Abstract
Chronic pain may alter both affect and value related behavior, which represents a potentially treatable aspect of the chronic pain experience. Current understanding of how chronic pain influences the function of brain reward systems, however, is limited. Using a monetary incentive delay (MID) task and functional magnetic resonance imaging (fMRI), we measured neural correlates of reward anticipation and outcome in female participants with the chronic pain condition of fibromyalgia (N=17) and age-matched, pain-free, female controls (N=15). We hypothesized that patients would demonstrate lower positive arousal, as well as altered reward anticipation and outcome activity within cortical-striatal circuits implicated in reward processing. Patients demonstrated lower arousal ratings as compared with controls, but no group differences were observed for valence, positive arousal or negative arousal ratings. Group fMRI analyses were conducted to determine predetermined region of interest (ROI), nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC), responses to potential gains, potential losses, reward outcomes, and punishment outcomes. Compared with controls, patients demonstrated similar, though slightly reduced, NAcc activity during gain anticipation. Conversely, patients demonstrated dramatically reduced mPFC activity during gain anticipation -- possibly related to lower estimated reward probabilities. Further, patients demonstrated normal mPFC activity to reward outcomes, but dramatically heightened mPFC activity to no-loss (non-punishment) outcomes. In parallel to NAcc and mPFC responses, patients demonstrated slightly reduced activity during reward anticipation in other brain regions, which included the ventral tegmental area, anterior cingulate cortex, and anterior insular cortex. Together, these results implicate altered cortical-striatal processing of monetary rewards in chronic pain.
Keywords: fibromyalgia, chronic pain, fMRI, BOLD, reward, value, punishment, anticipation, outcome, monetary incentive, arousal, valence, negative affect, depression, anxiety
INTRODUCTION
Chronic pain conditions embody, in part, dysfunctional neurophysiological and psychological processes [30]. Comorbid negative affective symptoms (e.g., anxiety and depression) are common in patients with chronic pain [62,78], and reward processing may also be altered in many patients with chronic pain [12]. Dysfunctional reward processing and motivational deficits may together play an important role in the maintenance of chronic pain and associated symptoms.
Neural processing of reward anticipation, choice, and outcomes recruit activity in deep subcortical structures and connected prefrontal and parietal cortical circuits [33]. A key neural component of reward processing involves dopaminergic projections from the midbrain ventral tegmental area (VTA) to the nucleus accumbens (NAcc). These neurons fire in the presence of reward cues, and NAcc activity is classically implicated in reward anticipation [40,74]. Brain reward circuits also include the medial prefrontal cortex (mPFC), which receives input from VTA and other cortical and subcortical regions [24]. mPFC activity is associated with evaluation of anticipated reward magnitude and probability [47,87], as well as reward outcomes and consumption [21,36,44]. Additional regions less directly involved in reward processing include the anterior insular cortex (aINS), and anterior cingulate cortex (ACC), among other regions [46]. Altered activity in these regions has been observed in individuals with chronic pain [48,58], further suggesting possible influences of altered reward processing in pain and comorbid affective disorders.
Neurobiological and clinical alterations exist and interact between brain reward systems, acute pain processing, and chronic pain (for reviews, see [10,12,54,66]). Relief from acute noxious stimuli is rewarding in itself and can increase NAcc activity [7]. Persons with chronic pain demonstrate altered brain responses to anticipated pain relief [56]. Persons with chronic low back pain demonstrate structural and functional alterations in reward pathways, particularly NAcc-mPFC connectivity [4], which correlate with pain chronicity [5]. Persons with chronic pain also show altered reward-seeking and risky behavior (e.g.,[2]), as well as altered brain dopaminergic activity [1,52,84,85]. Despite these findings, current understanding of dysfunctional reward processing in chronic pain is limited, and few studies have directly assessed neurobehavioral responses to non-nociceptive rewards and punishments in patients with chronic pain.
To enhance understanding of the neural processing of reward in chronic pain, patients with the chronic pain condition called fibromyalgia were evaluated using a monetary incentive delay (MID) task combined with functional magnetic resonance imaging (fMRI). The MID task is a validated experimental paradigm that quantifies anticipatory and outcome responses to varying levels of monetary reward and punishment [40,45]. Based on previous evidence of cortico-striatal alterations in chronic pain, we hypothesized that patients would demonstrate: 1) blunted positive arousal and reduced NAcc activity during reward anticipation and 2) altered mPFC activity during reward anticipation and outcome in comparison with controls. Post-hoc analyses were conducted on additional regions of interest (VTA, aINS, and ACC) as well as to identify relationships between neural activity and self-reported behavioral and clinical individual differences.
METHODS
Participants
Eighteen individuals diagnosed with fibromyalgia and 17 healthy individuals participated in the study. All patients met inclusion criteria as follows: modified American College of Rheumatology 2011 criteria for fibromyalgia [(1) widespread pain index (WPI) score ≥ 7 + symptom severity (SS) score ≥ 5, or WPI score 3–6 + SS score ≥ 9, (2) symptoms have been present at a similar level for at least 3 months, (3) the patient does not have a disorder that would otherwise explain the pain] [83]. Additionally, patients were required to have pain in all 4 quadrants of the body, have an average pain score of at least 2 (0–10 verbal scale) over the previous month, not be pregnant or nursing, have no MRI contraindications (e.g., metal in body, claustrophobia), and have no uncontrolled depression or anxiety. Because opioids can alter brain reward processing [79], patients were required to not be taking any opioid medications as part of their treatment regimen. Additionally, they were required not to have taken any opioids for a period of 90 days prior to study participation and never to have taken opioid medications for a period of greater than 30 days during their lifetime. Patients were allowed to continue their normal use of medications during participation in the study. Control participants met inclusion criteria for no chronic pain, not pregnant or nursing, no MRI contraindications, and no depression or anxiety. All participants signed written informed consent acknowledging that they were willing to participate in the study, understood all study procedures, and could withdraw from the study at any time. All study procedures were approved by the Stanford University Institutional Review Board.
Medication Usage
All patients reported less than 1 month of opioid use within their lifetime and no opioid use within 90 days prior to study participation. Control participants were taking no medications for pain at the time of study and had no history of chronic pain. Thirteen of the control participants reported not taking any medications for pain or mood-altering medications. One control participant with premenstrual symptoms (2 days per month) reported taking gabapentin (100 mg/day) and fluoxetine (40 mg/day), and another control participant reported taking celecoxib (200 mg) 3 weeks prior to the study visit due to a sports-related ankle injury. Exclusion of data from the 2 control subjects taking these medications did not significantly alter group results, and so these data were retained in the final analysis. Four of the patients were taking no pain or mood-altering medications. The remaining 13 patients were taking nonsteroidal anti-inflammatory drugs (NSAIDs, N = 7), serotonin-norepinephrine reuptake inhibitors (SNRIs, N = 4), selective serotonin reuptake inhibitors (SSRIs, N = 2), tricyclic antidepressants (TCAs, N = 3), other anxiolytics (e.g., buspirone hydrochloride, N=2), anticonvulsant drugs (N = 2), muscle relaxants (N = 2), gamma-aminobutyric acid (GABA) analogs (e.g., pregabalin and gabapentin, N = 6), low dose naltrexone (LDN, N = 2), medical cannabis (N = 1), and using topical lidocaine patches (N = 1). Exclusion of data from the patient taking medical cannabis did not significantly alter the group results and so these data remained in the final analysis.
Study Procedures
All study procedures were conducted at the Richard M. Lucas Center for Imaging at Stanford University. Prior to scanning, participants 1) were screened for any magnetic resonance contraindications, 2) received detailed instructions regarding the monetary incentive delay (MID) task, 3) practiced the MID task and the arousal and valence rating task, and 4) completed psychological, clinical, and behavioral questionnaires.
Instruction in and practice of the MID task was performed in a waiting room (outside of the MRI scanner) prior to the scan session. Participants practiced the MID task on a laptop computer for approximately 5 minutes. The practice was repeated if necessary until the participant was confident in her understanding of the task and able to respond to the target stimulus successfully. Also prior to the scanning session, participants were trained on the rating scales for arousal and valence. Written instructions were presented on the laptop screen and explained to the participants by trained research coordinators.
Patients completed questionnaires including the Beck Depression Inventory (BDI) [9], State-Trait Anxiety Inventory (STAI-State, STAI-Trait) [76], Behavioral Inhibition System/Behavioral Approach System (BIS/BAS Scales) [17], Profile of Mood States (POMS) [61], Positive and Negative Affect Schedule (PANAS) [82], Fibromyalgia Assessment Form (FAF) based on the Wolfe et al. 2011 Fibromyalgia Diagnostic Criteria [83], Brief Pain Inventory (BPI) [39], and PROMIS Fatigue [18]. Additional questionnaires were collected which were not included in the present analysis.
MRI Scans
MRI scans were conducted on a 3T General Electric scanner using an 8-channel head coil (GE Systems, Chicago, Illinois) at the Stanford University Richard M. Lucas Imaging Center. The scan session consisted of initial preparatory localizer and asset calibration scans, followed by 2 MID task fMRI scans. The 2 MID task scans were acquired sequentially with no breaks in between. The fMRI scan parameters were as follows: Gradient Echo Pulse Sequence using spiral in-out acquisition, flip angle 76°, echo time (TE) 30 seconds, repetition time (TR) 2 seconds, sequential descending slice order, 32 oblique slices, 4 mm slice thickness, 0.5 mm slice spacing (gap), pixel size 3.43 mm. The spiral in-out scan sequence reduces orbitofrontal signal drop-out [31] and was used to improve acquisition of medial prefrontal and orbitofrontal cortices. The MID 1 scan was 266 volumes and the MID 2 scan was 302 volumes, excluding lead-in (12 s) and lead-out times (8 s). A T1 anatomical scan [3D FSPGR (fast spoiled gradient-echo) IRprep BRAVO] was acquired for registration of functional images with parameters as follows: whole brain coverage including the brainstem and cerebellum, 1 mm slice thickness, 22 mm frequency field of view (FOV), frequency direction anterior/posterior, number of excitations (NEX) 2, flip angle 11°, TR 6.8, TE 2.6, frequency 256, phase 256, bandwidth 50.00.
