Abstract
BACKGROUND
Few effective treatment options exist for cannabis-using youth. This pilot study aimed to test Approach Avoidance Training to reduce cannabis use with non-treatment seeking adolescents.
METHODS
Eighty cannabis-using non-treatment-seeking adolescents (average age 19) were recruited from San Diego, California and Charleston, South Carolina, and randomized to complete either six sessions of Cannabis Approach Avoidance Task Training (CAAT-training) designed to reduce automatic approach biases for cannabis cues, or CAAT-sham training. Change in two primary outcome variables were examined: 1) cannabis approach bias and 2) percent cannabis use days over study enrollment. Change in percent alcohol use days over study enrollment was explored as a secondary outcome.
RESULTS
A mixed models repeated measures analysis confirmed the group by time interaction effect for approach bias failed to reach statistical significance (p=.06). Significant group by time interaction effects (ps<.05) predicted percent days of cannabis and alcohol use over study enrollment. Participants randomized to the avoid cannabis condition (CAAT-training) reported 7% less days of cannabis use compared to 0% change for sham; unexpectedly, those in the avoid cannabis condition reported 10% percent more alcohol use days compared to 3% more for sham.
CONCLUSIONS
Computerized cognitive bias modification paradigms may have utility in reducing adolescent cannabis use. Future work should consider developing a paradigm that addresses both cannabis and alcohol, as well as alternative computerized approaches for coping with addictive behavior in conjunction with bias modification.
ClinicalTrials.gov Identifier
1. Introduction
Adolescent cannabis use has become a focus of societal attention in recent years due to increasing rates of daily use among adolescents in the United States combined with increased access to cannabis and decreased legal restrictions on its use (Johnston et al., 2016). Cannabis use in adolescents is particularly concerning given its connection to altered brain development (Bava et al., 2013; Jacobus et al., 2009; Jacobus et al., 2015b; Jacobus et al., 2013) and vulnerability for future problematic substance use (Koob and Volkow, 2010; Paus et al., 2008). Therefore, it is imperative to both understand the cognitive processes underlying cannabis use and addiction and to test novel behavioral interventions that target these underlying neural circuit vulnerabilities (Boyce and Lynne-Landsman, 2013).
Incentive-salience models of addictive behavior suggest that automatic cognitive biases, including approach bias, attentional bias, and implicit memory, result from repeated substance use and altered dopaminergic systems; over time, substance users unconsciously become more reactive to substance cues (Berridge, 2007; Stacy and Wiers, 2010). These biases towards drug cues are particularly problematic for adolescents, who tend to exhibit poorer executive/regulatory control contributing to greater alcohol and substance use behavior (Everitt and Robbins, 2013; Larsen et al., 2014; Lisdahl et al., 2013; Peeters et al., 2013; Peeters et al., 2012; Thush et al., 2008).
Cognitive bias modification (CBM) is a growing area of addictions intervention research that aims to change maladaptive approach biases (i.e., automatic action tendencies to approach substance use cues) (Eberl et al., 2013, 2014; Wiers et al., 2011; Wiers et al., 2010); thereby diminishing use of the targeted substance (e.g., alcohol) (Eberl et al., 2013, 2014; Sharbanee et al., 2014; Wiers, C.E. et al., 2015a; Wiers et al., 2010) and potentially changing neural activation in reward-related brain regions (Wiers, C.E. et al., 2015a; Wiers, C.E. et al., 2015b). CBM paradigms have demonstrated positive outcomes in the treatment of anxiety symptoms (Rinck et al., 2013; See et al., 2009; Taylor and Amir, 2012) and problematic alcohol use in particular (Gladwin et al., 2016; Manning et al., 2016). Yet, CBM research exclusive to cannabis use, particularly in adolescents and young adults, is only beginning to be explored in the current literature. No studies to date have explored CBM as an intervention for adolescent cannabis use (Cousijn et al., 2012a; Cousijn et al., 2011; Cousijn et al., 2013a; Cousijn et al., 2013b), although a proof-of-concept study was recently completed on adults who met criteria for cannabis use disorder (Sherman et al., 2018). Notably, research has shown that cannabis-using youth display approach biases for cannabis cues, and this bias may predict future cannabis use (Cousijn et al., 2012a). Young cannabis users may be in greater need of this kind of intervention, as regular cannabis use has been found to increase cognitive vulnerabilities in adolescents through neurodevelopmental structural alterations (Filbey et al., 2015; Jacobus et al., 2016; Jacobus et al., 2015a), changes in emotion and reward processing (Cousijn et al., 2012b; de Graaf et al., 2010), and the potential for habitual drug use and emergence of more severe psychopathology into young adulthood (Everitt and Robbins, 2013; Rubino and Parolaro, 2008).
Approach-Avoidance Training (AAT) is a computerized approach to CBM that focuses on modifying approach bias, or the impulse to approach rather than avoid a substance cue, which has been associated with drug use in multiple studies (Gladwin et al., 2016). In AAT, participants are instructed to either pull a joystick towards themselves or push it away based on image format (e.g., the color of the border surrounding the image). A contingency is introduced such that the image format directs the individual to push the joystick on the majority of trials when a substance cue is presented, and pull the joystick when a non-substance cue is presented, thereby training the participant to “avoid” the targeted substance cue. This task has been successful in multiple trials with alcohol users by changing approach bias to avoid alcohol rather than approach it, as well as decreasing alcohol consumption (Wiers et al., 2010). Promising findings have been reported in studies of community populations (Sharbanee et al., 2014), clinical alcohol-dependent populations (Eberl et al., 2013, 2014; Wiers et al., 2011) and online interventions (Wiers, R.W. et al., 2015b), with reductions of up to 13% in alcohol relapse rates a full year after the training. In a proof-of-concept trial using cannabis AAT (CAAT) in adults with cannabis use disorder (N=33), participants randomized to the CAAT-training versus CAAT-sham condition showed blunted cannabis cue-induced craving post-training (Sherman et al., 2018). While no change in approach bias was found, sex-related reductions in cannabis use were found; specifically, men receiving CAAT-training reported fewer cannabis use sessions per day compared to women, with no sex or cannabis use differences observed in the sham condition.
Randomized controlled trials are needed to better understand the effectiveness of AAT for adolescent cannabis use. Targeting known cognitive vulnerabilities underlying substance addiction (Gladwin et al., 2016; Koob and Volkow, 2010) may enhance traditional behavioral interventions that focus on changing motivation or beliefs pertaining to substance use (Hogue et al., 2014). This proof-of-concept study is the first to test the CAAT paradigm in a randomized controlled trial of N=80 non-treatment-seeking adolescents who were frequent cannabis users between the ages of 17–21 and sampled across two sites. We predicted that individuals randomized to CAAT-training would show a decreased automatic approach bias toward cannabis stimuli and report fewer percent days of cannabis use after six sessions of CAAT-training compared to those randomized to the CAAT-sham condition. Motivation to change cannabis use was hypothesized to be a moderator of training effects on cannabis use outcomes; higher motivation levels were hypothesized to be associated with a greater reduction in cannabis approach bias and cannabis use. As cannabis and alcohol are co-used at high rates, exploratory analyses examined if change in percent days of alcohol use differed as a function of treatment group.
