Abstract
Interoception refers to the process by which the nervous system senses, interprets, and integrates signals originating from within the body, providing a moment-by-moment mapping of the body’s internal landscape across conscious and unconscious levels. Interoceptive signaling has been considered a component process of reflexes, urges, feelings, drives, adaptive responses, and cognitive and emotional experiences, highlighting its contributions to the maintenance of homeostatic functioning, body regulation, and survival. Dysfunction of interoception is increasingly recognized as an important component of different mental health conditions, including anxiety disorders, mood disorders, eating disorders, addictive disorders, and somatic symptom disorders. However, a number of conceptual and methodological challenges have made it difficult for interoceptive constructs to be broadly applied in mental health research and treatment settings. In November 2016, the Laureate Institute for Brain Research organized the first Interoception Summit, a gathering of interoception experts from around the world, with the goal of accelerating progress in understanding the role of interoception in mental health. The discussions at the meeting were organized around four themes: interoceptive assessment, interoceptive integration, interoceptive psychopathology, and the generation of a roadmap that could serve as a guide for future endeavors. This review article presents an overview of the emerging consensus generated by the meeting.
Keywords: Biomarker, Computational psychiatry, Interoception, Mental health, Research Domain Criteria, Treatment
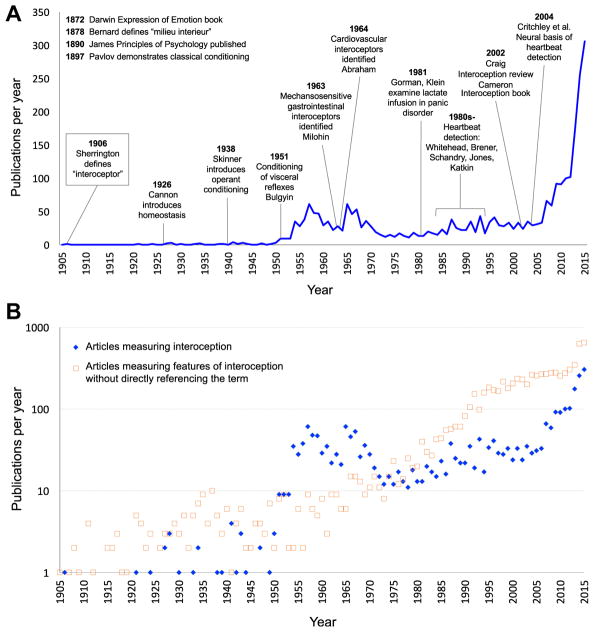
Interoception refers collectively to the processing of internal bodily stimuli by the nervous system. Parcellation of the nervous system’s processing of sensory signals into interoception, proprioception, and exteroception began more than 100 years ago (1), although it was predated by interest in linking body–brain interactions with conscious experience (2,3). Scientific interest in interoception has fluctuated (Figure 1A). During the 1980s, biological psychiatry was inundated with observations of interoceptive disturbances in panic disorder (4–7), although the trend receded after it became clear that the etiological mechanism was broader than a single molecular receptor target (8). Recent years have witnessed a surge of interest on the topic of interoception due in part to findings highlighting its integral role in emotional experience, self-regulation, decision making, and consciousness. Importantly, interoception is not limited to conscious perception or even unique to the human species. From this perspective, interdisciplinary efforts to understand different features of interoception have been essential for advancing progress in cognitive and clinical neuroscience (Figure 1B).
Figure 1.
(A) Number of English language publications per year on interoception from PubMed, PsycINFO, and Institute for Science Information Web of Knowledge. The timeline starts in 1905, one year before the publication of Charles Sherrington’s book, The Integrative Action of the Nervous System, which first defined the concept of interoception. Key historical events relevant to interoception science are superimposed. (B) Publications per year on interoception vs. those investigating features of interoception that do not specifically refer to the term. These latter publications are more numerous and arise mainly from basic neuroscience, physiology, and subspecialty disciplines within the biomedical field. Note the use of a logarithmic scale in the second panel. [Figure reproduced and modified with permission from Khalsa and Lapidus (33).]
ASSESSMENT
Body Systems of Interoception
Interoceptive processing occurs across all major biological systems involved in maintaining bodily homeostasis, including the cardiovascular (9,10), pulmonary (11), gastrointestinal (12,13), genitourinary (14), nociceptive (15), chemosensory (16), osmotic (17), thermoregulatory (18), visceral1 (19), immune (20,21), and autonomic systems (22,23) (Table 1). There has been relatively little focus overall on the integration across bodily systems; thus, it is not surprising that most investigations of the topic have been siloed within distinct research areas or scientific disciplines [see (24,25) for noteworthy exceptions].
Table 1.
Physiological Processes Often Ascribed to Interoception
| Nonpainful |
| Cardiovascular, respiratory, gastrointestinal (esophageal, gastric, intestinal, colorectal), bladder, hunger, thirst, blood/serum (pH, osmolality, glucose), temperature, vasomotor flush, air hunger, muscle tension, shudder, itch, tickle, genital sensation, sensual touch, fatigue |
| Painful |
| Visceral: kidney stone, pleuritic, angina, pericardial, bowel ischemia, pelvic, sickle crisis |
| Somatic: abscess/boil, bruising, myalgia, inflammation (systemic/laceration), headache |
| Skeletal: fractured/bruised bone, stress fracture, inflammatory/mechanical joint pain |
Several key distinctions are that interoceptive sensing 1) may be painful or nonpainful, 2) occurs across the spectra of high/low arousal and negative/positive valence, 3) usually occurs outside of conscious awareness (with the exception of pain sensations), and 4) is often (but not always) consciously experienced during instances of homeostatic perturbation.
Features of Interoception
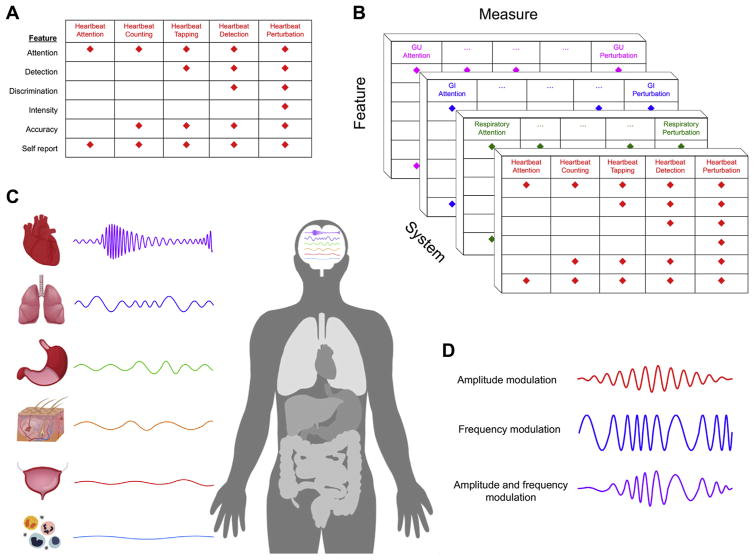
Interoception is not a simple process but rather has several facets (26). The act of sensing, interpreting, and integrating information about the state of inner body systems can be related to different elements such as interoceptive attention, detection, discrimination, accuracy, insight, sensibility, and self-report (Table 2). However, most interoceptive processes occur outside the realm of conscious awareness. Consciously experienced elements are measured clinically via subjective report, and there are few observable interoceptive signs (e.g., heart rate, respiration rate, pupillary dilation, flushing, perspiration, piloerection, nociceptive reflexes) (Table 3). Experimental approaches can quantify different body systems and features of interoceptive processing. Nevertheless, these measures are only partially overlapping and likely reflect somewhat distinct neural processes (27). Access to the full range of interoceptive signals often involves invasive approaches, which tend to elicit physiological perturbations and index more objectively measurable features (28). However, many insights have been gained by the application of noninvasive approaches within neuroscience and psychological assessment contexts (29) (see “Eavesdropping on Brain–Body Communication” section below).
Table 2.
Features of Interoceptive Awareness
| Feature | Definition |
|---|---|
| Attention | Observing internal body sensations |
| Detection | Presence or absence of conscious report |
| Magnitude | Perceived intensity |
| Discrimination | Localize sensation to a specific channel or organ system and differentiate it from other sensations |
| Accuracy (Sensitivity) | Correct and precise monitoring |
| Insight | Metacognitive evaluation of experience/performance (e.g., confidence–accuracy correspondence) |
| Sensibility | Self-perceived tendency to focus on interoceptive stimuli (trait measure) |
| Self-report Scales | Psychometric assessment via questionnaire (state/trait measure) |
For some examples of paradigms assessing each feature, see Supplemental Table S1.
Table 3.
