Abstract
BACKGROUD
Evidence is limited regarding the impact of healthy lifestyle practices on the risk of subsequent cardiovascular events among patients with diabetes.
OBJECTIVES
To examine the associations of an overall healthy lifestyle, defined by eating a high-quality diet (top two fifths of Alternative Healthy Eating Index), non-smoking, engaging in moderate- to vigorous-intensity physical activity (≥150 min/week), and drinking alcohol in moderation (5–15 g/day for women and 5–30 g/day for men), with the risk of developing cardiovascular disease (CVD) and CVD mortality among adults with type 2 diabetes (T2D).
METHODS
This prospective analysis included 11,527 participants with T2D diagnosed during follow-up (8,970 women from the Nurses’ Health Study and 2,557 men from the Health Professionals Follow-Up Study), who were free of CVD and cancer at the time of diabetes diagnosis. Diet and lifestyle factors before and after T2D diagnosis were repeatedly assessed every 2–4 years.
RESULTS
There were 2,311 incident CVD cases and 858 CVD deaths during an average of 13.3 years of follow-up. After multivariate adjustment of covariates, the low-risk lifestyle factors after diabetes diagnosis were each associated with a lower risk of CVD incidence and CVD mortality. The multivariate-adjusted hazard ratios (95% confidence intervals [CIs]) for participants with three or more low-risk lifestyle factors compared with zero was 0.48 (0.40–0.59) for total CVD incidence, 0.53 (0.42–0.66) for CHD incidence, 0.33 (0.21–0.51) for stroke incidence, and 0.32 (0.22–0.47) for CVD mortality (all P trend<0.001). The population-attributable-risk for poor adherence to the overall healthy lifestyle (<3 low-risk factors) was 40.9% (95% CI, 28.5%–52.0%) for CVD mortality. In addition, greater improvements in healthy lifestyle factors from pre- to post-diabetes diagnosis were also significantly associated with a lower risk of CVD incidence and CVD mortality. For each number increment in low-risk lifestyle factors, there were a 14% lower risk of incident total CVD, a 12% lower risk of CHD, a 21% lower risk of stroke, and a 27% lower risk of CVD mortality (all P <0.001). Similar results were observed when analyses were stratified by diabetes duration, sex/cohort, body mass index at diabetes diagnosis, smoking status, and lifestyle factors before diabetes diagnosis.
CONCLUSIONS
Greater adherence to an overall healthy lifestyle is associated with a substantially lower risk of CVD incidence and CVD mortality among adults with T2D. These findings further support the tremendous benefits of adopting a healthy lifestyle in reducing the subsequent burden of cardiovascular complications in patients with T2D.
Keywords: cardiovascular disease, diabetic patients, diet, healthy lifestyle, cohort study
Introduction
Type 2 diabetes (T2D) has become a global public health challenge, with approximately 422 million adults living with diabetes worldwide in 2014 (1). Cardiovascular disease (CVD) is the primary complication and the leading cause of deaths in patients with diabetes (2). It is of particular importance to identify cost-effective strategies to prevent or delay the development of cardiovascular complications among patients with T2D.
In addition to glycemic control, lifestyle modification is a fundamental component of diabetes self-management (3). Healthy lifestyle behaviours, including eating a high-quality diet, non-smoking, engaging in moderate to vigorous physical activity, and drinking alcohol in moderation, have been associated with a lower risk of cardiometabolic diseases and mortality in general populations (4,5), but the evidence regarding the impact of an overall healthy lifestyle after diabetes diagnosis on the risk of subsequent cardiovascular events is limited (6). Several trials that examined the efficacy of multi-component lifestyle interventions have demonstrated tremendous benefits on reducing T2D risk and improving cardiovascular health among high-risk individuals (7–12), although the long-term benefits on reducing CVD events among diabetic patients were less established (6). Prospective observational studies among diabetic patients thus far largely focused on associations between healthy lifestyle practices and total mortality (13–15), but data specific for CVD risk are lacking. Importantly, little is known regarding whether improvements in lifestyle from pre- to post-diabetes diagnosis may yield cardiovascular benefits.
To fill these critical knowledge gaps, we prospectively investigated healthy lifestyle practices after diabetes diagnosis, as well as changes in lifestyle factors before and after diabetes diagnosis, in relation to subsequent risk of CVD incidence and CVD mortality among patients with T2D participating in 2 large prospective cohort studies.
Methods
Study population
The Nurses’ Health Study (NHS) was established in 1976 with the enrollment of 121,700 U.S. female nurses aged 30 to 55 years from 11 U.S. states (16). The Health Professionals Follow-Up Study (HPFS) was initiated in 1986, enrolling 51,529 U.S. male health professionals aged 40 to 75 years from 50 U.S. states (17). The detailed information on dietary and lifestyle factors, medical history, and disease status was updated every 2–4 years through validated questionnaires (18). The cumulative response rate was over 90% for both cohorts. More details have been documented elsewhere (19,20).
In the current analysis, we included men and women with incident diabetes diagnosed during follow-up through 2012 (1980 for the NHS and 1986 for the HPFS as baseline, when validated food frequency questionnaires [FFQs] were first administered). Participants were excluded if they had existing T2D, CVD, or cancer at baseline, reported CVD or cancer before T2D diagnosis during follow-up, reported implausible daily caloric intake (<500 or >3,500 kcal/day for women, and <800 or >4,200 kcal/day for men), or had missing information on body mass index (BMI), smoking status, alcohol intake, physical activity, or dietary data at diabetes diagnosis. These exclusion criteria were based on considerations of minimizing reverse causation bias and reducing the impact of measurement errors and missing data. After exclusions, 8,970 women in the NHS and 2,557 men in the HPFS with incident T2D cases were included in the final analysis, with an average of 13.3 years of follow-up (Online Figure 1). When we modeled changes in lifestyle factors from pre- to post-diabetes diagnosis, participants with missing data of lifestyle factors assessed before diabetes diagnosis were further excluded. To increase statistical power, we pooled the participants from the two cohorts in the absence of heterogeneity of results.