2. Methods
2.1 Participants
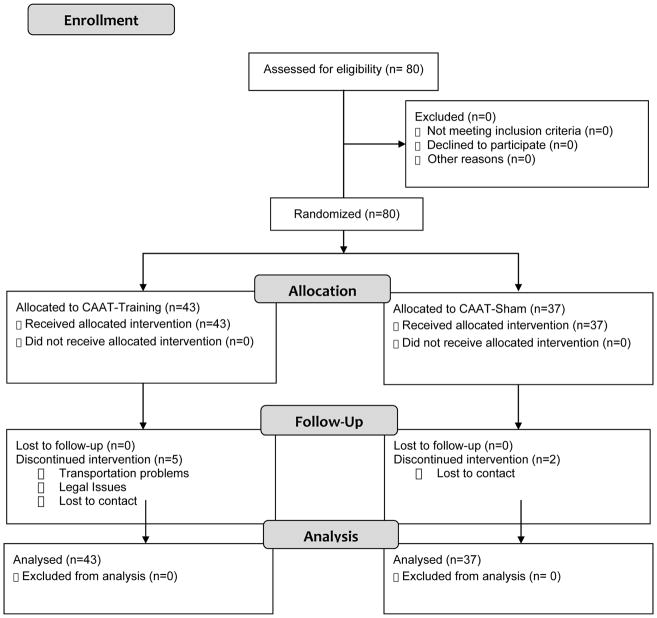
Participants (N=80, ages 17–21) were non-treatment-seeking cannabis users recruited from two geographic locations, San Diego, California (n=39) and Charleston, South Carolina (n=41), and completed identical protocols. Participants were recruited from fliers at local high school and college campuses and through online media advertising and were compensated for their time and participation. Site visits by the Principal Investigators and weekly meetings ensured recruitment and protocol administration were equivalent across sites. The computer-delivered nature of the protocol also helped ensure standardization. Seven individuals did not complete the protocol [4 from University of California, San Diego (UCSD) and 3 from Medical University of South Carolina (MUSC)]; therefore n=73 individuals completed the entire protocol, including 2 assessment and 6 training sessions. See Figure 1 for details regarding enrollment statistics and participants’ progress throughout the study. All individuals completing the in-person eligibility assessment after a pre-screen phone interview were enrolled in the study. Individuals with no history of cannabis use were not scheduled for an eligibility assessment. The study had institutional review board approval from the University of California, San Diego (UCSD) and Medical University of South Carolina (MUSC).
Figure 1.
Flow of participants through each state of study.
Inclusion and exclusion criteria were identical across sites. All participants were required to be between ages 16–21 and report a pattern of at least weekly cannabis use (≥1 use per week for 52 weeks, on average) and were non-treatment-seeking. Exclusion criteria at both data collection sites included inadequate English language skills; history of learning disability, pervasive developmental disorder or other condition requiring special education; non-correctable vision or hearing problems; history of serious medical problems that could affect brain development (as the MUSC site completed a neuroimaging substudy for all participants); major neurological problems and/or head trauma with loss of consciousness >10 minutes; prenatal exposure to alcohol (i.e., >2 drinks on an occasion or >4 drinks in a week) or any illicit drugs; premature birth weight (<5 lbs or born prior to 33 weeks gestation); use of other illicit substances (e.g., cocaine, methamphetamine) other than alcohol or cannabis > 100 times in the past year; and diagnosis of a current DSM-5 major psychiatric disorder (e.g., bipolar disorder, psychotic disorder).
2.2 Study Procedures
All adolescents (N=80) enrolled in the study were scheduled for an initial baseline assessment that collected background information, substance use history, substance use self-report questionnaires (e.g., cannabis craving, problems, motivation to quit), mental health functioning, and baseline automatic action tendencies to approach cannabis. Participants were then randomized within each site to complete six sessions of CAAT-training or CAAT-sham twice per week over the course of three weeks, and scheduled for a final appointment to re-assess approach bias (i.e., automatic actions tendencies to approach cannabis), and complete follow-up self-report substance use questionnaires. No other treatment was provided within the study.
2.3 Approach Bias Assessment and CAAT Intervention Conditions
2.3.1 Approach Bias Assessment
The approach bias computerized assessment task measured automatic approach tendencies toward cannabis stimuli before and after CAAT-training or sham. Participants viewed 8 cannabis-related images and 8 neutral images (i.e., nature picture, stationary materials; see supplemental materials for cannabis and neutral image examples) (Cousijn et al., 2011), and were instructed to push or pull a joystick in response to the border color of the image presented on the computer screen (i.e., blue or yellow) (Derntl et al., 2011; Seidel et al., 2010) and not the content of the image itself. They were instructed to pull the joystick towards themselves when the border was yellow and to push the joystick away when the border was blue. When the participant pushed the joystick the picture zoomed out and when the subject pulled the joystick the picture zoomed in, which created the visual impression that the pictures were moving away or coming closer, respectively. In the assessment task, participants were required to make an equal number of approach (pull) and avoidance (push) movements to both cannabis and neutral stimuli. The task was comprised of two sets of 8 pairs of validated cannabis and neutral pictures (sets A and B), counterbalanced across participants to assess generalizability of training effects. For example, participants assessed at baseline and trained on set A pictures were assessed on set B pictures post-intervention and vice versa. Each assessment block (either A or B, corresponding to assignment) comprised 192 total trials administered in two consecutive runs of 96 trials each with a short break in between: 8 pictures × 2 picture type (cannabis vs. neutral) × 2 border color (yellow vs. blue) × 3 repetitions × 2 runs. A brief practice (12 trials) preceded the task and included one cannabis and one neutral image not encountered during assessment or training. At the initial assessment, this practice trial was completed to ensure the participant was able to correctly distinguish yellow and blue colors from each other, as they were asked to respond to the color of the border. The same practice trial was done before each assessment session to ensure the participants understood the instructions before completing the assessment. This joystick task has been used extensively to study automatic action tendencies in both substance use and anxiety disorders (Gladwin et al., 2016; Kong et al., 2015; Rinck et al., 2013; Taylor and Amir, 2012; Wiers et al., 2011).
Reaction times were calculated based on the duration from the time the picture appeared on the screen to the time it disappeared. A cannabis approach bias score was computed by subtracting each participant’s median response latency for correct trials in the cannabis pull condition from his or her median response latency for correct trials in the corresponding cannabis push condition (i.e., cannabis push minus cannabis pull). A neutral image approach bias score was also computed (i.e., neutral image push minus neutral image pull). The primary outcome of interest, a comprehensive cannabis bias score, was calculated by subtracting the neutral image bias score from the cannabis image bias score (i.e., cannabis bias minus neutral image bias)(Wiers, C.E. et al., 2015b). Positive values indicate an approach bias (faster approach tendency) for cannabis cues whereas negative values indicate an avoidance bias.