Monetary Incentive Delay (MID) Task
The MID task was run in Matlab (Matlab R2012b, MathWorks, Natick, MA), using the Psychophysics Toolbox extensions for presentation of the visual stimuli (Psychtoolbox-3 [13]). A custom designed button box was used to collect participants’ responses. Participants viewed MID task stimuli via a mirror placed above the head coil; the mirror reflected images that were displayed via projector on a screen behind the scanner. Heart rate and respiration rate were collected using the MRI scanner pulse oximetry and plethysmography, respectively. Physiological data were not regressed due to an insufficient quantity of usable physiological data, however, previous MID task analyses do not typically regress out physiological data [47] (and a subset of data might be used in later validation analyses). The scanner sequence was programmed to send a “spacebar” to the Macintosh laptop running Matlab/Psychtoolbox to initiate the MID task.
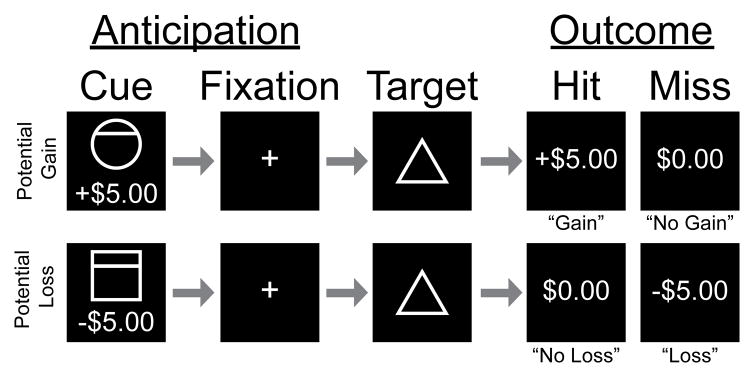
The two MID task scans together consisted of 90 trials, with a similar design as used previously [41] (Fig. 1). At the beginning of each trial, participants were shown a cue indicating the amount of money that they could either gain (+$5.00, +$1.00, +$0.00) or lose (−$5.00, −$1.00, −$0.00). Cue amounts were indicated with accompanying symbols (i.e., circles for gains, squares for losses with horizontal lines indicating the amount at stake). Symbols were used in addition to the monetary amounts to reduce the degree of potential confounds due to experience and/or learning that might occur with only numeric or word cues and to make the task more generalizable to people from different clinical groups and cultures. After each cue, a fixation cross was presented for a delay period of 2 seconds. Then, a target (triangle) was presented to initiate a response (button press) from the participants to either win (if the cue was positive) or avoid losing (if the cue was negative) the amount shown in the cue. Button presses were required to occur before target offset in order for participants to succeed (“hit”) on any given trial; if the button press occurred after target offset this resulted in a “miss”. The outcome was then presented as the amount gained (e.g., +$5.00), not gained (+$0.00), not lost (-$0.00), or lost (e.g., −$5.00). The target was presented for variable durations based on each participant’s calculated reaction time. An initial target duration was set at the beginning of each scan (typically 250 ms) and then adjusted continuously and automatically by the Matlab program to achieve a target “hit” rate of approximately 66% across trials.
Figure 1. Monetary Incentive Delay Task.
Example trials of the monetary incentive delay (MID) task depict both gain and loss, and anticipation and outcome phases. Trial periods were TR-locked so that for each trial TR 1 = Cue (Anticipation), TR 2 = Fixation (Anticipation), TR 3 = Target, TR 4 = Feedback (Outcome), TR(s) 5~7 = Variable Duration Inter-trial Interval. Cues (2 s duration) were circles (potential gains) or squares (potential losses) with monetary values under the shape images. Each fixation period (2 s duration) was followed by a target period (2 s duration) during which a triangle was presented for variable duration (~250 ms) depending on prior accuracy of responses to obtain an average 66% hit rate. Monetary gain or loss values were presented as win/loss feedback (2 s duration) at the end of each trial. Trials were separated by a black screen for a pseudo-randomized inter-trial interval period (2, 4, or 6 s durations).
After the MID task functional scans, all participants rated levels of arousal and valence for each cue. Ratings of arousal (low – medium – high) and valence (negative – neutral – positive) were presented on 7-point Likert scales. Button presses moved the rating selection left or right until participants selected a final rating for each cue. Each rating scale (arousal, valence) was presented once for each of the 6 cues (total = 12 ratings).
Before engaging in the MID task, all participants were informed that the task difficulty level was set to ensure a proportion of both gains and losses in order to determine how the brain responds to both gaining and losing money. To produce an experience of immediate (i.e., “today”) and tangible rewards [60], and to ensure incentive compatibility, participants were informed that an Amazon electronic gift card would be sent to their email address immediately after completion of the scan session in the amount of their cumulative gains and losses.
MID fMRI Scan: Preprocessing
All neuroimaging data were preprocessed using AFNI software (Analysis of Functional Neuroimages) [precompiled binary macosx_10.7_Intel_64: Jun 10 2016 (Version AFNI_16.1.21)] using custom scripts, and as performed previously [47]. Each MID scan was cropped to exclude 6 read-in and 9 read-out volumes. The MID1 and MID2 scans were then concatenated (3dTcat) into one image series of volumes to match the likewise concatenated trial output (generated by the Matlab script). Slice time correction (3dTshift -seqminus), transformation of oblique to axial slices (3dWarp – deoblique), and alignment of anatomical and functional images (align_epi_anat.py) were performed. Motion correction (3dvolreg) included 6 degrees of movement [translation (x, y, z) and rotation (roll, pitch, yaw)]. Functional images were spatially smoothed (3dmerge) with a 4 mm (full-width half-maximum, FWHM) Gaussian kernel [72]. Intensity normalization was applied by calculation of the percent signal change at each voxel and division by the average (3dcalc). A high-pass filter (to eliminate low-frequency noise/signal drift) was applied to the functional data with a threshold of 0.011 Hz (3dFourier). Anatomical images were warped to Talairach space and functional images were warped to the Talairach warped anatomical images (AFNI’s @auto_tlrc, 3drefit, and 3dfractionize). White matter (WM) and cerebrospinal fluid (CSF) masks were created for each subject’s Talairach-warped functional image based on seed loci in WM and CSF regions, respectively (3dmaskave).
fMRI Task Contrasts
As used previously [41,47], four separate orthogonal regressors were designed to contrast responses to gain and loss during anticipation and outcome versus neutral conditions (i.e., no-gain and no-loss anticipation and outcome): gain versus no-gain anticipation (GVNant), loss versus no-loss anticipation (LVNant), gain versus no-gain outcome (GVNout), and no-loss versus loss outcome (NVLout). Specifically, these orthogonal regressors contrasted the following trials and outcomes:
GVNant: gain (+$5 anticipation) trials versus no-gain (+/−$0 anticipation) trials during the anticipation period;
LVNant: loss (−$5 anticipation) trials versus no-loss (+/−$0 anticipation) trials during the anticipation period;
GVNout: gain (+$5 anticipation) trials: hits (+$5 outcome) versus misses (+$0 outcome) during the outcome period;
NVLout: loss (−$5 anticipation) trials: hits (−$0 outcome) versus misses (−$5 outcome) during the outcome period.
Although the LVNant regressor was included as part of the data processing pipeline, it was not included in either of the predetermined hypotheses. However, the LVNant contrast was analyzed for post-hoc ROI analyses of the aINS and ACC as described below. Additionally, the +$1 and −$1 trials were not included in the regressors because NAcc and mPFC activation is less pronounced in response to smaller incentives [44,47]. The regressors were convolved with a single gamma function for regressors of interest to approximate the hemodynamic response, with approximately 6 second delay. Motion censoring was applied to the regressors (1d_tool.py) to conservatively censor each volume (and preceding volume) showing motion greater than 0.5 mm. Resulting activation maps represented blood oxygenation level dependent (BOLD) signal (a correlate of blood oxygenation levels which are a correlate of neural activity in fMRI) response corresponding to the regressor for each contrast separately, within tested ROIs and masked regions as described further below. Therefore, “activity” used throughout the text refers to the measured BOLD fMRI signal.
Region of Interest Analyses
Region of interest (ROI) analyses were restricted to the NAcc and mPFC due to strong predetermined hypotheses that we would observe in patients: 1) decreased NAcc activity during gain anticipation (GVNant), and 2) altered mPFC activity during gain anticipation (GVNant) and in response to outcomes (GVNout and NVLout). To expand upon our interpretation of the main results, additional post-hoc ROI analyses included the midbrain ventral tegmental area (VTA) with GVNant contrast, anterior insular cortex (aINS) with GVNant and LVNant contrasts, and anterior cingulate cortex (ACC) with GVNant and LVNant contrasts.
The bilateral NAcc ROI was created from the Desai Atlas included in AFNI [23]. First, the DKD_Desai_MPM atlas was queried (AFNI’s whereami) for the “Left-Accumbens” and “Right-Accumbens”. Then, the two regions of interest were saved as image files and merged into one mask image file (AFNI’s 3dcalc). Finally, the mask image was resampled to functional image resolution (AFNI’s 3dresample) using a resampled standard space image as the template (TT_N27f+tlrc) resulting in a mask of 52 voxels.
