Boron promotes PIN2-based polar auxin transport in the apical transition zone of pea (Pisum sativum) roots, leading to root alkalization and thereby reducing aluminum toxicity.
Abstract
Boron (B) alleviates aluminum (Al) toxicity in higher plants; however, the underlying mechanisms behind this phenomenon remain unknown. Here, we used bromocresol green pH indicator, noninvasive microtest, and microelectrode ion flux estimation techniques to demonstrate that B promotes root surface pH gradients in pea (Pisum sativum) roots, leading to alkalization in the root transition zone and acidification in the elongation zone, while Al inhibits these pH gradients. B significantly decreased Al accumulation in the transition zone (∼1.0–2.5 mm from the apex) of lateral roots, thereby alleviating Al-induced inhibition of root elongation. Net indole acetic acid (IAA) efflux detected by an IAA-sensitive platinum microelectrode showed that polar auxin transport, which peaked in the root transition zone, was inhibited by Al toxicity, while it was partially recovered by B. Electrophysiological experiments using the Arabidopsis (Arabidopsis thaliana) auxin transporter mutants (auxin resistant1-7; pin-formed2 [pin2]) and the specific polar auxin transporter inhibitor1-naphthylphthalamic acid showed that PIN2-based polar auxin transport is involved in root surface alkalization in the transition zone. Our results suggest that B promotes polar auxin transport driven by the auxin efflux transporter PIN2 and leads to the downstream regulation of the plasma membrane-H+-ATPase, resulting in elevated root surface pH, which is essential to decrease Al accumulation in this Al-targeted apical root zone. These findings provide a mechanistic explanation for the role of exogenous B in alleviation of Al accumulation and toxicity in plants.
Aluminum (Al) toxicity is a limiting factor for plant growth in acidic soils (Kochian, 1995). As Al toxicity primarily affects cell elongation rather than cell division (Čiamporová, 2002; Ryan and Delhaize, 2010), the root elongation zone is usually considered to be affected by Al toxicity. More recently, the transition zone (also termed the distal transition zone or distal elongation zone), the zone between the meristem and elongation zone (Baluška et al., 2001, 2010; Verbelen et al., 2006), has been shown to be the most sensitive to Al, as evidenced by higher Al accumulation, higher callose induction, and more severe inhibition of root elongation (Sivaguru and Horst, 1998; Sivaguru et al., 1999; Kollmeier et al., 2000). The underlying reasons for this sensitivity to Al remain unknown, with alteration in microtubules and actin filaments (Sivaguru et al., 1999; Amenós et al., 2009), basipetal auxin transport (Kollmeier et al., 2000), and surface pH (Kollmeier et al., 2000) being potentially responsible.
Generally, the root apoplast is the target of Al accumulation, and the amount of surface Al is determined by two contributing factors, the content of unmethylated pectins for binding Al (Stass et al., 2007; Yang et al., 2008; Horst et al., 2010) and the amount (activity) of free Al3+ (Wagatsuma and Ezoe, 1985). The highest Al sensitivity is based on the highest content of unmethylated pectin in the root transition zone (Li et al., 2016). Therefore, factors affecting the amount of Al3+ available to bind to pectin in the cells in the root transition zone may determine the Al resistance or sensitivity of different cultivars (Degenhardt et al., 1998). The Al resistance is also based on the increased root surface pH, as shown in an aluminum-resistant (alr) chromosome 4 mutant, alr-104, of Arabidopsis (Arabidopsis thaliana; Degenhardt et al., 1998). This led to a conclusion that variations in the root surface pH contributed to the sensitivity or resistance to Al toxicity, as the activity of Al3+ is pH dependent (Wagatsuma and Ezoe, 1985).
As an essential micronutrient, boron (B) functions mainly in cross-linking two RGII pectins and thereby stabilizing cell wall structure (Kobayashi et al., 1996; O’Neill et al., 2001; Goldbach and Wimmer, 2007; Voxeur and Fry, 2014). The lack of B results in a disruption of cell wall formation and also in an inhibition of endocytosis and cell elongation (Fleischer et al., 1999; Yu et al., 2002; Pan et al., 2012). Boron may also play an important role in plasma membrane functions (Wimmer et al., 2009; Wang et al., 2010; Voxeur et al., 2014). As a result, B is essential for maintaining the function of the cell wall-plasma membrane-cytoskeleton continuum (Sardar et al., 2006), which is also the target of Al toxicity (Sivaguru et al., 1999; Baluška et al., 2003; Horst et al., 2010). Thus, it has been hypothesized that B supply may alleviate Al toxicity in higher plants (Lenoble et al., 1996a,b; Stass et al., 2007; Corrales et al., 2008; Yu et al., 2009). Surplus B alleviates Al toxicity in squash (Cucurbita sp.) and alfalfa (Medicago sativa; Lenoble et al., 1996a,b), while B deficiency aggravates Al toxicity in common bean (Phaseolus vulgaris; Stass et al., 2007). Boron pretreatment decreases root Al uptake and its translocation to the shoot in pea (Pisum sativum), thus alleviating Al toxicity (Yu et al., 2009). However, no such evidence was found for some monocotyledonous plants (e.g. maize [Zea mays; Wang et al., 2005] and, despite the less-severe Al toxicity observed in root tips of B-treated cucumber (Cucumis sativus), B-treated plants showed higher Al accumulation (Corrales et al., 2008).
The accumulation of Al is not uniform between functionally different root zones, and it is higher in the root transition zone, which is most sensitive to Al toxicity (Sivaguru and Horst, 1998). We thus speculate that B may affect the unequal Al accumulation in root zones and may possibly reduce Al accumulation in the root transition zone, as the content of unmethylated pectin is increased by B deficiency in common bean (Stass et al., 2007). Therefore, factors affecting the activity of Al3+ may be more crucial for Al accumulation in the root transition zone, especially in B-deficient plants. Organic acids and alkalization might be involved in deactivating Al3+ in the root transition zone. However, the effect of B in common bean could not be explained by the differences in citrate exudation, despite the latter being an essential mechanism conferring Al resistance in plants (Stass et al., 2007). On the other hand, the activity of Al3+ is directly related to the apoplastic pH (Wagatsuma and Ezoe, 1985; Wang et al., 2015). Apoplastic/surface pH differs between root zones in Arabidopsis and maize (Kollmeier et al., 2000; Staal et al., 2011). It is thus hypothesized that B may enhance Al tolerance by promoting root surface alkalization and limiting Al accumulation in the transition zone of pea.
Here, we present data supporting this notion. Our results suggest that B promotes root surface alkalization, which is regulated by polar auxin transport (PAT) and leads to the downstream regulation of plasma membrane (PM)-H+-ATPase, thus alleviating Al accumulation and toxicity in plants.
RESULTS
B Deficiency Disrupts Al-Induced Inhibition of Root Elongation by Increasing the Accumulation of Al in the Transition Zone of Lateral Pea Roots
In the lateral pea roots, the root transition zone extends between 1,188 ± 20 µm (mean ± se; n = 134) and 2,242 ± 41 µm (mean ± se; n = 109) from the tip of the root (Supplemental Fig. S1). Thus, the lateral root sections 0 to 1, 1 to 2.5, 2.5 to 5, and 5 to 10 mm represent the root cap and meristem, the transition zone, the elongation zone, and the mature zone, respectively.
The elongation rate of the lateral roots in B-deficient plants (−B) was several-fold smaller compared with the B-sufficient plants (+B; Fig. 1A). After being subjected to 15 µm AlCl3, root elongation was dramatically inhibited in both −B and +B plants (Fig. 1A). Under Al stress, the relative root elongation was only about 40% in −B plants, whereas it was 60% in +B plants (Fig. 1B), indicating a beneficial impact of B supply for plant resistance to Al stress.
