Short abstract
Postoperative pain remains a complex problem that is difficult to manage in the clinical context, seriously affecting rehabilitation and the quality of life of patients after surgery. Nociceptors, of which the cell bodies are located in the dorsal root ganglion, are crucial for initiating and conducting the pain signal. The peripheral voltage-gated sodium channels, including Nav1.7, which is mainly expressed in the dorsal root ganglion, are key to understanding the mechanism underlying postoperative pain. Nav1.7, in particular, of which mutations in the encoding gene (SCN9A) can determine whether pain occurs, has aroused most attention. Previous studies have shown that Nav1.7 in dorsal root ganglion is critical for the development of inflammatory pain and some neuropathic pain. However, the expression of Nav1.7 in the dorsal root ganglion after surgery and its role in postoperative pain hypersensitivity remain unclear. Therefore, in this study, in order to gain a better understanding of the role of dorsal root ganglion Nav1.7 in pain hypersensitivity following operation, we dynamically examined the pain-related behavior and expression of Nav1.7 in L4−L6 dorsal root ganglion before and after plantar incision in rats (an acute postoperative pain model). After plantar incision, the mechanical and thermal pain threshold decreased significantly, the cumulative pain score was increased significantly, meanwhile quantitative polymerase chain reaction and Western blotting results showed that expression of Nav1.7 in L4−L6 dorsal root ganglion was enhanced significantly. After pretreatment using SCN9A-RNAi-LV delivered via an intrathecal tube, immunohistochemistry showed that increased expression of Nav1.7 in L4−L6 dorsal root ganglion after plantar incision was inhibited, as also confirmed by quantitative polymerase chain reaction and Western blotting. Moreover, pain hypersensitivity was alleviated. These results suggested that Nav1.7 of L4−L6 dorsal root ganglion plays an important role in the development of pain hypersensitivity after plantar incision.
Keywords: Dorsal root ganglion, Nav1.7, pain hypersensitivity, plantar incision, postoperative pain
Introduction
Postoperative pain is the most common form of acute pain. In 2015, the annual global number of surgeries exceeded 310 millions.1 Although analgesic technologies have continually improved (e.g., multimodal analgesia, advanced analgesia, etc.), 39%−50% of surgical patients experience moderate to severe acute postoperative pain, which seriously affects the rehabilitation of these patients.2,3 Thus, there are tens of millions of patients who suffer from moderate to severe pain every year worldwide. Therefore, effective treatment of postoperative pain remains an urgent and key problem in clinical practice.
There is strong evidence that peripheral voltage-gated sodium channels, including Nav1.7, which is primarily expressed in the dorsal root ganglion (DRG), are associated with initiating and conducting pain signal. 4,5 In particular, tetrodotoxin-sensitive sodium channel Nav1.7, which is able to amplify sub-threshold excitability and regulate neuronal excitability as a threshold channel, has recently emerged as a potential analgesics target because mutation of the gene encoding Nav1.7 (SCN9A) can determine whether pain is present or not.6–9 In humans, gain-of-function mutations result in pain hypersensitivity (primary erythermalgia (PE),10 paroxysmal extreme pain disorder,11 and small-fiber neuropathy12), while loss-of-function mutations cause congenital insensitivity to pain.13 In addition, previous studies of conditional knockout mice and multiple rodent models of inflammatory and neuropathic pain have shown that Nav1.7 expressed in the DRG is crucial for generating and conducting pain signals, for example, inflammatory pain induced by formalin, carrageenan, nerve growth factor (NGF), or complete Freund’s adjuvant and the neuropathic pain induced by chronic constriction injury.6,14,15 Preoperative or perioperative use of nonselective sodium channel blockers (e.g., lidocaine) in patients can relieve postoperative pain sensitivity and reduce postoperative opioid consumption.16,17 Furthermore, our previous study confirmed that genetic polymorphisms in SCN9A might affect postoperative pain sensitivity in surgical patients.18,19 These findings suggested that Nav1.7 may play an important role in postoperative pain. However, its role in the development of postoperative pain remains unclear.
