Abstract
Background Ischemia-reperfusion injury (IRI) is a major risk factor for chronic renal failure. Here, we characterize the different modes of programmed cell death in the tubular and microvascular compartments during the various stages of IRI-induced AKI, and their relative importance to renal fibrogenesis.
Methods We performed unilateral renal artery clamping for 30 minutes and contralateral nephrectomy in wild-type mice (C57BL/6) or caspase-3−/− mice.
Results Compared with their wild-type counterparts, caspase-3−/− mice in the early stage of AKI had high urine cystatin C levels, tubular injury scores, and serum creatinine levels. Electron microscopy revealed evidence of tubular epithelial cell necrosis in caspase-3−/− mice, and immunohistochemistry showed upregulation of the necroptosis marker receptor-interacting serine/threonine-protein kinase 3 (RIPK3) in renal cortical sections. Western blot analysis further demonstrated enhanced levels of phosphorylated RIPK3 in the kidneys of caspase-3−/− mice. In contrast, caspase-3−/− mice had less microvascular congestion and activation in the early and extension phases of AKI. In the long term (3 weeks after IRI), caspase-3−/− mice had reduced microvascular rarefaction and renal fibrosis, as well as decreased expression of α-smooth muscle actin and reduced collagen deposition within peritubular capillaries. Moreover, caspase-3−/− mice exhibited signs of reduced tubular ischemia, including lower tubular expression of hypoxia-inducible factor-1α and improved tubular injury scores.
Conclusions These results establish the pivotal importance of caspase-3 in regulating microvascular endothelial cell apoptosis and renal fibrosis after IRI. These findings also demonstrate the predominant role of microvascular over tubular injury as a driver of progressive renal damage and fibrosis after IRI.
Keywords: ischemia-reperfusion, caspase-3, apoptosis, acute kidney injury
AKI is a major risk factor of progressive renal insufficiency. More than 20% of hospitalized adults worldwide experience some level of AKI,1–3 prompted most commonly by ischemia-reperfusion injury (IRI) and sepsis.4 The severity and number of AKI episodes in various patient cohorts predict progressive, long-term renal dysfunction.3,5 This association holds true regardless of whether patients presented with renal conditions before AKI.6 Also, in patients undergoing renal transplant, AKI at the time of transplantation portends reduced renal allograft survival independent from the risk of rejection.7
Programmed death of tubular epithelial cells (TECs) is a classic hallmark of AKI.4 Apoptotic renal tubular cells have been highlighted in animal models of AKI and human renal biopsy samples.8–12 The presence of renal tubular cells with a morphology suggestive of necrosis is also a classic finding in AKI.13,14 Although both apoptotic and necrotic TECs are present in the acute phase of AKI, mounting evidence suggests a predominant role for regulated necrosis or necroptosis in acute renal dysfunction. Receptor-interacting serine/threonine-protein kinases 1 and 3 (RIPK1 and RIPK3) and mixed lineage kinase domain–like protein (MLKL) regulate necroptosis in various cell types, including renal tubular cells.15 Inhibition of RIPK3 activation reduces early renal tubular injury and renal dysfunction in models of IRI or cisplatin-induced AKI.16,17
Although TEC injury has been recognized for decades as an important characteristic of AKI, renal microvascular injury is now appreciated as an important contributor to renal dysfunction.4,18–23 The molecular pathways and programs that control renal microvascular cell death and involution during the various stages of AKI remain debated. Upregulation of caspase-3, the main effector of apoptosis, has been described in both renal tubular and microvascular endothelial cells in the early stages of IRI-induced AKI.23,24 Caspase-3 inactivation with small interfering RNAs (siRNAs) before IRI yielded contradictory results in different animal models, with both cases of ameliorated and deteriorated renal dysfunction observed after IRI.24–26 In addition, the relative importance of caspase-3 in the regulation of different modes of cell death within renal tubular and microvascular compartments throughout the early, extension, and profibrotic phases of AKI remains to be characterized.
Here, we used caspase-3−/− mice to delineate the mechanisms of tubular and microvascular damage throughout the various phases of IRI-induced AKI. We report that early tubular injury and renal dysfunction are increased in caspase-3−/− mice, whereas microvascular integrity is ameliorated throughout the various phases. In the long term, caspase-3−/− mice show reduced microvascular dropout, decreased tubular ischemia, and reduced interstitial fibrosis, establishing a major role for caspase-3 activation in microvascular rarefaction and renal fibrosis.
Methods
Animals and Surgical Procedures
We purchased 6-to-8-week-old female C57BL/6 mice from Charles River Laboratories (Wilmington, MA). CASP3-deficient (caspase-3−/−) mice on a C57BL/6 congenic background, aged 6–8 weeks, were derived from breeding pairs of heterozygous CASP3-deficient (B6.129S1-C3tm1Flv/J) mice obtained from Jackson Laboratory (stock #006233; Bar Harbor, ME). Generation of these mice was previously described.27 These homozygotes mice are viable, reach adulthood, and show a variety of hyperplasias and disorganized cell deployment in the brain.28 They are also congenitally deaf29 and have cataracts at the anterior lens pole.30 All mice were kept in 12-hour light/dark cycles, with normal food provided ad libitum. IRI by unilateral renal artery clamping plus contralateral nephrectomy was performed as described previously.31 Detailed surgical procedures can be found in Supplemental Material. Mice were euthanized at presurgery or day 1, 2, 3, 7, or 21 postsurgery, and the left kidneys, sera, and urine were collected. Bone marrow was collected presurgery, and flow cytometry protocol can be found in Supplemental Material. All of the animal experimental protocols (document number for animal use approval: 4I14057MJHs) were reviewed and approved by the Centre hospitalier de l’Université de Montréal Comité Institutionnel de Protection des Animaux.
Biochemical Evaluation of Renal Function
Serum creatinine levels were determined using Vitro CREA slides and Vitro chemistry products (Vitro 250/350 Chemistry System; Ortho Clinical Diagnostics, Raritan, NJ).
Histopathological Examination
Tubular Injury Score
Tubular injury score was estimated in renal tissue stained with hematoxylin and eosin, as described previously.32 Renal tubular damage was graded on six levels on the basis of the loss of brush border, tubular dilation, cast formation, tubular necrosis, and neutrophil infiltration. Ten high-power fields (original magnification ×200) were chosen randomly; five of them were taken in the renal cortex and five at the cortico-medullary junction, and each field was scored from 0 to 5 (0: normal; 1: mild injury, involvement of 0%–10%; 2: moderate injury, involvement of 11%–25%; 3: severe injury, involvement of 26%–49%; 4: high severe injury, involvement of 50%–75%; 5: extensive injury, involvement of >75%). All assessments were done by two investigators blinded to experimental conditions.
Peritubular Capillary Vascular Congestion
On sections stained with hematoxylin and eosin, numbers of aggregated erythrocytes inside peritubular capillaries (PTCs) were counted in ten randomly chosen high-power fields by two investigators blinded to experimental conditions.
Immunohistochemistry
Ischemic kidneys were retrieved without perfusion presurgery or at days 1, 2, 3, 7, and 21 postsurgery, fixed in 10% formalin, embedded in paraffin, and subsequently cut into 4-μm slices. Immunohistochemistry staining was performed on paraffin-embedded slices as described previously.33 The antibodies used in this study were KIM-1 (AF1817; R&D systems), RIPK3 (ab152130; Abcam, Toronto, Canada), mouse endothelial cell antigen (MECA-32; 120501; Biolegend), HIF-1α (ab2185; Abcam), α-smooth muscle actin (α-SMA; clone 1A4; Dako), CD45 (550566; BD Bioscience), CD34 (QBend10 IR632; Dako) VCAM-1 (ab134047; Abcam), and caspase-3 (CP229; Biocare Medical). For all immunohistochemistry stainings, five randomly chosen high-power fields per mouse (original magnification ×200) were captured using the Leica DM4000B microscope (Leica Microsystems). Quantifications were done by two investigators blinded to experimental conditions.
