Abstract
Spinal cord injury (SCI), for which there currently is no cure, is a heavy burden on patient physiology and psychology. The microenvironment of the injured spinal cord is complicated. According to our previous work and the advancements in SCI research, ‘microenvironment imbalance’ is the main cause of the poor regeneration and recovery of SCI. Microenvironment imbalance is defined as an increase in inhibitory factors and decrease in promoting factors for tissues, cells and molecules at different times and spaces. There are imbalance of hemorrhage and ischemia, glial scar formation, demyelination and re-myelination at the tissue’s level. The cellular level imbalance involves an imbalance in the differentiation of endogenous stem cells and the transformation phenotypes of microglia and macrophages. The molecular level includes an imbalance of neurotrophic factors and their pro-peptides, cytokines, and chemokines. The imbalanced microenvironment of the spinal cord impairs regeneration and functional recovery. This review will aid in the understanding of the pathological processes involved in and the development of comprehensive treatments for SCI.
Keywords: spinal cord injury, microenvironment, imbalance, regeneration
Introduction
Spinal cord injury (SCI), a serious damage to the central nervous system, has historically been considered an incurable impairment worldwide. Patients with SCI suffer a lot both in terms of physiology and psychology1,2, and simultaneously, SCI has been a major burden on the society with increasing prevalence1.
Currently, the prevalence of SCI is approximately 180,000 cases worldwide, with numbers still rising. Our study found that the annual incidence was 23.7 cases per million population, and SCI was more common in older individuals in Tianjin, China3. Individuals with SCI had a higher rate of death than controls4. According to an epidemiological investigation of SCI, the most common causes of SCI are falls and traffic accidents5. Current treatments of SCI include traditional drug therapy1, surgery6,7, cell therapy8–10, gene therapy and tissue engineering11–13. However, these strategies cannot fully repair SCIs but can only improve symptoms and reduce complications.
The spinal cord consists of the gray and white matter which contains nerve cell bodies and ascending and descending tracts. Thus, the different locations and the extent of SCI can cause varying degrees of disability, from partial loss of sensory or motor function to complete paralysis below the injured location, as well as acute and chronic complications14. The poor prognosis of SCI is associated with the extremely weak regenerative capacity of the spinal cord; although there is some inherent regenerative capacity of the central nervous system, it is inadequate. The poor regenerative capacity of the spinal cord is further complicated by the fact that SCI is often accompanied by various molecular pathology cascades that interact with each other. Traditionally, the pathophysiology of SCI is divided into two phases: primary injury and secondary injury6,7,15,16. Recent studies have provided a more detailed description of these phases based on different times after injury reviewed in Rowland et al.17 and Hayta et al.18, and which have demonstrated the high complexity of SCI at different levels. The complicated pathophysiology of SCI includes cell death, axonal collapse and demyelination, glial scar formation, inflammation and other pathological defects. However, there is no systematic theory that can define these complicated pathophysiological processes and guide the development of therapies for SCI.
Thus, according to the developments in SCI research7,19–21 and our previous work, we defined ‘microenvironment imbalance after SCI’ as the imbalance of tissue-, cell- and molecule-promoting and inhibiting factors at different times and sites that aggravate and accelerate the course of SCI.
Microenvironment Imbalance After SCI
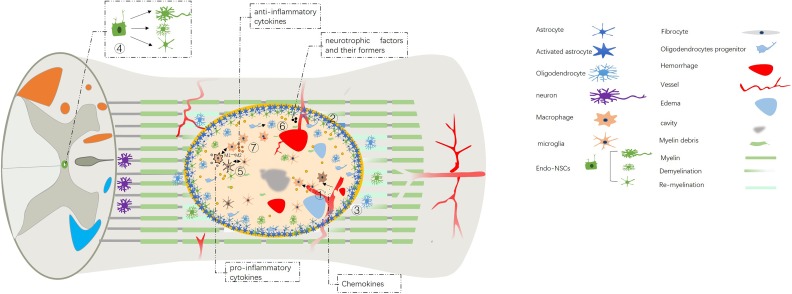
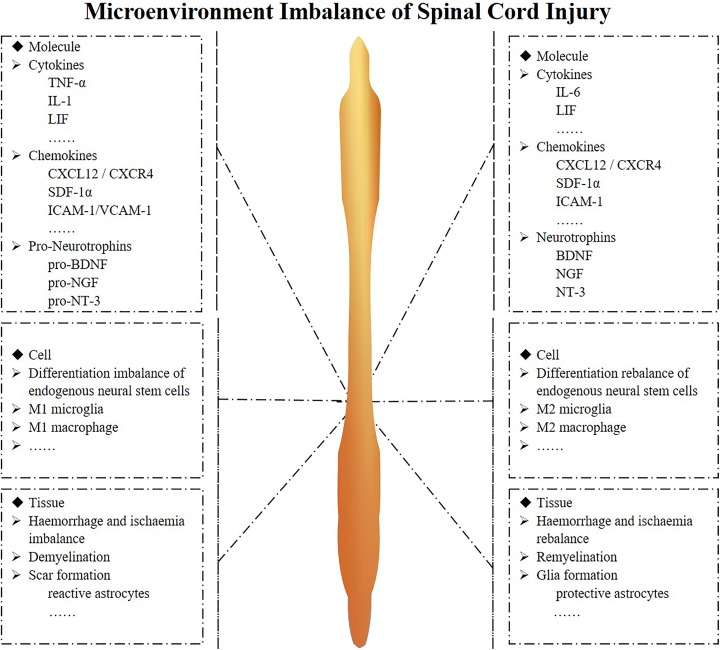
SCI can be divided into two categories: traumatic and non-traumatic spinal cord injury. Traumatic SCI is much more common and is typically caused by external physical impact1. Non-traumatic SCI is often caused by compression of tumor, vascular ischemia or congenital disease22. This review will focus on traumatic SCI. Following contusion injury, the balance of the spinal cord microenvironment is disrupted, which leads to a series of pathophysiological changes; beneficial factors become downregulated, and harmful factors become upregulated after SCI. The microenvironment imbalance consists of three levels at different times and sites: molecules, cells and tissues. The cellular level involves the activation of astrocytes, the differentiation of endogenous neural stem cells, oligodendrocyte progenitors and microglia, the infiltration of macrophages, etc. The tissue level involves hemorrhage and ischemia, glial scar formation, demyelination and re-myelination, etc. The molecular level involves the expression of neurotrophic factors and their pro-peptides, cytokines, chemokines, etc. These imbalances impair regeneration and functional recovery (Figs. 1 and 2).
Fig. 1.
Microenvironment imbalance of spinal cord injury.
① Hemorrhage and ischemia.
② Scar formation.
③ Demyelination and re-myelination.
④ Differentiation balance of endogenous neural stem cells.
⑤ Transformation of the phenotypes of microglia and macrophages.
⑥ Imbalance of neurotrophic factors and their pro-peptides.
⑦ Imbalance of the cytokines and chemokines.
Endo-NSC: endogenous neural stem cell.
Fig. 2.
Microenvironment imbalance of spinal cord injury at different level.
The microenvironment at the molecule, cell and tissue level is shown separately. The tissue level imbalance includes hemorrhage and ischemia, glial scar formation, demyelination and re-myelination; the cellular level imbalance involves an imbalance in the differentiation of endogenous stem cells and the transformation phenotypes of microglia and macrophages; the molecular level includes an imbalance of neurotrophic factors and their pro-peptides, cytokines, and chemokines.
BDNF: brain-derived neurotrophic factor; CXCR4: C-X-C chemokine receptor type 4; CXCL12: C-X-C motif chemokine 12; ICAM1: intercellular adhesion molecule; IL: interleukin; LIF: leukocyte inhibitory factor; NGF: nerve growth factor; NT-3: neurotrophin-3; SDF-1α: stromal cell-derived factor 1α; TNF-α: tumor necrosis factor alpha; VCAM1: vascular cell adhesion protein.