To explore the role of Nav1.7 in L4−L6 DRG in pain hypersensitivity after plantar incision, a rodent model of postoperative pain, we observed pain-related behavior and dynamically detected the expression of Nav1.7 in L4−L6 DRG before and after plantar incision in rats. Then, Nav1.7 in L4−L6 DRG was knocked down using SCN9A-RNAi-LV, administered via an intrathecal tube, and the effect on pain-related behavior after plantar incision was observed.
Materials and methods
Animals and groups
The study was approved by Animal Care and Use Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The protocols were conducted according to the ethical guidelines of the International Association for Pain Research. Male Sprague−Dawley rats, weighing 200 − 220 g, were provided by the Animal Center of Hubei Province, China. The rats were kept under controlled conditions, at 23 ± 1°C, with a 12-h light/dark schedule and had ad libitum access to food and water.
To detect Nav1.7 expression in the DRG of rats after plantar incision, the rats (N = 36) were randomly divided into two groups: control group (n = 6) and skin-fascia-muscle incision group (n = 30). The rats in the skin-fascia-muscle incision group were further divided into five groups: 2 h (n = 6, rats were sacrificed at 2 h), 24 h (n = 6, rats were sacrificed at 24 h), 48 h (n = 6, rats were sacrificed at 48 h), 72 h (n = 6, rats were sacrificed at 72 h), and 120 h (n = 6, rats were sacrificed at 120 h). Before 0 h and at 2 h, 24 h, 48 h, 72 h, and 120 h following surgery, the rats underwent behavior testing (withdrawal responses, thermal hyperalgesia, and cumulative pain score). Six rats from each of the incision and the control groups were used for quantitative polymerase chain reaction (Q-PCR) (n = 3 per group) and Western blotting analysis (n = 3 per group) after pain-related behavior.
To explore whether knockdown of SCN9A/Nav1.7 in DRG is effective in attenuating pain hypersensitivity after plantar incision, the rats (N = 48) were divided into four groups: a control group (n = 12), an incision group (n = 12), SCN9A-RNAi-LV + incision group (n = 12), and a negative control lentivirus (NC-LV) + incision group (n = 12). Before 0 h and at 2 h, 24 h, and 48 h following surgery, the rats were used for behavior testing. At 2 h following surgery, nine rats of each group were used for quantitative PCR (Q-PCR) (n = 3 per group), Western blotting analysis (n = 3 per group), and immunohistochemistry (n = 3 per group); the other rats (three rats of each group) were used for behavior testing until 48 h after surgery.
Construction of SCN9A-RNAi-LV and microinjection of virus via intrathecal catheter
For the knockdown experiment with SCN9A-RNAi-LV, the sequences of rat SCN9A siRNA lentivirus (SCN9A-RNAi-LV) were designed and synthesized using the following target sequences: 5′-ATCGAAGAAGCTAAACAGAAA-3′. The same vector without SCN9A siRNA was used as the NC-LV. The lentivirus vectors were constructed by Shanghai Genechem Co., Ltd. (Shanghai, China). The titer of the virus was 5 × 108 TU/ml and stored at −80°C.
According to previously described methods,20,21 intrathecal catheters (PE-10, Smiths Medical, England) were constructed, and 3 days before operation, 10 μl of SCN9A-RNAi-LV or NC-LV was administered intrathecally, followed by a 10-μl saline flush.
Establishment of incisional pain in rats
According to previously reported methods,22 the rats received general anesthesia with 2.5% isoflurane. Before surgery, each rat received 30,000 IU penicillin by intramuscular injection. Then, the left hindpaw plantar was sterilized with iodophor. A 1-cm longitudinal incision was made through the skin and underlying fascia (starting 0.5 cm from the proximal heel and extending toward the toes). The flexor digitorum brevis muscles of the plantar were exposed and incised longitudinally (leaving the muscle origin and insertion intact). After hemostasis by compression, the skin was sutured using 5–0 silk. The incision wound site was covered with penicillin ointment.
Measurement of pain behaviors
All behavior tests were performed under quiet conditions in the morning.
Measurement of mechanical hyperalgesia
The rats were habituated in plastic cages (10 × 10 × 19 cm), with a grid floor, for 30 min. Mechanical allodynia was measured using electronic von Frey filaments according the method of Vivancos et al.,23,24 the tip of the filament was applied perpendicularly to the mid-plantar with gradually increasing pressure; the pressure of the stimulus was recorded automatically when the paw was withdrawn. The tests were repeated until three similar measurements (within 10 g) were obtained; the test-free period was 10 min. The average of three measurements was considered as the mechanical withdrawal threshold.