Diagnostic Symptoms and Clinical Signs Indicating Interoceptive Dysfunction in Some Psychiatric Disorders
| Psychiatric Disorder | Symptoms | Signs | Sample Studies |
|---|---|---|---|
| Panic Disorder | Palpitations, chest pain, dyspnea, choking, nausea, dizziness, flushing, depersonalization/derealization | Elevated heart rate and/or blood pressure, exaggerated escape, startle, and flinching | (5,140,141) |
| Depression | Increased or decreased appetite, fatigue, lethargy | Weight gain, weight loss, psychomotor slowing | (142,143) |
| Eating Disorders | Hunger insensitivity, food anxiety, gastrointestinal complaints | Severe food restriction, severe weight loss, binging, purging, compulsive exercise | (72,98) |
| Somatic Symptom Disorders | Multiple current physical and nociceptive Symptoms | Medical observations do not correspond with symptom report | (144,145) |
| Substance Use Disorders | Physical symptoms associated with craving, intoxication, and/or withdrawal (drug specific) | Elevated/decreased: heart rate, respiratory rate, and/or blood pressure, pupil dilation/constriction, others (drug specific) | (101,146,147) |
| Posttraumatic Stress Disorder | Autonomic hypervigilance, depersonalization/derealization | Exaggerated startle, flinching, and/or escape responses, elevated heart rate and/or blood pressure | (148) |
| Generalized Anxiety Disorder | Muscle tension, headaches, fatigue, Gastrointestinal complaints, pain | Trembling, twitching, shaking, sweating, nausea, exaggerated startle | (149,150) |
| Depersonalization/Derealization Disorder | Detachment from one’s body, head fullness, tingling, lightheadedness | Physiological hyporeactivity to emotional stimuli | (151,152) |
| Autism Spectrum Disorders | Skin hypersensitivity | Selective clothing preferences | (107,153,154) |
Importance of an Interoceptive Taxonomy
There is no generally agreed-on taxonomy for interoception science. Variable definitions have made it difficult to identify the features under investigation, let alone evaluate the quality of the findings. Based on the number of physiological systems involved, it could be questioned whether the terms “interoception” and “interoceptive awareness” are too broad. Interoceptive awareness is an umbrella term that was first used to describe a self-report subscale (30), but it has subsequently been used to encompass any (or all) of the different interoception features accessible to conscious self-report. Researchers from different fields developed definitions that only partially overlapped, reflecting the need for operationalization in neuroscience (31,32) and clinical practice (33,34). Here we develop a more coherent nomenclature for its various components (Table 2), mirroring developments in other fields, especially pain (35). One key aspect is the importance of distinguishing sensation (i.e., the raw signals conveyed by bodily sensors) from perception (36,37). We return to this theme below.
Multilevel Investigations
While interoception research to date has typically focused on single organ systems, an expanded approach that assesses multiple interoceptive organ systems and/or elements is needed. Examples include targeting numerous interoceptive features simultaneously and employing different tasks that converge on the same feature (e.g., combining top-down assessments of interoceptive attention with bottom-up perturbation approaches in the same individual) (Figure 2A).
Figure 2.
(A) Cardiac interoceptive processing measures and feature loading. This illustrates how the most commonly used heartbeat perception tasks differentially measure interoceptive features. [Figure reproduced and modified with permission from Khalsa and Lapidus (33).] (B) Multisystem interoceptive processing measures and feature loadings: Example of hypothetical approaches to measuring interoceptive processing across multiple systems. Approaches that perturb the state of the body are recommended, as are convergent approaches such as combined assessments of interoceptive attention and perturbation. (C) Central neural integration of interoceptive rhythms. Interoceptive rhythms vary considerably across the different systems of the body. They exhibit complex characteristics and are hierarchically integrated within discrete regions of the central nervous system. [Figure modified, with permission, from Petzschner et al. (37).] (D) Interoceptive rhythms vary in both amplitude and frequency. The top trace illustrates a hypothetical example of an amplitude modulation signal superimposed on a static frequency. The middle trace illustrates a hypothetical frequency modulation signal superimposed on a static amplitude. The bottom trace illustrates a hypothetical signal change involving both amplitude and frequency modulations that are temporally correlated. GI, gastrointestinal; GU, genitourinary.
Sensing Perturbations
The inner and outer worlds of the body constantly fluctuate. The nervous system monitors these environmental changes and responds adaptively in order to maintain a homeostatic balance and promote survival. Because psychiatric disorders often promote or reflect the development of chronic homeostatic and allostatic disturbances (38), there is a need for methods capable of eliciting homeostatic perturbations in controlled settings, especially those assessing subjective and behavioral responses to valence and arousal deviations. However, interoception is not simply about afferent processing. The brain’s constant monitoring of the body occurs in service of optimizing homeostatic regulation. This efferent limb is understudied (39), and paradigms that can effectively measure visceromotor outputs will be critical to establish sensitive assays of dysfunctional interoception and homeostatic regulation (e.g., detection of visceromotor-efferent neural signals controlling baroreflex sensitivity during modulation of visceral-afferent input by sympathetic drugs). The reliability and validity of methods should be rigorously established.
INTEGRATION
Interoception and Domain Specificity Within the Brain
There are fundamentally differing ways to interpret the evolution of brain and body signaling in humans. The processing of interoceptive input could be domain specific, with modular processing occurring in specialized, encapsulated neural circuits [e.g., cardiac, respiratory, urinary, genital, chemical, hormonal; see (40) for a review of domain specificity] or functionally coupled (e.g., cardiorespiratory, genitourinary, chemohormonal) and integrated within a single neural circuit. Understanding the adaptive origins and functions of interoceptive domain specidicity (if present) could tell us how the implementation and deployment of interoceptive signals by the nervous system contributes to disordered mental health. Because interoceptive signaling involves afferent and efferent inputs across multiple hierarchies within the autonomic and central nervous systems, identifying where and how information processing dysfunctions negatively affect mental health represents a challenging problem.
Neural Pathways of Interoception
Several pathways have been implicated in the neural processing of interoceptive signals, beginning with a rich interface between autonomic afferents and the central nervous system. Relay pathways involve primarily spinal, vagal, and glossopharyngeal afferents, with multiple levels of processing and integration in autonomic ganglia and spinal cord (10,19,22,41). Several brainstem (nucleus of the solitary tract, parabrachial nucleus, and periaqueductal gray), subcortical (thalamus, hypothalamus, hippocampus, and amygdala), and cortical regions (insula and somatosensory cortices) represent key afferent processing regions (22,42,43). A complementary set of regions involved in visceromotor actions represents key efferent processing regions, including the anterior insula, anterior cingulate, subgenual cingulate, orbitofrontal, ventromedial prefrontal, supplementary motor, and premotor areas (44–46). It is noteworthy that these neural regions coincide closely with other sensory processing systems, especially the nociceptive and affective systems. The degree to which these represent distinct or overlapping systems is currently unclear.
Linking Paradigms Across Units of Analysis
A particular challenge when examining interoception is the fact that afferent sensory signals are integrated on several levels (peripherally, within the spinal cord, and supraspinally) to form sets of interoceptive maps across different body systems. The brain appears to integrate information representing particular states of multiple systems simultaneously (cardiac, respiratory, chemical, hormonal, nociceptive, etc.) (41), and it is imperative to be able to model and comparatively evaluate such mappings (Figure 2B). This poses many challenges. One approach might be to apply measures that assess multiple organ systems or interoceptive features simultaneously [see (42,47,48)] or to record activity across the brain, spinal cord, and peripheral organs (49). However, it is also possible that multisystem assessments may reduce specificity for certain disorders and therefore may be unnecessary. For example, some patients with panic disorder may experience dyspnea but not palpitations. Localizing and then targeting the dysfunctional interoceptive domain would become more useful than broad multisystem interventions.
Timing and Rhythm in Interoceptive Circuits
The physiological timescales and amplitudes of interoceptive signaling vary dramatically (e.g., heart rate [0.5–3.3 Hz], respiratory rate [0.08–1 Hz], gastric contractility [0.05–0.1 Hz], urinary frequency [0.000045–0.00012 Hz]), with even slower changes in humoral mediators (50) (Figure 2C, D). They also vary across individuals, and over the life span (e.g., increased heart rates in infants/children). Despite the variance, the brain tracks such changes in similar subregions, including the insula, somatosensory cortices, cingulate, amygdala, thalamus, and brainstem (42,43,51–53). Temporal synchrony or dyssynchrony between these systems may affect interoceptive experiences, affect, and behavior, although the exact mechanisms require further study (54). Repetitive events are another important element for learning, and while there are numerous classic studies on visceral learning at the peripheral organ system level (55,56), we know little about the central mapping of learned visceral memories, especially in psychiatric disorders (57).
How Can Animal Research Improve the Understanding of Human Interoceptive Processing?
