Key Points
Question
Are there additional genetic factors that contribute to the cardiomyopathic expression of MYBPC3Δ25bp variant, which is found in nearly 100 million individuals worldwide?
Findings
In this genotype-phenotype study, MYBPC3Δ25bp was found in 6% of 2401 South Asian individuals living in the United States, and genetic and phenotypic characterization of a subset identified distinct populations within this heterogeneous group. Specifically, 9.6% of MYBPC3Δ25bp carriers also had a novel MYBPC3 variant, D389V, on the same single allele, and this subset had hyperdynamic findings on echocardiogram.
Meaning
Additional genetic variants, specifically D389V, were associated with the variable cardiomyopathic findings linked to MYBPC3Δ25bp.
Abstract
Importance
The genetic variant MYBPC3Δ25bp occurs in 4% of South Asian descendants, with an estimated 100 million carriers worldwide. MYBPC3 Δ25bp has been linked to cardiomyopathy and heart failure. However, the high prevalence of MYBPC3Δ25bp suggests that other stressors act in concert with MYBPC3Δ25bp.
Objective
To determine whether there are additional genetic factors that contribute to the cardiomyopathic expression of MYBPC3Δ25bp.
Design, Setting, andParticipants
South Asian individuals living in the United States were screened for MYBPC3Δ25bp, and a subgroup was clinically evaluated using electrocardiograms and echocardiograms at Loyola University, Chicago, Illinois, between January 2015 and July 2016.
Main Outcomes and Measures
Next-generation sequencing of 174 cardiovascular disease genes was applied to identify additional modifying gene mutations and correlate genotype-phenotype parameters. Cardiomyocytes derived from human-induced pluripotent stem cells were established and examined to assess the role of MYBPC3Δ25bp.
Results
In this genotype-phenotype study, individuals of South Asian descent living in the United States from both sexes (36.23% female) with a mean population age of 48.92 years (range, 18-84 years) were recruited. Genetic screening of 2401 US South Asian individuals found an MYBPC3Δ25bpcarrier frequency of 6%. A higher frequency of missense TTN variation was found in MYBPC3Δ25bp carriers compared with noncarriers, identifying distinct genetic backgrounds within the MYBPC3Δ25bp carrier group. Strikingly, 9.6% of MYBPC3Δ25bp carriers also had a novel MYBPC3 variant, D389V. Family studies documented D389V was in tandem on the same allele as MYBPC3Δ25bp, and D389V was only seen in the presence of MYBPC3Δ25bp. In contrast to MYBPC3Δ25bp, MYBPC3Δ25bp/D389V was associated with hyperdynamic left ventricular performance (mean [SEM] left ventricular ejection fraction, 66.7 [0.7%]; left ventricular fractional shortening, 36.6 [0.6%]; P < .03) and stem cell–derived cardiomyocytes exhibited cellular hypertrophy with abnormal Ca2+ transients.
Conclusions and Relevance
MYBPC3Δ25bp/D389V is associated with hyperdynamic features, which are an early finding in hypertrophic cardiomyopathy and thought to reflect an unfavorable energetic state. These findings support that a subset of MYBPC3Δ25bp carriers, those with D389V, account for the increased risk attributed to MYBPC3Δ25bp.
This study determines whether there are additional genetic factors that contribute to the cardiomyopathic expression of MYBPC3Δ25bp.
Introduction
Hypertrophic cardiomyopathy (HCM) is a global genetic heart disease affecting approximately 600 000 people in the United States and 14.25 million people worldwide.1 Hypertrophic cardiomyopathy, characterized by excessive left ventricular thickening, features of diastolic dysfunction, left ventricular outflow obstruction, arrhythmia, myocardial ischemia, mitral regurgitation, and sudden death,2,3 is mainly caused by mutations in sarcomeric genes. Mutations in the thick filament protein-encoding genes MYH7 and MYBPC3 together constitute more than 80% of inherited cases.4,5 MYBPC3 encodes cardiac myosin binding protein-C (cMyBP-C), a vital protein for cardiac performance6 and regulator of cardiac contractility in response to adrenergic stimulation.7
A 25–base pair deletion within intron 32 of the MYBPC3 gene (MYBPC3Δ25bp) was previously described as a risk factor for cardiomyopathy8 and heart failure9 in South Asian individuals, with an odds ratio of 6.99 for cardiomyopathy in MYBPC3Δ25bp carriers.10 Although this genetic variant was discovered and examined in South Asian individuals living in Asia, it is estimated that 100 million people carry the MYBPC3Δ25bp variant.9 Genetically, MYBPC3Δ25bp is characterized by incomplete penetrance and variable expressivity.8,9 MYBPC3Δ25bp is associated with a range of outcomes from asymptomatic normal hearts to diastolic dysfunction; hypertrophic, dilated, and restrictive cardiomyopathies with tachyarrhythmias9; and left ventricular dysfunction in the setting of coronary artery disease.11,12 Cardiomyopathy features are increased when MYBPC3Δ25bp occurs in the presence of other sarcomere gene mutations,8,13,14 explaining some of the variable expression seen with MYBPC3Δ25bp. South Asian individuals have been the fastest-growing ethnic group in the world during the past decade, and defining genetic risk factors in this population has substantial clinical effect.15 The high prevalence of MYBPC3Δ25bp in South Asian individuals exceeds the incidence of cardiomyopathy and heart failure,8 indicating that MYBPC3Δ25bp on its own is not sufficient to cause cardiomyopathy and modifying factors add to its risk. Therefore, we undertook a systematic study of clinical and genetic screening to find MYBPC3Δ25bp carriers among South Asian individuals who migrated to the United States to better define the cardiovascular findings associated with MYBPC3Δ25bp.
