Abstract
This systematic review classifies smartwatch-based healthcare applications in the literature according to their application and summarizes what has led to feasible systems. To this end, we conducted a systematic review of peer-reviewed smartwatch studies related to health care by searching PubMed, EBSCOHost, Springer, Elsevier, Pro-Quest, IEEE Xplore, and ACM Digital Library databases to find articles between 1998 and 2016. Inclusion criteria were as follows: (1) a smartwatch was used, (2) the study was related to a healthcare application, (3) the study was a randomized controlled trial or pilot study, and (4) the study included human participant testing. Each article was evaluated in terms of its application, population type, setting, study size, study type, and features relevant to the smartwatch technology. After screening 1119 articles, 27 articles were chosen that were directly related to health care. Classified applications included activity monitoring, chronic disease self-management, nursing or home-based care, and healthcare education. All studies were considered feasibility or usability studies, and had limited sample sizes. No randomized clinical trials were found. Also, most studies utilized Android-based smartwatches over Tizen, custom-built, or iOS-based smartwatches, and many relied on the use of the accelerometer and inertial sensors to elucidate physical activities. The results show that most research on smartwatches has been conducted only as feasibility studies for chronic disease self-management. Specifically, these applications targeted various disease conditions whose symptoms can easily be measured by inertial sensors, such as seizures or gait disturbances. In conclusion, although smartwatches show promise in health care, significant research on much larger populations is necessary to determine their acceptability and effectiveness in these applications.
Keywords: Smartwatch, Health care, Remote health monitoring, Mobile health (mHealth), Systematic review
Background
Recent advances in smartwatches have led to several applications in remote health monitoring and mobile health (mHealth) [1]. The smartwatch is a new technology that combines features of smartphones with continuous data monitoring that promote health, such as step-tracking, heart rate monitoring, energy expenditure, and physical activity levels [2]. They can provide feedback to users that allow them to monitor their health, perform just-in-time interventions such as medication use based on symptoms, and direct communication with caregivers and physicians [3]. However, widespread adoption of the technology in health care and telemedicine is limited by barriers specific to smartwatches, such as cost, wearability, and battery life [4]. Thus, the following review examines smartwatch use in healthcare applications and the appropriate features that have led to feasible smartwatch-based remote health monitoring applications.
Health care and telemedicine have recently relied on the use of smartphones to enable remote health monitoring of patients in the community [5–12]. Examples of effective smartphone-based healthcare applications include those for the self-management of long-term illnesses [5], smoking cessation [6], family planning/contraception [7], and psychological therapies [8], among other clinical research applications [9–12]. Smartphones allow for continuous interactive communication from any location, computing power to support multimedia software applications, and continuous monitoring of individuals through wireless sensing technologies [12]. However, smartphones cannot be worn to provide certain continuous sensing information such as heart rate and are not always carried during behaviors of interest, such as during high levels of activity [13].
Besides the ability to wear smartwatches to collect continuous sensing data such as heart rate and activity, smartwatches have many other practical features that make them ideal platforms for healthcare applications [14]. First, unlike smartphones, smartwatches are ubiquitous in that they are typically worn even when at home and during nighttime. Also, similar to smartphones, smartwatches are able to combine sensor information such as accelerometers, gyroscopes, compasses, and heart rate, with global positioning satellite (GPS) data. This is particularly promising in applications that require continuous physical activity monitoring to identify unexpected changes in activity patterns and propose alarms and help based on the given localized area. Alarms and messages can also be more easily observed than those sent to smartphones, as individuals can receive vibrations, text, and sounds while wearing the watch. Finally, there is unlimited development potential regarding the use of smartwatches in healthcare applications, and the modularity of software applications (apps) allows for personalization to each individual’s healthcare needs.
Methods
Rationale
Previous reviews on smartwatches [1, 3] have found that although there have been several research studies that involve the use of smartwatches, very few have been tested beyond feasibility. In this review, we assess whether any studies have tested beyond feasibility and usability, and which healthcare applications have utilized smartwatches in their interventions. Furthermore, unlike in Lu et al. [1], this review looks further into the technology utilized behind each study by examining the smartwatch sensors, type of smartwatch used, and the features that were most important for classification. We will also examine the current smartwatches on the market to see what other sensing mechanisms and features of the watches may be beneficial to healthcare applications and how they fit in current clinical trial study design, as their fit in current clinical trials and the design for clinical interventions was not expanded upon in Reeder et al. [3].
Objectives
Given the above rationale, this review seeks to answer the following research questions:
[RQ1] Assess whether any studies related to smartwatches in healthcare applications have been tested beyond feasibility and usability.
[RQ2] Identify the types of healthcare applications in which smartwatches have been utilized.
[RQ3] Identify the populations and test conditions of previous healthcare studies that utilized smartwatches.
[RQ4] Compare the sensing technologies, classification features, and smartwatch features of the current smartwatch-based healthcare systems and identify the smartwatch technologies and features currently available on the market for future research applications.
The above research questions will determine how smartwatches fit in current clinical trial design for various healthcare applications. They will also help determine which healthcare applications can benefit from smartwatches in their system design, and which smartwatch and sensing technologies are most appropriate to use. In order to answer these questions, a systematic review analysis described below was performed to assess the healthcare application types, details of the testing conducted, and the features of smartwatch technologies that were most important. To compare the resulting technology features from the chosen articles, a brief search using a web search engine (Google Search) was performed to find currently available smartwatches on the market and their technological features. This search was performed to help guide future researchers on smartwatch selection in future healthcare applications and is included alongside the results of the systematic review described below on smartwatch studies related to health care.
Eligibility Criteria
A peer-reviewed study was included if (1) a smartwatch was used, (2) the scope of the study was related to a healthcare application, (3) the study was a randomized controlled trial or pilot study that evaluated the delivery of an intervention using a smartwatch, and (4) the study included testing on any human participants. Studies that used a combination of smartwatches and smartphones to deliver the intervention or application were included. Excluded studies were those that (1) solely relied on mobile phones or smartphones and a smartwatch was not used; (2) did not provide any specific intervention, education, or care aimed at improving health; (3) were considered a review paper on previously reported studies, a case report, poster, abstract, or editorials; (4) did not include testing on human participants; or (5) were not related to a healthcare application.
We searched articles published between 1998 and 2016, as the first wristwatch computer was invented and patented in 1998, a Linux-based wristwatch developed by Steve Mann [15]. Studies that were published in a language other than English were included if they met the above inclusion criteria and translated using a machine translation service (Google translate). Outcomes of the review included healthcare application type, population type the system was tested on, experimental setting (laboratory or community), number of participants, and the type of study (e.g., randomized controlled trial, feasibility study, usability study). In addition, details about the smartwatch use in the study, namely the operating system used, type of smartwatch, most important sensors used and classification features, and connectivity type (e.g., Bluetooth to smartphone connection), were reported for each study.
Information Sources
In order to find eligible intervention smartwatch studies for health care, we conducted a literature search for the use of smartwatches in health care in the PubMed, EBSCOHost, Springer, Elsevier, ProQuest, IEEE Xplore, and ACM Digital Library databases from 1998 to 2016, when the literature search was conducted. The search in each database was limited to English and those with English translations. The keywords we searched were “smartwatch application,” “smartwatch app,” “smartwatch apps,” and “smartwatch health care.” Note that we performed the same searches with the keyword “smart watch” instead of “smartwatch” for all of the above terms in each database; however, this did not produce any new results and often misinterpreted the search as a “watch” or “smart,” resulting in irrelevant articles. Selected articles from the literature search were screened for potential eligible studies based on the above criteria. Those titles and abstracts who fulfilled the inclusion criteria were sent to two independent reviewers who checked and verified the relevant material. In addition, to ensure literature saturation, reference lists from selected studies were included in the search and analyzed using the inclusion criteria.
