskolin can reduce this hyperpermeability, and an elevation of cAMP is naturally produced in response to reoxygenated microvascular endothelial cells. Importantly, in support of the previous study, EPAC1 activity was also found to be increased during reoxygenation, and loss of Rap1 abolished the cAMP-mediated restoration of barrier integrity. Thus together these conclusions suggest that EPAC1 is the predominant isoform regulating microvascular barrier permeability.
c) proliferation and angiogenesis.
In vascular systems, proliferation of cells must be tightly regulated to prevent hyperplasia of vessels and disruption of blood flow. However, upon injury to the vessel, these suppressive signaling pathways must be attenuated to promote healing and repair mechanisms. The repair process is commonly initiated by growth factor release and extracellular matrix remodeling to promote development of new vessel structures.
In VSMCs, the suppressive signal to reduce proliferation is attributed to cAMP inhibition of cell-cycle regulators, cyclin D and Skp2, with early reports describing PKA as the likely cAMP effector (456, 572, 1134). However, further investigation revealed that PKA inhibition incompletely restores Skp2 expression after cAMP stimulation, suggesting the full inhibition of cell cycle progression involves another cAMP-dependent signaling effector (417, 1134). Thus Hewer et al. (417) explored the potential position of EPAC to fill this role in cAMP-dependent cell-cycle arrest of VSMCs. Indeed, direct or constitutive activation of EPAC was found to synergize with PKA and augment cell cycle suppression to similar levels observed for nondiscriminant elevation of cAMP. Unexpectedly, investigation into the role of Rap1 in this pathway demonstrated that Rap1 actually appeared to promote cell-cycle progression in VSMCs, while cAMP-mediated growth arrest by EPAC and PKA occurs independent of Rap1. Rather, the activation of PKA and EPAC attenuates ERK1/2 and JNK signaling and disrupts actin cytoskeletal organization in VSMCs (417). Together, these effects could collectively induce antiproliferative effects in VSMCs. However, the mechanism of synergistic regulation for these antimitogenic regulators by concurrent EPAC and PKA stimulation remained elusive. Beginning to address this concern, a subsequent investigation supported the synergistic signaling of EPAC and PKA in cAMP-mediated VSMCs growth arrest, which was absent in endothelial cells suggesting a cell specific signaling pathway. In this study, EPAC and PKA activation reduced early growth response 1 (Egr1) expression, which is required for basal VSMC proliferation and regulated by cAMP to suppress proliferation. Cytoskeletal remodeling attributed to EPAC and PKA inhibition of Rac1 blocks nuclear entry of ERK1/2 preventing phosphorylation of Elk1 and subsequent transcription of Egr1 (527). Together, cAMP-mediated cell-cycle arrest in VSMCs appears to be a combination of effects facilitated by both EPAC and PKA to alter transcriptional regulation of mitogenic genes; however, the mechanism of overlapping regulatory roles for EPAC and PKA in these pathways requires more experimentation to be fully defined.
Aside from VSMCs, proliferation and migration of vascular endothelial cells is crucial for the angiogenesis process that is important to wound healing and promoting blood flow to hypoxic tissues. However, disrupted signaling can lead to either insufficient or excessive signaling and has implicated angiogenesis in pathologies such as cardiovascular disease and tumor progression. Therefore, the temporal activation and suppression of this signaling system is important to maintain low proliferation rates until injury stimuli are present.
Rap1A and Rap1B proteins are reported to be intricately involved in the process of angiogenesis, and genetic deletion of Rap1 genes exhibit defects in vessel formation (189, 576, 1149). Furthermore, loss of Rap1 was accompanied by reduced migration and proliferation of endothelial cells contributing to attenuation of aortic sprouts in response to growth factors, VEGF and fibroblast growth factor (FGF). At the molecular level, activity of ERK1/2, p38, and Rac were reduced in the absence of Rap1, suggesting the importance of these signaling components in the angiogenetic process (189, 1149). Further investigation into this pathway revealed that although Rap1B is activated by VEGF2 receptor, Rap1B also acts upstream of the VEGF2 receptor, in part, through αvβ3 integrin to promote full receptor activation, suggesting a positive-feedback mechanism (576). The functional consequence of Rap1 deletion leads to the thought that EPAC may also participate in angiogenesis through Rap1 signaling.
Surprisingly, in contrast to the Rap1-null animal studies, initial studies focusing on the angiogenesis of tumors found that in human microvascular endothelial cells (HMVECs) activation of EPAC or Rap1 attenuates VEGF-dependent angiogenesis. Microarray analysis identified inhibitor of differentiation 1 (Id1) to be upregulated with VEGF stimulation and consequently reduced in the presence of EPAC activation (248). One action of Id1 is to enhance tumor angiogenesis through suppression angiogenetic inhibitor thrombospondin 1 (TSP1) (644, 645, 1076). Indeed, activation of EPAC in endothelial cells attenuated VEGF induction of Id1 to increase TSP1 expression. Furthermore, the inhibitory signal of EPAC/Rap1 is abolished when TSP1 signaling is blocked, supporting TSP1 induction as the culprit in EPAC/Rap1 suppression of angiogenesis (248). Another investigation describes reduced proliferation and angiogenesis concurrent with PDE4 inhibition, suggesting that elevated levels of cAMP suppress angiogenesis in hypoxic tumor environments (843). These accounts may suggest potential environmental stimuli that determine the functional role of cAMP effectors during angiogenesis.
Following these controversial reports, subsequent studies have supported the Rap1-null animal results, suggesting that EPAC/Rap1 as well as PKA are involved in the induction of angiogenesis, and not suppression. Forskolin-mediated elevation was determined to increase angiogenesis similar to VEGF in vitro and in vivo (756). Activation of ERK (287, 756) and also the Akt/eNOS/NO pathway were associated with EPAC/Rap1 activity, and not PKA, which was linked with CREB activation and VEGF expression (337, 756). Silencing or truncation of EPAC1 abolished activation of these pathways and proliferation of endothelial cells supporting the role of EPAC1 in cAMP-mediated angiogenesis (287, 337, 756). These results suggest cAMP uses both PKA and EPAC to signal through multiple pathways and induce angiogenesis. Controversial results may represent differences in angiogenetic signaling dependent on the microenvironment of the endothelial cell. Thus, during typical vascular injury, the cAMP signal mediates a pro-angiogenic phenotype that is reliant on EPAC/Rap1 and PKA signaling. However, in the case of hypoxic tumor microenvironments, additional signal inputs and functions may relinquish the pools of EPAC and PKA to digress the cAMP signal attenuating this pathway of vessel development, while also potentially implicating alternative pathways corresponding to secreted growth factors by the tumor. More studies will be required to fully resolve these outcomes and define the entire role of EPAC in angiogenesis.
Currently the role of EPAC in the vascular system is undeniable. Under normal physiological conditions, cAMP enlists functional pathways of both EPAC and PKA to induce vasorelaxation and oppose RhoA-mediated contraction. This process involves modulation of both vascular smooth muscle cytoskeletal mechanics as well as endothelial secretion of vasodilatory molecules to enhance the relaxant effect. Intriguingly, under thermal stress, the function of EPAC/Rap1 appears to switch to support RhoA activation, although the nature and extent of this response in the macrovasculature is still unknown. EPAC/Rap1 is also reported to be crucial to barrier integrity, especially during reoxygenation after ischemia-reperfusion injury, and concurrent activation of EPAC and PKA pathways by cAMP is observed to induce angiogenesis. Similar to relaxation dynamics, stress conditions in microvascular cells invert the function of EPAC/Rap1 to attenuate angiogenesis; thus further investigation into influences derived from the microenvironment and vascular bed may provide fresh insight for this versatile cAMP cascade.
C. Endocrine and Reproductive Systems
The endocrine/hormonal system provides a critical mechanism for communications among various tissues and organs to maintain homeostasis within a living organism. cAMP was originally discovered to mediate the intracellular functions of epinephrine and glucagon in regulating sugar metabolism. The involvement of cAMP in a broad range of the hormonal regulation in all endocrine components is now clear. In this section, the roles of EPAC proteins in cAMP-mediated endocrinal functions will be discussed.
1. The hypothalamic-pituitary-adrenal axis
The hypothalamic-pituitary-adrenal (HPA) axis covers the functions of three endocrine glands, the hypothalamus, the pituitary, and the adrenal gland, as well as their interactions. The HPA axis is a major neuroendocrine system that controls many physiological processes and stress responses, including mood and emotions, energy balance, sexuality, and immune response. It is a major command center for organisms to integrate physical, psychosocial, and environmental cues to maintain physiological homeostasis and optimal survival.
a) hypothalamic functions.
The hypothalamus incorporates information from various sensory pathways and controls many physiological functions including appetite, energy balance, and circadian rhythms. The adipose-derived anorexigenic hormone leptin and the gastrointestinally derived orexigenic hormone ghrelin act in the hypothalamus to antagonistically regulate energy homeostasis (543, 755, 1055, 1196). Leptin binds the “long form” of the leptin receptor (OB-Rb or LepRb), a single-transmembrane-domain cytokine receptor expressed in neurons of the arcuate nucleus (Arc), and activates intracellular signaling cascades including the Janus kinase (JAK)/STAT3 pathway (63, 1055). Recent studies based on genetic and pharmacological manipulations have demonstrated EPAC/Rap1 as an important pathway for modulating leptin signaling in the hypothalamus. Elmquist and colleagues (322) demonstrate that cAMP-elevating agents can impair leptin-mediated STAT3 phosphorylation in hypothalamic brain slices. This inhibitory effect of cAMP is not affected by PKA inhibition, suggesting a potential PKA-independent event. Supporting this inhibitory function, direct activation of EPAC using an EPAC-selective cAMP analog 007 mimics the effect of cAMP, blunting leptin signaling in the hypothalamus, and impairs leptin-evoked depolarization of POMC neurons. Moreover, intracerebroventricular (ICV) infusion of 007 in mice blocks the anorexigenic actions of leptin (322). These findings suggest that pharmacological activation of the EPAC pathway in the hypothalamus leads to an attenuation of leptin signaling, a condition referred to as leptin resistance, which represents a key signaling defect underlying the development of obesity.
Genetic EPAC knockout mouse models have been generated to determine which EPAC isoform is involved in the cAMP-mediated leptin resistance. C57BL/6 EPAC1 null mice display enhanced hypothalamic leptin sensitivity as measured by STAT3 phosphorylation. These mice also exhibit reduced food intake, white adipose tissue (WAT) and plasma leptin levels, and enhanced resistance to high-fat diet (HFD)-induced obesity and glucose intolerance than wild-type counterparts. Consistent with the genetic knockout data, pharmacological inhibition of EPAC1, by the EPAC-selective inhibitor ESI-09, reduces plasma leptin levels in vivo and enhances leptin signaling in organotypic hypothalamic slices (1150). These data suggest that EPAC1 plays an important role in regulating adiposity and energy balance by modulating leptin signaling. A subsequent report using WAT-specific knockout EPAC1 mice further reveals EPAC1 engaging dual roles in leptin signaling, promoting leptin expression and secretion in peripheral WAT and suppressing leptin signaling in the CNS (444). The regulatory effects of EPAC on leptin signaling in the hypothalamus is most likely mediated by the small GTPase Rap1, as loss of neuronal Rap1 also protects against diet-induced obesity, glucose imbalance, and insulin resistance in the periphery while enhancing leptin sensitivity in hypothalamus (489). In addition, central infusion of an EPAC2-specific inhibitor, ESI-05 (1043), markedly enhances hypothalamic leptin sensitivity and reduces body weight in HFD-fed wild-type mice, but not in mice with CNS Rap1 ablation. These data suggest that EPAC2, acting through Rap1, is also involved in modulating leptin signaling, adiposity, and energy balance in the hypothalamus where an EPAC2 targeted inhibition may represent a promising antiobesity strategy (489). However, a recent study of EPAC2−/− mice demonstrates whole-body deletion of EPAC2A induces an impaired hypothalamic leptin signaling, early-onset increases in plasma leptin levels, and decreases in plasma adiponectin levels. Moreover, EPAC2 null mice are more prone to HFD-induced obesity presumably due to increased food intake and lower energy expenditure compared with HFD-fed wild-type mice (454). These data suggest a protective role of EPAC2 against obesity. It is important to note that EPAC2 is also expressed in other major endocrine organs, particularly in pancreatic β cells where the cAMP effector participates in insulin secretion. Therefore, the causes of the apparent obesogenic phenotype resulting from the whole-body EPAC2−/− mice may be complex in nature. Further studies using tissue-specific EPAC2−/− mouse models are needed to clarify the hypothalamic role of EPAC2 in leptin signaling.
In addition to modulating leptin signaling, EPAC proteins appear to be involved in gene transcriptional regulation in the hypothalamus. In immortalized murine hypothalamic cell lines, cAMP-stimulated proglucagon mRNA expression is reported to be mediated by the activation of EPAC, but not PKA (215). With the assistance of a CRE-dependent reporter, Breit and co-workers (348) show inhibition of EPAC proteins alleviates melanocortin 4 receptors (MC4R)-induced CRE reporter activation, whereas suppressing PKA activity has only weak or no effects on reporter gene expression in hypothalamic cell lines. Consistently, EPAC-mediated ERK activation, not PKA activation, is required for α-melanocyte-stimulating hormone (α-MSH)-induced CREB phosphorylation and CRE activation. Furthermore, EPAC inhibition mitigates α-MSH-induced expression of c-fos and thyrotropin-releasing hormone (TRH) mRNA, while inhibition of PKA blocks TRH expression alone. These findings reveal a salient role of EPAC in the regulation of hypothalamic gene expression (348).
b) pituitary functions.
cAMP signaling is previously reported to perform vital roles in various pituitary functions such as hormone production and cell proliferation. Dysregulation or mutational change of individual cAMP-associated components causes a spectrum of endocrine disorders (826). Extensive investigations have also established that EPAC proteins, working independently or alongside with PKA, contribute significantly to cAMP-mediated pituitary functions. For example, EPAC, likely EPAC2, has been shown to enhance the Ca2+-dependent secretory activity in mouse melanotrophs, neuroendocrine cells from the intermediate lobe of the pituitary that secrete α-MSH and β-endorphin which is derived from the precursor polypeptide proopiomelanocortin (POMC) in response to stimuli (929). The use of pharmacological agents selective for EPAC or PKA further demonstrated that EPAC activation boosts the exocytosis of low Ca2+ threshold, ATP-independent, and release-ready vesicles; however, PKA activation is required for augmentation of exocytosis for ATP-dependent secretory vesicles (929). A subsequent study using Rab3a knockout mice illustrates that deletion of Rab3, a small GTPase of the Rab3 subfamily critical to membrane fusion of transport vesicles during late stages of Ca2+-regulated exocytosis (219), abolishes cAMP-mediated enhancement of ATP-independent/EPAC2-dependent release of α-MSH. These data, coupled with the fact that Rab3 interacts with EPAC2 and Rim2 in regulated exocytosis (796), suggest that Rab3 promotes fusion of vesicles with the plasma membrane by interaction with EPAC2/Rim2 (928). On the contrary, cAMP enhances Ca2+-dependent action potentials and prolactin (PRL) release chiefly in an EPAC-independent and PKA-dependent manner in pituitary lactotrophs (354).
Hypothalamic neuropeptides regulate pituitary cell functions by generating different intracellular second messengers, including cAMP. Current evidence conveys that the functions of cAMP on regulating pituitary proliferation are cell-type specific. For instance, cAMP increases DNA synthesis and cyclin D1 expression in primary cells isolated from human somatotropinomas, while exerting opposing effects in cells derived from human prolactinomas and nonfunctioning adenomas. Similar mitogenic effects are observed in corresponding immortalized pituitary cell lines and shown to be mediated by both EPAC/Rap1/ERK and PKA/CREB pathways (994, 1074), respectively. Curiously, corticotropin releasing factor-induced ERK phosphorylation in AtT20 cell, a murine pituitary tumor cell line, proceeds via a cAMP-dependent mechanism mediated by the activation of EPAC2, but not PKA (1060).
c) adrenal functions.
A novel splicing variant of EPAC2, referred to as EPAC2B, is identified in mouse adrenal gland (766). EPAC2B is transcribed from intron 4 of the EPAC2 gene, and thus lacks the corresponding exons 1 to 4 of the full-length EPAC2A while also possessing an alternative exon 1b at the NH2 terminal. As such, EPAC2B has similar domain arrangement as EPAC1 without the first CBD and differs from the liver-specific EPAC2 isoform EPAC2C by preservation of the DEP domain. When ectopically expressed in HEK293 cells, EPAC2B retains the ability to activate downstream effector Rap1 in a cAMP-dependent manner. However, unlike EPAC2A, which is mainly associated with the plasma membrane, ectopically expressed EPAC2B is primarily localized in cytosol and incapable of stimulating hormone secretion in response to increased intracellular cAMP in MIN6 cells (766).
In the adrenal cortex, cAMP-induced steroidogenesis in response to trophic hormones and other external stimuli is largely mediated by the action of PKA, through acute regulation by means of phosphorylating hormone-sensitive lipase and steroidogenic acute regulatory protein (StAR). These PKA-mediated actions increase the availability of free cholesterol and its delivery to the inner mitochondrial membrane for steroid synthesis (27, 555). PKA signaling is also implicated in chronic steroidogenesis by transcriptional activation of other steroidogenic enzymes (815). A genome-wide cDNA microarray analysis for shifting mRNA expression in response to adrenocorticotropic hormone (ACTH) in mouse adrenal Y1 cells revealed that PKA activation is responsible for 56% of the ACTH transcriptional modulations, while PKC activation accounts for only 6% (912). Although this study demonstrates a dominant role of PKA in ACTH induced steroidogenesis, a large portion (~38%) of ACTH-mediated transcription remains unaccounted for. Could EPAC, particularly the adrenal specific EPAC2B, participate in these PKA and PKC-independent functions? To date, various studies suggest that besides mediating cAMP-induced effects on cytoskeletal integrity and cell migration, EPAC is not significantly implicated in many ACTH-mediated functions, such as steroidogenesis and ERK activation in adrenocortical cells (36, 888).
Adrenal zona glomerulosa (AZG) cells produce mineralocorticoids in response to various extracellular stimuli such as ACTH, angiotensin II (ANG II), and extracellular potassium, which activates two major intracellular second messenger systems: cAMP production and calcium mobilization. In primary rat and bovine AZG cells, activation of Ca2+/calmodulin-dependent protein kinases (CaMK) is essential for aldosterone production stimulated by ANG II, ACTH, and cAMP. Interestingly, cAMP-induced CaMK activation is mediated neither by PKA nor by EPAC in these cells. While PKA inhibition partially blocks cAMP-induced aldosterone production, activation of EPAC/Rap1/2 by 007 does not affect aldosterone secretion (331). These results reveal the complexity of second messenger signaling in AZG cells and point to the potential existence of an unidentified cAMP effector, in addition to PKA and EPAC, involved in modulating CaMK activity and aldosterone secretion.
Similarly demonstrated in adrenal zona fasciculata (AZF) cells, ACTH induces the transcriptional expression of TREK-1, a member of the two-pore, four-transmembrane-segment family of K+ channels, by a cAMP-dependent mechanism only partially blocked by PKA inhibition, and EPAC activation or Ca2+ signaling alone are insufficient in promoting TREK-1 expression. In AZF cells, ACTH also acutely inhibits TREK-1 current, resulting in membrane depolarization, Ca2+ entry, and cortisol secretion. Although PKA is known to inhibit TREK-1 channels by direct phosphorylation in neuronal cells (812), ACTH and cAMP-mediated inhibition of the adrenal TREK-1 channels are unaffected by inhibition of PKA. Moreover, intracellular administration of a selective EPAC agonist, 007, mimics the effects of cAMP by inhibiting TREK-1 channel activation, in an EPAC2-dependent manner (625). Using PKA-selective cAMP analogs modified at position 6 of the adenine ring, Enyeart et al. (626) reveal that intracellular administration of 6-benzoyl-cAMP or 6-monobutyryl-cAMP potently inhibit TREK-1 channels in bovine AZF cells. Surprisingly, the inhibition of TREK-1 mediated by these cAMP analogs does not require PKA activation as PKA inhibition by H-89, Rp-cAMP, or PKI has no effect on TREK-1 inhibition caused by N6-substituted cAMP analogs (626). These findings again point to the potential involvement of an unknown mediator associated with cAMP-related functions in adrenal cells.
Extensive studies have been performed to study cAMP signaling using the PC12 cell, a cell line derived from a pheochromocytoma of the rat adrenal medulla and widely used for investigations related to neuroendocrine as well as neuronal cell proliferation and differentiation (366). 5-Hydroxytryptamine (5-HT)-induced activation of ERK in PC12 cells transfected with the 5-HT7 receptor, a neuronal Gαs-coupled receptor, is shown to proceed through a cAMP-dependent pathway involving EPAC rather than PKA (618). Using cAMP agonists selective for PKA or EPAC, Kiermayer et al. (519) began to dissect the corresponding roles of EPAC and PKA in regulating ERK in PC12 cells revealing that stimulation of PKA is sufficient to evoke rapid and transient ERK activation, whereas activation of EPAC induces slower, more sustained ERK activation. Unlike PKA, EPAC activation alone fails to promote PC12 cell proliferation. Moreover, concomitant activation of EPAC suppresses PKA- and epidermal growth factor (EGF)-induced proliferation and promotes neurite outgrowth. These results suggest that activation of EPAC synergizes with PKA induction by extending the duration of ERK activation and switching a proliferation-promoting signal into a differentiation-stimulating one (519).
2. Thyroid functions
Cell signaling mediated by cAMP is also implicated in thyrocytes where the major function of TSH, the principal physiological regulator of the thyroid gland, is to increase intracellular cAMP. In primary cultures of canine thyrocytes, EPAC1 mRNA is highly expressed, and this expression is further enhanced in response to TSH stimulation, whereas EPAC2 mRNA is not detected by Northern blotting analyses in basal or stimulated dog thyrocytes (253). The expression of EPAC1 protein is also detected in dog thyrocytes using EPAC1 specific antibodies by immunoblot and immunofluorescence. Remarkably, despite the high EPAC1 expression and TSH responsiveness of mRNA levels, PKA-selective cAMP analogs appear to mimic all tested cAMP-dependent functions of TSH. Administration of EPAC-specific activator, 8-p-chloro-phenyl-thio-2′-O-methyl-cAMP, had no discernable effect, suggesting EPAC1 is not involved in mediation of currently known cAMP-dependent TSH effects (253). An additional report in rat thyroid cells describes a mediatory function for PKA, but not EPAC, in TSH/cAMP-stimulated ERK activation and cell proliferation (1079). Gene expression profile analyses of human primary cultured thyrocytes further support the conclusions with TSH, FSK, PKA activator N6-MB-cAMP treated cultures sharing similar mRNA expression profiles, whereas 007-treated cultures demonstrate distinct profiles. These data suggest that TSH-induced gene expression is mainly modulated by cAMP and PKA and not by EPAC (1062).
On the other hand, studies using PCCL3, a normal TSH-dependent rat thyroid follicular cell line, suggest that EPAC, in cooperation with PKA, is required for TSH-mediated cell proliferation. Supporting this claim, an EPAC1 mutant incapable of membrane interaction abolishes cAMP-mediated Rap1 activation and mitogenesis (422). In the same cell line, EPAC1 and PKA synergistically activate EPAC1-associated protein phosphatase 2A (PP2A) and promote dephosphorylation of Akt (433). A recent study, also suggesting TSH signaling through an EPAC pathway, shows that activation of EPAC, but not PKA, is responsible for TSH-stimulated efflux of taurine, a cellular osmolyte in rat thyrocytes, FRTL-5 (317).
Abnormal dysregulation of cAMP signaling caused by activating mutations in the thyrotropin receptor or in the corresponding Gαs subunit is responsible for 80% of the autonomous or hot adenomas, but not in cold thyroid follicular adenomas that do not take up radioactive iodide in the presence of normal serum TSH (990). Mutational analysis of the EPAC/Rap1 signaling pathway in cold thyroid follicular adenomas from 10 patients reveals no mutations in either EPAC or Rap1, suggesting that EPAC/Rap1 signaling pathway is unlikely involved in the generation of cold thyroid follicular adenomas in the thyroid gland (1065). Immunohistochemical analyses of EPAC1 in samples from patients with anaplastic thyroid carcinoma (ATC), poorly differentiated thyroid carcinoma (PDTC), papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and benign follicular adenoma reveal significant EPAC1 expression variations among thyroid tumor types with adenomas and FTC expressing high levels of EPAC1 while PTC, PDTC, and ATC expressing lower levels gradually. This pattern of strong expression in more differentiated thyroid tumor and weaker expression in less differentiated subtypes is consistent with the finding that activation of EPAC proteins stimulates the proliferation of a differentiated rat thyroid cell line, but has no effects on the proliferation of various thyroid carcinoma cell lines (109).
3. Sex reproductive systems
a) ovarian follicle development.
The development and maturation of ovarian follicles are tightly regulated by pituitary gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) (871). The binding of FSH and LH to their cognate GPCRs activates AC and stimulates the production of cAMP in ovarian granulosa cells, where both EPAC1 and 2 are expressed (355, 1101). In rat primary granulosa cells, FSH-mediated Akt and p38 phosphorylation are independent of PKA activation, while transcriptional induction and phosphorylation of serum and glucocorticoid-induced kinase (Sgk) by FSH requires PKA, as well as PI3K and p38 activation (355). The effects of FSH on Akt and p38 are likely mediated by EPAC. However, unlike FSH stimulation, 007 treatment only induces marginal to modest Akt and p38 phosphorylation, suggesting a more complex mechanism (1101).
In primary human granulosa cells, stimulation by LH, as well as forskolin and dibutyryl-cAMP, leads to progesterone secretion, an effect that can be dose-dependently suppressed by PKA-selective inhibitors. Interestingly, granulosa cells become less susceptible to PKA inhibition as luteinization progresses, a process coincident with increased EPAC1 expression, which is LH inducible. Moreover, activation of EPAC by 007 dose-dependently enhances progesterone secretion by luteinizing human granulosa cells (179). These results suggest that cAMP-induced progesterone secretion is mediated by both PKA and EPAC1, and while PKA plays a more predominant role in basal progesterone release, EPAC1 may be involved in sustained and chronic regulation of progesterone secretion.
Within the follicle, paracrine interactions between granulosa cells and the oocyte are also critical for oogenesis and folliculogenesis. Granulosa cell-derived ligand for the receptor tyrosine kinase KIT (KITLG) is important for multiple aspects of oocyte and follicle development, including primordial follicle activation, oocyte survival and growth, granulosa cell proliferation, and maintenance of meiotic arrest before germinal vesicle breakdown (255). In cultured zebrafish follicles, cAMP differentially regulates KITLG expression at different stages during folliculogenesis, promoting KITLG expression at the early vitellogenic stage, but suppressing the ligand at the full-grown stage. Importantly, it appears that PKA mediates cAMP’s stimulatory effect on KITLG expression, while EPAC activation suppresses the expression of KITLG (1159).
b) sperm activation and oocyte recognition.
During fertilization, cAMP plays critical roles in regulating sperm motility, capacitation, and acrosome reaction (358). EPACs, both EPAC1 and EPAC2 along with their downstream effector Rap1, are identified to be expressed in mouse male germ cells (8, 21, 74), as well as in stallion and boar spermatozoa (705). EPAC proteins exhibit differential cellular distribution patterns depending on the type of spermatogenic cells. As revealed by immunofluorescence analyses, under noncapacitating conditions EPAC1 is localized in the apical region of the acrosome and in the membrane of the tail mid-piece on boar spermatozoa, whereas EPAC2 is absent in the head and primarily localized to the connecting piece with overall weak expression along the mid, principal, and end pieces of the tail (705). Incubation of boar spermatozoa in capacitating conditions promotes accumulation of EPAC1 staining in the connection piece between the tail and head, as well as translocation to the post-acrosome region. Diverging redistribution of EPAC2 can be observed in the acrosome region, in addition to the connecting piece, under capacitating conditions (705). Similarly, activation of EPAC2 by 007 in mouse spermatozoa results in the translocation of EPAC2 from cytosolic towards the Golgi region, where EPAC2 interacts and activates Rap1 and RA-RhoGAP to promote the progression of spermatogenesis (8). Moreover, 007 treatment promotes calcium mobilization, sperm motility, acrosome reaction, plasma membrane phospholipids scrambling, and E-cadherin redistribution to colocalize with EPAC1. In hamster spermatozoa, the activation of EPAC, not PKA, is demonstrated to be responsible for cAMP-induced flagellar bending that generates the propulsive force for sperm motility (531). Taken together, these results suggest that EPAC proteins play an important role in mammalian gamete recognition and fertilization.
Further demonstration of the potential for EPAC proteins to exhibit these physiological functions is illustrated by expression of both EPAC1 and EPAC2 in human sperm (103, 705). Importantly, activation of EPAC by EPAC-specific cAMP analog in streptolysin O-permeabilized human sperm is sufficient to induce acrosome reaction, a regulated exocytotic process leading to fusion between the outer acrosomal and plasma membranes of sperm cells. The cAMP/EPAC-elicited acrosome reaction is insensitive to PKA inhibition and does not require extracellular Ca2+, but can be blocked by antibodies against components of the vesicle fusion machinery (Rab3A, NSF: N-ethylmaleimide-sensitive factor and α-SNAP: α-NSF attachment protein), intra-acrosomal Ca2+ chelators, or botulinum toxin E, a potent inhibitor of exocytosis (103). A subsequent study further reveals a bifurcated signaling pathway initiated by the activation of soluble ACs and EPAC proteins, which promote the concomitant activations of two small GTPase-mediated cascades, one involving direct activation of Rap1/PLC-ε and calcium release from an IP3-sensitive store and another involving indirect activation of Rab3A and the assembly of SNARE-related fusion machinery. The convergence of these two concerted cascades downstream of EPAC ultimately leads to the fusion of acrosomal and plasma membranes (102, 640, 889).
c) placenta development.
Immunohistochemistry analyses demonstrate the expression of both EPAC1 and EPAC2 in human placental tissue within the mononuclear villous cytotrophoblast (CTB) and the multinucleated syncytiotrophoblast (STB), which produces human chorionic gonadotropin (hCG) and progesterone (1174). Studies in BeWo cells, a well-characterized trophoblast-derived human choriocarcinoma cell line capable of differentiation and syncytialization, by selective activation EPAC or PKA using 007 or N6-phenyl-cAMP, respectively, reveal that although PKA activation is more predominantly involved in the production of hCG and progesterone, the cAMP-induced functional differentiation and syncytialization of human trophoblasts is mediated by the EPAC signaling pathway (1174). The importance of EPAC signaling in differentiation of the STB is further validated by two independent studies demonstrating that EPAC/Rap1 activation stimulates placental cell fusion in a PKA-independent, but GCM1 (glial cell missing 1)-dependent manner (157, 158). GCM1 is a placental transcription factor important for trophoblast fusion by regulating the expression of two fusogenic proteins, syncytin-1 and -2 (1175). Investigations by Chen and colleagues (157) demonstrate the cAMP/EPAC1/Rap1/CaMKI signaling cascade activates GCM1 by promoting its desumoylation and acetylation. Specifically, EPAC1-mediated CaMKI activation induces phosphorylation of Ser47 in GCM1 to facilitate interaction between the desumoylating enzyme SENP1 and GCM1. Desumoylation of GCM1 stimulates syncytin-1 and -2 gene expression (157). Moreover, EPAC1 stimulates histone deacetylase 5 (HDAC5) phosphorylation on Ser259 and Ser498 in a Rap1- and CaMKI-dependent manner. Phosphorylation of HDAC5 promotes nuclear export of the deacetylase to prevent interaction and suppressive deacetylation of GCM1 (158).
In addition to partaking in the regulation of the STB differentiation, EPAC proteins have also been implicated in cAMP-mediated decidualization of human endometrial stromal cells during pregnancy (568–571) and hCG-mediated leptin gene expression in human placental cells (669, 670). Overall, these studies depict the pervasive nature of EPAC signaling in the reproductive system including involvement in multiple stages of reproductive physiology including ovarian function, oocyte maturation, fertilization, embryo implantation, and development.
4. Pancreas
a) role in β-cells and insulin secretion.
The pancreas is a major endocrine organ producing several important hormones including insulin and glucagon that are critical for glucose and energy homeostasis. Insulin secretion from pancreatic β-cells is primarily controlled by plasma glucose levels. After entering β-cells, glucose is metabolized to produce ATP, which leads to the closure of KATP, and in turn triggers membrane depolarization and the opening of VDCCs. The elevation of intracellular Ca2+ stimulates the fusion of insulin granules and ultimately increases the rate of insulin release (546). In addition to Ca2+, cAMP signaling also play important role in regulating insulin secretion. The involvement of EPAC proteins, particularly EPAC2, in transducing cAMP-mediated potentiation of insulin exocytosis has been well documented by in vitro and in vivo analyses, and reviewed extensively (14, 427, 429, 594, 595, 648, 933, 1022). Therefore, the following section will focus mainly on the more recently published studies of the function of EPAC in pancreas.
Glucose-stimulated insulin secretion (GSIS) comprises a biphasic release of insulin with a rapid burst of insulin during the initial phase, followed by a second attenuated, but more sustained insulin release. The first phase arises from the readily releasable pool (RRP) of insulin: vesicles docked at the plasma membrane and primed for ready release, but the second phase requires mobilization and subsequent priming of insulin vesicles to replenish the depleted RRP (987). GSIS can be modulated by a number of factors, including hormones and neural inputs, as well as non-glucose nutrients. GSIS is strongly potentiated by incretin hormones such as GLP-1 and glucose-dependent insulinotropic peptide/gastric inhibitory polypeptide (GIP), which bind to their corresponding receptor on the surface of pancreatic β-cells to stimulate cAMP production (256, 291, 827). Senio and co-workers first demonstrated a direct role of EPAC2 in regulating exocytosis and incretin-potentiated insulin secretion by interacting with Rab3A and Rim family of small G proteins (499, 796), as well as the CAZ (cytomatrix at the active zone) protein Piccolo (318). In addition, a direct interaction between EPAC2 and the NBF-1 domain of SUR1, which interacts with Kir6.2 to form inwardly rectifying KATP, has been identified in a yeast two-hybrid screen and affinity pull-down assay using recombinant EPAC2 and NBF-1. This interaction is inhibited by high cAMP concentrations (955). These interactions among EPAC2, SUR1, Rim2, and Piccolo bring together sensors for cAMP, ATP, and calcium to coordinate insulin exocytosis in β-cells. Indeed, subsequent studies suggest that EPAC proteins act independently of PKA to mediate cAMP’s inhibitory effect on the KATP in pancreatic β-cells (492, 495).
GLP-1 is known to increase cytoplasmic calcium by mobilizing intracellular calcium storage in a cAMP-dependent manner in pancreatic β-cells (430). Indeed, in rat INS-1 β-cells, GLP-1-potentiated Ca2+-induced Ca2+ release (CICR) is mediated by EPAC2 and not PKA (491). In MIN6 β-cells, a slightly different effect is observed demonstrating EPAC2 and PKA synergistically mediating GLP-1 induced CICR and mitochondrial ATP synthesis by acting on IP3R or RyR, respectively (1046). Studies based on PLC-ε knockout mice indicate that EPAC2 stimulates IP3R-mediated Ca2+ mobilization through activation of PLC-ε (260), a known downstream effector of EPAC/Rap signaling (916). Calcium/calmodulin-dependent serine protein kinase (CASK), acting downstream of PKA, is also involved in GLP-1-induced insulin secretion in INS-1 cells (1212).
Studies performed by Holz and colleagues (177, 493, 494) using EPAC-selective cAMP analogs have provided convincing evidence supporting the activation of EPAC proteins stimulation of CICR and exocytosis in pancreatic β-cells. Furthermore, EPAC-selective cAMP analogs are capable of potentiating both first and second phases of GSIS in mouse and human islets (176, 515, 922). Surprisingly, inhibition of PKA activity virtually abolished the action of EPAC-selective cAMP analogs to potentiate GSIS in human islets, suggesting a complex mechanism in which PKA activation is permissive for the glucose-dependent and EPAC-regulated insulin secretion in these islets (176).
GLP-1 has also been shown to enhance glucose uptake, mitochondrial membrane potential, and cellular ATP production, as well as glucokinase (GK) activity in rat INS-1 and primary β-cells. GK is important for maintaining proper glucose sensitivity in β-cells. The effect of GLP-1 on GK is cAMP/EPAC-dependent, but PKA-independent. Silencing of EPAC2 or its interacting partners, Rim2 or Rab3A, obliterates the stimulatory effect of GLP-1 on glucose uptake, cellular ATP levels, and GK activity in INS-1 cells. These results suggest that GLP-1 can act through EPAC2 to further potentiate GSIS by stimulating GK activity and glucose metabolism (803). In addition to mediating GLP-1’s functions, EPAC2 is also important for prostacyclin- (385) or P2Y purinergic receptor (P2YR)-induced potentiation of GSIS (1195, 1197), as well as bile acid-stimulated insulin secretion in the pancreatic β-cells (566).
Further insight into EPAC2’s role in potentiating GSIS have been ascertained from studying knockout mouse models lacking the expression of EPAC2A, the major EPAC isoform expressed in the mouse pancreas (957, 968, 1189). Whole body deletion of EPAC2A has no effects on pancreas weight, β-cell mass, β-cell size, or insulin content. Moreover, although insulin secretion induced by glucose stimulation alone in pancreatic β-cells isolated from EPAC2A−/− mice were similar to wild-type mice, GSIS potentiation by the GLP-1 analog exendin-4 or cAMP analog 8-bromo-cAMP was significantly reduced in EPAC2A−/− cells (957, 968). The finding that EPAC2A deletion had no impact on β-cell GSIS is substantiated in vivo by the fact that EPAC2A−/− mice fed normal laboratory diet exhibited similar rates of plasma glucose clearance as wild-type mice after intraperitoneal glucose tolerance test (ipGTT), which, unlike oral glucose tolerance test (oGTT), does not invoke production of intestinal incretin hormones (6, 25). An intraperitoneal insulin tolerance test (ipITT) shows slightly increased insulin sensitivity in EPAC2A−/− mice as compared with wild-type animals. Administration of exendin-4 30 min before ipGTT resulted in an attenuated response to exendin-4-mediated GSIS potentiation and a slower decline of serum glucose levels in EPAC2A−/− mice as compared with wild-type controls. While the lack of EPAC2A had little impact on GSIS in lean mice, deletion of EPAC2A resulted in reduced GSIS and impaired glucose tolerance following a 4-wk HFD feeding regimen. These results suggest that EPAC2A primarily facilitates the effects of GLP-1 on GSIS and only significantly contributes to GSIS alone under high metabolic demand (968).
The unaffected oral glucose tolerance in EPAC2A null animals was surprising since oral administration of glucose at the utilized doses is known to elicit production of intestinal incretins such as GLP-1, and EPAC2A has been shown to be important to incretin’s secretagogue action (968). Hence, one would expect to observe a compromised glucose clearance during oGTT in EPAC2A null mice. One potential explanation for this discrepancy is that EPAC2A−/− mice exhibit improved insulin sensitivity, which could conceivably enable compensation for impaired insulin potentiation by incretins in the absence of EPAC2A (968). Another possibility is that deletion of EPAC2A outside of the β-cell may offset the effect of increased incretin levels on GSIS after an oGTT. This proposed explanation is likely as a recent study demonstrates compared with wild-type counterparts, EPAC2A−/− mice have increased levels of plasma leptin (454), which can stimulate GLP-1 secretion from rodent and human intestinal L cells (26).
In mouse and human pancreatic islets, EPAC2 is expressed at significantly higher levels than EPAC1 and is accepted as the main isoform that mediates the function of EPAC in regulating insulin secretion (176, 515). Furthermore, silencing of EPAC1 by shRNA affected neither basal insulin secretion, nor GSIS, nor its potentiation by exendin-4 in mouse islets even when EPAC2A is simultaneously suppressed (968). However, islets isolated from EPAC1 knockout mice have been shown to exert impaired GSIS despite normal basal insulin secretion. This apparent deficiency of GSIS could be an indirect effect related to the significant reduction in the expression of the glucose transporter Glut2 and the transcription factor PDX1, which are critical for β-cell function and insulin expression in these global EPAC1−/− mice (487). A recent report also describes that in INS-1 cells EPAC1, but not EPAC2, participates in glucose-stimulated ERK1/2 phosphorylation, which is important for maintaining β-cell mass and promoting insulin production in response to hyperglycemia (841).
b) epac2 and sulfonylureas.
It was observed that sulfonylurea-stimulated insulin secretion was reduced in EPAC2A knockout mice and that sulfonylureas were capable of activating an EPAC2-based FRET sensor in cells. The authors concluded that antidiabetic sulfonylurea drugs, with the exception of gliclazide, promoted insulin secretion by directly binding to EPAC2 (1189). A subsequent cell-based study showed that the effects of sulfonylureas were isoform specific. Only a sensor based on EPAC2, but not EPAC1, can be activated by sulfonylureas. In addition, an arginine residue (R448) at the switch-board region COOH terminal to the CBD-B in EPAC2 was identified to be critical for the interaction with sulfonylureas (412). Subsequent molecular docking proposes the sulfonylurea binding pocket within the CBD-A of EPAC2 and identifies a histidine residue (H124) predicted to mediate the interaction with sulfonylurea (1006). This residue is ~40 Å away from the R448 residue implicated previously for sulfonylurea interaction (412).
While these findings clearly demonstrate a potential role of EPAC2 in mediating the pharmacological effects of sulfonylureas and provided a rationale for new therapeutic strategies of combining sulfonylureas and incretin-related drugs (1004), they do not rule out the possibility that sulfonylureas exert their effects indirectly. Indeed, biochemical studies from two independent groups employing purified recombinant full-length EPAC2 conclude that sulfonylureas are not able to bind and activate EPAC2, casting doubts on the direct interaction between EPAC2 and sulfonylureas (859, 1041). Additionally, while gliclazide, unlike tolbutamide and other sulfonylureas, was shown to be ineffective in activating EPAC2-FRET sensors (412, 1189), gliclazide did potently inhibit KATP activity, and this action is facilitated by 007 as effectively as tolbutamide in INS-1 cells (465, 596). Neither tolbutamide nor gliclazide activated Rap1 in the same cells (596). Similar results were obtained using mouse islets. Moreover, unlike EPAC activators, tolbutamide was not able to enhance insulin secretion stimulated by gliclazide, and tolbutamide’s effect on GSIS was unaffected by ESI-05, an EPAC2-specific inhibitor (411). Finally, using islets from SUR1−/− mice, glibenclamide and tolbutamide were demonstrated to neither increase insulin secretion, as anticipated if activating EPAC2 directly, nor augment amplification of insulin secretion produced by an EPAC activator (761). Taken together, these data dismiss the possibility that direct activation of EPAC2 by sulfonylureas is responsible for the synergistic interaction of sulfonylureas and EPAC2 to inhibit KATP and promote insulin exocytosis.
c) Role in α-cells.
Glucagon, secreted predominantly from α-cells in pancreatic islets, is the most important hyperglycemic hormone and is critical in metabolic homeostasis by countering the effects of insulin. The expression and secretion of glucagon is tightly regulated by neuronal and hormonal inputs under the influence of both central and paracrine controls (742). A study by Ma et al. (649) illustrates that in addition to the orthodox systemic responses, glucagon release in mouse and rat α-cells can also be regulated in an autocrine/paracrine fashion, in which glucagon stimulates exocytosis in α-cells by binding to glucagon receptor and increasing intracellular cAMP concentration. Interestingly EPAC2 mediates the rapid component of glucagon-stimulated exocytosis in a SUR1-dependent manner (649). In rat primary pancreatic islets, cAMP acts through EPAC2 to stimulate the phosphorylation and nuclear exclusion of the transcriptional regulator Oct-1, a repressor of a proglucagon gene activator Cdx-2, thus promoting Gcg expression (1092). Using EPAC-selective cAMP analogs and a dominant negative EPAC2 mutant, Islam et al. (458) show that EPAC2 activation promotes proglucagon gene (Gcg) transcription and glucagon production, but not glucagon secretion, in the glucagonoma InR1-G9 pancreatic α-cell line. A detailed study using isolated mouse islets further reveals that cAMP can both inhibit and stimulate glucagon secretion depending on the ligand potency and occupancy of the Gαs-coupled membrane receptor evoked. Low intracellular cAMP mimics the inhibitory effect of GLP-1 on glucagon secretion while high cAMP levels, generated by high potency activators, GIP or epinephrine, promote glucagon secretion. Moreover, the inhibitory effects of low intracellular cAMP concentrations appear to be mediated by PKA activation, whereas the stimulatory effects of the higher concentrations of cAMP are mediated by EPAC2 (227).
Glucagon and GLP-1, along with glicentin-related pancreatic polypeptide (GRPP), GLP-2, glicentin, and oxyntomodulin (OXM), are all produced from a common precursor, proglucagon via alternative processing by prohormone convertase 2 (PC2) in pancreatic α-cells or PC1 in intestinal L cells, respectively (742). A recent study defines PC1 expression in mouse and human pancreatic islets, as well as in a glucagon-secreting pancreatic α cell line, αTC1–6. High glucose stimulation induces a significant upregulation of PC1 at both the mRNA and protein levels, as well as increasing GLP-1 secretions in human and mouse islets and αTC1–6 cells. Furthermore, activation of the G protein-coupled bile acid receptor 1 (GPBAR1 or TGR5) augments hyperglycemia-induced PC1 expression and GLP-1 secretion. Mechanistically, TGR5 activation enhances hyperglycemia-induced GLP-1 production by cAMP/PKA/CREB-dependent activation of PC1 expression, while also promoting GLP-1 release via an EPAC-mediated, PKA-independent mechanism in pancreatic α-cells (565). These results suggest that EPAC and PKA act synergistically downstream of TGR5 activation to coordinate cross-talk between α- and β-cells by balancing glucagon and GLP-1 release in α-cells to boost β-cell mass and function under hyperglycemic conditions.
5. Gut and intestinal functions
As mentioned in the pancreatic section above, cAMP signaling is known to regulate the expression of Gcg, in pancreatic islet α-cells, intestinal endocrine L cells, and certain brain neuroendocrine cells (458). Several in vitro studies over the years suggest the involvement of EPAC signaling in the regulation of Gcg gene expression (169, 215, 458, 637, 1092). In intestinal and pancreatic proglucagon-producing cell lines, cAMP acts through EPAC2 to regulate Gcg expression by stimulating ERK1/2 phosphorylation and Cdx-2 expression (169, 637). Conversely, a subsequent study demonstrates that unlike cAMP analogs that activate both PKA and EPAC, EPAC specific agonists fail to stimulate Gcg gene promoter activity in GLUTag, a GLP-1 secreting murine enteroendocrine L-cell line (175). Moreover, specific inhibition of EPAC2 has no effect on cAMP-induced Gcg expression. Furthermore, ectopic expression of either a dominant negative or constitutively active form of EPAC2 suppresses Gcg expression, suggesting previously observed inhibitory effects of dominant negative EPAC2 on Gcg expression (458) were likely caused by nonspecific suppression by overexpressing EPAC2. These results, coupled with the findings that EPAC-specific agonists still significantly increase Gcg mRNA and the EPAC2 inhibitor, ESI-05, reduces basal Gcg expression, challenge the notion that EPAC2 is involved in cAMP-mediated stimulation of Gcg expression, but do not exclude a basal function for EPAC2 involving enhanced stability of Gcg mRNA (175).
In addition to the potential regulation of GLP-1 production and secretion in intestinal endocrine cells, EPAC signaling has also been implicated in secretory regulation of other gastrointestinal hormones. In the human carcinoid endocrine BON cell line, EPAC and PKA synergistically mediate cAMP-induced neurotensin secretion (603). Emerging evidence also suggests that EPAC regulates the secretion of ghrelin, an orexigenic hormone secreted from the X/A-like endocrine cells of the stomach in response to low energy or fasting conditions (543, 980). In ghrelin-expressing rat stomach cell culture, glucagon stimulates ghrelin mRNA production and secretion by activating glucagon receptors. Surprisingly, glucagon-induced ghrelin is independent of PKA activation, but requires EPAC signaling (327); however, a previous report shows that PKA is required for norepinephrine-stimulated ghrelin secretion using the same cell model (328). Moreover, costimulation of glucagon with the sympathetic neurotransmitter norepinephrine further potentiates glucagon-induced ghrelin secretion. These findings suggest that PKA and EPAC synergistically integrate signal inputs from norepinephrine and glucagon, respectively, to coordinate ghrelin release (327). In another study, the ghrelinomas SG-1 cell line is used as a model system to investigate roles for Ca2+- and cAMP-mediated signaling in baseline and norepinephrine-induced ghrelin secretion. Basal ghrelin secretion was found to mainly be sustained by Ca2+ influx through L-type VDCCs, while norepinephrine-stimulated ghrelin secretion is predominantly mediated by cAMP/EPAC-dependent release of intracellular Ca2+ (658), supporting a distinct set of pathways for regulation of ghrelin secretion.
D. Digestive System
The digestive system includes the gastrointestinal track, which starts at the mouth and ends at the anus, as well as accessory digestive glands such as salivary gland, liver, gall bladder, and pancreas. Existing literature suggests that cAMP/EPAC signaling plays important roles in many of the organs involved in digestion.
1. Salivary glands
Saliva production is regulated by synergistic signaling cross-talk mediated by intracellular second messengers IP3 and cAMP, in response to autonomic neural inputs, to promote fluid secretion and protein exocytosis (7, 113). In isolated clusters of mouse parotid acinar cells, activation of β-adrenergic receptor enhances P2X4R- or P2X7R-mediated intracellular calcium influx. Although the augmentation of P2X4R-evoked Ca2+ signals by cAMP modulators is largely mediated by PKA, as the selective PKA-inhibitor PKI abolishes this effect, enhancement of Ca2+ changes by EPAC activation with 007 suggests a potential function for EPAC in P2XR signaling (79). Such a notion is consistent with the finding that isoproterenol-stimulated amylase secretion occurs via both an AKAP5/AC6/PKA complex and a PKA-independent, EPAC pathway in mouse parotid acini (1130).
2. EPAC proteins and hepatic functions
Both EPAC1 and EPAC2 have been identified to be expressed in liver (510). Interestingly, the size of the major EPAC2 transcripts (3.5 kb) in rodent and human liver are smaller than those detected in other tissues (4.2 kb), suggesting the existence of a putative liver-specific EPAC2 isoform (796, 1049). Cloning and exon-intron organizational studies, as well immunoblot analysis, identified a liver-specific 696-amino acid EPAC2 protein that initiates from the first ATG site in exon 10. Although this shorter, liver-specific form of EPAC2 lacks the first CNB domain and DEP domain, the cAMP-induced GEF activity toward Rap1 is retained, and therefore, this EPAC2 form may play a distinctive role in hepatic functions (1049). Furthermore, EPAC2 along with other cAMP-signaling components AC4/AC6/AC8, PKA RIβ/RIIα, and AKAP150 have been reported to colocalize with P2Y12 purinergic receptors, suggesting a P2Y12-mediated cAMP signaling collective in the primary cilia of cholangiocytes, the epithelial lining of intrahepatic bile ducts (664). However, it is still unclear whether the full-length version or the truncated liver-specific isoform of EPAC2 is predominant in the primary cilia of the cholangiocyte.
Currently, nearly all studies related to EPAC hepatic functions have been performed in vitro, using primary cell cultures or established cell lines coupled with pharmacological approaches. While these studies demonstrate that EPAC proteins exert hepatic functions by modulating multiple cell signaling pathways downstream of various physiological stimuli and xenobiotics, the dominant functional isoform of EPAC in these pathways in vivo remains to be described fully. One recent report depicts EPAC activation antagonizing acetaldehyde-induced activation and proliferation of hepatic stellate cell. However, only suppression of EPAC2, but not EPAC1, prevents the activation of hepatic stellate cell induced by acetaldehyde, suggesting differential hepatic functions between EPAC1 and EPAC2 (1156). Clearly, exploring the roles of EPAC proteins in liver using genetic approaches, such as tissue-specific and/or conditional EPAC knockout mouse models in an isoform-specific manner, will be a logical next step in defining hepatic roles for EPAC.
a) epac and glucagon mediated hepatic signaling.
Glucagon is an important hormone for maintaining glucose and energy homeostasis. By acting through classic Gαs-coupled receptors on the hepatocyte plasma membrane, glucagon increases intracellular cAMP and counters the effect of insulin by promoting glycogenolysis and gluconeogenesis while inhibiting glycolysis and glycogenesis. Glucagon also increases bile acid production and regulates hepatocellular volume by modulating membrane potential of hepatocytes (580, 742). In rat hepatocytes, glucagon activates a PLC-dependent inwardly rectifying Ca2+ current and an AC-dependent outwardly rectifying Cl- current. Interestingly, the AC/cAMP-dependent pathway does not involve PKA, but depends on EPAC (29).
Glucagon and cAMP suppress inducible nitric oxide synthase (iNOS) expression and activity in hepatocytes through a PKA-independent mechanism that involves JNK (1187). Consistent with these observations, a cAMP analog specific for EPAC, but not for PKA, inhibits cytokine-induced expression of iNOS in rat hepatocytes. Overexpression of the liver-specific EPAC2 isoform decreases iNOS expression and nitrite production induced by interleukin (IL)-1β and interferon-γ (IFN-γ). While activation of EPAC inhibits Raf1/MEK/ERK signaling in hepatocytes, this effect was not responsible for the suppression of iNOS expression by EPAC. Rather, EPAC-mediated hepatic iNOS suppression likely utilizes an Akt- and JNK-mediated signaling mechanism since inhibition of either Akt or JNK reverses the inhibitory effects of liver-specific EPAC2 on nitrite production and iNOS expression (1186). Also in primary murine hepatocytes, glucagon, acting through cAMP, negatively regulates IL-6-induced MAP kinase activation; however, this inhibitory effect appears to involve both EPAC and PKA (518).
SOCS3 is a negative-feedback regulator of several receptor signaling pathways including those mediated by IL-6 and leptin. In HUVEC, SOCS3 can be induced by a cAMP-dependent and PKA-independent pathway involving EPAC (905, 1160). The expression levels of Socs3 mRNA in fasted mice, as well as in primary hepatocytes treated with glucagon or EPAC selective agonist 007, increase significantly. EPAC activation and SOCS3 expression inhibit glucagon/cAMP-dependent PKA activation, CREB phosphorylation and gluconeogenic gene expression (339). These data indicate that glucagon signaling in hepatocytes involves cross-talk between two distinct cAMP-dependent branches, where the EPAC-dependent pathway negatively regulates the PKA-dependent pathway by inducing SOCS3. Unlike in the case of SOCS3, glucagon/cAMP increases hepatic FGF21 production at posttranscriptional levels via a cooperative mechanism. Activation of both EPAC/Rap1/AMPK and PKA/p38 signaling cascades contributes to glucagon-stimulated FGF21 secretion in hepatocytes (213).
b) epac and bile acids mediated signaling.
Bile acids are derivatives of cholesterols. Aside from the surfactant action that facilitates dietary lipid absorption and uptake of fat-soluble vitamins, bile acids also function as signaling ligands to regulate complex physiological processes (203, 1027). In the liver, bile acids are known to produce both beneficial and harmful effects by activating distinct intracellular signaling pathways. Moreover, EPAC proteins appear to be involved in modulating various bile acid-mediated hepatic effects.
Protection of hepatocytes against cell death induced by hydrophobic bile acids (1102), tumor necrosis factor (TNF)-α (604), and Fas ligand (304) can be attributed to cAMP signaling. In rat hepatocytes, cAMP-mediated protection against bile acid-induced apoptosis is PKA independent. Further supporting this shielding effect, the EPAC specific agonist 007 protects rat hepatocytes against bile acid-, Fas ligand-, and TNF-α-induced apoptosis by Src/PI3K-dependent activation of Akt (209, 338) or focal adhesion kinase and dephosphorylation of the proapoptotic JNK (1052). Subsequent studies reveal that glycogen synthase kinase 3β (GSK3β), downstream of PI3K/Akt, is responsible for EPAC-mediated protection of hepatocyte from bile acid-induced apoptosis. EPAC activation leads to Rap1-dependent GSK3β phosphorylation and inactivation. GSK inhibition prevents bile acid-induced phosphorylation of proapoptotic JNK and thus protects hepatocytes against bile acid-induced apoptosis (481). Furthermore, lipopolysaccharide (LPS) has been demonstrated to induce hepatocyte apoptosis through the activation of JNK and inhibition of Akt, whereas EPAC activation reverses the LPS-mediated activation of JNK and Akt to prevent LPS-induced apoptosis (836). Therefore, these studies suggest EPAC signaling may be implicated in a general cytoprotective role in liver cells. Such a notion is consistent with a recent study, which suggests that EPAC/Rap1-mediated Akt phosphorylation and mitochondrial respiration may contribute to cytoprotective effects against statin-induced toxicity in hepatocytes, the major site for statin inhibition of HMG-CoA (hydroxyl-methyl-glutaryl-coenzyme A) reductase, the rate-limiting step in cholesterol biosynthesis (741).
Polarization of hepatocytes, the primary epithelial cells present in the liver, is important for separating apical and basolateral membrane domains and forming the bile canaliculus (293). Assessing if bile acids potentially regulate hepatocyte polarization and canalicular formation, Fu et al. (316) ascertain the effect of bile acids on canalicular network formation using primary rat hepatocytes in collagen sandwich cultures. Their study reveals that taurocholate accelerates canalicular network formation. Moreover, taurocholate exerts its polarity-stimulating effect by increasing cellular cAMP and signals through EPAC/Rap1/MEK/LKB1/AMPK, but not PKA (316), suggesting a predominant role for EPAC in this process.
c) epac and cholestasis.
Bile salts and other solutes are secreted by hepatocytes into bile canaliculi primarily via ATP-dependent ABC transporters, including the bile salt export pump (Bsep, i.e., Abcb11) and the multidrug resistance-associated protein 2 (Mrp2, i.e., Abcc2) located in the apical membrane of the hepatocyte (212). The levels of expression, activity, and cellular localization of these transporters are critical in bile formation as transport of solutes through the canalicular membrane is the rate-limiting step of this process. Endocytic trafficking of Abcb11 and Abcc2 from the canalicular membrane into vesicular compartments has been associated with cholestasis, a pathological condition involving the impairment of bile secretion (212). Anticholestasis activity has been demonstrated by cAMP acting downstream of glucagon or epinephrine, which inhibits the internalization and promotes the reinsertion of ABC transporters to the canalicular pole of membrane in estrogen-induced cholestasis (207, 738).
In isolated rat hepatocyte couplets, glucagon and a β2 adrenergic agonist, salbutamol, increase intracellular cAMP where the ligands partially and additively reverse estradiol 17β-d-glucuronide (E17G)-induced impairment of biliary secretory function in a dose-dependent manner. While the protective effect of glucagon is dependent on PKA, but not EPAC, salbutamol’s effect is EPAC/MEK dependent and PKA independent. Mechanistically, salbutamol/EPAC promotes a microtubule-dependent and long-range trafficking of Abcb11/Abcc2-containing vesicles, whereas glucagon/PKA modulates the microfilament-mediated fusion of these vesicles with the apical membrane (1217). In a follow-up study, the preventive effects of salbutamol and glucagon were further validated in more physiological settings using perfused rat liver and in vivo alanine administration, which induces pancreatic glucagon secretion (1218).
3. Gastric functions
The endocrine function of bile acids is mainly mediated through activation of plasma membrane and nuclear receptors, including the GPCR TGR5 (509) and the nuclear farnesoid X receptor (FXR) or NR1H4 (nuclear receptor subfamily 1, group H, member 4) (655, 808, 1085). While TGR5 is highly expressed in liver, adipose tissue, and intestine and more selectively activated by secondary bile acid, lithocholic acid (509), TGR5 expression is also reported in the enteric nervous system where this receptor is involved in regulating gut motility (837). A study by Murthy and colleagues (850) show that TGR5 is present in gastric muscle cells where treatment with the TGR5-selective ligand oleanolic acid (OA) activated Gαs and increased cAMP levels. OA treatment does not cause gastric muscle cell contraction, but rather inhibits sustained cell contraction induced by carbachol, suggesting that OA provokes muscle cell relaxation. Carbachol-elicited contraction can also be partially blocked by EPAC activation, but not by activation of PKA. Interestingly, OA-induced relaxation is abolished in muscle cells isolated from tgr5−/− mice as anticipated, and PKA inhibition by myristoylated PKI partially suppresses this effect. At the molecular level, activation of TGR5 by OA or EPAC by 007 triggers Rap1 to inhibit carbachol-induced RhoA phosphorylation, while PKA inhibition partially reverses OA-mediated inhibition of RhoA phosphorylation. Taken together, these results show that although EPAC signaling sufficiently induces gastric muscle cell relaxation, PKA activation is involved but not sufficient to cause relaxation downstream of TGR5 activation (850).
4. Intestinal functions
The intestinal proton-coupled peptide transporter (PepT1) mediates the uptake of dipeptides and tripeptides, derived from digestion of dietary proteins, across the brush-border membranes into enterocytes (965). Earlier studies have established that glucose-dependent insulinotropic polypeptide (GIP), but not GLP-1, enhances PepT1-mediated peptide absorption in murine jejunum by a cAMP-dependent signaling pathway (201). Coon et al. (200) show that PepT1 and GIP receptor (GIPR) proteins are expressed in villus epithelial cells of jejunum where these membrane-bound proteins localize on the apical and basolateral membranes, respectively. The use of a nonhydrolyzable dipeptide, glycylsarcosine, permitted Coon’s group to further demonstrate that GIP activates PepT1 enhancing glycylsarcosine uptake in CDX2-transfected IEC-6 cells, an absorptive intestinal epithelial cell model. The augmentative effect of GIP can be blocked by Rp-cAMP, wortmannin, or an Akt inhibitor, but not by calphostin C, a PKC inhibitor, or BAPTA, an intracellular Ca2+ chelator. Importantly, activation of EPAC by 007 mimics GIP stimulation, dose-dependently enhances glycylsarcosine uptake, and increases the expression of PepT1 on apical membranes (200). These data suggest that EPAC and PI3K/Akt signaling pathways are involved in GIP-induced peptide uptake of the small intestine. Aside from regulating peptide reabsorption, EPAC, but not PKA, also is observed to mediate β-adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium (392).
5. Pancreatic functions
EPAC proteins, particularly EPAC2, are implicated in major regulatory functions related to the endocrine arm of the pancreas, which are detailed above (see sect. IVC4). Therefore, the roles of EPAC in zymogen secretion and activation in pancreatic acinar cells will be discussed below.
The GPCR and second messenger signaling system is crucial to the physiological stimulation for zymogen secretion in pancreatic acinar cells. An increase in cytosolic Ca2+ in response to muscarinic agonist carbachol stimulation is the principal stimulus for acinar cell secretion (1120), whereas cAMP synergizes with Ca2+ and potentiates digestive enzyme secretion and zymogen activation (161). In isolated pancreatic acinar cells, physiological concentrations of carbachol (~1 µM) lead to maximal amylase secretion and minimal to no zymogen activation while supraphysiological concentrations (1 mM) of carbachol attenuate secretion and cause intracellular zymogen activation. Stimulation by 8-Br-cAMP or the EPAC specific agonist 007 augments amylase secretion and enhances zymogen activation under both physiological and supraphysiological muscarinic agonist concentrations. PKA inhibition partially reverses the potentiation effects of 8-Br-cAMP, but not those of 007 (160). These results demonstrate that EPAC and PKA pathways are both involved in potentiating the secretion and activation of zymogen in the pancreatic acinar cells, through regulation of distinct zymogen pools in a similar manner to the regulation observed for insulin secretion by EPAC and PKA in β-cells of the pancreas (932).
To determine if activation of Rap1, a downstream effector of EPAC, is involved in amylase secretion and zymogen activation, Williams and colleagues (894) demonstrate that Rap1 and EPAC1, as well as CalDAG-GEFIII, a calcium- and DAG-sensitive Rap1 GEF, are all expressed in mouse pancreatic acini. Furthermore, Rap1 and EPAC1 are shown to integrally and peripherally associate with zymogen granule membranes, respectively. Secretagogues that activate second messengers DAG/IP3 or cAMP all induce robust Rap1 activation, which is required for stimulated amylase secretion. Moreover, supporting this principle function of Rap1, inhibition of Rap1 by ectopically overexpressing Rap1GAP antagonizes both the action of cAMP and Ca2+/DAG-stimulated amylase release in pancreatic acinar cells. These findings suggest the existence of two synergistic pathways where Rap1 is activated by both cAMP via EPAC1 and by Ca2+/DAG via CalDAG-GEFIII to provoke amylase release (894). An investigation of cross-talk between cAMP- and Ca2+-mediated pathways reveals that cAMP acts through EPAC and PKA independently to activate the RyRs which accelerate calcium waves in pancreatic acinar cells (944).
E. Renal Functions
1. Renal expression of EPAC protein
EPAC1 was most abundantly expressed in mouse kidney when initially discovered (229), suggesting it may play an important role in mediating cAMP functions in the kidney. Reverse transcription-PCR analysis of microdissected rat nephron segments confirms ubiquitous EPAC1 mRNA expression in glomerulus, proximal convoluted tubule (PCT), proximal straight tubule (PST), thin descending limbs of Henle's loop (TDL), ascending limbs of Henle's loop (ATL), medullary thick ascending limbs of Henle's loop (MTAL), cortical thick ascending limbs of Henle's loop (CTAL), cortical collecting duct (CCD), outer medullary duct (OMCD), and inner medullary collecting duct (IMCD) with the highest expression observed in the CCD (584). Furthermore, expression of EPAC1 protein in S1, S2, and S3 segments of mouse proximal tubules (PT) was demonstrated using an EPAC1-specific antibody (431).
Using EPAC1 and EPAC2 specific rabbit polyclonal antibodies 2293 and 2295, generated against recombinant human EPAC1 and mouse EPAC2 proteins, respectively, Li et al. (610) also revealed the abundant expression of EPAC2 protein in rat kidney. Coimmunohistochemical staining of rat renal sections for EPAC1 or 2 with individual nephron segment marker proteins further illustrated that EPAC1 and 2 have distinct distributions within kidney tissue. While EPAC1 staining appeared mostly confined to the brush-border membrane (BBM) of the cells in all tubules, EPAC2 expression was more dispersed throughout the cell, demonstrating positive staining mainly at the BBM, but also in the cytosol and at the basolateral side of these cells. Costaining of EPAC1 and aquaporin-1 (AQP1), a marker of PT/TDL, demonstrated colocalization in ~30% of AQP1-positive PT of the cortex, but not in the outer medulla and AQP1-positive TDL tubules of the inner medulla. Double labeling of EPAC1 with Tamm-Horsfall protein (THP), a marker of thick ascending limbs of Henle's loop (TAL), revealed that EPAC1 was expressed in all TAL cells, where EPAC1 staining was spread across the cells and slightly enriched at the apical side. The expression of EPAC1 in the convoluted tubule/connecting tubule (DCT/CNT), identified by positive staining of the Calbindin-D28k (Calb28K) protein, was weaker than that of the PT and mainly located to the apical region of the Calb28K-positive cells. In the collecting duct (CD), EPAC1 was found to be evenly distributed in intercalated cells of the cortex, and to some extent in (AQP2-positive) principal cells of the outer medulla, but mainly in AQP2-negative cells of the inner medulla. On the other hand, costaining of EPAC2 and AQP1 revealed robust EPAC2 expression in PT of the cortex and outer medulla, but was absent from AQP1-positive TDL tubules of the inner medulla. A strong, dispersed vesicular-like expression pattern for EPAC2 was observed and confined to the apical side of the cell body, in all THP-positive TAL cells. Clear apical and basolateral EPAC2 staining was also detected in all Calb28K-positive tubular cells and intercalated cells of the DCT and CNT. In the CD, EPAC2 staining was clearly visible in all AQP2-positive cells. In all segments, EPAC2 was again largely enriched at the apical region, with spread expression in the cytoplasm and at the basolateral side of both intercalated and principal cells. Furthermore, similar EPAC1 and 2 expression patterns were observed in the human kidney. This widespread distribution of EPAC1 and 2 expression in different tubular segments support the notion of potential major functions for both EPAC isoforms in cAMP-mediated tubular ion transport and cellular proliferation in the kidney. In the following subsections, we will discuss the physiological functions of EPAC proteins in regulating renal fluid and electrolyte balance as well as their pathophysiological roles in the development of kidney diseases (see sect. VF).
2. EPAC and H+-K+-ATPases
The first report of EPAC’s function in kidney cells was published in 2002 by Doucet and colleagues (584). In this study, they revealed that calcitonin-induced stimulation of H+-K+-ATPase in α-intercalated (Iα) cells of rat is mediated by EPAC1 (584). H+-K+-ATPases are ion pumps that couple the energy generated from ATP hydrolysis to the transport of H+ in exchange for K+ against their concentration gradients (380). In the kidney, H+-K+-ATPases are primarily localized to the CD and play important roles in K+ reabsorption, proton secretion, and acid-base balance (1214). The activity and membrane trafficking of H+-K+-ATPases are known to be regulated by signals that generate second messengers such as cAMP, Ca2+, and DAG (380). While both calcitonin and isoproterenol treatment led to a cAMP-dependent activation of the H+-K+-ATPase in microdissected rat CCD, only isoproterenol-induced H+-K+-ATPase stimulation was sensitive to inhibition of PKA. In contrast, administration of anti-EPAC1 antibodies in streptolysin-O-permeabilized CCDs blocked calcitonin-, but not isoproterenol-mediated H+-K+-ATPase activation. Furthermore, calcitonin-induced H+-K+-ATPase stimulation was accompanied by a PKA-independent ERK activation and could be suppressed by an ERK specific inhibitor U0126, as well as antibodies against Rap1 or Raf-B, but not Ras or Raf-1. These observations suggest that α-intercalated and β-intercalated cells utilize discrete cAMP signaling pathways in regulating H+-K+-ATPase activity, involving cAMP/EPAC I/Rap1/Raf-B/ERK or cAMP/PKA, respectively (584).
3. EPAC protein and Na+/H+ exchangers
The Na+/H+ exchanger 3 (NHE3), one of the nine isoforms of the mammalian NHE gene family, is expressed in the apical membrane of PT and critical for the reabsorption of sodium and the maintenance of acid-base homeostasis. The activity of this specific antiporter is tightly regulated by second messengers generated by a number of hormones (717). Agents known to increase intracellular cAMP concentration in renal and intestinal epithelia suppress NHE3 activity and decrease sodium absorption. To investigate the role of PKA and EPAC in the regulation of NHE3 by cAMP, selective PKA or EPAC cAMP analogs were used to stimulate opossum kidney cells and murine kidney slices. Activation of PKA or EPAC, by N6-MB-cAMP or 007 respectively, inhibited NHE3 activity without affecting the abundance of the NHE3 transporter in the BBM. PKA and EPAC appear to instigate independent activities on NHE3, as inhibition of NHE3 by PKA activation is accompanied by increased NHE3 phosphorylation, but inhibition of NHE3 by EPAC activation does not affect phosphorylation levels and is insensitive to H89. Moreover, inhibition of MEK1/2 by PD98059 completely blocked the inhibitory effect on NHE3 imposed upon by EPAC activation, while only partially attenuating the PKA-mediated effect. On the other hand, the consequence of PKA and EPAC activation imparted a different effect on the major renal sodium/phosphate cotransporter (NaPi-IIa), another Na+-dependent transporter expressed in proximal BBM. In the case of this transporter, activation of PKA, but not EPAC, inhibited NaPi-IIa activity by promoting NaPi-IIa internalization from BBM (431).
In addition to an incretin effect, the gut hormone GLP-1 has also been shown to have natriuretic and diuretic properties (386, 730). Studies based on pig kidney epithelial cell lines, LLC-PK1, suggest that GLP-1 most likely modulates sodium homeostasis in the kidney by suppressing NHE3 activity. Regulation of NHE3 activity by the GLP-1R agonist exendin-4 is associated with an increase of NHE3 phosphorylation without changes in surface expression of the transporter, and requires activation of both PKA and EPAC. Again, EPAC-dependent NHE3 inhibition is mediated by the MAPK pathway as pharmacological inhibition of MEK1/2 abolishes the effect conveyed by the EPAC activator (149).
ANG II is known to promote bicarbonate absorption in PCT by reducing intracellular cAMP via a Gαi-mediated signaling cascade (623). In LLC-PK1 cells, ANG II treatment led to a dose-dependent decrease in Rap1A activation and a concomitant increase in membrane translocation of NHE3. The effect of ANG II on NHE3 translocation was largely reduced with EPAC1 and PKA activators, as well as by constitutively activate Rap1 mutants, while opposing effects were observed in the presence of a dominant negative EPAC1 or Rap1A mutant. In parallel to NHE3 translocation, ANG II treatment also led to an induction of inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, in an EPAC1/Rap1-dependent manner. Remarkably, ANG II-mediated expression of proinflammatory cytokines could be blocked by a NHE3 inhibitor S3226 or with EPAC1 and PKA activators. These data reveal a novel EPAC1-Rap1A-NHE3 pathway that mediates ANG II-induced cytokine production in kidney epithelial cells and may have important implications in tubulointerstitial pathobiology relevant to various renal diseases (1137).
A family of NHE3-binding proteins, acting as cofactors, is required for cAMP-mediated NHE3 inhibition (251). The multi-PDZ domain containing NHERF1 binds NHE3 and transduces the cAMP-mediated NHE3-inhibitory effect (1108–1110, 1177). Studies using primary BBM isolated from the kidney cortex of NHERF1−/− mice have shown that NHERF1 is required for cAMP-mediated inhibition of NHE3 (1109). The role of NHERF1 in cAMP-mediated regulation of renal and intestinal NHE3 activity was examined using multi-photon microscopy coupled with a pH-sensitive dye, carboxyseminaphthorhodafluors-4F (SNARF-4F), during direct application of 8-BrcAMP to the ileum or kidney cortex isolated from wild-type and NHERF1−/− mice. 8-BrcAMP inhibited NHE3 activity in intact renal cortex of wild-type mice, but not in that of the NHERF1 null mice. Unexpectedly, deletion of NHERF1 failed to block cAMP’s inhibitory effect on NHE3 in the ileum. Consistent with previous findings by Honegger et al. (431), both PKA and EPAC were equally involved in cAMP-mediated NHE3 inhibition; however, the inhibitory effects of cAMP on ileal NHE3 were exclusively mediated by PKA, and independent of EPAC and NHERF1 (748). The mechanism for this apparent functional difference of NHERF1 in the cAMP regulation of NHE3 in ileum and PT is not clear, but one plausible explanation involves compensation by another NHERF family member, such as NHERF2, which is subapical in the kidney cortex, but both observed in the BBM and subapically in ileum. An extended analysis of NHERF1 in various segments of the intestine further reveals that although NHERF1 has segment-specific effects on intestinal salt absorption, NHE3 transport rates, and NHE3 membrane abundance, unlike NHERF3, NHERF1 is not required for NHE3 regulation by cAMP in intestine (110).