Methods
Institutional Review Board Approval
This study was performed in accordance with the Declaration of Helsinki. All experimental protocols were approved by the institutional review board of the host institutes (Loyola University Chicago institutional review board [LU207815 and 207359], Chicago, Illinois, and SingHealth institutional review board [CIRB 2015/2521], Singapore).
Sample Collection and Genotyping
Participants were prospectively recruited and enrolled through community engagement at local venues. Saliva samples (3-5 mL) were collected in either sterile 15-mL tubes and frozen at −20°C or using the Saliva DNA Sample Collection Kit (Cat OG-500, DNA Genotek) and stored at ambient temperature. Blood samples for genotyping were collected in EDTA vacutainers (Catalog No. 367861; BD Bioscience), and DNA was isolated using the Blood DNA Isolation Kit (Catalog No. 51104; Qiagen). Polymerase chain reaction amplification of MYBPC3Δ25bp (rs36212066) used the forward primer 5′-GTT TCC AGC CTT GGG CATAGT C-3′ and reverse primer 5′-GAG GAC AAC GGA GCA AAG CCC-3′, using REDTaq PCR master mix (Sigma-Aldrich) and 2.5% agarose gel analysis. DNA samples for sequencing were isolated from 10 mL of peripheral blood, as described previously, using the Blood DNA Isolation Kit (Catalog No. 51104; Qiagen). Participants in the United States who were positive for the MYBPC3Δ25bp variant, their first-degree relatives, or random control individuals were contacted in a single-blinded manner to participate in the follow-up genotype-phenotype study at Loyola University Chicago (LU207377 and LU207813), for which they provided additional written informed consent. Participants were provided a questionnaire pertaining to ethnic background, health history, family history, comorbid conditions, and current medications. The questionnaire was completed on a voluntary basis, and questions were answered only to the extent that each participant was willing.
Measuring Cardiac Function in US South Asian Individuals
Twelve-lead electrocardiograms with rhythm strips were performed at rest in a supine position using a MAC 5500 HD (GE Healthcare). Tracings were interpreted blinded to genotype. Two-dimensional (2-D) echocardiography and Doppler examinaton were performed from standard transthoracic windows using Acuson Sequoia (Siemens Medical Solutions) and Vivid 7 (GE Healthcare). Echocardiograms were interpreted by a reader blinded to genotype. Left ventricular (LV) internal diastolic diameter, end-diastolic thickness of the posterior wall and anterior interventricular septum were measured using 2-D echo. Left ventricular mass was determined using the Devereux formula and indexed for body surface area. Relative wall thickness was obtained using the formula (2 times posterior wall thickness in diastole) divided by LV diastolic dimension. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were calculated using Biplane Simpson method. Left ventricular diastolic function was evaluated using tissue Doppler velocities of mitral annulus as well as transmitral pulsed wave Doppler velocities. Medial and lateral tissue Doppler (e′) velocities were measured by placing the sample volume at the base of the interventricular septum and anterolateral wall in apical 4-chamber view immediately below the mitral annulus. Transmitral Doppler tracings were obtained by placing the sample volume in the left ventricle at the tip of the mitral valve leaflets. Early (E-wave) and late (A-wave) diastolic transmitral velocities, E to A ratio, and deceleration time were measured from transmitral Doppler tracings. Apical 4-chamber and 2-chamber views were used to determine left atrial area and length.
DNA Sequence Analysis
The TruSight Cardio Kit (Catalog No. FC-141-1011; Illumina) was used to sequence 174 cardiovascular disease genes (eTable 1 in the Supplement) on an Illumina MiSeq with 150–base pair paired-end reads. Sequences were aligned using the Burrows-Wheeler Aligner, and the GATK haplotype caller was applied to generate variant call files as described.16 Variants were interpreted with SnpEff, and HIGH (stop gain/loss, splice site, frameshifts) and MODERATE (missense) effect variants were curated.17 Variants were ranked by minor allele frequency based on data from ExAC.18 Variant call files were filtered for variants in 46 genes specifically involved in cardiomyopathy, with an ExAC frequency of 0.01 or less (eTable 1 in the Supplement). The number of variants identified in each gene was normalized to cohort size, and MYBPC3Δ25bp carriers and noncarriers were compared. TTN variant comparisons were made with an unpaired t test. Fisher exact test was used to compare individual variants in carriers vs noncarriers. A 1-way analysis of variance was used to compare functional cardiac measures in noncarriers, MYBPC3Δ25bp, and MYBPC3Δ25bp/D389V participant, followed by Tukey multiple comparisons test. Statistical analyses were performed using Prism 7 (GraphPad).