Sirius Red Staining
Sirius Red staining was carried out using the Picro-Sirius Red Stain Kit (Abcam) according to the manufacturer’s instructions. Five randomly chosen high-power fields per mouse (original magnification ×200) were captured using the Leica DM4000B microscope. Quantification was performed with ImageJ (National Institutes of Health) by two investigators blinded to experimental conditions.
ELISA
Serum levels of connective tissue growth factor (CTGF) and urinary levels of cystatin C were determined by ELISA, according to the manufacturer’s instructions: mouse CTGF (E-EL-M0340; Elabscience, Bethesda, MD), and mouse cystatin C (ab201280; Abcam).
Cell Culture and siRNAs
Human umbilical vascular endothelial cells (HUVECs, cat# 200p-05n) were purchased from Cell application Inc. (San Diego, CA), grown in endothelial cell basal medium (CC‐3121; Lonza, Basel, Switzerland) and used at passages three through four. PT-2 TECs (a kind gift of A. Jevnikar) is a human renal tubular cell line isolated and cloned from centrifuged urine, which was obtained from a transplant recipient undergoing acute rejection.34 Morphologically, PT-2 cells have a typical proximal tubular cell-like shape. They are γGTP-positive, cytokeratin-positive, and vimentin-positive, with high alkaline phosphatase activity, which is characteristic of epithelial cells. However, the expression of E-cadherin and aminopeptidase N (CD13) in PT-2 cells is low.34 PT-2 cells were cultured in k1 medium (details in Supplemental Material).
For hypoxia-reoxygenation treatment, HUVECs and PT-2 cells were grown in normal medium until 90% confluence, then exposed to serum-free medium (RPMI; Gibco, Carlsbad, CA) in a hypoxia incubator (5% O2, 5% CO2, 90% N2) for 4 or 24 hours, respectively. The cells were then changed with normal medium and incubate in normal oxygen incubator (21% O2, 5% CO2, 74% N2) for 1 hour.
For caspase-3 silencing, HUVECs and PT-2 cells grown in normal conditions were transfected with siCaspase-3 (L-004307–00–0005; Dharmacon), or siControl (D-001810–03; Dharmacon). We used the MATra si Reagent (iba Solutions for Life Sciences, Goettingen, Germany) according to the manufacturer’s guidelines. The final concentrations of siRNAs were 12 nM for HUVECs and 18 nM for PT-2 cells.
Fluorescence Microscopy to Quantify the Cells with Chromatin Condensation and Cell Membrane Permeabilization
Epifluorescence microscopy was performed using unfixed/unpermeabilized adherent cells. Cells were stained with Hoechst 33342 [2′‐(4‐ethoxyphenyl)‐5‐(4‐methyl‐1‐piperazinyl)‐2.5′‐bi‐1H‐benzimidazole] (Ho; Sigma Aldrich, ON, Canada) and propidium iodide (Invitrogen, Waltham, MA), as has been previously described.35
Immunoblotting
Homogenized renal tissue and cell lysate were separated onto 12% or 15% SDS-PAGE gels, transferred onto nitrocellulose or polyvinylidene difluoride (for procaspase-3 detection) membranes, and then probed by the following antibodies: anti-RIP3 (phospho S227) (ab209384; Abcam), HIF-1α (ab2185), α-SMA (clone 1A4), and procaspase-3 (31A893; Invitrogen).
Statistical Analyses
All data were expressed as means±SEM. Comparisons between groups were conducted with the unpaired t test using Prism 5 (GraphPad Software Inc.), with P<0.05 considered as significant for all tests.
Results
Caspase-3 Deficiency Aggravates Early Tubular Epithelial Injury after Ischemia-Reperfusion
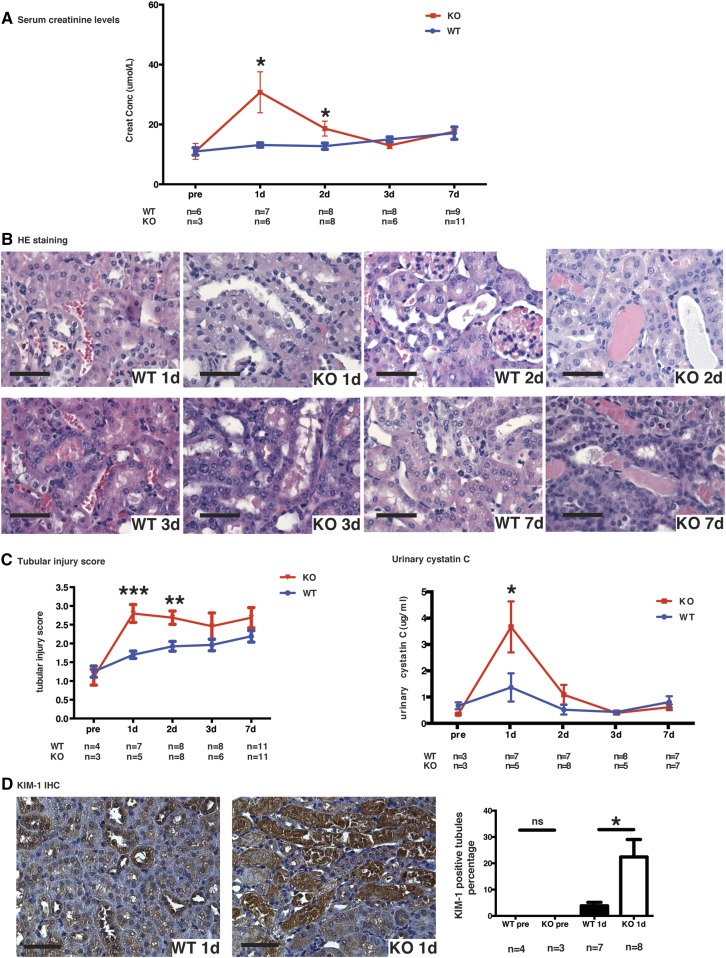
Renal artery clamping for 30 minutes followed by contralateral nephrectomy is a classic model of IRI. Unexpectedly, caspase-3−/− mice showed accentuated renal dysfunction post-IRI (Figure 1). Serum creatinine levels were significantly higher in caspase-3−/−mice at day 1 post-IRI, and rapidly returned to levels comparable with wild-type mice thereafter (Figure 1A). Renal tubular injury scores were significantly higher at 1 and 2 days post-IRI in caspase-3−/− mice (Figure 1, B and C), suggesting accentuation of tubular injury. Urinary cystatin C and immunostaining for KIM-1 were also increased in caspase-3−/− mice at day 1 post-IRI (Figure 1, C and D, Supplemental Figure 1), providing further evidence of enhanced epithelial injury in the absence of caspase-3. Immunostaining for activated caspase-3 was unsurprisingly present in wild-type mice post-IRI, but not in caspase-3−/− mice (Supplemental Figure 2). In wild-type mice, the increase in the number of caspase-3-positive cells was not statistically significant when compared with baseline in the first week post-IRI (Supplemental Figure 2). Furthermore, influx of CD45+ leukocytes was also higher in caspase-3−/− mice at 1 day post-IRI, demonstrating enhanced inflammation in association with increased tubular injury (Supplemental Figure 1C). Interestingly, influx of CD45+ leukocytes at 2 and 3 days post-IRI were not different between caspase-3−/− and wild-type mice.
Figure 1.
