Abstract
Polygonati rhizoma (PR), a traditional medicinal and edible product with various bioactive components (Polygonatum polysaccharides, saponins, phenols, and flavonoids), is widely consumed in China. However, other species with morphological characteristics similar to those of the actual components are being used to replace or adulterate PR, causing issues with quality control and product safety. The morphological similarity of PR and its substitutes makes classic morphological identification challenging. To address this issue, DNA barcoding-based identification using ITS2 and psbA-trnH sequences was applied in this study to evaluate the efficiency and accuracy of this approach in identifying PR samples collected from 39 different regions in China. The identification of PR by this method was confirmed by other methods (phylogeny-based and character-based methods), and all the samples were clearly and accurately distinguished. This study highlights the efficient and accurate nature of DNA barcoding in PR identification. Applying this technique will provide a means to differentiate PR from other altered formulations, thus improving product quality and safety for consumers of PR and its products.
Introduction
Polygonati rhizoma (PR) is a medicinal and edible product recognized by the China Food and Drug Administration. PR has been used during famine [1] and by Chinese Taoists and Buddhists for sustenance during extended fasting [2]. PR is composed of the dry rhizomes of Polygonatum sibiricum F. Delaroche, Polygonatum cyrtonema Hua, and Polygonatum kingianum Coll. et Hemsl., all of which are perennial herbs belonging to the Asparagaceae family [3]. These three species contain multiple bioactive components, such as Polygonatum polysaccharides [4], saponins [5], phenols [6], and flavonoids [7], which have various biological functions. Records in the Compendium of Materia Medica [8] describe PR as sweet and non-toxic. In China, people use PR as both a healthcare product and a food ingredient in daily meals. It appears to tonify the spleen and kidney, moisten the lungs, and quench thirst [9], according to the primary lung, kidney, and spleen channel classifications in traditional Chinese medicine theories. Moreover, modern pharmacological studies have shown that PR improves immunity [10] and memory [11]; reduces blood glucose [12] and fat levels [13]; and elicits antibacterial [14], antiviral [15], anticancer [16], and anti-aging [17] effects. As it has important edible value in both raw and processed forms, PR has been used to make various foods, such as cakes, biscuits, succade, preserved fruit, pastes, bread, tea, drinks, and wine, as well as products like soaps and shampoos.
Unfortunately, numerous rhizome species with similar morphological traits distributed in the southwest region of China are misused as PR components. These include the rhizomes of Polygonatum filipes Merr., Polygonatum punctatum, Polygonatum cathcartii Baker, Polygonatum verticillatum (L.) All., Disporopsis longifolia, and Gelsemium elegans (Gardn. & Champ.) Benth. Thus, efficiently and accurately distinguishing PR from other rhizome species is essential to assure product quality and consumer safety. The components of PR have particular characteristics, including time-consuming methods for breaking the dormancy [18] and growing the seedlings [19], as well as variable growth environments, all of which make classical identification techniques using herb morphology and biochemistry inefficient and often inaccurate. Therefore, other methodologies are required to effectively identify PR components and distinguish them from non-PR herbs. Molecular identification based on DNA barcoding is a recent tool that has been used to conduct species-level identification [20]. As an effective complement to traditional identification methods, DNA barcoding identification classifies species based on standardized, relatively short DNA sequences, which differ among species and are consistent regardless of environment. To date, DNA barcoding has been used to identify various plant species, such as lichens [21], fungi [22, 23], weeds [24], trees [25–28], and economically important plants such as crops [29] and medicinal and aromatic plants [30–35]. The DNA barcoding system and principles established by Chen [36] can be used to clearly identify Chinese medicinal materials found in various forms, including multi-component medicines, medicinal powders [37] and fragments [38], and the original herb [39]. This system uses the ITS2 and psbA-trnH sequences as the main and auxiliary sequences, respectively, and has successfully identified Dendrobium [40], Polygonaceae [41], Rosaceae [42], Araceae [43], and Fabaceae [44]. However, this DNA barcoding system has not been used to identify the components of PR.
In this study, we applied a DNA barcoding system with ITS2 and psbA-trnH sequences to identify PR samples collected from southern China. To validate this DNA barcoding-based method, identification using phylogeny-based and character-based methods was also conducted. To our knowledge, this is the first report demonstrating the use of this ITS2/psbA-trnH-based DNA barcoding system to efficiently and accurately identify PR. Importantly, our findings have immediate practical implications on the application of DNA barcoding to molecularly identify herbal medicines irrespective of their material state.
Materials and methods
Plant materials
In the present investigation, 39 samples were collected from different regions in 11 different provinces of China (Tables 1 and 2). Approximately 100 rhizomes were selected from each region and were maintained in PR GAP base (Buchang Pharma, Lveyang, Shaanxi, China).
Table 1. Details of the sampling areas of polygonati rhizoma from different regions in China.
| Provinces | Locations’ name | Geographic coordinates | Accession no. | Putative Species | NCBI ID (Top percent identity) |
Accession no. (GENE BANK) |
|---|---|---|---|---|---|---|
| Anhui | Chizhou Dajianshan |
117°36′32″E 30°39′36″N |
S1 | Polygonatum cyrtonema | Polygonatum cyrtonema (100) | KJ745884.1 |
| Luan Longxueshan |
116°42′51″E 31°43′27″N |
S2 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Qingyang Qingyuanshan |
118°02′6″E 30°41′9″N |
S3 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Jingxian Qinglongshan |
118°33′14″E 30°32′35″N |
S4 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Huangshan | 118°10′11″E 30°07′55″N |
S5 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Chongqing | Wulong Xiannvshan |
107°47′56″E 29°25′38″N |
S6 | P. cyrtonema | Polygonatum kingianum (100) | KJ745828.1 |
| Qijiang Huanggaoshan |
106°54′23″E 28°58′38″N |
S7 | P. cyrtonema | P. kingianum (100) | KJ745828.1 | |
| Fujian | Zhenghe Jinpingcun |
119°06′13″E 27°24′54″N |
S8 | P. cyrtonema |
Polygonatum curvistylum (99) Polygonatum cirrhifolium (99) Polygonatum franchetii (99) Polygonatum prattii (99) |
KJ745774.1 KJ745802.1 KJ745833.1 KJ745837.1 |
| Guangdong | Shaoguan Longdoushan |
113°55′33″E 24°43′21″N |
S9 | P. cyrtonema |
P. curvistylum (100) P. cirrhifolium (100) |
KJ745774.1 KJ745802.1 |
| Qingyuan Dawangshan |
113°22′34″E 23°44′4″N |
S10 | P. cyrtonema |
P. curvistylum (99) P. cirrhifolium (99) P. franchetii (99) |
KJ745774.1 KJ745802.1 KJ745833.1 |
|
| Guangxi | Chongzuo Shanglongxiang |
106°51′8″E 22°24′32″N |
S11 | P. cyrtonema | Disporopsis longifolia (100) | KJ745836.1 |
| Hezhou Pingshan |
111°26′21″E 24°24′51″N |
S12 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Yizhou Jiulongshan |
108°37′42″E 24°28′8″N |
S13 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Baise Jitishan |
106°41′29″E 23°55′32″N |
S14 | P. cyrtonema | P. kingianum (100) | KJ745828.1 | |
| Guizhou | Tongren Liulongshan |
109°16′22″E 27°38′25″N |
S15 | P. cyrtonema | P. kingianum (100) | KJ745828.1 |
| Zhenyuan Longtoushan |
108°25′59″E 27°02′22″N |
S16 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Dejiang Daxishan |
108°07′34″E 28°15′59″N |
S17 | P. cyrtonema | P. kingianum (100) | KJ745828.1 | |
| Guiyang Panlongdong |
106°28′5″E 26°40′44″N |
S18 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 |
Table 2. Details of the sampling areas of polygonati rhizoma from different regions in China.