Tissue Imbalance
Hemorrhage and Ischemia
Primary mechanical damage from SCI leads to the disruption of the topical capillaries and the blood–brain-spinal cord barrier (BSCB), which provides a specialized microenvironment for the spinal cord parenchyma. The imbalance of hemorrhage and ischemia was broken. (Fig. 1 ①) A direct rupture of the local capillaries induces bleeding into the parenchyma of the spinal cord, especially into the gray matter23, which could cause increased release of cytokines and chemokines from macrophages, microglia and astrocytes into the extracellular space. And the presence of red blood cells/heme in parenchyma, a rich source of iron, is likely to induce free radicals and be toxic24, which is a potential mechanism for ferroptosis. Venous stasis and distension would further cause the accumulation of proteinaceous fluid in the tissues, leading to edema25. On the other hand, neural tissue edema can also increase interstitial pressure, which would compress the surrounding vessels and subsequently cause ischemia26. In addition, damage to the BSCB can increase its permeability, which would cause macrophages infiltrated from injured blood vessels and accumulate in the microenvironment of the spinal cord, and the macrophages would express more cytokines and chemokines; in turn, this would further increase permeability of the BSCB. The lack of adenosine triphosphate (ATP) caused by ischemia and ion channel defects would result in an ion imbalance. Moreover, the accumulation of water in cells and the extracellular compartment worsens the neural tissue edema27.
Scar Formation
Glial scar formation is a vital part of the pathology of SCI, which includes fibrous and glial components. The fibrous component contains stromal cells at the center of the scar28, which are derived from the vascular-related type A pericytes; the glial component consists of astrocytes, which are derived from self-replicating astrocytes and endogenous neural stem cells (endo-NSCs), and the astrocytes from the endo-NSCs reinforce the glial scar29. The glial scar is also composed of microglia, macrophages and extracellular matrix and astrocytes. The extracellular matrix is mainly composed of chondroitin sulfate proteoglycans (CSPGs). From 2 days to 2 weeks after injury, astrocytes proliferate at the injured area, and their large cell bodies and protrusions closely link together to form glial scars; this separates the nerve tissue from the inflammatory cells and reduces the early stage of the neuroinflammatory response. From 2 weeks to 6 months after injury, the astrocyte scar is considered mature. Due to the presence of a glial scar and other inhibitory factors, such as CSPGs and myelin-associated protein, axon regeneration is limited. At 6 months after injury, the scar is continuously reinforced as cysts and cavities are gradually formed17,30. The scar forms a physical and molecular barrier, limiting the spread of inflammation; however, this also hinders axon regeneration and outgrowth.
Dual aspects of astrocytes
Damage to the BSCB causes macrophage infiltration and microglial activation, which could trigger the activation of local astrocytes. Furthermore, the resulting lack of oxygen and glucose and the increase in albumin would stimulate astrocyte accumulation in the center of the injury site. This would simultaneously alter the protein expression pattern of the astrocytes. Pekny et al. reviewed that glial fibrillary acidic protein (GFAP), a kind of III intermediate filamentous protein, was highly expressed in the reactive astrocytes, and vimentin, nestin, S100β were also upregulated, which would lead to cellular hypertrophy31. In addition, these astrocytes express inhibitory proteins that contribute to the formation of the glial scar31. The most important of these proteins, chondroitin sulfate proteoglycans (CSPGs)32, hinder axon outgrowth. Researchers at the Department of Cell Biology and Program in Neuroscience at Harvard Medical School, USA have reported that the tyrosine phosphatase receptor σ (PTPσ) is distributed on the surface of neurons. PTPσ is a CSPG receptor, and the PTPσ–CSPG interaction prevents axonal growth cone movement, thus inhibiting axons from passing through the glial scar33. This suggests that PTPσ functions in the spinal cord injury microenvironment as a ‘molecular switch’ to directly define the regenerative capacity of the axon. Our lab demonstrated that axons could bypass CSPG by inhibiting PTPσ34. The inhibitory proteins secreted by astrocytes combine with other cells to form a physical and molecular wall to prevent the expansion of the injury site into the intact area, and to inhibit the regenerative axons from passing through this barrier. Our team successfully established an isolation, culture and purification protocol for spinal cord-derived astrocytes in vitro, used small interfering RNA against PTPσ35 and conducted photodynamic therapy using upconverting nanoparticles to inhibit astrocytes36. These studies suggested that the inhibition of activated astrocytes at the subacute phase could be used as an effective repair strategy to rebalance the microenvironment in SCI patients.
Aside from the detrimental function, astrocytes play a critical role in the restriction of inflammation and the lesion area and contribute to endogenous neuroprotection. Sabelström et al. generated the FoxJ1-CreER mouse strain and demonstrated that astrocytes derived from endogenous stem cells are necessary to reinforce the scar and restrict the area of damaged tissue37. They further demonstrated that astrocytes derived from endogenous stem cells could express ciliary neurotrophic factor, hepatocyte growth factor, and insulin-like growth factor-1 (IGF-1)37. In addition, another study showed that astrocytes could also express brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), glial cell-derived neurotrophic factor (GDNF), basic fibroblast growth factor (FGF-2) and laminin and fibronectin38. In contrast, Anderson et al. reported that astrocytes promote axon regeneration, while fibroblasts inhibit axon passage through the glial scar39. Thus, the astrocytes from the endo-NSCs play a protective role in axon regeneration.
Altogether, the imbalance of dual aspects of astrocytes can be regulated by inhibiting the overactivation of astrocytes and maintaining the protective aspects to repair SCI (Fig. 1 ②).
Demyelination and re-myelination
In the central nervous system, each oligodendrocyte is responsible for generating and maintaining myelin segments of 30–80 distinct axons40,41. Myelin is essential to maintain the integrity of axons and could facilitate axon signal conduction. After SCI, direct damage and the imbalance of local microenvironment factors leads to demyelination (Fig. 1 ③). However, the mechanisms of demyelination are unclear. The necrosis and apoptosis of oligodendrocytes are potentially the leading causes of axonal demyelination. The level of oligodendrocyte apoptosis at the epicenter of a lesion peaks within a week of contusion injuries to the spinal cord42. This results in demyelination of the most injured axon; however, uninjured axons around the lesion remain myelinated43. Apoptosis of oligodendrocytes after SCI lasts for approximately 3 months, and then injured axons appear to become remyelinated. The continued loss of oligodendrocytes in the chronic phase of SCI is a major impediment to functional recovery44. Mechanical injury, ischemia, proinflammatory cytokines, oxidative stress, glutamate- and ATP-mediated excitotoxicity and autophagy45,46 can all potentially cause the death of oligodendrocytes due to the resulting imbalance of demyelination and re-myelination. Molecules involved in demyelination are potent inhibitors of axon regeneration, such as neurite outgrowth inhibitor A (Nogo-A), oligodendrocyte-myelin glycoprotein (OMgp) and myelin-associated glycoprotein (MAG)47, which cause growth cone collapse, neurite retraction and increases the risk of apoptosis. Thus, the process of demyelination inhibits the regeneration of axons.
