Abstract
The maturation of sleep regulatory systems during adolescence in combination with psychosocial and societal pressures culminate in a “Perfect Storm” of short and ill-timed sleep and the associated consequences for many youngsters. This model, first described by Carskadon in 2011, guides our current thinking of adolescent sleep behavior. Since the original description, the field has moved forward with remarkable pace, and this review aims to summarize recent progress and describe how this new work informs our understanding of sleep regulation and sleep behavior during this developmental time frame.
Keywords: sleep homeostasis, slow wave activity, circadian timing, light, school start time
A 2011 review paper (Carskadon, 2011) proposed that maturation of bioregulatory mechanisms in concert with psychosocial factors resulted in a “perfect storm” of short, ill-timed, and inadequate sleep in many teens. Two biological systems undergo modifications during adolescence. First, is the sleep/wake homeostatic process, which not only marks sleep recovery and restoration, but also signifies the speed with which the “pressure” for sleep and resulting deficits build across the day. Findings indicated that for more mature adolescents the recovery process did not accelerate, whereas the sleep pressure accumulation process decelerated, from which Carskadon inferred an ease of staying awake longer. The second process in this biological regulatory equation is the circadian timing system, which dictates the timing of many physiological and behavioral rhythms, including alertness and sleep propensity. Findings reviewed by Carskadon indicated a delayed shift in the intrinsic rhythmic system as adolescents mature, hence pushing alertness and bedtimes later into the evening and night and rising later into the morning. Data from the behavioral and psychosocial literature were sparse, yet led to a conclusion that evening light from devices with screens may activate the phase-delaying component of the circadian timing mechanism. Opposing these factors that drive the temporal placement of sleep later were such early morning events as sports practices and an early starting time for school.
In brief, the field has moved forward with remarkable pace since the earlier review was published (Carskadon, 2011), and we aim to detail this progress following a brief overview.
With regard to the homeostatic sleep “drive” process, for example, longitudinal studies have confirmed that the dissipation of sleep pressure does not change across adolescent development (Campbell, et al., 2011; Tarokh, Carskadon, & Achermann, 2012). Recommended sleep length guidelines have been proposed by the National Sleep Foundation and the American Academy of Sleep Medicine (Hirshkowitz, et al., 2015; Paruthi, et al., 2016), in both cases based upon expert judgment of the literature. We describe below more recent experimental findings that may provide evidence-based guidance on sleep duration for adolescents, concluding that about 9 to 9.25 hours a night is required for cognitive function/attention (Short, Weber, Reynolds, Coussens, & Carskadon, 2018) and for emotional regulation (Fuligni, Bai, Krull, & Gonzales, 2017). Yet, several studies from around the globe continue to report average sleep durations less than these healthful amounts. For example, in a recent meta-analysis of studies in which sleep behavior was measured from actigraphy in children and adolescents ranging in age from 3 to 18 years, total sleep time decreased with age, with pooled mean estimates in 12 to 18 year olds of about 7 h on school nights (Galland, et al., 2018). The disparity between sleep duration measured in the laboratory (9.25 hours) and the home environment (7 hours) reinforce an interplay between bioregulatory mechanisms and psychosocial factors.
We note with regard to the circadian timing system, a new study of a juvenile nonhuman mammal species, the marmoset, shows a delay of activity rhythms (Melo, Goncalves, Menezes, & Azevedo, 2016). This study seems to support suggestions of the earlier review that findings from nonhuman vertebrates help to substantiate the biological underpinnings of adolescent phase delay findings. Other new work detailed below has constructed a phase response curve (PRC) to light in late and post-pubertal adolescents and found a pattern that is contrary to predictions about how adolescents differ from adults (Crowley & Eastman, 2017; Crowley, unpublished). These data suggest that the circadian phase delay of late adolescence is not due to difference in circadian phase-shifting responses to light. Furthermore, an investigation of circadian period also showed no difference between older adolescents and adults (Crowley & Eastman, 2018). When combined with evidence for increased sensitivity to evening light (Crowley, Cain, Burns, Acebo, & Carskadon, 2015), as well as longer measured intrinsic period (Carskadon, Barker, Crowley, Rupp, & Van Reen, 2017) both in younger adolescents, a growing sense is emerging that biological changes affecting circadian timing arise earlier in development than previously predicted. One longitudinal study of circadian phase in adolescents (Crowley, et al., 2014) proposed a model that links the changes to sleep/wake homeostasis to circadian timing in a manner that helps to explain delayed sleep patterns as adolescents develop.
Materials described below extend the previous review paper significantly by detailing recent findings regarding adolescent sleep behaviors and nonbiological factors that affect sleep, the most prominent of which is the starting time of schools. A major advance with potential to influence the psychosocial landscape of adolescent sleep has been a forceful public health outcry and associated recommendations for delaying the starting times of schools as a means to alleviate both short duration sleep as well as ill-timed and irregular sleeping patterns. The American Academy of Pediatrics (Adolescent Sleep Working Group, Committee on Adolescence, & and Council on School Health, 2014), Centers for Disease Control (Wheaton, Chapman, & Croft, 2016), and the American Academy of Sleep Medicine (Watson, et al., 2017) have made the case, based largely on small cross-sectional studies linking school start time to such issues as driving crashes and large epidemiological studies that place short sleep duration of adolescents as a risk factor for serious behavioral and emotional consequences. This message has been taken up by a number of advocacy groups, including Start School Later (www.startschoollater.net), who have undertaken lobbying efforts to implement change. Although these efforts seem to target high school age students, the issue is relevant to and targets middle school students as well (cf, Temkin, Princiotta, Ryberg, & Lewin, 2018). As shown in the systematic review by Wheaton and colleagues (Wheaton, et al., 2016), much of the literature in support of delaying school start times has been gathered in the United States, though data from other regions including Hong Kong, China, Israel, Turkey, Switzerland, Spain, and New Zealand also show better sleep outcomes when schools start later. In a recent report from England (Kelley, Lockley, Kelley, & Evans, 2017), a school’s start time was shifted from 8:50 am to 10:00 am for 2 years and then shifted back to 8:50 am due to a change in local education administrators. This naturalistic experiment suggested that the recommended 8:30 am school start time may be too early as the number of sick days decreased and performance on general examinations improved during the 2 years in which school started later (Kelley, et al., 2017). Finally, Lo and colleagues (2018) showed increased total sleep and self-reported well-being 1 month following a 45-minute delay in school start time in female students at a Singapore high school, with changes sustained at a 9-month follow-up. Authors discussed how in a culture where academic achievement is high priority, delaying school start time was feasible, sustainable, and positively accepted by parents, students, and teachers.
A pattern of restricted sleep on school nights comes from the factors highlighted above that impinge on both ends of the sleep duration equation: bedtimes and wake-up times. Bedtimes on school nights shift later as youngsters transition through adolescence, while rise times remain stable or become earlier. While these age-related sleep timing changes across adolescence have been reviewed previously (Crowley, Acebo, & Carskadon, 2007; Gradisar, Gardner, & Dohnt, 2011), we describe below a more recent longitudinal study in the United States of two age cohorts spanning the second decade (9 to 19 years) that confirms these findings: sleep onset times measured from actigraphy shifted later as an adolescent got older, while wake-up times shifted earlier as an adolescent transitioned from middle school to high school (Crowley, et al., 2014). Interestingly, this paper showed that wake-up times shifted later as an adolescent transitioned out of high school, reflecting the societal pressure of school start time being lifted and wake-up times being less constrained.
Our introduction provides a quick overview and gist of the newer findings that now inform the Perfect Storm model. The details that follow allow for a more in-depth review of adolescent sleep biology and behavior.