Pain scoring based on weight bearing
According to a previously described method,22 the pain score was obtained based on the position of the operated paw: score = 0, the operated foot completely touched the mesh; score = 1, the wound touched the mesh and the foot distorted; and score = 2, the operated foot was kept completely off the mesh.
Measurement of thermal hyperalgesia
The rats were allowed to habituate in the test equipment for 30 min, after which thermal hyperalgesia was performed according to method of Hargreaves et al.25 A radiant light was focused on the hindpaw, and the response latency was automatically recorded when the rat lifted or licked its hindpaw, the average of three repeated measurements was considered the withdrawal latency (5-min intervals between measurements). To avoid tissue damage, a 20-s cutoff time was established.
Expression of Nav1.7 in the DRG
Quantitative PCR
Total RNA was extracted from the left L4−L5 DRG of the rats using Trizol (Invitrogen, Carlsbad, CA, USA) and 2 μg of total RNA was reverse-transcribed into cDNA using RevertAid Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using PCR system (ABI 7900/Viia, Foster City, CA, USA). SYBR Green was used to detect amplification of Nav1.7; reaction conditions were 95°C for 1 min (initial denaturation), followed by 40 cycles each consisting of 95°C for 30 s (denaturation) and at 60°C for 30 s (annealing extension). The primer sequences were as follows. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 5′-ACAGCAACAGGGTGGTGGAC-3′ (forward) and 5′-TTTGAGGGTGCAGCGAACTT-3′ (reverse), Nav1.7: 5′-CAGCCGCAGATAGCCGTCGT-3′ (forward) and 5′-GGGCGTCCGCAAAGTCAGAG-3′ (reverse). The data of Nav1.7 were normalized to the internal reference (GAPDH). The relative level of Nav1.7 mRNA was calculated using the comparative cycle threshold (CT) method (2−ΔΔCT). The samples were quantified in triplicate.
Western blotting
The DRGs were dissected from the left L4−L5 DRG of the rats and the total protein was extracted using RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China). The samples were separated by electrophoresis by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transfected to a 0.45-μm nitrocellulose membrane (Merck Millipore, Billerica, MA, USA), which were probed with primary antibodies (Nav1.7 rabbit polyclonal antibodies (1:200, Alomone Labs, Jerusalem, Israel) or GAPDH rabbit polyclonal antibody (1:1000, Abcam, Cambridge, MA, USA)) overnight at 4°C. Then, the nitrocellulose membranes were incubated with a secondary antibody for 2 h at room temperature. Positive signals were quantified using ImageJ software.
Immunohistochemistry
Under deep anesthesia with isoflurane, the rats were perfused intracardially with normal saline and subsequently fixed with 4% paraformaldehyde. Left L4−L5 DRGs were collected and postfixed in 4% paraformaldehyde for 4 h at room temperature. After dehydration, DRG sections (4 μm) were prepared and incubated in 5% bovine serum albumin for 30 min at room temperature and then incubated with Nav1.7 polyclonal antibodies (1:200) overnight at 4°C, followed by secondary antibody (1:500, Life Technologies, Carlsbad, CA, USA) for 90 min at 37°C. Images were captured with a Leica microscope (Welzar, Germany).
Statistical analysis
All data were described as means ± SD. Pain measurement data were analyzed by SPSS 19.0. Statistical analysis of Q-PCR, Western blotting, and immunostaining intensity was performed using GraphPad software. The statistically significant difference between groups was analyzed using one-way analysis of variance with Tukey’s post hoc test. For all analyses, P < 0.05 was considered as statistically significant difference.