The mPFC ROI was created with two conjoined 4mm radius spheres at +/−4, 50, −3 within Brodmann Area 10 (frontal pole). These coordinates were primarily based on coordinates from a previous MID task mPFC ROI assessed in controls and patients with major depressive disorder (MDD) (4, 50, −4) [41], and then slightly adjusted inferiorly to overlap a mPFC region of altered functional connectivity to the NAcc in chronic pain patients (+/−2, 52, −2) [5]. (We furthermore chose the more lateralized (x=+/−4) and less anterior (y=50) coordinates derived from the former (MDD) study to extend lateral coverage/reduce overlap of the spheres and avoid potential prefrontal (more anterior) signal dropout.) Despite the selected region being superior and anterior to previous meta-analytic findings [46], the selected ROI region was less susceptible to potential fMRI artifacts than regions in the orbitofrontal cortex [22]. The mPFC mask image was first created with spheres drawn in AFNI over the TT_N27+tlrc image. Then, the mask image was resampled to match the fMRI data image dimensions (AFNI’s 3dfractionize, clip 0.1) resulting in a mask of 42 voxels.
Statistical Analysis
Based on previous results of MID task fMRI data, peak NAcc reward anticipatory activation for +$5 versus +$0 results in a large reported effect size of f2 = 3.07, which requires at least 6 subjects to detect group effects at power equal to .80 (p < 0.05) [47,86]. Previous investigations have measured between-group differences with sample sizes ranging from 12 to 19 per group [8,41]. Therefore, our group sample sizes are large enough to detect group differences in fMRI activation in response to the MID task design.
After completing the MID task functional scans, participants rated their arousal and valence responses to each cue. Positive arousal and negative arousal were calculated from these ratings (i.e., mean-deviated and rotated) as described previously [47]. Group by cue ANOVAs were conducted separately for arousal, valence, positive arousal, and negative arousal (Matlab). Due to missing data, one control was excluded from analyses of arousal ratings and another control was excluded from analyses of valence ratings. This resulted in 2 control subjects being excluded from analyses of positive arousal and negative arousal.
Group and between-group level statistics for fMRI data were conducted by extracting averaged beta values (parameter estimates) for each ROI and contrast combination using custom Matlab scripts calling AFNI commands. The extracted beta values were compared between groups using unpaired t-tests in Matlab. The significance threshold was set at p < 0.0125 (initial p < 0.05 threshold Bonferroni corrected for 4 predetermined ROI x contrast comparisons: NAcc GVNant, mPFC GVNant, mPFC GVNout, mPFC NVLout).
For visualization of the fMRI data, AFNI’s 3dttest++ was used to create within-group and between-group activation maps. Activation maps of the resulting t-statistics for each regressor/ROI were transformed to z-scores, spatially smoothed (4mm), and warped to the standard Talairach template. Activation maps showing ROI group results and group differences were thresholded at a voxelwise threshold of p < 0.05 or p < 0.01 for visualization purposes.
Correlations between fMRI beta values and questionnaire measures were tested across both patient and control groups combined using SPSS (IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY). Correlations of ROI fMRI beta values were restricted to those ROIs/conditions that revealed group differences in the initial analysis comparing patients and controls: NAcc GVNant, mPFC GVNant, and mPFC NVLout. Behavioral and clinical variables included arousal (ratings to +$5 cues), behavioral drive (BIS/BAS, BAS drive subscale), behavioral reward responsiveness (BIS/BAS, BAS reward responsiveness subscale), behavioral fun seeking (BIS/BAS, BAS fun seeking subscale), behavioral inhibition (BIS/BAS, BIS subscale), positive affect (PANAS, PAS subscale), negative affect (PANAS, NAS subscale), total mood disturbance (POMS), depression (BDI), trait anxiety (STAI Trait), state anxiety (STAI State), pain severity (BPI), pain interference (BPI), and fatigue (PROMIS Fatigue). Correlations between ROI fMRI beta value measures resulted in 2 independent measures: (1) NAcc GVNant, mPFC GVNant (p = 0.025); (2) mPFC NVLout. Correlations between clinical/behavioral variables resulted in 3 independent measures: (1) arousal (not correlated with other measures); (2) BAS subscales (all p < 0.007); (3) BDI, STAI State, STAI Trait, BIS, PAS, NAS, POMS, BPI pain severity, BPI pain interference, and PROMIS Fatigue (all p < 0.025). Therefore, the sum of these independent measures was used to calculate corrections for multiple hypothesis testing, so that the final correlations tested were Bonferroni corrected for a total of 5 multiple comparisons and determined to be significant at the level of p < 0.01 (corrected threshold). Significant correlations (p < 0.01) from the initial across group analyses were then assessed post-hoc for within-group correlations separately for patients and controls. The standard BIS/BAS questionnaire uses a 4-point response scale (1 indicates strong agreement and 4 indicates strong disagreement) and no neutral response option. Our BIS/BAS questionnaire used unintentionally included a neutral response option resulting in a 5-point response scale; this discrepancy was realized after all data had been collected and thus our findings related to BIS/BAS measures may reflect central tendency influences (e.g., individuals may tend to select the neutral response option). All significant and nonsignificant associations are reported in Supplementary Table 1.
Additional ROIs for Post-hoc Analysis
Our predicted analyses resulted in unpredicted findings -- specifically with respect to similar NAcc reward anticipatory activity inpatients and controls. Because of this we implemented additional exploratory analyses (focused on specific questions) to test for potential mechanisms related to these unpredicted findings. For these post-hoc exploratory analyses, we selected three additional regions that directly project to NAcc (i.e., VTA, anterior insular cortex (aINS), and ACC), and we focused our analyses on specific ROIs within these regions based on previous studies [41].
The VTA projects directly to both the NAcc and mPFC [77]. We anticipated that altered VTA reward anticipatory response might be reduced in our patients. Hypothetically, such a finding might suggest reduced functionality of dopaminergic VTA-NAcc projections despite the overall unaltered NAcc reward anticipatory response in our patients (indicating possible increased inputs to NAcc from other sources (e.g., aINS or ACC)). The VTA ROI for post-hoc analysis was created previously based on structural landmark demarcation as part of a mask of the dopaminergic midbrain, which included both the VTA and substantia nigra, as used and described in detail in a previous publication [35]. The VTA subdivision of the original dopaminergic midbrain mask was used in the current analyses. The VTA ROI was resampled to functional image dimensions (3dfractionize, clip 0.1), and transformed back to Talairach space (AFNI’s adwarp).
The NAcc/ventral striatum also receives direct input from the aINS [19], therefore, we anticipated identifying altered (enhanced or reduced) reward anticipatory activity in the aINS that might potentially explain our observations in the NAcc. Additionally, the aINS is involved in risk processing [70], therefore we analyzed the loss anticipation response of the aINS to corroborate other post-hoc findings (i.e., the NAcc NVLant response). The bilateral aINS ROI for post-hoc analysis was created as from the DKD_Desai_MPM Atlas included in AFNI [23]. First, the atlas was queried (AFNI’s whereami) for the “Left-Anterior Insula” and “Right-Anterior Insula”. Then, the two ROIs were saved as image files and merged into one mask image file (AFNI’s 3dcalc). Finally, the mask image was resampled to functional image resolution (AFNI’s 3dresample) using a resampled standard space image as the template (TT_N27f+tlrc).
The ACC also sends direct projections to the NAcc [49], and we included this post-hoc ROI as a third region to probe our unexpected NAcc findings. Furthermore, due to the lack of initial observed correlations between activity in the predicted ROIs (NAcc and mPFC) with results and clinical/behavioral measures, we aimed to more directly compare our results with a previously published patient sample of unipolar depressed patients (MDD, [41]. Similar to the current findings, MDD patients showed similar NAcc reward anticipatory activity as controls but did show unexpectedly increased ACC reward anticipatory activity but decreased ACC loss anticipatory activity. Therefore, given the similarity of the current NAcc results to previous MDD NAcc results, we targeted the same ACC ROI to determine whether chronic pain patients might show a similar pattern.
The ACC ROI for post-hoc analysis was created with two 4mm radius spheres centered on the same coordinates identified in previous comparisons of MDD patients and controls (i.e., +/−8, 11, 34) [41]. The selected location of the ACC ROI is potentially relevant to pain because it co-localized near a peak focus of ACC activation in the Neurosynth.org “pain” reverse inference map (peak z-score = 11, coordinates 1, 11, 34) [88]. The mask image was created in AFNI with spheres drawn over the TT_N27+tlrc underlay image. Then, the edited mask image was resampled to match the fMRI image dimensions (AFNI’s 3dfractionize, clip 0.1).
Whole Brain Post-hoc Analysis
A post-hoc whole brain analysis was conducted to 1) confirm the ROI findings, and 2) potentially inform future research. A whole brain mask excluded all voxels outside of the brain fromstatistical comparison (TT_N27f.nii). Statistical analysis was performed as described above (AFNI’s 3dttest++ with 3D Clustsim). Resulting images were normalized to mean levels of activation using z-score transformation. Whole brain analysis results were initially thresholded at a level corresponding to p = 0.01 (z = 2.57). Cluster correction was set at a minimum cluster size of 20 voxels and required all voxels to share a face for inclusion in a cluster (NN set to = 1). Based on AFNI’s Clustsim algorithm results, any clusters exceeding an alpha < 0.05 (false positive rate) were excluded from the activation maps.
Expanded Anterior Medial Prefrontal Cortex Mask Post-hoc Analysis
A large anterior mPFC region, automatically functionally defined (by De La Vega et al.) [81], was used for post-hoc confirmatory and exploratory analyses. The mask was created using the following steps: The “kmeans_3.nii” image was downloaded from the cited Github source, converted to AFNI format (BRIK/HEAD), resampled to functional image dimensions (AFNI’s 3dfractionize, clip 0.1), and transformed into Talairach space (AFNI’s adwarp).
Results were initially thresholded at p < 0.05 (z = 1.975). This threshold was set to allow for detection of any between-group and within-group activations. Cluster correction was set at a minimum cluster size of 20 voxels and required all voxels to share a face for inclusion in a cluster (NN set to = 1). Based on the AFNI’s Clustsim algorithm results, any clusters exceeding alpha < 0.05 were excluded from the activation map.