Sleep Regulation
We begin with a quick overview of the guiding principles that structure the approach to assessing sleep regulation across adolescence. The Two-Process Model, first proposed by Borbély (1982), with its later refinements (Achermann, Dijk, Brunner, & Borbely, 1993; Borbely & Achermann, 1999; Borbely, Achermann, Trachsel, & Tobler, 1989; Daan, Beersma, & Borbely, 1984) and variations (Dijk & Czeisler, 1995; Edgar, Dement, & Fuller, 1993; Phillips, Chen, & Robinson, 2010; Phillips & Robinson, 2007) continues to guide our hypotheses and inform our understanding of developmental sleep regulatory changes that may explain sleep/wake behavior during development. The model’s two components consist of an approximate 24-hour (circadian) timing system, the brain center of which has been localized to the suprachiasmatic nucleus of the hypothalamus, and a sleep-wake pressure (homeostatic) system, for which a neuroanatomical locus still remains unknown. The genetically-regulated central circadian clock within the brain signals fluctuations of more or less sleep propensity across the 24-hour day regardless of prior sleep/wake duration. By contrast, the homeostatic sleep system favors sleep as wake is extended and favors wakefulness as sleep is prolonged. The homeostatic system is dependent on prior sleep/wake conditions and not the time of day. These two systems interact to regulate sleep duration and timing. As described in Carskadon’s original review paper (Carskadon, 2011), sleep homeostasis and circadian physiology are altered as youngsters progress through adolescence and partly explain developmental changes in sleep behavior.
Sleep Homeostasis during Adolescence
Our fundamental understanding of the developmental changes to the sleep homeostatic system during adolescence has not been altered by new studies; however, a handful of recent studies have reinforced and expanded upon previous findings since 2011. Inferences regarding the sleep homeostatic system are primarily derived from the incidence and amplitude of low-frequency high amplitude waves in the sleep EEG called slow waves. The mathematical quantification of these waves, called slow wave activity, shows homeostatic behavior, increasing during waking and dissipating during sleep (Achermann & Borbely, 2017). Slow wave activity is sensitive to prior sleep wake history and prolonged bouts of waking show a dose-dependent increase in this metric; thus slow wave activity has long been used as a measure of sleep’s homeostatic process.
Cross-sectional studies modeling the accumulation of sleep pressure have shown that sleep pressure builds more slowly in more mature adolescents (i.e., post pubertal) as compared to younger (pre-/early pubertal) adolescents (Jenni, Achermann, & Carskadon, 2005). The authors hypothesized that the slower accumulation of sleep pressure allowed older teens to better withstand increased sleep pressure and thus delay bedtimes (Jenni, et al., 2005). Indeed, in a study examining the speed of falling asleep following prolonged waking, pre-/early pubertal adolescents (mean age = 11.1 years) fell asleep faster than post-pubertal adolescents (mean age = 13.9 years) 14.5 and 16.5 hours after waking (Taylor, Jenni, Acebo, & Carskadon, 2005). Specifically, when both groups were woken at 8:00 A.M., post-pubertal adolescents tended to take longer to fall asleep from 10:30 P.M. to 2:30 A.M.
On the other hand, longitudinal and cross-sectional studies modeling the dissipation of sleep pressure based on sleep EEG slow wave activity have shown that the rate at which sleep pressure is dissipated does not change across adolescent development (Campbell, et al., 2011; Gaudreau, Carrier, & Montplaisir, 2001; Jenni, et al., 2005; Jenni & Carskadon, 2004; Tarokh, et al., 2012). The stability in the dissipation rate of sleep pressure implies that sleep need does not change across this developmental period, a finding that is in line with experimental evidence on sleep need in adolescence. Early evidence that sleep need does not change at the transition to adolescence came from a seminal study in which adolescents aged 10 to 17 years given a 10-hour sleep opportunity slept on average 9.25 hours irrespective of age or maturational stage (Carskadon, 1982).
This conclusion that the need for sleep does not diminish across adolescence is bolstered by two recent studies that examine behavioral outcomes rather than the sleeping EEG. In one, Short and colleagues (2018) computed the “dose response” of adolescents (ages 15 to 17 years) across 5 nights of 5, 7.5, or 10 hours of time in bed. Their computation estimated that the median sleep need of adolescents to sustain waking vigilance and alertness is 9.3 h, compared to 8.16 hours estimated for adults by Van Dongen and colleagues (2003). A field study of over 300 adolescents (ages 13–19 years) with daily self-reported measures of sleep, distress, and externalizing and internalizing symptoms concluded that the overall “optimum” sleep for daily mood was centered at about 9 hours across the sample (Fuligni, et al., 2017). This study showed a modest, statistically significant decline in optimum sleep need measured with these outcomes across this age range. Both studies echo the earlier work based on multiple sleep latency test (i.e., measuring the time taken to fall asleep on multiple occasions) findings, which concluded a stable sleep need of about 9 hours a night across adolescence (Carskadon, Orav, & Dement, 1983).
In addition to studies modeling the regulation of the homeostatic system during adolescence, a number of recent studies have examined the impact of sleep restriction on slow waves. One such cross-sectional study by Campbell and colleagues (Campbell, Kraus, Burright, & Feinberg, 2016) found that compared to a 10-hour condition, reducing time in bed to 8.5 or 7 hours for four nights did not impact slow wave activity in adolescents between the ages of 9.9 to 14.0 years when the same amount of non-REM sleep was included in the analysis. Another study which employed markedly more sleep restriction of five nights of 5 hours of time in bed in a sample of late adolescents (15 to 19 years) found substantial build-up of sleep pressure over the five days as indexed by slow wave energy (Ong, Lo, Gooley, & Chee, 2017). Slow wave energy takes into account the cumulative amount of slow wave activity across the entire sleep period, and therefore differs from measures of slow wave activity averaged across a given time interval. Interestingly, two nights of recovery sleep consisting of 9 hours of time in bed (akin to a weekend sleep-in) were insufficient to compensate for the lost slow wave energy over the restriction protocol (Ong, et al., 2017).
The above-mentioned studies (Campbell, et al., 2016; Ong, et al., 2017) are difficult to compare directly given the differences in the sleep restriction protocols and the metrics used to measure sleep homeostasis. Another important difference between the studies is the different ages studied (i.e., early versus late adolescence) as current evidence suggests that the rebound in slow wave activity following sleep deprivation/restriction undergoes maturation across the adolescent period. For example, a study in humans found that pre-/early pubertal adolescents showed a proportionally smaller increase in slow wave activity following one night of sleep deprivation as compared to post-pubertal teens (Jenni, et al., 2005). Similar findings were reported in a study of homeostasis in adolescent mice, which found an increase in slow wave activity after an acute sleep restriction (four hours) only in mice near the midpoint of adolescence (Nelson, Faraguna, Zoltan, Tononi, & Cirelli, 2013). This has led to the hypothesis that a ceiling effect may prevent slow wave activity from building in the younger brain (Jenni, et al., 2005; Nelson, et al., 2013). This diminished ability to recover from sleep restriction/deprivation with a rebound in slow wave activity, in combination with the faster build-up of sleep pressure may be the reason why young adolescents and mice have difficulty staying awake during periods of prolonged wakefulness (Jenni, et al., 2005). Indeed, in the study of adolescent mice, those in the early phase of adolescence made 21 sleep attempts during the four-hour deprivation phase as compared to seven attempts for the more mature adolescents (Nelson, et al., 2013). Therefore, the physiological response to sleep restriction may change progressively across adolescent development and has implications for the ability of the brain to recover from sleep loss.
To summarize, there are two arms to the sleep homeostatic system: one which is associated with the rate at which sleep pressure builds during waking periods and the other is a reflection of the rate of dissipation of sleep pressure. During adolescent development, sleep pressure builds more slowly allowing older teens to delay their bedtimes. On the other hand, the rate at which sleep pressure is dissipated does not change, thus sleep need does not change across the adolescent years (i.e., remains stable around 9.25 hours).
