Abstract
Chronic pancreatitis (CP) is an inflammatory disease that causes progressive damage to the pancreatic parenchyma with irreversible morphological changes and fibrotic replacement of the gland. The risk factors associated with developing CP have been described as toxic (e.g., alcohol and tobacco); idiopathic (e.g., unknown); genetic, autoimmune, recurrent acute pancreatitis, and obstructive (the TIGAR-O system). Upon histological screening of the pancreata from a cohort of CP patients who had undergone pancreatectomy for the treatment of intractable pain in Leicester, UK, one sample showed a striking change in the morphological balance toward an endocrine phenotype, most notably there was evidence of substantial α cell genesis enveloping entire cross sections of ductal epithelium and the presence of α cells within the ductal lumens. This patient had previously undergone a partial pancreatectomy, had severe sclerosing CP, an exceptionally low body mass index (15.2), and diabetes at the time the pancreas was removed, and although these factors have been shown to induce tissue remodeling, such high levels of α cells was an unusual finding within our series of patients. Due to the fact that α cells have been shown to be the first endocrine cell type that emerges during islet neogenesis, future research profiling the factors that caused such marked α cell genesis may prove useful in the field of islet transplantation.
Keywords: α cells, islet neogenesis, chronic pancreatitis, partial pancreatectomy, low BMI
Case History
Following a 30-mo period of worsening abdominal pain and fear of eating due to the associated epigastric pain and diarrhea, the male patient was diagnosed with severe chronic pancreatitis (CP; idiopathic). Between the onset of symptoms and the patient’s first partial pancreas resection, the patient’s weight had reduced from 108 kg (body mass index [BMI]: 36.5) to 63 kg (BMI: 21.3). At this stage, a Whipple procedure was performed, but only offered refractory relief from pain. Two years later, the patient was referred to Leicester, UK, and a completion pancreatectomy was carried out. At the time of the completion pancreatectomy, the patient was aged 55 y, and the CP was approaching end stage as evidenced by marked calcification of the remnant of the gland (confirmed by computed tomography scan and endoscopic retrograde cholangio-pancreatography assessment). Additionally, 3 mo before surgery, the patient showed “diabetic” endocrine function following an oral glucose tolerance test (5.55, 13, and 15.1 mmol/L at 0, 30 min and 2 h after a 75 g glucose bolus), again indicative of late-stage disease1,2, although the previous resection may have contributed to this. At the time of surgery, the patient weighed just 45 kg (BMI: 15.2) and required 150 mg of opiate equivalents per day in an attempt to control his pain.
At the time of the completion pancreatectomy, the patient was not suffering with any other comorbidities; however, 15 y prior to the onset of CP, the patient had a trauma-related injury of the lower leg which had resulted in a below-the-knee amputation. This was not deemed to be associated with the later onset of CP.
Materials and Methods
The pancreatectomy procedure has been previously described3. At the time of completion pancreatectomy, a specimen of pancreas was fixed for routine histological examination, although it must be noted that no samples were available for the first Whipple procedure.
In brief, specimens were sectioned and stained for insulin and glucagon in order to detect the prevalence of nonislet endocrine cells4, cytokeratin 7 (CK7), and cytokeratin 19 (CK19) to estimate the prevalence of ductal epithelial cells and pancreatic and duodenal homeobox 1 (PDX-1) as a marker of pluripotent cells, as it is known to be expressed during pancreatic development and in the maturation of β cells. Further staining for synaptophysin and somatostatin was also carried out on the case patient for characterization purposes. Staining within islets was excluded from the data reported in Table 1. All products were purchased from Dako (Glostrup, Denmark) with the exception of the insulin antibody (conditioned media derived from cell line HB124 of the Hybridoma Bank, Baltimore, MD, USA) and PDX-1 antibodies (R&D Systems, Abingdon, UK). Stained histological sections were assessed by analyzing 10 random fields (at 10× magnification) using Axiovision 4.7™ imaging software (Zeiss, Oberkochen, Germany) with the exception of glucagon. The Axiovision data presented here represent the mean percentage of positive pixels per field; however, it was recognized that the actual percentage of positive cells was likely to exceed the Axiovision figures quoted due to the fact that antibody staining was often restricted to either the nucleus or cytoplasm. Glucagon staining was carried out manually due to the fact that positive cells associated with the ductal epithelium needed to be counted separately, as such, glucagon data refer to the percentage of positive cells. The results of the case patient and the rest of the CP cohort (n = 22) and cadaveric pancreas samples (n = 8) are presented within Table 1. Medians and ranges were used as descriptive statistics.