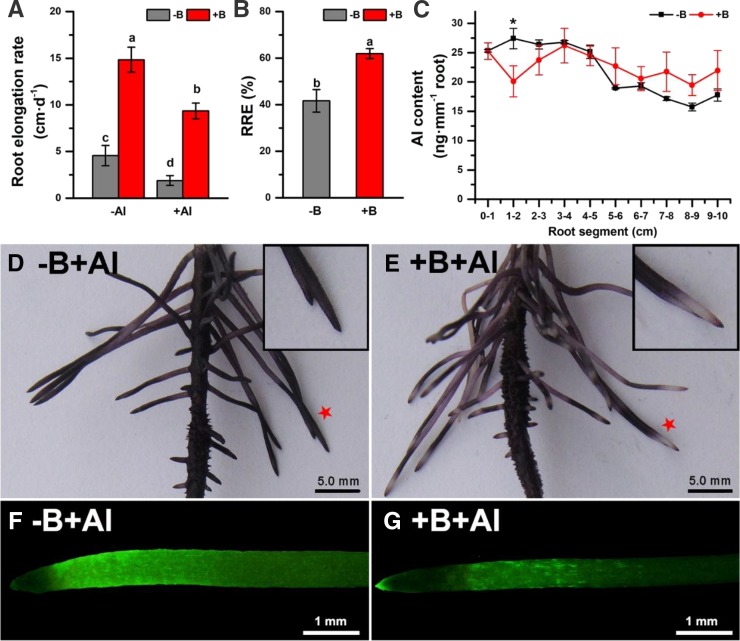
Figure 1.
Boron deficiency exacerbates Al-induced inhibition of root elongation by stimulating Al accumulation in the transition zone of the lateral roots of pea. Four-day-old seedlings were pretreated with two levels of boron (0 or 25 µm H3BO3; abbreviated as −B/+B, respectively) for 2 d and then exposed to either 0 or 15 μm AlCl3 solution (pH 4.0) for 24 h. A, Root elongation rate. B, The relative root elongation rate calculated as a rate of root elongation in plants exposed to Al treatment expressed as % of those without Al treatment. Mean ± se (n = 10–15). Different letters indicate significant differences between treatments at P < 0.05. C, Al content in the adjacent 1-mm-long sections within the 0- to 10-mm root tip segment. Mean ± se (n = 5 individual plants). An asterisk indicates a significant difference between −B and +B treatments at P < 0.05. D and E, Hematoxylin staining for the visualization of Al accumulation in various root zones. Insets are a magnification of some selected root tips (labeled with red asterisks). F and G, Morin staining for the visualization of the loosely bound Al accumulated in root zones. Four-day-old seedlings were pretreated with −B/+B (either 0 or 25 µm H3BO3) for 2 d and then exposed to either 0 or 30 μm AlCl3 solution (pH 4.5) for 12 h.
We then determined the content of Al in each 1-mm section in the 10-mm-long root apexes to investigate the effects of B on Al accumulation and distribution in functionally different root zones. The highest Al content was found in the root transition zone (1- to 2-mm segment from the apex) in −B plants, while in +B plants it was in the elongation zone (3- to 4-mm segment; Fig. 1C). The content of Al in the root transition zone was significantly higher in −B plants than in +B plants. Al content in the subsequent zones tended to be relatively higher in +B plants than in −B plants (although not significant at P < 0.05; Fig. 1C).
Hematoxylin staining was used to visualize the Al distribution in the roots. These experiments also disclosed similar distribution patterns of the total Al in the lateral roots. The root tip of −B plants was completely stained (Fig. 1D and inset) by hematoxylin; however, a clearly identifiable nonstained area (about 1.5 mm in length) overlapping with the root transition zone was present in +B plants (Fig. 1E and inset).
Morin staining was adopted to detect the loosely bound Al fraction in the cell wall (Eticha et al., 2005). Morin preferentially stained the cells in the root transition zone than in the other root zones (Fig. 1F), indicating that the loosely bound Al was mainly distributed in the root transition zone. Boron reduced the extent of morin staining in the root transition zone (Fig. 1G), supporting the notion that the presence of B reduces the amount of loosely bound Al in plant roots.
B Deficiency and Al Toxicity Decrease the Root Surface pH in the Root Transition Zone
To test whether the preferential Al distribution in the root transition zone was due to pH gradients observed in other plants (Staal et al., 2011), we detected changes in the rhizosphere pH using a bromocresol green. This pH indicator shows a color transition from blue at pH 5.4 to yellow at pH 3.8. The pH of the agar media was 4.0 at the beginning of the experiment. As shown in Figure 2A, the pH profiles along the root axis differed dramatically between the apical (blue color; indicating higher pH) and distal (yellow color; lower pH) root parts, indicating the process of alkalization and acidification, respectively. Less alkalization in the apical root parts occurred in −B plants than in +B plants in the absence of Al (Fig. 2A). Al inclusion inhibited the alkalization of the apical root parts and made the differences in pH profiles between the two root zones less obvious. However, the rhizosphere around the root apex was still alkalized more in +B plants than −B plants in the presence of Al (Fig. 2A).
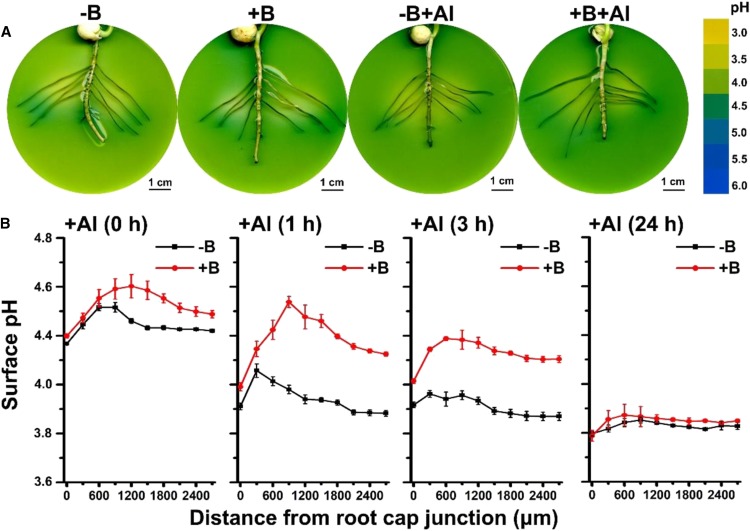
Figure 2.
Boron deficiency and Al toxicity modify the pH profile along the root axis and inhibit root surface alkalization in the transition zone of the lateral roots of pea. A, Rhizosphere alkalization in the apical root parts and acidification in the distal root parts. Four-day-old agar-grown seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 100 µm AlCl3 (pH 4.0) for 24 h. Changes in the rhizosphere pH were recorded by a pH indicator, bromocresol green. The colored scale on the right is a pH reference scale of the agar media measured in the absence of plants in a pH range from 3.0 to 6.0, at 0.5 pH unit intervals. B, Surface pH profile along pea root tips. Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 15 µm AlCl3 treatment (pH 4.0) for 0 h 1 h, 3 h, or 24 h. Root surface pH was measured at 300-μm intervals along root axis by a H+-selective microelectrode calibrated in a set of standards (pH 3.5–6.0) using a Noninvasive Micro-Test system (NMT100 Series; Younger). The data are means ± se (n = 4 to 5 individual plants).
We then used pH-sensitive microelectrodes (noninvasive microtest [NMT] technique) to measure the surface pH of the apical root parts every 300 µm along the root axis starting from the root cap junction and up to 2,700 µm (Fig. 2B). Under acidic adaptation, +B plants had a relatively higher surface pH value than −B plants in the root transition zone (Fig. 2B). Al exposure decreased the surface pH in both −B and +B plants in a time-dependent manner (Fig. 2B). The biggest difference in the surface pH profiles along the root axis between −B and +B plants was found after 1 h of Al exposure (Fig. 2B). A pH peak was present at about 900 µm after 1 h of Al exposure (Fig. 2B), which significantly dropped at 3 h in +B plants (Fig. 2B). For −B plants, only a small peak was detected at 300 µm along the root axis after 1 h of Al exposure (Fig. 2B); this peak disappeared after 3 h of Al treatment (Fig. 2B). All values of the pH profile of −B plants were lower than those of +B plants after 1 h and 3 h of Al exposure (Fig. 2B). However, there was little difference between −B and +B plants after 24 h of Al exposure (Fig. 2B). After 24 h of exposure, the pH value was rather uniform along the root profile, in contrast to the curved line with a peak in the transition zone observed at 1 h of Al exposure (Fig. 2). These results indicate that both B deficiency and Al toxicity inhibit surface alkalization in the root transition zone and that B helps maintain a higher surface pH under Al toxicity.