The present study was approved by the Institutional Review Boards at the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital, and the return of the questionnaires was considered implied consent.
Definition of low-risk lifestyle factors
In the current study, we considered four modifiable lifestyle factors—diet, smoking status, alcohol consumption, and physical activity. Details of the assessments of individual lifestyle factors can be found in Online Appendix. Given that weight change is one of the typical symptoms of diabetes, and treatment could result in weight change after diagnosis as well, BMI may no longer serve as a valid measure of adiposity and thus was not included in the lifestyle score to minimize reverse causation bias (21).
Diet quality was assessed using the 2010 Alternate Healthy Eating Index (AHEI) score (22), which was based on the U.S. Department of Agriculture Healthy Eating Index, designed to measure adherence to U.S. dietary guidelines (23). We included 10 dietary factors in the diet quality score: vegetables, fruits, whole grains, nuts, polyunsaturated fatty acids, long-chain omega-3 fatty acids, red and processed meats, trans fat, sugar sweetened beverages, and sodium. Each component was scored with a range from 0 to 10 based on consumption level, with 10 indicating a full adherence to the recommended consumption levels, 0 for the least adherence to the recommendation. In our analysis, AHEI was categorized in quintiles. A healthy diet was defined as an AHEI score in the top 40% of each cohort distribution (18,24). For smoking, we defined the low-risk as non-current smoking (24). We classified low-risk of physical activity as ≥ 150 min/week of moderate- or vigorous-intensity activities (defined as the intensity of activities ≥3 metabolic equivalents) (25,26). Low-risk alcohol consumption was defined as moderate alcohol consumption: 5–15 g/day for women and 5–30 g/day for men (27). Of note, few participants drank heavily in our cohorts; <1% of total participants drank alcohol >45 g/day at diabetes diagnosis.
For each low-risk lifestyle factor, the participant received 1 point if they met the criterion for the low-risk category, or 0 point otherwise. The sum of the four factors constituted a final low-risk lifestyle score of 0, 1, 2, 3, and 4 (higher score for a healthier lifestyle) (27).
Our primary exposures of interest were lifestyle factors assessed after diabetes diagnosis, and changes in lifestyle before and after diabetes diagnosis. The pre-diabetes lifestyle factors were assessed from the most recent questionnaires before diabetes was ascertained (the mean duration from questionnaire return to date of diagnosis was 11 months).
Ascertainment of T2D
Participants who reported a physician’s diagnosis of diabetes on any of the biennial questionnaires were mailed a validated supplementary questionnaire regarding diagnostic tests, symptoms, and hypoglycemic therapy. The National Diabetes Data Group and ADA criteria were applied to ascertain T2D diagnosis (Online Appendix). In our validation studies, 98% (61/62 cases) of diabetes cases confirmed by the supplementary questionnaire were re-confirmed by medical record review in the NHS, and 97% (57/59 cases) were re-confirmed in the HPFS (28,29).
Assessment of CVD and mortality
The outcomes of the current study were CVD incidence and CVD mortality. Incident CVD was defined as fatal and non-fatal CHD (including coronary artery bypass graft surgery [CABG] and non-fatal myocardial infarction [MI]) and fatal and non-fatal stroke. We requested permission to review medical records when participants reported cardiovascular events on any biennial questionnaires. Physicians blinded to the participant questionnaire data reviewed all medical records. Non-fatal MI was ascertained according to the World Health Organization criteria, including typical symptoms, elevated cardiac enzyme levels, and electrocardiographic findings (30). Non-fatal stroke was defined based on the National Survey of Stroke criteria, requiring evidence of neurologic deficits with sudden or rapid onset which persisted for at least 24 hours or until death (31). The diagnosis of CABG was based on self-report, for which the validity had been demonstrated (32). Deaths were identified by searching the National Death Index, or reports by next of kin or postal authorities. The follow-up rate for death in the NHS and HPFS was over 98%. Fatal CHD was defined if CHD was listed as the cause of death on the death certificate and the history of CHD was evident through reviewing hospital records or autopsy reports. Similarly, fatal stroke was identified and confirmed by reviewing death certificates, hospital records, or autopsy records. CVD mortality was defined using International Classification of Diseases, 8th Revision (ICD-8) codes of 390–458 or 795 (21).
Person-time was calculated from the date of a diabetes diagnosis to either the date of CVD diagnosis, or death, or the end of follow-up (June 30, 2014 for the NHS and January 30, 2014 for the HPFS), whichever came first. Cox proportional hazards models were applied to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of each individual lifestyle factor and the overall healthy lifestyle score (counted as the number of low-risk factors and categorized into 0, 1, 2, or ≥3) with the risk of total CVD, CHD, and stroke incidence, and CVD mortality. Individual lifestyle factors and the overall healthy lifestyle score were modeled as time-varying variables. Changes in lifestyle score from pre- to post-diabetes diagnosis were defined as the absolute difference of the low-risk lifestyle score (time-varying post-diabetes low-risk lifestyle score minus pre-diabetes low-risk lifestyle score). The linear trend was tested by assigning a median value to each category as a continuous variable. The time-varying covariates assessed during follow-up were adjusted in the multivariate models. Missing data of the exposure and covariates during follow-up were replaced by valid assessments in the previous one cycle only. In multivariate models, we adjusted for age, sex, ethnicity, diabetes duration, BMI at diabetes diagnosis, smoking status, alcohol consumption, physical activity, AHEI score, menopausal status (women only), family history of diabetes, family history of MI, current aspirin use, current multivitamin use, presence of hypertension or hypercholesterolemia, use of antihypertensive or cholesterol-lowering drugs, and diabetes medication use. To control for confounding by glucose control, the self-reported levels of HbA1c were further adjusted in a subset of the study participants (n=4,650). In the analysis of changes in lifestyle from pre- to post-diabetes diagnosis, the healthy lifestyle score before diabetes diagnosis was further adjusted for in the multivariate model. In the current study, the proportional hazards assumption was tested by using a likelihood ratio test comparing models with and without multiplicative interaction terms between exposure and calendar year, and we did not find evidence of violation of the proportional hazards assumption. We calculated population- attributable-risk (PAR) to estimate the percentage of CVD mortality in the study population that theoretically would not have occurred if all individuals had been in the low-risk category (≥3 low-risk factors) (33).