Motion Covariate Analysis
In addition to conservative motion scrubbing and correction applied as part of the preprocessing pipeline in AFNI, results were checked for effects of motion using motion as covariates in the fMRI and statistical analyses as follows. Euclidean norm (enorm, calculated as the square root of the sum of squares for values across 6 motion rigid-body motion parameters) values for each fMRI volume were generated in AFNI. These values were used to calculate average motion during the MID task scans for each participant. Motion estimates were included as a covariate of no interest in post-hoc fMRI group analyses (using 3dttest++ with covariate option). Statistical analysis of the extracted beta values were analyzed across groups using a univariate ANCOVA by group with motion estimate as the covariate of no interest (SPSS). Inclusion of the motion estimate as a covariate of no interest did not change the main findings of group fMRI results. Additionally, prior to plotting raw time course data, volumes with signal exceeding > 4 standard deviations from mean activity were removed from the raw preprocessed time course data [73].
RESULTS
Participants
Eighteen patients with fibromyalgia and 17 healthy control participants signed informed consent for the study. Data from three participants were excluded because of an incomplete scanning session due to artifacts (N = 1 patient) and excessive head motion (N = 2 controls). Thus, data from 17 patients and 15 controls were included in the final analysis and results presented (Table 1).
Table 1.
Participant Demographics.
| Patients | Controls | |
|---|---|---|
| Total Participants (all female) | 17 | 15 |
| Righthanded | 16 | 14 |
|
| ||
| Self-Identified Race | ||
| Asian | 2 | 6 |
| Caucasian | 13 | 8 |
| Other | 2 | 1 |
| Hispanic or Latina Ethnicity | 3 | 1 |
|
| ||
| Employment Status | ||
| Part-time employed | 3 | 2 |
| Full-time employed | 6 | 10 |
| Unemployed | 8 | 3 |
|
| ||
| Income Level | ||
| $0–$29,999 | 5 | 0 |
| $30,000–$59,999 | 3 | 2 |
| $60,000 or more | 8 | 11 |
|
| ||
| Education Level | ||
| High School | 3 | 0 |
| College/University | 11 | 7 |
| Advanced Degree | 3 | 8 |
One control participant did not indicate handedness, one patient was left handed. No participants were of race categories for African American, Pacific Islander or Alaskan, or Native American (i.e., “other” refers to race other than all of these categories). High school refers to “up to or through high school”, college/university refers to “up to or through college/university”, and advanced degrees refer to “any amount of education post college/university”.
Clinical, Behavioral, and Psychological Measures
Based on questionnaire data, and as expected, patients differed from controls on several behavioral measures including mood, fatigue, anxiety, depression, pain distribution across the body (i.e., number of painful body areas), pain severity, and pain interference (Table 2). Notably, however, most participants with fibromyalgia average BDI score was in the mild range. Furthermore, those with fibromyalgia did not demonstrate severe levels of depression (i.e., no BDI scores greater than 30) or severe levels of anxiety (i.e., no STAI scores greater than 62).
Table 2.
Clinical, Behavioral, and Psychological Measures.
| Patients | Controls | P-Value | |||
|---|---|---|---|---|---|
| N | Mean±sd | N | Mean±sd | ||
| Age | 17 | 48.1±9.6 | 15 | 48.1±10.2 | 0.997 |
| Positive Affect (PANAS) | 17 | 26.4±8.5 | 15 | 36.4±5.4 | < 0.001 |
| Negative Affect (PANAS) | 17 | 21.2±8.0 | 15 | 13.1±4.1 | 0.001 |
| Behavioral Reward (BAS) | 16 | 20.4±2.6 | 14 | 20.4±2.9 | 0.873 |
| Behavioral Drive (BAS) | 16 | 13.8±3.6 | 14 | 13.7±3.9 | 0.873 |
| Behavioral Fun (BAS) | 16 | 13.3±2.8 | 14 | 14.6±2.3 | 0.873 |
| Behavioral Inhibition (BIS) | 16 | 28.2±3.5 | 14 | 21.1±6.0 | 0.019 |
| Mood Disturbance (POMS) | 17 | 21.6±15.8 | 15 | −4.5±8.6 | < 0.001 |
| Fatigue (PROMIS) | 17 | 65.5±8.0 | 15 | 48±6.5 | < 0.001 |
| Trait Anxiety (STAI) | 17 | 49.7±8.5 | 15 | 34.9±8.3 | < 0.001 |
| State Anxiety (STAI) | 17 | 41.4±7.0 | 15 | 27.1±7.5 | < 0.001 |
| Depression (BDI) | 17 | 15.8±8.9 | 15 | 2.3±3.1 | < 0.001 |
| Number of Pain Areas (FAF) | 17 | 13.9±3.9 | 15 | 1.5±2.0 | < 0.001 |
| Pain Severity (BPI) | 17 | 5.7±2.1 | 15 | 0.4±1.0 | < 0.001 |
| Pain Interference (BPI) | 17 | 36.9±19.5 | 15 | 3.5±6.4 | < 0.001 |
Participant counts for each measure differ from the total number of participants (patients N=17, controls N=15) because some participants did not complete all questionnaires. Abbreviations: PANAS, Positive and Negative Affect Schedule; BIS/BAS, Behavioral Inhibition System/Behavioral Activation System; PROMIS, Patient-Reported Outcomes Measurement Information System; STAI, State-Trait Anxiety Inventory; FAF, Fibromyalgia Assessment Form; BDI, Beck Depression Inventory; POMS, Profile of Mood States; BPI, Brief Pain Inventory; sd, standard deviation. Data are presented for descriptive purposes only, therefore significance values (P-Value) shown are not corrected for multiple comparisons.
Patients’ reported painful areas spanned all regions of the body validating the presence of distributed pain across the body in our patient population (Supplementary Fig. 1). The number of painful areas per patient ranged from 9 – 19 out of a total 19 regions listed in the Fibromyalgia Assessment Form. The duration of pain symptoms reported by patients ranged from 2 – 28 years with an average duration of 11.5 years (standard deviation of 7.7 years).
Reaction Times and Accuracy Rates
As expected, more salient gain (+$5 anticipation) and loss (−$5 anticipation) trials elicited shorter reaction times [F(5,150)=9.9, p = 0.000] for both groups. No group [F(1,150)=0.0, p = 0.959] or group by trial interaction effects [F(5,150)=1.9, p = 0.101] were observed indicating similar performance and engagement between patients (N=17) and controls (N=15) across conditions.
The adaptive MID task algorithm targeted 66% accuracy rates (based on cumulatively tracked performance). Despite this adjustment, percent hits were greater for large gain (+$5 anticipation) and loss (−$5 anticipation) trials [F(5,150)=5.90, p < 0.000]. However, no group [F(1,150)=0.1, p = 0.805] or group by trial interaction effects [F(5,150)=1.0, p = 0.442] were observed, indicating objectively similar performance and engagement across patients (N=17) and controls (N=15). These results are consistent with previous implementations of the MID task in healthy and clinical populations [41], implying comparable levels of task engagement across groups.
Cue Ratings: Arousal, Valence, Positive Arousal, Negative Arousal
Arousal
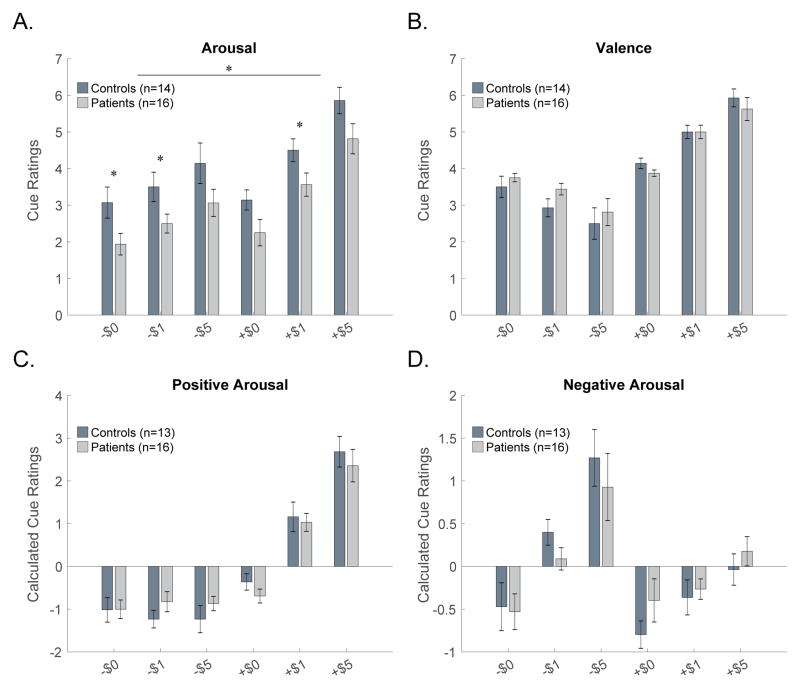
A group by cue ANOVA on arousal ratings revealed an effect of group [F(1,140)=8.7, p = 0.006], indicating lower arousal ratings overall in patients (N=16) relative to controls (N=14). A cue effect [F(5,140)=24.5, p = 0.000] was also observed for arousal, as expected, but no group by cue interaction was observed [F(5,140)=0.0, p = 0.999] (Fig. 2A).
Figure 2. Ratings of Arousal, Valence, Positive Arousal, Negative Arousal.
(A) Across all cues arousal ratings were lower in patients as compared with controls (p = 0.006). Post-hoc t-tests for individual cue between-group differences are marked with asterisks (p<0.05 uncorrected for multiple comparisons). Valence (B), positive arousal (C), and negative arousal (D) ratings were similar in patients and controls. One patient did not provide any ratings, one control subject was missing data for arousal, and a second control subject was missing data for valence; these subjects were excluded from the associated analyses.