Results
Pain-related behavior in rats following plantar incision
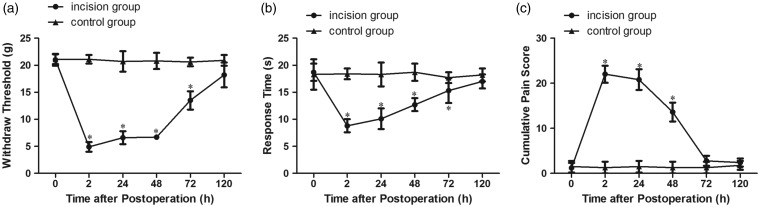
Pain-related behaviors were measured before and at 2 h, 24 h, 48 h, 72 h, and 120 h after plantar incision. Compared with the control group, the mechanical and thermal pain threshold decreased significantly from 2 h to 72 h after plantar incision (P < 0.05) and showed a minimum value at 2 h after the operation (Figure 1(a) and (b)). Cumulative pain scores were increased significantly as compared with the control group (P < 0.05) and reached a maximum value at 2 h after the operation (Figure 1(c)). These findings indicated that the model of plantar incision-related postoperative pain was established successfully. Subsequently, incision-related pain behaviors started to return to the preoperative value; although the pain threshold remained lower than that in the control group, no statistically significant differences (P > 0.05) were found between the two groups at 120 h after the operation (Figure 1(a) to (c)).
Figure 1.
Pain-related behavior in rats following plantar incision. (a) Mechanical hypersensitivity. (b) Thermal hypersensitivity. (c) Cumulative pain score. *P < 0.05 compared to the control group.
Dynamic expression of Nav1.7 in the DRG after plantar incision
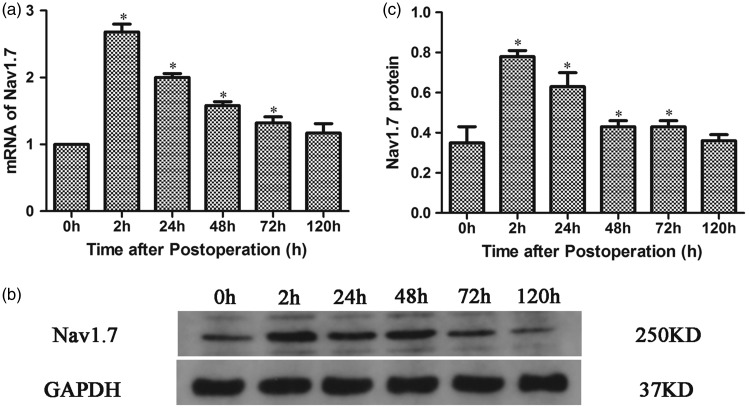
To analyze the expression of Nav1.7 in the DRG during pain hypersensitivity after plantar incision, the expression of Nav1.7 mRNA and Nav1.7 proteins in L4−L6 DRG of rats was detected by Q-PCR and Western blotting, respectively, before and at 2 h, 24 h, 48 h, 72 h, and 120 h after plantar incision. The results revealed that both Nav1.7 mRNA and Nav1.7 proteins in L4−L6 DRG significantly increased from 2 h to 72 h postoperatively and reached a maximum at 2 h after plantar incision. Subsequently, the expression began to return to preincision levels (Figure 2(a) to (c) and Figure S1).
Figure 2.
Dynamic expression of Nav1.7 in L4−L6 DRG of rats after plantar incision. (a) Quantitative analysis of Nav1.7 mRNA expression in the DRG after plantar incision. (b) The expression of Nav1.7 in the DRG as analyzed by Western blotting. (c) Quantitative analysis of Nav1.7 protein expression in the DRG after plantar incision. *P < 0.05 compared with controls.GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Nav1.7 expression in the DRG after plantar incision in rats pretreated with SCN9A-RNAi-LV
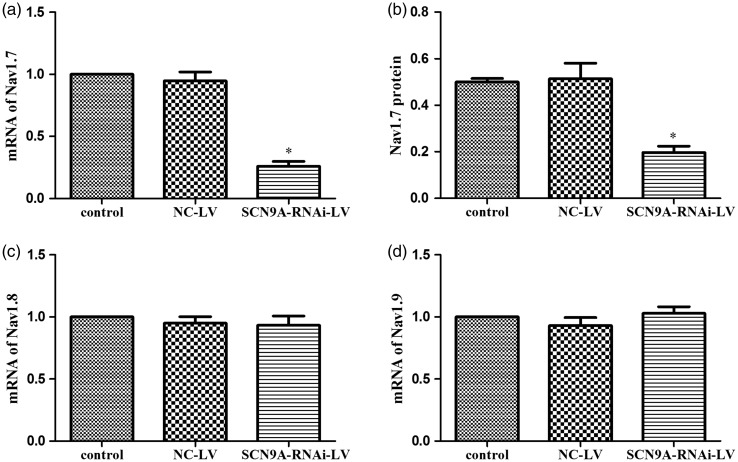
To detect whether SCN9A-RNAi-LV can knockdown Nav1.7 expression, mRNA of Nav1.7, Nav1.8, and Nav1.9 were examined by Q-PCR. We found that SCN9A-RNAi-LV significantly reduced mRNA levels of Nav1.7 (72.6% as compared to the NC-LV group) (Figure 3(a)), while the mRNA levels of Nav1.8 and Nav1.9 were not affected (Figure 3(c) and (d)). Nav1.7 protein levels were also reduced significantly, by 60.8%, by this treatment (Figure 3(b)). These results showed that SCN9A-RNAi-LV down-regulated Nav1.7 expression efficiently and specifically.