| Provinces | Locations’ name | Geographic coordinates | Accession no. | Putative Species | NCBI ID (Top percent identity) |
Accession no. (GENE BANK) |
|---|---|---|---|---|---|---|
| Henan | Lushi Wangjiashan |
111°05′51″E 34°02′8″N |
S19 | Polygonatum sibiricum | Polygonatum sibiricum (100) | KJ745880.1 |
| Nanzhao Dingjiazhuan |
112°22′10″E 33°28′56″N |
S20 | P. sibiricum | P. sibiricum (100) | KJ745880.1 | |
| Lingbao Sihecun |
111°05′12″E 34°28′48″N |
S21 | P. sibiricum | P. sibiricum (100) | KJ745880.1 | |
| Songxian Checunzhen |
112°05′2″E 33°48′38″N |
S22 | P. sibiricum | P. sibiricum (99) | KJ745880.1 | |
| Songxian Zhonghuangcun |
111°59′4″E 34°09′49″N |
S23 | P. sibiricum | P. cyrtonema (100) | KJ745884.1 | |
| Lingbao Sucun |
110°58′15″E 34°32′45″N |
S24 | P. sibiricum | P. sibiricum (100) | KJ745880.1 | |
| Shaanxi | Lveyang Wulongdong |
106°12′30″E 33°30′9″N |
S25 | P. sibiricum | P. sibiricum (100) | KJ745880.1 |
| Ankang Xiangxidong |
109°01′42″E 32°39′34″N |
S26 | P. sibiricum | P. sibiricum (100) | KJ745880.1 | |
| Sichuan | Yilong Shizishan |
106°16′39″E 31°15′46″N |
S27 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 |
| Yunan | Baoshan Qingshan |
99°15′36″E 25°04′6″N |
S28 | P. cyrtonema | P. kingianum (99) | KJ745828.1 |
| Honghe Mopanshan |
103°17′47″E 23°21′3″N |
S29 | Polygonatum kingianum | P. kingianum (100) | KJ745828.1 | |
| Dali Yuntaishan |
100°17′47″E 25°33′58″N |
S30 | P. cyrtonema | P. cyrtonema (99) | KJ745884.1 | |
| Mengzi Gaojiacun |
103°23′4″E 23°24′19″N |
S31 | P. kingianum | P. kingianum (100) | KJ745828.1 | |
| Mengzi Jixinshan |
103°20′42″E 23°25′42″N |
S32 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Yimen Shizishan |
102°08′45″E 24°39′15″N |
S33 | P. cyrtonema | P. kingianum (100) | KJ745828.1 | |
| Zhejiang | Huangyan Darenshan |
121°20′14″E 28°38′30″N |
S34 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 |
| Xianju Xiaoyaoxia |
120°36′43″E 28°41′10″N |
S35 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Kaihua Jiujiewu |
118°25′33″E 29°07′24″N |
S36 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Tongxiang Longwangmiao |
120°28′32″E 30°35′2″N |
S37 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Lishui Huangjiashan |
120°04′12″E 28°31′14″N |
S38 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 | |
| Tiantai Tiantaishan |
120°57′56″E 29°08′15″N |
S39 | P. cyrtonema | P. cyrtonema (100) | KJ745884.1 |
DNA extraction
Fresh rhizome tissue samples from each region were disinfected with 75% alcohol, frozen in liquid nitrogen, and preserved at −80°C. Total genomic DNA was extracted following the Doyle and Doyle method with little modification [45]. DNA was isolated from 0.5 g of rhizome tissue. Purified total DNA was quantified using a NanoDrop 2000 instrument (Thermo Fisher Scientific, Waltham, MA, USA). The DNA samples were then diluted to 30 ng/μL and stored at −20°C until ITS2 and psbA-trnH analysis.
ITS2 and psbA-trnH screening and amplification
Previously published ITS2 and psbA-trnH primers [32] were synthesized by Shanghai Sangon Biological Engineering Technology and Services (Shanghai, China). PCR amplification was performed with a Veriti 96-well Thermal Cycler (Applied Biosystems). Amplification reactions were conducted in 20 μL reaction volumes in 1.5 mL microfuge tubes with 10.0 μL of 2× Es Taq MasterMix (CWBIO, China), which contains Taq DNA polymerase, 2× Taq PCR buffer, 3 mM MgCl2, and 400 μM dNTP mix, along with 1.0 μL of 1 μM primer, 1.0 μL of 30 ng/μL DNA template, and 7.0 μL ddH2O (CWBIO). The PCR amplification procedure was set as follows: an initial denaturation of 5 min at 94°C; 30 cycles of 1 min denaturation at 94°C, 1 min annealing at 55°C, and 1.5 min extension at 72°C; and a final extension for 7 min at 72°C. All amplification products were separated on a 1.5% agarose gel using 1× TBE buffer by electrophoresis. The gel was stained with ethidium bromide and visualized with a gel documentation system (Bio-Rad Universal Hood II).
Data collection and analysis
PCR amplified products with high reproducibility and a clear single target band were recovered, and each product was sequenced by Shanghai Sangon Biological Engineering Technology and Services using a bidirectional sequencing method with the amplification primers. Sequences were proofread and spliced with DNAMAN 5.0 software, and the low-quality sequences and primer regions were removed. We blasted each sequence using NCBI BLAST software, and the top search hit was used as the reference sequence. Multiple sequence alignment using the ClustalW program, variable site analysis using the Data Explorer program, and genetic distance (GD) calculations using the Find Best DNA Models program were all conducted with MEGA 6.06 software. We used the nearest distance method to determine the “best close match” for species identification, using 95% intraspecific distance as the threshold [46]. Finally, a neighbor-joining (NJ) tree, which is closely related to the homology of the sequences, was estimated with the model selected by MEGA 6.06 using the GDs between the sampled sequences and the reference sequences calculated by the Compute Pairwise Distance program. A total of 1,000 bootstrap replicates were chosen to test the phylogeny for species identification. To further test the NJ tree, a maximum-likelihood (ML) tree was also estimated with the same models.
Phylogenetic analysis
DNA barcoding sequences of species in Polygonatum and other outgroups (Heteropolygonatum, Asparagus, and Zingiberaceae) were searched for in NCBI’s GenBank, which includes deposited data from other researchers and institutions. We collected all the sequences in these NCBI BLAST searches and organized them using the initial letter of their specific name as the norm. Two alignments were generated with the sequences within and among species. Sequences were aligned using ClustalW in the MEGA 6.06 software, which first processes the pairwise sequence alignment representing the relationship between them and then conducts multiple sequence alignments using an asymptotic approach. Thus, the GD among species precisely characterizes the genetic relationship among them.
The best-fit model of evolution and an optimal data-partitioning scheme were chosen using the Find Best DNA/Protein Models program in MEGA 6.06, with each codon position being chosen as an a priori data subset. ML was used as the statistical method using partial deletion gaps, with 95% site coverage as the cutoff for Gaps/Missing Data Treatment and moderate as the Branch Swap Filter. GDs were calculated using the Compute Pairwise Distances program with the Substitution Model selected and partial deletion gaps with 95% site coverage set as the cutoff. An ML tree was constructed in MEGA 6.06 using the models with the following settings: partitioning scheme selected, “Nearest-neighbor-interchange” chosen as the ML heuristic method, “Make initial tree automatically” as the initial tree for ML, and “Moderate” as the branch swap filter. A total of 1,000 bootstrap replicates were chosen to test the phylogeny illustrating phylogenetic relationships among species. Using the bootstrap values to test the credibility of the evolutionary tree branch, we can predicate its veracity.
