Abstract
According to the social intelligence hypothesis, understanding the cognitive demands of the social environment is key to understanding the evolution of intelligence. Many important socio-cognitive abilities, however, have primarily been studied in a narrow subset of the social environment—within-group social interactions—despite the fact that between-group social interactions often have a substantial effect on fitness. In particular, triadic awareness (knowledge about the relationships and associations between others) is critical for navigating many types of complex social interactions, yet no existing study has investigated whether wild animals can track associations between members of other social groups. We investigated inter-group triadic awareness in wild acorn woodpeckers (Melanerpes formicivorus), a socially complex group-living bird. We presented woodpeckers with socially incongruous playbacks that simulated two outsiders from different groups calling together, and socially congruous playbacks that simulated two outsiders from the same group calling together. Subjects responded more quickly to the incongruous playbacks, suggesting that they were aware that the callers belonged to two different groups. This study provides the first demonstration that animals can recognize associations between members of other groups under natural circumstances, and highlights the importance of considering how inter-group social selection pressures may influence the evolution of cognition.
Keywords: social cognition, triadic awareness, social intelligence hypothesis, vocal recognition, playback, acorn woodpecker
1. Introduction
Why do animal taxa vary so markedly in their cognitive abilities? The social intelligence hypothesis posits that the demands of the social environment are the primary driving force behind the evolution of complex cognition [1]. While abundant evidence supports a general positive association between social complexity and cognitive complexity [2,3], the ways in which different social selection pressures influence specific cognitive abilities are less clear. Understanding the evolution of a given cognitive ability requires investigating the full range of socio-ecological contexts in which the ability in question is used.
The social environment is not limited to the core social unit. In many taxa, successful mating [4], territorial defence [5], dispersal [6] and cooperation with kin [7] frequently depend on knowledge about members of other social groups. However, while a number of studies have shown that animals can recognize territorial neighbours [8,9], more complex forms of social cognition have primarily been studied within the confines of a single social group [10].
The ability to recognize and keep track of relationships and associations between others, known as triadic awareness or third-party knowledge, is critical for animals that engage in multi-way social interactions such as alliances and coalitions [10]. Primates can recognize several types of relationships between third parties, including kinship [11,12], dominance rank [13,14], male–female sexual relationships [15,16] and male–infant affiliative relationships [17]. Observational evidence suggests that spotted hyenas (Crocuta crocuta) are aware of the dominance and kin relationships between other individuals [18] and that rooks (Corvus frugilegus) recognize one another's preferred social affiliates [3], while common ravens (Corvus corax) [19] have been shown experimentally to recognize the dominance relationships between third parties.
Despite the prominence of triadic awareness within the social cognition literature, most studies have concentrated on whether animals have knowledge of the relationships between individuals within their own social group (intra-group triadic awareness). To our knowledge, the only unequivocal demonstration of inter-group triadic awareness, or knowledge about the relationships or associations between individuals in other groups, was in a single study of captive common ravens [19]. Moreover, this study was conducted with two groups of only eight birds each housed in very close proximity, where individual ravens had extensive opportunity to repeatedly observe interactions among the same conspecifics. Ravens in the wild live in much larger social networks with fission–fusion dynamics, where learning the relationships among outsiders would presumably be more cognitively challenging [20]. No previous study has examined whether animals are aware of the associations among individuals in other groups under natural conditions. Failing to investigate important cognitive abilities in the context of the full social environment risks ignoring major selection pressures on the evolution of social cognition. Thus, experimental studies of inter-group triadic awareness in an ecologically realistic context are needed to determine whether the benefits of this ability can outweigh the costs associated with its cognitive development.
We investigated inter-group triadic awareness in the acorn woodpecker (Melanerpes formicivorus), a cooperatively breeding bird with an unusually complex social system. Acorn woodpeckers in California live in family groups on stable, year-round territories with 1–4 joint-nesting females and 1–8 cobreeding males [21]. Breeders of the same sex are nearly always close relatives and opposite-sex breeders are unrelated; thus incest is rare [22]. Moreover, availability of suitable breeding locations is limited, and thus individuals of both sexes are often forced to delay dispersal and remain in their natal territory for several years as nonbreeding helpers [23]. Group members cooperate to raise offspring in a single nest, store acorns within a ‘granary’ tree, and defend resources from both conspecific and heterospecific intruders [24,25].