Although the inability to measure the subjective state of animals results in indirect inferences, well-established tasks exist [e.g., conditioned interoceptive place preference (58) and odor aversion (59)]. The principal utility of animal models is the hypothesis testing of mechanistic processes at the biological level independent of appraisal and cognition. These include examining effects of peripheral or central nervous system lesions on physiology/behavior, or mapping of peripheral/central interactions via stimulation of selective neurons/circuits using optogenetic methods (60,61), and targeted gene expression manipulation to test genetic hypotheses (62). Animal models are advantageous in that they allow for identification of neural mechanisms that may be distinct from higher cognitive processes (e.g., nonmammalian [reptiles/birds] vs. mammalian [mice/rats/monkeys/apes/chimpanzees], invertebrate [octopus] vs. vertebrate [fish/monkeys]). The study of interoception in nonhuman primates offers intriguing opportunities. Investigations in this area have been centered primarily on neural encoding of baroreceptor afferent stimulation (9) and neuroanatomical circuit tracing (63). Fewer studies have examined relationships between mechanistic manipulation of interoceptive experiences and neural representation in these animals [see (64,65) for exceptions].
Eavesdropping on Brain–Body Communications
Interoception is manifested by the conversation between the body and brain via multiple afferent and efferent feedback loops (41,66). Listening in on this process requires different approaches. Peripheral perturbations are often used to stimulate the afferent bottom-up transfer of information, usually of mechanical (28,47,52,53), chemical (67–69), or hormonal (70) origin (Supplemental Table S1). Central perturbations to probe efferent top-down processes have most typically involved selective regulation of attentional focus (29,71) and, less commonly, expectancy manipulations such as placebo/sham delivery (72). Functional magnetic resonance imaging (73), positron emission tomography (74), and electroencephalography (75,76) have provided the primary means of assessing neural circuitry. However, a host of novel tools are capable of inhibiting, stimulating, or modulating the activity of interoceptive brain networks. Noninvasive methods include the application of transcranial magnetic stimulation (77), transcranial direct and alternating current stimulation (78), low-intensity focused ultrasound (79), temporally interfering electric fields (80), transcutaneous vagus nerve stimulation (81), presentation of information during different phases of visceral rhythms (e.g., cardiac systole vs. diastole) (82), and assessment of corticocardiac signaling (83). An important point is that many of the critical brain structures are difficult to modulate noninvasively because they are located deep within the brain or near the midline. Invasive measures do not share this limitation, and while their implementation is driven by clinical concerns, they can provide important insights. These include implanted vagus nerve stimulation (84), direct brain stimulation (85), and intracranial electrode recordings (86,87). Beyond these perturbation tools, the use of experimental methods to modulate expectancies, such as placebo and sham interventions, is key. These methods will help to determine how sensitive psychiatric and other clinical patients’ afferent/efferent feedback loops are to processes requiring integrations of environmental context with body–brain signals (illustrated in the next section). Finally, neurofeedback (e.g., functional magnetic resonance imaging, electroencephalogram) represents an exciting opportunity to participate in the brain–body conversation by simultaneously measuring and modulating brain regions during treatment [for a noninteroceptive example, see (88)]. Equipped with these tools, the future looks promising, but to advance progress they need to be paired with better models of brain function.
Computational Theories of Interoception
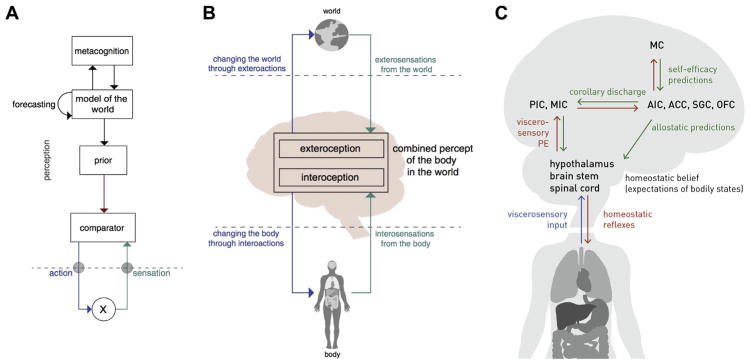
Identifying the state of the body represents a problem that cannot be solved by pure sensing because afferent signals from body sensors (interosensations) are not only noisy but often ambiguous (89). Recent computational theories suggest that interoception deploys Bayesian inference to address this challenge (36,37,44,45,90,91) (Figures 3 and 4). Specifically, the brain is assumed to construct a so-called generative model of interosensations that combines a predictive mapping (from hidden bodily states to interosensations) with prior information (beliefs or expectations about bodily states represented as probability distributions). This view is supported by findings that interoceptive perception is strongly shaped by expectations (41,72,92,93) and by theoretical arguments that suggest Bayesian inference as a unifying principle for interoception and exteroception (37,91).
Figure 3.
(A) Example of one possible form of a general inference–control loop illustrated within a hierarchical Bayesian model. (B) Highly schematic example of illustrating that both interoceptive information and exteroceptive information are concurrently integrated to inform perceptual representations and action selection with respect to internally directed (e.g., visceromotor, autonomic) and externally directed (e.g., skeletomotor) actions. (C) General nodes that comprise a peripheral and central neural circuit for hierarchically integrating afferent interoceptive information into homeostatic reflexes, sensory and meta-cognitive representations, and allostatic regulators (predictions). ACC, anterior cingulate cortex; AIC, anterior insular cortex; MC, metacognitive layer; MIC, midinsular cortex; OFC, orbitofrontal cortex; PE, prediction error; PIC, posterior insular cortex; SGC, subgenual cortex. [Panels (A) and (B) reproduced, with permission, from Petzschner et al. (37). Panel (C) adapted, with permission, from Stephan et al. (36).]
Figure 4.
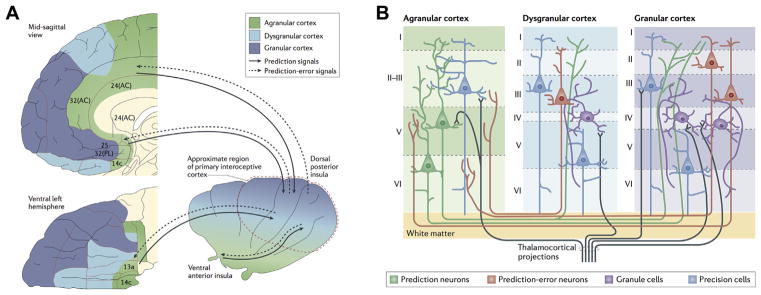
(A) Active inference implementation according to the Embodied Predictive Interoception Coding model. Agranular visceromotor cortices, including the cingulate cortex, posterior ventral medial prefrontal cortex, posterior orbitofrontal cortex, and ventral anterior insula, estimate the balance among autonomic, metabolic, and immunological resources available to the body and its predicted requirements. These agranular visceromotor cortices issue allostatic predictions to hypothalamus, brainstem, and spinal cord nuclei to maintain a homeostatic internal milieu and simultaneously to the primary interoceptive sensory cortex in the mid and posterior insula. The interoceptive sensory cortex in the granular mid and posterior insula sends reciprocal prediction error signals back to the agranular visceromotor regions to modify the predictions. Under usual circumstances, these agranular regions are relatively insensitive to such feedback, which explains why interoceptive predictions are fairly stable in the face of body fluctuations. One hypothesis of the role of interoception in mental illness is that interoceptive input (i.e., posteriors) becomes increasingly decoupled from interoceptive predictions issued by the agranular visceromotor cortex (priors), leading to increased interoceptive prediction error signals. This decoupling may present in the brain as “noisy afferent interoceptive inputs” (97). (B) Proposed intracortical architecture and intercortical connectivity for interoceptive predictive coding. The granular cortex contains six cell layers including granule cells, which are excitatory neurons that amplify and distribute thalamocortical inputs throughout the column. The granular cortex is structurally similar to the neocortex and therefore more recently evolved than the agranular and dysgranular cortices. Within the insula, the granular cortex is present in the mid and posterior sectors. AC, anterior cingulate; PL, prelimbic cortex. [Figures reproduced, with permission, from Barrett and Simmons (45).]
Another argument supporting a Bayesian view on interoception is its relation to what constitutes arguably the brain’s most fundamental task: the regulation (or control) of bodily states. Put simply, if the brain were unable to resolve the ambiguity of interosensations, it would face difficulties in choosing appropriate actions to protect homeostasis. In information-theoretic terms, the challenge of keeping bodily states within narrow homeostatic ranges corresponds to choosing actions that minimize the long-term average Shannon surprise (entropy) of interosensations (36,91). Solving this control problem requires knowledge or estimations of current and/or future bodily states and hence inference and predictions/forecasts—two natural domains of generative models.