2.3.2 CAAT Avoid Cannabis and CAAT-Sham Intervention Conditions
To modify automatic action tendencies to approach cannabis stimuli, the approach bias assessment was modified for the CAAT-training (i.e., avoid cannabis condition) such that the majority of cannabis pictures (92%) were presented in the push-format and 8% in the pull-format, with reversed contingencies for neutral pictures; the overall number of push- and pull-trials each remained 50%. The CAAT-sham condition was identical to the CAAT-training condition in every respect except there was no contingency between the presentation of cannabis stimuli and push vs. pull movements. Thus, participants were required to make an equal number of approach (pull) and avoidance (push) movements to both cannabis and neutral pictures. Each CAAT training or sham session comprised 384 trials administered in two consecutive runs of 192 trials each with a short break in between: 8 pictures × 2 picture type (cannabis vs. neutral) × 2 border color (yellow vs. blue) × 12 repetitions. The duration of each training session was approximately 15 minutes. All participants received identical computerized training tasks and standardized instructions for completing the tasks. A brief practice (12 trials) preceded the task and included one cannabis and one neutral image not encountered during training; this practice trial was done before each of the six training sessions to ensure the participants understood the instructions before completing the training.
2.4 Substance Use Outcome Measures
2.4.1 Primary and Secondary Substance Use Outcome Measures
Changes in approach bias and percent cannabis use days from baseline to post treatment were the primary outcomes of interest. Change in alcohol use was also explored as a secondary outcome given the high prevalence rates in adolescents (Miech et al., 2017). The Timeline Followback (Sobell and Sobell, 1992) was given at baseline to assess percent substance use days in the 30 days prior to the first appointment in the study. The Timeline Followback was administered at each of the six training appointments and post treatment to monitor substance use during study participation. Percent use days were calculated by dividing the number of use days by the number of days enrolled in the study at post treatment (enrollment was equal to 21 days, on average). Substance use questions from the Customary Drinking and Drug Use Record (CDDR) were asked to identify age of onset of alcohol and cannabis use, and cumulative lifetime use for each class of illicit substances (Brown et al., 1998).
2.4.2 Exploratory Outcome Measures
In line with research on cannabis interventions, we examined several stress and reward related symptoms (e.g., craving, withdrawal, etc.) (Koob and Volkow, 2010) that could impact the primary outcomes and change over study enrollment. Therefore, a series of self-report questionnaires were administered at baseline and post-treatment follow-up, including The Beck Depression Inventory-Second Edition (BDI-II) and Spielberger State Trait Anxiety Inventory (STAI) Trait scale (Beck et al., 1996; Spielberger et al., 1970). The Marijuana Ladder (score range 1–10) and Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES, score range 19–95) assessed motivation to change cannabis use (Miller and Tonigan, 1996; Slavet et al., 2006), with higher scores reflecting a desire for change; and the Self-Efficacy Questionnaire asked participants to rate their ability to resist the temptation to use cannabis in different situations (score range 1–7, higher scores relating to more confidence in their ability to resist using) (Steinberg et al., 2005; Stephens et al., 1994). Withdrawal, craving, and self-reported problems [Cannabis Withdrawal Scale (score range 0–190), Marijuana Craving Questionnaire-Short Form (score range 4–28), Marijuana Problems Scale (score range 0–38)] were assessed to identify any physiological and/or functional changes related to use (Allsop et al., 2011; Budney et al., 2001; Heishman et al., 2009; Rosenberg, 2009); higher scores on these scales represent more withdrawal and craving symptoms, and problems related to cannabis use. The Marijuana Effect Expectancy Questionnaire (MEEQ) provided a measure of appraisal on six subscales; this 48-item instrument asks participants to identify their beliefs about anticipated cannabis-related effects in several domains (e.g. relaxation/tension, cognitive/behavioral impairment)( (Schafer and Brown, 1991). MEEQ subscales can range from 5–50, and high scores reflect a high level of expectancy on the corresponding subscale. The Rutgers Alcohol Problem Index was utilized to assess at-risk drinking in the sample at baseline (White and Labouvie, 1989); scores range from 0–69 and higher scores indicate more reported drinking problems.
2.5 Data Analysis
2.5.1. Demographic comparisons
Analysis of variance (ANOVA) and Chi-square tests evaluated differences between groups on demographic and substance use variables to characterize the sample.
2.5.2 Primary and secondary outcomes
All analyses were intent-to-treat such that any randomized participant was included in the statistical models (N=80). A linear mixed model analysis of repeated measures using maximum likelihood estimation was tested in IBM SPSS Statistics version 24 to examine the effects of treatment group status on two primary outcome measures including 1) cannabis approach bias post-treatment and 2) percent cannabis use days during treatment, as well as one secondary outcome measure, 1) percent alcohol use days during treatment. A linear mixed-effects model with a random intercept and slope was selected to account for repeated measures and data missing at random (West et al., 2007). Fixed factors included time (repeated measures effect), treatment group status (CAAT-training vs. CAAT-sham), and the group by time interaction effect. Baseline motivation to change scores (i.e., SOCRATES) were explored as a moderator based on the research literature and potential for motivation level to differ between groups (Feaster et al., 2011; Gladwin et al., 2016); therefore SOCRATES score and the corresponding interaction with treatment condition and time was entered in the model to examine predictive value for cannabis and alcohol use outcome variables. Only factors that demonstrated a significant effect were retained in the model. Post hoc comparisons of estimated marginal means were used to compare the effects of group status on cannabis use over time.
2.5.3 Exploratory Analysis
Analysis of variance (ANOVA) examined between group differences at baseline and post treatment on cannabis and alcohol use self-report questionnaires reported in Table 2. We also explored bivariate correlations between change in approach bias and change in primary and secondary outcome measures.
Table 2.
Substance use self-report questionnaires, N=80.
| Cannabis & Alcohol Use Questionnaires | CAAT-Training M (SD) or % n=43 |
CAAT-Sham M (SD) or % n=37 |
|---|---|---|
| Marijuana Ladder, Baseline | 4.7 (2.7) | 4.5 (2.2) |
| Marijuana Ladder, Post Treatmenta | 4.6 (2.6) | 4.4 (2.4) |
| Cannabis Withdrawal Scale, Baseline | 23.2 (22.6) | 22.2 (21.0) |
| Cannabis Withdrawal Scale, Post Treatmentb | 28.8 (26.2) | 19.9 (22.2) |
| Marijuana Effect Expectancies, Baseline: | ||
| Cognitive and behavioral impairment | 30.1 (7.8) | 32.0 (7.8) |
| Relaxation and tension reduction | 30.3 (5.0) | 29.1 (5.5) |
| Social and sexual facilitation | 28.3 (6.2) | 27.6 (5.0) |
| Perceptual and cognitive enhancement | 27.8 (4.2) | 26.3 (4.5) |
| Global negative effect | 15.3 (4.7) | 14.6 (4.2) |
| Craving and physical effect | 24.4 (3.3) | 25.0 (3.6) |
| Marijuana Effect Expectancies, Post Treatment:a | ||
| Cognitive and behavioral impairment | 31.2 (7.9) | 31.0 (8.5) |
| Relaxation and tension reduction | 29.9 (4.4) | 29.7 (4.9) |
| Social and sexual facilitation | 28.9 (5.8) | 27.9 (4.9) |
| Perceptual and cognitive enhancement | 26.9 (4.3) | 26.4 (4.0) |
| Global negative effect | 16.0 (4.9) | 14.8 (4.5) |
| Craving and physical effect | 24.7 (3.3) | 24.8 (3.4) |
| Self-Efficacy Questionnaire, Baseline | 4.0 (1.3) | 4.6 (1.3) |
| Self-Efficacy Questionnaire, Post Treatmentb | 4.0 (1.3) | 4.5 (1.3) |
| Marijuana Craving Questionnaire- SF, Baseline | 13.7 (4.0) | 13.1 (4.6) |
| Marijuana Craving Questionnaire- SF, Post Treatmenta | 13.5 (5.3) | 12.5 (3.4) |
| Marijuana Problem Scale, Baseline | 5.3 (4.3) | 3.6 (3.3) |
| Marijuana Problem Scale, Post Treatmentb | 5.0 (4.2) | 3.5 (3.4) |
| SOCRATES Drug Use Questionnaire, Baseline* | 38.7 (12.0) | 33.2 (11.0) |
| SOCRATES Drug Use Questionnaire, Post Treatmentc | 35.7 (13.9) | 32.4 (10.5) |
| Rugters Alcohol Problem Index, Baseline | 6.9 (7.9) | 6.0 (5.2) |
p<.05;
n=72;
n=73;
n=70
3. Results
3.1 Demographic and Substance Use Characteristics
Minimal baseline differences were observed between CAAT-training and CAAT-sham groups on demographic characteristics (Table 1) and exploratory outcome measures (Table 2). However, significant between group differences at baseline were observed between percent cannabis days in the past 30 days (p=.02) and baseline SOCRATES score (p=.03). The CAAT-training group reported a greater percentage of cannabis use days driven primarily by between group differences at the UCSD site (p=.06); similarly, the CAAT-training group reported higher SOCRATES scores (greater motivation to change), also driven by differences at the UCSD site (p=.03); therefore site and quantity of cannabis use reported 30 days prior to baseline (measured in number of episodes of cannabis use), in addition to SOCRATES score, were further explored as possible moderators when examining relationships between group and percent days of substance use at follow-up.