The PM-H+-ATPase Is Involved in the Regulation of Surface Alkalization in the Root Transition Zone
Under acidic conditions, proton influx occurs in the root tips, peaking in the root transition zone, which coincides with the highest apoplastic pH in this zone (Newman, 2001; Staal et al., 2011). In this study, we used vanadate, a known inhibitor of PM-H+-ATPase, to investigate whether PM-H+-ATPase was involved in the regulation of surface alkalization in the root transition zone. After adding vanadate to the agar media, the apical root alkalization observed previously (Fig. 2A) disappeared, and much lower rhizosphere pH values were recorded (indicated by the yellow color of the bromocresol green dye; Fig. 3A). Also, root elongation was significantly inhibited in all treatments after the application of vanadate (Fig. 3B). This indicates that PM-H+-ATPase is involved in the regulation of surface alkalization in the root transition zone.
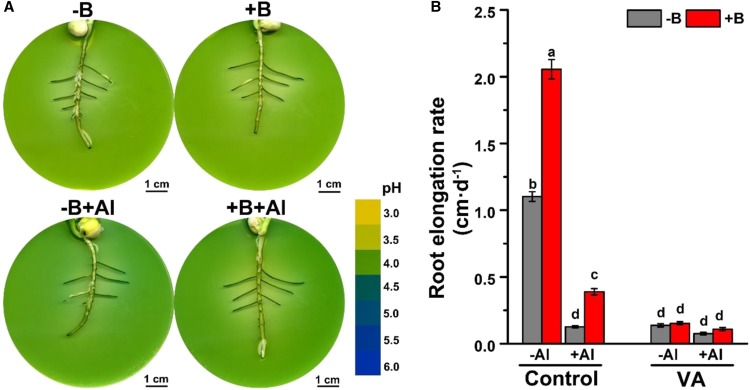
Figure 3.
Involvement of the PM-H+-ATPase in the regulation of the root surface pH in various zones of the lateral roots of pea. A, Inhibition of root apical alkalization by vanadate. Four-day-old agar-grown seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 100 µm AlCl3 (pH 4.0) for 24 h in the presence of vanadate (0.5 mm). Changes in the rhizosphere pH were recorded by a pH indicator, bromocresol green. The colored scale on the right is a pH reference scale of the agar media measured in the absence of plants in a pH range from 3.0 to 6.0, at 0.5 pH unit intervals. B, Inhibition of the root elongation by vanadate (VA). Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 15 µm AlCl3 treatment (pH 4.0) for 24 h in the absence or presence of vanadate (1.0 mm). The data are mean ± se (n = 6–8 individual plants). Different lowercase letters indicate significant differences between treatments at P < 0.05.
Polar Auxin Transport Is Involved in the Regulation of Root Surface Alkalization in the Root Transition Zone
Net IAA fluxes were determined in the root apices of pea in order to study whether polar auxin transport was involved in the regulation of surface alkalization in the root transition zone. The profiles of net IAA efflux were similar to the pH profiles along the root axis. Net IAA efflux peaked between 600 and 1,500 μm from the root cap junction, with the highest efflux detected at 900 μm in +B plants and 1,200 μm in −B plants (Fig. 4A). B deficiency significantly inhibited net IAA efflux in the meristem and the root transition zone. Al exposure for 3 h also significantly inhibited net IAA efflux in both −B and +B plants; this inhibition occurred predominately in the root transition zone (Fig. 4A). The net IAA efflux was lower in −B plants than that in +B plants in the meristem and transition zone after Al exposure. This indicates that Al toxicity inhibits IAA efflux in the root transition zone, while B deficiency inhibits IAA efflux in both the meristem and transition zones. B alleviates the Al-induced decrease of IAA efflux in the transition zone under Al toxicity.
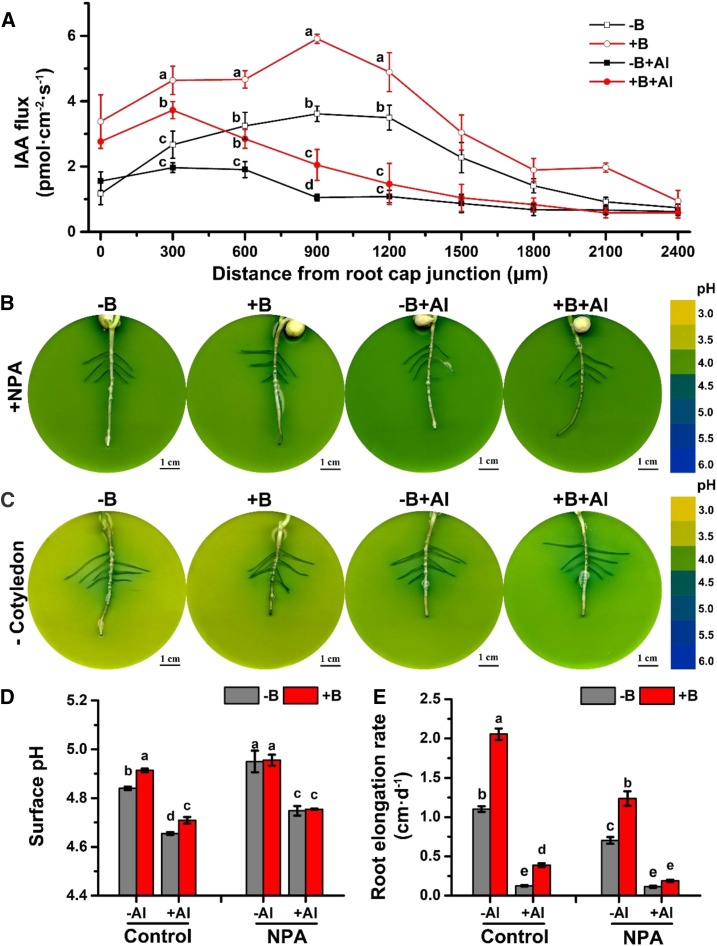
Figure 4.
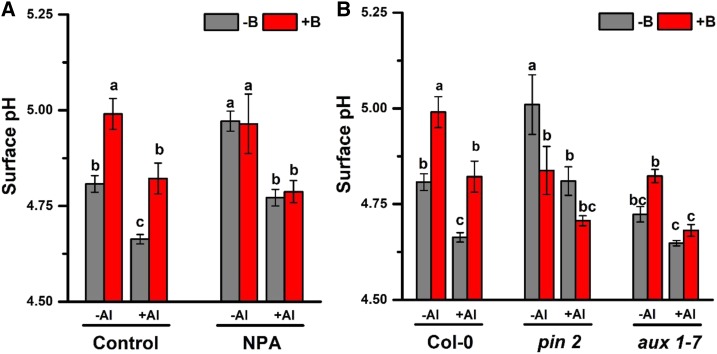
The impact of B and Al on polar auxin transport in the lateral root apices of pea (Pisum sativum). A, Inhibition of net IAA flux in pea root tips by Al toxicity and B deficiency. Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 15 µm AlCl3 solution (pH 4.0) for 3 h. IAA flux was measured at 300-μm intervals along the root axis by a platinum microelectrode using the NMT system. The data are means ± se (n = 4 individual plants). B and C, Changes in the rhizosphere pH recorded by a pH indicator, bromocresol green, in plants treated with 10 µm of NPA (1-naphthylphthalamic acid, a known inhibitor of the polar auxin transport), B, or triggered by the removal of the cotyledon, C. Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 100 µm AlCl3 treatment (pH 4.0) for 24 h either in the presence of NPA, B, or after the removal of the cotyledon, C. The colored scale on the right is a pH reference scale of the agar media measured in the absence of plants in a pH range from 3.0 to 6.0, at 0.5 pH unit intervals. D, Root surface pH in the transition root zone. Four-day-old seedlings were pretreated with −B/+B (either 0 or 25 µm H3BO3, respectively) for 36 h and then exposed to either 0 or 15 µm AlCl3 treatment (pH 4.0) for 3 h in the presence or absence of NPA (10 µm). The root surface pH was measured by the MIFE system. E, Inhibition of root elongation by NPA. Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 2 d and then exposed to either 0 or 15 µm AlCl3 treatment (pH 4.0) for 24 h in the absence and presence of NPA (10 µm). The data are means ± se (n = 10 individual plants). Different lowercase letters indicated significant differences between the treatments at P < 0.05.