Re-myelination naturally occurs after SCI. The process of re-myelination is mainly the process of replacement of oligodendrocytes45. The new oligodendrocytes have two sources: progenitor oligodendrocytes and endogenous neural stem cells. Progenitor oligodendrocytes become activated and convert to an immature state; following increased proliferation, these oligodendrocytes differentiate into myelinating oligodendrocytes, thus re-myelinating the spared and regenerated axons45. Endo-NSCs remain quiescent in the normal spinal cord, and become activated upon spinal cord damage; these cells primarily differentiate into astrocytes but also differentiate into oligodendrocytes to a lesser degree. This suppression of differentiation into oligodendrocytes is mainly due to the lack of growth factors that shift the balance to favor differentiation into oligodendrocytes. Epidermal growth factor (EGF), bFGF, and platelet-derived growth factor-AA (PDGF-AA) are important for oligodendrocyte differentiation, and Neuregulin-1 could promote oligodendrocyte progenitor cell (OPC) differentiation into mature myelinating oligodendrocytes. However, re-myelination-inhibiting factors are also present in the microenvironment44. Oligodendrocyte apoptosis causes the disruption of myelin, and the prolonged presence of myelin debris inhibits re-myelination. Wnt48 and LINGO1 signaling also inhibits re-myelination. As a result, the extent and quality of re-myelination are limited. Although spared and regenerated axons are myelinated, the conductive function of the axons does not change49. Thus, many studies demonstrated SCI repair and recovered spinal cord function through the restoration of the myelin sheath50–52. Our team transplanted autologous activated Schwann cells into the spinal cord, and we found relatively complete restoration of the myelin sheath and improved microenvironment balance53, and another study demonstrated similar results when co-transplanting human umbilical cord mesenchymal stem cells and human Schwann cells54. In addition, our clinical experiment showed functional recovery of the spinal cord following autologous activated Schwann cell transplantation55.
Cellular Imbalance
Differentiation Balance of Endogenous Neural Stem Cells
Traditionally, stem cells were thought to be absent from the mature central nervous system, especially from the spinal cord. In the mature spinal cord, OPCs and astrocytes are the main dividing cells29; SCI results in increased proliferation of OPCs and activation of astrocytes56. However, OPCs and activated astrocytes are not stem cells, as both lack pluripotency. Recent studies have revealed that in the central canal of the normal spinal cord, ependymal cells remain quiescent but have the ability to differentiate into astrocytes and oligodendrocytes. Johansson et al. reported that cells derived from ependymal cells could migrate to the olfactory bulb and differentiate into neurons, as well as migrate to the injured spinal cord and differentiate into astrocytes. Thus, the use of endo-NSCs of the spinal cord for the treatment of SCI attracted public attention57. Barnabe-Heider et al. further demonstrated that new glial cells were derived from ependymal cells using the construction of genetic fate mapping29. SCI caused a strong, persistent, long-distance proliferation of ependymal cells58, which peaked after 3–7 days, and there were 2 million new cells produced within 1 month at the injured site59. Ependymal cells rapidly divide, produce large amounts of astrocytes, and contribute to scar formation and the small amounts of oligodendrocytes. However, the activation of the ependymal stem cells is not sufficient to promote functional recovery due to the lack of neuronal differentiation.
As the imbalance in endo-NSC differentiation leads to the overall cellular imbalance in SCI, the rebalance of the cellular microenvironment of the injured spinal cord would improve SCI recovery (Fig. 1 ④).
The differentiation of neurons
The loss of neurons is the main reason for the limited recovery after SCI. The ratio of endo-NSCs to neurons directly impacts SCI recovery. The differentiation of endogenous stem cells can be impacted by inhibiting factors that are present in the microenvironment after SCI, which promote endogenous stem cell differentiation into more astrocytes. Thus, the population of neurons derived from endogenous stem cells is inadequate to reconstruct the synapses and nerve circle. Cell reprogramming technology is currently the principal strategy used to promote neuron differentiation via enhancing growth factors and decreasing inhibitors of the imbalance microenvironment. Recent studies have used reprogramming technology to convert endogenous glial cells into functional neurons within the brain and spinal cord60,61. The differentiation of endogenous neural progenitor cells into motor neurons is insufficient because the ratio of Ngn2/Olig2 for neural progenitor cells is 10 times lower than that for embryonic stem cells (ESCs)62. The ratio of Ngn2/Olig2 determines the differentiation of motor neurons and oligodendrocytes63. In the brain, the single transcription factor SOX2 was sufficient to reprogram the local astrocytes to neuroblasts, and these cells could further differentiate into functional neurons when combined with BDNF64. To decrease the impact of the inhibitors in the microenvironment, Fan et al. used a modified scaffold with a collagen-binding epidermal growth factor receptor (EGFR) antibody Fab fragment to neutralize myelin inhibitory molecules and repair SCI; they found that enhanced neurogenesis of endo-NSCs and neurons could reconnect the injured gap65. After transplantation into the injured spinal cord, an NT-3 chitosan biomaterial, which slowly releases NT-3, improved the local NT-3 concentration and attracted endo-NSCs to migrate towards the lesion epicenter and differentiate into neurons66. And there was study demonstrated that melatonin combined with exercise could also promoted endogenous stem cell differentiation into neurons after SCI67. Furthermore, our team used small molecules, valproic acid (VPA), combined with all-trans retinoic acid to promote neural stem cell differentiation into neurons in vitro. These results showed the promotion of neuron differentiation and the suppression of astrocyte differentiation68. For the imbalance of endo-NSC differentiation, the selective use of small molecules can effectively achieve its differentiation rebalance.
The differentiation of oligodendrocytes
Contusion or crushing injury of the spinal cord results in the loss of oligodendrocytes. After SCI, oligodendrocyte apoptosis at the center of the injury peaks within a week42, which leads to the demyelination of the most injured axon. Thus, the imbalance of differentiation of endo-NSCs can be caused by promoting oligodendrocyte differentiation to improve the re-myelination. Currently, there are limited studies focused on the differentiation of oligodendrocytes from endo-NSCs. The Nrg-ErbB network is essential for oligogenesis. Gauthier et al. demonstrated that Nrg-1 could enhance the differentiation of neural progenitor cells into oligodendrocytes in vitro. Administering rhNrg-1b1 in vivo increased the number of new oligodendrocytes and promoted the preservation of axons, whereas inhibiting its receptor, ErbB, had the opposite effect69. Insufficient oligodendrocyte differentiation was associated with the lack of neurotrophic factors in the microenvironment following SCI. A previous study transplanted human umbilical cord blood-derived mesenchymal stem cells into injured spinal cords and showed that cell transplantation enhanced the proliferation of endogenous neural stem cells and increased new oligodendrocytes70. This study suggested that the neuroprotective trophic factors secreted from graft cells contributed to the differentiation of oligodendrocytes. In addition, Karimi-Abdolrezaee et al. utilized chondroitinase and growth factors (EGF, bFGF and PDGF-AA) to repair SCI and demonstrated that this strategy promoted endogenous oligodendrocyte replacement and improved the microenvironment71. In addition, electroacupuncture was shown to promote the proliferation of endo-NSCs and oligodendrocytes72.
Transformation of the phenotypes of microglia and macrophages
Microglia are the resident macrophages of the central nervous system, and with regard to their cytokine production and immune function, they remain quiescent to a certain extent73. After SCI, the damaged neurons, astrocytes and other injured cells release cytokines and other factors such as interleukin (IL)-1β, tumor necrosis factor alpha (TNFα)74,75, signals of damage associated molecular patterns (DAMPs)76, interferon gamma (IFN-γ)77, ATP78,79, nitric oxide (NO)80, and growth factors81. The release of these cytokines induces the activation of microglia and, consequently, increases the proliferation of microglial cells. The number of activated microglia becomes elevated on the first day after SCI, and continues to increase within 7 days, until the cell population plateaus between 2–4 weeks82. In the central nervous system, activated microglia release trophic factors for the survival and proliferation of infiltrating cells as well as the growth and regeneration of axons in the lesion site during earlier stages of SCI83–85; moreover, microglial activation serves a protective role by limiting the expansion of the lesion site86. However, activated microglia can also express various proinflammatory cytokines, such as IL-1α, IL-1β and TNFα75. At 2–3 days after injury, microglia can induce macrophages from the peripheral circulation to infiltrate the injured site and trigger the inflammatory response through these cytokines. Macrophages can reach maximum numbers 7–10 days after SCI87 and persist in the lesion area for up to 42 days88,89. Macrophages, which are crucial for the inflammatory response in the spinal cord90,91, can be derived from two cell types: the resident microglial cells and the peripherally circulating macrophages. The latter originate from the bone marrow and infiltrate the injured site after SCI. However, the appropriate activation of macrophages can also aid in the repair and regeneration of the injured central nervous system87.