Opportunities for helpers to breed typically only occur when all the breeders of the opposite sex have died or disappeared either in their natal territory (in which they would inherit breeder status) or in another territory (to which they would disperse). Breeders may occasionally leave their current group to fill a breeding vacancy in a different territory [26]. Competition to fill breeding vacancies is intense, with fights for vacancies sometimes starting within a few hours of the disappearance of a breeder and lasting for several days [27,28]. Thus, reproductive success for both helper and breeder acorn woodpeckers often depends on being able to quickly identify when specific members of another group are missing, suggesting that individuals may routinely update knowledge about the members of other groups. When competing for breeding vacancies, acorn woodpeckers form coalitions with same-sex kin [27], so an awareness of the relationships among individuals in other groups would be likely to help acorn woodpeckers assess the potential allies of their competitors. Acorn woodpeckers make regular forays to territories up to several kilometres away (mean foray distance = 2.47 km for males, 4.98 km for females) [29], presumably allowing them to become familiar with a large number of individuals from other groups. We tested the hypothesis that acorn woodpeckers can determine whether two birds from outside the subject's own group belong to the same group as each other.
2. Material and methods
(a). Study site, subjects and dates
All data were collected at the Hastings Natural History Reservation in central coastal California, USA (36.379° N, 121.567° W). The acorn woodpecker population at this site has been the subject of a long-term study since 1968 [24,30], and more than 95% of the individuals are colour-banded. Approximately fifty social groups are monitored, and a census is taken of each group approximately every eight to ten weeks. Subjects for the study described included fifteen adult acorn woodpeckers, each from a different social group. To control for sex and reproductive status, all subjects were female breeders (as opposed to non-breeding helpers). The subjects' groups had a mean size of 5.9 (range 3–12) individuals (including the subject) at the time of the study. Experimental trials were conducted from 17 March to 15 May 2016. Unless otherwise stated, values given are mean ± standard deviation.
(b). Experimental protocol and playback stimuli
To test whether acorn woodpeckers could determine whether two individuals from outside their own group belonged to the same group as each other, we conducted a playback experiment using a violation-of-expectation paradigm [13,16]. Acorn woodpeckers frequently produce greeting calls known as ‘wakas’ in an overlapping chorus together with members of their own group, but not with members of other groups [24]. We presented subjects with playback stimuli consisting of waka calls recorded separately from two different individuals at our study site, artificially overlapped to simulate two birds calling together as if they were members of the same group (electronic supplementary material, figure S1). In all cases, the callers were not from the same group as the subject. In half the trials, the callers were from the same group as each other (socially congruous control stimulus), and in the other half of trials the callers were from two different groups (socially incongruous test stimulus). Each subject received both a test stimulus and a control, spaced apart by 2–5 days (median = 3) to reduce the chance of habituation. All observers were blind to stimulus identity until the trials were completed. A non-observer randomly assigned eight of the subjects to receive the test stimulus first and seven to receive the control stimulus first, and labelled the playback sound files with the appropriate subject's identification number.
We used a total of 13 waka calls recorded from 13 different callers to construct the playback stimuli, for a total of 12 unique test stimuli and five unique control stimuli. All of the calls were recorded at Hastings between January 2015 and March 2016 (see detailed methods in electronic supplementary material). Each stimulus (unique combination of two overlapping calls) was used in 1–5 playback trials (1.76 ± 0.33 s.e.). Each individual call was used as a component in 2–8 different stimuli (median = 4) (electronic supplementary material, table S1). The playback system was calibrated to a naturalistic amplitude (electronic supplementary material).
Each stimulus consisted of one call from a female bird and one call from a male bird, and in all cases but one control stimulus, the callers were both breeders. Because opposite-sex breeders in the same group are unrelated, this meant that the pair of callers in a given playback stimulus were unrelated to each other in all cases but one, even in the control stimuli where the two callers came from the same group.
For both test and control stimuli, callers were unrelated to the subject and the subject's current group members and had never lived in the same group as the subject or any of her current group members. This ensured the absence of an affiliative relationship between subjects and callers, which might have influenced the subjects' responses to the playbacks. Within the limits of this constraint, we presented each subject with calls recorded from the geographically closest group for which we had high-quality recordings to maximize the chance that subjects would be familiar with all the callers they were exposed to. The mean Euclidean distance between the granary of the subject's territory and the granary of the callers' territories was 430 ± 256 m (range 102–1136), which is well below the mean distance of extra-territorial forays in female acorn woodpeckers (4.98 km) [29]. Before each playback trial, we placed a Yamaha PDX-11 speaker (Hamamatsu, Shizuoka, Japan) 1–1.5 m off the ground in a tree within the subject's territory, approximately 40 m away from another tree near the centre of the territory (usually the granary tree), hereafter referred to as the ‘centre tree.’ We always placed the speaker in the same location for each trial with a given subject, and trials were only conducted when the subject was sitting in the centre tree. Because the anomalous ‘test’ stimuli consisted of calls recorded from two different groups, it was impossible to broadcast both calls from the direction of the callers' actual territories and still have them originate from a single location. Therefore, in both test and control trials the speaker was offset from the direction of either of the caller's territories so that the playback would originate from an unexpected direction. The angle between the speaker and either of the caller's territories (with the subject's centre tree as the vertex) was greater than 80° for all but one subject, for whom the speaker was offset by approximately 45° from the territories of all the callers used in both the test stimulus and the control stimulus. This ensured that any differential responses to test versus control stimuli would be due to recognition of the association between the callers and not whether the calls came from an unexpected direction.