Figure 3.
The efficient and special of SCN9A-RNAi-LV. (a) The influence of SCN9A-RNAi-LV on Nav1.7 mRNA in DRG. (b) The influence of SCN9A-RNAi-LV on Nav1.7 protein in the DRG. (c) The influence of SCN9A-RNAi-LV on Nav1.8 mRNA in the DRG. (d) The influence of SCN9A-RNAi-LV on Nav1.9 mRNA in the DRG. *P < 0.05 compared with NC-LV group. NC-LV: negative control lentivirus.
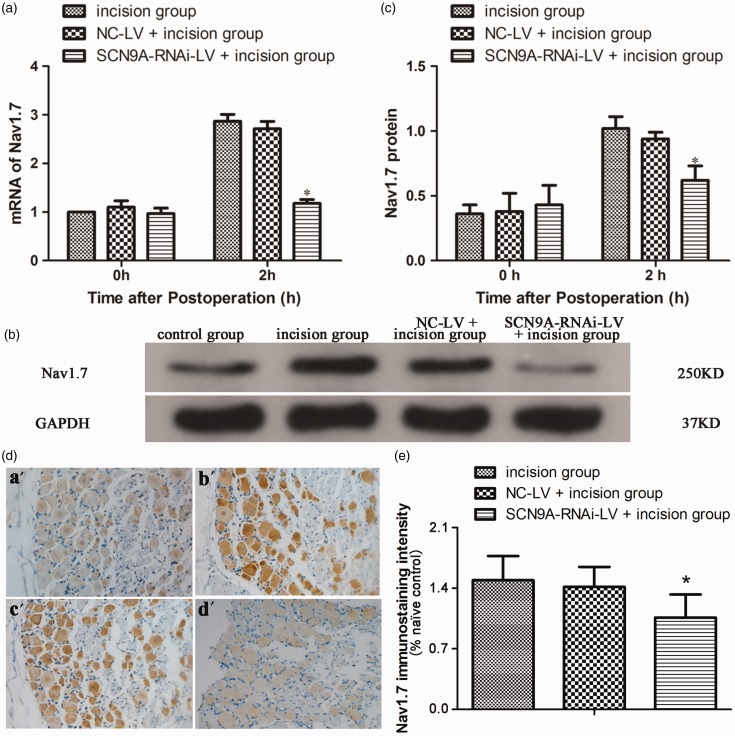
As the expression of Nav1.7 in L4−L5 DRG peaked at 2 h after plantar incision, when the postoperative pain sensation was most significant, we investigated Nav1.7 expression in the DRG at 2 h after plantar incision in rats pretreated with SCN9A-RNAi-LV. As shown in Figure 4(d) and Figure S2, compared with the NC-LV pretreatment group (c), the enhanced Nav1.7 expression in L4−L6 DRG was inhibited in the SCN9A-RNAi-LV group (d) after plantar incision (b).
Figure 4.