Search Strategy
Both qualitative and quantitative studies were included in the search, including case reports, review articles, posters, abstracts, book chapters, and editorials. However, the search was limited to those articles and findings that were peer-reviewed only. No study design or language limits were imposed, although only studies in languages other than English that could be translated using a machine translation service (Google translate) were included. A date range of 1998 to 2016 was used to restrict the search in each database from when smartwatches were first invented to when the literature review was performed. The search strategies were developed with input from both reviewers, and as relevant studies were identified, the reviewers checked for additional relevant cited and citing articles.
Study Records, Data Items, and Feature Extraction
All studies were extracted from the resulting publication search for each database and stored in a shared bibliography file (JabRef/BibTeX). The resulting studies were then analyzed by two independent reviewers. Specifically, they first underwent a screening process by reviewing the titles, followed by an abstract review and then a full-text review given the inclusion and exclusion criteria. If there was disagreement among the reviewers, these articles were discussed and the reasons for excluding studies were recorded. Neither of the review authors was blind to the journal titles or to the study authors or institutions. Furthermore, a systematic narrative synthesis was provided with information presented in the text to summarize and explain the characteristics and findings of the included studies.
To extract relevant features from the articles during the meta-analysis, each chosen article was reviewed in terms of its healthcare application type, population type, setting (clinic, laboratory, or community), number of participants, and study type (randomized controlled trial or pilot study). The healthcare application type was defined as the intended application type of the intervention and system described in the study, the population type included the disease type or healthy volunteers in which the system was tested on, and the setting and study type described the test conditions of the study. If the study was a randomized controlled trial, the study outcomes were also extracted. These features were extracted because they described the purpose and description of the smartwatch system in relation to the healthcare application.
Finally, features relevant to the smartwatch technology itself were also extracted for each study. These features included the operating system, type of smartwatch, battery type, connectivity type (e.g., WiFi, Bluetooth, and data communication locations), and its use in the health care-related study. In addition, we assessed the important sensors utilized for the intervention, as well as the important features for classification and use of the smartwatch sensors. These features determined which sensing mechanisms, data communication methods, and feedback types are used in smartwatch-based healthcare applications, as well as elucidated the desirable features of smartwatches.
Potential Biases
There were few potential biases associated with this review. First, since smartwatches have only begun to rise in popularity since 2010, studies prior to this date are unlikely. In addition, since the first iOS smartwatch, the Apple Watch (Apple Inc., Cupertino, CA) was not released until April 24, 2015 [16], there are likely to be more Android Wear and other operating systems (e.g., Tizen) used to develop smartwatch applications for health care. For pilot studies, it is likely that the sample sizes will be too small to assess anything further than feasibility and usability and thus will be subject to small sample bias. Finally, for randomized clinical trials, to determine whether reporting biases were present, we determined whether the protocol of the RCT was published before recruitment of patients of the study was started. The quality of evidence for all extracted features was judged using the Grading of Recommendations Assessment, Development and Evaluation working group methodology [17], and assessed across the domains biases.
Results
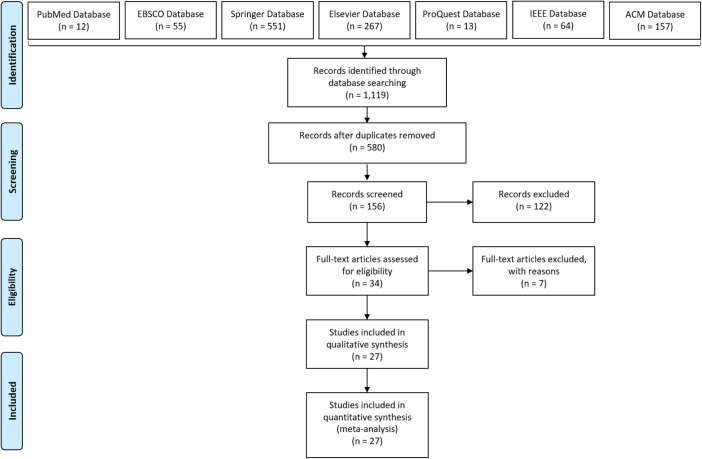
One thousand and one hundred nineteen articles were returned in the search (see Fig. 1 for a PRISMA process flow diagram of the search results). Five hundred and eighty unique articles remained after duplicates were removed. After reviewing the articles given the inclusion criteria above, 156 articles were selected for further review based on the above eligibility criteria. It was found during the screening process that out of the 122 articles that were excluded from further analysis, 21 articles were excluded because a smartwatch was not used, 50 articles were excluded because the scope of the study was not related to a healthcare application, 20 articles were excluded because they did not include testing on human participants, and 31 articles were excluded because they did not evaluate the delivery of an intervention. After performing an abstract review, 34 articles were chosen for full-text review, of which 27 were included in the final analysis. None of these articles were found to be sufficiently homogeneous in design to require a meta-analysis.
Fig. 1.
PRISMA process flow diagram of the literature search results
The seven articles that were removed from final review [18–24] were excluded because they used a hybrid fitness band and smartwatch, such as the Microsoft Band [18, 24], were unclear whether they tested using a smartwatch or used another wearable sensor [20, 21], or only discussed the integration of the system and did not clearly explain whether pilot data from human participants were obtained [22]. Furthermore, some systems did not specify which type of technology was utilized in the intervention [23] or allowed participants to use either a fitness band such as a Fit Bit or a smartwatch [24]. Fitness bands were excluded from review as they do not provide similar feedback and user interface features such as different available software apps as smartwatches.
The chosen articles were published in 2011 [25] (n = 1), 2014 [26–28] (n = 3), 2015 [29–41] (n = 13), and 2016 [42–51] (n = 10). Included articles were closely split between conferences [27, 28, 31–36, 38–42, 46, 47, 49–51] (n = 18) and journals [25, 26, 29, 30, 37, 43–45, 48] (n = 9) for publication venue. Not surprisingly, there were no articles that described a randomized clinical trial to assess safety and efficacy as none were prospective studies that compared the effect of an intervention against a control group [52]. In fact, only one study was defined as a prospective cohort study [45], while all other studies [25–44, 46–51] were pilot studies to assess feasibility and initial usability among a small number of participants.
Applications in Health care
Smartwatches have been tested in only a few aspects of healthcare research. Specifically, of the 27 articles reviews, most studies focused on using smartwatches for various types of activity monitoring [28, 30, 32, 33, 44, 47, 49] and chronic disease self-management applications [25–27, 29, 36, 37, 39–41, 43, 45, 46, 50, 51]. Other applications of smartwatch studies included those for nursing or home-based care monitoring [31, 35, 38, 42, 48] and healthcare education [34]. Note that activity monitoring included gait classification as well as classification of daily activities. In addition, chronic disease self-management studies were those that designed a smartwatch-based intervention to address a particular disease population. Some of these articles included sub-studies on health individuals that validated their activity recognition techniques if this variable was important in their intervention [36, 40, 43].
An overview of the smartwatch articles and their applications in health care is seen in Table 1. It can be seen from this table that several articles for chronic disease self-management applications focused on Parkinson’s disease [26, 27, 39, 40] or epilepsy [25, 45]. For the nursing or home-based care applications, the studies namely tested their systems on geriatrics [35] or those who suffer from dementia [31, 48]. In the activity classification studies, although most tested their systems on healthy individuals [30, 32, 33, 44, 47, 49], one study tested and directed towards applications for those with visual impairments [28]. Since majority of these studies were feasibility studies, it is not surprising that most studies across applications tested their algorithm and system design on healthy participants prior to future testing on targeted applications for health care. Furthermore, as seen in the table, these studies tested on an average of 11.52 ± 9.98 individuals, where the mode indicated that most studies tested their algorithm on only one participant.
Table 1.