Valence
A group by cue ANOVA for valence ratings revealed no significant effect of group [F(1,140)=0.3, p = 0.577] indicating similar valence ratings between patients (N=16) and controls (N=14). A cue effect [F(5,140)=43.7, p = 0.000] was observed for valence, as expected, but no group by cue interaction was observed [F(5,140)=0.9, p = 0.494] (Fig. 2B).
Positive Arousal
A group by cue ANOVA for positive arousal ratings revealed no effect of group [F(1,135)=0.0, p = 1.0], indicating similar positive arousal ratings in patients (N=16) and controls (N=13). A cue effect [F(5,135)=52.2, p = 0.000] was observed for positive arousal, as expected, but no group by cue interaction was observed [F(5,135)=0.6, p = 0.676] (Fig. 2C).
Negative Arousal
A group by cue ANOVA for negative arousal ratings revealed no effect of group [F(1,135)=0.6, p = 0.453] indicating similar negative arousal ratings in patients (N=16) and controls (N=13). A cue effect [F(5,135)=11.9, p = 0.000] was observed for negative arousal, as expected, but no group by cue interaction was observed [F(5,135)=0.7, p = 0.652] (Fig. 2D).
ROI Activation: Nucleus Accumbens
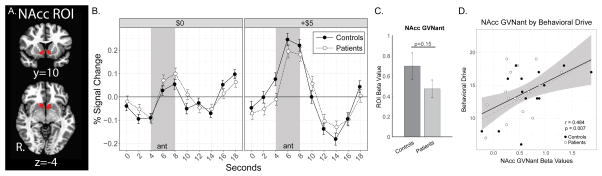
Both patient and control groups demonstrated similar NAcc responses. Robust NAcc activity was observed for both groups during gain anticipation (GVNant) (Fig. 3), consistent with extensive previous literature [46]. We had initially hypothesized that patients would demonstrate reduced NAcc activity during gain anticipation relative to controls. Our hypothesis was not supported by the results, however, slight reductions in NAcc BOLD signal (GVNant) were observed in patients [F(1,0)=1.9, p = 0.175], but these did not reach the corrected statistical threshold of p < 0.0125 (Fig. 3). GVNant fMRI beta values were positively correlated with behavioral drive across patient and control groups combined (BAS drive subscale, N = 32, r = 0.484, p = 0.007). Post-hoc within-group analyses identified positively correlated NAcc GVNant activity with behavioral drive in controls (r = 0.566, p = 0.035) but only trendwise in patients (r = 0.43, p = 0.097). Although NAcc activity has been observed in the context of avoiding anticipated losses (e.g., [16,42,50]), this activity typically does not scale with the magnitude of anticipated loss and is less than that observed for anticipation of gains of the same magnitude (also see Supplementary Fig. 5A). Post-hoc analyses confirmed no notable NAcc activity during loss anticipation (LVNant), gain outcome (GVNout), and no-loss outcome (NVLout) in either group, consistent with previous literature [40].
Figure 3. Nucleus Accumbens Activity during Reward Anticipation.
(A) Bilateral ROI of the NAcc. (B) Raw time course plots of NAcc ROI activity (group means and standard error) to $0 and +$5 anticipation trials. The shaded period of reward anticipation (ant) fMRI BOLD response was estimated to correspond to 4–8 seconds during the raw time course plots [presentation of cue and fixation during TRs 1 and 2 (0–4 seconds) plus 4 seconds to account for hemodynamic response function (HRF) delay]. (C) Contrast (GVNant) beta values extracted from the bilateral ROI for NAcc reward anticipatory activity. (D) Correlation between extracted NAcc GVNant beta values and behavioral drive (BAS drive subscale) for both groups (solid black line) with 95% confidence intervals (gray shading), and correlation in controls only (dashed gray line, confidence intervals not shown). All beta values shown as 10−3. BAS, Behavioral Activation System.
ROI Activation: Medial Prefrontal Cortex
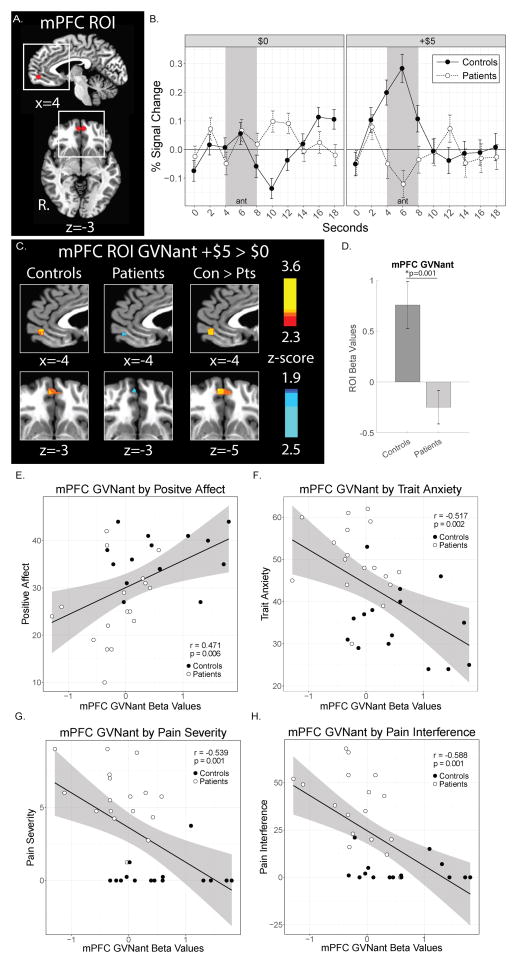
In the context of reward and value, mPFC activity is strongly associated with reward outcomes [43,47], but also with the estimated probability of expected rewards [40,47]. We hypothesized that mPFC might demonstrate altered activation during reward anticipation and reward outcomes in patients. For reward anticipation responses, increased mPFC activity during gain anticipation (GVNant) was observed in the control group, but this response was strikingly absent in the patient group (Fig. 4). The mPFC GVNant fMRI beta values were positively correlated with positive affect (PANAS, PAS subscale, r = 0.471, p = 0.006) across patient and control groups combined. mPFC GVNant fMRI beta values were also negatively correlated with trait anxiety (STAI Trait, r = −0.517, p = 0.002), pain severity (BPI, r = −0.539, p = 0.001), and pain interference (BPI, r −0.588, p = 0.001) across patient and control groups. Among these correlated measures, however, post-hoc analyses within groups revealed no significant correlations when patient and control groups were assessed separately for positive affect, trait anxiety, pain severity, or pain interference (see Supplementary Table 1). The mPFC GVNant beta values were not significantly correlated with depression (BDI) based on our predetermined significance criteria (i.e., p < 0.01 corrected) (Supplementary Table 1 and Supplementary Fig. 6A). Further analysis of the BDI scores indicated greater loading on the somatic factor of the BDI than the negative view of self factor as defined in Morley et al. [63] (Supplementary Fig. 6B). A separate analysis (i.e., two-sample t-test in AFNI) excluding 4 patients with moderate depressive symptoms (BDI > 19) provided similar mPFC ROI results as the original analysis (Supplementary Fig. 6C,D).
Figure 4. Medial Prefrontal Cortex Activity during Reward Anticipation.
(A) The bilateral mPFC ROI is shown in red. White boxes denote areas of magnification in sagittal and horizontal planes as depicted in later shown group activation maps. (B) Raw time course plots of the mPFC ROI activity (group means and standard error) to $0 and +$5 anticipation trials. The shaded period of reward anticipation (ant) fMRI BOLD response was estimated to correspond to 4–8 seconds during the raw time course plots [presentation of cue and fixation during TRs 1 and 2 (0–4 seconds) plus 4 seconds to account for hemodynamic response function (HRF) delay]. (C) Contrast (GVNant, +$5 > $0 trials) activation maps of mPFC ROI activity during reward anticipation (p < 0.05, uncorrected). (D) Extracted ROI beta values for mPFC reward anticipatory activity. (E, F, G, H) Correlation between extracted mPFC GVNant beta values and positive affect (PANAS), trait anxiety (STAI Trait), pain severity (BPI), and pain interference (BPI) for both groups (solid black line) with 95% confidence intervals (gray shading). All beta values shown as 10−3. PANAS, Positive Affect Negative Affect Schedule; STAI, State-Trait Anxiety Inventory, BPI, Brief Pain Inventory.
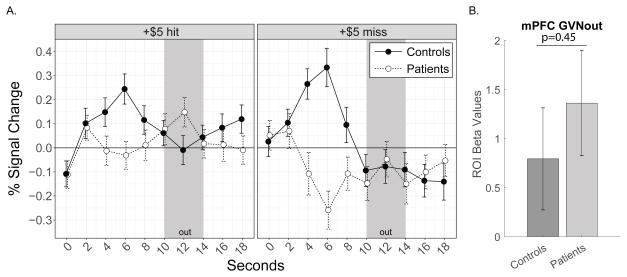
For reward outcome responses, both groups demonstrated increased mPFC BOLD signal in response to gain outcomes (GVNout). Thus, both groups showed responses consistent with previous evidence of mPFC in response to reward (gain) outcomes [44] (Fig. 5).
Figure 5. Medial Prefrontal Cortex Activity in Response to Reward Gain Outcomes.
(A) Raw time course plots of mPFC ROI activity (group means and standard error) split by outcomes (hits or misses) for +$5 anticipation trials. The period of reward outcome (out) fMRI BOLD response is estimated to correspond to 10–14 seconds during the raw time course plots [presentation of outcome and post-outcome during TRs 4 and 5 (6–10 seconds) plus 4 seconds to account for hemodynamic response function (HRF) delay]. (B) Contrast (GVNout) beta values extracted from the ROI for mPFC reward outcome activity.
