Abstract
Aiming to assess the association between measures of obesity and outcomes in coronary artery disease (CAD) patients. We included consecutive patients referred to cardiac rehabilitation because of prior CAD events, who were classified using BMI groups and sex-specific tertiles of waist-to-hip ratio (WHR). Follow-up was ascertained using a population-based, record linkage system that consists of complete data on all residents. A major cardiovascular event (MACE) was defined as the composite outcome including acute coronary syndromes, coronary revascularization, ventricular arrhythmias, stroke or death from any cause. The association between obesity measures and MACE was assessed using cox proportional hazards models adjusted for potential confounders. The cohort included 1529 patients (74% men) mean age ± SD 63.1±12.5 years, 40% were obese by BMI. Eighty-eight percent of men and 57% of women were classified as having central obesity by WHR. Median follow-up was 5.7 years and 415 patients had a MACE event. After adjustment, a high WHR tertile was a significant predictor for MACE in women (HR=1.85 [95% Cl: 1.16, 2.94]; p=0.01), but not in men (HR=0.92 [95%CI: 0.69, 1.22]; p=0.54). This relationship in women persisted after further adjustment for BMI (HR=1.75 [95%CI: 1.07, 2.87]; p=0.03). Obesity by BMI was not associated with MACE in either men (HR=1.07 [95%CI: 0.76, 1.51]; p=0.69) or women (HR=0.98 [95%CI: 0.62, 1.56]; p=0.95). In conclusion WHR is associated with a higher risk of MACE among women with CAD but not in men. There was no obesity paradox when assessing obesity by BMI and MACE in CAD patients when including non-fatal events.
Keywords: Obesity, Coronary artery disease, cardiac rehabilitation
Introduction
Assessing obesity with the body mass index (BMI) has limitations, not only because BMI does not perfectly correlate with body adiposity but also because BMI does not measure fat distribution. BMI has been paradoxically linked to lower total and cardiovascular mortality in patients with coronary artery disease (CAD)1 while measurements of central obesity such as waist circumference (WC) or waist-to-hip ratio (WHR) have shown conflictive results.2,3 Most of the evidence testing the obesity paradox or linking central obesity and outcomes in CAD patients has used mortality as the outcome of interest, with scant research testing the association between measures of obesity and major adverse cardiovascular events (MACE) including non-fatal clinical outcomes. In the general population WC and WHR are indirect estimations of visceral adiposity which is linked to insulin resistance, dyslipidemia, and increased cardiovascular risk.4 It is reasonable to believe that those mechanisms will continue to be relevant in the presence of CAD. In this study we test the hypotheses that in CAD patients, central obesity would be associated with increased MACE while obesity by BMI would either have none or paradoxical association with MACE.
Methods
We conducted a population-based, historical cohort study of all Olmsted County, Minnesota, residents over the age of 18 years that enrolled in phase II cardiac rehabilitation (CR) between the years 2002 and 2012 following a CAD event. Patients were identified using the resources of the Rochester Epidemiology Project (REP),5 a federally funded record linkage system that indexes medical records, medications, procedures, and other health related information from the primary providers of medical care in Olmsted County; Olmsted Medical Center, the Mayo Clinic and few other individual private providers. All tertiary care, cardiovascular procedures and CR occurred at Mayo Clinic during the study period. This serves as an ideal community-based infrastructure to investigate disease-associated risk factors and outcomes.5 The study protocol was reviewed and approved by the institutional review board of both the Mayo Clinic and Olmsted Medical Center. All included patients provided research authorization, as required by the state of Minnesota.
We identified patients referred for CR because of a myocardial infarction (MI) (ST or non-ST-segment elevation MI), stable or unstable angina and coronary revascularization by either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI). We excluded patients on whom anthropometric measurements were not performed. Baseline information was collected electronically from the Rochester Epidemiology Project (REP) within 3 months of CR entry using the International Classification of Diseases-9th revision; this approach has been previously validated.6 Demographic and clinical characteristics, laboratory values, blood pressure measurements and medications prescribed for the treatment of CAD were ascertained. For internal validation, a portion of this information was reviewed in duplicate by 2 investigators (J.M.I. and F.L.J.) who were masked to the baseline characteristics of patients.