Chosen studies and their features in relation to healthcare applications. Application type includes activity or gait monitoring, chronic disease self-management, nursing or home-based care, and healthcare education. Setting refers to testing the system in the laboratory, community, or hospital, while pilot study type refers to usability, feasibility, or prospective cohort study
| Article | Application | Population type tested | Setting | No. participants | Pilot study type |
|---|---|---|---|---|---|
| Lockman 2011 [25] | Chronic disease self-management | Epilepsy | Laboratory and community | 40 | Usability |
| Lopez 2014 [26] | Chronic disease self-management | Parkinson’s disease | Laboratory | 10 | Feasibility |
| Sharma 2014 [27] | Chronic disease self-management | Parkinson’s disease | Laboratory | 5 | Feasibility |
| Chippen-dale 2014 [28] | Activity monitoring | Visually impaired | Community | 1 | Feasibility |
| Årsand 2015 [29] | Chronic disease self-management | Diabetes | Community | 6 | Usability |
| Mortazavi 2015 [30] | Activity monitoring | Healthy | Laboratory | 20 | Feasibility |
| Boletsis 2015 [31] | Nursing or home-based care | Dementia | Community (nursing home) | 1 | Usability |
| Faye 2015 [32] | Activity monitoring | Healthy | Community | 13 | Feasibility |
| Haescher 2015 [33] | Activity monitoring | Healthy | Community | 14 | Feasibility |
| Jeong 2015 [34] | Healthcare education | Healthy | Laboratory | 1 | Feasibility |
| Panagop-oulos 2015 [35] | Nursing or home-based care | Geriatrics | Laboratory | 26 | Usability |
| Neto 2015 [36] | Chronic disease self-management | Healthy and visually impaired | Laboratory | 15 healthy, 11 low vision | Feasibility |
| Kalantarian 2015 [37] | Chronic disease self-management | Healthy | Laboratory | 10 | Feasibility |
| Vilarinho 2015 [38] | Nursing or home-based care | Healthy | Laboratory | 3 | Feasibility |
| Dubey 2015 [39] | Chronic disease self-management | Parkinson’s disease | Laboratory | 3 | Feasibility |
| Dubey 2015 [40] | Chronic disease self-management | Healthy and Parkinson’s disease | Laboratory | 3 healthy, 3 Parkinson’s disease | Feasibility |
| Thomaz 2015 [41] | Chronic disease self-management | Healthy | Laboratory and community | 20 | Feasibility |
| Ali 2016 [42] | Nursing or home-based care | Nursing home staff | Community | 1 | Usability |
| Banos 2016 [43] | Chronic disease self-management | Healthy and medical experts | Community | 10 healthy, 6 medical experts | Feasibility |
| Duclos 2016 [44] | Activity monitoring | Healthy | Laboratory and community | 16 | Feasibility |
| Velez 2016 [45] | Chronic disease self-management | Epilepsy | Hospital | 30 | Prospective cohort study |
| Hosseini 2016 [46] | Chronic disease self-management | Asthma | Community | 1 | Feasibility |
| Dobrican 2016 [47] | Activity monitoring | Healthy | Community | 5 | Feasibility |
| Thorpe 2016 [48] | Nursing or home-based care | Dementia | Laboratory and community | 5 | Usability |
| Nair 2016 [49] | Activity monitoring | Healthy | Laboratory | 10 | Feasibility |
| Micallef 2016 [50] | Chronic disease self-management | Stroke | Community | 15 | Usability |
| Ye 2016 [51] | Chronic disease self-management | Healthy | Community | 7 | Usability |
Activity and Gait Recognition
The activity monitoring studies that utilized smartwatches for feasibility testing of future healthcare applications [28, 30, 32, 33, 44, 47, 49] typically tested their systems on healthy individuals in the laboratory and community. However, one study [28] focused on activity classification for those with visual impairments. In Chippendale et al. [28], a smartwatch-based navigation system that aided visually impaired individuals by recognizing pose estimation of features in a scene to estimate the user’s location and pose. This was then used to provide audio and haptic feedback to the user of the current unfamiliar scene to aid them in performing daily activities such as shopping. To test this system design, the smartwatch navigation system was tested in a case study of one visually impaired individual while indoors and outdoors in a community-based scenario.
The other studies that focused on activity recognition tested the feasibility of these systems on healthy individuals [30, 32, 33, 44, 47, 49]. In these studies, feasibility testing was done on more than one participant, ranging from 5 to 20 participants, and these studies tested the system under both laboratory [30, 44, 49] and community [32, 33, 44, 47] conditions. Finally, the main goal of these studies was to test classification algorithms and platforms for various types of activities, such as sitting, standing lying down, running, jumping, cycling, and other daily activities such as working.
Self-Management of Chronic Diseases
Of the applications on chronic disease self-management interventions [25–27, 29, 36, 37, 39–41, 43, 45, 46, 50, 51], four studies focused on interventions for Parkinson’s disease [26, 27, 39, 40], and two studies focused on systems for epilepsy [25, 45]. The other studies focused on diabetes management [29], weight management and nutrition [37, 41, 43, 51], and interventions for those with visual impairments [36]. Specifically, in the studies that developed interventions for those with Parkinson’s disease [26, 27, 39, 40], Lopez et al. [26] attempted to use auditory signals to improve gait in patients via the smartwatch. Sharma et al. [27] attempted to leverage the smartwatch to monitor more multidimensional symptoms of Parkinson’s disease in addition to gait abnormalities, including facial tremors, dysfunctional speech, and limb dyskinesia. The other two articles [39, 40] used the smartwatch microphone to classify speech during vocal exercise in those who suffer from dysarthria. All these studies assessed their interventions in the laboratory in patients to assess the feasibility of detecting these symptoms from the smartwatch for future interventions and clinical trials.
For the studies that developed systems for epilepsy [25, 45], Lockman et al. [25] used smartwatches to detect tonic-clonic seizures in a hospital setting while patients underwent continuous video and electroencephalogram (EEG) monitoring in the hospital. Similarly, Velez et al. [45] also used smartwatches to detect tonic-clonic seizures during video and EEG monitoring. However, Velez et al. [45] studied 30 patients and measured these individuals over the course of several days in a prospective cohort study, while Lockman et al. [25] studied 40 individuals over the course of 24 h.
Banos et al. [43] focused on a general framework for all health and wellness smartwatch applications. However, this study tested the framework in a weight management app that utilized a smartwatch to classify activities associated with weight management, such as exercise and eating. Kalantarian and Sarrafzadeh [37] also attempted to utilize smartwatches for weight management, specifically by using the device’s built-in microphone to detect chews and swallows while eating. Their evaluation was successful in detecting different types of food chewing, such as apples and potato chips, in order to develop a heuristic estimation of caloric intake. Similar to this concept, Thomaz et al. [41] utilized the device’s microphone to detect chews and swallows; however, they also used inertial sensors and the built-in camera on the smartwatch to detect chews and swallows while eating. On the other hand, Ye et al. [51] did not detect chews and swallows; instead, they used inertial sensors to detect whether an individual was eating and then relied on the smartphone’s use of the Evernote app to take snapshots and self-report logs of their food.
For diabetes management, Årsand et al. [29] developed a diary application for a smartwatch that provided easy entry of relevant diabetes management information. Relevant information included carbohydrates, insulin, blood glucose, and physical activity type. This data was combined with step counts and transferred to the smartphone, which sent reminder notifications to the smartwatch every 90 min after meals to take blood glucose measurements. This was tested in six individuals, where two participants suffered from type 1 diabetes. However, although this application created a unique application for smartwatches, it relied heavily on user input and did not leverage sensor-based measures aside from step count.
The study conducted by Neto et al. [36] focused on a different need in the visually impaired community: face recognition during navigation. In this study is a scenario in which a visually impaired person needs to walk into a room in which he or she needs to recognize faces while silence and discretion is required, such as during a work meeting. To accomplish this task, the authors developed a smartwatch system in visually impaired individuals who were provided with information about their surroundings and faces via audio feedback. This was then tested in 15 healthy individuals who were blinded for feasibility, followed by usability testing in 11 low-vision individuals.