Analyses were further stratified by age at diabetes diagnosis (<65 years, ≥65 years), BMI at diabetes diagnosis (<25.0, 25.0–29.9, ≥30.0 kg/m2), smoking status after diabetes diagnosis (never smoker, past smoker, current smoker), diabetes duration (<5, 5–9, ≥10 years), sex/cohort (women/NHS, men/HPFS), and the lifestyle score before diabetes diagnosis. The P values for the product terms between the continuous lifestyle score and stratification variables were used to estimate the significance of interactions.
Several sensitivity/secondary analyses were conducted to demonstrate the robustness of our findings. First, we used cumulative averages of AHEI score, physical activity, and alcohol consumption since diabetes diagnosis to construct the overall healthy lifestyle score. Second, healthy body weight (18.5≤BMI<25.0 kg/m2) at diabetes diagnosis were included in the low-risk lifestyle score. Third, we examined the associations of different combinations of low-risk factors with CVD incidence and mortality. Fourth, the associations of low-risk lifestyle factors assessed before diabetes diagnosis with CVD incidence and mortality was analyzed. Fifth, we explored associations of healthy lifestyle practices with CVD incidence and mortality among diabetic patients with hypertension and/or hypercholesterolemia. Sixth, participants with missing data of exposure and covariates during follow-up were excluded and analyses were repeated. Seventh, we conducted analyses using data collected before and after 1998 (the median of follow-up time in HPFS), respectively. Lastly, we excluded deaths that occurred within 4 years after diabetes diagnosis to examine whether our analyses were impacted by reverse causation bias.
All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, North Carolina). Two-sided P<0.05 was considered statistically significant.
Results
The characteristics of the study participants at diabetes diagnosis are shown in Table 1. The proportion of participants with 0, 1, 2, ≥3 low-risk lifestyle factors at diabetes diagnosis was 6.6%, 45.9%, 34.6%, and 12.9% in women, and 3.0%, 31.7%, 37.8%, and 27.4% in men, respectively.
Table 1.
Characteristics of patients with type 2 diabetes at diagnosis
| NHS | HPFS | |
|---|---|---|
| (N=8,970) | (N=2,557) | |
| Age, years | 62.5 (9.1) | 63.0 (8.8) |
| White, % | 96.0 | 92.1 |
| Body Mass Index, kg/m2 | 30.9 (6.1) | 29.1 (4.6) |
| Alternate Healthy Eating Index score* | 46.0 (12.0) | 47.7 (11.0) |
| Physical activity, hours/week | 1.3 (2.4) | 2.5 (4.3) |
| Alcohol consumption, % | 49.2 | 75.9 |
| Alcohol consumption among drinkers, g/day† | 7.6 (2.5, 9.8) | 13.4 (3.0, 17.1) |
| Current smoker, % | 13.1 | 7.7 |
| Hypertension, % | 70.6 | 58.0 |
| Hypercholesterolemia, % | 58.1 | 52.2 |
| Family history of diabetes, % | 45.5 | 38.4 |
| Family history of MI, % | 28.1 | 33.2 |
| Aspirin use, % | 51.1 | 54.7 |
| Multivitamin use, % | 53.8 | 52.9 |
Values are means (SD) for continuous variables or percentages for categorical variables; NHS: Nurses’ Health Study; HPFS: Health Professionals Follow-Up Study; MI: myocardial infarction.
The Alternate Healthy Eating Index score ranged from 15.5 to 95.1, with a higher score indicating a healthier diet.
Medians (interquartile range).
A total of 2,311 incident CVD cases (including 498 stroke cases) and 858 CVD deaths were documented during a mean follow-up of 13.3 years. The multivariate-adjusted HRs of total CVD, CHD and stroke incidence by individual factors and the healthy lifestyle score are shown in Table 2. Comparing participants with zero low-risk lifestyle factor, the participants with three or more low-risk lifestyle factors had HRs (95% CIs) of 0.48 (0.40–0.59) for total CVD incidence, 0.53 (0.42–0.66) for CHD incidence, and 0.33 (0.21–0.51) for stroke incidence (all P trend<0.001).
Table 2.