Anthropometry at baseline was assessed during the CR entry evaluation according to the World Health Organization (WHO) Anthropometric Guidelines using structured protocols by trained nurses.7 Height without shoes was recorded to the nearest centimeter and weight was recorded to the nearest 0.1 kilogram using a stadiometer, BMI was calculated dividing weight in kilograms by height in meters squared. Hip circumference (HC) was measured at the widest portion of the buttocks with the tape horizontal in cm. WC was obtained at the midpoint between the lower margin of the lowest palpable rib and the top of the iliac crest in the mid-axillary line at the end of expiration. Measurements were performed standing. WHR was calculated by dividing WC by HC.
MACE included any of the following events: 1) any diagnosis of a new acute coronary syndrome, including both ST and non-ST-segment elevation myocardial infarction and unstable angina that required hospitalization; 2) coronary revascularization, including PCI and CABG; 3) stroke, including any non-traumatic brain hemorrhage or infarction; 4) ventricular arrhythmias warranting in-hospital management, and 6) death from any cause. Mortality information was obtained from the REP, which records vital status from federal and Minnesota state death registries. Each subject in the study sample was followed up from the date of cardiac rehabilitation entry (index date) until the occurrence of a first MACE event or date of last followup until December 1th 2014. All outcomes were passively followed through a review of the electronic medical records in the records-linkage system in duplicate by 2 physician investigators (J.M.I. and F.L.J.) who were blinded to baseline characteristics including WHR and BMI to assess inter-observer agreement. Consensus resolved all disagreements. We summarized baseline patient characteristics with frequencies and percentages, means ± standard deviations (SD), or medians and interquartile range (IQR), as appropriate. Patient characteristics were compared between men and women using, chi-square tests, fisher’s exact test or two-sample nonpaired t-tests, as appropriate. The Kappa (k) statistic was used to assess inter-observer agreement over comorbidities and also for each outcome composing MACE. BMI was analyzed both continuously and categorically using preestablished cutoffs.7 WHR was analyzed both continuously and categorically, using WHO criteria defining central obesity as WHR≥0.90 for men and WHR≥ 0.85 for women, and also using sex-adjusted tertiles due to differences in body composition by sex reported the literature demonstrating sex-specific outcomes when considering central obesity.8–10 The association between WHR and the time to first recorded MACE was assessed separately within men and women as a pre-specified analysis using Kaplan-Meier curves and Cox proportional hazards regression models. The models were adjusted for age, smoking, and history of heart failure, all known potential confounders in the association between obesity and Cardiovascular Disease (CVD) events. An additional model included BMI and an exploratory model adjusted for optimal medical therapy for CAD (defines as prescription of cardioprotective medication classes (statins, ACE inhibitors/angiotensin II receptor blockers, β-blockers, and antiplatelet agents. The multiplicative interaction between BMI and WHR was also assessed as a separate term in our overall model. We did not adjust for history of hypertension, diabetes or dyslipidemia because they are mechanistic factors linking obesity and CVD events, as generally accepted and recommended in obesity-related epidemiology. Findings were summarized using hazard ratios and 95% confidence intervals. The assumption of proportionality for the Cox proportional hazards models was assessed graphically and fulfilled. The functional form for WHR ratio (continuous) on the outcome was explored graphically with splines. Two-sided p-values less than 0.05 were considered statistically significant. All analyses were completed using JMP®, Version 12.1 and SAS® 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline patient characteristics are shown in Table 1. During a median follow-up of 5.7 years (IQR: 3.5–8.8), 415 patients (73% men) had at least 1 MACE,representing 9,586 person years, incidence of MACE was no different between sexes (p=0.10), as seen in Table 2. Central obesity measured using WHR tertiles was associated with an increased risk of MACE in women whose 3 year event-free-survival rates were 90.0, 84.5, 77.4% (log rank p=0.01); but not in men, in whom event-free survival rates were 84.8, 82.0, and 85.0% (log rank p=0.10), as observed in Figure 1-A/B.