In Hosseini et al. [46], a smartwatch application was designed to provide pediatrics who suffer from asthma with risk levels of having an asthma attack through the use of various physiological and environmental sensors as well as sensors on the smartwatch. The smartwatch sensors utilized included the heart rate sensor, accelerometer, gyroscope, and GPS to assess physical activity and location. The system was tested in one adult individual who suffers from asthma for feasibility to assess the application’s ability to assess asthma attack risk under different real-world conditions that can cause asthma exacerbations such as exposure to smoke and exercise.
The study described in Micallef [50] focused on the usability of an exercise reminder application to improve arm movement after stroke. A smartwatch, smartphone, and tablet were evaluated in terms of usability to provide reminders, instructions, goals, and self-reports through visual, audio, and tactile feedback. The application relied on the LCD screen, speaker, and vibrator to provide the feedback and tested the usability of these modalities in 15 stroke survivors. They also tested whether smartwatches were more desirable to use over smartphones and tablets by stroke individuals. It was found that many individuals preferred the use of a smartwatch as well as using all modalities to provide reminders.
The above studies that focused on chronic disease self-management demonstrated the ability to apply smartwatches for long-term use in self-management programs. In addition, although these studies applied smartwatches to different chronic disease conditions, there were significant similarities across each intervention. For instance, many studies provided some forms of gait monitoring. This was evident in several studies, such as in those that applied smartwatches to Parkinson’s disease self-management [26, 27], visual impairments [28, 36], asthma [46], or diabetes [29]. They also relied on sensors available on the smartwatch to assess gait throughout use of the intervention and typically assessed feasibility and usability issues for long-term use. These similarities are particularly important in smartwatch applications for health care, as they can elucidate whether the smartwatch application will improve adherence and continuous monitoring throughout a self-management and community-based intervention.
Nursing or Home-Based Care
The smartwatch studies that focused on nursing or home-based care monitoring [31, 35, 38, 42, 48] utilized smartwatches to monitor patients in nursing facilities and their homes for specific health events to improve quality of life. Specifically, the studies described in Boletsis et al. [31] and Thorpe et al. [48] evaluated a smartwatch intervention for those with dementia through small usability studies with one patient [31] and five patients [48] who suffered from dementia, respectively. These studies evaluated the smartwatch’s ability to provide tracking and interaction by measuring sleep disturbances [31], frequent toilet visits [31], day time snoozing [31], mobility [48], activity levels [48], and low sleep quality [31]. These systems, however, did not provide fall detection like the smartwatch system described in Vilarinho et al. [38]. Instead, they provided emergency call interactions in case of a fall. In Ali et al. [42], a similar small usability study to those described Boletsis et al. [31] and Thorpe et al. [48] was conducted but evaluated a smartwatch’s ability to integrate different call functions typically seen in nursing homes. This includes call light systems, chair and bed alarms, wander guards, and other informative alarms that allow nursing home staff to monitor their patients. Finally, in Panagopoulos et al. [35], an integrated home care smartwatch-based system that incorporates communication and health monitoring features, such as biosignal monitoring and reminder notifications for treatments based on physician-defined schedules, was studied. However, this study was much larger than the usability studies conducted in Boletsis et al. [31], Thorpe et al. [48], and Ali et al. [42], as it analyzed the system among 26 geriatrics in a laboratory-based setting.
Healthcare Education
The final application found for smartwatch-based healthcare studies focused on using smartwatches for healthcare education [34]. In particular, Jeong et al. [34] utilized smartwatches to evaluate and teach healthcare professionals how to perform cardio-pulmonary resuscitation (CPR). Through the measurement of chest compression depth from accelerometers on the smartwatch, the system was able to provide feedback to the user of the level of chest compression being performed, as well as guide them through the CPR process as defined by the American Heart Association guidelines. This study evaluated the system’s feasibility in one individual and found that a smartwatch was able to provide accurate estimates of chest compression. When compared to a smartphone application, it was found to be more convenient to use, as the individual did not need to grip the device and did not hand any visual interference with hands over the screen. This demonstrates some of the ideal features smartwatches have over smartphones for healthcare applications, which will be further evaluated below.
Ideal Smartwatch Features
Given the specific healthcare applications above, it is clear that smartwatches have several important features that make them ideal for use in health care over the use of smartphones. For instance, smartwatches are able to continuously collect physical activity data, as well as other biosensor data such as heart rate, which makes them ideal devices for interventions that focus on activity and gait recognition [28, 30, 32, 33, 44, 47, 49]. Furthermore, unlike smartphones which require individuals to carry the devices in their pocket or hands, smartwatches can be worn during physical activity and treatment interventions that may require exercise or a high level of exertion by the participant [14].
In addition to continuous biosignal monitoring, smartwatches are also able to be worn can smartwatches continuously in the community and home. However, studies reported limited battery life in field settings [30]. Furthermore, smartwatches can provide alarms and messages that are more easily seen by individuals during activities such as exercise or interventions, as they can use vibrations, text, and sound to alert the user and provide more immediate communication with healthcare professionals. Finally, after the recent 5.1.1 Android Wear update, smartwatches now have improved computation power and battery life, allowing them to be used throughout the day and night time to continuously capture information in the community. These features have allowed successful feasibility studies in the aforementioned research, and if battery life is improved, it can lead to useful applications that will be tested in clinical trial research.
Operating System
Of the studies examined, most healthcare smartwatch applications utilized Android-based smartwatches over iOS and Tizen-based smartwatches [27–30, 32–34, 36–40, 42, 44–48, 50, 51] (see Table 2). This is because the Android operating system is open source and the smartwatches that run on Android are low cost. In addition, as previously mentioned, Apple smartwatches that run on the iOS operating system did not make their debut until April 2015, so most studies did not have an iOS smartwatch available during time of publication [16]. Furthermore, the most common watch type that was researched and available during these studies was the Samsung Gear Live model [30, 32, 34, 36, 37, 42, 44, 46], which was used in 8 of 27 (30%) studies. This watch, along with most commercially available smartwatches, requires a paired smartphone or tablet for full functionality. However, this was before the 5.1.1 Android Wear update, which now allows for WiFi support and thus smartwatches can now be used as the sole device in large scale healthcare clinical trials.
Table 2.
