Abstract
Light is a fundamental driver of ecosystem dynamics, affecting the rate of photosynthesis and primary production. In spite of its importance, less is known about its community-scale effects on aquatic ecosystems compared with those of nutrient loading. Understanding light limitation is also important for ecosystem management, as human activities have been rapidly altering light availability to aquatic ecosystems. Here we show that decreasing light can paradoxically increase phytoplankton abundance in shallow lakes. Our results, based on field manipulation experiments, field observations and models, suggest that, under competition for light and nutrients between phytoplankton and submersed macrophytes, alternative stable states are possible under high-light supply. In a macrophyte-dominated state, as light decreases phytoplankton density increases, because macrophytes (which effectively compete for nutrients released from the sediment) are more severely affected by light reduction. Our results demonstrate how species interactions with spatial heterogeneity can cause an unexpected outcome in complex ecosystems. An implication of our findings is that partial surface shading for controlling harmful algal bloom may, counterintuitively, increase phytoplankton abundance by decreasing macrophytes. Therefore, to predict how shallow lake ecosystems respond to environmental perturbations, it is essential to consider effects of light on the interactions between pelagic and benthic producers.
Keywords: alternative stable states, asymmetry, competition, interspecific interactions, light environments, shallow lake
1. Introduction
Light intensity is a fundamental driver of ecosystem processes, affecting the rate of photosynthesis and primary production [1–3]. For example, Karlsson et al. [1] proposed that productivity in most unproductive lakes is limited by light and not by nutrients. In spite of its importance, however, the effect of light intensity in natural ecosystems has been relatively under-investigated compared with that of nutrients (but see [1,2,4,5]). Because human activities have altered light availability to aquatic ecosystems by riparian deforestation [6], changing the amount of clouds and aerosols with global climate change [7], reducing ice cover area over lakes with global warming [8], installing floating solar photovoltaic power plants [9], and changing inputs of terrestrial dissolved organic matter (DOM) [10], it is important to understand the effects of light availability on community dynamics and their implications for conservation and management.
Because of its requirement for photosynthesis, decreasing light availability is expected to reduce primary production [1,2]. However, the effects of light intensity on communities can be different from those of nutrient loading due to spatial heterogeneity. Light always comes from above (the Sun), thus the vertical distribution of species affects the outcome of competition for light: when species higher in the water column increase in biomass, the amount of light available for deeper species decreases. In shallow lakes containing phytoplankton, rooted macrophytes with floating leaves, submersed macrophytes and benthic algae (periphyton), community structure is predicted by theoretical studies [11–14] to show complicated dynamics such as hysteresis (i.e. alternative stable states) along light gradients. Therefore, the effects of light input on community structures must be examined in the context of the details of spatial heterogeneity and biological feedbacks.
To understand ecosystem responses to changing light intensity at a natural scale, we manipulated light input to experimental pond ecosystems (figure 1) and examined the effects of light availability on the plankton communities. Although previous studies have often used coloured DOM for manipulating underwater light conditions [5,15], this may affect phytoplankton abundance through addition of organic nutrients [16,17] and indirect effects on microbial activity [18]. Natural and anthropogenic environmental changes that affect light availability are in fact often tied to changes in nutrient concentrations [19] and other factors, however, simplifying the situation by solely manipulating light availability will be helpful for mechanistically understanding and predicting complex ecosystem dynamics [2,4,20,21]. In this study, by using partial shading with opaque floating swimming pool covers (figure 1b,c), we manipulated light input without changing the concentrations of DOM in the pond. We measured light penetration though the cover material and found that it removed greater than 95% of sunlight, although primary production still occurred because there was some light transmission into the ponds between the floating covers. In contrast to our intuition, we found that low light increased phytoplankton biomass (as measured by chlorophyll a concentration [22]). We also found a negative correlation between chlorophyll a and the abundance of submersed macrophytes, implying a potential competitive interaction between them. Although previous studies that examined the effects of light change by DOM have mainly focused on competition between pelagic phytoplankton and benthic periphyton [5,14], our simulations suggest that periphyton and macrophytes play a similar role to benthic producers in competition with pelagic phytoplankton. Our experimental results, field observations and a simulation model combine to demonstrate that shading can paradoxically increase phytoplankton abundance through the indirect effect of competition with benthic producers.
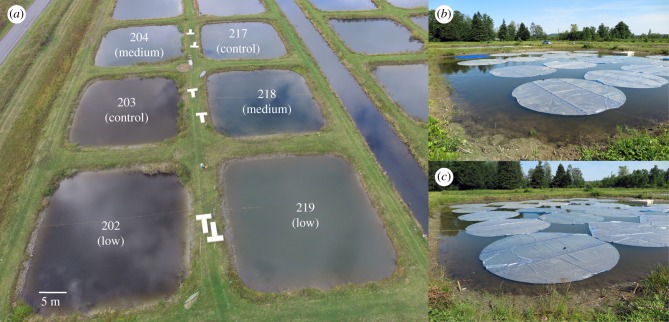
Figure 1.
Field site and shading treatments. (a) A bird's-eye view of the assigned treatments in CUEPF at the end of the experiment (after floating mats were removed). Control: high-light (0% shading), medium: medium-light (56.5% shading) and low: low-light (75.4% shading). T-shape floating docks (3 m × 1 m each), used for sampling, are visible on dikes between ponds. Floating mats during experiment in (b) a medium-light treatment (pond 218) and (c) a low-light treatment (pond 219).