Generation of Human-Induced Pluripotent Stem Cells and Cardiomyocyte Differentiation
Genotype-positive and genotype-negative participants were recruited to generate induced pluripotent stem cells (iPSCs). Peripheral blood mononuclear cells were isolated from whole blood (5 mL) using Ficoll-Paque (GE Healthcare Life Sciences) and cultured in peripheral blood mononuclear cell medium. Peripheral blood mononuclear cells were transduced using CytoTune-iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher Scientific), all performed according to the manufacturers’ instructions. Colonies that appeared underwent clonal expansion and were maintained in feeder-free conditions.19 Human iPSCs were differentiated into cardiomyocytes using an embryoid body–based protocol.20 Briefly, iPSCs were dissociated into single cells and aggregated into embryoid bodies with stage-specific media changes for the next 8 days. Embryoid bodies were plated on gelatin-coated dishes on day 8 and maintained in embryoid body–2 medium (Dulbecco modified eagle medium/F12 with 2% fetal bovine serum).
Imaging and Protein Analysis of Ca2+ Transients
Imaging of Ca2+ transients was performed on isolated single CMs generated from human iPSCs and seeded on matrix-coated slides using the Ca2+ probe fluo-4-acetoxymethyl ester (Fluo-4AM). Signals were captured using a high frame rate Cascade EMCCD camera (Photometrics) and analyzed using MetaMorph software, version 7 (Molecular Devices).
Statistical Analysis
Results were reported as mean (SE) of independent experiments. The comparison between 2 groups was performed using the t test, whereas to compare multiple groups, 1-way analysis of variance followed by Kruskal-Wallis analysis was used. A 2-sided P value less than .05 was considered statistically significant.
Results
Prevalence of MYBPC3Δ25bp US South Asian Individuals
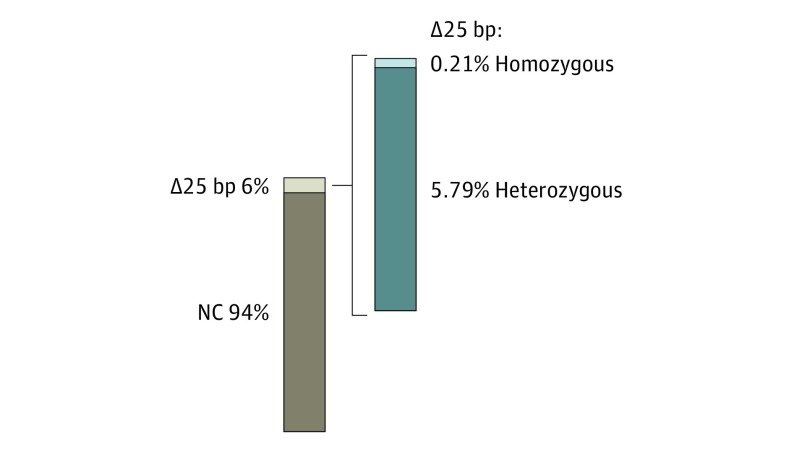
Most HCM-causing MYBPC3 mutations are dominantly inherited; however, MYBPC3Δ25bp has a more complex effect because its population prevalence significantly exceeds that of HCM.8,9 We randomly screened for the presence of MYBPC3Δ25bp among US South Asian individuals. During a 2-year period, we evaluated 2401 US South Asian participants from several cities in the United States. Using a polymerase chain reaction–based assay (eFigure 1A in the Supplement), the population frequency of the MYBPC3Δ25bp variant in US South Asian individuals was 6%, including both heterozygous (5.79%) and homozygous (0.21%) carriers (139 of 2401 and 5 of 2401, respectively) (Figure 1), as measured from a diverse age group (eFigure 1B in the Supplement). These values are higher than the previously reported prevalence of approximately 4.0% in South Asian individuals.9 The variant allele frequency was 3.14% with a Fisher exact test P value of .03, indicating that the variant’s distribution follows Hardy-Weinberg equilibrium.
Figure 1. Prevalence and Incomplete Penetrance of MYBPC3Δ25bp in South Asian Individuals From the United States .
Genotype distribution of 2401 US South Asian participants. Among carriers of the MYBPC3Δ25bp variant, 5.79% (139, dark color sector) were heterozygous, and 0.21% (5, light color sector) were homozygous. bp Indicates base pair; NC, noncarrier.
Clinical Findings in US South Asian MYBPC3Δ25bp Carriers
To further characterize the association between the MYBPC3Δ25bp variant and the presence of cardiomyopathy in US South Asian individuals, cardiac phenotype was assessed using 12-lead electrocardiogram and echocardiogram in carriers (n = 47, 43 heterozygous and 4 homozygous) and noncarriers (n = 35) at a single time. Mean (SE) age at the time of study in noncarriers and carriers was 45.1 (1.71) years and 47.6 (1.73) years (P = .31), respectively (Table). Blood pressure was not significantly different between noncarriers and carriers (Table), and both noncarriers and carriers showed normal correlation between mean arterial pressure and body surface area (eFigure 1C in the Supplement). Comparing echocardiographic parameters indicative of HCM, such as relative wall thickness, intraventricular septal thickness, LV posterior wall thickness, and mitral inflow pattern (E/e′ ratio) (eFigure 1D-G in the Supplement) revealed no significant differences between the groups. The percent LVFS (eFigure 1H in the Supplement), but not LVEF (eFigure 1I in the Supplement), showed a small but significant increase between MYBPC3Δ25bp carriers and noncarriers. Collectively, these data demonstrate that the MYBPC3Δ25bp variant alone does not elicit an overt HCM phenotype.