Caspase-3 deficiency aggravates IRI-induced tubular injury. (A) Serum creatinine levels in wild-type (WT) and caspase-3−/− (KO) mice at baseline (pre-op), and 1, 2, 3, and 7 days post-IRI. (B) Representative hematoxylin and eosin (H&E)–stained renal sections from WT and KO mice at 1, 2, 3, and 7 days post-IRI (original magnification ×200). (C) Left panel: mean tubular injury scores of ten randomly chosen high-power fields (original magnification ×200) in mice kidney sections post-IRI. Right panel: urinary levels of cystatin C in WT and KO mice at baseline (pre-op) and 1, 2, 3, or 7 days post-IRI. (D) Left panels: representative Kidney Injury Molecule 1 (KIM-1) immunohistochemistry in renal cortical sections from WT and KO mice at day 1 post-IRI (magnification 200×). Right panel: quantification of KIM-1 immunohistochemistry-stained murine renal cortical sections at baseline (pre-op) and 1 day post-IRI. All scale bars, 50 μm. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
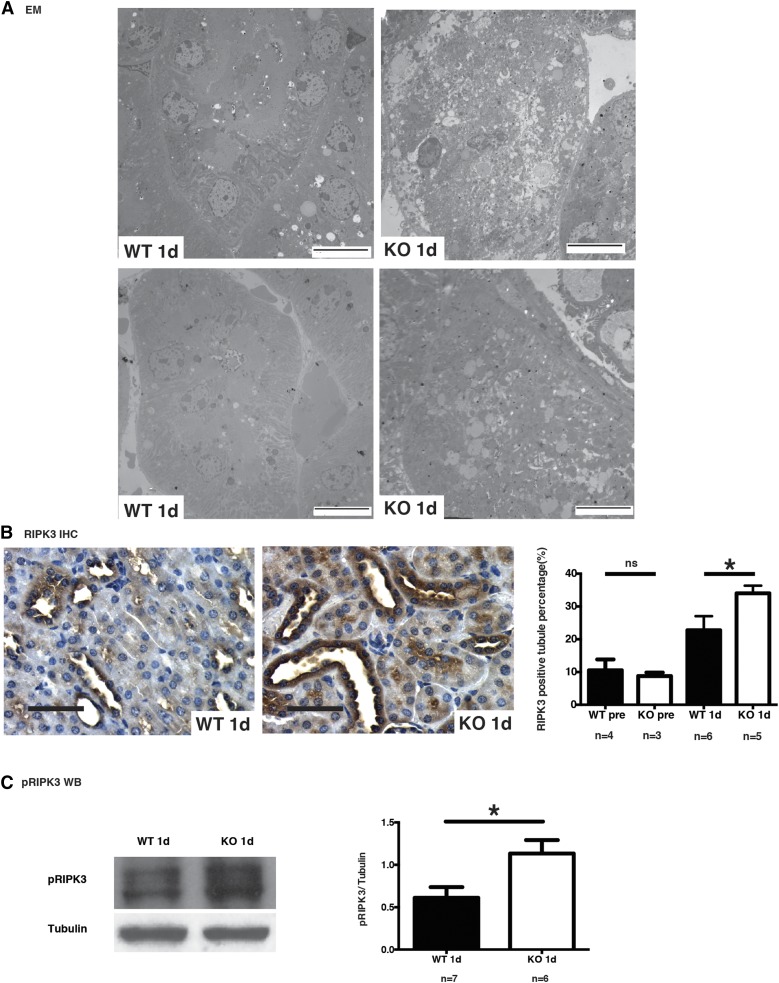
We then investigated the morphologic characteristics of tubular cell death at day 1 post-IRI. Light microscopy revealed severe features of acute TECs necrosis in caspase-3−/− mice, including cell swelling and sloughing, whereas features of apoptosis were absent (Figure 1B). Electron microscopy confirmed the presence of necrosis with degenerative damages in mitochondria, intense vacuolization and membrane rupture whereas in wild-type mice, TEC injury was less prominent (Figure 2A). These findings suggested enhanced necrosis in TECs of caspase-3−/− mice. RIPK3 is a marker of necroptosis, a genetically regulated form of necrosis. RIPK3 immunostaining was found to be enhanced in kidney sections from caspase-3−/− mice at 1 day post-IRI, consistent with accentuation of tubular necroptosis in caspase-3−/− mice after IRI (Figure 2B). We also found significantly increased pRIPK3 levels in whole kidney extracts from caspase-3−/− mice (Figure 2C). We also evaluated the effect of the pan-caspase inhibitor ZVAD-FMK in wild-type and caspase-3−/− mice. Injection of ZVAD-FMK significantly increased serum creatinine levels at day 1 post-IRI in caspase-3−/− mice but not in wild-type controls (Supplemental Figure 3), suggesting that caspases other than caspase-3 may contribute to the response to IRI in caspase-3-deficient animals. Collectively, these results demonstrate that caspase-3 deficiency increases the propensity to tubular injury in the early stage of IRI-induced AKI by enhancing the activation of the necroptotic response.
Figure 2.
Caspase-3 deficiency increases IRI-induced necroptosis in tubular cells. (A) Representative electron microscopy (EM) showing renal tubules from wild-type (WT) (left) and caspase-3−/− (KO) (right) mice that underwent IRI and were euthanized at 1 day post-IRI (original magnification ×1000). Scale bars, 10 μm. Tubules from KO mice show severe necrotic changes with loss or tubular cell membrane integrity and widespread accumulation of cellular debris within tubules. (B) Left panels: representative RIPK3 immunohistochemistry (IHC) in renal cortical sections from WT and KO mice that underwent IRI and were euthanized at 1 day post-IRI (original magnification ×200). Right panel: quantification of RIPK3 IHC in murine renal cortical sections at baseline (pre-op) and 1 day post-IRI. Scale bars, 50 μm. (C) Left panel: representative Western blot (WB) of phosphorylated RIPK3 (pRIPK3) in renal tissue from WT and KO mice that underwent IRI and were euthanized at 1 day post-IRI. Right panel: quantification of pRIPK3 WB at day 1 post-IRI. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
Caspase-3 Deficiency Attenuates Microvascular Injury and Preserves Microvascular Integrity
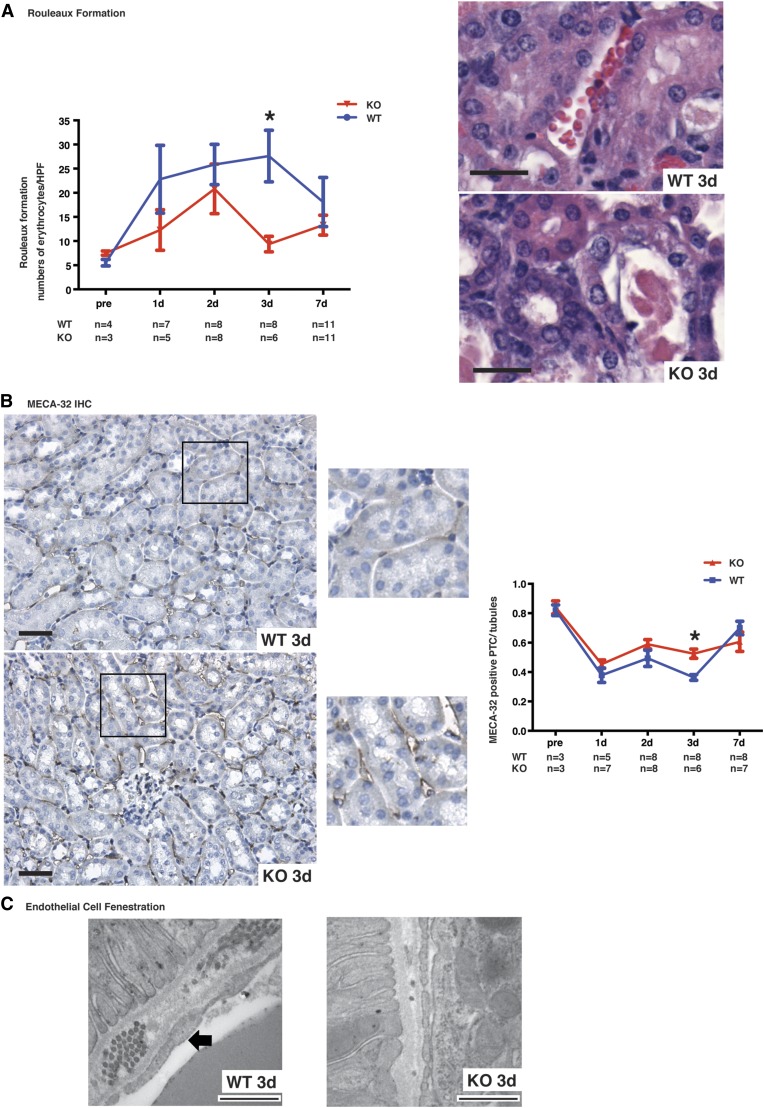
We then evaluated whether microvascular endothelial cells were similarly affected by caspase-3 deficiency. Intriguingly, rouleaux formation, a read-out of microvascular congestion, tended to be attenuated in caspase-3−/− mice at day 1 and 2, but was significantly reduced at day 3 (Figure 3A). CD34, a marker of endothelial progenitor cells,36 was significantly higher 1 day post-IRI (Supplemental Figure 4A). There was no difference at baseline in the number of bone marrow CD34+/CD45+ bone marrow cells in caspase-3−/− and wild-type mice (Supplemental Figure 4B). As evidence of better preservation of microvascular integrity in caspase-3−/− mice during the extension phase of AKI, staining of MECA-32, a marker of microvascular endothelial cells,37 was found to be significantly increased within PTCs in caspase-3−/− mice compared with wild-type mice at day 3 post-IRI (Figure 3B). Electron microscopy results were also consistent with this observation. Wild-type mice showed widespread signs of peritubular microvascular damage with reduced endothelial fenestration, and irregularities in the endothelium and basement membrane at day 3 post-IRI. In contrast, these changes were uncommon in caspase-3−/− mice, where fenestration of peritubular endothelial cells was preserved (Figure 3C). Collectively, these results suggest that caspase-3 deficiency aggravates tubular injury and concomitantly preserves the integrity of microvasculature.