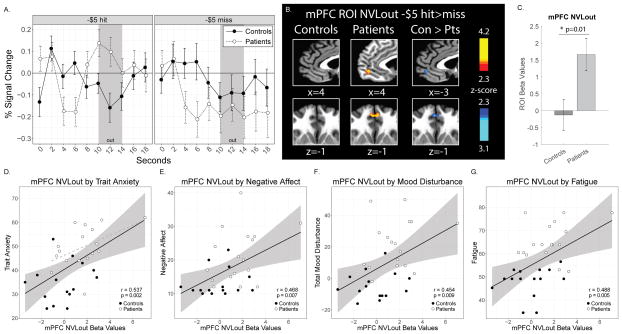
Consistent with previous observations, mPFC activity in controls did not significantly change in response to no-loss versus loss outcomes (NVLout). In sharp contrast, mPFC activity in patients robustly increased in response to no-loss outcomes (NVLout) (Fig. 6). NVLout extracted beta values were positively correlated with trait anxiety (STAI Trait, N = 32, r = 0.537, p = 0.002), negative affect (PANAS subscale, N = 32, r = 0.468, p = 0.007), mood disturbance (POMS, N=32, r = 0.454, p = 0.009), and fatigue (PROMIS Fatigue, r = 0.488, p = 0.005) across patient and control groups combined. Among these correlated measures, post-hoc within-group analyses identified a positive correlation trend for mPFC NVLout activity with trait anxiety in patients (r = 0.482, p = 0.05), but no other within-group correlations were observed. As expected, post-hoc analyses confirmed minimal changes in mPFC activity during loss anticipation (LVNant) in both groups.
Figure 6. Medial Prefrontal Cortex Activity in Response to Reward No-Loss Outcomes.
(A) Raw time course plots of mPFC ROI activity (group means and standard error) split by outcomes (hits or misses) for −$5 anticipation trials. The period of reward outcome (out) fMRI BOLD response is estimated to correspond to 10–14 seconds during the raw time course plots [presentation of outcome and post-outcome during TRs 4 and 5 (6–10 seconds) plus 4 seconds to account for hemodynamic response function (HRF) delay]. (B) Contrast (NVLout)) activation maps of mPFC ROI during reward outcome (hits versus misses) to −$5 anticipation trials (p < 0.05, uncorrected). (C) Contrast (NVLout) beta values extracted from the ROI for mPFC reward outcome activity. (D, E, F, G) Correlations between mPFC NVLout extracted beta values and trait anxiety (STAI Trait), negative affect (PANAS subscale), mood disturbance (POMS), and fatigue (PROMIS Fatigue) for both groups (solid black lines) with 95% confidence intervals (gray shading), and in patients only (dashed gray line in trait anxiety plot, confidence intervals not shown). All beta values shown as 10−3. STAI, State Trait Anxiety Inventory; PANAS, Positive and Negative Affect Schedule; POMS, Profile of Mood States.
Post-hoc fMRI Analysis
Across all additional ROIs investigated (VTA, aINS, ACC), activity during gain anticipation (GVNant) was slightly reduced in patients relative to controls, as described in detail below (Supplementary Fig. 2).
The VTA sends direct projections to the NAcc [77] and the mPFC [24,27]. As a post-hoc analysis, to better understand altered mPFC activity in patients, we conducted an ROI analysis on the VTA for the GVNant contrast. Extracted beta values from the VTA ROI revealed no significant differences between patients and controls during anticipation of gains (p=0.24) (Supplementary Fig. 2).
The anterior insula (aINS) is involved in value processing, arousal, and risk assessment [70] and projects directly to the NAcc [19]. Post-hoc ROI analyses of the bilateral aINS were conducted for GVNant and LVNant contrasts. Extracted beta values from the aINS ROI revealed no significant differences between patients and controls during anticipation of gains (p =0.09) (Supplementary Fig. 2) or anticipation of losses (p = 0.76).
The anterior cingulate cortex (ACC) is involved in pain [28,68] and value processing [15] and also directly projects to the NAcc [49]. Post-hoc ROI analyses of ACC activity were conducted for GVNant and LVNant contrasts to better understand our NAcc findings and to test for a pattern observed in previous studies of depressed individuals. Specifically, patients with MDD show enhanced ACC response during gain anticipation but reduced ACC response during loss anticipation during the MID task [41]. In contrast to the ACC alterations observed in MDD, extracted beta values from the ACC ROI revealed reduced ACC activity in patients relative to controls during anticipation of gains (p = 0.04) (Supplementary Fig. 2) and no group difference during anticipation of losses (p = 0.94). Additionally, GVNant ACC extracted beta values were not correlated with depression (BDI) among patients and controls combined (BDI, r = −0.118, p = 0.520), among patients (BDI, r = −0.357, p = 0.159), or among controls (BDI, r = −0.091, p = 0.748), thus supporting distinct alterations in neural correlates in reward processing in our chronic pain patient sample relative to previously studied MDD patients.
Confirming the findings of the ROI analyses, whole brain analyses revealed group differences in the extent of activation during gain anticipation (GVNant) across multiple brain regions (Supplementary Fig. 3). No clusters with alpha < 0.05 were identified within whole brain activation maps for LVNant, GVNout, and NVLout contrasts, therefore the results for these post-hoc analyses are not displayed or discussed further.
The expanded mPFC mask analysis revealed that mPFC activation extended beyond the boundaries of the initially targeted ROI for both GVNant and NVLout contrasts (Supplementary Fig. 4).
DISCUSSION
We identified altered neural correlates of monetary rewards and non-punishments in patients with fibromyalgia. Considerable alterations in mPFC activity to both reward anticipation and outcome were observed and may reflect dysregulated reward processing in chronic pain. These observations were not observed in NAcc activity, which showed a slight but nonsignificant blunting to anticipated reward also observed in ACC activity. Together, these observations indicate that brain reward processing in response to non-drug rewards, and apart from the context of pain itself, are altered in individuals with fibromyalgia.
Reduced Brain and Behavioral Response to Anticipated Rewards
In the presence of increased negative affective symptoms prevalent in chronic pain [29], our patients demonstrated decreased arousal to all monetary cues. Despite not observing differences in both arousal and valence as initially hypothesized, lower arousal ratings (in particular to less salient +$1 cues) in patients may represent a reduction in the valuation threshold in patients with chronic pain and may parallel decreases in motivation in chronic pain as observed in rodent models [75].
Previous studies demonstrate a direct relationship between positive arousal and expected value (e.g., increasing monetary rewards) which is tracked by NAcc activity [46]. We did not observe this relationship, potentially due to the retrospective nature of arousal and valence ratings. However, NAcc gain anticipatory activity was correlated with behavioral drive implicating motivation (e.g., individual differences in behavioral activation; as in [86]. Thus, our hypothesis that patients would demonstrate reduced positive arousal and reduced NAcc reward anticipatory activity was only partially supported. Instead, patients demonstrated reduced arousal to all cues and only slightly reduced NAcc activity during reward anticipation that did not meet our pre-established significance criteria. Similarly reduced reward anticipatory response was observed in the ACC, with a trend for a similar pattern in the aINS, but no reduction in the VTA.
The mPFC encodes probabilistic aspects of the expected value of reward [47], and several potential explanations exist for mPFC recruitment during reward anticipation. Although reward anticipation has more commonly been linked to NAcc dopamine release (e.g., [11]), the mPFC also receives dopaminergic projections from the VTA [33]. Our observation of reduced mPFC reward anticipatory activity in patients relative to controls is consistent with previous evidence of altered cortical and subcortical dopamine function in fibromyalgia [84,85]. VTA-mPFC projections excite GABAergic mPFC interneurons but inhibit pyramidal mPFC neurons, and loss of this inhibitory circuit can increase perseverative behavior with no changes in attention or impulsivity in mice [37]. Our observation of reduced mPFC activity during reward anticipation may represent reduced VTA-mPFC input in patients, which could be compensatory to maintain perseverance during the task. Alternatively, reduced mPFC reward anticipatory activity may reflect inflexibility in updating reward estimates [42] which might influence approach behavior in chronic pain [46]. Subjective value and working memory demands are reflected by mPFC activity during intertemporal choice tasks [38], and our findings may also relate to deficits in working memory and extending subjective value over time. Lastly, the group differences might reflect higher reward probability estimates in the controls, since reward probability can modulate mPFC activity [47]. This account is consistent with the positive association of mPFC gain anticipatory activity with positive affect and negatively correlated with trait anxiety. Nonetheless, future investigations will need to determine the relative plausibility of these hypotheses.
Increased Brain Response to No-Loss Outcomes
Reward outcome and related consumption behaviors represent the terminal components of reward processing and also recruit the mPFC [36,44]. Similar mPFC reward outcome activity between patients and controls suggests intact processing of reward gains in patients. In contrast, in response to no-loss outcomes, patients demonstrated robust increases in mPFC activity relative to controls. Specifically, controls appeared to process no-loss outcomes as “zero sum” (i.e., no net gain/loss) while patients appeared to process no-loss outcomes as a reward, relief, or surprise. Additionally, across both groups, mPFC no-loss outcome response was correlated with individual differences in negative affect, mood disturbance, and trait anxiety suggesting a contribution of negative expectations to the mPFC response during loss avoidance. Counterfactual processes involve regions of prefrontal cortex that include the mPFC [6,44,80]. Therefore, another possibility is that no-loss outcomes evoked thoughts about contrasting past or future events or imagined alternative outcomes in patients. Together, these observations suggest an altered experience of outcomes in patients, specifically related to avoidance of punishment. Previous investigations have demonstrated analgesia by monetary reward in circuits including the medial and orbitofrontal cortex [10]. Based on our observations, unexpected no-loss outcomes, if indeed perceived as relief or escape from punishment, may interact with regions along the medial wall of the frontal cortex regions to decrease the experience of pain.