Table. Sample Cohort, Clinical Risk Factors, ECG, and Echocardiographic Data.
| Variable | Noncarriers, Mean (SEM) | No. | Carriers, Mean (SEM) | No. | P Valuea |
|---|---|---|---|---|---|
| Male, % | 57.14 | 20 | 48.94 | 23 | 0.51 |
| Female, % | 42.86 | 15 | 51.06 | 24 | |
| Age, y | 45.1 (1.71) | 35 | 47.6 (1.73) | 47 | .31 |
| Blood pressure, mm Hg | |||||
| Diastolic | 74.33 (1.78) | 33 | 74.24 (1.73) | 37 | .93 |
| Systolic | 122.1 (2.29) | 33 | 127 (2.64) | 39 | .12 |
| Body surface index, % | 1.81 (0.03) | 35 | 1.81 (0.03) | 42 | .97 |
| ECG characteristicsb | |||||
| QTc | 407.9 (3.38) | 35 | 416.4 (3.96) | 38 | .10 |
| Echocardiographic characteristicsb | |||||
| LVEF, % | 60.52 (1.19) | 35 | 62.78 (1.08) | 41 | .28 |
| LVFS, % | 32.27 (0.81) | 36 | 34.92 (0.81) | 41 | .04 |
| LV mass index | 63.32 (2.55) | 36 | 66.74 (2.37) | 42 | .32 |
| IVS, cm | 0.91 (0.04) | 36 | 0.89 (0.03) | 42 | .84 |
| LVPW, cm | 0.82 (0.02) | 36 | 0.86 (0.03) | 42 | .11 |
| RWT, cm | 0.38 (0.01) | 36 | 0.41 (0.01) | 42 | .21 |
| LA Volume 4C mod, mL | 38.22 (1.80) | 36 | 36.52 (1.56) | 37 | .48 |
| LA Volume index | 24.9 (0.88) | 32 | 24.05 (1.05) | 40 | .53 |
| LVOT Gradient, mm Hg | 4.24 (0.24) | 36 | 4.33 (0.21) | 40 | .84 |
| E/A Ratio | 1.44 (0.07) | 36 | 1.32 (0.07) | 42 | .16 |
| E/e' Ratio | 8.7 (0.43) | 36 | 6.78 (0.34) | 37 | .49 |
| Clinical presentation | |||||
| Family history, % | 22.22 | 8 | 26.19 | 11 | NA |
| Hypertension, % | 13.89 | 5 | 26.19 | 11 | NA |
| Diabetes | 2.78 | 1 | 11.9 | 5 | NA |
| Hyperlipidimic | 13.89 | 5 | 16.8 | 7 | NA |
Abbreviations: ECG, electrocardiogram; E/A ratio, ratio of early transmitral flow to late flow owing to atrial contraction; E/e′ Ratio, ratio of early transmitral flow to left ventricular early diastolic velocity; IVS, interventricular septal thickness; LA, left atrium; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVOT, left ventricular outflow tract; LVPW, left ventricular posterior wall thickness; NA, not applicable; QTc, corrected QT interval; RWT, relative wall thickness; 4C, four-chamber view.
Statistical significance was calculated using unpaired t test or χ2 test for categorical variables.
Electrocardiogram and echocardiography measurements were taken under normal and conscious conditions.
Genetically Distinct Subgroups Among MYBPC3Δ25bp Carriers
Because population reports of MYBPC3Δ25bp carriers suggest incomplete penetrance and an increased frequency among bona fide HCM patients,9 we evaluated the possibility of other cardiomyopathy variants existing in MYBPC3Δ25bp carriers using targeted sequencing on 72 participants. A panel of 174 cardiovascular disease–associated genes was sequenced and evaluated for protein-altering variants with moderate and high effect, as interpreted by snpEff.17 Forty-six genes from this panel are specifically linked to cardiomyopathy. We analyzed protein-altering variants including nonsense and nonsynonymous changes in only these 46 cardiomyopathy genes (eTable 1 in the Supplement). Protein-altering variant numbers were normalized to cohort size; the MYBPC3Δ25bp carrier group had a 1.4-fold excess of rare variants compared with the noncarrier group; this difference was not significant in the context of the small sample size. The genes with variants are depicted in eFigure 2A in the Supplement, with rare being defined as a global ExAC minor allele frequency of 0.01 or less. MYBPC3Δ25bp carriers had 4.8 rare protein-altering variants per individual, while noncarriers had 3.4, consistent with a distinct genetic background between MYBPC3Δ25bp carriers and noncarriers (eTables 2-3 in the Supplement).
