Abstract
The currently recommended approach to managing cancer risk for patients with Barrett’s esophagus is endoscopic surveillance including a biopsy protocol to sample the esophageal tissue randomly to detect dysplasia. However, there are numerous limitations in this practice that relies on the histopathological grading of dysplasia alone to make clinical decisions. The availability of in silico models demonstrating the potential cost-effectiveness of biomarker-based stratification have increased interest in finding a clinically relevant “Barrett’s biomarker”. The success of endoscopic eradication therapy in preventing neoplastic progression of dysplastic Barrett’s esophagus has promoted the desire to stratify non-dysplastic Barrett’s esophagus to those with “high risk” that may benefit from endotherapy. Furthermore, on the other end of the spectrum, there is interest in searching for a “low risk” marker that may identify those that would not likely benefit from endoscopy screening or surveillance. This review highlights recent data from the genomics (r)evolution revealing new genetic biomarkers of susceptibility to the development of Barrett’s esophagus and novel pathways for its neoplastic progression, addresses the development of new modes of tissue sampling and imaging to detect early neoplasia in Barrett’s esophagus, and discusses current progress in moving biomarkers from the laboratory into clinical practice in the era of precision medicine.
INTRODUCTION
For years, investigators have been searching for the best “Barrett’s biomarker” without much success. Recently, however, identification of such a biomarker has become enticing due to the success of endoscopic therapy in preventing neoplastic progression of dysplastic Barrett’s esophagus. In the past decade, two randomized, controlled clinical trials have demonstrated the efficacy of radiofrequency ablation (RFA), with endoscopic mucosal resection of any nodular areas, in Barrett’s patients with low grade and high grade dysplasia in preventing progression to higher grades of dysplasia and/or esophageal adenocarcinoma.1,2 A biomarker that could reliably indicate the likelihood of neoplastic progression, rather than relying only on the histopathologic diagnosis of dysplasia which itself is fraught with poor intra-observer agreement3–5, could be used to trigger endoscopic intervention.
For the general population of patients with non-dysplastic Barrett’s esophagus, the yearly risk of developing esophageal adenocarcinoma is low, between 0.12% and 0.33% per year.6,7 Although some experts have proposed compelling arguments for endoscopic eradication for non-dysplastic Barrett’s esophagus8, there are complications, albeit low, with this procedure and real costs, incurred by the patient and medical system, with its delivery.9,10 Thus, gastrointestinal society guidelines recommend against endoscopic ablative therapy in the non-dysplastic population of Barrett’s esophagus and instead recommend endoscopic surveillance.7,11,12 However, surveillance has limited effectiveness, as rates of esophageal adenocarcinoma have continued to rise despite the widespread practice of Barrett’s surveillance.13,14 However, if a biomarker could clearly indicate that a patient was at high risk for neoplastic progression, then the balance between risks and benefits would be tipped in favor of endoscopic ablation for these selected patients. Moreover, it is conceivable that those non-dysplastic Barrett’s patients who are negative for the biomarker could benefit from longer intervals in endoscopic surveillance or even discontinuation of surveillance entirely.
The following sections will highlight proof-of principle studies demonstrating the potential clinical utility of biomarkers for basing decisions on therapy for patients with non-dysplastic Barrett’s esophagus, recent genomic discoveries on pathways to carcinogenesis and genetic susceptibility to Barrett’s esophagus and esophageal adenocarcinoma, and recently published, key, proof-of principle studies demonstrating where we are with using biomarkers in clinical practice, and where we are headed with novel methods of biomarker detection to predict neoplastic risk in patients with Barrett’s esophagus.