2. Methods
(a). Light manipulation and sampling at experimental ponds
We used the ponds at the Cornell University Experimental Ponds Facility (CUEPF) in Ithaca, NY, USA (42°30′ N, 76°26′ W). At the Neimi Road Site (Unit 2) of CUEPF, 50 ponds were built in 1964 and have been used for various field experiments in aquatic ecology (e.g. [23–25]). We conducted our experiment at six ponds (pond ID: 202, 203, 204, 217, 218 and 219; figure 1) from 3 July to 28 September 2015. Each pond is 0.09 ha surface area (30 × 30 m) with a 0.04ha central deep area (20 × 20 m) at 1.5 m depth. Slopes from the shore to the deep area occupy approximately 0.05 ha yielding a water body of 950 m3 volume. In June 2015, to equalize initial conditions among ponds, we pumped the water down to a depth of 10 cm, chlorinated the remaining water with granular (swimming pool) sodium dichloroisocyanurate, and then refilled the ponds with filtered water from a reservoir source (through a 1 mm mesh). Pond 219 had unusually high initial densities of macrophytes (mostly Potamogeton crispus), so we further removed those manually to be roughly equivalent to those in other ponds. To prevent prevalence of remaining small zooplanktivorous cyprinid fish (mainly fathead minnow, Pimephales promelas), we added five to six juvenile largemouth bass (Micropterus salmoides; electronic supplementary material, table S1) to each pond on 3 July 2015. We manipulated light supply to the ponds by using opaque floating swimming pool covers (6 m diameter; Solar-cell SunBlanket, Century Products, Inc., Georgia, USA). The mats are dark blue on one side and silvered and reflective on the other. They were installed silvered side up to reflect light and minimize heating of the water (figure 1). We randomly assigned the six ponds to low-light treatment (75.4% of pond surface area covered with 24 covers/pond), medium-light treatment (56.5% shading with 18 covers/pond) and high-light (control) treatment (0% shading with 0 cover). Ponds 202 and 219 were low-light treatments, ponds 204 and 218 were medium-light treatments, and ponds 203 and 217 were controls (figure 1). Dissolved oxygen (DO) data (electronic supplementary material, figure S1) did not indicate any dramatic differences between the treatments, suggesting that gas exchange with the atmosphere was less important than internal dynamics of oxygen production and consumption.
Sampling, performed once every two weeks during the experiment, consisted of collecting 10-l pond water from the bottom to surface by repeated deployment of a 2.2-l tube sampler. The water samples were kept in the dark, brought to the laboratory within 2 h and analysed for chlorophyll a, total phosphorus (TP) and total nitrogen (TN). Subsamples were preserved for analysis of phytoplankton biovolume and composition. Zooplankton samples were collected by filtering a total of 22-l pond water from five sites in each pond with a 100 µm mesh net, and fixed with 99% ethanol for enumeration. Benthic primary producers were collected in triplicate on 16 August 2015 using an Ekman-Birge grab sampler (15 × 15 cm) from floating docks deployed in each pond (figure 1). We measured the dry weight of benthic producers after drying for 24 h at 105°C. We found that submersed macrophyte, Chara vulgaris, was dominant in the ponds. Thus, to quantify the area dominated by macrophytes, we took photos of the six experimental ponds from above using an unmanned aerial vehicle (UAV, i.e. a drone) with a video camera (Phantom 3, DJI, Shenzhen, China) on 7 October 2015 after the experiment had been terminated and the floating swimming pool covers removed. Methods for measuring chlorophyll a, TP and TN, and for enumerating and estimating biomass of phytoplankton and zooplankton are given in electronic supplementary material, appendix S1.
(b). Pattern of primary producers in 35 unmanipulated ponds
In addition to the experimental manipulation, we surveyed 35 CUEPF ponds that were not used for our shading experiments on 27–28 July and on 11–12 August 2016 to understand patterns of pond communities without shading. For this survey, at each pond, we collected 7-l pond water for chlorophyll a analysis from below the surface using a 1.4-l weighted throw bottle and quantified as described in electronic supplementary material, appendix S1. For macrophytes, we took aerial photos by the UAV and quantified the area dominated by macrophytes using imagej software [26]. We used the fraction of pond area covered by macrophytes as a proxy for macrophyte abundance as these measures were positively correlated in our six experimental ponds in 2015 (electronic supplementary material, figure S2). Averaged values of chlorophyll a and macrophyte abundance of the two samplings were used for the subsequent analyses.
(c). Statistical analyses
For the six experimental ponds manipulated in 2015, we conducted linear mixed-effect model (LMM) analyses for log-scaled chlorophyll a data except for the initial date, 24 June, because it was a transitory period (figure 2a). We set sampling date as the random effects and used ‘lme’ function of the ‘nlme' package in R [27]. Also, we statistically compared the differences in log-scaled chlorophyll a among the study ponds as a post-hoc multiple comparison of LMM using the ‘multicomp' function of the ‘multicomp' package in R [27].