Figure 3.
Caspase-3 deficiency attenuates IRI-induced microvascular injury. (A) Left panel: quantification of rouleaux formation in hematoxylin and eosin (H&E)–stained kidney sections at baseline (pre-op) and from mice that underwent IRI and were euthanized at 1, 2, 3, or 7 days post-IRI. Right panel: representative H&E-stained murine renal cortical sections at 3 days post-IRI (original magnification ×400). Scale bars, 20 μm. (B): Left panel: representative images of MECA-32 immunohistochemistry (IHC) in renal cortical sections from wild-type (WT) and caspase-3−/− (KO) mice that underwent IRI and were euthanized at 3 days post-IRI (original magnification ×200 and ×400). Right panel: quantification of MECA-32 in murine renal cortical medullary junction sections at baseline (pre-op) and 1, 2, 3, or 7 days post-IRI. Scale bars, 50 μm. (C) Representative electron microscopy images of renal endothelial cells in WT (left) and KO (right) mice that underwent IRI and were euthanized at 3 days post-IRI. Loss of endothelial fenestration is found in WT mice (arrow), whereas KO show preserved fenestrae (original magnification ×1000). Scale bars, 500 nm. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
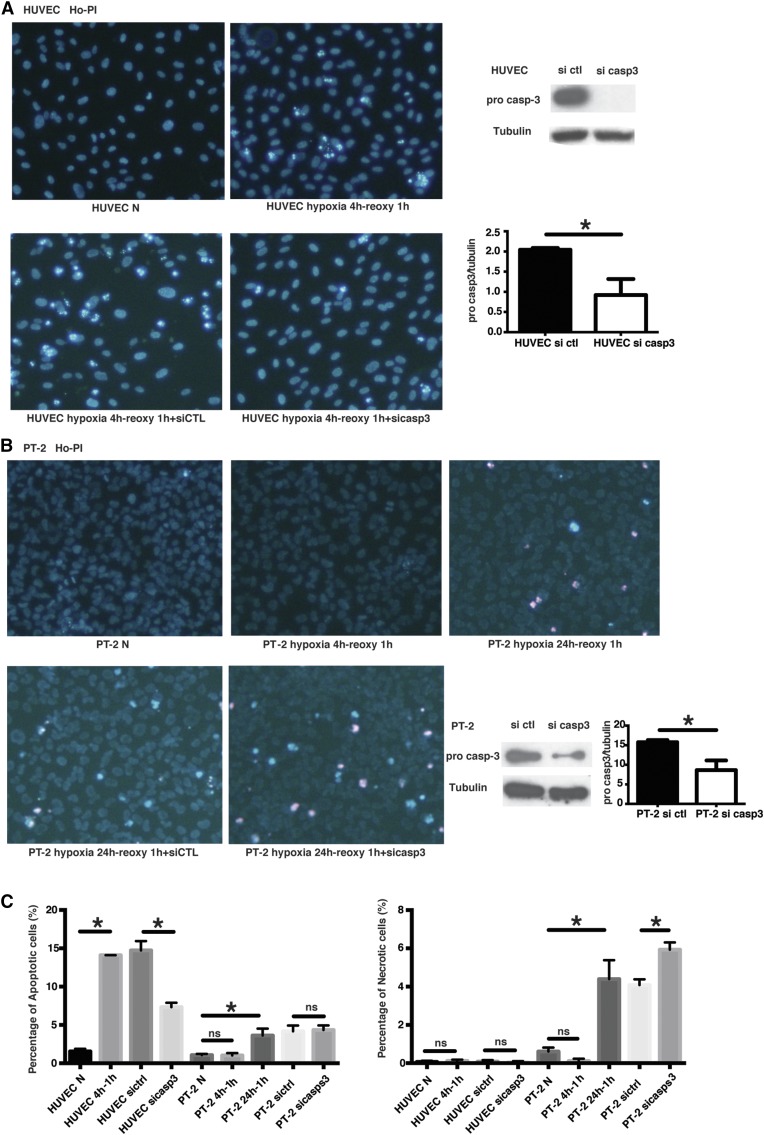
To further assess this finding, we evaluated kinetics and modes of cell death in human endothelial and renal TECs exposed to oxygen and serum deprivation in vitro. Exposure to hypoxia and serum deprivation for 4 hours followed by reoxygenation led to a significant increase in apoptosis in endothelial cells but not in epithelial cells (Figure 4). Only after 24 hours of hypoxia and serum starvation did we find a similar level of cell death in renal epithelial cells. However, this was associated with a concomitant and significant increase in the number of necrotic cells (Figure 4C). Silencing caspase-3 in endothelial cells significantly reduced the number of apoptotic cells without increasing necrosis. In epithelial cells, however, caspase-3 silencing did not affect levels of apoptosis but significantly increased the number of necrotic cells. Collectively, these results confirm that different types of death programs and different kinetics of cell death are activated in endothelial and renal TECs on oxygen and serum deprivation.
Figure 4.