Nav1.7 expression in the DRG after plantar incision in rats pretreated with SCN9A-RNAi-LV. (a) The influence of SCN9A-RNAi-LV on Nav1.7 mRNA in the DRG after plantar incision. (*P < 0.05 compared with incision group.). (b) The influence of SCN9A-RNAi-LV on Nav1.7 proteins in the DRG after plantar incision. (c) Quantitative analysis of Nav1.7 protein expression in the DRG after plantar incision. (*P < 0.05 compared with incision group.). (d) Representative immunohistochemical images showed the Nav1.7 expression in DRG of naïve control group (a′), incision group (b′), NC-LV + incision group (c′), and SCN9A-RNAi-LV + incision group (d′). Nav1.7-positive neurons exhibited a brown color. The naïve control group is the section of DRG from naïve rat, in which anti-Nav1.7antibodies were pre-incubated with Nav1.7. (e) Quantification of Nav1.7 labelling intensity in normalized to naïve control values. The increased expression of Nav1.7 in L4−L6 DRG after plantar incision was inhibited by SCN9A-RNAi-LV. *P < 0.05 compared with incision group. GAPDH: glyceraldehyde-3-phosphate dehydrogenase; NC-LV: negative control lentivirus.
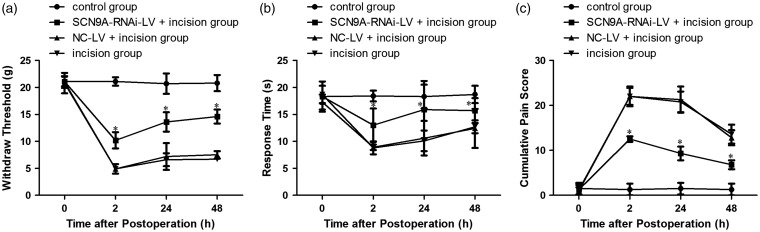
Effect of SCN9A-RNAi-LV pretreatment in incision-related pain behavior
Pretreatment with SCN9A-RNA-LV significantly inhibited the enhanced expression of Nav1.7 in L4−L6 DRG after plantar incision (Figure 5(a) to (c)). As compared with the NC-LV + incision group, the mechanical pain threshold was significantly increased by 108% (10.2 ± 1.5 vs. 4.9 ± 0.9), 88% (13.6 ± 1.8 vs. 7.2 ± 2.5), and 91% (14.3 ± 1.6 vs. 7.5 ± 0.7) at 2 h, 24 h, and 48 h, respectively (Figure 5(a)). In the pretreated group, the thermal pain threshold was significantly increased by 46% (13.0 ± 3.1 vs. 8.9 ± 0.5), 50% (15.9 ± 5.3 vs. 10.6 ± 3.2), and 27% (15.7 ± 2.3 vs. 12.4 ± 3.6) at 2 h, 24 h and 48 h, respectively (Figure 5(b)). The cumulative pain score in the pretreated group also significantly declined by 43% (12.5 ± 0.6 vs. 22.0 ± 2.2), 56% (9.3 ± 1.5 vs. 21.3 ± 2.9), 47% (6.8 ± 1.0 vs. 12.8 ± 1.7) at 2 h, 24 h and 48 h, respectively (Figure 5(c)).
Figure 5.
The effect of SCN9A-RNAi-LV pretreatment in incision-related pain behavior. (a) Mechanical hypersensitivity. (b) Pain score. (c) Thermal hypersensitivity. *P < 0.05 compared with the incision group. NC-LV: negative control lentivirus.
Discussion
The rat model of plantar incision-related pain is a reliable model for simulating postoperative pain in humans.22 It can be used to facilitate understanding of the mechanism underlying postoperative pain and to explore new treatments for postoperative pain. In the present study, after plantar incision, the rats developed pain sensitization; the mechanical and thermal pain thresholds were significantly decreased and cumulative pain scores were significantly increased (from 2 h to 72 h after plantar incision). The mechanical and thermal pain thresholds were lowest and the cumulative pain scores were highest at 2 h after the operation and then subsequently recovered gradually. Nav1.7 expression in L4−L6 DRG increased significantly and reached a peak at 2 h after plantar incision and then recovered from 72 h after plantar incision. However, when the rats were pretreated with SCN9A-RNAi-LV to inhibit the enhancement of Nav1.7 expression in the DRG, the pain hypersensitivity after plantar incision was alleviated.
Voltage-gated sodium channel Nav1.7 plays an important role in the generation and transmission of pain signals.26 Electrophysiological studies confirmed that Nav1.7 is able to amplify sub-threshold excitability and regulate neuronal excitability.4,27 The increased Nav1.7 expression in DRG suggests hyperexcitability of DRG neurons and significant stimulate synaptic transmission following plantar incision, which may contribute to hyperalgesia after plantar incision. These findings demonstrate that the increased Nav1.7 in the DRG is very important for postoperative pain hypersensitivity after plantar incision.