We separately considered protein-altering variants in TTN, which encodes the giant protein titin. TTN truncating mutations are linked to heart failure and cardiomyopathy.21,22 TTN is also uniquely enriched with an extraordinarily high degree of missense variants of unclear functional impact. We reasoned that the signature of TTN variation could be used to identify subgroups within the cohort of MYBPC3Δ25bp carriers. MYBPC3Δ25bp carriers had an excess of rare TTN variants (3.2 counts), compared with noncarriers (2.4 counts) per participant (eFigure 2B in the Supplement). Using the South Asian (SAS) population frequencies from ExAC,18 population frequency differed for TTN variants in MYBPC3Δ25bp carriers compared with the noncarrier group. The mean (SE) ExAC_SAS frequency of TTN variants in MYBPC3Δ25bp carriers was 0.010 (0.0009) compared with 0.005 (0.001) in noncarriers (P < .001) (eFigure 2C in the Supplement), suggesting a distinct genetic landscape between MYBPC3Δ25bp carriers and noncarriers. When using the Non-Finnish European ExAC population frequencies, we found no difference between carriers and noncarriers for TTN variants. In addition, when evaluating other cardiomyopathy genes, we saw no significant difference between the carriers and noncarriers using ExAC_SAS frequencies. Thus, the distinct TTN landscape in MYBPC3Δ25bp carriers is both population- and gene-specific (eFigure 2 in the Supplement). Because TTN truncating variants are highly enriched in dilated cardiomyopathy,23 we separately evaluated TTN truncating variants. Two TTN truncating variants were identified in the cohort: a frameshift variant in a MYBPC3Δ25bp carrier and an early termination variant in a noncarrier, both of which are part of exon 48 of the Novex-3 transcript and unlikely to be important for the pathogenesis of cardiac dysfunction, suggesting that TTN truncating variants do not account for differences between MYBPC3Δ25bp carriers and noncarriers. Overall, the TTN genetic signature in MYBPC3Δ25bp carriers marks population stratification within the analyzed US South Asian cohort.
A Novel Second Variant in MYBPC3 Identified in MYBPC3Δ25bp Carriers
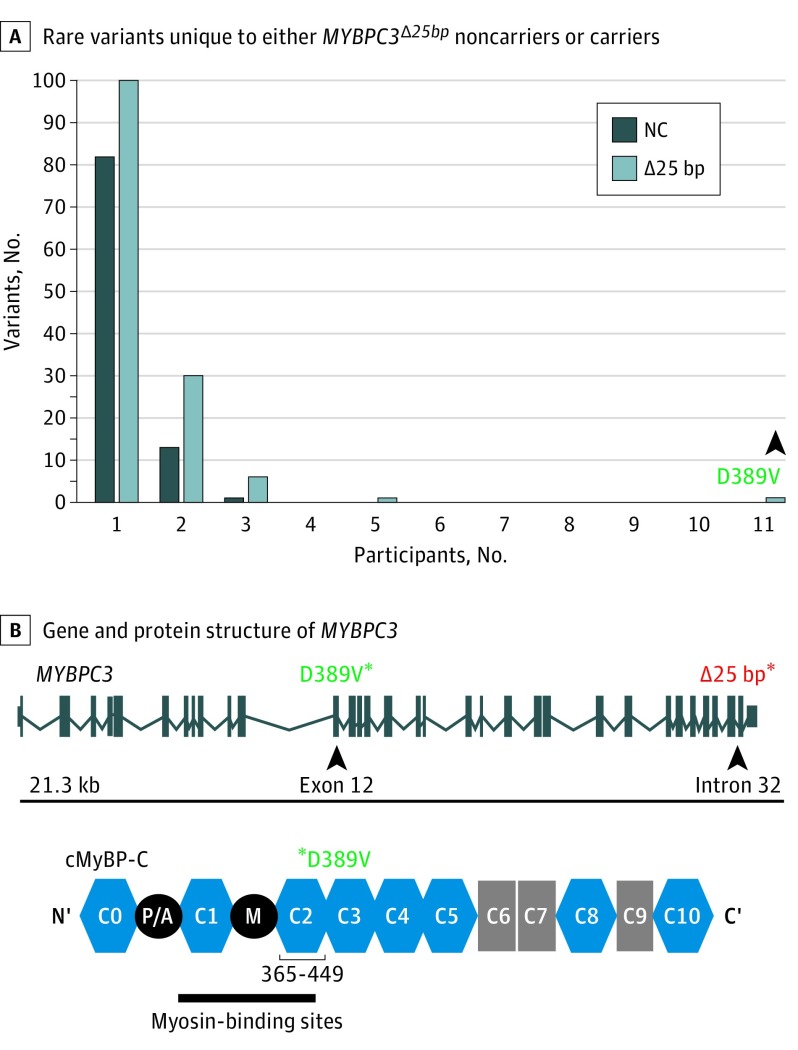
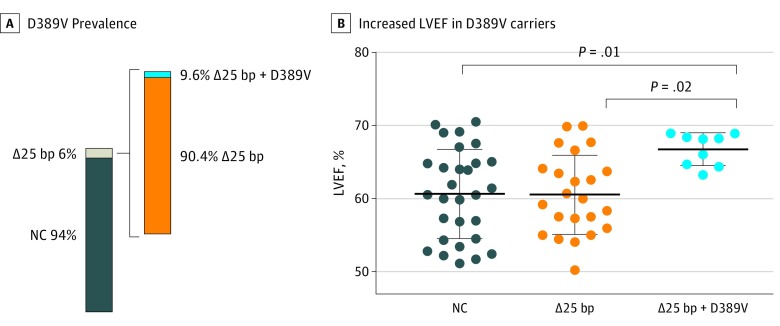
We found a novel MYBPC3 missense single-nucleotide polymorphism in a subset of MYBPC3Δ25bp carriers encoding an A to T nucleotide change at chr11:47365100, producing a missense aspartic acid (D) to valine (V) variant at amino acid 389 in cMyBP-C (D389V) (Figure 2A and eFigure 3A in the Supplement). D389V was the only variant found to be significantly enriched in MYBPC3Δ25bp carriers and was not identified in noncarriers (Fisher exact test 0.0019, eTable 4 in the Supplement). D389V localizes in MYBPC3 exon 12 at the interface between C2 domain of the cMyBP-C and the S2 domain of myosin (Figure 2B and eFigure 3B in the Supplement). This domain interface directly regulates the superrelaxed state of cardiac contraction.24 The glutamic acid in position 389 of the protein has been conserved among various species (eFigure 3C in the Supplement). We next screened specifically for the D389V variant and determined its presence in 13 of 136 MYBPC3Δ25bp carriers (9.6%) and absence in 1488 US South Asian individuals who do not carry the MYBPC3∆25 allele (Figure 3A). D389V variant was carried in tandem on the same allele as MYBPC3Δ25bp, as confirmed by family studies (eFigure 4A in the Supplement). D389V variant was never seen in the absence of MYBPC3Δ25bp. Thus, it is likely that MYBPC3D389V arose on the background of the MYBPC3Δ25bp allele. We reevaluated the echocardiographic findings, excluding those with confounding genetic variants or confounding comorbidities (eTable 5 in the Supplement). Left ventricular ejection fraction and LVFS were significantly increased in those carrying D389V plus MYBPC3Δ25bp (mean [SD] LVEF, 66.7% [0.7%]; LVFS, 36.6% [0.6%]; P = .03; n = 9) compared with MYBPC3Δ25bp carriers (mean [SD] LVEF, 61.7 [1.2], LVFS, 34.3 [1.2]) and noncarriers (mean [SD] LVEF, 60.6 [1.1]; LVFS, 32.3 [0.8]) (Figure 3B; eFigure 4B in the Supplement), consistent with a hyperdynamic state and similar to what has been described in early HCM.25
Figure 2. Representation of D389V Rare Variant in MYBPC3Δ25bp Carriers.