Once the focal breeder female was located in the centre tree, we played the appropriate playback file and one observer began filming the focal female with a Panasonic SDR-H80 video camera (Kadoma, Osaka, Japan). The playback file contained one min of background noise before the calls, which served as a pre-playback period. At the same time, a second observer watched the space between the centre tree and the speaker tree and verbally noted on a digital recorder the times at which any acorn woodpecker flew from the centre tree towards the speaker, the distance between the birds and the speaker, and when possible, the identity of the approaching individuals. We also placed a Sennheiser ME62 omnidirectional microphone (Wedemark, Germany) connected to a Roland R26 (Hamamatsu, Shizuoka, Japan) or a Fostex FR2 (Akishima City, Tokyo, Japan) digital recorder between the centre tree and the speaker tree, to record any vocalizations produced by the subject's group (48 kHz sampling rate, 24 bits of amplitude resolution). Filming and behavioural observations continued for at least 10 min after the playback ended, but only the first 3 min of the playback and post-playback period were considered for analysis, as the woodpeckers almost always returned to baseline behaviour in less than 3 min.
(c). Response variables
Using the video and audio recordings of each playback trial, we measured the latency to the focal female's first ‘positive’ flight, defined as flying up to a higher vantage point or flying closer to the speaker. In addition, we measured the following five response variables but did not include them in analysis because they were highly correlated (|Pearson's r| ≥ 0.7) with latency to first positive flight: latency to the focal female's initial flight in any direction, latency to her first ‘reaction’ (positive flight or vocalization, whichever came first), latency to her closest approach to the speaker, distance of her closest approach to the speaker, and direction of her initial flight on an ordinal scale (−1 = away from the speaker, 0 = parallel to speaker with no height gain, 1 = to a higher vantage point without getting closer or further from the speaker, 2 = towards the speaker).
To assess the response of other group members, we measured the latency to the first vocalization by any group member, the distance of the closest approach to the speaker by any bird other than the focal female, the change in the group's waka call rate between the minute immediately preceding the start of the playback and the minute immediately following the start of the playback, and the proportion of the subject's group members who approached the speaker. We also measured the following variables that were highly correlated with other response variables and therefore excluded from analysis: latency to the first flight from the centre tree towards the speaker by any bird other than the focal female, latency to the closest approach to the speaker by any bird other than the focal female, and the number of birds who gathered around the focal female following the playback. For all latency response variables, if the behaviour of interest did not occur within the first 3 min after the start of the playback, the latency was assigned the maximum possible value of 180 s and marked as ‘censored’. All response variables included in the paper and their definitions are described in table 1.
Table 1.
Definitions of the response variables measured during each playback trial that were not highly correlated (|Pearson's r|<0.7) with any other response variables included in the analysis.
| response variable | definition |
|---|---|
| focal bird latency to first ‘positive’ flight | time in seconds from the start of the first call in the playback file until the focal female flew up and landed at a higher vantage point or flew closer to the speaker (censored after 3 min) |
| latency to first call by any individual | time in seconds from the start of the first call in the playback file until the first vocalization of any call type by any member of the group (censored after 3 min) |
| non-focal birds closest approach distance | the closest distance between any bird other than the focal female and the speaker at any point in time within the 3 min after the start of the first call in the playback file (closest approach distance by the focal female was excluded from analysis because of correlation with other response variables) |
| proportion of group members approaching | proportion of group members who flew out of the centre tree towards the speaker within 3 min after the start of the first call in the playback file |
| change in group waka call rate | number of waka calls produced by any group member in the first min after the start of the first call in the playback file, minus the number of waka calls produced in the min preceding the first call in the playback file |
(d). Predictions
As both the test and control stimuli represented a territorial intrusion by outside birds, we expected some degree of aggressive response to both conditions. However, we predicted that if acorn woodpeckers can determine whether two individuals from outside their own group belong to the same group as each other, then they should react more strongly to test playbacks than to controls, reflecting a ‘violation of expectation’ caused by the socially incongruous test stimuli, and also potentially reflecting the higher threat level associated with a simultaneous intrusion by two groups instead of only one [13,31]. Specifically, we predicted that subjects would exhibit shorter response latencies and approach the speaker more closely (smaller approach distance) in the test condition than in the control condition. We also predicted that a greater proportion of the subject's group would approach the speaker in the test condition than in the control condition, and that the group's waka call rate would increase more (from pre-playback to post-playback period) after test playbacks than after control playbacks.