4. EPAC and urea transporter UT-A1
Urea transporters (UTs) are pivotal in the concentration of urine and maintaining body fluid balance. The UT-A1 is expressed in the terminal IMCD and is the major and most important UT in kidney among six known UT-A transcriptional variants derived from alternative promoters or splicing. Vasopressin, acting through cAMP, increases permeability of IMCD to urea by regulating the cell surface expression and phosphorylation of UT-A1 (89, 1188). Traditionally, speculation of vasopressin’s effects on UT-A1 was mainly attributed to PKA. However, urea flux stimulated by vasopressin and forskolin was only partially blocked by pharmacological inhibition of PKA, suggesting a PKA-independent cAMP component downstream of arginine vasopressin (AVP) (314). Sands and colleagues (1097) were the first to show that selective activation of EPAC in isolated rat terminal IMCD significantly increased urea permeability. The stimulatory effect of EPAC activation was accompanied by an increase in UT-A1 phosphorylation and accumulation of the transporter in the plasma membrane (1097). Interestingly, while PKA phosphorylation sites on UT-A1 have been mapped to S486, S499, and S84 (56, 89, 455), the phosphorylation sites regulated by EPAC appear to be different and are not currently mapped (420). A PKC-dependent, hypertonicity-stimulated UT-A1 phosphorylation at site S494 was recently identified (88). Phosphorylation of S494 was independent of the cAMP pathway as activation of PKA and EPAC was without effect on UT-A1 phosphorylation at S494. Unlike cAMP-mediated UT-A1 phosphorylation, phosphorylation of S494 by PKC did not directly promote UT-A1 trafficking to the apical membrane. Nevertheless, a phosphorylation incompetent mutation S494A decreased UT-A1 abundance in the membrane. These results suggest that PKC phosphorylation of S494 may be important for the retention of UT-A1 in the plasma membrane.
5. EPAC and aquaporins
Aquaporins (AQPs), also known as water channels, belong to a family of integral membrane pore proteins that selectively channel water across the cell membrane (528). At least seven aquaporin channels are known to be expressed in human kidney, where these pore proteins participate in important functions to maintain body water homeostasis (774). Among these renal AQPs, AQP2, an AVP-sensitive water channel expressed in the principal cells of the CD, is the best known and most studied. Dehydration-induced secretion of AVP, a peptide hormone in the posterior pituitary, results in activation of the Gαs-coupled basolateral V2-vasopressin receptors in the basolateral plasma membrane of the CD. Consequently, induction of this receptor initiates the transcription, translocation, and fusion of AQP2-containing intracellular vesicles to the apical plasma membrane, where water is reabsorbed by AQP2 from the urine into the cell and then transported across the basolateral membrane by AQP3 and AQP4 (765). The effect of AVP on AQP2 was originally proposed to be mediated by PKA through direct phosphorylation at residue Ser256 (326, 503, 769, 842, 1057) and requires cAMP-dependent intracellular calcium mobilization (184). Surprisingly, treatment with PKA inhibitors, H89, KT-5710 and Rp-cAMP, at concentrations known to inhibit AQP2 phosphorylation failed to block AVP-induced Ca2+ mobilization and [Ca2+]i oscillations in perfused IMCD; conversely, the EPAC-selective cAMP agonist 007 indeed mimicked AVP in triggering ryanodine-sensitive Ca2+ mobilization and [Ca2+]i oscillations, triggered apical exocytosis, and induced apical translocation of AQP2 in perfused IMCD. These results reveal a previously unrecognized mechanism in which activation of EPAC, not PKA, mediates AVP-induced intracellular Ca2+ mobilization and apical exocytotic membrane fusion of AQP2 in IMCD (1167). Since both EPAC1 and 2 are expressed in the CD, the determination of which EPAC isoform, as well as potential downstream effectors, are involved in AVP-induced AQP2 translocation will be interesting. Potential candidates of Ca2+ signaling molecules that may be modulated by EPAC and have been previously implicated in the regulation of AQP2 trafficking include calmodulin, myosin light-chain kinase, calmodulin kinase II, and calcineurin (44).
In addition to acute regulation of AQP2 trafficking, AVP is also known to promote a sustained transcriptional activation of AQP2 expression (402, 1024) in a cAMP/CREB-dependent manner (443, 665, 1161). To determine if PKA activation is required for AVP-induced AQP2 expression, Umenishi et al. (1051) examined the AVP-mediated expression of AQP2 in an immortalized mouse CCD cell line, mpkCCDC14, and demonstrated that PKA inhibitors do not affect AVP-mediated, dose-dependent expression of AQP2 expression and CREB phosphorylation. However, inhibition of ERK blocked CREB phosphorylation and attenuated upregulation of AQP2 by AVP. These results suggest that AVP-induced AQP2 expression involves ERK activation but is independent of the PKA pathway (1051). Another in-depth investigation of this mechanism responsible for AVP-mediated AQP2 transcriptional activation again employed mpkCCD cells, which endogenously express EPAC1 and 2, and revealed that stimulation with 1-desamino-8-d-arginine vasopressin (dDAVP), robustly elevated intracellular cAMP concentration at 30 min. Although steady reduction was observed after 1 and 4 days of dDAVP incubation, cAMP levels remained significantly higher than basal concentrations. Additionally, while the level of pS133 CREB followed a similar pattern with that of cAMP, peaking at 30 min and fading below baseline levels at day 1 and 4, AQP2 expression kinetics followed a different pattern with a clear increase at day 1 and reaching maximal abundance at day 4. Interestingly, administration of dDAVP upregulated the expression of EPAC1, while downregulating EPAC2 in mpkCCD cells. Importantly, while dDAVP-induced AQP2 transcription, monitored by an AQP2 promoter-luciferase reporter assay, coadministration of H89 with dDAVP at day 1 could partially block this effect, but inhibition of PKA after 3 days of dDAVP stimulation did not suppress AQP2 transcription. Conversely, incubation with a specific EPAC agonist 3 days after dDAVP pretreatment increased both AQP2 transcription and abundance compared with sole dDAVP treatment. Thus these results demonstrate that the PKA/CREB pathway is partially responsible for the AVP-mediated acute expression of AQP2, but not for the sustained AQP2 expression, which may involve the activation of EPAC (552).
6. Roles of EPAC versus PKA
As discussed above, extensive studies have demonstrated a clear involvement of EPAC signaling in major renal functions undergoing cAMP-dependent regulation. Although the focus of the above subsections portrayed an importance of EPAC in modulating some of these functions, not all cAMP-mediated functions in kidney are associated with EPAC. For example, cAMP stimulates apical exocytic insertion of the renal Na+-K+-2Cl- cotransporter (NKCC2) in the thick ascending limb is highly dependent on PKA but not EPAC (128). Similarly, studies depict that PTH- or dopamine-mediated regulation of the sodium-dependent phosphate transporter (Npt2a) is dependent on PKC or PKC and PKA, respectively, but EPAC and MAPK activation in mouse PT cells are uninvolved (211). Furthermore, the kidney regulates K+ homeostasis by modulating the activity of K+ secretory channels, including the renal outer medullary potassium channel (ROMK), in the apical membrane of distal nephron segments. While ANG II inhibits ROMK channel activity by activating the ANG II type 1 receptor (AT1R) and the PLC/PKC pathway under conditions of dietary K+ restriction (1106), ANG II acts on the ANG II type 2 receptor (AT2R) to activate ROMK channels in CCDs isolated from high potassium fed rats via a NO/cGMP pathway involving cAMP/PKA-dependent signaling in an EPAC-independent manner (1105).
Clearly, the cAMP-mediated signaling system is very complex, and our ability to dissect the respective contributions of EPAC and PKA in a spatial and temporal manners is very limited. Currently, most knowledge regarding EPAC/PKA-mediated signaling and function are based on in vitro analyses in cell cultures. Technically, distinguishing the contribution of each cAMP effector in vivo, even in intact tissues, with the use of conventional genetic or pharmacological approaches is still quite challenging. As an initial attempt to overcome this challenge, Schwärzel and colleagues (265) apply an elegant approach utilizing transgenic bacterial photoactive AC (bPAC) that can be activated to rapidly and reversibly generate cAMP pulses in cell-type-specific manners. This optogenetic approach, coupled with genetic and pharmacological manipulation of either PKA or EPAC, allows greater exploration into the individual functional roles of PKA and EPAC between two major cell types in Drosophila Malpighian tubules, the principal cell and the stellate cell. Results from these studies reveal that although both EPAC and PKA are involved in cAMP-mediated signaling in Drosophila renal tubules, each cAMP effector provides distinct functions in modulating fluid secretion in a cell-type-specific manner. EPAC is important in stimulated fluid secretion of both cell types; but in contrast, PKA is only essential for basal secretion in principal cells that actively transport potassium from basolateral to apical surfaces and provide an electrochemical potential for fluid secretion, or for stimulated fluid secretion in stellate cells that control water and anions conductance (265). This study demonstrates the potential of applying optogenetics as a pertinent approach in dissecting the complex cAMP signaling network in vivo. The use of bPAC properly targeted to intracellular microdomains or further extension of similar approaches into higher organisms could significantly advance our understanding of cAMP signaling with unprecedented spatial and temporal resolutions.
F. Pulmonary
In the lung, cAMP signaling has been implicated in signaling pathways responsible for the mitogenic and contractile state of the tissue. Discerning these functions for cAMP signaling could be fundamental in formulating next generation therapies for pathologies such as asthma and chronic obstructive pulmonary disease (COPD) that affect large populations of patients. With the failure of contemporary treatments to prevent long-term damage to the pulmonary tissue and only suppress acute symptoms, the pathways governing these maladies must be further explored. Below we will discuss the current knowledge of EPAC and PKA functions associated with lung physiology.
1. Airway smooth muscle proliferation
The physiological nature of airway smooth muscle is a dynamic state between contractile and synthetic phenotypes important to growth and repair mechanisms. The phenotypic plasticity of airway smooth muscle is tightly regulated, shifting to a synthetic phenotype when mitogenic cues are present, but returning to contractile states in the absence of growth factors and in the presence of insulin or TGF-β (232, 391, 909). Improper stimuli affecting phenotype switching in airway smooth muscle can lead to irreversible airway remodeling in patients where excessive proliferation increases the thickness of the smooth muscle through both hyperplasia and hypertrophy, as is the case for many chronic airway disease states such as asthma and COPD (22, 231, 261). In addition, the presence of growth factors, such as EGF, can synergize with GPCR agonists such as lysophosphatidic acid (LPA) to augment the proliferative effect yielding greater hyperplasia (156, 264, 344). Commonly β2-AR agonists are used to induce smooth muscle relaxation in the airways of asthmatic patients, invoking a similar relaxation effect observed in the vasculature. Although this attenuates the bronchoconstriction of the airway in these patients, no effective therapies for reversing the remodeling are in the clinic. However, anti-proliferative signaling by PGE2-induced elevation of cAMP may suggest a regulatory mechanism for cell proliferation agents that increase cAMP and could be exploited for therapeutic benefit (305, 1033). Induction of relaxation and attenuation of airway smooth muscle cell proliferation would be effective at treating both short- and long-term symptoms of proliferative airway pathologies.
As such, initial studies investigating the mechanism of β2-AR agonists to reduce proliferation of airway smooth muscle noted that EGF-stimulated proliferation was attenuated nearly 40% by isoproterenol, a β-AR agonist, and this inhibition was also around 50% with specific β2-AR agonists, albuterol and salmeterol. Together these results suggest β2-AR is the predominant receptor responsible for the suppression of EGF-induced proliferation (500). Although both β2-AR agonists only partially induce cAMP and PKA activation, induction of cAMP by Gαs-coupled agonist, such as PGE2, or forskolin mimics the effects of β2-AR agonists (417, 456, 500, 881, 927, 1032). Forskolin stimulation demonstrates the greatest inhibition of EGF-dependent mitogenic signaling. These results are consistent with rapid desensitization of β2-AR and decreased maximal signaling, with forskolin bypassing the receptor input. Furthermore, in the case of sufficient relaxation induced by β2-AR agonists, amplification of the signal to compensate for the lower levels of cAMP may exist. With multiple pathways that elevate cAMP all converging to inhibit EGF-induced proliferation, involvement of cAMP is apparent (500).
Investigation into downstream elements of cAMP signaling involved in EGF-induced proliferation, measured by DNA synthesis and cell number, demonstrated that two PKA-selective agonists were unable to effectively reduce proliferation by EGF stimulation, whereas selective PKA-independent EPAC activation imitated the β2-AR agonist anti-proliferative effect. Similar conclusions were obtained in lung fibroblasts where the inhibitory potency of cAMP signaling was attenuated by genetic knockdown of EPAC1 and expression of Rap1 inactivating constructs, while pharmacological inhibitors of PKA were ineffective (387, 447). Further supporting this conclusion, ectopic expression of the constitutively active C subunit of PKA was also unable to inhibit proliferation in these cells, suggesting a less dominant role for PKA (447). This effect was observed again in LPA-induced proliferation and in the synergetic proliferative signal between LPA and EGF, demonstrating attenuated mitogenic effect with direct activation of EPAC or forskolin, but not PKA. These reagents exhibited greater inhibitory effects than β2-AR agonists, presumably due to high β2-AR internalization following simulation. Interestingly, activation of both EPAC and PKA was 10-fold less potent as compared with selective EPAC activation. Together these results depict an essential role for EPAC in the inhibition of EGF-stimulated proliferation by β2-AR agonists (500).
Although EPAC appears to be a major regulator for the inhibitory effect of cAMP elevating agents such as β2-AR agonists, an independent investigation sought to provide evidence of PKA requirement in the anti-mitogenic effects of these agents. β2-AR agonists reduced EGF-stimulated DNA synthesis and proliferation by 25–30%, and PGE2 further potentiated this effect in agreement with the above results; however, expression of the PKA inhibitory peptide PKI or a mutant PKA R subunit blocked anti-mitogenic effect of β2-AR agonists, suggesting a role for PKA in this regulation. Surprisingly, loss of PKA function greatly potentiated the ERK1/2 activity and proliferation in airway smooth muscle cells treated with PGE2. This may suggest an unknown modulatory role for PKA specifically in PGE2 anti-mitogenic signaling or may be resultant of highly elevated cAMP levels induced by PGE2 compared with those by β2-AR agonist counterparts (1148). Thus, even though EPAC is crucial in this process, the inhibitory effect of β2-AR agonists and PGE2 requires input from the PKA arm of cAMP signaling.
Indeed, subsequent studies using treatment with PGE2 demonstrates a synergistic signaling of both EPAC and PKA to modulate platelet-derived growth factor (PDGF)-induced proliferation. In bovine tracheal smooth muscle (881) and human smooth muscle (884), PDGF-induced proliferative, hypo-contractile phenotype is blocked by activation of either PKA or EPAC mimicking the PGE2 treatment. Both sustained ERK and p70S6K activation are required for proliferation in airway smooth muscle. Activation of either EPAC or PKA diminished PDGF-induced ERK activity, but surprisingly only PKA activation could block p70S6K phosphorylation (881). In human airway smooth muscle cells, flow cytometric cell cycle analysis revealed that PDGF stimulated promotion of G1 to S phase transition is blocked by activation of EPAC or PKA arresting these cells in G0/G1 phase and preventing S phase entrance. Further investigation revealed this inhibitory function of EPAC or PKA is accomplished by suppression of both cyclin D1 expression and subsequent phosphorylation of the restriction protein retinoblastoma (Rb) (884). Supporting this function, an independent study also reported G1 phase arrest in airway smooth muscle after cAMP elevation reduced cell cycle regulators cyclin D1 and Rb phosphorylation (983).
Further delving into the regulation of the contractile machinery demonstrated that activation of either PKA or EPAC protected the expression of α-SMA and smooth muscle myosin heavy chain which are typically decreased in the presence of PDGF (881, 884). This suggests that EPAC and PKA are important in maintenance of the normal contractile phenotype of airway smooth muscle in pro-mitogenic environments. With multiple pathways involved in phenotypic switch of airway smooth muscle cells, additional studies will be needed to fully characterize the cAMP signaling cascade invoking the observed inhibitory effect. Clearer is the existence of divergent functions for both EPAC and PKA in certain aspects of β2-AR and PGE2 signaling where the summation of these signaling pathways inevitably coalesces to inhibit mitogenic signaling.
The inability of β2-AR agonists to sustain cAMP signaling accompanied by increased proliferative stimuli in patients and in vivo models of proliferative airway diseases may underlie the ineffective prevention of smooth muscle remodeling by β2-AR agonists. Indeed, PGE2 selective activation of EP2 receptor exhibits greater resistance to desensitization compared with β2-AR (817), elevates cAMP for hours as compared with minutes (1148), and inhibits PDGF-stimulated proliferation (881, 884). The sustained cAMP increase may be important for adequate activation of both EPAC and PKA to more completely inhibit growth factor-stimulated mitogenic effects in airway smooth muscle. Therefore, with no effective therapies for reversing airway remodeling in chronic pathologies, and unfavorable side effects of PDE inhibitors, potential use of EPAC or PKA agonists may provide substantial value not only in promoting acute bronchodilation for symptom alleviation, but also in long-term regimens for proliferative airway diseases by effectively controlling hyperplasia. Although the complete functions of EPAC in airway smooth muscle anti-mitogenic signaling continues to be debated, dissecting precise roles for EPAC and PKA in both proliferation and contractility will prove essential for deciphering and specifically exploiting key regulatory targets of this pathway to prevent, or even reverse, the effects of airway remodeling in these patients.
2. Airway relaxation
Inopportune bronchoconstriction is associated with COPD and asthma. The hyperresponsive property of airway smooth muscle in these airway diseases can be linked to improper regulation on the molecular level. Regulation of the airway smooth muscle tone is attributed to the interaction of actin and myosin regulated by the phosphorylation of the myosin light chain (MLC), a key component in this dynamic signaling of muscle tone in the airway. Modulation of the phosphorylation state of MLC is conducted by a pair of enzymes: MLC kinase and phosphatase activity. The balance of RhoA versus Rac1 activity directly affects the activation of these enzymes where RhoA leads to constriction by MLC phosphatase inhibition and subsequent increased MLC phosphorylation (526), while Rac1 activity contrastingly decreases MLC phosphorylation by triggering p21-activated kinase to phosphorylate and inhibit MLC kinase (351, 903).
Commonly β2-AR agonists are used to suppress bronchoconstriction, although the precise mechanism of action has remained elusive. With the β2-AR being a Gαs-coupled receptor, activation results in the elevation of cAMP to induce EPAC and PKA signaling. Investigation into the PKA-mediated effect of isoproterenol on relaxation demonstrated minor suppression of isoproterenol-induced response when PKA was inhibited, suggesting the involvement of additional major, PKA-independent mechanisms (883). An earlier report also suggested relaxation induced by β-AR stimulation of guinea pig trachea occur through an unknown, PKA-independent effect (972). Thus distinguishing alternative cAMP pathways in airway smooth muscle cells revealed that selective activation of EPAC, independent of PKA signaling, successfully relaxed methacholine-treated guinea pig and human tracheal tissue (883).
In other systems, such as endothelial barrier function, EPAC/Rap1 modulation of the balance between RhoA and Rac1 activity have demonstrated activation of EPAC modulates MLC phosphorylation through a shift towards Rac1 activation (82, 83). Exploring the mechanism of EPAC-dependent relaxation of methacholine-induced constriction, ascertained that methacholine increased RhoA dependent stress fiber generation and RhoA-GTP levels while inhibiting Rac1-GTP; however, EPAC activation effectively attenuated each of these effects thus skewing the balance from RhoA to Rac1 activity (883). The regulation of this RhoA/Rac1 balance by cAMP was also apparent in bronchial smooth muscle cells where the β2-AR agonist salbutamol effectively blocked calpeptin-induced RhoA activation and plasma membrane translocation, essential for activity of the small GTPase. More importantly, these inhibitory effects were reproduced by activation of EPAC and PKA, corroborating this shift to Rac1 signaling and attenuation of RhoA with elevated cAMP (306). Further support for EPAC signaling through Rac1 was observed using Clostridium difficile toxin B-1470 that selectively inhibits Rac1 through monoglucosylation (917) or pharmacological inhibition of Rac1, both of which prevented the relaxant effect of EPAC activation. These results suggest that this EPAC-dependent relaxation, at least in part, is reliant on Rac1 activity (883).
Moreover, activation of EPAC was found to specifically attenuate the two- to threefold increase in MLC phosphorylation induced my methacholine (883), presumably by Rac1-dependent inhibition of MLC kinase (351, 903). The unaffected basal phosphorylation suggests a regulatory role for EPAC in the dynamic signaling of airway smooth muscle contraction (883). Additionally, the modulation of Ca2+-dependent or ATP-sensitive channels by EPAC activation could also contribute to the relaxation observed in this signaling pathway prompting further investigation into the regulatory function of EPAC in airway smooth muscle cells (844, 982).
Contrary to the aforementioned function of EPAC in airway smooth muscle relaxation, an independent study found that 71% reduction of EPAC by siRNA was unable to prevent isoproterenol-induced decrease in cell stiffness, as a measure of relaxation, directly contrasting the requirement of EPAC in this relaxant effect. Also, loss of PKA function resulted in unregulated histamine-induced phosphorylation of MLC-20 by β-AR agonist. Knockdown of EPAC1 resulted in retained regulation by isoproterenol, but did decrease basal phosphorylation of MLC-20 in the presence of histamine (731). These results suggest that PKA is involved in the relaxation effect of β-AR agonists, but also depict differential signaling roles for PKA and EPAC that require further study.
Another receptor class that has been shown to be efficacious in bronchodilation in asthmatic patients are dopamine receptors. The expression of dopamine D1 receptors in airway smooth muscle cells prompted the function of these receptors in relaxation mechanisms seen in patients. Similar to β2-AR, dopamine D1 receptors are Gαs-coupled receptors where activation leads to elevation of cAMP. Indeed, comparable with β2-AR activation, agonist activation of D1 receptors, with dopamine or D1 receptor-like agonists (A68930 or SKF38393), leads to promotion of significant relaxation in guinea pig tracheal rings treated with acetylcholine to induce contraction. While the D1 agonists paralleled the relaxant effect with elevated cAMP, endogenous dopamine promotes relaxation without changes to cAMP. Rather, the ability of dopamine to induce cAMP is masked by concomitant activation of the Gαi-coupled dopamine D2 receptor. Indeed, blocking D2 receptor activation reveals cAMP production by endogenous dopamine, but also potentiated relaxation invoked by D1 receptor agonists which suggests D1 receptor agonists may also exhibit minor activation of dopamine D2 receptors (716).
Another interesting result demonstrated rapid relaxation rates induced by endogenous dopamine compared with reduced rates in response to D1 receptor agonists (716). Although this could represent different pharmacokinetics of the synthetic agonist, endogenous dopamine may alternatively function on parallel pathways, including cAMP-independent pathways, not affected by D1 receptor agonist stimulation. Further investigation is still needed to fully elucidate the actions of dopamine versus the D1 receptor agonist to improve the therapeutic potential of this class of reagents.
Interestingly in terms of exploration of downstream effectors for dopamine-mediated airway smooth muscle relaxation, PKA inhibition with Rp-cAMPS suppresses relaxation, while EPAC activation is not involved in relaxation mediated by dopamine (716). These data suggest potentially distinct Gαs-coupled pathways through β2-AR and dopamine D1 receptor which may activate unique, parallel pathways for relaxation in airway smooth muscle.
3. Paracrine and endocrine inputs
In airway epithelium, GABA release augments β-AR relaxant effects (329, 330). Surprisingly, activation of PKA reduces GABA release and reuptake by these airway cells. Joining these two concepts, the β2-AR agonist terbutaline reduced methacholine-induced GABA release, and expression of PKI rescued this secretion. Further supporting the role of PKA in this signaling system, treatment of these airway epithelial cells with forskolin replicated the β2-AR agonist suppression, while EPAC activation was without effect (216). Although these results describe a pro-contractile function of PKA through reduction of GABA release, the common observed effect of β2-AR agonist treatment is relaxation of the airway smooth muscle; therefore, overall β2-AR relaxant effects may far outweigh this anti-relaxant effect, which could also be a contributing factor to the subpar clinical effects observed with long-term β-AR agonist therapies.
Additionally, the effects of sex hormones are noted in the progression of asthma. In particular, prepubescent child asthma is more common in boys, but postpuberty asthma becomes more prevalent in women until post-menopause (828). Although direct roles of estrogen in modulation of the airways still require further attention, estrogen signaling does demonstrate elevation of cAMP and augmentation of β-AR signaling leading to reduction of intracellular calcium influx in airway smooth muscle cells to promote acute bronchodilation (1037, 1093). Furthermore, EPAC1 and EPAC2 expression were reduced by estrogen treatment, suggested to be associated with observed decreases in brain-derived neurotrophic factor (BDNF) exocytosis from airway smooth muscle by estrogen (1093). In support of this conclusion, maximal secretion of BDNF was found to rely on EPAC2 expression in hyperoxic environments (1028). These initial results propose a direct role of estrogen on the modulation of airway constriction/relaxation by altering cAMP signaling components, although the full extent of hormone-induced action is still undergoing investigation.
Overall, the antimitogenic and airway relaxant roles of EPAC in pulmonary signaling present a unique opportunity to target this specific downstream effector exhibiting two beneficial responses. With known drawbacks associated with β-AR agonists and PDE inhibitors, a more precise strategy to alleviate acute bronchoconstriction and prevent pulmonary tissue remodeling could significantly improve quality of life for asthmatic and COPD patients.
G. Musculature
The body’s musculature is important for many physiological processes including, but not limited to, maintaining blood flow and pressure, basic and dexterous motor skills, ingestion and digestion, and focusing the iris in your eye. Much effort has been directed to the role of EPAC in cardiac muscle, but roles for EPAC in other muscle groups have slowly begun to emerge. Below we discuss currently reported functions of EPAC in different muscle groups.
1. Calcium handling
Stressful conditions or exercise invoke increased contraction of cardiac muscles to increase blood flow and oxygen delivery to exhausted tissues. The importance of Ca2+ mobilization from the SR or the extracellular environment in the process of muscular excitation-contraction coupling cannot be overstated. Furthermore, the proper signaling of this system requires rapid induction and quick reboot for subsequent contractions. Additionally, the system must be tightly regulated to adjust to changes in conditions where greater or faster contraction/relaxation is needed, such as during the fight or flight response.
During physiological pumping of blood from the heart, contraction of cardiomyocytes is reliant on the activation of L-type VDCCs to elevate intracellular Ca2+ levels during action potential propagation. This influx of Ca2+ triggers RyR activation on the SR to flood the cell with stored Ca2+ reserves. This process is known as CICR and is necessary to induce myofilament contraction. Between depolarizations, the cytoplasm must then reduce the levels of Ca2+ to invoke the maximal response with the subsequent potential. This occurs through reloading of the SR via the Ca2+-ATPase SERCA by removing the inhibitory PLN, and by efflux of Ca2+ out of the cell through the Na+/Ca2+ exchanger.
Thus many regulatory pathways act on modulatory proteins involved in the release and sequestration of Ca2+ during the contraction/relaxation of the myocyte. During a stressful situation, activation of cAMP signaling pathways through the β-AR system results in a net positive ionotropic effect. For example, PKA is known to act on L-type Ca2+ channels, RyRs, and PLN to amplify Ca2+ influx, release, and SR loading to generate stronger signals and thereby contraction. The role of EPAC in Ca2+ handling has been more recently described, and interestingly a great deal of effort in determination of this function has been conducted in cardiomyocytes.
Acute stimulation of β-AR induces cAMP and activates downstream effectors PKA and EPAC to initiate CICR. In the case of EPAC, loss of PLC-ε expression or inhibition attenuated the electrically induced Ca2+ transient amplitude in response to 007 stimulation. The reintroduction of PLC-ε rescued the increased Ca2+ induction, suggesting the importance of PLC-ε in this pathway (778, 779). This increase in Ca2+ transient amplitude was also found to be independent of SR loading or Ca2+ clearance (779). Rather, the enhanced SR Ca2+ release is associated with PLC-ε-dependent DAG generation leading to PKC-ε activation and subsequent CaMKII Thr286 phosphorylation, while no effect of IP3 on electrically stimulated Ca2+ release was observed. Activation of EPAC or upstream β-adrenergic signaling increased membrane localized PKC-ε, where inhibition of PKC-ε completely abolished EPAC-induced CaMKII activation, but only partially diminished the effect of isoproterenol, suggesting additional pathways involved in maximal CaMKII activation. Activation of EPAC further demonstrates actions of CaMKII to phosphorylate RyR2 Ser2815 which induces Ca2+ efflux from the SR, and also phosphorylation of PLN Thr17, enhancing resequestration of Ca2+ into the SR. Paired together, the increased sequestration and release can lead to the larger Ca2+ transients that are observed (778). Increased intracellular Ca2+ could also occur through alteration of L-type VDCC currents; however, induction of the β-AR/EPAC/PLC-ε/PKC-ε did not measurably alter L-type Ca2+ in the presence or absence of PLC-ε (778, 820, 964).
Although multiple groups agree that EPAC activation modulates the Ca2+ transient, the direction of modulation is still debated (778, 779, 819, 820). For example, in contrast to the results mentioned above, 007 activation of EPAC signaling reduced SR Ca2+ load correlating with decreased Ca2+ transients (154, 208, 820). Ergo, the level of Ca2+ sparks were measured to simulate spontaneous Ca2+ released from the SR by RyR activation which alter the magnitude of Ca2+ released during electrical stimulation. EPAC activation increased the frequency of these sparks in a PLC-dependent manner, but in turn decreased the amplitude which further supports a role of EPAC on RyRs (820, 821). Interestingly in a multicellular cardiac preparation, these effects were only observed in the absence of near-physiological extracellular Ca2+ levels, suggesting this pathway might only be fully activated under conditions of stress where the driving force of Ca2+ entry is from intracellular stores (504). Moreover, these changes in intracellular Ca2+ influx are consistently found to be blocked by the CaMKII inhibitor (KN93), and RyR2 Ser2815 phosphorylation was again greater after EPAC activation explaining the increased Ca2+ leak and decreased Ca2+ transient (820). One proposed explanation for EPAC-mediated decreases in Ca2+ transients suggests the global activation of β-AR acting on EPAC, as well as PKA, induces increased Ca2+ transients through paired increases in both SR Ca2+ load and leak, while specific activation of EPAC alone only increases Ca2+ leak leading to decreased load and thus decreased transients. Explanations for these discrepancies described above could also be attributed to differences in experimental procedures leading to altered SR Ca2+ leak or spark generation thereby affecting the transient magnitude. Regardless, each study agrees that EPAC activation leads to sensitization of RyR receptors through CaMKII-dependent phosphorylation.
To distinguish independent functions of EPAC on Ca2+ leak and Ca2+ sensitivity of the contractile machinery, cardiomyocytes were permeabilized to hold intracellular levels of Ca2+ constant. In this scenario, activation of EPAC revealed a leftward shift of intracellular Ca2+ required to generate tension by myofilaments, directly opposing the effect of PKA activation. This suggests contrasting roles for PKA and EPAC in alteration of Ca2+ sensitivity. Indeed, EPAC activation was also observed to increase phosphorylation of cardiac myosin binding protein-C Ser282 (cMyBP-C) and cardiac troponin I (cTn1) at a PKA-independent site (154). These phosphorylation events were unaffected by inhibition of Rap1, but rather were determined to be sensitive to inhibition of PLC, PKC, and CaMKII (154, 504). Thus, during low ionotropic states, increased Ca2+ sensitivity by selective EPAC activation could be potentially useful to increase contraction strength (504).
With these initial studies paving the way for EPAC’s function in Ca2+ handling, the requirement for either EPAC isoform over the other was questioned. Keeping this question in mind, the absence of EPAC1 caused observable decreases in basal and peak Ca2+ transients, attributed to a reduction in SR stored Ca2+. Phosphorylation of PLN Ser16, a previously identified PKA site, was also found to be decreased in EPAC1-null animals revealing cross-talk between EPAC1 and PKA. Furthermore, the convergent actions of EPAC1 and PKA at PLN Ser16 were determined to be additive in nature indicating maximal PLN phosphorylation requires activation of both cAMP sensors. The phosphorylation of PLN at Thr17, a CaMKII site, was also initially observed to be regulated by EPAC activation in vitro, but EPAC1-null heart homogenates did not portray altered phosphorylation of PLN Thr17. This may represent potential differences in the knockout efficiency of siRNA versus genomic knockout, or alternative pathways activated in vivo to activate CaMKII which can circumvent the loss of EPAC1. Additionally, reduction of RyR2 Ser2808/2814 phosphorylation in response to isoproterenol by ~69 and ~50%, respectively, was observed in the absence of EPAC1 further signifying the cooperative and interlinked signaling pathways of EPAC1, PKA, and CaMKII (785). In contrast, another group of investigators observed no appreciable effect on basal Ca2+ handling with loss of either EPAC1 or EPAC2, suggesting the modulatory roles for EPACs are only present during times of stress with agonist mediated cAMP induction. Interestingly, in this study the increased incidence of Ca2+ sparks observed after EPAC activation in previous studies was attributed as an EPAC2 function in accordance with the loss of this effect only in EPAC2-knockout mice (819). Additional experimentation also revealed more specific participants in this signaling system demonstrating that the SR Ca2+ leak involves β1-adrenergic signaling via EPAC2/CaMKIIδ/RyR2 Ser2814 phosphorylation (819). Thus both isoforms of EPAC are involved in Ca2+ handling of myocytes in the intact animal, although the exact contribution of each isoform is still being explored.
There were also questions about the function of EPAC on Ca2+ handling in terms of chronic stimulation. In contrast to acute activation, chronic activation of EPAC in vitro or in vivo by Sp-8pCPT, thus far, only promotes increased Ca2+ transients demonstrated with rapid caffeine application and found to be associated with larger SR Ca2+ loads, whereas EPAC knockdown abolished the agonist effects. Investigation into the mechanism of increased SR load revealed that in the presence of caffeine, the decay of the Ca2+ transient was stunted suggestive that the Na+/Ca2+ exchanger is suppressed during EPAC activation. Furthermore, activation of EPAC increased the phosphorylation of the CaMKII site Thr17 on PLN, which as previously mentioned induces greater sequestration of Ca2+. When paired with attenuated cytosolic Ca2+ depletion of the Na+/Ca2+ exchanger, the increased sequestration enhances the Ca2+ load in the SR even further (890).
The increased levels of Ca2+ may also be resultant of increased Ca2+ intake through altered L-type VDCC properties. Although EPAC was not found to alter the maximal amplitude of the Ca2+ current induced by these channels, the activation curve was shifted to hyperpolarized potentials after EPAC activation with no impact on inactivation curves. The added inflow of Ca2+ at lower potentials could further elevate the SR load in these cells and explain the increased transient generation (890).
Interestingly, the effects of EPAC were completely abolished when transcriptional activity was inhibited, suggesting the importance of this activity in maintenance of the regulation of Ca2+ handling imparted by chronic EPAC activation. One potential transcript found to be increased with chronic EPAC activation was calmodulin, which have been shown to inhibit RyRs (53, 1145). Again, a discrepancy from acute EPAC activation was revealed demonstrating that Ca2+ spark frequency was unaltered during chronic activation of the cAMP sensor, whereas acute stimulation increased the spark occurrence. The upregulation of a negative regulator for RyRs may be indicative of a negative-feedback mechanism that is activated to diminish arrhythmias (890). Indeed, during acute activation of EPAC in paced whole hearts, arrhythmias such as spontaneous ventricular tachycardia develop (439). These EPAC-dependent arrhythmias were attributable to increased diastolic Ca2+ leak and were observed to be CaMKII dependent (439). Additionally, these spontaneous events of Ca2+ release implicated in arrhythmia induction can be linked with the previously observed increase in Ca2+ spark generation of isolated cardiomyocytes (820, 821). These data suggest that increased Ca2+ influx by EPAC activation can hyperpolarize myocytes to increase frequency of action potentials, resulting in arrhythmic events.
2. Contraction/relaxation
One well-studied area for muscle contraction/relaxation is the smooth muscles involved in vasculature to modulate arterial contraction dynamics detailed above (see sect. IVB2a). Briefly, cAMP acts as a relaxant for vascular smooth muscles through inhibition of the contractile forces generated by RhoA. Downstream cAMP effectors PKA and EPAC are implicated in the attenuation of RhoA-mediated contractile force in smooth muscles by a number of mechanisms including inhibitory phosphorylation of RhoA, disruption of SR calcium induction, or calcium desensitization of MYPT1 and subsequent activation of MLCP (208, 272, 749, 1125, 1133). In support of these actions by EPAC, the antagonistic modulation of RhoA activity by EPAC activation is lost in Rap1B-null animals (1213). Also, as noted in the above subsection on Ca2+ handling, EPAC is implicated in the regulation of cytosolic Ca2+ levels in cardiomyocytes. Interestingly, during relaxation of vascular smooth muscles, EPAC activation is implicated in reduction of cytosolic Ca2+ by increasing BKCa channels activity (878) or slowly leaking cytosolic Ca2+ from the SR to reduce the SR load (208), consequently hyperpolarizing the membrane and attenuating contractile states, respectively. EPAC activation is also associated with the activation of eNOS, along with PKA, in endothelial cells to produce NO and induce relaxation of nearby muscle (336, 878). During stress however, EPAC/Rap1 initiates a RhoA-dependent mechanism to translocate α2c-adrenoreceptors to the cell surface and induce vasoconstriction (182, 183, 471, 472, 737). Therefore, these conclusions in vascular smooth muscle depict EPAC/Rap1 and PKA cooperatively signaling through pathways that induce vasorelaxation, except in the presence of stressful stimuli where EPAC/Rap1 switches to modulate constriction of the vessels.
Furthermore, in EPAC1 knockout mice, the functional consequence for loss of EPAC1 in intact cardiac muscles was investigated. Cardiac function in these animals was found to be attenuated including the left ventricular ejection fraction, suggesting a potential contractility dysregulation. Myocytes from these animals demonstrate reduced basal force of contraction and increased relaxation period by 90%. Treatment with isoproterenol effectively reversed these effects, suggesting the presence of compensatory pathways (785). Investigation into the action of EPAC1 in cardiomyocytes from EPAC1−/− mice attributed the above phenotypes to decreased phosphorylation of RyR2 and PLN resulting in attenuated Ca2+ induction from the SR and Ca2+ sequestration, respectively (785).
In sharp contrast to the cardiac and vascular smooth muscles, β-adrenergic stimulation of cAMP in a skeletal muscle cell line does not appear to induce PKA, PI3K, PKC, PKG, or EPAC-dependent signaling systems to inhibit contractility and detachment, but rather activates K+ channels for extracellular Ca2+ depletion-induced ROCK-dependent contractility (776).
Although most of the known roles of EPAC in contraction are related to cardiovascular smooth muscles, EPAC’s function in other smooth muscle groups such as those in the gut and lower urinary tract have recently been investigated. One initial study, investigating the action of cAMP on gastric smooth muscle relaxation, determined that blocking PKA’s phosphorylation of RhoA Ser188 could not completely recover ROCK activity. Indeed, direct activation of EPAC/Rap1 was also determined to inhibited ROCK activity resulting in muscle relaxation in a PKA-independent manner. The partial inhibition of the RhoA/ROCK signaling system by inhibition of PKA and EPAC activation suggests cooperative roles for the cAMP sensors (850). A more confined role for EPAC was identified in prostate, smooth muscle where tone is regulated by α1-AR mediated contraction and cAMP mediated relaxation. Direct activation of EPAC did in fact reduce phenylephrine-induced contraction, but only in the presence of the cyclooxygenase (COX) inhibitor indomethacin, and no observable activity was detected after norepinephrine treatment regardless of COX inhibition. Potentially, this represents a masking effect where EPAC is maximally activated following COX or β-AR induction of cAMP; thus the action of EPAC is only observable after inhibition of these components (410). In agreement with the results in prostate, the role of EPAC in human detrusor smooth muscle revealed only a minor role for EPAC activation in relaxation when compared with PKA stimulation. Increased relaxation was shown following cAMP inhibition of M3 receptor Ca2+ sensitization. The relaxant action of cAMP, mainly PKA, in this system is observed to be limited to regulation of ROCK, while PKC induction of Ca2+ sensitization is independent of cAMP intervention (405).
These results may be reflective of a more modulatory role of EPAC, compared with PKA, in physiological conditions, whereas the function of EPAC may be exemplified in times of stress for many smooth muscles groups. Although EPAC may only have a minor role in contraction in these muscles, the activation of EPAC was unexpectedly linked to transcriptional regulation through phosphorylation of Elk-1 in prostate which may indicate a connection between muscle tone and growth to be determined by future studies (410). Thus, as with other aspects of cAMP signaling that were initially reserved for PKA activation, further investigation is required to identify relevant and potentially alternative mechanisms that may involve EPAC modulation of these signaling systems.
3. Proliferation/differentiation
The role of cAMP in myocytes extends beyond contraction and relaxation mechanics, but also serve different functions in the proliferation and differentiation of these cells. For example, in skeletal muscle, adult muscle stem cells (satellite cells) are maintained in a niche between the sarcolemma and basal lamina, where these cells are preserved in a G0-phase quiescent state until activated for regeneration due to injury. Proliferation and muscle regeneration are linked to the function of calcitonin receptors to maintain quiescence, as described by Fukada et al. (321), but this effect was also attributed to PKA activity alone (1144). Interestingly, both EPAC and PKA activation were determined to suppress the emergence of muscle satellite cells from below the niche basal lamina (1144). This suggests that although EPAC is not directly involved in the maintenance of G0 quiescence in these satellite cells, it indirectly modulates stem cell activity by preventing premature escape from the niche. The mechanism for this modulation still requires further attention, but may provide insight into use of EPAC inhibitors for decreased recovery time during extensive muscle injuries or degenerations.
Another studied headed by Hewer and colleagues (417) sought to dissect individual roles of PKA and EPAC in vascular smooth muscle cAMP-dependent cell-cycle arrest. Although activation of PKA or EPAC alone was determined to not inhibit proliferation, rigorous testing demonstrated that concurrent activation of both PKA and EPAC could promote quiescence through inhibition of ERK1/2 and JNK pathways. This effect of EPAC is Rap1-independent since loss of Rap1 did not alter the level of proliferation in these cells (417). A follow-up study further illustrated the synergistic effect of PKA and EPAC activation by highlighting the decrease in Egr1 expression, an important protein for VSMC proliferation. Inhibition of Egr1 expression is coordinated by inhibition of Rac1 resulting in cytoskeletal remodeling and consequential attenuation of nuclear localization of ERK1/2. Nuclear export of ERK1/2, in turn, prevents phosphorylation of Elk1, a cofactor of SRE binding element, resulting in subsequent decrease in Egr1 (527). Proliferation was again attenuated by EPAC in human coronary artery smooth muscle acting downstream of α2B-AR on NR4A1 in the presence of PDGF stimulation (668). Taken together, pharmacological EPAC activation appears to promote quiescence in VSMCs, but the general mechanism of action has not been completely elucidated for different cell types.
In a myocardial environment, infarction or hypertension can initiate differentiation of cardiac fibroblasts into smooth muscle-like myofibroblasts forming fibrotic scar tissue, where stiffness increases and cardiac function decreases (824, 995). The role of EPAC1/Rap1 activation was previously determined to be inhibitory to collagen synthesis in rat cardiac fibroblasts (1071, 1171), thus EPAC may be responsible for the regulation of fibrotic scarring of the heart either through this mechanism or promotion of smooth muscle-like myofibroblasts. The level of PDE1A was elevated in fibrotic areas of the heart as well as activated myofibroblasts. Therefore, similar to the reduction in EPAC1 expression by TGF-β1 (1171), a reduction in activity of cAMP sensors, like EPAC, is observed at reduced cAMP levels in response to elevated PDE1A (700). In support of this suppressive function of EPAC1/Rap1, collagen synthesis was higher following EPAC1 or Rap1 siRNA treatment (700). A potential role of EPAC in myofibroblast formation is also supported since elevated cAMP effectively inhibited the formation of cardiac myofibroblasts (999), although the direct action of EPAC in decreased differentiation of myofibroblasts has not been pursued. The suppressive role of EPAC signaling was also examined in epicardium epithelial-to-mesenchymal transition (EMT). The induction of this process could induce neovascularization to promote self-healing of an injured heart after an infarction (68). Defining the role for EPAC in the transition of epicardial cells to coronary smooth muscle cells demonstrated a dependence on RhoA/ROCK activity (638) and a similar inhibitory nature of cAMP activation to attenuate TGF-β1-induced EMT (545, 906). In support of a suppressive role for EPAC/Rap1 in EMT, TGF-β1 treatment is observed to increase PDE4D mRNA while decreasing Rap1A and EPAC expression (34, 1171). In contrast to TGF-β1 stimulation, direct EPAC activation in epicardial cells suppressed markers of smooth muscle, smooth muscle protein 22 (SM22), and smooth muscle actin (SMA) and maintained cell-cell contacts suggesting prevention of EMT. These effects were echoed by the inhibition of PDE (34). Thus the suppressive action of cAMP on EMT and EPAC/Rap1 roles could again be targeted to decrease fibrosis and promote healing of affected hearts.
4. Muscle anabolism
An important process in muscle tissue is the balance between protein synthesis and proteolysis. This physiological balance is known to be regulated by insulin and catecholamine signaling although the precise mechanisms for protein metabolism, synthesis, and degradation are still actively studied (758, 760, 818, 1094). With all three β-AR isoforms being expressed in skeletal muscle, initial investigations focused on the β2-AR which accounts for the majority of the isoforms in skeletal muscle. Thus, to define the role of β-ARs in muscle anabolism, early investigators utilized clenbuterol, a first-generation epinephrine derivative selective for β2-AR, to effectively observe increases of muscle growth by 27–41% and decreases of muscle loss by 10–20% (341, 540). Signaling through β2-AR can lead to PI3K activation, subsequent Akt phosphorylation, and mTOR activation which is involved in muscle hypertrophy. Moreover, further investigation determined that β2-AR activation led to phosphorylation of Akt and two downstream targets of mTOR, ribosomal protein S6K1/p70s6k and 4E-BP1/PHAS-1, involved in regulating protein synthesis (105, 480, 540, 780).
However, Brennesvik et al. (105) previously ascertained in rat soleus muscles that catecholamine signaling alone was insufficient to alter Akt phosphorylation, but rather Akt phosphorylation is triggered by insulin signaling and potentiated by epinephrine through an EPAC-dependent signaling pathway. With the knowledge that epinephrine predominantly functions through cAMP-mediated downstream signaling and insulin activates Akt, relevant cross-talk between these two signaling pathways during hypertrophy was postulated (105). Indeed, epinephrine activity increased cAMP-induced EPAC activation and Akt phosphorylation was enhanced in the presence of insulin stimuli (105). Support for this conclusion was further developed with the use of the EPAC agonist 007 to induce phosphorylation of Akt and Foxo3a resulting in subsequent decreased proteolysis in muscles (66). Ohnuki et al. (780), a few years later, added to this support by using EPAC1-knockout mice to demonstrate loss of EPAC1 inhibited clenbuterol-induced muscle hypertrophy measured by suppression of masseter muscle mass, fiber diameter, tibial length, and cross-sectional area. These effects were mediated by the prevention of complete Akt phosphorylation, inhibition of downstream S6K1 and 4E-BP1 phosphorylation, as well as decreased phosphorylation of CaMKII/HDAC4 (Ser246) known to induce skeletal muscle hypertrophy (628, 780). The group also noted that loss of EPAC1 presented with increased Erk1/2 phosphorylation upon clenbuterol treatment, suggesting that EPAC1 may typically be involved in the suppression of Erk1/2 activation (780).
Furthermore, Akt activation is not only associated with muscle hypertrophy, but also in suppressing muscle atrophy as extensively discussed in previous reviews (315, 750). In the case of insulin stimulation, induction of muscle-sparing is accomplished by inhibition of the ubiquitin proteasome system through a PI3K/PDK-1/Akt pathway. Briefly, insulin stimulates production of insulin receptor substrate (IRS) by activation of the tyrosine kinase receptor. This leads to the recruitment of PI3K by IRS and subsequent phosphoinositide-dependent kinase-1 (PDK-1) activation of both Akt phosphorylation sites (Thr308 and Ser473). Akt then phosphorylates Foxo1/3a transcription factors to occlude their nuclear translocation, thus disrupting transcription of E3 ubiquitin ligases atrogin-1/MAFbx and MuRF-1 that are important to the protein degradation process (66, 91, 904, 984). As a side note, although the PI3K inhibitor wortmannin attenuated both insulin and epinephrine-induced Akt phosphorylation, this inhibition failed to completely reverse the proteolysis inhibition (66). Some resolution to these results can be explained by wortmannin’s ability to block lysosomal proteolytic pathways as has been described in previous studies (87, 220, 284). Indeed, epinephrine can also potentiate the phosphorylation of Akt and Foxo3a, and this effect is observed at concentrations correlated to those found to inhibit proteolytic actions in muscle (760). Further support of the involvement of β-adrenergic signaling in proteolytic inhibition is evident with clenbuterol treatment leading to suppression of the E3 ubiquitin ligase transcripts, MuRF1 and MAFbx, and this effect was attenuated in the presence of rapamycin suggesting the requirement of both Akt and mTOR activation and downstream targets for suppressing muscle loss (540). Previous studies also demonstrated the cooperative nature of insulin and epinephrine signaling in acutely diabetic sympathectomized rats, where both insulin and catecholamine signaling is disrupted. These animals exhibited increased activity of ubiquitin proteasome system in soleus muscles of rats concurrent with decreased phosphorylation of Akt and Foxo3a (67) that is not recapitulated in individual loss of either hormone (759, 818).
The use of pentoxifylline, a nonselective PDE inhibitor, gave investigators some insight into the action of cAMP sensors where inhibition of PDEs lead to a decrease in activity of both Ca2+-dependent and proteasome-dependent proteolytic processes suggesting potential roles for PKA or EPAC (65). Braviera et al. (66) first demonstrated the role of EPAC in the proteolytic process during insulin/epinephrine simulation to determine the effect of EPAC on Akt signaling. Indeed, the selective EPAC agonist 007 was found to activate both phosphorylation sites of Akt (Thr308 and Ser473) and subsequently phosphorylate Foxo3a (Thr32) to prevent nuclear transport (66). Monitoring the action of PKA during this process revealed activation to have a similar anti-proteolytic effect to that of EPAC activation; however, this regulation occurs independent of Akt or Foxo3a suggesting an alternative pathway which PKA can employ for inhibition of proteolysis (66). Thus PKA may employ alternative pathways to EPAC, but reach a comparable result, potentially through Ca2+-dependent proteolysis in a capacity independent of Akt signaling (66). Supporting this notion, PKA inhibition with H89 illustrates increased Akt activation after epinephrine stimuli in the presence of insulin, while decreasing glycogen phosphorylase activation as anticipated (66, 105). This suggests a potential shift of cAMP balance readily available for EPAC activation and potentiation of downstream signaling in skeletal muscle.
In a recent study considering the timing of the above-mentioned protein phosphorylation events and ubiquitin ligase expression, a rapid yet transient profile was discovered. The phosphorylation of β2-AR, CREB, Akt, 4E-BP1, and Foxo3a were at the highest deviations from normal after a few days and returned to basal after 10 days. This would suggest that with continual β2-AR activation other pro-anabolic pathways would need to be induced to sustain the positive effect. This altered signaling may be caused by receptor desensitization or decreased receptor density observed on the 10th day in the study (480). Overall, the combination of increased protein synthesis and attenuated protein degradation can seemingly induce transient increases to protein translation and begin to explain the muscle mass enhancement. A curious question would be whether the levels of active EPAC and PKA are altered during this time, and if so how does altered cAMP signaling effect short-term anabolism versus sustained muscle growth.
Interestingly, this anabolic effect is noted in fast-twitch muscles, but is less effective in slow-twitch muscles where the β2-AR levels are more abundant. This paradoxical effect was recently investigated and revealed that slow-twitch muscles had ~12-fold increase to PDE4 levels. The increased PDE4 was suggested to effectively reduce the available cAMP to insufficient levels to activate EPAC, and thus prevent Akt activation and CaMKII-mediated HDAC phosphorylation that induce hypertrophic signaling in these muscles (781). In further support of this conclusion, reestablishment of cAMP concentrations by inhibition of PDE4 activity in a rat burn-injury model resulted in reversal of proteolysis in skeletal muscles. PDE4 inhibition enhanced EPAC expression, increased Akt activation and normalized Foxo1 and 4E-BP1 levels in skeletal muscles and myotubes of these animals. Inhibition of PI3K or EPAC reversed the effect of the PDE4 inhibitor, suggesting that EPAC is required for the anabolic effect in these animals after serious injury (483).
Therefore, although the role of EPAC in the musculature has predominantly been uncovered through work in cardiovascular muscles groups, efforts to unveil the nature of these signaling pathways in other muscle groups, such as skeletal muscle mentioned above, continue to expand. Indeed, Ca2+ mobilization is an essential process for the proper contraction/relaxation dynamics in all muscle groups and this function appears to be intricately tied with cAMP-dependent EPAC and PKA pathways. Furthermore, spontaneous Ca2+ release also appears connected to EPAC-induced arrhythmias suggesting a potential targeting strategy for reducing these occurrences. EPAC and PKA are also reported to cooperatively suppress RhoA/ROCK-mediated contraction, although the role of such functions during conditional stresses requires further attention. And finally, the promotion of Akt-dependent pathways by EPAC to stimulate muscle anabolism, while attenuating proteolysis, implicates EPAC-targeted therapies in alleviation of serious skeletal muscle injuries and warrants greater effort in future studies.
H. Skeletal
1. Osteogenesis
Terminal differentiation and lineage determination of mesenchymal cells are controlled by multiple mechanisms involving the cytoskeletal assembly of focal adhesions and tension as well as cell shape. Many of these mechanisms share a common signaling component through utilization of the RhoA/ROCK pathway (674, 693). In a perfusion culture system to simulate fluid shear stress and induce osteogenesis, expression of EPAC1 mRNA and protein were maintained suggesting a potential role in this signaling system for the cAMP sensor. Initial EPAC activation was found to downregulate gene expression of several pro-osteogenic genes including alkaline phosphatase (ALP), type I collagen, and osteocalcin, while only attenuating the phosphorylation level of Runt-related transcription factor 2 (Runx2), known to be a triggering factor for the osteogenesis lineage determination. Activation of EPAC was also found to directly oppose the activation of RhoA, observed as reduction in RhoA and resultant focal adhesion kinase (FAK) activities, which translates to disruption of both actin and tubulin arrangement in the cytoskeleton and diminished focal adhesion number and size. FAK activity can activate the downstream MEK/ERK pathway to increase phosphorylation Runx2 and thereby direct stimulation of osteogenic genes. Indeed, in the presence of active EPAC signaling, ERK and Runx2 also exhibited decreased phosphorylation, suggesting an inhibitory mechanism for EPAC (1010).
Additional confirmation of the anti-osteogenic role of EPAC on RhoA signaling cascade was demonstrated by expression of a constitutively active RhoA, which completely abolished the effects of EPAC activation. Unregulated positive osteogenic activity of RhoA rescued the phosphorylation of FAK and subsequent phosphorylation of ERK and Runx2, while also restoring osteogenic properties of the cytoskeleton (1010). A potential link in the mechanism for the inhibitory action of EPAC towards RhoA is PI3K activity concurrent with Rap1 activation. Plausibly, PI3K can generate PIP3 to stimulate translocation of ARAP3 (ArfGAP With RhoGAP Domain, Ankyrin Repeat and PH Domain 3), which function as GTPase accelerating proteins (GAPs) for RhoA, whereas Rap1 activates ARAP3 (560). Indeed, inhibition of PI3K or ARAP3 siRNA effectively reversed the effects of EPAC in a manner similar to the expression of a RhoA activating mutation, thus corroborating functions of PI3K and ARAP3 in this pathway (1010).
2. Hormone-induced proliferation
Osteoblast cell differentiation and proliferation are also modulated by parathyroid hormone and the highly homologous parathyroid hormone related protein (PTHrP) acting on the parathyroid hormone 1 receptor (PTH-1R). Signaling pathways involving these hormones are highly regarded as the most important in the process of bone growth and resorption, where the bidirectional signal is attributed to a heterogeneous microenvironment within a diverse population of osteoblasts (133, 498, 581, 582). In this signaling cascade, activation of PTH receptor stimulates AC to produce cAMP which can signal to downstream cAMP sensors, namely, PKA and EPAC, to activate or repress specific genes important to the maturation process. Similarly, PTHrP stimulation induces selective alteration of response genes and is dependent on the heterogeneity of maturation states within the culture of cells. For example, PTHrP activation decreased osteocalcin by 90%, while also acting on the AP-1 promoter (−1200 to −225) predominantly to induce IL-6, an important factor for osteoclastic bone resorption, in a PKA-dependent manner (164). Another major component implicated in the effects of PTH signaling and situated downstream of cAMP is ERK activation, which is commonly observed to promote proliferation and bone growth. Indeed, ERK inhibition prevents cAMP-induced proliferation in bone cells (319), but surprisingly loss of MAPK signaling also blocks PTHrP-induced IL-6 expression by 42% without altering osteocalcin expression. However, even with the reduction to IL-6 expression, loss of ERK signaling was unable to directly inhibit PTH-induced AP-1 activity at the transcriptional level, suggesting a potential indirect mechanism regulating this pathway.
PKA is the most likely candidate for cAMP-regulated IL-6 expression and, as anticipated, inhibition of PKA did markedly reduce the levels of IL-6 expression through CREB activity with inhibition of ERK and PKC mirroring this reduction to lesser extents (164). A conclusion further supported in mouse calvarial osteoblasts where VIP stimulation, mimicked by elevated intracellular cAMP activation of PKA, leads to IL-6 expression through increased phosphorylation of CREB by PKA, consequential C/EBP DNA binding and depression of AP-1 activity (823). The activity of PKA was also reported to play a partial role in inhibiting expression of osterix, an important osteoblast specific transcription factor, in an EPAC-independent manner (435). Investigating the MAPK-dependent effect, direct activation of EPAC did induce ERK phosphorylation rapidly after stimulation, but this activity was also unable to enhance the IL-6 promoter (164, 823). Taken together, the role of cAMP on IL-6 production appears to function through PKA with minor involvement of PKC and ERK, although the divergent regulation by MAPK parallel signaling pathways requires further study (164). Dual pharmacological manipulation of both PKA and EPAC in these systems may also reveal synergistic activities to explain residual expression after either particular protein is inhibited. Additionally, other genes selectively regulated by PTH/PTHrP and MAPK such as bone sialoprotein (BSP) and fra-2 should be examined to determine if the dominant PKA regulation is indeed the general mechanism for these genes.
PTHR signaling also induces proliferation in cells of bone origin, and with PKA being commonly decorated as the primary signal transducer for PTHrP-induced osteoblast differentiation through PKC and MAPK (148, 1135), one could anticipate PKA inhibition would decrease bone growth. In contrast, blocking PKA does not affect basal proliferation and only enhanced mitogenic action with increasing cAMP levels (319). After a threshold level, further elevation of cAMP even began to revert proliferation in the presence of endogenous PKA, whereas this stabilizing effect was lost with inhibition of PKA (164, 319). A negative-feedback mechanism by PKA could be attributed to PKA countering MAPK activation through phosphorylation of Raf-1 and subsequent inactivation of Ras/Raf-1/MEK signaling as previously noted (199, 364, 942, 1131). Furthermore, even partial involvement of MAPK could suggest a potential, unexplored role for EPAC in this mitogenic process through modulation of Rap1/B-Raf/MEK pathways (319, 510, 847, 1078, 1173). The expression of B-Raf switches the function of cAMP from an inhibitory proliferative signal to a stimulatory one (258, 652, 783, 784). In developing osteoblasts, a dependency on the expression of two major splice variants of B-Raf, 95 kDa and 62 kDa, appear to be a key component to the duality of PTH signal propagation. Rap1 activation of ERK is exhibited in osteoblasts expressing the 96-kDa B-Raf, while this effect is lost in cells predominately expressing the 62-kDa B-Raf. In agreement with these results, B-Raf expressing cells with a constitutively active Rap1V12 mutant increase ERK activity and subsequent cell growth in a manner similar to forskolin treatment, and these effects were completely abolished in the presence of a dominant-negative Rap1N17 mutant. As anticipated, absence of B-Raf reversed the action of cAMP to inhibit mitogenic signaling with reduction of ERK activity, thus illustrating the important molecular switch function of B-Raf for cAMP signaling in osteoblasts (319). In fact, PTHrP even demonstrates a bidirectional effect on ERK activity with elevated ERK phosphorylation in undifferentiated cells and repressed activity post-differentiation (164). Another group also commented on this effect where ERK activity is gradually decreased in maturing osteoblasts (319). Taken together, these results depict a signaling pathway through EPAC/Rap1/B-Raf to induce bone growth, but also illustrate the input of multiple regulatory brakes, such as PKA activity and B-Raf expression, to stabilize MAPK signaling during the maturation process of osteoblasts.
Aside from PTH/PTHrP signaling, prostaglandins (PGs) are also implicated in growth plate chondrocyte development and long bone growth through potentiation of insulin-like growth factor I (IGF-I) stimulation. Mice treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or the cyclooxygenase (COX)-2 inhibitor celecoxib, at similar doses given to children for chronic inflammatory conditions, exhibit dwarfism, in line with the negative action of these processes on bone development. Inhibition of COX enzymes also negatively impacted ERK activity stimulated by IGF-I. Exploring the role of PGs in the inhibitory effect of COX enzyme inhibitors revealed that PGI2 was the predominant PG released in growth plate chondrocytes, greater than that of PGE2. Thus an analog of PGI2, iloprost, was used to treat chondrocytes in the presence of celecoxib, and successfully restored ERK activity and proliferation reversing the effect of the COX inhibitor. PGE2 also reversed the effects of celecoxib, but to a lesser extent. Furthermore, the effect of iloprost was blocked by AC inhibition, but mimicked by elevation of cAMP (453). The action of AC infers that PGI2 is signaling through the prostacyclin IP receptor present in osteoblasts (307). As reported in osteoblast cells above, loss of PKA function revealed a potential negative regulation of PKA on ERK activity, while EPAC activation and cAMP elevation completely restored the effects induced by celecoxib, a similar agent to iloprost (453). One major question following these results is why children receiving these treatments do not exhibit larger growth impairment as observed in murine models. Interestingly, COX-2-null and prostacyclin receptor-null mice grow normally, suggesting a potential compensatory mechanism, either through PGE2 or COX-1 pathways yet to be determined (243, 745). Thus the relevance of the PKA-independent PGI2/EPAC/Rap1/MEK pathway induction of bone growth remains to be resolved.
3. Adhesion
Adhesion of osteoblasts is clearly important in the development of bone regeneration technology. This often requires integrin-induced adhesion to biodegradable scaffolding. Indeed, adhesion to poly-l-lysine-coated plates in serum-starved conditions and the use of a minimal integrin-binding region peptide (arginine, glycine, aspartic acid, serine; RGDS) competition assay both demonstrated reduction in cAMP-induced adhesion supporting that integrin-ECM association is required (631). Although with growing evidence in various cell types for a role of EPAC/Rap1 in cAMP-induced integrin adhesion (97, 117, 145, 855), the role of adhesion in osteoblasts appears to be dominated by PKA regulation. Exploring the roles for the two cAMP sensors revealed that PKA activation, but not an EPAC agonist, induced adhesion similar to that seen with elevated cAMP, while inhibition of PKA reversed these effects (631). Thus selective activation of PKA may prove to be effective in enhancing bone regenerative technology.
4. Osteoclastogenesis
Similar to the differentiation of osteoblasts, osteoclastogenesis involves a highly regulated cascade of signaling systems to coordinate the processes of differentiation, fusion, and function. Bone is dynamically regulated and in constant flux to maintain balance between growth and resorption, but when this balance is tipped off, maladies begin to emerge. For example, acceleration of osteoporosis is common following loss of ovarian function and has been attributed to both decreased estrogen and elevation of FSH. Osteoporosis can be defined as excessive catabolic function resulting from increased populations or improved survival of osteoclasts. FSH lowers cAMP to enhance osteoclast differentiation and function, stimulating increased bone loss (993). In opposition to the activity of FSH, elevation of cAMP by PDE inhibition is observed to augment bone mass (512, 530, 807, 1143, 1198).
In agreement with these results, the increase in osteoclasts following an ovariectomy was effectively blocked by cilostazol, a PDE3 inhibitor. Also supporting reduced osteoclast populations, osteoclast function was notably diminished by cilostazol treatment, demonstrated by decreased dentine pit formation. Exploring the mechanism of cAMP action exhibited by knockdown of PKA or EPAC1 revealed that loss of either increased the number of tartrate-resistant acid phosphatase (TRAP+) multinucleated osteoclasts after cilostazol treatment. This suggests a cooperative involvement of both PKA and EPAC1 downstream of the negative regulator cAMP in this inhibitory pathway (512). Receptor activator of nuclear factor-κB ligand (RANKL)-induced reactive oxygen species (ROS) production is another essential factor of osteoclast differentiation (525, 591), and with cilostazol’s antioxidative properties (588), the heightened level of ROS after ovariectomy or RANKL/macrophage-colony stimulating factor (M-CSF) stimulation was observed to be reduced with PDE inhibition. Indeed, forskolin imitated the effect of cilostazol and more importantly loss of PKA and EPAC1 effectively blocked impairment of osteoclasts both alone and in tandem. Knockdown of NAPDH oxidase by siRNA also directly decreased the number of osteoclasts, unable to be further depleted by cilostazol, corroborating the essential role for ROS in osteoclast formation (512).
Moreover, cilostazol’s elevation of cAMP affected the transcriptional regulation of the osteoclast precursors. Differentiation of osteoclasts occurs through stimulation with M-CSF and RANKL to activate necessary transcription factors NFκB and NFAT2. Both RANKL-induced NFκB and NFAT2 activity were decreased with elevation of cAMP. Directly blocking phosphorylation of IκBα to inhibit NFκB activity mimicked effects of cilostazol and interestingly, in the presence of the PDE inhibitor, further decreased osteoclast populations and ROS production. This suggests a multifaceted nature of inhibition for cilostazol on osteoclastogenesis (512). Being that NFAT2-null stem cells cannot fully differentiate into osteoclasts (1007), the loss of NFAT2 nuclear translocation by cilostazol is a major deficiency for the differentiation process. In the presence of elevated cAMP, direct phosphorylation sites of NFAT2 by PKA are increased, which can prevent RANKL-induced nuclear translocation and subsequent activity (512, 1172). Generally, in cardiomyocytes, EPAC activation is also alleged to increase NFAT transcriptional activity (729). This suggests EPAC may act on NFAT through an alternative pathway in osteoclast differentiation or that PKA is the dominant mediator of NFAT transcriptional activity in this system. These results collectively suggest that elevated cAMP inhibits multiple central pathways preventing osteoclastogenesis, and PDE inhibitors may be a prospective therapeutic target for bone loss.
Further support of cAMP inhibition of osteoclastogenesis is observed in M-CSF/RANKL-induced osteoclast differentiation that was blocked by calcitonin in a highly enriched (CD45R-CD3-CD115+) population of osteoclast progenitor cells. Unexpectedly, the direct action of calcitonin on osteoclast progenitors demonstrated no effect on mRNA expression important to osteoclast differentiation and function, including RANKL and M-CSF receptors, NFAT2, c-Fos, c-Jun, IκBα, TRAP, cathepsin K, or integrins to name a few (363, 1021). In support of this lack of inhibition on transcriptional activity, calcitonin was found to be effective at inhibiting osteoclast development only 24 h before completion of 96-h cultures, suggesting the effect occurs at a very late stage. The large population of mononucleated TRAP+ precursors also led to an investigation of calcitonin’s involvement in multinucleation, a late-stage process of osteoclast differentiation. These cells revealed that mRNA expression of multinucleation components such as FcRγ and DAP12 with coupled receptors (452, 798) or DC-STAMP and ATP6v0d2 with downstream effectors (592, 1142) were not perturbed by calcitonin (363). Similar to calcitonin stimulation, elevation of cAMP with forskolin directly mimicked this inhibitory effect on osteoclasts. Thus, exploring a potential mechanism for PKA and EPAC using selective activators illustrates parallel mechanisms of inhibition of TRAP+ multinucleated osteoclasts.
However, the role of cAMP in osteoclastogenesis remains controversial with others observing a potential positive effect on osteoclast formation. For instance, selective EPAC activation enhanced osteoclast RANKL-induced differentiation by 13–20% and pharmacological inhibition prevented this increase. Further RANKL stimulation demonstrated Rap1 activity dependent on the presence of EPAC1 and EPAC2, while in the absence of EPACs, morphometric measurements of toluidine blue-stain dentine pit areas are decreased. Contrasting the above results where PDE inhibitors decreased NFκB and NFAT2 activity, EPAC1 or EPAC2 knockdown blocked NFκB p50/p105 translocation, as well as abolished the three- to fivefold RANKL-induced increase in mRNA expression of NFAT2, cathepsin K, and osteopontin. Additionally, loss of EPAC1 versus EPAC2 exhibited differences in translocation and activation of NFκB, suggesting potentially distinct roles for each isoform that require closer attention (682). In contrast to the above investigations, where transcriptional regulation was not modified by calcitonin, potential off-target effects of the pharmacological agents could partially explain some of the observed discrepancies. With these conclusions from current investigations, the regulatory mechanisms of cAMP governing bone dynamics remain open; however, initial results suggest that exploitation of such a process could prove to be beneficial in stimulating bone growth for patients with bone regression diseases such as osteoporosis (363).
I. Innate Immunity
When someone catches a cold for the first time or steps on a rusty nail, the body initiates steps to respond to any potentially harmful invading pathogen. Although there are many lines of defense against these events such as skin, mucosal production, stomach acid, and a host’s normal flora to name a few, many microbes have developed unique ways to infiltrate these barriers. Thus the body has evolved a cell-based defense network to control the spread of pathogens throughout the body. Once the antigenic organisms are past the chemical and physical fortifications and enter the tissue/blood of the host, the innate immunity is primarily accomplished by specialized cells such as neutrophils, macrophages, natural killers, and dendritic cells. These cells mount a formidable defense system, and further reinforce the host against future attacks by training the adaptive immune response for subsequent infections. Signaling through cAMP is known to be important for many functions of these innate immune cells. Thus discrimination for roles of EPAC and PKA is needed to determine if selective modulation of these effectors would be beneficial for targeted therapies in patients with dysfunctional innate immunity. Below we discuss current knowledge of physiological functions for EPAC in each cell type of the innate immune system.
1. Neutrophils
Host defense against foreign pathogens is initialized by release of chemoattractants functioning as a beacon to signal to innate immunity first responders such as neutrophils. Upon sensing a local elevation of circulating chemokines, neutrophils adhere to the resident vessel endothelium and extravasate from the bloodstream through a tightly regulated process to direct the migration of the professional sentries to the source of inflammation. Once at the site of infection, these cells act as the front-line infantry of the host, phagocytizing the invading pathogens and killing them through harsh enzymatic digestion. Neutrophils have always been considered to be short-lived guard cells, although more recent studies employing heavy water labeling for in vivo 2H incorporation demonstrated an unexpected lifespan of ~5.4 days (829). Thus understanding regulatory signaling pathways of these neutrophilic defenders is essential in further defining initial protective functions of the innate arm of the immune system and developing systematic strategies to shape host defense in patients where this system is malfunctioning.
For the first decade of this century, neutrophils were reported to not express EPAC1 (634, 1030), but selective EPAC agonist induced activation of Rap1 in differentiated neutrophil-like cell lines and human polymorphonuclear (PMN) leukocytes began to alter this concept (223). These observations were ultimately supported by visual mRNA and protein levels of EPAC1 in human PMN cells (223). The authors conclude that since inclusion of serine protease inhibitors was required for visualization of EPAC, in earlier studies EPAC may have been subject to degradation by granular proteases released from neutrophils during cellular disruption (223).
a) integrin activation.
Although there is some controversy over the action of cAMP on adhesion properties of neutrophils (86, 140, 235), activation of Rap1 by cytokines and bacterial fMLP (N-formylmethionyl-leucyl-phenylalanine), a chemotactic peptide (646) and targeting Rap1 to the plasma membrane is important for Rap1-GTP-interacting adaptor molecule (RIAM) recruitment (240, 394, 573, 587) and induction 223 of “inside-out” signaling. Rap1-dependent adhesion then invokes talin to stimulate β-integrin conformational changes exposing a high-affinity ligand binding site and activating the integrin (1001). Furthermore, Rap1 is reported to induce activation of αMβ2 integrins in macrophages (147) and may hint to a selective functional role for Rap1 in activation of the predominant β2 integrins in neutrophils through EPAC signaling. Indeed, direct activation of EPAC does enhance adhesion to fibronectin, but not BSA, for leukemic cell lines differentiated into PMN cells, suggesting a specific activation of β2 integrins by this signaling pathway; but surprisingly in human PMN, induction of EPAC was without effect on adhesion. In cell lines, fMLP and EPAC agonist induce a slight conformational bias for the high-affinity binding state of LFA-1 (CD11a subunit), while this is absent in human PMN. Taken together, the activation of EPAC/Rap1 in human neutrophils is not sufficient to induce the conformational switch to a high-affinity binding state of β2 integrins and enhance adhesion, while adhesion of the in vitro cell model of PMN cells was activated by EPAC stimulation (223). This could suggest that although Rap1 is active in human neutrophils, translocation of Rap1 requires further signals that are either basally, or exogenously, activated in the leukemic cell line, or endogenous functions of nontransformed neutrophils that the cellular model is incapable of recapitulating.
b) phagocytosis.