Hazard ratio (95% CI) of CVD, CHD, and stroke incidence according to individual and combined lifestyle factors after diabetes diagnosis
| Person Years | CVD Incidence | CHD Incidence | Stroke Incidence | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Cases | HR* (95% CI) | Cases | HR* (95% CI) | Cases | HR* (95% CI) | ||
| Cigarette smoking | |||||||
| Never | 66079 | 902 | 1.00 (ref) | 696 | 1.00 (ref) | 217 | 1.00 (ref) |
| Past | 72009 | 1108 | 1.09 (0.99–1.19) | 917 | 1.14 (1.03–1.26) | 207 | 0.91 (0.75–1.11) |
| Current 1–14 cigarettes/day | 5669 | 124 | 1.78 (1.47–2.16) | 94 | 1.74 (1.40–2.17) | 31 | 1.88 (1.28–2.76) |
| Current ≥15 cigarettes/day | 7209 | 177 | 2.09 (1.76–2.47) | 137 | 2.09 (1.73–2.54) | 43 | 2.08 (1.47–2.95) |
| Alcohol consumption (g/day) | |||||||
| 0 | 81615 | 1286 | 1.00 (ref) | 1015 | 1.00 (ref) | 288 | 1.00 (ref) |
| 1.0–4.9 | 37982 | 533 | 0.89 (0.80–0.99) | 423 | 0.86 (0.77–0.97) | 116 | 0.99 (0.79–1.24) |
| 5.0–14.9 | 18333 | 304 | 0.93 (0.81–1.06) | 249 | 0.90 (0.78–1.05) | 61 | 1.06 (0.79–1.41) |
| ≥15.0 † | 13035 | 188 | 0.73 (0.62–0.86) | 157 | 0.71 (0.59–0.85) | 33 | 0.78 (0.53–1.14) |
| Physical activity (hours/week) | |||||||
| 0 | 85436 | 1421 | 1.00 (ref) | 1112 | 1.00 (ref) | 326 | 1.00 (ref) |
| 0.1–0.9 | 28895 | 396 | 0.94 (0.84–1.06) | 323 | 0.98 (0.86–1.11) | 78 | 0.82 (0.63–1.06) |
| 1.0–3.4 | 14680 | 220 | 1.02 (0.88–1.18) | 183 | 1.05 (0.90–1.24) | 39 | 0.87 (0.62–1.23) |
| ≥3.5 | 21955 | 274 | 0.87 (0.76–0.99) | 226 | 0.88 (0.76–1.02) | 55 | 0.86 (0.64–1.17) |
| Alternative healthy eating index (quintiles) | |||||||
| Q 1 | 31223 | 517 | 1.00 (ref) | 400 | 1.00 (ref) | 121 | 1.00 (ref) |
| Q 2 | 30389 | 487 | 0.97 (0.86–1.11) | 397 | 1.02 (0.89–1.18) | 100 | 0.87 (0.66–1.13) |
| Q 3 | 30208 | 437 | 0.89 (0.79–1.02) | 348 | 0.91 (0.79–1.05) | 92 | 0.83 (0.63–1.09) |
| Q 4 | 30604 | 474 | 0.93 (0.82–1.06) | 379 | 0.96 (0.83–1.10) | 101 | 0.88 (0.67–1.15) |
| Q 5 | 28542 | 396 | 0.84 (0.74–0.97) | 320 | 0.87 (0.75–1.02) | 84 | 0.78 (0.59–1.04) |
| Number of low-risk factors ‡ | |||||||
| None | 6443 | 148 | 1.00 (ref) | 113 | 1.00 (ref) | 38 | 1.00 (ref) |
| One | 63036 | 987 | 0.62 (0.52–0.74) | 782 | 0.64 (0.52–0.78) | 212 | 0.52 (0.36–0.74) |
| Two | 56610 | 840 | 0.55 (0.46–0.66) | 658 | 0.56 (0.45–0.69) | 199 | 0.54 (0.38–0.78) |
| Three or more | 24877 | 336 | 0.48 (0.40–0.59) | 291 | 0.53 (0.42–0.66) | 49 | 0.33 (0.21–0.51) |
Adjusted for age (years), sex (men or women), ethnicity (Caucasian, African American, Hispanic, or Asian), body mass index at diabetes diagnosis (<25.0, 25.0–29.9, 30.0–34.9, ≥35.0 kg/m2), menopausal status (women only), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), current aspirin use (yes/no), current multivitamin use (yes/no), and diabetes duration (years). Individual lifestyle factors were mutually adjusted.
Less than 1% of the patients had alcohol consumption >45 g/day at diabetes diagnosis.
Low-risk lifestyle factors: non-smoking, moderate to vigorous physical activity (≥150 min/week), high quality diet (top two fifths of Alternative Healthy Eating Index), and moderate alcohol consumption (5–15 g/day for women and 5–30 g/day for men).
For low-risk lifestyle factors and CVD mortality, a similar pattern of association was observed (Table 3). Comparing participants who adhered to ≥3 low-risk lifestyle factors with those who adhered to none, the HR (95% CI) was 0.32 (0.22–0.47) for CVD mortality (P trend<0.001). The PAR (95% CI) for poor adherence to an overall healthy lifestyle (defined as <3 low-risk factors) was 40.9% (28.5%–52.0%) for CVD mortality.
Table 3.
Hazard ratio (95% CI) of CVD mortality according to individual and combined lifestyle factors after diabetes diagnosis
| Person Years | CVD Mortality | ||
|---|---|---|---|
|
| |||
| Cases | HR* (95% CI) | ||
| Cigarette smoking | |||
| Never | 72333 | 320 | 1.00 (ref) |
| Past | 79909 | 457 | 1.27 (1.09–1.47) |
| Current 1–14 cigarettes/day | 6284 | 35 | 1.57 (1.10–2.24) |
| Current ≥15 cigarettes/day | 7989 | 46 | 2.21 (1.60–3.04) |
| Alcohol consumption (g/day) | |||
| 0 | 92009 | 555 | 1.00 (ref) |
| 1.0–4.9 | 41150 | 163 | 0.82 (0.69–0.99) |
| 5.0–14.9 | 19652 | 73 | 0.62 (0.48–0.80) |
| ≥15.0 | 13705 | 67 | 0.81 (0.62–1.07) |
| Physical activity (hours/week) | |||
| 0 | 95684 | 629 | 1.00 (ref) |
| 0.1–0.9 | 31717 | 121 | 0.79 (0.65–0.97) |
| 1.0–3.4 | 15973 | 46 | 0.65 (0.48–0.88) |
| ≥3.5 | 23142 | 62 | 0.62 (0.47–0.82) |
| Alternative healthy eating index (quintiles) | |||
| Q 1 | 34514 | 213 | 1.00 (ref) |
| Q 2 | 33691 | 188 | 0.97 (0.79–1.19) |
| Q 3 | 33219 | 168 | 0.90 (0.74–1.11) |
| Q 4 | 33781 | 158 | 0.84 (0.68–1.04) |
| Q 5 | 31312 | 131 | 0.77 (0.62–0.97) |
| Number of low-risk factors† | |||
| None | 7215 | 47 | 1.00 (ref) |
| One | 70510 | 442 | 0.63 (0.46–0.86) |
| Two | 62348 | 294 | 0.46 (0.34–0.64) |
| Three or more | 26443 | 75 | 0.32 (0.22–0.47) |
| PAR‡, % | 40.9 (28.5–52.0) | ||
Adjusted for age (years), sex (men or women), ethnicity (Caucasian, African American, Hispanic, or Asian), body mass index at diabetes diagnosis (<25.0, 25.0–29.9, 30.0–34.9, ≥35.0 kg/m2), menopausal status (women only), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), current aspirin use (yes/no), current multivitamin use (yes/no), and diabetes duration (years). Individual lifestyle factors were mutually adjusted.