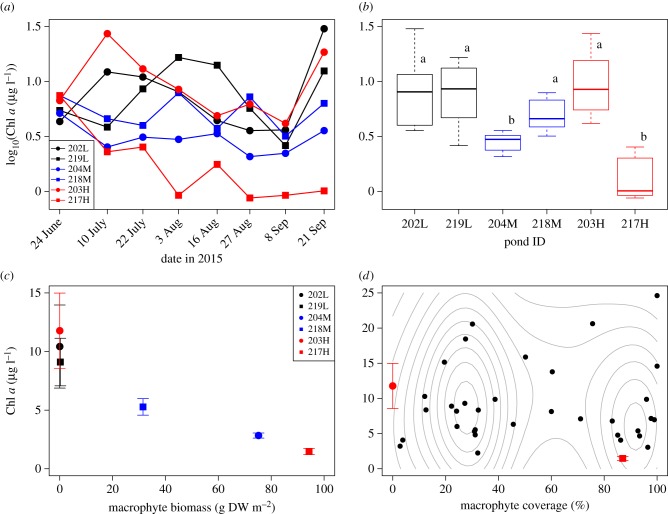
Figure 2.
Experimental and observational results. (a) Time-series and (b) boxplot of log-scaled chlorophyll a (Chl a) under three light intensities. The bold line in the box indicates the median value. Lower/upper limits of the box are the first/third quartiles, respectively. Lower/upper whisker plots represent the minimum/maximum values within the first/third quartiles minus/plus 1.5× interquartile range, respectively. The different characters on the box indicate significant differences among the ponds using a post-hoc multiple comparison for LMM (p < 0.002). (c) A correlation between chlorophyll a (mean and standard error, s.e.) and submersed macrophyte biomass (Spearman's ρ = –0.99; p = 0.00031). Red: control, blue: medium-light, black: low-light. Circle: road-side ponds, square: channel-side ponds. (d) Macrophyte coverage (%) and chlorophyll a concentration in 34 non-shaded ponds in 2016 (we removed a pond with an outlier value of chlorophyll a, see text). Black points indicate mean values at two sampling times in 2016. Red points and error bars indicate mean and s.e. values of non-shaded control ponds in 2015. Grey contours indicate a two-dimensional binned kernel density estimation.
We examined correlations between chlorophyll a and macrophyte biomass (figure 2c), macrophyte biomass and areal coverage (electronic supplementary material, figure S2), attenuation coefficients, chlorophyll a, total biovolume of phytoplankton and seston carbon (electronic supplementary material, figure S3), chlorophyll a, TP, TN, zooplankton biomass and zooplankton body length (electronic supplementary material, figure S4), chlorophyll a and phytoplankton biovolume (electronic supplementary material, figure S5a), phytoplankton biovolume and macrophyte biomass (electronic supplementary material, figure S8c), seston carbon and macrophyte biomass (electronic supplementary material, figure S9c), and zooplankton body length, phytoplankton biovolume and seston carbon (electronic supplementary material, figure S12) using Spearman's rank correlation tests. We used the data from all sampling dates of the experiment in electronic supplementary material, figures S3 and S5a, whereas we used mean values of all the data except for the initial date, 24 June (a transitory period), in electronic supplementary material, figures S4 and S12.
For the survey of 35 CUEPF ponds in 2016, the chlorophyll a and macrophyte coverage data were plotted against each other (figure 2d, note that we removed a pond with an outlier value of mean chlorophyll a, 46.9 µg l−1, with 100% macrophyte coverage) and subsequently analysed for unimodality by using Hartigans' dip test (‘dip.test' function of the ‘diptest' package in R [27]) where the alternative hypothesis is non-unimodal. A two-dimensional binned kernel density estimation for drawing contours (figure 2d) was calculated using the ‘bkde2D' function of the ‘KernSmooth' package with bandwidth (10, 10) in R.
(d). Theoretical model
Because we found that most benthic producers in these ponds were submersed macrophytes (the macroalga Chara), we developed a dynamic model of phytoplankton biomass Ai (g DW m–3), macrophyte biomass S (g DW m–3) and nutrient concentration in a water column Ri (g m–3) with vertical spatial heterogeneity to explore the mechanistic relationship between phytoplankton and macrophyte densities, as determined by light and nutrient resources. Similar models have been developed for describing competition among phytoplankton [28], between floating and submersed plants [11], and between pelagic and benthic algae [5,14]. Dynamics are represented as
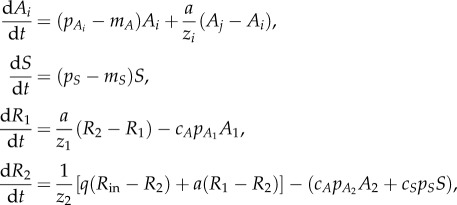 |
2.1 |
where i, j = 1, 2. We assume that nutrients and phytoplankton occur in the pelagic (1) and the benthic (2) habitats with exchange rate a. Here pi is the growth rate of the primary producers (i = Aj or S), mi is loss rate, q is the nutrient loading rate from sediments, z1 is depth of the pelagic habitat, z2 is depth of the benthic habitat, Rin is the concentration of incoming nutrients and ci is the nutrient to dry weight ratio (electronic supplementary material, table S2). The growth rate is co-limited by nutrient (R) and light (I) as pi = fi(Ri)gi(I)pmax,i, where pmax,i is maximum growth rate. The nutrient limitation factor, fi(Ri), is a saturating function, 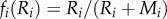 , where Mi is a half-saturation constant. The light limitation factor, gi(I), is obtained by integrating over the depth as
, where Mi is a half-saturation constant. The light limitation factor, gi(I), is obtained by integrating over the depth as
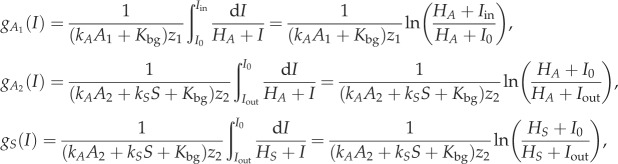 |
2.2 |
where ki is a light attenuation coefficient, Hi is a half-saturation constant for light and Kbg is a background light attenuation [28]. Here Iin is surface light intensity, I0 is light intensity below the pelagic habitat and above the benthic habitat and Iout is light intensity below the benthic habitat. They depend on the light attenuation by water and producer biomass (electronic supplementary material, figure S3), according to Lambert–Beer's Law
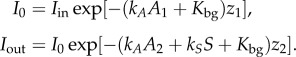 |
2.3 |
Based on field observation, we set z1 = 1.4 and z2 = 0.1. We conducted numerical simulations based on parameter values obtained primarily from previous studies (electronic supplementary material, table S2) [11,14].