A, Sequencing was used to identify rare protein-altering variation in 46 cardiomyopathy genes. An excess of unique singleton rare variants occurred in both MYBPC3Δ25bp carriers (light bar) and noncarriers (dark bar). D389V (arrowhead) was the only variant present in more than 5 carriers and 0 noncarriers (NCs). B, The MYBPC3 gene and cMyBP-C protein structure with the positions of D389V (green asterisks) and MYBPC3Δ25bp (red asterisk) indicated. Within the MYBPC3 gene, these 2 variants are separated by 12 kilobases (kb) (top). D389V falls in the C2 domain of cMyBP-C in a region implicated in binding myosin heavy chain (bottom).
Figure 3. Association of MYBPC3Δ25bp and D389V in Tandem on the Same Allele With a Hyperdynamic State.
A, Prevalence of the D389V genotype as a subset of the MYBPC3Δ25bp carriers among all samples. B, Left ventricular ejection fraction was significantly elevated (66.7 [0.7]) in participants who carry MYBPC3Δ25bp/D389V compared with noncarriers and those with MYBPC3Δ25bp alone (1-way analysis of variance and Tukey post hoc test). bp Indicates base pair; NC, noncarrier.
Human-Induced Pluripotent Stem Cell–Derived Cardiomyocytes Carrying D389V Recapitulated HCM-Like Phenotypes
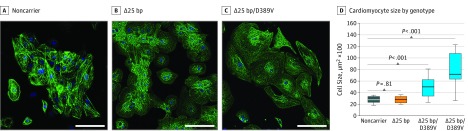
To better understand the cellular phenotype linked to MYBPC3Δ25bp/D389V, we examined human cardiomyocytes derived from iPSCs. Strikingly, cardiomyocytes derived from 2 individuals with MYBPC3Δ25bp/D389V were significantly more hypertrophic compared with control cardiomyocytes and cardiomyocytes with MYBPC3Δ25bp alone (Figure 4A and B), consistent with a hypertrophic cellular phenotype. An increased frequency of arrhythmic cells as well as ectopic Ca2+ transients were observed, in cardiomyocytes with MYBPC3Δ25bp/D389V compared with MYBPC3Δ25bp alone or control cells (eFigure 4C and D in the Supplement). Collectively, these data show that the single MYBPC3Δ25bp/D389V variant allele is associated with a hyperdynamic, hypertrophic, and arrhythmogenic state, consistent with an HCM-like substrate.25
Figure 4. Cellular Hypertrophy of Cardiomyocytes With MYBPC3Δ25bp/D389V Variants.
Cardiomyocytes were differentiated from induced pluripotent stem cells (iPSCs) from (A) a noncarrier control individual, (B) a MYBPC3Δ25bp carrier (Δ25bp), and (C) a carrier of MYBPC3Δ25bp/D389V (Δ25bp/D389V). Cells stained for cardiac α-actinin (green) and nuclei (blue) were compared, and those with MYBPC3Δ25bp/D389V were larger than controls and MYBPC3Δ25bp alone. Scale bar: 100 μm. D, Graphic representation of cell size (n = 27 per cell line). One-way analysis of variance and Tukey post hoc test were used. bp Indicates base pair.
Discussion
The MYBPC3Δ25bp variant is carried by 5% to 6% of the large and diverse South Asian population.9,26 Because this exceeds the incidence of heart failure, other factors, both genetic and environmental, were hypothesized to contribute to risk associated with MYBPC3Δ25bp.9,11,12 Armed with deep sequencing of additional cardiomyopathy genes from MYBPC3Δ25bp carriers and noncarriers, we identified a unique signature of TTN missense variation between MYBPC3Δ25bp carriers and noncarriers, suggesting discrete cohorts within the MYBPC3Δ25bp carrier group. The pathophysiologic significance of the TTN variation, if any, is not known. Other cardiomyopathy genes did not display this same pattern of enrichment in MYBPC3Δ25bp carriers compared with noncarriers, with the exception of MYBPC3 itself.