Table 1.
Patient and Histological Data Relating to the Case Patient and the Remaining Chronic Pancreatitis (CP) Cohort.
| Case Patient | Remaining CP Cohort | Control Cohort | |
|---|---|---|---|
| N | 1 | 22 | 8 |
| Age (y) | 55 | 43 (21–62) | 54 (17–67) |
| Previous pancreatic surgery | 1 | 0 | 0 |
| Etiology of CP | |||
| Alcohol | — | 8 | — |
| Idiopathic | 1 | 11 | — |
| Small duct disease | — | 1 | — |
| Pancreas divisum | — | 1 | — |
| Gallstones | — | 1 | — |
| Smoker | 1 | 11 | 2 |
| Weight (kg) | 45 | 61 (41–92) | ND |
| BMI (kg/m2) | 15.2 | 22.0 (17.2–31.0) | 28.6 (23.6–29.3) |
| Insulin | 1.6 | 0.8 (0.2–4.5) | 0.3 (0.2–0.5) |
| Glucagon | 30.4 | 1.08 (0–3.9) | 1.4 (0.5–3.1) |
| Glucagon (ducts) | 13.9 | 0.1 (0–1.5) | 0.1 (0–0.6) |
| PDX-1 | 28.2 | 19.2 (7.10–34.0) | 9 (4.6–17.1) |
| CK7 | 27.9 | 21.7 (12.9–41.0) | 17.6 (8.7–38.7) |
| CK19 | 69.3 | 33.0 (8.4–75.4) | 17.7 (13.1–20.3) |
Note: Stained sections were assessed using Axiovision™ software, and the data reported refer to the percentage of positive pixels. The exception is glucagon that was manually counted due to the fact that the glucagon-positive cells associated with the ductal epithelium needed to be counted separately, as such these data refer to the percentage of glucagon-positive cells. Glucagon ducts refers to glucagon staining closely associated with the ductal epithelium. All staining reported refers to staining outside the islets of Langerhans. Data are described as medians (range). Etiology, previous pancreatic surgery, and smoker are presented as a proportion of the total.
Results
Low weight is a signature of CP, however, the case patient fell well within the underweight category with a BMI of 15.25. Due to a previous Whipple procedure and atrophy caused by the CP, the remnant pancreas weighed just 22 g and exhibited a rigid and sclerotic gross morphology with visible calcium deposits.
Upon screening, the pancreatic biopsy showed marked replacement of acinar parenchyma with fibrotic tissue. Fibrotic areas were largely rich in islets (Fig. 1a); however, within the fibrotic areas, the islet morphology was often distorted with islets exhibiting a flattened appearance (Fig. 1c). Quantification of the immunohistochemistry staining showed higher levels of PDX-1-positive cells, ductal cells, and nonislet insulin-positive cells than the median values of the rest of the CP cohort (Table 1). This is suggestive of significant acinar atrophy or high levels of regeneration within the case sample6–8, although it was the prevalence of nonislet glucagon-positive cells that was particularly upregulated. In this patient, glucagon-positive cells were frequently shown to envelope medium-sized ducts (Fig. 2a), and approximately 30-fold more were found in this patient than the median of the rest of the CP cohort (Table 1).
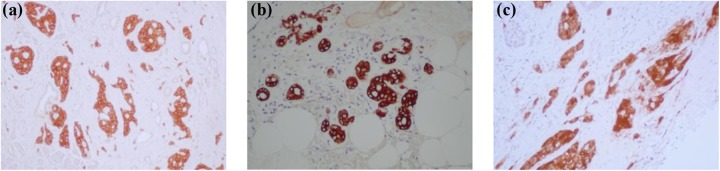
Fig. 1.
The abnormal architecture of the case patient sample. Staining with synaptophysin (pan-endocrine marker) showed that the tissue was largely islet rich (a and b), although in many areas the islets appeared crushed by the surrounding fibrotic tissue (c).