Low-risk lifestyle factors: non-smoking, moderate to vigorous physical activity (≥150 min/week), high quality diet (top two fifths of Alternative Healthy Eating Index), and moderate alcohol consumption (5–15 g/day for women and 5–30 g/day for men).
PAR: population attributable risk, theoretically attributable to non-adherence to three or more low-risk lifestyle factors.
The results were largely unchanged with further adjustment of presence of hypertension or hypercholesterolemia and use of antihypertensive or cholesterol-lowering drugs (Online Table 1). In addition, the results did not materially change when diabetes medication use and HbA1c levels were further controlled for in a subset of the study participants (Online Table 2). No significant interaction was observed between lifestyle factors and HbA1c levels.
Greater improvements in lifestyle factors from pre- to post-diabetes diagnosis were also significantly associated with a lower risk of CVD incidence and mortality (Table 4). Comparing participants without changes in lifestyle, the participants who improved their lifestyle had an HR (95% CI) of 0.79 (0.70–0.89) for total CVD incidence, 0.82 (0.72–0.94) for CHD incidence, 0.68 (0.52–0.89) for stroke incidence, and 0.80 (0.66–0.96) for CVD mortality (all P trend<0.001). For each number increment in low-risk lifestyle factors, there was a 14% lower risk of total CVD incidence, a 12% lower risk of CHD incidence, a 21% lower risk of stroke incidence, and a 27% lower risk of CVD mortality (all P <0.001) (Table 4). Similar results were observed when a different reference group was used: compared with the diabetic patients who maintained a lifestyle score <2 from pre- to post-diabetes diagnosis, those who changed the lifestyle score from <2 to ≥2 before and after diabetes diagnosis had a 19% (95% CI: 8%–29%) lower risk of CVD incidence and a 20% (95% CI: 2%–34%) lower risk of CVD mortality.
Table 4.
Hazard ratio (95% CI) of CVD incidence and mortality according to changes in healthy lifestyle score from pre- to post-diabetes diagnosis
| CVD Incidence | CHD Incidence | Stroke Incidence | CVD Mortality | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Cases* | HR† (95% CI) | Cases | HR† (95% CI) | Cases | HR† (95% CI) | Cases* | HR† (95% CI) | |
| Changes in lifestyle score (range) | ||||||||
| Decreased (−3, −1) | 468 | 1.13 (1.00–1.27) | 364 | 1.10 (0.96–1.25) | 112 | 1.24 (0.97–1.59) | 275 | 1.61 (1.34–1.93) |
| Unchanged (0, 0) | 1278 | 1.00 (ref) | 1008 | 1.00 (ref) | 284 | 1.00 (ref) | 411 | 1.00 (ref) |
| Increased (1, 3) | 427 | 0.79 (0.70–0.89) | 357 | 0.82 (0.72–0.94) | 76 | 0.68 (0.52–0.89) | 95 | 0.80 (0.66–0.96) |
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| HR‡ continuous | 0.86 (0.80–0.92) | 0.88 (0.82–0.95) | 0.79 (0.68–0.91) | 0.73 (0.66–0.82) | ||||
| Pcontinuous | <0.001 | <0.001 | <0.001 | <0.001 | ||||
After excluding the participants with missing information on lifestyle before and after diabetes diagnosis (n=375), there were 2173 CVD incident cases and 781 CVD deaths.
Low-risk lifestyle factors: non-smoking, moderate to vigorous physical activity (≥150 min/week), high quality diet (top two fifths of Alternative Healthy Eating Index), and moderate alcohol consumption (5–15 g/day for women and 5–30 g/day for men). The values were adjusted for age (years), sex (men or women), ethnicity (Caucasian, African American, Hispanic, or Asian), body mass index at diabetes diagnosis (<25.0, 25.0–29.9, 30.0–34.9, ≥35.0 kg/m2), menopausal status (women only), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), current aspirin use (yes/no), current multivitamin use (yes/no), diabetes duration (years), and healthy lifestyle score before diabetes diagnosis.
For per one number increment in low-risk lifestyle factors.
Consistent results were observed when analyses were stratified by age at diabetes diagnosis, BMI at diabetes diagnosis, smoking status after diabetes diagnosis (smoking status was excluded from the healthy lifestyle score in this analysis), diabetes duration, sex/cohort, and number of low-risk lifestyle factors before diabetes diagnosis (Table 5). No significant interactions were detected between these stratifying variables and the low-risk lifestyle score (all P interaction>0.1).
Table 5.