Potential for Biomarker-Based Risk Stratification to Change the Current Management of Patients with Non-Dysplastic Barrett’s Esophagus
For patients with non-dysplastic Barrett’s esophagus, gastrointestinal society guidelines recommend endoscopic surveillance using high-definition white light endoscopy with biopsy of all visible lesions and 4-quadrant, random biopsies obtained every 2 cm as a means to detect dysplasia and early cancers.7,11,12 Unfortunately, this practice is not cost-effective.15 Although not currently recommended by gastrointestinal societies, endoscopic therapy using RFA for all patients with non-dysplastic Barrett’s esophagus is also not cost-effective.16 Recently, Das et al. used a Markov model to assess cost-effectiveness of RFA in selected patients with non-dysplastic Barrett’s esophagus who were deemed to be at “high risk for cancer progression” based on a biomarker panel. The management strategies that were compared in this analysis included biomarker-based RFA ablation of high risk patients with non-dysplastic Barrett’s esophagus, no surveillance, the current American College of Gastroenterology (ACG) recommended surveillance guidelines, and RFA of all patients with non-dysplastic Barrett’s esophagus.17 The investigators modeled a cohort of 50 year-old white men with non-dysplastic Barrett’s esophagus recently diagnosed by endoscopy, and followed them over their lifetimes. For the biomarker, they selected a commercially available panel that assesses genomic instability and microsatellite instability across a group of genes that have been implicated in facilitating neoplastic progression of Barrett’s esophagus. In the biomarker-based RFA strategy, patients with non-dysplastic Barrett’s esophagus who were negative for the biomarker underwent endoscopic surveillance every 10 years rather than every 3 years. During the lifetime of the cohort, this model predicted that the strategy of biomarker-based RFA dominated the other strategies, yielding the highest number of quality of life years at the lowest cost.17 Moreover, using a Monte Carlo simulation, they found that treatment with RFA based on a high-risk biomarker profile resulted in fewer cancers being diagnosed. Compared with no surveillance, the relative risk (RR) of cancer development with the biomarker-based strategy was 0.48, and the number needed to treat (NNT) to prevent cancer was only 23. Furthermore, compared with ACG guideline-recommended endoscopic surveillance, the RR of cancer development with the biomarker-based strategy was 0.49, with a NNT of 24.17 Based on these findings, it is easy to appreciate the potential clinical utility of using biomarker-based risk stratification to guide clinical decision making on the management of patients with non-dysplastic Barrett’s esophagus.
Concepts in Biomarker Identification: Cancer Hallmarks
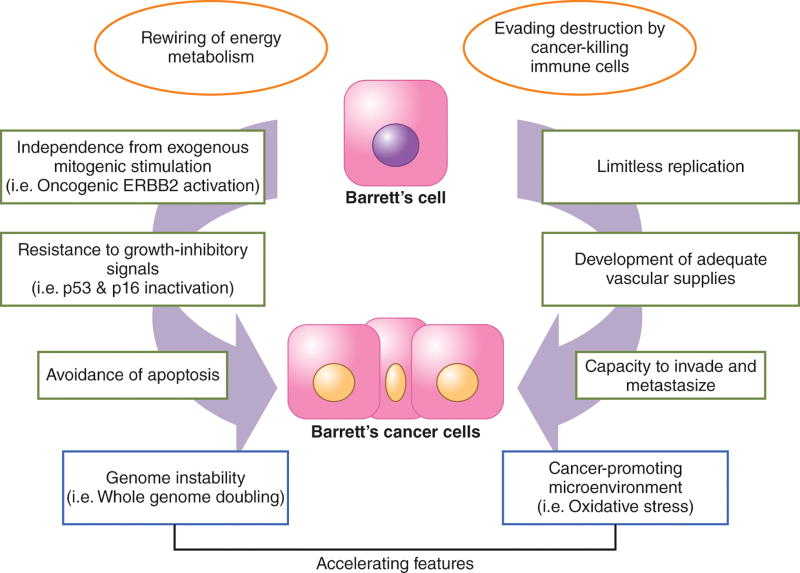
Biomarkers might be used in a number of different ways, including assessment of the risk of cancer development, prediction of response to treatment, and estimation of prognosis.18 Biomarkers can be factors that simply correlate with a disease, or factors that have a functional, mechanistic effect that causes the disease. In general, many of the causative biomarkers can be categorized according to the physiologic properties they provide to normal cells that enable them to become cancer cells. In 2000, Hanahan and Weinberg proposed the concept that several distinct physiologic properties distinguish the behavior of cancer cells from that of normal cells, and that genetic alterations within several key molecular pathways allow normal cells to acquire these essential cancer properties (i.e. hallmarks; Figure 1).19 These acquired cancer hallmarks include independence from exogenous mitogenic stimulation, resistance to growth-inhibitory signals, avoidance of apoptosis, limitless replication, development of adequate vascular supplies, and the capacity to invade and metastasize. In 2011, the same authors proposed two additional hallmarks: 1) rewiring of energy metabolism to support enhanced proliferation of cancer cells and 2) evading destruction by cancer-killing immune cells (Figure 1).20 Moreover, the acquisition of these cancer hallmarks can be accelerated by: 1) genome instability (which can reflected by aneuploidy or whole genome doubling) and mutation, and 2) a cancer-promoting microenvironment.20,21 In metaplastic Barrett’s tissue specimens, genetic alterations in genes that facilitate the acquisition of these cancer hallmarks have been described even before the tissues exhibit any histological features of dysplasia (Reviewed in22), suggesting that a biomarker(s) is out there that could be used in clinical management strategies for patients with non-dysplastic Barrett’s esophagus.