Smartwatch used for each chosen study, including operating system, type of smartwatch used, most important sensors on the smartwatch that were utilized, features used for classification, connectivity type, and application. Note that some studies developed custom-built smartwatches for their applications, and all studies utilized a Bluetooth connection as WiFi was unavailable in the chosen smartwatches
| Article | Operating system | Smartwatch used | Important sensors used | Classification features | Connectivity type | Application |
|---|---|---|---|---|---|---|
| Lockman 2011 [25] | Android Wear, iOS | Smart Monitor SmartWatch | Accelerometer (± 4G) | Duration, frequency, intensity | Bluetooth to PC | Chronic disease self-management |
| Lopez 2014 [26] | N/A—not mentioned | N/A—not mentioned | Accelerometer (range not available) | Intensity, direction | N/A—not mentioned | Chronic disease self-management |
| Sharma 2014 [27] | Android Wear | Pebble | Accelerometer (± 4G) | Mean, energy, high frequency energy content, entropy | Bluetooth to mobile | Chronic disease self-management |
| Chippen-dale 2014 [28] | Android Wear | Sony smartwatch | Microphone, gyroscope (± 8G) | Vertical angular acceleration matched to stepping | WiFi to mobile | Activity monitoring |
| Årsand 2015 [29] | Android Wear | Pebble | LCD screen, accelerometer (± 4G) | Built-in step count | Bluetooth to mobile | Chronic disease self-management |
| Mortaz-avi 2015 [30] | Android Wear | Samsung Gear Live | Accelerometer (± 2G), gyroscope (± 300°/s) | Difference, gyroscope intensity, mean, sum, dominant frequency | Bluetooth to PC | Activity monitoring |
| Boletsis 2015 [31] | Custom | Basis B1 | Heart rate, accelerometer, temperature, skin impedance (recalled, range not available) | Change in heart rate, movement intensity, sweat levels, body temperature, ambient temperature | Bluetooth to PC | Nursing or home-based care |
| Faye 2015 [32] | Android Wear | Samsung Gear Live | Heart rate, pedometer, accelerometer (± 2G) | Average wrist velocity, maximum wrist velocity, heart rate | Bluetooth to mobile | Activity monitoring |
| Haescher 2015 [33] | Android Wear | Simvalley Mobile AW-420 RX | Accelerometer (± 2G), gyroscope (± 256°/s), microphone | Magnitude area, energy, mean crossing rate, dominant frequency, movement intensity | Bluetooth to mobile | Activity monitoring |
| Jeong 2015 [34] | Android Wear | Samsung Gear Live | LCD screen, accelerometer (± 2G) | Positive peak accelerations | Bluetooth to PC | Healthcare education |
| Panagop-oulos 2015 [35] | Android Wear, iOS | N/A—not mentioned | LCD screen, accelerometer (range not available) | N/A—not mentioned | Bluetooth to mobile | Nursing or home-based care |
| Neto 2015 [36] | Android Wear | Samsung Gear Live | Camera, microphone | Face recognition, temporal coherence across video frames | Bluetooth to mobile | Chronic disease self-management |
| Kalantar-ian 2015 [37] | Android Wear | Samsung Gear Live | Microphone | Audio frequency distribution | Bluetooth to mobile | Chronic disease self-management |
| Vilarinho 2015 [38] | Android Wear | LG G Watch R2 | Accelerometer, gyroscope (1G–3G) | Total vectorial acceleration, fall index, absolute vertical acceleration | Bluetooth to mobile | Nursing or home-based care |
| Dubey 2015 [39] | Android Wear | ASUS ZenWatch | Microphone | Kurtosis, negentropy | Bluetooth to PC | Chronic disease self-management |
| Dubey 2015 [40] | Android Wear | ASUS ZenWatch | Microphone | Loudness, fundamental frequency | Bluetooth to PC | Chronic disease self-management |
| Thomaz 2015 [41] | iOS | Pebble | Accelerometer (± 4G) | mean, variance, skewness, kurtosis, root mean square | Bluetooth to mobile | Chronic disease self-management |
| Ali 2016 [42] | Android Wear | Samsung Gear Live | Microphone, LCD screen | None—used as feedback only | N/A—not mentioned | Nursing or home-based care |
| Banos 2016 [43] | N/A—not mentioned | N/A—not mentioned | Accelerometer, gyroscope (range not available) | N/A—not mentioned | Bluetooth to Cloud | Chronic disease self-management |
| Duclos 2016 [44] | Android Wear | Samsung Gear Live | Accelerometer (± 2G) | Acceleration vector variance, relative magnitude | Bluetooth to mobile | Activity monitoring |
| Velez 2016 [45] | Android Wear, iOS | Smart Monitor Smartwatch | Accelerometer (± 4G), microphone | Mean shake frequency and amplitude | Bluetooth to PC | Chronic disease self-management |
| Hosseini 2016 [46] | Android Wear | Samsung Gear Live | Accelerometer (± 2G), heart rate, GPS, LCD screen | Heart rate, total energy expenditure | Bluetooth to Cloud | Chronic disease self-management |
| Dobrican 2016 [47] | Android Wear | Samsung Gear S2 | Heart rate | Change in heart rate | Bluetooth to mobile | Activity monitoring |
| Thorpe 2016 [48] | Android Wear | Sony smartwatch | LCD screen | N/A—not applicable | Bluetooth to mobile | Nursing or home-based care |
| Nair 2016 [49] | Tizen | Samsung Gear S and S2 | Accelerometer (± 2G), heart rate, GPS | Magnitude, standard deviation, signal strength from 0.6–2.5 Hz, dominant frequency | Bluetooth to Cloud | Activity monitoring |
| Micallef 2016 [50] | Android Wear | Samsung Gear S | Microphone, speakers, LCD screen | N/A—not applicable, no classification performed | N/A—no data collection | Chronic disease self-management |
| Ye 2016 [51] | Android Wear | Pebble | Accelerometer (± 4G) | Gravitational field vector, pitch, roll | Bluetooth to mobile | Chronic disease self-management |
N/A not available
Sensors
Of the different features available on the smartwatch, the ability to continuously monitor individuals through various sensing mechanisms is the most important feature for use in healthcare applications. Specifically, after reviewing the smartwatch features utilized in the examined studies (Table 2), the use of accelerometers and other inertial sensors was most important, as they elucidated individual physical activities [25–35, 38, 41, 43–46, 49, 51]. Particularly, several studies relied solely on accelerometer sensors [25–27, 29, 34, 35, 44, 51] rather than other sensing mechanisms such as gyroscopes to classify activities such as seizures [25], gait [26, 27], or other types of physical activity [44]. Also, it was found that the use of the liquid crystal display (LCD) screens and microphones [28, 29, 33–37, 39, 40, 42, 45, 46, 48, 50] were of great importance in smartwatch research for healthcare applications, as these features were typically used to provide feedback to the user or allow the user to provide information and voice commands. However, some systems used the microphone feature to classify activities such as eating [37] or speech [39, 40].
In addition to the above important sensors utilized across studies, the features required for classification were typically in the time domain (Table 2). Several studies relied on accelerometer intensity, duration, and direction [25, 26, 30, 31, 33, 38, 41, 44, 49] to classify activities. In addition, frequency domain features such as energy [27, 33], entropy [27, 39], dominant frequency [25, 30, 33, 40, 45, 49], and frequency distribution [37] were sometimes utilized for classification. To improve the accuracy of physical activity classification in some studies, heart rate was also included as a feature [31, 32, 46]. Finally, some studies relied on built-in classifiers of commercially available watches such as step counters to extract information related to their applications [29, 32]. This, however, can be problematic, as commercially driven classifiers are designed using healthy populations and have been found to be inaccurate among target health care-related populations such as the elderly [53, 54].
As seen in Table 3, current commercially available smartwatches contain a wide range of sensors. Available sensors include accelerometers, gyroscopes, proximity sensors, magnetometers, pedometers, heart rate sensors (which relies on photoplethysmography, PPG, sensors), barometers, altimeters, compasses, GPS, and microphones. Furthermore, the BLOCKS Modular smartwatch described in Table 3, a custom Android-based smartwatch that allows researchers to choose which sensing mechanisms they would like available on the watch, allows for even more sensor types important for health monitoring. These sensors include pulse oximeters, electrocardiogram (ECG), skin temperature, and perspiration sensors. This smartwatch may become particularly useful in future healthcare applications, as it allows for physiological sensors not typically seen in smartwatches to continuously monitor individuals in the community and thus allow for more potential health conditions to be monitored and interventions to be explored.
Table 3.