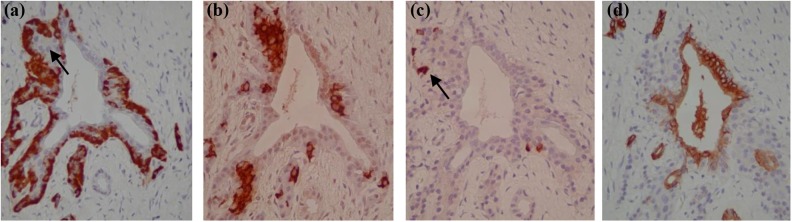
Fig. 2.
Hormone-positive cells associated with the ductal epithelium (consecutive sections). Glucagon-positive cells could be seen to completely envelope certain ducts and also surround budding structures that appeared to be new islets (a). Figure (b) and (c) show that insulin (b) and less frequently somatostatin-positive cells (c) were present within the budding structures. Figure (d) verifies that the structure is ductal through CK19 positivity.
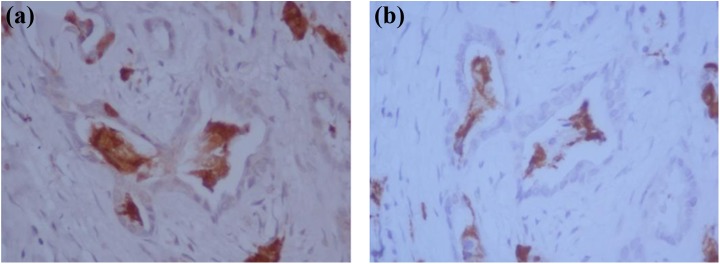
Additionally, within certain areas, ducts were shown to express significant levels of glucagon-positive cells (both viable and damaged) within their lumens (Fig. 3). Further assessments of consecutive tissue sections showed that cells within the ductal lumens were negative for CK7, CK19, and insulin but positive for the pan-endocrine marker (synaptophysin). The presence of hormone-positive cells with duct lumens was not found in any other CP samples assessed.
Fig. 3.
Hormone-positive cells within the duct lumen. Figure (a) shows hormone-positive (synaptophysin) cells (both viable and damaged) within the duct lumens. The consecutive sections show positive glucagon staining (b); however, the cells in the lumens were negative for insulin, somatostatin, and CK7 and CK19 staining (not shown).
Discussion
The pancreas of this patient demonstrated marked fibrosis but was also islet rich, and there were extremely high levels of glucagon associated with the ductal epithelium. The patient has a number of traits that may have contributed to this peculiar histological phenotype. CP6,9,10, previous pancreatectomy/injury11,12, and the onset of diabetes13 create an ideal milieu for regeneration, and in particular CP is associated with an increase in the prevalence of ductal cells, PDX-1-positive cells, and intermediate cells6–8. Likewise, extreme low weight (caused by anorexia nervosa coupled with Crohn’s disease) has also been reported to induce a metaplastic change from the acinar phenotype into ductal cells14. Although CP has certainly been reported to induce islet neogenesis, particularly in the later stages of the disease15, in our experience, the level of glucagon-positive cells found surrounding the ductal epithelium was unusual. Based on the severe cachexia shown by the patient at the time of pancreatectomy, one could hypothesize that the apparent nesidioblastosis may represent an evolutionary attempt to maximize insulin output, thus increasing glucose uptake during times of severe starvation.