(e). Statistical analyses
We conducted all statistical analyses in R v. 3.4.3 [32]. We constructed separate models for each response variable to facilitate interpretation of the results. We used mixed-effects Cox proportional hazards regression models in the R package ‘coxme’ [33] to analyse the latency to the focal female’s first positive flight and the latency to the first vocalization by any group member. We used linear mixed-effects models in the R package ‘lme4’ [34] to analyse the closest approach distance by any bird other than the focal female and the change in waka call rate before and after the playback. As the residuals were not normally distributed for the change in waka call rate, we square root transformed this response variable. We used a generalized linear mixed model with a binomial distribution in the R package ‘lme4’ to analyse the proportion of the subject's group who approached the speaker.
In each model, we included treatment (test or control) as a fixed effect, and subject ID and stimulus ID (unique combination of two callers) as random effects. The recorded calls used as playback stimuli differed in duration, inter-note spacing, and the distance between the caller's territory and the territory of the subject who heard the playback (which could potentially affect the subject's familiarity with the caller). To ensure that these factors could not explain any difference in response to test and control stimuli, we re-ran the same models with stimulus duration, three metrics of the degree of synchrony between the two overlapping callers [35,36], and inter-territorial distance added as covariates (see electronic supplementary material). Alpha level was set to 0.05 for all tests, and all tests were two-tailed.
3. Results
With three factors, five covariates and a sample size of 15 individuals, the full models were likely over-parametrized, and the addition of the five covariates did not change the significance of Treatment in any model. Therefore, we present the results of the more parsimonious models here (table 2) and include the results of the full models in the electronic supplementary material (electronic supplementary material, table S2).
Table 2.
Summary of each model. Parameter estimates (coefficients), test-statistics and p-values are displayed for treatment. The variance component for each random effect (individual ID and stimulus ID) is also displayed.
| treatment |
|||||||
|---|---|---|---|---|---|---|---|
| response variable | model type | n | coef. | test statistic | p-value | indiv. ID variance | stim. ID variance |
| focal latency to first positive flight | mixed-effects Cox model | 15 | 1.20 | χ2 = 7.91 | 0.005* | 1.19 | 0.00 |
| latency to first call by any individual | mixed-effects Cox model | 15 | −0.20 | χ2 = 0.32 | 0.57 | 0.09 | 0.00 |
| non-focal birds closest approach distance | linear mixed model | 15 | 2.32 | F1,14.2 = 0.16 | 0.69 | 52.29 | 0.00 |
| change in group waka call rate | linear mixed model | 15 | −0.04 | F1,9.7 = 0.01 | 0.92 | 0.08 | 0.43 |
| prop. of birds approaching | binomial GLMM | 14 | 0.32 | χ2 = 0.41 | 0.52 | 0.32 | 0.13 |
*Significant at 5% level.
(a). Response of the focal breeder female
The latency to the focal female's first positive flight was significantly shorter (faster response) in the test condition than in the control condition (Cox regression, n = 15, β = 1.2, χ2 = 7.91, p = 0.005; figure 1).
Figure 1.
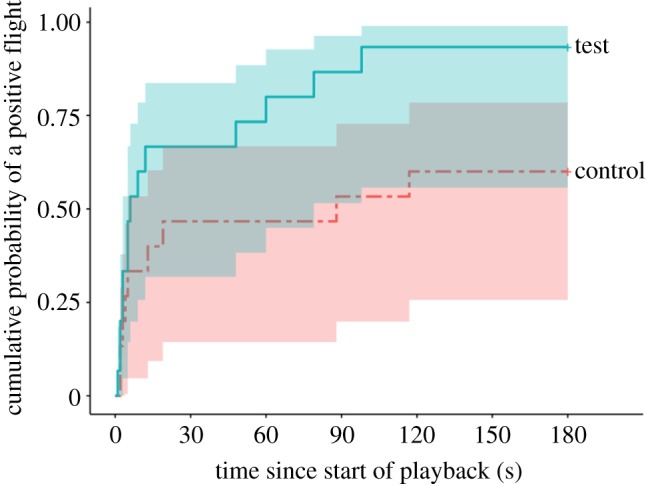
Kaplan–Meier survival curves for the latency to the focal female's first positive flight in socially congruous control playbacks (dashed red line) versus socially incongruous test playbacks (solid blue line) (n = 15). The lines indicate the cumulative probability that a ‘positive’ flight (flight to a higher vantage point or towards speaker) will occur by a given point in time. The colour-coded shaded areas around each line represent 95% confidence intervals. The curve for the test treatment rises significantly faster than the curve for the control, indicating a faster response time to the socially incongruous test stimuli (p = 0.005). (Online version in colour.)