Table 1.
Baseline patient characteristics
| Variable | All (n= 1529) |
Men (N=1133) |
Women (N=396) |
|---|---|---|---|
| Age (years) | 63.20 ±12.5 | 62.10 ±11.87 | 66.24 ± 13.58 § |
| Non-Hispanic white | 1472(96.3%) | 1090(96.2%) | 382(96.5%) |
| Black or African American | 24(1.6%) | 16(1.4%) | 8(2.0%) |
| Asian | 29(1.9%) | 23(2.0%) | 7(1.8%) |
| American Indian or Alaska Native | 3(0.2%) | 3(0.3%) | 0 |
| Native Hawaiian/Pacific Islander | 1(0.1%) | 1(0.1%) | 0 |
| Heart failure | 274 (17.9%) | 180 (15.9%) | 94 (23.7%) § |
| Ever smokers | 901 (58.9%) | 716 (63.2%) | 185 (46.7%) § |
| Diabetes mellitus | 683 (44.7%) | 509 (44.9%) | 174 (43.9%) |
| Hypercholesterolemia | 1419 (92.8%) | 1059 (93.5%) | 360 (90.9%) |
| Hypertension | 894 (58.5%) | 636 (56.1%) | 258 (65.2%) § |
| Beta blockers use | 1107 (72.4%) | 816 (72.0%) | 291 (73.5%) |
| Angiotensin converting enzyme inhibitor use | 642 (42.0%) | 472 (41.7%) | 170 (42.9%) |
| Calcium channel blockers | 166 (10.9%) | 110 (9.7%) | 56 (14.1%) § |
| Diuretics | 447 (29.2%) | 295 (26.0%) | 152 (38.4%) § |
| Waist to hip ratio | 0.95 (0.1) | 0.98 (0.1) | 0.86 (0.1)§ |
| Waist to hip ratio sex-adjusted tertile1 | |||
| Low | 0.91 (0.05) | 0.79 (0.05) | |
| Middle | 0.98 (0.03) | 0.86 (0.04) | |
| High | 1.04 (0.06) | 0.94 (0.07) | |
| Central obesity | 1229 (80.4%) | 1003 (88.5%) | 226 (57.1%) § |
| Body mass index (Kg/m2) | 29.7 ±5.7 | 29.9 ±5.5 | 28.9 ±6.2 |
Values are mean ± standard deviation SD or n (%), unless otherwise indicated.
Denotes statistical significance <0.05, compares males vs females.
Median and (Interquartile range)
Waist to hip ratio sex-adjusted tertiles (male Low Tertile: <0.94, Middle Tertile: 0.94 to <1.01, High Tertile: ≥1.01; women Low Tertile : <0.83, Middle Tertile: 0.83 to <0.89, High Tertile: ≥0.89).
Table 2.
Major adverse Cardiovascular Events by Gender
| Overall N=1529 |
Men N=1133 |
Women N=396 |
|
|---|---|---|---|
| # Major Adverse Cardiovascular Events per Total person years | 584/9586 | 430/7333 | 154/2253 |
| Major adverse cardiovascular events | 415 (27.1%) | 307 (27.1%) | 108 (27.3%) |
| Total years of follow up (median) | 5.7 (3.58.4) | 5.9 (3.8–9.2) | 4.9 (3.2–7.7) |
| Percutaneous Coronary Intervention | 133 (32.0%) | 101 (32.9%) | 32 (29.6%) |
| Death | 107 (25.8%) | 77 (25.0%) | 30 (27.8%) |
| Myocardial Infarction | 58 (14.0%) | 39 (12.7%) | 19 (17.6%) |
| Stroke | 42(10.0%) | 27 (8.8%) | 15 (14.0%) |
| Coronary Artery Bypass Graft | 34 (8.3%) | 33(10.8%) | 1 (0.9%) |
| Angina | 33 (8.1%) | 24 (7.8%) | 9 (8.3%) |
| Ventricular Arrhythmia | 8 (1.8%) | 6 (2.0%) | 2 (1.8%) |
Values presented are frequencies and percentages.