Topology tests
There are two types of errors in phylogenetic trees: topological errors and branch length errors. Topological difference tests are needed to determine tree reliability, and branch length errors can also be tested using bootstrap tests. When the number of sequences is large and the extent of sequence divergence is low, it is generally difficult to reconstruct the true tree by any method. However, the bootstrap consensus tree often gives a reasonably good tree. Although weakly supported interior branches might differ, the bootstrap consensus tree obtained with the NJ method is usually similar to that obtained with the ML method. Therefore, we chose the NJ method to reconstruct the NJ tree and verify the topological tree constructed with the ML method.
Character-based tests
According to our BLAST searches, Polygonatum species have low psbA-trnH diversity. This makes distance-based identification more challenging, because a query sequence can have a nearly identical distance to multiple, different reference sequences. To circumvent this issue, we used the previously published character-based identification key named “characteristic attributes” (CAs) [47], which consists of 14 nucleotide characters (Tables 3 and 4) at specific positions across the psbA-trnH barcoding region that were used to identify PR species. This CA system was developed from an alignment of 32 reference sequences with diagnostic states that are specific to each PR species.
Table 3. Character based identification for samples.
| Menu listing | Character positions (22, 27, 65, 67, 103, 104,125, 127, 128, 129, 130, 132, 172, 429) |
Species identified |
|---|---|---|
| S1 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S2 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S3 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S4 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S5 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S6 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S7 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S8 | CGCACTGTTTCTTA | Incomplete certain (multi-species) |
| S9 | CGCACTGTTTCTTA | Incomplete certain (multi-species) |
| S10 | CGCACTGTTTCTTA | Incomplete certain (multi-species) |
| S11 | CGCGTCGTTTCTTC | Non-retrieved |
| S12 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S13 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S14 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S15 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S16 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S17 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S18 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S19 | CGCGTCGTTTCTCA | Polygonatum sibiricum |
| S20 | CGCGTCGTTTCTCA | Polygonatum sibiricum |
| S21 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S22 | CGCGTCGTTTCTCC | Non-retrieved |
Table 4. Character based identification for samples.
| Menu listing | Character positions (22, 27, 65, 67, 103, 104,125, 127, 128, 129, 130, 132, 172, 429) |
Species identified |
|---|---|---|
| S23 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S24 | CGCGTCGTTTCTCA | Polygonatum sibiricum |
| S25 | CGCGTCGTTTCTCA | Polygonatum sibiricum |
| S26 | CGCGTCGTTTCTCA | Polygonatum sibiricum |
| S27 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S28 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S29 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S30 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S31 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S32 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S33 | CGCGCTAGAAACTC | Polygonatum kingianum |
| S34 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S35 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S36 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S37 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S38 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
| S39 | CGCGTCGTTTCTTA | Polygonatum cyrtonema |
Results
Sequence amplification, data collation, and preliminary analysis
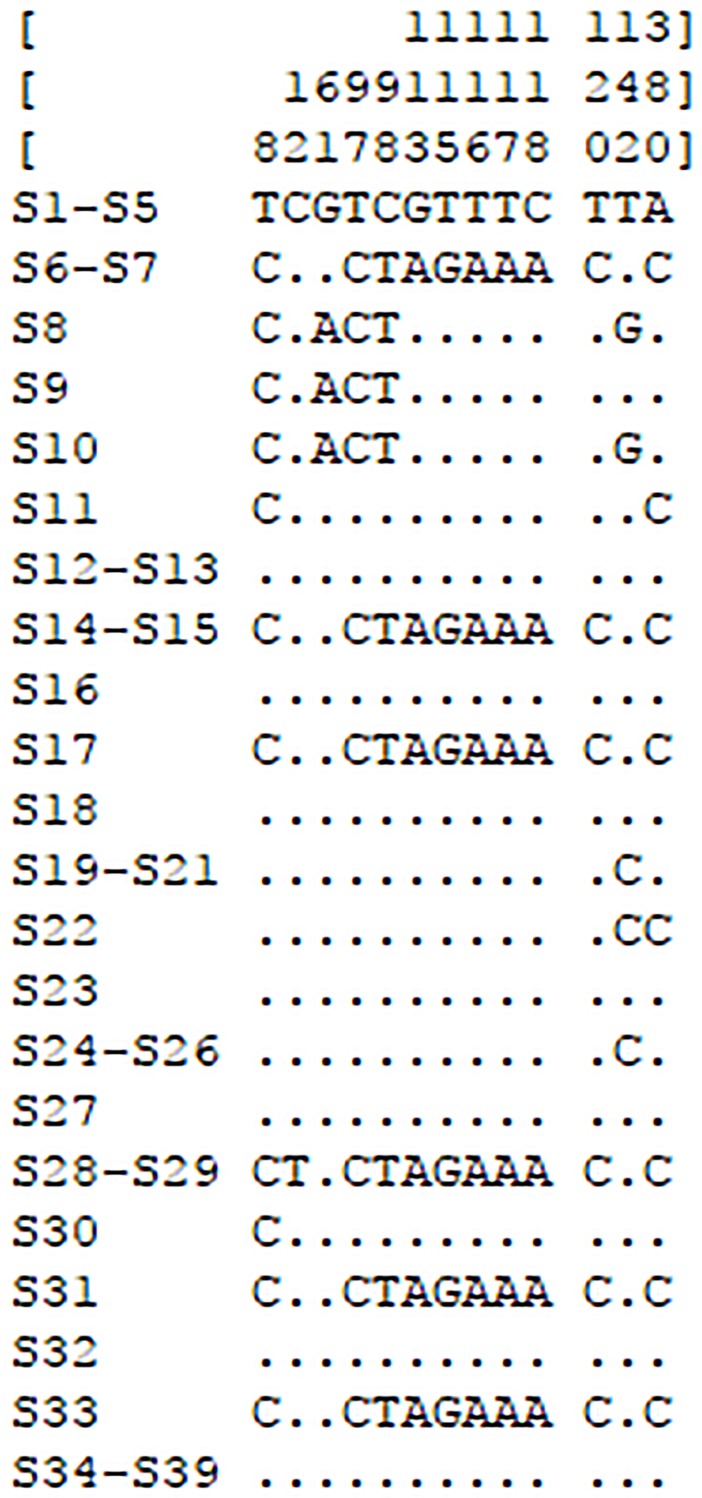
In our analysis using ITS2 and psbA-trnH primers, ITS2 sequences with polymorphic bands (S1A Fig) were not favorable for PR species identification. However, psbA-trnH sequences (approximately 650 bp) were clear and formed single bands, which could be used to identify PR species (S1B Fig). After each band was recovered and amplified, the samples were labeled and sequenced. After proofreading, aligning, and removing the low-quality sequences and primer sequences, sequence length varied from 529 to 603 bp, with the G+C and A+T content ranging from 34.8% to 35.6% and 64.4% to 65.2%, respectively (Table 5). All the sequences showed a total of 13 variable sites among the samples in the multiple sequence alignment (Fig 1).