Eliciting surprise-minimizing (homeostasis-restoring) actions changes the bodily state and thus interosensations. This means that inference and control of bodily states form a closed loop. Inference–control loops that minimize interoceptive surprise can be cast as hierarchical Bayesian models (HBMs). Anatomically, HBMs are plausible candidates given that interoceptive circuitry is structured hierarchically (45,94). Under general assumptions, HBMs employ a small set of computational quantities—predictions, prediction errors, and precisions (37,95). These quantities can support surprise minimization in two ways: by adjusting beliefs (probability distributions) throughout the hierarchy [predictive coding (95)] or engaging actions that fulfill beliefs about bodily states [active inference (96)].
HBMs support both homeostatic (reactive) and allostatic (prospective) control. Reconsidering classical homeostatic set points as beliefs (i.e., probabilistic representations of expected/desired bodily states) enables reactive regulation at the bottom of the hierarchy (36,91); here, prediction errors elicit reflex-like actions that minimize momentary interoceptive surprise. Allostatic regulation at longer time scales is achieved through modulation of homeostatic beliefs by inferred or forecast states signaled from higher hierarchical levels (36). Importantly, belief precision determines the force/pace of corrective actions—that is, the tighter the expected range of bodily state, the more vigorous the elicited regulatory action. This offers a novel explanation for psychosomatic phenomena and placebo effects (37).
In summary, a hierarchical Bayesian perspective unifies interoception and homeostatic/allostatic control under the same computational principles. This provides a conceptual foundation for computational psychosomatics and supports a taxonomy of disease processes (37). One caveat is that the empirical evidence for hierarchical Bayesian principles of interoception and homeostatic/allostatic control is indirect so far. Studies designed to probe hierarchical Bayesian processes under experimentally controlled homeostatic perturbations will be crucial for finessing (or refuting) current computational concepts of interoception.
PSYCHOPATHOLOGY
Interoceptive Psychopathology
Several conceptual and heuristic models have linked dysfunctions of interoception to mental health conditions. Specifically, mood and anxiety disorders have been linked to failures to appropriately anticipate changes in interoceptive states (97). Eating disorders show behavioral and neural abnormalities in interoceptive processing, particularly in the context of caloric anticipation (72,98–100), although it remains unclear whether this is due to altered afferent signaling, altered central sensory processing, abnormal temperament, and/or metacognition. Drug addiction, another condition marked by interoceptive disturbances, has an overlapping neural circuitry and abnormal responses to interoceptive cues (101–104). Interoceptive dysfunction also likely plays a role in conditions such as posttraumatic stress disorder and somatic symptom disorders (33). Other disorders also have interoceptive symptom overlap; however, the specific feature involved may differ according to the disorder or affected individual [e.g., chronic pain (105,106), Tourette’s syndrome and other tic disorders, borderline personality disorder, obsessive-compulsive disorder, autism spectrum disorder (107), functional developmental disorders (108)]. Table 3 lists diagnostic symptoms and clinical signs indicative of interoceptive dysfunction in several psychiatric disorders. Conditions that have a psychiatric component include fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and functional disorders within medicine (e.g., noncardiac chest pain, functional dysphagia) as well as certain medical disorders (e.g., gastroesophageal reflux, asthma).
Alternatively, one can use a dimensional psychopathology approach to link processes underlying interoceptive dysfunction to psychiatric disorders. Transdiagnostic perspectives such as those provided by the Research Domain Criteria (109) may be particularly helpful in identifying the potential role played by various interoceptive processes because several of these might not be readily identified at the symptom report level relied on by clinicians and, accordingly, might not have entered into the diagnostic specifications for DSM. This would allow for identification of mechanistic dysfunctions across units of analyses and might bridge the biological gap in current diagnostic classification frameworks by directly probing the links between physiological and psychological dysfunctions. Interoceptive investigations in mental health populations might reveal evidence of 1) attentional bias (e.g., hypervigilance), 2) distorted physiological sensitivity (e.g., blunted or heightened magnitude estimation in response to a perturbation), 3) cognitive bias (e.g., catastrophizing in response to an anticipated stimulus), 4) abnormal sensibility (e.g., tendency to label one’s experiences in a particular way), and 5) impaired insight (e.g., poor confidence–accuracy correspondence on a task).
Determining whether interoceptive processes are a cause or consequence of developmental psychopathology, and which factors might affect this development (such as early life stress or pain), will be an important area for future research. Such studies may benefit from the examination of younger (110,111) or older (112,113) samples and premorbid identification and longitudinal tracking of individuals (114). Investigating the role of social cognition/theory of mind in clinically relevant interoceptive inference generation represents another ripe opportunity (115).
Interoceptive Tests and/or Biomarkers
Because interoception is fundamentally a process linking body and brain, it is conceivable that objective measures of this process could serve as biological indicators of disease states. However, there is currently limited evidence for interoceptive predictors of diagnostic, prognostic, or treatment status (33,116,117). Biomarkers, such as those derived from neuroimaging or blood measurements, should be sensitive, specific, and unaffected by cognitive and emotional influences. However, it seems conceivable that the most clinically sensitive interoceptive measures might derive from probes that perturb physiological functions to engage specific metacognitive beliefs and/or expectations about bodily states. Such measures could facilitate differential diagnosis testing by revealing the presence of interoceptive dysfunction of biological (within a physiological system or systems), psychological (e.g., overly precise expectations about bodily states), or metacognitive (e.g., discrepant self-efficacy beliefs with regard to homeostatic/allostatic regulation) origin (37). This approach could be seen as analogous to a cardiac stress test, such that adequate engagement of the system under ecologically valid conditions is required in order to measure its dysfunction.
The most common application of interoceptive evaluation in current clinical practice occurs during interoceptive exposure psychotherapy for panic disorder (118). During this procedure, patients self-induce varieties of interoceptive symptoms via low-arousal manipulations (e.g., hyperventilation, performing jumping jacks, spinning in a chair, breathing through a straw) while the clinician monitors their subjective distress level. Unfortunately these manipulations often fail to adequately reproduce the fear response, possibly because the patient retains full control over the stimulation (the patient can quit at any time) and the perturbation remains predictable with minimal uncertainty, raising the question of whether modulating both physiological homeostasis and the perception of controllability might further improve the ecological validity and efficacy of interoceptive exposures (119). A test to verify successful interoceptive exposure therapy for panic disorder involves completion of a standardized behavioral avoidance paradigm (120). In this setting, the degree of tolerance to being enclosed in a small dark chamber for 10 minutes might provide behavioral evidence verifying tolerance to triggers of interoceptive dysregulation. There is also experimental evidence that pharmacological interoceptive exposure therapy can reduce anxiety disorder symptom severity either as monotherapy (7,121–123) or as an augmentative approach (124). However, there are few studies of these procedures to date, the impact of such interventions on longer term outcomes (e.g., 6 months or beyond) are unknown, and none of these approaches has translated into clinical practice.
Current Treatments Relevant to Interoception
Among the currently available therapies with an interoceptive basis are pharmacotherapies directly modulating interoceptive physiology. Examples include adrenergic blockade (e.g., propranolol) or agonism (e.g., yohimbine), stimulants (e.g., methylphenidate), benzodiazepines, muscle relaxants, and opioids. A second example is cognitive behavioral therapy with exposure and response prevention to reverse or attenuate conditioned fears or form new learned associations. It is helpful in ameliorating cognitive biases in numerous disorders, including depression, obsessive-compulsive disorder, posttraumatic stress disorder (specifically prolonged exposure therapy), irritable bowel syndrome, and chronic pain. Interoceptive exposure is a special example demonstrated to be effective in specific disorders (especially panic disorder). Behavioral activation therapy for depression sometimes includes exposure to experiences with positive interoceptive value. A third example is capnometry-assisted respiratory training. Based on the assumption that sustained hypocapnia resulting from hyperventilation is a key mechanism in the production and maintenance of panic, carbon dioxide capnography-assisted therapy aims to help patients voluntarily increase end-tidal partial pressure of carbon dioxide and tolerate physiological variability associated with panic attacks (125,126). As a fourth example, mindfulness-based stress reduction, yoga, and other meditation/movement-based treatments may be aimed at improving metacognitive awareness of mind–body connections by systematically attending to sensations of breathing, cognitions, and/or other modulated body states (e.g., muscle stretching) (127).
Interoceptive Treatments on the Horizon
Several emerging technologies may have relevance for interoception and mental health, including Floatation-REST (reduced environmental stimulation therapy) and perturbation approaches.
Floatation-REST
This intervention, which systematically attenuates exteroceptive sensory input to the nervous system, also appears to noninvasively enhance exposure to interoceptive sensations such as the breath and heartbeat (128). Preliminary data suggest that a single 1-hour session has a short-term anxiolytic and antidepressant effect in patients with comorbid anxiety and depression (129), but further research is needed to evaluate the safety, feasibility, and potential for long-term efficacy in psychiatric populations.