Current smartwatches available on the market in 2016, their manufacturer, sensors available, operating system, version of Bluetooth available (where N/A is not available), battery rating, and average price in 2016
| Name of watch | Manufacturer | Sensors available | Operating system | Bluetooth version | Battery rating (mAh) | Price (USD) |
|---|---|---|---|---|---|---|
| LG Watch Urbane 2nd Edition LTE | LG Electronics | Accelerometer, gyroscope, proximity, heart rate, barometer, GPS, LTE communication | Android Wear | 4.1 | 570 | 500 |
| LG Watch Urbane W150 | LG Electronics | Accelerometer, gyroscope, proximity, heart rate | Android Wear | 4.1 | 410 | 450 |
| LG G Watch R W110 | LG Electronics | Accelerometer, gyroscope, proximity, heart rate, barometer | Android Wear | 4.0 | 410 | 300 |
| LG Gizmo Gadget | LG Electronics | GPS | Android Wear, iOS | N/A | 510 | 150 |
| Huawei Watch | Huawei Technologies Co., Ltd. | Accelerometer, altimeter, gyroscope, heart rate | Android Wear | 4.1 | 300 | 350 |
| Pebble Classic Smartwatch | Pebble Technology | Accelerometer, compass, microphone | Android Wear | 4.0 | 140 | 100 |
| Pebble Steel Smartwatch | Pebble Technology | Accelerometer, compass, microphone | Android Wear | 4.0 | 150 | 150 |
| Pebble Time Round Watch | Pebble Technology | Accelerometer, gyroscope, compass, magnetometer, pedometer, microphone | Android Wear | 4.0 | 150 | 200 |
| Apple Watch | Apple | Accelerometer, gyroscope, heart rate, microphone | iOS | 4.0 | 250 | 600 |
| Motorola Moto 360 Sport | Motorola | Accelerometer, gyroscope, proximity, heart rate, barometer, GPS | Android Wear | 4.0 | 300 | 300 |
| BLOCKS Modular Smartwatch | BLOCKS | Accelerometer, gyroscope, heart rate, GPS, altimeter, pulse oxygen level, ECG, temperature, perspiration, microphone | Android Wear (custom) | 4.0 | 300 | 330 |
| Sony Smartwatch 3 | Sony | Accelerometer, gyroscope, compass | Android Wear | 4.0 | 420 | 130 |
| Samsung Gear Live | Samsung | Accelerometer, gyroscope, heart rate, compass, camera | Android Wear | 4.0 | 300 | 100 |
| Basis B1 Band | Basis | Accelerometer, heart rate | N/A | 4.0 | 190 | Recalled |
| Simvalley Mobile AW-420 | Simvalley Mobile | Accelerometer, gyroscope, compass, GPS, camera | Android Wear | 4.0 | 600 | 250 |
| Smart Monitor Smartwatch | Smart Monitor | Accelerometer, GPS | Android Wear, iOS | N/A | N/A | 150 + $30 monthly fee |
Discussion
It is evident from the findings of this study that most research on smartwatches has been conducted as feasibility studies for chronic disease-self management [26, 27, 36, 37, 39–41, 43, 46] (see Sect. 3.1). Furthermore, most studies were conducted in 2015 [29–41]. This is expected, as smartwatches have not become popular until 2010, when they have been improved to run mobile apps and function as wearable computers. Furthermore, in June 2014, the Android Wear platform was introduced [55]. This year was also when a number of commercial smartwatches became available [56], such as the Samsung Gear Live, Moto 360, and Sony Smartwatch 3. In April 2015, the first Apple Watch was released, which uses the iOS operating system [16].
The feasibility studies on chronic disease self-management applications of health care [25–27, 29, 36, 37, 39–41, 43, 45, 46, 50, 51] utilized similar smartwatch features. Specifically, these applications targeted various disease conditions that exhibited behaviors measurable by inertial sensors (e.g., accelerometers, gyroscopes), such as seizures in those who suffer from epilepsy [25, 45] or gait disturbances in those who suffer from Parkinson’s disease [26, 27]. Thus, it can be inferred from these previous studies that smartwatches may have a future niche in healthcare applications as continuous monitoring of physical activity when smartphones are unable to measure the behavior or are not being worn (e.g., during exercise, walking, or sleep).
In addition to inertial sensing mechanisms, of the smartwatch features presented in Sect. 3.2, only a few other types of sensing mechanisms were utilized in healthcare applications (see Table 2 in Sect. 3.2). For example, several studies [28, 29, 34–37, 39, 40, 42, 46, 48, 50] took advantage of the LCD screen and microphone to provide continuous feedback to individuals throughout their studies. Others utilized the smartwatches’ ability to record heart rate [31, 32, 46, 47, 49], temperature [31], skin impedance [31], or take images using the camera [36]. In addition, most applications mainly used Android-based watches, such as the Samsung Gear Live [30, 32, 34, 36, 37, 42, 44, 46], as they are more affordable, have open-source code and documentation, and allow for WiFi support. They also contain the same built-in sensing mechanisms as iOS and custom-built watches, such as the above desirable inertial sensors and other sensors utilized in the studies examined.
Although many of the above features are available in smartphones, smartwatches were utilized in these studies for several reasons. First, as previously mentioned, the smartwatches utilized in the chronic disease self-management studies [25–27, 29, 36, 37, 39–41, 43, 45, 46, 50, 51] were able to monitor physical activities and behaviors from inertial sensors when smartphones would not be worn, such as at the bedside during a hospital visit or during exercise. This is particularly important in future applications of smartwatches, as it has been found that certain populations such as children do not wear smartphones during exercise [13] or those who are lying in a hospital bed [25, 45]. In addition, these studies were able to capitalize on the use of the LCD screen and speaker to provide feedback to individuals, or ask input from users. The more diverse sensors such as microphones [36, 37, 39, 40, 45, 50], cameras [36], heart rate [46], temperature [31], and skin impedance [31] sensors available in smartwatches allowed these studies to classify more complex behaviors than just physical activity, such as type of food being eaten [37, 39, 40] or face recognition during navigation [36]. These sensors and complex classifications will be important in future clinical trials that require more diverse behaviors to be monitored for individualized interventions of multifaceted disease conditions.
In the future, further research is necessary to assess the efficacy of the healthcare interventions described in these studies. Specifically, randomized controlled trials in much larger populations are needed to determine the safety and efficacy of using smartwatches in interventions among specific populations and disease conditions. This is typically done through phase I, II, and III clinical trials, where a phase I trial is first performed on healthy volunteers to assess safety, a phase II trial is next performed to collect preliminary data on the effectiveness of the intervention, and a phase III trial is performed to gather more information about the safety and effectiveness of the intervention [57]. These typically range from 20 to 100 participants in a phase I trial, 100–300 participants in a phase II trial, and > 1000 participants in a phase III trial and assess safety and efficacy over long periods of time. If successfully performed for any of the aforementioned applications, it may lead to widespread adoption of smartwatch use in health care for continuous monitoring and real-time feedback driven interventions.
Limitations
There are several limitations to smartwatches that have prevented widespread adoption in healthcare research. First, since smartwatches have only begun to rise in popularity since 2010, and were not improved to run mobile apps and function as wearable computers since 2014, very little time has passed for them to become popular in the general market, let alone in the healthcare market. Also, there has been little to no validation of using smartwatch sensors to collect physiological and environmental data by comparing smartwatch sensors against clinical measures. In order to maintain Health Insurance Portability and Accountability Act (HIPAA) compliance during such validation studies, security and privacy of smartwatch data must also be maintained throughout the transmission process. This can be difficult, especially since many current smartwatches are still tied to smartphones to allow for data transmission to a secure server if they do not have the 5.1.1 Android Wear update.
In addition to the above study design challenges, there are several technological challenges in smartwatches that still exist. For example, although the battery life of smartwatches has vastly improved in recent years, they still require to be charged after a day or few days of use depending on the computation being performed and amount of LCD screen feedback being provided. This may make compliance to use of the smartwatch in interventions challenging, as individuals may prefer to wear the smartwatches throughout the intervention without having to remember to charge the device. Finally, due to the small LCD screen on a smartwatch, these devices have very limited capabilities in terms of the amount of feedback and interaction with the individual. Thus, many future interventions should rely on other sensing mechanisms available in smartwatches, such as microphones or video cameras that interface with the interventionist, caregiver, or clinician. Future advancements in how these feedback mechanisms are provided in the context of human machine interfacing is required, however, to improve compliance and adherence to the intervention and use of the smartwatch.
Conclusions
This paper found that in that of 1119 articles on the use of smartwatches, there were only 27 studies that are directly related to health care. Furthermore, these studies had limited applications, which included activity monitoring, chronic disease self-management, nursing or home-based care, and healthcare education. All studies were considered feasibility or usability studies and thus had a very limited number of study subjects tested. Since no randomized clinical trial research was found, it is suggested that significant further research on much larger populations is needed. This will assess the efficacy of using smartwatches in healthcare interventions and may eventually lead to widespread adoption of the technology in this field.