Outcomes ascertained electronically: Myocardial infarction ICD-9,410. X; Unstable Angina ICD-9,411.X; Percutaneous Coronary Intervention CPT/ ICD-9, 92980– 92982/V45.82; Coronary Artery Bypass Graft CPT/ ICD-9 337700–337735/V45.81; Ventricular arrhythmias ICD-9,427.X; Stroke ICD-9, 433.X
Figure 1A.
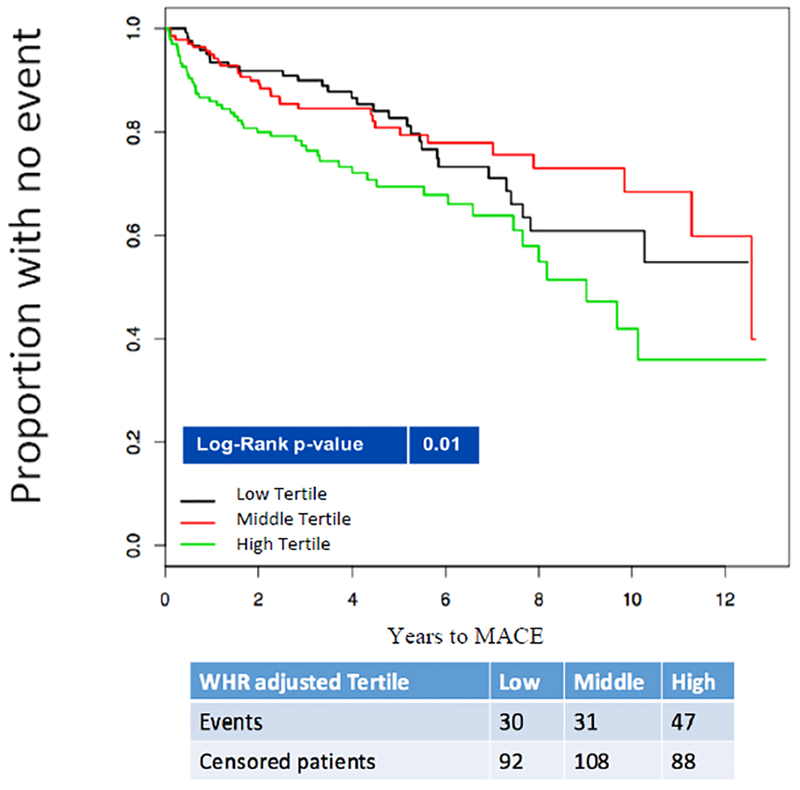
Kaplan-Meier curves indicating the relationship between waist to hip ratio tertiles and major adverse cardiovascular events among Women.
Figure 1B.

Kaplan-Meier curves indicating the relationship between waist to hip ratio tertiles and major adverse cardiovascular events among men.