In root tips, auxin transport is regulated by IAA influx (AUX/LIKE-AUXIN RESISTANT, AUX/LAX family) and efflux (PIN/ATP-binding cassette-B family) transporters (Tromas and Perrot-Rechenmann, 2010; Band et al., 2014). Polar auxin transport is controlled by auxin efflux transporters (PINs), which are predominantly expressed in the basal ends of epidermal cells (Habets and Offringa, 2014; Adamowski and Friml, 2015). When we applied the inhibitor of the auxin efflux transporter (1-naphthylphthalamic acid [NPA]; Ma and Robert, 2014; Zhu et al., 2016) to the agar media, both root apical alkalization and distal acidification, previously observed in root tips (Fig. 2A), were not present anymore (Fig. 4B). The entire root surface was stained blue (indicating high apoplastic pH), and the pH gradients between various root zones disappeared in all treatments (Fig. 4B). These observations were further quantified by measuring the surface pH in NPA-treated roots (Fig. 4D). A similar effect was observed when we prevented the supply of auxin to roots by excising the cotyledons before placing the seedling on the agar (Fig. 4C). Root elongation was arrested by the inhibition of PAT by the application of NPA in all the treatments except in plants with both −B and +Al (Fig. 4E). This indicates that the PAT-induced surface pH gradients (root apical alkalization and distal acidification) are essential for root elongation.
Although NPA inhibits polar auxin transport in the root and was expected to reduce auxin content in the cytoplasm (Di Mambro et al., 2017) in both −B and +B plants, the cytoplasmic auxin concentration was already intrinsically low in the root transition zone of +B plants, as B promotes PAT in the absence of NPA. The surface pH in the transition zone, detected by microelectrode ion flux estimation (MIFE), increased in −B plants in either −Al or +Al treatments after the addition of NPA (Fig. 4D). However, there was little difference in the root surface pH between −B and +B plants in either −Al or +Al treatments after the addition of NPA (Fig. 4D). Al toxicity decreased surface pH in both −B and +B plants under the NPA treatment (Fig. 4D), most likely due to the stimulation of auxin biosynthesis in the root transition zone induced by Al toxicity (Yang et al., 2014).
Further support for B promoting PAT and surface alkalization in the root transition zone under Al toxicity were obtained in experiments with Arabidopsis (Fig. 5A), where the beneficial effects of B on the alkalization of wild-type roots disappeared in the presence of NPA.
Figure 5.
Influence of the polar auxin transport on the surface pH of Arabidopsis roots exposed to B and Al treatments. A, Variation of the surface pH in the root transition zone of Arabidopsis (wild type Col-0). B, Variation in the surface pH in the root transition zone of Arabidopsis wild type (Col-0) and impaired auxin transport mutants pin2 and aux1-7. PIN2, auxin efflux transporter; AUX1, auxin influx transporter. Four-day-old seedlings were pretreated with −B/+B (0 or 25 µm H3BO3, respectively) for 24 h. The root surface pH in the transition zone was measured before or 30 min after the addition of 15 µm AlCl3 solution (pH 4.5), in the absence or presence of NPA (10 μm). The data are means ± se (n = 5 to 8 roots). Different lowercase letters indicate significant differences between treatments at P < 0.05.
We then adopted Arabidopsis aux1-7 and pin2 mutants, lacking functional PIN2 and AUX genes, to investigate whether auxin transporters are involved in the regulation of surface alkalization in the root transition zone. The surface pH of pin2 plants varied depending on the B level (Fig. 5B). The lack of PIN2 blocks auxin transport from the quiescent center to the root transition zone, thus resulting in a decreased auxin content and increased pH in B-deficient pin2 plants. Since B promotes PAT, this results in relatively higher surface pH in the root transition zone of plants with fully functional PIN2 (e.g. wild type). In pin2 mutants, however, B supply did not increase but instead decreased the root surface pH (Fig. 5B), possibly due to the enhanced function of auxin influx transporters AUX1 by B (Camacho-Cristóbal et al., 2015). Thus, the surface pH of pin2 mutants was much higher in −B plants than in +B plants (Fig. 5B). However, in aux1-7 mutants, B and Al had similar but reduced effects compared to the wild-type plants. Al toxicity decreased surface pH in both pin2 and aux1-7 mutants (Fig. 5B). This suggests that the mutation of AUX does not affect the root surface pH, while the mutation of PIN2 does. These results demonstrate that PAT driven by PIN2 is involved in the regulation of root surface alkalization in the root transition zone.
DISCUSSION
The results presented in this work suggest that B promotes PAT driven by the auxin efflux transporter PIN2 and leads to the downstream regulation of the PM-H+-ATPase, creating an elevated root surface pH that is essential to decrease Al accumulation in this Al-targeted apical root zone. These findings provide a mechanistic explanation for the role of exogenous B in alleviation of Al accumulation and toxicity in plants.
B Deficiency Enhances the Sensitivity of the Root Transition Zone to Al Toxicity
While the important role of B in preventing Al-induced inhibition of root growth is widely accepted (Lenoble et al., 1996a,b; Stass et al., 2007; Corrales et al., 2008; Yu et al., 2009), the underlying mechanisms remain elusive.
Both hematoxylin staining and inductively coupled plasma atomic emission spectroscopy analysis of the total Al content, as well as morin staining for loosely bound Al in the cell wall (Eticha et al., 2005), show the uneven accumulation of Al in various root zones, with the highest Al levels observed in the root transition zone under B deficiency (Fig. 1, C and D). These results are consistent with the bulk of published literature (Sivaguru and Horst, 1998; 1999; Kollmeier et al., 2000; Illés et al., 2006), considering that B was usually not added to the experimental media in most studies. However, a significantly lower Al content was found in the root transition zone after B treatment, while the highest content of Al was actually in the elongation zone (Fig. 1, C and D). This indicates that the root transition zone is sensitive to both Al toxicity and B deficiency, displaying the highest sensitivity to Al toxicity under B deficiency.
Aluminum accumulation in the elongation zone inhibited root elongation; however, this inhibition was lower in B-sufficient compared to B-deficient roots (Fig. 1, A and B). This demonstrates that the transition zone of pea roots is more sensitive to Al toxicity than the elongation zone, as was found in maize (Sivaguru and Horst, 1998). One possible reason is that there is a higher content of loosely bound Al by the cell wall (Eticha et al., 2005) in the transition zone than in the elongation zone, especially after addition of B (Fig. 1E). This Al fraction has more possibility to move to the cytosol and induce severe cellular damage. Thus, the binding strength of Al modifies Al sensitivity, which is affected by the content of either demethylated (Kollmeier et al., 2000) or alkalized pectin (Li et al., 2017) and by the formation of complexes of silicon with Al (Wang et al., 2004). Boron decreases both total Al accumulation and the loosely bound Al in the root transition zone of pea, thus alleviating Al-induced inhibition of root elongation.
Boron decreases Al accumulation in the transition zone but not the following root zones, explaining the contradictory results with cucumber where B was found to increase Al accumulation in 10-mm-long root tip segments (Corrales et al., 2008) that included a mix of functionally different root tissues. This apparent controversy draws attention to the fact that the data obtained from studies involving entire root apices should be treated with caution, as they ignore the differences in Al sensitivity between different root zones and the tissue specificity of Al accumulation. As shown here, the root transition zone is the most sensitive zone to Al toxicity, and this sensitivity can be ameliorated by treatment with B.
Boron Is Essential for Maintaining the Alkalization of the Root Surface in the Transition Zone, in Order to Alleviate Al Toxicity
Cells in the transition zone are in a critical preparatory phase for the deposition of cell wall material, wherein they adjust growth from the slow mitotic mode to a rapid mode of elongation (Baluška et al., 1996). Therefore, roots must have evolved a mechanism to restrict cell elongation in the transition zone while promoting cell elongation in the elongation zone. The mechanism is the pH gradient in root zones, with the highest surface pH in the transition zone and the lowest surface pH in the elongation zone reported for several plant species, including pea (Fig. 2A; Kollmeier et al., 2000; Staal et al., 2011; Barbez et al., 2017). However, root elongation was severely inhibited once the pH gradient in the root zones was disturbed/eliminated by the application of either vanadate (Fig. 3A) or NPA (Fig. 4B) or by removing the cotyledons (Fig. 4C). This demonstrates that pH gradients are essential for root growth. The increase of surface pH in the elongation zone correlates with root growth inhibition under salt (Pitann et al., 2009), anoxia (Felle, 2006), drought (Felle and Hanstein, 2002), and water stresses (Ehlert et al., 2011), and here we show that the decrease in the surface pH in the transition zone correlates with the inhibition of root elongation under B deficiency and Al toxicity (Figs. 1 and 2).