3. Results
(a). Field manipulation experiments
In contrast to the previous studies where an increase in light availability led to an increase in phytoplankton biomass (e.g. [20,29,30]), in our field experiments, phytoplankton biomass instead increased with decreasing light (figure 2a,b). We found that chlorophyll a concentration increased in low-light ponds (mean values from 10 July to 21 September: 10.4 and 9.11 µg l−1) relative to medium-light ponds (2.83 and 5.28 µg l−1). In addition, whereas, consistent with this trend, one of the control (no shading) ponds had the lowest mean chlorophyll a (1.47 µg l−1), the other had mean chlorophyll a as high as in the low-light ponds (11.8 µg l−1). We found a statistically significant difference between ponds (202L, 219L, 218M, 203H) and (204M, 217H) (figure 2b, p < 0.002). The pattern except for the control ponds is consistent and is apparently driven by the light manipulation, because chlorophyll a concentrations were similar among the ponds before the manipulation (24 June of figure 2a) and the differences among the ponds were roughly consistent throughout the experimental period (10 July to 21 September of figure 2a).
To understand the mechanism behind the unexpected pattern, we measured a range of relevant biological and environmental variables (electronic supplementary material, appendix S1) and found that phytoplankton chlorophyll a concentration was significantly and negatively correlated with the areal biomass of submersed macrophytes (primarily Chara vulgaris in these ponds) (Spearman's ρ = –0.99; p = 0.00031; figure 2c). Because all the ponds began with the equivalent macrophyte conditions, probably macrophyte biomass changed over the summer to produce the observed pattern. We also found indications of positive correlations between chlorophyll a and nutrients (Spearman's ρ = 0.66, p = 0.18 for TP, and ρ = 0.77, p = 0.10 for TN; electronic supplementary material, figures S4a and S4b, respectively). On the other hand, the relationships between chlorophyll a and zooplankton biomass and body length suggest much less clear patterns (Spearman's ρ = –0.14 and –0.029 with p = 0.80 and p > 0.99 for biomass and body length, respectively; electronic supplementary material, figures S4c, S4d).
(b). A simulation model
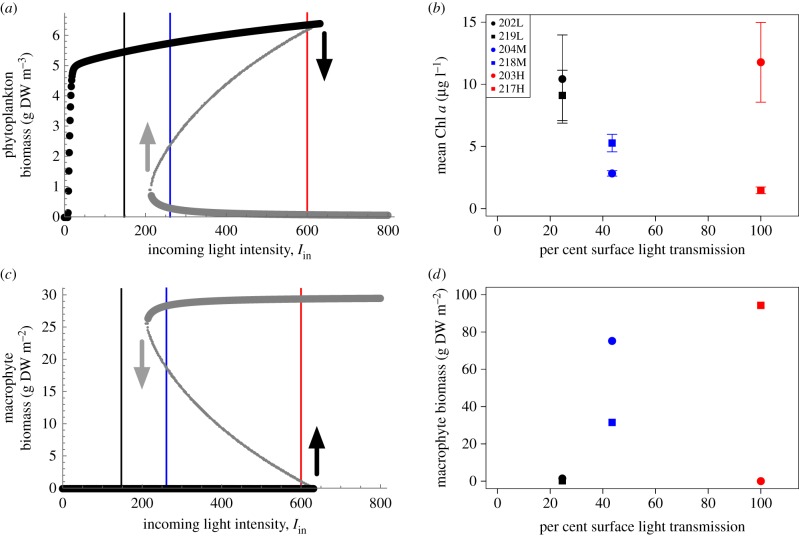
Because the observed negative correlation between phytoplankton and submersed macrophytes suggests competition between these groups, we analysed a competition model in which the growth rates of these primary producers are co-limited by light and nutrients. By numerical simulation, we found that macrophytes should dominate under high-light supply, whereas phytoplankton should dominate under low-light supply, and there are alternative stable states at intermediate light (figure 3a,c). The alternative stable states have one attractor at which phytoplankton and macrophytes coexist (the grey dots in figure 3a,c). If the system is at that attractor, phytoplankton can increase under low-light, because, due to the stratified community structure in a water column, decreasing surface light has a greater influence on macrophytes than on phytoplankton suspended in the water column (figure 3). The simulation results (figure 3a,c) were consistent with experimental results (figure 3b,d) when the light intensity of control (no shading) ponds is assumed to be 600 µmol m–2 s–1. This could be taken as a rough approximation of the average irradiance during a 24 h day–night cycle, because the mean light intensity at the pond surface between 10.00 and 12.00 during the study period was 1231 µmol m–2 s–1 (see electronic supplementary material, appendix S1).