Table 5. Sequences length and codon content of psbA-trnH sequences.
| Regions | T(%) | C(%) | A(%) | G(%) | Length (bp) |
Regions | T(%) | C(%) | A(%) | G(%) | Length (bp) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S21 | 35.0 | 16.8 | 30.2 | 18.0 | 529 |
| S2 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S22 | 35.0 | 17.0 | 30.1 | 18.0 | 529 |
| S3 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S23 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S4 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S24 | 35.0 | 16.8 | 30.2 | 18.0 | 529 |
| S5 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S25 | 35.0 | 16.8 | 30.2 | 18.0 | 529 |
| S6 | 34.0 | 17.2 | 30.5 | 18.2 | 603 | S26 | 35.0 | 16.8 | 30.2 | 18.0 | 529 |
| S7 | 34.0 | 17.2 | 30.5 | 18.2 | 603 | S27 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S8 | 34.4 | 17.1 | 30.1 | 18.4 | 602 | S28 | 34.0 | 17.2 | 30.5 | 18.2 | 603 |
| S9 | 34.7 | 17.1 | 30.0 | 18.2 | 603 | S29 | 34.0 | 17.2 | 30.5 | 18.2 | 603 |
| S10 | 34.4 | 17.1 | 30.1 | 18.4 | 602 | S30 | 34.6 | 17.1 | 30.1 | 18.3 | 602 |
| S11 | 34.5 | 17.1 | 29.9 | 18.5 | 595 | S31 | 34.0 | 17.2 | 30.5 | 18.2 | 603 |
| S12 | 34.5 | 17.1 | 29.9 | 18.5 | 595 | S32 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S13 | 34.5 | 17.1 | 29.9 | 18.5 | 595 | S33 | 34.0 | 17.2 | 30.5 | 18.2 | 603 |
| S14 | 34.5 | 17.1 | 29.9 | 18.5 | 595 | S34 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S15 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S35 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S16 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S36 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S17 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S37 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S18 | 34.7 | 16.9 | 30.1 | 18.3 | 602 | S38 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S19 | 35.0 | 16.8 | 30.2 | 18.0 | 529 | S39 | 34.7 | 16.9 | 30.1 | 18.3 | 602 |
| S20 | 35.0 | 16.8 | 30.2 | 18.0 | 529 |
Fig 1. Variation sites of psbA-trnH sequences of samples.
DNA barcoding identification
Tables 1 and 2 shows the top hits from the BLAST alignment selected as the reference sequences. Sequences from samples collected from six regions (S19, S20, S21, S24, S25, and S26) were identified as Polygonatum sibiricum, sharing 100% identity, while sequences from samples collected from S22 shared 99% identity. Furthermore, sample sequences obtained from 19 regions (S1−S5, S12, S13, S16, S18, S23, S27, S32, S34, and S35−S39) were identified as P. cyrtonema, and with the exception of S30 (which shared 99% identity), all the sequences shared 100% identity. Sample sequences from nine regions (S6, S7, S14, S15, S17, S28, S29, S31, and S33) are relatively similar to that of P. kingianum, while the sequence from S11 shared 100% identity with that of Disporopsis longifolia. Interestingly, sample sequences from three regions (S8, S9, and S10) shared 99% identity with different reference sequences. Hence, four species were identified in our analysis: P. sibiricum (7), P. cyrtonema (19), P. kingianum (9), and D. longifolia (1). The other three samples cannot be identified clearly at present.
We used MEGA 6.0 in association with the Tamura 3-parameter model to calculate the GDs within and among sample sequences and reference sequences. GDs between samples ranged from 0.000 to 0.023 (S2 Fig), with 0.000–0.002 indicating being present within species and 0.002–0.023 meaning being present among species. According to study by Meier [48], barcoding gap of sequences is quantified as 0.000, which indicates that barcoding gap does not exist in our samples. P. sibiricum indicated a close GD with P. cyrtonema (0.002) and was the farthest from P. kingianum (0.021–0.023). In addition, P. sibiricum showed moderate GDs with P. odoratum (0.004), P. filipes (0.004), P. curvistylum (0.010), P. cirrhifolium (0.010), P. prattii (0.010), P. franchetii (0.008), and D. longifolia (0.006). The GD between P. cyrtonema and P. kingianum was 0.019, while the GDs varied for P. cyrtonema with P. odoratum (0.002), P. filipes (0.002), P. curvistylum (0.008), P. cirrhifolium (0.008), P. prattii (0.008), P. franchetii (0.006), and D. longifolia (0.004). Among the Polygonatum species, P. kingianum had the farthest GD with P. odoratum (0.021), followed by P. filipes (0.021), P. curvistylum (0.015), P. cirrhifolium (0.015), P. prattii (0.015), P. franchetii (0.013), and D. longifolia (0.015). Therefore, P. kingianum has a closer relationship with P. cyrtonema than with P. sibiricum. According to the nearest distances method, we verified that sample sequences from 18 regions are classified as P. cyrtonema; those from nine regions, as P. kingianum; those from seven regions, as P. sibiricum; and that from one region, as D. longifolia. Among these, the sample sequences from S30 had equal GDs with P. cyrtonema and D. longifolia, while sample sequences from S8 and S10 had equal GDs with P. curvistylum, P. cirrhifolium, and P. prattii. Sequences from the S9 region also have the same GD with P. curvistylum and P. cirrhifolium. Therefore, this analysis using the nearest distances method supports our results from the BLAST analysis, with the exception of sample sequences from the S30 region.
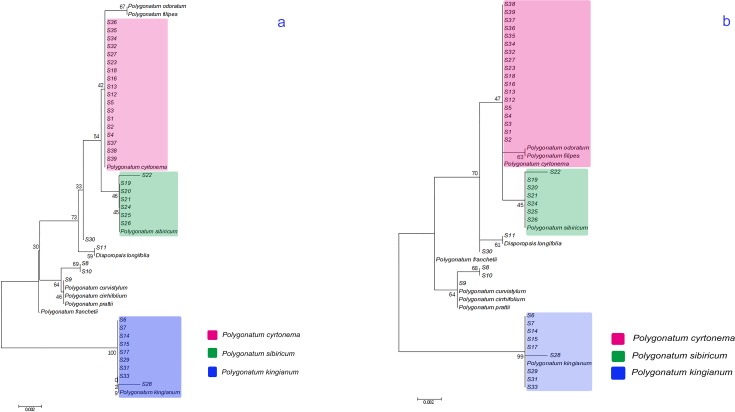
To visualize these results, an NJ tree was generated with the GDs using MEGA 6.06 (Fig 2A). All regions were divided into two clusters with a support rate of 100%. One cluster contained nine regions belonging to P. kingianum, while another cluster contained the remaining regions. To further verify this NJ tree, an ML tree was also generated (Fig 2B). In this tree, all the sampled regions were clustered into two clusters with a 99% support rate. Notably, the ML tree revealed more accurate genetic relationships compared to the NJ tree.
Fig 2.
Neighbour-joining tree (a) and maximum likelihood tree (b) constructed based on psbA-trnH sequences.
Phylogeny-based tests
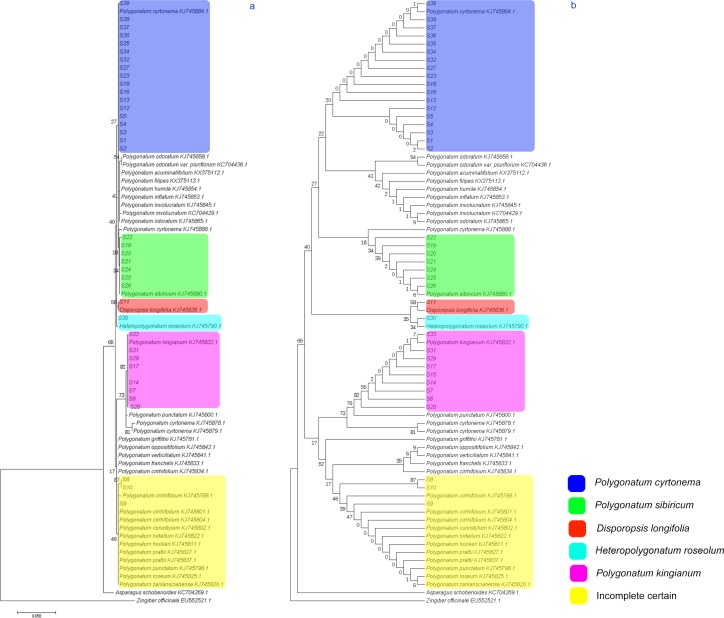
A total of 207 psbA-trnH sequences from Polygonatum were downloaded from NCBI’s GenBank. After identical sequences deposited by different researchers were removed using ClustalW, 32 sequences were selected (S1 Table). Through data collection and analysis, we found that the reference sequences of psbA-trnH from the same species were not the same in P. cyrtonema and P. odoratum. This phenomenon may be related to the different regions of origin. In our sampled sequences, 32 reference sequences and four outgroup sequences, including D. longifolia (GenBank No.: KJ745836.1), Heteropolygonatum (GenBank No.: KJ745790.1), Asparagus (GenBank No.: KC704269.1), and Zingiberaceae (GenBank No.: EU552521.1), were used to construct the ML tree.