Macrophages and microglia both have the ability to become polarized92–94. There are two main polarization phenotypes, M1 and M2 (Fig. 1 ⑤); additionally, the M2 phenotype can be divided into M2a, M2b and M2c. The ratio of M1 to M2 determines the homeostasis of the local microenvironment. During the acute response to trauma, high levels of reactive oxygen species (ROS) are detectable. With the stimulation of these factors, the M1 macrophage/microglia in SCI occupy a predominant state, which is detrimental to the repair of SCI95. This ratio results in the production of proinflammatory cytokines, such as IL-6, IFN-γ, IL-12, IL-23, IL-1β, and TNFα96. M1 macrophages are converted to the M2 phenotype with the phagocytosis of myelin phenotype. M2 macrophages are anti-inflammatory cells that exhibit tissue repair properties (i.e. high production of IL-10 and transforming growth factor beta (TGFβ)), exhibit defective nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, upregulate arginase 1 and downregulate the expression of proinflammatory cytokines94. Although apoptotic neutrophils and red blood cells (RBCs) can transform the phenotype of macrophages from M1 to M2, the number of M2 macrophages was low at the early stage of SCI and further decreased after 7 days93,97. The microenvironment post-SCI is unfavorable for M2 macrophages, such that the high expression of TNF would inhibit the transformation of M1 to M291.
Molecular Imbalance
Imbalance of Neurotrophic Factors and Their Pro-Peptides
There is an imbalance between growth promoting molecules and growth inhibiting molecules among the SCI microenvironment, where growth inhibitors occupy the dominant position (Fig. 1⑥). This results in the death of neurons and oligodendrocytes as well as the degeneration of axons. Among the growth promoting molecules, neurotrophic factors play a critical role in the development, maintenance and survival of cells in the central and peripheral nervous system98,99. Neurotrophic factors significantly promote the survival and proliferation of different cells and axon regeneration after SCI100,101. The neurotrophic factor family consists of BDNF, NGF, and neurotrophin-3 (NT-3), neurotrophin-4/5 (NT-4/5). Recently, several studies have showed that the pro-neurotrophins of NGF, BDNF and NT-3 are also present and play a vital role in the cell death102. NGF, BDNF and NT-3 are synthesized as uncleaved pro-peptides (proNGF, proBDNF and proNT-3), which are either secreted from cells or cleaved intracellularly into mature neurotrophic factors103,104. The balance of proneurotrophins and neurotrophins is disrupted after SCI, and the resulting elevated expression of proneurotrophins accelerates apoptosis, reduces synaptic plasticity, increases the inflammatory response and induces degeneration105,106.
BDNF versus proBDNF
BDNF is mostly involved in the repair of SCI. BDNF was first extracted from the brain by Barde et al. in 1982107. BDNF can combine with the receptor of tropomyosin receptor kinase (Trk)B and promote the outgrowth of axons and survival of dorsal root ganglion neurons,108,109 as well as promote the regeneration of axons of corticospinal tract. In addition, BDNF can promote myelination, regulate synaptic plasticity and affect synaptic transmission110. Several studies have used BDNF to repair SCI by direct administration, transplantation of cells overexpressing BDNF and release by scaffold111; these studies demonstrated a neuroprotective role of BDNF. Our team used BDNF, NGF genetically modified Schwann cells and fetal spinal cord cell suspension to repair SCI in rats. This combination treatment elicited a robust growth response of corticospinal axons and significant functional recovery112. Following SCI, Wong et al. found that proBDNF levels were upregulated in the spinal cord 1 to 3 days after injury but downregulated after 7 days105. Moreover, the inhibition of proBDNF promoted an increase in the number of neurons and improved the functional recovery of the animals. Our team used proBDNF-specific antibodies to antagonize the inhibitory effect of proBDNF. This resulted in increased proliferation of OPCs and cell division activity and promoted the function of the animal113. These results suggested that proBDNF in the spinal cord microenvironment suppressed the survival of neurons and that, through the use of proBDNF-specific antibodies, microenvironment rebalance can be achieved.
NGF versus proNGF
NGF has historically been thought to function only in the peripheral nervous system. However, recent studies have demonstrated a similar role for NGF in the central nervous system. NGF binds to the Trk receptors and pan-neurotrophin receptors (p75NTR) to maintain and promote the survival of neural cells, which was reviewed by Richner et al.114. Several studies have demonstrated that NGF promotes the regeneration of axons and improves the functional recovery. Romero et al. used conditional expression of NGF in the adult rat spinal cord and found that the expression of NGF could promote the axonal sprouting of the sensory afferents and achieve better behavioral outcomes115. In addition, our team prepared genetically modified Schwann cells overexpressing NGF to repair SCI in rats, which improved hind limb movement116. The precursor of NGF could interact with Sortilin and p75 to form a complex to lead to an apoptotic cascade117,118. Thus, the balance between NGF and proNGF could determine the balance of cell survival and death. Harrington et al. demonstrated that proNGF was increased in the brain injuries and SCIs102. ProNGF is the predominant form of NGF expressed in nearly all brain tissue in mice, rat and human. Beattie et al. reported that the expression of NGF and proNGF were both upregulated in contusion SCIs and that the expression of proNGF was equivalent to or higher than that of NGF. They also demonstrated that proNGF induced the p75NTR-mediated decrease in the number of oligodendrocytes106. In addition, proNGF was detected in the GFAP-positive cells in the brain. Domeniconi et al. also demonstrated that astrocytes from neonatal spinal cord could express proNGF with stimulation, which resulted in neuron death when cultured in vitro, suggesting that astrocytes are potentially the major source of proNGF119.
NT-3 versus proNT-3
The neurotrophin NT-3, plays an important role in the development of the nervous system. The mRNA of NT-3 is mainly expressed in the developing brain and motor neurons of the spinal cord, whereas the expression of NT-3 is low in the adult spinal cord. After SCI, the expression of NT-3 dropped rapidly in the first 6 hours and recovered to normal levels by 12 hours120. Thus, follow-up studies utilized NT-3 to repair SCI and obtained functional recovery116,121. The pro-peptide of NT-3 is proneurotrophin-3 (proNT-3). Tauris et al. demonstrated that proNT-3 induces the neuron death in the inner ear using Sortilin122. Furthermore, this study demonstrated that recombinant proNT-3 could induce sympathetic neuron death through a p75NTR- and a Sortilin-dependent mechanism. However, the role of proNT-3 in the process of SCI is unknown. Thus, much remains to be understood about the balance of NT-3 and proNT-3 in the SCI microenvironment.
