Obesity is a major risk factor for diabetes and cardiovascular disease. The societal cost of obesity is keeping pace with the growing scope of the epidemic, estimated to account for approximately 17% of all US health expenditures by 2030.1 The clinical burden of obesity-related cardiovascular disease is profound; recent data suggest that severe obesity [body mass index (BMI) > 35 kg/m2] is associated with nearly a 4-fold increased risk of incident heart failure and approximately 2-fold increased risk of incident coronary heart disease and stroke.2 Given the strong associations between obesity and hypertension, diabetes, and dyslipidemia, it is understandable that much of the obesity-related coronary heart disease risk is attributable to concurrent traditional risk factors such as hypertension, diabetes, and dyslipidemia. However, data suggest a persistent risk association between obesity and coronary heart disease that is not explained by conventional risk factors, thereby spawning extensive investigations to identify the culprit mechanisms.3 Over the past several decades, the research focus has shifted to the adipose tissues themselves, and more specifically the adipocyte, as a key link between obesity and cardiovascular disease risk.
Adipose tissue functions primarily as a storage depot for excess calories and thus plays an important physiologic role to maintain metabolic homeostasis. In healthy (non-obese) states, adipocytes participate in metabolic regulation not only by storing excess calories as lipid, but also by secreting hormones such as leptin that regulate food intake to maintain proper energy balance. When this balance is perturbed in favor of chronic caloric excess, the adipocytes primarily expand in size (hypertrophy) to provide sufficient storage capacity. As obesity progresses over time, the hypertrophied adipocytes become mechanically stressed, leading to local and systemic inflammation and insulin resistance, a key component of the metabolic syndrome. Moreover, the heightened systemic inflammation in obesity may promote atherosclerosis, which is itself an inflammatory disorder.4 Indeed, levels of C-reactive protein track closely with BMI and parameters of metabolic syndrome, suggesting that obesity is a key mechanism driving inflammation in coronary heart disease.5
Since the first adipokine, adipsin, was discovered in 1987,6 various pro-inflammatory adipokines, such as tumor necrosis factor (TNF)-α, interleukin-6, and monocyte chemoattractant protein (MCP)-1 have been implicated in the pathogenesis of obesity-related disease. Increased levels of these mediators in obese adipose tissues can promote recruitment of inflammatory cells, perturb insulin signaling, and act on the liver to augment systemic inflammation.7 Moreover, adipokines can potentially disrupt vascular homeostasis and promote neointima formation, a process which is prevalent in obese diabetic patients.8
Neointima formation is characterized by inflammatory cell recruitment and enhanced vascular smooth muscle cell (VSMC) proliferation and migration into the intimal layer. Adipose-derived molecules implicated primarily in inflammation and angiogenesis, such as MCP-1 and vascular endothelial growth factor, may also play a paracrine role to promote VSMC migration and proliferation.9, 10 These findings are illustrative of the complex interactions between adipose tissues and the blood vessel wall in obesity. To add yet another layer of complexity, adipose tissues also produce an array of adipokines that have beneficial effects on the blood vessel wall to reduce inflammation and VSMC proliferation.11 The most well described of these beneficial adipokines is adiponectin, whose anti-inflammatory and vasculoprotective effects are diminished in obesity owing to reduced production by adipocytes.12 Moreover, the actions of certain adipokines may result in part from local production by perivascular adipose tissue (PVAT), which directly abuts most large arteries and likely plays an important local role in vascular homeostasis and disease.13
In this issue of Circulation, Wang et al. report the discovery of a novel secreted adipokine, “family with sequence similarity 19 (chemokine (C-C motif)-like) member A (FAM19A),” that negatively regulates VSMC proliferation and migration into the neointima, which further supports the importance of adipose tissue as an endocrine organ affecting the vasculature.14 FAM19A5 has previously been identified as a brain-specific chemokine that inhibits RANKL-induced osteoclast formation and induces macrophage migration through formyl peptide receptor 2. Wang et al. show for the first time that FAM19A5 is abundantly expressed in and released from adipose tissues of lean mice, and that its expression is markedly diminished in obesity. Local perivascular expression of FAM15A5 by adenoviral-mediated gene transduction, or transgenic overexpression of FAM15A5 in adipose tissues, protected against neointima formation in vivo. Importantly, the plasma levels of FAM15A5 achieved in FAM19A5 transgenic mice fed a high-fat western diet were restored approximately to the physiological levels observed in wild-type mice fed a regular chow diet, which supports the hypothesis that reduced FAM15A5 may contribute to obesity-related vascular disease. In vitro studies indicated a direct effect of FAM19A5 to inhibit VSMC proliferation and migration. The authors further identified the sphingosine-1-phosphate receptor 2 (S1PR2) as a novel receptor responsible for FAM19A5 binding, and G-12/13 and RhoA as key downstream signaling molecules to regulate VSMC proliferation and migration (Figure). Additionally, the authors detected decreased levels of CD45-positive leukocytes infiltrating into femoral arteries of FAM19A5 transgenic mice post injury. Although the impact of FAM19A5 overexpression on monocyte recruitment was not examined, the authors commented that recombinant FAM19A5 was chemoattractant for monocytes in vitro, in keeping with its predicted chemokine-like properties. Limited data suggested that transgenic FAM19A5 overexpression did not impact body weight, fasting glucose or lipid levels. Thus, FAM19A5 is an adipokine that potentially can regulate VSMC proliferation and inflammation via multiple mechanisms in the context of obesity-related cardiovascular disease.