Figure 1.
Cancer Hallmarks. The essential properties of cancer cells are shown in the green boxes. In general, activation of oncogenes such as ERBB2 is the way in which Barrett’s cells can proliferate without exogenous stimulation and inactivation of tumor suppressor genes such as p53 and p16 is a common way in which Barrett’s cells resist growth-inhibitory signals. The two additional cancer hallmarks proposed in 2011 are shown in the orange circles. The rounded boxes in blue are the accelerating features such as whole genome doubling and a microenvironment rich in oxidative stress as a result of chronic GERD that allow Barrett’s cells faster acquisition of the cancer hallmarks.
The “omics” (R)Evolution on Pathways of Neoplastic Progression in Barrett’s Esophagus
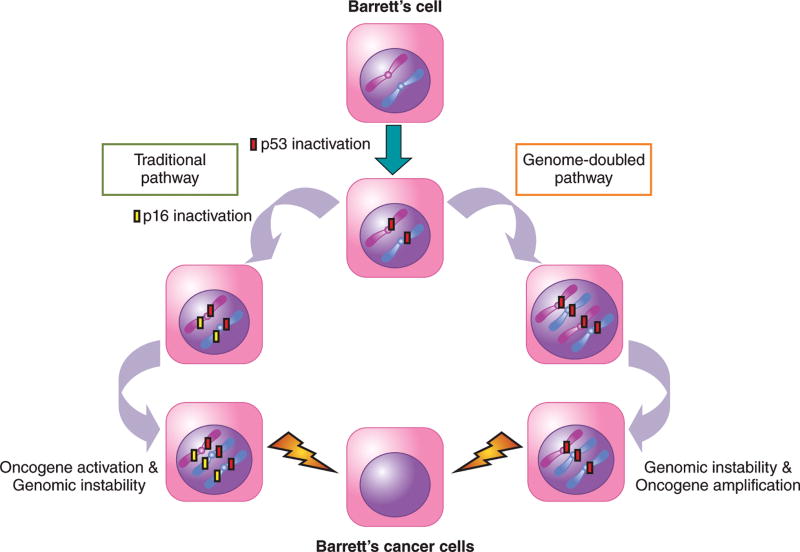
Incredible advances in “omics” techniques such as whole-genome sequencing and whole-exome sequencing (i.e. sequencing limited only to coding regions of genes) have revolutionized our understanding of how premalignant Barrett’s cells become tumor cells. For example, Stachler et al. performed whole-exome sequencing on DNA extracted from matching (i.e. from the same patient) tissue specimens of Barrett’s metaplasia and esophageal adenocarcinoma to identify somatic (i.e. acquired, not inherited) alterations involved in neoplastic progression.23 Using complex bioinformatics analyses, the investigators found that non-dysplastic Barrett’s esophagus has a high frequency of somatic mutations ranging from 1.3 to 5.4 mutations per megabase of DNA, rates higher than those found in cancers of the prostate and breast.23 Dysplastic Barrett’s esophagus and esophageal adenocarcinoma had similar somatic mutational frequencies, both of which were higher than those found in non-dysplastic Barrett’s esophagus. Moreover, the mutational signature in non-dysplastic Barrett’s esophagus was commonly a transversion from an adenine-adenine pair to an adenine-cytosine pair (AA>AC), a type of mutational pattern indicative of genotoxic damage from oxidative stress which can result from chronic GERD.23 They found that p53 mutations were the earliest shared somatic mutations, often preceding inactivation of p16, in non-dysplastic Barrett’s metaplasia and its matching esophageal adenocarcinoma. Although inactivation of the tumor suppressor genes p53 and p16 were early alterations found in non-dysplastic Barrett’s tissues, activation of oncogenes such as ERBB2 occurred in the later dysplastic stage suggesting a role in neoplastic transformation.23 Although this study showcases the spectacular molecular and bioinformatics techniques that characterize modern day genomics, the study findings primarily just support conclusions of earlier genomic investigations that used less “flashy” techniques regarding the traditional pathway of carcinogenesis (Reviewed in24). Interestingly, however, this study found that only a minority of tumors progressed along this traditional pathway, which involves the step-wise accumulation of alterations in the p53 and p16 tumor suppressor genes, followed by oncogene activation, and then development of genomic instability. Rather, the study found that the majority (62.5%) of tumors in Barrett’s esophagus develop though a “genome-doubled pathway” (Figure 2).23
Figure 2.