Figure 3.
Effects of light on phytoplankton and submersed macrophytes. Bifurcation analyses of (a) phytoplankton biomass (averaged over the pelagic and the benthic habitats), (z1A1 + z2A2)/(z1 + z2), and (c) macrophyte biomass, z2S, along incoming light intensity. Large black and grey dots represent stable equilibria and smaller grey dots represent unstable equilibria. Black and grey arrows indicate abrupt shifts due to alternative stable states. Red, blue and black vertical lines indicate light conditions for control, medium-light and low-light ponds, respectively, when the light intensity of control ponds is assumed to be 600 (µmol m–2 s–1). The experimental results of (b) chlorophyll a and (d) macrophyte biomass along per cent surface light transmission.
The results based on nitrogen (electronic supplementary material, figure S6) showed qualitatively the similar bifurcation pattern to that of phosphorus (figure 3), though our data show TN : TP in all the ponds ranging between 49 : 1 and 73 : 1 (electronic supplementary material, figure S4), well above the Redfield ratio and indicating general phosphorus limiting conditions [31]. In our simulations, nutrients in the pelagic habitat (R1 + cAA1) are also dynamic response variables that depend on many drivers, and we can examine the relationship between phytoplankton and nutrients in our framework. As long as the incoming light intensity is not very small, there is a positive correlation between phytoplankton and nutrients (electronic supplementary material, figure S7), and hence the observed positive correlations (electronic supplementary material, figure S4a and S4b) are consistent with our simulations.
(c). Field survey
To explore whether the bistability is a more general pattern beyond our experimental ponds, we looked for it in 35 ponds at the CUEPF in 2016, by measuring in each pond both chlorophyll a and the fraction of pond area covered by macrophytes as a proxy for macrophyte abundance (electronic supplementary material, figure S2). The bimodal frequency distribution of states in real ecosystems has been used to demonstrate the existence of alternative stable states [13]. We found, as predicted, a bimodal distribution in macrophyte coverage (figure 2d, p = 0.0030 with Hartigans' dip test). Interestingly, we did not find a concomitant bimodal pattern in chlorophyll a (figure 2d, p = 0.85). The absence of the clear bimodal pattern in chlorophyll a in 2016 might be because temporal variance of chlorophyll a was so high (figure 2a) that a single sampling was not representative of average chlorophyll a over a long period.
4. Discussion
(a). A shady phytoplankton paradox
Although our experimental observations seem at first to be paradoxical (figure 2), our mechanistic model, parametrized for phytoplankton and submersed macrophytes (electronic supplementary material, table S2), successfully replicates the pattern observed, in particular, the increase of phytoplankton abundance under low light, the negative correlation between phytoplankton and macrophytes, and the possibility of alternative stable states (figure 3) [14]. The concordance between our observations and model supports our conclusion that competition for light and nutrients is a crucial driver inducing complex community dynamics. Previous theoretical studies have predicted a similar pattern [11,14], but this prediction has not been generally recognized, or explored experimentally, because most empirical studies have focused primarily on the effects of nutrients on lake ecosystems [1] and experimental studies manipulating light conditions often have not typically considered the response of benthic producers (e.g. [20,32]). In shallow lakes, however, submersed macrophytes are common primary producers and can affect ecosystem dynamics [33]. Together with previous studies [12,34], our results suggest that submersed macrophytes play a pivotal role in phytoplankton dynamics in shallow lentic freshwater systems such as the ones we studied.
In another recent study using field mesocosms to examine competition between pelagic and benthic algae, Vasconcelos et al. [5] demonstrated that light attenuation in high DOM treatments decreased benthic algae and increased pelagic algae. Because DOM can affect phytoplankton not only through decreased light availability but also via addition of organic matter [15–17] that stimulates microbial activity [18], our result helps to separate the mechanisms by ensuring that only light availability affects competition in this light-stratified community. Because we reduced light supply (Iin in our model), whereas previous studies with DOM increased the background light attenuation (Kbg), direct comparison is difficult. However, future studies should try teasing apart the parallel shading and fertilizing effects of DOM addition on community dynamics.
Shading has often been proposed as a management tool to regulate both blooms of nuisance and harmful algae in reservoirs [35] and macrophytes in ponds [36]. However, our study indicates that partial light-shading as a management tool to reduce harmful algae might instead result in the opposite outcome, especially in shallow lakes where submersed macrophytes are initially abundant. Recently, installations of floating solar photovoltaic systems at the surface of lakes have been touted an emerging ‘attractive option' to ‘conserve the valuable land and water' [9], but this interception of light reaching the lake surface risks increasing phytoplankton abundance and thus degrading water quality in ponds and reservoirs. Although we have focused here on primary producers, it will also be important to consider the broader food webs in the ponds, including zooplankton and zooplanktivorous fish, in order to obtain more general conclusions.