As seen in Table 3, multivariate modeling demonstrated that WHR tertile remained a significant predictor for MACE for women; The risk of MACE for women in the highest WHR tertile was almost two-fold higher when compared to those in the lowest tertile. This relationship did not change after further adjusting for BMI (HR=1.75 [95%CI: 1.07, 2.87]; p=0.03). For men, the adjusted risk of MACE for those in the highest WHR tertile was no different when compared to the lowest tertile, after adjusting for BMI (Adjusted HR=1.09, [95%CI: 0.79, 1.50] p=0.58). When assessing WHR as a continuous variable, a 0.10 increase in WHR was associated with a 32% increase in risk of MACE among women,but not among men, as seen in Table 3.This relationship remained constant after further adjusting for optimal medical therapy for CAD ( Data not shown). Obesity by BMI was not a significant predictor for MACE in either the whole cohort (HR 0.99, [95%CI: 0.76, 1.31] p=0.99) or in either men (HR=1.07 [95%CI: 0.76, 1.51]; p=0.69) or women (HR=0.98 [95%CI: 0.62, 1.56]; p=0.95) when analyzed separately. This association remained constant when only considering waist circumference, as seen in Appendix 1.
Table 3.
Adjusted Cox proportional hazard models testing the association between Waist to Hip Ratio tertiles and major adverse cardiovascular events among Men and Women.
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| Measure | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic |
| Waist to hip ratio sex-adjusted tertiles* | ||||||||||||
| Low | Referent | Referent | Referent | Referent | ||||||||
| Middle | 1.27 | 0.54 | 1.21 | 0.58 | 0.94 | 0.58 | 0.99 | 0.62 | ||||
| (0.97,1.66) | (0.92,1.58) | (0.57, 1.56) | (0.60,1.64) | |||||||||
| High | 0.97 (0.73, 1.29) | 0.11 | 0.92 | 0.12 | 1.77 | 0.008 | 1.85 | 0.007 | ||||
| (0.69,1.22) | (1.12–2.82) | (1.16, 2.94) | ||||||||||
| Waist to hip ratio continuous, for 0.10 increase | 1.00 (0.88,1.15) | 0.96 | 0.54 | 0.98 (0.85, 1.13) | 0.81 | 0.58 | 1.30 (1.07,1.58) | 0.008 | 0.58 | 1.32 (1.08, 1.61) | 0.007 | 0.61 |
Cl= confidence interval
Model 1: Adjusted for age, Model 2: Adjusted for Age, smoking and history of heart failure.
Interaction between Body mass index and Waist to hip ratio (in a model with Body mass index and waist hip ratio), was not statistically significant (p=>0.05).
Waist to hip ratio sex-adjusted tertiles (male Low Tertiles: <0.94, Middle Tertile: 0.94 to <1.01, High Tertile: ≥1.01; women Low Tertile : <0.83, Middle Tertile: 0.83 to <0.89, High Tertile: ≥0.89)
Discussion
In this historical cohort study of patients with known CAD attending CR, we found that there is no obesity paradox when assessing the association between BMI and MACE, as seen for mortality.1 However, while WHR is not related to MACE in men, we observed that in women, a higher WHR was related to a higher risk of MACE, independent of BMI. Few studies have tested the association between obesity and MACE in CAD patients, while this is the first study to assess the risk of MACE when compared by WHR in patients with CAD.
Several studies have suggested that measures of central obesity, particularly WHR, provide incremental information beyond BMI, particularly in women.5,11–14 Cerhan et al.13 and Lassalle C et al.14 have shown the value of WC over BMI in prediction of total mortality even with BMI units or categories, while Li et al.10 and Sahakyan et al.8 found that WHR relates to increased cardiovascular and mortality risk in women in all BMI categories. This was only seen in men with normal body weight.12 We found no significant association between central obesity and MACE in men; a finding that may be related to the high prevalence of central obesity in men, with only 12% with a normal WHR, so the statistical and discriminatory power to detect associations was limited (Unadjusted C-statistic, for Men=0.50 and for Women=0.60). These results are consistent with other cohorts where this relationship has also been considered modest.11,15 In the case of CAD patients undergoing cardiac rehabilitation, sex differences also appear to contribute additionally to the effect of obesity on clinical outcomes8,10,16. This relationship was demonstrated in a meta-analysis by Coutinho et al.9 that concluded that in patients with CAD with either normal or high BMI, measures of central obesity were directly associated with an increased mortality rates and that this association was significantly greater in women.