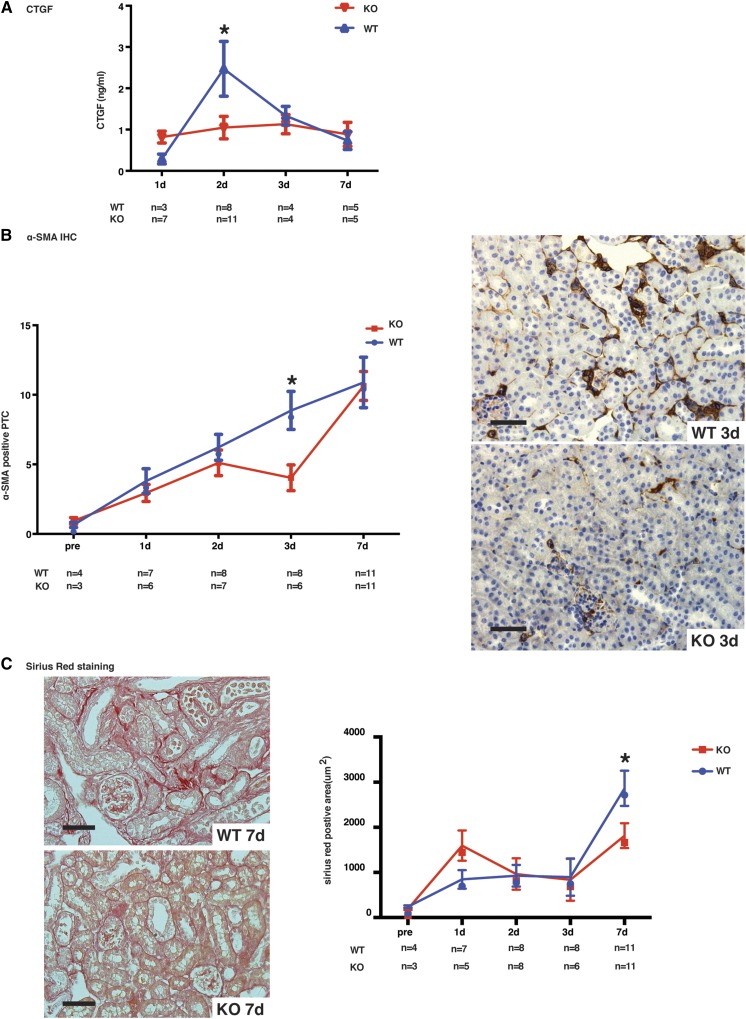
Caspase-3 deficiency attenuates IRI-induced upregulation of profibrotic markers. (A) Serum levels of CTGF in wild-type (WT) and caspase-3−/− (KO) mice that underwent IRI and were euthanized at 1, 2, 3, or 7 days post-IRI. (B) Left panel: quantification of α-SMA immunohistochemistry (IHC) in renal cortical sections from WT and KO mice at baseline (pre) or at 1, 2, 3, or 7 days post-IRI (original magnification X200). Right panels: representative α-SMA staining in murine renal cortical sections at day 3 after surgery. Scale bars, 50 μm. (C) Left panels: representative images of Sirius Red staining in renal cortical medullary junction sections from WT (top panel) and KO (bottom panel) mice that underwent IRI and were euthanized at 7 days post-IRI (original magnification X200). Scale bars, 50 μm. Right panel: quantification of Sirius Red staining in murine renal cortical medullary junction sections at baseline and 1, 2, 3, or 7 days post-IRI. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
Caspase-3 Deficiency Attenuates Activation of Fibrogenic Pathways Post-IRI
Previously, we showed that caspase-3 activation within apoptotic endothelial cells led to the release of the fibrogenic mediator CTGF.38 Higher circulating levels of CTGF predict renal fibrosis and reduced renal allograft function in renal transplant patients.39,40 We found a surge in circulating CTGF levels at day 2 post-IRI, whereas CTGF remained stable in caspase-3−/− mice (Figure 5A). Microvascular injury and dysfunction is known to lead to increased α-SMA expression.41 α-SMA upregulation increased steadily in the first week post-IRI in wild-type mice, whereas caspase-3−/− mice showed a slower increase with significantly less peritubular α-SMA staining at day 3 post-IRI (Figure 5B). Collectively, these results confirm better preservation of microvascular integrity in the early and extension stages of AKI in caspase-3−/− mice.
Figure 5.
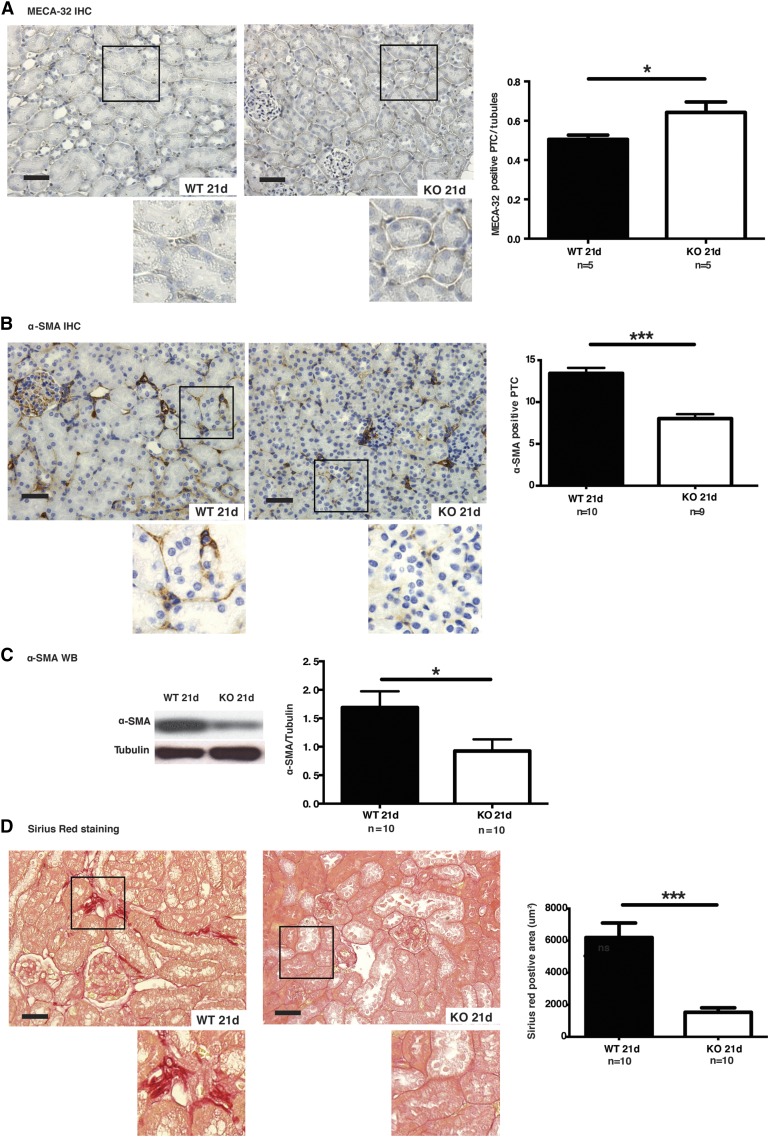
Caspase-3 deficiency attenuates IRI-induced microvascular rarefaction and fibrosis. (A) Left panels: representative images of MECA-32 immunohistochemistry (IHC) in cortical medullary junction sections from wild-type (WT) and caspase-3−/− (KO) mice that underwent IRI and were euthanized at 21 days post-IRI (original magnification ×200 and ×400). Right panel: quantification of MECA-32 staining in murine renal cortical sections at 21 days post-IRI. Scale bars, 50 μm. (B) Left panels: representative image of α-SMA IHC of renal cortical sections from WT and KO mice that underwent IRI and were euthanized at 21 days post-IRI (n=10 per group; original magnification X200 and X400). Right panel: quantification of α-SMA staining in PTCs in murine renal cortical sections at 21 days post-IRI. Scale bars, 50 μm. (C) Left panel: representative Western blot (WB) of α-SMA from WT and KO mice that underwent IRI and were euthanized at 21 days post-IRI. Right panel: quantification of α-SMA WB at 21 day post-IRI. (D) Left panels: representative image of Sirius Red staining of renal cortical medullary junction sections from WT and KO mice that underwent IRI and were euthanized at 21 days post-IRI (original magnification ×200 and ×400). Right panel: quantification of Sirius Red staining of murine renal cortical medullary junction sections at 21 days post-IRI. Scale bars, 50 μm. Values are mean±SEM. *P<0.05; ***P<0.001, compared between WT and KO at the same time point.