There are several possible negative consequences of the decrease in the root surface pH in the transition zone induced by Al toxicity under B deficiency. First, such acidification will increase the activity of Al3+ (Wagatsuma and Ezoe, 1985), as shown by the strong fluorescence of the morin stain in the transition zone (Fig. 1; Yang et al., 2014), thus making it the most sensitive root zone to Al accumulation (Sivaguru and Horst, 1998; Sivaguru et al., 1999; Kollmeier et al., 2000). Second, the acidification in the root transition zone results in unwanted cell growth, which usually tears the rigidified cell wall bound with more Al, resulting in the typical symptoms of Al injuries (radial swelling behind the apex and rupturing of the rhizodermis and outer cortex; Kopittke et al., 2015). Boron promotes surface alkalization, which counteracts the effects of Al in reducing the surface pH, thus maintaining the cell state of restrained elongation in the transition zone thereby alleviating Al toxicity.
The PM H+-ATPase Is Involved in Proton Exudation and Control of Surface pH in the Root Transition Zone under B and Al Stress
Protons passively diffuse into the cell or are actively pumped out of a cell by PM-H+-ATPase (Palmgren and Harper, 1999; Kühlbrandt et al., 2002). In our study, the pH gradient and root surface alkalization in the transition zone of pea roots, promoted by B and inhibited by Al toxicity, was abolished by the vanadate application in all the treatments of B and Al (Fig. 3). This indicates that PM-H+-ATPase is involved in root surface alkalization in the transition zone.
Polar Auxin Transport Is Essential for Root Surface Alkalization, Which Is Inhibited by B Deficiency and Al Toxicity
Auxin is an important hormonal signal that affects cell growth by inducing cell wall acidification through the PM H+-ATPase (Ryan et al., 1992; Sze et al., 1999). Recent studies showed that polar auxin transport and auxin gradients in root zones are important signals that are required for root growth (for review, see Pan et al., 2015; Kopittke, 2016; Naramoto, 2017; Yang et al., 2017; Leyser, 2018). Here, we have shown an existence of the intermediate link between auxin signals and root growth, namely the surface alkalization of the root transition zone.
The pattern of IAA flux along the root axis reported in our study showing the maximum efflux in the root transition zone (Fig. 4A) is consistent with previously published results on maize and Arabidopsis (Mancuso et al., 2005; Schlicht et al., 2006; Shen et al., 2008; Wan et al., 2012). This indicates that auxin is transported acropetally from the quiescent center to the elongation zone through the root transition zone in pea. This polar auxin transport matches the pH gradients in root zones, where the highest IAA efflux corresponds to the highest pH in the root transition zone and the lowest IAA efflux corresponds to the lowest pH in the elongation zone (Figs. 2 and 4). The highest IAA efflux in the root transition zone points to the auxin minimum in the cytoplasm, which leads to root surface alkalization through the downstream regulation of PM-H+-ATPase (Sabatini et al., 1999; Di Mambro et al., 2017). This also suggests that auxin is transported to the elongation zone through the transition zone to provide cytosolic auxin concentrations optimal for cell elongation (Staal et al., 2011). These pH gradients between the root zones disappeared in plants treated with NPA or when the supply of IAA was compromised by removing the cotyledons (Fig. 4), indicating that polar auxin transport is essential to maintain the pH gradients through the downstream regulation of PM-H+-ATPase.
Polar auxin transport is specifically inhibited by Al toxicity in the root transition zone and by B deficiency in the meristem and the transition zone (Fig. 4), mimicking the effect in plants with the application of IAA transporter inhibitors (Mancuso et al., 2005; Schlicht et al., 2006; Shen et al., 2008). Boron promotes PAT and sustains the auxin minimum in the root transition zone (Sabatini et al., 1999; Di Mambro et al., 2017), which maintains surface alkalization in the root transition zone under Al toxicity (Fig. 4).
Cell elongation is regulated by the apoplastic pH (Monshausen et al., 2011; Shih et al., 2015; Barbez et al., 2017) by modulating cell wall extensibility (Tanimoto, 2005). Shih et al. (2015) reported that auxin stimulated the activity of Cyclic Nucleotide-Gated Ion Channels14, triggering Ca2+ influx, which in turn resulted in cell wall alkalization and inhibition of cell elongation. We observed compromised PAT by Al toxicity, which is aggravated by B deficiency in pea, which disturbs the auxin and pH gradients in the transition and elongation zones. This results in abnormal cell expansion in the root transition zone and the inhibition of root elongation (Fig. 1).
Our pharmacological experiments using the auxin transport inhibitor NPA (Figs. 4 and 5), as well as the studies on Arabidopsis mutants with an impaired auxin transporter (aux1-7, pin2; Fig. 5B; Enders and Strader, 2015), reveals that the auxin efflux transporter, PIN2, is involved in PAT through the root transition zone, which results in surface alkalization in the transition zone (Xu et al., 2012). This process is promoted by B and inhibited by Al toxicity, explaining why B alleviates Al-induced root growth inhibition (Figs. 1 and 3–5). This is corroborated by the findings of the higher expression of PIN carriers in the root transition zone than in the elongation zone that favor endosomal/vesicular IAA maximum in these cells due to endocytosis (Mettbach et al., 2017). Consistent with this notion, both B deficiency and Al toxicity inhibit endocytotic events in cells of the apical transition zone (Yu et al., 2002; Shen et al., 2008; Baluška and Mancuso, 2013). Thus, it is plausible that B promotes PAT via PIN2 endocytosis, thereby promoting surface alkalization in the root transition zone and alleviating Al toxicity.
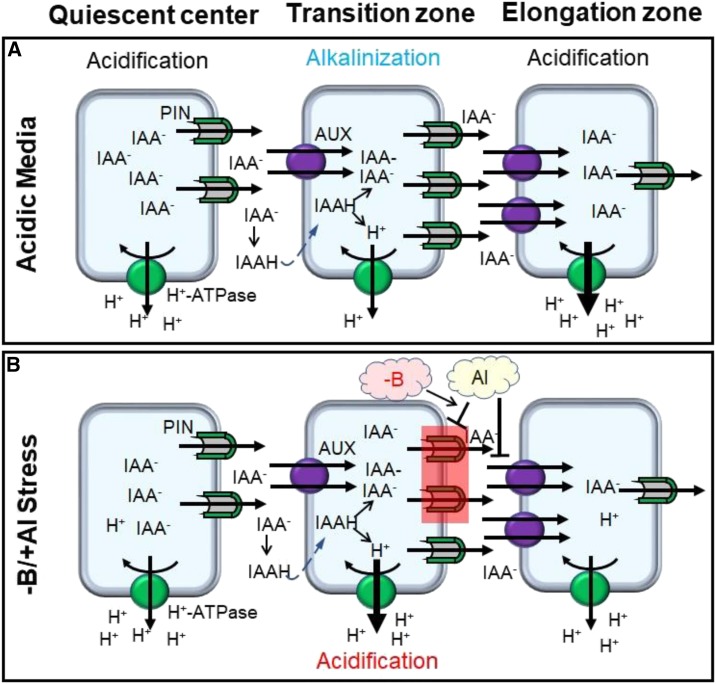
The following model can be suggested (Fig. 6): Under acidic conditions (pH < 5, no Al, sufficient B; Fig. 6A), some fraction of IAA− in the cell wall becomes protonated (IAAH) and can move into the next cell by passive diffusion. Polar auxin transport between the quiescent center and the elongation zone is mediated by the differential expression of auxin efflux (PIN) proteins. It is postulated that these proteins are expressed in a polar manner and are present at a higher density at the transverse side of the PM in the root transition zone than in the elongation zone. This difference in the functional expression of PIN proteins favors a preferential accumulation of IAA in the elongation zone, where it activates H+-ATPase and acidifies the cell wall, promoting elongation. At the same time, cells in the transition zone operate mainly as a “transfer center” and do not accumulate large amounts of auxin. Consequently, H+-ATPase activity here is relatively low, and cell wall pH is more alkaline compared to the elongation zone.
Figure 6.