Comparing Brain Responses to Pain Offset and Monetary Reward
Altered reward processing may interact with the experience of both acute and chronic pain. A balanced relationship between pain and reward circuits may be evident at the confluence of painful and pleasurable experience [54] as well as the combination of descending networks which can inhibit and facilitate pain [26,53]. Overall, the altered mPFC response in patients, reduced during gain anticipation but increased in response to loss avoidance, may be related to reduced expectation of reward but enhanced relief to avoiding punishment. These responses may relate to the chronic pain experience – both expectations of reduced reward and enhanced surprise at relief from punishment. Other research suggests that midbrain (i.e., VTA) and striatal (i.e., NAcc) fMRI responses are inverted in patients in response to painful heat onset and offset [7,56]. Pain relief may induce a reward experience via opioidergic and dopaminergic responses in the ventral striatum [65]. Further, chronic pain patients show reduced pain relief to small decreases in noxious heat intensity [67] suggesting altered adjustments to changes in nociceptive information [59,89]. While pain relief related to these small decreases is largely opioid-independent [57,67] and involves central and supraspinal processes [64], it remains to be determined how brain reward systems and associated expectations can modulate the experience of pain in healthy and chronic pain populations.
Limitations and Future Directions
The present study represents one of the first studies using the MID task in chronic pain patients. Given the importance of scientific reproducibility [69], the validity of these initial results will need to be confirmed in additional studies, as well as in other chronic pain patient samples of varying ethnicities, and races. Both reduced [84,85] and increased [52] striatal dopamine response has been observed in patients with fibromyalgia. While our observations indicate relatively normal striatal reward anticipatory response in our patients, we did not observe correlations between striatal reward anticipatory response and positive arousal ratings. Future investigations with peripheral physiological measures of arousal (e.g., skin conductance recordings, pupillometry) may more robustly document these relationships than retrospectively reported arousal and valence ratings.
Our interpretation of mPFC reward anticipation response is limited because we did not explicitly ask our participants about perceived probability of reward, assess working memory function, or directly measure brain dopamine response in the present study. However, behavioral drive was correlated with mPFC (as well as NAcc) anticipatory responses, imparting a motivational relevance for these findings. Future investigations could include task designs involving varying levels of reward probability and distinguishing between valence and motivation (e.g., [71]).
Additionally, the present findings may be influenced by comorbid affective conditions and medications, although it should be noted that our patients BDI scores averaged in the mild range. Reward processing may be altered by depression and anxiety [3,14,34], and sleep disturbance [32,55]. However, our observations in patients with fibromyalgia contrast with previous findings in affective disorders, since our fibromyalgia patients demonstrated an opposite ACC response to anticipated gain than MDD [41]. Thus, these contrasting results support distinctly altered reward processing in fibromyalgia as compared with affective disorders.
Lastly, we cannot infer directionality of neural alterations observed from the current data. Altered top-down cortical influences from the mPFC to NAcc may drive cortico-striatal circuit dysfunction [66] [25]. Alternatively, bottom-up subcortical influences from the VTA may modulate mPFC activity and are relevant for motivational and affective disorders [20]. Further, the VTA extends distinct projections to NAcc versus mPFC regions [51]. Future investigations in humans using higher resolution fMRI and complementary animal models could elucidate specific mechanisms that contribute to the presently observed alterations [25].
Conclusions
Our findings begin to fill a critical gap in the chronic pain literature distinguishing anticipation and outcomes of both gains and losses. We directly probed reward circuits using a validated task and predetermined hypotheses in patients with fibromyalgia. Altered reward processing may interact with chronic pain to initiate and maintain maladaptive and pain-enhancing brain and behavioral processes. Ultimately, increased understanding of the bidirectional influences of reward processing and chronic pain may improve treatment options and selection. Further study of brain value systems in chronic pain may determine whether circuits modulated by reward, such as the mPFC, may provide potential neural targets for behavioral and pharmacological therapies designed to treat chronic pain.
Supplementary Material
(A) Distribution of painful areas is shown across the 19 body regions listed in the Fibromyalgia Assessment Form. Each patient confirmed the presence or absence of pain in each region. The number of patients indicating pain in each region is shown (total N = 17 patients). Bars are differently grayscale shaded to cluster among similar regions of the body to enhance readability of results and shading does not represent additional data. (B) The number of painful body regions indicated by each individual patient is shown (maximum number of body regions on the Fibromyalgia Assessment Form = 19). Each patient is represented by a number and bar of the graph. Patients are ordered by low to high number of painful body regions to enhance readability of the results.
ROIs (top panel) and ROI extracted beta values (bottom panel) for the ventral tegmental area (VTA), anterior insular cortex (aINS), and anterior cingulate cortex (ACC) are presented for the gain versus no-gain anticipation (GVNant) contrast. Analyses were conducted post-hoc and not corrected for multiple comparisons. LVNant contrast results for aINS and ACC ROIs were similar across groups (not shown). White boxes depict alternate ROI views. VTA, ventral tegmental area; aINS, anterior insular cortex; ACC, anterior cingulate cortex; R., right; ROI, region of interest.
A post-hoc whole brain analysis was conducted to inform future analyses. Group activation maps during reward anticipation (gain versus no-gain anticipation, GVNant) are shown for control group and patient group separately (p < 0.01, corrected alpha < 0.05). No other contrasts elicited whole brain activation clusters exceeding p < 0.05, with corrected alpha < 0.05. R, right.
(A) Expanded mask of the anterior medial prefrontal cortex created from a previous automated parcellation of functional data by De La Vega et al., see text for details. Activation maps showing group and group differences of mPFC activity for (B) reward anticipation (GVNant) and (C) no-loss outcome (NVLout) contrasts (p < 0.01, corrected alpha < 0.05).
(A) Comparison of Nacc activity time courses during the anticipation phase (yellow shading, estimated 2 – 6 seconds) for $0, +$5, and −$5 trials. These demonstrate distinct activity levels for GVNant (+$5 versus $0) and LVNant contrasts (−$5 versus $0) in the NAcc. (B) Comparison of mPFC activity time courses during the anticipation phase (yellow shading, estimated 2 – 6 seconds) for $0, +$5, and −$5 trials. These demonstrate distinct activity levels for GVNant (+$5 versus $0) and less distinct activity for LVNant contrasts (−$5 versus $0) in the mPFC. (C) Comparison of mPFC activity time courses during the outcome phase (yellow shading, estimated 10 – 14 seconds) for +$5 trials (left panel) and −$5 trials (right panel). Across both trial types, mPFC demonstrates greater activity during the outcome phase to +$5 outcome (hits) as compared with all other outcomes.
To further determine the potential contributions of depression in our patient sample we provide additional information on the data from the Beck Depression Inventory (BDI). (A) Scatter plot of the relationship between mPFC GVNant activity and depression (measured with the BDI) shows a significant negative correlation across both groups combined (as expected due to significant group differences in both mPFC activity and depression), but no within group correlation. (B) Bar graph of BDI factors (negative view of self factor, somatic factor, additional items contributing to an overall measure of negative affect) shows greater loading on the somatic factor in our fibromyalgia sample. Means (and standard deviation in parentheses) for each factor are shown below each factor label. Patient numbers highlighted in red text indicate individuals with BDI > 19 (i.e., moderate depressive symptoms). (C, D) Results for mPFC ROI for controls (N=15) versus fibromyalgia with mild depressive symptoms (N=13, excludes 4 patients with moderate depressive symptoms) for (C) GVNant contrast and (D) NVLout contrast (p < 0.05, uncorrected).
Participant counts for each tested correlation differ from the total number of participants (N = 32; patients N=17, controls N=15) because some participants did not complete all questionnaires. Arousal equals post-scan arousal rating to +$5 cue. Abbreviations: PANAS, Positive and Negative Affect Schedule; BIS/BAS, Behavioral Inhibition System/Behavioral Activation System; PROMIS, Patient-Reported Outcomes Measurement Information System; STAI, State-Trait Anxiety Inventory; BDI, Beck Depression Inventory; POMS, Profile of Mood States; BPI, Brief Pain Inventory; p, p value; r, Pearson correlation value; N, number of participants included in each analysis. Bold text indicates between group results p < 0.01 corrected for multiple comparisons and associated post-hoc within group results p < 0.05 uncorrected.
Acknowledgments
The authors would like to thank Erin Perrine, Christina Cojocaru, and Elizabeth Cha for assistance with recruitment, data collection, data organization, and analysis. We thank the Stanford University Richard M. Lucas Center for Imaging, Dr. Gary Glover, Kevin Epperson, and Anne Sawyer for their expert assistance. Lastly, we thank the study participants for their time and contribution to advance clinical research.
Funding Sources:
For this study the authors received funding from the National Institutes of Health K99 DA040154 (KTM) and K24 DA029262 (SCM), Redlich Research Endowment (SCM), and the Stanford Neuroscience Institutes’ Neurochoice Initiative (BK).
Footnotes
Conflict of Interest Statement:
Other than the cited funding sources, the authors have no financial interests to disclose.