The most marked finding of the study was the total envelopment of sections of duct with glucagon-positive cells within areas of fibrosis (Fig. 2a). Furthermore, glucagon-positive cells were frequently observed lining protrusions of the ductal epithelium which were frequently positive for both insulin and somatostatin (Fig. 2b and c), suggesting that these “lobular” ducts lined with glucagon-positive cells represent zones of islet generation. Currently, there is much debate as to whether the mature ductal epithelium gives rise to new islet cells. As this histological study merely forms a snapshot of pancreas immunoreactivity, it cannot definitively prove that the duct epithelium gave rise to the hormone-positive cells. However, the involvement of the ductal epithelium can be in little doubt and this study, similar to numerous others, demonstrates a clear accumulation of islet cells in the close proximity to the ductal epithelium during pancreas regeneration16–20. Additionally, in line with a case study by Soltani and colleagues, within this case study there was evidence of hormone-positive cells (in this case, glucagon-positive cells) within the lumen of some ducts9. This mode of cell shedding is reminiscent of the disease course of pancreatic cancer where it is caused by enhanced proliferation rates of the ductal epithelium (reviewed by Jura and colleagues21); however, the finding was unexpected, mainly due to the fact that no glucagon-positive cells were observed either intercalated with the ductal epithelial cells or attached to the luminal surface of the ductal epithelium (Fig. 3). In line with the findings of Soltani and colleagues (2011) who reported insulin-positive cells within the duct lumens of a patient with severe CP9, this finding suggests that ductal epithelium itself gives rise to or transdifferentiates into glucagon-positive cells. This theory is contentious, particularly as several lineage tracing studies in mice have shown that the ductal epithelium plays a negligible role in the neogenesis of new β cells22–25. However, the findings of these studies are balanced by lineage tracing studies by the Bonner-Weir group who have eloquently shown that labeled pancreatic ductal cells acted as progenitors that gave rise to new islets and acini both after birth and following injury (ductal ligation)26,27.
Interestingly, while the case patient showed high levels of nonislet glucagon-positive cells, levels of nonislet insulin-positive cells were relatively moderate (Table 1). This finding may reflect the fact that glucagon-positive cells have been shown to be the first hormone-positive cells to emerge during islet neogenesis28,29, while based on our limited observations, insulin-positive cells emerged adjacent to the ductal epithelium as “islet-like clusters” surrounded by a layer of glucagon-positive cells (Fig. 2a). It could be hypothesized that the case patient had undergone marked proliferation of both glucagon and insulin-positive cells, but the insulin cells emerged later in the islet neogenesis process and are therefore largely contained within the new islets. Indeed this hypothesis is supported by the fact that the pancreatic tissue analyzed is particularly islet dense (Fig. 1).
The fact that whole cross sections of duct were surrounded by α cells demonstrates that under defined conditions the ductal epithelium has substantial potential for islet neogenesis. Future research defining the profile of factors causing new islet growth is an exciting possibility and could substantially contribute to studies assessing the potential of growing islets in vitro for transplantation purposes.
Conclusion
In line with previous studies, this case study demonstrates the increased prevalence of ductal cells, PDX-1-positive cells, endocrine cells in response to severe mechanical insults (CP and partial pancreatectomy) and possibly physiological influences such as low body mass and the onset of diabetes. More specifically, this study highlights the substantial potential for α cell genesis close to the ductal epithelium. Knowledge of the factors involved in such marked α cell genesis may prove useful for growing pseudoislets in vitro for transplantation purposes.
Acknowledgments
I would like to thank Professor Michael Nicholson for the use of his research facilities and Dr. Severine Illouz and Mrs. Cristina Pollard for sample and data collection.
Authors’ Note: The views expressed in this manuscript are the authors' and do not represent the official position of The University Hospitals of Leicester, NHS Trust.
Ethical Approval: Ethical approvals for this study were obtained from the Cambridge and Trent Multi-Centre Ethics Committees of the United Kingdom (UK).
Statement of Human Rights: All procedures outlined in this study were conducted in accordance with guidelines of the Cambridge Ethics Committee, UK (approval number: 07/H0304/65) and Trent-Multi Centre Research Ethics Committee (approval number: 04/MRE04/77).
Statement of Informed Consent: For chronic pancreatitis samples (07/H0304/65), informed consent for patient information to be published in this article was not legally required due to the fact the tissue had been collected prior to the UK Human Tissue Act of September 2006. For cadaveric pancreata (04/MRE0477), written informed consent was obtained from a legally authorized representative for anonymised patient information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The University Hospitals of Leicester, NHS Trust.