Phagocytosis of invading microbes is one of the fundamental roles for neutrophils, macrophages, and dendritic cells in the innate immune response. Dysfunction of this signaling system in phagocytic cells can delay clearance of infections as well as promote persistence of these cells provoking undesired inflammatory events leading to tissue remodeling, which could lead to opportunistic hospital-acquired infections in critically ill patients present with decreased neutrophil function (732). Therefore, understanding the mechanisms that reduce neutrophil function are relevant in preventing secondary infections leading to morbidity and mortality in patient populations.
A large population of patients that are treated for COPD and asthma are often prescribed β2-agonists and corticosteroids that effectively induce smooth muscle relaxation, but can also persistently suppress neutrophil function. Indeed, the β2-agonist salbutamol and several glucocorticoids are observed to attenuate neutrophil phagocytosis (925). Engulfment of a foreign agent involves reorganization of the actin cytoskeleton to envelop the particle, and this action can be regulated by RhoA which is observed as a central mediator of complement- and immunoglobulin-induced phagocytosis (146, 732, 925). As expected, stimulation with β2-agonist impaired RhoA activity and phagocytic levels in neutrophils. In support of a cAMP signaling system being stimulated by β2-agonist to inhibit RhoA, pharmacological inhibition of PKA as well as blocking PKA compartmentalization with the AKAP disrupting peptide St-Ht31 both partially rescue phagocytosis, demonstrating PKA signaling as a major regulator of RhoA suppression. However, an exciting result demonstrated protective actions of EPAC activation opposing the function of PKA. Restoration of neutrophil phagocytosis in the presence of β2-agonist or corticosteroids by EPAC was attributed to increased Rap1 activity to effectively bypass the RhoA requirement in this process (925). Supporting this claim, existence of an independent pathway for EPAC activation of Rap1 to rescue phagocytosis is also observed in macrophages (FIGURE 9) (521). Furthermore, the restorative properties of EPAC activity could even rescue phagocytic characteristics in dysfunctional neutrophils from critically ill patients, further implicating EPAC as a potential clinical target (925). With the prevalence of nosocomial infections continuing to rise globally and emergence of more antibiotic-resistant microbes, the demand for nonantibiotic therapies to restore or boost the immune system against resistant bacteria will be highly coveted in the coming years.
FIGURE 9.
Select physiological signals involving EPAC1 in immune cells. Depicted are currently reported signaling cascades involving EPAC1 in complement-mediated (top right) and IgG-mediated (bottom right) phagocytosis as well as reactive oxygen species production in bacterial killing (left middle) of macrophages and neutrophils. See section IVI for additional details and abbreviations.
Additionally, β2 integrins are also associated with complement-induced phagocytosis of neutrophils and macrophages. Similar to activation of β2 integrins in adhesion, activation involves the recruitment of talin to the cytoplasmic tail of the β2 integrin subunit. This regulation of “inside-out” signaling to enhance binding affinity of integrins is attributed to Rap1 activity. Coprecipitation of talin with active Rap1 and recruitment of talin to αMβ2 integrin (complement receptor 3) at the phagocytic cup by active Rap1 in macrophages only further support involvement of this pathway in complement-dependent phagocytosis (613). Additionally, this recruitment is detected in neutrophil-like HL60 cells. Further investigation into the molecular mechanism for Rap1-induced phagocytosis reveals RIAM is integral to the complement-mediated phagocytosis process in HL60 cells. Inflammatory stimulation continues to activate Rap1 in RIAM knockdown HL60 cells, but impairs αM integrin high-affinity switching and attenuates 50–75% of fibrinogen binding attributed to αMβ2 integrins. RIAM silencing also reduces EPAC/Rap1-dependent enhancement of complement-opsonized phagocytosis by 50%. Additionally, coimmunoprecipitation demonstrates an EPAC/Rap1-dependent complex of talin, RIAM, and the β2 integrin, corroborated by microscopy results of talin recruitment to phagocytic cups. The complex formation and recruitment is lost with RIAM silencing, suggesting that RIAM is crucial for “inside-out” signaling of Rap1 and is involved in recruitment of talin for subsequent activation of β2 integrins to induce “outside-in” signaling and initiate the engulfment process (FIGURE 9) (684). Taken together, EPAC activation of Rap1 is implicated in phagocytic signaling that could be a relevant target for enhancement of the innate immune system in patients that have neutrophil dysfunction or are susceptible to hospital-acquired infections.
c) apoptosis.
As mentioned, neutrophils are somewhat short-lived immune cells participating in seeking out and eliminating any pathogens they encounter. Subsequently, these front-line defense cells die to resolve chronic inflammation and make room for a fresh battalion of neutrophils. For some time, elevation in cAMP has been known to delay the onset of apoptosis in neutrophils where cAMP and PG mimetics attenuate spontaneous and TNF-α-induced apoptosis (773, 887). Investigation into the selective roles of PKA and EPAC in neutrophil apoptosis demonstrated that EPAC agonists are ineffective at blocking apoptotic signals, but the protective effect bestowed by cAMP is mimicked by PKA activation and lost with PKA inhibition (557, 773, 887). Similarly, a recent study investigating neutrophil-derived microparticles, which can be released as a form of communication during activation or apoptosis of neutrophils, sought to determine if cAMP affected the production or release of these microparticles. Supporting a potential role of PKA in delaying apoptosis, the cAMP analog and PKA activator decreased the level of microparticles in an EPAC-independent manner (697).
Together, these studies depict the complex nature of cAMP signaling in neutrophils and demonstrate selective activation of cAMP sensors for varying effects. With increasing efforts to distinguish EPAC and PKA signaling pathways, the unique functions of these cAMP sensors will only continue to be further defined for neutrophils. Moreover, conclusions to date demonstrate discriminatory targeting of PKA and EPAC may modulate neutrophil responses in ways that are clinically advantageous, and the nature of such strategies should be carefully considered in upcoming years.
2. Macrophages
Monocytes and macrophages are one branch of mobile phagocytes implemented as first line defenders by host organisms and can function to present antigens, secrete cytokines, or engulf pathogens. Myeloid progenitors mature into monocytes which circulate in the blood for a short time before taking residence in peripheral tissues and lymph nodes where they differentiate further into macrophages. In these tissue, macrophages are responsible for sifting the blood for infectious particles and cellular debris to alert the immune system to potential threats and provide maintenance for the system.
Similar to neutrophils, macrophages and other phagocytic cells were initially reported to not express EPAC1 transcripts (1030); however, described below is the demonstration of functional roles that are now attributed to EPAC in macrophage cells. Although monocytes do express EPAC1, many functions including cytokine production, adhesion and chemotaxis, phagocytosis, and oxidative burst are reported to be regulated by cAMP/PKA signaling and not EPAC1/Rap1 (120). On the contrary, monocyte maturation into macrophages induces threefold induction of EPAC1 expression (120), further corroborated in macrophage cell lines and alveolar macrophage preparations as well (31, 710, 727). With the diverse role of macrophages in inflammation and implication in autoimmune diseases and cancer microenvironments as tumor-associated macrophages, understanding underlying signaling mechanisms is crucial to development of next line therapies.
a) transcriptional regulation.
Immune function and differentiation of specific immune cell subpopulations requires transcriptional modification often induced by an ever-changing cytokine milieu. Cytokines, such as TGF-β, are commonly implicated in transcriptional regulation in the immune system and can have multiple effects depending on the cellular state and ligand isoform. Pathophysiological hijacking of these regulatory processes can also be rapidly detrimental in patients generating improper responses such as in leukemias or autoimmune diseases. For example, TGF-β1 can suppress or accelerate cell growth in cancerous cells, while upregulation of TGF-β2 is observed in invasive macrophages and aggressive tumors. This could suggest an analogous signaling pathway involved in transcriptional regulation of these cells.
Another major regulatory agent that affects transcription in immunological cells is LPS. LPS promotes signaling through Toll-like receptor 4 (TLR4) to activate NFκB which is typically kept inactive in complex with inhibitor factor kappa B (IκB). Upon stimulation, IκB is phosphorylated to remove the inhibition of NFκB heterodimers and promote nuclear influx to initiate transcription. As phosphorylation of IκB is lessened or more is produced de novo, IκB re-associates with NFκB abolishing the DNA binding and thus inactivating the system (28, 41, 174, 448). Curiously, the role of cAMP and PKA on NFκB regulation remains controversial, with some reporting that IκB degradation activates PKA to phosphorylate p65 to increase binding sites for co-activators CBP/p300 and enhance transcriptional regulation (1201, 1202), and others suggesting that PKA modification of the COOH-terminal transactivation domain of p65 inhibits NFκB activity (1005).
Investigation into the role of EPAC in this regulatory pathway implicated EPAC/Rap1 signaling in the induction of NFκB activity. Initial approaches defined that LPS induced cAMP and Rap1 activity to a similar extent as direct stimulation of EPAC. The NFκB activity induced by LPS and EPAC activation was also abolished in the presence of a dominant-negative Rap1-N17 construct. Study of the promotion of murine B cell activating factor (mBAFF) by LPS-stimulation of macrophages have also illustrated similar results. In this case, the stimulation of PKA of EPAC effectively upregulates the promoter activity, transcription, and translation of mBAFF (723, 725). Initial exploration into this transcriptional regulation revealed that inhibiting CREB signaling reduced mBAFF (725), but NFκB regulation was also implicated in a follow-up report (723). Indeed, LPS-stimulated macrophages exhibit decreased cytoplasmic IκB with consequential increase in nuclear localization of NFκB and abrogated phosphorylation of the PKA-regulated site Ser276 of the p65 subunit reduced mBAFF promoter activity (723). However, LPS activation of TLR4 receptors also initiates ROS production which can potentially affect NFκB induction of mBAFF (723, 724). Conflicting with a lone action of PKA on p65 Ser276 phosphorylation, H2O2 treatment enhanced mBAFF expression while attenuating phosphorylation of Ser276 and exhibiting enhanced CREB Ser133 and p65 Ser536 phosphorylation. Furthermore, EPAC/Rap1 activation was observed to phosphorylate both p65 sites at Ser276 as well as Ser536. Thus the activation of NFκB by LPS stimulation appears to involve the combined action of PKA to phosphorylate p65 Ser276 and ROS/EPAC/Rap1 to phosphorylate p65 Ser276 and Ser536 (723), supporting the initial report that mBAFF expression is reliant on PKA and EPAC activation (725). These conclusions do indeed suggest that EPAC/Rap1 are involved in the regulation of NFκB (723, 725, 727); however, the verdict is still out on how this regulation of EPAC/Rap1 phosphorylates NFκB, what additional modulators like IκB or potential kinases may be involved, and also the extent, order, synergy, or cross-talk of EPAC/Rap1 and PKA effects on the final NFκB activity.
Interestingly, these transcriptional regulatory mechanisms may also be dependent on the presence of additional cues in the extracellular environments. Contrasting the role of EPAC1 and PKA in promoting NFκB activity above, β2-AR stimulation is commonly reported to suppress proinflammatory cytokines through NFκB inhibition (288, 542, 934, 1059). Since β2-AR signaling also induces cAMP elevation, this presents a new conundrum for the roles of cAMP sensors EPAC and PKA in these signaling pathways. A study by Tan et al. (1008) suggests that the inhibitory signal by β2-AR may be relative to the overall environmental signaling around the macrophage. This is because in the absence of pro-inflammatory stimuli, β2-AR activation unexpectedly increases IL-1β and IL-6 pro-inflammatory cytokine production in murine and human macrophage cells. This signaling was attributed to EPAC activation as 007 treatment mimicked the β2-AR induction, with 30-fold induction of IL-1β and 3-fold induction of IL-6, while PKA and NFκB blockade was without effect (1008).
Furthermore, the mechanism of induction for IL-1β and IL-6 in a null-inflammatory environment was explored, revealing an ERK1/2- and p38-mediated phosphorylation of activating transcription factor (ATF) 1 and 2 (1008), similar to previous reports on the phosphorylation of ATF1 (382) and ATF2 (734). Activation of ATF transcription factors initiates the association with CRE-binding sites and promotes transcription of the cytokines. Activities of other transcriptional regulators on IL-1β and IL-6 expression including C/EBPβ and CREB were largely unaffected by β2-AR stimulation. This suggests the major inducers of IL-1β and IL-6 to be ATFs in this signaling system, while CREB, which was also phosphorylated during β2-AR stimulation, may act on other cytokine promoters (1008). Of future interest would be potential cross-talk between EPAC and MAPK pathways in this signaling system, being that Rap1 has been observed to crosstalk with MAPK signaling through B-Raf activation (584, 1173).
These conclusions point to an environmentally defined signal propagation for cytokine production dependent on the collective of external stimuli. Pleiotropic signaling of β2-AR could help to clarify the suppressive action during active inflammation, while pro-inflammatory signaling dominates under conditions where the extracellular environment is immunologically unchallenged. The latter of these two scenarios involves active EPAC to stimulate the transcriptional machinery and MAPK-mediated activation of ATF1 and ATF2, while PKA inhibition augments the production of IL-1β and IL-6 suggesting a distinctive, potentially antagonistic inhibition of EPAC signaling by PKA. The full extent of this regulation and definition of environments lending to this specific pathway remain to be fully appreciated.
The distinct roles of the cAMP effectors in transcriptional regulation of macrophages during elevated cAMP have been observed for other genes such as ARG1 (388) and REDD1 (1151) that demonstrate EPAC-independent regulation by PKA or other undetermined cAMP-dependent mechanisms, respectively. Thus, in macrophages, the transcriptional regulation of genes is differentially regulated in response to cAMP levels and environmental stimuli leading to cooperative or antagonistic EPAC and PKA pathways.
b) cytokine production.
The transcriptional regulation of cytokines is of high importance in macrophage cells, enabling the ability to signal additional support from the immune system to properly respond to foreign pathogens. Macrophage stimulation with PGs like PGE2 induces cAMP to regulate cytokine profiles which typically results in a suppression of inflammatory cytokines. However, this cAMP-mediated cytokine regulation in macrophages is dependent on PKA, not EPAC, in contrast to DCs where EPAC and PKA are both important mediators. In support of this, LPS-mediated elevation of TNF-α is suppressed by PKA agonist or induction of cAMP in alveolar (31, 32), peritoneal (32), monocyte-derived (120), and cell line (37, 976) macrophages. Similar results were observed for LPS-induced MIP1α (CCL3) induction (32) and leukotriene B4 (31). PKA activation is also attributed to augment IL-10 and IL-6 in the presence of LPS (32, 37), while EPAC agonist was without effect. Furthermore, during an infection with visceral leishmaniasis, peritoneal macrophages treated with PKA inhibitors demonstrated inversed regulation of TNF-α and IL-10 as observed with cAMP/PKA activation and PGE2 treatment. This further supports PKA as the dominant regulator of cytokines in macrophages. Although the authors describe the suppression of CCL3 to implicate EPAC, the use of a more specific inhibitor to validate this conclusion is warranted (895).
An independent study did explore a possible regulatory role for EPAC1 in chemokine production, and surprisingly concluded that EPAC1 did indeed induce the chemokines CCL2, CXCL5, and CXCL7 that are upregulated with PGE2 treatment or elevated cAMP levels, while PKA activation had no measurable effect. Interestingly, inhibition of IκB kinases blocks the forskolin stimulation of these chemokine genes implicating a role of NFκB in this regulation (416). Along with the aforementioned role of EPAC and Rap1 in LPS-mediated transcriptional regulation by NFκB (727), these results may establish plausibility of chemokine regulation by EPAC depicting a set of genes that are regulated by EPAC. Furthermore, in a recent study investigating apremilast, a PDE4 inhibitor used in the treatment of psoriatic arthritis, silencing PKA, EPAC1 or EPAC2 expression was found to impede the protective effect of the compound, increasing TNF-α and depleting IL-10 release (822). Although these results are quite interesting, one could be curious to determine whether the knockdown of PKA or EPACs distinctly affects transcriptional regulation or secretion of these cytokines to support or refute previous reports. Therefore, current conclusions bolster that transcriptional regulation of cytokines appears specifically dominated by PKA signaling, while any EPAC regulatory activity may be reserved for specific sets of chemokines that are not modulated by PKA.
c) migration and adhesion.
Migration of macrophages to the site of inflammation through the vasculature and host tissue is extremely important to function of these inflammatory mediators where perturbation underlies pathologies with ineffective immunological response or exaggerated tissue-resident leukocytes. Upon receiving an inflammatory chemokine signal, monocytes respond by rolling along the vascular endothelia, adhering to local vascular endothelial cells through integrin CD11b/ICAM interactions and proceeding through the process of diapedesis to enter the stressed tissue. Once infiltrated into the tissue, CD11b’s role shifts to locate C3b complement-opsonized particles and induce phagocytosis. Full function of β2 integrins in adhesion requires “inside-out” signaling to switch to high-affinity receptors through signaling of RIAM, EPAC1/Rap1, and talin as described in the neutrophils section and discussed below (613, 684).
TGF-β1 is an immunological regulator typically found in a latent form in the blood, but upon activation this protein is commonly associated with a myriad of anti-inflammatory functions. In peripheral blood monocytes, prolonged exposure to TGF-β1 attenuates EPAC1 levels and Rap1 activation by MCP-1 (CCL2) and MIP1α (CCL3), subsequently reducing CD11b integrin surface expression and activation. This inhibition can be reversed by expression of a constitutively activate Rap1 mutant (61). An independent investigation also reported that VIP induction of EPAC/Rap1 signals through PI3K/Akt and ERK to enhance CD11b surface expression in monocytes (269). Interestingly, the positive role of EPAC/Rap1 in monocyte migration induced by TGF-β1 was observed to be inversed in macrophages. Suppressed chemotaxis following TGF-β1 treatment is suggested to be modulated by EPAC/Rap1 activation of ARAP3 and subsequent inactivation of RhoA (728). Discrepancies between these conclusions could be due to cell-type-specific effects in response to increased EPAC1 expression upon maturation, or differences in the TGF-β1 stimulation times used between the studies.
Interestingly, similar to TGF-β1 stimulation, the response of myeloid cells to Toll-like receptor activation by LPS is attenuation of chemotaxis and tissue recruitment with increased cell spreading. Indeed, monocytes treated initially with LPS lose their chemotactic capacity, while those treated with chemokine then subsequently with LPS retain initial chemotactic potential. This suggests that the order of stimuli matters to determine the monocyte response, potentially due to temporal rearrangement in the surface expression of adhesion molecules or overlapping use of signaling components between the two pathways. Regardless, in the presence of chemokines, Rap1 and EPAC1 are found to localize to the leading edge of the monocyte to presumably continually activate β integrins, but this polarized expression is attenuated with LPS treatment. Additionally, combined inhibition of Rap1 and p38 is required to reverse the LPS-induced migratory arrest supporting a cooperative role for EPAC/Rap1 signaling in this process (1165). This may seem counterintuitive as the cell is trying to reach the site of infection, but the functional relevance of such a regulatory signal may be vital to extend time of contact for phagocytic cells upon reaching the infectious site. Initial signals in circulation will exhibit minimal LPS with greater quantities of chemokine not to impede chemotaxis, but as the cell approaches the main site where levels of LPS are persistent and at their highest levels, migration arrests while cell adhesion and spreading are initiated. Furthermore, rather than centralizing complement-mediated phagocytosis, discussed below, to the leading edge of the cells, LPS-mediated dispersion of EPAC/Rap1 signaling may function to increase the effectiveness of this process throughout the cell periphery.
Although Rap1 is often reported to mediate β2 integrin activation, EPAC/Rap1 has also been reported to enhance β1 integrin surface expression leading to increased cell adhesion and chemotaxis in monocytes. Furthermore, EPAC was implicated in polarization of these cells, and microscopic analysis of EPAC illustrates relocalization of EPAC to the uropod during this process. Interestingly, serotonin, which stimulates cAMP induction, mimics the enhanced adhesion and chemotaxis of EPAC activation independent of PKA in monocytes (634). The reason behind the apparent lack of regulation of EPAC on β2 integrin activation in this study remains unclear.
d) proliferation and polarization.
As in many other cells, the function of cAMP on proliferation through PKA and EPAC has been reported for macrophages. Observed by Misra and Pizzo (710), the elevation of cAMP by forskolin increases activation of Akt to promote proliferation of macrophages, enhancing CREB phosphorylation to suggest a role for PKA and increasing EPAC1 levels with subsequent Rap1 activity. Indeed, inhibition of PKA or knockdown of CREB by 60–70% effectively attenuates the mitogenic effect of forskolin, but PKA-dependent signaling is unable to account for the enhanced phosphorylation of Akt. In fact, inhibition of PKA further augmented the phosphorylation of Akt Ser473, suggesting PKA may inhibit this activity (710).
Rather, activation of Akt by forskolin is attributed to PI3K signaling, and it has been previously shown that EPAC1 activation of Rap1 can cross-talk with PI3K/Akt signaling pathways in PKA-independent manners (150, 584, 687, 710, 711, 1173). Rapid induction of PI3K by forskolin was retained for several minutes matching the kinetics of pAkt Ser473 during this stimulation, unlike pAkt Thr308. Differential results for the two Akt phosphorylation events were also observed with PI3K inhibition only accounting for 50% of Thr308 inhibition, suggesting involvement of a secondary kinase for this regulation and thereby full activation of Akt. Intriguingly, EPAC1 coprecipitates with Rap1, Akt phosphorylated at Ser473 and Thr308, and integrin-linked kinase (ILK) in the presence of forskolin (708, 710). This could depict a functional role for EPAC1 in targeting Akt to the membrane for phosphorylation by mitogen-activated kinases (710), such as ILK that is implicated in phosphorylation of Ser473 (1038). Additionally, a follow-up study depicts the association of T-cell leukemia protein 1 (TCL1) in complex with EPAC1 and Akt, where TCL1 binds and enhances Akt activity (709). Subcellular fractionation analysis further supports that EPAC1/Rap1 facilitates activation of Akt by PI3K on the plasma membrane and extends this regulation to include relocalization of active Akt to the nucleus (708, 709). Many unanswered mechanisms remain regarding this pathway. Confirmation for the identity and association of the kinases responsible for Akt phosphorylation, how association with active EPAC/Rap1 and TCL1 enhances Akt phosphorylation and promotes nuclear translocation, and the sequence/timing of Akt phosphorylation and association with components of this complex all remain a challenge and are yet to be resolved.
However, the activation of Akt is observed to propagate pro-survival signals by elevating X-linked inhibitor of apoptosis protein (XIAP) levels to inhibit caspase-9 and caspase-3 cleavage. In addition to blocking caspase cleavage, to ensure the anti-apoptotic environment, cAMP-mediated induction of CREB upregulates Bcl-2 expression (710) through CRE-binding elements in the Bcl-2 promoter (1122). Furthermore, the pro-apoptotic protein Bad was phosphorylated by elevated cAMP at both PKA- and Akt-mediated sites (710), Ser155 (629) and Ser136 (90, 224), respectively. The phosphorylation of these sites abrogates the association of Bad with Bcl-2 further promoting an anti-apoptotic signal which is reversed by 50% in the presence of PKA or Akt inhibitors. PI3K/Akt signaling also reduced induction of apoptotic genes by increasing pGSK3β Ser9 to suppress many transcription factors aside from CREB (898), and nuclear exclusion of FOXO1 by enhancing phosphorylation of Ser256 (710).
With these actions for PI3K/Akt in the presence of elevated cAMP and the association of EPAC1/Rap1 with activated Akt, a regulatory role of EPAC1 is implicated by targeting of Akt to a pro-activation complex and promotion of proliferation/survival. Surprisingly, aside from reducing forskolin-induced proliferation, silencing CREB expression also lessened phosphorylation of both Akt sites independent of PKA, which is unexpected since CREB is typically downstream of Akt (710). This potential interrelated pathway requires further exploration, but these conclusions suggest a potential cross-talk between PKA- and EPAC-dependent signaling pathways in the mediation of murine macrophage proliferation and survival.
Macrophages polarize into a number of different phenotypes to successively carry out their primary missions in host defense. The most associated phenotype is M1 macrophages, which are responsible for acute inflammation, including recruitment of neutrophils and employment of phagocytosis to clear foreign pathogens. M2 macrophages, on the other hand, are observed during the end of inflammation or during chronic inflammatory environments to attempt to subvert the inflammatory state. These two phenotypes are the most commonly described, although it is alleged that others exist between these states, such as regulatory, alveolar, Kupffer, and foam macrophages cells.
Although the role of EPAC on macrophage polarization has not been well defined, one study by Wang et al. (1099) identified miR-21 to be implicated in suppression of M2 differentiation of macrophages. Interestingly, PGE2 as well as EPAC and PKA activation effectively depleted miR-21 expression, suggesting cAMP signaling skews macrophages to an M2 phenotype, which is exaggerated in the absence of miR-21. The authors further examine miR-21 targeting to demonstrate suppression of STAT3 by miR-21 blocks M2 polarization by PGE2 (1099). This would be in agreement with increased IL-10 and IL-6 production observed with LPS-mediated elevation of cAMP, but the involvement of EPAC in this regulation is lacking (32, 37). Thus investigation into specific roles and mechanisms employed by PKA and EPAC in M2 polarization of macrophages is necessary, as well as defining the potential anti-inflammatory properties of individual EPAC and PKA agonists alone and in combination.
e) phagocytosis.
Similar to neutrophils, macrophages are professional phagocytizing cells, clearing cellular debris from sites of inflammation and infection as well as physiological maintenance throughout the body. Therefore, it is not surprising that similar mechanisms between these cell types may be conserved, while others are altered. Enhancement of particles targeted for phagocytosis by opsins, such as complement components or IgG antibodies, are used to activate the phagocytic machinery in macrophages. With enhanced expression of EPAC1 after macrophage maturation, the presence of this cAMP effector may be important to macrophage phagocytic pathways. Interestingly enough, EPAC appears to have opposing roles in the distinct phagocytic pathways. In macrophages undergoing complement-mediated phagocytosis, serum-opsonized zymosan particle uptake is enhanced by EPAC/Rap1 activation (521, 684).
Rearrangement of the actin cytoskeleton by RhoA signaling is commonly associated with phagocytosis in general (146, 732, 925), and as anticipated, inhibition with Tat-C3 toxin or dominant negative RhoA N19 expression attenuate complement-mediated phagocytosis in macrophages. Strikingly, in the presence of these RhoA inhibitors, activation of EPAC or expression of constitutively active Rap1 V12 bypasses the nonfunctional RhoA signaling to induce phagocytosis (521).
Further investigation into this regulatory pathway determined that EPAC activation enhances F-actin content and rescues Tat-C3 toxin-dependent depletion of this filamentous structure (521). The stabilization of actin can be affected by profilin-1 and -2 which were previously linked to RhoA and Rap1 signaling (92, 214, 573) and observed to co-immunoprecipitate with both Rap1 and RhoA (521). Attenuated phagocytosis with siRNA knockdown of profilin illustrates that both RhoA and EPAC/Rap1 pathways require downstream profilin activation, whereas activation of neither small GTPase could rescue the abolished phagocytosis. Additionally, EPAC/Rap1 phagocytosis does not appear to utilize the known RhoA downstream kinases, ROCK and LIMK (LIM domain kinase), to phosphorylate cofilin and form filaments, suggesting completely independent pathways (521).
As observed in neutrophils, RIAM expression is upregulated during the differentiation of macrophages, and this protein is found to be essential in Rap1-regulated phagocytosis. Supporting this claim, RIAM is reported to precipitate and colocalized with Rap1, talin, and β2 integrin at phagocytic cups (613, 684). Loss of this complex by RIAM knockdown in human macrophage cells reduces basal complement-mediated phagocytosis and abrogates increases in EPAC-stimulated uptake resulting in ~50% reduction in the phagocytic index. Furthermore, primary human monocyte-derived macrophages with lentiviral knockdown of RIAM reproduce this defective signaling demonstrating decreased “inside-out” signaling with reduced αMβ2 integrin activation and attenuated phagocytic activity in the presence of activated EPAC (FIGURE 9) (684). This signifies the importance of RIAM in the regulation of EPAC/Rap1-dependent phagocytosis in biologically relevant cells.
The conclusion that Rap1 can rescue or replace RhoA signaling and inability of Rap1 to modulate F-actin content with the same molecular kinases as RhoA suggests a collective regulation of complement-mediated phagocytosis by two independent pathways. In endogenous systems, cross-talk between Rap1 “inside-out” signaling and increased RhoA activation in “outside-in” signaling may also be present, but such feed-forward mechanisms in macrophages are not yet described. Additionally, the redundancy between two such signaling systems or natural divergence for specialized use of RhoA versus EPAC/Rap1 signaling in this context remains to be explored.
Interestingly, in antibody-induced phagocytosis of macrophages, the role of EPAC is completely inverted, following the historically reported inhibition of phagocytosis by cAMP induction. IgG-opsonized debris and microbes are opsonized with antibody containing Fc domains, and phagocytosis of these particles is initiated through activation of FcγR clusters on the surface of phagocytic cells such as macrophages.
In monocyte-derived macrophages and rat alveolar macrophages, important to the distal lung surveillance, PGE2 stimulation elevates cAMP and subsequently attenuates FcγR-mediated phagocytosis of IgG-opsonized Escherichia coli (30, 31, 120). Although activation of PKA does not suppress phagocytosis and inhibition of PKA fails to reduce the suppression by more than 10%, activation of EPAC mimics the effect of cAMP, suggesting that EPAC is involved in the cAMP-dependent suppression of phagocytosis in alveolar macrophages (31). Interestingly, in human monocyte-derived macrophages, activation of either EPAC or PKA could recapitulate the PGE2-mediated suppression of phagocytosis implicating both cAMP sensors (120). These observed discrepancies are likely resultant of experimental conditions such as agonist concentration or time allotted for phagocytosis. However, variable roles of PKA between macrophage subtypes or species could be another contributing factor.
Unlike complement-mediated phagocytosis where Rap1 is implicated as a positive regulator and observed to colocalize with late endocytic/phagocytic compartments (831), altered Rap1 expression does not affect FcγR-mediated phagocytosis (147), suggesting the inhibitory nature of EPAC occurs through Rap1-independent mechanisms (31). Further support for Rap1-independent suppression of phagocytosis is observed with the localization of EPAC1 and Rap1 in the presence of PGE2 during phagocytosis. Although EPAC1 typically localizes to punctate membranes throughout the alveolar macrophages, including the perinuclear region and MTOC, stimulation by PGE2 promotes a redistribution of EPAC1 to the nuclear envelope and away from the MTOC, while Rap1 does not follow suit. This loss of MTOC localization may lead to the suppression of phagocytosis through altered function of EPAC on microtubule dynamics, although the mechanism behind this EPAC1 function is not clear in phagocytizing cells. Also interesting is that after PGE2 stimulation or direct activation of EPAC by 007, EPAC1 is found to visually and physically associate with maturing, late phagosomes, while only weak visual evidence of Rap1 with these structures can be defined (108). This could suggest that Rap1 is not a major target of EPAC in this signaling pathway, but rather EPAC may merely signal through Rap1 for other unidentified signaling cascades concurrent with inhibition of phagocytosis of the particle.
Instead, FcγR-mediated phagocytosis requires the activation of Akt and ERK1/2, which is known to be suppressed by tensin homolog deleted on chromosome 10 (PTEN) (144, 522, 639). PGE2 also inhibits activation of Akt and ERK1/2 in alveolar macrophages in a PTEN-dependent manner, suggesting that PTEN may be involved in the suppressive function of PGE2/EPAC on IgG-induced phagocytosis. Corroborating the potential role of PTEN in this process, blocking PTEN rescues phagocytosis and bacterial killing from PGE2/EPAC-dependent suppression and reverses the PGE2 inhibition of Akt activation (141). Furthermore, PGE2 treatment and EPAC agonist treatment attenuates tyrosine phosphorylation of PTEN, which is inversely correlated with enhanced PTEN lipid phosphatase activity and antagonizes PI3K/Akt signaling (141, 639). Dephosphorylation of PTEN in this system implicates Src homology 2-containing protein tyrosine phosphatase-1 (SHP-1) as the middle-man where inhibition of SHP-1 returns tyrosine phosphorylation of PTEN to basal levels even in the presence of PGE2. Although the mechanisms governing the activation of SHP-1 by PGE2 and involvement of EPAC in this process remain unclear, the suppressive function of PGE2/EPAC on FcγR-mediated phagocytosis is observed to be reliant on SHP-1 dephosphorylation of PTEN to consequently abrogate PI3K/Akt signaling and disrupt microbial engulfment and killing (FIGURE 9) (141).
Further support of the notion that EPAC and PKA are involved in the negative regulation of FcγR-mediated phagocytosis comes with the action of the etiological agent of anthrax, Bacillus anthracis. These microbes produce an edema toxin that exhibits a Ca2+/calmodulin-dependent AC activity to induce a massive influx of cAMP after 6–24 h and disrupt the physiological homeostasis in the cell. The immense induction of cAMP would be anticipated to act on cAMP effectors such as PKA and EPAC to enact the toxin’s effect in the cell. Indeed, implication of EPAC and PKA in toxin signaling mechanisms is revealed by maximal activation of either cAMP effector, to transduce the effect of anthrax AC, which reveals coactivation of both augments the loss of phagocytosis. Being that edema toxin reduced ingestion of E. coli to a similar degree as another toxin, cytochalasin B, that disrupts actin filaments, the role of EPAC and PKA in toxin-mediated cytoskeletal remodeling was explored. Full activation of PKA and EPAC is observed to recapitulate the effects of the toxin including reduced cell spreading, filopodia formation, F-actin content, and phagocytosis. This suggests that the edema toxin, acting through PKA and EPAC, regulates actin dynamics to enact its detrimental effects on motility and phagocytosis (1164).
Thus far, only a single instance has reported that EPAC did not alter phagocytosis in macrophages (609). Taken together, one can then conclude that two independent systems for phagocytosis by macrophages are currently perceivable. One involving EPAC activation of Rap1 to enhance “inside-out” signaling of integrins and modulate cytoskeletal rearrangements promoting complement-mediated phagocytosis. While on the other hand, EPAC is differentially regulated by PGE2 to decrease PTEN phosphorylation, presumably through a SHP-1-dependent mechanism, consequently attenuating FcγR-mediated macrophage ingestion by PTEN-dependent inhibition of PI3K/Akt. Edema toxin treatment also supports the action of EPAC and PKA in attenuating phagocytosis and the involvement of these regulators on cytoskeletal remodeling. Thus the effect of EPAC inhibitors in animal models exhibiting suppressed macrophage functions could be interesting to investigate and potentially restore partial macrophage functions.
f) reactive oxygen species production.
Professional phagocytes, like macrophages and neutrophils, consume pathogens and damaged cell particulates; therefore, bactericidal environments in the phagosome are important to kill microbes to clear infections. Activation of EPAC or PKA by elevated cAMP has been reported to suppress bactericidal activity through attenuating H2O2 production to a similar degree as PGE2-mediated inhibition, around 30–40% (31, 234). Comparable results are witnessed in malaria-infested Kupffer cells where sporozoites defend themselves against these macrophages through a PKA-independent cAMP/EPAC signal to abrogate respiratory burst (1053). In addition, PGE2 and 007 treatment do not alter Rap1 localization in rat alveolar macrophages compared with EPAC phagosome localization which could alter EPAC function in these cells (108). The localization of constitutively active Rap1 compared with dominant-negative Rap1 N17 would also be of interest to determine if small populations of active Rap1-GTP are transiently associating with the phagosome and were undetected in the mentioned study, potentially explaining the discrepancy between microscopic and immunoprecipitation observations. Furthermore, reduced bacterial killing of these alveolar macrophages may only, in part, be related to the production of H2O2 while additional mechanisms involving EPAC or PKA regulation of bactericidal activity remain to be explored.
Interestingly, contrasting the suppressive role for EPAC in H2O2 production and bactericidal activity in alveolar macrophages, FcγR-mediated phagocytosis in mouse macrophage cells induces superoxide production to generate an oxidative burst which is dependent on NADPH oxidase assembly in the phagosome membrane through association with active Rac1. Genetic deletion of RhoA or Rap1 attenuates superoxide production ~40% and can be recovered by activation of the other pathway (609). Toxicological and dominant-negative functional depletion of RhoA presented similar reductions in superoxide levels that are effectively restored with EPAC activation (521). Also notable, treatment with EPAC agonist was observed to augment superoxide production beyond control levels in the presence of IgG-opsonized particles (521, 609). This suggests that the oxidative process is directed by additive actions of RhoA and Rap1 in the activation and translocation of Rac1 to enhance association with the NADPH oxidase component p22phox (609).
Supporting a cooperative function, loss of Rac1 activity and translocation by genetic knockdown of either RhoA or Rap1 in macrophage cells is restored with LPA or EPAC agonist activation of the complementary small GTPase in the presence of IgG-opsonized particles. However, in the absence of particle stimulation, activation of RhoA is ineffective at enhancing Rac1 activity, while EPAC/Rap1 activation of Rac1 is retained. Thus the mechanism of Rac1 activation by RhoA and Rap1 may function through independent regulators where RhoA signaling may require additional input from upstream pathways stimulated by IgG-opsonized particles (609). Indeed, Rap1 has been observed to activate Rac1 through Rac1-specific GEFs such as Vav2 and Tiam-1 (35, 695, 770). In this signaling system, the sole knockdown of Vav2 by siRNA effectively reduced Rac1 activation by EPAC/Rap1 suggesting Vav2 as the major Rac1 GEF in this process (609).
During FcγR-mediated phagocytosis, increasing levels of RhoA phosphorylation and RhoA-RhoGDI complexes are observed that are connected to competitive relief in RhoGDI binding of Rac1 (98, 609, 879). RhoGDI precipitation of Rac1 appears inversely related to IgG-induced phagocytosis and RhoA-RhoGDI complex formation. Furthermore, minimal levels of Rac1 are complexed with RhoGDI in the presence of 007 (609), which suggests a supplemental role for EPAC activation of Rap1 in activating ARAP3 to reduce active RhoA and promote RhoA-RhoGDI association (470, 560). Additionally, Rap1 is not reported to affect NAPDH oxidase activity (418), but is observed to translocate with Rac1 to associate with p22phox, whether this interaction is direct, serving as a coordination point for the formation of a larger complex of regulators to assemble NADPH oxidase, or is depicting an unidentified functional role for Rap1 remains to be explored (FIGURE 9). Thus the discrepancy between H2O2 and superoxide production by EPAC/Rap1 signaling could be conditional to the presence of PGE2 stimulation in these macrophages or differences attributed to species and macrophage types used in these investigations.
Conclusively, although initial exploration into the role of EPAC in macrophages was more gradual, the extensive nature of this signaling molecule in macrophages has begun to be appreciated as studies pertaining to this topic continue to grow. Moreover, while beneficial effects can be demonstrated with modulation of EPAC activation in various macrophage functions, caution may be in order with differential effects observed dependent on the macrophage phenotype. Rather, combinational therapy to skew macrophages to a desired phenotype followed by EPAC modulation may be more effective in producing the sought response. Indeed, treatments in cancer biology may benefit from applying the findings of these studies to tumor-associated macrophages to potentially enhance re-education to M1 phenotypes and FcγR-mediated phagocytosis by EPAC inhibition. As more studies become available, our insight into the full extent of EPAC signaling in macrophages and targeting strategies for this signaling system will become more apparent.
3. Dendritic cells
Dendritic cells (DCs) are uniquely poised between the innate and adaptive immune responses patrolling the body in search of foreign antigens and acting as protective sentinels for the host organism. Upon contacting antigenic particles in the peripheral tissues of the body, DCs mature by upregulating surface expression of MHC class II to present exogenous antigen along with upregulated costimulatory molecules. Additionally, mature DCs migrate to draining lymph nodes by upregulation of chemokine receptors to more readily initiate activation of the adaptive immune response during pathogenic events. Furthermore, following clearance of the pathogen, the host must induce anti-inflammatory actions through suppressive mediators, such as cytokines and prostaglandins, to decrease the activities of T-effector cells and antigen-presenting cells re-establishing homeostasis of the system. Dysfunction of the maturation process can result in failure to activate T cells and subsequent T-cell tolerance (239, 403). Overactivation of DCs is also detrimental to the host and upsets the balance in the opposing direction leading to uncontrolled inflammation and autoimmunity. Thus understanding the mechanisms that govern the maturation process can assist in priming of the immune system or alternatively inducing tolerance, both of which are highly coveted therapeutic effects.
a) maturation/transcriptional regulation.
A variety of cAMP stimulating ligands potentiate the maturation of DCs, but PKA and EPAC are observed to partake in differential roles in this process. With DC maturation being a distinct process, there are several hallmarks of proper execution, including upregulation of costimulatory and antigen presenting molecules on the cell surface, increased migration towards lymph nodes, decreased endocytosis, T-cell activation, and altered cytokine production. Selective activation of PKA, but not EPAC, demonstrated amplified surface expression of costimulatory molecules CD80 and CD86, the maturation marker CD83, antigen presentation complex MHC class II, and CXCR4 in DCs (335). In support of this effect, a cAMP-responsive element is located within the CXCR4 promoter producing a prime target for CREB, a major PKA effector (206). Moreover, dendritic cells costimulated with EPAC and PKA activators exhibit reduced surface expression of costimulatory molecules, MHC class II, and CXCR4 where the reduced expression of this chemokine receptor is attributed to an EPAC-dependent interference of PKA/CREB signaling resulting in 20% loss of CREB phosphorylation (335).
b) maturation/chemotaxis.
Furthermore, DCs utilize chemotactic migration for lymph node homing to increase frequency of T-cell encounters and transmission of the activation signal to immune effector cells. Interestingly, activation of PKA not only enhances intrinsic DC migratory capacity observed as increased chemotaxis towards CXCL12, concomitant to increased CXCR4 surface expression, but also augments random migration and chemokinesis (335). These nongradient processes of motility are alleged to be important to the stochastic, nonaggregatory nature of T-cell activation by DCs in the lymph node where chemokine gradients would be less effective (701). In support of the action of EPAC on CXCR4 expression, EPAC activation in tandem with PKA suppresses CXCL12-induced chemotaxis, but surprisingly random migration and chemokinesis are unaffected by the action of EPAC. These results suggest that while EPAC activity interferes with PKA-mediated chemotactic migration to CXCL12, presumably through decreased CXCR4 expression, PKA enlists other signaling pathways to differentially regulate alternate motilities in DCs that are not affected by EPAC (335).
With the myriad of chemoattractant signals, the function of EPAC in DC migration in relation to other DC functions should not be completely dismissed. A recent study observed the migration of DCs towards adenosine specifically generated by regulatory T (Treg) cells in Boyden chambers. This action is dependent on adenosine A2A receptor expression in DCs which promotes cAMP production and subsequent activation of EPAC and Rap1 in the presence of Treg cells. Supporting an EPAC-mediated signal, inhibition of EPAC with ESI-09 reduced DC aggregation around Treg cells. In addition, coculture of DCs with Treg cells or adenosine stimulation increased Rap1 activation similar to direct stimulation of EPAC. Furthermore, coculture with Treg cells induced redistribution of Rap1 to the actin cytoskeleton in DCs where it functions to stabilize F-actin. This action of Rap1 suggests that Treg generated adenosine can induce cytoskeletal reorganization in DCs to promote directed motility and enhance contact with Treg cells (873). This function may be relevant during minor injuries or cell death where levels of purines are elevated, but Treg cell conversion of ATP to adenosine can attract and circumvent unnecessary DC-mediated immunological responses to such nonthreatening events. Although Rap1 signaling is linked with immunosuppressive actions of DCs such as migration to Treg cells (873) and suppressed cytokine release (479), CTLA-4 induced signaling relies on Rap1 to initiate disruption of the immune synapse between antigen presenting cells (APCs) and Treg cells (396). These results may suggest differential roles of Rap1 dependent on the initiating and ongoing signals to alter cellular function. However, whether these signals result in feedback mechanisms to avoid oversuppression or mechanisms to ensure adequate timing and efficiency for Treg cells to modulate larger populations of DCs remains to be elucidated.
c) maturation/endocytosis.
Suppressing immature DC functions such as endocytosis for antigen uptake is another common marker for DC maturation. In line with the above characteristics of PKA activation being sufficient to induce DC maturation, PKA agonists did indeed reduce FITC-dextran uptake by 50%, while addition of EPAC activity perturbed this effect resulting in only 26% reduction. This further supports EPAC interference of PKA-mediated endocytosis and maturation of DCs. Activation of specific T-cell populations is an end goal of DC maturation and, not surprisingly, coactivation of EPAC also antagonizes PKA-mediated induction of allogenic T-cell proliferation (335). Thus PKA activation appears sufficient to induce DC maturation, whereas the function of EPAC is commonly implicated as a direct hindrance of this PKA-induced process. Importantly, the use of cAMP analogs selective for EPAC and PKA in many studies involving dendritic cell maturation and function signify regulatory actions of EPAC on PKA signaling are independent of cAMP competition between the two sensors, although defining the elements involved in such regulatory pathways for each process require further attention. Taken together, EPAC appears to crosstalk to select PKA signaling pathways and is responsible for keeping PKA activity in check to prevent over-activation of T cells.
d) maturation/chemokine secretion.
Another important aspect to accomplish maturation and function of DCs is skewing chemokine release profiles in conjunction with antigen presentation, costimulatory molecule expression, and effector cell contact. The role of PKA activity in this process is attenuation of TNF-α, TGF-β, IL-18, and IL-10 mRNA transcription. Cotreatment with EPAC agonist selectively reversed only expression of IL-10 and IL-18 partially, again suggesting that only some of the PKA-regulated pathways are also sensitive to EPAC (335). Interestingly, in an independent investigation, immature murine DCs briefly pretreated with PKA or EPAC agonists before LPS-induced maturation demonstrate attenuation of MIP1α, IL-6, and TNF-α release with activation of either cAMP effector, potentially suggesting differential regulatory mechanisms for transcription, translation, and/or exocytic release (32).
As mentioned, PGs are often reported to mediate anti-inflammatory actions in dendritic cells including regulation of chemokine release. In LPS-stimulated DCs, the presence of PGE2 reduces pro-inflammatory chemokines IL-12p70 and IL-12p40, while inversely promoting IL-10 expression (398, 488). Furthermore, PGE2 also inhibits MIP1α (CCL3) and MIP1β (CCL4) expression through EP2 receptor activation of EPAC in a PKA-independent mechanism. EPAC subsequently activates Akt to phosphorylate and inactivate GSK-3, abolishing endogenous inhibition of CCAAT displacement protein (CDP) repressor. CDP repressor binding to MIP1α/β promoters then attenuates transcription (478, 479). Together, these results implicate EPAC activation in suppression of chemokine expression and release, promoting anti-inflammatory signaling.
However, the role for PGs is often confronted with contradictions in the literature and some researchers report paradoxical, selective promotion of pro-inflammatory chemokines by PGE2 in DCs, such as IL-23, while supporting the reduction of others including IL-12 expression (541, 832). In this case, activation of EP4 receptors in LPS- or TNF-α-stimulated DCs promotes EPAC and PKA mediated phosphorylation of C/EBPβ and CREB, respectively, inducing IL-23p19 expression and release (541). This dichotomy in the role for PGE2 may be reflective of a regulatory function dependent on differential activation of PG receptors or inconsistencies of stimulatory times in these studies. In fact, notable support for differential regulation of PG receptors by PGE2 was explored by Poloso and colleagues through selective pharmacological discrimination of each PG receptor (EP1–4) (832). In LPS-stimulated human DCs, PGE2 and the EP2 receptor agonist effectively inhibited pro-inflammatory chemokines and induced IL-10, suggesting an anti-inflammatory effect as previously observed (398, 488, 832), whereas an EP4 receptor agonist imitated this chemokine profile except for one reversal, inducing IL-23 production (541, 832, 919). EP1 and EP3 receptor agonists are without effect. Since the endogenous PGE2 ligand can stimulate any of the PG receptors, coupling the activation studies above with antagonist experiments in the presence of PGE2 corroborate differential signaling observed between EP2 and EP4 receptors in a more natural signaling environment. Additional investigation into the regulatory mechanism of these PG receptors on IL-23 production revealed a dose-dependent signaling pathway where low EP4 agonist or PGE2 levels induce IL-23p19 expression, but the production of IL-23 is attenuated with increasing EP4 activation levels. Moreover, the induction of IL-23 is completely abolished with the addition of EP2 agonist or simply increasing PGE2 to relevant physiological levels between 50 and 100 nM. At these higher PGE2 concentrations, the subunit shared between IL-12 and IL-23, the IL-12p40 subunit, is reduced and limits the release of both IL-12 and IL-23. Moreover, downstream effectors PKA and EPAC were again implicated in the regulation of these chemokines. Direct activation of EPAC or low levels of PKA activation are observed to enhance IL-23 expression, in accordance with previous accounts, suggesting that EPAC and PKA function cooperatively in this signaling pathway. Interestingly, cAMP production was not detected with EP4 activation, but only after EP2 agonist stimulation. Thus the cAMP sensors may function at cAMP levels below the detection limit to promote IL-23, but elevation of cAMP appears to primarily promote an inhibitory mechanism (832).
Thus selective activation of the higher affinity EP4 receptors may initially dictate minute generation of cAMP to promote production and release of IL-23 from LPS-stimulated DCs, but as PG levels increase, the action of EP4 receptors is masked by the activation of the predominately expressed EP2 receptors (541, 832). Activation of EP2 receptors increases cAMP to inhibitory levels, shutting down IL-23 production and release, thereby inducing an anti-inflammatory signal. Taken together, these results help to mend controversial reports of PGs in immunity and depict a fluid regulatory role for PGE2 that permits DCs to employ PGs as molecular sensors detecting inflammatory levels and acting as regulatory switches to suppress overreactive responses.
Although cAMP is one of a plethora of signals being integrated by DCs during the maturation process, specific targeting of either cAMP sensor may be advantageous for therapeutic benefit. With EPAC predominately suppressing the maturation process, selective activation of EPAC could lead to tolerance induction and attenuate allergic and autoimmune disorders. On the other hand, in the development of ex vivo conditioning strategies for cancer vaccines and immunotherapy, enhancement of DC maturation by PKA activation may be further augmented by cotreatment with an EPAC antagonist to prevent any remnant suppression of PKA agonist effects, thus maximize T-cell priming.
J. Adaptive Immunity
In an effort to make immunity of the host more efficient in eliminating reoccurring pathogens, a separate, yet interconnected, defense network evolved referred to as the adaptive immune system. Upon contact with an unknown microbe, APCs of the innate immune system will report to specific locales rich in adaptive immune cells. There the APCs present specific antigens of the persistent threat and the adaptive cells mature into cells that specifically recognize this pathogen, while also creating memory cells that can be reactivated if the same antigen is recognized again. Returning pathogens will then be met with a more potent and swift response from the combined host immune systems. Therefore, the adaptive immune system is crucial for long-lasting protection from specific microbes and can be mobilized to assist an overwhelmed innate immune system during infections. In the following subsections, we will summarize reported functions of EPAC in T and B cells of adaptive immunity, which could be beneficial to current immunotherapy-based strategies.
1. T cells
T cells are a dynamically regulated set of immune cells belonging primarily to the adaptive immune system. In the circumstance when the initial acute inflammatory response is unable to effectively clear an infection, APCs from the site of infection will migrate to local lymph nodes to initiate activation of T cells. Engagement of T-cell receptors (TCR) with antigen presentation of MHC class II and costimulatory molecules initiates T-cell differentiation into one of several subtypes depending on the cytokines, chemokines, and soluble lipid molecules secreted by the APCs during this process. During active infections, APCs can also induce rapid clonal expansion of antigen-specific T cells to promote T-cell-mediated cytokine secretions and help facilitate the immunological clearance of the contagion as well as attenuation of the immune response after the pathogen has been eradicated. Following the induction of T cells there is a period of cellular contraction through controlled cell death, and also the formation of memory T cells for future induction. Therefore, to systematically control the immune response in all facets of the inflammatory process, the large functional repertoire of T cells is dispersed between multiple T cell types including, but not limited to, cytotoxic T cells, T helper cells, T regulatory cells, memory T cells, or natural killer T cells. Below the role of cAMP activation of EPAC will be discussed in various aspects of T cell functions.
a) proliferation.
T-cell proliferation and expansion is pivotal to heightened immune responses in host defense. Intriguingly, the mitogenic action of cAMP is often dependent on the cell type in question. For instance, cAMP induction in macrophages induces proliferation and pro-survival signals (710), but elevated cAMP acts to enhance p27kip1 expression and inhibit cyclin-dependent cell cycle progression in T cells (118, 324, 501, 1061). Although initial reports dismissed the expression of EPAC in T cells (1030), subsequent investigation reassessed these conclusions determining EPAC expression in most immune cells, including T cells (634). Investigation into the role of EPAC in growth arrest of Jurkat T cells demonstrated that PKA was responsible for increase in G1-phase cells, while activation of EPAC was without effect (324). Thus current conclusions dismiss an EPAC component in the antimitogenic action of cAMP in T cells, but results for the function of EPAC in this process are based on the leukemic Jurkat cell line and should be validated in bona fide T cells.
b) transcriptional regulation.
Another aspect of T-cell function, as in most immune cells, is transcriptional regulation which is crucial to activation, cytokine expression, and proper function. In other cells, cAMP is implicated in these regulatory processes with PKA commonly described as the major mediator in T cells through CREB activity. However, a microarray analysis reveals EPAC activity can also control transcriptional regulation in Jurkat T cells. Additionally, activation of EPAC is observed to suppress c-Jun binding of AP-1 promoter elements in a PKA-independent fashion, while PKA acts independently on CREB and ATF2 (324). Supporting the potential roles of cAMP sensors on these transcription factors, an independent investigation observed increased CREB and AP-1 activation with parallel attenuation of NFκB and NFAT activity in the presence of elevated cAMP. This led to abrogated cytokine expression in the T cells, but not all cytokines were sensitive to PKA inhibition, suggesting the role for a cAMP-sensitive, PKA-independent mechanism such as EPAC (477). Although these results may support the function of EPAC in transcriptional modulation of T cells, lack of AP-1 elements in the promoters of genes identified suggests additional transcription factors specifically regulated by EPAC that are currently unreported (324). Indeed, EPAC activation was also implicated in induction of p38 phosphorylation in leukocytes (345, 346), defining a potential link to other transcriptional regulators aside from c-Jun, but a complete list of genes regulated by this action is not defined.
Although the regulators of gene transcription by EPAC are not fully established, functional consequences of cAMP signaling on T-cell cytokine production is observed in airway inflammation. PGE2 elevation of cAMP in this tissue coincides with reduced IL-13 and IFN-γ production in wild-type splenocytes to maintain inflammatory status quo, but knockout of the EP2 receptor for PGE2 increases the expression of these cytokines, signifying an enhanced inflammatory response. More importantly, agonist stimulation of PKA and EPAC both reduced IL-13 expression in splenocytes and CD4+ T cells similar to PGE2; and further, the inhibitory actions of EPAC and PKA were retained with deletion of the EP2 receptor in T cells, suggesting the cAMP effectors activity is downstream of the EP2 receptor. PGE2 was also observed to reduce STAT6 phosphorylation, suggesting a potential link for EPAC and PKA in regulation of cytokine suppression by STAT6 activity (1183). Thus PGE2 and downstream effectors PKA and EPAC appear vital to the innate suppression of allergic inflammation by CD4+ T cells and enhancing these signals could be pertinent to alleviating airway inflammation in patients.
c) activation and suppression.
The immune system exists in a balance between induction and suppression of effector cells. Many cells exhibit regulatory functions to assist in the maintenance of this equilibrium. DCs are the major cell type implicated in the education and induction of T cells in the body forming a bridge between the innate and adaptive immune systems. Although the activation of PKA appears to induce DC-mediated CD3+ T-cell proliferation, EPAC activity does not, and even partially blocks the PKA-mediated proliferation (335). Moreover, elevation of cAMP by PGE2 is also reported to induce IL-23 in DCs mediating Th17 differentiation as well as promotion of Th1 differentiation (59, 180, 1158). This suggests PKA may function to promote T-cell expansion, while EPAC prevents excessive signal propagation by indirectly interfering with PKA action in DCs. Interestingly, cholera toxin promotes induction of cAMP in Jurkat and CD4+ T cells and directly stimulates IL-17A production, suggesting a functional role for cAMP effectors in the activation of Th17 cells (1039). A more recent report determined that the IL-17A production was in part mediated by PKA, but the potential role for EPAC was not explored (583). Intriguingly, this cAMP-dependent effect was observed to be inversed during differentiation of naive T cells into Th17 cells in response to PGE2 or Leishmania donovani stimulation (895, 1056). This suggests cAMP may have distinct roles in the differentiation or activation of Th17 cells dependent on the maturity of the cell, again attributing these actions to PKA without exploring potential EPAC modulatory roles in this pathway (895). Therefore, to conclude the functional relevance for EPAC within T cells specifically during differentiation or activation will require further attention. However, with the inhibitory nature of EPAC in DCs to block maturation and attenuate T-cell induction, another regulatory cell responsible for the suppression of T-cell function was investigated.
Opposing induction of T-cell immunity, Treg cells are a major cell type involved in the suppression and induction of tolerance in peripheral immune cells. Suppression of different aspects of the immune system requires a plethora of signaling pathways invoked by Treg cells to inhibit proliferation and/or activation of other immune cells. Tregs can secrete immunosuppressive cytokines or signal through direct contact of TGF-β1 or cytotoxic T-lymphocyte protein 4 (CTLA-4) to suppress proliferation or induce anergy (661, 754, 1002, 1194). Therapeutic manipulation of Treg function is coveted due to their ability to govern immunosuppression throughout the body. This could introduce a potentially valid approach to suppress autoimmune disorders, host defensive against organ transplant or rejection of primed T-cell immunotherapy, as well as enhance responses to infection and cancer. However, understanding of the mechanisms behind these Treg suppressive functions must be delineated to explore effective targeting of this system.
Defining a role for EPAC in this immunoregulation, Almahariq et al. (16) recently reported that suppression of CD4+ T-cell proliferation and IL-2 production was attenuated with genetic deletion of EPAC1 in Treg cells and similar deletion in CD4+ T cells partially protects from wild-type Treg-mediated suppression . A similar observation was reported in an independent study, demonstrating EPAC activation as an alternate regulator of CD4+ T-cell suppression. Additionally, the authors dismiss a redundant mechanism of PKA through inducible cAMP early repressor (ICER), being that suppression by PKA is unaltered regardless of ICER expression (1063). Investigation into the mechanism of EPAC in suppressive regulation of Tregs revealed that TGF-β1 signaling was attenuated in EPAC1−/− CD4+ T cells attributing to the loss of IL-2 production. Indeed, TGF-β1 suppression of IL-2 was partially negated by loss of EPAC1 function. Furthermore, absence of EPAC1 resulted in diminished activation of Smad2, a downstream effector of TGF-β1, and reduced Smad4 expression crucial for nuclear translocation of active Smad2/3. Deficiency in TGF-β1 activation was augmented with concomitant increase in the inhibitory component Smad7 (16). Induction of Smad7 and reduction in TGF-β1 sensitivity are both associated with STAT3 activation (469, 643), and interestingly, phosphorylation of STAT3 was enhanced in both Treg and CD4+ T cells in the absence of EPAC1. Furthermore, inhibition of STAT3 restores Treg suppression even in the absence of EPAC1, suggesting that STAT3 is downstream of EPAC1 signaling (16). This is consistent with reports of negative regulation of STAT3 by EPAC/SOCS3 in non-immune cells (905, 1150), and regulation of the related transcription factor STAT6 in splenocytes and CD4+ T cells (1183). With the levels of typical modulators of STAT3 activation, namely SOCS3, JAK2, and LCK unaffected in the absence of EPAC1, relevant regulators in this pathway are still currently unclear and under investigation. Functional consequence of EPAC deletion in an animal model immunized with oral ovalbumin demonstrated increased ovalbumin IgG antibodies, further supporting an attenuation of Treg function in these animals. Pharmacological inhibition of EPAC by ESI-09 reproduced the suppressed Treg function and corroborate the role of EPAC to boost Treg suppression through TGF-β1 signaling in CD4+ T cells (16).
Direct suppression of T-helper cells activity by cAMP is also reported with treatment of the PDE4 inhibitor rolipram. Concomitant increases in cAMP levels in purified T cells abrogates production of Th1 cytokines IFN-γ, IL-2, and TNF-α, as well as Th2 cytokines IL-5 and IL-10. However, inhibition of PKA was only able to reverse the attenuation of Th1 cytokines by rolipram, while the Th2 cytokine IL-10 was insensitive to loss of PKA. This suggests the possibility that EPAC may be involved in the regulation of Th2 cytokines to influence T-cell activation, but additional studies are needed to confirm this function (477).
d) activation-induced cell death.
Following an immune response, the population of T cells is too large for the body to sustain indefinitely and thus controlled cell death is induced to pave room for the next response with fresh antigen-specific T cells. The controlled death of autoreactive and chronically active T cells is also important to the peripheral tolerance of the host to attenuate autoimmunity. This process is tightly controlled through the activation-induced cell death (AICD) process and is invoked by interaction of Fas ligand (FasL/CD95L) with Fas receptor (CD95) to form the death-inducing signaling complex. Continued TCR/CD3-mediated activation of T cells regulates this action through increased surface expression of CD95L/FasL and consequent AICD-mediated cellular suicide. However, during an active infection, this suppressive system needs to be circumvented to allow for both activation and expansion of antigen-specific T-cell clones. Thus APCs responsible for the T-cell activation are in a prime position to also contribute to survival of T cells during activation.
Indeed, investigation into the role of APCs in attenuation of the AICD process demonstrates the secretion of PGE2 as a protective factor imparting T-cell survival in the presence of the immune-stimulatory agent LPS (1107). Furthermore, PGE2-dependent attenuation of AICD is attributed to the downregulation of CD95L/FasL expression (839, 1107). This action of PGE2 occurs via EP2 and EP4 which elevate cAMP and activate EPAC and PKA. Reduced CD95L/FasL surface expression and associated T-cell death is observed to be predominately linked to PKA activity; however, forskolin treatment is observed to further reduce the CD95L/FasL surface levels and concurrent EPAC activation with PKA does augment the suppression of AICD. Taken together, this PGE2-initiated synergistic signal from cAMP employs PKA and complementary EPAC cascades to suppress the AICD pathway to maintain active T cells during infections (1107). The precise mechanism by which EPAC can influence PKA-mediated CD95L/FasL induction remains to be defined as in many signaling pathways. Supporting these conclusions, autoimmune diseases such as rheumatoid arthritis and type 1 diabetes exhibit increased PGE2 (159, 332, 551) and reduced CD95L/FasL levels. Modulating expression of these two components attenuates AICD in T cells (33, 230) leading to prolonged autoimmune states.
Overall, the role of EPAC in T cells is only beginning to be revealed. Many current conclusions depict modulatory roles for transcriptional suppression of cytokines although the exact mechanism for this action remains elusive. Also, although alone EPAC is ineffective in altering PGE2-dependent AICD, activation of EPAC does enhance the protective effect of PKA suggesting a role in maintaining the inflammatory state. Further exploration into the precise roles of EPAC in these immunological cells may prove important in developing more effective targeting strategies for immunotherapy and treatment of autoimmune diseases.
2. B cells
Developing B cells, undergo negative selection to eliminate self-reacting cells being released into circulation that could provoke autoimmune processes. The process induces cell cycle arrest and apoptosis signals in the autoreactive cell. The B-cell antigen receptor (BCR) signaling is crucial for this homeostatic process and eventually leading to functional mature cells. The consequential signaling invoked by activation of BCR signaling is dependent on the signal strength, duration, and maturity of the B cell. This leads to a shift in balance between the two states of survival and apoptosis. As mentioned, in immature B cells BCR signaling promotes G1 phase arrest and subsequent cell death for self-reacting cells.
The survival and apoptosis signals pivot on a sensitive balance attributed to pro-apoptotic ERK1/2 activity or pro-survival PI3K-dependent Akt activity. BCR signaling is implicated in the shift of balance towards cell cycle arrest and apoptosis in immature B cells, and cAMP is observed to mimic these molecular cues. In a cell line phenotypically similar to immature B cells, WEHI-231 cells, α-IgM ligation of BCR induces a rapid yet transient generation of cAMP and promotes growth arrest and apoptosis. Direct stimulation of AC by forskolin or treatment with the cAMP analog 8-BrcAMP replicated the processes induced by BCR ligation. Exploring the functional consequence of elevated cAMP levels in immature B cells illustrated that cAMP exhibits conflicting effects on phosphorylation of ERK1/2 and Akt Ser473 in the presence of BCR signaling presumably by activation of either PKA or EPAC. Pharmacological inhibition of PKA was not observed to alter ERK or Akt phosphorylation during BCR ligation, thus the role of EPAC/Rap in this signaling paradigm was explored (360). Interestingly, initial studies depicting the role of Rap, the major downstream target of EPAC, in BCR-induced activation of ERK and Akt are controversial. One investigation in B lymphocytes found that attenuated Rap signaling solely enhanced Akt activity (187), while a more recent study found that Rap was essential for both Akt and ERK1/2 activation in response to particulate antigens (616). In agreement with the latter, direct stimulation of EPAC revealed an enhancement of both Akt and ERK1/2 activity, with a preference for ERK2, within the initial few minutes of α-IgM-induced BCR ligation. Supporting the downstream activity of Rap in this signal cascade, all effects of EPAC on Akt and ERK1/2 activation were highly reduced with inhibition of the Ras family, including Rap, by treatment with C. sordellii toxin. Curiously, although activation of EPAC alone is enough to induce Akt and ERK1/2 phosphorylation, the action of EPAC in growth arrest and apoptosis is only observed in the presence of α-IgM-induced BCR ligation where the apoptotic effect is augmented by ~35%. Further supporting this conclusion, IgM treatment or individual activation of EPAC results in low induction of caspase 3 and PARP cleavage, while the combination of the two markedly amplifies the Rap-dependent cleavage, suggesting promotion of apoptosis. Thus, in immature B cells, the pro-apoptotic signaling propagation appears reliant on modulation by EPAC/Rap activation during late BCR signaling events (360). This also suggests that α-IgM-induced BCR ligation induces not only the cAMP/EPAC/Rap signaling cascade to facilitate rapid Akt and ERK1/2 activation, but also other cross-talking pathways to promote BCR-induced growth arrest and apoptosis.
Defective signaling of cascades in the immune system often leads to dysfunction in growth arrest, selection, and apoptosis. These altered pathways are commonly responsible for lymphoproliferative disorders and immunodeficiency in patients. Understanding the underlying mechanisms modulating these processes is crucial in treating many disease states including multiple leukemias and lymphomas, but may also be exploited to boost the immune system in immunocompromised patients undergoing stringent treatments. As mentioned, the complexity of signal transduction in the immune system often leads to differential signaling cascades highly dependent on the combined effect of a myriad of stimuli, along with the intensity and duration of these signals. Furthermore, the response observed during these processes can be cell-type specific creating discrepancies for the function of such signaling cascades. Indeed, this divergent signaling is observed in B cells and B-cell chronic lymphocytic leukemia (B-CLL) cells as compared with other cell types including T cells (1030). B-CLL cells undergo apoptosis when exposed to cAMP elevating agents such as PDE inhibitors and forskolin (520). Interestingly, the anti-apoptotic effect is predominantly dependent on PDE4 while other isoforms, such as PDE3, may work loosely in tandem with PDE4 (721, 726). In support of a pro-survival role for PDE4, elevation of cAMP by blocking PDE4 induced PP2A activity and subsequently downregulated Bad Ser112 phosphorylation, resulting in mitochondrial translocation. This signal then cascades, setting apoptotic events into motion including promotion of mitochondrial depolarization, cytochrome c release, and caspase cleavage. Similarly, forskolin treatment mimicked the effects of PDE4 inhibition, while blocking PP2A returns cytochrome c release and Bad phosphorylation to baseline (726). Importantly, the action of cAMP in B-cell survival implicates a cAMP sensor in the mediation of these apoptotic processes.
Peripheral B cells and B-CLL cells were observed to express increased levels of EPAC1 transcript alluding to a potential role for EPAC in this signaling cascade (539, 1030). Indeed, PDE4 inhibition with rolipram effectively induces greater activity of Rap1 in B-CLL cells than peripheral B cells. But surprisingly, unlike overall elevation of cAMP, direct activation of EPAC reduced basal apoptosis by ~25%. This suggests the apoptotic signal is most likely funneling through a PKA-mediated pathway, and this hypothesis is further upheld by attenuated apoptosis in the presence of a PKA antagonist (1030). Thus, with the PKA signal overriding the less pronounced modulatory role of EPAC, the purpose of increased EPAC1 transcripts in these B-CLL cells is still unclear. Use of EPAC inhibitors or siRNA could facilitate exploration of the significance for increased EPAC expression in B-CLL cells. Potentially, the smaller anti-apoptotic signal of EPAC1 may be upregulated to balance the predominant PKA signal and impart a survival advantage for transformed B-CLL cells over nontransformed B cells. Potential agents targeting such discrepancies between transformed, malignant cells and normal cells could be vital in development of future targeted-therapeutic strategies.
V. ROLES IN DISEASE DEVELOPMENT AND PROGRESSION
In agreement with the multitude functional roles of EPAC proteins in physiological systems, extensive studies using various animal disease models, as well as related clinical samples, have also implicated the potential involvements of EPAC proteins in the development and progression of a plethora of human diseases (FIGURE 10). A list of studies reporting the involvement of EPAC proteins in human patient pathologies are summarized in TABLE 2.
FIGURE 10.
Overview of the involvement of EPAC proteins in major pathophysiology in various organ/tissue systems.
Table 2.
Involvement of EPAC proteins in human patient pathologies
| Pathology | EPAC1 Expression or Mutation | EPAC2 Expression or Mutation |
|---|---|---|
| Alzheimer’s disease and autism | ↑AD: prefrontal cortex/hippocampus (680) | ↓AD: prefrontal cortex/hippocampus (680) |
| ↑AD: 3 SNPs (1207) | ||
| ↑Autism: 4 missense mutations (40, 1044) | ||
| Depression | SNP (696) | ↑Prefrontal cortex/hippocampus of suicide victims (259) |
| Heart failure | ↑Left ventricle (692) | |
| COPD/cigarette smoke | ↓Lung tissue (786) | |
| Thyroid malignancies | ↑Differentiated (109), ↓undifferentiated (109) | |
| Cancer | ↑Gastric cancer (991), ↑breast cancer (567), ↑pancreatic cancer (636) |
AD, Alzheimer’s disease; SNP, single nucleotide polymorphism. Reference numbers are in parentheses.
A. Neurological Disorders
1. Alzheimer’s disease
Alzheimer’s disease is the most common cause of dementia in patients and presents with a detrimental pathology that encompasses amyloid β peptides aggregations that progress to senile plaques in the brain where consequential learning and memory deficits transpire. Amyloid β peptides are generated by proteolytic cleavage of the type I integral membrane glycoprotein, amyloid precursor protein (APP), diverging into either the amyloidogenic or nonamyloidogenic pathway. The amyloidogenic pathway is initiated by β-secretases cleaving the NH2 terminal and γ-secretases clipping the COOH terminal of APP to generate proaggregative amyloid β peptides. Alternatively, the nonamyloidogenic pathway produces the soluble form of APP (sAPPα), by cleaving and releasing the extracellular domain from APP into the extracellular space. This APP processing is carried out by membrane-anchored α-secretases of the ADAM protein family including ADAM9, ADAM10, and ADAM17/TACE (TNF-α converting enzyme) referred to as α-secretase (12, 436). Shedding of APP through the nonamyloidogenic pathway cleaves APP in the middle of the amyloid β peptide sequence suggests enhanced α-secretase expression/function would be a viable strategy to reduce the pathogenic amyloid peptide, although increased sAPPα is not always directly correlated with decreased amyloid β peptide production (876). Interestingly though, induction of sAPPα release does indeed exhibit neuroprotective effects (325, 667, 893) and potential memory improvement (419, 694, 699, 874, 1017).
Thus the mechanisms underlying the nonamyloidogenic pathway are of great interest as a potential therapeutic target for neurodegenerative diseases. In brains from post-mortem Alzheimer’s patients, cAMP signaling has been found to be perturbed (308). Additionally, although α-secretases cleave and induce sAPPα secretion basally, activation of specific GPCRs, including serotonin 5-HT4 receptor, are shown to enhance this effect through cAMP-dependent and PKA-independent mechanisms (602, 877). With inhibition of PKA at best enhancing the levels of sAPPα secreted (602, 877), investigation into possible functions for EPAC in the APP processing cascade was pursued. In support of a role for EPACs, Alzheimer’s patients demonstrate altered EPAC1 and EPAC2 expression in the frontal cortex and hippocampus, but not in resistant regions such as the cerebellum (680). Additionally, one of three single-nucleotide polymorphisms in EPAC2 gene (Rapgef4) ia found near the catalytic domain and associated with risk of Alzheimer’s disease in a population of Chinese patients (1207).
Selective EPAC activation supports a positive role for enhancing the cleavage of APP in primary and neuronal cell lines (654, 876). Further exploration into the action of cAMP on sAPPα secretion though EPAC demonstrated a requirement for Rac GTPase activation by upstream Rap1 through a PKA- and Ras-independent signal (654). This important and interesting crosstalk between Rap1 and Rac in cAMP-dependent APP processing suggests the existence of an adaptor protein that could effectively activate Rac. Considering this molecular rapport between the two small GTPases, the association of GEFs that are considered Rac-specific, such as Vav1, Tiam-1, and STEF (438, 666, 695, 770), was investigated. Successive isolated domain pulldowns revealed that the TSS region of STEF associates with constitutively active Rap1, but none of the Vav1 or Tiam-1 constructs could bind. Additionally, elevation of cAMP or direct activation of EPAC in cells ectopically expressing STEF further increased Rac1 activation and subsequent sAPPα secretion without altering Rap1 activation; moreover, the mutant STEF lacking the TSS domain completely abolished these effects (1182). Collectively these studies depict a predominant role of EPAC in the processing of APP through association of Rap1 with STEF to initiate crosstalk with the Rac1 pathway and increase secretion of sAPPα.