This is in agreement with greatly increased Nav1.7 expression and pain hypersensitivity in preclinical studies from rodent model of inflammatory pain and peripheral neuropathic pain.6,14,28,29 A previous study has shown that the expression of Nav1.7 is regulated by inflammatory mediators, such as NGF and prostaglandin E2 via the protein kinase B/Akt pathway.30 Pronociceptive factors (e.g., NGF) are increased after plantar incision.31 These findings dovetail well with previous results and show that NGF signaling may be important in the up-regulation of Nav1.7 in postoperative pain.
In addition, in clinical context, postoperative pain is categorized as at-rest pain and movement-induced pain. In previous studies, the mechanical and the thermal pain thresholds were quantified by the von Frey filaments method and thermal radiation, respectively, these pain sensations are similar to pain evoked by coughing or movement. However, in this study, we also used a cumulative pain score, which reflects a kind of nonevoked pain and is based on the position chosen by the rats after the incision, and which is similar to surgical limb protection behavior of patients, to assist in the analysis of mechanical sensitivity.16,19
In summary, this study confirms that Nav1.7 in DRG plays an important role in a preclinical rodent model of acute postoperative pain and suggests that Nav1.7-targeted analgesics currently under development may be effective in patients with acute postoperative pain.
Supplemental Material
Supplemental material, Supplementary Figures for Increased Nav1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats by Jiaoli Sun, Ningbo Li, Guangyou Duan, Yi Liu, Shanna Guo, Cong Wang, Changmao Zhu and Xianwei Zhang in Molecular Pain
Author Contributions
Xianwei Zhang conceived the study and supervised project, Xianwei Zhang and Jiaoli Sun designed the experiment, analyzed the data, and wrote the manuscript. Ningbo Li and Guangyou Duan were responsible for establishment of incisional pain in rats. Yi Liu and Shanna Guo conducted the measurement of pain behaviors. Cong Wang and Changmao Zhu carried out Q-PCR, Western blotting, and immunohistochemistry analysis. All authors approved the version to be published.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Nature Science Foundation of China (grants 81271235).
Supplemental Material
Supplementary material is available for this article online.
References
- 1.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, Fu R, Azad T, Chao TE, Berry WR, Gawande AA. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015; 385: S11. [DOI] [PubMed] [Google Scholar]
- 2.Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014; 30: 149–160. [DOI] [PubMed] [Google Scholar]
- 3.Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet 2011; 377: 2215–2225. [DOI] [PubMed] [Google Scholar]
- 4.Habib AM, Wood JN, Cox JJ. Sodium channels and pain. Handb Exp Pharmacol 2015; 227: 39–56. [DOI] [PubMed] [Google Scholar]
- 5.Dib-Hajj SD, Geha P, Waxman SG. Sodium channels in pain disorders: pathophysiology and prospects for treatment. Pain 2017; 158: S97–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minett MS, Falk S, Santana-Varela S, Bogdanov YD, Nassar MA, Heegaard A-M, Wood JN. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep 2014; 6: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer NJ. Switching off pain at the source: is this the end for opioid pain relief ? Pain Manag 2016; 6: 39–47. [DOI] [PubMed] [Google Scholar]
- 8.Vetter I, Deuis JR, Mueller A, Israel MR, Starobova H, Zhang A, Rash LD, Mobli M. NaV1.7 as a pain target - From gene to pharmacology. Pharmacol Ther 2017; 172: 73–100. [DOI] [PubMed] [Google Scholar]
- 9.Zakrzewska JM, Palmer J, Morisset V, Giblin GM, Obermann M, Ettlin DA, Cruccu G, Bendtsen L, Estacion M, Derjean D, Waxman SG, Layton G, Gunn K, Tate S. Safety and efficacy of a Nav1.7 selective sodium channel blocker in patients with trigeminal neuralgia: a double-blind, placebo-controlled, randomised withdrawal phase 2a trial. Lancet Neurol 2017; 16: 291–300. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet 2004; 41: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fertleman CR, Baker MD, Parker KA, Moffatt S, Elmslie FV, Abrahamsen B, Ostman J, Klugbauer N, Wood JN, Gardiner RM, Rees M. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 2006; 52: 767–774. [DOI] [PubMed] [Google Scholar]