Circadian Timing during Adolescence
Early studies suggested that the central circadian clock shifts later (phase delay) with the progression of puberty, findings that were inferred from subjective measures initially (Andrade, Benedito-Silva, Domenice, Arnhold, & Menna-Barreto, 1993; Carskadon, Vieira, & Acebo, 1993) and then later confirmed with objective measures of circadian phase using salivary melatonin (Carskadon, Acebo, & Jenni, 2004; Carskadon, Acebo, Richardson, Tate, & Seifer, 1997). The circadian phase delay seen in humans is also observed in nonhuman mammals, including rhesus monkey, degu, laboratory rat, laboratory mouse, and the fat sand rat (Hagenauer, Perryman, Lee, & Carskadon, 2009). A more recent study adds to this list as it shows delayed activity rhythms in juvenile marmosets relative to their adult parents (Melo, et al., 2016), and further substantiates the biological underpinnings of adolescent phase delay findings in humans.
Two primary hypotheses that could explain the puberty-related circadian delay of adolescents have guided recent work in this area. The first hypothesis posited that the endogenous circadian period – the internal day length of the circadian system – may lengthen over the course of puberty. The majority of adult humans show an intrinsic circadian period a little longer than 24 hours (Duffy & Wright, 2005); for example, the average ranges from about 24.2 to 24.3 h (Burgess & Eastman, 2008; Czeisler, et al., 1999; Duffy, et al., 2011; Duffy, Rimmer, & Czeisler, 2001; Eastman, Molina, Dziepak, & Smith, 2012; Eastman, Suh, Tomaka, & Crowley, 2015; Eastman, Tomaka, & Crowley, 2017; Kitamura, et al., 2012; Lazar, et al., 2012; Smith, Burgess, Fogg, & Eastman, 2009; Wright, Hughes, Kronauer, Dijk, & Czeisler, 2001). If the system was left to run at its own pace (“free run”), sleep/wake patterns of those with circadian periods longer than 24 hours would progressively get later and later each day. The process of entrainment (synchronizing internal rhythms with the external 24-hour day) occurs primarily though light/dark exposure. The length of the free-running circadian period predicts how the circadian system entrains to the 24-hour day (Burgess & Eastman, 2008; Duffy, Dijk, Hall, & Czeisler, 1999; Wright, Gronfier, Duffy, & Czeisler, 2005; Wright, et al., 2001); a longer circadian period is associated with more owl-like evening tendencies, whereas a short circadian period is associated with larkish morning-type tendencies (Duffy, et al., 2001). A lengthening of the endogenous circadian period would be consistent with the evening-type sleep/wake behavior of older more mature adolescents than say their pre-pubertal siblings or their parents. Preliminary evidence from animal (McGinnis, Lumia, Tetel, Molenda-Figueira, & Possidente, 2007) and human (Carskadon & Acebo, 2005) studies supported this hypothesis as these data suggested that adolescents have a longer circadian period than adults. A recent study by Crowley and Eastman do not support this hypothesis, however, as they show similar free-running circadian periods in late- and post-pubertal adolescents (Tanner stage 4 or 5; 14.3 – 17.8 years) compared to adults (30.8 – 45.8 years) when run in the same laboratory protocol (Crowley & Eastman, 2018). In this study, both age groups showed an average circadian period of about 24.2 hours. Similar to previous studies in adults (Eastman, et al., 2012; Eastman, et al., 2017; Smith, et al., 2009), ancestry differences were noted; free-running circadian period was shorter in African-American participants compared to those of other ancestries (mostly White), but this was primarily driven by the adult group as the mean ancestry differences in the adolescents was smaller by comparison. Sex differences in both age groups were also noted, though not at a statistically reliable level. These recent results would indicate that late and post-pubertal adolescents have adult-like free-running circadian periods, and therefore, do not provide support for a coincident change in free-running circadian period and sleep behavior during late adolescence. New data from the Carskadon laboratory (Carskadon, et al., 2017) seems to indicate that changes to free-running circadian period may be occurring earlier in development, though analyses that consider ancestry and sex differences in these developmental patterns are needed.
The second mechanism proposed to explain a delayed circadian phase with pubertal development was an altered circadian response to light. The system’s response to light is phase-dependent and predictable; in general, light exposure to the eyes in the evening or first part of habitual sleep shifts the circadian system later (phase delay) and light exposure in the second half of habitual sleep or shortly after waking shifts circadian rhythms earlier (phase advance). These responses are experimentally derived and illustrated as a phase response curve (PRC) to light. A delayed circadian system as seen in older adolescents could be explained by a blunted response to morning phase advancing light, an exaggerated response to evening phase delaying light, or a combination of both. Crowley and colleagues (2015) tested these hypotheses in a group of pre- to post-pubertal adolescents aged 9.1 – 15.9 years. Circadian sensitivity to light was assessed using melatonin suppression in response to 0, 15, 150, or 500 lux of light shown in the evening (between 23:00 – midnight) or the morning (03:00 – 04:00). In the evening, early to mid-pubertal adolescents (Tanner Stage 1–3) showed greater sensitivity to light compared to the late and post-pubertal adolescents (Tanner Stage 4 and 5), which contradicted the original evening light sensitivity hypothesis. Indeed, the younger group was incredibly sensitive to evening light such that responses were observed in light levels (~15 lux) far less than normal room lighting in most homes (~100 lux). The morning light sensitivity hypothesis showed some support; 500 lux of light initially suppressed melatonin more in the early- to mid-pubertal group compared to the late and post-pubertal group, but this difference was not statistically reliable (p=.06) and percent suppression was similar between groups at the end of the 1-h light exposure. These data, which showed no sex differences, suggested an overall reduction of the circadian clock’s sensitivity to light, particularly in the evening as adolescents mature. Taken together with findings showing robust evening melatonin suppression in young children (4.3 ± 1.1 years) (Akacem, Wright, & LeBourgeois, 2018) and greater melatonin suppression in the evening in school-aged children (7.4 ± 1.8 years) compared to their parents (41.2 ± 4.8 years) (Higuchi, Nagafuchi, Lee, & Harada, 2014), this decline of evening light sensitivity is likely an age-related phenomenon and not related to puberty.
Melatonin suppression is one method to assess circadian light sensitivity; however, phase shifts (changes in the timing of the circadian rhythm) in response to light may be a more relevant outcome to test the hypothesis that adolescent sleep behavior is associated with altered responses to light. In particular, evening light may produce larger phase delay shifts as adolescents mature or phase advance shifts are attenuated in response to morning light. As reviewed previously (Carskadon, 2011), one animal study of female mice provides some support for this hypothesis as pre-pubertal (49 days) animals delayed more in response to a 15-min 150-lux light pulse compared to adults (140 days) (Weinert & Kompauerova, 1998). In an attempt to test this hypothesis in humans, Crowley and Eastman (2017) constructed a phase response curve to light in late and post-pubertal (Tanner Stage 4 and 5) adolescents aged 14.3 to 17.8 years. The adolescent phase response curve to light showed a predictable pattern with the largest delay shifts occurring in the hours straddling habitual bedtime, and the largest advance shifts occurring in the hours straddling habitual wake-up time. Moreover, the phase delay and phase advance regions of the adolescent phase response curve were symmetrical, suggesting that the phase delay shift in response to bright light is not exaggerated, and the phase advance response to bright light is not attenuated as predicted, in late- and post-pubertal adolescents. Also, inconsistent with previous animal data, the older adolescent phase response curve does not differ from a phase response curve constructed in adults (30–45 years) using the same protocol (Crowley, unpublished). Whether phase shift responses of older adolescents differ from their younger peers remains unknown.
These recent studies (Crowley, et al., 2015; Crowley & Eastman, 2017) do not support the hypothesis that the circadian system of older adolescents are intrinsically more sensitive to evening light. What is more likely is the opportunity for light exposure in the evening increase as adolescents get older. Crowley and colleagues (2014) found that young adolescents (9 –13 years) fell asleep about 1 h after the onset of their biological night, defined by the secretion of melatonin. Older adolescents (15 to 18 years), however, fell asleep 2 h into their biological night. The authors proposed that this difference in when they fell asleep with respect to the onset of melatonin secretion is due to a slowed accumulation of waking homeostatic sleep pressure allowing older adolescents to stay awake later into their biological night. Recent modeling work provides support for this hypothesis (Skeldon, Derks, & Dijk, 2015).