Author Contributions
MS had the idea to write a systematic review on smartwatches in health care, CEK and MS performed the review and analysis, CEK wrote the article, and MS is the guarantor of the review.
Funding Information
This work was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) Los Angeles Pediatric Research using Integrated Sensor Monitoring Systems (PRISMS) Center: The Biomedical REAl-Time Health Evaluation (BREATHE) Platform, NIH/NIBIB U54 award no. EB022002.
Compliance with Ethical Standards
Conflict of Interests
The authors declare that they have no conflict of interest.
Contributor Information
Christine E. King, Phone: (310) 794-5616, Email: cking0130@gmail.com
Majid Sarrafzadeh, Email: majid@cs.ucla.edu.
References
- 1.Lu TC, Fu CM, Ma MM, Fang CC, Turner AM. Healthcare applications of smart watches. Appl Clin Inform. 2016;7(3):850–869. doi: 10.4338/ACI-2016-03-R-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glowacki EM, Zhu Y, Hunt E (2016) Magsamen-Conrad K, Bernhardt JM Facilitators and barriers to smartwatch use among individuals with chronic diseases: a qualitative study. University of Texas, Austin
- 3.Reeder B, David A. Health at hand: a systematic review of smart watch uses for health and wellness. J Biomed Eng Inform. 2016;63:269–276. doi: 10.1016/j.jbi.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 4.James DC, Harville C (2015) Barriers and motivators to participating in mHealth research among African American men. Am J Mens Health. 10.1177/1557988315620276 [DOI] [PMC free article] [PubMed]
- 5.De Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, Atun R (2012) Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 10.1002/14651858.CD007459.pub2 [DOI] [PMC free article] [PubMed]
- 6.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2009;4(4):CD006611. doi: 10.1002/14651858.CD006611.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, Gold J, Ngo TD, Sumpter C, Free C (2015) Mobile phone-based interventions for improving contraception use. Cochrane Database Syst Rev. 10.1002/14651858.CD011159.pub2 [DOI] [PMC free article] [PubMed]
- 8.Fisher E, Law E, Palermo TM, Eccleston C (2015) Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 10.1002/14651858.CD011118.pub2 [DOI] [PMC free article] [PubMed]
- 9.Marcano Belisario JS, Jamsek J, Huckvale K, O'Donoghue J, Morrison CP, Car J (2015) Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev. 10.1002/14651858.MR000042.pub2 [DOI] [PMC free article] [PubMed]
- 10.Ozdalga E, Ozdalga A, Ahuja N. The smartphone in medicine: a review of current and potential use among physicians and students. JMIR. 2012;14(5):e128. doi: 10.2196/jmir.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webb T, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. JMIR. 2010;12(1):e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of mhealth technologies for improving health and health services: a systematic review protocol. BMC Res Notes. 2010;3(1):250. doi: 10.1186/1756-0500-3-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunton GF, Liao Y, Intille SS, Spruijt-Metz D, Pentz M. Investigating children’s physical activity and sedentary behavior using ecological momentary assessment with mobile phones. Obesity. 2011;19(6):1205–1212. doi: 10.1038/oby.2010.302. [DOI] [PubMed] [Google Scholar]
- 14.Ehrler F, Lovis C. Supporting elderly homecare with smartwatches: advantages and drawbacks. Stud Health Technol Inform. 2014;205:667–671. [PubMed] [Google Scholar]
- 15.Mann S Wristwatch-based videoconferencing system. http://brevets-patents.ic.gc.ca/opic-cipo/cpd/eng/patent/2275784/summary.html?type=number_search&tabs1Index=tabs1_1. Accessed 11 January 2017
- 16.Kastrenakes J (2015) Apple Watch release date is April 24th, with pricing from $349 to over $10,000 (March 2015). https://www.theverge.com/2015/3/9/8162455/apple-watch-price-release-date-2015. Accessed 4 August 2017
- 17.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ, pp 336–924. http://www.ebcp.com.br/simple/upfiles/pdfs/2009BrozekPart1.pdf. Accessed 19 July 2016 [DOI] [PMC free article] [PubMed]
- 18.Gutierrez MA, Fast ML, Ngu AH, Gao BJ (2015) Real-time prediction of blood alcohol content using smartwatch sensor data. In Proc ICSH, pp 175–186. https://link.springer.com/chapter/10.1007/978-3-319-29175-8_16. Accessed 19 July 2016
- 19.Iakovakis DE, Papadopoulou FA, Hadjileontiadis LJ. Fuzzy logic-based risk of fall estimation using smartwatch data as a means to form an assistive feedback mechanism in everyday living activities. Healthc Technol Lett. 2016;3(4):263–268. doi: 10.1049/htl.2016.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelling C, Pitaro D, Rantala J (2016) Good vibes: the impact of haptic patterns on stress levels. In Proc Academic Mindtrek Conference, pp 130–136. https://dl.acm.org/citation.cfm?id=2994368. Accessed 6 July 2016
- 21.Moder T, Wisiol K, Wieser M (2016 Walking aid identification using wearables. In Proc Comut Help People Spec Needs, pp 335–341. https://link.springer.com/chapter/10.1007/978-3-319-41264-1_46. Accessed 19 August 2016
- 22.Prange A, Sonntag D (2015) Easy deployment of spoken dialogue technology on smartwatches for mental healthcare. In Proc Pervasive Computing Paradigms for Mental Health, pp 150–156. 10.1007/978-3-319-32270-4_15. Accessed 19 July 2016
- 23.Berrocal J, Garcia-Alonso J, Murillo J, Canal C (2017) Rich contextual information for monitoring the elderly in an early stage of cognitive impairment. Pervasive Mob Comput 34:106–125. http://www.sciencedirect.com/science/article/pii/S1574119216300499. Accessed 19 August 2016
- 24.Blaauw F, Schenk H, Jeronimus B, van der Krieke L, de Jonge P, Aiello M. Let’s get physiqual—an intuitive and generic method to combine sensor technology with ecological momentary assessments. J Bioed Eng Inform. 2016;63:141–149. doi: 10.1016/j.jbi.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Lockman J, Fisher RS, Olson DM. Detection of seizure-like movements using a wrist accelerometer. Epilepsy Behav. 2011;20(4):638–641. doi: 10.1016/j.yebeh.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Lopez WOC, Higuera CAE, Fonoff ET, de Oliveira Souza C, Albicker U, Martinez JAE. Listenmee and listenmee smartphone application: synchronizing walking to rhythmic auditory cues to improve gait in Parkinson’s disease. Hum Mov Sci. 2014;37:147–156. doi: 10.1016/j.humov.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Sharma V, Mankodiya K, De La Torre F, Zhang A, Ryan N, Ton TG, Gandhi R, Jain S (2014) SPARK: personalized Parkinson disease interventions through synergy between a smartphone and a smartwatch. In Proc The Design, User Experience, and Usability: User Experience Design for Everyday Life Applications and Services. 103–114, 10.1007/978-3-319-07635-5_11
- 28.Chippendale P, Tomaselli V, DAlto V, Urlini G, Modena CM, Messelodi S, Strano SM, Alce G, Hermodsson K, Razafimahazo M, Michel T (2014) Personal shopping assistance and navigator system for visually impaired people. In Proc Comput Vis ECCV, pp 375–390. https://hal.inria.fr/hal-01102707/. Accessed 19 July 2016