Perturbation Approaches
Minimally invasive tools capable of systematically modulating interoceptive processing, such as inspiratory breathing loads (130), core body thermomodulation (131,132), and transcutaneous vagus nerve stimulation (133), are several approaches awaiting further investigation. Given the hypothesis of noisy baseline afferent signaling, these approaches may systematically enhance the signal-to-noise ratio and facilitate interoceptive learning. A key aspect in discerning clinical efficacy of any perturbation may be the extent to which the patient perceives controllability over the intervention and is willing/able to surrender this parameter in treatment. Interventions in which escape or active avoidance behaviors are directly measurable may provide especially meaningful information (134).
ROADMAP
The Road Ahead
Beyond the issues outlined previously, progress in determining the relevance of interoception for mental health relies on emphasizing the features that distinguish it from other sensory modalities. Interoception seemingly involves a high degree of connectivity within the brain (135). It appears to be tightly linked to the self and survival through homeostatic maintenance of the body, and by helping us to represent how things are going in the present with respect to the experienced past and the anticipated future. These computations may depend on what has occurred to shape the body’s internal landscape, and it is in this regard that learning, and malleability of representations over time, could play important roles.
The conceptual framework for investigating interoception may overlap with other processes, including emotion (136) and pain (137), because each is integral for maintaining bodily homeostasis. An important endeavor may involve the identification of which neural systems for interoception, emotion, cognition, and pain are overlapping, interdigitating, or even possibly identical. Additional effort is needed to define the neurophysiological nomenclature, core criteria, common features, developmental aspects, modulating factors, functional consequences, and putative pathophysiologic mechanisms of interoception in mental health disorders.
The current work offers some conceptual distinctions and some mutually agreed-on terminology, with many others still needed. Several low-hanging fruits, as well as promising emerging technologies and tools, have been mentioned. Further empirical work will be critical to delineate how interoception can be mapped to mental health measures, models, and approaches, and benchmarks for success/failure need to be established. Models of interoceptive processing that improve on the traditional stimulus, sensorimotor processing, and response function concepts have been described, but these models remain theoretical and await further testing. Therefore, the current document is best viewed as a work in progress.
Supplementary Material
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURES
We express our sincere appreciation to the William K. Warren Foundation for supporting the Interoception Summit 2016 and to all of the Laureate Institute for Brain Research staff members for their assistance with facilitating the meeting.
AEM reports the following disclosures: research/grant: National Institutes of Health (NIH) Grant No. 1U01EB021952-01; scientific advisory board: Anxiety and Depression Association of America. AvL reports the following disclosures: research/grants: Research Fund KU Leuven, Belgium (Grant Nos. STRT/13/002 and DBOF/14/021), an infrastructure grant from the Herculesstichting, Belgium (Grant No. AKUL/13/07), “Asthenes” long-term structural funding Methusalem grant (Grant No. METH/15/011) by the Flemish Government, Belgium. CBN reports the following disclosures: research/grant: NIH, Stanley Medical Research Institute; consulting (last 3 years): Xhale, Takeda, Taisho Pharmaceutical, Inc., Prismic Pharmaceuticals, Bracket (Clintara), Total Pain Solutions, Gerson Lehrman Group Healthcare & Biomedical Council, Fortress Biotech, Sunovion Pharmaceuticals, Inc., Sumitomo Dainippon Pharma, Janssen Research & Development, LLC, Magstim, Inc., Navitor Pharmaceuticals, Inc., TC MSO, Inc.; stockholder: Xhale, Celgene, Seattle Genetics, Abbvie, OPKO Health, Inc., Bracket Intermediate Holding Corporation, Network Life Sciences, Inc., Antares; scientific advisory boards: American Foundation for Suicide Prevention (AFSP), Brain and Behavior Research Foundation (formerly National Alliance for Research on Schizophrenia and Depression [NARSAD]), Xhale, Anxiety and Depression Association of America (ADAA), Skyland Trail, Bracket (Clintara), RiverMend Health, LLC, Laureate Institute for Brain Research, Inc.; board of directors: AFSP, Gratitude America, ADAA; income sources or equity of $10,000 or more: American Psychiatric Publishing, Xhale, Bracket (Clintara), CME Outfitters, Takeda; patents: method and devices for transdermal delivery of lithium (Patent No. US 6,375,990B1), method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (Patent No. US 7,148,027B2). HDC reports the following disclosures: research/grants: European Research Council Horizon 2020 Proof of Concept Grant “HeartRater: Tools for the systematic evaluation of interoceptive ability,” Medical Research Council (UK) MRC Confidence in Concept Grant “Identifying neural, cognitive, and phenomenological markers of auditory verbal hallucinations in borderline personality,” MQ (Mental Health) PsyImpact “Aligning Dimensions of Interoceptive Experience (ADIE) to prevent development of anxiety disorders in autism,” Dr. Mortimer and Theresa Sackler Foundation Sackler Centre for Consciousness Science, University of Sussex, BIAL Foundation Bursary “Microneurography as a tool for consciousness science”; scientific advisory boards: Emteq, Ltd., unpaid governor on board of charity “Reflecting nature in art & science”; board of directors: Codirector of Sackler Centre for Consciousness Science, University of Sussex. KES reports the following disclosures: research/grants: Deutsche Forschungsgemeinschaft, Transregional Collaborative Research Centre, “Ingestive Behaviour: Homeostasis and Reward,” René and Susanne Braginsky Foundation. LPS reports the following disclosures: scientific advisory board: Laureate Institute for Brain Research, Inc. JDF reports the following disclosures: research/grants: NIH Grant Nos. R01MH105662, R21MH110865, and R01HD087712; scientific advisory boards: International Obsessive-Compulsive Disorder Foundation Clinical and Scientific Advisory Board. JLR reports the following disclosures: research/grants: NIH Grant No. R01MD007807 and Oklahoma Center for the Advancement of Science and Technology Grant No. HR15-079. JSF reports the following disclosures: research/grants: NIH/National Institute of General Medical Sciences (NIGMS) Grant No. P20GM121312, Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator Award. MBS reports the following disclosures: scientific advisory board: Laureate Institute for Brain Research, Inc.; editorial board: Depression and Anxiety, Biological Psychiatry. MPP reports the following disclosures: research/grants: the William K. Warren Foundation and NIH Grant No. R01DA016663, NIH/NIGMS Grant No. P20DA027834, and NIH Grant Nos. R01DA027797, R01DA018307, U01DA041089, and 1R01MH101453; consulting (last 3 years): has received royalties for an article about methamphetamine use disorder from UpToDate. OVdB reports the following disclosures: scientific advisory boards: Research Training Group 2271 of the Deutsche Forschungsgemeinschaft on “Expectation maintenance vs. change in the context of expectation violations: Connecting different approaches,” University of Marburg. RDL reports the following disclosures: research/grant: NIH. SSK reports the following disclosures: research/grants: NIH/National Institute of Mental Health Grant No. K23MH112949, NIH/NIGMS Grant No. P20GM121312, William K. Warren Foundation, Brain and Behavior Foundation (formerly NARSAD) Young Investigator Award. WEM reports the following disclosures: research/grants: NCIRE (Veterans Health Research Institute, San Francisco, California), Alzheimer’s Association, Mental Insight Foundation, the Pepper Foundation, University of California, San Francisco, Resource Allocation Program. WKS reports the following disclosures: research/grants: NIH Grant No. P20GM121312, Brain and Behavior Foundation (formerly NARSAD) Young Investigator Award. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Visceroception has classically referred to the perception of bodily signals arising specifically from visceral organs, such as the heart, lungs, stomach, intestines, and bladder, along with other internal organs in the trunk of the body (19). It did not include organs such as the skin and skeletal muscle, in contrast to contemporary definitions of interoception that typically encompasses signals from both the viscera and all other tissues that relay a signal to the central nervous system about the current state of the body, including the skin and skeletal/smooth muscle fibers, via lamina I spinothalamic afferents (41,138,139).
For the Interoception Summit 2016 participants, see below and Supplement.