Our sex-specific differences in the relationship between WHR and MACE provide some insight into the effects that predict long-term outcomes, and expands on what we and other authors have found before.10,11,15,17,18 First, women are at higher risk in other populations, including post-myocardial infarction in-hospital mortality,19 incident CVD,10 total mortality in elderly patients,15,16 and CAD patients.9 These sex differences could be due to several physiologic, metabolic and hormonal differences between sexes. Second, fat and muscle distribution differs by sex. Men store more visceral fat20 while women with greater fat in pelvis and gluteo-femoral muscle 17,21 The distribution in women changes on the post-menopausal state due to changes in steroid hormones, specifically estrogen.22,23 maintaining after this stage their pre-menopausal BMI concomitant with an increase in WC and decrease in HC leading to an increase in WHR.22 Third, those with enlarged abdominal adiposity may have an increased amount of androgen production and thus higher risk of CVD.23 Further studies are needed to confirm these hypotheses.
Importantly, we did not observe the “obesity paradox”, where individuals classified as overweight or obese by BMI have better survival than those with normal weight. Results that may be justified because of the inability of BMI to discriminate between fat mass and lean mass and so it leads to misclassification regarding CVD risk.11 Studies showing the obesity paradox have only included mortality as outcome.
This study has several strengths. The infrastructure of the REP provides excellent generalization to the US caucasian population in a community-based setting. Complete ascertainment of outcomes leads to minimization of loss to follow-up since they are applied to a relatively stable population,5 thereby reducing referral bias and increasing the generalizability of the results. Our blinded investigators independently verified comorbidities and outcomes improving the validity of our data. Our sex-stratified analysis evaluated the association of WHR and the composite endpoint of MACE, an important and widely used measure since it has a significant impact on medical decision making for cardiovascular patient care and treatment. MACE offers more information when it is included as an outcome, because it provides a better idea of safety, effectiveness of treatments, disability and reduction of quality of life, along with costs through the evaluation of non-fatal events, which has not been extensively studied. Lastly, our study had a considerably longer follow-up compared to previous studies about prognosis of patients with CAD.9
This study is susceptible to a number of sources of bias. First, by limiting our sample exclusively to patients with CAD and measures of central obesity, we might introduce selection bias and limit the generalizability of our results . Second, our sample included mostly non-Hispanic white men, given the target population and the well-known lack of CR participation among women.24 Third, we could not account for additional confounders (genetic susceptibility, nutritional quality, physical activity, daily environment, sedentary behaviors, cardiorespiratory fitness, sleep disorders, duration of obesity, socio-economic status along with others).Fourth, because our estimates are based on a single measure of WHR as the exposure variable, assuming that it will not change through follow-up, misclassification bias could potentially affect our sample. Finally, subjects without central obesity who develop CAD do so as a result of other risk factors and therefore their risk for recurrent events would theoretically depend on the persistance of those non-obesity related factors. Although such sources of bias can limit causal inferences, the results of our study are still informative.
These results expand upon studies that have found a relationship between WHR and cardiovascular risk factors,25 metabolic syndrome,26 total and cardiovascular mortality,8,27 and other CVD outcomes.18,27,28 Our study has potential implications for clinical care of individuals with CAD referred for CR. An early assessment of central adiposity in addition to BMI and application of lifestyle changes focused on caloric balance, healthy nutrition and tailored exercise prescription may have a positive impact on these patients as MACE events.
In conclusion, WHR was associated with a higher risk of MACE among women with CAD undergoing CR. Measuring WHR could be used as an additional measure to BMI for assessment of cardiovascular event risk in CAD patients with a higher impact among women.
Highlights.
Women with CAD attending cardiac rehabilitation with higher WHR had a greater long-term risk of major adverse cardiovascular events when compared to those with a lower WHR.
A higher WHR was not associated with major adverse cardiovascular events in men.
BMI was not associated with major cardiovascular events.