Unexpectedly, we identified a novel variant in MYBPC3 that cosegregates with the MYBPC3Δ25bp variant. This new variant, D389V (chr11:47365100A>T), was seen in 13 of 136 (9.6%) of MYBPC3Δ25bp carriers, putting prevalence of the MYBPC3Δ25bp/D389V allele at 1 in 200 South Asian individuals. MYBPC3 variants have been described in combination with other genetic variants in the setting of dilated cardiomyopathy and heart failure, consistent with an oligogenic model of disease.27,28,29,30 However, distinct from these previous studies, D389V and MYBPC3Δ25bp are found together on the same single allele, and D389V was never observed in the absence of MYBPC3Δ25bp. D389V maps near the beginning of immunoglobulin-like domain C2 in cMyBP-C protein, a region that is known to directly interact with the neck region of β-myosin heavy chain (S2-region), effectively regulating the engagement and interaction of myosin heads with actin filaments.31 In silico tools predict D389V to be highly disruptive to protein function owing to the exchange of the highly conserved charged amino acid for a nonpolar moiety.
Limitations
This study specifically focused on 48 genes, mutations in which are known to cause cardiomyopathy. Systematic analysis of the additional sequenced genes could yield other cosegregating gene variations. The sample size in this study is relatively small, but proportional considering South Asian individuals represent about 1.0% of the United States population.
Conclusions
MYBPC3Δ25bp in the context of D389V may be an important marker of cardiac health. MYBPC3Δ25bp/D389V, especially when combined with additional cardiac stressors, could prove suitable substrate for cardiomyopathy development and heart failure. Specifically, those with single MYBPC3Δ25bp/D389V allele had increased LVEF. The hyperdynamic phase of HCM is recognized as an early feature of HCM, and despite an appearance of hyperperformance is one that is deceivingly energetically unfavorable.25,32,33 At a cellular level, iPSC-derived cardiomyocytes with MYBPC3Δ25bp/D389V had hypertrophy and increased frequency of abnormal Ca2+ transients. Ca2+ hypersensitivity and hyperdynamic ventricles are viewed as an early compensation or adaptation for the unfavorable energetic state and altered myocyte signaling.34 Importantly, this hyperdynamic state in HCM is viewed as a target for drug development and intervention.35
eFigure 1. Genotype-Phenotype Features of the MYBPC3Δ25bp Variant Carriers
eFigure 2. Evaluation of Rare Protein-Altering Variation in 46 Cardiomyopathy Genes in 72 US-SAs Who Were Carriers or Noncarriers of the MYBPC3Δ25bp Variant
eFigure 3. MYBPC3D389V is Conserved and Enriched in MYBPC3Δ25bp Gene Carriers.
eFigure 4. D389V is Carried on the Same Single Allele as MYBPC3Δ25bp and is Associated With Increased LVFS and Irregular Ca2+ Transients in iPSC-derived Cardiomyocytes
eTable 1. Illumina TruSight Cardio Panel of 174 Cardiovascular Disease Genes
eTable 2. Rare Variants in the 46-gene Cardiomyopathy Panel Found in Southeast Asian Cohort, Including TTN Variants
eTable 3. Rare Variants in the 46-gene Cardiomyopathy Panel Found in Southeast Asian Cohort, Excluding TTN Variants
eTable 4. Fisher’s Exact Test of MYBPC3Δ25bp Carriers vs Noncarriers
eTable 5. Subjects Excluded From Analysis and Rationale
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: an important global disease. Am J Med. 2004;116(1):63-65. [DOI] [PubMed] [Google Scholar]
- 2.Gersh BJ, Maron BJ, Bonow RO, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142(6):e153-e203. [DOI] [PubMed] [Google Scholar]
- 3.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287(10):1308-1320. [DOI] [PubMed] [Google Scholar]
- 4.Richard P, Charron P, Carrier L, et al. ; EUROGENE Heart Failure Project . Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227-2232. [DOI] [PubMed] [Google Scholar]
- 5.Kensler RW, Shaffer JF, Harris SP. Binding of the N-terminal fragment C0-C2 of cardiac MyBP-C to cardiac F-actin. J Struct Biol. 2011;174(1):44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruen M, Gautel M. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286(3):933-949. [DOI] [PubMed] [Google Scholar]
- 7.Sadayappan S, Osinska H, Klevitsky R, et al. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci U S A. 2006;103(45):16918-16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waldmüller S, Sakthivel S, Saadi AV, et al. Novel deletions in MYH7 and MYBPC3 identified in Indian families with familial hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2003;35(6):623-636. [DOI] [PubMed] [Google Scholar]
- 9.Dhandapany PS, Sadayappan S, Xue Y, et al. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41(2):187-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuster DW, Sadayappan S. MYBPC3's alternate ending: consequences and therapeutic implications of a highly prevalent 25 bp deletion mutation. Pflugers Arch. 2014;466(2):207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Garg N, Mittal T, et al. Association of 25 bp deletion in MYBPC3 gene with left ventricle dysfunction in coronary artery disease patients. PLoS One. 2011;6(9):e24123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Mishra A, Srivastava A, et al. Role of common sarcomeric gene polymorphisms in genetic susceptibility to left ventricular dysfunction. J Genet. 2016;95(2):263-272. [DOI] [PubMed] [Google Scholar]