Changes to sleep physiology propping up evening alertness also increases the likelihood of light exposure later into the evening, a time when the circadian system is particularly sensitive to phase delaying light (Crowley & Eastman, 2017). Not to mention, an increasing number of adolescents access screens at night before bedtime (Gradisar, et al., 2013; Hale, et al., 2018), and growing evidence suggests that prolonged screen light exposure (i.e., ≥ 1.5 hours) can feed back into the circadian and homeostatic sleep systems (Cajochen, et al., 2011; van der Lely, et al., 2015; Wood, Rea, Plitnick, & Figueiro, 2013) to reinforce this evening alertness. It should be noted, however, that the assumption to avoid bright screens in the hour before bed has not yet shown to significantly attenuate the natural rise in melatonin (van der Lely, et al., 2015; Wood, et al., 2013) nor meaningfully influence the subsequent sleep of adolescents (Heath, et al., 2014; van der Lely, et al., 2015). Two meta-analyses of the effect of screen time on young people’s sleep have been conducted since the 2011 Carskadon review (Bartel, Gradisar, & Williamson, 2015; Carter, Rees, Hale, Bhattacharjee, & Paradkar, 2016). When considering only portable interactive devices (i.e, smartphones, tablets) there is a two-fold increased effect on inadequate sleep (Carter, et al., 2016). Yet when considering all devices, the associations with sleep duration are small (Bartel, et al., 2015). Nevertheless, extrinsic alerting factors can interact with bioregulatory systems to perpetuate a cycle of delayed sleep timing and restricted sleep duration.
In sum, new data suggest that changes in central circadian physiology may be occurring earlier in development, while older adolescents are showing adult-like circadian physiology. It is still unclear whether the described changes in circadian physiology early in development provide the impetus for a delayed system in the later years of adolescence. Persistence of delayed and shortened sleep as adolescents mature may be ascribed to bioregulatory sleep changes and environmental factors that both reinforce evening alertness and delayed sleep onset.
Schools Start Times, Cognitive Functioning, and Academic Performance
Bioregulatory and psychosocial factors together create a deficient environment for adolescents’ sleep health and the consequences that follow (Carskadon, 2011). Negative health outcomes include sleepiness mood disturbances, mental health difficulties, behavioral problems, substance use and abuse, weight gain, accidents including motor vehicles, and immune system challenges. School-related negative consequences include inattention, school absenteeism and tardiness, learning and memory difficulties, and poor academic grades. Likewise, a number of psychosocial factors affect sleep patterns in adolescents and contribute to the delay in sleep timing and insufficient sleep including school start times, socioeconomic status, digital media use, social engagements, and caffeine intake (Calamaro, Mason, & Ratcliffe, 2009; LeBourgeois, et al., 2017; Ludden & Wolfson, 2010; Marco, Wolfson, Sparling, & Azuaje, 2011; Minges & Redeker, 2016). With one of the hallmark environmental constraints for adolescent sleep being the starting time of school, and keeping in mind that, on average, children spend 1,000 hours of instructional time per year in school (13,000 hours from kindergarten through 12th grade), in the section below we review the research on school start times (constraint) and academic/cognitive functioning (consequence) associated with insufficient, delayed, and erratic sleep.
Early school schedules significantly contribute to insufficient sleep for adolescents in middle and high school. In a now hallmark study, Carskadon and colleagues examined sleep, sleepiness, and circadian rhythms in adolescents in a school district that advanced school start times from 8:25AM to 7:20AM from 9th (end of junior high school) to 10th grade or high school (Carskadon, Wolfson, Acebo, Tzischinsky, & Seifer, 1998). Using actigraphically estimated sleep, findings demonstrated that although the adolescents didn’t go to sleep earlier, they woke up significantly earlier on school days when school start times were advanced, 10th versus 9th grade, and therefore obtained less sleep. Furthermore, comparing a 9th grade overnight assessment vs. 10th grade, these adolescents demonstrated increased daytime sleepiness, shortened sleep onset latency, and nearly 50 percent of the 10th graders had REM sleep on the first morning (8:30am) assessment within only 10 or 15 minutes of sleep onset, (i.e., a finding seen in a narcolepsy diagnosis (Mignot, et al., 2006)). Moreover, this study revealed that the melatonin onset phase (i.e., the circadian timing system) for these adolescents was 40 minutes later in 10th versus 9th grade (Carskadon, et al., 1998). In other words, these adolescents biologically needed to be sleeping at the time that they were required to be in school ready to tackle any number of high school subjects such as calculus, literature, and chemistry. Over the last 25 years, more attention has been directed toward delaying middle and high school start times because many school districts in the U.S. and around the world start the school day earlier as children progress through school, thus requiring adolescents to get up at an early hour juxtaposed to their bedtimes, circadian phases, and need for sleep. Since Carskadon and colleagues’ study, school start time outcome research from a range of disciplines and methodologies: circadian rhythms to economics, and across different countries, have investigated the impact of delaying school start times on adolescents’ health and academic performance as well as on the ramifications for school districts (Hafner, Stepanek, & Troxel, 2017; Lee, Nolan, Lockley, & Pattison, 2017; Owens, Wang, Lewin, Skora, & Baylor, 2016; Wahlstrom, 2016).
Wahlstrom’s study of over 18,000 high school students was one of the first to demonstrate that delaying school start times increased attendance, improved high school enrollment, and showed some improvement in grades (Wahlstrom, 2002; Wahlstrom, 1999). Moreover, these students had similar bedtimes to students from other earlier starting schools, despite the delay in school start time. Moreover, on average, students at the later starting high schools got almost one hour more sleep each school night. Since Wahlstrom’s study, numerous studies have examined various aspects of the impact of delaying school start times on adolescents’ sleep, health, daytime functioning, and academic performance along with the economic and policy implications (Adolescent Sleep Working Group, et al., 2014; Hafner, et al., 2017; Troxel & Wolfson, 2017). Three recent reviews document the body of work conducted over the last 25 years on school start times and adolescents’ sleep. Using a meta-analysis approach, Minges and Redecker and Bowers and Moyer examined the effects of delayed school start time on adolescents’ sleep, health, and academic outcomes (Bowers & Moyer, 2017; Minges & Redeker, 2016). Based on Minges and Redecker’s meta-analysis, school start times were delayed 25–60 min, and correspondingly, total sleep time increased from 25 to 77 min per weeknight. In addition, studies reported reduced daytime sleepiness, depression, caffeine use, and tardiness (Minges & Redeker, 2016). Bowers and Moyer, similarly, concluded that later starting school times are associated with longer sleep durations, less daytime sleepiness, and tardiness to school (Bowers & Moyer, 2017). Additionally, Wheaton and colleagues (Wheaton, et al., 2016) systematically reviewed 38 studies, captured through PubMed and Scopus that focused on the association between school start times, sleep, and other behavioral outcomes. They concluded that there is significant evidence that delaying school start time increases school-night sleep duration by at least 30 minutes, primarily by delaying rise times; and that later start times generally correspond to improved attendance, less tardiness, decreased incidence of falling asleep in class, fewer motor vehicle crashes, and improved grades (Wheaton, et al., 2016).
Understanding the impact of delayed school start times on academic performance specifically, as well as related factors such as tardiness and absenteeism is timely. Yet, as Wheaton and colleagues discuss, it is challenging to assess for several reasons: 1) grading is not standardized and varies by subject, teacher, and school; 2) achievement tests vary across school districts and standardized tests (e.g., Secondary School Admission Test (SSAT), Preliminary Scholastic Aptitude Test (PSAT), or the SAT) are not taken by all students; 3) variables associated with struggling in school are complex and multifaceted; and 4) it is challenging to assess the impact of delaying school start times for high achieving students as they have less room for improvement in this realm even though there are overall health benefits (Wheaton, et al., 2016). Nonetheless, studies conclude that later school start times have a positive impact on academic performance and behavior. For example, in Wahlstrom’s more recent multisite study of eight school districts, researchers concluded that the majority of schools reported an increase in GPA with a delay in school start times (Wahlstrom, et al., 2014). Another study identified the effect of later school start times on academic performance in 1999–2006 data from middle school students in a large U.S. school district. Using variation in middle school start times both within and across schools, this analysis revealed that a delay in start times by 1 hour lead to a 3-percentile point gain in both math and reading test scores for the average student. In the study’s longitudinal analysis, the impact of delayed middle school start times on test scores persisted into the 10th grade with a larger effect for the lower end of the test score distribution.