Acknowledgements:
This work was supported in part by the European Regional Development Fund-FNUSA-ICRC (No. Z.1.05/1.1.00/02.0123) by project no. LQ1605 from the National Program of Sustainability II (MEYS CR), by the project ICRC-ERA-Human Bridge (No. 316345) funded by the 7th Framework Programme of the European Union. National Institute of Health (NIH) grants (R01HL65176 and R01HL114024 to VKS). Dr. Batsis is supported in part by the National Institute on Aging under award number K23AG051681 with administrative support from the Dartmouth Health Promotion and Disease Prevention Research Center (Cooperative Agreement Number U48DP005018) from the Centers for Disease Control and Prevention. This publication was made possible in part by CTSA Grant Number UL1TR000135 from the National Center for Advancing Translational Sciences (NCATS), and resources of the Rochester Epidemiology Project (REP), which is supported by the National Institute of Aging under Award Number R01AG034676. Both are components of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources.
Appendix 1. Adjusted Cox proportional hazard models testing the association between waist circumference tertiles and major adverse cardiovascular events among Men and Women.
| Men (N=807) |
Women (N=309) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||
| Measure | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic | Hazard Ratio (95%CI) | P Value | C-Statistic |
| Waist circumference sex-adjusted tertiles* | ||||||||||||
| Low | Referent | Referent | Referent | Referent | ||||||||
| Middle | 1.27 (0.89,1.83) | 0.50 | 1.24 (0.86,1.78) | 0.61 | 1.57 (0.79, 3.15) | 0.61 | 1.57 (0.78,3.17) | 0.63 | ||||
| High | 1.30 (0.91, 1.85) | 0.25 | 1.21 (0.84, 1.73) | 0.40 | 3.28 (1.75,6.36) | 0.0008 | 3.42 (1.80, 6.74) | 0.0006 | ||||
| 0.50 | 0.61 | 0.61 | 0.62 | |||||||||
| Waist circumference continuous, for 1 cm increase | 1.00 (0.99,1.01) | 0.16 | 1.00 (0.99, 1.01) | 0.26 | 1.02 (1.01,1.04) | 0.0003 | 1.02 (1.01, 1.03) | 0.0006 | ||||
Cl= confidence interval
Model 1: Adjusted for age, Model 2: Adjusted for Age, smoking and history of heart failure. (The variable Waist circumference was available in less subjects than those included in table 3)
Interaction between Body mass index and waist circumference (in a model with Body mass index and waist circumference), was not statistically significant (p=>0.05).
Waist circumference sex-adjusted tertiles [Men Low: <98 cms (N=291 ,MACE=64), Middle: 98 cms to <108.56 cms (N=247, MACE=58), High: ≥108.56 cms (N=269, MACE=60); Women Low: 87 cms (N=106,MACE=19), Middle: 87 cms to <101 cms (N=100,MACE=18), High: ≥101 cms (N=103,MACE=29)j
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet;368:666.-. [DOI] [PubMed] [Google Scholar]
- 2.de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. European heart journal 2007;28:850–856. [DOI] [PubMed] [Google Scholar]
- 3.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr 2001;74:315–321. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Annals of the New York Academy of Sciences 1995;748:399–406. [DOI] [PubMed] [Google Scholar]
- 5.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Pankratz JJ, Brue SM, Rocca WA. Data Resource Profile: The Rochester Epidemiology Project (REP) medical records-linkage system. International Journal of Epidemiology 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. The American journal of medicine 2015;128:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee., 1995. [PubMed]
- 8.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, Coutinho T, Jensen MD, Roger VL, Singh P, Lopez-Jimenez F. Normal Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Annals of internal medicine 2015;163:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho T, Goel K, Correa de Sa D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol 2011;57:1877–1886. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Engstrom G, Hedblad B, Calling S, Berglund G, Janzon L. Sex differences in the relationships between BMI, WHR and incidence of cardiovascular disease: a population-based cohort study. Int J Obes (Lond) 2006;30:1775–1781. [DOI] [PubMed] [Google Scholar]