Table 1.
Demographic characteristics of sample, (N=80).
| Demographics | CAAT-Training M (SD) or % n=43 |
CAAT-Sham M (SD) or % n=37 |
|---|---|---|
| Age, Baseline | 19.7 (1.0) | 19.5 (0.9) |
| % Male | 53.5% | 59.5% |
| % Caucasian | 72.1% | 64.9% |
| Education (grade, in years) | 12.7 (1.0) | 12.8 (0.8) |
| BDI-II, Baseline | 8.0 (8.3) | 5.6 (5.2) |
| BDI-II, Post Treatmenta | 6.7 (6.6) | 4.4 (5.7) |
| STAI, Baseline | 31.0 (7.9) | 30.3 (7.7) |
| STAI, Post Treatmentb | 29.6 (7.8) | 29.9 (7.7) |
| Internalizing | 50.0 (11.4) | 47.8 (9.2) |
| Externalizing | 53.3 (8.1) | 52.9 (7.8) |
| % AUD | 35.7% | 32.4% |
| % CUD | 73.8% | 59.5% |
| Age of cannabis use onset | 15.9 (1.2) | 16.0 (1.3) |
| Age of cannabis use onset, regular use | 17.2 (1.2) | 17.5 (1.0) |
| Cannabis use episodes, past year | 349.8 (251.3) | 286.7 (268.7) |
| % cannabis use days, past 30 days, Baseline* | 71.9 (32.7) | 55.0 (33.2) |
| % cannabis use days, Post Treatment | 65.0 (33.9) | 55.2 (32.6) |
| Days since last use of cannabis, Baseline | 2.1 (3.9) | 2.9 (4.1) |
| Days since last use of cannabis, Post Treatmentb | 1.7 (3.3) | 4.3 (7.9) |
| % alcohol use days, past 30 days, Baseline | 17.4 (17.2) | 20.5 (14.2) |
| % alcohol Use Days, Post Treatmenta | 27.1 (22.6) | 23.3 (16.9) |
| Days since last use of alcohol, Baseline | 14.0 (22.3) | 6.7 (11.0) |
| Days since last use of alcohol, Post Treatmentc | 8.3 (23.2) | 8.5 (18.5) |
| Average Tobacco Use episodes per month, Baselined | 75.8 (95.3) | 25.1 (44.1) |
p<.05; Beck Depression Inventory-II (BDI-II); State-Trait Anxiety Inventory (STAI) Trait version; Alcohol Use Disorder (AUD); Cannabis Use Disorder (CUD); regular use, >1/week for 52 weeks;
n=73,
n=72;
n=71;
n=22
3.2 Cannabis Approach Bias Tendencies
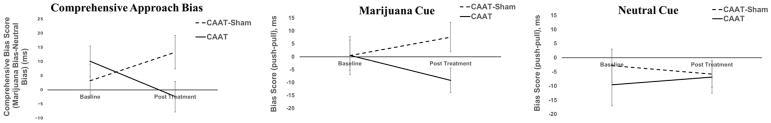
Examining the relationship between treatment group, time, and their interaction, the group by time interaction effect on comprehensive approach bias scores (cannabis image bias minus neutral image bias) did not reach statistical significance (p=.06). Findings suggest the experimental manipulation generated an anticipated small to medium sized reduction (Cohen’s dz = 0.42) in approach bias for cannabis cues for those randomized to the CAAT-training condition, even though the effect failed to reach significance (p=.06). Table 3 provides parameter estimates, significance levels, and confidence intervals, and suggests that change in cannabis approach bias slopes from baseline to post treatment is positive for CAAT-sham (i.e., an increased approach bias) and negative for CAAT-training (i.e., change to avoidance bias) (β=−22.62, SE=12.20, 95% CI: −46.92, 1.67, p=.06). Follow-up analyses examined approach bias for each cue type, although no significant effects were found when cue type was examined independently. For cannabis stimuli only, the CAAT-sham group showed a non-significant increase in their approach bias for cannabis stimuli, whereas the CAAT-training group demonstrated a non-significant change toward an avoidance bias (p=.13). Minimal between group and within group differences were observed in approach biases for neutral cues, as both groups showed an avoidance bias at baseline and post treatment (ps>0.50).
Table 3.
Final linear mixed model estimation of comprehensive cannabis approach bias score by time and treatment group (N=80).