The constructed ML phylogeny tree (Fig 3A) indicates that our sample sequences can be classified into six groups, which supports our species identification with the DNA barcoding-based method. In this tree, psbA-trnH sequences of some species do not appear to be unique, such as those for P. cyrtonema (4), P. odoratum (2), P. cirrhifolium (4), and P. prattii (2). The reason for this might stem from similar sequences being uploaded by different researchers at different institutions. This would result in the sequences being slightly different (individual base changes) because of the plants’ different growth environments or small interspecific divergences at the psbA-trnH region. By the ML tree method, the samples identified as P. cyrtonema showed a closer evolutionary relationship with P. cyrtonema, having a 19% support rate, compared with the DNA barcoding-based method. Samples identified as P. sibiricum appeared to have a close evolutionary relationship with both P. sibiricum and P. cyrtonema, with support rates of 26% and 14%, respectively. Furthermore, the ML tree showed 82% and 79% support rates for evolutionary relationships among the samples identified as P. kingianum, P. punctatum, P. cyrtonema, and P. cyrtonema. The topology of the ML tree (Fig 3B) also supports the conclusions drawn from our phylogeny tree.
Fig 3.
Phylogeny tree (a) and its topology (b) of maximum likelihood tree constructed based on psbA-trnH sequences.
Finally, to test the authenticity and accuracy of our ML tree, an NJ tree was constructed. The NJ tree (S3A Fig) and its topology (S3B Fig) showed the same results as those of the ML tree. Thus, the authenticity and accuracy of the ML tree constructed based on the psbA-trnH sequences is supported by conventional phylogeny-based identification methods, indicating that DNA barcoding using psbA-trnH sequences can be used to identify PR species.
Character-based tests
A total of 14 variable positions at sites 22, 27, 65, 67, 103, 104,125, 127, 128, 129, 130, 132, 172, and 429 psbA-trnH sequences (S2 Table) were used to identify Polygonatum species. The combination of these 14 sites formed 11 compound CAs used to identify 22 species distributed in the sampling areas. Apart from P. cyrtonema (3), P. cirrhifolium (2), P. odoratum (2), P. punctatum (2), and P. involucratum (2), which had multiple corresponding CAs, the other 17 species were each associated with a single CA for species identification. Among these, only seven CAs were specific solely to their own species. P. kingianum (B17) and P. sibiricum (B26) had specific CAs, whereas three CAs (B5, B6, and B7) were found for P. cyrtonema. A single CA (B15) was observed for P. involucratum, while another (B24) was found for P. punctatum. Furthermore, two nonspecific CAs (B16 and B23) were also observed for P. involucratum and P. punctatum, but this association requires further confirmation.
These constructed CAs were then used to verify the identity of our 39 experimental samples (Tables 3 and 4). We found that our samples were differentiated in a manner that is similar to that observed using the nearest distance method corresponding to the “best close match.” Notably, the S11 and S22 CAs were not retrieved in this CA analysis, whereas they were identified as D. longifolia and P. cyrtonema, respectively, by the nearest distance method and phylogeny-based analysis. Thus, while we can precisely differentiate PR from other Polygonatum species and genera with this character-based method, discrepancies among the identification methods used in this study do exist.
Discussion
Consumers purchase and consume PR based on its rhizome composition, which is largely identified on the basis of morphological characteristics. However, the rhizomes of other Polygonatum species and Asparagaceae genera are similar to those constituting PR, making them difficult to differentiate and easy to substitute into this herbal formulation. This can affect the biological effectiveness and safety of PR. Unfortunately, an efficient and accurate identification method has yet to be established for these types of medicinal rhizome formulations. In this study, we used three different methodologies to identify PR. Notably, all three enabled accurate identification of the PR components. Our results are the first to report an efficient approach, combining the three methods, for the characterization of PR, which can be used to discern the identity of PR components in formulations in the market.
DNA barcoding-based identification of samples from different regions
DNA barcoding is an efficient and accurate method for true product identification that is not affected by the condition of the sample material. Barcoding gap, quantified as the difference between intraspecific and the smallest interspecific distance, has been used to evaluate DNA barcoding [49,50] and define new species [51], studies reported earlier showed that it is an artifact of insufficient sampling across taxa [52] and no distinct or sufficiently sized global barcoding gap exists [53]. Thus, it is useless and unworthiness for PR identification at species level due to inexistence of barcoding gap in PR samples in our study. This may because the number of sequences per species is small, and the study reported earlier supports this result [54]. ITS2 and psbA-trnH as recommended DNA barcoding genes have been used to identify plants at the species level based on their high resolution [55] and fast evolutionary rate [56]. The ITS2 sequence has been considered an ideal DNA barcoding sequence for species identification of fungi and higher plants [57], which revealed a 92.7% of resolution success rate at the species level [44, 58]. Among genes used for DNA barcoding in plants, rpoB, rpoC1, matK, trnH-psbA, rbcL, ITS, accD, nhdJ, YCF5, UPA, atpF-atpH, and psbK-psbI, psbA-trnH have demonstrated the best amplification success rates and species identification rates [59, 60]. However, the success rate of ITS2 amplification is comparatively lower, and the sequencing of cITS2 sequences is a little difficult [55], thereby limiting its application. In addition, a large number of insertions/deletions in the psbA-trnH sequence makes BLAST searches among species in different genera challenging. In this study, ITS2 sequences amplified from samples using universal primers yielded polymorphic DNA bands, not as psbA-trnH. This fact may because universal primer of ITS2 is not specific for PR and Polygonatum species. Study reported by Li [61] showed that ITS2 regions was very low due to failure in PCR amplification for Taxillus chinensis, and this fact could support our result. As a matter of course, the reasons need further investigation. Although ITS2 sequences were unsuitable for PR identification, the higher resolving power and accurate discrimination of PR obtained using the psbA-trnH sequence in this study indicates that this DNA barcoding system can be used to differentiate PR from other Polygonatum species and genera. Our results reflect similar findings reported earlier [62]. According to this method, our 39 samples were divided into five groups: P. sibiricum (7), P. cyrtonema (19), P. kingianum (9), D. longifolia (1), and undetermined (3). Notably, the undetermined groups could not be identified with the same GDs, and the “best close match” was observed for multiple species. This could be due to the low level of variation in the psbA-trnH sequence among these species. However, it is clear that the identity of the P. punctatum samples corresponded to their geographical origin.
Phylogeny-based tests of psbA-trnH sequences
Phylogenetic tree construction can reveal interrelations among different species and can be used to judge the relationships between sample sequences and reference sequences based on their psbA-trnH sequence. These relationships can then be used to accurately identify the samples. Among the multiple phylogenetic trees constructed, the ML tree was considered to be the tree closest to the true tree for our samples. In fact, the ML tree based on the psbA-trnH sequences of our samples reflected the results based on the DNA barcoding system. In the ML tree, S30 was identified as P. cyrtonema and had a close relationship with Heteropolygonatum roseolum. Because three psbA-trnH sequences were downloaded from NCBI’s GenBank for P. cyrtonema, the samples identified as P. sibiricum were observed to have a close relationship with P. cyrtonema (KJ745888.1). This may again be due to the low level of variation in the psbA-trnH sequences of these two species. S8, S9, and S10 were also incompletely identified owing to their close relationships with numerous species. This indicates that the psbA-trnH sequences had a low identification efficiency for these species. The topology of the ML tree confirmed these results. Moreover, the NJ tree constructed in this study and its topology were also used to verify the reliability of the ML tree and the accuracy of our results. Our phylogeny-based tests revealed that DNA barcoding identification is an accurate method and can also be used to distinguish PR from adulterants or imitations.