We focused on submersed macrophytes (Chara, a benthic macroalga) in this study, because we found that Chara was dominant in benthic samples. Previous studies that manipulated light inputs by adding DOM have mainly focused on the effects on benthic microalgae (periphyton) [5,14]. However, considering competition with pelagic phytoplankton, macrophytes (whether rooted plants or macroalgae) and periphyton should play a similar role as they have limited access to light and they can easily obtain nutrients from sediments or near-sediment sources. Indeed, a model for competition between pelagic and benthic algae [14] showed very similar patterns to our model for competition between pelagic algae and submersed macrophytes. Thus, we expect to see the increase of phytoplankton under low light even with periphyton. Periphyton can be key primary producers in lakes [37,38], they may contribute to the establishment of alternative equilibria [37,39], and periphyton production can be the primary regulator of nutrient loading from the sediments [40]. Therefore, future studies should further explore the interaction between pelagic phytoplankton, benthic periphyton and submersed macrophytes under changing inputs of light and nutrients.
(b). Other possible explanations
Though our experimental manipulation, field observations and theoretical modelling suggest that competition between phytoplankton and submersed macrophytes for light and nutrients can explain the patterns we observed, alternative explanations may be possible. Here we discuss the following three possibilities.
First, the observed increase of chlorophyll a concentration under low light might have been due to compensation for photosynthesis (e.g. [2]). To explore this possibility, we calculated total phytoplankton biovolume from cell abundance and the biovolumes of each taxon. We found that chlorophyll a is proportional to total biovolume of phytoplankton across all the experimental ponds (electronic supplementary material, figure S5a) and the ratio of the two indices did not show consistent changes through time (electronic supplementary material, figure S5b). Furthermore, using phytoplankton biovolume and seston carbon instead of chlorophyll a produces qualitatively the same patterns as figures 2a–c and 3b (electronic supplementary material, figures S8 and S9). In addition, phytoplankton community composition did not show a consistent pattern in response to the light manipulation (electronic supplementary material, figures S10 and S11), suggesting that species replacement did not occur due to compensation or photoinhibition. Therefore, chlorophyll a concentration is a good proxy for the phytoplankton biomass in this study.
Second, fish predation is unlikely to cause the observed divergence between ponds as we introduced piscivorous largemouth bass to each pond in July. Although there were zooplanktivorous fathead minnow and banded killifish (Fundulus diaphanus) in ponds 217 (control) and 218 (medium-light), these ponds had relatively low chlorophyll a, which is opposite to the pattern expected from a trophic cascade [41]. In addition, the chlorophyll a concentration, phytoplankton biovolume and seston carbon were not related to either zooplankton biomass or body length (electronic supplementary material, figures S2c, S2d and S12). These results indicate that the influence of predation (top-down effects) was very small in our experiment, although the trophic cascade was strong in previous studies at the CUEPF in which high fish stoking densities were used [23,25].
Finally, because our experimental ponds have had long and pond-specific treatment histories dating from when they were constructed in 1964 (e.g. [24]), it was difficult to equalize the initial conditions among the ponds. Although we removed as many of the macrophytes as we could before establishing our experiments, we cannot exclude the possibility that differences in physical and biological conditions at the start might have differently affected effect sizes of the experimental manipulations. Indeed, we observed the divergent pattern in the control ponds (figure 2). While the bimodal distribution of macrophyte coverage in the neighbouring ponds (figure 2d) and simulations (figure 3) were consistent with the idea that alternative stable states are responsible for the divergence, the distribution of chlorophyll a in the neighbouring ponds (figure 2d) was not consistent, suggesting the potential role of initial conditions. Nevertheless, the observed patterns of phytoplankton and macrophytes were strikingly consistent between replicates in low- and medium-light ponds (figure 2), suggesting a stronger influence of shading than pond history on the patterns observed.
5. Conclusion
Although phytoplankton and submersed macrophytes are the major groups of primary producers in lakes, few studies have examined their competitive interactions under different light conditions. This study suggests strongly that phytoplankton abundance increased with decreasing light input as a result of competition for light and nutrients. In addition, it is plausible that this competitive interaction promoted distinct phytoplankton communities with either high or low abundances even under the same environmental conditions, indicating the possibility of alternative stable states. It is essential to take into account the effects of the balance between inputs of light and nutrients on biological interactions to predict how pelagic phytoplankton and benthic producers respond to perturbations in shallow lake ecosystems. Although our simulations are able to provide a plausible explanation for the pond community dynamics, nature is always more complex than models. In future studies, therefore, it will be interesting to address what kinds of basin shapes and lake sizes will exhibit these alternate stable-state dynamics due to the interaction between rooted submersed macrophytes, periphyton and phytoplankton.
Supplementary Material
Acknowledgements
We thank N. Hamm and R. L. Johnson for managing the experimental ponds, L. R. Schaffner and X. Yin for helping with field and laboratory work, B. E. Miner for his helpful advice, and T. Fukami, S. Diehl and two anonymous reviewers for helpful comments on the earlier version of the manuscript.
Ethics
This research was performed in accordance with the laws, guidelines and ethical standards of USA, where the research was performed.
Data accessibility
Data and R codes were deposited in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.bc60cd5) [42].