| Independent Variables | β | SE | p | 95% CI | |
|---|---|---|---|---|---|
| Time | 9.84 | 8.87 | 0.27 | −7.84 | 27.53 |
| Treatment Group | 6.89 | 8.78 | 0.44 | −10.58 | 24.35 |
| Time x Treatment Group | −22.62 | 12.20 | 0.06 | −46.92 | 1.67 |
3.3 Primary and Secondary Substance Use Outcomes
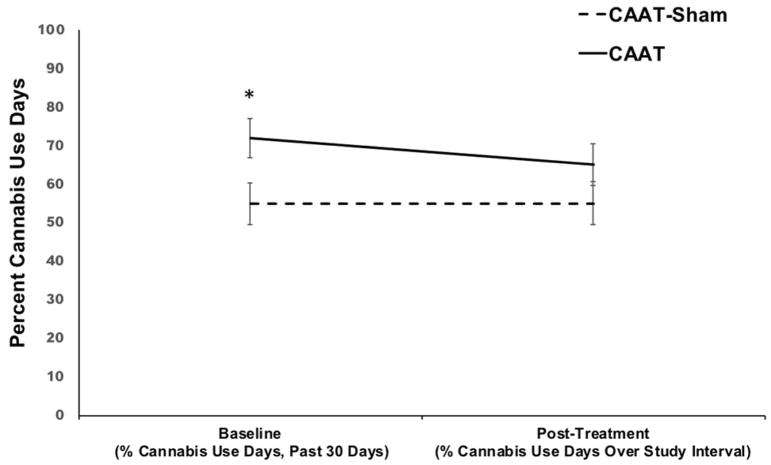
To test our hypothesis that participants randomized to the CAAT-training condition would report fewer percent cannabis use days during treatment, we examined the relationships between group, time, and SOCRATES score at baseline (motivation to change cannabis use behaviors). Table 4 presents parameter estimates, significance levels, and confidence intervals for the final significant model. We found a significant group by time interaction predicting percent cannabis use days over study enrollment (β=−8.96, SE=4.33, 95% CI: −17.58, −0.33, p=.04). SOCRATES score was not a significant predictor and/or moderator (ps>.80) and therefore was not retained in the final model. Treatment site and quantity of cannabis use reported in the 30 days prior to baseline (measured in number of episodes of cannabis use) were further explored as potential moderators, but did not significantly interact with treatment condition and/or time (ps=.54–.81) and therefore were not included in the final model. While individuals randomized to the CAAT-training condition reported more percent days of cannabis use at baseline, their percent days of cannabis use decreased over treatment; no change was observed in the sham group. Participants randomized to the avoid cannabis condition (CAAT-training) reported 7% less days of cannabis use over study enrollment compared to 0% change in the CAAT-sham condition.
Table 4.
Final linear mixed model estimation of percent cannabis use days over study interval by time and treatment group (N=80).
| Independent Variables | β | SE | p | 95% CI | |
|---|---|---|---|---|---|
| Time | 0.96 | 3.13 | 0.76 | −5.27 | 7.19 |
| Treatment Group | 16.81 | 7.38 | 0.02 | 2.15 | 31.48 |
| Time x Treatment Group | −8.96 | 4.33 | 0.04 | −17.58 | −0.33 |
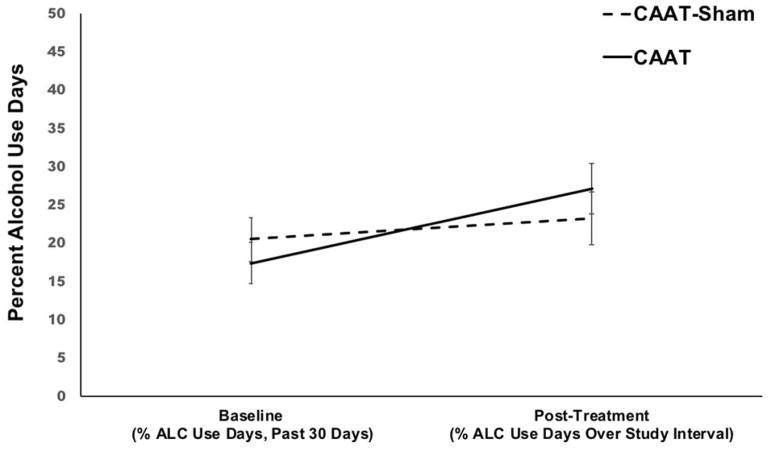
Percent alcohol use days was also explored (see Table 5 for parameter estimates, significance levels, and confidence intervals), and the increase in percent alcohol use days from baseline to post treatment was significantly greater for the CAAT-training group compared to CAAT-sham (β=6.31, SE=3.13, 95% CI: 0.06, 12.55, p=0.04). Unexpectedly, those in the avoid cannabis condition reported 10% percent more alcohol use days during study enrollment compared to 3% more for sham.
Table 5.
Final linear mixed model estimation of percent alcohol use days over study interval by time and treatment group (N=80).
| Independent Variables | β | SE | p | 95% CI | |
|---|---|---|---|---|---|
| Time | 3.01 | 2.27 | 0.19 | −1.50 | 7.53 |
| Treatment Group | −3.12 | 3.96 | 0.43 | −10.96 | 4.75 |
| Time x Treatment Group | 6.31 | 3.13 | 0.04 | 0.06 | 12.55 |
3.4 Exploratory Analysis
No between group differences were observed at baseline or post treatment in the cannabis and alcohol self-report questionnaires (ps>.05) other than for SOCRATES score as reported above. The correlation between change in approach bias and change in percent days of cannabis use (r=.21, p=.07) was not statistically significant. While the magnitude of the relationship is small, it suggests decreases in percent days of cannabis use over study enrollment were associated with an increasing avoid cannabis bias from baseline to post treatment (i.e., increasing negative bias values). No other correlations were significant or trending toward significance (ps>.31). Notably, we did not find evidence that changes in alcohol use were related to changes in marijuana use in the intervention group.
4. Discussion
Three key findings from this multisite proof-of-concept study with frequent cannabis-using adolescents suggest: 1) the cannabis approach-avoidance training paradigm may alter approach biases for cannabis stimuli in the anticipated direction, as those randomized to the experimental manipulation exhibited a small observed effect (Cohen’s dz = 0.42) in avoidance bias for cannabis-related stimuli, despite not reaching statistical significance (p=.06), 2) those randomized to the experimental condition reduced cannabis use (percent use days) during the intervention with 7% less cannabis use days in the experimental condition versus no change in the control condition, and 3) unexpectedly, those completing the cannabis intervention increased their alcohol use with 10% more alcohol use days in the experimental condition versus 3% in the control condition. Greater cannabis avoidance bias from baseline to post treatment was related to decreased percent days of cannabis use during treatment, although the effect size was small (r=.21) and the relationship did not meet statistical threshold (p=.07). Importantly, change in cannabis use, which was targeted in the intervention, was found in non-treatment seeking adolescents in the absence of adjunctive behavioral and pharmacological interventions (unlike other studies that found significant effects of AAT on substance use in the context of co-occurring treatment; e.g., (Eberl et al., 2013; Wiers et al., 2011). Findings provide evidence that multisite clinical trials with adolescent cannabis users can be completed successfully, and provide a framework for follow-up studies aimed at testing novel computerized interventions, such as AAT. Multisite trials not only help increase external validity, but allow for standardized methods of data collection with more statistical power and larger sample sizes in shorter periods of time, and ultimately more timely and informative results (Boyce and Lynne-Landsman, 2013; Cristea et al., 2016; Rinck, 2017; Weinberger et al., 2001).
Approach avoidance training paradigms have shown promising results for alcohol misuse, with participants randomized to alcohol AAT training showing 10 to 13% greater abstinence rates a year post-training compared to sham groups (Eberl et al., 2013; Wiers et al., 2011), yet very few studies have examined approach biases to cannabis stimuli. Current research suggests a cannabis approach bias in heavy cannabis users, and a positive correlation between approach bias and future use (i.e., stronger cannabis approach biases associated with heavier cannabis use at follow-up) (Cousijn et al., 2012a; Cousijn et al., 2011). While previous findings have shown reduction in cannabis craving after four sessions of CAAT in adult cannabis users (Sherman et al., 2018), no studies to date have tested a cannabis training paradigm as a targeted intervention for adolescents. Our cannabis findings are largely consistent with the alcohol use literature and suggest that in six training sessions, a modest reduction (small to medium sized effect) in automatic approach tendencies toward substance use stimuli as well as substance use outcomes can be found (Eberl et al., 2013, 2014; Rinck, 2017; Wiers et al., 2011; Wiers et al., 2010). Motivation to quit was not found to be a predictive factor in our non-treatment-seeking sample, despite it being suggested to be a potential moderator of clinical outcomes (Gladwin et al., 2016).