Stratified analysis of the association of CVD incidence and mortality with per one number increment in low-risk lifestyle factors*
| CVD Incidence | CVD Mortality | |||
|---|---|---|---|---|
|
|
||||
| Cases | HR† (95% CI) | Cases | HR† (95% CI) | |
| Age at diabetes diagnosis | ||||
| <65 | 1299 | 0.80 (0.74–0.86) | 435 | 0.65 (0.56–0.74) |
| ≥65 | 1012 | 0.92 (0.85–1.00) | 420 | 0.78 (0.68–0.90) |
| BMI at diabetes diagnosis | ||||
| <25.0 | 465 | 0.85 (0.76–0.95) | 245 | 0.64 (0.53–0.76) |
| 25.0–29.9 | 882 | 0.87 (0.80–0.95) | 306 | 0.77 (0.66–0.90) |
| ≥30.0 | 964 | 0.79 (0.72–0.87) | 307 | 0.68 (0.57–0.81) |
| Smoking status‡ | ||||
| Never | 902 | 0.86 (0.78–0.95) | 320 | 0.70 (0.58–0.84) |
| Past | 1108 | 0.94 (0.86–1.02) | 457 | 0.73 (0.64–0.85) |
| Current | 301 | 0.98 (0.82–1.18) | 81 | 0.78 (0.53–1.15) |
| Diabetes duration (years) | ||||
| <5 | 1093 | 0.85 (0.79–0.92) | 160 | 0.64 (0.52–0.80) |
| 5–9 | 586 | 0.87 (0.78–0.98) | 210 | 0.73 (0.60–0.89) |
| ≥10 | 632 | 0.81 (0.73–0.90) | 488 | 0.70 (0.61–0.80) |
| Sex/cohort | ||||
| Women (NHS) | 1624 | 0.81 (0.76–0.86) | 648 | 0.67 (0.60–0.75) |
| Men (HPFS) | 687 | 0.92 (0.84–1.02) | 210 | 0.81 (0.68–0.97) |
| Lifestyle score before T2D diagnosis§ | ||||
| <2 | 944 | 0.80 (0.72–0.89) | 348 | 0.82 (0.69–0.98) |
| ≥2 | 1229 | 0.90 (0.84–0.96) | 433 | 0.70 (0.62–0.79) |
Low-risk lifestyle factors: non-smoking, moderate to vigorous physical activity (≥150 min/week), high quality diet (top two fifths of Alternative Healthy Eating Index), and moderate alcohol consumption (5–15 g/day for women and 5–30 g/day for men). BMI: body mass index.
Adjusted for age (years), sex (men or women), ethnicity (Caucasian, African American, Hispanic, or Asian), body mass index at diabetes diagnosis (<25.0, 25.0–29.9, ≥30.0 kg/m2), current menopausal hormone use (yes/no), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), current aspirin use (yes/no), current multivitamin use (yes/no), and diabetes duration (years). The strata variable was not included in the model when stratifying by itself.
Smoking status was not included in the low-risk lifestyle factors.
After excluding the participants without data of covariates before diabetes diagnosis, the total number of incident CVD cases and CVD deaths was 2173 and 781, respectively.
In secondary analyses, similar results were observed when we used cumulative averages of AHEI score, physical activity, and alcohol consumption to compute the overall healthy lifestyle score. Comparing participants who adhered to ≥3 low-risk lifestyle factors with those who adhered to none, the HRs (95% CIs) were 0.46 (0.39–0.58) for CVD incidence and 0.31 (0.21–0.46) for CVD mortality. When 18.5≤BMI<25.0 kg/m2 at diabetes diagnosis was also included in the low-risk lifestyle score, the results did not change materially (Online Table 3). The different combinations of low-risk lifestyle factors in relation to CVD incidence and CVD mortality are demonstrated in Online Table 4. When we only considered diet and alcohol consumption in the healthy lifestyle score, comparing ≥1 with 0 low-risk lifestyle factors, the HRs (95% CIs) were 0.91 (0.84, 1.00) for CVD incidence and 0.81 (0.71, 0.94) for CVD mortality. When moderate physical activity was further included in the score, comparing ≥2 with 0 low-risk factors, the HRs (95% CIs) were 0.85 (0.75, 0.96) for CVD incidence and 0.53 (0.42, 0.66) for CVD mortality. Further adding non-smoking to the lifestyle score yielded the same estimates of associations as in Tables 2 and 3. For the associations of the lifestyle factors before diabetes diagnosis with CVD incidence and mortality, similar results were observed (Online Table 5). Online Figure 2 shows that adherence to a healthy lifestyle is significantly associated with a lower risk of subsequent CVD events among diabetic patients with hypertension and/or hypercholesterolemia. The results remained similar when analyses were stratified before/after 1998, when complete data were used, or when deaths occurred within 4 years of diabetes diagnosis were excluded.
Discussion
In these two large prospective cohort studies among U.S. men and women with incident diabetes, we found that an overall healthy lifestyle after diabetes diagnosis, defined as eating a high quality diet, non-smoking, engaging in moderate- to vigorous-intensity physical activity, and drinking alcohol in moderation, was significantly associated with a lower risk of CVD incidence and CVD mortality. This association was independent of established CVD risk factors, including diabetes duration, BMI, medication use, and lifestyle before diabetes diagnosis. In addition, greater improvements in these lifestyle factors from pre- to post-diabetes diagnosis were also significantly associated with a lower risk of subsequent CVD events.