With the action of serotonin 5-hydroxytryptamine (5-HT4) receptors in learning and memory (218, 630, 660, 848) and the regulatory action of these receptors in APP processing (602, 877), the downstream role of EPAC in agonist-stimulated protease activity was examined. Activation of 5-HT4 receptors enhanced sAPPα secretion as previously described, but this increase was effectively blocked by zinc metalloprotease inhibitors, suggesting the involvement of α-secretases in cAMP-induced cleavage. Pharmacological activation of EPAC as well as expression of constitutively active EPAC or Rac also mimicked these results, supporting the role of an EPAC/Rap1/Rac/ADAM nonamyloidogenic signaling cascade (876). Amyloid β peptide levels were preserved with receptor or EPAC activation, suggesting that this pathway is independent of the β-, γ-secretase pathways that regulate production of amyloid β peptide (876). Thus serotonin 5-HT4 receptor activation directly increases α-secretase activity presumably through an EPAC/Rap1 pathway.
The nonamyloidogenic pathway also constitutively releases sAPPα, and perturbation of this action may lead to early onset of disease. Investigating the role of 5-HT4 receptors in this pathway in cortical neurons revealed unstimulated release of sAPPα was cAMP-independent and rather was associated with increased expression of 5-HT4 receptors (192); additionally, stimulation of muscarinic M3 receptors or PAC1 receptors was ineffective in increasing sAPPα release supporting the distinct role for 5-HT4 receptors (544, 771). To distinguish the metalloproteinase responsible for the constitutive secretion, siRNA targeting of ADAM10 specifically diminished overall sAPPα release both in the absence and presence of increased 5-HT4 receptors (192). Additional support for ADAM10 as the major α-secretase in APP shedding was observed by RNAi-mediated knockdown in primary neurons (564), conditional knockout mice (482), and mutational prevalence of ADAM10 in late-onset Alzheimer’s disease patients (524). Curiously, ADAM10 demonstrated selective association with 5-HT4 receptors in coprecipitation assays that were unaffected by activation state of the receptor. Furthermore, this association appears to be unique to 5-HT4 receptors as other GPCRs failed to associate with ADAM10 (192). Interestingly, reduction of 5-HT4 receptor binding, and presumably associated expression of membrane receptor levels, in post mortem brains of AD patients could suggest that decreased trafficking of ADAM10 to the membrane is implicated in the progression of the disease (869). Thus the full nature for the activation of ADAM10 by association with 5-HT4 receptors still requires further attention to determine if this regulation assists in maturation of ADAM10 by translocating the protease to the membrane or if the receptor functions to activate the protease through an unknown mechanism. Together, these results depict two independent APP processing mechanisms. Agonist-mediated release requiring canonical signaling through cAMP/EPAC and an agonist-independent mechanism implicating a minimal protein complex between 5-HT4 receptors and ADAM10, but most likely including additional, yet to be identified, interactions with proteins that can modulate ADAM10 activity and initiate sAPPα shedding (192).
Initial attempts at targeting APP processing pursued γ-secretase inhibition to attenuate amyloid β peptides production, but these trials have been plagued with detrimental side effects predominately involving Notch pathway inhibition. Perturbation of the Notch pathway by these compounds further decrease cognitive performance and are proving more problematic than predicted (reviewed in Refs. 651, 797, 801). A new grasp at this strategy involves development of γ-secretase modulators by targeting proteins including GSAP (γ-secretase activating protein) which selectively prevents amyloid β peptides generation without affecting Notch signaling (407, 651). Moreover, with the production of amyloid β peptide unaffected by activation of EPAC and no observable effect of β- or γ-secretase inhibitors on the basal sAPPα shedding, the two APP processing pathways may be regulated independently of one another and potentially amendable for combinational therapy (192, 876). Thus, although increased α-secretase release of sAPPα does not always decrease amyloid β levels (876), therapeutics to augment this process are also being developed (651). Interestingly, retinoid-induced heightened expression of ADAM10 does attenuate amyloid β peptide production and as described above is important in the constitutive release of sAPPα additionally supporting the continued advancement of these compounds (466, 1029).
As mentioned, dementia is also a common symptom of Alzheimer’s disease, linked to memory formation which has been associated with EPAC as discussed above (see sect. IVA3a). Thus altered EPAC levels observed in Alzheimer’s patients may lead to the induction of dementia through disrupting memory formation (680, 1207). Progressive memory decline in Alzheimer’s and dementia have also been linked with reduced melatonin levels and in some cases hormonal supplementation appears to suppress dementia (116, 1047, 1206, 1209). In support of the effect of melatonin in Alzheimer’s related dementia, a case study depicting a set of monozygotic twins were differentially treated with melatonin. The patient receiving the supplementation exhibited diminished impairment of memory, suggesting attenuated cognitive deterioration (116). To explore the potential role of EPAC in melatonin-induced memory retention, Wang et al. (1096) employed a scopolamine-induced memory impairment animal model. Scopolamine nonselectively inhibits muscarinic receptors blocking cholinergic signaling to induce short-term amnesia and can be used as a tool to model dementia invoked by dysfunction of the cholinergic system. In these animals, EPAC1, EPAC2, and Egr1 expression were impaired by scopolamine treatment, and the expression of miR-124 is inverted, as similarly described for EPAC-null animals (see also sect. IVA3a) (1096, 1155). This result suggests that EPAC may be involved in memory loss prompted by cholinergic dysfunction. Moreover, increasing melatonin levels in the presence of scopolamine could reverse these effects, recovering EPAC expression and subsequently decreasing miR-124 expression. Reduction of the inhibitory miR-124 also lifts the targeted inhibition of Egr1 expression (1096). On the other hand, chronic reduction of cerebral blood flow can also lead to ischemic/hypoxic events and consequent neuronal damage leading to cognitive defects, dementia, and an increased risk of developing Alzheimer’s disease. Again miR-124, among other miRNAs, is not only implicated in hypoxic stress response, but also potentially targets β-site amyloid precursor protein cleaving enzyme 1 (BACE1, β-secretase 1) reasonably positioning miR-124 to regulate the progression of memory dysfunction during chronic cerebral hypoperfusion (286). In a rat model of hypoperfusion, miR-124 levels were attenuated, and in accordance BACE1 and amyloid β peptide production were augmented in the hippocampus. In this animal model, levels of EPAC1 and EPAC2 were markedly increased and subsequently active Rap1 was elevated by ~30%. Similar results were obtained with intrahippocampal injections of amyloid β peptide. Consistent with previous reports, activation of EPAC effectively attenuated miR-124 levels, but the magnitude at which EPAC activation or the increased EPAC expression affects BACE1 was not determined (1193).
Although tempting to conclude that the additive reduction of miR-124 by decreased cerebral blood flow along with amyloid β peptide elevation may signify some sort of tipping point in disease progression, further analysis is required to fully understand this process. This is based on the conclusion that BACE1 expression is not solely regulated by miR-124 since overexpression of the microRNA was unable to completely block BACE1 induction in response to hypoxia or amyloid β peptide insults (1193). Although the precise mechanism for miR-124 remains elusive, chronic cerebral hypoperfusion and amyloid β peptide both promote suppression of miR-124 and may, in part, augment BACE1 expression through a positive feedback mechanism; however, still to be determined is the more complex network regulating the β-secretase.
Furthermore, the role of EPAC expression and function in Alzheimer’s disease models requires greater attention. Discrepancies between the elevation of EPAC1 and EPAC2 in brains of Alzheimer’s patients described by McPhee et al. (680) should be corroborated with miR-124 expression in specific brain regions and in larger patient cohorts followed by associative comparison with current animal models to study Alzheimer’s disease-induced dementia. These results would assist in distinguishing potential deviating roles for EPAC1 and EPAC2 while also exploring potential redundancy in the action of the two isoforms in these pathological processes. Additionally, investigation into the molecular etiology of altered EPAC levels in this signaling system would be valuable in determining when dysregulation signaling begins and whether the perturbed cascade can be countered before clinical symptoms manifest.
In line with these notions, a promising recent study by Zhou et al. (1207) observed Alzheimer’s patients that exhibited single nucleotide polymorphisms in the EPAC2 gene Rapgef4 near the catalytic domain sequences and was correlated with both increased risk for cognitive decline and with apathy and mood disturbances in these patients. These effects were not attributed to mutations in the EPAC1 gene, suggesting a predominant role of EPAC2 in mood control and cognitive function. Similar to Alzheimer’s patients, EPAC2-null mice have a wide range of mood disorders including anxiety and depression. Animals not expressing EPAC2 also demonstrated attenuated learning and memory not replicated in EPAC1-null animals. These behaviors were paired with decreased hippocampal neurogenesis and were effectively reversed by treatment with fluoxetine, a selective serotonin reuptake inhibitor (1207). This study illustrates the importance of EPAC2 in Alzheimer’s patients, but again a larger patient pool is warranted to appreciate the full extent and consequences of these polymorphisms in the Alzheimer’s population. Furthermore, are these mutations predictive of early disease and can we develop a platform to screen susceptible patients as a means of earlier detection?
2. Autism and neurodevelopmental disorders
Autism is a neurodevelopmental disorder occurring early in childhood and commonly associated with impaired social and communication development as well as other characteristic behaviors that vary dependent on the autistic phenotype. Genetic mutations have been associated with autistic spectrum disorders including FMR1 and TSC1/2 genes presenting as fragile X syndrome and tuberous sclerosis, respectively. However, the vast majority of individuals do not exhibit these mutations, suggesting that additional genes may be involved in the development of these disorders. Hence, several genome analyses have identified chromosome 2q21-q33 as a region harboring potential autism susceptibility genes (40, 126, 457, 947). Initial exploration into specific genes in this locus responsible for the development of autism identified EPAC2 as a contributor in two independent studies (40, 1044). One study further detected the expression of four nonsynonymous EPAC2 missense mutations in five different families that appeared to be segregated with the autistic phenotype of the individuals. Surprisingly, an additional group of German families exhibiting autism did not exhibit the presence of these EPAC2 variants. This suggests that although EPAC2 may not be the major contributor to autism, loss of function of this protein may increase susceptibility to developing autism in certain populations (40). Moreover, these mutations may be critical in the development of specific phenotypes of autism; thus it is important to define how these mutations affect protein function and neuronal activity.
During early development, connectivity of the neuronal circuitry through initial axon and dendrite formation is paramount; however, maintenance of dendrites is essential throughout early childhood for synaptic remodeling and neuronal plasticity leading to learning, memory formation, and development of complex behaviors. In autistic individuals, dendrite levels are suppressed in both the cortex and hippocampal regions, suggesting a potential dysfunction in dendrite maintenance (740, 857). As described previously, EPAC2 has a functional role in synapse destabilization of the dendritic spine to promote synaptic remodeling, and disruption of this pathway leads to improper basal dendritic arbor upkeep in vivo and in vitro (see sect. IVA2) (975, 1127). Further implicating the cAMP effector, EPAC2 is shown to interact with NL3, another proposed contributor to autism (462, 1127). Thus the identification of genetic mutations of EPAC2 in autistic families sparked an association between this cAMP receptor and the development of autism. Excitingly, probing the molecular consequence for overexpressing the G706R missense mutation, located in the RA domain of EPAC2, demonstrated attenuation of dendritic length and complexity near the neuronal cell body without affecting apical regulation in cortical pyramidal neurons (975). Additionally, expression of this EPAC2 coding variant suppressed the interaction of EPAC2 with Ras in this pathway, while preserving basal Rap1 activity (975, 1127). These results position EPAC2 G706R as key variant leading to loss of function and consequent suppression of basal dendrite maintenance which could manifest autistic characteristics.
Individuals with autism can also exhibit spontaneous epileptic seizures. Excessive excitatory stimuli associated with augmented glutamate release is often associated with seizure vulnerability, and intriguingly, EPAC is observed to enhance glutamate transmitter release in the presynaptic neurons by enhancing vesicle localization to the synaptic active zone through regulated localization of RIM1α/Rab3 and Munc13–1 (see sect. IVA1a) (300, 301). Furthermore, loss of EPAC1/2 in mice is observed to attenuate glutamate release in presynaptic terminals (1155). In dentate granule cell presynaptic terminals, EPAC2 is observed to directly bind SUR1, which also associates with Kir6.1 to form the neuronal-type KATP, to enhance glutamate vesicle release (1199), similar to binding of EPAC2/SUR1 in the pancreas to increase insulin secretion (1189). Specific binding of EPAC2 (residues 351–458), a region of the CNB-B, to an intracellular loop of SUR1 at residues 859–881 (FIGURE 4B), inhibits the KATP function by suppressing the open probability and efflux of K+, promoting depolarization and neurotransmitter release (FIGURE 8); however, deletion of EPAC1/2 is observed to dysregulate KATP opening and suppress the voltage-dependent Ca2+ channel activation inducing a hyperpolarized state with attenuated glutamate release and reduced seizure susceptibility (1199). In support of this mechanism, Kir6.1-null or SUR1-null animals are susceptible to epileptic seizures (969), but deletion of EPAC1/2 expression is protective against seizure development (1199). Thus this protective phenotype is curious being that autistic individuals can be susceptible to seizure development, while loss of EPAC appears to be protective. Several questions regarding these conclusions include: can mutated EPAC variants partake in functions involving postsynaptic receptor regulation or alternatively lower the resistance of postsynaptic neuron depolarization to further enhance neuronal firing during an epileptic event? Do these mutant EPAC2 variants retain, or even enhance, inhibition of KATP while the same mutation disrupts dendritic maintenance by an independent signaling cascade? Are individuals harboring the missense mutations in EPAC2 resistant to epileptic seizures as compared with autistic patients lacking these mutations?
Thus, although the action of EPAC2 in autism is still in its infancy, the exploration and clarification of the genetic and molecular cues regulating dendritic maintenance and channel regulation by EPAC2 may be crucial in developing directed therapies for neurological disorders stemming from disruptions of this process. The extent of EPAC regulation has also begun to extend to other disorders involving neuronal development, similar to autism. For example, the occurrence of a mutation in a purine generating enzyme, hypoxanthine-guanine phosphoribosyl transferase (HPRT), leads to a neurological disorder known as Lesch-Nyhan syndrome (LNS). Improper neurodevelopment in these patients is implicated in behavioral, cognitive, and motor defects. Interestingly, loss of the HPRT enzyme modifies microRNA (miRNA) expression and transcriptional regulation in dopaminergic neuron pathways that are important in normal neurodevelopment (155, 376, 377). More recently, the absence of HPRT in dopaminergic neurons, LNS fibroblasts, and distinct murine brain regions was mapped to the dysregulated expression of the miRNA-17 family cluster. Furthermore, the action of these modulatory miRNAs was associated with dysregulation of EPAC1/2 expression, demonstrating attenuation in human neuronlike cells and the striatum while enhancement was observed in the midbrain (EPAC1/2) and cortex (EPAC1), suggesting potential region-specific regulation by the miRNA profiles. Moreover, LNS patient-derived fibroblasts have reduced EPAC1/2 expression and Rap1 activity, which is restored with supplementation of HPRT enzyme (378). Although the function of EPAC2 is implicated in memory and behavior potentially associated with dendrite stability and subsequent synaptic plasticity (462, 974, 975, 1127), EPAC/Rap1 signaling is also involved in the migratory and adhesive properties of multiple cell types through modulation of actin cytoskeletal dynamics. Indeed, HPRT-deficient neurons appear to exhibit disorganized F-actin architecture along the cell periphery in accordance with reduced EPAC/Rap1 signaling, and thus enhanced neuronal migration is observed (378). The increased motility of neurons during early neurodevelopment could ostensibly affect the initial neuronal circuitry connections. Paired with disrupted synaptic plasticity, these functions of EPAC may begin to shed light on the molecular components involved in the onset of LNS. Major questions still remain in defining this signaling pathway including: Why and how does loss of HPRT affect the miRNA-17 family cluster levels differentially in distinct brain regions? Which miRNA(s) are directly involved in the regulation of EPAC1 and EPAC2 expression? What are the roles for the other dysregulated miRNAs, and what proteins are these miRNAs affecting to attribute to the LNS phenotype? Does loss of EPAC also affect neurotransmitter release or dendrite plasticity in these HPRT-null neurons (FIGURE 8)? Can the EPAC pathway be targeted and amplified in early development to reverse the effects of HPRT deficiency?
3. Behavioral disorders
a) anxiety and depression.
Depression-like behavior in rats was attenuated with inhibition of PKA, which unexpectedly increases hippocampal levels of cAMP and CREB phosphorylation. These observations suggest that alternative pathways are activated by cAMP to exert antidepressant activity (612). The use of the selective serotonin reuptake inhibitor (SSRI) fluoxetine, Prozac, has proven to be a great tool in the battle of depression and has shown similar increases in cAMP levels subsequently enhancing PKA/CREB activity for altered hippocampal neurogenesis (310, 611, 657). Not surprisingly, anxiety and depressive-like behaviors associated with impaired hippocampal neurogenesis were discovered in genetically depleted EPAC2, but not apparent in EPAC1, knockout models. However, fluoxetine could effectively reverse the effects from EPAC2 deletion by increasing neurogenesis and bestowing an antidepressant activity in these mice, suggesting fluoxetine functions by stimulating parallel pathways independent of EPAC2. Indeed, supporting divergent pathways for fluoxetine action, PKA-mediated events including phosphorylation of CREB and brain-derived neurotrophic factor (BDNF) expression are unchanged in response to EPAC2 deletion, but appear increased with fluoxetine treatment (1207).
Moreover, investigating the levels of EPACs and Rap1 activation in the prefrontal cortex and hippocampus regions of depressed suicide victims illustrated reduction in Rap1 expression leading to attenuated activation and an increase in EPAC2 levels in these victims. This could suggest the brain’s attempt to compensate for the decreased activation of Rap1 by enhancing expression of EPAC2, thus demonstrating that potential disconnections between EPAC2 and Rap1 signaling may project as depression in humans (259). Further implication of EPACs in human subjects was found in another epidemiological study that determined single nucleotide polymorphisms in EPAC1 of a Dutch population presented with altered levels of depression and anxiety (696). This function of EPAC in anxiety and depression is contradicted in a couple of reports where EPAC2 knockout and intrahippocampal EPAC activation did not affect anxiety of the animal (793, 974). One potential explanation for these differences could involve EPACs acting as stress regulators for the system, and thereby symptoms including depression and anxiety only manifest in response to environmental stimuli or differences in experimental procedures performed to measure these neurological characteristics. The application of a CNS-specific conditional knockout mouse model for EPAC1, EPAC2, and the combination of both proteins may assist in defining precise roles for EPACs in the development of these disorders.
b) drug-seeking and dependence.
Given the role of EPAC in synaptic transmission and learning as described above, these proteins are well-positioned to modulate drug-induced reinforcement training and plasticity. A few studies have begun to determine the cellular and behavioral functions for EPACs in seeking substances of abuse.
Cocaine is known to increase expression of GluR2-lacking AMPARs on the synapse of dopamine neurons in the ventral tegmental area (VTA) (71, 356), whereas EPAC activation reduces the surface GluR2/3 AMPARs (1127). Considering this regulation of AMPAR subunit composition in dopamine neurons of the VTA after a single exposure of cocaine, Liu et al. (627) demonstrated the effect of cocaine on redistribution of GluR2-lacking AMPARs was dependent on the presence of EPAC2, and furthermore, EPAC2 activation could mimic the effects of a single cocaine dose in altering AMPAR subunits. Cue-induced administration of cocaine in rats was decreased after EPAC activation in the basolateral amygdala, suggesting an inhibitory role for EPAC in this behavior (1083). An interesting followup to these studies could explore how complete loss of EPAC2 affects drug-associated memory reconsolidation. Also, the notion that activation of EPAC in differing regions of the brain may participate in functionally diverse roles during drug-seeking could be of great interest in delineating potential addiction mechanisms to therapeutically target.
In relation to nicotine addiction, a microarray analysis determined that EPAC expression was upregulated in the prefrontal cortex of rats exposed to chronic nicotine self-administration (547). Further investigation into the effect of single nucleotide polymorphisms of the EPAC gene in human subjects found a correlation between the presence of three SNPs and nicotine dependence, while one was associated with smoking initiation (171). Although there is still much to explore to fully elucidate EPAC mechanisms in drug-seeking and dependency, the function of EPACs in learning and synaptic plasticity represent a potential connection between addictive behaviors and these cAMP effectors.
In summary, reports describing the role of EPAC in neurological disorders have already demonstrated great forward progression in defining pathways that EPACs are associated with, especially EPAC2. As mentioned, altered EPAC expression or mutations are identified in populations of patients suffering from Alzheimer’s disease, autism, LNS, anxiety and depression, and drug dependence. Currently, expansive functions of EPAC are characterized to regulate multiple pathways related to these disorders including increased α-secretase activity and sAPPα cleavage, disruption of dendrite synaptic plasticity, excessive migration of neurons during development of neural circuitry, and reward pathways in response to substance abuse. Furthermore, animal models deficient in EPAC2 or both EPAC1/2 exhibit many behavioral, social, and mood disorders commonly sustained by these patients, suggesting the intricate involvement of EPAC proteins in these neural disease pathways that require further investigation.
B. Role of EPAC in Cancers
While the involvement of cAMP/PKA signaling in cancer development and prognosis has long been established, the associations of EPAC proteins, particularly EPAC1, with cancer are emerging and have been summarized in a recent review (15). In general, EPAC and PKA mediated signaling pathways function antagonistically, independently or synergistically in rare occasions to modulate cancer cell proliferation, apoptosis, adhesion, and migration (193, 361, 473, 474, 722, 1030, 1073). Activation of cAMP/EPAC1 signaling pathways has also been reported to sensitize refractory cancer cells to oncolytic virotherapy (605, 606).
The effects of EPAC1 on cancer cell proliferation and survival are shown to be cell-type and context dependent. While EPAC1 inhibits cell proliferation in clear renal cell carcinoma (cRCC) A498 cells (1054), EPAC1 activity stimulates cell proliferation and survival by upregulating Ras/MAPK and PI3K/Akt/mTOR signaling in prostate cancer cells (295, 303, 712). Likewise, EPAC1 expression is shown to be increased in human ovarian cancer cells where silencing EPAC1 by small interfering RNA inhibits proliferation and induces cell cycle arrest in vitro, as well as suppresses tumor growth in vivo in xenograft nude mouse models. At the level of cell signaling, downregulation of EPAC1 in ovarian cancer cells significantly decreases resulting pAkt, cyclin D1, and CDK4 (334). EPAC1 may also provide growth and survival advantages to cancer cells through metabolic reprograming as a recent study implicates the EPAC1/Rap1 signaling pathway in promoting oncogenesis via upregulating aerobic glycolysis (792).
A large number of studies have established a critical role for EPAC1 in the invasion and metastasis of several cancers including melanoma (45–50, 333), prostate cancer (42, 362, 688, 711, 713), ovarian cancer (62, 855), pancreatic cancer (13, 17, 123, 636, 1095), cervical cancer (589), fibrosarcoma (399), and lung cancer (463, 614). While the majority of reports suggest that EPAC1 activation promotes cancer cell migration and metastasis, few studies, using an EPAC-selective agonist 007, suggest that activation of EPAC1 inhibits cancer cell migration (62, 362). However, some argue the inhibitory effect observed for EPAC1, in the aforementioned contradictory studies, was actually due to indirect activation of PKA because the 007-mediated inhibitory effects could be rescued by PKA inhibitors, H89 and PKI, but remained unaffected by silencing EPAC1 and EPAC2 expression with siRNA (688). In melanoma, levels of EPAC1 expression are upregulated in metastatic melanoma compared with primary melanoma, and EPAC1 expression is positively correlated with the expression profile of N-deacetylase/N-sulfotransferase-1 (NDST-1) and heparan sulfate (HS), a major component of extracellular matrix (49). Additionally, EPAC-induced cell migration is associated with the production of HS in response to increased NDST-1. Furthermore, translocation of syndecan-2, a cell-surface HS proteoglycan, to lipid rafts is also regulated by EPAC1/PI3K-dependent tubulin polymerization (46). A role of PLC/IP3 receptor-dependent intracellular Ca2+ signaling and actin assembly in EPAC1-induced melanoma cancer cell migration has also been demonstrated (45). Similarly, EPAC1 is overexpressed in pancreatic cancer (636). Genetic and pharmacological studies demonstrate that EPAC1 promotes pancreatic cancer cell migration and invasion through stimulating activation and trafficking of integrin β1 (13). Recent studies also suggest that EPAC signaling can stimulate the migration of cancer cells by inducing histone deacetylase 6 (HDAC6) expression (614), or by enhancing β-catenin nuclear translocation and β-catenin-dependent transcription (463) in lung cancer cells. While a diverse array of signaling pathways are involved in EPAC1-mediated cancer cell migration and invasion, most may ultimately converge and attribute this EPAC1 function to an integrin-dependent mechanism in various cancers (13, 42, 45, 333, 855).
Currently, the majority of evidence implicating EPAC’s involvement in cancer is based on studies performed using cancer cell lines or xenograft tumor growth in animal models. Most recently, a retrospective cohort study of 141 patients with gastric cancer shows that EPAC1 expression is upregulated in gastric cancer cells and tissues. Importantly, overexpression of EPAC1 is associated with several clinicopathological parameters such as the depth of invasion, cancer stage, and vascular invasion. A Kaplan-Meier analysis further demonstrates that upregulation of EPAC1 is significantly associated with poorer overall survival, as well as disease-free survival, suggesting that EPAC1 can be used as a prognostic marker for gastric cancer (991). Similarly, in 51 cases of invasive ductal esophagus cancer tissues, positive EPAC1 expression was detected in 63% of the samples (32/51), significantly higher than the 20% positive expression rate (2/10) detected in the para-carcinoma tissues (375). A recent analysis of the Cancer Genome Atlas (TCGA) data set reveals that EPAC1, along with PKA, AKAP9, and other cAMP signaling components, is amplified in breast cancer patients, and the amplification of this gene set is associated with poor survival. In addition, pharmacological inhibition of EPAC1 by ESI-09 suppresses cell proliferation and migration, while inducing cell cycle arrest and apoptosis in breast cancer cells (567). Increased expression of EPAC1 in 58% of breast cancer patients (29/50) was reported, as compared with a 10% positive rate (1/10) in controls (446). These studies demonstrate fresh and exciting potentials for novel therapeutic strategies targeting EPAC1 in breast and gastric/esophagus cancer treatments.
Thus far, the association of the EPAC signaling with cancer is almost exclusively attributed to EPAC1. However, one recent study reported that cAMP upregulated the expression of HDAC8 and augmented cisplatin-induced apoptosis in H1299 lung cancer cells in a PKA-independent and EPAC-dependent manner. Interestingly, the effect of cAMP was mediated by EPAC2, not EPAC1, since silencing EPAC1 using an EPAC1-specific shRNA did not block the cAMP-induced HDAC8 expression, but EPAC2 shRNA or an EPAC2-specific inhibitor, ESI-05, abolished cAMP-induced enhancement of HDAC8 expression. Mechanistically, the authors propose that activation of EPAC2 inhibits the PI3K/Akt/MKK4/JNK1 pathway, which in turn suppresses HDAC8 protein degradation to augment cisplatin-induced apoptosis by repressing the TOR signaling pathway regulator-like (TIPRL) protein expression in lung cancer cells (804). While these data are interesting, the main conclusions of the paper are based on results collected from one cancer cell line, the human non-small cell lung cancer cell H1299, and representative changes in HDAC8 expression depicted in the paper were modest. Clearly, additional research is needed to ascertain if dysregulation of EPAC2, similar to that of EPAC1, is implicated in playing a role in cancer.
C. Cardiovascular Disease
1. Cardiomyopathies
Cardiovascular disease is the leading cause of morbidity and mortality in the world, commonly occurring in response to perturbations of signaling in the heart and vessels of the body. The role of EPAC in cardiac pathologies is often associated with the increased expression of EPAC in isoproterenol- and pressure overload-induced cardiac hypertrophy, an indicative marker for cardiac diseases such as arrhythmias, cardiomyopathies, and heart failure (692, 1050). This function of EPAC in promoting hypertrophic signaling is well supported in the literature (75, 586, 692, 729, 916, 1050, 1191, 1192). However, genetic knockout studies revealed isoproterenol-induced hypertrophy was attenuated in EPAC1-null animals (586), but in other studies loss of EPAC1 did not alter heart size after isoproterenol or pressure overload (785, 819). Interestingly, even with these controversial results over the hypertrophic signaling of EPAC, EPAC-null hearts appear to be protected against many stress-induced myopathies and arrhythmias (785, 819). Extensive effort has been invested into the determination of the EPAC-mediated signaling mechanisms in cardiomyopathies, arrhythmias, and heart failure which has been meticulously reviewed recently by two independent groups (320, 601). Rather than redundantly reviewing these functions of EPAC, we refer the reader to these excellent reviews, and will instead focus on more recently identified roles for EPACs in vascular disease progression.
2. Vascular disease
During vascular injury, contractile differentiated VSMCs undergo a phenotypic switch to a synthetic phenotype, migrate from the tunica media to the site of injury, and begin the process of vessel remodeling to heal the wounded vessel (763, 867). Excessive retention of VSMCs in the synthetic phenotype outside of the healing process can lead to promotion of neointimal hyperplasia in the vessel and consequential development of restenosis and atherosclerotic pathologies. Instigation of the majority of VSMC migration during acute vascular injury is attributed to PDGF-BB stimulation (296, 467), and this signaling pathway is observed to induce cAMP production (96, 365). Thus the role for the cAMP effector EPAC1 in VSMC migration and neointimal development was investigated.
Reports by Yokoyama and co-workers (1168, 1169) investigating the role of EPAC1 in VSMC migration and intimal formation initially described a potential PKA-independent function for EPAC in neointimal thickening. Following vascular injury, mRNA expression of EPAC1 was observed to increase in rat VSMCs, while EPAC2 levels remained unaltered and PKA regulatory and catalytic subunit expression were attenuated. This led to the notion that during injury and vessel remodeling, EPAC1 could be preferentially activated over other cAMP effectors and may be responsible for enhanced migration leading to neointima formation. Indeed, migration of VSMCs to PDGF stimuli was markedly improved with activation or overexpression of EPAC1 and inversely diminished with PKA activation, suggesting EPAC and PKA act in opposing fashions during the migration of VSMCs (1168). The intimal cushion formation during the closure of the ductus arteriosus is a physiological model system of neointima formation where PGE2 induction of cAMP is observed to be a central mediator (1169, 1170). Intriguingly, during this process, and similar to injured rat VSMCs, EPAC1 and EPAC2 mRNA expression are reported to be enhanced. Also supporting the role in EPAC in promoting migratory properties, SMCs from the ductus arteriosus exhibit enhanced migration following EPAC stimulation or overexpression, while silencing EPAC1 expression reduced migration of these rat SMCs (1169). In both studies, EPAC activation altered the morphology of the SMCs promoting a migratory-like phenotype displaying broadened cells with dendrite-like protrusions, enhanced focal adhesions, and organized actin-stress fibers. Stimulation of the SMCs with a PKA-specific agonist, however, yielded stellate-shaped cells (1168, 1169). Aside from SMC migration, cAMP signaling was also previously implicated in the closure of the ductus arteriosus through initiation of hyaluronan (HA) production by a PGE2/cAMP/PKA signaling cascade (1170). However, direct activation of EPAC does not appear to alter HA production in these cells, further supporting the opposing nature of PKA and EPAC in this signaling pathway (1169). Moreover, neointimal formation in the injured femoral artery or the ductus arteriosus was augmented by transduced EPAC1 overexpression, reinforcing the role of this cAMP effector both in physiological and pathological neointimal thickening (1168, 1169).
Two recent investigations focused on the role of EPAC1 in vascular disease to further build a strong case for the cAMP effector in the development of neointimal hyperplasia and potentially define a valuable therapeutic target (502, 1088). The results of these studies demonstrate that the migratory and invasive response of VSMCs to PDGF is greatly reduced in the absence of EPAC1, although cAMP and PKA activity is unaltered (502, 1088). The suppressed migration of EPAC1-null VSMCs was investigated by Kato and colleagues, demonstrating perturbation of cytosolic Ca2+ release and subsequent dephosphorylation of cofilin in response to PDGF (225, 502, 1035). In the presence of elevated phosphorylated cofilin, lamellipodia formation and consequent migration is attenuated. Further experimentation determined that silencing Rap1A affected lamellipodia formation in wild-type VSMCs and not EPAC1-null cells, suggesting this process is dictated by EPAC1/Rap1A to modulate VSMC migration (FIGURE 11) (502).
FIGURE 11.
Summarized pathways implicating EPAC1 in vascular diseases. Illustrated representation of the pathological progression of neointimal hyperplasia in arteries (top panel) and EPAC1-mediated pathways during phenotypic switch of contractile VSMCs to synthetic VSMCs to promote hyperproliferation and migration/invasion (bottom panel). See section VC for additional details and abbreviations.
The effects of EPAC1 on phenotypic switching and proliferation were also assessed in EPAC1-knockout animals. Both in vivo and ex vivo experiments demonstrate that deletion of EPAC1 reduces mitogenic signaling in VSMCs and neointima areas. Investigating PDGF-induced proliferative pathways revealed that although the MAPK pathway was unaltered by deletion of EPAC1, activation of the PI3K/Akt pathway was attenuated in the absence of EPAC1 (1088). Indeed, drug-eluting stents coated with rapamycin to inhibit mTOR downstream of Akt are effective strategies to reduce restenosis rates in the clinic (735, 739). However, mitochondrial dynamics are also known to participate in the induction of VSMC phenotype switching (615, 663, 1090). As it happens, EPAC1 contains a mitochondrial targeting sequence and colocalizes with mitochondria both in cell fractionation and live cell imaging experiments (845, 1088). Interestingly, EPAC1 was determined to regulate the dynamic phosphorylation of dynamin-1-like protein (DRP1) which is crucial for mitochondrial fission/fusion balance. The pro-fission phosphorylation site Ser616 was induced by EPAC activation and suppressed by EPAC inhibition, while a fission-suppressant site, Ser637, was inversely modulated by EPAC activity. Mitochondrial morphology and ROS production in VSMCs stimulated with PDGF followed in suit, exhibiting reduced levels of mitochondrial circularity and ROS production in EPAC1 knockout cells, suggesting a reduction in mitochondrial fission (FIGURE 11) (1088).
Two separate models of neointimal hyperplasia have been explored in EPAC1-null animals with similar results (502, 1088). First, a wire injury model was employed to induce mechanical injury to mice femoral arteries. Excitingly, this model demonstrated the neointimal thickening was drastically attenuated in EPAC1-null animals. Furthermore, cofilin phosphorylation was enhanced in the neointimal areas of EPAC1 knockout arteries, supporting the reduction in VSMC phenotype switching with subsequent migration and invasion in response to injury. Importantly, bone marrow-derived cells were not observed to participate in the generation of the neointimal area, but perivascular fibroblast migration was observed to be attenuated in EPAC1 knockout animals. Therefore, perivascular fibroblasts could potentially support the formation of the neointima hyperplasia (502). In favor of these results, the second model of neointimal hyperplasia was conducted using carotid artery ligation. Staining of the neointimal area supports the invasion of VSMCs and illustrates reduced proliferation of cells further complementing the reduction in synthetic VSMCs growth. Most importantly, pharmacological treatment of wild-type mice with the EPAC inhibitor ESI-09 directly phenocopied the genetic deletion of EPAC1, suggesting that targeting EPAC1 is an effective strategy in the attenuation of neointima hyperplasia (1088). Taken together, these investigations illustrate that EPAC1 is involved in PDGF-induced phenotypic switching of VSMCs in response to injury and production of neointima hyperplasia during persistent injury to the artery. Furthermore, targeting this signaling pathway may have therapeutic potential for patients suffering from restenosis or other vascular proliferative diseases.
Contrasting these studies, a study investigating the action of roflumilast, a PDE4 inhibitor, on VSMC migration was observed to reduce TNF-α-induced expression of cell adhesion molecule VCAM-1 through an EPAC1 mechanism. This EPAC1 function was associated with epigenetic regulation of histone H3-lysine 4 demethylation (H3K4me2) at the VCAM-1 promoter. Indeed, monocyte recruitment was suppressed with treatment of roflumilast or EPAC activator, and neointima formation was reduced (597). These results illustrate the complexity of the cAMP signaling system where multiple cell types can be affected to produce a net effect in seemingly opposite directions. Another investigation also concluded that activation of EPAC and PKA by betaprost, a prostacyclin analog, attenuates migration of human saphenous vein VSMCs and serum-induced thickening of saphenous vein walls. This is suggested to occur via EPAC/Rap1 inhibition of RhoA to disrupt the actin cytoskeletal reorganization required for migration at the leading edge (678). Although this model of neointima formation is not reliant on the common pathological trigger of vascular injury, but rather serum induction, these observations may reveal an alternate pathway for migration dependent on the phenotype of the SMC. Indeed, EPAC and PKA expression levels are reported to change after injury to VSMCs (1168), and unaltered expression of the cAMP effectors may, in part, be responsible for the inverted results obtained in this study. Also, these observations may suggest differential signaling mechanisms between vasculature of separate species or VSMCs harvested from vein versus artery. Taken together, these conclusions in some ways contrast the recent studies conducted in EPAC1-null animals and wild-type mice treated with an EPAC specific inhibitors; however, rather than dismissing these seemingly opposite findings, we should evaluate the results further for alternative signaling systems that may be relevant in certain disease processes. Importantly, EPAC as well as PKA may have critical functions specific to venous versus arterial signaling that have yet to be described, and roles for EPAC in epigenetic modulation and governing VSMC phenotypic switching have only begun to be realized.
Conclusively, EPAC is known to be involved in cardiac hypertrophy, arrhythmias, and heart failure; however, the role of EPAC in vascular pathologies has been less resolved until recent years. Indeed, vascular injury is reported to enhance EPAC1 expression in VSMCs where phenotypic switching to a synthetic phenotype augments migration/invasion, proliferation, mitochondrial fission, and ROS production in response to PDGF promoting the formation of neointimal hyperplasia. Furthermore, these results are supported by three independent models of neointima formation including the intimal cushion formation during the closure of the ductus arteriosus, mechanical injury to the femoral artery, and ligation of the carotid artery, all bolstering similar phenotypes where EPAC expression promotes formation of the hyperplasia. Some controversy remains, but these conflicting results may represent distinct pathways in veins and arteries as well as different pathological insults that warrant further investigation.
D. Chronic Pain
Pain is an agonizing, but necessary, avoidance response to noxious stimuli or internal damage. Acute (normal) pain represents an important protective mechanism by preventing an organism from further harm and initiating the healing process. However, under pathological conditions, disruption of the normal pain circuits leads to heightened excitability of the sensory neurons resulting in painful reaction to innocuous stimuli (allodynia) or amplified pain sensitivity (hyperalgesia) (60). If such conditions are not resolved, chronic pain can ensue, defined as pain persisting over 12 wk. Dissecting the signaling networks, as well as the signal rewiring underlying the transition from acute to chronic pain sensation, is key to understanding the mechanisms of pain hypersensitivity and identifying novel therapeutic targets for the treatment of chronic pain.
1. Inflammatory pain
Proinflammatory signals released during tissue damage are major triggers of pain hypersensitivity. These factors bind directly to the surface receptors on nociceptive neurons to enhance their excitability. PGs are important inflammatory mediators implicated in the sensitization of nociceptors, and consequentially common pain medications, such as aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), block the synthesis of PGs by inhibiting cyclooxygenases (196, 299, 506). PGE2 directly activates specific GPCRs linked through Gαs and AC to increase intracellular cAMP (106, 298, 508, 1003). The acute effects of PGE2 are mediated by the classic cAMP receptor PKA, which can be blocked by PKA inhibitors (10, 633). On the other hand, hyperalgesia induced by repeated exposure to PGE2 is not blocked by PKA inhibition, but rather attenuated by inhibition of the PKC pathway (11, 802). The mechanism of this signaling switch from PKA to PKC during inflammation-induced hyperalgesia is not well understood.
The connection between EPAC signaling and pain sensing was first made in 2005 by Levine and colleagues (451). This investigative group revealed that in cultured DRG neurons, β2-AR/cAMP signaling acts through EPAC, but not PKA, to induce membrane translocation of the PKC-ε, in a PLC- and phospholipases D (PLD)-dependent manner (FIGURE 12). Consistent with the role of PKC-ε as a key signaling molecule important for nociceptor sensitization and the transition from acute to chronic pain, pharmacological activation of EPAC using a EPAC-selective agonist led to robust reduction of paw-withdrawal threshold in vivo. Furthermore, EPAC-mediated mechanical hyperalgesia could be blocked by individually distinct pharmacological inhibitors of PKC-ε or PLC or PLD (451).
FIGURE 12.
Schematic overview of the cAMP signaling cascades in sensory neurons. See text for additional details and abbreviations.
Notably, the aforementioned study was performed using male Sprague-Dawley rats, which is of interest since a previous study revealed that PKC-ε was found to mediate β-adrenergic-induced mechanical hyperalgesia only in male, but not female rats (242). In humans, sex differences in pain sensing and tolerance have been well-documented, although the underlying mechanism remains elusive (718, 719). To further understand this sexual dimorphism, Hucho et al. (450) demonstrate that β2-AR agonist isoproterenol induces PKC-ε membrane translocation only in cultured DRG neurons derived from male rats, and not female. Furthermore, estrogen above 10 nM abrogates β2-AR- and EPAC-mediated activation of PKC-ε in male DRG neurons. The antagonistic effect of estrogen is rapid, indicative of a transcriptional-independent mechanism. Consistent with in vitro observations, preinjection of estrogen merely 1 h before intradermal injection of the EPAC activator 007 on hindpaws of male rats suppresses EPAC-mediated mechanical hyperalgesia in a binary mode-like dose-response manner, namely, complete inhibition at 100 ng estrogen with no effects observed at doses below 10 ng. Paradoxically, estrogen alone promotes a brief membrane translocation of PKC-ε in male DRG neurons and induces PKC-ε-dependent hyperalgesia in male rats when injected intradermally (450). A subsequent study demonstrated that estrogen acts through G protein-coupled estrogen receptor 1 (GPER, also known as GPCR 30, GPR30), and not the classical estrogen receptors (ER), ERα and ERβ, to induce PKC-ε membrane translocation and subsequent hyperalgesia in DRG neurons and in vivo, respectively (563). While these results implicate estrogen as a powerful modulator in rewiring the sensory nervous system and EPAC/PKC-ε signaling as significant contributors to estrogen-mediated sex dimorphism in nociception, important questions remain. For example, are signaling components related to GPR30, EPAC, and PKC-ε differentially expressed in male and female sensory neurons; is EPAC1, EPAC2, or both isoforms involved and do other PKC isoforms also participate in this signaling pathway?
As an initial approach to explore the potential mechanism governing two sensitizing signals, such as estrogen and β2-AR agonist, acting antagonistically against the other in modulating PKC-ε and pain sensing, Hucho et al. (449) investigated the consequence of consecutive isoproterenol treatments of the β2-AR. Resultant observations determined that only the first stimulation led to transient PKC-ε membrane translocation in cultured nociceptive neurons, while secondary stimulation inhibits subsequent and ongoing PKC-ε translocation events. The apparent inhibition of PKC-ε translocation is not caused by receptor desensitization as stimulating the downstream component EPAC directly using 007 after an initial isoproterenol treatment also fails to produce a subsequent PKC-ε translocation event. Interestingly, reversing the order of treatment by activating EPAC first mirrors the first isoproterenol treatment and antagonizes a second PKC-ε translocation, suggesting the inhibition is initiated at or downstream of EPAC. These findings characterize a model in which a signaling fork is observed to act like a “railroad switch,” relaying a distinct signaling event onto one of two cascades with opposing readouts dependent on the initiating signaling component and history of the cell (449). At the center of this switch mechanism lies EPAC and its downstream effector PLC (916), which cleaves the phospholipid phosphatidylinositol-4,5-bisphosphate (PIP2) into DAG and inositol 1,4,5-trisphosphate (IP3), two important second messengers that activate PKC and induce calcium release via activating IP3R, respectively. Although IP3-induced calcium release is not expected to modulate PKC-ε activation, since it lacks a calcium-binding domain, surprisingly, pharmacological inhibition of IP3Rs reverses the secondary PKC-ε translocation blockage. Supporting this unexpected result, direct activation of IP3R or RyR blocks subsequent PKC-ε translocation. Furthermore, blocking calcium release or calcium calmodulin-dependent kinase (CaMKII) also reverses the inhibition of PKC-ε translocation in response to the application of a second stimulus. Thus these results suggest that Ca2+/CaMKII plays a key role in switching nociceptor-sensitizing stimuli into desensitizing stimuli. Consequently, in behavioral experiments, activation of calcium stores switches sensitizing stimuli into desensitizing stimuli in a CaMKII-dependent manner. Based on these observations, the authors conclude that initial activation of Gαs-coupled GPCRs results in hyperalgesia via EPAC/PLC/PKC-ε signaling; however, concurrent activation of the EPAC/PLC/CaMKII arm resets the signaling network from pro- to anti-algetic. Thereby, subsequent stimulation of the EPAC pathway is rerouted to anti-algetic signaling. Although the precise mechanism defining how activation of the Ca2+/CaMKII pathway switches the route of signaling from pro- to anti-algetic is not clear, one potential benefit of this intricate regulation is to provide an effective means to prevent nociceptive system overload in response to repeated noxious stimuli. Exploration into the Ca2+/CaMKII-mediated rerouting mechanism and disruption of such a system in various abnormal chronic pain conditions comprises an interesting area for upcoming investigations.
Potentiation of the purinergic P2X3 receptor (P2X3R) contributes to injury-induced exaggerated pain responses. Activation of the Gαs-coupled EP3C receptor by inflammatory stimulus PGE2 potentiates homomeric P2X3R-mediated, fast-inactivating ATP currents in DRG neurons. Although under basal conditions the effect of PGE2 on P2X3 sensitization is mediated by PKA only, the P2X3-mediated ATP current in response to PGE2 is greatly enhanced and supplemented by EPAC/PKC-ε-dependent signaling consequent to complete Freund’s adjuvant (CFA)-induced inflammation in DRG neurons. Therefore, EPAC1 appears to play an important role in the transition from the basal PKA-dependent pathway to combined induction of both EPAC/PKC- and PKA-dependent pathways following inflammation injury. Further support of this conclusion is evident with increased EPAC1 protein levels observed in CFA-treated rat DRG neurons and 007-mediated effects mimicking PGE2 actions on ATP current in a PKC-dependent manner (1084). Subsequent studies by the same group reveal that in addition to EPAC1, the expression of EPAC2 and the level of cAMP in DRGs are also significantly enhanced after CFA treatment in DRG neurons. Furthermore, administration of the EPAC specific agonist 007 in control rats before α,β-meATP injection resembles a CFA response, inducing a twofold enhancement in flinch responses, but pharmacological inhibition of EPAC1 and EPAC2, using isoform-selective EPAC antagonists, substantially blocks α, β-meATP-evoked flinch responses. These data suggest that EPAC1 and EPAC2 are essential in meditating P2X3R-induced hyperalgesia after inflammatory injury. Moreover, both CFA and 007 treatment enhances translocation and phosphorylation of PKC-α and PKC-ε in DRG neurons. Using isoform selective inhibitors, the authors (373) demonstrate that inhibition of PKC-α or PKC-ε successfully blocks EPAC agonist-induced increases to flinch responses. Mechanistically, activation of EPAC proteins induces a PKC-dependent increase in F-actin and promotes membrane partitioning of P2X3Rs in DRG neurons, promoting sensitization of P2X3Rs and abnormal pain behaviors (374). These studies significantly expand previous findings by implicating EPAC2 and PKC-α, in addition to EPAC1 and PKC-ε, in inflammation-induced hyperalgesia. Curious would be if there is a specific, yet to be determined pattern of wiring among EPAC1, EPAC2, PKC-α, and PKC-ε signaling.
Vasko and colleagues (1068) led the investigation questioning if NGF, an inflammatory mediator known to induce sensory hypersensitivity (600, 1126), participates in the sensitizing actions of PGE2 on the transition from activation of PKA to EPACs. Findings from these studies in isolated sensory neurons reveal that the ability of PGE2 to enhance capsaicin-evoked release of calcitonin gene-related peptide (CGRP) and to increase the number of action potentials (APs) evoked by a ramp of depolarizing current is mediated by PKA activation in the absence of NGF, but not in the presence of 30 ng/ml NGF. Interestingly, chronic exposure to NGF dose-dependently increases the expression of EPAC2 mRNA and protein, but not EPAC1, in cultured sensory neurons. Moreover, intradermal injections of CFA into the rat hindpaw quadruples the expression of EPAC2, but not EPAC1, in the DRG and dorsal spinal cord in a NGF-dependent manner. This enhanced EPAC2 expression can be effectively blocked by intraplantar administration of NGF antibodies. Silencing EPAC2 expression by siRNA abolishes the ability of PGE2 to augment capsaicin and potassium-evoked CGRP release and also increases the number of APs generated by a ramp of current in sensory neurons grown with added NGF, but not in the absence of NGF. In contrast, EPAC1 siRNAs did not attenuate PGE2-induced sensitization. These results demonstrate that NGF rewires the signaling cascade through increasing EPAC2 expression to heighten PGE2-induced hypersensitivity during inflammation (1068).
Determining the signaling pathways downstream of EPACs that contribute to persistent sensitization of sensory neurons, Shariati et al. (949) first demonstrated that selective activation of EPACs with 007-AM increases the number of APs generated by a ramp of depolarizing currents and augments the evoked release of the neuropeptide CGRP from isolated rat sensory neurons. Subsequently, the authors show that the EPAC agonist 007-AM activates both Rap1 and Ras small GTPases in cultured sensory neurons. Interestingly, inhibition of Rap1, a known EPAC effector, by shRNA or through internal perfusion of a Rap1-neutralizing antibody, does not attenuate the sensitization generated by EPAC activation. On the other hand, overexpression of dominant negative Ras (DN-Ras) or internal perfusion of a Ras-neutralizing antibody blocks the 007-AM-induced sensitization of cultured sensory neurons. Therefore, a novel interplay between EPACs and Ras appears relevant to this pathway, rather than the canonical EPACs and Rap1 pathway, where the Ras small GTPase is critical for mediating EPAC-induced sensitization in small-diameter capsaicin-sensitive sensory neurons (949). However, unclear is the precise identity of the Ras isoform activated by the EPAC specific agonist, and also which EPAC isoform is responsible, as well as whether EPAC proteins are capable of activating Ras directly as Ras-GEFs.
In addition to PKA and PKC, GRK2, which desensitizes GPCR-mediated signaling by phosphorylating the receptor, has also been implicated in chronic pain (505, 538, 1086). A recent study by Eijkelkamp et al. (268) showed that GRK2 protein levels in rat DRG neurons were reduced during peripheral paw inflammation. Downregulation of GRK2 in Nav1.8-positive sensory neuron tissue-specific GRK2 heterozygous knockout (SNS-GRK2+/−) mice prolonged PGE2- and cAMP-induced hyperalgesia in a PKA-independent, but EPAC-dependent manner, while PGE2- or cAMP-induced hyperalgesia in wild-type mice is PKA-mediated. These results demonstrate that GRK2 contributes to PGE2-induced hyperalgesia. Furthermore, activation of EPAC by 007 was sufficient to induce prolonged hyperalgesia in SNS-GRK2+/− mice, whereas PKA activity is not required for hyperalgesia in SNS-GRK2+/− mice. In the cell, GRK2 acts as a binding partner for EPAC1 where reduced GRK2 expression enhances the activation of EPAC, Rap1, and ERK. Consequently, aggravated hyperalgesia is observed in vivo during inflammatory response via biased cAMP signaling shifting from PKA to EPAC1/Rap1, ERK/PKC-ε pathways (268). This association of GRK2 attenuation in nociceptors with enhanced inflammatory hyperalgesia was further confirmed in a rat model using the Randall-Selitto paw pressure test (854). Intrathecal administration of GRK2 antisense oligonucleotide (ODN) led to a heightened and prolonged hyperalgesia in response to PGE2, epinephrine, and carrageenan by activation of the cAMP-dependent pathways downstream of GPCRs (297).
Using two mouse models of hyperalgesic priming with an intraplantar injection of carrageenan or ΨεRACK, Wang et al. (1087) showed that induction of GRK2 expression in vivo by viral-based gene transfer or reduction of EPAC1 in mice via EPAC1 ODN and gene knockout approaches prevented the development PGE2-induced chronic hyperalgesia in response to carrageenan and ΨεRACK priming. In addition, increasing GRK2 or decreasing EPAC1 also inhibited chronic pain in the CFA chronic inflammatory pain model (1087). Subsequent studies further define a molecular mechanism by which GRK2 inhibits EPAC1-to-Rap1 signaling by phosphorylating EPAC1 at Ser-108 in the DEP domain. GRK2 phosphorylation of EPAC1 does not affect its ability to activate Rap1 biochemically, but inhibits EPAC1-mediated cellular activation of Rap1 by blocking agonist-induced plasma membrane translocation of EPAC1. GRK2 inhibits EPAC1-mediated sensitization of the mechanosensor Piezo2, which contributes to inflammatory mechanical hyperalgesia. In vivo, EPAC1−/− mice are protected against inflammatory hyperalgesia in the CFA model. Moreover, the EPAC-specific inhibitor ESI-09 inhibits established CFA-induced mechanical hyperalgesia without affecting normal mechanical sensitivity. Mechanistically, CFA increased activity of the EPAC target Rap1 in dorsal root ganglia of wild-type, but not of EPAC1−/− mice. Moreover, using sensory neuron-specific overexpression of GRK2 or its kinase-dead mutant in vivo demonstrated that GRK2 inhibits CFA-induced hyperalgesia in a kinase activity-dependent manner. Taken together, these results convincingly demonstrate that the balance between GRK2 and EPAC1 signaling is crucial in pain sensitivity. Therefore, maintaining GRK2/EPAC1 equilibrium may represent a promising therapeutic strategy for the prevention and treatment of abnormal pain (962).
2. Neuropathic pain
Studies using EPAC1 knockout mouse models provide the first direct evidence that EPAC1 is involved in a physiological role in the development of mechanical allodynia during neuropathic pain (267). During the development of neuropathic pain, the expression levels of EPAC1 mRNA and protein, but not those of EPAC2, are significantly increased in DRG innervating the ipsilateral paw after a unilateral L5 spinal nerve transection (SNT) in comparison with DRG innervating the contralateral paw from sham-operated mice. Conversely, SNT-induced mechanical allodynia is greatly reduced in EPAC−/− and EPAC+/− mice when compared with wild-type counterparts. Application of the EPAC-selective agonist 007 enhances sensory neuron mechanotransduction as demonstrated by increasing the rapidly adapting (RA) mechanically evoked peak current, while decreasing the activation threshold in vitro and inducing long-lasting mechanical allodynia in vivo. Moreover, intrathecal EPAC1 ODN administration almost completely reverses the development of mechanical sensitization induced by 007. These results suggest that EPAC1 is critical for the development of chronic neuropathic pain in a mouse model. Mechanistic analysis further reveals that activation of EPAC1, but not EPAC2, boosts mechanically evoked currents mediated by Piezo2, a well-studied mechanotransducer involved in the development of allodynia. Importantly, EPAC-mediated allodynia is Piezo2 dependent and does not require Nav1.8-positive sensory neurons, which comprise 85% of nociceptors and are essential for the development of inflammatory hyperalgesia, but not neuropathic pain (1).
A recent study, employing a rat chronic postoperative pain model of skin/muscle incision and retraction (SMIR), demonstrates enhanced EPAC1 signaling may also contribute to postoperative chronic pain. The expression of EPAC1 protein is significantly upregulated in both the lumbar spinal cord and DRG 7 days after SMIR. Intraplantar administration of 007 dramatically decreased the mechanical withdrawal threshold while the levels of VEGF and pERK in the lumbar spinal cord are increased in response to EPAC agonist in naive rats. On the other hand, intrathecal injection of EPAC1 siRNA 7 days after SMIR suppresses mechanic allodynia induced by this process, while also reducing the levels of VEGF and pERK in both the lumbar spinal cord and DRG. These results suggest that EPAC1 plays a role in pain sensitization during postoperative recovery and is a promising, potential therapeutic target for the prevention and treatment of chronic postoperative pain (142). In a similar study using a rat SCI model, Wei et al. (1104) examine the role of PKA and EPAC signaling in neuroplasticity associated with recovery after SCI. cAMP-mediated signaling, particularly PKA activation, has been previously implicated in promoting neuron sprouting and neurite extension. Surprisingly, inhibiting PKA activity in the forelimb sensory motor cortex contralateral to the spinal lesion, and in combination with rehabilitative training, significantly enhances the recovery in a single pellet skilled reaching paradigm, and is found to promote collateral sprouting of injured corticospinal tract axons, an indicator of neuroplasticity. Likewise, activation or inhibition of PKA by selective agonist N6-MB-cAMP or Rp-cAMPS, respectively, has no effect on the phosphorylation of CREB in cultured, conditionally immortalized mouse striatal STHdh neurons. In contrast, activation of EPAC using 007 leads to increased pCREB levels and promotes neurite outgrowth of the DRG neurons, while combination treatment with Rp-cAMPS and ESI-05, an EPAC2 selective inhibitor, inhibits neurite outgrowth and pCREB. These results suggest a complex role of PKA and EPAC2 in neuroplasticity after SCI (1104). The authors propose that EPAC2 activation may be involved in promoting neuroplasticity and CREB phosphorylation, while PKA inhibition synergizes with EPAC2 activation by increasing the readily available cAMP pool. However, inhibition of EPAC2 alone by ESI-05 is not sufficient to affect either the neurite outgrowth or pCREB level, suggesting the involvement of additional factors, such as EPAC1, which were not investigated. In addition, in vivo experiments directly testing the role of EPAC2 in neuroplasticity after SCI using genetic or pharmacological approaches are lacking.
In the last few years, the essential roles of EPAC proteins in the development of hyperalgesia and/or allodynia in various rodent models of inflammatory, neuropathic, and postoperative pain are becoming clearer and clearer. Pathway analyses using genetic and pharmacological approaches have also firmly established EPAC signaling as an important component of the nociceptive pain transmission and regulation network. While the cellular and molecular details remain to be fully elucidated, a delicate balance between EPAC and PKA, two major intracellular cAMP sensors, has become evident for proper signal propagation of noxious stimuli in sensory neurons to maintain homeostasis. In general, PKA signaling predominates under the acute pain state, while the EPAC pathway enhances pain signals and produces hypersensitivity (FIGURE 13). Uncovering the mechanism of this signaling switch during the transition from acute to chronic pain will likely provide profound insight into understanding of neuroplasticity in the nociceptive circuit associated with the development of chronic pain, and may one day yield novel therapeutics for prevention and treatment for this ever-growing patient population. This is of particular importance considering the ongoing crisis of the “opioid epidemic” (125). Therapeutics targeting EPAC proteins may circumvent some of the undesirable effects of opioid-based medications that rely on systematic blockage of the entire μ-opioid receptor signaling pathway at the receptor level.
FIGURE 13.
Role of EPAC and PKA of the cAMP signaling cascades in sensory neurons during transition from acute to chronic pain. See text for additional details and abbreviations.
E. Infection and Inflammation
cAMP is a potent regulator of host immune functions. In general, an increase in intracellular cAMP suppresses innate immune functions by modulating the production of pro- and anti-inflammatory mediators and by inhibiting phagocytosis and microbicidal functions (940). Early investigations of EPAC functions in the immune system have been reported previously (347, 559, 656, 958, 1141) and discussed extensively in section IVI. The specific roles of EPAC in infection and inflammation are discussed below.
1. EPAC and infection
a) bacterial pathogens.
In human and rat alveolar macrophages, activation of either PKA or EPAC1 inhibits H2O2 production and killing of immune serum-opsonized K. pneumoniae. Furthermore, activation of EPAC1, but not PKA, suppresses IgG-mediated phagocytosis (31). Taskén and colleagues (120) demonstrated that in human peripheral blood monocytes activation processes such as production of cytokines/chemokines, stimulation of cell adhesion, chemotaxis, phagocytosis, and respiratory burst are all suppressed by PKA activation, but not by EPAC. However, in monocyte-derived macrophages, the level of EPAC1 is upregulated and both EPAC and PKA are involved in PGE2/cAMP-mediated inhibition of phagocytosis of IgG-opsonized E. coli (120). While PKA is solely responsible for the regulation of most cytokines and chemokines produced in monocytes and macrophages by cAMP, both EPAC1 and PKA activation contribute to regulating inflammatory mediators produced in dendritic cells (32). A subsequent study reveals that EPAC1, but not Rap1, accumulates on maturing phagosomes in response to PGE2 or 007 treatment. This association of EPAC1 with phagosomes might contribute to its functions in suppressing H2O2 production and bactericidal activity (108). Further delving into the molecular mechanisms governing the inhibitory effect of PGE2/cAMP/EPAC1 on FcγR phagocytosis in primary macrophages revealed that activation of EPAC1 stimulates SHP-1, thereby enhancing PTEN activity to counter the PI3K/Akt-dependent innate immune signaling (141).
Phagocytosis mediated by the complement receptor CR3 (also known as integrin αMβ2 or Mac-1) is known to be regulated by RhoA GTPase and actin dynamics. Unlike FcγR-mediated phagocytosis described above, activation of EPAC/Rap1 by 007 stimulates complement-mediated phagocytosis (684) and restores CR3-dependent phagocytosis suppressed by Rho GTPase inhibition (521). Furthermore, EPAC/Rap1 appears to regulate CR3-mediated phagocytosis through RIAM, which activates CR3 by recruiting talin to the cytoplasmic tail of the β2 integrin subunit (684). In addition, EPAC activation induces CR3-mediated phagocytosis by promoting the formation of filamentous actin through interactions among Rap1, profilin, and actin (521).
Using a visceral leishmaniasis animal model system, infection of macrophages in vitro and in vivo by Leishmania donovani, an intracellular parasitic protozoan, is demonstrated to induce increased expression of COX2 elevating PGE2 production. In turn, raised PGE2 increases cAMP levels in macrophages through G protein-coupled E-series prostanoid (EP) receptor activation. Among four different EPs (EP1–4), an increased expression of EP2 is observed during L. donovani infection. These results demonstrate that Leishmania can manipulate host macrophages to modify immunological defense and facilitate pathogen survival by inducing EP2-mediated cAMP signaling through EPAC and PKA (895). Similarly, upregulation of TGF-β signaling in Theileria annulata-infected macrophages induces COX2 and EP4 expression, which leads to a PGE2/EP4 auto-stimulatory loop, subsequent high intracellular cAMP levels, and cooperative activation of PKA and EPAC to induce the CREB-mediated transcriptional program in infected macrophages (390). On the other hand, a closely related parasite, Plasmodium falciparum, increases intracellular cAMP levels of Kupffer cells, resident macrophages of the liver, by activating ACs in a low-density lipoprotein receptor-related protein (LRP-1)-dependent manner (1053). Also of interest, malaria sporozoites can pass through Kupffer cells to reach hepatocytes, employing these resident macrophages to serve as initial sites of multiplication in the mammalian host (312). Thus this clever mechanism of sporozoites involves elevating intracellular cAMP, which acts through EPAC, but not PKA, to suppress the major defense mechanism in Kupffer cells, the respiratory burst, permitting safe passage through the professional phagocytes and invasion into neighboring hepatocytes (1053).
β2-Agonist treatment of neutrophils isolated from healthy volunteers dose-dependently impairs neutrophil phagocytosis, an effect that can be reversed by a specific AC inhibitor, suggesting that β2-agonist-induced impairment of neutrophil phagocytosis is mediated through cAMP. Phagocytic impairment induced by β2-agonists is associated with a PKA-dependent reduction in RhoA activity that can be reversed by PKA inhibition. Surprisingly, activation of EPAC by 007 can reverse neutrophil dysfunction induced by β2-agonists or by Rho kinase inhibition, despite 007 treatment alone reducing RhoA activity by 63% and having no effect on β2-agonist-induced inhibition of Rho activity. Importantly, EPAC activation significantly restored the β2-agonist-suppressed capacity of neutrophils to kill laboratory and clinical strains of gram-positive and gram-negative pathogens, Staphylococcus aureus and Pseudomonas aeruginosa, respectively (925). Therefore, the functional roles of EPAC1 phagocytes are complex, cell-type specific, and even seemly contradictory. Additional studies using genetic and pharmacological approaches in defined animal models will be required to fully elucidate the responsible cAMP receptor in these mechanisms.
While initially discovered in eukaryotic organisms, cAMP signaling is also a prevailing second messenger critical for sensing environmental cues in prokaryotes. In particular, many microbial pathogens are known to use cAMP as a “weapon” to enhance their infectious virulence by controlling the expression of virulence genes important for regulating carbon metabolism, biofilm formation, and the type III secretion system (677). Furthermore, numerous microbial pathogens can “highjack” host cell cAMP signaling pathways as an effective virulence strategy. There are several routes by which bacterial pathogens can manipulate the host cAMP levels. A primary strategy employed by several pathogens is the secretion of ACs directly and/or AC modulating toxins into host cells. For example, anthrax toxin edema factor (EF) is a highly active calmodulin-dependent AC that can dramatically increase the intracellular level of cAMP in host cells.
While the cAMP produced by EF toxin is known to impair macrophage and T-cell functions by activating PKA and promoting immunological evasion for the bacteria, the roles of EPAC in this defensive mechanism were less clear. Rosner and colleagues (432) showed that treatment of HMVECs, which are enriched in anthrax receptor expression, with EF increased cellular cAMP concentration, induced cytoskeletal rearrangement, and blocked VEGF-mediated chemotaxis, but did not inhibit cell proliferation and survival. Interestingly, the changes in cell morphology and motility induced by EF are mainly mediated by the cAMP/EPAC/Rap1 signaling pathway (432). Similarly, in human macrophages, EF induces cytoskeletal changes, including decreased cell spreading as well as reduced filopodia generation and F-actin content, while also significantly suppressing phagocytosis. Importantly, EF treatment modulated both protein levels and activity of EPAC1 and PKA, and selective activation of EPAC or PKA recapitulated EF’s cellular effects. These results demonstrate that the anthrax EF can disrupt macrophage functions via signaling through EPAC and PKA (1164).
In contrast to anthrax EF toxin, the main virulence factor of S. aureus, the pore-forming toxin α-hemolysin (Hla), has been shown to activate an alternative autophagic pathway independent of both PI3K activity and Beclin 1 by decreasing intracellular cAMP levels (689). S. aureus, traditionally considered an extracellular pathogen, can invade various types of nonprofessional phagocytic cells, disrupt the host cell autophagic pathway, and induce caspase-independent cell death (918). While Hla toxin is required for the activation of the autophagic pathway in infected host cells (691), treatment of host cells with a membrane-permeable cAMP analog inhibits Hla-induced autophagy in a PKA-independent manner. In light of this conclusion, EPAC and Rap2B, acting through calpain, are involved in the suppression of Hla-mediated autophagy induction. Moreover, treatment of cells with purified Hla toxin or S. aureus results in decreased intracellular cAMP levels, which stimulates autophagy induction and promotes pathogen intracellular survival (689, 690).
Rickettsiae are obligate intracellular bacteria that infect hosts by targeting the endothelial lining of the blood vessels. Considering the important roles EPAC1/Rap1 perform in endothelial functions, it was hypothesized that EPAC1 might be involved in the development of rickettsioses. Indeed, deletion of the Rapgef3 gene protects mice from an ordinarily lethal dose of rickettsia by suppressing bacterial adhesion and invasion into endothelial cells. Consistent with the genetic manipulation, pharmacological inhibition of EPAC1 in vivo using an EPAC specific small molecule inhibitor, ESI-09, recapitulates the EPAC1 knockout phenotype. ESI-09 treatment dramatically decreases the morbidity and mortality associated with fatal spotted fever rickettsiosis (353). These results establish a novel signaling mechanism for host-pathogen interactions and EPAC1 as a target for the prevention and treatment of fatal rickettsioses. Most importantly, this study demonstrates a new strategy for combating bacterial infection by targeting a host molecule, EPAC1 in the case of rickettsiosis, while conventional antibiotic treatment acts on the pathogen-associated molecules. This new approach may help combat the current “antibiotic resistance” crisis, notably as treatment targeting host molecules does not apply selective pressure directly on pathogen survival and is therefore less likely to provoke the development of resistance.
b) viral pathogens.
In addition to infections associated with bacterial pathogens, EPAC signaling has also been implicated in viral infections. Human immunodeficiency virus type 1 (HIV-1) infection is known to trigger an inflammatory response and stimulate the expression of proinflammatory genes such as COX2, which induces an increased production of PGE2 and ROS in HIV-infected patients (270, 825). PGE2 treatment inhibits HIV replication in infected human peripheral blood lymphocytes without affecting viral entry, retrotranscriptase or integrase activity, and viral protein synthesis. Furthermore, the effects of PGE2 can be mimicked by a specific EPAC agonist, 007. Mechanistic analysis suggests that PGE2, through EPAC/Rap activation, suppresses RhoA activity to reduce actin polymerization, thus disrupting the transport and assembly of new virons and subsequently inhibiting HIV-1 release and transmission (191). EPAC1 has also been shown to regulate the replication of both Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV) in a cell type-independent manner. Specifically, ESI-09 treatment or silencing EPAC1 gene expression rendered cells resistant to viral infection (1013).
2. EPAC and inflammation
Insults by foreign pathogens are classically detrimental to host organisms, and thus systems have evolved to protect the body against these invaders and preserve host function. The initiation of the inflammatory response begins in the injured tissue where endothelial cells and smooth muscle cells respond by secreting chemoattractants to summon resident innate immune cells. Indeed, transcriptional regulation of cytokines is found to be attenuated by cAMP signaling, especially EPAC mediated, suggesting an anti-inflammatory role for this cAMP effector (702, 905, 911, 1160).
Upon moving into the site of inflammation, first responder cells including neutrophils and macrophages begin to seek out and consume foreign particles, while also recruiting additional immune cells to the site. The movement of immune cells in tissue or from the bloodstream requires migratory signals as well as reduction in cell-cell junctions to promote tissue traversal and diapedesis, respectively. Vascular endothelial barrier integrity also implicates EPAC and PKA in preservation of barrier function and adhesion between endothelial cells (83, 210, 323, 548, 762, 930). Additionally, alcohol-mediated enhancement of vascular permeability, which rapidly reduces active Rac1, is quickly restored to baseline with activation of EPAC1 (249). Regulation of EPAC/Rap1 signaling is also implicated in the fine balance between migration and stagnation of these first responding immunological cells across endothelial monolayers discussed previously (see sect. IVI) (61, 728). Furthermore, pathogen engulfment and clearance are regulated by cAMP induction of EPAC, although curiously dependent on the opsonization of the particle (31, 521, 684, 925). The diversity of the EPAC/Rap1 cascade during induction of the inflammatory response illustrates the importance of maintaining proper signaling to accomplish the primary objective, eradication of invading pathogens.
Following elimination of the inflammatory stimulus, the resolution phase begins to circumvent excessive tissue damage and eliminate large unsustainable populations of immune cells. Pro-inflammatory cytokine production is suspended, and many innate leukocytes undergo apoptosis followed by subsequent macrophage-mediated efferocytosis. During this phase, anti-inflammatory signaling mediated by cAMP/EPAC signaling may be specifically upregulated to promote inflammatory clearing. Professional APCs from the site of insult, such as DCs and macrophages, will also migrate towards draining lymph nodes after, and potentially during, resolution in a phase termed post-resolution. Once there, these regulatory APCs prepare consumed particles to express the processed antigens through MHC class II molecules and initiate education of B and T cells to the recently encountered pathogen. Distinct cytokine secretions from the APCs during this interaction with adaptive immune cells will constitute a population of memory cells that are well adapted to instigate a more rapid and aggressive immune response if the pathogen is encountered by the innate defenses again. During this phase, the population of resident macrophages that underwent apoptosis during resolution will also begin to be repopulated in the peripheral tissue. Thus cAMP/EPAC signaling is observed to be implicated in several pathways of inflammatory regulation endorsing the importance of the cAMP signaling pathway in physiological inflammation.
Although inflammation is a proper physiological response of the host, perturbations leading to excessive stimulation can lead to detrimental disease states. Indeed, elevation of cAMP by PDE4 inhibition or A2A receptor stimulation inhibited inflammation through depletion of TNF-α and amplified IL-10 secretion. These effects of PDE4 inhibition were attributed to PKA, EPAC1, and EPAC2 activation by cAMP since the secretion of TNF-α and IL-10 was reversed after silencing each of the individual cAMP effectors. Furthermore, in vivo analysis revealed that PDE4 inhibition reduced leukocyte infiltration and TNF-α secretion, implying that modulation of cAMP and subsequent PKA and EPAC1/2 signaling can effectively control the inflammatory state (822). This also suggests that perturbation of these cAMP pathways could reveal mechanisms present in chronic inflammatory pathologies that could be developed for therapeutic targeting. Below we cover the identified roles of EPAC in vascular and pulmonary pathologies that have been initially explored in the past decade.
a) vascular inflammation.
Inflammation in the vasculature is modulated by the immune system in response to vessel wall injury or pathogen stimulation. This response is supposed to be short-lived, clearing damaged cells or microbes then being repressed to allow initiation of repair mechanisms by the smooth muscle and endothelial cells. However, excessive inflammation incurred at the site of injury can lead to detrimental consequences and if left unchecked can develop into chronic pathologies in the host including atherosclerosis and hypertension.
Initiation of the inflammatory process is commonly mediated by peripheral tissues and cells exposed to the initial insult. Endothelial cells are well positioned to take on this role through secretion of chemokines to attract immunological mediators to the problematic tissue site in a host organism. However, in pathological conditions, chronic secretion of cytokines by vascular endothelial cells in response to injury induces enhanced adhesion of monocytes and T cells promoting the formation of atherosclerotic plaques, when in the presence of unhealthy lipid levels. One cytokine secretion linked with heart disease and involved in the initiation of an inflammatory response is IL-6 from endothelial, smooth muscle, and macrophage cells in the affected area. Endothelial cell stimulation with increasing levels of IL-6 and soluble IL-6R promotes production of CCL2 (MCP-1) and cell adhesion molecules to enhance recruitment of inflammatory cells (880).