It is important to acknowledge that some degree of doubt has been raised on the effectiveness of Approach-Avoidance Training for addiction problems (Cristea et al., 2016), largely due to the lack of existing data available and select negative findings reported in the substance use literature for both alcohol and tobacco-related stimuli (Cousijn et al., 2014; Cousijn et al., 2015; Field et al., 2017; Larsen et al., 2014; Lindgren et al., 2015). Despite this, examining cognitive bias modification (e.g., approach-avoidance task training) for intervention purposes is an important exploration, as it utilizes a growing body of neuroscience knowledge on adolescent brain development and addictions, and applies it for intervention purposes (Boyce and Lynne-Landsman, 2013; O’Brien, 2015; Onken, 2015). The general pattern of findings across the literature support Approach-Avoidance Training for modulation of biases and improving clinical effects (Gladwin et al., 2016), and future study with larger sample sizes that will yield even greater informational value on the magnitude of treatment effect (Rinck, 2017). Cognitive bias modification is largely based on dual process models of addiction (Everitt and Robbins, 2013; Robinson and Berridge, 2008; Wiers et al., 2007), and these models parallel the vast majority of neuroimaging research with adolescent cannabis as well as alcohol users, in which alterations in corticostriatal circuit integrity are associated with addictive behaviors and loss of behavioral control for drugs and drug-related stimuli (Jacobus and Tapert, 2013; Wiers, C.E. et al., 2015b). In fact, support exists for functional brain changes in reward-related brain regions following completion of Approach-Avoidance Training, both in response to activation during approach trials and in response to cue-evoked activation of the target stimuli following training completion (Wiers, C.E. et al., 2015a; Wiers, C.E. et al., 2015b; Wiers et al., 2014; Wiers, R.W. et al., 2015a).
More traditional behavioral intervention approaches (e.g., Cognitive-Behavioral Therapy (CBT)) have demonstrated improvements in changing problematic substance use with adolescents and young adults (Budney et al., 2007; Copeland and Swift, 2009; Hettema et al., 2005; Martin and Copeland, 2008), but none have proven to be superior in treating cannabis use disorder (de Gee et al., 2014; Waldron and Turner, 2008). By targeting reward-related mechanisms that produce problematic cannabis use, CAAT-training could complement or synergistically improve existing substance use treatment programs. The study presented is not only a step in bridging the gap between translating neuroscience research into novel interventions for cannabis use treatment (Onken, 2015), but an important piece of evidence that the CAAT can be successfully tested in a multisite trial with adolescent cannabis users. Nevertheless, some limitations of the current study are important to clearly address. The study includes a modest sample size and limited follow-up data, and longer-term effects of this intervention may be missed and/or interact with factors not measured in this study. Similarly, test-retest reliability of approach bias assessment is still undetermined (Janssen et al., 2015) and more work is needed to better understand the psychometrics of these types of tasks (Reinecke et al., 2010). It is also important to note that we cannot rule out regression to the mean as a possible explanation for changes observed given the differences in cannabis use at baseline, although quantity of cannabis use reported 30 days prior to baseline was not found to be a significant predictor and/or moderator of treatment outcome (Kraemer, 2015). Further, earlier research has indicated that it is important that the irrelevant feature of cognitive bias modification tasks should not be too easy, as participants can easily ignore the contents of the pictures ((De Houwer, 2003). Hence, it is possible that the current irrelevant feature used (e.g., border color) is not optimal.
Unexpectedly, we found that youth in the CAAT group increased their alcohol use during treatment. This may suggest a “substitution effect” was present, wherein cannabis reduction or abstinence appeared to increase the use of other substances of abuse, which is consistent with previous literature (Chaloupka and A, 1997; Copersino et al., 2006; Schaub et al., 2010). These findings highlight the need for additional studies designed to hypothesize and test how the CAAT influences both alcohol and cannabis use, with the ultimate goal of reducing use of both substances given the high rates of co-use during adolescence. Finally, the smaller effect sizes in this pilot investigation need to be replicated in a large sample study. This study used the CAAT as a standalone treatment. Future studies should also examine the CAAT in motivated treatment-seekers or as an adjunctive treatment to existing computerized and non-computerized substance use interventions (Olmos et al., 2017).
Future directions for our work include development of multisite protocols and paradigms that combine alcohol and cannabis stimuli, given the high prevalence rates of these two substances during adolescence (Miech et al., 2017) and the potential for alcohol use as a substitute coping behavior in the presence of reduced cannabis use. Likewise, a better understanding of cognitive biases as they relate to healthier coping behaviors often explored in treatment as usual (e.g., eating, exercise) may improve approach-avoidance training paradigms and provide ideas for future task components (Wiers et al., 2013). Web-based paradigms have been explored with mixed effects, with some limitations including drop-out and trouble capturing motivations for participating and quitting (Wiers, R.W. et al., 2015b). Introduction of gaming elements could be a logical next step to increase task motivation (Boendermaker et al., 2016), although more work is needed to understand if gaming elements add or detract from the effectiveness of the paradigm. Computerized interventions like CAAT have many advantages, as they are relatively quick, inexpensive, and easy to administer, thereby increasing the likelihood they would be utilized by care providers compared to more time-intensive behavioral interventions that require extensive training (e.g., Cognitive Behavioral Therapy) (Eberl et al., 2013). Ongoing exploration is needed to determine if CBM will increase the efficacy of empirically-supported intervention strategies and improve treatment outcomes. As the literature on neurobiological vulnerabilities and neurodevelopmental consequences of cannabis misuse grow, development of novel treatment approaches will be important for the future.
Figure 2.
Mean approach bias scores measured at baseline and post-treatment follow-up for CAAT-training and CAAT-Sham. Comprehensive bias scores are presented, as well as approach bias scores as a function of cue type (i.e., cannabis and neutral).
Figure 3.
Percent cannabis use days measured at baseline (past 30 days) and post-treatment follow-up (days over study enrollment) for CAAT-training and CAAT-Sham, N=80.
Figure 4.
Percent alcohol use days at baseline (past 30 days) and follow-up (days over study enrollment) for CAAT-training and CAAT-Sham, N=80.