(b). Responses of other birds
Treatment was not significantly related to the latency to the first vocalization by any group member (Cox regression, n = 15, β = −0.20, χ2 = 0.32, p = 0.57). It was also not significantly related to the closest approach distance by a non-focal bird (LMM, β = 2.32, F1,14.2 = 0.16, p = 0.69), the change in the group's waka call rate (LMM, n = 15, β = −0.04, F1,9.7 = 0.010, p = 0.92), or the proportion of group members who approached the speaker (binomial GLM, n = 14, β = 0.32, χ2 = 0.41, p = 0.52).
4. Discussion
Our results indicate that breeder female acorn woodpeckers are capable of determining whether or not two individuals from outside their own group belong to the same group as each other. Many territorial animals can indirectly assess the group membership of their neighbours by associating individual neighbours with particular territories [5,8]. However, because we broadcast our playbacks from a different direction than the territories of the callers, the subjects' knowledge of the group membership of others did not depend on associating callers with particular locations. Instead, our results suggest that the subjects were able to infer the association (shared group membership) between callers from other groups.
Many songbirds can glean information about territorial neighbours by eavesdropping on their vocal interactions with others, but in most cases, the birds could be cueing in on the aggressiveness or win–loss record of each individual conspecific in isolation, without necessarily knowing anything about dyadic relationships [5,37]. Great tits (Parus major) [38], pinyon jays (Gymnorhinus cyanocephalus) [39] and Burton's mouthbrooders (Astatotilapia burtoni) [40] were able to learn the dominance relationship between two conspecifics after witnessing a single interaction between them, independent of the absolute aggressiveness or win–loss record of each individual. In contrast to our study, however, these animals were provided with information about the relationships between others by the experimenters, so it is not clear whether they were aware of third-party relationships under natural conditions. Nonetheless, the rapidity with which all three of these species learned the relative dominance ranks of unfamiliar individuals suggests that many species may have the capacity to learn the relationships between members of other groups, despite the fact that interaction with non-group members is less frequent than interaction with members of one's own group. Thus, inter-group triadic awareness may be more common than current research suggests.
There are at least two mechanisms by which the acorn woodpeckers in our study could have determined whether two given callers were from the same group. One possibility is that waka calls carry an acoustic signature of group identity [41]. If such a group signature exists, acorn woodpeckers could determine whether two conspecifics belong to the same group by comparing their calls and assessing whether the signatures match, without necessarily recognizing either bird or having any prior knowledge about their association.
The existence of acoustic group signatures, however, is unlikely in acorn woodpeckers. A previous study examining the acoustic structure of waka calls in the acorn woodpeckers at our study site found that while wakas were individually distinct, there was no evidence of group signatures [42]. This study also found that individuals treated playbacks of their own waka calls like the waka calls of outsiders, rather than like the waka calls of fellow group members, which strongly suggests that recognition is not based on a group signature [42]. Moreover, over 70% of individuals who eventually attain breeding status do so via dispersal to a different group [43] with a minority of dispersers changing groups twice or more during their lifetime, and opposite-sex breeders within the same group virtually always come from different natal territories [22]. Consequently, any acoustic signature shared by all members of a group could only be maintained via open-ended vocal production learning. While vocal learning has not been studied in any woodpecker species, it is currently only known in three avian orders (Passeriformes, Apodiformes and Psittaciformes), and open-ended vocal learning is relatively rare even in taxa where vocal learning exists [44,45]. Our results cannot be explained by the existence of genetically determined kin signatures, because in all but one case, our playback stimuli (both test and control) consisted of the calls of one male breeder and one female breeder who were unrelated (electronic supplementary material, table S1). Although shared group membership could potentially be signalled by the degree of synchronicity of a waka chorus, this is unlikely to explain our results because none of the metrics of synchronicity that we measured (electronic supplementary material) were significantly related to response latency.
We believe the more plausible explanation for our results is that acorn woodpeckers recognize the calls of individual members of other groups, and can integrate this information with knowledge about which group each caller belongs to in order to infer the association between two callers. This mechanism implies a more complex mental representation of the associations between third parties than the group signature hypothesis. Regardless of the underlying cognitive mechanism, however, this study demonstrates that wild acorn woodpeckers recognize associations between members of other social groups without being artificially primed with information about those associations.