Authors' contributions
J.U., M.Y., I.K., H.D., T.Y. and N.G.H. designed research. All authors performed fieldwork, T.K. and K.T. quantified aquatic organisms and analysed water chemistry, and M.Y. analysed the theoretical model and wrote the first draft. All authors contributed to the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (KAKENHI) 15H02642 to J.U., M.Y., I.K., H.D. and T.Y., 16K18618 and 16H04846 to M.Y., 15K00596 to I.K., 25281052 and 26291088 to T.Y., and 25291094 and 16H02522 to J.U. M.Y. was supported by Hakubi Center for Advanced Research and John Mung Program of Kyoto University.
References
- 1.Karlsson J, Byström P, Ask J, Ask P, Persson L, Jansson M. 2009. Light limitation of nutrient-poor lake ecosystems. Nature 460, 506–509. ( 10.1038/nature08179) [DOI] [PubMed] [Google Scholar]
- 2.Hill WR, Ryon MG, Schilling EM. 1995. Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76, 1297–1309. ( 10.2307/1940936) [DOI] [Google Scholar]
- 3.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 4.Collins SM, et al. 2016. Fish introductions and light modulate food web fluxes in tropical streams: a whole-ecosystem experimental approach. Ecology 97, 3154–3166. ( 10.1002/ecy.1530) [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos FR, Diehl S, Rodríguez P, Hedström P, Karlsson J, Byström P. 2016. Asymmetrical competition between aquatic primary producers in a warmer and browner world. Ecology 97, 2580–2592. ( 10.1002/ecy.1487) [DOI] [PubMed] [Google Scholar]
- 6.Sweeney BW, Bott TL, Jackson JK, Kaplan LA, Newbold JD, Standley LJ, Hession WC, Horwitz RJ. 2004. Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc. Natl Acad. Sci. USA 101, 14 132–14 137. ( 10.1073/pnas.0405895101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IPCC. 2013. Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change, p. 1535 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Fritsen CH, Priscu JC. 1999. Seasonal change in the optical properties of the permanent ice cover on Lake Bonney, Antarctica: consequences for lake productivity and phytoplankton dynamics. Limnol. Oceanogr. 44, 447–454. ( 10.4319/lo.1999.44.2.0447) [DOI] [Google Scholar]
- 9.Sahu A, Yadav N, Sudhakar K. 2016. Floating photovoltaic power plant: a review. Renewable Sustainable Energy Rev. 66, 815–824. ( 10.1016/j.rser.2016.08.051) [DOI] [Google Scholar]
- 10.Solomon CT, et al. 2015. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems 18, 376–389. ( 10.1007/s10021-015-9848-y) [DOI] [Google Scholar]
- 11.van Gerven LPA, de Klein JJM, Gerla DJ, Kooi BW, Kuiper JJ, Mooij WM. 2015. Competition for light and nutrients in layered communities of aquatic plants. Am. Nat. 186, 72–83. ( 10.1086/681620) [DOI] [PubMed] [Google Scholar]
- 12.Seto M, Takamura N, Iwasa Y. 2013. Individual and combined suppressive effects of submerged and floating-leaved macrophytes on algal blooms. J. Theor. Biol. 319, 122–133. ( 10.1016/j.jtbi.2012.11.016) [DOI] [PubMed] [Google Scholar]
- 13.Scheffer M, Szabó S, Gragnani A, van Nes EH, Rinaldi S, Kautsky N, Norberg J, Roijackers RMM, Franken RJM. 2003. Floating plant dominance as a stable state. Proc. Natl Acad. Sci. USA 100, 4040–4045. ( 10.1073/pnas.0737918100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jäger CG, Diehl S. 2014. Resource competition across habitat boundaries: asymmetric interactions between benthic and pelagic producers. Ecol. Monogr. 84, 287–302. ( 10.1890/13-0613.1) [DOI] [Google Scholar]
- 15.Vasconcelos FR, Diehl S, Rodríguez P, Karlsson J, Byström P. In press Effects of terrestrial organic matter on aquatic primary production as mediated by pelagic–benthic resource fluxes. Ecosystems ( 10.1007/s10021-017-0217-x) [DOI] [Google Scholar]
- 16.Kissman CEH, Williamson CE, Rose KC, Saros JE. 2013. Response of phytoplankton in an alpine lake to inputs of dissolved organic matter through nutrient enrichment and trophic forcing. Limnol. Oceanogr. 58, 867–880. ( 10.4319/lo.2013.58.3.0867) [DOI] [Google Scholar]
- 17.Seekell DA, Lapierre JF, Ask J, Bergström AK, Deininger A, Rodríguez P, Karlsson J. 2015. The influence of dissolved organic carbon on primary production in northern lakes. Limnol. Oceanogr. 60, 1276–1285. ( 10.1002/lno.10096) [DOI] [Google Scholar]
- 18.Ducklow HW, Carlson CA. 1992. Oceanic bacterial production. In Advances in microbial ecology (ed. Marshall KC.), pp. 113–181. Boston, MA: Springer. [Google Scholar]
- 19.Staehr PA, Brighenti LS, Honti M, Christensen J, Rose KC. 2016. Global patterns of light saturation and photoinhibition of lake primary production. Inland Waters 6, 593–607. ( 10.1080/IW-6.4.888) [DOI] [Google Scholar]
- 20.Urabe J, Kyle M, Makino W, Yoshida T, Andersen T, Elser JJ. 2002. Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology 83, 619–627. ( 10.2307/3071868) [DOI] [Google Scholar]