Polar auxin transport between the quiescent center and the elongation zone and its regulation of the root surface pH in the transition zone in the root apex, in either acidic media or conditions of B deficiency and Al toxicity. A, Under acidic conditions (pH < 5, no Al, sufficient B supply) the polar auxin transport between the quiescent center and the elongation zone is mediated by the differential localization of the auxin efflux (PIN) proteins. These proteins are expressed in a polar manner and are present in a higher density at the transverse side of the plasma membrane in the root transition zone than in the elongation zone. This difference favors a preferential accumulation of IAA in the elongation zone, where it activates H+-ATPase and acidifies the cell wall, promoting elongation growth. At the same time, cells in the transition zone operate mainly as “transfer center” and do not accumulate large amounts of auxin. Consequently, the H+-ATPase activity here is relatively low, and the cell wall pH is more alkaline, as compared to the elongation zone. B, The presence of Al in the growth media inhibits activity of PIN efflux carriers and results in a substantial increase in the cytoplasmic free IAA in cells of the root transition zone. This leads to a substantial activation of H+-ATPase and acidification of the cell wall in this region, exacerbating effects of Al toxicity, which is further aggravated by B deficiency. Taken together, these two processes result in higher accumulation of (loosely bound) Al in the cell wall and more severe inhibition of the root elongation.
The presence of Al in the growth media (Fig. 6B) inhibits the activity of PIN efflux carriers (Shen et al., 2008) and results in a substantial increase of free cytoplasmic IAA accumulated in cells of the root transition zone. This leads to a substantial activation of H+-ATPases (Shen et al., 2008; Guo et al., 2013; Wang et al., 2016) and acidification of the cell wall in this region, exacerbating the effects of Al toxicity. This toxicity is further enhanced by B deficiency, thus resulting in a higher accumulation of (loosely bound) Al in the cell wall and stronger inhibition of root elongation.
MATERIALS AND METHODS
Culture of Pea Seedlings for B and Al Treatments
Pea (Pisum sativum cv Zhongwan no. 5) seeds were obtained from the pea seed breeding center in China (Gu’an, Hebei). Plants were grown in a liquid culture using a one-fourth-strength modified Hoagland nutrient solution (pH 5.5) in a growth chamber under 14 h light (100 µmol photons m−2 s−1, 25°C) and 10 h dark (15°C) with 75% relative humidity. A relatively low amount of B (0.25 µm) was applied at the first stage of culture (the first 2 d) in order to diminish B variations in seeds. Uniform 4-d-old seedlings were pretreated with 25 µm H3BO3 (B treatment) in the modified Hoagland nutrient solution, transferred to 0.5 mm CaCl2 solution (pH 4.0 or 4.5) for acidic adaptation for at least 2 h and then exposed to 0, 15, or 30 µm AlCl3 (pH 4.0 or 4.5) solution for up to 24 h.
Root Elongation Measurements
Plants were pretreated with either 0 (−B) or 25 µm (+B) H3BO3 for 36 h, followed by 12 h in the “acidic adaptation” solution (pH 4.0; 0.5 mm CaCl2) and then exposed to either 0 (control) or 15 µm AlCl3 (pH 4.0) for 24 h. The lateral roots were scanned before and after the acidic adaptation, as well as after the Al treatment by an Epson Expression 11000XL scanner. The length of the lateral roots was then determined using WinRhizo-Pro software (Reagent Instruments). The absolute and relative (percentage of non-Al-treated controls) root elongation rates were quantified.
Hematoxylin and Morin Staining
Hematoxylin stain was applied to the lateral roots of the seedlings treated with −B or +B for 36 h, followed by 12 h of acidic adaptation (pH 4.0; 0.5 mm CaCl2) and then exposed to either 0 or 15 µm AlCl3 solution (pH 4.0) for 24 h. For staining, the whole roots were immersed in 0.2% (w/v) hematoxylin (Sigma-Aldrich) solution (containing 0.02% [w/v] KI) for 15 min, thoroughly rinsed, and then photographed by a Sony Cybershot DSC T2 camera. The morin stain was applied to the lateral roots exposed to 30 μm AlCl3 (pH 4.5) for 12 h. Root tips were stained in 0.01% morin (Sigma-Aldrich) for 30 min and then observed under a fluorescent stereo microscope (Olympus SZX16).
Determination of Al in 1-mm Lateral Root Sections
Lateral roots of the seedlings were treated with −B or +B for 36 h, followed by 12 h of acidic adaptation (pH 4.0; 0.5 mm CaCl2) and then exposed to 15 µm AlCl3 solution (pH 4.0) for 24 h. Lateral root tips (0–10 mm) were then cut into sequential 1-mm-long sections after three rinses in ultrapure water. The Al contained in the root sections was extracted by immersing segments in 2 m HCl for 48 h, and the Al concentration in the extract was measured by inductively coupled plasma atomic emission spectroscopy (IRIS-Advantage, Thermo Elemental; Yu et al., 2009).
Root Surface pH Measurements by NMT and MIFE Systems
Pea plants pretreated with −B or +B for 36 h were transferred to 0.5 mm CaCl2 solution (pH 4.0) for acidic adaptation for 12 h and then exposed to 0, 15, or 30 µm AlCl3 (pH 4.0) solution for various durations (up to 24 h). The surface pH was measured by a H+-selective microelectrode using a noninvasive microtest technique system (NMT100 Series; Younger) as described previously (Shabala et al., 1997; Sun et al., 2009). H+-selective microelectrodes were pulled from the borosilicate glass capillaries with an external tip diameter of 1.5 ± 0.5 μm (XY-DJ-02; Younger) and filled with an ion-selective H+ liquid exchanger (LIX; catalog no. 95293; Sigma-Aldrich). The electrodes were calibrated before and after measurement using a set of pH standards (pH 3.5–6.0). Fresh lateral roots were equilibrated for 15 min in 4 mL measuring solution (in mm): 0.1 KCl, 0.1 CaCl2, 0.1 MgCl2, 0.5 NaCl, 0.2 Na2SO4, and 0.3 MES, pH 4.0. The microelectrode was placed 10 μm above the root surface, and the root surface pH was measured at 300-μm intervals starting from the quiescent center (0 μm) up to 2,700 μm along the root axis in order to obtain the surface pH pattern.
We then measured the surface pH in the root transition zone. The lateral roots of pea pretreated with −B or +B for 24 h were transferred to 0.5 mm CaCl2 solution (pH 4.5) for acidic adaptation for 12 h and then exposed to 0 or 15 µm AlCl3 solution (pH 4.5) for either 0 or 3 h (including 40 min conditioning). The lateral roots were then fixed on the polymethyl methacrylate by Parafilm and conditioned in BSM solution (0.1 mm CaCl2 and 0.5 mm KCl, pH 4.5), with or without Al for 40 min. The root surface pH was measured by an H+ selective microelectrode calibrated with a set of standards (pH 4.0–6.0) using the MIFE system (University of Tasmania, Hobart, Australia). The microelectrode tip was placed 10 µm above the epidermal cells of the root transition zone.
Arabidopsis (Arabidopsis thaliana) wild type Columbia-0 (Col-0) and a mutant line auxin resistant1-7 (aux1-7; lack of an auxin influx transporter) were kindly provided by Dr. Robert Tegg, University of Tasmania. Another Arabidopsis mutant, pin2 (knockout of the PIN-FORMED2, an auxin efflux transporter), was received from Dr. Jinxiang Wang (South China Agricultural University). The seeds were surface sterilized in a mixture of 1% (v/v) NaClO and 0.01% (v/v) Triton X-100 and planted on half-strength Murashige and Skoog media (pH 5.5) with 12.5 µm H3BO3, 0.5% (w/v) Phytagel, and 0.5% (w/v) Suc. Four-day-old seedlings were pretreated with −B or +B (0 or 25 µm H3BO3, respectively) in 0.5 strength Murashige and Skoog media (pH 5.5) for 24 h. Plants were then immobilized on polymethyl methacrylate by Parafilm and conditioned in BSM solution for 40 min. The surface pH of Arabidopsis roots was measured at the root transition zone before or 30 min after the addition of 15 µm AlCl3 solution (pH 4.5), similar to measurements on pea roots.
Pharmacological Treatments
NPA (10 μm; Sigma-Aldrich) or vanadate (0.5 mm Na3VO4 for root surface pH measurement, 1 mm Na3VO4 for root elongation measurement; Sigma-Aldrich) was applied to pea seedlings for the first 3 h of the 12-h-long acidic acclimatization. The surface pH of the root transition and elongation zones were measured after 3 h and 24 h of Al treatment, respectively. NPA (10 μm) was applied to Arabidopsis for 30 min before the conditioning, and the surface pH was determined in the root transition zone before or 30 min after the addition of 15 µm AlCl3 solution (pH 4.5).