Acorn woodpecker knowledge of the group membership of others most likely extends beyond immediate neighbours. Out of the fifteen subjects in our study, only three were presented exclusively with calls from territories that were immediately adjacent to their own. The remainder received at least one playback involving calls recorded from a non-adjacent territory, and for nine subjects both the test and control stimuli contained at least one call from a non-adjacent territory. Thus, it is likely that breeder female acorn woodpeckers can recognize the group membership of at least some of the birds in groups two or more territories away from their own. Furthermore, given the regular long-distance forays made by acorn woodpeckers [29], it is possible that they recognize birds much further away than two territories. Additional work is necessary to determine the geographical extent of vocal recognition and triadic awareness in this species.
We originally focused on breeder females, as opposed to another sex or reproductive class, for practical reasons unrelated to this experiment. Nonetheless, inter-group social knowledge may be more important for female acorn woodpeckers, because females are less likely than males to inherit a breeding position in their natal territory, and are thus more dependent on being able to identify breeding vacancies in other groups [27]. Inter-group social information might also be more relevant to helpers than to breeders, because helpers are more likely to need to disperse.
Acorn woodpeckers have a social system in which knowledge about the associations among members of other groups could be particularly beneficial, both for identifying breeding opportunities and for predicting the size and membership of rival coalitions. We have found evidence that at least breeder female acorn woodpeckers can determine whether two individuals from other groups have an associative relationship. This finding supports the prediction of the social intelligence hypothesis that a species's cognitive abilities will be adapted to its social environment. This study also highlights the importance of accounting for social selection pressures external to the core social group when investigating the evolution of social cognition in general. Future work on social cognition should consider the cognitive demands of inter-group social interactions as well as intra-group interactions.
Supplementary Material
Acknowledgements
We thank V. Voegeli for lending us a loudspeaker and providing extensive logistical support, the Macaulay Library at the Cornell Lab of Ornithology for lending us recording equipment, the Bioacoustics Research Program at the Cornell Lab of Ornithology for providing access to an anechoic chamber, and UC Berkeley for permission to conduct research at Hastings Reserve. We also thank S. Pardo for assistance with the statistical analyses, M. Pesendorfer for blinding the playback stimuli and for helpful comments on the manuscript, Ç. Akçay for advice on the experimental design, and two anonymous reviewers for helpful comments on the manuscript.
Ethics
This work was approved under Cornell University IACUC protocol 2008-0185, State of California scientific collecting permit SC-1457, and US federal banding permit 21508.
Data accessibility
Data and R code available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.7mf14t0 [46].
Authors' contributions
M.A.P. conceived of and designed the study, collected field data, analysed the data and drafted the manuscript; E.A.S. and T.S.K. collected field data; N.D.H. colour-banded subjects and provided data on the distances between groups; E.L.W. and W.D.K. supervised the study, colour-banded subjects, provided long-term demographic data and contributed to the writing of the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
M.A.P. was supported by a Graduate Research Fellowship from the National Science Foundation (NSF) and by a grant from the Department of Neurobiology and Behavior at Cornell University. The project was supported by NSF grant no. IOS-1455900 to E.L.W. and NSF grant no. IOS-1455881 to W.D.K.
References
- 1.Humphrey NK. 1976. The social function of intellect. In Growing points in ethology (eds Bateson PPG, Hinde RA), pp. 303–317. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347. ( 10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 3.Emery NJ, Seed AM, von Bayern AM, Clayton NS. 2007. Cognitive adaptations of social bonding in birds. Phil. Trans. R. Soc. B 362, 489–505. ( 10.1098/rstb.2006.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21. ( 10.1006/anbe.2000.1726) [DOI] [Google Scholar]
- 5.Akçay Ç, Reed VA, Campbell SE, Templeton CN, Beecher MD. 2010. Indirect reciprocity: song sparrows distrust aggressive neighbours based on eavesdropping. Anim. Behav. 80, 1041–1047. ( 10.1016/j.anbehav.2010.09.009) [DOI] [Google Scholar]
- 6.Jungwirth A, Walker J, Taborsky M. 2015. Prospecting precedes dispersal and increases survival chances in cooperatively breeding cichlids. Anim. Behav. 106, 107–114. ( 10.1016/j.anbehav.2015.05.005) [DOI] [Google Scholar]
- 7.Dickinson JL, Koenig WD, Pitelka FA. 1996. Fitness consequences of helping behavior in the western bluebird. Behav. Ecol. 7, 168–177. ( 10.1093/beheco/7.2.168) [DOI] [Google Scholar]