The “omics” (R)Evolution on Pathways of Neoplastic Progression in Barrett’s Esophagus. Metaplastic Barrett’s cells first acquire a mutation leading to inactivation of p53. The traditional pathway involves the step-wise accumulation of alterations in tumor suppressor genes such as p16, followed by oncogene activation, and genomic instability, finally resulting in cancer formation. In the genome-doubled pathway, the p53-mutant Barrett’s cells undergo whole genome doubling, followed by genomic instability and oncogene amplification, resulting in cancer formation. It has been proposed that the genome-doubled pathway may be a more rapid pathway to cancer development, and may possibly explain the failure of endoscopic surveillance to detect early cancer progression in Barrett’s esophagus.
In this genome-doubled pathway, p53 mutation was acquired first, followed by whole genome doubling, an alteration primarily detected in areas of dysplasia. Genomic instability and oncogene amplification developed after whole genome doubling, followed by malignancy (Figure 2). In an earlier study using whole genome sequencing on esophageal adenocarcinomas, Nones et al. reported evidence of genomic catastrophes that caused structural genomic rearrangements leading to large increases in oncogene amplification.25 The occurrence of whole genome doubling, which facilitates the acquisition of catastrophic genomic events, early in the process of Barrett’s carcinogenesis might explain this earlier finding by Nones et al.25 Stachler et al. postulated that the genome-doubled pathway may be a more rapid pathway to cancer development, and may possibly explain the failure of endoscopic surveillance to detect rapid cancer progression in Barrett’s esophagus.23 It is clear that incorporation of “omics” data such as those discussed above will continue to (r)evolutionize our understanding of how premalignant Barrett’s cells become tumor cells.
The “omics” (R)Evolution of Biomarker Identification
Germline Susceptibility
Incredible advances in genomics techniques have also revolutionized our identification of potential biomarkers for predicting risk of developing Barrett’s esophagus and esophageal adenocarcinoma. Genome-wide association studies (GWAS) compare genome-wide genetic variations among individuals who have the disease of interest with controls subjects. The genetic variants studied usually focus on a single-nucleotide polymorphism (SNP) at a specific location in the genome that can then be mapped to a specific gene. Unlike the studies discussed above in which genomics were performed on tissue specimens to identify somatic mutations, GWAS studies are usually performed on whole-blood DNA to identify germline (i.e. inherited) alterations. Ek et al. used the Barrett’s and Esophageal Adenocarcinoma Consortium (BEACON) dataset, which contains clinical information and blood samples from subjects enrolled in 14 epidemiological studies from three countries.26 From this dataset, the investigators selected a subset of subjects with white-European ancestry (including 1509 patients with esophageal adenocarcinoma, 2383 patients with Barrett’s esophagus, and 2170 control subjects), and performed GWAS on whole-blood DNA samples obtained from these subjects. Using complex bioinformatics analyses, they estimated that 25% of esophageal adenocarcinoma and 35% of Barrett’s esophagus cases have a polygenic component underlying disease risk.26 A polygenic trait is defined as a trait influenced by many genes and, indeed, this study found that many common variant SNPs (any one of which did not increase risk of disease) together accounted for the increased risk of Barrett’s esophagus and esophageal adenocarcinoma. Moreover, these unrelated subjects with Barrett’s esophagus and esophageal adenocarcinoma were found to have a substantial overlap of SNPs, suggesting a shared genetic susceptibility for the two disorders.26 GWAS studies have shown that many of the SNPs are located in or around genes that regulate esophageal development (i.e. FOXF1, FOXP1), the cystic fibrosis transmembrane conductance regulator (CFTR) gene, and the major histocompatibility complex (MHC) locus that regulates activation of the immune system.27–29