Character-based tests of psbA-trnH sequences
Character-based tests showed the same results as the DNA barcoding system and phylogeny-based methods. In this study, P. cyrtonema, P. cirrhifolium, P. odoratum, P. punctatum, and P. involucratum all had more than one CA, which could lead to mistakes in identifying PR. However, three CAs of P. cyrtonema were specific and could be used for identification. Thus, character-based tests of the psbA-trnH sequences can be further used to distinguish PR. Similar findings reported in other species [63] support our results. Compared with DNA barcoding-based and phylogeny-based methods, a character-based method has advantages for identifying species with lower variation in DNA barcoding. DNA barcoding-based and phylogeny-based methods are the main and universal methods, while phylogeny-based methods can identify components at not only the genus and family level but also the species level. Thus, combining all three methods would render our results more accurate.
Application of DNA barcoding for the identification of PR
DNA barcoding has been used for identifying medicinal plants [64] and industrial quality assurance [65], such as for Smithia conferta Sm. [66], turmeric [67], Crocus sativus [68, 69], Peucedanum praeruptorum [70], radix astragali [71, 72], Cinnamomum verum [73], Sabia parviflora [74], Valeriana jatamansi [75], sandalwood [76], and Hippophae [30], which supports our findings. To date, it has been difficult to completely authenticate PR and its related products without relying on morphological characterization. Furthermore, the authenticity of raw materials is essential to guarantee product quality and consumer safety. DNA barcoding can efficiently and accurately identify products [23] regardless of their form. Thus, the method used in this study has immediate practical implications and can be quickly applied to molecularly identify PR.
However, for all Polygonatum species, DNA barcoding based on psbA-trnH sequences is limited due to lower genetic diversity, which might make inaccurate identification. Identification with more DNA barcodes or complete chloroplast genome and whole genome sequences would provide an effective method for Polygonatum species authentication. In addition, morphological characteristics of medicinal herbs plants can also be used to correctly classify species when they does not vary under different growing environment. Thus, more researches are needed to optimize and improve the method to molecularly identify Polygonatum species.
Conclusions
A total of five species were identified in the 39 samples we analyzed from different growing regions: P. sibiricum, P. cyrtonema, P. kingianum, D. longifolia, and P. punctatum. Samples collected from four regions, S8−S11, were misidentified based on the morphological characteristics of their rhizomes. Our study indicates that this DNA barcoding identification method based on psbA-trnH sequences can efficiently and precisely differentiate PR from other species with the same rhizome characteristics. With this technology, PR quality can be preserved and improved for consumer consumption.
Supporting information
Gel electrophoresis images of PCR products of ITS2 (a) and psbA-trnH (b).
(TIF)
(TIF)
Phylogeny tree (a) and its topology (b) of neighbour-joining tree constructed based on psbA-trnH sequences in Polygonatum and outgroup.
(TIF)
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Huimin Plan of Ministry of Science and Technology [grant numbers 2012GS610102] and the Support Plan Project of the state Ministry of Science & Technology [grant number 2015BAC01B03]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wujisguleng WJGL, Liu YJ, Long CL. Ethnobotanical review of food uses of Polygonatum (Convallariaceae) in China. Acta Soc Bot Pol. 2012;81(4):239–44. 10.5586/asbp.2012.045 [DOI] [Google Scholar]
- 2.Jin JN. Verificine of polygonati rhizoma in connection with Buddhism and Taoism. National botany BBS in asia-pacific region; Nanjing2006. p. 323–9.
- 3.Chinese PC. Pharmacopoeia of China. Beijing: China Medical Science Press; 2015. 306–7 p. [Google Scholar]
- 4.Jiang QG, Lv YX, Dai WD, Miao XY, Zhong DW. Extraction and bioactivity of Polygonatum polysaccharides. Int J Biol Macromol. 2013;54:131–5. 10.1016/j.ijbiomac.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Ma K, Huang XF, Kong LY. Steroidal saponins from Polygonatum cyrtonema. Chem Nat Compd+. 2013;49(5):888–91. 10.1007/s10600-013-0770-2 [DOI] [Google Scholar]
- 6.Lan GS, Chen HX, Chen SH, Tian JG. Chemical composition and physicochemical properties of dietary fiber from Polygonatum odoratum as affected by different processing methods. Food Res Int. 2012;49(1):406–10. 10.1016/j.foodres.2012.07.047 [DOI] [Google Scholar]
- 7.Gvazava LN, Kikoladze VS. Flavonoids from the plants Polygonatum polyanthemum and P. glaberrimum. Chem Nat Compd+. 2011;47(5):818–9. 10.1007/s10600-011-0072-5 [DOI] [Google Scholar]
- 8.Li S. Compendium of Materia Medica. Shanghai: Shanghai science and technology press; 1993. [Google Scholar]
- 9.Zhao XY, Li J. Chemical constituents of the genus Polygonatum and their role in medicinal treatment. Nat Prod Commun. 2015;10(4):683–8. [PubMed] [Google Scholar]
- 10.Du L, Nong MN, Zhao JM, Peng XM, Zong SH, Zeng GF. Polygonatum sibiricum polysaccharide inhibits osteoporosis by promoting osteoblast formation and blocking osteoclastogenesis through Wnt/beta-catenin signalling pathway. Sci Rep-Uk. 2016;6 Artn 32261 10.1038/Srep32261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo S, Liu C, Liu S, Guan X, Guo L, Jia F, et al. Streptomyces polygonati sp. nov., an endophytic actinomycete isolated from a root of Polygonatum odoratum (Mill.). International journal of systematic and evolutionary microbiology. 2016. 10.1099/ijsem.0.000906 [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Lu CS, Liu DY, Xu YT, Zhu Y, Wu HH. Constituents from Polygonatum sibiricum and their inhibitions on the formation of advanced glycosylation end products. J Asian Nat Prod Res. 2016;18(7):697–704. 10.1080/10286020.2015.1135905 [DOI] [PubMed] [Google Scholar]
- 13.Lu JM, Wang YF, Yan HL, Lin P, Gu W, Yu J. Antidiabetic effect of total saponins from Polygonatum kingianum in streptozotocin-induced daibetic rats. J Ethnopharmacol. 2016;179:291–300. 10.1016/j.jep.2015.12.057 [DOI] [PubMed] [Google Scholar]
- 14.Yan HL, Lu JM, Wang YF, Gu W, Yang XX, Yu J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine. 2017;26:45–54. 10.1016/j.phymed.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 15.Khan H, Saeed M, Muhammad N, Perviz S. Phytochemical analysis, antibacterial, and antifungal assessment of aerial parts of Polygonatum verticillatum. Toxicol Ind Health. 2016;32(5):841–7. 10.1177/0748233713512362 [DOI] [PubMed] [Google Scholar]