- 8.Cheney DL, Seyfarth RM. 1982. Recognition of individuals within and between groups of free-ranging vervet monkeys. Am. Zool. 22, 519–529. ( 10.1093/icb/22.3.519) [DOI] [Google Scholar]
- 9.Stoddard PK. 1996. Vocal recognition of neighbors by territorial passerines. In Ecology and evolution of acoustic communication in birds (eds Kroodsma DE, Miller EH), pp. 356–374. Ithaca, NY: Cornell University Press. [Google Scholar]
- 10.Seyfarth RM, Cheney DL. 2015. Social cognition. Anim. Behav. 103, 191–202. ( 10.1016/j.anbehav.2015.01.030) [DOI] [Google Scholar]
- 11.Cheney DL, Seyfarth RM. 1980. Vocal recognition in free-ranging vervet monkeys. Anim. Behav. 28, 362–367. ( 10.1016/S0003-3472(80)80044-3) [DOI] [Google Scholar]
- 12.Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. 2007. Kin-mediated reconciliation substitutes for direct reconciliation in female baboons. Proc. R. Soc. B 274, 1109–1115. ( 10.1098/rspb.2006.0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheney DL, Seyfarth RM, Silk JB. 1995. The responses of female baboons (Papio cynocephalus ursinus) to anomalous social interactions: evidence for causal reasoning? J. Comp. Psychol. 109, 134–141. ( 10.1037/0735-7036.109.2.134) [DOI] [PubMed] [Google Scholar]
- 14.Borgeaud C, van de Waal E, Bshary R. 2013. Third-party ranks knowledge in wild vervet monkeys (Chlorocebus aethiops pygerythrus). PLoS ONE 8, e58562 ( 10.1371/journal.pone.0058562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann C, Kummer H. 1980. Male assessment of female choice in hamadryas baboons. Behav. Ecol. Sociobiol. 6, 315–321. ( 10.1007/BF00292774) [DOI] [Google Scholar]
- 16.Crockford C, Wittig RM, Seyfarth RM, Cheney DL. 2007. Baboons eavesdrop to deduce mating opportunities. Anim. Behav. 73, 885–890. ( 10.1016/j.anbehav.2006.10.016) [DOI] [Google Scholar]
- 17.Kubenova B, Konecna M, Majolo B. 2017. Triadic awareness predicts partner choice in male–infant–male interactions in Barbary macaques. Anim. Cogn. 20, 221–232. ( 10.1007/s10071-016-1041-y) [DOI] [PubMed] [Google Scholar]
- 18.Engh AL, Siebert ER, Greenberg DA, Holekamp KE. 2005. Patterns of alliance formation and postconflict aggression indicate spotted hyaenas recognize third-party relationships. Anim. Behav. 69, 209–217. ( 10.1016/j.anbehav.2004.04.013) [DOI] [Google Scholar]
- 19.Massen JJM, Pašukonis A, Schmidt J, Bugnyar T. 2014. Ravens notice dominance reversals among conspecifics within and outside their social group. Nat. Commun. 5, 3679 ( 10.1038/ncomms4679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun A, Bugnyar T. 2012. Social bonds and rank acquisition in raven nonbreeder aggregations. Anim. Behav. 84, 1507–1515. ( 10.1016/j.anbehav.2012.09.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koenig WD, Walters EL, Haydock J. 2016. Acorn woodpeckers: helping at the nest, polygynandry, and dependence on a variable acorn crop. In Cooperative breeding: studies of ecology, evolution, and behavior (eds Koenig WD, Dickinson JL), pp. 217–236. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Koenig WD, Haydock J, Stanback MT. 1998. Reproductive roles in the cooperatively breeding acorn woodpecker: incest avoidance versus reproductive competition. Am. Nat. 151, 243–255. ( 10.1086/286115) [DOI] [PubMed] [Google Scholar]
- 23.Koenig WD, Walters EL, Haydock J. 2011. Variable helper effects, ecological conditions, and the evolution of cooperative breeding in the acorn woodpecker. Am. Nat. 178, 145–157. ( 10.1086/660832) [DOI] [PubMed] [Google Scholar]
- 24.MacRoberts MH, MacRoberts BR. 1976. Social organization and behavior of the acorn woodpecker in central coastal California. Ornithol. Monogr. 21, 1–115. [Google Scholar]
- 25.Koenig WD, Walters EL. 2016. Provisioning patterns in the cooperatively breeding acorn woodpecker: does feeding behaviour serve as a signal? Anim. Behav. 119, 125–134. ( 10.1016/j.anbehav.2016.06.002) [DOI] [Google Scholar]
- 26.Koenig WD, Mumme RL. 1987. Population ecology of the cooperatively breeding acorn woodpecker. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Hannon SJ, Mumme RL, Koenig WD, Pitelka FA. 1985. Replacement of breeders and within-group conflict in the cooperatively breeding acorn woodpecker. Behav. Ecol. Sociobiol. 17, 303–312. ( 10.1007/BF00293208) [DOI] [Google Scholar]
- 28.Koenig WD. 1981. Space competition in the acorn woodpecker: power struggles in a cooperative breeder. Anim. Behav. 29, 396–409. ( 10.1016/S0003-3472(81)80099-1) [DOI] [Google Scholar]
- 29.Koenig WD, Van Vuren D, Hooge PN. 1996. Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol. Evol. 11, 514–517. ( 10.1016/S0169-5347(96)20074-6) [DOI] [PubMed] [Google Scholar]
- 30.Koenig WD. 1981. Reproductive success, group size, and the evolution of cooperative breeding in the acorn woodpecker. Am. Nat. 117, 421–443. ( 10.1086/521238) [DOI] [Google Scholar]
- 31.Radford AN. 2003. Territorial vocal rallying in the green woodhoopoe: influence of rival group size and composition. Anim. Behav. 66, 1035–1044. ( 10.1006/anbe.2003.2292) [DOI] [Google Scholar]
- 32.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 33.Therneau TM.2018. coxme: Mixed effects cox models. R package version 2, .2-7. See https://CRAN.R-project.org/package=coxme .