Interoception Summit 2016 participant coauthors: Vivien Ainley, Obada Al Zoubi, Robin Aupperle, Jason Avery, Leslie Baxter, Christoph Benke, Laura Berner, Jerzy Bodurka, Eric Breese, Tiffany Brown, Kaiping Burrows, Yoon-Hee Cha, Ashley Clausen, Kelly Cosgrove, Danielle Deville, Laramie Duncan, Patrice Duquette, Hamed Ekhtiari, Thomas Fine, Bart Ford, Indira Garcia Cordero, Diamond Gleghorn, Yvette Guereca, Neil A. Harrison, Mahlega Hassanpour, Tanja Hechler, Aaron Heller, Natalie Hellman, Beate Herbert, Behnaz Jarrahi, Kara Kerr, Namik Kirlic, Megan Klabunde, Thomas Kraynak, Michael Kriegsman, Juliet Kroll, Rayus Kuplicki, Rachel Lapidus, Trang Le, Kyle Logie Hagen, Ahmad Mayeli, Amanda Morris, Nasir Naqvi, Kristina Oldroyd, Christiane Pané-Farré, Raquel Phillips, Tasha Poppa, Willliam Potter, Maria Puhl, Adam Safron, Margaret Sala, Jonathan Savitz, Heather Saxon, Will Schoenhals, Colin Stanwell-Smith, Adam Teed, Yuri Terasawa, Katie Thompson, Marisa Toups, Satoshi Umeda, Valerie Upshaw, Teresa Victor, Christina Wierenga, Colleen Wohlrab, Hung-wen Yeh, Adrian Yoris, Fadel Zeidan, Vadim Zotev, and Nancy Zucker.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2017.12.004.
References
- 1.Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale University Press; 1906. [Google Scholar]
- 2.Cameron OG. Visceral Sensory Neuroscience: Interoception. New York: Oxford University Press; 2002. [Google Scholar]
- 3.Adam G. Interoception and Behaviour. Budapest, Hungary: Akadémiai Kiadó; 1967. [Google Scholar]
- 4.Gorman JM, Fyer AF, Gliklich J, King D, Klein DF. Effect of sodium lactate on patients with panic disorder and mitral valve prolapse. Am J Psychiatry. 1981;138:247–249. doi: 10.1176/ajp.138.2.247. [DOI] [PubMed] [Google Scholar]
- 5.Pohl R, Yeragani VK, Balon R, Rainey JM, Lycaki H, Ortiz A, et al. Isoproterenol-induced panic attacks. Biol Psychiatry. 1988;24:891–902. doi: 10.1016/0006-3223(88)90224-7. [DOI] [PubMed] [Google Scholar]
- 6.Woods SW, Charney DS, Loke J, Goodman WK, Redmond DE, Heninger GR. Carbon dioxide sensitivity in panic anxiety: Ventilatory and anxiogenic response to carbon dioxide in healthy subjects and patients with panic anxiety before and after alprazolam treatment. Arch Gen Psychiatry. 1986;43:900–909. doi: 10.1001/archpsyc.1986.01800090090013. [DOI] [PubMed] [Google Scholar]
- 7.van den Hout MA, van der Molen GM, Griez E, Lousberg H, Nansen A. Reduction of CO2-induced anxiety in patients with panic attacks after repeated CO2 exposure. Am J Psychiatry. 1987;144:788–791. doi: 10.1176/ajp.144.6.788. [DOI] [PubMed] [Google Scholar]
- 8.Margraf J, Ehlers A, Roth WT. Sodium lactate infusions and panic attacks: A review and critique. Psychosom Med. 1986;48:23–51. doi: 10.1097/00006842-198601000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Oppenheimer S, Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol. 2016;6:1081–1133. doi: 10.1002/cphy.c140076. [DOI] [PubMed] [Google Scholar]
- 10.Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594:3911–3954. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Leupoldt A, Chan PY, Esser RW, Davenport PW. Emotions and neural processing of respiratory sensations investigated with respiratory-related evoked potentials. Psychosom Med. 2013;75:244–252. doi: 10.1097/PSY.0b013e31828251cf. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain–gut axis: From basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 14.Drake MJ, Fowler CJ, Griffiths D, Mayer E, Paton JF, Birder L. Neural control of the lower urinary and gastrointestinal tracts: Supraspinal CNS mechanisms. Neurourol Urodyn. 2010;29:119–127. doi: 10.1002/nau.20841. [DOI] [PubMed] [Google Scholar]
- 15.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav Rev. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattie E, Li A. Central chemoreceptors: Locations and functions. Compr Physiol. 2012;2:221–254. doi: 10.1002/cphy.c100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson RJ, Mahmut M, Rooney K. Individual differences in the interoceptive states of hunger, fullness and thirst. Appetite. 2015;95:44–57. doi: 10.1016/j.appet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Fealey RD. Interoception and autonomic nervous system reflexes thermoregulation. Handb Clin Neurol. 2013;117:79–88. doi: 10.1016/B978-0-444-53491-0.00007-9. [DOI] [PubMed] [Google Scholar]
- 19.Janig W. Neurobiology of visceral afferent neurons: Neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- 20.Capuron L, Miller AH. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin BR. Interoception. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. New York: Cambridge University Press; 2000. pp. 1039–1506. [Google Scholar]
- 24.Tsakiris M, Critchley H. Interoception beyond homeostasis: Affect, cognition and mental health. Philos Trans R Soc Lond B Biol Sci. 2016;371:20160002. doi: 10.1098/rstb.2016.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane RD, Wager TD. Introduction to a special issue of NeuroImage on brain–body medicine. Neuro Image. 2009;47:781–784. doi: 10.1016/j.neuroimage.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Vaitl D. Interoception. Biol Psychol. 1996;42:1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- 27.Baranauskas M, Grabauskaite A, Griskova-Bulanova I. Brain responses and self-reported indices of interoception: Heartbeat evoked potentials are inversely associated with worrying about body sensations. Physiol Behav. 2017;180:1–7. doi: 10.1016/j.physbeh.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Int J Psychophysiol. 2009;72:34–45. doi: 10.1016/j.ijpsycho.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 30.Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexianervosa and bulimia. Int J Eat Disord. 1983;2:15–34. [Google Scholar]
- 31.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Forkmann T, Scherer A, Meessen J, Michal M, Schachinger H, Vogele C, et al. Making sense of what you sense: Disentangling interoceptive awareness, sensibility and accuracy. Int J Psychophysiol. 2016;109:71–80. doi: 10.1016/j.ijpsycho.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Khalsa SS, Lapidus RC. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front Psychiatry. 2016;7:121. doi: 10.3389/fpsyt.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehling W. Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philos Trans R Soc Lond B Biol Sci. 2016;371:20160013. doi: 10.1098/rstb.2016.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Association for the Study of Pain. [Accessed November 20, 2017];IASP taxonomy. 2012 Available at: http://www.iasp-pain.org/Taxonomy?navItemNumber=576.
- 36.Stephan KE, Manjaly ZM, Mathys CD, Weber LA, Paliwal S, Gard T, et al. Allostatic self-efficacy: A metacognitive theory of dyshomeostasis-induced fatigue and depression. Front Hum Neurosci. 2016;10:550. doi: 10.3389/fnhum.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petzschner FH, Weber LAE, Gard T, Stephan KE. Computational psychosomatics and computational psychiatry: Toward a joint framework for differential diagnosis. Biol Psychiatry. 2017;82:421–430. doi: 10.1016/j.biopsych.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Sterling P. Homeostasis vs allostasis: Implications for brain function and mental disorders. JAMA Psychiatry. 2014;71:1192–1193. doi: 10.1001/jamapsychiatry.2014.1043. [DOI] [PubMed] [Google Scholar]
- 39.Schulz A, Vogele C. Interoception and stress. Front Psychol. 2015;6:993. doi: 10.3389/fpsyg.2015.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spunt RP, Adolphs R. A new look at domain specificity: Insights from social neuroscience. Nat Rev Neurosci. 2017;18:559–567. doi: 10.1038/nrn.2017.76. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 42.Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus R, Bodurka J, et al. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology. 2018;43:426–434. doi: 10.1038/npp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci. 2009;12:1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dum RP, Levinthal DJ, Strick PL. Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla. Proc Natl Acad Sci U S A. 2016;113:9922–9927. doi: 10.1073/pnas.1605044113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, et al. Dyspnea and pain share emotion-related brain network. Neuro Image. 2009;48:200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 48.De Cort K, Schroijen M, Hurlemann R, Claassen S, Hoogenhout J, Van den Bergh O, et al. Modeling the development of panic disorder with interoceptive conditioning. Eur Neuropsychopharmacol. 2017;27:59–69. doi: 10.1016/j.euroneuro.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Khan HS, Stroman PW. Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience. 2015;307:231–241. doi: 10.1016/j.neuroscience.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 50.Savitz J, Harrison NA. Interoception and inflammation in psychiatric disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:514–524. doi: 10.1016/j.bpsc.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, et al. A cortical potential reflecting cardiac function. Proc Natl Acad Sci U S A. 2007;104:6818–6823. doi: 10.1073/pnas.0609509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, et al. Cortical processing of human somatic and visceral sensation. J Neurosci. 2000;20:2657–2663. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, et al. Gastric distention activates satiety circuitry in the human brain. Neuro Image. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Wittmann M. The inner experience of time. Philos Trans R Soc Lond B Biol Sci. 2009;364:1955–1967. doi: 10.1098/rstb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ádám G. Visceral Perception: Understanding Internal Cognition. New York: Plenum; 1998. [Google Scholar]