Imbalance of the Cytokines and Chemokines
Cytokines
Cytokines can be divided into proinflammatory or anti-inflammatory proteins that participate in neuroinflammation, neurodegeneration, neuropathic pain123,124. After SCI, neurons in the spinal cord express these cytokines within 30 min, and microglia express these cytokines 5 hours later; however, the expression of both decreases by the second day125. In addition, TNFα and IL-6 can be secreted by other cells in the central nervous system (CNS), such as astrocytes and epidermal cells126. Several cytokines, such as IL-1, IL-6, TNFα, granulocyte-macrophage colony-stimulating factor (GM-CSF) and leukocyte inhibitory factor (LIF), participate in the dynamic changes of the SCI microenvironment26,127. Some proinflammatory cytokines have protective qualities at low concentrations due to their induction of neurotrophin expression as well as the induction of adhesion molecules in the cell surface, which mediates leukocyte activation/recruitment to the injury site128. Proinflammatory cytokines also activate endogenous stem cells. However, the main function of these cytokines, as proinflammatory molecules, leads to neuronal damage and destruction when their concentration exceeds a certain threshold. At higher concentrations, these proinflammatory cytokines activate transcription factors (ATF) as well as factors that stimulate the expression of neurotoxic genes, including cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), and proinflammatory proteases such as thrombin in different target cells129,130. Accumulation of IL-1 in the spinal cord leads to enhanced vascular permeability and lymphocyte recruitment. Moreover, the release of IL-6 has been found to promote the activation and infiltration of macrophages and microglia. Several studies have revealed that the continuous inhibition of IL-6 is detrimental to functional recovery because it also participates in axonal regeneration and gliosis131,132. TNFα is significantly upregulated in neurons, glia, and endothelial cells following SCI133. In addition, TNFα could recruit neutrophils to the site of the lesion by the induction of adhesion molecules such as intercellular adhesion molecule (ICAM-1) and vascular cell adhesion protein (VCAM-1)134. With the increased level of TNFα, the permeability of endothelial cells is altered, which further resulted in the disabling of the blood–spinal cord barrier. TNFα could induce cell death in oligodendrocytes135 and lead to demyelination; and the suppression of TNFα resulted in decreased demyelination136. Neutralizing antibodies against TNFα improved functional neurological recovery following SCI137. However, TNFα signaling has also been demonstrated to have a neuroprotective role in vitro138 and promote functional recovery following SCI139.
Chemokines
There are complex changes in the levels of a variety of important chemokines at different times and sites after SCI, among which stromal cell-derived factor 1α (SDF-1α) binds to G-protein-coupled C-X-C chemokine receptor type 4 (CXCR4) and plays an important role in the repair of SCI. Kucia et al. reviewed that SDF-1-CXCR4 axis could regulate stem/progenitor cell trafficking and the metastatic behavior of tumor cells140. Our team used immunohistochemistry to observe the changes in CXCR4 expression in spinal cord tissue141. It was found that the expression of CXCR4 in neurons, glial cells, macrophages and ependymal cells in spinal gray matter was increased on the third day after SCI in rats. The number of CXCR4-positive cells peaked in the gray matter of spinal cord. In addition, a previous study showed that the CXCR4-mediated stem cell migration to the injured area to repair the injured spinal cord tissue142. In addition, the expression of SDF-1α in the proximal and distal SCI centers was significantly increased143. The number of SDF-1α-positive cells in the spinal cord tissue began to rise at 1 day after SCI, reaching its peak at 2 days after injury. The number of proximal SDF-1α-positive cells at 7 days after injury was significantly higher than the numbers in normal and sham-operated groups. The results showed that proliferative astrocytes could release trophic factors to promote damaged axon repair and regeneration, high levels of expression of SDF-1α can strongly stimulate the proliferation of astrocytes and play a role in repairing the spinal cord. Thus, 48 h after acute SCI, continuous local intrathecal injection of SDF-1α to restore local SDF-1α concentrations to a high level may be a viable option for early treatment of SCI.
Ion Imbalance
In the pathological process of SCI, ion imbalance plays a fundamental role in regulating other pathological changes. The most important ions are K+, Na+, and Ca2+. After SCI, the selectivity of the K+, Na+, and Ca2+ channels is altered due to damage to the membrane of cells and the release of proinflammatory factors by different cells. Subsequently, the cellular and extracellular homeostasis of K+, Na+, and Ca2+ is disrupted. Finally, the concentrations of Na+ and Ca2+ are upregulated in cells, while the concentrations of K+ and Mg2+ are upregulated extracellularly144. With the Na+ influx into the cell, water gradually accumulates in the cell, which leads to cytotoxic cellular edema. This further leads to the stimulation of intracellular phospholipase activity and promotion of intracellular acidosis145. There were studies utilizing Na+ channel inhibitors, such as tetrodotoxin146,147, riluzole148,149, and phenytoin150,151, to repair SCI, and these study demonstrated that inhibition of the Na+ channel has a neural protective effect. As Ca2+ participates in several pathological processes (e.g. synaptic transmission), Ca2+ plays a vital role in responding to injuries of the central nervous system. After SCI, the concentration of Ca2+ was increased within 1 min after SCI and reached its peak at 8 hours; moreover, this high concentration of Ca2+ persisted for 2 weeks. The high concentration of Ca2+ in cells could cause apoptosis or necrosis through increasing the activation of cellular enzymes, mitochondrial damage, acidosis, and the production of free radicals145, and it could also further impact the white matter after SCI152. The K+ channel is the most extensively studied ion channel in SCI myelination153. The myelin sheath of axons in spinal cord is disrupted after SCI, which exposes K+ channels and disrupts K+ channel distribution45. This could result in a number of detrimental effects, including conduction failure and demyelination. The conduction failure is ascribed to the increased activity of K1 channels. In contrast, the voltage-gated K+ channels are also important for re-myelination. There was a study that injected 4-aminopyridine (4-AP), a K+ channel antagonist, subcutaneously into adult male C57BL/6 mice, and found that 4-AP could decrease re-myelination in the corpus callosum154.
Recently, iron has been shown to play a vital role in the maintenance of the normal function of the CNS155,156. After SCI, the accumulation of iron in the extracellular space is caused by the influx of RBCs due to hemorrhage. Liu et al. demonstrated that the level of iron was increased at 0.5 h157. In addition, Liu et al. demonstrated that iron was rapidly increased within 20 min155. Iron plays a significant role in glutamate excitotoxicity, the formation of ROSs and the production of free radicals158,159, which inhibit the regeneration of SCI. Our team utilized deferoxamine (DFO), an iron chelator, in the repair of SCI. We found that the application of DFO could decrease the total iron ion level, TNFα, IL1-β and caspase-3 expression and glial scar formation after SCI and promote the survival of cells and recovery of motor function24.
Conclusions
The theory of ‘microenvironment imbalance after SCI’ describes the imbalance of molecules, cells and tissues in the spinal cord following injury. This theory explains the complicated intercorrelation of each level, which will provide guidance for the understanding of pathological process and treatment of SCI.
Footnotes
Authors’ Note: Baoyou Fan and Zhijian Wei contributed equally to this work.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: NSFC programme (81330042, 81620108018, 81672171), Ministry of Science and Technology, China (2014DFR31210), and Tianjin Science and Technology Committee, China (13RCGFSY19000, 14ZCZDSY00044).