During the extension and repair phases of AKI, microvascular rarefaction plays a key role in renal fibrogenesis.4,42,43 We therefore evaluated the effect of caspase-3 invalidation on microvascular rarefaction and fibrogenesis. Collagen deposition, as evaluated with Sirius Red staining, was significantly lower in caspase-3−/− mice at day 7 post-IRI compared with wild-type mice. In the latter, intense Sirius Red staining was present within peritubular spaces, whereas glomerular and macrovascular compartments were largely negative (Figure 5D).
We then evaluated whether the positive effect of caspase-3 invalidation on preservation of microvascular integrity was sustained in the long term. Peritubular microvascular density, as assessed with MECA-32 staining, was significantly higher at 21 days post-IRI in caspase-3−/− mice (Figure 5A, Supplemental Figure 5A). Electron microscopy also highlighted preservation of microvascular integrity in caspase-3−/− mice (Figure 6). In wild-type mice, PTCs showed characteristic apoptotic ultrastructural changes within PTCs, such as chromatin condensation and formation of apoptotic bodies (Figure 6). These changes were absent in caspase-3−/− mice. In addition, α−SMA staining within PTCs was significantly reduced in caspase-3−/− mice at day 21 post-IRI, consistent with better microvascular homeostasis and reduced fibrogenesis (Figure 5, B and C, Supplemental Figure 6). α-SMA levels in whole kidney extracts were also evaluated by Western blotting and confirmed reduced α-SMA levels in caspase-3−/− mice (Figure 5C, Supplemental Figure 6B). However, the kinetics of glomerular α-SMA staining were similar at all time points in caspase-3−/− mice and wild-type controls Supplemental Figure 5C. This suggested a major role for caspase-3 within PTCs but not in glomeruli. In support of this conclusion, Sirius Red staining was also significantly reduced within PTCs in caspase-3−/− mice, indicating reduced collagen deposition (Figure 5D). Collectively, these results demonstrate a sustained advantage for caspase-3−/− mice in preserving the integrity of the PTC network and reducing fibrosis post-IRI, and highlight a novel role for caspase-3 in the regulation of microvascular rarefaction.
Figure 6.
Caspase-3 deficiency prevents IRI-induced long-term endothelial cell death. (A) Representative electronic microscopic images of endothelial cell apoptotic death from wild-type (WT) and caspase-3−/− (KO) mice that underwent IRI and were euthanized at 21 days post-IRI. Red arrows indicate apoptotic bodies and blue arrows indicate exosome-like membrane vesicles (original magnification ×1000). Scale bars, 500 nm. (B) Quantification of apoptotic endothelial cells (ECs) in WT and KO mice that underwent IRI and were euthanized at 21 days post-IRI. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
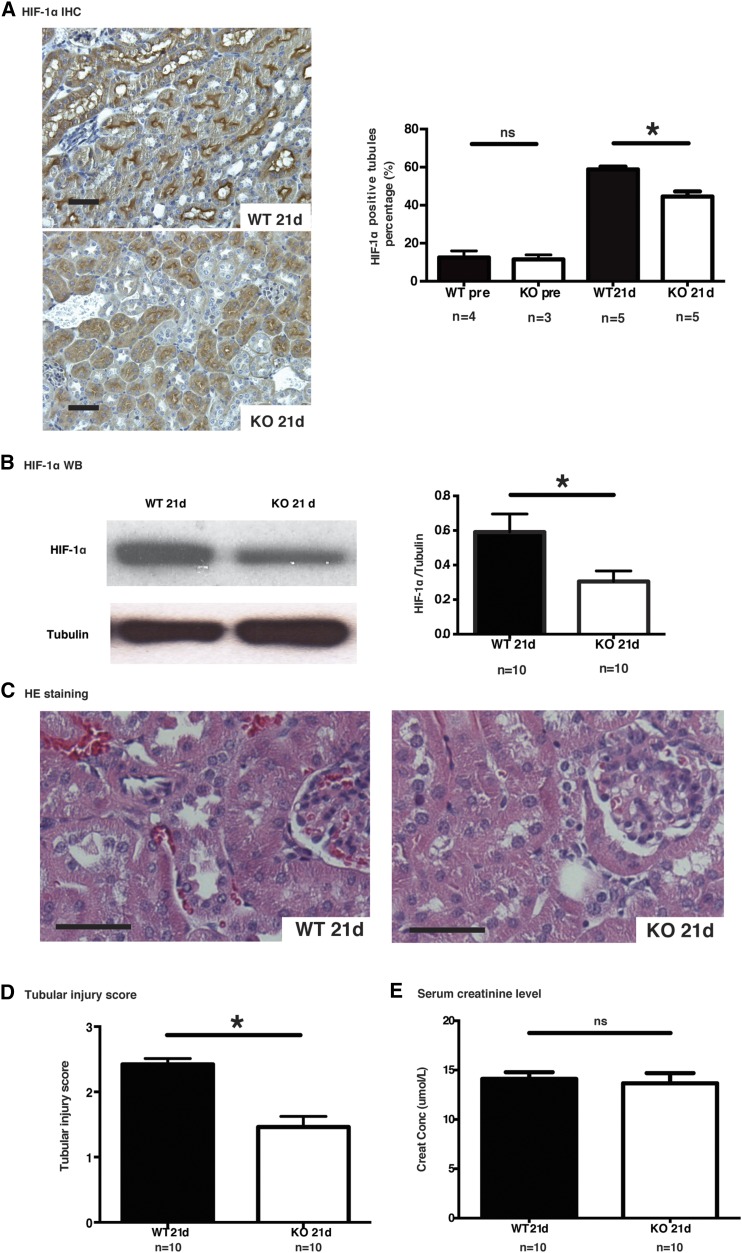
Microvascular dropout can lead to a state of tubular ischemia characterized by HIF-1α overexpression, which in turn favors tubular atrophy and renal dysfunction.42 Immunohistochemistry and Western blotting showed reduced expression of tubular HIF-1α in caspase-3−/− mice at 21 days post-IRI (Figure 7, A and B). Immunostaining for activated caspase-3 showed that the number of caspase-3-positive cells was maximal at day 21 post-IRI in wild-type mice, suggesting persistent cell death over time (Supplemental Figure 2). Tubular injury scores rose steadily post-IRI in wild-type mice whereas caspase-3−/− mice showed a sharper decline after the initial surge with significantly lower levels at 21 days post-IRI compared with wild-type mice (Figure 7, C and D, Supplemental Figures 7C, 8). Serum creatinine levels were not different in wild-type and caspase-3−/− mice at day 21 post-IRI, highlighting the known lack of sensitivity of creatinine as a biomarker of renal fibrosis.14 Collectively, these results suggest that preservation of microvascular integrity in caspase-3-deficient mice with lower levels of tubular ischemia in the long term benefits tubular homeostasis and prevents peritubular fibrosis (Figure 9).
Figure 7.
Caspase-3 deficiency prevents IRI-induced long-term tubular injury. (A) Left panels: representative images of HIF-1α staining in murine renal cortical sections 21 days after surgery. Right panel: quantification of HIF-1α immunohistochemistry (IHC) in renal cortical sections from wild-type (WT) and caspase-3−/− (KO) mice at baseline and 21 days post-IRI (original magnification ×200). (B) Left panel: representative Western blot (WB) images of HIF-1α in renal tissue 21 days post-IRI. Right panel: quantification of HIF-1α WB detection at 21 days post-IRI. (C) Representative hematoxylin and eosin (H&E)–stained murine renal cortical sections 21 days post-IRI (original magnification ×200). (D) Mean tubular injury scores of ten randomly chosen high-power fields in wild-type (WT) and caspase-3−/− (KO) mice that underwent IRI and were euthanized at 21 days post-IRI. (E) Serum creatinine levels in WT and KO mice that underwent IRI and were euthanized at 21 days post-IRI. All bar scales=50 μm. Values are mean±SEM. *P<0.05, compared between WT and KO at the same time point.