ORCID iD: M’Balu Alena Webb  http://orcid.org/0000-0002-5633-6211
http://orcid.org/0000-0002-5633-6211
References
- 1. Larsen S. Diabetes mellitus secondary to chronic pancreatitis. Dan Med Bull. 1993;40(2):153–162. [PubMed] [Google Scholar]
- 2. Talamini G, Bassi C, Butturini G, Falconi M, Casetti L, Gumbs AA, Carrara S, Fantin A, Pederzoli P. Outcome and quality of life in chronic pancreatitis. JOP. 2001;2(4):117–123. [PubMed] [Google Scholar]
- 3. White SA, Davies JE, Pollard C, Swift SM, Clayton HA, Sutton CD, Weymss-Holden S, Musto PP, Berry DP, Dennison AR. Pancreas resection and islet autotransplantation for end-stage chronic pancreatitis. Ann Surg. 2001;233(3):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Szynaka B, Szynaka P, Andrzejewska A, Puchalski Z. Intermediate cells in chronic pancreatitis ultrastructural observations. Rocz Akad Med Bialymst. 1997; 42(2 Suppl):26–33. [PubMed] [Google Scholar]
- 5. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(2 Suppl):51S–209S. [PubMed] [Google Scholar]
- 6. Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133(6):1999–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Jr, Wright CV, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117(6):1416–1426. [DOI] [PubMed] [Google Scholar]
- 8. Szynaka B, Zimnoch L, Puchalski Z. Ultrastructural observations of intermediate cells in chronic pancreatitis. Hepatogastroenterology. 2002;49(46):1120–1123. [PubMed] [Google Scholar]
- 9. Soltani SM, O’Brien TD, Loganathan G, Bellin MD, Anazawa T, Tiwari M, Papas KK, Vickers SM, Kumaravel V, Hering BJ, Sutherland DE, Balamurugan AN. Severely fibrotic pancreases from young patients with chronic pancreatitis: evidence for a ductal origin of islet neogenesis. Acta Diabetol. 2011;50(5):807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webb MA, Chen JJ, Illouz SC, Pollard CA, Dennison B, West KP, James RF, Dennison AR. The impact of potential islet precursor cells on islet autotransplantation outcomes. Cell Transplant. 2013;22(6):1041–1051. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi KY, Tamaki H, Handa K, Takahashi T, Kakita A, Yamashina S. Differentiation and proliferation of endocrine cells in the regenerating rat pancreas after 90% pancreatectomy. Arch Histol Cytol. 2003;66(2):163–174. [DOI] [PubMed] [Google Scholar]
- 12. Ye L, Robertson MA, Hesselson D, Stainier DYR, Anderson RM. Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development. 2015;142(8):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Defronzo RA. Banting lecture from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evert M, Seiler C, Dombrowski F. Aberrant acinar cell CA 19-9 expression and peri-insular acinar cell alterations in an adult human pancreas. Virchows Arch. 2005;446(1):68–72. [DOI] [PubMed] [Google Scholar]
- 15. Kloppel G, Bommer G, Commandeur G, Heitz P. The endocrine pancreas in chronic pancreatitis. Immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1978;377(2):157–174. [DOI] [PubMed] [Google Scholar]
- 16. Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-g transgenic mice. Development. 1993;118(1):33–46. [DOI] [PubMed] [Google Scholar]
- 17. Wang RN, Bouwens L, Kloppel G. Beta-cell growth in adolescent and adult rats treated with streptozotocin during the neonatal period. Diabetologia. 1996;39(5):548–557. [DOI] [PubMed] [Google Scholar]
- 18. Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47(2):259–265. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg L. In vivo cell transformation: neogenesis of beta cells from pancreatic ductal cells. Cell Transplant. 1995;4(4):371–383. [DOI] [PubMed] [Google Scholar]
- 20. Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. [DOI] [PubMed] [Google Scholar]
- 21. Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15(1):72–77. [DOI] [PubMed] [Google Scholar]
- 22. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. [DOI] [PubMed] [Google Scholar]
- 24. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. [DOI] [PubMed] [Google Scholar]
- 25. Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. [DOI] [PubMed] [Google Scholar]
- 26. Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36(Pt 3):353–356. [DOI] [PubMed] [Google Scholar]
- 27. Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105(50):19915–19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29(4):436–467. [DOI] [PubMed] [Google Scholar]
- 29. Rall LB, Pictet RL, Williams RH, Rutter WJ. Early differentiation of glucagon-producing cells in embryonic pancreas: a possible developmental role for glucagon. Proc Natl Acad Sci U S A. 1973;70(12):3478–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]