Germline Susceptibility and Environmental Factors
More recent studies have analyzed selected SNPs within different inflammatory pathways to derive information on the contribution of genetic susceptibility within these pathways to the risk of developing Barrett’s esophagus and esophageal adenocarcinoma. Buas et al. selected 5 different inflammation-related pathways that have been previous linked with Barrett’s esophagus and esophageal adenocarcinoma including cyclooxygenase (COX), pro-inflammatory and anti-inflammatory cytokines, oxidative stress, human leukocyte antigen, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB).30 They found a significant association for germline variations only in the COX pathway (specifically in the antioxidant microsomal glutathione S-transferase 1 [MGST1] gene), with risk for Barrett’s esophagus and the combined outcome of Barrett’s esophagus and esophageal adenocarcinoma; none of the pathways were associated with risk of esophageal adenocarcinoma alone.30 MGST1 belongs to the GST gene family that encodes proteins responding to oxidative stress. In esophageal squamous and Barrett’s epithelial cells, oxidative stress can be induced by GERD.31 Up to 40% of adult Westerners report symptoms of GERD, but only a minority of those individuals develop Barrett’s esophagus.32 Acid and bile salts, the main noxious components of refluxed gastric juice, induce oxidative stress via the production of reactive oxygen species (ROS). 31 Huo et al. have recently shown that the intensity of NF-κB pathway activation caused by acid and bile salt-induced ROS is substantially greater in esophageal squamous cells from patients with Barrett’s esophagus than from GERD patients without Barrett’s esophagus.33 Only in squamous cells of the Barrett’s patients is the NF-κB activation sufficient to induce expression of caudal-related homeobox transcription factor 2 (CDX2), a key developmental transcription factor that determines the formation of intestinal epithelium and that is frequently found in Barrett’s esophagus.33 Therefore, germline mutations in genes that modify inflammatory responses to oxidative stress, such as MGST1, might help to explain differences in intensity of NF-κB activation in esophageal squamous cells from GERD with and without Barrett’s esophagus, and perhaps why Barrett’s esophagus develops in only some GERD patients.
Other studies have analyzed the interaction between SNPs and the well-known epidemiologic risk factors for Barrett’s esophagus including GERD, cigarette smoking, and body-mass index (BMI). For example, Dai et al. used SNPs located in or near the FOXF1, TBX5, GDF7, MHC, CRTC1, BARX1, and FOXP1 genes (which have been shown in GWAS studies to be significantly associated with the risk of Barrett’s esophagus27,34,35), and assessed the association of these SNPs with epidemiologic risk factors (GERD, cigarette smoking, and BMI) and with the risk of developing Barrett’s esophagus and esophageal adenocarcinoma.36 They found having a SNP in the FOXP1 gene in the absence of reflux symptoms had an odds ratio (OR) of developing Barrett’s esophagus of 1.5; the highest OR of 6.0 was found for patients with reflux symptoms who had no SNP in their FOXP1 gene.36 The presence of a SNP in the FOXP1 gene combined with at least weekly reflux symptoms significantly decreased the risk of Barrett’s esophagus from an OR of 6.0 to 5.44.36 No significant associations were found for any of the 7 SNPs with BMI or cigarette smoking and risk for Barrett’s esophagus.36 Furthermore, no significant interactions between any of these SNPs and the epidemiologic risk factors were found for esophageal adenocarcinoma alone. Although the basics of the findings generated from “omics” studies are not surprising (i.e. genetic susceptibility, inflammatory pathway signaling, and GERD influence the risk of developing Barrett’s esophagus and esophageal adenocarcinoma), the large scale of data generated and the identification of novel gene-environment interactions (MGST1 and FOXP1 SNPs) that modulate disease risk, clearly exemplify the magnitude with which biomarker identification has been (r)evolutionized.