The involvement of cAMP signaling in inflammatory initiation by IL-6 is observed to be suppressive. IL-6-mediated CCL2 induction is inhibited by cAMP activation of the downstream EPAC target SOCS3 which blocks JAK/STAT3 phosphorylation implicating EPAC in the negative-feedback loop for IL-6 and promoting anti-inflammatory effects in endothelial cells (905, 908). Further investigations connect EPAC1 activation with SOCS3 transcriptional regulation by C/EBPβ binding the SOCS3 promoter (1160) and associate EPAC1 activation of PLC-ε and downstream signaling mediators, PKC-α and/or -δ isoforms, to induce MAPK-dependent phosphorylation of C/EBPβ Thr235 (93, 702, 1128), albeit the precise mechanism of induction by cAMP/EPAC is still investigated.
Indeed, natural lowering of lipids and avoidance of risk factors would be the ideal option for prevention and/or treatment of these disease states, but with patient noncompliance and genetic predisposition, attempts to modulate lipid levels have been explored. Pharmacological manipulation with the use of statins has proven unreliable, with patients showing resistance or intolerance to the therapy and potentially incurring myopathies. Thus a more direct targeting approach may be beneficial in overcoming some of these issues.
Although IL-6 is a prominent mediator of inflammation, other signaling molecules are also involved in the induction of inflammatory events. Hyaluronan (HA) is one such component upregulated in response to vascular injury and inflammation in the case of neointima and atherosclerotic lesion development whose accumulation proceeds inflammatory cell recruitment. HA acts to form a permissive matrix for proliferation, migration of smooth muscle cells (SMCs) and adhesion of immunological cells (281, 282, 872, 1069, 1119). Furthermore, the action of HA is reported to be dependent on the molecular weight of the stimulating factor, with low-molecular-weight HA fragments promoting inflammation and high-molecular-weight HA bestowing a suppressive effect (138, 139, 475, 517, 679). Signaling of low-molecular-weight HA through CD44/TLR4 is also implicated in the activation of NFκB and production of pro-inflammatory cytokines (134, 135, 138, 437, 475). Surprisingly, inhibition of the synthase for HA does not provide protection against atherosclerotic development, but rather initiates a pro-inflammatory effect with enhanced macrophage recruitment attributed to endothelial glycocalyx injury (753), suggesting the regulation of inflammation by HA is more complex.
Like HA levels, adenosine signaling accumulates during tissue injury and inflammation where it is associated with SMC proliferation, initially reported to induce anti-mitogenic effects, but disputed by reports of pro-mitogenic effects. These discrepancies may be attributable to different receptor isoform profiles of individual cells with A1 receptor mediating proliferation and A2A/A2B receptor responsible for reduced proliferation, respectively (950). Additionally, in opposition to the low-molecular-weight HA, adenosine is reported to suppress proinflammatory cytokine production (1066). Fittingly with the characteristic anti-inflammatory role of adenosine signaling, loss of adenosine production in ApoE−/− or A2B receptor-knockout mice exacerbates a pro-atherogenic phenotype (122, 1152). Intriguingly, although loss of A2A receptor also demonstrates an overzealous inflammatory response to minor infections (782), supporting an atherogenic effect as anticipated, unexpectedly, atherosclerotic lesion development is attenuated in mice lacking both ApoE and A2A receptors observed by increased apoptosis of foam cells (1089). These results could suggest a potential role of adenosine to potentiate or suppress HA signaling dependent on the activation of specific adenosine receptor isoforms.
Exploring a potential connection between adenosine signaling and HA production in coronary artery smooth muscle cells demonstrated that pan adenosine receptor agonists increase HA synthase 1 and subsequent HA generation with matrix formation in a biphasic pattern, with initial increases after 6 h and a lesser induction at 24 h. Furthermore, differential receptors are involved in signal propagation at these distinct times with A2B receptors important during early induction of HA, and A2A and A3 receptors important for the later response. Interestingly, A2B receptor-mediated HA synthase induction was relieved in a PKA-independent manner by activation of EPAC following adenosine stimulation or by inhibition of either PI3K or MAPK signaling (359). A previous independent study supports this conclusion reporting that A2B receptor transcriptional regulation is mediated through a cAMP/EPAC pathway (668). Additionally, in chondrocytes, A2A receptor activation suppresses HA-dependent NFκB activity similar to blocking CD44/TLR4 signaling, suggesting that the A2A receptors act as negative feedback signals for HA transcriptional regulation. Furthermore, during HA stimulation of NFκB activity and the pro-inflammatory cytokine TNF-α, the A2A receptor agonist-mediated inhibition is blocked by silencing EPAC expression, suggesting a negative regulatory role of EPAC on HA signaling (136, 137). Taken together, EPAC appears to have divergent modulatory roles for adenosine-mediated transcriptional regulation dependent potentially on the receptor isoform specificity, timing of the signal, or cell type.
Functionally, adenosine receptor stimulation induces formation of a rich HA matrix to “catch” inflammatory cells by promoting adhesion of monocytes and a pro-migratory environment for smooth muscle cell migration demonstrating pro-atherogenic signaling (359). This suggests that EPAC may initially act to inhibit the promotion of pro-atherogenic characteristics of SMCs, but eventually falters as the cell switches to an EPAC-independent signaling pathway. Indeed, these observations depict differential activation of adenosine receptor isoforms that could be relevant in unraveling the in vivo observations described above, where adenosine is working in a multilevel signaling system. In such a case, complete loss of signaling by all adenosine receptor isoforms or the initial induction of HA by A2B receptor would produce a distinct response in comparison to cells where only the latter induction of the A2A receptor is disrupted. Thereby temporal activation of adenosine receptor isoforms may indeed act to modulate the level of cAMP and regulate disease progression.
Once the inflammatory cells are summoned to the site of inflammation, another major instigator of atherosclerotic plaque development is the generation of lipid-laden macrophages or foam cells. The modification of low-density lipoprotein (LDL) into oxidized LDL is responsible for the generation of these cells, which engulf the oxidized lipid and become occupants of the lesion. Cells typically remove excess cytoplasmic lipids through reverse cholesterol transport using active transporter proteins such as ABCA1 and ABCG1 in the presence of acceptor molecules such as apoA-I and high-density lipoprotein (HDL). Therefore, mechanisms causing perturbations of these cholesterol transporters are of great interest to discern a potential target for recovering the lipid-loaded cells. Indeed, extracellular stimuli like IFN-γ demonstrate regulatory properties on macrophage cholesterol efflux by reducing ABCA1 transporters and promoting atherosclerosis development (80, 800). Supporting this function of the pro-inflammatory cytokine, knockout of IFN-γ in ApoE−/− mice, which are prone to atherogenesis, demonstrates 60% attenuation of lesion burden (384). Furthermore, stimulation of A2A receptor suppresses foam cells formation through enhanced ABCA1 transporter expression in the presence of IFN-γ. Moreover, this effect was reproduced with specific agonist activation of EPAC or PKA (80). ABCA1 phosphorylation was also increased in the presence of active EPAC, adding to the previously reported effect of cAMP through PKA on ABCA1 phosphorylation and increased cholesterol efflux (80, 389). This anti-inflammatory signal was further extended to demonstrate that A2A receptor activation of EPAC or PKA increases apoA-I-mediated cholesterol efflux, subsequently attenuating foam cell morphogenesis by 60–80%. The A2A receptor pathway is specific for ABCA1 enhanced activity, as these effects are lost with the knockdown of ABCA1, but are retained in the absence of another cholesterol transporter ABCG1 (80). However, in the presence of co-suppression of ApoE and A2A receptors, foam cells are reported to have increased apoptosis conferring a protective effect against lesion development (1089). Although one may predict that the loss of A2A receptor signaling would only augment the lesion development, this is clearly not the case. Thus consequential loss of A2A receptors may function in surrounding cells to trigger the apoptosis of foam cells or perhaps simultaneous loss of both proteins sends these cells into an overloaded state where apoptosis pathways are initiated, although it is currently unclear the mechanism that lies behind this phenotype.
b) pulmonary inflammation.
Inflammatory mediators can participate in a number of functions in tissue to perturb or enhance inflammation. Excessive inflammation in the lung contributes to pathologies including pulmonary fibrosis and COPD. Furthermore, extraneous inhaled agents, such as cigarette smoke, can expedite the process by invoking unnecessary inflammation. In pulmonary inflammation, major actions of inflammatory mediators consist of altered endothelial barrier integrity, secretion of pro-inflammatory cytokines, and induction of adhesion molecules to recruit excessive inflammatory cells. Regulation of the semi-permeable barrier of the pulmonary vascular endothelium and recovery of this barrier following injury are crucial factors during inflammation of the lung. Dysfunction of the barrier to increase permeability can create a portal for excessive inflammatory invasion or pulmonary edema.
Multiple results attribute barrier regulation to PG stimulation of cAMP reporting enhanced vascular endothelial barrier function. Moreover, cAMP activation of both PKA and EPAC/Rap1 is implicated in this process, appearing to activate Rac1 to subsequently decrease RhoA activation and modulate cytoskeletal dynamics (81, 83, 210, 323, 548). Furthermore, a recent study investigating the effects of prostacyclin treatment after LPS-mediated lung injury to disrupt endothelial barrier function also demonstrates the requirement of cAMP signaling in the barrier recovery process. Prostacyclin suppression of p38 MAPK and NFκB signaling is observed to be crucial to reduce recruitment of inflammatory cells by attenuating production and release of IL-8 and soluble ICAM-1. These reductions are recapitulated with direct activation of EPAC and lost with EPAC inhibition or Rap1 knockdown, suggesting the importance of EPAC/Rap1 in this prostacyclin-mediated suppression of inflammation. Additionally, although Rap1 is often associated with the activation of Rac1 to modulate RhoA in barrier function, the small GTPase also targets a scaffolding protein known as afadin, which is involved in cell-cell adherens junction formation. Indeed, targeted knockdown of afadin was sufficient to abolish the protective effects of prostacyclin and EPAC activation on restoration of the barrier, and suggest Rap1 may participate in a set of signaling events that additively affect barrier function (81). Further supporting the role of EPAC/Rap1 signaling in barrier recovery, EPAC activation is ineffective in recuperating barrier integrity in Rap1−/− mice after LPS-induced lung injury (81).
Similar to prostacyclin, ANP, which exhibits increased circulation level during lung injury and inflammation, also provides protection against thrombin-induced pulmonary endothelial barrier dysfunction. Intriguingly, the pathway invoked by ANP to decrease barrier permeability parallels the prostacyclin signaling pathway with elevation of cAMP and activation of EPAC/Rap1 and PKA. In this model, the action of Rap1 mediates induction of Rac1-specific GEFs, Vav2, and Tiam1 to activate Rac1. Supporting the activation of Rac by ANP, the Rac effector protein PAK1 is phosphorylated by ANP treatment, and inhibition or silencing of PKA, EPAC1, Rap1, Rac, or either Rac-specific GEF abolished the effect of ANP on PAK1 activation. The protective role of Rac activation in suppressing endothelial barrier dysfunction may be linked to reduced contraction of the cells by attenuating RhoA/ROCK-mediated phosphorylation of myosin light chain. Indeed, ANP treatment antagonized thrombin-induced phosphorylation of the myosin light chain in a Rap1/Rac- and PKA-dependent manner. The presence of ANP was also reported to act through PAK1 to repress association of RhoA with p115-RhoGEF, subsequently resulting in decreased RhoA activation (82). Continued investigation into protective signaling instilled by ANP during inflammatory-mediated barrier disruption with LPS or TNF-α illustrates enhanced adherens junction remodeling along with reduction of p38 MAPK and NFκB activation similar to prostacyclin (1138). These studies depict parallel pathways for PGs and natriuretic peptides utilized by endothelial cells demonstrating redundant mechanisms to achieve regulation of pulmonary endothelial barriers. Taken together, these conclusions support coordinated signaling for EPAC/Rap1/Rac and PKA in preserving pulmonary endothelial barrier integrity through increased junction formation, reduced cellular contraction, and diminished inflammatory responses.
Many signals are responsible for the suppression of airway inflammation, but the implication of cAMP signaling is observed by actue bronchoconstriction experienced by a group of asthmatics taking aspirin or NSAIDS. Identification of the mechanism stimulating this undesirable response is warranted, and reproduction of COX depletion in animal models demonstrates an increased allergic inflammatory state that could be the root of the problem (340, 816). The COX enzymes are responsible for the production of PGs such as PGE2 which has anti-inflammatory effects when signaling through EP2 receptors to elevate cAMP in inflammatory cells including T cells (30, 757, 1184). In the case of airway inflammation, EP2 receptor-null mice exhibit increased airway inflammation, suggesting the suppressive role of PGE2 in this process. Supporting this notion, Th2 CD4+ T-cell secretion of allergic inflammatory cytokines, such as IL-13, is increased in EP2-null animals and also wild-type mice following adoptive transfer of CD4+ T cells from EP2-deficient animals. This function of PGE2 is attributed to the induction of cAMP to attenuate transcriptional regulation of cytokines in T cells. Indeed, STAT6 activation by CD3/CD28 stimulation of CD4+ T cells is impeded by PGE2 stimulation. Furthermore, loss of EP2 blocks PGE2 antagonism of IL-13 in CD4+ T cells, but the suppressive action of EPAC or PKA activation is retained, suggesting the cAMP sensors function downstream of EP2 stimulation. Collectively, these results suggest that PGE2 stimulation of EP2 on Th2 cells naturally represses allergic airway inflammatory cytokines through an EPAC- and PKA-dependent inhibitory mechanism, and this action is a major factor to maintaining reduced inflammatory response in the lungs (1183).
With endogenous suppressive signals also emanates signals that stimulate the inflammatory response naturally, but can partake in excessive signaling during pathological states. In the lung, endothelin-1 (ET-1) is expressed by an abundance of cells and can enhance collagen synthesis and fibroblast activation to induce fibrosis (806, 951, 1140). Therefore, targeting of this pathway may diminish fibrotic development. Although initial trials targeting ET-1 receptors in pulmonary fibrosis did not meet expectations (529), genetic suppression of endothelin-converting enzyme-1 (ECE-1), the major enzyme responsible for ET-1 activation, in mice is observed to decrease inflammation and pulmonary fibrosis (401). Additionally, the concentration of CGRP was elevated with partial loss of ECE-1 (401), which is described to elevate cAMP through calcitonin receptor-like receptor (CRLR) and receptor-activity-modifying protein-1 (RAMP-1) (415, 511). Furthermore, 2 wk after bleomycin-induced lung injury, wild-type mice show decreased EPAC and PKA expression, while the expression of these proteins is retained in ECE-1 heterozygous mice. Supporting a potential suppressive function of EPAC and PKA, following chronic inflammation of induced lung injury, CRLR and RAMP-1 are induced in M2 macrophages for CGRP to signal to raise cAMP levels and activate the preserved EPAC and PKA whose anti-inflammatory effects in macrophages are discussed at length in section IVI2 (401). Thus loss of ECE-1 and subsequent ET-1 could ostensibly limit inflammation and fibrosis through the action of CGRP on EPAC- and PKA-mediated regulation of macrophage functions.
As mentioned in the previous subsection, regulation of low-molecular-weight HA fragment signaling has profound effects on the induction of inflammatory cytokines. During pulmonary inflammation, adenosine signaling acts to negatively regulate a variety of inflammatory mediating cells expressing A2A receptors. This ubiquitous anti-inflammatory role of A2A receptors is further supported by A2A receptor null animals exhibiting augmented inflammation, HA accumulation, and tissue damage leading to fibrosis and death in response to lung injury (911).
Investigation into the mechanism of A2A receptor suppression in pulmonary inflammation demonstrated HA-treated alveolar macrophages with suppressed pro-inflammatory cytokines, such as TNF-α, in response to A2A stimulation (911). With a potential convergence point for HA and adenosine at the level of transcriptional regulation through the transcription factor NFκB (134–138, 437, 475), A2A stimulation was shown to attenuate HA-induced NFκB activity by 55%. Furthermore, adenosine suppression of TNF-α was found to require expression of EPAC, while activation of EPAC successfully replicated the suppressive cytokine profile of adenosine on HA-mediated cytokine production. Together these observations support HA-dependent TNF-α transcription is, in part, due to NFκB activity, and furthermore, the adenosine pathway activation of EPAC attenuates HA cytokine induction by suppressing NFκB (911).
Curiously, in alveolar macrophages, A2A receptor and EPAC activation positively regulate a single HA-induced cytokine, IL-12. This synergistic enhancement by HA and A2A receptors was attributed to increased stability of the IL-12 mRNA (911). Therefore, although A2A receptor stimulation typically suppresses HA-mediated transcriptional regulation, additional undiscovered regulatory pathways may modulate posttranscriptional events and affect net protein translation during inflammation.
Additionally, in lungs of patients exhibiting chronic airway disease, levels of chemokines and cytokines are often elevated by the airway epithelium promoting inflammation of the area and contributing to pathological progression. One prime proinflammatory chemokine observed to be elevated in bronchial epithelial cells, bronchoalveolar lavage fluid, and sputum of patients suffering from asthma or COPD is IL-8 (662, 959, 1146). Corresponding to the increased IL-8, bradykinin is reported to be elevated in asthmatic patients and function as a modulator of IL-8 release from airway smooth muscle (188, 406, 799). Secretion of IL-8 by bradykinin is further augmented by treatment with the β2-AR agonist fenoterol, and this enhanced secretion is reproduced with pharmacological activation of both EPAC and PKA in a cooperative manner. The action of bradykinin on IL-8 secretion is also dependent on the MAPK signaling cascade (406). Therefore, pursuit of functional roles for EPAC and PKA within this signaling pathway revealed bradykinin-induced phosphorylation of ERK1/2 was further elevated by both EPAC and PKA activation, while secretion of IL-8 induced by bradykinin or by direct EPAC/PKA activation was completely abolished with U0126-mediated MEK inhibition. Silencing of EPAC1 and EPAC2 or pharmacological inhibition of PKA effectively perturbs stimulated release of IL-8 providing additional support for the requirement of EPAC and PKA in this pro-inflammatory mechanism (882).
Furthermore, we have known for many years that inhalation of cigarette smoke can also be attributed to heightened airway inflammation by inducing pro-inflammatory cytokines such as IL-8 (704, 733, 777, 791, 1154) and therefore may contribute to COPD initiation and progression (513, 704, 1011, 1146). Cigarette smoke extract elevates IL-8 through activation of IKK which phosphorylates the inhibitory component IκBα to subsequently dissociates from NFκB allowing nuclear translocation and initiation of transcriptional activity (733, 777, 1154). The activation of NFκB by cigarette smoke extract in airway smooth muscle cells is countered by the presence of a β2-agonist or specific EPAC agonist, acting in an inverted manner to that of bradykinin, suggesting the involvement of cAMP in negative regulation of IL-8 production. Concurrent siRNA to EPAC1 and EPAC2 or PKA impairs the ability of cAMP or EPAC and PKA agonists to attenuate IL-8. Moreover, cigarette smoke stimulation is observed to deplete EPAC1 expression in cells and COPD patients, suggesting a loss of this negative-feedback mechanism correlated with advancement of disease (786). This regulation of EPAC1 expression by cigarette smoke extract may also be connected to elevated expression of miRNA-7 in COPD patient serum, which is a predicted posttranslational regulator of EPAC1 (788). Exploring this possibility, the expression of EPAC1, but not EPAC2, is reduced in human airway smooth muscle cells correlating with an increase in miRNA-7 by cigarette smoke extract. Relating back to COPD patients, although laser dissection of bronchial smooth muscle cells from these patients exhibited increased miRNA-7, the levels of EPAC1 in these dissections were not discussed (788). The potential loss of pulmonary EPAC1 by elevation of miRNA-7 in this pathology may, in part, explain increased inflammation, proliferation, and constriction of smooth muscle cells in COPD, as well as other chronic airway diseases. Accordingly, EPAC may act as a gatekeeper for immunosuppression in COPD. While cAMP/EPAC signaling attempts to calm the immune response, as the expression of EPAC1 falls with increased cigarette stimulation, the protective effect is lost, and consequently inflammatory cells begin to pour-in causing tissue damage and reduced airflow. Thus deeper dissection of these relevant signaling cascades and potential differences in triggering the switch from pro- to anti-inflammatory is warranted. This knowledge will be critical in determining precise actions of EPAC and PKA in airway diseases as well as development of next generation therapies for these patients.
Moreover, a follow-up study investigating the action of EPACs in cigarette-induced inflammation also reveals differential roles for EPAC1 and EPAC2 in this process. Genetic knockout mice for EPAC1, EPAC2, or PLC-ε were used to demonstrate that EPAC2/PLC-ε appear to be involved in pro-inflammatory signaling such as immune cell recruitment and cytokine production, while EPAC1 appears implicated in suppressing matrix secretion and remodeling of the lung (787). The precise mechanism by which EPAC2 enhances immune cells to contribute to this phenotype is still unclear, especially with many functions of cAMP and EPAC signaling reported to be anti-inflammatory. Furthermore, the final cAMP readout may depend on additional factors including, but not limited to, cell type-dependent expression of isoforms, redundancy between isoforms, net outcome from EPAC1/EPAC2/PKA signaling pathways, or compartmentalization of signaling complexes. This divergence of EPAC1 and EPAC2 inflammatory regulation may also be accountable for the inversed expression of IL-12 in alveolar macrophages in the presence of HA and A2A stimulation mentioned previously (911). Such results demonstrate the necessity of future studies to better characterize the distinct physiological and pathophysiological functions of EPAC1 and EPAC2 so appropriate therapeutic interventions can be developed to target these important signaling switches in different disease states.
In summary, with the roles of EPAC being implicated in a multitude of functions in cells of the immune system, one would expect inflammatory responses to be reliant on proper EPAC signaling and perturbations of this pathway to result in pathologies. Within the vasculature, chronic inflammation leading to atherosclerosis and hypertension are leading causes of morbidity and mortality in the world. In these processes, EPAC commonly confers an anti-inflammatory signal through mediation of SOCS3 expression subsequently reducing expression of inflammatory cytokines, such as IL-6. Temporal-mediated adenosine signaling is also involved in modulation of proinflammatory cytokines during chronic inflammation through EPAC-mediated mechanisms, where activation of distinct isoform populations may confer differential regulation of cAMP levels to modulate EPAC and PKA during different stages of the inflammatory process, and consequently disease progression during chronic stimulation. Additionally, EPAC and PKA are observed to enhance ABCA1 transporter activity, increasing cholesterol efflux and thereby attenuating foam cell formation. However, inconsistencies observed in adenosine A2A receptor genetic knockout models warrant additional studies exploring potential effects attributed to the surrounding cellular environment of foam cells. Similar to vascular inflammation, EPAC signaling is also consistently implicated in anti-inflammatory effects in the lungs. In this tissue, the effects of EPAC/Rap1 on Rac1/RhoA cytoskeletal dynamics well-positions this signaling cascade in modulating barrier integrity, and indeed EPAC activation does protect against barrier dysfunction following lung injury. Furthermore, EPAC is observed to suppress inflammatory cytokine production employing similar pathways implicated in vascular inflammation, and interestingly chronic insult by agents such as cigarette smoke attenuates EPAC1 expression thus removing the anti-inflammatory “brake” to ostensibly promote the onset of COPD. Continued studies of EPAC-mediated effects, particularly isoform-specific functions, in chronic inflammatory disease progression will undoubtedly further elucidate the role of EPAC signaling and lead to the exploration of EPAC as a therapeutic target and/or cotreatment in many of these immunologically related pathologies.
F. EPAC and Kidney Diseases
cAMP is involved in all aspect of renal physiology, and abnormal cAMP signaling has been linked to various kidney disorders including polycystic kidney diseases (PKDs), chronic kidney fibrosis, and renal failure (914, 1036). PKDs are a family of genetic disorders involving the formation of noncancerous cyst clusters within a patient’s kidney. cAMP is implicated in playing pivotal roles of cyst development by stimulating proliferation of PKD cystic epithelial cells and secretion of transepithelial fluid (1080). In the PCK rat model, a well-characterized animal model of autosomal recessive polycystic kidney disease (ARPKD) linked to mutations in the polycystic kidney and hepatic disease 1 (PKHD1) gene, LaRusso and colleagues (54) demonstrate that cholangiocyte-derived liver cysts are associated with increased intracellular levels of cAMP and upregulation of EPAC1, EPAC2, and PKA regulatory subunit Iβ expression, as well as decreased intracellular calcium levels. Activation of EPAC and PKA both result in increased cell proliferation of PCK cholangiocytes and expansion of PCK-cholangiocyte derived cysts via the MEK/ERK1/2 pathway. Interestingly, while PKA-associated effects can be inhibited with restoration of intracellular Ca2+ levels, EPAC-mediated proliferation of PCK cholangiocytes is calcium-independent. These data demonstrate that EPAC and PKA both contribute to the hepatic cystogenesis of ARPKD (54).
Ischemic acute kidney injury is a common clinical event leading to the development of chronic kidney diseases and eventual transition to end-stage renal disease (948). Endothelial progenitor cells (EPCs) can migrate into postischemic kidneys and protect them from acute ischemic damage (814). The therapeutic benefit of EPC administration is further shown to be inversely correlated with the length of the ischemic damage, diminishing during prolonged ischemia. In animals with ischemia lasting longer than 40 min, conventional EPC infusion does not prevent acute renal failure; however, animals infused with EPCs that are prestimulated with an EPAC specific agonist, 007 in vitro, are protected against acute renal failure. Furthermore, the protective effect of 007 is reversed by cotreatment with the integrin binding antagonist cyclic Arg-Gly-Asp peptide (cRGD). Although activation of EPCs by 007 does not affect the overall expression of integrins, an augmented induction in membrane translocation of β1-integrins is observed. Thus these findings suggest that the EPAC agonist 007 enhances the anti-ischemic potential of EPCs through activation of the cell-cell and cell-matrix adhesion molecule β1-integrins (813).
Ischemia-reperfusion injury is frequently associated with microvascular damage caused by inflammation and oxidative stress when blood supply returns following a period of ischemic hypoxia. Loss of tubular epithelial cell-cell and cell-matrix interactions associated with ischemia-reperfusion injury contributes to renal failure. Therefore, a membrane-permeable EPAC specific agonist was used by Stokman et al. (986) to demonstrate that pharmacological activation of EPAC/Rap signaling preserves cell adhesions during hypoxia in vitro, maintaining the barrier function of the epithelial monolayer. Furthermore, in a mouse model for ischemia-reperfusion injury, intrarenal administration of 007 preserves renal function and reduces tubular epithelial cell stress as indicated by preserved lateral expression of β-catenin, but decreased expression of the tubular epithelial damage marker clusterin-α after ischemia. These data suggest that enhancing tubular epithelial cell function by EPAC during renal ischemia may represent a new therapeutic strategy for reducing kidney injury (986). Subsequent studies further reveal that activation of EPAC by 007 preserves mouse proximal tubular epithelial cell junctions and protects against cisplatin-associated cell apoptosis (846). Mechanistically, activation of EPAC/Rap signaling reduces reactive oxygen species after hypoxia by decreasing mitochondrial superoxide production, a common feature in ischemia-reperfusion renal injury and cisplatin nephrotoxicity (985).
Prolonged activation of the renin-angiotensin system contributes to the progression of chronic kidney diseases, such as diabetic nephropathy accounting for over 30% of the end-stage renal diseases, in part by ANG II-induced activation of NADPH oxidase and ROS production. A study by Fang et al. (285) depicts adiponectin attenuating ANG II-mediated superoxide formation and oxidative stress in primary human renal proximal tubule cells, which express both adiponectin receptor 1 and 2 (adipoR1 and R2). The effect of adiponectin is dependent on adipoR1 and AMPK activation and can also be mimicked by cAMP analogs. Furthermore, this cAMP-associated effect of adiponectin is mediated by EPAC, not PKA. This conclusion was reached from the observations that adiponectin does not activate PKA in renal tubular cells, and a PKA specific inhibitor is unable to block adiponectin’s inhibitory effect on ANG II-induced ROS production, but like adiponectin, activation of EPAC by 007 reduces NADPH oxidase activity induced by ANG II. These findings indicate that adiponectin, acting through EPAC/AMPK, exerts renal protective effects by attenuating ANG II-induced oxidative stress in the kidney (285). Interestingly, EPAC1 expression, as well as the downstream effector Rap1B, are increased in diabetic mice (617, 992). In vitro analyses of EPAC1 gene expression further reveal two glucose response elements (GREs) within the EPAC1 promoter and increased EPAC1 expression under high glucose ambience in human proximal tubular HK-2 cells. Activation of EPAC1 or high glucose stimulation leads to a hypertrophic response along with increased protein synthesis in HK-2 cells, which can be abridged by EPAC1-siRNA, but not the PKA inhibitor H89. High glucose-induced hypertrophic response is associated with an EPAC1-dependent upregulation of the cyclin-dependent kinase inhibitors p21 and p27 and phosphorylation of Akt at Ser473, accompanied by reduced cyclin-dependent kinase 4 activity to promote cell-cycle arrest at G0/G1 phase. These data suggest a role of EPAC1 in high glucose-induced cellular hypertrophy during the development of diabetic nephropathy (992). Furthermore, EPAC signaling also appears directly involved in glucose reabsorption in kidney. The filtered glucose in the glomerulus is reabsorbed by sodium-dependent glucose cotransporters (SGLTs) located in the renal PT cells. SGLTs are regulated by various signals, including agents that stimulate intracellular cAMP (840). In primary rabbit renal PT cell cultures, cAMP-mediated induction of EPAC and PKA was observed to stimulate α-methyl-d-glucopyranoside (α-MG) uptake by promoting SGLT1 and 2 expression with subsequent translocation to caveolin-1-enriched lipid rafts (593). Taken together, these studies suggest a positive-feedback loop between glucose and EPAC since high glucose induces EPAC1 expression, which may promote glucose reuptake by activating SGLT1 and 2.
In addition to inducing oxidative stress, ANG II is also reported to activate mesangial cells (MCs) and stimulate the synthesis of extracellular matrix components, including collagens, a hallmark of glomerular diseases (486). Two independent studies using a mouse mesangial cell line, MES-13, demonstrate that ANG II induces collagen synthesis in MCs by activating the ANG II/AT1R/PI3K/Akt pathway in a cAMP/EPAC-dependent, but PKA-independent manner (121, 1157). While one study suggests that EPAC activates PI3K/Akt signaling via the Src kinase and EGFR pathway (1157), the second report suggests a role of TGF-βRI transactivation to mediate this pathway (121).
An in-depth account by Tao et al. (1012) utilizes an adriamycin (ADR) nephrosis mouse model to show that forskolin pretreatment significantly alleviates albuminuria in ADR-treated mice while reversing ADR-induced widening of the foot processes of podocytes. Additionally, forskolin pretreatment in this study induced downregulation in synaptopodin, nephrin, phosphorylated ERM, podocalyxin, and chloride intracellular channel 5 (CLIC5) expression. In vitro podocyte injury assays reveal that puromycin aminonucleoside (PAN) treatment attenuates ERM phosphorylation and inhibits CLIC5 expression. Pharmacological analyses using cAMP analogs selective for EPAC or PKA indicate that although EPAC activation prevents PAN-induced ERM dephosphorylation, selective PKA activation rescues PAN-induced downregulation of CLIC5 (1012). These data suggest both EPAC and PKA may contribute to attenuated albuminuria in ADR-induced nephrosis mice.
VI. POTENTIAL TARGETING STRATEGIES
Given the crucial roles for EPAC proteins in various physiological and pathophysiological functions, great effort has been dedicated to the development of small molecule EPAC modulators as pharmacological probes for dissecting EPAC-mediated cell signaling, as well as further development of potential therapeutics targeting EPAC. Currently reported EPAC modulators can be categorized into two major classes on the basis of their chemical structures: 2'-O-alkyl-based cAMP analogs and non-cyclic nucleotide small molecules. Predictably, a cAMP-like EPAC modulator commonly acts as an EPAC agonist, whereas most of the non-cyclic nucleotide ligands tend to behave as EPAC inhibitors or antagonists (FIGURE 14).
FIGURE 14.
Representative chemical structures of EPAC agonists and antagonists.
A. EPAC Specific Agonists
With the discovery of the EPAC family of cAMP sensors, the need for selective EPAC pharmacological modulators to better understand and discern the independent roles of EPAC- or PKA-mediated cAMP signaling became immediately imperative within the research field. While numerous cyclic-nucleotide analogs had been synthesized and used as biochemical or pharmacological tools (923), none of these conventional analogs exhibited selectivity towards EPAC. Through a comparative sequence analysis, it was revealed that a conserved glutamic acid residue of the CNB domain of PKA and CNG/HCN is absent in the EPAC protein. This glutamic acid forms a hydrogen bond with the 2'-OH group of the cAMP ribose moiety and is required for high-affinity cAMP binding for PKA and CNG/HCN (FIGURE 15) (989, 1181). This discovery guided the identification of a cAMP analog, 8-(4-chloro-phenylthio)-2'-O-methyladenosine-3′,5′-cyclic monophosphate (8-pCPT-2'-O-Me-cAMP, i.e., 007) that exhibits ~100-fold EPAC to PKA selectivity (185, 274, 865). Further modifications and optimizations generated more membrane-permeable and PDE-resistant EPAC-specific agonists (838, 1075). While these 2'-O-alkyl-based cAMP analogs have been widely employed as pharmacological tools to probe EPAC-specific functions (191, 290, 451, 627), extensive studies also document that these EPAC-specific cAMP analogs, as well as their cellular metabolites, display activity towards multiple cellular targets, thereby leading to cross-target activities and off-target effects (275–277, 413, 414, 624, 838, 902). These findings raise significant concerns over the specificity of 8-CPT-cAMP analogs as cAMP mimetics and the applications of 8-CPT-2'-O-Me-cAMP class compounds as EPAC selective activators. Therefore, extreme caution should be taken when applying these cAMP analog-based EPAC selective agonists in dissecting EPAC-mediated signaling events. Results obtained from application of these analogs should be verified using complementary approaches, such as CRISP/Cas9 and RNAi. Greater details about cAMP analog-based EPAC agonists have been previously discussed extensively (168, 428, 1091).
FIGURE 15.
Side-chain interactions important for cAMP binding in PKA (A) and EPAC2 (B). Structures of PKA and EPAC2 are based on PDB files 1RGS and 3CF6, respectively.
Designing cAMP mimetics with isoform selectivity for EPAC1 and 2 has been rather challenging, in accordance with the high sequence and structural homology of the CNB domain between them. A recent structure-based design of selective EPAC1 and EPAC2 agonists has shown promising initial results. While 007 activates both EPAC1 and EPAC2, the activity of the agonist leans towards EPAC1 activation with an AC50 of 1.8 μM and relative maximal activity (rkmax) of 3.3 compared with EPAC2 (AC50 = 3.5 μM; rkmax = 0.8). Structural analyses reveal that a single amino acid determines the differential activity of 007 toward EPAC1 and EPAC2, as well as PKA. In PKA, the 2′-OH group of cAMP forms a hydrogen bond with the side chain of an aforementioned conserved glutamic acid, and the corresponding residue in EPAC1 or EPAC2 is a glutamine or a lysine residue, respectively. Swapping the residues in EPAC1 and 2 leads to a corresponding switch in relative selectivity of 007 (921). On the other hand, a cAMP mimetic with an axial sulfur in the cyclic phosphate along with a benzylthio group in position 8 (Sp-8-Bnt-Me-cAMPS) is more selective for EPAC2 with AC50 of 2.1 μM and rkmax of 6.6 compared with that of EPAC1 (AC50 = 13 μM; rkmax = 0.3). Again, structural analysis reveals that the preference of Sp-8-Bnt-Me-cAMPS is attributable to a single amino acid residue, Lys450 in EPAC2 and corresponding Glu315 in EPAC1 (921). Although this study does provide a structure-based rationale for further designing cAMP mimetics selective for either EPAC1 or EPAC2, developing cyclic nucleotides that are completely isoform selective by targeting the highly homologous CNB remains an intrinsically difficult task.
B. EPAC Specific Antagonists
While the structure-based approach has been quite effective in designing cAMP analogs capable of activating EPAC selectively, an inherent limitation of this approach hinders the usefulness in crafting EPAC antagonists since cyclic nucleotide analogs will likely interfere with PDEs, the enzymes critical for metabolizing and desensitizing cyclic nucleotide signaling. Inhibiting PDEs increases cyclic nucleotide concentrations in cells, countering the intended antagonistic effects. Therefore, major efforts in discovering EPAC inhibitors have been centered on de novo high throughput screening (HTS) of diverse non-cyclic nucleotide small molecule libraries. One effective EPAC HTS assay involves the application of a fluorescence cAMP analog, 8-NBD-cAMP. Binding of 8-NBD-cAMP to full-length EPAC2 induces a dramatic increase in fluorescent intensity, providing a robust readout for the identification of small molecule hits that compete with cAMP binding to EPAC2 (1042). The use of this simple “mix-and-read” HTS assay promoted the discovery of a panel of EPAC specific inhibitors (ESIs) from a 14,400 diverse druglike compound library. These ESIs completely inhibit EPAC2 GEF activities at 25 µM in the presence of the same concentration of cAMP (17, 1043).
Remarkably, two of the ESIs, ESI-05 and ESI-07, exhibit isoform specific activity exclusively for EPAC2 with no measurable activity towards EPAC1. A DXMS approach was used to determine the potential inhibitory mechanism of ESI-07, permitting Tsalkova et al. (1043) to reveal that ESI-07 exerts its inhibitory function by binding to a previously unidentified allosteric site, the interface between the two CNB domains of EPAC2. Binding of ESI-07 locks the EPAC2 protein in its autoinhibitory conformation (1043), which explains why these inhibitors are EPAC2 specific as EPAC1 only has one CNB domain. This allosteric mechanism of action for ESI-05 and ESI-07 function has been validated by subsequent findings demonstrating that ESI-05 does not inhibit EPAC2 deletion mutants lacking the NH2-terminal CNB-A domain (860). In addition, molecular docking analyses suggest EPAC2 specific inhibitors cluster at an allosteric site to interact with key residues from both CNB domains: Lys42 of CNB-A and His335 of CNB-B, respectively (1118). Medicinal chemistry optimization efforts have also introduced additional EPAC2 specific antagonists consisting of three different scaffolds of diarylsulfones, N,N-diarylamines and arylsulfonamides. Several newly synthesized EPAC2-specific antagonists are highly potent with relative binding affinities 100-fold stronger than the natural cAMP ligand (166, 1118). “Clearly, the development of the first antagonists with (subtype) selectivity for EPAC represents another research milestone to ascribe biologic responses to EPAC” (915). Indeed, these selective EPAC2 inhibitors are becoming effective tools in probing isoform-specific EPAC functions (18, 47, 130, 175, 226, 250, 302, 411, 463, 565, 682, 743, 841, 896, 961, 1114).
Not surprisingly, the majority of hit compounds from the HTS perform as pan competitive EPAC antagonists inhibiting cAMP-induced activation of both EPAC1 and EPAC2 (17, 1043). Improved chemical synthesis and structure-activity relationship (SAR) analysis have resulted in the identification of more potent dual EPAC antagonists (165, 167, 1162, 1163, 1210). In particular, structural optimization of hit ESI-09 reveals that the 3-, 4-, and 5-positions of the phenyl scaffold and the 5-position of the isoxazole moiety are highly tunable for improving compound potency. A simple addition of a Cl atom at the 5-position of the phenyl ring leads to a fivefold improvement in potency, and another Cl addition at the 4-position generates the potent compound NY0123 with a relative binding affinity more than 35-fold higher than cAMP (1162, 1210). On the other hand, the isoxazole ring can tolerate chemical modifications with either introduction of flexible electron-donating substitutions or structurally restrictive fusing with a phenyl ring to generate potent dual inhibitors with apparent IC50 values in the low micromolar range (1163). These SAR data are consistent with the molecular modeling results illustrating that while the isoxazole moiety docks into a hydrophobic pocket and interacts with residues Phe367, Leu406, Ala407, and Ala415, the phenyl ring forms hydrophobic interactions with Val386 and Leu397 within the CNB-B of EPAC2 (1162).
The use of a functional fluorescence-based Rap1 exchange assay identified another EPAC specific antagonist, a tetrahydroquinoline known as CE3F4, from a screen of 640 chemically diverse compounds (204). This compound does not compete for binding of cAMP and acts as an uncompetitive inhibitor for EPAC. A SAR analysis further revealed that the substituents on the tetrahydroquinoline pharmacophore are essential for antagonizing EPAC activity, and the (R)-enantiomer (R)-CE3F4 is an even more potent EPAC antagonist than racemic CE3F4 and (S)-CE3F4. Moreover, (R)-CE3F4 exhibits ~10-fold selectivity for EPAC1 over EPAC2 (205). Subsequently, noncompetitive EPAC1 inhibitors (compounds 5225554 and 5376753) have also been identified through a virtual compound screening of a library from the Chembridge database against the apo-EPAC structural model (111, 112). More recently, Parnell et al. (809) applied the 8-NBD-cAMP displacement HTS assay to isolated CNB domains of EPAC1 or EPAC2 and identified additional compounds I178, I288, and I942 that could modestly suppress cAMP-mediated EPAC1 and EPAC2 activation. Interestingly, when tested in the absence of cAMP, I942 was able to weakly activate EPAC1 with apparent AC50 values of 40 μM and a maximal activity around 10% compared with cAMP, suggesting that I942 may act as a partial agonist for EPAC1 (809). Additional pharmacological characterization and medicinal chemistry optimization are necessary to improve the potency and utility of these compounds. The further development of novel medium- to high-throughput assays, especially those cell-based in nature and with the ability to test isoform-specific EPAC functions, will greatly facilitate this process and benefit the development of EPAC-based therapeutics (1211).
The discovery of first-in-class EPAC specific inhibitors not only provides us with the ability to pharmacologically dissect the functional roles of EPAC isoforms, but also offers golden opportunities for developing potential therapeutics specifically targeting diseases where dysregulation of EPAC signaling is implicated (810, 1091). In particular, one family of ESI-09-based analogs has been shown to exert excellent in vivo PK and toxicity profiles and is promising lead compounds for therapeutic applications. For example, mice with genetic knockout of EPAC1 gene are resistant to fatal rickettsioses, inflammation-induced mechanical hyperalgesia, and neointima formation induced by vascular injury. Astonishingly, administration of ESI-09 in vivo completely recapitulates these EPAC1 knockout phenotypes (353, 962, 1088). ESI-09 has also been shown to be effective in suppressing pancreatic cancer metastasis in vivo in an orthotopic metastatic mouse model (13). To date, ESI-09 has been used extensively to study EPAC functions in various systems both in vitro and in vivo, boasting more than 100 related publications in the literature since the initial launch merely a few years ago. Active efforts in preclinical evaluation of ESI-09-based therapeutics, with the ultimate goal of developing IND-enabling leads for the treatment of human diseases, are also currently ongoing.
VII. PERSPECTIVES
Nearly two decades of intensive research since the initial discovery of EPAC family proteins in 1998 has led to major advances in our understanding of the structure and function of this important family of molecular switches, as well as in the development of molecular, cellular, and pharmacological toolkits for probing cAMP signaling in vitro and in vivo. These advances have rejuvenated the cAMP signaling research field by significantly expanding its scope and complexity in terms of function and regulation. Now clear is the expansive, meaningful roles that EPAC proteins partake in almost all tissues under a diverse array of physiological settings, where EPACs work cooperatively with other cAMP effectors to coordinate intracellular cAMP functions. Between the two families of most widely expressed mammalian cAMP sensors, PKA and EPAC often exhibit divergent tissue/cellular distributions and spatiotemporal regulation, which result in complex stress-response readouts where EPAC and PKA can act antagonistically, synergistically, or independently to each other during the modulation of a specific cellular function in response to environmental cues. Also, signal cross-talk between EPAC and PKA appear to be highly dynamic and can undergo robust rewiring when transitioning from acute to chronic stress conditions. Within the EPAC family, the two mammalian EPAC isoforms, EPAC1 and EPAC2, by and large exert diverse and discrete physiological functions by assuming different tissue distributions and partaking in distinct intracellular signalosomes.
In addition to participating in important functions that regulate various physiological processes, EPAC family members also contribute to the pathogenesis of numerous disease states, which offer exciting opportunities for developing new therapeutic interventions. The cAMP signaling cascade is one of the most pharmaceutically targeted pathways. For example, pharmacological inhibition of β-ARs using β-blockers is a treatment option for cardiac arrhythmias and heart failure (311), while opioid-based medications are the most potent pain-management drugs available (978). However, β-blockers are difficult to use in patients with COPD or severe heart failure, and opioid usage is often associated with constipation and respiratory depression, as well as addiction, which is the major cause of the current “opioid crisis” in the United States (371). As discussed extensively in this review, there is sufficient scientific evidence to indicate that β-blockers and opioids may exert their therapeutic efficacy at least in part through EPAC signaling. Therefore, EPAC-based therapeutics represent promising alternatives for the management of cardiovascular diseases and chronic pain. In fact, there are several potential advantages associated with targeting EPAC signaling. Pharmacological modulation of EPAC, downstream of β-blockers and opioids in the adrenergic and opioid receptor pathways, respectively, may overcome the limitations of these therapeutics by providing more specific and improved treatment options. Moreover, EPAC proteins are not essential for normal development and survival as EPAC1, EPAC2, and EPAC1/EPAC2 knockout mice show no overt physiological abnormalities, indicating that the on-target toxicity of inhibiting EPAC will likely be minimal. These observations suggest that EPAC proteins are excellent targets for developing therapeutics for chronic diseases like cardiovascular diseases and chronic pain.
Despite recent significant advances, major gaps in our understanding of EPAC-mediated signaling remain. Dissecting and distinguishing physiological functions of EPAC1 and EPAC2 continue to present an ongoing challenge. The study of tissue-specific and conditional EPAC knockout animal models will help to further define the physiological functions of EPAC isoforms. At the molecular and cellular levels, the dissection of discrete EPAC signalosomes in situ within the context of compartmentalized cAMP signaling will lead to further elucidation of EPAC specific signaling networks. Many studies still lean on the exclusive use of EPAC-selective cAMP analogs and do not validate the functional readouts with genetic approaches. The rapid advancement in the genome editing field using CRISPR-Cas9 approaches should facilitate efforts to overcome this problem. In addition, further development of EPAC-specific pharmacological probes, particularly novel, bioactive isoform-specific small molecule EPAC modulators will provide additional toolkits, complementing genetic approaches, for interrogating the physiological functions of the EPAC isoforms. Currently, very little is known about the regulation of EPAC proteins at the transcriptional, translational, and posttranslational levels. Besides limited studies on EPAC2 liver- and adrenal-specific splicing variants, sparse information about the function and regulation of various EPAC splicing variants, particularly for EPAC1, is available. Importantly, human genetic information and related clinical data for EPAC proteins remain scant, which presents a major barrier for the medical translation of EPAC-based technologies. Nonetheless, if the past is a prologue, the future looks bright: our knowledge in the field should advance at an accelerated rate, which will not only further promote our basic understanding of cAMP/EPAC signaling, but also facilitate the development of new diagnostics and therapeutics specifically targeting this important family of proteins.
GRANTS
This work was supported by National Institute of General Medical Sciences Grants R01GM066170 and R35GM122536 and by National Institute of Allergy and Infectious Diseases Grant R01AI111464.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: X. Cheng, Dept. of Integrative Biology and Pharmacology, The University of Texas Health Science Center, 6431 Fannin St., Houston, TX 77030-1051 (e-mail: xiaodong.cheng@uth.tmc.edu).
REFERENCES
- 1.Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321: 702–705, 2008. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 2.Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature 349: 694–697, 1991. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal SR, MacDougall DA, Tyser R, Pugh SD, Calaghan SC, Harvey RD. Effects of cholesterol depletion on compartmentalized cAMP responses in adult cardiac myocytes. J Mol Cell Cardiol 50: 500–509, 2011. doi: 10.1016/j.yjmcc.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal SR, Yang PC, Rice M, Singer CA, Nikolaev VO, Lohse MJ, Clancy CE, Harvey RD. Role of membrane microdomains in compartmentation of cAMP signaling. PLoS One 9: e95835, 2014. doi: 10.1371/journal.pone.0095835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agircan FG, Schiebel E, Mardin BR. Separate to operate: control of centrosome positioning and separation. Philos Trans R Soc Lond B Biol Sci 369: 20130461, 2014. doi: 10.1098/rstb.2013.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahrén B, Winzell MS, Pacini G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J Endocrinol 197: 181–187, 2008. doi: 10.1677/JOE-07-0460. [DOI] [PubMed] [Google Scholar]
- 7.Ahuja M, Jha A, Maléth J, Park S, Muallem S. cAMP and Ca2+ signaling in secretory epithelia: crosstalk and synergism. Cell Calcium 55: 385–393, 2014. doi: 10.1016/j.ceca.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aivatiadou E, Ripolone M, Brunetti F, Berruti G. cAMP-Epac2-mediated activation of Rap1 in developing male germ cells: RA-RhoGAP as a possible direct down-stream effector. Mol Reprod Dev 76: 407–416, 2009. doi: 10.1002/mrd.20963. [DOI] [PubMed] [Google Scholar]
- 9.Alenkvist I, Gandasi NR, Barg S, Tengholm A. Recruitment of Epac2A to Insulin Granule Docking Sites Regulates Priming for Exocytosis. Diabetes 66: 2610–2622, 2017. doi: 10.2337/db17-0050. [DOI] [PubMed] [Google Scholar]
- 10.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci 19: 2181–2186, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 20: 4680–4685, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J Neurosci Res 74: 342–352, 2003. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- 13.Almahariq M, Chao C, Mei FC, Hellmich MR, Patrikeev I, Motamedi M, Cheng X. Pharmacological inhibition and genetic knockdown of exchange protein directly activated by cAMP 1 reduce pancreatic cancer metastasis in vivo. Mol Pharmacol 87: 142–149, 2015. doi: 10.1124/mol.114.095158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almahariq M, Mei FC, Cheng X. Cyclic AMP sensor EPAC proteins and energy homeostasis. Trends Endocrinol Metab 25: 60–71, 2014. doi: 10.1016/j.tem.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almahariq M, Mei FC, Cheng X. The pleiotropic role of exchange protein directly activated by cAMP 1 (EPAC1) in cancer: implications for therapeutic intervention. Acta Biochim Biophys Sin (Shanghai) 48: 75–81, 2016. doi: 10.1093/abbs/gmv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almahariq M, Mei FC, Wang H, Cao AT, Yao S, Soong L, Sun J, Cong Y, Chen J, Cheng X. Exchange protein directly activated by cAMP modulates regulatory T-cell-mediated immunosuppression. Biochem J 465: 295–303, 2015. doi: 10.1042/BJ20140952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83: 122–128, 2013. doi: 10.1124/mol.112.080689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso B, Bartolomé-Martín D, Ferrero JJ, Ramírez-Franco J, Torres M, Sánchez-Prieto J. CB1 receptors down-regulate a cAMP/Epac2/PLC pathway to silence the nerve terminals of cerebellar granule cells. J Neurochem 142: 350–364, 2017. doi: 10.1111/jnc.14059. [DOI] [PubMed] [Google Scholar]
- 19.Altschuler D, Lapetina EG. Mutational analysis of the cAMP-dependent protein kinase-mediated phosphorylation site of Rap1b. J Biol Chem 268: 7527–7531, 1993. [PubMed] [Google Scholar]
- 20.Altschuler DL, Peterson SN, Ostrowski MC, Lapetina EG. Cyclic AMP-dependent activation of Rap1b. J Biol Chem 270: 10373–10376, 1995. doi: 10.1074/jbc.270.18.10373. [DOI] [PubMed] [Google Scholar]
- 21.Amano R, Lee J, Goto N, Harayama H. Evidence for existence of cAMP-Epac signaling in the heads of mouse epididymal spermatozoa. J Reprod Dev 53: 127–133, 2007. doi: 10.1262/jrd.18077. [DOI] [PubMed] [Google Scholar]
- 22.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med 157: 1907–1912, 1998. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 23.Anciaux K, Van Dommelen K, Nicolai S, Van Mechelen E, Slegers H. Cyclic AMP-mediated induction of the glial fibrillary acidic protein is independent of protein kinase A activation in rat C6 glioma. J Neurosci Res 48: 324–333, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 24.Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to Rab3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13-1 and ubMunc13-2. J Biol Chem 281: 19720–19731, 2006. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- 25.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 26.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52: 252–259, 2003. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 27.Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF III. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272: 32656–32662, 1997. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- 28.Arenzana-Seisdedos F, Thompson J, Rodriguez MS, Bachelerie F, Thomas D, Hay RT. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol Cell Biol 15: 2689–2696, 1995. doi: 10.1128/MCB.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aromataris EC, Roberts ML, Barritt GJ, Rychkov GY. Glucagon activates Ca2+ and Cl- channels in rat hepatocytes. J Physiol 573: 611–625, 2006. doi: 10.1113/jphysiol.2006.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 173: 559–565, 2004. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 31.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174: 595–599, 2005. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 32.Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res 26: 827–833, 2006. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- 33.Arreaza G, Salojin K, Yang W, Zhang J, Gill B, Mi QS, Gao JX, Meagher C, Cameron M, Delovitch TL. Deficient activation and resistance to activation-induced apoptosis of CD8+ T cells is associated with defective peripheral tolerance in nonobese diabetic mice. Clin Immunol 107: 103–115, 2003. doi: 10.1016/S1521-6616(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 34.Artamonov MV, Jin L, Franke AS, Momotani K, Ho R, Dong XR, Majesky MW, Somlyo AV. Signaling pathways that control rho kinase activity maintain the embryonic epicardial progenitor state. J Biol Chem 290: 10353–10367, 2015. doi: 10.1074/jbc.M114.613190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol 167: 111–122, 2004. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aumo L, Rusten M, Mellgren G, Bakke M, Lewis AE. Functional roles of protein kinase A (PKA) and exchange protein directly activated by 3′,5′-cyclic adenosine 5′-monophosphate (cAMP) 2 (EPAC2) in cAMP-mediated actions in adrenocortical cells. Endocrinology 151: 2151–2161, 2010. doi: 10.1210/en.2009-1139. [DOI] [PubMed] [Google Scholar]
- 37.Avni D, Philosoph A, Meijler MM, Zor T. The ceramide-1-phosphate analogue PCERA-1 modulates tumour necrosis factor-alpha and interleukin-10 production in macrophages via the cAMP-PKA-CREB pathway in a GTP-dependent manner. Immunology 129: 375–385, 2010. doi: 10.1111/j.1365-2567.2009.03188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baameur F, Singhmar P, Zhou Y, Hancock JF, Cheng X, Heijnen CJ, Kavelaars A. Epac1 interacts with importin β1 and controls neurite outgrowth independently of cAMP and Rap1. Sci Rep 6: 36370, 2016. doi: 10.1038/srep36370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bacallao K, Monje PV. Opposing roles of PKA and EPAC in the cAMP-dependent regulation of schwann cell proliferation and differentiation [corrected] PLoS One 8: e82354, 2013. doi: 10.1371/journal.pone.0082354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bacchelli E, Blasi F, Biondolillo M, Lamb JA, Bonora E, Barnby G, Parr J, Beyer KS, Klauck SM, Poustka A, Bailey AJ, Monaco AP, Maestrini E; International Molecular Genetic Study of Autism Consortium (IMGSAC) . Screening of nine candidate genes for autism on chromosome 2q reveals rare nonsynonymous variants in the cAMP-GEFII gene. Mol Psychiatry 8: 916–924, 2003. doi: 10.1038/sj.mp.4001340. [DOI] [PubMed] [Google Scholar]
- 41.Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 53: 211–217, 1988. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 42.Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res 69: 4962–4968, 2009. doi: 10.1158/0008-5472.CAN-08-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci USA 96: 11241–11246, 1999. doi: 10.1073/pnas.96.20.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balasubramanian L, Sham JS, Yip KP. Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflugers Arch 456: 747–754, 2008. doi: 10.1007/s00424-007-0371-7. [DOI] [PubMed] [Google Scholar]
- 45.Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS, Nowycky MC, Iwatsubo K. Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res 70: 5607–5617, 2010. doi: 10.1158/0008-5472.CAN-10-0056. [DOI] [PubMed] [Google Scholar]
- 46.Baljinnyam E, Iwatsubo K, Kurotani R, Wang X, Ulucan C, Iwatsubo M, Lagunoff D, Ishikawa Y. Epac increases melanoma cell migration by a heparan sulfate-related mechanism. Am J Physiol Cell Physiol 297: C802–C813, 2009. doi: 10.1152/ajpcell.00129.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baljinnyam E, Umemura M, Chuang C, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Whitelock JM, Iwatsubo K. Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling. Pigment Cell Melanoma Res 27: 611–620, 2014. doi: 10.1111/pcmr.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baljinnyam E, Umemura M, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Iwatsubo K. Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment Cell Melanoma Res 24: 680–687, 2011. doi: 10.1111/j.1755-148X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- 50.Baljinnyam E, Umemura M, De Lorenzo MS, Xie LH, Nowycky M, Iwatsubo M, Chen S, Goydos JS, Iwatsubo K. Gβγ subunits inhibit Epac-induced melanoma cell migration. BMC Cancer 11: 256, 2011. doi: 10.1186/1471-2407-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ballanyi K, Mückenhoff K, Bellingham MC, Okada Y, Scheid P, Richter DW. Activity-related pH changes in respiratory neurones and glial cells of cats. Neuroreport 6: 33–36, 1994. doi: 10.1097/00001756-199412300-00010. [DOI] [PubMed] [Google Scholar]
- 52.Ballinger ML, Blanchette AR, Krause TL, Smyers ME, Fishman HM, Bittner GD. Delaminating myelin membranes help seal the cut ends of severed earthworm giant axons. J Neurobiol 33: 945–960, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 53.Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276: 20144–20153, 2001. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 54.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD). Hepatology 49: 160–174, 2009. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banerjee U, Cheng X. Exchange protein directly activated by cAMP encoded by the mammalian rapgef3 gene: structure, function and therapeutics. Gene 570: 157–167, 2015. doi: 10.1016/j.gene.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, Wang G, Knepper MA. Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol 21: 303–315, 2010. doi: 10.1681/ASN.2009070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA 95: 15020–15025, 1998. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barak LS, Salahpour A, Zhang X, Masri B, Sotnikova TD, Ramsey AJ, Violin JD, Lefkowitz RJ, Caron MG, Gainetdinov RR. Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol Pharmacol 74: 585–594, 2008. doi: 10.1124/mol.108.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrie A, Khare A, Henkel M, Zhang Y, Barmada MM, Duerr R, Ray A. Prostaglandin E2 and IL-23 plus IL-1β differentially regulate the Th1/Th17 immune response of human CD161(+) CD4(+) memory T cells. Clin Transl Sci 4: 268–273, 2011. doi: 10.1111/j.1752-8062.2011.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basoni C, Nobles M, Grimshaw A, Desgranges C, Davies D, Perretti M, Kramer IM, Genot E. Inhibitory control of TGF-beta1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J 19: 822–824, 2005. doi: 10.1096/fj.04-3085fje. [DOI] [PubMed] [Google Scholar]
- 62.Bastian P, Balcarek A, Altanis C, Strell C, Niggemann B, Zaenker KS, Entschladen F. The inhibitory effect of norepinephrine on the migration of ES-2 ovarian carcinoma cells involves a Rap1-dependent pathway. Cancer Lett 274: 218–224, 2009. doi: 10.1016/j.canlet.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA 93: 8374–8378, 1996. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumer Y, Spindler V, Werthmann RC, Bünemann M, Waschke J. Role of Rac 1 and cAMP in endothelial barrier stabilization and thrombin-induced barrier breakdown. J Cell Physiol 220: 716–726, 2009. doi: 10.1002/jcp.21819. [DOI] [PubMed] [Google Scholar]
- 65.Baviera AM, Zanon NM, Carvalho Navegantes LC, Migliorini RH, Kettelhut IC. Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. Am J Physiol Endocrinol Metab 292: E702–E708, 2007. doi: 10.1152/ajpendo.00147.2006. [DOI] [PubMed] [Google Scholar]
- 66.Baviera AM, Zanon NM, Navegantes LC, Kettelhut IC. Involvement of cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Mol Cell Endocrinol 315: 104–112, 2010. doi: 10.1016/j.mce.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Baviera AM, Zanon NM, Navegantes LC, Migliorini RH, Kettelhut IC. Chemical sympathectomy further increases muscle protein degradation of acutely diabetic rats. Muscle Nerve 38: 1027–1035, 2008. doi: 10.1002/mus.21018. [DOI] [PubMed] [Google Scholar]
- 68.Bax NA, van Oorschot AA, Maas S, Braun J, van Tuyn J, de Vries AA, Groot AC, Goumans MJ. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res Cardiol 106: 829–847, 2011. doi: 10.1007/s00395-011-0181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z. Differential modulation of adenylyl cyclases I and II by various G beta subunits. J Biol Chem 273: 2273–2276, 1998. doi: 10.1074/jbc.273.4.2273. [DOI] [PubMed] [Google Scholar]
- 70.Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3: 133–141, 2000. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 71.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9: 636–641, 2006. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 72.Berman HM, Ten Eyck LF, Goodsell DS, Haste NM, Kornev A, Taylor SS. The cAMP binding domain: an ancient signaling module. Proc Natl Acad Sci USA 102: 45–50, 2005. doi: 10.1073/pnas.0408579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA 94: 7041–7046, 1997. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berruti G. CAMP activates Rap1 in differentiating mouse male germ cells: a new signaling pathway mediated by the cAMP-activated exchange factor Epac? Cell Mol Biol (Noisy-le-grand) 49: 381–388, 2003. [PubMed] [Google Scholar]
- 75.Berthouze-Duquesnes M, Lucas A, Saulière A, Sin YY, Laurent AC, Galés C, Baillie G, Lezoualc’h F. Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell Signal 25: 970–980, 2013. doi: 10.1016/j.cellsig.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Berthouze-Duquesnes M, Lucas A, Saulière A, Sin YY, Laurent AC, Galés C, Baillie G, Lezoualc’h F. Specific interactions between Epac1, β-arrestin2 and PDE4D5 regulate β-adrenergic receptor subtype differential effects on cardiac hypertrophic signaling. Cell Signal 25: 970–980, 2013. doi: 10.1016/j.cellsig.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 8: 451–463, 2007. doi: 10.1038/nrm2180. [DOI] [PubMed] [Google Scholar]
- 78.Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron 30: 183–196, 2001. doi: 10.1016/S0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharya S, Imbery JF, Ampem PT, Giovannucci DR. Crosstalk between purinergic receptors and canonical signaling pathways in the mouse salivary gland. Cell Calcium 58: 589–597, 2015. doi: 10.1016/j.ceca.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bingham TC, Fisher EA, Parathath S, Reiss AB, Chan ES, Cronstein BN. A2A adenosine receptor stimulation decreases foam cell formation by enhancing ABCA1-dependent cholesterol efflux. J Leukoc Biol 87: 683–690, 2010. doi: 10.1189/jlb.0709513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Birukova AA, Meng F, Tian Y, Meliton A, Sarich N, Quilliam LA, Birukov KG. Prostacyclin post-treatment improves LPS-induced acute lung injury and endothelial barrier recovery via Rap1. Biochim Biophys Acta 1852: 778–791, 2015. doi: 10.1016/j.bbadis.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bisserier M, Blondeau JP, Lezoualc’h F. Epac proteins: specific ligands and role in cardiac remodelling. Biochem Soc Trans 42: 257–264, 2014. doi: 10.1042/BST20140033. [DOI] [PubMed] [Google Scholar]
- 85.Blirando K, Blaise R, Gorodnaya N, Rouxel C, Meilhac O, Vincent P, Limon I. The stellate vascular smooth muscle cell phenotype is induced by IL-1β via the secretion of PGE2 and subsequent cAMP-dependent protein kinase A activation. Biochim Biophys Acta 1853: 3235–3247, 2015. doi: 10.1016/j.bbamcr.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 86.Bloemen PG, van den Tweel MC, Henricks PA, Engels F, Kester MH, van de Loo PG, Blomjous FJ, Nijkamp FP. Increased cAMP levels in stimulated neutrophils inhibit their adhesion to human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 272: L580–L587, 1997. [DOI] [PubMed] [Google Scholar]
- 87.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243: 240–246, 1997. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 88.Blount MA, Cipriani P, Redd SK, Ordas RJ, Black LN, Gumina DL, Hoban CA, Klein JD, Sands JM. Activation of protein kinase Cα increases phosphorylation of the UT-A1 urea transporter at serine 494 in the inner medullary collecting duct. Am J Physiol Cell Physiol 309: C608–C615, 2015. doi: 10.1152/ajpcell.00171.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008. doi: 10.1152/ajprenal.00102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blume-Jensen P, Janknecht R, Hunter T. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr Biol 8: 779–785, 1998. doi: 10.1016/S0960-9822(98)70302-1. [DOI] [PubMed] [Google Scholar]
- 91.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 92.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci USA 97: 9064–9069, 2000. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borland G, Bird RJ, Palmer TM, Yarwood SJ. Activation of protein kinase Calpha by EPAC1 is required for the ERK- and CCAAT/enhancer-binding protein beta-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J Biol Chem 284: 17391–17403, 2009. doi: 10.1074/jbc.M109.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borland G, Gupta M, Magiera MM, Rundell CJ, Fuld S, Yarwood SJ. Microtubule-associated protein 1B-light chain 1 enhances activation of Rap1 by exchange protein activated by cyclic AMP but not intracellular targeting. Mol Pharmacol 69: 374–384, 2006. doi: 10.1124/mol.105.016337. [DOI] [PubMed] [Google Scholar]
- 95.Börner S, Schwede F, Schlipp A, Berisha F, Calebiro D, Lohse MJ, Nikolaev VO. FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat Protoc 6: 427–438, 2011. doi: 10.1038/nprot.2010.198. [DOI] [PubMed] [Google Scholar]
- 96.Bornfeldt KE, Campbell JS, Koyama H, Argast GM, Leslie CC, Raines EW, Krebs EG, Ross R. The mitogen-activated protein kinase pathway can mediate growth inhibition and proliferation in smooth muscle cells. Dependence on the availability of downstream targets. J Clin Invest 100: 875–885, 1997. doi: 10.1172/JCI119603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F, Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans 31: 83–86, 2003. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- 98.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 12: 477–483, 2010. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem 5: 365–374, 1998. [PMC free article] [PubMed] [Google Scholar]
- 100.Boute N, Jockers R, Issad T. The use of resonance energy transfer in high-throughput screening: BRET versus FRET. Trends Pharmacol Sci 23: 351–354, 2002. doi: 10.1016/S0165-6147(02)02062-X. [DOI] [PubMed] [Google Scholar]
- 101.Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol 7: 397–403, 1997. doi: 10.1016/S0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- 102.Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VE, Treviño CL, Darszon A, Mayorga LS, Tomes CN. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J Biol Chem 284: 24825–24839, 2009. doi: 10.1074/jbc.M109.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Branham MT, Mayorga LS, Tomes CN. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J Biol Chem 281: 8656–8666, 2006. doi: 10.1074/jbc.M508854200. [DOI] [PubMed] [Google Scholar]
- 104.Bregman DB, Hirsch AH, Rubin CS. Molecular characterization of bovine brain P75, a high affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II beta. J Biol Chem 266: 7207–7213, 1991. [PubMed] [Google Scholar]
- 105.Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal 17: 1551–1559, 2005. doi: 10.1016/j.cellsig.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 106.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690, 2001. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 107.Brock M, Fan F, Mei FC, Li S, Gessner C, Woods VL Jr, Cheng X. Conformational analysis of Epac activation using amide hydrogen/deuterium exchange mass spectrometry. J Biol Chem 282: 32256–32263, 2007. doi: 10.1074/jbc.M706231200. [DOI] [PubMed] [Google Scholar]
- 108.Brock TG, Serezani CH, Carstens JK, Peters-Golden M, Aronoff DM. Effects of prostaglandin E2 on the subcellular localization of Epac-1 and Rap1 proteins during Fcgamma-receptor-mediated phagocytosis in alveolar macrophages. Exp Cell Res 314: 255–263, 2008. doi: 10.1016/j.yexcr.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Broecker-Preuss M, Baten J, Sheu-Grabellus SY, Görges R, Bockisch A, Schmid KW, Führer D, Mann K. Expression of the cAMP binding protein EPAC1 in thyroid tumors and growth regulation of thyroid cells and thyroid carcinoma cells by EPAC proteins. Horm Metab Res 47: 200–208, 2015. doi: 10.1055/s-0034-1390484. [DOI] [PubMed] [Google Scholar]
- 110.Broere N, Chen M, Cinar A, Singh AK, Hillesheim J, Riederer B, Lünnemann M, Rottinghaus I, Krabbenhöft A, Engelhardt R, Rausch B, Weinman EJ, Donowitz M, Hubbard A, Kocher O, de Jonge HR, Hogema BM, Seidler U. Defective jejunal and colonic salt absorption and alteredNa(+)/H (+) exchanger 3 (NHE3) activity in NHE regulatory factor 1 (NHERF1) adaptor protein-deficient mice. Pflugers Arch 457: 1079–1091, 2009. doi: 10.1007/s00424-008-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown LM, Rogers KE, Aroonsakool N, McCammon JA, Insel PA. Allosteric inhibition of Epac: computational modeling and experimental validation to identify allosteric sites and inhibitors. J Biol Chem 289: 29148–29157, 2014. doi: 10.1074/jbc.M114.569319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown LM, Rogers KE, McCammon JA, Insel PA. Identification and validation of modulators of exchange protein activated by cAMP (Epac) activity: structure-function implications for Epac activation and inhibition. J Biol Chem 289: 8217–8230, 2014. doi: 10.1074/jbc.M114.548636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem 277: 1340–1348, 2002. doi: 10.1074/jbc.M106609200. [DOI] [PubMed] [Google Scholar]
- 114.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science 194: 1178–1181, 1976. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 115.Brunton LL, Hayes JS, Mayer SE. Functional compartmentation of cyclic AMP and protein kinase in heart. Adv Cyclic Nucleotide Res 14: 391–397, 1981. [PubMed] [Google Scholar]
- 116.Brusco LI, Márquez M, Cardinali DP. Monozygotic twins with Alzheimer’s disease treated with melatonin: case report. J Pineal Res 25: 260–263, 1998. doi: 10.1111/j.1600-079X.1998.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 117.Bruzzone A, Saulière A, Finana F, Sénard JM, Lüthy I, Galés C. Dosage-dependent regulation of cell proliferation and adhesion through dual β2-adrenergic receptor/cAMP signals. FASEB J 28: 1342–1354, 2014. doi: 10.1096/fj.13-239285. [DOI] [PubMed] [Google Scholar]
- 118.Bryce PJ, Dascombe MJ, Hutchinson IV. Immunomodulatory effects of pharmacological elevation of cyclic AMP in T lymphocytes proceed via a protein kinase A independent mechanism. Immunopharmacology 41: 139–146, 1999. doi: 10.1016/S0162-3109(98)00060-5. [DOI] [PubMed] [Google Scholar]