- 56.Dworkin BR. Learning and Physiological Regulation. Chicago: University of Chicago Press; 1993. [Google Scholar]
- 57.DeVille DC, Kerr KL, Avery JA, Burrows K, Bodurka J, Feinstein JS, et al. The neural bases of interoceptive encoding and recall in healthy adults and adults with depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:546–554. doi: 10.1016/j.bpsc.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schechter MD, Calcagnetti DJ. Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev. 1998;22:827–846. doi: 10.1016/s0149-7634(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 59.Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- 60.Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell. 2015;161:622–633. doi: 10.1016/j.cell.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, et al. Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc Natl Acad Sci U S A. 2017;114:E2739–E2747. doi: 10.1073/pnas.1620507114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mesulam MM, Mufson EJ. Insula of the old world monkey: I. Architectonics in the insulo-orbito-temporal component of the para-limbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 64.Mitz AR, Chacko RV, Putnam PT, Rudebeck PH, Murray EA. Using pupil size and heart rate to infer affective states during behavioral neurophysiology and neuropsychology experiments. J Neurosci Methods. 2017;279:1–12. doi: 10.1016/j.jneumeth.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudebeck PH, Putnam PT, Daniels TE, Yang T, Mitz AR, Rhodes SE, et al. A role for primate subgenual cingulate cortex in sustaining autonomic arousal. Proc Natl Acad Sci U S A. 2014;111:5391–5396. doi: 10.1073/pnas.1317695111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 67.Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, et al. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53:601–607. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulz A, Strelzyk F, Ferreira de Sa DS, Naumann E, Vogele C, Schachinger H. Cortisol rapidly affects amplitudes of heartbeat-evoked brain potentials—Implications for the contribution of stress to an altered perception of physical sensations? Psychoneuroendocrinology. 2013;38:2686–2693. doi: 10.1016/j.psyneuen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 71.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. Altered interoceptive awareness in anorexia nervosa: Effects of meal anticipation, consumption and bodily arousal. Int J Eat Disord. 2015;48:889–897. doi: 10.1002/eat.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schulz SM. Neural correlates of heart-focused interoception: A functional magnetic resonance imaging meta-analysis. Philos Trans R Soc Lond B Biol Sci. 2016;371:20160018. doi: 10.1098/rstb.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cameron OG, Huang GC, Nichols T, Koeppe RA, Minoshima S, Rose D, et al. Reduced gamma-aminobutyric acidA-benzodiazepine binding sites in insular cortex of individuals with panic disorder. Arch Gen Psychiatry. 2007;64:793–800. doi: 10.1001/archpsyc.64.7.793. [DOI] [PubMed] [Google Scholar]
- 75.Pollatos O, Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology. 2004;41:476–482. doi: 10.1111/1469-8986.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 76.von Leupoldt A, Keil A, Chan PY, Bradley MM, Lang PJ, Davenport PW. Cortical sources of the respiratory-related evoked potential. Respir Physiol Neurobiol. 2010;170:198–201. doi: 10.1016/j.resp.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pollatos O, Herbert BM, Mai S, Kammer T. Changes in interoceptive processes following brain stimulation. Philos Trans R Soc Lond B Biol Sci. 2016;371:20160016. doi: 10.1098/rstb.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fox KC, Christoff K. Transcranial direct current stimulation to lateral prefrontal cortex could increase meta-awareness of mind wandering. Proc Natl Acad Sci U S A. 2015;112:E2414. doi: 10.1073/pnas.1504686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM. Non-invasive, ultrasonic thalamic stimulation in disorders of consciousness after severe brain injury: A first-in-man report. Brain Stimul. 2016;9:940–941. doi: 10.1016/j.brs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell. 2017;169:1029–1041. doi: 10.1016/j.cell.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antonino D, Teixeira AL, Maia-Lopes PM, Souza MC, Sabino-Carvalho JL, Murray AR, et al. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: A randomized placebo-controlled trial. Brain Stimul. 2017;10:875–881. doi: 10.1016/j.brs.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Azevedo RT, Garfinkel SN, Critchley HD, Tsakiris M. Cardiac afferent activity modulates the expression of racial stereotypes. Nat Commun. 2017;8:13854. doi: 10.1038/ncomms13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panitz C, Hermann C, Mueller EM. Conditioned and extinguished fear modulate functional corticocardiac coupling in humans. Psychophysiology. 2015;52:1351–1360. doi: 10.1111/psyp.12498. [DOI] [PubMed] [Google Scholar]
- 84.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 85.Mazzola L, Mauguiere F, Isnard J. Electrical stimulations of the human insula: Their contribution to the ictal semiology of insular seizures. J Clin Neurophysiol. 2017;34:307–314. doi: 10.1097/WNP.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 86.Kern M, Aertsen A, Schulze-Bonhage A, Ball T. Heart cycle-related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuro Image. 2013;81:178–190. doi: 10.1016/j.neuroimage.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 87.Park HD, Bernasconi F, Salomon R, Tallon-Baudry C, Spinelli L, Seeck M, et al. Neural sources and underlying mechanisms of neural responses to heartbeats, and their role in bodily self-consciousness: An intracranial EEG study. Cereb Cortex. 2017 doi: 10.1093/cercor/bhx136. published online ahead of print Jun 7. [DOI] [PubMed] [Google Scholar]
- 88.Young KD, Misaki M, Harmer CJ, Victor T, Zotev V, Phillips R, et al. Real-time functional magnetic resonance imaging amygdala neurofeedback changes positive information processing in major depressive disorder. Biol Psychiatry. 2017;82:578–586. doi: 10.1016/j.biopsych.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petersen S, Schroijen M, Molders C, Zenker S, Van den Bergh O. Categorical interoception: Perceptual organization of sensations from inside. Psychol Sci. 2014;25:1059–1066. doi: 10.1177/0956797613519110. [DOI] [PubMed] [Google Scholar]
- 90.Seth AK, Critchley HD. Extending predictive processing to the body: Emotion as interoceptive inference. Behav Brain Sci. 2013;36:227–228. doi: 10.1017/S0140525X12002270. [DOI] [PubMed] [Google Scholar]
- 91.Pezzulo G, Rigoli F, Friston K. Active inference, homeostatic regulation and adaptive behavioural control. Prog Neurobiol. 2015;134:17–35. doi: 10.1016/j.pneurobio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: A predictive coding perspective. Neuron. 2014;81:1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 93.Seth AK. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci. 2013;17:565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Smith R, Thayer JF, Khalsa SS, Lane RD. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev. 2017;75:274–296. doi: 10.1016/j.neubiorev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 95.Friston K. Hierarchical models in the brain. PLoS Comput Biol. 2008;4:e1000211. doi: 10.1371/journal.pcbi.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Friston K. The free-energy principle: A unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 97.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berner LA, Simmons AN, Wierenga CE, Bischoff-Grethe A, Paulus MP, Bailer UF, et al. Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychol Med. 2018;48:142–154. doi: 10.1017/S0033291717001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology. 2016;41:521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frank GK. Advances from neuroimaging studies in eating disorders. CNS Spectr. 2015;20:391–400. doi: 10.1017/S1092852915000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front Psychiatry. 2013;4:137. doi: 10.3389/fpsyt.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avery JA, Burrows K, Kerr KL, Bodurka J, Khalsa SS, Paulus MP, et al. How the brain wants what the body needs: The neural basis of positive alliesthesia. Neuropsychopharmacology. 2017;42:822–830. doi: 10.1038/npp.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hechler T, Endres D, Thorwart A. Why harmless sensations might hurt in individuals with chronic pain: About heightened prediction and perception of pain in the mind. Front Psychol. 2016;7:1638. doi: 10.3389/fpsyg.2016.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Lernia D, Serino S, Riva G. Pain in the body: Altered interoception in chronic pain conditions: A systematic review. Neurosci Biobehav Rev. 2016;71:328–341. doi: 10.1016/j.neubiorev.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 107.Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, Critchley HD. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biol Psychol. 2016;114:117–126. doi: 10.1016/j.biopsycho.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Flack F, Pane-Farre CA, Zernikow B, Schaan L, Hechler T. Do interoceptive sensations provoke fearful responses in adolescents with chronic headache or chronic abdominal pain? A preliminary experimental study. J Pediatr Psychol. 2017;42:667–678. doi: 10.1093/jpepsy/jsw108. [DOI] [PubMed] [Google Scholar]