References
- 1.Albrecht DS, MacKie PJ, Kareken DA, Hutchins GD, Chumin EJ, Christian BT, Yoder KK. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016;10:829–839. doi: 10.1007/s11682-015-9459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, Robbins TW, Fletcher PC, Murray GK. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol. 2015;6:1280. doi: 10.3389/fpsyg.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbey AK, Krueger F, Grafman J. Structured event complexes in the medial prefrontal cortex support counterfactual representations for future planning. Philos Trans R Soc Lond B Biol Sci. 2009;364:1291–1300. doi: 10.1098/rstb.2008.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 10.Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Soc Cogn Affect Neurosci. 2017;12:651–661. doi: 10.1093/scan/nsw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 12.Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev. 2016;68:282–297. doi: 10.1016/j.neubiorev.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 14.Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, Klumpp H. Neural Reactivity to Reward as a Predictor of Cognitive Behavioral Therapy Response in Anxiety and Depression. Depress Anxiety. 2016;33:281–288. doi: 10.1002/da.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter RM, Macinnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front Behav Neurosci. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994:319–333. [Google Scholar]
- 18.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, Devellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cromwell HC, Panksepp J. Rethinking the cognitive revolution from a neural perspective: how overuse/misuse of the term “cognition” and the neglect of affective controls in behavioral neuroscience could be delaying progress in understanding the BrainMind. Neurosci Biobehav Rev. 2011;35:2026–2035. doi: 10.1016/j.neubiorev.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–879. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 23.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981;1:1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fields HL. Understanding How Opioids Contribute to Reward and Analgesia. Reg Anesth Pain Med. 2007;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014;8:35. doi: 10.3389/fnint.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaskin ME, Greene AF, Robinson ME, Geisser ME. Negative affect and the experience of chronic pain. J Psychosom Res. 1992;36:707–713. doi: 10.1016/0022-3999(92)90128-o. [DOI] [PubMed] [Google Scholar]
- 30.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 31.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 32.Gujar N, Yoo S-S, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry. 2009;66:201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennigan K, D’Ardenne K, McClure SM. Distinct midbrain and habenula pathways are involved in processing aversive events in humans. J Neurosci. 2015;35:198–208. doi: 10.1523/JNEUROSCI.0927-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horst NK, Laubach M. Reward-related activity in the medial prefrontal cortex is driven by consumption. Front Neurosci. 2013;7:56. doi: 10.3389/fnins.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabanova A, Pabst M, Lorkowski M, Braganza O, Boehlen A, Nikbakht N, Pothmann L, Vaswani AR, Musgrove R, Di Monte DA, Sauvage M, Beck H, Blaess S. Function and developmental origin of a mesocortical inhibitory circuit. Nat Neurosci. 2015;18:872–882. doi: 10.1038/nn.4020. [DOI] [PubMed] [Google Scholar]
- 38.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 43.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 44.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 45.Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology. 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 46.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2016;18:20–30. doi: 10.1038/nrn.2016.162. [DOI] [PubMed] [Google Scholar]
- 49.Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- 50.Lamm C, Benson BE, Guyer AE, Perez-Edgar K, Fox NA, Pine DS, Ernst M. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain Cogn. 2014;89:51–60. doi: 10.1016/j.bandc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledermann K, Jenewein J, Sprott H, Hasler G, Schnyder U, Warnock G, Johayem A, Kollias S, Buck A, Martin-Soelch C. Altered Dopamine Responses to Monetary Rewards in Female Fibromyalgia Patients with and without Depression: A [11C]Raclopride Bolus-plus-Infusion PET Study. Psychother Psychosom. 2017;86:181–182. doi: 10.1159/000455927. [DOI] [PubMed] [Google Scholar]
- 53.Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. The importance of context: when relative relief renders pain pleasant. Pain. 2013;154:402–410. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 55.Libedinsky C, Smith DV, Teng CS, Namburi P, Chen VW, Huettel SA, Chee MWL. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front Behav Neurosci. 2011;5:70. doi: 10.3389/fnbeh.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loggia ML, Berna C, Kim J, Cahalan CM, Gollub RL, Wasan AD, Harris RE, Edwards RR, Napadow V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014;66:203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martucci KT, Eisenach JC, Tong C, Coghill RC. Opioid-independent mechanisms supporting offset analgesia and temporal sharpening of nociceptive information. Pain. 2012;153:1232–1243. doi: 10.1016/j.pain.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martucci KT, Mackey SC. Neuroimaging of Pain: Human Evidence and Clinical Relevance of Central Nervous System Processes and Modulation. Anesthesiology. 2018 doi: 10.1097/ALN.0000000000002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martucci KT, Yelle MD, Coghill RC. Differential effects of experimental central sensitization on the time-course and magnitude of offset analgesia. Pain. 2012;153:463–472. doi: 10.1016/j.pain.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 61.McNair DM, Lorr M, Droppleman L. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 62.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 63.Morley S, Williams AC, de C, Black S. A confirmatory factor analysis of the Beck Depression Inventory in chronic pain. Pain. 2002;99:289–298. doi: 10.1016/s0304-3959(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 64.Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain. 2014;155:2491–2501. doi: 10.1016/j.pain.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navratilova E, Atcherley CW, Porreca F. Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci. 2015;38:741–750. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014;17:1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology. 2011;115:1063–1071. doi: 10.1097/ALN.0b013e31822fd03a. [DOI] [PubMed] [Google Scholar]
- 68.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 69.Poldrack RA, Farah MJ. Progress and challenges in probing the human brain. Nature. 2015;526:371–379. doi: 10.1038/nature15692. [DOI] [PubMed] [Google Scholar]
- 70.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 72.Sacchet MD, Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage. 2013;66:270–277. doi: 10.1016/j.neuroimage.2012.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sawe N, Knutson B. Neural valuation of environmental resources. Neuroimage. 2015;122:87–95. doi: 10.1016/j.neuroimage.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 74.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science. 2014;345:535–542. doi: 10.1126/science.1253994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. 1970 Available: https://ubir.buffalo.edu/xmlui/handle/10477/2895.
- 77.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 78.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GLG, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine J-P, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Hoeck N, Ma N, Ampe L, Baetens K, Vandekerckhove M, Van Overwalle F. Counterfactual thinking: an fMRI study on changing the past for a better future. Soc Cogn Affect Neurosci. 2013;8:556–564. doi: 10.1093/scan/nss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-Scale Meta-Analysis of Human Medial Frontal Cortex Reveals Tripartite Functional Organization. J Neurosci. 2016;36:6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 83.Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 84.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 86.Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. Neuroimage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yelle MD, Rogers JM, Coghill RC. Offset analgesia: a temporal contrast mechanism for nociceptive information. Pain. 2008;134:174–186. doi: 10.1016/j.pain.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Distribution of painful areas is shown across the 19 body regions listed in the Fibromyalgia Assessment Form. Each patient confirmed the presence or absence of pain in each region. The number of patients indicating pain in each region is shown (total N = 17 patients). Bars are differently grayscale shaded to cluster among similar regions of the body to enhance readability of results and shading does not represent additional data. (B) The number of painful body regions indicated by each individual patient is shown (maximum number of body regions on the Fibromyalgia Assessment Form = 19). Each patient is represented by a number and bar of the graph. Patients are ordered by low to high number of painful body regions to enhance readability of the results.
ROIs (top panel) and ROI extracted beta values (bottom panel) for the ventral tegmental area (VTA), anterior insular cortex (aINS), and anterior cingulate cortex (ACC) are presented for the gain versus no-gain anticipation (GVNant) contrast. Analyses were conducted post-hoc and not corrected for multiple comparisons. LVNant contrast results for aINS and ACC ROIs were similar across groups (not shown). White boxes depict alternate ROI views. VTA, ventral tegmental area; aINS, anterior insular cortex; ACC, anterior cingulate cortex; R., right; ROI, region of interest.
A post-hoc whole brain analysis was conducted to inform future analyses. Group activation maps during reward anticipation (gain versus no-gain anticipation, GVNant) are shown for control group and patient group separately (p < 0.01, corrected alpha < 0.05). No other contrasts elicited whole brain activation clusters exceeding p < 0.05, with corrected alpha < 0.05. R, right.
(A) Expanded mask of the anterior medial prefrontal cortex created from a previous automated parcellation of functional data by De La Vega et al., see text for details. Activation maps showing group and group differences of mPFC activity for (B) reward anticipation (GVNant) and (C) no-loss outcome (NVLout) contrasts (p < 0.01, corrected alpha < 0.05).
(A) Comparison of Nacc activity time courses during the anticipation phase (yellow shading, estimated 2 – 6 seconds) for $0, +$5, and −$5 trials. These demonstrate distinct activity levels for GVNant (+$5 versus $0) and LVNant contrasts (−$5 versus $0) in the NAcc. (B) Comparison of mPFC activity time courses during the anticipation phase (yellow shading, estimated 2 – 6 seconds) for $0, +$5, and −$5 trials. These demonstrate distinct activity levels for GVNant (+$5 versus $0) and less distinct activity for LVNant contrasts (−$5 versus $0) in the mPFC. (C) Comparison of mPFC activity time courses during the outcome phase (yellow shading, estimated 10 – 14 seconds) for +$5 trials (left panel) and −$5 trials (right panel). Across both trial types, mPFC demonstrates greater activity during the outcome phase to +$5 outcome (hits) as compared with all other outcomes.
To further determine the potential contributions of depression in our patient sample we provide additional information on the data from the Beck Depression Inventory (BDI). (A) Scatter plot of the relationship between mPFC GVNant activity and depression (measured with the BDI) shows a significant negative correlation across both groups combined (as expected due to significant group differences in both mPFC activity and depression), but no within group correlation. (B) Bar graph of BDI factors (negative view of self factor, somatic factor, additional items contributing to an overall measure of negative affect) shows greater loading on the somatic factor in our fibromyalgia sample. Means (and standard deviation in parentheses) for each factor are shown below each factor label. Patient numbers highlighted in red text indicate individuals with BDI > 19 (i.e., moderate depressive symptoms). (C, D) Results for mPFC ROI for controls (N=15) versus fibromyalgia with mild depressive symptoms (N=13, excludes 4 patients with moderate depressive symptoms) for (C) GVNant contrast and (D) NVLout contrast (p < 0.05, uncorrected).
Participant counts for each tested correlation differ from the total number of participants (N = 32; patients N=17, controls N=15) because some participants did not complete all questionnaires. Arousal equals post-scan arousal rating to +$5 cue. Abbreviations: PANAS, Positive and Negative Affect Schedule; BIS/BAS, Behavioral Inhibition System/Behavioral Activation System; PROMIS, Patient-Reported Outcomes Measurement Information System; STAI, State-Trait Anxiety Inventory; BDI, Beck Depression Inventory; POMS, Profile of Mood States; BPI, Brief Pain Inventory; p, p value; r, Pearson correlation value; N, number of participants included in each analysis. Bold text indicates between group results p < 0.01 corrected for multiple comparisons and associated post-hoc within group results p < 0.05 uncorrected.