Keeping in mind that being present in school is normally required for academic success, studies have examined sleep duration and attendance along with the effects of delaying school start times on attendance and tardiness. In a population-based study of over 8,000 high school age adolescents, after adjusting for gender and socioeconomic status, short sleep duration and poor sleep efficiency were the sleep variables with the highest odds of non-attendance (Hysing, Haugland, Stormark, Boe, & Sivertsen, 2015). Likewise, improved absenteeism and particularly tardiness records stand out as one of the key findings in many of the school start time studies (e.g., (Wolfson, Spaulding, Dandrow, & Baroni, 2007)). For example, one study examined sleep patterns and school performance of early adolescents attending two urban, public middle schools with early (7:15 a.m.) versus late (8:37 a.m.) start times early and later in the year. At both times, students at the late-starting school had transcript based tardiness records that were four times lower and reported waking up over 1 hour later on school mornings, obtaining 50 minutes more sleep each night, and less daytime sleepiness than students at the early school (Wolfson, et al., 2007).
In summary, the three reviews of non-experimental studies demonstrate that delaying school start times improves sleep and consequential daytime behaviors for middle and high school age adolescents. Furthermore, the studies in Sleep Health’s special issue (e.g., (Troxel & Wolfson, 2017)) on school start times and sleep explored some of the ongoing research questions including economic and legal questions and benefits, cross cultural questions, assessment, and implementation.
Undoubtedly, later school start times create the opportunity for adolescents to obtain sufficient sleep and to avoid social jet lag (i.e., experience like jet lag where one’s regular sleep-wake schedule is misaligned with one’s biological clock) with positive implications for academic performance. In turn, there is growing body of work that has looked directly at the impact of circadian timing and sleep duration on academic and cognitive performance. Studies have utilized both naturalistic, survey methodologies as well as experimental, laboratory-based approaches in a variety of research settings and from adolescents living in the U.S., as well as a wide range of other countries. Reviews of this scholarship continue to enhance our understanding of the findings, the limitations, and future research questions (Abraham & Scaria, 2015; Curcio, Ferrara, & De Gennaro, 2006; Dewald, Meijer, Oort, Kerkhof, & Bogels, 2010; Tonetti, Natale, & Randler, 2015; Wolfson & Carskadon, 2003). Taking both approaches into account, findings demonstrate that poor sleep quality, daytime sleepiness, insufficient sleep, and evening circadian preference are correlated with decreased academic performance; sleep loss is associated with poor declarative and procedural learning; and laboratory-based sleep restriction might negatively affect neurobehavioral and cognitive performance (e.g., psychomotor reaction times, reduced awareness of the extent of sleep loss, impulsivity, and sustained attention, (Curcio, et al., 2006; Fallone, Acebo, Arnedt, Seifer, & Carskadon, 2001; Randazzo, Muehlbach, Schweitzer, & Walsh, 1998; Sadeh, Gruber, & Raviv, 2003)).
In particular, studies have examined the associations between circadian preference, phase, cognitive functioning, and academic performance, including two meta-analytic reviews. Preckel and colleagues (Preckel, Lipnevich, Schneider, & Roberts, 2011) conducted four meta-analyses: morningness and cognitive ability measures (e.g., memory, processing speed verbal/quantitative reasoning), eveningness and cognitive ability, morningness and academic achievement (e.g., GPA), and eveningness and academic achievement. In the four analyses, associations between chronotype and cognitive ability as well as academic achievement were significant (Preckel, et al., 2011). Eveningness was positively correlated with adolescents’ cognitive ability, yet negatively associated with academic achievement measures. In contrast, morningness was negatively associated with cognitive ability and positively correlated with academic measures. Although a weak association, the authors suggest that evening types might be more cognitively adept and make use of a range of times to carry out their studies, particularly the late evening hours, a time when there are fewer distractions (Preckel, et al., 2011). These findings, however, raise questions and require further research. In another meta-analysis, researchers examined circadian phase preference and academic performance (transcript, teacher or self-report GPA and/or standardized test scores) in studies that focused on “school” versus “university” age participants (Tonetti, et al., 2015). Overall, there was a higher effect size in school-level compared to university-level studies. Also, self-report measures of grades revealed a stronger effect size in comparison to more objective measures. Similar to Preckel and colleagues’ findings, this meta-analysis concluded that evening preference is associated with poorer academic performance, both in school and university populations. As the authors highlight, university students or older adolescents often have more independence and flexibility in creating their class schedule than middle or high school students allowing them to select classes that fit their own sleep-wake schedules, to avoid social jetlag, and to obtain more sleep (Tonetti, et al., 2015). One clear limitation, however, is that it is unclear as to the age of the participants included in the studies categorized as “school” level in this systematic review.
There have been a few new and significant studies that engaged self-report methodologies, data base analyses, and experimental designs. Historically, sleep schedule regularity and academic performance has not been as well examined. Wolfson and Carskadon found that students who described themselves as struggling or failing school (C’s, D’s/F’s) reported greater differences between their weekend and school-night sleep schedules (i.e., 2.3-hour vs. 1.8-hour difference between weekend and school-night bedtimes) than A and B students (Wolfson & Carskadon, 1998). Using a new approach to sleep regularity, Phillips and colleagues assessed 61 undergraduates for 30 days using sleep diaries and quantified sleep regularity using the sleep regularity index (Phillips, et al., 2017). The sleep regularity index calculates the probability of an individual being asleep or awake at any two time-points 24 hours apart, scaled so that an individual who is asleep or awake at the same times each day scores 100 versus an individual who sleeps randomly scores zero. Not surprising, a higher regularity index was associated with better academic performance (Phillips, et al., 2017).
Using another novel approach, Smarr and Schirmer (Smarr & Schirmer, 2018) analyzed two years of data (N = 14,894) from a large US university’s learning management system to examine the impact of misalignment between circadian rhythms and older adolescents’ course schedules (i.e., social jet lag) on academic performance (GPA). After categorizing the students into “night owls,” “daytime finches,” and “morning larks,” based on their learning management system activity on non-class days, researchers compared their class times to their academic outcomes. Forty percent of the students learning management activity was synchronized with their class times; fifty percent were taking classes before they were fully alert, and 10 percent had already peaked by the time their classes started. Moreover, 60 percent of the students experienced more than 30 minutes of social jet lag, with greater social jetlag associated with lower semester GPAs, especially for owls (Smarr & Schirmer, 2018). In other words, when students’ course schedules were aligned with their circadian schedule based on learning management activity, they were more likely to do better academically.
Recent experimental, laboratory-based studies further contribute to our understanding of the impact of sleep restriction, napping, and sleep timing on adolescents’ cognitive functioning. Lo and colleagues simulated the school-night sleep loss and weekend night catch up common to many adolescents (Lo, et al., 2017). In their design, they examined adolescents’ neurobehavioral functioning during two periods of restricted and recovery sleep, as well as the effectiveness of afternoon naps in ameliorating neurobehavioral deficits associated with multiple nights of sleep restriction. Fifty-seven male adolescents (ages 15–19) experienced two cycles of 5 hours time in bed for three nights with a 9-hour time in bed recovery for two nights per cycle; half of the participants were given the opportunity for a 1-hour afternoon nap following each sleep-restriction night. They found that participants in the non-nap condition showed a progressive decline in sustained attention, working memory, and speed of processing that did not return to baseline after two nights of recovery sleep. Napping attenuated but did not eliminate the adolescents’ declining performance. In contrast, in Lo and colleagues’ previous study, they compared a similar group of adolescent males, randomly assigned to sleep restriction or control groups. Participants underwent a 2-week protocol consisting of three baseline nights (time in bed = 9 hours), seven nights of sleep opportunity manipulation (time in bed = 5 hours for sleep restriction and 9 hours for controls) with three nights of recovery sleep (time in bed = 9 hours) at a boarding school (Lo, Ong, Leong, Gooley, & Chee, 2016). In this case, the sleep restriction group demonstrated poorer sustained attention, working memory, and executive function; whereas, the control group was able to maintain cognitive performance levels.