Figure 9.
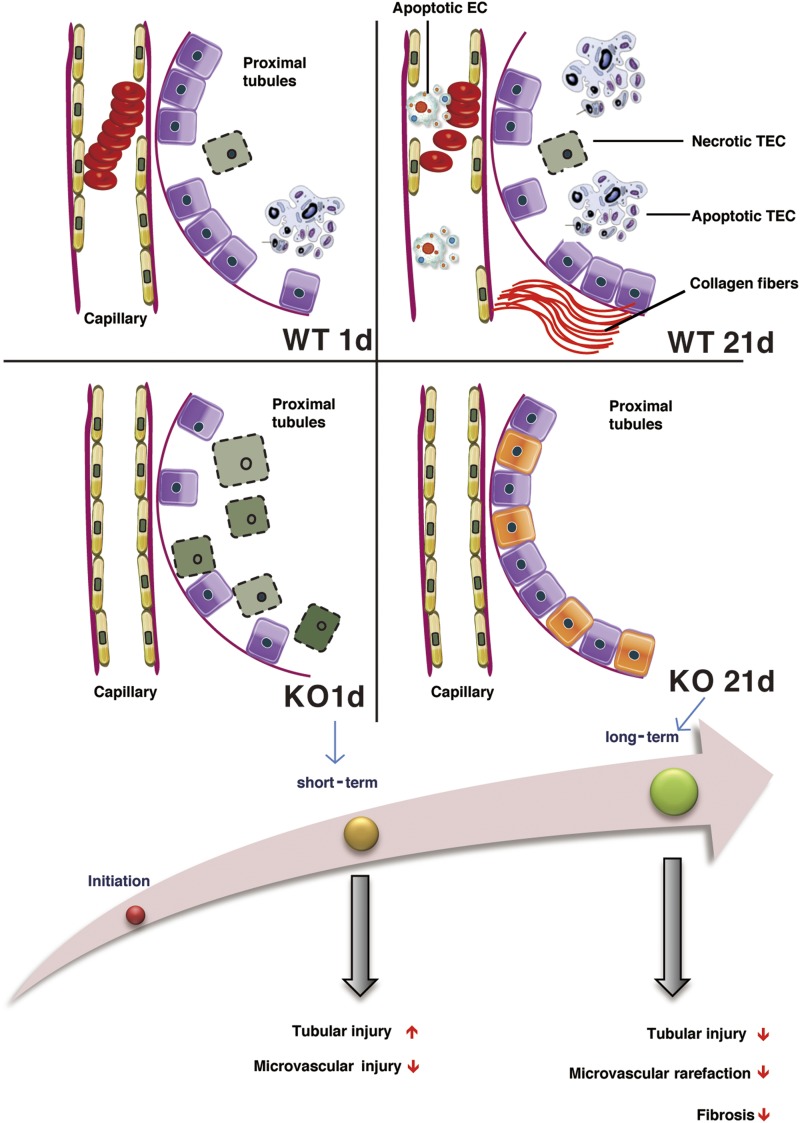
Schematic representation of the effect of caspase-3 deficiency on microvascular rarefaction and renal dysfunction post-IRI. Caspase-3 is a pivotal regulator of PTC rarefaction and renal dysfunction. Early tubular injury and renal dysfunction are increased in caspase-3−/− (KO) mice, whereas microvascular integrity is ameliorated throughout the various phases. In the long term, KO mice show reduced microvascular dropout, decreased tubular injury, and reduced interstitial fibrosis. EC, endothelial cell; WT, wild type.
Figure 8.
Caspase-3 silencing decreases apoptosis in endothelial cells but increases necrosis in TECs submitted to hypoxia plus serum starvation and reoxygenation. (A) Left panels: representative images of Hoechst 33342 and propidium iodide (Ho-PI) staining in HUVECs exposed to hypoxia (5% O2) for 4 hours, followed by reoxygenation for 1 hour in serum-free medium. Right panels: representative images of western blot (top panel) and quantification (bottom panel) for pro-caspase-3 in HUVECs transfected with siRNA control (si ctl) or caspase-3 (si casp3; n=3 independent experiments). (B) Left panels: representative images of Hoechst 33342 and propidium iodide (Ho-PI) staining of human PT-2 TECs exposed to hypoxia (5% O2) for 4 or 24 hours followed by reoxygenation for 1 hour in serum-free medium. Right panels: representative images of Western blot and quantification for pro-caspase-3 in PT-2 cells transfected with si ctl or caspase-3 (n=3 independent experiments). (C) Left panel: quantification of apoptotic death in HUVECs and PT-2 cells exposed to hypoxia reoxygenation in serum-free medium. Right panel: quantification of necrotic death in HUVECs and PT-2 cells exposed to hypoxia reoxygenation in serum-free medium. Values are mean±SEM. *P<0.05.
Discussion
IRI is one of the most common causes of AKI and a major risk factor for chronic renal failure. In this study, we identified caspase-3 as a central regulator of PTC injury, microvascular rarefaction and fibrosis post-IRI.
Intriguingly, our results showed a deleterious effect of caspase-3 invalidation on renal function and TEC injury in the early stage of AKI. Caspase-3−/− mice displayed enhanced tubular injury scores, increased urinary cystatin C, increased KIM-1 levels, and deteriorated serum creatinine at 1 day post-IRI. We demonstrated, by electron microscopy, RIPK3 staining, and evaluation of phosphorylated RIPK3 levels, that absence of caspase-3 redirects tubular cell death toward necroptosis. These results are consistent with mounting evidence highlighting a major role for necroptosis as the predominant type of renal tubular demise in the early stage of AKI.13,15,44,45 Our results also highlight that failure to activate tubular apoptosis on IRI favors RIPK3 activation and leads to accentuated renal dysfunction in the early stage of AKI.
However, our results are in stark contrast with a previous report by Zhang et al.24 describing a protective role for siRNA targeting caspase-3 when infused intravenously, before renal IRI. One major difference between the two studies is the method of caspase-3 invalidation. It is possible that intravenous infusion of caspase-3 siRNA before IRI in the study by Zhang et al. could have led to preferential caspase-3 silencing within the vasculature. The relative levels of caspase-3 silencing in renal tubules compared with the microvasculature were not addressed in that study. Nonetheless, this prompted us to evaluate the relative contributions of caspase-3 in both cellular compartments in our system.
In the early stage of AKI, caspase-3−/− mice showed enhanced tubular injury, yet reduced microvascular damage as demonstrated by rouleaux formation, staining for CD34 and MECA-32, and electron microscopy. Collectively, our results highlight different modes of regulated cell death in renal tubules compared with PTCs post-IRI. Although necroptotic pathways have been shown to play a predominant role in regulated epithelial cell death post-IRI,16,44 our results show that microvascular endothelial cell injury occurs through apoptosis and is under the control of caspase-3. They also suggest that necroptosis does not represent a major cell death pathway for microvascular cells because the absence of caspase-3 does not enhance necrotic cell death within the microvascular compartment post-IRI in vivo or in cultured endothelial cells on exposure to oxygen and serum deprivation in vitro. This highlights the complexity and potential crosstalk between various modes of cell death. For example, caspase-3 is a classic effector of apoptosis but, in certain conditions, can also trigger necrosis by cleaving the gasdermin GSDME.46 Also, caspases are increasingly appreciated as regulators of multiple functions in addition to cell death.47 In this study, we demonstrated reduced endothelial peritubular cell death in caspase-3-deficient mice. However, this does not exclude the potential contribution of other caspase-3-dependent pathways in the preservation of PTC density. Whether absence of caspase-3 could favor progenitor cell trafficking and microvascular repair is a possibility that should be addressed in future studies.