References
- 1. Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. [DOI] [PubMed] [Google Scholar]
- 2. Furlan JC, Noonan V, Singh A, Fehlings MG. Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. J Neurotrauma. 2011;28(8):1445–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ning GZ, Yu TQ, Feng SQ, Zhou XH, Ban DX, Liu Y, Jiao XX. Epidemiology of traumatic spinal cord injury in Tianjin, China. Spinal Cord. 2011;49(3):386–390. [DOI] [PubMed] [Google Scholar]
- 4. Krause JS, Sternberg M, Lottes S, Maides J. Mortality after spinal cord injury: an 11-year prospective study. Arch Phys Med Rehabil. 1997;78(8):815–821. [DOI] [PubMed] [Google Scholar]
- 5. Wu Q, Li YL, Ning GZ, Feng SQ, Chu TC, Li Y, Hao Y, Wu QL. Epidemiology of traumatic cervical spinal cord injury in Tianjin, China. Spinal Cord. 2012;50(10):740–744. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz T, Kaptanoglu E. Current and future medical therapeutic strategies for the functional repair of spinal cord injury. World J Orthop. 2015;6(1):42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80(suppl 3):S9–S22. [DOI] [PubMed] [Google Scholar]
- 8. Sabapathy V, Tharion G, Kumar S. Cell therapy augments functional recovery subsequent to spinal cord injury under experimental conditions. Stem Cells Int. 2015;2015:132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A, Asady H, Razavi Tousi SM, Hosseini M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience. 2016;322:377–97. [DOI] [PubMed] [Google Scholar]
- 10. Ide C, Kanekiyo K. Points regarding cell transplantation for the treatment of spinal cord injury. Neural Regen Res. 2016;11(7):1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin XY, Lai BQ, Zeng X, Che MT, Ling EA, Wu W, Zeng YS. Cell transplantation and neuroengineering approach for spinal cord injury treatment: a summary of current laboratory findings and review of literature. Cell Transplant. 2016;25(8):1425–1438. [DOI] [PubMed] [Google Scholar]
- 12. Raspa A, Pugliese R, Maleki M, Gelain F. Recent therapeutic approaches for spinal cord injury. Biotechnol Bioeng. 2016;113(2):253–259. [DOI] [PubMed] [Google Scholar]
- 13. Dumont CM, Margul DJ, Shea LD. Tissue engineering approaches to modulate the inflammatory milieu following spinal cord injury. Cells Tissues Organs. 2016;202(1–2):52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Post MW, Bussmann JB. Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. J Rehabil Med. 2007;39(5):393–398. [DOI] [PubMed] [Google Scholar]
- 15. Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- 16. Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10(5):726–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 18. Hayta E, Elden H. Acute spinal cord injury: a review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. [DOI] [PubMed] [Google Scholar]
- 19. Zhao Y, Xiao Z, Chen B, Dai J. The neuronal differentiation microenvironment is essential for spinal cord injury repair. Organogenesis. 2017;13(3):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21(5):941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alizadeh A, Karimi-Abdolrezaee S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. 2016;594(13):3539–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKinley WO, Seel RT, Hardman JT. Nontraumatic spinal cord injury: incidence, epidemiology, and functional outcome. Arch Phys Med Rehabil. 1999;80(6):619–623. [DOI] [PubMed] [Google Scholar]
- 23. Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44(5):1027–1039; discussion 1039–1040. [DOI] [PubMed] [Google Scholar]
- 24. Hao J, Li B, Duan HQ, Zhao CX, Zhang Y, Sun C, Pan B, Liu C, Kong XH, Yao X, Feng SQ. Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Regen Res. 2017;12(6):959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandler AN, Tator CH. Effect of acute spinal cord compression injury on regional spinal cord blood flow in primates. J Neurosurg. 1976;45(6):660–676. [DOI] [PubMed] [Google Scholar]
- 26. Mortazavi MM, Verma K, Harmon OA, Griessenauer CJ, Adeeb N, Theodore N, Tubbs RS. The microanatomy of spinal cord injury: a review. Clin Anat. 2015;28(1):27–36. [DOI] [PubMed] [Google Scholar]
- 27. Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des. 2005;11(11):1353–1389. [DOI] [PubMed] [Google Scholar]
- 28. Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333(6039):238–242. [DOI] [PubMed] [Google Scholar]
- 29. Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–482. [DOI] [PubMed] [Google Scholar]
- 30. Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:15–54. [DOI] [PubMed] [Google Scholar]
- 31. Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–434. [DOI] [PubMed] [Google Scholar]
- 32. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. [DOI] [PubMed] [Google Scholar]
- 33. Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou HX, Li XY, Li FY, Liu C, Liang ZP, Liu S, Zhang B, Wang TY, Chu TC, Lu L, Ning GZ, Kong XH, Feng SQ. Targeting RPTPsigma with lentiviral shRNA promotes neurites outgrowth of cortical neurons and improves functional recovery in a rat spinal cord contusion model. Brain Res. 2014;1586:46–63. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Feng S, Xia R, Yan J. Influence of small interfering RNA-mediated inhibiting Vimentin on reactive astrogliosis and its effect in formation of glial scar. Chin J Exp Surg. 2014;31(12):2758–2760. [Google Scholar]
- 36. Ma C, Feng S, Wei J, Zhang Z, Zhang J, Ban D, Liu Y. The photodynamic effects mediated by upconversion nanoparticles on rat astrocytes in vitro. Chin J Orthop. 2015;35(4):450–455. [Google Scholar]
- 37. Sabelstrom H, Stenudd M, Reu P, Dias DO, Elfineh M, Zdunek S, Damberg P, Goritz C, Frisen J. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342(6158):637–640. [DOI] [PubMed] [Google Scholar]
- 38. Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S. Concise review: reactive astrocytes and stem cells in spinal cord injury: good guys or bad guys? Stem Cells. 2015;33(4):1036–1041. [DOI] [PubMed] [Google Scholar]
- 39. Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77(5):873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chong SYC, Rosenberg SS, Fancy SPJ, Zhao C, Shen YAA, Hahn AT, Mcgee AW, Xu XM, Zheng BH, Zhang LI, Rowitch DH, Franklin RJM, Lu QR, Chan JR. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA. 2012;109(4):1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2(4):299–315. [DOI] [PubMed] [Google Scholar]
- 44. Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. [DOI] [PubMed] [Google Scholar]
- 45. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Myelin damage and repair in pathologic CNS: challenges and prospects. Front Mol Neurosci. 2015;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Almad A, Sahinkaya FR, Mctigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8(2):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kastin AJ, Pan WH. Targeting neurite growth inhibitors to induce CNS regeneration. Curr Pharm Des. 2005;11(10):1247–1253. [DOI] [PubMed] [Google Scholar]
- 48. Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28(33):8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Powers BE, Lasiene J, Plemel JR, Shupe L, Perlmutter SI, Tetzlaff W, Horner PJ. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J Neurosci. 2012;32(15):5120–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoon H, Radulovic M, Walters G, Paulsen A, Drucker K, Starski P, Wu J, Fairlie D, Scarisbrick I. Protease activated receptor 2 controls myelin development, resiliency and repair. Glia. 2017;65(12):2070–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assinck P, Duncan G, Hilton B, Plemel J, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–647. [DOI] [PubMed] [Google Scholar]
- 52. Ineichen BV, Kapitza S, Bleul C, Good N, Plattner PS, Seyedsadr MS, Kaiser J, Schneider MP, Zorner B, Martin R, Linnebank M, Schwab ME. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017:134(3):423–440. [DOI] [PubMed] [Google Scholar]
- 53. Ban DX, Kong XH, Feng SQ, Ning GZ, Chen JT, Guo SF. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat’s spinal cord injury. Brain Res. 2009;1256:149–61. [DOI] [PubMed] [Google Scholar]
- 54. Wu Q, Chen Y, Ning G, Feng S, Han J, Wu Q, Li Y, Wu H, Shi H. The role of hSCs in promoting neural differentiation of hUC-MSCs in spinal cord injury. J Neurorestoratol. 2013;1:55–61. [Google Scholar]