Figure. Proposed mechanisms whereby a novel adipokine, FAM19A5, regulates neointima formation.
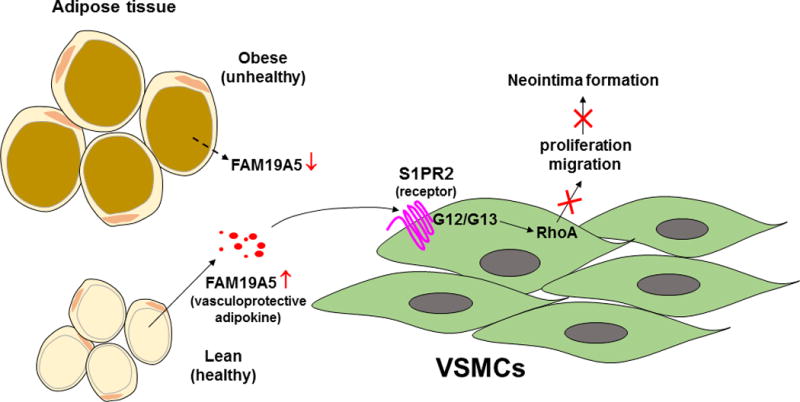
FAM19A5 secreted from healthy adipocytes binds to the S1PR2 receptor in vascular smooth muscle cells (VSMCs), which triggers downstream G12/G13 and RhoA signaling pathway to inhibit VSMC proliferation and migration, thereby protecting against neointima formation. In obesity, impaired FAM19A5 release from dysfunctional adipocytes fails to restrain VSMC growth, thus leading to enhanced neointima formation.
The authors detected abundant secretion of FAM19A5 by subcutaneous, brown, epididymal, and perirenal adipose tissues; expression in PVAT was also detected though not quantitated. Notably, FAM19A5 is expressed not only in murine adipocytes, but also in human adipocytes, as previously reported15 and demonstrated in the present study by immunohistochemistry. Interestingly, FAM19A5 levels in human adipocytes were significantly downregulated by TNF-α-induced inflammation,15 suggesting that pro-inflammatory cytokines produced during obesity may cause FAM19A5 downregulation. However, differential expression of FAM19A5 in lean and obese humans has not been reported yet, and FAM19A5’s potential to serve as a biomarker or target of obesity-related cardiovascular disease in humans remains to be determined. Thus, the present study raises additional questions that will require future investigations.
The mechanistic role of adipocytes in promoting vascular homeostasis has received relatively little attention to date. The study by Wang et al. provides support for the notion that anti-inflammatory adipokines produced from healthy adipocytes may function to protect against vascular disease. The authors provide proof-of-principle that disrupted levels of FAM19A5 during obesity can potentially augment VSMC proliferation and migration following vascular injury. Understanding the mechanisms that perturb adipokine balance may provide new insight into the causes and treatment of obesity-related cardiovascular disease.
Acknowledgments
Sources of Funding
Dr. Weintraub is supported by grants HL126949, HL134354 and AR070029 from the National Institutes of Health.
Footnotes
Disclosures
None.
References
- 1.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity. 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 2.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E, Folsom AR, Coresh J. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc. 2016;5:e003921. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 4.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 5.Rogowski O, Shapira I, Toker S, Melamed S, Shirom A, Berliner S, Zeltser D. Obesity-related correlation between C-reactive protein and the calculated 10-y Framingham Coronary Heart Disease Risk Score. Int J Obes. 2005;29:772–777. doi: 10.1038/sj.ijo.0802939. [DOI] [PubMed] [Google Scholar]
- 6.Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anders H Berg, Philipp E Scherer. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 9.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 10.Schlich R, Willems M, Greulich S, Ruppe F, Knoefel WT, Ouwens DM, Maxhera B, Lichtenberg A, Eckel J, Sell H. VEGF in the Crosstalk between Human Adipocytes and Smooth Muscle Cells: Depot-Specific Release from Visceral and Perivascular Adipose Tissue. Mediators Inflamm. 2013;2013:982458. doi: 10.1155/2013/982458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Fuster JJ, Ouchi N, Gokce N, Walsh K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 2014;34:1631–1636. doi: 10.1161/ATVBAHA.114.303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Chen D, Zhang Y, Wang P, Zheng C, Zhang S, Yu B, Zhang L, Zhao G, Ma B, Cai Z, Xie N, Huang S, Liu Z, Mo X, Guan Y, Wang X, Fu Y, Ma D, Wang Y, Kong W. A Novel Adipokine, FAM19A5, Inhibits Postinjury Neointima Formation through Sphingosine-1-Phosphate Receptor 2. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.117.032398. CIRCULATIONAHA.117.032398. [DOI] [PubMed] [Google Scholar]
- 15.Tourniaire F, Romier-Crouzet B, Lee JH, Marcotorchino J, Gouranton E, Salles J, Malezet C, Astier J, Darmon P, Blouin E, Walrand S, Ye J, Landrier JF. Chemokine expression in inflamed adipose tissue is mainly mediated by NF-κB. PLoS One. 2013;8:e66515. doi: 10.1371/journal.pone.0066515. [DOI] [PMC free article] [PubMed] [Google Scholar]