- 12.Faber CG, Hoeijmakers JGJ, Ahn H-S, Cheng X, Han C, Choi J-S, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JPH, Waxman SG, Merkies ISJ. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012; 71: 26–39. [DOI] [PubMed] [Google Scholar]
- 13.Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature 2006; 444: 894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J-H, Park C-K, Chen G, Han Q, Xie R-G, Liu T, Ji R-R, Lee S-Y. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 2014; 157: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Nassar MA, Stirling LC, Forlani G, Baker MD, Matthews EA, Dickenson AH, Wood JN. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA 2004; 101: 12706–12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MH, Kim MS, Lee JH, Kim ST, Lee JR. Intravenously administered lidocaine and magnesium during thyroid surgery in female patients for better quality of recovery after anesthesia. Anesth Analg. Epub ahead of print 9 January 2018. DOI: 10.1213/ANE.0000000000002797 [DOI] [PubMed]
- 17.Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology 2017; 126: 729–737. [DOI] [PubMed] [Google Scholar]
- 18.Duan G, Xiang G, Guo S, Zhang Y, Ying Y, Huang P, Zheng H, Zhang M, Li N, Zhang X. Genotypic analysis of SCN9A for prediction of postoperative pain in female patients undergoing gynecological laparoscopic surgery. Pain Physician 2016; 19: E151–E162. [PubMed] [Google Scholar]
- 19.Duan G, Xiang G, Zhang X, Yuan R, Zhan H, Qi D. A single-nucleotide polymorphism in SCN9A may decrease postoperative pain sensitivity in the general population. Anesthesiology 2013; 118: 436–442. [DOI] [PubMed] [Google Scholar]
- 20.Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods 1999; 90: 81–86. [DOI] [PubMed] [Google Scholar]
- 21.Ke CB, He WS, Li CJ, Shi D, Gao F, Tian YK. Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience 2012; 227: 80–89. [DOI] [PubMed] [Google Scholar]
- 22.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 1996; 64: 493–501. [DOI] [PubMed] [Google Scholar]
- 23.Vivancos GG, Verri Jr WA, Cunha TM, Schivo IRS, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for rats. Braz J Med Biol Res 2004; 37: 391–399. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari LF, Lotufo CM, Araldi D, Rodrigues MA, Macedo LP, Ferreira SH, Parada CA. Inflammatory sensitization of nociceptors depends on activation of NMDA receptors in DRG satellite cells. Proc Natl Acad Sci USA 2014; 111: 18363–18368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 26.Black JA, Frézel N, Dib-Hajj SD, Waxman SG. Expression of Nav1.7 in DRG neurons extends from peripheral terminals in the skin to central preterminal branches and terminals in the dorsal horn. Mol Pain 2012; 8: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci 2013; 14: 49–62. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh M-C, Ho Y-C, Lai C-Y, Wang H-H, Lee A-S, Cheng J-K, Chau Y-P, Peng H-Y. Bromodomain-containing protein 4 activates voltage-gated sodium channel 1.7 transcription in dorsal root ganglia neurons to mediate thermal hyperalgesia in rats. Anesthesiology 2017; 127: 862–877. [DOI] [PubMed] [Google Scholar]
- 29.Chang W, Berta T, Kim YH, Lee S, Lee S-Y, Ji R-R. Expression and role of voltage-gated sodium channels in human dorsal root ganglion neurons with special focus on Nav1.7, species differences, and regulation by paclitaxel. Neurosci Bull 2018; 34: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang L, Fan L, Tao B, Yaster M, Tao Y-X. Protein kinase B/Akt is required for complete Freund’s adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons. J Pain 2013; 14: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spofford CM, Brennan TJ. Gene expression in skin, muscle, and dorsal root ganglion after plantar incision in the rat. Anesthesiology 2012; 117: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Figures for Increased Nav1.7 expression in the dorsal root ganglion contributes to pain hypersensitivity after plantar incision in rats by Jiaoli Sun, Ningbo Li, Guangyou Duan, Yi Liu, Shanna Guo, Cong Wang, Changmao Zhu and Xianwei Zhang in Molecular Pain