Observation of the Rhizosphere pH by Bromocresol Green
The changes in the rhizosphere pH were monitored according to the method of Yan et al. (2002). Uniform seedlings with six to eight lateral roots were pretreated with −B or +B for 48 h and gently pressed into agar in petri dishes containing 0.6% (w/v) agar (Sigma-Aldrich; purified agar), 0.006% (w/v) bromocresol green (Sigma-Aldrich), 0.5 mm KCl, and 0.5 mm CaCl2 (pH 4.0). Treatments of B (0 or 25 µm H3BO3) and Al (0 or 100 μm AlCl3) were applied to the agar sheet. The petri dishes were then incubated in the growth chamber for 24 h. Vanadate (1 mm) or NPA (10 µm) were added to the agar sheet in pharmacological experiments. The color change of the agar was recorded by an Epson Expression 11000XL scanner (Reagent Instruments). The pH value was compared with a standard colored scale in the same plate measured in the absence of plants (pH range between 3.0 and 6.0, at 0.5 pH unit intervals).
Detection of Net IAA Flux along Root Tips by the NMT System
Pea seedlings were pretreated by −B or +B for 48 h, then acclimated to acidic solution (0.5 mm CaCl2 solution, pH 4.0) for 3 h and subsequently exposed to either 0 or 15 µm AlCl3 solution (pH 4.0) for 3 h. Net IAA flux was measured from the lateral roots by the NMT technique (NMT100 Series; Younger), used by the Xuyue Science and Technology, before and after Al treatment using a platinum microelectrode, as described (McLamore et al., 2010). The microelectrodes were calibrated in a set of IAA standard solutions ranging from 0 to 10 μm. Fresh lateral roots were equilibrated for 15 min in a 4 mL measuring solution (in mm): 0.1 KCl, 0.1 CaCl2, 0.1 MgCl2, 0.5 NaCl, 0.2 Na2SO4, and 0.3 MES, pH 4.0. The microelectrode was placed 10 μm above the root surface and moved with a travel range of 30 μm in a square-wave manner (5.4-s cycle). IAA fluxes were recorded continuously for 5 min to yield a steady mean value. At least four individual plants were measured for each treatment. The IAA flux profile was drawn from the discrete measurements taken at 300-μm intervals starting from the quiescent center (0 μm) and up to 2,400 μm along the root axis. The positive and negative values indicate net IAA efflux and influx, respectively.
Statistical Analysis
Duncan’s multiple range test was used to test differences among the treatments at P < 0.05 using Statistical Analysis Systems (SAS 9.13) programs.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: AUX1, AT2G38120; and PIN2, AT5G57090
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Morphology of pea (Pisum sativum) root and its zonation.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Prof. Heiner E. Goldbach (Plant Nutrition-Institute of Crop Science and Resource Conservation, University of Bonn, Germany) and Prof. Lijun Wang (Microelement Research Center, Huazhong Agricultural University, Wuhan, China) for their critical reviews and comments on the manuscript. We also thank the editors and reviewers for their critical, constructive, and helpful comments and revisions to the manuscript.
Footnotes
This work was supported by the National Natural Science Foundation of China (31672228), the Key Project of Department of Education of Guangdong Province (2014KZDXM061), the Provincial National Science Foundation of Guangdong Province (2015A030313637 and 2016A030313379), the Science and Technology Project of Guangdong Province (2015A040404048), and the Foundation for Distinguished Young Talents in Higher Education of Guangdong (LYM11125 and 2015KQNCX174), and by the Australian Research Council (DP150101663).
Articles can be viewed without a subscription.
References
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenós M, Corrales I, Poschenrieder C, Illés P, Baluška F, Barceló J (2009) Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant Cell Physiol 50: 528–540 [DOI] [PubMed] [Google Scholar]
- Baluška F, Mancuso S (2013) Root apex transition zone as oscillatory zone. Front Plant Sci 4: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Volkmann D, Barlow PW (1996) Specialized zones of development in roots: view from the cellular level. Plant Physiol 112: 3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Volkmann D, Barlow PW (2001) A polarity crossroad in the transition growth zone of maize root apices: cytoskeletal and developmental implications. J Plant Growth Regul 20: 170–181 [Google Scholar]
- Baluška F, Samaj J, Wojtaszek P, Volkmann D, Menzel D (2003) Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol 133: 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Mancuso S, Volkmann D, Barlow PW (2010) Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 15: 402–408 [DOI] [PubMed] [Google Scholar]
- Band LR, Wells DM, Fozard JA, Ghetiu T, French AP, Pound MP, Wilson MH, Yu L, Li W, Hijazi HI, et al. (2014) Systems analysis of auxin transport in the Arabidopsis root apex. Plant Cell 26: 862–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbez E, Dünser K, Gaidora A, Lendl T, Busch W (2017) Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc Natl Acad Sci USA 114: E4884–E4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J, González-Fontes A (2015) Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J Exp Bot 66: 3831–3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čiamporová M. (2002) Morphological and structural responses of plant roots to aluminium at organ, tissue, and cellular levels. Biol Plant 45: 161–171 [Google Scholar]
- Corrales I, Poschenrieder C, Barceló J (2008) Boron-induced amelioration of aluminium toxicity in a monocot and a dicot species. J Plant Physiol 165: 504–513 [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol 117: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mambro R, De Ruvo M, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, et al. (2017) Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA 114: E7641–E7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert C, Plassard C, Cookson SJ, Tardieu F, Simonneau T (2011) Do pH changes in the leaf apoplast contribute to rapid inhibition of leaf elongation rate by water stress? Comparison of stress responses induced by polyethylene glycol and down-regulation of root hydraulic conductivity. Plant Cell Environ 34: 1258–1266 [DOI] [PubMed] [Google Scholar]
- Enders TA, Strader LC (2015) Auxin activity: Past, present, and future. Am J Bot 102: 180–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D, Stass A, Horst WJ (2005) Localization of aluminium in the maize root apex: Can morin detect cell wall-bound aluminium? J Exp Bot 56: 1351–1357 [DOI] [PubMed] [Google Scholar]
- Felle HH. (2006) Apoplastic pH during low-oxygen stress in Barley. Ann Bot 98: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH, Hanstein S (2002) The apoplastic pH of the substomatal cavity of Vicia faba leaves and its regulation responding to different stress factors. J Exp Bot 53: 73–82 [PubMed] [Google Scholar]
- Fleischer A, O’Neill MA, Ehwald R (1999) The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol 121: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach HE, Wimmer MA (2007) Boron in plants and animals: Is there a role beyond cell wall structure? J Plant Nutr Soil Sci 170: 39–48 [Google Scholar]
- Guo CL, Chen Q, Zhao XL, Chen XQ, Zhao Y, Wang L, Li KZ, Yu YX, Chen LM (2013) Al-enhanced expression and interaction of 14-3-3 protein and plasma membrane H+-ATPase is related to Al-induced citrate secretion in an Al-resistant Black Soybean. Plant Mol Biol Report 31: 1012–1024 [Google Scholar]
- Habets MEJ, Offringa R (2014) PIN-driven polar auxin transport in plant developmental plasticity: A key target for environmental and endogenous signals. New Phytol 203: 362–377 [DOI] [PubMed] [Google Scholar]
- Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illés P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovečka M (2006) Aluminium toxicity in plants: Internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot 57: 4201–4213 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110: 1017–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ (2000) Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol 122: 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM. (2016) Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ 39: 2319–2328 [DOI] [PubMed] [Google Scholar]
- Kopittke PM, Moore KL, Lombi E, Gianoncelli A, Ferguson BJ, Blamey FPC, Menzies NW, Nicholson TM, McKenna BA, Wang P, et al. (2015) Identification of the primary lesion of toxic aluminum in plant roots. Plant Physiol 167: 1402–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W, Zeelen J, Dietrich J (2002) Structure, mechanism, and regulation of the Neurospora plasma membrane H+-ATPase. Science 297: 1692–1696 [DOI] [PubMed] [Google Scholar]
- Lenoble ME, Blevins DG, Miles RJ (1996a) Prevention of aluminium toxicity with supplemental boron. 2. Stimulation of root growth in an acidic, high-aluminium subsoil. Plant Cell Environ 19: 1143–1148 [Google Scholar]