Acknowledgments
This study was supported by National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Grants R01 AA013419, U01 DA041089, K12 DA031794, K23 AA025399; National Center for Advancing Translational Sciences KL2 TR001444; National Institute of Mental Health R00 MH090243; and the Society for Clinical Neuropsychology, Division 40 of the American Psychological Association
References
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, RAS, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Boendermaker WJ, Sanchez Maceiras S, Boffo M, Wiers RW. Attentional Bias Modification With Serious Game Elements: Evaluating the Shots Game. JMIR Serious Games. 2016;4:e20. doi: 10.2196/games.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce CA, Lynne-Landsman SD. Integrating translational neuroscience to improve drug abuse treatment for adolescents. Psychol Addict Behav. 2013;27:547–551. doi: 10.1037/a0032434. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Roffman R, Stephens RS, Walker D. Marijuana dependence and its treatment. Addict Sci Clin Pract. 2007;4:4–16. doi: 10.1151/ascp07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloupka FJ, AL Do youths substitute alcohol and marijuana? Some economic evidence. Eastern Economic Journal. 1997:23. [Google Scholar]
- Copeland J, Swift W. Cannabis use disorder: epidemiology and management. International Review of Psychiatry. 2009;21:96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15:297–302. doi: 10.1080/10550490600754341. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective FMRI study. PLoS One. 2012a;7:e42394. doi: 10.1371/journal.pone.0042394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Wiers RW. Reaching out towards cannabis: approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction. 2011;106:1667–1674. doi: 10.1111/j.1360-0443.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Luijten M, Wiers RW. Mechanisms underlying alcohol-approach action tendencies: the role of emotional primes and drinking motives. Front Psychiatry. 2014;5:44. doi: 10.3389/fpsyt.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Snoek RW, Wiers RW. Cannabis intoxication inhibits avoidance action tendencies: a field study in the Amsterdam coffee shops. Psychopharmacology (Berl) 2013a;229:167–176. doi: 10.1007/s00213-013-3097-6. [DOI] [PubMed] [Google Scholar]
- Cousijn J, van Benthem P, van der Schee E, Spijkerman R. Motivational and control mechanisms underlying adolescent cannabis use disorders: A prospective study. Dev Cogn Neurosci. 2015;16:36–45. doi: 10.1016/j.dcn.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Watson P, Koenders L, Vingerhoets WA, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. 2013b;38:2825–2832. doi: 10.1016/j.addbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Porrino LJ, Goudriaan AE. Individual differences in decision making and reward processing predict changes in cannabis use: a prospective functional magnetic resonance imaging study. Addict Biol. 2012b doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P. The Effectiveness of Cognitive Bias Modification Interventions for Substance Addictions: A Meta-Analysis. PLoS One. 2016;11:e0162226. doi: 10.1371/journal.pone.0162226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gee EA, Verdurmen JE, Bransen E, de Jonge JM, Schippers GM. A randomized controlled trial of a brief motivational enhancement for non-treatment-seeking adolescent cannabis users. J Subst Abuse Treat. 2014;47:181–188. doi: 10.1016/j.jsat.2014.05.001. [DOI] [PubMed] [Google Scholar]
- de Graaf R, Radovanovic M, van Laar M, Fairman B, Degenhardt L, Aguilar-Gaxiola S, Bruffaerts R, de Girolamo G, Fayyad J, Gureje O, Haro JM, Huang Y, Kostychenko S, Lepine JP, Matschinger H, Mora ME, Neumark Y, Ormel J, Posada-Villa J, Stein DJ, Tachimori H, Wells JE, Anthony JC. Early cannabis use and estimated risk of later onset of depression spells: Epidemiologic evidence from the population-based World Health Organization World Mental Health Survey Initiative. Am J Epidemiol. 2010;172:149–159. doi: 10.1093/aje/kwq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J. The Extrinsic Affective Simon Task. Exp Psychol. 2003;50:77–85. doi: 10.1026//1618-3169.50.2.77. [DOI] [PubMed] [Google Scholar]
- Derntl B, Seidel EM, Eickhoff SB, Kellermann T, Gur RC, Schneider F, Habel U. Neural correlates of social approach and withdrawal in patients with major depression. Soc Neurosci. 2011;6:482–501. doi: 10.1080/17470919.2011.579800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J. Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J. Implementation of approach bias re-training in alcoholism-how many sessions are needed? Alcohol Clin Exp Res. 2014;38:587–594. doi: 10.1111/acer.12281. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Feaster DJ, Mikulich-Gilbertson S, Brincks AM. Modeling site effects in the design and analysis of multi-site trials. Am J Drug Alcohol Abuse. 2011;37:383–391. doi: 10.3109/00952990.2011.600386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Di Lemma L, Christiansen P, Dickson J. Automatic avoidance tendencies for alcohol cues predict drinking after detoxification treatment in alcohol dependence. Psychol Addict Behav. 2017;31:171–179. doi: 10.1037/adb0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. 2015;16:16–22. doi: 10.1016/j.dcn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Wiers CE, Wiers RW. Cognitive neuroscience of cognitive retraining for addiction medicine: From mediating mechanisms to questions of efficacy. Prog Brain Res. 2016;224:323–344. doi: 10.1016/bs.pbr.2015.07.021. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hogue A, Henderson CE, Ozechowski TJ, Robbins MS. Evidence base on outpatient behavioral treatments for adolescent substance use: updates and recommendations 2007–2013. J Clin Child Adolesc Psychol. 2014;43:695–720. doi: 10.1080/15374416.2014.915550. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Castro N, Squeglia LM, Meloy MJ, Brumback T, Huestis MA, Tapert SF. Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol Teratol. 2016;57:20–29. doi: 10.1016/j.ntt.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Castro N, Brumback T, Meruelo AD, Tapert SF. Neuropsychological Performance in Adolescent Marijuana Users With Co-Occurring Alcohol Use: A Three-Year Longitudinal Study. Neuropsychology. 2015a doi: 10.1037/neu0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015b doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Effects of Cannabis on the Adolescent Brain. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013;27:431–442. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen T, Larsen H, Vollebergh WA, Wiers RW. Longitudinal relations between cognitive bias and adolescent alcohol use. Addict Behav. 2015;44:51–57. doi: 10.1016/j.addbeh.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2015: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- Kong G, Larsen H, Cavallo DA, Becker D, Cousijn J, Salemink E, Collot D’Escury-Koenigs AL, Morean ME, Wiers RW, Krishnan-Sarin S. Re-training automatic action tendencies to approach cigarettes among adolescent smokers: a pilot study. Am J Drug Alcohol Abuse. 2015;41:425–432. doi: 10.3109/00952990.2015.1049492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC. A Source of False Findings in Published Research Studies: Adjusting for Covariates. JAMA Psychiatry. 2015;72:961–962. doi: 10.1001/jamapsychiatry.2015.1178. [DOI] [PubMed] [Google Scholar]
- Larsen H, Kong G, Becker D, Cousijn J, Boendermaker W, Cavallo D, Krishnan-Sarin S, Wiers R. Implicit motivational processes underlying smoking in american and dutch adolescents. Front Psychiatry. 2014;5:51. doi: 10.3389/fpsyt.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KP, Wiers RW, Teachman BA, Gasser ML, Westgate EC, Cousijn J, Enkema MC, Neighbors C. Attempted Training of Alcohol Approach and Drinking Identity Associations in US Undergraduate Drinkers: Null Results from Two Studies. PLoS One. 2015;10:e0134642. doi: 10.1371/journal.pone.0134642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning V, Staiger PK, Hall K, Garfield JB, Flaks G, Leung D, Hughes LK, Lum JA, Lubman DI, Verdejo-Garcia A. Cognitive Bias Modification Training During Inpatient Alcohol Detoxification Reduces Early Relapse: A Randomized Controlled Trial. Alcohol Clin Exp Res. 2016;40:2011–2019. doi: 10.1111/acer.13163. [DOI] [PubMed] [Google Scholar]