Another recent study focused on a laboratory model for understanding the impact of cramming for exams instead of sleeping (Huang, et al., 2016). In this case, researchers assessed the interaction of study timing and time in bed on vocabulary learning. Fifty-six adolescents (ages 15–19) were randomly assigned to a week of either 5 (sleep restricted) or 9-hour time in bed as part of a 14-day protocol at a boarding school. Participants studied 40 Graduate Record Examination (GRE)-type words on digital flashcards with word pairs spaced over four consecutive days or all at once during a single study session. Recall was examined immediately, 24 hours, and 120 hours (i.e., 5 days) after items were studied. For all retention intervals, recall was more impaired for adolescents in the sleep restriction condition. Cued recall on spaced items, however, was similar for the five and nine-hour time in bed groups. Paced learning seemed to mitigate the effects of sleep restriction on vocabulary recall, whereas students with insufficient sleep were more likely to forget items studied over short time intervals. Taken together, these experimental, laboratory studies demonstrate that for sleep-restricted adolescents, weekend “catch-up sleep,” even when combined with napping on school days, is inferior to obtaining sufficient and regular school-night sleep (Lo, et al., 2017; Lo, et al., 2016). Furthermore, adolescents with insufficient sleep who cram for exams might be particularly prone to having difficulty remembering new material; however, learning deficits might be minimized when students study over several days (Huang, et al., 2016).
Finally, few studies have tried to improve adolescents’ sleep hygiene practices and patterns as way of also improving or maintaining academic performance during middle school, a time when personal responsibility for learning and sleep habits is significant. Using a social learning model, the Sleep Smart Program aimed to improve sleep health behaviors and secondarily improve academic performance and behavioral well-being. A diverse group of seventh graders from two urban, middle schools were randomly assigned to a Sleep Smart program or a comparison group. In addition to improved sleep hygiene, more time in bed, and earlier bedtimes, sleep-smart participants also reported sustained academic performance over 7th grade whereas the comparison group’s academic performance declined (Wolfson, Harkins, Johnson, & Marco, 2015).
The Perfect Storm: Insufficient and Ill-timed Sleep and the Troubling Consequences
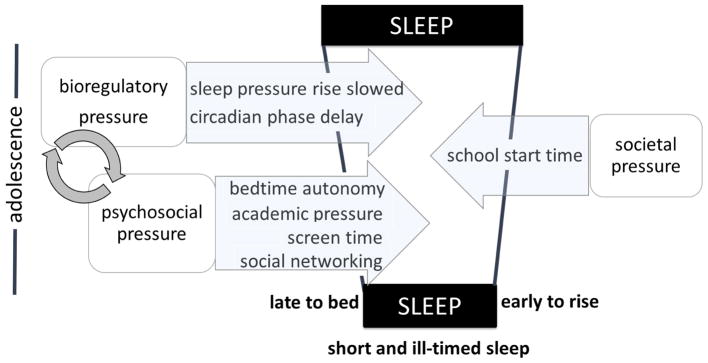
Figure 1 illustrates a summary of the several factors discussed in this review that conspire to produce a Perfect Storm of insufficient and inappropriately-timed sleep. Bioregulatory pressures sustain evening alertness later into the night in maturing adolescents, at the same time parent-set bedtimes wane, academic demands increase, and social networks expand. Activities that are potentially stimulating or produce additional light stimuli can reinforce evening alertness and perpetuate a cycle of late sleep onsets as adolescents mature. Although there has been progress in recent years in the call for action to delay school start times, the major societal constraint on adolescents’ sleep continues to be school start times. Other morning activities unique to individual families or cultures, such as religious activities or early sports practices, may also be considered. Although this review focused primarily on the school-related negative consequences of this Perfect Storm of insufficient and ill-timed sleep, other serious consequences for adolescents include mood disturbances, behavioral problems, weight gain, and motor vehicle accidents.
Figure 1.
The Perfect Storm model, first presented by Carskadon (2011), illustrates several factors described in the text that contribute to changes in sleep behavior over the course of adolescent development. Bedtime and thus sleep onset is shifted later due to bioregulatory changes to the homeostatic sleep system and the circadian timing system. Psychosocial factors also contribute to delayed bedtimes. What we add here is the likely interaction between bioregulatory and psychosocial pressures (arrows). Changes to sleep physiology propping alertness later into the evening likely facilitates engaging in other activities besides sleep. Activities that are stimulating (e.g., engaging in their social network) or provide light exposure (e.g., from screens) during a time that delays circadian rhythms can exacerbates late sleep onset, but also feedback on the systems regulating sleep and wake. The length of sleep is not affected by these factors, it is only when societal pressures – the most notable being school start time – forces adolescents awake earlier than spontaneous arousal with an alarm clock.
Adolescence is an important developmental stage where sleep is strained both in duration and timing by a host of factors, some modifiable others not. Evidence for a relatively stable need for about 9 hours of sleep per day on average, maturational modifications in bioregulatory processes, sleep homeostasis and circadian timing system, frame the nonmodifiable challenges. That said, appropriate lighting conditions (for example reduced light in the evening and increased light in the morning) can affect circadian timing in a positive way; however, feasible strategies to implement these circadian-based treatment strategies are still needed in this age group. Modifications of the school bell schedule can also loosen societal constraints on sleep duration and sleep irregularity, and many continue to work toward this goal. Moving forward, there is also a need to investigate these bioregulatory and psychosocial factors at an individual level to understand and identify individual differences in developmental trajectories that may predispose young people to more or less “stormy” sleep conditions. More work is also needed in identifying other exposures that could inform the Perfect Storm model, such as substance use (caffeine, alcohol, and marijuana), trauma, and economic disadvantage. Furthermore, molecular genetics including genotyping and epigenetic approaches may provide further insights into the bioregulatory processes. Thus, while the overall issues and concerns remain, the more that is learned through research supported by the National Institutes of Health or Center for Disease Control and Prevention in the US and other agencies and entities across the world, the better able adolescents, parents, families, teachers, administrators, pediatricians, and legislators will be to improve the sleep circumstances and sleep patterns of adolescents.
Acknowledgments
The work described in this paper was supported by the National Institutes of Health (R01HL105395 to SJC; R01HD047928 to ARW; MH45945, MH52415, MH58879, HL71120, MH076969, MH079179 to MAC) and the Periodic Breathing Foundation (MAC).