- 29.Årsand E, Muzny M, Bradway M, Muzik J, Hartvigsen G. Performance of the first combined smartwatch and smartphone diabetes diary application study. J Diabetes Sci Technol. 2015;9(3):556–563. doi: 10.1177/1932296814567708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortazavi B, Nemati E, VanderWall K, Flores-Rodriguez HG, Cai JYJ, Lucier J, Naeim A, Sarrafzadeh M. Can smartwatches replace smartphones for posture tracking? Sensors. 2015;15(10):26783–26800. doi: 10.3390/s151026783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boletsis C, McCallum S, Landmark BF (2015) The use of smartwatches for health monitoring in home-based dementia care. In Proc Conf Hum Aspects IT Aged Pop, pp 15–26.https://link.springer.com/chapter/10.1007/978-3-319-20913-5_2. Accessed 19 July 2016
- 32.Faye S, Frank R, Engel T (2015) Adaptive activity and context recognition using multimodal sensors in smart devices. In Proc MobiCase, pp 33–50. https://link.springer.com/chapter/10.1007/978-3-319-29003-4_3. Accessed 6 July 2016
- 33.Haescher M, Trimpop J, Matthies DJ, Bieber G, Urban B, Kirste T (2015) aHead: considering the head position in a multi-sensory setup of wearables to recognize everyday activities with intelligent sensor fusions. In Proc Mobile HCI, pp 741–752. https://link.springer.com/chapter/10.1007/978-3-319-20916-6_68. Accessed 19 July 2016
- 34.Jeong Y, Chee Y, Song Y, Koo K (2015) Smartwatch app as the chest compression depth feedback device. In Proc World Congress on Medical Physics and Biomedical Engineering, pp 1465–1468. https://link.springer.com/chapter/10.1007/978-3-319-19387-8_357. Accessed 19 July 2016
- 35.Panagopoulos C, Kalatha E, Tsanakas P, Maglogiannis I (2015) Evaluation of a mobile home care platform. In Proc ECAI, pp 328–343. https://link.springer.com/chapter/10.1007/978-3-319-26005-1_22. Accessed 18 July 2016
- 36.Neto LdSB, Maike VRML, Koch FL, Baranauskas MCC, de Rezende Rocha A, Goldenstein SK (2015) A wearable face recognition system built into a smartwatch and the blind and low vision users. In Proc ICEIS, pp 515–528. https://link.springer.com/chapter/10.1007/978-3-319-29133-8_25. Accessed 19 August 2017
- 37.Kalantarian H, Sarrafzadeh M. Audio-based detection and evaluation of eating behavior using the smartwatch platform. Comput Biol Med. 2015;65:1–9. doi: 10.1016/j.compbiomed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Vilarinho T, Farshchian B, Bajer DG, Dahl OH, Egge I, Hegdal SS, Lønes A, Slettevold JN, Weggersen SM (2015) A combined smartphone and smartwatch fall detection system. In Proc IEEE Int Conf CIT UCC DASC PICOM, pp 1443–1448. http://ieeexplore.ieee.org/abstract/document/7363260/?reload=true. Accessed 28 July 2016
- 39.Dubey H, Goldberg JC, Mankodiya K, Mahler L (2015) A multi-smartwatch system for assessing speech characteristics of people with dysarthria in group settings. In Proc Int Conf HealthCom, pp 528–533. http://ieeexplore.ieee.org/abstract/document/7454559/. Accessed 6 July 2016
- 40.Dubey H, Goldberg JC, Abtahi M, Mahler L, Mankodiya K. EchoWear: smartwatch technology for voice and speech treatments of patients with Parkinson’s disease. Proc Wireless Health. 2015;15:1–15. [Google Scholar]
- 41.Thomaz E, Essa I, Abowd GD (2015) A practical approach for recognizing eating moments with wrist-mounted inertial sensing. In Proc 2015 ACM UbiComp, pp 1029–1040. https://dl.acm.org/citation.cfm?id=2807545. Accessed 6 July 2016 [DOI] [PMC free article] [PubMed]
- 42.Ali H, Li H (2016) Designing a smart watch interface for a notification and communication system for nursing homes. In Proc Conf Hum Aspects IT Aged Pop, pp 401–411. https://link.springer.com/chapter/10.1007/978-3-319-39943-0_39. Accessed 19 July 2016
- 43.Banos O, Amin MB, Khan WA, Afzal M, Hussain M, Kang BH, Lee S. The mining minds digital health and wellness framework. Biomed Eng Online. 2016;15(Suppl. 1):S76. doi: 10.1186/s12938-016-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duclos M, Fleury G, Lacomme P, Phan R, Ren L, Rousset S. An acceleration vector variance based method for energy expenditure estimation in real-life environment with a smartphone/smartwatch integration. Expert Syst Appl. 2016;63:435–449. doi: 10.1016/j.eswa.2016.07.021. [DOI] [Google Scholar]
- 45.Velez M, Fisher RS, Bartlett V, Le S. Tracking generalized tonic-clonic seizures with a wrist accelerometer linked to an online database. Seizure. 2016;39:13–18. doi: 10.1016/j.seizure.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Hosseini A, Buonocore CM, Hashemzadeh S, Hojaiji H, Kalantarian H, Sideris C, Bui AA, King CE, Sarrafzadeh M (2016) Hipaa compliant wireless sensing smartwatch application for the self-management of pediatric asthma. In Proc 2016 I.E. Int Conf BSN, pp 49–54. http://ieeexplore.ieee.org/abstract/document/7516231/. Accessed 28 July 2016 [DOI] [PMC free article] [PubMed]
- 47.Dobrican RA, Zampunieris D (2016) A proactive solution, using wearable and mobile applications, for closing the gap between the rehabilitation team and cardiac patients. In Proc 2016 I.E. Int Conf ICHI. 146–155
- 48.Thorpe JR, Rønn-Andersen KVH, Bień P, Özkil AG, Forchhammer BH, Maier AM. Pervasive assistive technology for people with dementia: a UCD case. Healthc Technol Lett. 2016;3(4):297–302. doi: 10.1049/htl.2016.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair S, Kheirkhahan M, Davoudi A, Rashidi P, Wanigatunga AA, Corbett DB, Manini TM, Ranka S (2016) Roamm: a software infrastructure for real-time monitoring of personal health. In Proc Int Conf HealthCom, pp 1–6. http://ieeexplore.ieee.org/abstract/document/7749479/. . Accessed 28 July 2016
- 50.Micallef N, Baillie L, Uzor S (2016) Time to exercise!: an aide-memoire stroke app for post-stroke arm rehabilitation. In Proc Int Conf MobileHCI, pp 112–123. https://dl.acm.org/citation.cfm?id=2935338. Accessed 6 July 2016
- 51.Ye X, Chen G, Gao Y, Wang H, Cao Y (2016) Assisting food journaling with automatic eating detection. In Proc 2016 CHI, pp 3255–3262. https://dl.acm.org/citation.cfm?id=2892426. Accessed 19 August 2017
- 52.Portney LG, Watkins MP (2015) Foundations of clinical research: applications to practice. FA Davis. https://www.fadavis.com/product/physical-therapy-foundations-clinical-research-portney-3?&RequestId=1574275166. Accessed 1 January 2017
- 53.Singh AK, Farmer C, Van Den Berg ML, Killington M, Barr CJ. Accuracy of the FitBit at walking speeds and cadences relevant to clinical rehabilitation populations. Disabil Health J. 2016;9(2):320–323. doi: 10.1016/j.dhjo.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Bassett DR, Toth LP, LaMunion SR, Crouter SE (2016) Step counting: a review of measurement considerations and health-related applications. Sports Med:1–13 [DOI] [PMC free article] [PubMed]
- 55.Egan M The 5 most exciting announcements from Google i/o 2014: Android l, android wear news, android auto, chromebooks, and android tv. http://www.pcadvisor.co.uk/feature/google-android/5-most-exciting-announcements-from-google-i-o-2014-3524194/. Accessed 11 January 2017
- 56.Dawson T Top 10 best smartwatches buyers guide: October 2014 edition. 2017-01-11. http://www.androidheadlines.com/2014/10/top-10-best-smartwatches-buyers-guide-october-2014-edition.html. Accessed 11 January 2017
- 57.Introduction to Clinical Trials (1998) In: Friedman LM, Furberg C, DeMets DL (eds.) Fundamentals of Clinical Trials. Springer, New York, pp 1–18 http://www.springer.com/us/book/9781441915863. Accessed 12 August 2017