The Future of Biomarkers in the “omics” (R)Evolution
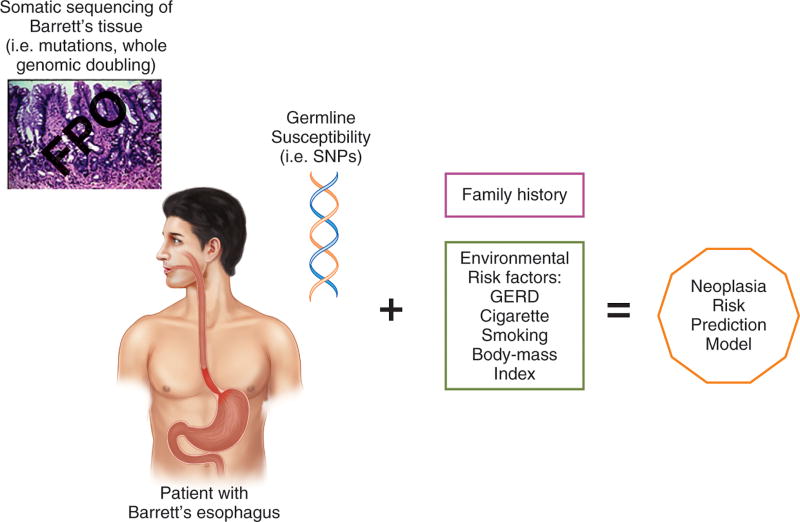
During his state-of-the-union address in 2015, President Barack Obama announced the United States Precision Medicine Initiative, whose goal is to improve health by collecting clinical and biomarker data on a massive scale, analyzing the data, and developing prevention and treatment strategies for specific groups of individuals.18,37 This initiative envisions collecting longitudinal data from a large (≈1 million) cohort of Americans (similar to other large cohorts in the United Kingdom, in Denmark, and in Germany), and using the American cohort to address issues that currently hinder biomarker development such as logistical issues of sample size, disease outcomes, and the need for a separate validation cohort.37 Biomarker development is also subject to regulatory guidelines, and the Precision Medicine Initiative includes regulatory framework alterations designed to help hasten the translation of biomarker discoveries into the clinic.37 As noted in the studies discussed above, modern “omics” technologies have provided insights into pathways of neoplastic progression in Barrett’s esophagus through acquired somatic mutations and genome wide doubling, and have increased our understanding of the contribution that inherited, genetic variation may play in modifying susceptibility to Barrett’s esophagus and esophageal adenocarcinoma. Until recently, most biomarker studies selected a single somatic biomarkers based on the biology underlying cancer development (i.e cancer hallmarks). In contrast, recent studies attempting to determine the risk of neoplasia in patients with Barrett’s esophagus have used panels comprising multiple different biomarkers.38–40 In the era of precision medicine, it is conceivable that genetics, somatic sequencing of esophageal tissue, mutational signatures, pathway signatures, and environmental risk factors will all be synthesized into a single, risk-prediction model for Barrett’s esophagus and esophageal adenocarcinoma, a model that can be “tested” in the large national US cohort established by the Precision Medicine Initiative. (Figure 3)
Figure 3.
The Future of Biomarkers in the “omics” (R)Evolution. In the era of precision medicine, it is conceivable that patients with Barrett’s esophagus would have testing for germline susceptibility (SNPs) performed on whole-blood DNA, somatic sequencing for mutations or whole genomic doubling performed on Barrett’s tissues specimens acquired by endoscopic biopsy, a family history, and an assessment for environmental risk factors that will all be synthesized into a single, risk prediction model for neoplastic progression.