Another important finding that stems from this study is the different effects of tubular versus endothelial cell death on progressive renal dysfunction. Progression from AKI to CKD is a common clinical event. Large cohort studies have highlighted the robust predictive effect of AKI on risk of chronic renal failure.6 The precise cellular mechanisms explaining the major role of AKI in progressive renal failure are still debated. Our work showed increased tubular damage concomitant with microvascular protection early post-IRI in caspase-3−/− mice, allowing us to discriminate the relative importance of both cell compartments in progressive renal dysfunction. Early accentuation of renal tubular injury did not portend negative long-term renal outcomes in caspase-3−/− mice. Preservation of microvascular integrity was present both in the early and late phases of AKI in caspase-3−/− mice. In late stages, better preservation of microvascular integrity protected tubules from ischemia, with reduced HIF-1α expression and reduced tubular injury scores. These results are in keeping with recent elegant studies demonstrating a pivotal role for PTC damage in progressive renal fibrosis and dysfunction.37,48 Our work suggests that, on renal injury, the peritubular microvasculature undergoes significant ultrastructural and functional changes of importance in the development of fibrosis.37,48
Collectively, our results provide novel insights into the molecular mechanisms controlling microvascular dropout, and identify caspase-3 as a novel and pivotal upstream regulator of microvascular rarefaction and renal fibrogenesis post-IRI. They also demonstrate a predominant role for microvascular versus epithelial injury in regulating the development of fibrosis after renal IRI.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the J.-L. Levesque Foundation for renewed support, and Antony M. Jevnikar from the Lawson Health Research Institute (Western University, London, Ontario), who kindly provided PT-2 cells. We thank the Centre hospitalier de l'Université de Montréal (CHUM) Research Centre’s cytometry, cell imaging, and molecular pathology core facility for their technical help, and Ms. Josée-Marie Dubé for her help taking electron microscopy photos.
B.Y., S.L., and M.-J.H. designed experiments, B.Y., S.L., M.-J.H., and N.P. analyzed the data, and wrote the manuscript. B.Y., S.L., A.K.-R., J.-P.S.-V., J.T., and S.Q. performed experiments. M.D. participated in analyzing the data and the preparation of the manuscript. L.G. performed KIM-1 immunohistochemical staining.
This work was supported by research grants from the Canadian Institutes of Health Research (MOP-123436 and PJT-148884). B.Y. is a recipient of a research fellowship from the Université de Montréal Nephrology Research Consortium and is a Canadian National Transplant Research Program (CNTRP) student. S.L. is a recipient of the merit scholarship for foreign students from Fonds de recherche du Québec – Nature et technologies and is a CNTRP student. M.-J.H. holds the Shire Chair in Nephrology, Transplantation, and Renal Regeneration of Université de Montréal.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017050581/-/DCSupplemental.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al.: Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedford M, Farmer C, Levin A, Ali T, Stevens P: Acute kidney injury and CKD: Chicken or egg? Am J Kidney Dis 59: 485–491, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. : Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE: Outcomes following diagnosis of acute renal failure in U.S. veterans: Focus on acute tubular necrosis. Kidney Int 76: 1089–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, et al. : Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steegh FM, Gelens MA, Nieman FH, van Hooff JP, Cleutjens JP, van Suylen RJ, et al. : Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol 22: 1024–1029, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nogae S, Miyazaki M, Kobayashi N, Saito T, Abe K, Saito H, et al.: Induction of apoptosis in ischemia-reperfusion model of mouse kidney: Possible involvement of Fas. J Am Soc Nephrol 9: 620–631, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Toronyi E, Lord R, Bowen ID, Perner F, Szende B: Renal tubular cell necrosis and apoptosis in transplanted kidneys. Cell Biol Int 25: 267–270, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Jaffe R, Ariel I, Beeri R, Paltiel O, Hiss Y, Rosen S, et al.: Frequent apoptosis in human kidneys after acute renal hypoperfusion. Exp Nephrol 5: 399–403, 1997 [PubMed] [Google Scholar]
- 11.Havasi A, Borkan SC: Apoptosis and acute kidney injury. Kidney Int 80: 29–40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishino M, Yukawa K, Hoshino K, Kimura A, Shirasawa N, Otani H, et al.: Deletion of the kinase domain in death-associated protein kinase attenuates tubular cell apoptosis in renal ischemia-reperfusion injury. J Am Soc Nephrol 15: 1826–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Kers J, Leemans JC, Linkermann A: An overview of pathways of regulated necrosis in acute kidney injury. Semin Nephrol 36: 139–152, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Moledina DG, Parikh CR: Phenotyping of acute kidney injury: Beyond serum creatinine. Semin Nephrol 38: 3–11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, et al. : Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81: 751–761, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al. : A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharfuddin AA, Molitoris BA: Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Basile DP: The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 72: 151–156, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, et al. : Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Basile DP, Donohoe DL, Roethe K, Mattson DL: Chronic renal hypoxia after acute ischemic injury: Effects of L-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, et al. : Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293: F688–F695, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Zheng X, Sun H, Feng B, Chen G, Vladau C, et al.: Prevention of renal ischemic injury by silencing the expression of renal caspase 3 and caspase 8. Transplantation 82: 1728–1732, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Jia Y, Zhao T, Xue Y, Zhao Z, Zhang J, et al. : Naked caspase 3 small interfering RNA is effective in cold preservation but not in autotransplantation of porcine kidneys. J Surg Res 181: 342–354, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Zhao T, Zhao Z, Jia Y, Li L, Zhang Y, et al. : Serum-stabilized naked caspase-3 siRNA protects autotransplant kidneys in a porcine model. Mol Ther 22: 1817–1828, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, et al.: Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384: 368–372, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Leonard JR, Klocke BJ, D’Sa C, Flavell RA, Roth KA: Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol 61: 673–677, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Kamiya K, Urase K, Suga M, Takizawa T, Mori H, et al.: Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res 894: 359–367, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Zeiss CJ, Neal J, Johnson EA: Caspase-3 in postnatal retinal development and degeneration. Invest Ophthalmol Vis Sci 45: 964–970, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wei Q, Dong Z: Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am J Physiol Renal Physiol 303: F1487–F1494, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, et al.: Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos-Vara JA: Principles and methods of immunohistochemistry. Methods Mol Biol 691: 83–96, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Zhang ZX, Yin Z, Liu W, Garcia B, Huang X, et al.: Anti-IL-2 receptor antibody decreases cytokine-induced apoptosis of human renal tubular epithelial cells (TEC). Nephrol Dial Transplant 26: 2144–2153, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, Dieudé M, et al.: A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13: 1108–1120, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, et al.: Expression of the CD34 gene in vascular endothelial cells. Blood 75: 2417–2426, 1990 [PubMed] [Google Scholar]
- 37.Babickova J, Klinkhammer BM, Buhl EM, Djudjaj S, Hoss M, Heymann F, et al. : Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int 91: 70–85, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Laplante P, Sirois I, Raymond MA, Kokta V, Beliveau A, Prat A, et al. : Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ 17: 291–303, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Cheng O, Thuillier R, Sampson E, Schultz G, Ruiz P, Zhang X, et al. : Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant 6: 2292–2306, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Mannon RB, Fairchild R: Allograft fibrosis--unmasking the players at the dance. Am J Transplant 10: 201–202, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. : Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, et al. : Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest 124: 2396–2409, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dagher PC, Hato T, Mang HE, Plotkin Z, Richardson QV, Massad M, et al. : Inhibition of toll-like receptor 4 signaling mitigates microvascular loss but not fibrosis in a model of ischemic acute kidney injury. Int J Mol Sci 17(5): 647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, et al. : Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A 110: 12024–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linkermann A: Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int 89: 46–57, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. : Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547: 99–103, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Julien O, Wells JA: Caspases and their substrates. Cell Death Differ 24: 1380–1389, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehling J, Babickova J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, et al. : Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol 27: 520–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.