- 21.Diehl S, Berger S, Wöhrl R. 2005. Flexible nutrient stoichiometry mediates environmental influences on phytoplankton and its resources. Ecology 86, 2931–2945. ( 10.1890/04-1512) [DOI] [Google Scholar]
- 22.Wetzel RG. 2001. Limnology: lake and river ecosystems, 3rd edn. San Diego, CA: Academic Press. [Google Scholar]
- 23.Hambright KD. 1994. Morphological constraints in the piscivore–planktivore interaction: implications for the trophic cascade hypothesis. Limnol. Oceanogr. 39, 897–912. ( 10.4319/lo.1994.39.4.0897) [DOI] [Google Scholar]
- 24.Hall DJ, Cooper WE, Werner EE. 1970. An experimental approach to the production dynamics and structure of freshwater animal communities. Limnol. Oceanogr. 15, 839–928. ( 10.4319/lo.1970.15.6.0839) [DOI] [Google Scholar]
- 25.Hambright KD, Hairston NG Jr, Schaffner WR, Howarth RW. 2007. Grazer control of nitrogen fixation: synergisms in the feeding ecology of two freshwater crustaceans. Fundam. Appl. Limnol. Arch. Hydrobiol. 170, 89–101. ( 10.1127/1863-9135/2007/0170-0089) [DOI] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 28.Huisman J, Weissing FJ. 1994. Light-limited growth and competition for light in well-mixed aquatic environments: an elementary model. Ecology 75, 507–520. ( 10.2307/1939554) [DOI] [Google Scholar]
- 29.Huisman J. 1999. Population dynamics of light-limited phytoplankton: microcosm experiments. Ecology 80, 202–210. ( 10.1890/0012-9658(1999)080%5B0202:PDOLLP%5D2.0.CO;2) [DOI] [Google Scholar]
- 30.Berger SA, Diehl S, Kunz TJ, Albrecht D, Oucible AM, Ritzer S. 2006. Light supply, plankton biomass, and seston stoichiometry in a gradient of lake mixing depths. Limnol. Oceanogr. 51, 1898–1905. ( 10.4319/lo.2006.51.4.1898) [DOI] [Google Scholar]
- 31.Hecky RE, Kilham P. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33, 796–822. ( 10.4319/lo.1988.33.4part2.0796) [DOI] [Google Scholar]
- 32.Dickman EM, Newell JM, González MJ, Vanni MJ. 2008. Light, nutrients, and food-chain length constrain planktonic energy transfer efficiency across multiple trophic levels. Proc. Natl Acad. Sci. USA 105, 18 408–18 412. ( 10.1073/pnas.0805566105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheffer M. 2004. Ecology of shallow lakes. Berlin, Germany: Springer. [Google Scholar]
- 34.Kéfi S, Holmgren M, Scheffer M. 2016. When can positive interactions cause alternative stable states in ecosystems? Funct. Ecol. 30, 88–97. ( 10.1111/1365-2435.12601) [DOI] [Google Scholar]
- 35.Chen X-C, Kong H-N, He S-B, Wu D-Y, Li C-J, Huang X-C. 2009. Reducing harmful algae in raw water by light-shading. Process Biochem. 44, 357–360. ( 10.1016/j.procbio.2008.11.002) [DOI] [Google Scholar]
- 36.Zhu B, Ellis MS, Fancher KL, Rudstam LG. 2014. Shading as a control method for invasive European frogbit (Hydrocharis morsus-ranae L.). PLoS ONE 9, e98488 ( 10.1371/journal.pone.0098488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genkai-Kato M, Vadeboncoeur Y, Liboriussen L, Jeppesen E. 2012. Benthic–planktonic coupling, regime shifts, and whole-lake primary production in shallow lakes. Ecology 93, 619–631. ( 10.1890/10-2126.1) [DOI] [PubMed] [Google Scholar]
- 38.Brothers SM, Hilt S, Meyer S, Köhler J. 2013. Plant community structure determines primary productivity in shallow, eutrophic lakes. Freshw. Biol. 58, 2264–2276. ( 10.1111/fwb.12207) [DOI] [Google Scholar]
- 39.Roberts E, Kroker J, Körner S, Nicklisch A. 2003. The role of periphyton during the re-colonization of a shallow lake with submerged macrophytes. Hydrobiologia 506, 525–530. ( 10.1023/B:HYDR.0000008560.73832.1c) [DOI] [Google Scholar]
- 40.Brothers S, Köhler J, Attermeyer K, Grossart HP, Mehner T, Meyer N, Scharnweber K, Hilt S. 2014. A feedback loop links brownification and anoxia in a temperate, shallow lake. Limnol. Oceanogr. 59, 1388–1398. ( 10.4319/lo.2014.59.4.1388) [DOI] [Google Scholar]
- 41.Carpenter SR, Kitchell JF. 1996. The trophic cascade in lakes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 42.Yamamichi M, Kazama T, Tokita K, Katano I, Doi H, Yoshida T, Hairston NG Jr., Urabe J. 2018. Data from: A shady phytoplankton paradox: when phytoplankton increases under low light. Dryad Digital Repository ( 10.5061/dryad.bc60cd5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Yamamichi M, Kazama T, Tokita K, Katano I, Doi H, Yoshida T, Hairston NG Jr., Urabe J. 2018. Data from: A shady phytoplankton paradox: when phytoplankton increases under low light. Dryad Digital Repository ( 10.5061/dryad.bc60cd5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and R codes were deposited in the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.bc60cd5) [42].