- 11.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, Lopez-Jimenez F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010;34:791–799. [DOI] [PubMed] [Google Scholar]
- 12.Tice JA, Kanaya A, Hue T, Rubin S, Buist DS, Lacroix A, Lacey JV Jr., Cauley JA, Litwack S, Brinton LA, Bauer DC. Risk factors for mortality in middle-aged women. Arch Intern Med 2006;166:2469–2477. [DOI] [PubMed] [Google Scholar]
- 13.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, Ebbert JO, English DR, Gapstur SM, Giles GG, Horn-Ross PL, Park Y, Patel AV, Robien K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hartge P, Bernstein L, Berrington de Gonzalez A. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinic proceedings 2014;89:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der AD, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado-Yohar SM, Engstrom G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina-Portillo E, Moreno-Iribas C, Norberg M, Overvad K, Panico S, Quiros JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjonneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. European heart journal 2017. [DOI] [PMC free article] [PubMed]
- 15.Sharma S, Batsis JA, Coutinho T, Somers VK, Hodge DO, Carter RE, Sochor O, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Lopez-Jimenez F. Normal-Weight Central Obesity and Mortality Risk in Older Adults With Coronary Artery Disease. Mayo Clinic proceedings 2016;91:343–351. [DOI] [PubMed] [Google Scholar]
- 16.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 2013;112:1592–1598. [DOI] [PubMed] [Google Scholar]
- 17.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986;10:53–67. [PubMed] [Google Scholar]
- 18.Lee HW, Hong TJ, Hong JY, Choi JH, Kim BW, Ahn J, Park JS, Oh JH, Choi JH, Lee HC, Cha KS. Waist-hip ratio and 1-year clinical outcome in patients with non-ST-elevation myocardial infarctions. Coron Artery Dis 2016;27:357–364. [DOI] [PubMed] [Google Scholar]
- 19.Shiraki T, Saito D. Sex difference of in-hospital mortality in patients with acute myocardial infarction. Acta Med Okayama 2011;65:307–314. [DOI] [PubMed] [Google Scholar]
- 20.Blaak E Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001;4:499–502. [DOI] [PubMed] [Google Scholar]
- 21.Kvist H, Chowdhury B, Sjostrom L, Tylen U, Cederblad A. Adipose tissue volume determination in males by computed tomography and 40K. Int J Obes 1988;12:249–266. [PubMed] [Google Scholar]
- 22.Bjorkelund C, Lissner L, Andersson S, Lapidus L, Bengtsson C. Reproductive history in relation to relative weight and fat distribution. Int J Obes Relat Metab Disord 1996;20:213–219. [PubMed] [Google Scholar]
- 23.Haffner SM, Katz MS, Dunn JF. Increased upper body and overall adiposity is associated with decreased sex hormone binding globulin in postmenopausal women. Int J Obes 1991;15:471–478. [PubMed] [Google Scholar]
- 24.Supervia M, Medina-Inojosa JR, Yeung C, Lopez-Jimenez F, Squires RW, Perez-Terzic CM, Brewer LC, Leth SE, Thomas RJ. Cardiac Rehabilitation for Women: A Systematic Review of Barriers and Solutions. Mayo Clinic proceedings 2017. [DOI] [PMC free article] [PubMed]
- 25.See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: the Dallas Heart Study. J Am Coll Cardiol 2007;50:752–759. [DOI] [PubMed] [Google Scholar]
- 26.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet;365:1415–1428. [DOI] [PubMed] [Google Scholar]
- 27.Song X, Jousilahti P, Stehouwer CD, Soderberg S, Onat A, Laatikainen T, Yudkin JS, Dankner R, Morris R, Tuomilehto J, Qiao Q. Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutr Metab Cardiovasc Dis 2015;25:295–304. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr., Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–1649. [DOI] [PubMed] [Google Scholar]