- 120.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Taskén K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol 176: 7361–7370, 2006. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 121.Bu L, Qu S, Gao X, Zou JJ, Tang W, Sun LL, Liu ZM. Enhanced angiotensin-converting enzyme 2 attenuates angiotensin II-induced collagen production via AT1 receptor-phosphoinositide 3-kinase-Akt pathway. Endocrine 39: 139–147, 2011. doi: 10.1007/s12020-010-9435-0. [DOI] [PubMed] [Google Scholar]
- 122.Buchheiser A, Ebner A, Burghoff S, Ding Z, Romio M, Viethen C, Lindecke A, Köhrer K, Fischer JW, Schrader J. Inactivation of CD73 promotes atherogenesis in apolipoprotein E-deficient mice. Cardiovasc Res 92: 338–347, 2011. doi: 10.1093/cvr/cvr218. [DOI] [PubMed] [Google Scholar]
- 123.Burdyga A, Conant A, Haynes L, Zhang J, Jalink K, Sutton R, Neoptolemos J, Costello E, Tepikin A. cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: effects of PKA and EPAC. Biochim Biophys Acta 1833: 2664–2672, 2013. doi: 10.1016/j.bbamcr.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burger MM, Bombik BM, Breckenridge BM, Sheppard JR. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol 239: 161–163, 1972. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- 125.Burke DS. Forecasting the Opioid Epidemic. New York: Science, 2016, p. 529. [DOI] [PubMed] [Google Scholar]
- 126.Buxbaum JD, Silverman JM, Smith CJ, Kilifarski M, Reichert J, Hollander E, Lawlor BA, Fitzgerald M, Greenberg DA, Davis KL. Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. Am J Hum Genet 68: 1514–1520, 2001. doi: 10.1086/320588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 258: 10233–10239, 1983. [PubMed] [Google Scholar]
- 128.Caceres PS, Ares GR, Ortiz PA. cAMP stimulates apical exocytosis of the renal Na(+)-K(+)-2Cl(-) cotransporter NKCC2 in the thick ascending limb: role of protein kinase A. J Biol Chem 284: 24965–24971, 2009. doi: 10.1074/jbc.M109.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22: 89–101, 1999. doi: 10.1016/S0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 130.Calderón-Sánchez E, Díaz I, Ordóñez A, Smani T. Urocortin-1 Mediated Cardioprotection Involves XIAP and CD40-Ligand Recovery: Role of EPAC2 and ERK1/2. PLoS One 11: e0147375, 2016. doi: 10.1371/journal.pone.0147375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 7: e1000172, 2009. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269: 12190–12195, 1994. [PubMed] [Google Scholar]
- 133.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest 107: 277–286, 2001. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie 92: 204–215, 2010. doi: 10.1016/j.biochi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 135.Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol 80: 480–490, 2010. doi: 10.1016/j.bcp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 136.Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S. The stimulation of adenosine 2A receptor reduces inflammatory response in mouse articular chondrocytes treated with hyaluronan oligosaccharides. Matrix Biol 31: 338–351, 2012. doi: 10.1016/j.matbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Nastasi G, Calatroni A, Campo S. Inhibition of the hyaluronan oligosaccharides inflammatory response: reduction of adenosine 2A receptor activation by EPAC and PKA. Cell Biochem Funct 32: 692–701, 2014. doi: 10.1002/cbf.3073. [DOI] [PubMed] [Google Scholar]
- 138.Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, Calatroni A, Campo S. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem 113: 1852–1867, 2012. doi: 10.1002/jcb.24054. [DOI] [PubMed] [Google Scholar]
- 139.Campo GM, Avenoso A, Nastasi G, Micali A, Prestipino V, Vaccaro M, D’Ascola A, Calatroni A, Campo S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta 1812: 1170–1181, 2011. doi: 10.1016/j.bbadis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 140.Canalli AA, Franco-Penteado CF, Traina F, Saad ST, Costa FF, Conran N. Role for cAMP-protein kinase A signalling in augmented neutrophil adhesion and chemotaxis in sickle cell disease. Eur J Haematol 79: 330–337, 2007. doi: 10.1111/j.1600-0609.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 141.Canetti C, Serezani CH, Atrasz RG, White ES, Aronoff DM, Peters-Golden M. Activation of phosphatase and tensin homolog on chromosome 10 mediates the inhibition of FcgammaR phagocytosis by prostaglandin E2 in alveolar macrophages. J Immunol 179: 8350–8356, 2007. doi: 10.4049/jimmunol.179.12.8350. [DOI] [PubMed] [Google Scholar]
- 142.Cao S, Bian Z, Zhu X, Shen SR. Effect of Epac1 on pERK and VEGF Activation in Postoperative Persistent Pain in Rats. J Mol Neurosci 59: 554–564, 2016. doi: 10.1007/s12031-016-0776-x. [DOI] [PubMed] [Google Scholar]
- 143.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature 401: 286–290, 1999. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 144.Cao X, Wei G, Fang H, Guo J, Weinstein M, Marsh CB, Ostrowski MC, Tridandapani S. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol 172: 4851–4857, 2004. doi: 10.4049/jimmunol.172.8.4851. [DOI] [PubMed] [Google Scholar]
- 145.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 111: 2640–2646, 2008. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 146.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282: 1717–1721, 1998. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 147.Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol 10: 974–978, 2000. doi: 10.1016/S0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 148.Carpio L, Gladu J, Goltzman D, Rabbani SA. Induction of osteoblast differentiation indexes by PTHrP in MG-63 cells involves multiple signaling pathways. Am J Physiol Endocrinol Metab 281: E489–E499, 2001. doi: 10.1152/ajpendo.2001.281.3.E489. [DOI] [PubMed] [Google Scholar]
- 149.Carraro-Lacroix LR, Malnic G, Girardi AC. Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297: F1647–F1655, 2009. doi: 10.1152/ajprenal.00082.2009. [DOI] [PubMed] [Google Scholar]
- 150.Cass LA, Summers SA, Prendergast GV, Backer JM, Birnbaum MJ, Meinkoth JL. Protein kinase A-dependent and -independent signaling pathways contribute to cyclic AMP-stimulated proliferation. Mol Cell Biol 19: 5882–5891, 1999. doi: 10.1128/MCB.19.9.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cassel D, Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci USA 75: 4155–4159, 1978. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castillo PE, Schoch S, Schmitz F, Südhof TC, Malenka RC. RIM1alpha is required for presynaptic long-term potentiation. Nature 415: 327–330, 2002. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 153.Cazillis M, Gonzalez BJ, Billardon C, Lombet A, Fraichard A, Samarut J, Gressens P, Vaudry H, Rostène W. VIP and PACAP induce selective neuronal differentiation of mouse embryonic stem cells. Eur J Neurosci 19: 798–808, 2004. doi: 10.1111/j.0953-816X.2004.03138.x. [DOI] [PubMed] [Google Scholar]
- 154.Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc’h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci USA 106: 14144–14149, 2009. doi: 10.1073/pnas.0812536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ceballos-Picot I, Mockel L, Potier MC, Dauphinot L, Shirley TL, Torero-Ibad R, Fuchs J, Jinnah HA. Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: implications for Lesch-Nyhan disease pathogenesis. Hum Mol Genet 18: 2317–2327, 2009. doi: 10.1093/hmg/ddp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cerutis DR, Nogami M, Anderson JL, Churchill JD, Romberger DJ, Rennard SI, Toews ML. Lysophosphatidic acid and EGF stimulate mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 273: L10–L15, 1997. [DOI] [PubMed] [Google Scholar]
- 157.Chang CW, Chang GD, Chen H. A novel cyclic AMP/Epac1/CaMKI signaling cascade promotes GCM1 desumoylation and placental cell fusion. Mol Cell Biol 31: 3820–3831, 2011. doi: 10.1128/MCB.05582-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chang CW, Cheong ML, Chang GD, Tsai MS, Chen H. Involvement of Epac1/Rap1/CaMKI/HDAC5 signaling cascade in the regulation of placental cell fusion. Mol Hum Reprod 19: 745–755, 2013. doi: 10.1093/molehr/gat050. [DOI] [PubMed] [Google Scholar]
- 159.Chase HP, Williams RL, Dupont J. Increased prostaglandin synthesis in childhood diabetes mellitus. J Pediatr 94: 185–189, 1979. doi: 10.1016/S0022-3476(79)80819-7. [DOI] [PubMed] [Google Scholar]
- 160.Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 292: G1403–G1410, 2007. doi: 10.1152/ajpgi.00478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chaudhuri A, Kolodecik TR, Gorelick FS. Effects of increased intracellular cAMP on carbachol-stimulated zymogen activation, secretion, and injury in the pancreatic acinar cell. Am J Physiol Gastrointest Liver Physiol 288: G235–G243, 2005. doi: 10.1152/ajpgi.00334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chavez-Noriega LE, Stevens CF. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J Neurosci 14: 310–317, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen-Goodspeed M, Lukan AN, Dessauer CW. Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem 280: 1808–1816, 2005. doi: 10.1074/jbc.M409172200. [DOI] [PubMed] [Google Scholar]
- 164.Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D’Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J Biol Chem 279: 29121–29129, 2004. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- 165.Chen H, Ding C, Wild C, Liu H, Wang T, White MA, Cheng X, Zhou J. Efficient Synthesis of ESI-09, A Novel Non-cyclic Nucleotide EPAC Antagonist. Tetrahedron Lett 54: 1546–1549, 2013. doi: 10.1016/j.tetlet.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem 56: 952–962, 2013. doi: 10.1021/jm3014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen H, Tsalkova T, Mei FC, Hu Y, Cheng X, Zhou J. 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg Med Chem Lett 22: 4038–4043, 2012. doi: 10.1016/j.bmcl.2012.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen H, Wild C, Zhou X, Ye N, Cheng X, Zhou J. Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC). J Med Chem 57: 3651–3665, 2014. doi: 10.1021/jm401425e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen L, Wang P, Andrade CF, Zhao IY, Dubé PE, Brubaker PL, Liu M, Jin T. PKA independent and cell type specific activation of the expression of caudal homeobox gene Cdx-2 by cyclic AMP. FEBS J 272: 2746–2759, 2005. doi: 10.1111/j.1742-4658.2005.04694.x. [DOI] [PubMed] [Google Scholar]
- 170.Chen MC, Lin H, Hsu FN, Huang PH, Lee GS, Wang PS. Involvement of cAMP in nerve growth factor-triggered p35/Cdk5 activation and differentiation in PC12 cells. Am J Physiol Cell Physiol 299: C516–C527, 2010. doi: 10.1152/ajpcell.00534.2009. [DOI] [PubMed] [Google Scholar]
- 171.Chen X, Wu B, Kendler KS. Association study of the Epac gene and tobacco smoking and nicotine dependence. Am J Med Genet B Neuropsychiatr Genet 129B: 116–119, 2004. doi: 10.1002/ajmg.b.30040. [DOI] [PubMed] [Google Scholar]
- 172.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 173.Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106, 2001. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- 174.Cheng Q, Cant CA, Moll T, Hofer-Warbinek R, Wagner E, Birnstiel ML, Bach FH, de Martin R. NK-kappa B subunit-specific regulation of the I kappa B alpha promoter. J Biol Chem 269: 13551–13557, 1994. [PubMed] [Google Scholar]
- 175.Chepurny OG, Bertinetti D, Diskar M, Leech CA, Afshari P, Tsalkova T, Cheng X, Schwede F, Genieser HG, Herberg FW, Holz GG. Stimulation of proglucagon gene expression by human GPR119 in enteroendocrine L-cell line GLUTag. Mol Endocrinol 27: 1267–1282, 2013. doi: 10.1210/me.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab 298: E622–E633, 2010. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem 284: 10728–10736, 2009. doi: 10.1074/jbc.M900166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cheung U, Atwood HL, Zucker RS. Presynaptic effectors contributing to cAMP-induced synaptic potentiation in Drosophila. J Neurobiol 66: 273–280, 2006. doi: 10.1002/neu.20218. [DOI] [PubMed] [Google Scholar]
- 179.Chin EC, Abayasekara DR. Progesterone secretion by luteinizing human granulosa cells: a possible cAMP-dependent but PKA-independent mechanism involved in its regulation. J Endocrinol 183: 51–60, 2004. doi: 10.1677/joe.1.05550. [DOI] [PubMed] [Google Scholar]
- 180.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood 112: 3696–3703, 2008. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Choi EJ, Xia Z, Storm DR. Stimulation of the type III olfactory adenylyl cyclase by calcium and calmodulin. Biochemistry 31: 6492–6498, 1992. doi: 10.1021/bi00143a019. [DOI] [PubMed] [Google Scholar]
- 182.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 183.Chotani MA, Mitra S, Eid AH, Han SA, Flavahan NA. Distinct cAMP signaling pathways differentially regulate alpha2C-adrenoceptor expression: role in serum induction in human arteriolar smooth muscle cells. Am J Physiol Heart Circ Physiol 288: H69–H76, 2005. doi: 10.1152/ajpheart.01223.2003. [DOI] [PubMed] [Google Scholar]
- 184.Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. doi: 10.1074/jbc.M005552200. [DOI] [PubMed] [Google Scholar]
- 185.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278: 35394–35402, 2003. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 187.Christian SL, Lee RL, McLeod SJ, Burgess AE, Li AH, Dang-Lawson M, Lin KB, Gold MR. Activation of the Rap GTPases in B lymphocytes modulates B cell antigen receptor-induced activation of Akt but has no effect on MAPK activation. J Biol Chem 278: 41756–41767, 2003. doi: 10.1074/jbc.M303180200. [DOI] [PubMed] [Google Scholar]
- 188.Christiansen SC, Proud D, Sarnoff RB, Juergens U, Cochrane CG, Zuraw BL. Elevation of tissue kallikrein and kinin in the airways of asthmatic subjects after endobronchial allergen challenge. Am Rev Respir Dis 145: 900–905, 1992. doi: 10.1164/ajrccm/145.4_Pt_1.900. [DOI] [PubMed] [Google Scholar]
- 189.Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC II, Vansluys J. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood 111: 2647–2656, 2008. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002. doi: 10.1083/jcb.200204022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clemente MI, Álvarez S, Serramía MJ, Martínez-Bonet M, Muñoz-Fernández MÁ. Prostaglandin E2 reduces the release and infectivity of new cell-free virions and cell-to-cell HIV-1 transfer. PLoS One 9: e85230, 2014. doi: 10.1371/journal.pone.0085230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cochet M, Donneger R, Cassier E, Gaven F, Lichtenthaler SF, Marin P, Bockaert J, Dumuis A, Claeysen S. 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci 4: 130–140, 2013. doi: 10.1021/cn300095t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Coelho M, Soares-Silva C, Brandão D, Marino F, Cosentino M, Ribeiro L. β-Adrenergic modulation of cancer cell proliferation: available evidence and clinical perspectives. J Cancer Res Clin Oncol 143: 275–291, 2017. doi: 10.1007/s00432-016-2278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cohen P. The origins of protein phosphorylation. Nat Cell Biol 4: E127–E130, 2002. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 195.Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol 9: 216–221, 1999. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 196.Collier HO, Schneider C. Nociceptive response to prostaglandins and analgesic actions of aspirin and morphine. Nat New Biol 236: 141–143, 1972. doi: 10.1038/newbio236141a0. [DOI] [PubMed] [Google Scholar]
- 197.Conrotto P, Yakymovych I, Yakymovych M, Souchelnytskyi S. Interactome of transforming growth factor-beta type I receptor (TbetaRI): inhibition of TGFbeta signaling by Epac1. J Proteome Res 6: 287–297, 2007. doi: 10.1021/pr060427q. [DOI] [PubMed] [Google Scholar]
- 198.Consonni SV, Gloerich M, Spanjaard E, Bos JL. cAMP regulates DEP domain-mediated binding of the guanine nucleotide exchange factor Epac1 to phosphatidic acid at the plasma membrane. Proc Natl Acad Sci USA 109: 3814–3819, 2012. doi: 10.1073/pnas.1117599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science 262: 1069–1072, 1993. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 200.Coon SD, Rajendran VM, Schwartz JH, Singh SK. Glucose-dependent insulinotropic polypeptide-mediated signaling pathways enhance apical PepT1 expression in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 308: G56–G62, 2015. doi: 10.1152/ajpgi.00168.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Coon SD, Schwartz JH, Rajendran VM, Jepeal L, Singh SK. Glucose-dependent insulinotropic polypeptide regulates dipeptide absorption in mouse jejunum. Am J Physiol Gastrointest Liver Physiol 305: G678–G684, 2013. doi: 10.1152/ajpgi.00098.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cooper DM, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374: 421–424, 1995. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 203.Copple BL, Li T. Pharmacology of bile acid receptors: evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol Res 104: 9–21, 2016. doi: 10.1016/j.phrs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Courilleau D, Bisserier M, Jullian JC, Lucas A, Bouyssou P, Fischmeister R, Blondeau JP, Lezoualc’h F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J Biol Chem 287: 44192–44202, 2012. doi: 10.1074/jbc.M112.422956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Courilleau D, Bouyssou P, Fischmeister R, Lezoualc’h F, Blondeau JP. The (R)-enantiomer of CE3F4 is a preferential inhibitor of human exchange protein directly activated by cyclic AMP isoform 1 (Epac1). Biochem Biophys Res Commun 440: 443–448, 2013. doi: 10.1016/j.bbrc.2013.09.107. [DOI] [PubMed] [Google Scholar]
- 206.Cristillo AD, Highbarger HC, Dewar RL, Dimitrov DS, Golding H, Bierer BE. Up-regulation of HIV coreceptor CXCR4 expression in human T lymphocytes is mediated in part by a cAMP-responsive element. FASEB J 16: 354–364, 2002. doi: 10.1096/fj.01-0744com. [DOI] [PubMed] [Google Scholar]
- 207.Crocenzi FA, Mottino AD, Cao J, Veggi LM, Pozzi EJ, Vore M, Coleman R, Roma MG. Estradiol-17beta-d-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol 285: G449–G459, 2003. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- 208.Cuíñas A, García-Morales V, Viña D, Gil-Longo J, Campos-Toimil M. Activation of PKA and Epac proteins by cyclic AMP depletes intracellular calcium stores and reduces calcium availability for vasoconstriction. Life Sci 155: 102–109, 2016. doi: 10.1016/j.lfs.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 209.Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 287: G334–G343, 2004. doi: 10.1152/ajpgi.00517.2003. [DOI] [PubMed] [Google Scholar]
- 210.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood 105: 1950–1955, 2005. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 211.Cunningham R, Biswas R, Brazie M, Steplock D, Shenolikar S, Weinman EJ. Signaling pathways utilized by PTH and dopamine to inhibit phosphate transport in mouse renal proximal tubule cells. Am J Physiol Renal Physiol 296: F355–F361, 2009. doi: 10.1152/ajprenal.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cuperus FJ, Claudel T, Gautherot J, Halilbasic E, Trauner M. The role of canalicular ABC transporters in cholestasis. Drug Metab Dispos 42: 546–560, 2014. doi: 10.1124/dmd.113.056358. [DOI] [PubMed] [Google Scholar]
- 213.Cyphert HA, Alonge KM, Ippagunta SM, Hillgartner FB. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9: e94996, 2014. doi: 10.1371/journal.pone.0094996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, Dotti CG. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J Cell Biol 162: 1267–1279, 2003. doi: 10.1083/jcb.200304021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dalvi PS, Erbiceanu FD, Irwin DM, Belsham DD. Direct regulation of the proglucagon gene by insulin, leptin, and cAMP in embryonic versus adult hypothalamic neurons. Mol Endocrinol 26: 1339–1355, 2012. doi: 10.1210/me.2012-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Danielsson J, Zaidi S, Kim B, Funayama H, Yim PD, Xu D, Worgall TS, Gallos G, Emala CW. Airway Epithelial Cell Release of GABA is Regulated by Protein Kinase A. Lung 194: 401–408, 2016. doi: 10.1007/s00408-016-9867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, Martinez A, Døskeland SO. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem 281: 21500–21511, 2006. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- 218.Darcet F, Gardier AM, David DJ, Guilloux JP. Chronic 5-HT4 receptor agonist treatment restores learning and memory deficits in a neuroendocrine mouse model of anxiety/depression. Neurosci Lett 616: 197–203, 2016. doi: 10.1016/j.neulet.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 219.Darchen F, Goud B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie 82: 375–384, 2000. doi: 10.1016/S0300-9084(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 220.Dardevet D, Sornet C, Vary T, Grizard J. Phosphatidylinositol 3-kinase and p70 s6 kinase participate in the regulation of protein turnover in skeletal muscle by insulin and insulin-like growth factor I. Endocrinology 137: 4087–4094, 1996. doi: 10.1210/endo.137.10.8828461. [DOI] [PubMed] [Google Scholar]
- 221.Das R, Chowdhury S, Mazhab-Jafari MT, Sildas S, Selvaratnam R, Melacini G. Dynamically driven ligand selectivity in cyclic nucleotide binding domains. J Biol Chem 284: 23682–23696, 2009. doi: 10.1074/jbc.M109.011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Das R, Mazhab-Jafari MT, Chowdhury S, SilDas S, Selvaratnam R, Melacini G. Entropy-driven cAMP-dependent allosteric control of inhibitory interactions in exchange proteins directly activated by cAMP. J Biol Chem 283: 19691–19703, 2008. doi: 10.1074/jbc.M802164200. [DOI] [PubMed] [Google Scholar]
- 223.Dash-Koney M, Deevi RK, McFarlane C, Dib K. Exchange protein directly activated by cAMP 1 (Epac1) is expressed in human neutrophils and mediates cAMP-dependent activation of the monomeric GTPase Rap1. J Leukoc Biol 90: 741–749, 2011. doi: 10.1189/jlb.0211108. [DOI] [PubMed] [Google Scholar]
- 224.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241, 1997. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 225.Davidson MM, Haslam RJ. Dephosphorylation of cofilin in stimulated platelets: roles for a GTP-binding protein and Ca2+. Biochem J 301: 41–47, 1994. doi: 10.1042/bj3010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dawn A, Singh S, More KR, Siddiqui FA, Pachikara N, Ramdani G, Langsley G, Chitnis CE. The central role of cAMP in regulating Plasmodium falciparum merozoite invasion of human erythrocytes. PLoS Pathog 10: e1004520, 2014. doi: 10.1371/journal.ppat.1004520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.De Marinis YZ, Salehi A, Ward CE, Zhang Q, Abdulkader F, Bengtsson M, Braha O, Braun M, Ramracheya R, Amisten S, Habib AM, Moritoh Y, Zhang E, Reimann F, Rosengren A, Shibasaki T, Gribble F, Renström E, Seino S, Eliasson L, Rorsman P. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab 11: 543–553, 2010. doi: 10.1016/j.cmet.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 275: 20829–20836, 2000. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 229.De Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 230.Decallonne B, van Etten E, Giulietti A, Casteels K, Overbergh L, Bouillon R, Mathieu C. Defect in activation-induced cell death in non-obese diabetic (NOD) T lymphocytes. J Autoimmun 20: 219–226, 2003. doi: 10.1016/S0896-8411(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 231.Dekkers BG, Maarsingh H, Meurs H, Gosens R. Airway structural components drive airway smooth muscle remodeling in asthma. Proc Am Thorac Soc 6: 683–692, 2009. doi: 10.1513/pats.200907-056DP. [DOI] [PubMed] [Google Scholar]
- 232.Dekkers BG, Schaafsma D, Tran T, Zaagsma J, Meurs H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am J Respir Cell Mol Biol 41: 494–504, 2009. doi: 10.1165/rcmb.2008-0251OC. [DOI] [PubMed] [Google Scholar]
- 233.Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron 69: 317–331, 2011. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dent G, Giembycz MA, Rabe KF, Wolf B, Barnes PJ, Magnussen H. Theophylline suppresses human alveolar macrophage respiratory burst through phosphodiesterase inhibition. Am J Respir Cell Mol Biol 10: 565–572, 1994. doi: 10.1165/ajrcmb.10.5.8179921. [DOI] [PubMed] [Google Scholar]
- 235.Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol 154: 308–317, 1995. [PubMed] [Google Scholar]
- 236.Dessauer CW, Tesmer JJ, Sprang SR, Gilman AG. Identification of a Gialpha binding site on type V adenylyl cyclase. J Biol Chem 273: 25831–25839, 1998. doi: 10.1074/jbc.273.40.25831. [DOI] [PubMed] [Google Scholar]
- 237.Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, Seifert R. International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases. Pharmacol Rev 69: 93–139, 2017. doi: 10.1124/pr.116.013078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem 267: 5108–5113, 1992. [PubMed] [Google Scholar]
- 239.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193: 233–238, 2001. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dib K, Axelsson L, Andersson T. Beta2 integrins target Rap GTPases to the plasma membrane by means of degranulation. Biochem Biophys Res Commun 376: 642–646, 2008. doi: 10.1016/j.bbrc.2008.08.160. [DOI] [PubMed] [Google Scholar]
- 241.Diel S, Klass K, Wittig B, Kleuss C. Gbetagamma activation site in adenylyl cyclase type II. Adenylyl cyclase type III is inhibited by Gbetagamma. J Biol Chem 281: 288–294, 2006. doi: 10.1074/jbc.M511045200. [DOI] [PubMed] [Google Scholar]
- 242.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci 13: 2227–2233, 2001. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 243.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 244.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA 101: 16513–16518, 2004. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci 114: 1431–1437, 2001. [DOI] [PubMed] [Google Scholar]
- 246.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437: 574–578, 2005. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, Houslay MD, Langeberg LK, Scott JD. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J 20: 1921–1930, 2001. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Doebele RC, Schulze-Hoepfner FT, Hong J, Chlenski A, Zeitlin BD, Goel K, Gomes S, Liu Y, Abe MK, Nor JE, Lingen MW, Rosner MR. A novel interplay between Epac/Rap1 and mitogen-activated protein kinase kinase 5/extracellular signal-regulated kinase 5 (MEK5/ERK5) regulates thrombospondin to control angiogenesis. Blood 114: 4592–4600, 2009. doi: 10.1182/blood-2009-04-217042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Doggett TM, Breslin JW. Acute alcohol intoxication-induced microvascular leakage. Alcohol Clin Exp Res 38: 2414–2426, 2014. doi: 10.1111/acer.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Domínguez-Rodríguez A, Ruiz-Hurtado G, Sabourin J, Gómez AM, Alvarez JL, Benitah JP. Proarrhythmic effect of sustained EPAC activation on TRPC3/4 in rat ventricular cardiomyocytes. J Mol Cell Cardiol 87: 74–78, 2015. doi: 10.1016/j.yjmcc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 251.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007. doi: 10.1152/physrev.00030.2006. [DOI] [PubMed] [Google Scholar]
- 252.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, Mangeat PH, Goldenring JR. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J 16: 35–43, 1997. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dremier S, Milenkovic M, Blancquaert S, Dumont JE, Døskeland SO, Maenhaut C, Roger PP. Cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases, but not exchange proteins directly activated by cAMP (Epac), mediate thyrotropin/cAMP-dependent regulation of thyroid cells. Endocrinology 148: 4612–4622, 2007. doi: 10.1210/en.2007-0540. [DOI] [PubMed] [Google Scholar]
- 254.Dremier S, Pohl V, Poteet-Smith C, Roger PP, Corbin J, Doskeland SO, Dumont JE, Maenhaut C. Activation of cyclic AMP-dependent kinase is required but may not be sufficient to mimic cyclic AMP-dependent DNA synthesis and thyroglobulin expression in dog thyroid cells. Mol Cell Biol 17: 6717–6726, 1997. doi: 10.1128/MCB.17.11.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Driancourt MA, Reynaud K, Cortvrindt R, Smitz J. Roles of KIT and KIT LIGAND in ovarian function. Rev Reprod 5: 143–152, 2000. doi: 10.1530/ror.0.0050143. [DOI] [PubMed] [Google Scholar]
- 256.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 257.Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron 49: 81–94, 2006. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 258.Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, Gutmann DH. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem 274: 25842–25848, 1999. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- 259.Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, Conley RR, Pandey GN. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch Gen Psychiatry 63: 639–648, 2006. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- 260.Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, Lu Y, Schwede F, Genieser HG, Smrcka AV, Holz GG. Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets 3: 121–128, 2011. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 148: 720–726, 1993. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 262.Eddleman CS, Ballinger ML, Smyers ME, Godell CM, Fishman HM, Bittner GD. Repair of plasmalemmal lesions by vesicles. Proc Natl Acad Sci USA 94: 4745–4750, 1997. doi: 10.1073/pnas.94.9.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Eddleman CS, Bittner GD, Fishman HM. Barrier permeability at cut axonal ends progressively decreases until an ionic seal is formed. Biophys J 79: 1883–1890, 2000. doi: 10.1016/S0006-3495(00)76438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ediger TL, Toews ML. Synergistic stimulation of airway smooth muscle cell mitogenesis. J Pharmacol Exp Ther 294: 1076–1082, 2000. [PubMed] [Google Scholar]
- 265.Efetova M, Petereit L, Rosiewicz K, Overend G, Haußig F, Hovemann BT, Cabrero P, Dow JA, Schwärzel M. Separate roles of PKA and EPAC in renal function unraveled by the optogenetic control of cAMP levels in vivo. J Cell Sci 126: 778–788, 2013. doi: 10.1242/jcs.114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Eid AH, Chotani MA, Mitra S, Miller TJ, Flavahan NA. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha2C-adrenoceptors. Am J Physiol Heart Circ Physiol 295: H266–H272, 2008. doi: 10.1152/ajpheart.00084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Minett MS, Hong GS, Lee E, Oh U, Ishikawa Y, Zwartkuis FJ, Cox JJ, Wood JN. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 4: 1682, 2013. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Eijkelkamp N, Wang H, Garza-Carbajal A, Willemen HL, Zwartkruis FJ, Wood JN, Dantzer R, Kelley KW, Heijnen CJ, Kavelaars A. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci 30: 12806–12815, 2010. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.El Zein N, Badran B, Sariban E. VIP differentially activates beta2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol 83: 972–981, 2008. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- 270.Elbim C, Pillet S, Prevost MH, Preira A, Girard PM, Rogine N, Hakim J, Israel N, Gougerot-Pocidalo MA. The role of phagocytes in HIV-related oxidative stress. J Clin Virol 20: 99–109, 2001. doi: 10.1016/S1386-6532(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 271.Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 121: 181–197, 2003. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031, 2003. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 273.Emery AC, Eiden MV, Eiden LE. Separate cyclic AMP sensors for neuritogenesis, growth arrest, and survival of neuroendocrine cells. J Biol Chem 289: 10126–10139, 2014. doi: 10.1074/jbc.M113.529321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906, 2002. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 275.Enyeart JA, Enyeart JJ. Metabolites of an Epac-selective cAMP analog induce cortisol synthesis by adrenocortical cells through a cAMP-independent pathway. PLoS One 4: e6088, 2009. doi: 10.1371/journal.pone.0006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Enyeart JA, Liu H, Enyeart JJ. 8-Phenylthio-adenines stimulate the expression of steroid hydroxylases, Cav3.2 Ca2+ channels, and cortisol synthesis by a cAMP-independent mechanism. Am J Physiol Endocrinol Metab 301: E941–E954, 2011. doi: 10.1152/ajpendo.00282.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Enyeart JA, Liu H, Enyeart JJ. cAMP analogs and their metabolites enhance TREK-1 mRNA and K+ current expression in adrenocortical cells. Mol Pharmacol 77: 469–482, 2010. doi: 10.1124/mol.109.061861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Espinoza S, Masri B, Salahpour A, Gainetdinov RR. BRET approaches to characterize dopamine and TAAR1 receptor pharmacology and signaling. Methods Mol Biol 964: 107–122, 2013. doi: 10.1007/978-1-62703-251-3_8. [DOI] [PubMed] [Google Scholar]
- 279.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA 101: 2993–2998, 2004. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Esseltine JL, Scott JD. AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol Sci 34: 648–655, 2013. doi: 10.1016/j.tips.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19: 1004–1013, 1999. doi: 10.1161/01.ATV.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 282.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol 31: 90–100, 2012. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Evron T, Daigle TL, Caron MG. GRK2: multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol Sci 33: 154–164, 2012. doi: 10.1016/j.tips.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fang CH, Li BG, James JH, King JK, Evenson AR, Warden GD, Hasselgren PO. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor I and glycogen synthase kinase-3beta inhibitors. Endocrinology 146: 3141–3149, 2005. doi: 10.1210/en.2004-0869. [DOI] [PubMed] [Google Scholar]
- 285.Fang F, Liu GC, Kim C, Yassa R, Zhou J, Scholey JW. Adiponectin attenuates angiotensin II-induced oxidative stress in renal tubular cells through AMPK and cAMP-Epac signal transduction pathways. Am J Physiol Renal Physiol 304: F1366–F1374, 2013. doi: 10.1152/ajprenal.00137.2012. [DOI] [PubMed] [Google Scholar]
- 286.Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, Ling S, Chen W, Han S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209: 94–105, 2012. doi: 10.1016/j.toxlet.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 287.Fang Y, Olah ME. Cyclic AMP-dependent, protein kinase A-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: role of exchange protein activated by cAMP 1 (Epac1). J Pharmacol Exp Ther 322: 1189–1200, 2007. doi: 10.1124/jpet.107.119933. [DOI] [PubMed] [Google Scholar]
- 288.Farmer P, Pugin J. Beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am J Physiol Lung Cell Mol Physiol 279: L675–L682, 2000. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 289.Fazal L, Laudette M, Paula-Gomes S, Pons S, Conte C, Tortosa F, Sicard P, Sainte-Marie Y, Bisserier M, Lairez O, Lucas A, Roy J, Ghaleh B, Fauconnier J, Mialet-Perez J, Lezoualc’h F. Multifunctional Mitochondrial Epac1 Controls Myocardial Cell Death. Circ Res 120: 645–657, 2017. doi: 10.1161/CIRCRESAHA.116.309859. [DOI] [PubMed] [Google Scholar]
- 290.Fechner L, Baumann O, Walz B. Activation of the cyclic AMP pathway promotes serotonin-induced Ca2+ oscillations in salivary glands of the blowfly Calliphora vicina. Cell Calcium 53: 94–101, 2013. doi: 10.1016/j.ceca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 291.Fehmann HC, Göke R, Göke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr Rev 16: 390–410, 1995. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 292.Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol 308: 99–114, 2001. doi: 10.1006/jmbi.2001.4585. [DOI] [PubMed] [Google Scholar]
- 293.Feracci H, Connolly TP, Margolis RN, Hubbard AL. The establishment of hepatocyte cell surface polarity during fetal liver development. Dev Biol 123: 73–84, 1987. doi: 10.1016/0012-1606(87)90429-5. [DOI] [PubMed] [Google Scholar]
- 294.Fernandes HB, Riordan S, Nomura T, Remmers CL, Kraniotis S, Marshall JJ, Kukreja L, Vassar R, Contractor A. Epac2 Mediates cAMP-Dependent Potentiation of Neurotransmission in the Hippocampus. J Neurosci 35: 6544–6553, 2015. doi: 10.1523/JNEUROSCI.0314-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Fernández-Martínez AB, Carmena MJ, Bajo AM, Vacas E, Sánchez-Chapado M, Prieto JC. VIP induces NF-κB1-nuclear localisation through different signalling pathways in human tumour and non-tumour prostate cells. Cell Signal 27: 236–244, 2015. doi: 10.1016/j.cellsig.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 296.Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science 253: 1129–1132, 1991. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 297.Ferrari LF, Bogen O, Alessandri-Haber N, Levine E, Gear RW, Levine JD. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience 222: 392–403, 2012. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ferreira SH. Local control of inflammatory pain. Agents Actions 11: 636–638, 1981. doi: 10.1007/BF01978773. [DOI] [PubMed] [Google Scholar]
- 299.Ferreira SH. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol 240: 200–203, 1972. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- 300.Ferrero JJ, Alvarez AM, Ramírez-Franco J, Godino MC, Bartolomé-Martín D, Aguado C, Torres M, Luján R, Ciruela F, Sánchez-Prieto J. β-Adrenergic receptors activate exchange protein directly activated by cAMP (Epac), translocate Munc13-1, and enhance the Rab3A-RIM1α interaction to potentiate glutamate release at cerebrocortical nerve terminals. J Biol Chem 288: 31370–31385, 2013. doi: 10.1074/jbc.M113.463877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ferrero JJ, Ramírez-Franco J, Martín R, Bartolomé-Martín D, Torres M, Sánchez-Prieto J. Cross-talk between metabotropic glutamate receptor 7 and beta adrenergic receptor signaling at cerebrocortical nerve terminals. Neuropharmacology 101: 412–425, 2016. doi: 10.1016/j.neuropharm.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 302.Fields DP, Springborn SR, Mitchell GS. Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114: 2015–2022, 2015. doi: 10.1152/jn.00374.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Flacke JP, Flacke H, Appukuttan A, Palisaar RJ, Noldus J, Robinson BD, Reusch HP, Zippin JH, Ladilov Y. Type 10 soluble adenylyl cyclase is overexpressed in prostate carcinoma and controls proliferation of prostate cancer cells. J Biol Chem 288: 3126–3135, 2013. doi: 10.1074/jbc.M112.403279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Fladmark KE, Gjertsen BT, Døskeland SO, Vintermyr OK. Fas/APO-1(CD95)-induced apoptosis of primary hepatocytes is inhibited by cAMP. Biochem Biophys Res Commun 232: 20–25, 1997. doi: 10.1006/bbrc.1997.6214. [DOI] [PubMed] [Google Scholar]
- 305.Florio C, Martin JG, Styhler A, Heisler S. Antiproliferative effect of prostaglandin E2 in cultured guinea pig tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 266: L131–L137, 1994. [DOI] [PubMed] [Google Scholar]
- 306.Fogli S, Stefanelli F, Battolla B, Bianchi F, Breschi MC, Mattii L. Salbutamol inhibits RhoA activation in normal but not in desensitized bronchial smooth muscle cells. J Pharm Pharmacol 67: 1416–1420, 2015. doi: 10.1111/jphp.12444. [DOI] [PubMed] [Google Scholar]
- 307.Fortier I, Patry C, Lora M, Samadfan R, de Brum-Fernandes AJ. Immunohistochemical localization of the prostacyclin receptor (IP) human bone. Prostaglandins Leukot Essent Fatty Acids 65: 79–83, 2001. doi: 10.1054/plef.2001.0292. [DOI] [PubMed] [Google Scholar]
- 308.Fowler CJ, Cowburn RF, Garlind A, Winblad B, O’Neill C. Disturbances in signal transduction mechanisms in Alzheimer’s disease. Mol Cell Biochem 149-150: 287–292, 1995. doi: 10.1007/BF01076590. [DOI] [PubMed] [Google Scholar]
- 309.Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. A novel lipid-anchored A-kinase Anchoring Protein facilitates cAMP-responsive membrane events. EMBO J 17: 2261–2272, 1998. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Frechilla D, Otano A, Del Río J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 22: 787–802, 1998. doi: 10.1016/S0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- 311.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 318: 1730–1737, 1999. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Frevert U, Engelmann S, Zougbédé S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. Intravital observation of Plasmodium berghei sporozoite infection of the liver. PLoS Biol 3: e192, 2005. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260: 1661–1664, 1993. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 314.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006. doi: 10.1152/ajpcell.00413.2005. [DOI] [PubMed] [Google Scholar]
- 315.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol (1985) 103: 378–387, 2007. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 316.Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA 108: 1403–1408, 2011. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Fugelli K. Effects of sodium ions on rat thyrocyte (FRTL-5 cells) swelling- and thyrotropin-activated taurine efflux dependent on cAMP and Epac. Amino Acids 48: 763–777, 2016. doi: 10.1007/s00726-015-2124-9. [DOI] [PubMed] [Google Scholar]
- 318.Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem 277: 50497–50502, 2002. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 319.Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem 277: 22191–22200, 2002. doi: 10.1074/jbc.M110364200. [DOI] [PubMed] [Google Scholar]
- 320.Fujita T, Umemura M, Yokoyama U, Okumura S, Ishikawa Y. The role of Epac in the heart. Cell Mol Life Sci 74: 591–606, 2017. doi: 10.1007/s00018-016-2336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25: 2448–2459, 2007. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 322.Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metab 13: 331–339, 2011. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Fuld S, Borland G, Yarwood SJ. Elevation of cyclic AMP in Jurkat T-cells provokes distinct transcriptional responses through the protein kinase A (PKA) and exchange protein activated by cyclic AMP (EPAC) pathways. Exp Cell Res 309: 161–173, 2005. doi: 10.1016/j.yexcr.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 325.Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem 67: 1882–1896, 1996. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 326.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 327.Gagnon J, Anini Y. Glucagon stimulates ghrelin secretion through the activation of MAPK and EPAC and potentiates the effect of norepinephrine. Endocrinology 154: 666–674, 2013. doi: 10.1210/en.2012-1994. [DOI] [PubMed] [Google Scholar]
- 328.Gagnon J, Anini Y. Insulin and norepinephrine regulate ghrelin secretion from a rat primary stomach cell culture. Endocrinology 153: 3646–3656, 2012. doi: 10.1210/en.2012-1040. [DOI] [PubMed] [Google Scholar]
- 329.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW. Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 295: L1040–L1047, 2008. doi: 10.1152/ajplung.90330.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gallos G, Townsend E, Yim P, Virag L, Zhang Y, Xu D, Bacchetta M, Emala CW. Airway epithelium is a predominant source of endogenous airway GABA and contributes to relaxation of airway smooth muscle tone. Am J Physiol Lung Cell Mol Physiol 304: L191–L197, 2013. doi: 10.1152/ajplung.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Gambaryan S, Butt E, Tas P, Smolenski A, Allolio B, Walter U. Regulation of aldosterone production from zona glomerulosa cells by ANG II and cAMP: evidence for PKA-independent activation of CaMK by cAMP. Am J Physiol Endocrinol Metab 290: E423–E433, 2006. doi: 10.1152/ajpendo.00128.2005. [DOI] [PubMed] [Google Scholar]
- 332.Ganapathy V, Gurlo T, Jarstadmarken HO, von Grafenstein H. Regulation of TCR-induced IFN-gamma release from islet-reactive non-obese diabetic CD8(+) T cells by prostaglandin E(2) receptor signaling. Int Immunol 12: 851–860, 2000. doi: 10.1093/intimm/12.6.851. [DOI] [PubMed] [Google Scholar]
- 333.Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L, Linette G, Zhao H, Cornelius LA. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res 66: 7880–7888, 2006. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- 334.Gao M, Ma Y, Bast RC Jr, Li Y, Wan L, Liu Y, Sun Y, Fang Z, Zhang L, Wang X, Wei Z. Epac1 knockdown inhibits the proliferation of ovarian cancer cells by inactivating AKT/Cyclin D1/CDK4 pathway in vitro and in vivo. Med Oncol 33: 73, 2016. doi: 10.1007/s12032-016-0786-0. [DOI] [PubMed] [Google Scholar]
- 335.Garay J, D’Angelo JA, Park Y, Summa CM, Aiken ML, Morales E, Badizadegan K, Fiebiger E, Dickinson BL. Crosstalk between PKA and Epac regulates the phenotypic maturation and function of human dendritic cells. J Immunol 185: 3227–3238, 2010. doi: 10.4049/jimmunol.0903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.García-Morales V, Cuíñas A, Elíes J, Campos-Toimil M. PKA and Epac activation mediates cAMP-induced vasorelaxation by increasing endothelial NO production. Vascul Pharmacol 60: 95–101, 2014. doi: 10.1016/j.vph.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 337.Garg J, Feng YX, Jansen SR, Friedrich J, Lezoualc’h F, Schmidt M, Wieland T. Catecholamines facilitate VEGF-dependent angiogenesis via β2-adrenoceptor-induced Epac1 and PKA activation. Oncotarget 8: 44732–44748, 2017. doi: 10.18632/oncotarget.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Gates A, Hohenester S, Anwer MS, Webster CR. cAMP-GEF cytoprotection by Src tyrosine kinase activation of phosphoinositide-3-kinase p110 beta/alpha in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol 296: G764–G774, 2009. doi: 10.1152/ajpgi.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Gaudy AM, Clementi AH, Campbell JS, Smrcka AV, Mooney RA. Suppressor of cytokine signaling-3 is a glucagon-inducible inhibitor of PKA activity and gluconeogenic gene expression in hepatocytes. J Biol Chem 285: 41356–41365, 2010. doi: 10.1074/jbc.M110.159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Gavett SH, Madison SL, Chulada PC, Scarborough PE, Qu W, Boyle JE, Tiano HF, Lee CA, Langenbach R, Roggli VL, Zeldin DC. Allergic lung responses are increased in prostaglandin H synthase-deficient mice. J Clin Invest 104: 721–732, 1999. doi: 10.1172/JCI6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Gehrig SM, Koopman R, Naim T, Tjoakarfa C, Lynch GS. Making fast-twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am J Pathol 176: 29–33, 2010. doi: 10.2353/ajpath.2010.090760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci 28: 7991–8002, 2008. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ, Nguyen PV. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem 15: 403–411, 2008. doi: 10.1101/lm.830008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, Natarajan V. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy 37: 311–322, 2007. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 345.Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R. Multiple cAMP-induced signaling cascades regulate prolactin expression in T cells. Cell Mol Life Sci 63: 92–99, 2006. doi: 10.1007/s00018-005-5433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gerlo S, Verdood P, Hooghe-Peters EL, Kooijman R. Multiple, PKA-dependent and PKA-independent, signals are involved in cAMP-induced PRL expression in the eosinophilic cell line Eol-1. Cell Signal 17: 901–909, 2005. doi: 10.1016/j.cellsig.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 347.Gerlo S, Verdood P, Kooijman R. Modulation of cytokine production by cyclic adenosine monophosphate analogs in human leukocytes. J Interferon Cytokine Res 30: 883–891, 2010. doi: 10.1089/jir.2009.0021. [DOI] [PubMed] [Google Scholar]
- 348.Glas E, Mückter H, Gudermann T, Breit A. Exchange factors directly activated by cAMP mediate melanocortin 4 receptor-induced gene expression. Sci Rep 6: 32776, 2016. doi: 10.1038/srep32776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Gloerich M, Ponsioen B, Vliem MJ, Zhang Z, Zhao J, Kooistra MR, Price LS, Ritsma L, Zwartkruis FJ, Rehmann H, Jalink K, Bos JL. Spatial regulation of cyclic AMP-Epac1 signaling in cell adhesion by ERM proteins. Mol Cell Biol 30: 5421–5431, 2010. doi: 10.1128/MCB.00463-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Gloerich M, Vliem MJ, Prummel E, Meijer LA, Rensen MG, Rehmann H, Bos JL. The nucleoporin RanBP2 tethers the cAMP effector Epac1 and inhibits its catalytic activity. J Cell Biol 193: 1009–1020, 2011. doi: 10.1083/jcb.201011126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem 275: 18366–18374, 2000. doi: 10.1074/jbc.M001339200. [DOI] [PubMed] [Google Scholar]
- 352.Goedhart J, van Weeren L, Hink MA, Vischer NO, Jalink K, Gadella TW Jr. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat Methods 7: 137–139, 2010. doi: 10.1038/nmeth.1415. [DOI] [PubMed] [Google Scholar]
- 353.Gong B, Shelite T, Mei FC, Ha T, Hu Y, Xu G, Chang Q, Wakamiya M, Ksiazek TG, Boor PJ, Bouyer DH, Popov VL, Chen J, Walker DH, Cheng X. Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc Natl Acad Sci USA 110: 19615–19620, 2013. doi: 10.1073/pnas.1314400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gonzalez-Iglesias AE, Jiang Y, Tomić M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signaling pathway in pituitary lactotrophs. Mol Endocrinol 20: 2231–2246, 2006. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- 355.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14: 1283–1300, 2000. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- 356.Good CH, Lupica CR. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J Neurosci 30: 7900–7909, 2010. doi: 10.1523/JNEUROSCI.1507-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature 418: 93–96, 2002. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- 358.Goupil S, Maréchal L, El Hajj H, Tremblay ME, Richard FJ, Leclerc P. Identification and Localization of the Cyclic Nucleotide Phosphodiesterase 10A in Bovine Testis and Mature Spermatozoa. PLoS One 11: e0161035, 2016. doi: 10.1371/journal.pone.0161035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Grandoch M, Hoffmann J, Röck K, Wenzel F, Oberhuber A, Schelzig H, Fischer JW. Novel effects of adenosine receptors on pericellular hyaluronan matrix: implications for human smooth muscle cell phenotype and interactions with monocytes during atherosclerosis. Basic Res Cardiol 108: 340, 2013. doi: 10.1007/s00395-013-0340-6. [DOI] [PubMed] [Google Scholar]
- 360.Grandoch M, López de Jesús M, Oude Weernink PA, Weber AA, Jakobs KH, Schmidt M. B cell receptor-induced growth arrest and apoptosis in WEHI-231 immature B lymphoma cells involve cyclic AMP and Epac proteins. Cell Signal 21: 609–621, 2009. doi: 10.1016/j.cellsig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 361.Grandoch M, López de Jesús M, Oude Weernink PA, Weber AA, Jakobs KH, Schmidt M. B cell receptor-induced growth arrest and apoptosis in WEHI-231 immature B lymphoma cells involve cyclic AMP and Epac proteins. Cell Signal 21: 609–621, 2009. doi: 10.1016/j.cellsig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 362.Grandoch M, Rose A, ter Braak M, Jendrossek V, Rübben H, Fischer JW, Schmidt M, Weber AA. Epac inhibits migration and proliferation of human prostate carcinoma cells. Br J Cancer 101: 2038–2042, 2009. doi: 10.1038/sj.bjc.6605439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Granholm S, Lundberg P, Lerner UH. Calcitonin inhibits osteoclast formation in mouse haematopoetic cells independently of transcriptional regulation by receptor activator of NF-kappaB and c-Fms. J Endocrinol 195: 415–427, 2007. doi: 10.1677/JOE-07-0338. [DOI] [PubMed] [Google Scholar]
- 364.Graves LM, Bornfeldt KE, Raines EW, Potts BC, Macdonald SG, Ross R, Krebs EG. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci USA 90: 10300–10304, 1993. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Graves LM, Bornfeldt KE, Sidhu JS, Argast GM, Raines EW, Ross R, Leslie CC, Krebs EG. Platelet-derived growth factor stimulates protein kinase A through a mitogen-activated protein kinase-dependent pathway in human arterial smooth muscle cells. J Biol Chem 271: 505–511, 1996. doi: 10.1074/jbc.271.1.505. [DOI] [PubMed] [Google Scholar]
- 366.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73: 2424–2428, 1976. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Greengard P, Kuo JF, Miyamoto E. Studies on the mechanism of action of cyclic AMP in nervous and other tissues. Adv Enzyme Regul 9: 113–125, 1971. doi: 10.1016/S0065-2571(71)80040-7. [DOI] [PubMed] [Google Scholar]
- 368.Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science 271: 1718–1723, 1996. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- 369.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 276: 29188–29194, 2001. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- 370.Grimes MT, Powell M, Gutierrez SM, Darby-King A, Harley CW, McLean JH. Epac activation initiates associative odor preference memories in the rat pup. Learn Mem 22: 74–82, 2015. doi: 10.1101/lm.037101.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Grosser T, Woolf CJ, FitzGerald GA. Time for nonaddictive relief of pain. Science 355: 1026–1027, 2017. doi: 10.1126/science.aan0088. [DOI] [PubMed] [Google Scholar]
- 372.Grove BD, Bruchey AK. Intracellular distribution of gravin, a PKA and PKC binding protein, in vascular endothelial cells. J Vasc Res 38: 163–175, 2001. doi: 10.1159/000051043. [DOI] [PubMed] [Google Scholar]
- 373.Gu Y, Li G, Chen Y, Huang LY. Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 157: 1541–1550, 2016. doi: 10.1097/j.pain.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gu Y, Wang C, Li G, Huang LY. EXPRESS: F-actin links Epac-PKC signaling to purinergic P2X3 receptors sensitization in dorsal root ganglia following inflammation. Mol Pain 12: 1744806916660557, 2016. doi: 10.1177/1744806916660557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Guan Z, Zhuang W, Lei H, Wang D, Yao Y, Guo D, Sun Q, Chen Y, Chen X, Lin H, Teng B, Zhang Y. Epac1, PDE4, and PKC protein expression and their correlation with AKAP95 and Cx43 in esophagus cancer tissues. Thorac Cancer 8: 572–576, 2017. doi: 10.1111/1759-7714.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Guibinga GH, Hrustanovic G, Bouic K, Jinnah HA, Friedmann T. MicroRNA-mediated dysregulation of neural developmental genes in HPRT deficiency: clues for Lesch-Nyhan disease? Hum Mol Genet 21: 609–622, 2012. doi: 10.1093/hmg/ddr495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Guibinga GH, Hsu S, Friedmann T. Deficiency of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) dysregulates neurogenesis. Mol Ther 18: 54–62, 2010. doi: 10.1038/mt.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Guibinga GH, Murray F, Barron N, Pandori W, Hrustanovic G. Deficiency of the purine metabolic gene HPRT dysregulates microRNA-17 family cluster and guanine-based cellular functions: a role for EPAC in Lesch-Nyhan syndrome. Hum Mol Genet 22: 4502–4515, 2013. doi: 10.1093/hmg/ddt298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Guinzberg R, Díaz-Cruz A, Acosta-Trujillo C, Vilchis-Landeros MM, Vázquez-Meza H, Lozano-Flores C, Chiquete-Felix N, Varela-Echavarría A, Uribe-Carvajal S, Riveros-Rosas H, Piña E. Newly synthesized cAMP is integrated at a membrane protein complex signalosome to ensure receptor response specificity. FEBS J 284: 258–276, 2017. doi: 10.1111/febs.13969. [DOI] [PubMed] [Google Scholar]
- 380.Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS. The renal H+-K+-ATPases: physiology, regulation, and structure. Am J Physiol Renal Physiol 298: F12–F21, 2010. doi: 10.1152/ajprenal.90723.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gupta M, Yarwood SJ. MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance Rap1 GTPase activity and cell adhesion. J Biol Chem 280: 8109–8116, 2005. doi: 10.1074/jbc.M413697200. [DOI] [PubMed] [Google Scholar]
- 382.Gupta P, Prywes R. ATF1 phosphorylation by the ERK MAPK pathway is required for epidermal growth factor-induced c-jun expression. J Biol Chem 277: 50550–50556, 2002. doi: 10.1074/jbc.M209799200. [DOI] [PubMed] [Google Scholar]
- 383.Gupta R, Qualls-Creekmore E, Yoshimura M. Real-time monitoring of intracellular cAMP during acute ethanol exposure. Alcohol Clin Exp Res 37: 1456–1465, 2013. doi: 10.1111/acer.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest 99: 2752–2761, 1997. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Gurgul-Convey E, Hanzelka K, Lenzen S. Mechanism of prostacyclin-induced potentiation of glucose-induced insulin secretion. Endocrinology 153: 2612–2622, 2012. doi: 10.1210/en.2011-2027. [DOI] [PubMed] [Google Scholar]
- 386.Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061, 2004. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 387.Haag S, Warnken M, Juergens UR, Racké K. Role of Epac1 in mediating anti-proliferative effects of prostanoid EP(2) receptors and cAMP in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol 378: 617–630, 2008. doi: 10.1007/s00210-008-0334-3. [DOI] [PubMed] [Google Scholar]
- 388.Haffner I, Teupser D, Holdt LM, Ernst J, Burkhardt R, Thiery J. Regulation of arginase-1 expression in macrophages by a protein kinase A type I and histone deacetylase dependent pathway. J Cell Biochem 103: 520–527, 2008. doi: 10.1002/jcb.21422. [DOI] [PubMed] [Google Scholar]
- 389.Haidar B, Denis M, Krimbou L, Marcil M, Genest J Jr. cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J Lipid Res 43: 2087–2094, 2002. doi: 10.1194/jlr.M200235-JLR200. [DOI] [PubMed] [Google Scholar]
- 390.Haidar M, Echebli N, Ding Y, Kamau E, Langsley G. Transforming growth factor β2 promotes transcription of COX2 and EP4, leading to a prostaglandin E2-driven autostimulatory loop that enhances virulence of Theileria annulata-transformed macrophages. Infect Immun 83: 1869–1880, 2015. doi: 10.1128/IAI.02975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol 270: L1040–L1051, 1996. [DOI] [PubMed] [Google Scholar]
- 392.Halm ST, Zhang J, Halm DR. beta-Adrenergic activation of electrogenic K+ and Cl- secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol 299: G81–G95, 2010. doi: 10.1152/ajpgi.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Han J, Han L, Tiwari P, Wen Z, Zheng JQ. Spatial targeting of type II protein kinase A to filopodia mediates the regulation of growth cone guidance by cAMP. J Cell Biol 176: 101–111, 2007. doi: 10.1083/jcb.200607128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16: 1796–1806, 2006. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 395.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41: 145–174, 2001. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 396.Hara S, Nakaseko C, Yamasaki S, Hattori M, Bos JL, Saito Y, Minato N, Saito T. Involvement of Rap-1 activation and early termination of immune synapse in CTLA-4-mediated negative signal. Hematology 14: 150–158, 2009. doi: 10.1179/102453309X402241. [DOI] [PubMed] [Google Scholar]
- 397.Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE 2006: pl6, 2006. doi: 10.1126/stke.3532006pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin E(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol 168: 2255–2263, 2002. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 399.Harper K, Arsenault D, Boulay-Jean S, Lauzier A, Lucien F, Dubois CM. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res 70: 4634–4643, 2010. doi: 10.1158/0008-5472.CAN-09-3813. [DOI] [PubMed] [Google Scholar]
- 400.Harper SM, Wienk H, Wechselberger RW, Bos JL, Boelens R, Rehmann H. Structural dynamics in the activation of Epac. J Biol Chem 283: 6501–6508, 2008. doi: 10.1074/jbc.M707849200. [DOI] [PubMed] [Google Scholar]
- 401.Hartopo AB, Emoto N, Vignon-Zellweger N, Suzuki Y, Yagi K, Nakayama K, Hirata K. Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am J Respir Cell Mol Biol 48: 465–476, 2013. doi: 10.1165/rcmb.2012-0354OC. [DOI] [PubMed] [Google Scholar]
- 402.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- 403.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med 194: 769–779, 2001. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol 530: 193–205, 2001. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hayashi M, Kajioka S, Itsumi M, Takahashi R, Shahab N, Ishigami T, Takeda M, Masuda N, Yamaguchi A, Naito S. Actions of cAMP on calcium sensitization in human detrusor smooth muscle contraction. BJU Int 117: 179–191, 2016. doi: 10.1111/bju.13180. [DOI] [PubMed] [Google Scholar]
- 406.Hayashi R, Yamashita N, Matsui S, Fujita T, Araya J, Sassa K, Arai N, Yoshida Y, Kashii T, Maruyama M, Sugiyama E, Kobayashi M. Bradykinin stimulates IL-6 and IL-8 production by human lung fibroblasts through ERK- and p38 MAPK-dependent mechanisms. Eur Respir J 16: 452–458, 2000. doi: 10.1034/j.1399-3003.2000.016003452.x. [DOI] [PubMed] [Google Scholar]
- 407.He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature 467: 95–98, 2010. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Heinick A, Husser X, Himmler K, Kirchhefer U, Nunes F, Schulte JS, Seidl MD, Rolfes C, Dedman JR, Kaetzel MA, Gerke V, Schmitz W, Müller FU. Annexin A4 is a novel direct regulator of adenylyl cyclase type 5. FASEB J 29: 3773–3787, 2015. doi: 10.1096/fj.14-269837. [DOI] [PubMed] [Google Scholar]
- 409.Heldsinger A, Grabauskas G, Wu X, Zhou S, Lu Y, Song I, Owyang C. Ghrelin induces leptin resistance by activation of suppressor of cytokine signaling 3 expression in male rats: implications in satiety regulation. Endocrinology 155: 3956–3969, 2014. doi: 10.1210/en.2013-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Hennenberg M, Strittmatter F, Schmetkamp H, Rutz B, Walther S, Stief CG, Gratzke C. The cAMP effector EPAC activates Elk1 transcription factor in prostate smooth muscle, and is a minor regulator of α1-adrenergic contraction. J Biomed Sci 20: 46, 2013. doi: 10.1186/1423-0127-20-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Henquin JC, Nenquin M. Activators of PKA and Epac distinctly influence insulin secretion and cytosolic Ca2+ in female mouse islets stimulated by glucose and tolbutamide. Endocrinology 155: 3274–3287, 2014. doi: 10.1210/en.2014-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Herbst KJ, Coltharp C, Amzel LM, Zhang J. Direct activation of Epac by sulfonylurea is isoform selective. Chem Biol 18: 243–251, 2011. doi: 10.1016/j.chembiol.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Herfindal L, Krakstad C, Myhren L, Hagland H, Kopperud R, Teigen K, Schwede F, Kleppe R, Døskeland SO. Introduction of aromatic ring-containing substituents in cyclic nucleotides is associated with inhibition of toxin uptake by the hepatocyte transporters OATP 1B1 and 1B3. PLoS One 9: e94926, 2014. doi: 10.1371/journal.pone.0094926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Herfindal L, Nygaard G, Kopperud R, Krakstad C, Døskeland SO, Selheim F. Off-target effect of the Epac agonist 8-pCPT-2′-O-Me-cAMP on P2Y12 receptors in blood platelets. Biochem Biophys Res Commun 437: 603–608, 2013. doi: 10.1016/j.bbrc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 415.Héroux M, Breton B, Hogue M, Bouvier M. Assembly and signaling of CRLR and RAMP1 complexes assessed by BRET. Biochemistry 46: 7022–7033, 2007. doi: 10.1021/bi0622470. [DOI] [PubMed] [Google Scholar]
- 416.Hertz AL, Bender AT, Smith KC, Gilchrist M, Amieux PS, Aderem A, Beavo JA. Elevated cyclic AMP and PDE4 inhibition induce chemokine expression in human monocyte-derived macrophages. Proc Natl Acad Sci USA 106: 21978–21983, 2009. doi: 10.1073/pnas.0911684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Hewer RC, Sala-Newby GB, Wu YJ, Newby AC, Bond M. PKA and Epac synergistically inhibit smooth muscle cell proliferation. J Mol Cell Cardiol 50: 87–98, 2011. doi: 10.1016/j.yjmcc.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Heyworth PG, Knaus UG, Xu X, Uhlinger DJ, Conroy L, Bokoch GM, Curnutte JT. Requirement for posttranslational processing of Rac GTP-binding proteins for activation of human neutrophil NADPH oxidase. Mol Biol Cell 4: 261–269, 1993. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Hick M, Herrmann U, Weyer SW, Mallm JP, Tschäpe JA, Borgers M, Mercken M, Roth FC, Draguhn A, Slomianka L, Wolfer DP, Korte M, Müller UC. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol 129: 21–37, 2015. doi: 10.1007/s00401-014-1368-x. [DOI] [PubMed] [Google Scholar]
- 420.Hoban CA, Black LN, Ordas RJ, Gumina DL, Pulous FE, Sim JH, Sands JM, Blount MA. Vasopressin regulation of multisite phosphorylation of UT-A1 in the inner medullary collecting duct. Am J Physiol Renal Physiol 308: F49–F55, 2015. doi: 10.1152/ajprenal.00642.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Hochbaum D, Barila G, Ribeiro-Neto F, Altschuler DL. Radixin assembles cAMP effectors Epac and PKA into a functional cAMP compartment: role in cAMP-dependent cell proliferation. J Biol Chem 286: 859–866, 2011. doi: 10.1074/jbc.M110.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Hochbaum D, Hong K, Barila G, Ribeiro-Neto F, Altschuler DL. Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis. J Biol Chem 283: 4464–4468, 2008. doi: 10.1074/jbc.C700171200. [DOI] [PubMed] [Google Scholar]
- 423.Hochbaum D, Tanos T, Ribeiro-Neto F, Altschuler D, Coso OA. Activation of JNK by Epac is independent of its activity as a Rap guanine nucleotide exchanger. J Biol Chem 278: 33738–33746, 2003. doi: 10.1074/jbc.M305208200. [DOI] [PubMed] [Google Scholar]
- 424.Hoivik EA, Witsoe SL, Bergheim IR, Xu Y, Jakobsson I, Tengholm A, Doskeland SO, Bakke M. DNA methylation of alternative promoters directs tissue specific expression of Epac2 isoforms. PLoS One 8: e67925, 2013. doi: 10.1371/journal.pone.0067925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci 15: 988–997, 2012. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Holtmaat A, Caroni P. Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 19: 1553–1562, 2016. doi: 10.1038/nn.4418. [DOI] [PubMed] [Google Scholar]
- 427.Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53: 5–13, 2004. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20: 10–20, 2008. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol 577: 5–15, 2006. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37). J Biol Chem 274: 14147–14156, 1999. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, Murer H, Hernando N. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC). Proc Natl Acad Sci USA 103: 803–808, 2006. doi: 10.1073/pnas.0503562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang WJ, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem 282: 19781–19787, 2007. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- 433.Hong K, Lou L, Gupta S, Ribeiro-Neto F, Altschuler DL. A novel Epac-Rap-PP2A signaling module controls cAMP-dependent Akt regulation. J Biol Chem 283: 23129–23138, 2008. doi: 10.1074/jbc.M800478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Hong KP, Spitzer NC, Nicol X. Improved molecular toolkit for cAMP studies in live cells. BMC Res Notes 4: 241, 2011. doi: 10.1186/1756-0500-4-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Hong SH, Lu X, Nanes MS, Mitchell J. Regulation of osterix (Osx, Sp7) and the Osx promoter by parathyroid hormone in osteoblasts. J Mol Endocrinol 43: 197–207, 2009. doi: 10.1677/JME-09-0012. [DOI] [PubMed] [Google Scholar]
- 436.Hooper NM, Turner AJ. The search for alpha-secretase and its potential as a therapeutic approach to Alzheimer s disease. Curr Med Chem 9: 1107–1119, 2002. doi: 10.2174/0929867023370121. [DOI] [PubMed] [Google Scholar]
- 437.Horton MR, Boodoo S, Powell JD. NF-kappa B activation mediates the cross-talk between extracellular matrix and interferon-gamma (IFN-gamma) leading to enhanced monokine induced by IFN-gamma (MIG) expression in macrophages. J Biol Chem 277: 43757–43762, 2002. doi: 10.1074/jbc.M206007200. [DOI] [PubMed] [Google Scholar]
- 438.Hoshino M, Sone M, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem 274: 17837–17844, 1999. doi: 10.1074/jbc.274.25.17837. [DOI] [PubMed] [Google Scholar]
- 439.Hothi SS, Gurung IS, Heathcote JC, Zhang Y, Booth SW, Skepper JN, Grace AA, Huang CL. Epac activation, altered calcium homeostasis and ventricular arrhythmogenesis in the murine heart. Pflugers Arch 457: 253–270, 2008. doi: 10.1007/s00424-008-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci 35: 91–100, 2010. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 441.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 370: 1–18, 2003. doi: 10.1042/bj20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Houslay MD, Baillie GS, Maurice DH. cAMP-specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res 100: 950–966, 2007. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 443.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996. doi: 10.1152/ajpcell.1996.270.6.C1695. [DOI] [PubMed] [Google Scholar]
- 444.Hu Y, Robichaux WG III, Mei FC, Kim ER, Wang H, Tong Q, Jin J, Xu M, Chen J, Cheng X. Role of Exchange Protein Directly Activated by Cyclic AMP Isoform 1 in Energy Homeostasis: Regulation of Leptin Expression and Secretion in White Adipose Tissue. Mol Cell Biol 36: 2440–2450, 2016. doi: 10.1128/MCB.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Huang CC, Hsu KS. Presynaptic mechanism underlying cAMP-induced synaptic potentiation in medial prefrontal cortex pyramidal neurons. Mol Pharmacol 69: 846–856, 2006. doi: 10.1124/mol.105.018093. [DOI] [PubMed] [Google Scholar]
- 446.Huang P, Sun Q, Zhuang W, Peng K, Wang D, Yao Y, Guo D, Zhang L, Shen C, Sun M, Tang C, Teng B, Zhang Y. Epac1, PDE4, and PKC protein expression and their association with AKAP95, Cx43, and cyclinD2/E1 in breast cancer tissues. Thorac Cancer 8: 495–500, 2017. doi: 10.1111/1759-7714.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol 39: 482–489, 2008. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA 97: 1014–1019, 2000. doi: 10.1073/pnas.97.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Hucho T, Suckow V, Joseph EK, Kuhn J, Schmoranzer J, Dina OA, Chen X, Karst M, Bernateck M, Levine JD, Ropers HH. Ca++/CaMKII switches nociceptor-sensitizing stimuli into desensitizing stimuli. J Neurochem 123: 589–601, 2012. doi: 10.1111/j.1471-4159.2012.07920.x. [DOI] [PubMed] [Google Scholar]
- 450.Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci 24: 527–534, 2006. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 451.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 25: 6119–6126, 2005. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Humphrey MB, Ogasawara K, Yao W, Spusta SC, Daws MR, Lane NE, Lanier LL, Nakamura MC. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J Bone Miner Res 19: 224–234, 2004. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 453.Hutchison MR, White PC. Prostacyclin regulates bone growth via the Epac/Rap1 pathway. Endocrinology 156: 499–510, 2015. doi: 10.1210/en.2014-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Hwang M, Go Y, Park JH, Shin SK, Song SE, Oh BC, Im SS, Hwang I, Jeon YH, Lee IK, Seino S, Song DK. Epac2a-null mice exhibit obesity-prone nature more susceptible to leptin resistance. Int J Obes 41: 279–288, 2017. doi: 10.1038/ijo.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Hwang S, Gunaratne R, Rinschen MM, Yu MJ, Pisitkun T, Hoffert JD, Fenton RA, Knepper MA, Chou CL. Vasopressin increases phosphorylation of Ser84 and Ser486 in Slc14a2 collecting duct urea transporters. Am J Physiol Renal Physiol 299: F559–F567, 2010. doi: 10.1152/ajprenal.00617.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Indolfi C, Avvedimento EV, Di Lorenzo E, Esposito G, Rapacciuolo A, Giuliano P, Grieco D, Cavuto L, Stingone AM, Ciullo I, Condorelli G, Chiariello M. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med 3: 775–779, 1997. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 457.International Molecular Genetic Study of Autism Consortium (IMGSAC) A genomewide screen for autism: strong evidence for linkage to chromosomes 2q, 7q, and 16p. Am J Hum Genet 69: 570–581, 2001. doi: 10.1086/323264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Islam D, Zhang N, Wang P, Li H, Brubaker PL, Gaisano HY, Wang Q, Jin T. Epac is involved in cAMP-stimulated proglucagon expression and hormone production but not hormone secretion in pancreatic alpha- and intestinal L-cell lines. Am J Physiol Endocrinol Metab 296: E174–E181, 2009. doi: 10.1152/ajpendo.90419.2008. [DOI] [PubMed] [Google Scholar]
- 459.Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. Regulation of adenylyl cyclase by protein kinase A. J Biol Chem 270: 12481–12484, 1995. doi: 10.1074/jbc.270.21.12481. [DOI] [PubMed] [Google Scholar]
- 460.Jacobs S, Calebiro D, Nikolaev VO, Lohse MJ, Schulz S. Real-time monitoring of somatostatin receptor-cAMP signaling in live pituitary. Endocrinology 151: 4560–4565, 2010. doi: 10.1210/en.2010-0341. [DOI] [PubMed] [Google Scholar]
- 461.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA 100: 10676–10681, 2003. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, Gillberg C, Råstam M, Gillberg C, Nydén A, Söderström H, Leboyer M, Betancur C, Philippe A, Giros B, Colineaux C, Cohen D, Chabane N, Mouren-Siméoni M-C, Brice A, Sponheim E, Spurkland I, Skjeldal OH, Coleman M, Pearl PL, Cohen IL, Tsiouris J, Zappella M, Menchetti G, Pompella A, Aschauer H, Van Maldergem L; Paris Autism Research International Sibpair Study . Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34: 27–29, 2003. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Jansen SR, Poppinga WJ, de Jager W, Lezoualc’h F, Cheng X, Wieland T, Yarwood SJ, Gosens R, Schmidt M. Epac1 links prostaglandin E2 to β-catenin-dependent transcription during epithelial-to-mesenchymal transition. Oncotarget 7: 46354–46370, 2016. doi: 10.18632/oncotarget.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol 21: 1387–1395, 2003. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- 465.Jarrard RE, Wang Y, Salyer AE, Pratt EP, Soderling IM, Guerra ML, Lange AM, Broderick HJ, Hockerman GH. Potentiation of sulfonylurea action by an EPAC-selective cAMP analog in INS-1 cells: comparison of tolbutamide and gliclazide and a potential role for EPAC activation of a 2-APB-sensitive Ca2+ influx. Mol Pharmacol 83: 191–205, 2013. doi: 10.1124/mol.112.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Jarvis CI, Goncalves MB, Clarke E, Dogruel M, Kalindjian SB, Thomas SA, Maden M, Corcoran JP. Retinoic acid receptor-α signalling antagonizes both intracellular and extracellular amyloid-β production and prevents neuronal cell death caused by amyloid-β. Eur J Neurosci 32: 1246–1255, 2010. doi: 10.1111/j.1460-9568.2010.07426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest 89: 507–511, 1992. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Jayaraman S, Haggie P, Wachter RM, Remington SJ, Verkman AS. Mechanism and cellular applications of a green fluorescent protein-based halide sensor. J Biol Chem 275: 6047–6050, 2000. doi: 10.1074/jbc.275.9.6047. [DOI] [PubMed] [Google Scholar]
- 469.Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, Inglese M, McLoughlin RM, Jones SA, Topley N, Baumann H, Judd LM, Giraud AS, Boussioutas A, Zhu HJ, Ernst M. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med 11: 845–852, 2005. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 470.Jeon CY, Kim HJ, Morii H, Mori N, Settleman J, Lee JY, Kim J, Kim SC, Park JB. Neurite outgrowth from PC12 cells by basic fibroblast growth factor (bFGF) is mediated by RhoA inactivation through p190RhoGAP and ARAP3. J Cell Physiol 224: 786–794, 2010. doi: 10.1002/jcp.22184. [DOI] [PubMed] [Google Scholar]
- 471.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol 60: 1195–1200, 2001. doi: 10.1124/mol.60.6.1195. [DOI] [PubMed] [Google Scholar]
- 472.Jeyaraj SC, Unger NT, Eid AH, Mitra S, Paul El-Dahdah N, Quilliam LA, Flavahan NA, Chotani MA. Cyclic AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am J Physiol Cell Physiol 303: C499–C511, 2012. doi: 10.1152/ajpcell.00461.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X. Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. J Biol Chem 282: 37370–37377, 2007. doi: 10.1074/jbc.M703697200. [DOI] [PubMed] [Google Scholar]
- 474.Ji Z, Mei FC, Miller AL, Thompson EB, Cheng X. Protein kinase A (PKA) isoform RIIbeta mediates the synergistic killing effect of cAMP and glucocorticoid in acute lymphoblastic leukemia cells. J Biol Chem 283: 21920–21925, 2008. doi: 10.1074/jbc.M803193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 476.Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, Fraser I, Sternweis PC. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282: 10576–10584, 2007. doi: 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Jimenez JL, Punzón C, Navarro J, Muñoz-Fernández MA, Fresno M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J Pharmacol Exp Ther 299: 753–759, 2001. [PubMed] [Google Scholar]
- 478.Jing H, Vassiliou E, Ganea D. Prostaglandin E2 inhibits production of the inflammatory chemokines CCL3 and CCL4 in dendritic cells. J Leukoc Biol 74: 868–879, 2003. doi: 10.1189/jlb.0303116. [DOI] [PubMed] [Google Scholar]
- 479.Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem 279: 55176–55186, 2004. doi: 10.1074/jbc.M409816200. [DOI] [PubMed] [Google Scholar]
- 480.Joassard OR, Amirouche A, Gallot YS, Desgeorges MM, Castells J, Durieux AC, Berthon P, Freyssenet DG. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. Int J Biochem Cell Biol 45: 2444–2455, 2013. doi: 10.1016/j.biocel.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 481.Johnston A, Ponzetti K, Anwer MS, Webster CR. cAMP-guanine exchange factor protection from bile acid-induced hepatocyte apoptosis involves glycogen synthase kinase regulation of c-Jun NH2-terminal kinase. Am J Physiol Gastrointest Liver Physiol 301: G385–G400, 2011. doi: 10.1152/ajpgi.00430.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Jorissen E, Prox J, Bernreuther C, Weber S, Schwanbeck R, Serneels L, Snellinx A, Craessaerts K, Thathiah A, Tesseur I, Bartsch U, Weskamp G, Blobel CP, Glatzel M, De Strooper B, Saftig P. The disintegrin/metalloproteinase ADAM10 is essential for the establishment of the brain cortex. J Neurosci 30: 4833–4844, 2010. doi: 10.1523/JNEUROSCI.5221-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Joshi R, Kadeer N, Sheriff S, Friend LA, James JH, Balasubramaniam A. Phosphodiesterase (PDE) inhibitor torbafylline (HWA 448) attenuates burn-induced rat skeletal muscle proteolysis through the PDE4/cAMP/EPAC/PI3K/Akt pathway. Mol Cell Endocrinol 393: 152–163, 2014. doi: 10.1016/j.mce.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 484.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA 93: 295–299, 1996. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144: 282–295, 2011. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest 93: 2431–2437, 1994. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, Yeung PK, Tam S, Wang J, Lam KS, Vanhoutte PM, Bos JL, Chung SS, Xu A, Chung SK. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J 27: 4122–4135, 2013. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 488.Kaliński P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97: 3466–3469, 2001. doi: 10.1182/blood.V97.11.3466. [DOI] [PubMed] [Google Scholar]