We thank our colleagues, students, staff, and the families who have contributed to the research described in this paper. We are also grateful to Michael Gradisar, PhD for his thoughtful feedback on and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Scaria J. Influence of sleep in academic performance–an integrated review of literature. Journal of Nursing and Health Science. 2015;4:78–81. [Google Scholar]
- Achermann P, Borbely AA. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6. Philadelphia, PA: Elsevier Saunders; 2017. pp. 377–387. [Google Scholar]
- Achermann P, Dijk DJ, Brunner DP, Borbely AA. A model of human sleep homeostasis based on EEG slow-wave activity: Quantitative comparison of data and simulations. Brain Research Bulletin. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- Adolescent Sleep Working Group, Committee on Adolescence and Council on School Health. School start times for adolescents. Pediatrics. 2014;134:642–649. doi: 10.1542/peds.2014-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akacem LD, Wright KP, Jr, LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiological Reports. 2018;6:1–10. doi: 10.14814/phy2.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MM, Benedito-Silva AA, Domenice S, Arnhold IJ, Menna-Barreto L. Sleep characteristics of adolescents: A longitudinal study. Journal of Adolescent Health. 1993;14:401–406. doi: 10.1016/s1054-139x(08)80016-x. [DOI] [PubMed] [Google Scholar]
- Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: A meta-analytic review. Sleep Medicine Reviews. 2015;21:72–85. doi: 10.1016/j.smrv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. Journal of Biological Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Achermann P, Trachsel L, Tobler I. Sleep initiation and initial sleep intensity: Interactions of homeostatic and circadian mechanisms. Journal of Biological Rhythms. 1989;4:149–160. [PubMed] [Google Scholar]
- Bowers JM, Moyer A. Effects of school start time on students’ sleep duration, daytime sleepiness, and attendance: A meta-analysis. Sleep Health. 2017;3:423–431. doi: 10.1016/j.sleh.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. Journal of Biological Rhythms. 2008;23:374–376. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Frey S, Anders D, Spati J, Bues M, Pross A, Mager R, Wirz-Justice A, Stefani O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. Journal of Applied Physiology. 2011;110:1432–1438. doi: 10.1152/japplphysiol.00165.2011. [DOI] [PubMed] [Google Scholar]
- Calamaro CJ, Mason TB, Ratcliffe SJ. Adolescents living the 24/7 lifestyle: Effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics. 2009;123:e1005–1010. doi: 10.1542/peds.2008-3641. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, Feinberg I. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91. doi: 10.1093/sleep/34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Kraus AM, Burright CS, Feinberg I. Restricting time in bed in early adolescence reduces both NREM and REM sleep but does not increase slow wave eeg. Sleep. 2016;39:1663–1670. doi: 10.5665/sleep.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA. The second decade. In: Guilleminault C, editor. Sleep and waking disorders: Indications and techniques. Menlo Park: Addison Wesley; 1982. pp. 99–125. [Google Scholar]
- Carskadon MA. Sleep in adolescents: The perfect storm. Pediatric Clinics North America. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. Intrinsic circadian period in adolescents versus adults from forced desynchrony. Sleep. 2005;28:A71. [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: Implications for behavior. Annals of the New York Academy of Sciences. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. Journal of Biological Rhythms. 1997;12:278–289. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Barker D, Crowley SJ, Rupp TL, Van Reen E. Changes to the circadian timing system may arise in early adolescence. 9th biennial Pediatric Sleep Medicine Conference; Amelia Island, Florida. 2017. [Google Scholar]
- Carskadon MA, Orav EJ, Dement WC. Evolution of sleep and daytime sleepiness in adolescents. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: Natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 201–216. [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- Carter B, Rees P, Hale L, Bhattacharjee D, Paradkar MS. Association between portable screen-based media device access or use and sleep outcomes: A systematic review and meta-analysis. JAMA Pediatrics. 2016;170:1202–1208. doi: 10.1001/jamapediatrics.2016.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Medicine. 2007;8:602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid puberty. Journal of Clinical Endocrinology & Metabolism. 2015;100:4067–4073. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Eastman CI. Human adolescent phase response curves to bright white light. J Biol Rhythms. 2017;32:334–344. doi: 10.1177/0748730417713423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Eastman CI. Free-running circadian period in adolescents and adults. Journal of Sleep Research. 2018 doi: 10.1111/jsr.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, Barker DH, Carskadon MA. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS ONE. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Medicine Reviews. 2006;10:323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. American Journal of Physiology. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Medicine Reviews. 2010;14:179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Jr, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. Journal of Investigative Medicine. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behavioral Neuroscience. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Wright KP. Entrainment of the human circadian system by light. Journal of Biological Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Molina TA, Dziepak ME, Smith MR. Blacks (african americans) have shorter free-running circadian periods than whites (caucasian americans) Chronobiology International. 2012;29:1072–1077. doi: 10.3109/07420528.2012.700670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Suh C, Tomaka VA, Crowley SJ. Circadian rhythm phase shifts and endogenous free-running circadian period differ between african-americans and european-americans. Scientific Reports. 2015;5:8381. doi: 10.1038/srep08381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. Sex and ancestry determine the free-running circadian period. Journal of Sleep Research. 2017;26:547–550. doi: 10.1111/jsr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of scn lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. Journal of Neuroscience. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Perceptual and Motor Skills. 2001;93:213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Bai S, Krull JL, Gonzales NA. Individual differences in optimum sleep for daily mood during adolescence. Journal of Clinical Child & Adolescent Psychology. 2017:1–11. doi: 10.1080/15374416.2017.1357126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland BC, Short MA, Terrill P, Rigney G, Haszard JJ, Coussens S, Foster-Owens M, Biggs SN. Establishing normal values for pediatric nighttime sleep measured by actigraphy: A systematic review and meta-analysis. Sleep. 2018;41 doi: 10.1093/sleep/zsy017. [DOI] [PubMed] [Google Scholar]
- Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of nrem sleep eeg: From childhood to middle age. Journal of Sleep Research. 2001;10:165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Medicine. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of americans: Findings from the national sleep foundation’s 2011 sleep in america poll. Journal of Clinical Sleep Medicine. 2013;9:1291–1299. doi: 10.5664/jcsm.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Stepanek M, Troxel WM. The economic implications of later school start times in the united states. Sleep Health. 2017;3:451–457. doi: 10.1016/j.sleh.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience. 2009;31:276–284. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Kirschen GW, LeBourgeois MK, Gradisar M, Garrison MM, Montgomery-Downs H, Kirschen H, McHale SM, Chang AM, Buxton OM. Youth screen media habits and sleep: Sleep-friendly screen behavior recommendations for clinicians, educators, and parents. Child and Adolescent Psychiatric Clinics of North America. 2018;27:229–245. doi: 10.1016/j.chc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath M, Sutherland C, Bartel K, Gradisar M, Williamson P, Lovato N, Micic G. Does one hour of bright or short-wavelength filtered tablet screenlight have a meaningful effect on adolescents’ pre-bedtime alertness, sleep, and daytime functioning? Chronobiology International. 2014;31:496–505. doi: 10.3109/07420528.2013.872121. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. Journal of Clinical Endocrinology & Metabolism. 2014;99:3298–3303. doi: 10.1210/jc.2014-1629. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Ohayon MM, Peever J, Rawding R, Sachdeva RC, Settters B, Vitiello MV, Ware JC, Hillard PJA. National sleep foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Huang S, Deshpande A, Yeo SC, Lo JC, Chee MW, Gooley JJ. Sleep restriction impairs vocabulary learning when adolescents cram for exams: The need for sleep study. Sleep. 2016;39:1681–1690. doi: 10.5665/sleep.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysing M, Haugland S, Stormark KM, Boe T, Sivertsen B. Sleep and school attendance in adolescence: Results from a large population-based study. Scandinavian Journal of Public Health. 2015;43:2–9. doi: 10.1177/1403494814556647. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- Kelley P, Lockley SW, Kelley J, Evans MDR. Is 8:30 a.M. Still too early to start school? A 10:00 a.M. School start time improves health and performance of students aged 13–16. Frontiers in Human Neuroscience. 2017;11:1–10. doi: 10.3389/fnhum.2017.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S, Hida A, Enomoto M, Watanabe M, Katayose Y, Nozaki K, Aritake S, Higuchi S, Moriguchi Y, Kamei Y, Mishima K. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biological Psychiatry. 2012;73:63–69. doi: 10.1016/j.biopsych.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Lazar AS, Santhi N, Hasan S, Lo JC, Johnston JD, Von Schantz M, Archer SN, Dijk DJ. Circadian period and the timing of melatonin onset in men and women: Predictors of sleep during the weekend and in the laboratory. Journal of Sleep Research. 2012;22:155–159. doi: 10.1111/jsr.12001. [DOI] [PubMed] [Google Scholar]