- 16.Tai Y, Sun YM, Zou X, Pan Q, Lan YD, Huo Q, et al. Effect of Polygonatum odoratum extract on human breast cancer MDA-MB-231 cell proliferation and apoptosis. Exp Ther Med. 2016;12(4):2681–7. 10.3892/etm.2016.3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng MJ, Zhang YP, Shi SY. Separation of polar antioxidants from Rhizoma Polygonatum Odorati by high-speed counter-current chromatography with a hydrophilic solvent system. J Liq Chromatogr R T. 2016;39(4):171–7. 10.1080/10826076.2016.1141298 [DOI] [Google Scholar]
- 18.Rhie YH, Lee SY, Park JH, Kim KS. Scarification and gibberellic acid affecting to dormancy breaking of Variegated Solomon's Seal (Polygonatum odoratum var. pluriflorum 'Variegatum'). Korean J Hortic Sci. 2014;32(3):296–302. 10.7235/hort.2014.13146 [DOI] [Google Scholar]
- 19.Takagi H. Breaking of two types of dormancy in seeds of edible Polygonatum macranthum. J Jpn Soc Hortic Sci. 2001;70(4):424–30. [Google Scholar]
- 20.Hebert PDN, Cywinska A, Ball SL, DeWaard JR. Biological identifications through DNA barcodes. P Roy Soc B-Biol Sci. 2003;270(1512):313–21. 10.1098/rspb.2002.2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu MNA, Heidmarsson S, Thorsteinsdottir M, Eiriksson FF, Omarsdottir S, Olafsdottir ES. DNA barcoding and LC-MS metabolite profiling of the lichen-forming genus Melanelia: Specimen identification and discrimination focusing on Icelandic taxa. Plos One. 2017;12(5). doi: ARTN e0178012 10.1371/journal.pone.0178012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberlies N. How do you know that material is what it says it is? DNA barcoding for the taxonomic identification of fungi. Toxicol Lett. 2017;280:S39–S40. [Google Scholar]
- 23.Raja HA, Baker TR, Little JG, Oberlies NH. DNA barcoding for identification of consumer-relevant mushrooms: A partial solution for product certification? Food Chem. 2017;214:383–92. 10.1016/j.foodchem.2016.07.052 [DOI] [PubMed] [Google Scholar]
- 24.Tang YL, Wu YS, Huang RS, Chao NX, Liu Y, Xu P, et al. Molecular identification of Uncaria (Gouteng) through DNA barcoding. Chin Med-Uk. 2016;11. doi: ARTN 3 10.1186/s13020-015-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Liu K, Zhou L, Zhao L, Liu SQ. Testing three proposed DNA barcodes for the wood identification of Dalbergia odorifera T. Chen and Dalbergia tonkinensis Prain. Holzforschung. 2016;70(2):127–36. 10.1515/hf-2014-0234 [DOI] [Google Scholar]
- 26.Nithaniyal S, Parani M. Evaluation of chloroplast and nuclear DNA barcodes for species identification in Terminalia L. Biochem Syst Ecol. 2016;68:223–9. [Google Scholar]
- 27.Han YW, Duan D, Ma XF, Jia Y, Liu ZL, Zhao GF, et al. Efficient identification of the forest tree species in Aceraceae using DNA barcodes. Front Plant Sci. 2016;7. doi: ARTN 1707 10.3389/fpls.2016.01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enan MR, Ahmed A. Cultivar-level phylogeny using chloroplast DNA barcode psbK-psbI spacers for identification of Emirati date palm (Phoenix dactylifera L.) varieties. Genet Mol Res. 2016;15(3). doi: ARTN 15038470 10.4238/gmr.15038470. [DOI] [PubMed] [Google Scholar]
- 29.Mahadani P, Sharma GD, Ghosh SK. Identification of ethnomedicinal plants (Rauvolfioideae: Apocynaceae) through DNA barcoding from northeast India. Pharmacogn Mag. 2013;9(35):255–63. 10.4103/0973-1296.113284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Xiang L, Zhang Y, Lai XR, Xiong C, Li JJ, et al. DNA barcoding based identification of Hippophae species and authentication of commercial products by high resolution melting analysis. Food Chem. 2018;242:62–7. 10.1016/j.foodchem.2017.09.040 [DOI] [PubMed] [Google Scholar]
- 31.Zhang DQ, Mo XC, Xiang JY, Zhou N. Molecular identification of original plants of fritillariae cirrhosae bulbus, a tradtional Chinese medicine (Tcm) using plant DNA barcoding. Afr J Tradit Complem. 2016;13(6):74–82. 10.21010/ajtcam.v13i6.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv TW, Teng RD, Shao QS, Wang HZ, Zhang WS, Li MY, et al. DNA barcodes for the identification of Anoectochilus roxburghii and its adulterants. Planta. 2015;242(5):1167–74. 10.1007/s00425-015-2353-x [DOI] [PubMed] [Google Scholar]
- 33.Moon BC, Kim WJ, Ji Y, Lee YM, Kang YM, Choi G. Molecular identification of the traditional herbal medicines, Arisaematis Rhizoma and Pinelliae Tuber, and common adulterants via universal DNA barcode sequences. Genet Mol Res. 2016;15(1). UNSP gmr.15017064 10.4238/gmr.15017064. [DOI] [PubMed] [Google Scholar]
- 34.Kim WJ, Ji Y, Choi G, Kang YM, Yang S, Moon BC. Molecular identification and phylogenetic analysis of important medicinal plant species in genus Paeonia based on rDNA-ITS, matK, and rbcL DNA barcode sequences. Genet Mol Res. 2016;15(3). [DOI] [PubMed] [Google Scholar]
- 35.Hirsch AM, Moraes DC. Identification of the south American medicinal plant Baccharis genistelloides ("carqueja") using DNA barcodes. Abstr Pap Am Chem S. 2014;248. [Google Scholar]
- 36.Chen XC, Xiang L, Shi LC, Li G, Yao H, Han JP, et al. Identification of crude drugs in the Japanese pharmacopoeia using a DNA barcoding system. Sci Rep-Uk. 2017;7 ARTN 42325 10.1038/srep42325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song XN, Li YP, Xu GJ, Liu CS, Liu Y, Zhang XQ, et al. Identification of Notoginseng powder based on similarity to "DNA Barcoding Core-genotype''. Mitochondrial DNA A. 2017;28(3):355–7. 10.3109/19401736.2015.1122777 [DOI] [PubMed] [Google Scholar]
- 38.Song M, Dong GQ, Zhang YQ, Liu X, Sun W. Identification of processed Chinese medicinal materials using DNA mini-barcoding. Chin J Nat Medicines. 2017;15(7):481–6. 10.1016/S1875-5364(17)30073-0 [DOI] [PubMed] [Google Scholar]
- 39.Nithaniyal S, Vassou SL, Poovitha S, Raju B, Parani M. Identification of species adulteration in traded medicinal plant raw drugs using DNA barcoding. Genome. 2017;60(2):139–46. 10.1139/gen-2015-0225 [DOI] [PubMed] [Google Scholar]
- 40.Yao H, Song JY, Ma XY, Liu C, Li Y, Xu HX, et al. Identification of Dendrobium species by a candidate DNA barcode sequence: The chloroplast psbA-trnH intergenic region. Planta Med. 2009;75(6):667–9. 10.1055/s-0029-1185385 [DOI] [PubMed] [Google Scholar]
- 41.Song JY, Yao H, Li Y, Li XW, Lin YL, Liu C, et al. Authentication of the family Polygonaceae in Chinese pharmacopoeia by DNA barcoding technique. J Ethnopharmacol. 2009;124(3):434–9. 10.1016/j.jep.2009.05.042 [DOI] [PubMed] [Google Scholar]
- 42.Pang XH, Chen SL. Using DNA barcodes to identify Rosaceae. Planta Med. 2009;75(4):417–. [Google Scholar]
- 43.Luo K, Chen SL, Chen KL, Song JY, Yao H. Application of DNA barcoding to the medicinal plants of the Araceae family. Planta Med. 2009;75(4):416–. [Google Scholar]
- 44.Gao T, Chen SL. Authentication of the medicinal plants in Fabaceae by DNA barcoding technique. Planta Med. 2009;75(4):417–. [Google Scholar]
- 45.He LH, Zhao Y, Chen MJ, Pan YJ. An efficient method for DNA extraction from compost. Acta microbiologica Sinica. 2006;46(1):162–5. [PubMed] [Google Scholar]
- 46.Ross HA, Murugan S, Li WLS. Testing the reliability of genetic methods of species identification via simulation. Systematic Biol. 2008;57(2):216–30. [DOI] [PubMed] [Google Scholar]