- 489.Kaneko K, Xu P, Cordonier EL, Chen SS, Ng A, Xu Y, Morozov A, Fukuda M. Neuronal Rap1 Regulates Energy Balance, Glucose Homeostasis, and Leptin Actions. Cell Reports 16: 3003–3015, 2016. doi: 10.1016/j.celrep.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci 24: 5202–5208, 2004. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol 536: 375–385, 2001. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol 573: 595–609, 2006. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol 566: 173–188, 2005. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278: 8279–8285, 2003. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J Physiol 586: 1307–1319, 2008. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Kannan N, Wu J, Anand GS, Yooseph S, Neuwald AF, Venter JC, Taylor SS. Evolution of allostery in the cyclic nucleotide binding module. Genome Biol 8: R264, 2007. doi: 10.1186/gb-2007-8-12-r264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J Cell Sci 112: 2725–2736, 1999. [DOI] [PubMed] [Google Scholar]
- 498.Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 8: 277–289, 1994. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- 499.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276: 46046–46053, 2001. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 500.Kassel KM, Wyatt TA, Panettieri RA Jr, Toews ML. Inhibition of human airway smooth muscle cell proliferation by beta 2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol 294: L131–L138, 2008. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- 501.Kato JY, Matsuoka M, Polyak K, Massagué J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79: 487–496, 1994. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 502.Kato Y, Yokoyama U, Yanai C, Ishige R, Kurotaki D, Umemura M, Fujita T, Kubota T, Okumura S, Sata M, Tamura T, Ishikawa Y. Epac1 Deficiency Attenuated Vascular Smooth Muscle Cell Migration and Neointimal Formation. Arterioscler Thromb Vasc Biol 35: 2617–2625, 2015. doi: 10.1161/ATVBAHA.115.306534. [DOI] [PubMed] [Google Scholar]
- 503.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997. [PubMed] [Google Scholar]
- 504.Kaur S, Kong CH, Cannell MB, Ward ML. Depotentiation of intact rat cardiac muscle unmasks an Epac-dependent increase in myofilament Ca(2+) sensitivity. Clin Exp Pharmacol Physiol 43: 88–94, 2016. doi: 10.1111/1440-1681.12504. [DOI] [PubMed] [Google Scholar]
- 505.Kavelaars A, Eijkelkamp N, Willemen HL, Wang H, Carbajal AG, Heijnen CJ. Microglial GRK2: a novel regulator of transition from acute to chronic pain. Brain Behav Immun 25: 1055–1060, 2011. doi: 10.1016/j.bbi.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 506.Kawabata A. Prostaglandin E2 and pain–an update. Biol Pharm Bull 34: 1170–1173, 2011. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- 507.Kawabe J, Iwami G, Ebina T, Ohno S, Katada T, Ueda Y, Homcy CJ, Ishikawa Y. Differential activation of adenylyl cyclase by protein kinase C isoenzymes. J Biol Chem 269: 16554–16558, 1994. [PubMed] [Google Scholar]
- 508.Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta 1851: 414–421, 2015. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 509.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem 278: 9435–9440, 2003. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 510.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 511.Kawase T, Okuda K, Burns DM. Immature human osteoblastic MG63 cells predominantly express a subtype 1-like CGRP receptor that inactivates extracellular signal response kinase by a cAMP-dependent mechanism. Eur J Pharmacol 470: 125–137, 2003. doi: 10.1016/S0014-2999(03)01763-1. [DOI] [PubMed] [Google Scholar]
- 512.Ke K, Safder AM, Sul OJ, Suh JH, Joe Y, Chung HT, Choi HS. Cilostazol attenuates ovariectomy-induced bone loss by inhibiting osteoclastogenesis. PLoS One 10: e0124869, 2015. doi: 10.1371/journal.pone.0124869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 153: 530–534, 1996. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 514.Kelleher RJ III, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116: 467–479, 2004. doi: 10.1016/S0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 515.Kelley GG, Chepurny OG, Schwede F, Genieser HG, Leech CA, Roe MW, Li X, Dzhura I, Dzhura E, Afshari P, Holz GG. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM. Islets 1: 260–265, 2009. doi: 10.4161/isl.1.3.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Kelly MP, Stein JM, Vecsey CG, Favilla C, Yang X, Bizily SF, Esposito MF, Wand G, Kanes SJ, Abel T. Developmental etiology for neuroanatomical and cognitive deficits in mice overexpressing Galphas, a G-protein subunit genetically linked to schizophrenia. Mol Psychiatry 14: 398–415, 2009. doi: 10.1038/mp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Kessler S, Rho H, West G, Fiocchi C, Drazba J, de la Motte C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin Transl Sci 1: 57–61, 2008. doi: 10.1111/j.1752-8062.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Khouri C, Dittrich A, Sackett SD, Denecke B, Trautwein C, Schaper F. Glucagon counteracts interleukin-6-dependent gene expression by redundant action of Epac and PKA. Biol Chem 392: 1123–1134, 2011. doi: 10.1515/BC.2011.171. [DOI] [PubMed] [Google Scholar]
- 519.Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S, Piiper A. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell 16: 5639–5648, 2005. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Kim DH, Lerner A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood 92: 2484–2494, 1998. [PubMed] [Google Scholar]
- 521.Kim JG, Moon MY, Kim HJ, Li Y, Song DK, Kim JS, Lee JY, Kim J, Kim SC, Park JB. Ras-related GTPases Rap1 and RhoA collectively induce the phagocytosis of serum-opsonized zymosan particles in macrophages. J Biol Chem 287: 5145–5155, 2012. doi: 10.1074/jbc.M111.257634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Kim JS, Peng X, De PK, Geahlen RL, Durden DL. PTEN controls immunoreceptor (immunoreceptor tyrosine-based activation motif) signaling and the activation of Rac. Blood 99: 694–697, 2002. doi: 10.1182/blood.V99.2.694. [DOI] [PubMed] [Google Scholar]
- 523.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 19: 567–575, 2013. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 524.Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, Norton D, Tesco G, Elliott K, Wagner SL, Moir RD, Becker KD, Tanzi RE. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuate alpha-secretase activity. Hum Mol Genet 18: 3987–3996, 2009. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, Muallem S, Shin DM. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem 285: 6913–6921, 2010. doi: 10.1074/jbc.M109.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273: 245–248, 1996. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 527.Kimura TE, Duggirala A, Hindmarch CC, Hewer RC, Cui MZ, Newby AC, Bond M. Inhibition of Egr1 expression underlies the anti-mitogenic effects of cAMP in vascular smooth muscle cells. J Mol Cell Cardiol 72: 9–19, 2014. doi: 10.1016/j.yjmcc.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5: 687–698, 2004. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 529.King TE Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, Valeyre D, Leconte I, Morganti A, Roux S, Behr J. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 184: 92–99, 2011. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 530.Kinoshita T, Kobayashi S, Ebara S, Yoshimura Y, Horiuchi H, Tsutsumimoto T, Wakabayashi S, Takaoka K. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone 27: 811–817, 2000. doi: 10.1016/S8756-3282(00)00395-1. [DOI] [PubMed] [Google Scholar]
- 531.Kinukawa M, Oda S, Shirakura Y, Okabe M, Ohmuro J, Baba SA, Nagata M, Aoki F. Roles of cAMP in regulating microtubule sliding and flagellar bending in demembranated hamster spermatozoa. FEBS Lett 580: 1515–1520, 2006. doi: 10.1016/j.febslet.2006.01.078. [DOI] [PubMed] [Google Scholar]
- 532.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell 56: 77–84, 1989. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 533.Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci USA 88: 9307–9310, 1991. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K. Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity. PLoS One 10: e0122513, 2015. doi: 10.1371/journal.pone.0122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Klarenbeek J, Jalink K. Detecting cAMP with an EPAC-based FRET sensor in single living cells. Methods Mol Biol 1071: 49–58, 2014. doi: 10.1007/978-1-62703-622-1_4. [DOI] [PubMed] [Google Scholar]
- 536.Klarenbeek JB, Goedhart J, Hink MA, Gadella TW, Jalink K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PLoS One 6: e19170, 2011. doi: 10.1371/journal.pone.0019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271: 1589–1592, 1996. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 538.Kleibeuker W, Ledeboer A, Eijkelkamp N, Watkins LR, Maier SF, Zijlstra J, Heijnen CJ, Kavelaars A. A role for G protein-coupled receptor kinase 2 in mechanical allodynia. Eur J Neurosci 25: 1696–1704, 2007. doi: 10.1111/j.1460-9568.2007.05423.x. [DOI] [PubMed] [Google Scholar]
- 539.Klein U, Tu Y, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, Inghirami G, Cro L, Baldini L, Neri A, Califano A, Dalla-Favera R. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med 194: 1625–1638, 2001. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Kline WO, Panaro FJ, Yang H, Bodine SC. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol (1985) 102: 740–747, 2007. doi: 10.1152/japplphysiol.00873.2006. [DOI] [PubMed] [Google Scholar]
- 541.Kocieda VP, Adhikary S, Emig F, Yen JH, Toscano MG, Ganea D. Prostaglandin E2-induced IL-23p19 subunit is regulated by cAMP-responsive element-binding protein and C/AATT enhancer-binding protein β in bone marrow-derived dendritic cells. J Biol Chem 287: 36922–36935, 2012. doi: 10.1074/jbc.M112.402958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Koff WC, Fann AV, Dunegan MA, Lachman LB. Catecholamine-induced suppression of interleukin-1 production. Lymphokine Res 5: 239–247, 1986. [PubMed] [Google Scholar]
- 543.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 544.Kojro E, Postina R, Buro C, Meiringer C, Gehrig-Burger K, Fahrenholz F. The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J 20: 512–514, 2006. doi: 10.1096/fj.05-4812fje. [DOI] [PubMed] [Google Scholar]
- 545.Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, Seeger W, Banat GA, Schermuly RT, Pullamsetti SS. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell 20: 4751–4765, 2009. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Komatsu M, Takei M, Ishii H, Sato Y. Glucose-stimulated insulin secretion: a newer perspective. J Diabetes Investig 4: 511–516, 2013. doi: 10.1111/jdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Konu O, Kane JK, Barrett T, Vawter MP, Chang R, Ma JZ, Donovan DM, Sharp B, Becker KG, Li MD. Region-specific transcriptional response to chronic nicotine in rat brain. Brain Res 909: 194–203, 2001. doi: 10.1016/S0006-8993(01)02685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett 579: 4966–4972, 2005. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 549.Kopperud RK, Rygh CB, Karlsen TV, Krakstad C, Kleppe R, Hoivik EA, Bakke M, Tenstad O, Selheim F, Lidén Å, Madsen L, Pavlin T, Taxt T, Kristiansen K, Curry FE, Reed RK, Døskeland SO. Increased microvascular permeability in mice lacking Epac1 (Rapgef3). Acta Physiol (Oxf) 219: 441–452, 2017. doi: 10.1111/apha.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Korayem AH, Mujica PE, Aramoto H, Durán RG, Nepali PR, Kim DD, Harris AL, Sánchez FA, Durán WN. Endothelial cAMP deactivates ischemia-reperfusion-induced microvascular hyperpermeability via Rap1-mediated mechanisms. Am J Physiol Heart Circ Physiol 313: H179–H189, 2017. doi: 10.1152/ajpheart.00002.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Korotkova M, Westman M, Gheorghe KR, af Klint E, Trollmo C, Ulfgren AK, Klareskog L, Jakobsson PJ. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum 52: 3439–3447, 2005. doi: 10.1002/art.21390. [DOI] [PubMed] [Google Scholar]
- 552.Kortenoeven ML, Trimpert C, van den Brand M, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol 302: F1395–F1401, 2012. doi: 10.1152/ajprenal.00376.2011. [DOI] [PubMed] [Google Scholar]
- 553.Kou R, Shiroto T, Sartoretto JL, Michel T. Suppression of Gαs synthesis by simvastatin treatment of vascular endothelial cells. J Biol Chem 287: 2643–2651, 2012. doi: 10.1074/jbc.M111.303594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Kraemer A, Rehmann HR, Cool RH, Theiss C, de Rooij J, Bos JL, Wittinghofer A. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J Mol Biol 306: 1167–1177, 2001. doi: 10.1006/jmbi.2001.4444. [DOI] [PubMed] [Google Scholar]
- 555.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43: 1585–1594, 2002. doi: 10.1194/jlr.R200009-JLR200. [DOI] [PubMed] [Google Scholar]
- 556.Krähling AM, Alvarez L, Debowski K, Van Q, Gunkel M, Irsen S, Al-Amoudi A, Strünker T, Kremmer E, Krause E, Voigt I, Wörtge S, Waisman A, Weyand I, Seifert R, Kaupp UB, Wachten D. CRIS-a novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet 9: e1003960, 2013. doi: 10.1371/journal.pgen.1003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Krakstad C, Christensen AE, Døskeland SO. cAMP protects neutrophils against TNF-alpha-induced apoptosis by activation of cAMP-dependent protein kinase, independently of exchange protein directly activated by cAMP (Epac). J Leukoc Biol 76: 641–647, 2004. doi: 10.1189/jlb.0104005. [DOI] [PubMed] [Google Scholar]
- 558.Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci 14: 6638–6651, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Kreckler LM, Gizewski E, Wan TC, Auchampach JA. Adenosine suppresses lipopolysaccharide-induced tumor necrosis factor-alpha production by murine macrophages through a protein kinase A- and exchange protein activated by cAMP-independent signaling pathway. J Pharmacol Exp Ther 331: 1051–1061, 2009. doi: 10.1124/jpet.109.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Krugmann S, Williams R, Stephens L, Hawkins PT. ARAP3 is a PI3K- and rap-regulated GAP for RhoA. Curr Biol 14: 1380–1384, 2004. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 561.Krupinski J, Coussen F, Bakalyar HA, Tang WJ, Feinstein PG, Orth K, Slaughter C, Reed RR, Gilman AG. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science 244: 1558–1564, 1989. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- 562.Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol 110: 1097–1106, 2013. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci 27: 1700–1709, 2008. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 564.Kuhn PH, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF. ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J 29: 3020–3032, 2010. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Kumar DP, Asgharpour A, Mirshahi F, Park SH, Liu S, Imai Y, Nadler JL, Grider JR, Murthy KS, Sanyal AJ. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis. J Biol Chem 291: 6626–6640, 2016. doi: 10.1074/jbc.M115.699504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Kumar DP, Rajagopal S, Mahavadi S, Mirshahi F, Grider JR, Murthy KS, Sanyal AJ. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun 427: 600–605, 2012. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Kumar N, Gupta S, Dabral S, Singh S, Sehrawat S. Role of exchange protein directly activated by cAMP (EPAC1) in breast cancer cell migration and apoptosis. Mol Cell Biochem 430: 115–125, 2017. doi: 10.1007/s11010-017-2959-3. [DOI] [PubMed] [Google Scholar]
- 568.Kusama K, Yoshie M, Tamura K, Daikoku T, Takarada T, Tachikawa E. Possible roles of the cAMP-mediators EPAC and RAP1 in decidualization of rat uterus. Reproduction 147: 897–906, 2014. doi: 10.1530/REP-13-0654. [DOI] [PubMed] [Google Scholar]
- 569.Kusama K, Yoshie M, Tamura K, Imakawa K, Tachikawa E. EPAC2-mediated calreticulin regulates LIF and COX2 expression in human endometrial glandular cells. J Mol Endocrinol 54: 17–24, 2015. doi: 10.1530/JME-14-0162. [DOI] [PubMed] [Google Scholar]
- 570.Kusama K, Yoshie M, Tamura K, Kodaka Y, Hirata A, Sakurai T, Bai H, Imakawa K, Nishi H, Isaka K, Nagai T, Nagao T, Tachikawa E. Regulation of decidualization in human endometrial stromal cells through exchange protein directly activated by cyclic AMP (Epac). Placenta 34: 212–221, 2013. doi: 10.1016/j.placenta.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 571.Kusama K, Yoshie M, Tamura K, Nakayama T, Nishi H, Isaka K, Tachikawa E. The role of exchange protein directly activated by cyclic AMP 2-mediated calreticulin expression in the decidualization of human endometrial stromal cells. Endocrinology 155: 240–248, 2014. doi: 10.1210/en.2013-1478. [DOI] [PubMed] [Google Scholar]
- 572.L’Allemain G, Lavoie JN, Rivard N, Baldin V, Pouyssegur J. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene 14: 1981–1990, 1997. doi: 10.1038/sj.onc.1201038. [DOI] [PubMed] [Google Scholar]
- 573.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell 7: 585–595, 2004. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 574.Lai HL, Yang TH, Messing RO, Ching YH, Lin SC, Chern Y. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2a-adenosine receptor-mediated cAMP response. J Biol Chem 272: 4970–4977, 1997. doi: 10.1074/jbc.272.8.4970. [DOI] [PubMed] [Google Scholar]
- 575.Lai TW, Lin SZ, Lee HT, Fan JR, Hsu YH, Wang HJ, Yu YL, Shyu WC. HIF-1α binding to the Epac1 promoter recruits hematopoietic stem cells to the ischemic brain following stroke. J Mol Cell Biol 4: 184–187, 2012. doi: 10.1093/jmcb/mjs009. [DOI] [PubMed] [Google Scholar]
- 576.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin αvβ3. Blood 118: 2015–2026, 2011. doi: 10.1182/blood-2011-04-349282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Lakshmikanthan S, Zieba BJ, Ge ZD, Momotani K, Zheng X, Lund H, Artamonov MV, Maas JE, Szabo A, Zhang DX, Auchampach JA, Mattson DL, Somlyo AV, Chrzanowska-Wodnicka M. Rap1b in smooth muscle and endothelium is required for maintenance of vascular tone and normal blood pressure. Arterioscler Thromb Vasc Biol 34: 1486–1494, 2014. doi: 10.1161/ATVBAHA.114.303678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Laliberté F, Liu S, Gorseth E, Bobechko B, Bartlett A, Lario P, Gresser MJ, Huang Z. In vitro PKA phosphorylation-mediated human PDE4A4 activation. FEBS Lett 512: 205–208, 2002. doi: 10.1016/S0014-5793(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 579.Landa LR Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280: 31294–31302, 2005. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 581.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest 104: 399–407, 1999. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Jüppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273: 663–666, 1996. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 583.Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. J Immunol 194: 3829–3839, 2015. doi: 10.4049/jimmunol.1401633. [DOI] [PubMed] [Google Scholar]
- 584.Laroche-Joubert N, Marsy S, Michelet S, Imbert-Teboul M, Doucet A. Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J Biol Chem 277: 18598–18604, 2002. doi: 10.1074/jbc.M201868200. [DOI] [PubMed] [Google Scholar]
- 585.Lastres-Becker I, Fernández-Pérez A, Cebolla B, Vallejo M. Pituitary adenylate cyclase-activating polypeptide stimulates glial fibrillary acidic protein gene expression in cortical precursor cells by activating Ras and Rap1. Mol Cell Neurosci 39: 291–301, 2008. doi: 10.1016/j.mcn.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 586.Laurent AC, Bisserier M, Lucas A, Tortosa F, Roumieux M, De Régibus A, Swiader A, Sainte-Marie Y, Heymes C, Vindis C, Lezoualc’h F. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc Res 105: 55–64, 2015. doi: 10.1093/cvr/cvu242. [DOI] [PubMed] [Google Scholar]
- 587.Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem 284: 5119–5127, 2009. doi: 10.1074/jbc.M807117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Lee JH, Oh GT, Park SY, Choi JH, Park JG, Kim CD, Lee WS, Rhim BY, Shin YW, Hong KW. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-alpha formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther 313: 502–509, 2005. doi: 10.1124/jpet.104.079780. [DOI] [PubMed] [Google Scholar]
- 589.Lee JW, Lee J, Moon EY. HeLa human cervical cancer cell migration is inhibited by treatment with dibutyryl-cAMP. Anticancer Res 34: 3447–3455, 2014. [PubMed] [Google Scholar]
- 590.Lee K, Kobayashi Y, Seo H, Kwak JH, Masuda A, Lim CS, Lee HR, Kang SJ, Park P, Sim SE, Kogo N, Kawasaki H, Kaang BK, Itohara S. Involvement of cAMP-guanine nucleotide exchange factor II in hippocampal long-term depression and behavioral flexibility. Mol Brain 8: 38, 2015. doi: 10.1186/s13041-015-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106: 852–859, 2005. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 592.Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, Lorenzo J, Choi Y. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12: 1403–1409, 2006. doi: 10.1038/nm1514. [DOI] [PubMed] [Google Scholar]
- 593.Lee YJ, Kim MO, Ryu JM, Han HJ. Regulation of SGLT expression and localization through Epac/PKA-dependent caveolin-1 and F-actin activation in renal proximal tubule cells. Biochim Biophys Acta 1823: 971–982, 2012. doi: 10.1016/j.bbamcr.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 594.Leech CA, Chepurny OG, Holz GG. Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm 84: 279–302, 2010. doi: 10.1016/B978-0-12-381517-0.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG, Holz GG. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol 107: 236–247, 2011. doi: 10.1016/j.pbiomolbio.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. Facilitation of ß-cell K(ATP) channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets 2: 72–81, 2010. doi: 10.4161/isl.2.2.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Lehrke M, Kahles F, Makowska A, Tilstam PV, Diebold S, Marx J, Stöhr R, Hess K, Endorf EB, Bruemmer D, Marx N, Findeisen HM. PDE4 inhibition reduces neointima formation and inhibits VCAM-1 expression and histone methylation in an Epac-dependent manner. J Mol Cell Cardiol 81: 23–33, 2015. doi: 10.1016/j.yjmcc.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 598.Lerosey I, Pizon V, Tavitian A, de Gunzburg J. The cAMP-dependent protein kinase phosphorylates the rap1 protein in vitro as well as in intact fibroblasts, but not the closely related rap2 protein. Biochem Biophys Res Commun 175: 430–436, 1991. doi: 10.1016/0006-291X(91)91582-W. [DOI] [PubMed] [Google Scholar]
- 599.Lessmann V, Heumann R. Cyclic AMP endogenously enhances synaptic strength of developing glutamatergic synapses in serum-free microcultures of rat hippocampal neurons. Brain Res 763: 111–122, 1997. doi: 10.1016/S0006-8993(97)00406-X. [DOI] [PubMed] [Google Scholar]
- 600.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci 13: 2136–2148, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Lezoualc’h F, Fazal L, Laudette M, Conte C. Cyclic AMP Sensor EPAC Proteins and Their Role in Cardiovascular Function and Disease. Circ Res 118: 881–897, 2016. doi: 10.1161/CIRCRESAHA.115.306529. [DOI] [PubMed] [Google Scholar]
- 602.Lezoualc’h F, Robert SJ. The serotonin 5-HT4 receptor and the amyloid precursor protein processing. Exp Gerontol 38: 159–166, 2003. doi: 10.1016/S0531-5565(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 603.Li J, O’Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM Jr, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol 21: 159–171, 2007. doi: 10.1210/me.2006-0340. [DOI] [PubMed] [Google Scholar]
- 604.Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. J Biol Chem 275: 13026–13034, 2000. doi: 10.1074/jbc.275.17.13026. [DOI] [PubMed] [Google Scholar]
- 605.Li K, Liang J, Lin Y, Zhang H, Xiao X, Tan Y, Cai J, Zhu W, Xing F, Hu J, Yan G. A classical PKA inhibitor increases the oncolytic effect of M1 virus via activation of exchange protein directly activated by cAMP 1. Oncotarget 7: 48443–48455, 2016. doi: 10.18632/oncotarget.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Li K, Zhang H, Qiu J, Lin Y, Liang J, Xiao X, Fu L, Wang F, Cai J, Tan Y, Zhu W, Yin W, Lu B, Xing F, Tang L, Yan M, Mai J, Li Y, Chen W, Qiu P, Su X, Gao G, Tai PW, Hu J, Yan G. Activation of Cyclic Adenosine Monophosphate Pathway Increases the Sensitivity of Cancer Cells to the Oncolytic Virus M1. Mol Ther 24: 156–165, 2016. doi: 10.1038/mt.2015.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Li S, Tsalkova T, White MA, Mei FC, Liu T, Wang D, Woods VL Jr, Cheng X. Mechanism of intracellular cAMP sensor Epac2 activation: cAMP-induced conformational changes identified by amide hydrogen/deuterium exchange mass spectrometry (DXMS). J Biol Chem 286: 17889–17897, 2011. doi: 10.1074/jbc.M111.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Li Y, Asuri S, Rebhun JF, Castro AF, Paranavitana NC, Quilliam LA. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J Biol Chem 281: 2506–2514, 2006. doi: 10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- 609.Li Y, Kim JG, Kim HJ, Moon MY, Lee JY, Kim J, Kim SC, Song DK, Kim YS, Park JB. Small GTPases Rap1 and RhoA regulate superoxide formation by Rac1 GTPases activation during the phagocytosis of IgG-opsonized zymosans in macrophages. Free Radic Biol Med 52: 1796–1805, 2012. doi: 10.1016/j.freeradbiomed.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 610.Li Y, Konings IB, Zhao J, Price LS, de Heer E, Deen PM. Renal expression of exchange protein directly activated by cAMP (Epac) 1 and 2. Am J Physiol Renal Physiol 295: F525–F533, 2008. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- 611.Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang HT. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34: 2404–2419, 2009. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Liebenberg N, Müller HK, Fischer CW, Harvey BH, Brink CB, Elfving B, Wegener G. An inhibitor of cAMP-dependent protein kinase induces behavioural and neurological antidepressant-like effects in rats. Neurosci Lett 498: 158–161, 2011. doi: 10.1016/j.neulet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 613.Lim J, Dupuy AG, Critchley DR, Caron E. Rap1 controls activation of the α(M)β(2) integrin in a talin-dependent manner. J Cell Biochem 111: 999–1009, 2010. doi: 10.1002/jcb.22788. [DOI] [PubMed] [Google Scholar]
- 614.Lim JA, Juhnn YS. Isoproterenol increases histone deacetylase 6 expression and cell migration by inhibiting ERK signaling via PKA and Epac pathways in human lung cancer cells. Exp Mol Med 48: e204, 2016. doi: 10.1038/emm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Lim S, Lee SY, Seo HH, Ham O, Lee C, Park JH, Lee J, Seung M, Yun I, Han SM, Lee S, Choi E, Hwang KC. Regulation of mitochondrial morphology by positive feedback interaction between PKCδ and Drp1 in vascular smooth muscle cell. J Cell Biochem 116: 648–660, 2015. doi: 10.1002/jcb.25016. [DOI] [PubMed] [Google Scholar]
- 616.Lin KB, Freeman SA, Zabetian S, Brugger H, Weber M, Lei V, Dang-Lawson M, Tse KW, Santamaria R, Batista FD, Gold MR. The rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity 28: 75–87, 2008. doi: 10.1016/j.immuni.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 617.Lin S, Chugh S, Pan X, Wallner EI, Wada J, Kanwar YS. Identification of up-regulated Ras-like GTPase, Rap1b, by suppression subtractive hybridization. Kidney Int 60: 2129–2141, 2001. doi: 10.1046/j.1523-1755.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- 618.Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem 87: 1076–1085, 2003. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- 619.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem 278: 15922–15926, 2003. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 620.Liu C, Takahashi M, Li Y, Dillon TJ, Kaech S, Stork PJ. The interaction of Epac1 and Ran promotes Rap1 activation at the nuclear envelope. Mol Cell Biol 30: 3956–3969, 2010. doi: 10.1128/MCB.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Liu C, Takahashi M, Li Y, Song S, Dillon TJ, Shinde U, Stork PJ. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol 28: 7109–7125, 2008. doi: 10.1128/MCB.01060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Liu FC, Takahashi H, McKay RD, Graybiel AM. Dopaminergic regulation of transcription factor expression in organotypic cultures of developing striatum. J Neurosci 15: 2367–2384, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Liu FY, Cogan MG. Angiotensin II stimulates early proximal bicarbonate absorption in the rat by decreasing cyclic adenosine monophosphate. J Clin Invest 84: 83–91, 1989. doi: 10.1172/JCI114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Liu H, Enyeart JA, Enyeart JJ. ACTH induces Cav3.2 current and mRNA by cAMP-dependent and cAMP-independent mechanisms. J Biol Chem 285: 20040–20050, 2010. doi: 10.1074/jbc.M110.104190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Liu H, Enyeart JA, Enyeart JJ. ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways. J Gen Physiol 132: 279–294, 2008. doi: 10.1085/jgp.200810003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Liu H, Enyeart JA, Enyeart JJ. N6-substituted cAMP analogs inhibit bTREK-1 K+ channels and stimulate cortisol secretion by a protein kinase A-independent mechanism. Mol Pharmacol 76: 1290–1301, 2009. doi: 10.1124/mol.109.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Liu X, Chen Y, Tong J, Reynolds AM, Proudfoot SC, Qi J, Penzes P, Lu Y, Liu QS. Epac Signaling Is Required for Cocaine-Induced Change in AMPA Receptor Subunit Composition in the Ventral Tegmental Area. J Neurosci 36: 4802–4815, 2016. doi: 10.1523/JNEUROSCI.3186-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Liu Y, Schneider MF. Opposing HDAC4 nuclear fluxes due to phosphorylation by β-adrenergic activated protein kinase A or by activity or Epac activated CaMKII in skeletal muscle fibres. J Physiol 591: 3605–3623, 2013. doi: 10.1113/jphysiol.2013.256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J 349: 547–557, 2000. doi: 10.1042/bj3490547. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 630.Lo AC, De Maeyer JH, Vermaercke B, Callaerts-Vegh Z, Schuurkes JA, D’Hooge R. SSP-002392, a new 5-HT4 receptor agonist, dose-dependently reverses scopolamine-induced learning and memory impairments in C57Bl/6 mice. Neuropharmacology 85: 178–189, 2014. doi: 10.1016/j.neuropharm.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 631.Lo KW, Ashe KM, Kan HM, Lee DA, Laurencin CT. Activation of cyclic amp/protein kinase: a signaling pathway enhances osteoblast cell adhesion on biomaterials for regenerative engineering. J Orthop Res 29: 602–608, 2011. doi: 10.1002/jor.21276. [DOI] [PubMed] [Google Scholar]
- 632.Lobo MJ, Amaral MD, Zaccolo M, Farinha CM. EPAC1 activation by cAMP stabilizes CFTR at the membrane by promoting its interaction with NHERF1. J Cell Sci 129: 2599–2612, 2016. doi: 10.1242/jcs.185629. [DOI] [PubMed] [Google Scholar]
- 633.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 18: 6081–6092, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80: 1542–1552, 2006. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- 636.Lorenz R, Aleksic T, Wagner M, Adler G, Weber CK. The cAMP/Epac1/Rap1 pathway in pancreatic carcinoma. Pancreas 37: 102–103, 2008. doi: 10.1097/MPA.0b013e318160748f. [DOI] [PubMed] [Google Scholar]
- 637.Lotfi S, Li Z, Sun J, Zuo Y, Lam PP, Kang Y, Rahimi M, Islam D, Wang P, Gaisano HY, Jin T. Role of the exchange protein directly activated by cyclic adenosine 5′-monophosphate (Epac) pathway in regulating proglucagon gene expression in intestinal endocrine L cells. Endocrinology 147: 3727–3736, 2006. doi: 10.1210/en.2006-0056. [DOI] [PubMed] [Google Scholar]
- 638.Lu J, Landerholm TE, Wei JS, Dong XR, Wu SP, Liu X, Nagata K, Inagaki M, Majesky MW. Coronary smooth muscle differentiation from proepicardial cells requires rhoA-mediated actin reorganization and p160 rho-kinase activity. Dev Biol 240: 404–418, 2001. doi: 10.1006/dbio.2001.0403. [DOI] [PubMed] [Google Scholar]
- 639.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, McMurray JS, Fang X, Yung WK, Siminovitch KA, Mills GB. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem 278: 40057–40066, 2003. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 640.Lucchesi O, Ruete MC, Bustos MA, Quevedo MF, Tomes CN. The signaling module cAMP/Epac/Rap1/PLCε/IP3 mobilizes acrosomal calcium during sperm exocytosis. Biochim Biophys Acta 1863: 544–561, 2016. doi: 10.1016/j.bbamcr.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 641.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther 109: 366–398, 2006. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 642.Lum H, Del Vecchio PJ, Schneider AS, Goligorsky MS, Malik AB. Calcium dependence of the thrombin-induced increase in endothelial albumin permeability. J Appl Physiol (1985) 66: 1471–1476, 1989. doi: 10.1152/jappl.1989.66.3.1471. [DOI] [PubMed] [Google Scholar]
- 643.Luwor RB, Baradaran B, Taylor LE, Iaria J, Nheu TV, Amiry N, Hovens CM, Wang B, Kaye AH, Zhu HJ. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene 32: 2433–2441, 2013. doi: 10.1038/onc.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201, 2001. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 645.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401: 670–677, 1999. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 646.M’Rabet L, Coffer P, Zwartkruis F, Franke B, Segal AW, Koenderman L, Bos JL. Activation of the small GTPase rap1 in human neutrophils. Blood 92: 2133–2140, 1998. [PubMed] [Google Scholar]
- 647.Ma N, Abel T, Hernandez PJ. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem 16: 367–370, 2009. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Ma X, Guan Y, Hua X. Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells. J Diabetes 6: 394–402, 2014. doi: 10.1111/1753-0407.12161. [DOI] [PubMed] [Google Scholar]
- 649.Ma X, Zhang Y, Gromada J, Sewing S, Berggren PO, Buschard K, Salehi A, Vikman J, Rorsman P, Eliasson L. Glucagon stimulates exocytosis in mouse and rat pancreatic alpha-cells by binding to glucagon receptors. Mol Endocrinol 19: 198–212, 2005. doi: 10.1210/me.2004-0059. [DOI] [PubMed] [Google Scholar]
- 650.MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1). Br J Pharmacol 136: 421–433, 2002. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.MacLeod R, Hillert EK, Cameron RT, Baillie GS. The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer’s disease. Future Sci OA 1: fso.15.9, 2015. doi: 10.4155/fso.15.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.MacNicol MC, MacNicol AM. Nerve growth factor-stimulated B-Raf catalytic activity is refractory to inhibition by cAMP-dependent protein kinase. J Biol Chem 274: 13193–13197, 1999. doi: 10.1074/jbc.274.19.13193. [DOI] [PubMed] [Google Scholar]
- 653.Magiera MM, Gupta M, Rundell CJ, Satish N, Ernens I, Yarwood SJ. Exchange protein directly activated by cAMP (EPAC) interacts with the light chain (LC) 2 of MAP1A. Biochem J 382: 803–810, 2004. doi: 10.1042/BJ20040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, Fischmeister R, Lezoualc’h F. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol 5: 633–639, 2003. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 655.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 656.Makranz C, Cohen G, Reichert F, Kodama T, Rotshenker S. cAMP cascade (PKA, Epac, adenylyl cyclase, Gi, and phosphodiesterases) regulates myelin phagocytosis mediated by complement receptor-3 and scavenger receptor-AI/II in microglia and macrophages. Glia 53: 441–448, 2006. doi: 10.1002/glia.20303. [DOI] [PubMed] [Google Scholar]
- 657.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Mani BK, Chuang JC, Kjalarsdottir L, Sakata I, Walker AK, Kuperman A, Osborne-Lawrence S, Repa JJ, Zigman JM. Role of calcium and EPAC in norepinephrine-induced ghrelin secretion. Endocrinology 155: 98–107, 2014. doi: 10.1210/en.2013-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Manning DR, Gilman AG. The regulatory components of adenylate cyclase and transducin. A family of structurally homologous guanine nucleotide-binding proteins. J Biol Chem 258: 7059–7063, 1983. [PubMed] [Google Scholar]
- 660.Marchetti E, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS. Differential modulation of the 5-HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39: 2017–2027, 2000. doi: 10.1016/S0028-3908(00)00038-1. [DOI] [PubMed] [Google Scholar]
- 661.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 201: 1061–1067, 2005. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Marini M, Vittori E, Hollemborg J, Mattoli S. Expression of the potent inflammatory cytokines, granulocyte-macrophage-colony-stimulating factor and interleukin-6 and interleukin-8, in bronchial epithelial cells of patients with asthma. J Allergy Clin Immunol 89: 1001–1009, 1992. doi: 10.1016/0091-6749(92)90223-O. [DOI] [PubMed] [Google Scholar]
- 663.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Masyuk AI, Gradilone SA, Banales JM, Huang BQ, Masyuk TV, Lee SO, Splinter PL, Stroope AJ, Larusso NF. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol 295: G725–G734, 2008. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 666.Matsuo N, Hoshino M, Yoshizawa M, Nabeshima Y. Characterization of STEF, a guanine nucleotide exchange factor for Rac1, required for neurite growth. J Biol Chem 277: 2860–2868, 2002. doi: 10.1074/jbc.M106186200. [DOI] [PubMed] [Google Scholar]
- 667.Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10: 243–254, 1993. doi: 10.1016/0896-6273(93)90315-I. [DOI] [PubMed] [Google Scholar]
- 668.Mayer P, Hinze AV, Harst A, von Kügelgen I. A2B receptors mediate the induction of early genes and inhibition of arterial smooth muscle cell proliferation via Epac. Cardiovasc Res 90: 148–156, 2011. doi: 10.1093/cvr/cvq371. [DOI] [PubMed] [Google Scholar]
- 669.Maymó JL, Pérez Pérez A, Dueñas JL, Calvo JC, Sánchez-Margalet V, Varone CL. Regulation of placental leptin expression by cyclic adenosine 5′-monophosphate involves cross talk between protein kinase A and mitogen-activated protein kinase signaling pathways. Endocrinology 151: 3738–3751, 2010. doi: 10.1210/en.2010-0064. [DOI] [PubMed] [Google Scholar]
- 670.Maymó JL, Pérez Pérez A, Maskin B, Dueñas JL, Calvo JC, Sánchez Margalet V, Varone CL. The alternative Epac/cAMP pathway and the MAPK pathway mediate hCG induction of leptin in placental cells. PLoS One 7: e46216, 2012. doi: 10.1371/journal.pone.0046216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Mazhab-Jafari MT, Das R, Fotheringham SA, SilDas S, Chowdhury S, Melacini G. Understanding cAMP-dependent allostery by NMR spectroscopy: comparative analysis of the EPAC1 cAMP-binding domain in its apo and cAMP-bound states. J Am Chem Soc 129: 14482–14492, 2007. doi: 10.1021/ja0753703. [DOI] [PubMed] [Google Scholar]
- 672.Mazina O, Reinart-Okugbeni R, Kopanchuk S, Rinken A. BacMam system for FRET-based cAMP sensor expression in studies of melanocortin MC1 receptor activation. J Biomol Screen 17: 1096–1101, 2012. doi: 10.1177/1087057112449862. [DOI] [PubMed] [Google Scholar]
- 673.McAfee DA, Schorderet M, Greengard P. Adenosine 3′,5′-monophosphate in nervous tissue: increase associated with synaptic transmission. Science 171: 1156–1158, 1971. doi: 10.1126/science.171.3976.1156. [DOI] [PubMed] [Google Scholar]
- 674.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6: 483–495, 2004. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 675.McCann FE, Palfreeman AC, Andrews M, Perocheau DP, Inglis JJ, Schafer P, Feldmann M, Williams RO, Brennan FM. Apremilast, a novel PDE4 inhibitor, inhibits spontaneous production of tumour necrosis factor-alpha from human rheumatoid synovial cells and ameliorates experimental arthritis. Arthritis Res Ther 12: R107, 2010. doi: 10.1186/ar3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.McClatchey AI, Fehon RG. Merlin and the ERM proteins–regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol 19: 198–206, 2009. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 677.McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10: 27–38, 2012. doi: 10.1038/nrmicro2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.McKean JS, Murray F, Gibson G, Shewan DA, Tucker SJ, Nixon GF. The cAMP-producing agonist beraprost inhibits human vascular smooth muscle cell migration via exchange protein directly activated by cAMP. Cardiovasc Res 107: 546–555, 2015. doi: 10.1093/cvr/cvv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest 98: 2403–2413, 1996. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, Cooreman A, MacKenzie SJ. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem Soc Trans 33: 1330–1332, 2005. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- 682.Mediero A, Perez-Aso M, Cronstein BN. Activation of EPAC1/2 is essential for osteoclast formation by modulating NFκB nuclear translocation and actin cytoskeleton rearrangements. FASEB J 28: 4901–4913, 2014. doi: 10.1096/fj.14-255703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Medraño-Fernandez I, Reyes R, Olazabal I, Rodriguez E, Sanchez-Madrid F, Boussiotis VA, Reche PA, Cabañas C, Lafuente EM. RIAM (Rap1-interacting adaptor molecule) regulates complement-dependent phagocytosis. Cell Mol Life Sci 70: 2395–2410, 2013. doi: 10.1007/s00018-013-1268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 686.Mei FC, Cheng X. Interplay between exchange protein directly activated by cAMP (Epac) and microtubule cytoskeleton. Mol Biosyst 1: 325–331, 2005. doi: 10.1039/b511267b. [DOI] [PubMed] [Google Scholar]
- 687.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277: 11497–11504, 2002. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 688.Menon J, Doebele RC, Gomes S, Bevilacqua E, Reindl KM, Rosner MR. A novel interplay between Rap1 and PKA regulates induction of angiogenesis in prostate cancer. PLoS One 7: e49893, 2012. doi: 10.1371/journal.pone.0049893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Mestre MB, Colombo MI. cAMP and EPAC are key players in the regulation of the signal transduction pathway involved in the α-hemolysin autophagic response. PLoS Pathog 8: e1002664, 2012. doi: 10.1371/journal.ppat.1002664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Mestre MB, Colombo MI. Staphylococcus aureus promotes autophagy by decreasing intracellular cAMP levels. Autophagy 8: 1865–1867, 2012. doi: 10.4161/auto.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Mestre MB, Fader CM, Sola C, Colombo MI. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 6: 110–125, 2010. doi: 10.4161/auto.6.1.10698. [DOI] [PubMed] [Google Scholar]
- 692.Métrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc’h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 102: 959–965, 2008. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 693.Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J Bone Miner Res 20: 1858–1866, 2005. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA 95: 12683–12688, 1998. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375: 338–340, 1995. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 696.Middeldorp CM, Vink JM, Hettema JM, de Geus EJ, Kendler KS, Willemsen G, Neale MC, Boomsma DI, Chen X. An association between Epac-1 gene variants and anxiety and depression in two independent samples. Am J Med Genet B Neuropsychiatr Genet 153B: 214–219, 2010. doi: 10.1002/ajmg.b.30976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Midura EF, Prakash PS, Johnson BL III, Rice TC, Kunz N, Caldwell CC. Impact of caspase-8 and PKA in regulating neutrophil-derived microparticle generation. Biochem Biophys Res Commun 469: 917–922, 2016. doi: 10.1016/j.bbrc.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Milde M, Werthmann RC, von Hayn K, Bünemann M. Dynamics of adenylate cyclase regulation via heterotrimeric G-proteins. Biochem Soc Trans 42: 239–243, 2014. doi: 10.1042/BST20130280. [DOI] [PubMed] [Google Scholar]
- 699.Mileusnic R, Lancashire CL, Rose SP. The peptide sequence Arg-Glu-Arg, present in the amyloid precursor protein, protects against memory loss caused by A beta and acts as a cognitive enhancer. Eur J Neurosci 19: 1933–1938, 2004. doi: 10.1111/j.1460-9568.2004.03276.x. [DOI] [PubMed] [Google Scholar]
- 700.Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, Fujiwara K, Yan C. Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol 106: 1023–1039, 2011. doi: 10.1007/s00395-011-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA 101: 998–1003, 2004. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Milne GR, Palmer TM, Yarwood SJ. Novel control of cAMP-regulated transcription in vascular endothelial cells. Biochem Soc Trans 40: 1–5, 2012. doi: 10.1042/BST20110606. [DOI] [PubMed] [Google Scholar]
- 703.Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron 19: 1225–1235, 1997. doi: 10.1016/S0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 704.Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med 155: 1770–1776, 1997. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 705.Miro-Moran A, Jardin I, Ortega-Ferrusola C, Salido GM, Peña FJ, Tapia JA, Aparicio IM. Identification and function of exchange proteins activated directly by cyclic AMP (Epac) in mammalian spermatozoa. PLoS One 7: e37713, 2012. doi: 10.1371/journal.pone.0037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Mironov SL, Skorova E, Taschenberger G, Hartelt N, Nikolaev VO, Lohse MJ, Kügler S. Imaging cytoplasmic cAMP in mouse brainstem neurons. BMC Neurosci 10: 29, 2009. doi: 10.1186/1471-2202-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Mironov SL, Skorova EY. Stimulation of bursting in pre-Bötzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J Neurochem 117: 295–308, 2011. doi: 10.1111/j.1471-4159.2011.07202.x. [DOI] [PubMed] [Google Scholar]
- 708.Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal 20: 1459–1470, 2008. doi: 10.1016/j.cellsig.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Misra UK, Kaczowka SJ, Pizzo SV. Interaction between TCL1 and Epac1 in the activation of Akt kinases in plasma membranes and nuclei of 8-CPT-2-O-Me-cAMP-stimulated macrophages. Cell Signal 20: 130–138, 2008. doi: 10.1016/j.cellsig.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem 280: 38276–38289, 2005. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- 711.Misra UK, Pizzo SV. Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J Cell Biochem 108: 998–1011, 2009. doi: 10.1002/jcb.22333. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 712.Misra UK, Pizzo SV. Evidence for a pro-proliferative feedback loop in prostate cancer: the role of Epac1 and COX-2-dependent pathways. PLoS One 8: e63150, 2013. doi: 10.1371/journal.pone.0063150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 713.Misra UK, Pizzo SV. Upregulation of mTORC2 activation by the selective agonist of EPAC, 8-CPT-2Me-cAMP, in prostate cancer cells: assembly of a multiprotein signaling complex. J Cell Biochem 113: 1488–1500, 2012. doi: 10.1002/jcb.24018. [DOI] [PubMed] [Google Scholar]
- 714.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164: 567–574, 1989. doi: 10.1016/0006-291X(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 715.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci USA 96: 2135–2140, 1999. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 716.Mizuta K, Zhang Y, Xu D, Mizuta F, D’Ovidio F, Masaki E, Emala CW. The dopamine D1 receptor is expressed and facilitates relaxation in airway smooth muscle. Respir Res 14: 89, 2013. doi: 10.1186/1465-9921-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol 10: 2412–2425, 1999. [DOI] [PubMed] [Google Scholar]
- 718.Mogil JS. Perspective: Equality need not be painful. Nature 535: S7, 2016. doi: 10.1038/535S7a. [DOI] [PubMed] [Google Scholar]
- 719.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13: 859–866, 2012. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 720.Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem 104: 74–88, 2008. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Moon E, Lee R, Near R, Weintraub L, Wolda S, Lerner A. Inhibition of PDE3B augments PDE4 inhibitor-induced apoptosis in a subset of patients with chronic lymphocytic leukemia. Clin Cancer Res 8: 589–595, 2002. [PubMed] [Google Scholar]
- 722.Moon EY, Lee GH, Lee MS, Kim HM, Lee JW. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci 90: 373–380, 2012. doi: 10.1016/j.lfs.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 723.Moon EY, Lee JH, Lee JW, Song JH, Pyo S. ROS/Epac1-mediated Rap1/NF-kappaB activation is required for the expression of BAFF in Raw264.7 murine macrophages. Cell Signal 23: 1479–1488, 2011. doi: 10.1016/j.cellsig.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 724.Moon EY, Lee JH, Oh SY, Ryu SK, Kim HM, Kwak HS, Yoon WK. Reactive oxygen species augment B-cell-activating factor expression. Free Radic Biol Med 40: 2103–2111, 2006. doi: 10.1016/j.freeradbiomed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 725.Moon EY, Lee YS, Choi WS, Lee MH. Toll-like receptor 4-mediated cAMP production up-regulates B-cell activating factor expression in Raw264.7 macrophages. Exp Cell Res 317: 2447–2455, 2011. doi: 10.1016/j.yexcr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 726.Moon EY, Lerner A. PDE4 inhibitors activate a mitochondrial apoptotic pathway in chronic lymphocytic leukemia cells that is regulated by protein phosphatase 2A. Blood 101: 4122–4130, 2003. doi: 10.1182/blood-2002-10-3208. [DOI] [PubMed] [Google Scholar]
- 727.Moon EY, Pyo S. Lipopolysaccharide stimulates Epac1-mediated Rap1/NF-kappaB pathway in Raw 264.7 murine macrophages. Immunol Lett 110: 121–125, 2007. doi: 10.1016/j.imlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 728.Moon MY, Kim HJ, Kim JG, Lee JY, Kim J, Kim SC, Choi IG, Kim PH, Park JB. Small GTPase Rap1 regulates cell migration through regulation of small GTPase RhoA activity in response to transforming growth factor-β1. J Cell Physiol 228: 2119–2126, 2013. doi: 10.1002/jcp.24383. [DOI] [PubMed] [Google Scholar]
- 729.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompré AM, Vandecasteele G, Lezoualc’h F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res 97: 1296–1304, 2005. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 730.Moreno C, Mistry M, Roman RJ. Renal effects of glucagon-like peptide in rats. Eur J Pharmacol 434: 163–167, 2002. doi: 10.1016/S0014-2999(01)01542-4. [DOI] [PubMed] [Google Scholar]
- 731.Morgan SJ, Deshpande DA, Tiegs BC, Misior AM, Yan H, Hershfeld AV, Rich TC, Panettieri RA, An SS, Penn RB. β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J Biol Chem 289: 23065–23074, 2014. doi: 10.1074/jbc.M114.557652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Morris AC, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, McCulloch C, Barr LC, McDonald NA, Dhaliwal K, Jones RO, Mackellar A, Haslett C, Hay AW, Swann DG, Anderson N, Laurenson IF, Davidson DJ, Rossi AG, Walsh TS, Simpson AJ. C5a-mediated neutrophil dysfunction is RhoA-dependent and predicts infection in critically ill patients. Blood 117: 5178–5188, 2011. doi: 10.1182/blood-2010-08-304667. [DOI] [PubMed] [Google Scholar]
- 733.Mortaz E, Rad MV, Johnson M, Raats D, Nijkamp FP, Folkerts G. Salmeterol with fluticasone enhances the suppression of IL-8 release and increases the translocation of glucocorticoid receptor by human neutrophils stimulated with cigarette smoke. J Mol Med (Berl) 86: 1045–1056, 2008. doi: 10.1007/s00109-008-0360-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Morton S, Davis RJ, Cohen P. Signalling pathways involved in multisite phosphorylation of the transcription factor ATF-2. FEBS Lett 572: 177–183, 2004. doi: 10.1016/j.febslet.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 735.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE; SIRIUS Investigators . Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315–1323, 2003. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 736.Motawea HK, Blazek AD, Zirwas MJ, Pleister AP, Ahmed AA, McConnell BK, Chotani MA. Delocalization of Endogenous A-kinase Antagonizes Rap1-Rho-α2C-Adrenoceptor Signaling in Human Microvascular Smooth Muscle Cells. J Cytol Mol Biol 1: 1000002, 2014. [PMC free article] [PubMed] [Google Scholar]
- 737.Motawea HK, Jeyaraj SC, Eid AH, Mitra S, Unger NT, Ahmed AA, Flavahan NA, Chotani MA. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle α2C-adrenoceptors through the actin-binding protein filamin-2. Am J Physiol Cell Physiol 305: C829–C845, 2013. doi: 10.1152/ajpcell.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Mottino AD, Cao J, Veggi LM, Crocenzi F, Roma MG, Vore M. Altered localization and activity of canalicular Mrp2 in estradiol-17beta-d-glucuronide-induced cholestasis. Hepatology 35: 1409–1419, 2002. doi: 10.1053/jhep.2002.33327. [DOI] [PubMed] [Google Scholar]
- 739.Mourani PM, Garl PJ, Wenzlau JM, Carpenter TC, Stenmark KR, Weiser-Evans MC. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation 109: 1299–1306, 2004. doi: 10.1161/01.CIR.0000118462.22970.BE. [DOI] [PubMed] [Google Scholar]
- 740.Mukaetova-Ladinska EB, Arnold H, Jaros E, Perry R, Perry E. Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathol Appl Neurobiol 30: 615–623, 2004. doi: 10.1111/j.1365-2990.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 741.Mullen PJ, Zahno A, Lindinger P, Maseneni S, Felser A, Krähenbühl S, Brecht K. Susceptibility to simvastatin-induced toxicity is partly determined by mitochondrial respiration and phosphorylation state of Akt. Biochim Biophys Acta 1813: 2079–2087, 2011. doi: 10.1016/j.bbamcr.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 742.Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschöp MH. The New Biology and Pharmacology of Glucagon. Physiol Rev 97: 721–766, 2017. doi: 10.1152/physrev.00025.2016. [DOI] [PubMed] [Google Scholar]
- 743.Muñoz-Llancao P, de Gregorio C, Las Heras M, Meinohl C, Noorman K, Boddeke E, Cheng X, Lezoualc’h F, Schmidt M, Gonzalez-Billault C. Microtubule-regulating proteins and cAMP-dependent signaling in neuroblastoma differentiation. Cytoskeleton 74: 143–158, 2017. doi: 10.1002/cm.21355. [DOI] [PubMed] [Google Scholar]
- 744.Muñoz-Llancao P, Henríquez DR, Wilson C, Bodaleo F, Boddeke EW, Lezoualc’h F, Schmidt M, González-Billault C. Exchange Protein Directly Activated by cAMP (EPAC) Regulates Neuronal Polarization through Rap1B. J Neurosci 35: 11315–11329, 2015. doi: 10.1523/JNEUROSCI.3645-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 745.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, Ueno A, Oh-ishi S, Narumiya S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature 388: 678–682, 1997. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 746.Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci 38: 578–588, 2008. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 747.Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci 29: 15434–15444, 2009. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007. doi: 10.1074/jbc.M701910200. [DOI] [PubMed] [Google Scholar]
- 749.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 284: G1006–G1016, 2003. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- 750.Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol 37: 1985–1996, 2005. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 751.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90, 2002. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 752.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559, 2004. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Nagy N, Freudenberger T, Melchior-Becker A, Röck K, Ter Braak M, Jastrow H, Kinzig M, Lucke S, Suvorava T, Kojda G, Weber AA, Sörgel F, Levkau B, Ergün S, Fischer JW. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation 122: 2313–2322, 2010. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 754.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194: 629–644, 2001. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 756.Namkoong S, Kim CK, Cho YL, Kim JH, Lee H, Ha KS, Choe J, Kim PH, Won MH, Kwon YG, Shim EB, Kim YM. Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal 21: 906–915, 2009. doi: 10.1016/j.cellsig.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 757.Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J Clin Invest 108: 1229–1235, 2001. doi: 10.1172/JCI200113640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Catecholamines inhibit Ca2+-dependent proteolysis in rat skeletal muscle through beta(2)-adrenoceptors and cAMP. Am J Physiol Endocrinol Metab 281: E449–E454, 2001. doi: 10.1152/ajpendo.2001.281.3.E449. [DOI] [PubMed] [Google Scholar]
- 759.Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Effect of guanethidine-induced adrenergic blockade on the different proteolytic systems in rat skeletal muscle. Am J Physiol 277: E883–E889, 1999. [DOI] [PubMed] [Google Scholar]
- 760.Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Role of adrenoceptors and cAMP on the catecholamine-induced inhibition of proteolysis in rat skeletal muscle. Am J Physiol Endocrinol Metab 279: E663–E668, 2000. doi: 10.1152/ajpendo.2000.279.3.E663. [DOI] [PubMed] [Google Scholar]
- 761.Nenquin M, Henquin JC. Sulphonylurea receptor-1, sulphonylureas and amplification of insulin secretion by Epac activation in β cells. Diabetes Obes Metab 18: 698–701, 2016. doi: 10.1111/dom.12607. [DOI] [PubMed] [Google Scholar]
- 762.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res 101: 768–776, 2007. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- 763.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol 190: 300–309, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 764.Nguyen MP, Bittner GD, Fishman HM. Critical interval of somal calcium transient after neurite transection determines B 104 cell survival. J Neurosci Res 81: 805–816, 2005. doi: 10.1002/jnr.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 766.Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol 219: 652–658, 2009. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- 767.Nikolaev VO, Boivin V, Störk S, Angermann CE, Ertl G, Lohse MJ, Jahns R. A novel fluorescence method for the rapid detection of functional beta1-adrenergic receptor autoantibodies in heart failure. J Am Coll Cardiol 50: 423–431, 2007. doi: 10.1016/j.jacc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 768.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279: 37215–37218, 2004. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 769.Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol 276: F254–F259, 1999. [DOI] [PubMed] [Google Scholar]
- 770.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7: 270–277, 2005. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 771.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258: 304–307, 1992. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 772.Niu TK, Pfeifer AC, Lippincott-Schwartz J, Jackson CL. Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol Biol Cell 16: 1213–1222, 2005. doi: 10.1091/mbc.E04-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 773.Niwa M, Hara A, Kanamori Y, Matsuno H, Kozawa O, Yoshimi N, Mori H, Uematsu T. Inhibition of tumor necrosis factor-alpha induced neutrophil apoptosis by cyclic AMP: involvement of caspase cascade. Eur J Pharmacol 371: 59–67, 1999. doi: 10.1016/S0014-2999(99)00145-4. [DOI] [PubMed] [Google Scholar]
- 774.Noda Y, Sohara E, Ohta E, Sasaki S. Aquaporins in kidney pathophysiology. Nat Rev Nephrol 6: 168–178, 2010. doi: 10.1038/nrneph.2009.231. [DOI] [PubMed] [Google Scholar]
- 775.Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 136: 1869–1878, 2009. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Öberg AI, Dehvari N, Bengtsson T. β-Adrenergic inhibition of contractility in L6 skeletal muscle cells. PLoS One 6: e22304, 2011. doi: 10.1371/journal.pone.0022304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 777.Oenema TA, Kolahian S, Nanninga JE, Rieks D, Hiemstra PS, Zuyderduyn S, Halayko AJ, Meurs H, Gosens R. Pro-inflammatory mechanisms of muscarinic receptor stimulation in airway smooth muscle. Respir Res 11: 130, 2010. doi: 10.1186/1465-9921-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 778.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem 284: 1514–1522, 2009. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 779.Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem 282: 5488–5495, 2007. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- 780.Ohnuki Y, Umeki D, Mototani Y, Jin H, Cai W, Shiozawa K, Suita K, Saeki Y, Fujita T, Ishikawa Y, Okumura S. Role of cyclic AMP sensor Epac1 in masseter muscle hypertrophy and myosin heavy chain transition induced by β2-adrenoceptor stimulation. J Physiol 592: 5461–5475, 2014. doi: 10.1113/jphysiol.2014.282996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Ohnuki Y, Umeki D, Mototani Y, Shiozawa K, Nariyama M, Ito A, Kawamura N, Yagisawa Y, Jin H, Cai W, Suita K, Saeki Y, Fujita T, Ishikawa Y, Okumura S. Role of phosphodiesterase 4 expression in the Epac1 signaling-dependent skeletal muscle hypertrophic action of clenbuterol. Physiol Rep 4: e12791, 2016. doi: 10.14814/phy2.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916–920, 2001. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 783.Ohtsuka T, Shimizu K, Yamamori B, Kuroda S, Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J Biol Chem 271: 1258–1261, 1996. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- 784.Okada T, Hu CD, Jin TG, Kariya K, Yamawaki-Kataoka Y, Kataoka T. The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases. Mol Cell Biol 19: 6057–6064, 1999. doi: 10.1128/MCB.19.9.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Okumura S, Fujita T, Cai W, Jin M, Namekata I, Mototani Y, Jin H, Ohnuki Y, Tsuneoka Y, Kurotani R, Suita K, Kawakami Y, Hamaguchi S, Abe T, Kiyonari H, Tsunematsu T, Bai Y, Suzuki S, Hidaka Y, Umemura M, Ichikawa Y, Yokoyama U, Sato M, Ishikawa F, Izumi-Nakaseko H, Adachi-Akahane S, Tanaka H, Ishikawa Y. Epac1-dependent phospholamban phosphorylation mediates the cardiac response to stresses. J Clin Invest 124: 2785–2801, 2014. doi: 10.1172/JCI64784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.Oldenburger A, Roscioni SS, Jansen E, Menzen MH, Halayko AJ, Timens W, Meurs H, Maarsingh H, Schmidt M. Anti-inflammatory role of the cAMP effectors Epac and PKA: implications in chronic obstructive pulmonary disease. PLoS One 7: e31574, 2012. doi: 10.1371/journal.pone.0031574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Oldenburger A, Timens W, Bos S, Smit M, Smrcka AV, Laurent AC, Cao J, Hylkema M, Meurs H, Maarsingh H, Lezoualc’h F, Schmidt M. Epac1 and Epac2 are differentially involved in inflammatory and remodeling processes induced by cigarette smoke. FASEB J 28: 4617–4628, 2014. doi: 10.1096/fj.13-248930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 788.Oldenburger A, van Basten B, Kooistra W, Meurs H, Maarsingh H, Krenning G, Timens W, Schmidt M. Interaction between Epac1 and miRNA-7 in airway smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol 387: 795–797, 2014. doi: 10.1007/s00210-014-1015-z. [DOI] [PubMed] [Google Scholar]
- 789.Oliveira RF, Terrin A, Di Benedetto G, Cannon RC, Koh W, Kim M, Zaccolo M, Blackwell KT. The role of type 4 phosphodiesterases in generating microdomains of cAMP: large scale stochastic simulations. PLoS One 5: e11725, 2010. doi: 10.1371/journal.pone.0011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Olmedo I, Muñoz C, Guzmán N, Catalán M, Vivar R, Ayala P, Humeres C, Aránguiz P, García L, Velarde V, Díaz-Araya G. EPAC expression and function in cardiac fibroblasts and myofibroblasts. Toxicol Appl Pharmacol 272: 414–422, 2013. doi: 10.1016/j.taap.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 791.Oltmanns U, Chung KF, Walters M, John M, Mitchell JA. Cigarette smoke induces IL-8, but inhibits eotaxin and RANTES release from airway smooth muscle. Respir Res 6: 74, 2005. doi: 10.1186/1465-9921-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Onodera Y, Nam JM, Bissell MJ. Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest 124: 367–384, 2014. doi: 10.1172/JCI63146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Ostroveanu A, van der Zee EA, Eisel UL, Schmidt M, Nijholt IM. Exchange protein activated by cyclic AMP 2 (Epac2) plays a specific and time-limited role in memory retrieval. Hippocampus 20: 1018–1026, 2010. doi: 10.1002/hipo.20700. [DOI] [PubMed] [Google Scholar]
- 794.Ou Y, Chan G, Zuo J, Rattner JB, van der Hoorn FA. Purinergic A2b receptor activation by extracellular cues affects positioning of centrosome and nucleus and causes reduced cell migration. J Biol Chem 291: 15388–15403, 2016. doi: 10.1074/jbc.M116.721241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Ouyang M, Zhang L, Zhu JJ, Schwede F, Thomas SA. Epac signaling is required for hippocampus-dependent memory retrieval. Proc Natl Acad Sci USA 105: 11993–11997, 2008. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2: 805–811, 2000. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 797.Palmer AM. Neuroprotective therapeutics for Alzheimer’s disease: progress and prospects. Trends Pharmacol Sci 32: 141–147, 2011. doi: 10.1016/j.tips.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 798.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med 198: 669–675, 2003. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Pang L, Knox AJ. Bradykinin stimulates IL-8 production in cultured human airway smooth muscle cells: role of cyclooxygenase products. J Immunol 161: 2509–2515, 1998. [PubMed] [Google Scholar]
- 800.Panousis CG, Zuckerman SH. Interferon-gamma induces downregulation of Tangier disease gene (ATP-binding-cassette transporter 1) in macrophage-derived foam cells. Arterioscler Thromb Vasc Biol 20: 1565–1571, 2000. doi: 10.1161/01.ATV.20.6.1565. [DOI] [PubMed] [Google Scholar]
- 801.Panza F, Frisardi V, Imbimbo BP, Capurso C, Logroscino G, Sancarlo D, Seripa D, Vendemiale G, Pilotto A, Solfrizzi V. REVIEW: γ-Secretase inhibitors for the treatment of Alzheimer’s disease: The current state. CNS Neurosci Ther 16: 272–284, 2010. doi: 10.1111/j.1755-5949.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain 113: 185–190, 2005. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 803.Park JH, Kim SJ, Park SH, Son DG, Bae JH, Kim HK, Han J, Song DK. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic β-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology 153: 574–582, 2012. doi: 10.1210/en.2011-0259. [DOI] [PubMed] [Google Scholar]
- 804.Park JY, Juhnn YS. cAMP signaling increases histone deacetylase 8 expression via the Epac2-Rap1A-Akt pathway in H1299 lung cancer cells. Exp Mol Med 49: e297, 2017. doi: 10.1038/emm.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Park KH, Park HJ, Shin KS, Lee MK. Multiple treatments with l-3,4-dihydroxyphenylalanine modulate dopamine biosynthesis and neurotoxicity through the protein kinase A-transient extracellular signal-regulated kinase and exchange protein activation by cyclic AMP-sustained extracellular signal-regulated kinase signaling pathways. J Neurosci Res 92: 1746–1756, 2014. doi: 10.1002/jnr.23450. [DOI] [PubMed] [Google Scholar]
- 806.Park SH, Saleh D, Giaid A, Michel RP. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med 156: 600–608, 1997. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 807.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148: 421–433, 2012. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science 284: 1365–1368, 1999. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 809.Parnell E, McElroy SP, Wiejak J, Baillie GL, Porter A, Adams DR, Rehmann H, Smith BO, Yarwood SJ. Identification of a Novel, Small Molecule Partial Agonist for the Cyclic AMP Sensor, EPAC1. Sci Rep 7: 294, 2017. doi: 10.1038/s41598-017-00455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Parnell E, Palmer TM, Yarwood SJ. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci 36: 203–214, 2015. doi: 10.1016/j.tips.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Parnell E, Smith BO, Yarwood SJ. The cAMP sensors, EPAC1 and EPAC2, display distinct subcellular distributions despite sharing a common nuclear pore localisation signal. Cell Signal 27: 989–996, 2015. doi: 10.1016/j.cellsig.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Patel AJ, Honoré E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J 17: 4283–4290, 1998. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Patschan D, Patschan S, Wessels JT, Becker JU, David S, Henze E, Goligorsky MS, Müller GA. Epac-1 activator 8-O-cAMP augments renoprotective effects of syngeneic murine EPCs in acute ischemic kidney injury. Am J Physiol Renal Physiol 298: F78–F85, 2010. doi: 10.1152/ajprenal.00485.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Patschan D, Plotkin M, Goligorsky MS. Therapeutic use of stem and endothelial progenitor cells in acute renal injury: ça ira. Curr Opin Pharmacol 6: 176–183, 2006. doi: 10.1016/j.coph.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 815.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25: 947–970, 2004. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 816.Peebles RS Jr, Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, Christman JW, Kang KH, Jarzecka K, Furlong J, Mitchell DB, Talati M, Graham BS, Sheller JR. Selective cyclooxygenase-1 and -2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am J Respir Crit Care Med 165: 1154–1160, 2002. doi: 10.1164/ajrccm.165.8.2106025. [DOI] [PubMed] [Google Scholar]
- 817.Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA Jr, Benovic JL. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem 276: 32648–32656, 2001. doi: 10.1074/jbc.M104143200. [DOI] [PubMed] [Google Scholar]
- 818.Pepato MT, Migliorini RH, Goldberg AL, Kettelhut IC. Role of different proteolytic pathways in degradation of muscle protein from streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab 271: E340–E347, 1996. [DOI] [PubMed] [Google Scholar]
- 819.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM. Epac2 mediates cardiac β1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation 127: 913–922, 2013. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 820.Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Bénitah JP, Lezoualc’h F, Gómez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol 583: 685–694, 2007. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Pereira L, Ruiz-Hurtado G, Morel E, Laurent AC, Métrich M, Domínguez-Rodríguez A, Lauton-Santos S, Lucas A, Benitah JP, Bers DM, Lezoualc’h F, Gómez AM. Epac enhances excitation-transcription coupling in cardiac myocytes. J Mol Cell Cardiol 52: 283–291, 2012. doi: 10.1016/j.yjmcc.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 822.Perez-Aso M, Montesinos MC, Mediero A, Wilder T, Schafer PH, Cronstein B. Apremilast, a novel phosphodiesterase 4 (PDE4) inhibitor, regulates inflammation through multiple cAMP downstream effectors. Arthritis Res Ther 17: 249, 2015. doi: 10.1186/s13075-015-0771-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Persson E, Voznesensky OS, Huang YF, Lerner UH. Increased expression of interleukin-6 by vasoactive intestinal peptide is associated with regulation of CREB, AP-1 and C/EBP, but not NF-kappaB, in mouse calvarial osteoblasts. Bone 37: 513–529, 2005. doi: 10.1016/j.bone.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 824.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 39: 258–263, 2002. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 825.Pettersen FO, Torheim EA, Dahm AE, Aaberge IS, Lind A, Holm M, Aandahl EM, Sandset PM, Taskén K, Kvale D. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downregulated immune activation and improved T cell-dependent vaccine responses. J Virol 85: 6557–6566, 2011. doi: 10.1128/JVI.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Peverelli E, Mantovani G, Lania AG, Spada A. cAMP in the pituitary: an old messenger for multiple signals. J Mol Endocrinol 52: R67–R77, 2013. doi: 10.1530/JME-13-0172. [DOI] [PubMed] [Google Scholar]
- 827.Phillips LK, Prins JB. Update on incretin hormones. Ann N Y Acad Sci 1243: E55–E74, 2011. doi: 10.1111/j.1749-6632.2012.06491.x. [DOI] [PubMed] [Google Scholar]
- 828.Pignataro FS, Bonini M, Forgione A, Melandri S, Usmani OS. Asthma and gender: The female lung. Pharmacol Res 119: 384–390, 2017. doi: 10.1016/j.phrs.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 829.Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, Borghans JA, Tesselaar K, Koenderman L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116: 625–627, 2010. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 830.Pírez N, Christmann BL, Griffith LC. Daily rhythms in locomotor circuits in Drosophila involve PDF. J Neurophysiol 110: 700–708, 2013. doi: 10.1152/jn.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Pizon V, Desjardins M, Bucci C, Parton RG, Zerial M. Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the Golgi complex. J Cell Sci 107: 1661–1670, 1994. [DOI] [PubMed] [Google Scholar]
- 832.Poloso NJ, Urquhart P, Nicolaou A, Wang J, Woodward DF. PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol Immunol 54: 284–295, 2013. doi: 10.1016/j.molimm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 833.Pon LA. Organelle transport: mitochondria hitch a ride on dynamic microtubules. Curr Biol 21: R654–R656, 2011. doi: 10.1016/j.cub.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 834.Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol 29: 2521–2531, 2009. doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep 5: 1176–1180, 2004. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 836.Ponzetti K, King M, Gates A, Anwer MS, Webster CR. Cyclic AMP-guanine exchange factor activation inhibits JNK-dependent lipopolysaccharide-induced apoptosis in rat hepatocytes. Hepat Med 2010: 1–11, 2010. doi: 10.2147/HMER.S7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, 2010. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 5: 277–278, 2008. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- 839.Porter BO, Malek TR. Prostaglandin E2 inhibits T cell activation-induced apoptosis and Fas-mediated cellular cytotoxicity by blockade of Fas-ligand induction. Eur J Immunol 29: 2360–2365, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 840.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24: 463–469, 2015. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Pratt EP, Salyer AE, Guerra ML, Hockerman GH. Ca2+ influx through L-type Ca2+ channels and Ca2+-induced Ca2+ release regulate cAMP accumulation and Epac1-dependent ERK 1/2 activation in INS-1 cells. Mol Cell Endocrinol 419: 60–71, 2016. doi: 10.1016/j.mce.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Procino G, Carmosino M, Marin O, Brunati AM, Contri A, Pinna LA, Mannucci R, Nielsen S, Kwon TH, Svelto M, Valenti G. Ser-256 phosphorylation dynamics of Aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J 17: 1886–1888, 2003. doi: 10.1096/fj.02-0870fje. [DOI] [PubMed] [Google Scholar]
- 843.Pullamsetti SS, Banat GA, Schmall A, Szibor M, Pomagruk D, Hänze J, Kolosionek E, Wilhelm J, Braun T, Grimminger F, Seeger W, Schermuly RT, Savai R. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene 32: 1121–1134, 2013. doi: 10.1038/onc.2012.136. [DOI] [PubMed] [Google Scholar]
- 844.Purves GI, Kamishima T, Davies LM, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J Physiol 587: 3639–3650, 2009. doi: 10.1113/jphysiol.2009.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 845.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem 277: 26581–26586, 2002. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 846.Qin Y, Stokman G, Yan K, Ramaiahgari S, Verbeek F, de Graauw M, van de Water B, Price LS. cAMP signalling protects proximal tubular epithelial cells from cisplatin-induced apoptosis via activation of Epac. Br J Pharmacol 165, 4b: 1137–1150, 2012. doi: 10.1111/j.1476-5381.2011.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Qiu W, Zhuang S, von Lintig FC, Boss GR, Pilz RB. Cell type-specific regulation of B-Raf kinase by cAMP and 14-3-3 proteins. J Biol Chem 275: 31921–31929, 2000. doi: 10.1074/jbc.M003327200. [DOI] [PubMed] [Google Scholar]
- 848.Quiedeville A, Boulouard M, Hamidouche K, Da Silva Costa-Aze V, Nee G, Rochais C, Dallemagne P, Fabis F, Freret T, Bouet V. Chronic activation of 5-HT4 receptors or blockade of 5-HT6 receptors improve memory performances. Behav Brain Res 293: 10–17, 2015. doi: 10.1016/j.bbr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 849.Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res 94: 1359–1366, 2004. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 850.Rajagopal S, Kumar DP, Mahavadi S, Bhattacharya S, Zhou R, Corvera CU, Bunnett NW, Grider JR, Murthy KS. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol Gastrointest Liver Physiol 304: G527–G535, 2013. doi: 10.1152/ajpgi.00388.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Rall TW, Sutherland EW. Formation of a cyclic adenine ribonucleotide by tissue particles. J Biol Chem 232: 1065–1076, 1958. [PubMed] [Google Scholar]
- 852.Ramírez-Franco J, Bartolomé-Martín D, Alonso B, Torres M, Sánchez-Prieto J. Cannabinoid type 1 receptors transiently silence glutamatergic nerve terminals of cultured cerebellar granule cells. PLoS One 9: e88594, 2014. doi: 10.1371/journal.pone.0088594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Rampersad SN, Ovens JD, Huston E, Umana MB, Wilson LS, Netherton SJ, Lynch MJ, Baillie GS, Houslay MD, Maurice DH. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem 285: 33614–33622, 2010. doi: 10.1074/jbc.M110.140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 854.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111: 409–419, 1957. [PubMed] [Google Scholar]