- 55. Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen JT, Zheng YF, Ban DX, Liu T, Li H, Wang P. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: six cases, more than five years of follow up. Cell Transplant. 2012;21(suppl 1):S39–S47. [DOI] [PubMed] [Google Scholar]
- 56. Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81(2):229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. [DOI] [PubMed] [Google Scholar]
- 58. Gregoire CA, Goldenstein BL, Floriddia EM, Barnabe-Heider F, Fernandes KJ. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63(8):1469–1482. [DOI] [PubMed] [Google Scholar]
- 59. Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol 2015;72(2):235–237. [DOI] [PubMed] [Google Scholar]
- 60. Heinrich C, Bergami M, Gascón S, Lepier A, Viganò F, Dimou L, Sutor B, Berninger B, Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep. 2014;3(6):1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grande A, Sumiyoshi K, López-Juárez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Comm. 2013;4(4):2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M. Challenges of stem cell therapy for spinal cord injury: human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells. 2010;28(1):93–99. [DOI] [PubMed] [Google Scholar]
- 63. Li H, Chen G. In Vivo Reprogramming for CNS Repair: Regenerating Neurons from Endogenous Glial Cells. Neuron. 2016;91(4):728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan C, Li X, Xiao Z, Zhao Y, Liang H, Wang B, Han S, Li X, Xu B, Wang N, Liu S, Xue W, Dai J. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–316. [DOI] [PubMed] [Google Scholar]
- 66. Yang Z, Zhang A, Duan H, Zhang S, Hao P, Ye K, Sun YE, Li X. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci USA. 2015;112(43):13354–13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee Y, Lee S, Lee SR, Park K, Hong Y, Lee M, Park S, Jin Y, Chang KT, Hong Y. Beneficial effects of melatonin combined with exercise on endogenous neural stem/progenitor cells proliferation after spinal cord injury. Int J Mol Sci. 2014;15(2):2207–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu L, Zhou H, Pan B, Li X, Fu Z, Liu J, Shi Z, Chu T, Wei Z, Ning G, Feng S. c-Jun amino-terminal kinase is involved in valproic acid-mediated neuronal differentiation of mouse embryonic NSCs and neurite outgrowth of NSC-derived neurons. Neurochem Res. 2017;42(4):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gauthier MK, Kosciuczyk K, Tapley L, Karimi-Abdolrezaee S. Dysregulation of the neuregulin-1-ErbB network modulates endogenous oligodendrocyte differentiation and preservation after spinal cord injury. Eur J Neurosci. 2013;38(5):2693–2715. [DOI] [PubMed] [Google Scholar]
- 70. Park SI, Lim JY, Jeong CH, Kim SM, Jun JA, Jeun SS, Oh WI. Human umbilical cord blood-derived mesenchymal stem cell therapy promotes functional recovery of contused rat spinal cord through enhancement of endogenous cell proliferation and oligogenesis. J Biomed Biotechnol. 2012;2012:362473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karimi-Abdolrezaee S, Schut D, Wang J, Fehlings MG. Chondroitinase and growth factors enhance activation and oligodendrocyte differentiation of endogenous neural precursor cells after spinal cord injury. PLoS One. 2012;7(5):e37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu HY, Hu M, Yuan DK, Wu HY, Wang YH, Wang J, Li T, Qian CY, Yu HL. Electroacupuncture promotes the proliferation of endogenous neural stem cells and oligodendrocytes in the injured spinal cord of adult rats. Neural Regen Res. 2012;7(15):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. David S, Greenhalgh AD, Kroner A. Macrophage and microglial plasticity in the injured spinal cord. Neuroscience. 2015;307:311–318. [DOI] [PubMed] [Google Scholar]
- 74. Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500(2):267–285. [DOI] [PubMed] [Google Scholar]
- 75. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12(7):388–399. [DOI] [PubMed] [Google Scholar]
- 76. Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193(6):2615–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, Fujimoto M, Takeda K, Naka T, Yamashita T. IFN-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 2013;4:e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA. 2012;109(16):6265–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huang C, Han X, Li X, Lam E, Peng W, Lou N, Torres A, Yang M, Garre JM, Tian GF, Bennett MVL, Nedergaard M, Takano T. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci. 2012;32(10):3333–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, Neusch C, Kirchhoff F. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58(9):1133–1144. [DOI] [PubMed] [Google Scholar]
- 81. Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7(6 Pt B):574–585. [DOI] [PubMed] [Google Scholar]
- 82. Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–464. [DOI] [PubMed] [Google Scholar]
- 83. Schwartz M. Sell Memorial Lecture. Helping the body to cure itself: immune modulation by therapeutic vaccination for spinal cord injury. J Spinal Cord Med. 2003;26(suppl 1):S6–S10. [PubMed] [Google Scholar]
- 84. Hashimoto M, Nitta A, Fukumitsu H, Nomoto H, Shen L, Furukawa S. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16(2):99–102. [DOI] [PubMed] [Google Scholar]
- 85. Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, Hart RP, Schwartz M. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2005;92(5):997–1009. [DOI] [PubMed] [Google Scholar]
- 86. Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia. 2009;57(15):1610–1618. [DOI] [PubMed] [Google Scholar]
- 87. Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma. 2006;23(3–4):360–370. [DOI] [PubMed] [Google Scholar]
- 88. Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. 2014;34(18):6316–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mawhinney LA, Thawer SG, Lu WY, Rooijen N, Weaver LC, Brown A, Dekaban GA. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol. 2012;71(3):180–197. [DOI] [PubMed] [Google Scholar]
- 90. Anwar MA, Al Shehabi TS, Eid AH. Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci. 2016;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. [DOI] [PubMed] [Google Scholar]
- 92. Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60(5):717–727. [DOI] [PubMed] [Google Scholar]
- 93. Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83(5):1098–1116. [DOI] [PubMed] [Google Scholar]
- 94. Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–355. [DOI] [PubMed] [Google Scholar]
- 95. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. [中国神经再生研究(英文版)] 2014;9(20):1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Filardy AA, Pires DR, Nunes MP, Takiya CM, Freire-de-Lima CG, Ribeiro-Gomes FL, DosReis GA. Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol. 2010;185(4):2044–2050. [DOI] [PubMed] [Google Scholar]
- 98. Ichim G, Tauszig-Delamasure S, Mehlen P. Neurotrophins and cell death. Exp Cell Res. 2012;318(11):1221–1228. [DOI] [PubMed] [Google Scholar]
- 99. Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Widenfalk J, Lundstromer K, Jubran M, Brene S, Olson L. Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. J Neurosci. 2001;21(10):3457–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 2017;18(3):E548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl L, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA. 2004;101(16):6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33(1):9–12. [DOI] [PubMed] [Google Scholar]
- 104. Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. [DOI] [PubMed] [Google Scholar]
- 105. Wong I, Liao H, Bai XS, Zaknic A, Zhong JH, Guan Y, Li HY, Wang YJ, Zhou XF. ProBDNF inhibits infiltration of ED1+macrophages after spinal cord injury. Brain Behav Immun. 2010;24(4):585–597. [DOI] [PubMed] [Google Scholar]
- 106. Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36(3):375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6(10):3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rodriguez-Tebar A, Jeffrey PL, Thoenen H, Barde YA. The survival of chick retinal ganglion cells in response to brain-derived neurotrophic factor depends on their embryonic age. Dev Biol. 1989;136(2):296–303. [DOI] [PubMed] [Google Scholar]
- 110. Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. 2001;14(11):1800–1808. [DOI] [PubMed] [Google Scholar]