Comparison with other studies
It is well-established that a healthy lifestyle is associated with a lower risk of cardiometabolic diseases and mortality in largely healthy populations (4,5). Several lifestyle intervention trials among individuals at an elevated risk of developing diabetes or CVD demonstrated beneficial effects of lifestyle modification on reducing diabetes risk and improving cardiovascular health (8–12). For example, in the China Da Qing Diabetes Prevention Study, lifestyle interventions through improving diet quality and increasing physical activities over 6 years substantially reduced the incidence of diabetes, CVD, and total mortality among individuals with impaired glucose tolerance (9,10). In the Diabetes Prevention Program trial and the Finnish Diabetes Prevention Study, both conducted among individuals at high risk of diabetes, lifestyle interventions, including improving diet quality and promoting moderate intensity physical activity, significantly improved CVD risk profiles and reduced diabetes incidence (8,12,34), although CVD incidence was not significantly reduced (34,35), probably due to relatively short follow-up duration
Regarding associations between lifestyle and health outcomes among diabetes patients, previous studies largely focused on associations of adhering to an overall healthy lifestyle with total mortality (13–15). Data linking lifestyle with incident CVD events are sparse in observational studies, and existing evidence from intervention studies in this regard is somewhat mixed (6,41,42). For instance, in the Steno-2 Study among 160 patients with T2D and microalbuminuria, behaviour modification (i.e., improvements in diet quality and physical activity) together with use of medications (i.e., antihypertensive drugs and aspirin) significantly reduced the risk of CVD events after an average of 7.8 years of follow-up (41). In contrast, in the Look AHEAD trial among 5145 overweight or obese patients with T2D, an intensive lifestyle intervention focusing on weight loss through decreased caloric intake and increased physical activity resulted in significant improvements in body weight, HbA1c, systolic blood pressure, and HDL-cholesterol at 4 years of follow-up (43), but the risk of cardiovascular events did not change significantly (42). For these intervention studies among diabetic patients, the relatively short intervention or follow-up duration, small between-group differences in lifestyle change, or the varied adherence of the participants may at least partially explain the inconsistent findings.
In the current study, we addressed a few major limitations in previous studies by using repeated assessments (every 2–4 years) of dietary and lifestyle factors to capture potential variations of lifestyle practices, examining both CHD and stroke incidence and CVD mortality, and evaluating associations of an overall healthy lifestyle. Moreover, we further illustrated that improvements in lifestyle behaviour from pre- to post-diabetes diagnosis were also associated with a significantly lower risk of subsequent CVD events. Our findings were in line with those observed in the ADDITION-Cambridge study among 867 newly diagnosed diabetic patients, where a greater number of healthy behaviour changes within the first year of diagnosis were associated with a lower risk of cardiovascular outcomes (44). Overall, our findings and existing evidence suggest that adhering to an overall healthy lifestyle before and after diabetes diagnosis can significantly aid in the prevention of CVD complications among patients with T2D.
Strengths and limitations
The strengths of our study included a relatively large sample size, long-term follow-up with a high follow-up rate (>90%), repeated assessments of dietary and lifestyle factors before and after diabetes diagnosis, and adjudicated disease outcomes.
Several limitations should be discussed as well. First, the study participants were all health professionals, and most were Caucasians. Although the relative homogeneity could alleviate confounding by socioeconomic status, caution must be taken when generalizing the findings to other ethnic groups. Second, the diabetic patients in our study were diagnosed during an extended period of time since 1980s. The risk profile of diabetic patients might significantly change over time due to better control of blood lipids and other risk factors in recent years, although similar results were found in analyses stratified before/after 1998. Third, measurement errors in self-reported assessments of dietary and lifestyle factors were inevitable, although our validation studies demonstrated reasonable validity of questionnaire assessments of these factors. In addition, such measurement errors were likely to be non-differential in this prospective study and thus would be more likely to bias the associations toward the null. Fourth, our study did not have direct measurements of glycemic control and severity of diabetes. However, the results remained similar when we further adjusted for duration of diabetes, use of insulin and hypoglycemic medications, and self-reported HbA1c levels, suggesting that our findings are unlikely explained by confounding due to the severity of diabetes. Fifth, the PAR calculation was based on the assumption of a causal relationship between the low-risk lifestyle factors and CVD risk, which may not hold true in observational studies. In particular, we could not exclude the role of confounding by genetic susceptibility, medication use, or psychosocial stress, residual confounding due to measurement errors of covariates, or chance in the current study. Lastly, the low-risk lifestyle factors considered in the current analysis may not necessarily represent all healthy behaviors.
Implications of findings
Our study has provided further evidence to suggest that adopting an overall healthy lifestyle, consisting of eating a high-quality diet, non-smoking, engaging in moderate to vigorous physical activity, and drinking alcohol in moderation, could be an affordable and effective prevention strategy for patients with T2D to reduce the risk of developing cardiovascular complications.
Conclusions
Our findings indicate that adherence to a healthy diet and lifestyle after diabetes diagnosis is associated with a substantially lower risk of CVD incidence and CVD mortality among adults with incident diabetes. In addition, greater improvements in lifestyle behaviour from pre- to post-diabetes diagnosis are also associated with a lower risk of subsequent CVD events. These findings further support the current recommendation that patients with diabetes should practice a healthy lifestyle to improve their health and maintain a lower risk of developing cardiovascular complications.
Supplementary Material
Central Illustration. Healthy lifestyle and CVD Events among Diabetic Patients.