- 855.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol 160: 487–493, 2003. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Raymond DR, Wilson LS, Carter RL, Maurice DH. Numerous distinct PKA-, or EPAC-based, signalling complexes allow selective phosphodiesterase 3 and phosphodiesterase 4 coordination of cell adhesion. Cell Signal 19: 2507–2518, 2007. doi: 10.1016/j.cellsig.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 857.Raymond GV, Bauman ML, Kemper TL. Hippocampus in autism: a Golgi analysis. Acta Neuropathol 91: 117–119, 1995. doi: 10.1007/s004010050401. [DOI] [PubMed] [Google Scholar]
- 858.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106: 157–169, 2001. doi: 10.1016/S0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 859.Rehmann H. Epac2: a sulfonylurea receptor? Biochem Soc Trans 40: 6–10, 2012. doi: 10.1042/BST20110640. [DOI] [PubMed] [Google Scholar]
- 860.Rehmann H. Epac-inhibitors: facts and artefacts. Sci Rep 3: 3032, 2013. doi: 10.1038/srep03032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 455: 124–127, 2008. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- 862.Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 439: 625–628, 2006. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- 863.Rehmann H, Prakash B, Wolf E, Rueppel A, de Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol 10: 26–32, 2003. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 864.Rehmann H, Rueppel A, Bos JL, Wittinghofer A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem 278: 23508–23514, 2003. doi: 10.1074/jbc.M301680200. [DOI] [PubMed] [Google Scholar]
- 865.Rehmann H, Schwede F, Døskeland SO, Wittinghofer A, Bos JL. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem 278: 38548–38556, 2003. doi: 10.1074/jbc.M306292200. [DOI] [PubMed] [Google Scholar]
- 866.Rehmann H, Wittinghofer A, Bos JL. Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat Rev Mol Cell Biol 8: 63–73, 2007. doi: 10.1038/nrm2082. [DOI] [PubMed] [Google Scholar]
- 867.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15: 100–108, 2007. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Renström E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol 502: 105–118, 1997. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Reynolds GP, Mason SL, Meldrum A, De Keczer S, Parnes H, Eglen RM, Wong EH. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol 114: 993–998, 1995. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.Rich TC, Tse TE, Rohan JG, Schaack J, Karpen JW. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J Gen Physiol 118: 63–78, 2001. doi: 10.1085/jgp.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 871.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev 15: 725–751, 1994. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 872.Riessen R, Wight TN, Pastore C, Henley C, Isner JM. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation 93: 1141–1147, 1996. doi: 10.1161/01.CIR.93.6.1141. [DOI] [PubMed] [Google Scholar]
- 873.Ring S, Pushkarevskaya A, Schild H, Probst HC, Jendrossek V, Wirsdörfer F, Ledent C, Robson SC, Enk AH, Mahnke K. Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J Immunol 194: 3735–3744, 2015. doi: 10.4049/jimmunol.1401434. [DOI] [PubMed] [Google Scholar]
- 874.Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27: 7817–7826, 2007. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 875.Rizzo MA, Springer GH, Granada B, Piston DW. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol 22: 445–449, 2004. doi: 10.1038/nbt945. [DOI] [PubMed] [Google Scholar]
- 876.Robert S, Maillet M, Morel E, Launay JM, Fischmeister R, Mercken L, Lezoualc’h F. Regulation of the amyloid precursor protein ectodomain shedding by the 5-HT4 receptor and Epac. FEBS Lett 579: 1136–1142, 2005. doi: 10.1016/j.febslet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 877.Robert SJ, Zugaza JL, Fischmeister R, Gardier AM, Lezoualc’h F. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J Biol Chem 276: 44881–44888, 2001. doi: 10.1074/jbc.M109008200. [DOI] [PubMed] [Google Scholar]
- 878.Roberts OL, Kamishima T, Barrett-Jolley R, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) induces vascular relaxation by activating Ca2+-sensitive K+ channels in rat mesenteric artery. J Physiol 591: 5107–5123, 2013. doi: 10.1113/jphysiol.2013.262006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 879.Rolli-Derkinderen M, Toumaniantz G, Pacaud P, Loirand G. RhoA phosphorylation induces Rac1 release from guanine dissociation inhibitor alpha and stimulation of vascular smooth muscle cell migration. Mol Cell Biol 30: 4786–4796, 2010. doi: 10.1128/MCB.00381-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6: 315–325, 1997. doi: 10.1016/S1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 881.Roscioni SS, Dekkers BG, Prins AG, Menzen MH, Meurs H, Schmidt M, Maarsingh H. cAMP inhibits modulation of airway smooth muscle phenotype via the exchange protein activated by cAMP (Epac) and protein kinase A. Br J Pharmacol 162: 193–209, 2011. doi: 10.1111/j.1476-5381.2010.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Roscioni SS, Kistemaker LE, Menzen MH, Elzinga CR, Gosens R, Halayko AJ, Meurs H, Schmidt M. PKA and Epac cooperate to augment bradykinin-induced interleukin-8 release from human airway smooth muscle cells. Respir Res 10: 88, 2009. doi: 10.1186/1465-9921-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Roscioni SS, Maarsingh H, Elzinga CR, Schuur J, Menzen M, Halayko AJ, Meurs H, Schmidt M. Epac as a novel effector of airway smooth muscle relaxation. J Cell Mol Med 15: 1551–1563, 2011. doi: 10.1111/j.1582-4934.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 884.Roscioni SS, Prins AG, Elzinga CR, Menzen MH, Dekkers BG, Halayko AJ, Meurs H, Maarsingh H, Schmidt M. Protein kinase A and the exchange protein directly activated by cAMP (Epac) modulate phenotype plasticity in human airway smooth muscle. Br J Pharmacol 164: 958–969, 2011. doi: 10.1111/j.1476-5381.2011.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16: 1197–1207, 1996. doi: 10.1016/S0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 886.Ross SH, Post A, Raaijmakers JH, Verlaan I, Gloerich M, Bos JL. Ezrin is required for efficient Rap1-induced cell spreading. J Cell Sci 124: 1808–1818, 2011. doi: 10.1242/jcs.079830. [DOI] [PubMed] [Google Scholar]
- 887.Rossi AG, Cousin JM, Dransfield I, Lawson MF, Chilvers ER, Haslett C. Agents that elevate cAMP inhibit human neutrophil apoptosis. Biochem Biophys Res Commun 217: 892–899, 1995. doi: 10.1006/bbrc.1995.2855. [DOI] [PubMed] [Google Scholar]
- 888.Roy S, Pinard S, Chouinard L, Gallo-Payet N. Adrenocorticotropin hormone (ACTH) effects on MAPK phosphorylation in human fasciculata cells and in embryonic kidney 293 cells expressing human melanocortin 2 receptor (MC2R) and MC2R accessory protein (MRAP)β. Mol Cell Endocrinol 336: 31–40, 2011. doi: 10.1016/j.mce.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 889.Ruete MC, Lucchesi O, Bustos MA, Tomes CN. Epac, Rap and Rab3 act in concert to mobilize calcium from sperm’s acrosome during exocytosis. Cell Commun Signal 12: 43, 2014. doi: 10.1186/s12964-014-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Ruiz-Hurtado G, Domínguez-Rodríguez A, Pereira L, Fernández-Velasco M, Cassan C, Lezoualc’h F, Benitah JP, Gómez AM. Sustained Epac activation induces calmodulin dependent positive inotropic effect in adult cardiomyocytes. J Mol Cell Cardiol 53: 617–625, 2012. doi: 10.1016/j.yjmcc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 891.Ruiz-Hurtado G, Morel E, Domínguez-Rodríguez A, Llach A, Lezoualc’h F, Benitah JP, Gomez AM. Epac in cardiac calcium signaling. J Mol Cell Cardiol 58: 162–171, 2013. doi: 10.1016/j.yjmcc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 892.Ruppelt A, Mosenden R, Grönholm M, Aandahl EM, Tobin D, Carlson CR, Abrahamsen H, Herberg FW, Carpén O, Taskén K. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J Immunol 179: 5159–5168, 2007. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- 893.Ryan MM, Morris GP, Mockett BG, Bourne K, Abraham WC, Tate WP, Williams JM. Time-dependent changes in gene expression induced by secreted amyloid precursor protein-alpha in the rat hippocampus. BMC Genomics 14: 376, 2013. doi: 10.1186/1471-2164-14-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem 283: 23884–23894, 2008. doi: 10.1074/jbc.M800754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Saha A, Biswas A, Srivastav S, Mukherjee M, Das PK, Ukil A. Prostaglandin E2 negatively regulates the production of inflammatory cytokines/chemokines and IL-17 in visceral leishmaniasis. J Immunol 193: 2330–2339, 2014. doi: 10.4049/jimmunol.1400399. [DOI] [PubMed] [Google Scholar]
- 896.Sahr A, Wolke C, Maczewsky J, Krippeit-Drews P, Tetzner A, Drews G, Venz S, Gürtler S, van den Brandt J, Berg S, Döring P, Dombrowski F, Walther T, Lendeckel U. The Angiotensin-(1-7)/Mas Axis Improves Pancreatic β-Cell Function in Vitro and in Vivo. Endocrinology 157: 4677–4690, 2016. doi: 10.1210/en.2016-1247. [DOI] [PubMed] [Google Scholar]
- 897.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature 424: 775–778, 2003. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 898.Salas TR, Reddy SA, Clifford JL, Davis RJ, Kikuchi A, Lippman SM, Menter DG. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J Biol Chem 278: 41338–41346, 2003. doi: 10.1074/jbc.M302972200. [DOI] [PubMed] [Google Scholar]
- 899.Salonikidis PS, Niebert M, Ullrich T, Bao G, Zeug A, Richter DW. An ion-insensitive cAMP biosensor for long term quantitative ratiometric fluorescence resonance energy transfer (FRET) measurements under variable physiological conditions. J Biol Chem 286: 23419–23431, 2011. doi: 10.1074/jbc.M111.236869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Salonikidis PS, Zeug A, Kobe F, Ponimaskin E, Richter DW. Quantitative measurement of cAMP concentration using an exchange protein directly activated by a cAMP-based FRET-sensor. Biophys J 95: 5412–5423, 2008. doi: 10.1529/biophysj.107.125666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ Res 95: 1012–1018, 2004. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 902.Sand C, Grandoch M, Börgermann C, Oude Weernink PA, Mahlke Y, Schwindenhammer B, Weber AA, Fischer JW, Jakobs KH, Schmidt M. 8-pCPT-conjugated cyclic AMP analogs exert thromboxane receptor antagonistic properties. Thromb Haemost 103: 662–678, 2010. doi: 10.1160/TH09-06-0341. [DOI] [PubMed] [Google Scholar]
- 903.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 283: 2083–2085, 1999. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 904.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol 26: 6333–6346, 2006. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Santibáñez JF, Olivares D, Guerrero J, Martínez J. Cyclic AMP inhibits TGFbeta1-induced cell-scattering and invasiveness in murine-transformed keratinocytes. Int J Cancer 107: 715–720, 2003. doi: 10.1002/ijc.11457. [DOI] [PubMed] [Google Scholar]
- 907.Sarkar D, Erlichman J, Rubin CS. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J Biol Chem 259: 9840–9846, 1984. [PubMed] [Google Scholar]
- 908.Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 4: 339–351, 1999. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- 909.Schaafsma D, McNeill KD, Stelmack GL, Gosens R, Baarsma HA, Dekkers BG, Frohwerk E, Penninks JM, Sharma P, Ens KM, Nelemans SA, Zaagsma J, Halayko AJ, Meurs H. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol 293: C429–C439, 2007. doi: 10.1152/ajpcell.00502.2006. [DOI] [PubMed] [Google Scholar]
- 910.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem 6: 97–110, 1999. [PMC free article] [PubMed] [Google Scholar]
- 911.Scheibner KA, Boodoo S, Collins S, Black KE, Chan-Li Y, Zarek P, Powell JD, Horton MR. The adenosine a2a receptor inhibits matrix-induced inflammation in a novel fashion. Am J Respir Cell Mol Biol 40: 251–259, 2009. doi: 10.1165/rcmb.2008-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Schimmer BP, Cordova M, Cheng H, Tsao A, Goryachev AB, Schimmer AD, Morris Q. Global profiles of gene expression induced by adrenocorticotropin in Y1 mouse adrenal cells. Endocrinology 147: 2357–2367, 2006. doi: 10.1210/en.2005-1526. [DOI] [PubMed] [Google Scholar]
- 913.Schindler RF, Brand T. The Popeye domain containing protein family–A novel class of cAMP effectors with important functions in multiple tissues. Prog Biophys Mol Biol 120: 28–36, 2016. doi: 10.1016/j.pbiomolbio.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 914.Schinner E, Wetzl V, Schlossmann J. Cyclic nucleotide signalling in kidney fibrosis. Int J Mol Sci 16: 2320–2351, 2015. doi: 10.3390/ijms16022320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 65: 670–709, 2013. doi: 10.1124/pr.110.003707. [DOI] [PubMed] [Google Scholar]
- 916.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol 3: 1020–1024, 2001. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 917.Schmidt M, Voss M, Thiel M, Bauer B, Grannass A, Tapp E, Cool RH, de Gunzburg J, von Eichel-Streiber C, Jakobs KH. Specific inhibition of phorbol ester-stimulated phospholipase D by Clostridium sordellii lethal toxin and Clostridium difficile toxin B-1470 in HEK-293 cells. Restoration by Ral GTPases. J Biol Chem 273: 7413–7422, 1998. doi: 10.1074/jbc.273.13.7413. [DOI] [PubMed] [Google Scholar]
- 918.Schnaith A, Kashkar H, Leggio SA, Addicks K, Krönke M, Krut O. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J Biol Chem 282: 2695–2706, 2007. doi: 10.1074/jbc.M609784200. [DOI] [PubMed] [Google Scholar]
- 919.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood 105: 1582–1589, 2005. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 920.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 415: 321–326, 2002. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 921.Schwede F, Bertinetti D, Langerijs CN, Hadders MA, Wienk H, Ellenbroek JH, de Koning EJ, Bos JL, Herberg FW, Genieser HG, Janssen RA, Rehmann H. Structure-guided design of selective Epac1 and Epac2 agonists. PLoS Biol 13: e1002038, 2015. doi: 10.1371/journal.pbio.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Schwede F, Chepurny OG, Kaufholz M, Bertinetti D, Leech CA, Cabrera O, Zhu Y, Mei F, Cheng X, Manning Fox JE, MacDonald PE, Genieser HG, Herberg FW, Holz GG. Rp-cAMPS Prodrugs Reveal the cAMP Dependence of First-Phase Glucose-Stimulated Insulin Secretion. Mol Endocrinol 29: 988–1005, 2015. doi: 10.1210/me.2014-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Schwede F, Maronde E, Genieser H, Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther 87: 199–226, 2000. doi: 10.1016/S0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- 925.Scott J, Harris GJ, Pinder EM, Macfarlane JG, Hellyer TP, Rostron AJ, Conway Morris A, Thickett DR, Perkins GD, McAuley DF, Widdrington JD, Wiscombe S, Baudouin SV, Roy AI, Linnett VC, Wright SE, Ruchaud-Sparagano MH, Simpson AJ. Exchange protein directly activated by cyclic AMP (EPAC) activation reverses neutrophil dysfunction induced by β2-agonists, corticosteroids, and critical illness. J Allergy Clin Immunol 137: 535–544, 2016. doi: 10.1016/j.jaci.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 926.Scott JD, Dessauer CW, Taskén K. Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol 53: 187–210, 2013. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Scott PH, Belham CM, al-Hafidh J, Chilvers ER, Peacock AJ, Gould GW, Plevin R. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J 318: 965–971, 1996. doi: 10.1042/bj3180965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Sedej S, Klemen MS, Schlüter OM, Rupnik MS. Rab3a is critical for trapping alpha-MSH granules in the high Ca2+-affinity pool by preventing constitutive exocytosis. PLoS One 8: e78883, 2013. doi: 10.1371/journal.pone.0078883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929.Sedej S, Rose T, Rupnik M. cAMP increases Ca2+-dependent exocytosis through both PKA and Epac2 in mouse melanotrophs from pituitary tissue slices. J Physiol 567: 799–813, 2005. doi: 10.1113/jphysiol.2005.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 930.Sehrawat S, Cullere X, Patel S, Italiano J Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell 19: 1261–1270, 2008. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 931.Sehrawat S, Ernandez T, Cullere X, Takahashi M, Ono Y, Komarova Y, Mayadas TN. AKAP9 regulation of microtubule dynamics promotes Epac1-induced endothelial barrier properties. Blood 117: 708–718, 2011. doi: 10.1182/blood-2010-02-268870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 932.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303–1342, 2005. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 933.Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes Metab 11, Suppl 4: 180–188, 2009. doi: 10.1111/j.1463-1326.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 934.Sekut L, Champion BR, Page K, Menius JA Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol 99: 461–466, 1995. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Seldon PM, Barnes PJ, Meja K, Giembycz MA. Suppression of lipopolysaccharide-induced tumor necrosis factor-alpha generation from human peripheral blood monocytes by inhibitors of phosphodiesterase 4: interaction with stimulants of adenylyl cyclase. Mol Pharmacol 48: 747–757, 1995. [PubMed] [Google Scholar]
- 936.Selvaratnam R, Chowdhury S, VanSchouwen B, Melacini G. Mapping allostery through the covariance analysis of NMR chemical shifts. Proc Natl Acad Sci USA 108: 6133–6138, 2011. doi: 10.1073/pnas.1017311108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 937.Selvaratnam R, Mazhab-Jafari MT, Das R, Melacini G. The auto-inhibitory role of the EPAC hinge helix as mapped by NMR. PLoS One 7: e48707, 2012. doi: 10.1371/journal.pone.0048707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Selvaratnam R, VanSchouwen B, Fogolari F, Mazhab-Jafari MT, Das R, Melacini G. The projection analysis of NMR chemical shifts reveals extended EPAC autoinhibition determinants. Biophys J 102: 630–639, 2012. doi: 10.1016/j.bpj.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 939.Seo H, Lee K. Epac2 contributes to PACAP-induced astrocytic differentiation through calcium ion influx in neural precursor cells. BMB Rep 49: 128–133, 2016. doi: 10.5483/BMBRep.2016.49.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 940.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39: 127–132, 2008. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Sette C, Conti M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J Biol Chem 271: 16526–16534, 1996. doi: 10.1074/jbc.271.28.16526. [DOI] [PubMed] [Google Scholar]
- 942.Sevetson BR, Kong X, Lawrence JC Jr. Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc Natl Acad Sci USA 90: 10305–10309, 1993. doi: 10.1073/pnas.90.21.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron 58: 223–237, 2008. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Shah AU, Grant WM, Latif SU, Mannan ZM, Park AJ, Husain SZ. Cyclic AMP accelerates calcium waves in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 294: G1328–G1334, 2008. doi: 10.1152/ajpgi.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945.Shakiryanova D, Zettel GM, Gu T, Hewes RS, Levitan ES. Synaptic neuropeptide release induced by octopamine without Ca2+ entry into the nerve terminal. Proc Natl Acad Sci USA 108: 4477–4481, 2011. doi: 10.1073/pnas.1017837108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572, 2004. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 947.Shao Y, Raiford KL, Wolpert CM, Cope HA, Ravan SA, Ashley-Koch AA, Abramson RK, Wright HH, DeLong RG, Gilbert JR, Cuccaro ML, Pericak-Vance MA. Phenotypic homogeneity provides increased support for linkage on chromosome 2 in autistic disorder. Am J Hum Genet 70: 1058–1061, 2002. doi: 10.1086/339765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 948.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 949.Shariati B, Thompson EL, Nicol GD, Vasko MR. Epac activation sensitizes rat sensory neurons through activation of Ras. Mol Cell Neurosci 70: 54–67, 2016. doi: 10.1016/j.mcn.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 950.Shen J, Halenda SP, Sturek M, Wilden PA. Novel mitogenic effect of adenosine on coronary artery smooth muscle cells: role for the A1 adenosine receptor. Circ Res 96: 982–990, 2005. doi: 10.1161/01.RES.0000165800.81876.52. [DOI] [PubMed] [Google Scholar]
- 951.Shi-Wen X, Renzoni EA, Kennedy L, Howat S, Chen Y, Pearson JD, Bou-Gharios G, Dashwood MR, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endogenous endothelin-1 signaling contributes to type I collagen and CCN2 overexpression in fibrotic fibroblasts. Matrix Biol 26: 625–632, 2007. doi: 10.1016/j.matbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 952.Shi GX, Jin L, Andres DA. Pituitary adenylate cyclase-activating polypeptide 38-mediated Rin activation requires Src and contributes to the regulation of HSP27 signaling during neuronal differentiation. Mol Cell Biol 28: 4940–4951, 2008. doi: 10.1128/MCB.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Shi GX, Jin L, Andres DA. Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation. Mol Biol Cell 21: 1597–1608, 2010. doi: 10.1091/mbc.E09-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Shi GX, Rehmann H, Andres DA. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol 26: 9136–9147, 2006. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem 279: 7956–7961, 2004. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 956.Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes 53, Suppl 3: S59–S62, 2004. doi: 10.2337/diabetes.53.suppl_3.S59. [DOI] [PubMed] [Google Scholar]
- 957.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104: 19333–19338, 2007. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Shirshev SV. Role of Epac proteins in mechanisms of cAMP-dependent immunoregulation. Biochemistry (Mosc) 76: 981–998, 2011. doi: 10.1134/S000629791109001X. [DOI] [PubMed] [Google Scholar]
- 959.Shute JK, Vrugt B, Lindley IJ, Holgate ST, Bron A, Aalbers R, Djukanović R. Free and complexed interleukin-8 in blood and bronchial mucosa in asthma. Am J Respir Crit Care Med 155: 1877–1883, 1997. doi: 10.1164/ajrccm.155.6.9196089. [DOI] [PubMed] [Google Scholar]
- 960.Simon K, Hennen S, Merten N, Blättermann S, Gillard M, Kostenis E, Gomeza J. The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Gαi/o and Its Downstream Effector Molecules. J Biol Chem 291: 705–718, 2016. doi: 10.1074/jbc.M115.683953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961.Simon K, Hennen S, Merten N, Blättermann S, Gillard M, Kostenis E, Gomeza J. The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Gαi/o and Its Downstream Effector Molecules. J Biol Chem 291: 705–718, 2016. doi: 10.1074/jbc.M115.683953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 962.Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Zhu Y, Cheng X, Hawke D, Mayor F Jr, Murga C, Heijnen CJ, Kavelaars A. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci USA 113: 3036–3041, 2016. doi: 10.1073/pnas.1516036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Sivertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol 9: 209–222, 2012. doi: 10.1038/nrcardio.2011.211. [DOI] [PubMed] [Google Scholar]
- 964.Smani T, Calderón-Sanchez E, Gómez-Hurtado N, Fernández-Velasco M, Cachofeiro V, Lahera V, Ordoñez A, Delgado C. Mechanisms underlying the activation of L-type calcium channels by urocortin in rat ventricular myocytes. Cardiovasc Res 87: 459–466, 2010. doi: 10.1093/cvr/cvq063. [DOI] [PubMed] [Google Scholar]
- 965.Smith DE, Clémençon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Aspects Med 34: 323–336, 2013. doi: 10.1016/j.mam.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 966.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature 388: 275–279, 1997. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 967.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol 9: 355–363, 1999. doi: 10.1016/S0959-4388(99)80052-X. [DOI] [PubMed] [Google Scholar]
- 968.Song WJ, Mondal P, Li Y, Lee SE, Hussain MA. Pancreatic β-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A. Diabetes 62: 2796–2807, 2013. doi: 10.2337/db12-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969.Soundarapandian MM, Wu D, Zhong X, Petralia RS, Peng L, Tu W, Lu Y. Expression of functional Kir6.1 channels regulates glutamate release at CA3 synapses in generation of epileptic form of seizures. J Neurochem 103: 1982–1988, 2007. doi: 10.1111/j.1471-4159.2007.04883.x. [DOI] [PubMed] [Google Scholar]
- 970.Spaeth CS, Boydston EA, Figard LR, Zuzek A, Bittner GD. A model for sealing plasmalemmal damage in neurons and other eukaryotic cells. J Neurosci 30: 15790–15800, 2010. doi: 10.1523/JNEUROSCI.4155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Spaeth CS, Spaeth EB, Wilcott RW, Fan JD, Robison T, Bittner GD. Pathways for plasmalemmal repair mediated by PKA, Epac, and cytosolic oxidation in rat B104 cells in vitro and rat sciatic axons ex vivo. Dev Neurobiol 72: 1399–1414, 2012. doi: 10.1002/dneu.20998. [DOI] [PubMed] [Google Scholar]
- 972.Spicuzza L, Belvisi MG, Birrell MA, Barnes PJ, Hele DJ, Giembycz MA. Evidence that the anti-spasmogenic effect of the beta-adrenoceptor agonist, isoprenaline, on guinea-pig trachealis is not mediated by cyclic AMP-dependent protein kinase. Br J Pharmacol 133: 1201–1212, 2001. doi: 10.1038/sj.bjp.0704213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, Nikolaev VO. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat Commun 6: 6965, 2015. doi: 10.1038/ncomms7965. [DOI] [PubMed] [Google Scholar]
- 974.Srivastava DP, Jones KA, Woolfrey KM, Burgdorf J, Russell TA, Kalmbach A, Lee H, Yang C, Bradberry MM, Wokosin D, Moskal JR, Casanova MF, Waters J, Penzes P. Social, communication, and cortical structural impairments in Epac2-deficient mice. J Neurosci 32: 11864–11878, 2012. doi: 10.1523/JNEUROSCI.1349-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 975.Srivastava DP, Woolfrey KM, Jones KA, Anderson CT, Smith KR, Russell TA, Lee H, Yasvoina MV, Wokosin DL, Ozdinler PH, Shepherd GM, Penzes P. An autism-associated variant of Epac2 reveals a role for Ras/Epac2 signaling in controlling basal dendrite maintenance in mice. PLoS Biol 10: e1001350, 2012. doi: 10.1371/journal.pbio.1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976.Stafford JB, Marnett LJ. Prostaglandin E2 inhibits tumor necrosis factor-alpha RNA through PKA type I. Biochem Biophys Res Commun 366: 104–109, 2008. doi: 10.1016/j.bbrc.2007.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Steegborn C. Structure, mechanism, and regulation of soluble adenylyl cyclases–similarities and differences to transmembrane adenylyl cyclases. Biochim Biophys Acta 1842, Pt B: 2535–2547, 2014. doi: 10.1016/j.bbadis.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 978.Stein C. Opioid Receptors. Annu Rev Med 67: 433–451, 2016. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- 979.Steiner D, Saya D, Schallmach E, Simonds WF, Vogel Z. Adenylyl cyclase type-VIII activity is regulated by G(betagamma) subunits. Cell Signal 18: 62–68, 2006. doi: 10.1016/j.cellsig.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 980.Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol Rev 97: 411–463, 2017. doi: 10.1152/physrev.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, Fagni L. Epac mediates PACAP-dependent long-term depression in the hippocampus. J Physiol 587: 101–113, 2009. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Ster J, De Bock F, Guérineau NC, Janossy A, Barrère-Lemaire S, Bos JL, Bockaert J, Fagni L. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci USA 104: 2519–2524, 2007. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Stewart AG, Harris T, Fernandes DJ, Schachte LC, Koutsoubos V, Guida E, Ravenhall CE, Vadiveloo P, Wilson JW. Beta2-adrenergic receptor agonists and cAMP arrest human cultured airway smooth muscle cells in the G(1) phase of the cell cycle: role of proteasome degradation of cyclin D1. Mol Pharmacol 56: 1079–1086, 1999. doi: 10.1124/mol.56.5.1079. [DOI] [PubMed] [Google Scholar]
- 984.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 985.Stokman G, Qin Y, Booij TH, Ramaiahgari S, Lacombe M, Dolman ME, van Dorenmalen KM, Teske GJ, Florquin S, Schwede F, van de Water B, Kok RJ, Price LS. Epac-Rap signaling reduces oxidative stress in the tubular epithelium. J Am Soc Nephrol 25: 1474–1485, 2014. doi: 10.1681/ASN.2013070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Stokman G, Qin Y, Genieser HG, Schwede F, de Heer E, Bos JL, Bajema IM, van de Water B, Price LS. Epac-Rap signaling reduces cellular stress and ischemia-induced kidney failure. J Am Soc Nephrol 22: 859–872, 2011. doi: 10.1681/ASN.2010040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab Res Rev 18: 451–463, 2002. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 988.Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol 6: 941–953, 2004. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- 989.Su Y, Dostmann WR, Herberg FW, Durick K, Xuong NH, Ten Eyck L, Taylor SS, Varughese KI. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science 269: 807–813, 1995. doi: 10.1126/science.7638597. [DOI] [PubMed] [Google Scholar]
- 990.Suárez HG. Genetic alterations in human epithelial thyroid tumours. Clin Endocrinol (Oxf) 48: 531–546, 1998. doi: 10.1046/j.1365-2265.1998.00443.x. [DOI] [PubMed] [Google Scholar]
- 991.Sun DP, Fang CL, Chen HK, Wen KS, Hseu YC, Hung ST, Uen YH, Lin KY. EPAC1 overexpression is a prognostic marker and its inhibition shows promising therapeutic potential for gastric cancer. Oncol Rep 37: 1953–1960, 2017. doi: 10.3892/or.2017.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 992.Sun L, Kondeti VK, Xie P, Raparia K, Kanwar YS. Epac1-mediated, high glucose-induced renal proximal tubular cells hypertrophy via the Akt/p21 pathway. Am J Pathol 179: 1706–1718, 2011. doi: 10.1016/j.ajpath.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell 125: 247–260, 2006. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 994.Sun W, Jiao W, Huang Y, Li R, Zhang Z, Wang J, Lei T. Exchange proteins directly activated by cAMP induce the proliferation of rat anterior pituitary GH3 cells via the activation of extracellular signal-regulated kinase. Biochem Biophys Res Commun 485: 355–359, 2017. doi: 10.1016/j.bbrc.2017.02.075. [DOI] [PubMed] [Google Scholar]
- 995.Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol 28: 851–858, 1996. doi: 10.1006/jmcc.1996.0080. [DOI] [PubMed] [Google Scholar]
- 996.Sunahara RK, Dessauer CW, Whisnant RE, Kleuss C, Gilman AG. Interaction of Gsalpha with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem 272: 22265–22271, 1997. doi: 10.1074/jbc.272.35.22265. [DOI] [PubMed] [Google Scholar]
- 997.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 232: 1077–1091, 1958. [PubMed] [Google Scholar]
- 998.Sutherland EW, Rall TW, Menon T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem 237: 1220–1227, 1962. [PubMed] [Google Scholar]
- 999.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 102: 437–442, 2005. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000.Swärd K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol 522: 33–49, 2000. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science 302: 103–106, 2003. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 1002.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 119: 5155–5163, 2012. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience 32: 577–580, 1989. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 1004.Takahashi H, Shibasaki T, Park JH, Hidaka S, Takahashi T, Ono A, Song DK, Seino S. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes 64: 1262–1272, 2015. doi: 10.2337/db14-0576. [DOI] [PubMed] [Google Scholar]
- 1005.Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem 269: 4559–4565, 2002. doi: 10.1046/j.1432-1033.2002.03157.x. [DOI] [PubMed] [Google Scholar]
- 1006.Takahashi T, Shibasaki T, Takahashi H, Sugawara K, Ono A, Inoue N, Furuya T, Seino S. Antidiabetic sulfonylureas and cAMP cooperatively activate Epac2A. Sci Signal 6: ra94, 2013. doi: 10.1126/scisignal.2004581. [DOI] [PubMed] [Google Scholar]
- 1007.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901, 2002. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 1008.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal 19: 251–260, 2007. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 1009.Tang WJ, Gilman AG. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 254: 1500–1503, 1991. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- 1010.Tang Z, Shi D, Jia B, Chen J, Zong C, Shen D, Zheng Q, Wang J, Tong X. Exchange protein activated by cyclic adenosine monophosphate regulates the switch between adipogenesis and osteogenesis of human mesenchymal stem cells through increasing the activation of phosphatidylinositol 3-kinase. Int J Biochem Cell Biol 44: 1106–1120, 2012. doi: 10.1016/j.biocel.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 1011.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 57: 405–411, 2002. doi: 10.1136/thorax.57.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Tao H, Li X, Wei K, Xie K, Ni Z, Gu L. Cyclic AMP prevents decrease of phosphorylated ezrin/radixin/moesin and chloride intracellular channel 5 expressions in injured podocytes. Clin Exp Nephrol 19: 1000–1006, 2015. doi: 10.1007/s10157-015-1102-6. [DOI] [PubMed] [Google Scholar]
- 1013.Tao X, Mei F, Agrawal A, Peters CJ, Ksiazek TG, Cheng X, Tseng CT. Blocking of exchange proteins directly activated by cAMP leads to reduced replication of Middle East respiratory syndrome coronavirus. J Virol 88: 3902–3910, 2014. doi: 10.1128/JVI.03001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Taussig R, Iñiguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi alpha. Science 261: 218–221, 1993. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 1015.Taussig R, Quarmby LM, Gilman AG. Regulation of purified type I and type II adenylylcyclases by G protein beta gamma subunits. J Biol Chem 268: 9–12, 1993. [PubMed] [Google Scholar]
- 1016.Taussig R, Tang WJ, Hepler JR, Gilman AG. Distinct patterns of bidirectional regulation of mammalian adenylyl cyclases. J Biol Chem 269: 6093–6100, 1994. [PubMed] [Google Scholar]
- 1017.Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis 31: 250–260, 2008. doi: 10.1016/j.nbd.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 1018.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59: 971–1005, 1990. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 1019.Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 36: 65–77, 2011. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1020.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta 1834: 1271–1278, 2013. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet 4: 638–649, 2003. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 1022.Tengholm A. Cyclic AMP dynamics in the pancreatic β-cell. Ups J Med Sci 117: 355–369, 2012. doi: 10.3109/03009734.2012.724732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1023.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol 175: 441–451, 2006. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1024.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol Renal Physiol 271: F414–F422, 1996. [DOI] [PubMed] [Google Scholar]
- 1025.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science 278: 1907–1916, 1997. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 1026.Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem 257: 3284–3290, 1982. [PubMed] [Google Scholar]
- 1027.Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7: 678–693, 2008. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 1028.Thompson MA, Britt RD Jr, Kuipers I, Stewart A, Thu J, Pandya HC, MacFarlane P, Pabelick CM, Martin RJ, Prakash YS. cAMP-mediated secretion of brain-derived neurotrophic factor in developing airway smooth muscle. Biochim Biophys Acta 1853, Pt A: 2506–2514, 2015. doi: 10.1016/j.bbamcr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J 23: 1643–1654, 2009. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- 1030.Tiwari S, Felekkis K, Moon EY, Flies A, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL uniquely expresses functional EPAC1, but EPAC1-mediated Rap1 activation does not account for PDE4 inhibitor-induced apoptosis. Blood 103: 2661–2667, 2004. doi: 10.1182/blood-2003-06-2154. [DOI] [PubMed] [Google Scholar]
- 1031.Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci 119: 2780–2786, 2006. doi: 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]
- 1032.Tomlinson PR, Wilson JW, Stewart AG. Inhibition by salbutamol of the proliferation of human airway smooth muscle cells grown in culture. Br J Pharmacol 111: 641–647, 1994. doi: 10.1111/j.1476-5381.1994.tb14784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1033.Tomlinson PR, Wilson JW, Stewart AG. Salbutamol inhibits the proliferation of human airway smooth muscle cells grown in culture: relationship to elevated cAMP levels. Biochem Pharmacol 49: 1809–1819, 1995. doi: 10.1016/0006-2952(94)00532-Q. [DOI] [PubMed] [Google Scholar]
- 1034.Topell S, Hennecke J, Glockshuber R. Circularly permuted variants of the green fluorescent protein. FEBS Lett 457: 283–289, 1999. doi: 10.1016/S0014-5793(99)01044-3. [DOI] [PubMed] [Google Scholar]
- 1035.Torres RA, Drake DA, Solodushko V, Jadhav R, Smith E, Rocic P, Weber DS. Slingshot isoform-specific regulation of cofilin-mediated vascular smooth muscle cell migration and neointima formation. Arterioscler Thromb Vasc Biol 31: 2424–2431, 2011. doi: 10.1161/ATVBAHA.111.232769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1036.Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25: 18–32, 2014. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1037.Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol 303: L923–L928, 2012. doi: 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1038.Troussard AA, Mawji NM, Ong C, Mui A, St -Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 278: 22374–22378, 2003. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- 1039.Tsai HC, Wu R. Cholera toxin directly enhances IL-17A production from human CD4+ T cells. J Immunol 191: 4095–4102, 2013. doi: 10.4049/jimmunol.1301079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040.Tsalkova T, Blumenthal DK, Mei FC, White MA, Cheng X. Mechanism of Epac activation: structural and functional analyses of Epac2 hinge mutants with constitutive and reduced activities. J Biol Chem 284: 23644–23651, 2009. doi: 10.1074/jbc.M109.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Tsalkova T, Gribenko AV, Cheng X. Exchange protein directly activated by cyclic AMP isoform 2 is not a direct target of sulfonylurea drugs. Assay Drug Dev Technol 9: 88–91, 2011. doi: 10.1089/adt.2010.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1042.Tsalkova T, Mei FC, Cheng X. A fluorescence-based high-throughput assay for the discovery of exchange protein directly activated by cyclic AMP (EPAC) antagonists. PLoS One 7: e30441, 2012. doi: 10.1371/journal.pone.0030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL Jr, Cheng X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci USA 109: 18613–18618, 2012. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1044.Tsang KM, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, Yoshida CK, Zerbo O, Weiss LA. A genome-wide survey of transgenerational genetic effects in autism. PLoS One 8: e76978, 2013. doi: 10.1371/journal.pone.0076978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Tsien RY. The green fluorescent protein. Annu Rev Biochem 67: 509–544, 1998. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 1046.Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J 369: 287–299, 2003. doi: 10.1042/bj20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047.Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res 717: 154–159, 1996. doi: 10.1016/0006-8993(96)00086-8. [DOI] [PubMed] [Google Scholar]
- 1048.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 1049.Ueno H, Shibasaki T, Iwanaga T, Takahashi K, Yokoyama Y, Liu LM, Yokoi N, Ozaki N, Matsukura S, Yano H, Seino S. Characterization of the gene EPAC2: structure, chromosomal localization, tissue expression, and identification of the liver-specific isoform. Genomics 78: 91–98, 2001. doi: 10.1006/geno.2001.6641. [DOI] [PubMed] [Google Scholar]
- 1050.Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, Hirotani S, Ishikawa Y. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol 293: H1662–H1672, 2007. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- 1051.Umenishi F, Narikiyo T, Vandewalle A, Schrier RW. cAMP regulates vasopressin-induced AQP2 expression via protein kinase A-independent pathway. Biochim Biophys Acta 1758: 1100–1105, 2006. doi: 10.1016/j.bbamem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 1052.Usechak P, Gates A, Webster CR. Activation of focal adhesion kinase and JNK contributes to the extracellular matrix and cAMP-GEF mediated survival from bile acid induced apoptosis in rat hepatocytes. J Hepatol 49: 251–261, 2008. doi: 10.1016/j.jhep.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1053.Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol 9: 2610–2628, 2007. doi: 10.1111/j.1462-5822.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 1054.Vacas E, Fernández-Martínez AB, Bajo AM, Sánchez-Chapado M, Schally AV, Prieto JC, Carmena MJ. Vasoactive intestinal peptide (VIP) inhibits human renal cell carcinoma proliferation. Biochim Biophys Acta 1823: 1676–1685, 2012. doi: 10.1016/j.bbamcr.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 1055.Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14: 95–97, 1996. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 1056.Valdez PA, Vithayathil PJ, Janelsins BM, Shaffer AL, Williamson PR, Datta SK. Prostaglandin E2 suppresses antifungal immunity by inhibiting interferon regulatory factor 4 function and interleukin-17 expression in T cells. Immunity 36: 668–679, 2012. doi: 10.1016/j.immuni.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002. doi: 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- 1058.Van der Krogt GN, Ogink J, Ponsioen B, Jalink K. A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example. PLoS One 3: e1916, 2008. doi: 10.1371/journal.pone.0001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun 62: 2046–2050, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1060.Van Kolen K, Dautzenberg FM, Verstraeten K, Royaux I, De Hoogt R, Gutknecht E, Peeters PJ. Corticotropin releasing factor-induced ERK phosphorylation in AtT20 cells occurs via a cAMP-dependent mechanism requiring EPAC2. Neuropharmacology 58: 135–144, 2010. doi: 10.1016/j.neuropharm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 1061.Van Oirschot BA, Stahl M, Lens SM, Medema RH. Protein kinase A regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem 276: 33854–33860, 2001. doi: 10.1074/jbc.M104395200. [DOI] [PubMed] [Google Scholar]
- 1062.Van Staveren WC, Beeckman S, Tomás G, Dom G, Hébrant A, Delys L, Vliem MJ, Trésallet C, Andry G, Franc B, Libert F, Dumont JE, Maenhaut C. Role of Epac and protein kinase A in thyrotropin-induced gene expression in primary thyrocytes. Exp Cell Res 318: 444–452, 2012. doi: 10.1016/j.yexcr.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 1063.Vang AG, Housley W, Dong H, Basole C, Ben-Sasson SZ, Kream BE, Epstein PM, Clark RB, Brocke S. Regulatory T-cells and cAMP suppress effector T-cells independently of PKA-CREM/ICER: a potential role for Epac. Biochem J 456: 463–473, 2013. doi: 10.1042/BJ20130064. [DOI] [PubMed] [Google Scholar]
- 1064.VanSchouwen B, Selvaratnam R, Fogolari F, Melacini G. Role of dynamics in the autoinhibition and activation of the exchange protein directly activated by cyclic AMP (EPAC). J Biol Chem 286: 42655–42669, 2011. doi: 10.1074/jbc.M111.277723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1065.Vanvooren V, Allgeier A, Nguyen M, Massart C, Parma J, Dumont JE, Van Sande J. Mutation analysis of the Epac–Rap1 signaling pathway in cold thyroid follicular adenomas. Eur J Endocrinol 144: 605–610, 2001. doi: 10.1530/eje.0.1440605. [DOI] [PubMed] [Google Scholar]
- 1066.Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, Borea PA. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther 13: R197, 2011. doi: 10.1186/ar3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Vardjan N, Kreft M, Zorec R. Dynamics of β-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia 62: 566–579, 2014. doi: 10.1002/glia.22626. [DOI] [PubMed] [Google Scholar]
- 1068.Vasko MR, Habashy Malty R, Guo C, Duarte DB, Zhang Y, Nicol GD. Nerve growth factor mediates a switch in intracellular signaling for PGE2-induced sensitization of sensory neurons from protein kinase A to Epac. PLoS One 9: e104529, 2014. doi: 10.1371/journal.pone.0104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1069.Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A, Clerici M, Hascall VC, De Luca G, Passi A. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem 283: 4448–4458, 2008. doi: 10.1074/jbc.M709051200. [DOI] [PubMed] [Google Scholar]
- 1070.Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, Eliasson L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab 297: E452–E461, 2009. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- 1071.Villarreal F, Epperson SA, Ramirez-Sanchez I, Yamazaki KG, Brunton LL. Regulation of cardiac fibroblast collagen synthesis by adenosine: roles for Epac and PI3K. Am J Physiol Cell Physiol 296: C1178–C1184, 2009. doi: 10.1152/ajpcell.00291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283: 2949–2961, 2008. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 1073.Vitali E, Cambiaghi V, Spada A, Tresoldi A, Zerbi A, Peverelli E, Carnaghi C, Mantovani G, Lania AG. cAMP effects in neuroendocrine tumors: The role of Epac and PKA in cell proliferation and adhesion. Exp Cell Res 339: 241–251, 2015. doi: 10.1016/j.yexcr.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 1074.Vitali E, Peverelli E, Giardino E, Locatelli M, Lasio GB, Beck-Peccoz P, Spada A, Lania AG, Mantovani G. Cyclic adenosine 3′-5′-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). Mol Cell Endocrinol 383: 193–202, 2014. doi: 10.1016/j.mce.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 1075.Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. ChemBioChem 9: 2052–2054, 2008. doi: 10.1002/cbic.200800216. [DOI] [PubMed] [Google Scholar]
- 1076.Volpert OV, Pili R, Sikder HA, Nelius T, Zaichuk T, Morris C, Shiflett CB, Devlin MK, Conant K, Alani RM. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell 2: 473–483, 2002. doi: 10.1016/S1535-6108(02)00209-X. [DOI] [PubMed] [Google Scholar]
- 1077.Von Hayn K, Werthmann RC, Nikolaev VO, Hommers LG, Lohse MJ, Bünemann M. Gq-mediated Ca2+ signals inhibit adenylyl cyclases 5/6 in vascular smooth muscle cells. Am J Physiol Cell Physiol 298: C324–C332, 2010. doi: 10.1152/ajpcell.00197.2009. [DOI] [PubMed] [Google Scholar]
- 1078.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89: 73–82, 1997. doi: 10.1016/S0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 1079.Vuchak LA, Tsygankova OM, Prendergast GV, Meinkoth JL. Protein kinase A and B-Raf mediate extracellular signal-regulated kinase activation by thyrotropin. Mol Pharmacol 76: 1123–1129, 2009. doi: 10.1124/mol.109.060129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1080.Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta 1812: 1291–1300, 2011. doi: 10.1016/j.bbadis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1081.Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependent protein kinase from rabbit skeletal muscle. J Biol Chem 243: 3763–3765, 1968. [PubMed] [Google Scholar]
- 1082.Wan G, Zhou L, Lim Q, Wong YH, Too HP. Cyclic AMP signalling through PKA but not Epac is essential for neurturin-induced biphasic ERK1/2 activation and neurite outgrowths through GFRα2 isoforms. Cell Signal 23: 1727–1737, 2011. doi: 10.1016/j.cellsig.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 1083.Wan X, Torregrossa MM, Sanchez H, Nairn AC, Taylor JR. Activation of exchange protein activated by cAMP in the rat basolateral amygdala impairs reconsolidation of a memory associated with self-administered cocaine. PLoS One 9: e107359, 2014. doi: 10.1371/journal.pone.0107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1084.Wang C, Gu Y, Li GW, Huang LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol 584: 191–203, 2007. doi: 10.1113/jphysiol.2007.135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1085.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3: 543–553, 1999. doi: 10.1016/S1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 1086.Wang H, Heijnen CJ, Eijkelkamp N, Garza Carbajal A, Schedlowski M, Kelley KW, Dantzer R, Kavelaars A. GRK2 in sensory neurons regulates epinephrine-induced signalling and duration of mechanical hyperalgesia. Pain 152: 1649–1658, 2011. doi: 10.1016/j.pain.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 1087.Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A, Eijkelkamp N, Kavelaars A. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 123: 5023–5034, 2013. doi: 10.1172/JCI66241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1088.Wang H, Robichaux WG, Wang Z, Mei FC, Cai M, Du G, Chen J, Cheng X. Inhibition of Epac1 suppresses mitochondrial fission and reduces neointima formation induced by vascular injury. Sci Rep 6: 36552, 2016. doi: 10.1038/srep36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1089.Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen JF, Linden J, Wu C, Huo Y. Inactivation of the adenosine A2A receptor protects apolipoprotein E-deficient mice from atherosclerosis. Arterioscler Thromb Vasc Biol 29: 1046–1052, 2009. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1090.Wang L, Yu T, Lee H, O’Brien DK, Sesaki H, Yoon Y. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc Res 106: 272–283, 2015. doi: 10.1093/cvr/cvv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1091.Wang P, Liu Z, Chen H, Ye N, Cheng X, Zhou J. Exchange proteins directly activated by cAMP (EPACs): emerging therapeutic targets. Bioorg Med Chem Lett 27: 1633–1639, 2017. doi: 10.1016/j.bmcl.2017.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Wang P, Wang Q, Sun J, Wu J, Li H, Zhang N, Huang Y, Su B, Li RK, Liu L, Zhang Y, Elsholtz HP, Hu J, Gaisano HY, Jin T. POU homeodomain protein Oct-1 functions as a sensor for cyclic AMP. J Biol Chem 284: 26456–26465, 2009. doi: 10.1074/jbc.M109.030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093.Wang SY, Freeman MR, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Sex Steroids Influence Brain-Derived Neurotropic Factor Secretion From Human Airway Smooth Muscle Cells. J Cell Physiol 231: 1586–1592, 2016. doi: 10.1002/jcp.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 1095.Wang X, Luo C, Cheng X, Lu M. Lithium and an EPAC-specific inhibitor ESI-09 synergistically suppress pancreatic cancer cell proliferation and survival. Acta Biochim Biophys Sin (Shanghai) 49: 573–580, 2017. doi: 10.1093/abbs/gmx045. [DOI] [PubMed] [Google Scholar]
- 1096.Wang X, Wang ZH, Wu YY, Tang H, Tan L, Wang X, Gao XY, Xiong YS, Liu D, Wang JZ, Zhu LQ. Melatonin attenuates scopolamine-induced memory/synaptic disorder by rescuing EPACs/miR-124/Egr1 pathway. Mol Neurobiol 47: 373–381, 2013. doi: 10.1007/s12035-012-8355-9. [DOI] [PubMed] [Google Scholar]
- 1097.Wang Y, Klein JD, Blount MA, Martin CF, Kent KJ, Pech V, Wall SM, Sands JM. Epac regulates UT-A1 to increase urea transport in inner medullary collecting ducts. J Am Soc Nephrol 20: 2018–2024, 2009. doi: 10.1681/ASN.2008121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1098.Wang Y, Sugita S, Sudhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem 275: 20033–20044, 2000. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- 1099.Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A, Serezani CH. MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PLoS One 10: e0115855, 2015. doi: 10.1371/journal.pone.0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1100.Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, Caubere C, Rouet P, Gomez AM, Vandecasteele G, Fischmeister R, Brenner C. A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death Dis 7: e2198, 2016. doi: 10.1038/cddis.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1101.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21: 1940–1957, 2007. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 1102.Webster CR, Anwer MS. Cyclic adenosine monophosphate-mediated protection against bile acid-induced apoptosis in cultured rat hepatocytes. Hepatology 27: 1324–1331, 1998. doi: 10.1002/hep.510270519. [DOI] [PubMed] [Google Scholar]
- 1104.Wei D, Hurd C, Galleguillos D, Singh J, Fenrich KK, Webber CA, Sipione S, Fouad K. Inhibiting cortical protein kinase A in spinal cord injured rats enhances efficacy of rehabilitative training. Exp Neurol 283, Pt A: 365–374, 2016. doi: 10.1016/j.expneurol.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 1105.Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K+ channel activity in the renal cortical collecting duct during high dietary K+ adaptation. Am J Physiol Renal Physiol 307: F833–F843, 2014. doi: 10.1152/ajprenal.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1106.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1107.Weinlich R, Bortoluci KR, Chehab CF, Serezani CH, Ulbrich AG, Peters-Golden M, Russo M, Amarante-Mendes GP. TLR4/MYD88-dependent, LPS-induced synthesis of PGE2 by macrophages or dendritic cells prevents anti-CD3-mediated CD95L upregulation in T cells. Cell Death Differ 15: 1901–1909, 2008. doi: 10.1038/cdd.2008.128. [DOI] [PubMed] [Google Scholar]
- 1108.Weinman EJ, Steplock D, Shenolikar S. cAMP-mediated inhibition of the renal brush border membrane Na+-H+ exchanger requires a dissociable phosphoprotein cofactor. J Clin Invest 92: 1781–1786, 1993. doi: 10.1172/JCI116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Weinman EJ, Steplock D, Shenolikar S. NHERF-1 uniquely transduces the cAMP signals that inhibit sodium-hydrogen exchange in mouse renal apical membranes. FEBS Lett 536: 141–144, 2003. doi: 10.1016/S0014-5793(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 1110.Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na+-H+ exchanger. J Clin Invest 95: 2143–2149, 1995. doi: 10.1172/JCI117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111.Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265: 1878–1882, 1994. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 1112.Werthmann RC, Lohse MJ, Bünemann M. Temporally resolved cAMP monitoring in endothelial cells uncovers a thrombin-induced [cAMP] elevation mediated via the Ca2+-dependent production of prostacyclin. J Physiol 589: 181–193, 2011. doi: 10.1113/jphysiol.2010.200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1113.Werthmann RC, von Hayn K, Nikolaev VO, Lohse MJ, Bünemann M. Real-time monitoring of cAMP levels in living endothelial cells: thrombin transiently inhibits adenylyl cyclase 6. J Physiol 587: 4091–4104, 2009. doi: 10.1113/jphysiol.2009.172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114.Wewer Albrechtsen NJ, Albrechtsen R, Bremholm L, Svendsen B, Kuhre RE, Poulsen SS, Christiansen CB, Jensen EP, Janus C, Hilsted L, Deacon CF, Hartmann B, Holst JJ. Glucagon-like Peptide 1 Receptor Signaling in Acinar Cells Causes Growth-Dependent Release of Pancreatic Enzymes. Cell Reports 17: 2845–2856, 2016. doi: 10.1016/j.celrep.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 1115.Whisnant RE, Gilman AG, Dessauer CW. Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc Natl Acad Sci USA 93: 6621–6625, 1996. doi: 10.1073/pnas.93.13.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1116.Whitaker CM, Wei H. An alternate cAMP pathway Epac promotes hippocampal long-term depression. J Physiol 587: 3067–3068, 2009. doi: 10.1113/jphysiol.2009.175216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1117.White MA, Li S, Tsalkova T, Mei FC, Liu T, Woods VL Jr, Cheng X. Structural analyses of a constitutively active mutant of exchange protein directly activated by cAMP. PLoS One 7: e49932, 2012. doi: 10.1371/journal.pone.0049932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118.Wild CT, Zhu Y, Na Y, Mei F, Ynalvez MA, Chen H, Cheng X, Zhou J. Functionalized N,N-Diphenylamines as Potent and Selective EPAC2 Inhibitors. ACS Med Chem Lett 7: 460–464, 2016. doi: 10.1021/acsmedchemlett.5b00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1119.Wilkinson TS, Bressler SL, Evanko SP, Braun KR, Wight TN. Overexpression of hyaluronan synthases alters vascular smooth muscle cell phenotype and promotes monocyte adhesion. J Cell Physiol 206: 378–385, 2006. doi: 10.1002/jcp.20468. [DOI] [PubMed] [Google Scholar]
- 1120.Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol 63: 77–97, 2001. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- 1121.Willoughby D, Cooper DM. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci 119: 828–836, 2006. doi: 10.1242/jcs.02812. [DOI] [PubMed] [Google Scholar]
- 1122.Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol 16: 5546–5556, 1996. doi: 10.1128/MCB.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1123.Wilson LS, Baillie GS, Pritchard LM, Umana B, Terrin A, Zaccolo M, Houslay MD, Maurice DH. A phosphodiesterase 3B-based signaling complex integrates exchange protein activated by cAMP 1 and phosphatidylinositol 3-kinase signals in human arterial endothelial cells. J Biol Chem 286: 16285–16296, 2011. doi: 10.1074/jbc.M110.217026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23: 787–798, 1999. doi: 10.1016/S0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 1125.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 1126.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 62: 327–331, 1994. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 1127.Woolfrey KM, Srivastava DP, Photowala H, Yamashita M, Barbolina MV, Cahill ME, Xie Z, Jones KA, Quilliam LA, Prakriya M, Penzes P. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci 12: 1275–1284, 2009. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Woolson HD, Thomson VS, Rutherford C, Yarwood SJ, Palmer TM. Selective inhibition of cytokine-activated extracellular signal-regulated kinase by cyclic AMP via Epac1-dependent induction of suppressor of cytokine signalling-3. Cell Signal 21: 1706–1715, 2009. doi: 10.1016/j.cellsig.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 1129.Wright PT, Nikolaev VO, O’Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol 67: 38–48, 2014. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Wu CY, DiJulio DH, Jacobson KL, McKnight GS, Watson EL. The contribution of AKAP5 in amylase secretion from mouse parotid acini. Am J Physiol Cell Physiol 298: C1151–C1158, 2010. doi: 10.1152/ajpcell.00382.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262: 1065–1069, 1993. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 1132.Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, Levin LR, Jaffrey SR. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci 9: 1257–1264, 2006. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133.Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J Biol Chem 273: 11362–11369, 1998. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- 1134.Wu YJ, Bond M, Sala-Newby GB, Newby AC. Altered S-phase kinase-associated protein-2 levels are a major mediator of cyclic nucleotide-induced inhibition of vascular smooth muscle cell proliferation. Circ Res 98: 1141–1150, 2006. doi: 10.1161/01.RES.0000219905.16312.28. [DOI] [PubMed] [Google Scholar]
- 1135.Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res 17: 101–110, 2002. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
- 1136.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, Gossen JA, Esposito G, van Duin M, Conti M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol 296: 353–362, 2006. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 1137.Xie P, Joladarashi D, Dudeja P, Sun L, Kanwar YS. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Renal Physiol 306: F1260–F1274, 2014. doi: 10.1152/ajprenal.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1138.Xing J, Birukova AA. ANP attenuates inflammatory signaling and Rho pathway of lung endothelial permeability induced by LPS and TNFalpha. Microvasc Res 79: 56–62, 2010. doi: 10.1016/j.mvr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Xu N, Engbers J, Khaja S, Xu L, Clark JJ, Hansen MR. Influence of cAMP and protein kinase A on neurite length from spiral ganglion neurons. Hear Res 283: 33–44, 2012. doi: 10.1016/j.heares.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1140.Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem 279: 23098–23103, 2004. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- 1141.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol 180: 2125–2131, 2008. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, Oike Y, Takeya M, Toyama Y, Suda T. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202: 345–351, 2005. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.Yamagami H, Nishioka T, Ochiai E, Fukushima K, Nomura M, Kasugai S, Moritani S, Yokogawa K, Miyamoto K. Inhibition of osteoclastogenesis by a phosphodiesterase 4 inhibitor XT-611 through synergistic action with endogenous prostaglandin E2. Biochem Pharmacol 66: 801–807, 2003. doi: 10.1016/S0006-2952(03)00409-X. [DOI] [PubMed] [Google Scholar]
- 1144.Yamaguchi M, Watanabe Y, Ohtani T, Uezumi A, Mikami N, Nakamura M, Sato T, Ikawa M, Hoshino M, Tsuchida K, Miyagoe-Suzuki Y, Tsujikawa K, Takeda S, Yamamoto H, Fukada S. Calcitonin Receptor Signaling Inhibits Muscle Stem Cells from Escaping the Quiescent State and the Niche. Cell Reports 13: 302–314, 2015. doi: 10.1016/j.celrep.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 1145.Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca(2+) release channel (ryanodine receptor). J Biol Chem 278: 23480–23486, 2003. doi: 10.1074/jbc.M301125200. [DOI] [PubMed] [Google Scholar]
- 1146.Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K, Narita N. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest 112: 505–510, 1997. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- 1147.Yammamoto H, Tanaka S, Tanaka A, Hide I, Seki T, Sakai N. Long-term exposure of RN46A cells expressing serotonin transporter (SERT) to a cAMP analog up-regulates SERT activity and is accompanied by neural differentiation of the cells. J Pharmacol Sci 121: 25–38, 2013. doi: 10.1254/jphs.12229FP. [DOI] [PubMed] [Google Scholar]
- 1148.Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. Anti-mitogenic effects of β-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J 25: 389–397, 2011. doi: 10.1096/fj.10-164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149.Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol 28: 5803–5810, 2008. doi: 10.1128/MCB.00393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, Patrikeev I, Hao D, Stutz SJ, Dineley KT, Motamedi M, Hommel JD, Cunningham KA, Chen J, Cheng X. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol 33: 918–926, 2013. doi: 10.1128/MCB.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1151.Yanagawa Y, Hiraide S, Matsumoto M, Togashi H. Rapid induction of REDD1 gene expression in macrophages in response to stress-related catecholamines. Immunol Lett 158: 109–115, 2014. doi: 10.1016/j.imlet.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 1152.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116: 1913–1923, 2006. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153.Yang HJ, Vainshtein A, Maik-Rachline G, Peles E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun 7: 10884, 2016. doi: 10.1038/ncomms10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 1155.Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Xu X, Tian Q, Zhang J, Qian K, Wang YX, Petralia RS, Tu W, Zhu LQ, Wang JZ, Lu Y. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron 73: 774–788, 2012. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156.Yang Y, Yang F, Wu X, Lv X, Li J. EPAC activation inhibits acetaldehyde-induced activation and proliferation of hepatic stellate cell via Rap1. Can J Physiol Pharmacol 94: 498–507, 2016. doi: 10.1139/cjpp-2015-0437. [DOI] [PubMed] [Google Scholar]
- 1157.Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng YT. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem 282: 18819–18830, 2007. doi: 10.1074/jbc.M610537200. [DOI] [PubMed] [Google Scholar]
- 1158.Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med 15: 633–640, 2009. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 1159.Yao K, Ge W. Differential regulation of kit ligand A (kitlga) expression in the zebrafish ovarian follicle cells–evidence for the existence of a cyclic adenosine 3′,5′ monophosphate-mediated binary regulatory system during folliculogenesis. Mol Cell Endocrinol 402: 21–31, 2015. doi: 10.1016/j.mce.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 1160.Yarwood SJ, Borland G, Sands WA, Palmer TM. Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem 283: 6843–6853, 2008. doi: 10.1074/jbc.M710342200. [DOI] [PubMed] [Google Scholar]
- 1161.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 1162.Ye N, Zhu Y, Chen H, Liu Z, Mei FC, Wild C, Chen H, Cheng X, Zhou J. Structure-Activity Relationship Studies of Substituted 2-(Isoxazol-3-yl)-2-oxo-N'-phenyl-acetohydrazonoyl Cyanide Analogues: Identification of Potent Exchange Proteins Directly Activated by cAMP (EPAC) Antagonists. J Med Chem 58: 6033–6047, 2015. doi: 10.1021/acs.jmedchem.5b00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Ye N, Zhu Y, Liu Z, Mei FC, Chen H, Wang P, Cheng X, Zhou J. Identification of novel 2-(benzo[d]isoxazol-3-yl)-2-oxo-N-phenylacetohydrazonoyl cyanide analoguesas potent EPAC antagonists. Eur J Med Chem 134: 62–71, 2017. doi: 10.1016/j.ejmech.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1164.Yeager LA, Chopra AK, Peterson JW. Bacillus anthracis edema toxin suppresses human macrophage phagocytosis and cytoskeletal remodeling via the protein kinase A and exchange protein activated by cyclic AMP pathways. Infect Immun 77: 2530–2543, 2009. doi: 10.1128/IAI.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Yi L, Chandrasekaran P, Venkatesan S. TLR signaling paralyzes monocyte chemotaxis through synergized effects of p38 MAPK and global Rap-1 activation. PLoS One 7: e30404, 2012. doi: 10.1371/journal.pone.0030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Ying Y, Li L, Cao W, Yan D, Zeng Q, Kong X, Lu L, Yan M, Xu X, Qu J, Su Q, Ma X. The microtubule associated protein syntabulin is required for glucose-stimulated and cAMP-potentiated insulin secretion. FEBS Lett 586: 3674–3680, 2012. doi: 10.1016/j.febslet.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 1167.Yip KP. Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]
- 1168.Yokoyama U, Minamisawa S, Quan H, Akaike T, Jin M, Otsu K, Ulucan C, Wang X, Baljinnyam E, Takaoka M, Sata M, Ishikawa Y. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle cell migration. Am J Physiol Heart Circ Physiol 295: H1547–H1555, 2008. doi: 10.1152/ajpheart.01317.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169.Yokoyama U, Minamisawa S, Quan H, Akaike T, Suzuki S, Jin M, Jiao Q, Watanabe M, Otsu K, Iwasaki S, Nishimaki S, Sato M, Ishikawa Y. Prostaglandin E2-activated Epac promotes neointimal formation of the rat ductus arteriosus by a process distinct from that of cAMP-dependent protein kinase A. J Biol Chem 283: 28702–28709, 2008. doi: 10.1074/jbc.M804223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1170.Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, Iwasaki S, Iwamoto M, Misra S, Tamura K, Hori H, Yokota S, Toole BP, Sugimoto Y, Ishikawa Y. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest 116: 3026–3034, 2006. doi: 10.1172/JCI28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 105: 6386–6391, 2008. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1172.Yoon SH, Ryu J, Lee Y, Lee ZH, Kim HH. Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway. J Bone Miner Res 26: 1217–1229, 2011. doi: 10.1002/jbmr.310. [DOI] [PubMed] [Google Scholar]
- 1173.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392: 622–626, 1998. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 1174.Yoshie M, Kaneyama K, Kusama K, Higuma C, Nishi H, Isaka K, Tamura K. Possible role of the exchange protein directly activated by cyclic AMP (Epac) in the cyclic AMP-dependent functional differentiation and syncytialization of human placental BeWo cells. Hum Reprod 25: 2229–2238, 2010. doi: 10.1093/humrep/deq190. [DOI] [PubMed] [Google Scholar]
- 1175.Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD, Chen H. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem 277: 50062–50068, 2002. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 1176.Yu S, Fan F, Flores SC, Mei F, Cheng X. Dissecting the mechanism of Epac activation via hydrogen-deuterium exchange FT-IR and structural modeling. Biochemistry 45: 15318–15326, 2006. doi: 10.1021/bi061701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1177.Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178.Zaccolo M, De Giorgi F, Cho CY, Feng L, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nat Cell Biol 2: 25–29, 2000. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- 1179.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 1180.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296: 913–916, 2002. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 1181.Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425: 200–205, 2003. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- 1182.Zaldua N, Gastineau M, Hoshino M, Lezoualc’h F, Zugaza JL. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett 581: 5814–5818, 2007. doi: 10.1016/j.febslet.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 1183.Zasłona Z, Okunishi K, Bourdonnay E, Domingo-Gonzalez R, Moore BB, Lukacs NW, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol 133: 379–387.e1, 2014. doi: 10.1016/j.jaci.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1184.Zaslona Z, Serezani CH, Okunishi K, Aronoff DM, Peters-Golden M. Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase A signaling. Blood 119: 2358–2367, 2012. doi: 10.1182/blood-2011-08-374207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Zhai K, Hubert F, Nicolas V, Ji G, Fischmeister R, Leblais V. β-Adrenergic cAMP signals are predominantly regulated by phosphodiesterase type 4 in cultured adult rat aortic smooth muscle cells. PLoS One 7: e47826, 2012. doi: 10.1371/journal.pone.0047826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1186.Zhang B, Nweze I, Lakshmanan J, Harbrecht BG. Activation of a cyclic AMP-guanine exchange factor in hepatocytes decreases nitric oxide synthase expression. Shock 39: 70–76, 2013. doi: 10.1097/SHK.0b013e3182760530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1187.Zhang B, Perpetua M, Fulmer M, Harbrecht BG. JNK signaling involved in the effects of cyclic AMP on IL-1beta plus IFNgamma-induced inducible nitric oxide synthase expression in hepatocytes. Cell Signal 16: 837–846, 2004. doi: 10.1016/j.cellsig.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 1188.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. doi: 10.1152/ajprenal.0054.2001. [DOI] [PubMed] [Google Scholar]
- 1189.Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325: 607–610, 2009. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 1190.Zhang G, Liu Y, Ruoho AE, Hurley JH. Structure of the adenylyl cyclase catalytic core. Nature 386: 247–253, 1997. doi: 10.1038/386247a0. [DOI] [PubMed] [Google Scholar]
- 1191.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem 286: 23012–23021, 2011. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192.Zhang L, Malik S, Pang J, Wang H, Park KM, Yule DI, Blaxall BC, Smrcka AV. Phospholipase Cε hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell 153: 216–227, 2013. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Zhang X, Huang X, Fang C, Li Q, Cui J, Sun J, Li L. miR-124 Regulates the Expression of BACE1 in the Hippocampus Under Chronic Cerebral Hypoperfusion. Mol Neurobiol 54: 2498–2506, 2017. doi: 10.1007/s12035-016-9845-y. [DOI] [PubMed] [Google Scholar]
- 1194.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol 167: 4245–4253, 2001. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 1195.Zhang Y, Guo Q, Li X, Gao J, Liu Y, Yang J, Li Q. P2Y purinergic receptor-regulated insulin secretion is mediated by a cAMP/Epac/Kv channel pathway. Biochem Biophys Res Commun 460: 850–856, 2015. doi: 10.1016/j.bbrc.2015.03.121. [DOI] [PubMed] [Google Scholar]
- 1196.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 1197.Zhang Y, Wang H, Guo Q, Li X, Gao J, Liu Y, Yang C, Niu L, Yang J. PI3K is involved in P2Y receptor-regulated cAMP /Epac/Kv channel signaling pathway in pancreatic β cells. Biochem Biophys Res Commun 465: 714–718, 2015. doi: 10.1016/j.bbrc.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 1198.Zhao H, Li X, Li N, Liu T, Liu J, Li Z, Xiao H, Li J. Long-term resveratrol treatment prevents ovariectomy-induced osteopenia in rats without hyperplastic effects on the uterus. Br J Nutr 111: 836–846, 2014. doi: 10.1017/S0007114513003115. [DOI] [PubMed] [Google Scholar]
- 1199.Zhao K, Wen R, Wang X, Pei L, Yang Y, Shang Y, Bazan N, Zhu LQ, Tian Q, Lu Y. EPAC inhibition of SUR1 receptor increases glutamate release and seizure vulnerability. J Neurosci 33: 8861–8865, 2013. doi: 10.1523/JNEUROSCI.5686-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Zhao M, Li Y, Peng L. FPGA-based multi-channel fluorescence lifetime analysis of Fourier multiplexed frequency-sweeping lifetime imaging. Opt Express 22: 23073–23085, 2014. doi: 10.1364/OE.22.023073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1201.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89: 413–424, 1997. doi: 10.1016/S0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 1202.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1: 661–671, 1998. doi: 10.1016/S1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 1203.Zhong N, Beaumont V, Zucker RS. Calcium influx through HCN channels does not contribute to cAMP-enhanced transmission. J Neurophysiol 92: 644–647, 2004. doi: 10.1152/jn.00112.2004. [DOI] [PubMed] [Google Scholar]
- 1204.Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neuromuscular junction. J Neurosci 25: 208–214, 2005. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1205.Zhong N, Zucker RS. Roles of Ca2+, hyperpolarization and cyclic nucleotide-activated channel activation, and actin in temporal synaptic tagging. J Neurosci 24: 4205–4212, 2004. doi: 10.1523/JNEUROSCI.0111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1206.Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res 35: 125–130, 2003. doi: 10.1034/j.1600-079X.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 1207.Zhou L, Ma SL, Yeung PK, Wong YH, Tsim KW, So KF, Lam LC, Chung SK. Anxiety and depression with neurogenesis defects in exchange protein directly activated by cAMP 2-deficient mice are ameliorated by a selective serotonin reuptake inhibitor, Prozac. Transl Psychiatry 6: e881, 2016. doi: 10.1038/tp.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1208.Zhou Z, Tanaka KF, Matsunaga S, Iseki M, Watanabe M, Matsuki N, Ikegaya Y, Koyama R. Photoactivated adenylyl cyclase (PAC) reveals novel mechanisms underlying cAMP-dependent axonal morphogenesis. Sci Rep 6: 19679, 2016. doi: 10.1038/srep19679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1209.Zhu LQ, Wang SH, Ling ZQ, Wang DL, Wang JZ. Effect of inhibiting melatonin biosynthesis on spatial memory retention and tau phosphorylation in rat. J Pineal Res 37: 71–77, 2004. doi: 10.1111/j.1600-079X.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 1210.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G, Zhou J, Cheng X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci Rep 5: 9344, 2015. doi: 10.1038/srep09344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1211.Zhu Y, Mei F, Luo P, Cheng X. A cell-based, quantitative and isoform-specific assay for exchange proteins directly activated by cAMP. Sci Rep 7: 6200, 2017. doi: 10.1038/s41598-017-06432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212.Zhu ZQ, Wang D, Xiang D, Yuan YX, Wang Y. Calcium/calmodulin-dependent serine protein kinase is involved in exendin-4-induced insulin secretion in INS-1 cells. Metabolism 63: 120–126, 2014. doi: 10.1016/j.metabol.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 1213.Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, Somlyo AV. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem 286: 16681–16692, 2011. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1214.Zies DL, Gumz ML, Wingo CS, Cain BD. The renal H+,K+-ATPases as therapeutic targets. Expert Opin Ther Targets 11: 881–890, 2007. doi: 10.1517/14728222.11.7.881. [DOI] [PubMed] [Google Scholar]
- 1215.Zimmermann G, Taussig R. Protein kinase C alters the responsiveness of adenylyl cyclases to G protein alpha and betagamma subunits. J Biol Chem 271: 27161–27166, 1996. doi: 10.1074/jbc.271.43.27161. [DOI] [PubMed] [Google Scholar]
- 1216.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82–84, 2003. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 1217.Zucchetti AE, Barosso IR, Boaglio A, Pellegrino JM, Ochoa EJ, Roma MG, Crocenzi FA, Sánchez Pozzi EJ. Prevention of estradiol 17beta-d-glucuronide-induced canalicular transporter internalization by hormonal modulation of cAMP in rat hepatocytes. Mol Biol Cell 22: 3902–3915, 2011. doi: 10.1091/mbc.E11-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1218.Zucchetti AE, Barosso IR, Boaglio AC, Luquita MG, Roma MG, Crocenzi FA, Sánchez Pozzi EJ. Hormonal modulation of hepatic cAMP prevents estradiol 17β-d-glucuronide-induced cholestasis in perfused rat liver. Dig Dis Sci 58: 1602–1614, 2013. doi: 10.1007/s10620-013-2558-4. [DOI] [PubMed] [Google Scholar]
- 1219.Zufall F, Shepherd GM, Barnstable CJ. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr Opin Neurobiol 7: 404–412, 1997. doi: 10.1016/S0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]