- 13.Bashyam MD, Purushotham G, Chaudhary AK, et al. A low prevalence of MYH7/MYBPC3 mutations among familial hypertrophic cardiomyopathy patients in India. Mol Cell Biochem. 2012;360(1-2):373-382. [DOI] [PubMed] [Google Scholar]
- 14.Tanjore RR, Rangaraju A, Kerkar PG, Calambur N, Nallari P. MYBPC3 gene variations in hypertrophic cardiomyopathy patients in India. Can J Cardiol. 2008;24(2):127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraker J, Viswanathan SK, Knöll R, Sadayappan S. Recent advances in the molecular genetics of familial hypertrophic cardiomyopathy in South Asian descendants. Front Physiol. 2016;7:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puckelwartz MJ, Pesce LL, Nelakuditi V, et al. Supercomputing for the parallelization of whole genome analysis. Bioinformatics. 2014;30(11):1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cingolani P, Platts A, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lek M, Karczewski KJ, Minikel EV, et al. ; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta A, Chung Y, Sequiera GL, Wong P, Liew R, Shim W. Pharmacoelectrophysiology of viral-free induced pluripotent stem cell-derived human cardiomyocytes. Toxicol Sci. 2013;131(2):458-469. [DOI] [PubMed] [Google Scholar]
- 20.Mehta A, Ramachandra CJ, Sequiera GL, et al. Phasic modulation of Wnt signaling enhances cardiac differentiation in human pluripotent stem cells by recapitulating developmental ontogeny. Biochim Biophys Acta. 2014;1843(11):2394-2402. [DOI] [PubMed] [Google Scholar]
- 21.Roberts AM, Ware JS, Herman DS, et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci Transl Med. 2015;7(270):270ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM. Population-based variation in cardiomyopathy genes. Circ Cardiovasc Genet. 2012;5(4):391-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herman DS, Lam L, Taylor MR, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara JW, Li A, Lal S, et al. MYBPC3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS One. 2017;12(6):e0180064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho CY, Day SM, Colan SD, et al. ; HCMNet Investigators . The burden of early phenotypes and the influence of wall thickness in hypertrophic cardiomyopathy mutation carriers: findings from the HCMNet Study. JAMA Cardiol. 2017;2(4):419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonson TS, Zhang Y, Huff CD, et al. Limited distribution of a cardiomyopathy-associated variant in India. Ann Hum Genet. 2010;74(2):184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zahka K, Kalidas K, Simpson MA, et al. Homozygous mutation of MYBPC3 associated with severe infantile hypertrophic cardiomyopathy at high frequency among the Amish. Heart. 2008;94(10):1326-1330. [DOI] [PubMed] [Google Scholar]
- 28.Dellefave LM, Pytel P, Mewborn S, et al. Sarcomere mutations in cardiomyopathy with left ventricular hypertrabeculation. Circ Cardiovasc Genet. 2009;2(5):442-449. [DOI] [PubMed] [Google Scholar]
- 29.Finsterer J, Zarrouk-Mahjoub S. Left ventricular noncompaction associated with a compound heterozygous MYBPC3 mutation. Eur J Med Genet. 2014;57(7):349. [DOI] [PubMed] [Google Scholar]
- 30.Wessels MW, Herkert JC, Frohn-Mulder IM, et al. Compound heterozygous or homozygous truncating MYBPC3 mutations cause lethal cardiomyopathy with features of noncompaction and septal defects. Eur J Hum Genet. 2015;23(7):922-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem. 2011;286(14):12650-12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rüssel IK, Brouwer WP, Germans T, et al. Increased left ventricular torsion in hypertrophic cardiomyopathy mutation carriers with normal wall thickness. J Cardiovasc Magn Reson. 2011;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmer SA, Germans T, Brouwer WP, et al. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur J Heart Fail. 2011;13(12):1283-1289. [DOI] [PubMed] [Google Scholar]
- 34.Knöll R. Myosin binding protein C: implications for signal-transduction. J Muscle Res Cell Motil. 2012;33(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green EM, Wakimoto H, Anderson RL, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Genotype-Phenotype Features of the MYBPC3Δ25bp Variant Carriers
eFigure 2. Evaluation of Rare Protein-Altering Variation in 46 Cardiomyopathy Genes in 72 US-SAs Who Were Carriers or Noncarriers of the MYBPC3Δ25bp Variant
eFigure 3. MYBPC3D389V is Conserved and Enriched in MYBPC3Δ25bp Gene Carriers.
eFigure 4. D389V is Carried on the Same Single Allele as MYBPC3Δ25bp and is Associated With Increased LVFS and Irregular Ca2+ Transients in iPSC-derived Cardiomyocytes
eTable 1. Illumina TruSight Cardio Panel of 174 Cardiovascular Disease Genes
eTable 2. Rare Variants in the 46-gene Cardiomyopathy Panel Found in Southeast Asian Cohort, Including TTN Variants
eTable 3. Rare Variants in the 46-gene Cardiomyopathy Panel Found in Southeast Asian Cohort, Excluding TTN Variants
eTable 4. Fisher’s Exact Test of MYBPC3Δ25bp Carriers vs Noncarriers
eTable 5. Subjects Excluded From Analysis and Rationale