From Laboratory to Clinical Practice
Endoscopic surveillance to detect dysplasia in Barrett’s esophagus is the cancer preventive strategy currently endorsed by gastrointestinal societies.7,11,12 Data from a number of observational studies have demonstrated that patients with Barrett’s esophagus enrolled in surveillance programs have cancers detected at an earlier stage associated with improved survival (Reviewed in7,11). Endoscopic surveillance uses high-definition white light endoscopy to visually inspect the mucosa, coupled with systematic, four-quadrant biopsies obtained at 1–2 cm intervals along the length of the Barrett’s metaplasia, with areas of mucosal irregularity sampled separately, preferably by endoscopic mucosal resection (EMR).7,11 Subsequent surveillance intervals or interventions are determined by the finding of dysplasia in biopsies so obtained.11 Endoscopic surveillance programs are expensive, labor-intensive, and time consuming both for patients and physicians.7,11 Furthermore, physician adherence to this surveillance protocol is far from ideal, ranging from 30–51%.41,42 Even if strict adherence to the biopsy protocol is followed, there remain troublesome issues of biopsy sampling error and disagreement among pathologists in the histological interpretation of dysplasia.4,43 To overcome these problems, biomarkers may provide a diagnostic or predictive yield over traditional histology. Specifically, p53 immunostaining has been proposed as an adjunct to routine histologic assessment of dysplasia. Also, newer imaging modalities are being technologically advanced to distinguish dysplasia from the surrounding areas of non-dysplastic Barrett’s mucosa in vivo so that “targeted” rather than random biopsies can be obtained. Futhermore, new modes of sampling are being developed (tethered devices and breath tests) and, if proven to be reliable, are non-endoscopic tissue sampling methods that may accurately select who may benefit from endoscopy. The studies discussed below demonstrate where we are with adding biomarkers to current clinical practice and where we may be headed in efforts to predict cancer risk in patients with Barrett’s esophagus or even the general population at large. For now, however, the American College of Gastroenterology and American Gastroenterological Association recommend against the routine use of biomarkers in management of Barrett’s esophagus.7,11
p53 Immunostain: Closer Than You May Think
The tumor suppressor gene p53, which is activated by DNA injury, decreases cell proliferation, thus enabling time for DNA repair and preventing DNA-damaged cells from undergoing mitosis and replicating the DNA damage. If the DNA injury is severe and beyond repair, however, then p53 induces cell death through apoptosis. Therefore, inactivation of p53 allows cells to acquire two essential cancer hallmarks - the ability to resist growth-inhibitory signals such as DNA damage and the avoidance of apoptosis. Moreover, inactivation of p53 is one of the earliest somatic events in the neoplastic progression of Barrett’s esophagus (Reviewed in22,23). In general, p53 inactivation occurs by mutation in one allele of the gene accompanied by genomic loss of the other copy (i.e. loss of heterozygosity). Wild-type p53 protein is rapidly degraded, but some p53 mutations render the protein stable so that it accumulates and becomes easily detectable in tissue samples by immunostaining. In contrast, other mutations in p53 lead to loss of its expression in tissue samples, resulting in loss of immunostaining.
Aberrant p53 expression (overexpression or loss of expression) has been shown in recent studies to be a useful indicator of neoplastic progression in Barrett’s esophagus. A case-control study performed in the Netherlands determined p53 immunostaining in more than 12,000 biopsy specimens taken from 586 controls (patients with Barrett’s esophagus who had no neoplastic progression during follow-up) and from 49 cases (patients with Barrett’s esophagus who progressed to high-grade dysplasia or cancer during follow-up).44 Aberrant p53 expression was detected in 14% of biopsies from controls, and in 49% of biopsies from the cases with neoplastic progression. In the cases, aberrant p53 expression was associated with an overall relative risk (RR) of 6.4 for neoplastic progression after adjusting for age, gender, length of Barrett’s esophagus, and esophagitis.44 In patients with non-dysplastic Barrett’s esophagus at baseline, aberrant p53 expression was associated with an adjusted RR of 4.3 for neoplastic progression, whereas the adjusted RR for neoplastic progression in those with low grade dysplasia at baseline was 12.2.44
More recently, aberrant p53 expression was prospectively evaluated in patients with Barrett’s esophagus enrolled in a surveillance program as a predictor of progression to high grade dysplasia (HGD) or esophageal adenocarcinoma.45 Immunostaining for p53 was performed on biopsy specimens from Barrett’s patients without HGD or esophageal adenocarcinoma, and they were followed for a median period of 71 months. Among the 91 patients, 11 (12%) progressed to HGD or cancer during follow-up.45 Aberrant p53 expression was found significantly more often in the patients who progressed to HGD or cancer (63.6%) than in patients who did not progress (7.5%).45 Multivariate analysis demonstrated that aberrant p53 expression detected by immunostaining was a significant (hazard ratio, HR 17) and independent predictor of neoplastic progression.45
Recent studies such as these suggest that detecting aberrant p53 expression by immunohistochemistry on non-dysplastic and low-grade dysplastic biopsies of Barrett’s esophagus may be a clinically useful predictor of neoplastic progression. In fact, the guidelines from the British Society for Gastroenterology state that “the addition of a p53 immunostain to the histopathological assessment may improve the diagnostic reproducibility of a diagnosis of dysplasia in Barrett’s oesophagus and should be considered as an adjunct to routine clinical diagnosis.”12 Essentially this means using the p53 immunostain as a biomarker of neoplasia, and so doing may enhance the “performance” of the histologic diagnosis of dysplasia to risk stratify Barrett’s esophagus patients.