- Martin G, Copeland J. The adolescent cannabis check-up: randomized trial of a brief intervention for young cannabis users. J Subst Abuse Treat. 2008;34:407–414. doi: 10.1016/j.jsat.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use, 1975–2016; Volume I, Secondary school students. Ann Arbor: Institute for Social Research; 2017. University of Michigan. [Google Scholar]
- Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagnerness Scale (SOCRATES) Psychol Addict Behav. 1996;10:81–89. [Google Scholar]
- O’Brien C. In treating alcohol use disorders, why not use evidence-based treatment? Am J Psychiatry. 2015;172:305–306. doi: 10.1176/appi.ajp.2014.14111413. [DOI] [PubMed] [Google Scholar]
- Olmos A, Tirado-Munoz J, Farre M, Torrens M. The efficacy of computerized interventions to reduce cannabis use: A systematic review and meta-analysis. Addict Behav. 2017;79:52–60. doi: 10.1016/j.addbeh.2017.11.045. [DOI] [PubMed] [Google Scholar]
- Onken LS. Cognitive training: Targeting Cognitive Processes in the Development of Behavioral Interventions. Clinical Psychological Science. 2015;3:39–44. [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, van de Schoot RA, Janssen T, Vollebergh WA, Wiers RW. Automatic processes and the drinking behavior in early adolescence: a prospective study. Alcohol Clin Exp Res. 2013;37:1737–1744. doi: 10.1111/acer.12156. [DOI] [PubMed] [Google Scholar]
- Peeters M, Wiers RW, Monshouwer K, van de Schoot R, Janssen T, Vollebergh WA. Automatic processes in at-risk adolescents: the role of alcohol-approach tendencies and response inhibition in drinking behavior. Addiction. 2012;107:1939–1946. doi: 10.1111/j.1360-0443.2012.03948.x. [DOI] [PubMed] [Google Scholar]
- Reinecke A, Becker ES, Rink M. Three indirect tasks assessing implicit threat associations and behavioral response tendencies: Test-retest reliability and validity. Zeitschrift für Psychologie/Journal of Psychology. 2010;218:4–11. [Google Scholar]
- Rinck M. CBM research needs more power: Commentary on the special issue on cognitive bias modification. J Behav Ther Exp Psychiatry. 2017;57:215. doi: 10.1016/j.jbtep.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Rinck M, Telli S, Kampmann IL, Woud ML, Kerstholt M, Te Velthuis S, Wittkowski M, Becker ES. Training approach-avoidance of smiling faces affects emotional vulnerability in socially anxious individuals. Front Hum Neurosci. 2013;7:481. doi: 10.3389/fnhum.2013.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clin Psychol Rev. 2009;29:519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108–113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Schafer J, Brown SA. Marijuana and cocaine effect expectancies and drug use patterns. Journal of Consulting and Clinical Psychology. 1991;59:558–565. doi: 10.1037//0022-006x.59.4.558. [DOI] [PubMed] [Google Scholar]
- Schaub M, Gmel G, Annaheim B, Mueller M, Schwappach D. Leisure time activities that predict initiation, progression and reduction of cannabis use: a prospective, population-based panel survey. Drug Alcohol Rev. 2010;29:378–384. doi: 10.1111/j.1465-3362.2009.00156.x. [DOI] [PubMed] [Google Scholar]
- See J, MacLeod C, Bridle R. The reduction of anxiety vulnerability through the modification of attentional bias: a real-world study using a home-based cognitive bias modification procedure. J Abnorm Psychol. 2009;118:65–75. doi: 10.1037/a0014377. [DOI] [PubMed] [Google Scholar]
- Seidel EM, Habel U, Finkelmeyer A, Schneider F, Gur RC, Derntl B. Implicit and explicit behavioral tendencies in male and female depression. Psychiatry Res. 2010;177:124–130. doi: 10.1016/j.psychres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Sharbanee JM, Hu L, Stritzke WG, Wiers RW, Rinck M, MacLeod C. The effect of approach/avoidance training on alcohol consumption is mediated by change in alcohol action tendency. PLoS One. 2014;9:e85855. doi: 10.1371/journal.pone.0085855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, Squeglia LM, McRae-Clark AL. Approach bias modification for cannabis use disorder: A proof-of-principle study. J Subst Abuse Treat. 2018;87:16–22. doi: 10.1016/j.jsat.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavet JD, Stein LA, Colby SM, Barnett NP, Monti PM, Golembeske C, Jr, Lebeau-Craven R. The Marijuana Ladder: measuring motivation to change marijuana use in incarcerated adolescents. Drug Alcohol Depend. 2006;83:42–48. doi: 10.1016/j.drugalcdep.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. A technique for assessing self-reported alcohol consumption. Humana Press; New York, NY: 1992. Timeline follow back. [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behavior. Annu Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, Kadden R, Duresky D, Stephens R. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Treating adult marijuana dependence: a test of the relapse prevention model. J Consult Clin Psychol. 1994;62:92–99. doi: 10.1037//0022-006x.62.1.92. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Amir N. Modifying automatic approach action tendencies in individuals with elevated social anxiety symptoms. Behav Res Ther. 2012;50:529–536. doi: 10.1016/j.brat.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Interactions between implicit and explicit cognition and working memory capacity in the prediction of alcohol use in at-risk adolescents. Drug Alcohol Depend. 2008;94:116–124. doi: 10.1016/j.drugalcdep.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HB, Turner CW. Evidence-based psychosocial treatments for adolescent substance abuse. J Clin Child Adolesc Psychol. 2008;37:238–261. doi: 10.1080/15374410701820133. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Oddone EZ, Henderson WG, Smith DM, Huey J, Giobbie-Hurder A, Feussner JR. Multisite randomized controlled trials in health services research: scientific challenges and operational issues. Med Care. 2001;39:627–634. doi: 10.1097/00005650-200106000-00010. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: A practical guide using statistical software. 2. Chapman & Hall; Boca Raton, FL: 2007. [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Ludwig VU, Gladwin TE, Park SQ, Heinz A, Wiers RW, Rinck M, Lindenmeyer J, Walter H, Bermpohl F. Effects of cognitive bias modification training on neural signatures of alcohol approach tendencies in male alcohol-dependent patients. Addict Biol. 2015a doi: 10.1111/adb.12221. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Gladwin TE, Park SQ, Pawelczack S, Gawron CK, Stuke H, Heinz A, Wiers RW, Rinck M, Lindenmeyer J, Walter H, Bermpohl F. Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am J Psychiatry. 2015b;172:335–343. doi: 10.1176/appi.ajp.2014.13111495. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, Bermpohl F. Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology. 2014;39:688–697. doi: 10.1038/npp.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels RC, Sher KJ, Grenard J, Ames SL, Stacy AW. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Boelema SR, Nikolaou K, Gladwin TE. On the Development of Implicit and Control Processes in Relation to Substance Use in Adolescence. Curr Addict Rep. 2015a;2:141–155. doi: 10.1007/s40429-015-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–497. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Hofmann W, Salemink EK, Ridderinkhof KR. Cognitive bias modification and cognitive control training in addiction and related psychopathology. Clinical Psychological Science. 2013;1:192–212. [Google Scholar]
- Wiers RW, Houben K, Fadardi JS, van Beek P, Rhemtulla M, Cox WM. Alcohol cognitive bias modification training for problem drinkers over the web. Addict Behav. 2015b;40:21–26. doi: 10.1016/j.addbeh.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]