- 34.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 35.Elie JE, Mariette MM, Soula HA, Griffith SC, Mathevon N, Vignal C. 2010. Vocal communication at the nest between mates in wild zebra finches: a private vocal duet? Anim. Behav. 80, 597–605. ( 10.1016/j.anbehav.2010.06.003) [DOI] [Google Scholar]
- 36.Maynard DF, Ward K-AA, Doucet SM, Mennill DJ. 2012. Calling in an acoustically competitive environment: duetting male long-tailed manakins avoid overlapping neighbours but not playback-simulated rivals. Anim. Behav. 84, 563–573. ( 10.1016/j.anbehav.2012.06.008) [DOI] [Google Scholar]
- 37.Toth CA, Mennill DJ, Ratcliffe LM. 2012. Evidence for multicontest eavesdropping in chickadees. Behav. Ecol. 23, 836–842. ( 10.1093/beheco/ars038) [DOI] [Google Scholar]
- 38.Peake TM, Terry AMR, Mcgregor PK, Dabelsteen T. 2002. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proc. R. Soc. Lond. B 269, 1925–1929. ( 10.1098/rspb.2002.2112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paz-y-Miño CG, Bond AB, Kamil AC, Balda RP. 2004. Pinyon jays use transitive inference to predict social dominance. Nature 430, 778–781. ( 10.1038/nature02720.1) [DOI] [PubMed] [Google Scholar]
- 40.Grosenick L, Clement TS, Fernald RD. 2007. Fish can infer social rank by observation alone. Nature 445, 429–432. ( 10.1038/nature05511) [DOI] [PubMed] [Google Scholar]
- 41.Price JJ. 1999. Recognition of family-specific calls in stripe-backed wrens. Anim. Behav. 57, 483–492. ( 10.1006/anbe.1998.1018) [DOI] [PubMed] [Google Scholar]
- 42.Yao Y. 2008. Studies of vocal communications in cooperatively breeding acorn woodpeckers (Melanerpes formicivorus). PhD dissertation, UCLA, Los Angeles, CA. [Google Scholar]
- 43.Koenig WD, Hooge PN, Stanback MT, Haydock J. 2000. Natal dispersal in the cooperatively breeding acorn woodpecker. Condor 102, 492–502. ( 10.1650/0010-5422(2000)102%5B0492:NDITCB%5D2.0.CO;2) [DOI] [Google Scholar]
- 44.Nottebohm F. 1972. The origins of vocal learning. Am. Nat. 106, 116–140. ( 10.2307/2678832) [DOI] [Google Scholar]
- 45.Baptista LF, Schuchmann KL. 1990. Song learning in the Anna Hummingbird (Calypte anna). Ethology 84, 15–26. ( 10.1111/j.1439-0310.1990.tb00781.x) [DOI] [Google Scholar]
- 46.Pardo MA, Sparks EA, Kuray TS, Hagemeyer ND, Walters EL, Koenig WD. 2018. Data from: Wild acorn woodpeckers recognize associations between individuals in other groups Dryad Digital Repository. ( 10.5061/dryad.7mf14t0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Pardo MA, Sparks EA, Kuray TS, Hagemeyer ND, Walters EL, Koenig WD. 2018. Data from: Wild acorn woodpeckers recognize associations between individuals in other groups Dryad Digital Repository. ( 10.5061/dryad.7mf14t0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R code available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.7mf14t0 [46].