- 109.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC) Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 110.Koch A, Pollatos O. Cardiac sensitivity in children: Sex differences and its relationship to parameters of emotional processing. Psychophysiology. 2014;51:932–941. doi: 10.1111/psyp.12233. [DOI] [PubMed] [Google Scholar]
- 111.Maister L, Tang T, Tsakiris M. Neurobehavioral evidence of interoceptive sensitivity in early infancy. eLife. 2017;6:e25318. doi: 10.7554/eLife.25318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy J, Brewer R, Catmur C, Bird G. Interoception and psychopathology: A developmental neuroscience perspective. Dev Cogn Neurosci. 2017;23:45–56. doi: 10.1016/j.dcn.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khalsa SS, Rudrauf D, Tranel D. Interoceptive awareness declines with age. Psychophysiology. 2009;46:1130–1136. doi: 10.1111/j.1469-8986.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Leupoldt A, Mangelschots E, Niederstrasser NG, Braeken M, Billiet T, Van den Bergh BRH. Prenatal stress exposure is associated with increased dyspnoea perception in adulthood. Eur Respir J. 2017;50:1700642. doi: 10.1183/13993003.00642-2017. [DOI] [PubMed] [Google Scholar]
- 115.Ondobaka S, Kilner J, Friston K. The role of interoceptive inference in theory of mind. Brain Cogn. 2017;112:64–68. doi: 10.1016/j.bandc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ehlers A. A 1-year prospective study of panic attacks: Clinical course and factors associated with maintenance. J Abnorm Psychol. 1995;104:164–172. doi: 10.1037//0021-843x.104.1.164. [DOI] [PubMed] [Google Scholar]
- 117.Sundermann B, Bode J, Lueken U, Westphal D, Gerlach AL, Straube B, et al. Support vector machine analysis of functional magnetic resonance imaging of interoception does not reliably predict individual outcomes of cognitive behavioral therapy in panic disorder with agoraphobia. Front Psychiatry. 2017;8:99. doi: 10.3389/fpsyt.2017.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Craske MG, Barlow DH. Mastery of Your Anxiety and Panic: Therapist Guide. 4. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 119.Abelson JL, Khan S, Liberzon I, Erickson TM, Young EA. Effects of perceived control and cognitive coping on endocrine stress responses to pharmacological activation. Biol Psychiatry. 2008;64:701–707. doi: 10.1016/j.biopsych.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richter J, Hamm AO, Pane-Farre CA, Gerlach AL, Gloster AT, Wittchen HU, et al. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: Implications for the etiology of panic disorder. Biol Psychiatry. 2012;72:512–520. doi: 10.1016/j.biopsych.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 121.Beck JG, Shipherd JC, Zebb BJ. How does interoceptive exposure for panic disorder work? An uncontrolled case study. J Anxiety Disord. 1997;11:541–556. doi: 10.1016/s0887-6185(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 122.Forsyth JP, Lejuez CW, Finlay C. Anxiogenic effects of repeated administrations of 20% CO2-enriched air: Stability within sessions and habituation across time. J Behav Ther Exp Psychiatry. 2000;31:103–121. doi: 10.1016/s0005-7916(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 123.Deacon B, Kemp JJ, Dixon LJ, Sy JT, Farrell NR, Zhang AR. Maximizing the efficacy of interoceptive exposure by optimizing inhibitory learning: A randomized controlled trial. Behav Res Ther. 2013;51:588–596. doi: 10.1016/j.brat.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: A randomized controlled trial. Biol Psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 125.Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of endtidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meuret AE, Ritz T, Wilhelm FH, Roth WT, Rosenfield D. Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:539–545. doi: 10.1016/j.bpsc.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farb N, Daubenmier J, Price CJ, Gard T, Kerr C, Dunn BD, et al. Interoception, contemplative practice, and health. Front Psychol. 2015;6:763. doi: 10.3389/fpsyg.2015.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feinstein JS, Khalsa SS, Yeh H, Al Zoubi O, Arevian AC, Wohlrab C, et al. The elicitation of relaxation and interoceptive awareness using Floatation therapy in individuals with high anxiety sensitivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:555–562. doi: 10.1016/j.bpsc.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feinstein JS, Khalsa SS, Yeh HW, Wohlrab C, Simmons WK, Stein MB, Paulus MP. Examining the short-term anxiolytic and antidepressant effect of Floatation-REST. PLoS One. 2018;13:e0190292. doi: 10.1371/journal.pone.0190292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Diest I, Davenport P, Van den Bergh O, Miller S, Robertson E. Interoceptive fear conditioning to an inspiratory load using 20% CO2 inhalation as an unconditioned stimulus. Biol Psychol. 2007;75:211. [Google Scholar]
- 131.Janssen CW, Lowry CA, Mehl MR, Allen JJ, Kelly KL, Gartner DE, et al. Whole-body hyperthermia for the treatment of major depressive disorder: A randomized clinical trial. JAMA Psychiatry. 2016;73:789–795. doi: 10.1001/jamapsychiatry.2016.1031. [DOI] [PubMed] [Google Scholar]
- 132.Kox M, van Eijk LT, Zwaag J, van den Wildenberg J, Sweep FC, van der Hoeven JG, et al. Voluntary activation of the sympathetic nervous system and attenuation of the innate immune response in humans. Proc Natl Acad Sci U S A. 2014;111:7379–7384. doi: 10.1073/pnas.1322174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.De Couck M, Cserjesi R, Caers R, Zijlstra WP, Widjaja D, Wolf N, et al. Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton Neurosci. 2017;203:88–96. doi: 10.1016/j.autneu.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Krause E, Benke C, Koenig J, Thayer JF, Hamm AO, Pané-Farré CA. Dynamics of defensive response mobilization to approaching external versus interoceptive threat. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:525–538. doi: 10.1016/j.bpsc.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, et al. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav. 2017;1:0069. doi: 10.1038/s41562-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kent ML, Tighe PJ, Belfer I, Brennan TJ, Bruehl S, Brummett CM, et al. The ACTTION-APS-AAPM Pain Taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. J Pain. 2017;18:479–489. doi: 10.1016/j.jpain.2017.02.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cameron OG. Interoception: The inside story—A model for psychosomatic processes. Psychosom Med. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 139.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 140.Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: Evidence for a central fear mechanism. Arch Gen Psychiatry. 2001;58:125–131. doi: 10.1001/archpsyc.58.2.125. [DOI] [PubMed] [Google Scholar]
- 141.Stein MB, Asmundson GJ. Autonomic function in panic disorder: Cardiorespiratory and plasma catecholamine responsivity to multiple challenges of the autonomic nervous system. Biol Psychiatry. 1994;36:548–558. doi: 10.1016/0006-3223(94)90619-x. [DOI] [PubMed] [Google Scholar]
- 142.Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, et al. Depression-related increases and decreases in appetite: Dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry. 2016;173:418–428. doi: 10.1176/appi.ajp.2015.15020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nierenberg AA, Pava JA, Clancy K, Rosenbaum JF, Fava M. Are neurovegetative symptoms stable in relapsing or recurrent atypical depressive episodes? Biol Psychiatry. 1996;40:691–696. doi: 10.1016/0006-3223(96)00029-7. [DOI] [PubMed] [Google Scholar]
- 144.Dimsdale JE, Creed F, Escobar J, Sharpe M, Wulsin L, Barsky A, et al. Somatic symptom disorder: An important change in DSM. J Psychosom Res. 2013;75:223–228. doi: 10.1016/j.jpsychores.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 145.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16:266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glenn DE, Acheson DT, Geyer MA, Nievergelt CM, Baker DG, Risbrough VB, et al. High and low threshold for startle reactivity associated with PTSD symptoms but not PTSD risk: Evidence from a prospective study of active duty Marines. Depress Anxiety. 2016;33:192–202. doi: 10.1002/da.22475. [DOI] [PubMed] [Google Scholar]
- 149.Pluess M, Conrad A, Wilhelm FH. Muscle tension in generalized anxiety disorder: A critical review of the literature. J Anxiety Disord. 2009;23:1–11. doi: 10.1016/j.janxdis.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 150.Rapaport MH, Schettler P, Larson ER, Edwards SA, Dunlop BW, Rakofsky JJ, et al. Acute Swedish massage monotherapy successfully remediates symptoms of generalized anxiety disorder: A proof-of-concept, randomized controlled study. J Clin Psychiatry. 2016;77:e883–e891. doi: 10.4088/JCP.15m10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sedeno L, Couto B, Melloni M, Canales-Johnson A, Yoris A, Baez S, et al. How do you feel when you can’t feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS One. 2014;9:e98769. doi: 10.1371/journal.pone.0098769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schulz A, Koster S, Beutel ME, Schachinger H, Vogele C, Rost S, et al. Altered patterns of heartbeat-evoked potentials in depersonalization/derealization disorder: Neurophysiological evidence for impaired cortical representation of bodily signals. Psychosom Med. 2015;77:506–516. doi: 10.1097/PSY.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 153.Schauder KB, Mash LE, Bryant LK, Cascio CJ. Interoceptive ability and body awareness in autism spectrum disorder. J Exp Child Psychol. 2015;131:193–200. doi: 10.1016/j.jecp.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci Biobehav Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.