- LeBourgeois MK, Hale L, Chang AM, Akacem LD, Montgomery-Downs HE, Buxton OM. Digital media and sleep in childhood and adolescence. Pediatrics. 2017;140:S92–S96. doi: 10.1542/peds.2016-1758J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Nolan DM, Lockley SW, Pattison B. Law-based arguments and messages to advocate for later school start time policies in the united states. Sleep Health. 2017;3:486–497. doi: 10.1016/j.sleh.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Lo JC, Lee SM, Lee XK, Sasmita K, Chee N, Tandi J, Cher WS, Gooley JJ, Chee MWL. Sustained benefits of delaying school start time on adolescent sleep and well-being. Sleep. 2018 doi: 10.1093/sleep/zsy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Lee SM, Teo LM, Lim J, Gooley JJ, Chee MW. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017:40. doi: 10.1093/sleep/zsw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: The need for sleep study. Sleep. 2016;39:687–698. doi: 10.5665/sleep.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden AB, Wolfson AR. Understanding adolescent caffeine use: Connecting use patterns with expectancies, reasons, and sleep. Health Education & Behavior. 2010;37:330–342. doi: 10.1177/1090198109341783. [DOI] [PubMed] [Google Scholar]
- Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behavioral Sleep Medicine. 2011;10:70–80. doi: 10.1080/15402002.2012.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiology & Behavior. 2007;92:1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo PR, Goncalves BS, Menezes AA, Azevedo CV. Circadian activity rhythm in pre-pubertal and pubertal marmosets (callithrix jacchus) living in family groups. Physiology & Behavior. 2016;155:242–249. doi: 10.1016/j.physbeh.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Mignot E, Lin L, Finn L, Lopes C, Pluff K, Sundstrom ML, Young T. Correlates of sleep-onset rem periods during the multiple sleep latency test in community adults. Brain. 2006;129:1609–1623. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- Minges KE, Redeker NS. Delayed school start times and adolescent sleep: A systematic review of the experimental evidence. Sleep Medicine Reviews. 2016;28:86–95. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Faraguna U, Zoltan JT, Tononi G, Cirelli C. Sleep patterns and homeostatic mechanisms in adolescent mice. Brain Sciences. 2013;3:318–343. doi: 10.3390/brainsci3010318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JL, Lo JC, Gooley JJ, Chee MWL. EEG changes accompanying successive cycles of sleep restriction with and without naps in adolescents. Sleep. 2017:40. doi: 10.1093/sleep/zsx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J, Wang G, Lewin D, Skora E, Baylor A. Association between short sleep duration and risk behavior factors in middle school students. Sleep. 2016:40. doi: 10.1093/sleep/zsw004. [DOI] [PubMed] [Google Scholar]
- Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, Malow BA, Maski K, Nichols C, Quan SF, Rosen CL, Troester MM, Wise MS. Consensus statement of the american academy of sleep medicine on the recommended amount of sleep for healthy children: Methodology and discussion. Journal of Clinical Sleep Medicine. 2016;12:1549–1561. doi: 10.5664/jcsm.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AJ, Chen PY, Robinson PA. Probing the mechanisms of chronotype using quantitative modeling. Journal of Biological Rhythms. 2010;25:217–227. doi: 10.1177/0748730410369208. [DOI] [PubMed] [Google Scholar]
- Phillips AJ, Robinson PA. A quantitative model of sleep-wake dynamics based on the physiology of the brainstem ascending arousal system. Journal of Biological Rhythms. 2007;22:167–179. doi: 10.1177/0748730406297512. [DOI] [PubMed] [Google Scholar]
- Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, Lockley SW, Klerman EB, Czeisler CA. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Scientific Reports. 2017;7:3216. doi: 10.1038/s41598-017-03171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel F, Lipnevich AA, Schneider S, Roberts RD. Chronotype, cognitive abilities, and academic achievement: A meta-analytic investigation. Learning and Individual Differences. 2011;21:483–492. [Google Scholar]
- Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10–14. Sleep. 1998;21:861–868. [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Short MA, Weber N, Reynolds C, Coussens S, Carskadon MA. Estimating adolescent sleep need using dose-response modelling. Sleep. 2018:41. doi: 10.1093/sleep/zsy011. [DOI] [PubMed] [Google Scholar]
- Skeldon AC, Derks G, Dijk DJ. Modelling changes in sleep timing and duration across the lifespan: Changes in circadian rhythmicity or sleep homeostasis? Sleep Medicine Reviews. 2015;28:92–103. doi: 10.1016/j.smrv.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Schirmer AE. 3.4 million real-world learning management system logins reveal the majority of students experience social jet lag correlated with decreased performance. Scientific Reports. 2018;8:4793. doi: 10.1038/s41598-018-23044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS ONE. 2009;4:e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–177. doi: 10.1016/j.neuroscience.2012.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: Insights into adolescent sleep regulation and behavior. Journal of Sleep Research. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Temkin DA, Princiotta D, Ryberg R, Lewin DS. Later start, longer sleep: Implications of middle school start times. Journal of School Health. 2018;88:370–378. doi: 10.1111/josh.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti L, Natale V, Randler C. Association between circadian preference and academic achievement: A systematic review and meta-analysis. Chronobiology International. 2015;32:792–801. doi: 10.3109/07420528.2015.1049271. [DOI] [PubMed] [Google Scholar]
- Troxel WM, Wolfson AR. The intersection between sleep science and policy: Introduction to the special issue on school start times. Sleep Health. 2017;3:419–422. doi: 10.1016/j.sleh.2017.10.001. [DOI] [PubMed] [Google Scholar]
- van der Lely S, Frey S, Garbazza C, Wirz-Justice A, Jenni OG, Steiner R, Wolf S, Cajochen C, Bromundt V, Schmidt C. Blue blocker glasses as a countermeasure for alerting effects of evening light-emitting diode screen exposure in male teenagers. Journal of Adolescent Health. 2015;56:113–119. doi: 10.1016/j.jadohealth.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Wahlstrom K. Changing times: Findings from the first longitudinal study of later high school start times. NASSP Bulletin. 2002;86:3–21. [Google Scholar]
- Wahlstrom K, Dretzke W, Gordon MF, Peterson K, Edwards K, Gdula J. Examining the impact of later high school start times on the health and academic performance of high school students: A multi-site study. St Paul, MN: Center for Applied Research and Educational Improvement, University of Minnesota; 2014. [Google Scholar]
- Wahlstrom KL. The prickly politics of school starting times. Phi Delta Kappan. 1999;80:344. [Google Scholar]
- Wahlstrom KL. Later start time for teens improves grades, mood, and safety. Phi Delta Kappan. 2016;98:8–14. [Google Scholar]
- Watson NF, Martin JL, Wise MS, Carden KA, Kirsch DB, Kristo DA, Malhotra RK, Olson EJ, Ramar K, Rosen IM, Rowley JA, Weaver TE, Chervin RD American Academy of Sleep Medicine Board of D. Delaying middle school and high school start times promotes student health and performance: An american academy of sleep medicine position statement. Journal of Clinical Sleep Medicine. 2017;13:623–625. doi: 10.5664/jcsm.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert D, Kompauerova V. Light-induced phase and period reponses of circadian activity rhythms in laboratory mice of different age. Zoology. 1998;101:45–52. [Google Scholar]
- Wheaton AG, Chapman DP, Croft JB. School start times, sleep, behavioral, health, and academic outcomes: A review of the literature. Journal of School Health. 2016;86:363–381. doi: 10.1111/josh.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Understanding adolescents’ sleep patterns and school performance: A critical appraisal. Sleep Medicine Reviews. 2003;7:491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Harkins E, Johnson M, Marco C. Effects of the young adolescent sleep smart program on sleep hygiene practices, sleep health efficacy, and behavioral well-being. Sleep Health. 2015;1:197–204. doi: 10.1016/j.sleh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson AR, Spaulding NL, Dandrow C, Baroni EM. Middle school start times: The importance of a good night’s sleep for young adolescents. Behavioral Sleep Medicine. 2007;5:194–209. doi: 10.1080/15402000701263809. [DOI] [PubMed] [Google Scholar]
- Wood B, Rea MS, Plitnick B, Figueiro MG. Light level and duration of exposure determine the impact of self-luminous tablets on melatonin suppression. Applied Ergonomics. 2013;44:237–240. doi: 10.1016/j.apergo.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Wright KP, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. Journal of Biological Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]