- Lenoble ME, Blevins DG, Sharp RE, Cumbie BG (1996b) Prevention of aluminium toxicity with supplemental boron. 1. Maintenance of root elongation and cellular structure. Plant Cell Environ 19: 1132–1142 [Google Scholar]
- Leyser O. (2018) Auxin signaling. Plant Physiol 176: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li Y, Qu M, Xiao H, Feng Y, Liu J, Wu L, Yu M (2016) Cell wall pectin and its methyl-esterification in transition zone determine al resistance in cultivars of pea (Pisum sativum). Front Plant Sci 7: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XW, Liu JY, Fang J, Tao L, Shen RF, Li YL, Xiao HD, Feng YM, Wen HX, Guan JH, et al. (2017) Boron supply enhances aluminum tolerance in root border cells of pea (Pisum sativum) by interacting with cell wall pectins. Front Plant Sci 8: 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Robert S (2014) Auxin biology revealed by small molecules. Physiol Plant 151: 25–42 [DOI] [PubMed] [Google Scholar]
- Mancuso S, Marras AM, Magnus V, Baluška F (2005) Noninvasive and continuous recordings of auxin fluxes in intact root apex with a carbon nanotube-modified and self-referencing microelectrode. Anal Biochem 341: 344–351 [DOI] [PubMed] [Google Scholar]
- McLamore ES, Diggs A, Calvo Marzal P, Shi J, Blakeslee JJ, Peer WA, Murphy AS, Porterfield DM (2010) Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J 63: 1004–1016 [DOI] [PubMed] [Google Scholar]
- Mettbach U, Strnad M, Mancuso S, Baluška F (2017) Immunogold-EM analysis reveal brefeldin a-sensitive clusters of auxin in Arabidopsis root apex cells. Commun Integr Biol 10: e1327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011) Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J 65: 309–318 [DOI] [PubMed] [Google Scholar]
- Naramoto S. (2017) Polar transport in plants mediated by membrane transporters: focus on mechanisms of polar auxin transport. Curr Opin Plant Biol 40: 8–14 [DOI] [PubMed] [Google Scholar]
- Newman IA. (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24: 1–14 [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849 [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50: 883–893 [Google Scholar]
- Pan X, Chen J, Yang Z (2015) Auxin regulation of cell polarity in plants. Curr Opin Plant Biol 28: 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Wang Z, Yang L, Wang Z, Shi L, Naran R, Azadi P, Xu F (2012) Differences in cell wall components and allocation of boron to cell walls confer variations in sensitivities of Brassica napus cultivars to boron deficiency. Plant Soil 354: 383–394 [Google Scholar]
- Pitann B, Kranz T, Mühling KH (2009) The apoplastic pH and its significance in adaptation to salinity in maize (Zea mays L.): Comparison of fluorescence microscopy and pH-sensitive microelectrodes. Plant Sci 176: 497–504 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E (2010) The convergent evolution of aluminium resistance in plants exploits a convenient currency. Funct Plant Biol 37: 275–284 [Google Scholar]
- Ryan PR, Shaff JE, Kochian LV (1992) Aluminum toxicity in roots: correlation among ionic currents, ion fluxes, and root elongation in aluminum-sensitive and aluminum-tolerant wheat cultivars. Plant Physiol 99: 1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sardar HS, Yang J, Showalter AM (2006) Molecular interactions of arabinogalactan proteins with cortical microtubules and F-actin in Bright Yellow-2 tobacco cultured cells. Plant Physiol 142: 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht M, Strnad M, Scanlon MJ, Mancuso S, Hochholdinger F, Palme K, Volkmann D, Menzel D, Baluška F (2006) Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav 1: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Hou N, Schlicht M, Wan Y, Mancuso S, Baluska F (2008) Aluminium toxicity targets PIN2 in Arabidopsis root apices: effects on PIN2 endocytosis, vesicular recycling, and polar auxin transport. Chin Sci Bull 53: 2480–2487 [Google Scholar]
- Shih HW, DePew CL, Miller ND, Monshausen GB (2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25: 3119–3125 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ (1998) The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol 116: 155–163 [Google Scholar]
- Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ (1999) Impacts of aluminum on the cytoskeleton of the maize root apex. Short-term effects on the distal part of the transition zone. Plant Physiol 119: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal M, De Cnodder T, Simon D, Vandenbussche F, Van der Straeten D, Verbelen JP, Elzenga T, Vissenberg K (2011) Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid. Plant Physiol 155: 2049–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stass A, Kotur Z, Horst WJ (2007) Effect of boron on the expression of aluminium toxicity in Phaseolus vulgaris. Physiol Plant 131: 283–290 [DOI] [PubMed] [Google Scholar]
- Sun J, Chen S, Dai S, Wang R, Li N, Shen X, Zhou X, Lu C, Zheng X, Hu Z, et al. (2009) NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol 149: 1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases. Regulation and biosynthesis. Plant Cell 11: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto E. (2005) Regulation of root growth by plant hormones—roles for auxin and gibberellin. Crit Rev Plant Sci 24: 249–265 [Google Scholar]
- Tromas A, Perrot-Rechenmann C (2010) Recent progress in auxin biology. C R Biol 333: 297–306 [DOI] [PubMed] [Google Scholar]
- Verbelen JP, De Cnodder T, Le J, Vissenberg K, Baluška F (2006) The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav 1: 296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, Fry SC (2014) Glycosylinositol phosphorylceramides from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J 79: 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagatsuma T, Ezoe Y (1985) Effect of pH on ionic species of aluminum in medium and on aluminum toxicity under solution culture. Soil Sci Plant Nutr 31: 547–561 [Google Scholar]
- Wan Y, Jasik J, Wang L, Hao H, Volkmann D, Menzel D, Mancuso S, Baluška F, Lin J (2012) The signal transducer NPH3 integrates the phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 24: 551–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yu W, Zhang J, Rengel Z, Xu J, Han Q, Chen L, Li K, Yu Y, Chen Q (2016) Auxin enhances aluminium-induced citrate exudation through upregulation of GmMATE and activation of the plasma membrane H+-ATPase in soybean roots. Ann Bot 118: 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao XQ, Chen RF, Dong XY, Lan P, Ma JF, Shen RF (2015) Altered cell wall properties are responsible for ammonium-reduced aluminium accumulation in rice roots. Plant Cell Environ 38: 1382–1390 [DOI] [PubMed] [Google Scholar]
- Wang Y, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136: 3762–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Stass A, Horst WJ (2005) Assessing the effect of boron on aluminium resistance in maize (Zea mays L.). In Li CJ, ed, Plant Nutrition for Food Security, Human Health and Environmental Protection. Tsinghua University Press, Beijing, pp 314–315 [Google Scholar]
- Wang Z, Wang Z, Shi L, Wang L, Xu F (2010) Proteomic alterations of Brassica napus root in response to boron deficiency. Plant Mol Biol 74: 265–278 [DOI] [PubMed] [Google Scholar]
- Wimmer MA, Lochnit G, Bassil E, Mühling KH, Goldbach HE (2009) Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant Cell Physiol 50: 1292–1304 [DOI] [PubMed] [Google Scholar]
- Xu W, Jia L, Baluška F, Ding G, Shi W, Ye N, Zhang J (2012) PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. J Exp Bot 63: 6105–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Zhu Y, Müller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129: 50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z (2014) TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 26: 2889–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZB, Liu G, Liu J, Zhang B, Meng W, Müller B, Hayashi KI, Zhang X, Zhao Z, De Smet I, et al. (2017) Synergistic action of auxin and cytokinin mediates aluminum-induced root growth inhibition in Arabidopsis. EMBO Rep 18: 1213–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Shen R, Xiao H, Xu M, Wang H, Wang H, Zeng Q, Bian J (2009) Boron alleviates aluminum toxicity in pea (Pisum sativum). Plant Soil 314: 87–98 [Google Scholar]
- Yu Q, Hlavacka A, Matoh T, Volkmann D, Menzel D, Goldbach HE, Baluška F (2002) Short-term boron deprivation inhibits endocytosis of cell wall pectins in meristematic cells of maize and wheat root apices. Plant Physiol 130: 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Bailly A, Zwiewka M, Sovero V, Di Donato M, Ge P, Oehri J, Aryal B, Hao P, Linnert M, et al. (2016) TWISTED DWARF1 Mediates the action of auxin transport inhibitors on actin cytoskeleton dynamics. Plant Cell 28: 930–948 [DOI] [PMC free article] [PubMed] [Google Scholar]