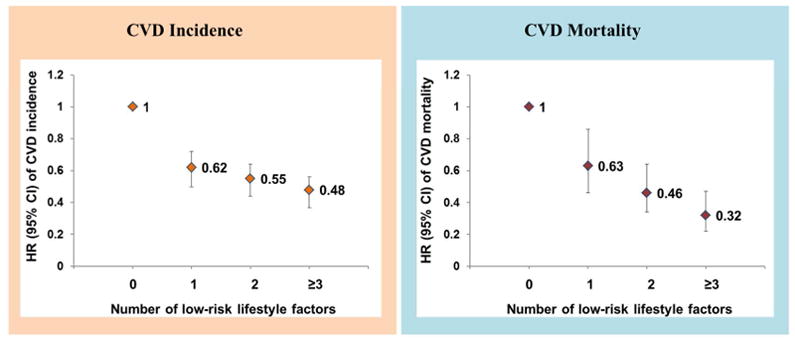
Hazard ratio (95% CI) of CVD incidence and CVD mortality according to number of low-risk lifestyle factors among patients with type 2 diabetes. Low-risk lifestyle factors: non-smoking, moderate to vigorous physical activity (≥150 min/week), high-quality diet (top two fifths of Alternative Healthy Eating Index), and moderate alcohol consumption (5–15 g/day for women and 5–30 g/day for men). Multivariable model was adjusted for age (years), sex (men or women), ethnicity (Caucasian, African American, Hispanic, or Asian), body mass index at diabetes diagnosis (<25.0, 25.0–29.9, 30.0–34.9, ≥35.0 kg/m2), menopausal status (women only), family history of diabetes (yes/no), family history of myocardial infarction (yes/no), current aspirin use (yes/no), current multivitamin use (yes/no), and diabetes duration (years).
CLINICAL PERSPECTIVES.
Competency in Patient Care
Adherence to a healthy diet and lifestyle and correction of past risk-prone behavior are associated with substantially lower risks of cardiovascular disease and related mortality among patients with type 2 diabetes mellitus.
Translational Outlook
Further research is needed to identify the most effective strategies to encourage patients with diabetes to adopt and maintain a healthy lifestyle.
Acknowledgments
Funding: This study was sponsored by the National Institutes of Health, CA186107, CA176726, CA167552, DK082486, HL35464, DK058845, and HL034594.
ABBREVIATIONS AND ACRONYMS
- AHEI
alternate healthy eating index
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CI
confidence interval
- HR
hazard ratio
- MI
myocardial infarction
- T2D
type 2 diabetes
Footnotes
Disclosures: We declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardiovascular Disease and Risk Management. Sec. 9 In Standards of Medical Care in Diabetes-2017. Diabetes Care. 2017;40:S75–S87. doi: 10.2337/dc17-S012. [DOI] [PubMed] [Google Scholar]
- 3.Lifestyle Management. Sec. 4. Lifestyle Management Diabetes Care. 2017;40:S33–S43. doi: 10.2337/dc17-S007. [DOI] [PubMed] [Google Scholar]
- 4.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163–70. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:543–51. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–9. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 9.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–9. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 10.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–80. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 11.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 12.Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–75. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CC, Li CI, Liu CS, et al. Impact of lifestyle-related factors on all-cause and cause-specific mortality in patients with type 2 diabetes: the Taichung Diabetes Study. Diabetes Care. 2012;35:105–12. doi: 10.2337/dc11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel YR, Gadiraju TV, Gaziano JM, Djousse L. Adherence to healthy lifestyle factors and risk of death in men with diabetes mellitus: The Physicians' Health Study. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.11.003. pii: S0261-5614:31317–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NÖTHLINGS Ute, FORD Earl S, KRÖGER Janine, BOEING H. Lifestyle factors and mortality among adults with diabetes: findings from the European Prospective Investigation into Cancer and Nutrition–Potsdam study. J Diabetes. 2010;2:112–117. doi: 10.1111/j.1753-0407.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317:1303–9. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- 17.Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67:933–8. doi: 10.1016/0002-9149(91)90163-f. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 21.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–8. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Committee Report 2008. Washington, DC: United States Department of Health and Human Services; 2008. p. 683. [Google Scholar]
- 27.Veronese N, Li Y, Manson JE, Willett WC, Fontana L, Hu FB. Combined associations of body weight and lifestyle factors with all cause and cause specific mortality in men and women: prospective cohort study. BMJ. 2016;355:i5855. doi: 10.1136/bmj.i5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–8. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 30.Rose GA. Cardiovascular survey methods. Geneva Albany, NY: World Health Organization; WHO Publications Centre distributor; 1982. [Google Scholar]
- 31.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings Stroke. 1981;12:I13–44. [PubMed] [Google Scholar]
- 32.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 33.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risk in cohort studies: Examples and Software. Cancer Causes and Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 34.Ratner R, Goldberg R, Haffner S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28:888–94. doi: 10.2337/diacare.28.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uusitupa M, Peltonen M, Lindstrom J, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS One. 2009;4:e5656. doi: 10.1371/journal.pone.0005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonaccio M, Di Castelnuovo A, Costanzo S, et al. Adherence to the traditional Mediterranean diet and mortality in subjects with diabetes. Prospective results from the MOLI-SANI study. Eur J Prev Cardiol. 2016;23:400–7. doi: 10.1177/2047487315569409. [DOI] [PubMed] [Google Scholar]
- 37.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49:648–52. doi: 10.1007/s00125-005-0127-x. [DOI] [PubMed] [Google Scholar]
- 38.Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation. 2015;132:1795–804. doi: 10.1161/CIRCULATIONAHA.115.017926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu G, Eriksson J, Barengo NC, et al. Occupational, commuting, and leisure-time physical activity in relation to total and cardiovascular mortality among Finnish subjects with type 2 diabetes. Circulation. 2004;110:666–73. doi: 10.1161/01.CIR.0000138102.23783.94. [DOI] [PubMed] [Google Scholar]
- 40.Balducci S, Zanuso S, Cardelli P, et al. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES) Diabetes Care. 2012;35:1347–54. doi: 10.2337/dc11-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 42.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long GH, Cooper AJ, Wareham NJ, Griffin SJ, Simmons RK. Healthy behavior change and cardiovascular outcomes in newly diagnosed type 2 diabetic patients: a cohort analysis of the ADDITION-Cambridge study. Diabetes Care. 2014;37:1712–20. doi: 10.2337/dc13-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