- 111. Ji WC, Zhang XW, Qiu YS. Selected suitable seed cell, scaffold and growth factor could maximize the repair effect using tissue engineering method in spinal cord injury. World J Exp Med. 2016;6(3):58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Feng SQ, Kong XH, Guo SF, Wang P, Li L, Zhong JH, Zhou XF. Treatment of spinal cord injury with co-grafts of genetically modified Schwann cells and fetal spinal cord cell suspension in the rat. Neurotox Res. 2005;7(1–2):169–177. [DOI] [PubMed] [Google Scholar]
- 113. Shen L, Shi-qing F, Xian-hu Z, Guang-zhi N, Chao Z. Effects of precursor of brain-derived neurotrophic factor on the survival and growth of oligodendrocytes after spinal cord injury. Chin J Exp Surg. 2013;30(12):2668–2670. [Google Scholar]
- 114. Richner M, Ulrichsen M, Elmegaard SL, Dieu R, Pallesen LT, Vaegter CB. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol Neurobiol. 2014;50(3):945–970. [DOI] [PubMed] [Google Scholar]
- 115. Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20(12):4435–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feng SQ, Kong XH, Liu Y, Ban DX, Ning GZ, Chen JT, Guo SF, Wang P. Regeneration of spinal cord with cell and gene therapy. Orthop Surg. 2009;1(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–1948. [DOI] [PubMed] [Google Scholar]
- 118. Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–848. [DOI] [PubMed] [Google Scholar]
- 119. Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M. Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci Lett. 2008;441(3):261–266. [DOI] [PubMed] [Google Scholar]
- 121. Gu YL, Yin LW, Zhang Z, Liu J, Liu SJ, Zhang LF, Wang TH. Neurotrophin expressions in neural stem cells grafted acutely to transected spinal cord of adult rats linked to functional improvement. Cell Mol Neurobiol. 2012;32(7):1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tauris J, Gustafsen C, Christensen EI, Jansen P, Nykjaer A, Nyengaard JR, Teng KK, Schwarz E, Ovesen T, Madsen P, Petersen CM. Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons. Eur J Neurosci. 2011;33(4):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta Mol Cell Res. 2014;1843(11):2563–2582. [DOI] [PubMed] [Google Scholar]
- 125. Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. (Phila Pa 1976) 2004;29(9):966–971. [DOI] [PubMed] [Google Scholar]
- 126. Xia M, Zhu Y. The regulation of Sox2 and Sox9 stimulated by ATP in spinal cord astrocytes. J Mol Neurosci. 2015;55(1):131–140. [DOI] [PubMed] [Google Scholar]
- 127. Jin X, Yamashita T. Microglia in central nervous system repair after injury. J Biochem. 2016;159(5):491–496. [DOI] [PubMed] [Google Scholar]
- 128. Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang X, Barone FC, Feuerstein GZ. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29(8):1698–1706; discussion 1707. [DOI] [PubMed] [Google Scholar]
- 129. Li Y, Liu L, Barger SW, Mrak RE, Griffin WS. Vitamin E suppression of microglial activation is neuroprotective. J Neurosci Res. 2001;66(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu NK, Xu XM. Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regen Res. 2012;7(26):2051–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, Iwanami A, Ikegami T, Ohsugi Y, Tsuji O. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp Neurol. 2010;224(2):403. [DOI] [PubMed] [Google Scholar]
- 132. Guerrero AR, Uchida K, Nakajima H, Watanabe S, Nakamura M, Johnson WE, Baba H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 2012;9(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yan P, Li Q, Kim GM, Xu J, Hsu CY, Xu XM. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma. 2001;18(5):563–568. [DOI] [PubMed] [Google Scholar]
- 134. Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016;2016:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Paintlia MK, Paintlia AS, Singh AK, Singh I. Synergistic activity of interleukin-17 and tumor necrosis factor-alpha enhances oxidative stress-mediated oligodendrocyte apoptosis. J Neurochem. 2011;116(4):508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wang L, Wei FX, Cen JS, Ping SN, Li ZQ, Chen NN, Cui SB, Wan Y, Liu SY. Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res. 2014;1575:87–100. [DOI] [PubMed] [Google Scholar]
- 137. Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16(10):851–863. [DOI] [PubMed] [Google Scholar]
- 138. Glazner GW, Mattson MP. Differential effects of BDNF, ADNF9, and TNF alpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000;161(2):442–452. [DOI] [PubMed] [Google Scholar]
- 139. Kim GM, Xu J, Xu J, Song SK, Yan P, Ku G, Xu XM, Hsu CY. Tumor necrosis factor receptor deletion reduces nuclear factor-kappaB activation, cellular inhibitor of apoptosis protein 2 expression, and functional recovery after traumatic spinal cord injury. J Neurosci. 2001;21(17):6617–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35(3):233–245. [DOI] [PubMed] [Google Scholar]
- 141. Zhang Y, Feng S, Wang Q, Zhang L, Wang T, Zhang B, Yao L. Temporal and spatial distributions of CXC chemokine receptor 4 after spinal cord injury in Wistar rats. Chin J Exp Surg. 2013;30(7):1406–1408. [Google Scholar]
- 142. Li J, Guo W, Xiong M, Han H, Chen J, Mao D, Tang B, Yu H, Zeng Y. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36(5):1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yao L, Feng S, Kong X, Zhang L, Wang Q, Zhang B, Zhang Y. Temporal and spatial distributions of stromal cell-derived factor-1α(SDF-1α) in spinal cord tissue after spinal cord injury in rat. Chin J Spine Spinal Cord. 2013;23(9):842–848. [Google Scholar]
- 144. Lemke M, Demediuk P, Mcintosh TK, Vink R, Faden AI. Alterations in tissue Mg ++, Na + and spinal cord edema following impact trauma in rats. Biochem Biophys Res Commun. 1987;147(3):1170–1175. [DOI] [PubMed] [Google Scholar]
- 145. Liu WM, Wu JY, Li FC, Chen QX. Ion channel blockers and spinal cord injury. J Neurosci Res. 2011;89(6):791–801. [DOI] [PubMed] [Google Scholar]
- 146. Rosenberg LJ, Teng YD, Wrathall JR. Effects of the sodium channel blocker tetrodotoxin on acute white matter pathology after experimental contusive spinal cord injury. J Neurosci. 1999;19(14):6122–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Black JA, Cummins TR, Yoshimura N, de Groat WC, Waxman SG. Tetrodotoxin-resistant sodium channels Na-v 1.8/SNS and Na-v 1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res. 2003;963(1–2):132–138. [DOI] [PubMed] [Google Scholar]
- 148. Vasconcelos NL, Gomes ED, Oliveira EP, Silva CJ, Lima R, Sousa N, Salgado AJ, Silva NA. Combining neuroprotective agents: effect of riluzole and magnesium in a rat model of thoracic spinal cord injury. Spine J. 2016;16(8):1015–1024. [DOI] [PubMed] [Google Scholar]
- 149. Satkunendrarajah K, Nassiri F, Karadimas SK, Lip A, Yao G, Fehlings MG. Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Exp Neurol. 2016;276:59–71. [DOI] [PubMed] [Google Scholar]
- 150. Kaptanoglu E, Solaroglu I, Surucu HS, Akbiyik F, Beskonakli E. Blockade of sodium channels by phenytoin protects ultrastructure and attenuates lipid peroxidation in experimental spinal cord injury. Acta Neurochir. (Wien.) 2005;147(4):405–412. [DOI] [PubMed] [Google Scholar]
- 151. Hains BC, Saab CY, Lo AC, Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol. 2004;188(2):365–377. [DOI] [PubMed] [Google Scholar]
- 152. Agrawal SK, Nashmi R, Fehlings MG. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience. 2000;99(1):179–188. [DOI] [PubMed] [Google Scholar]
- 153. Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Rev. 2001;38(1–2):165–91. [DOI] [PubMed] [Google Scholar]
- 154. Bacia A, Wollmann R, Soliven B. K+ channel blockade impairs remyelination in the cuprizone model. Glia. 2004;48(2):156–65. [DOI] [PubMed] [Google Scholar]
- 155. Liu DX, Liu J, Sun DC, Alcock NW, Wen J. Spinal cord injury increases iron levels: Catalytic production of hydroxyl radicals. Free Radic Biol Med. 2003;34(1):64–71. [DOI] [PubMed] [Google Scholar]
- 156. Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51(6):157–170. [DOI] [PubMed] [Google Scholar]
- 157. Liu JB, Tang TS, Xiao DS. Changes of free iron contents and its correlation with lipid peroxidation after experimental spinal cord injury. Chin J Traumatol. 2004;7(4):229–232. [PubMed] [Google Scholar]
- 158. Routhe LJ, Moos T. Handling iron in restorative neuroscience. Neural Regen Res. 2015;10(10):1558–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55(6):333–339. [DOI] [PubMed] [Google Scholar]