- 47.Lowenstein JH, Amato G, Kolokotronis SO. The real maccoyii: identifying tuna sushi with DNA barcodes—contrasting characteristic attributes and genetic distances. PloS one. 2009;4(11):e7866 10.1371/journal.pone.0007866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier R, Zhang G, Ali F. The use of mean instead of smallest interspecific distances exaggerates the size of the “barcoding gap” and leads to misidentification. Systematic Biol. 2008;57(5):809–13. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Rannala B. Bayesian species identification under the multispecies coalescent provides significant improvements to DNA barcoding analyses. Mol Ecol. 2017;26(11):3028–36. 10.1111/mec.14093 [DOI] [PubMed] [Google Scholar]
- 50.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012;21(8):1864–77. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- 51.Hebert PD, Stoeckle MY, Zemlak TS, Francis CM. Identification of birds through DNA barcodes. Plos Biology. 2004;2(10):e312 10.1371/journal.pbio.0020312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiemers M, Fiedler K. Does the DNA barcoding gap exist?–a case study in blue butterflies (Lepidoptera: Lycaenidae). Front Zool. 2007;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kvist S. Does a global DNA barcoding gap exist in Annelida? Mitochondrial DNA A. 2016;27(3):2241–52. 10.3109/19401736.2014.984166 [DOI] [PubMed] [Google Scholar]
- 54.Chen J, Zhao J, Erickson DL, Xia N, Kress WJ. Testing DNA barcodes in closely related species of Curcuma (Zingiberaceae) from Myanmar and China. Mol Ecol Resour. 2015;15(2):337–48. 10.1111/1755-0998.12319 [DOI] [PubMed] [Google Scholar]
- 55.Ren BQ, Xiang XG, Chen ZD. Species identification of Alnus (Betulaceae) using nrDNA and cpDNA genetic markers. Mol Ecol Resour. 2010;10(4):594–605. 10.1111/j.1755-0998.2009.02815.x [DOI] [PubMed] [Google Scholar]
- 56.Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, et al. The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am J Bot. 2005;92(1):142–66. 10.3732/ajb.92.1.142 [DOI] [PubMed] [Google Scholar]
- 57.Yao H, Song JY, Liu C, Luo K, Han JP, Li Y, et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PloS one. 2010;5(10):-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S, Yao H, Han J, Liu C, Song J, Shi L, et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. Plos One. 2010;5(1):e8613 10.1371/journal.pone.0008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. Plos One. 2007;2(6):e508 10.1371/journal.pone.0000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fazekas AJ, Burgess KS, Kesanakurti PR, Graham SW, Newmaster SG, Husband BC, et al. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. Plos One. 2008;3(7):e2802 10.1371/journal.pone.0002802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li YH, Jinlan R, Chen SL, Song JY, Luo K, Dong L, et al. Authentication of Taxillus chinensis using DNA barcoding technique. J Med Plants Res. 2010;4(24):2706–9. [Google Scholar]
- 62.Yang P, Zhou H, Xin T, Ma S, Duan B, Yao H. Identification study of DNA barcode sequences in the medicinal plants of Polygonatum. World Chinese Medicine. 2015;10(8):1173–6. [Google Scholar]
- 63.Zou SM, Li Q. Pay attention to the overlooked cryptic diversity in existing barcoding data: the case of Mollusca with character-based DNA barcoding. Mar Biotechnol. 2016;18(3):327–35. 10.1007/s10126-016-9692-x [DOI] [PubMed] [Google Scholar]
- 64.Gao ZT, Liu Y, Wang XY, Song JY, Chen SL, Ragupathy S, et al. Derivative technology of DNA barcoding (nucleotide signature and SNP double peak methods) detects adulterants and substitution in Chinese patent medicines. Sci Rep-Uk. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sgamma T, Lockie-Williams C, Kreuzer M, Williams S, Scheyhing U, Koch E, et al. DNA barcoding for industrial quality assurance. Planta Med. 2017;83(14–15):1117–29. 10.1055/s-0043-113448 [DOI] [PubMed] [Google Scholar]
- 66.Umdale SD, Kshirsagar PR, Lekhak MM, Gaikwad NB. Molecular authentication of the traditional medicinal plant "Lakshman Booti" (Smithia conferta Sm.) and its adulterants through DNA barcoding. Pharmacogn Mag. 2017;13(50):S224–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parvathy VA, Swetha VP, Sheeja TE, Sasikumar B. Detection of plant-based adulterants in turmeric powder using DNA barcoding. Pharm Biol. 2015;53(12):1774–9. 10.3109/13880209.2015.1005756 [DOI] [PubMed] [Google Scholar]
- 68.Huang WJ, Li FF, Liu YJ, Long CL. Identification of Crocus sativus and its adulterants from Chinese markets by using DNA barcoding technique. Iran J Biotechnol. 2015;13(1):36–42. ARTN e1034 10.15171/ijb.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang W, Li F, Liu Y, Long C. Identification of Crocus sativus (Iridaceae) and its adulterants by using DNA barcoding technique. Planta Med. 2014;80(10):846–. [Google Scholar]
- 70.Zhou J, Wang WC, Liu MQ, Liu ZW. Molecular authentication of the traditional medicinal plant Peucedanum praeruptorum and its substitutes and adulterants by DNA—barcoding technique. Pharmacogn Mag. 2014;10(40):385–90. 10.4103/0973-1296.141754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng SH, Liu DW, Ren WG, Fu J, Huang LF, Chen SL. Integrated analysis for identifying Radix Astragali and its adulterants based on DNA barcoding. Evid-Based Compl Alt. 2014. Artn 843923 10.1099/ijsem.0.000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo HY, Wang WW, Yang N, Guo BL, Zhang S, Yang RJ, et al. DNA barcoding provides distinction between Radix Astragali and its adulterants. Sci China Life Sci. 2010;53(8):992–9. 10.1007/s11427-010-4044-y [DOI] [PubMed] [Google Scholar]
- 73.Swetha VP, Parvathy VA, Sheeja TE, Sasikumar B. DNA barcoding for discriminating the economically important Cinnamomum verum from its Adulterants. Food Biotechnol. 2014; 28(3):183–94. 10.1080/08905436.2014.931239 [DOI] [Google Scholar]
- 74.Sui XY, Huang YA, Tan Y, Guo Y, Long CL. Molecular authentication of the ethnomedicinal plant Sabia parviflora and its adulterants by DNA barcoding technique. Planta Med. 2011;77(5):492–6. 10.1055/s-0030-1250468 [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, Zhai YH, Liu T, Zhang FM, Ji YH. Detection of Valeriana jatamansi as an adulterant of medicinal Paris by length variation of chloroplast psbA-trnH region. Planta Med. 2011;77(1):87–91. 10.1055/s-0030-1250072 [DOI] [PubMed] [Google Scholar]
- 76.Dev SA, Muralidharan EM, Sujanapal P, Balasundaran M. Identification of market adulterants in east Indian sandalwood using DNA barcoding. Ann Forest Sci. 2014;71(4):517–22. 10.1007/s13595-013-0354-0 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gel electrophoresis images of PCR products of ITS2 (a) and psbA-trnH (b).
(TIF)
(TIF)
Phylogeny tree (a) and its topology (b) of neighbour-joining tree constructed based on psbA-trnH sequences in Polygonatum and outgroup.
(TIF)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.