Potential for Acquiring Tissue for Biomarkers Without Endoscopy
Endoscopic surveillance has the burden of cost, time, and risks associated both the procedure and the sedation. A non-endoscopic tissue acquisition tool may enable sampling for biomarkers without the need for endoscopy. One tool that has been studied is the Cytosponge, which is a capsule containing a sponge tethered by a string. The swallowed capsule dissolves in the stomach, releasing the sponge that is withdrawn by the string through the esophagus where it collects cells that can be processed for immunocytochemistry staining and molecular analysis. Initial studies examined tissue collection with the Cytosponge and immunocytochemistry analysis for tissue trefoil factor 3 for screening purposes.46,47 Among 1,110 subjects in a case control study, the ability to detect Barrett’s esophagus was 79.9% overall, and was 87.2% among those patients with long segment Barrett’s esophagus (≤ 3 cm). Additional validation is needed in the general primary care population.47 A more recent study explored using clinical and demographic data along with Cytosponge tissue samples analyzed for a molecular biomarker panel including protein biomarkers (P53, c-Myc, Aurora kinase A), methylation markers (MYOD1, RUNX3), glandular atypia, and TP53 mutation to classify patients with Barrett’s esophagus into low-, moderate-, or high-risk categories.48 The initial discovery cohort consisted of 468 patients with non-dysplastic or high grade dysplastic Barrett’s esophagus and esophageal adenocarcinoma; validation was performed on an additional cohort of 65 non-dysplastic and high grade dysplastic Barrett’s patients. The initial findings suggest that this non-endoscopic sampling tool combined with biomarker analyses can stratify Barrett’s patients who are at low risk for progression, as well as those who already harbor neoplasia to minimize low yield endoscopic surveillance.48
Real Time Acquisition of Biomarkers: In vivo Imaging
Currently, most biomarkers require tissue sampling that then requires various fixation, processing, and interpretation procedures for any potential clinical application. Innovations in in vivo advanced endoscopic imaging are moving beyond providing a surrogate for histology to providing immediately-available information beyond histology that may be either molecular or dynamic in nature. Confocal laser endomicroscopy (CLE) provides in vivo imaging at the level of microarchitecture with high resolution. Various criteria have been proposed and investigated to differentiate nondysplastic tissue from dysplastic tissue.49,50 In the gastrointestinal tract, CLE requires the use of a fluorophore, most often the nonspecific intravenously administered fluorescein. However, this platform may be used with more specific targets such as labeled peptides or antibodies to target molecular markers of interest in vivo. A proof of concept study established that a FITC labeled peptide that was associated with esophageal neoplasia could be imaged in vivo in human subjects.51 The ASYNYDA peptide was labeled with FITC and tested in vitro, ex vivo, and in vivo. After binding, this peptide could be detected with confocal laser endomicroscopy. In a study involving 25 patients with sites varying from squamous, Barrett’s esophagus, high grade dysplasia, and esophageal cancer, the receiver operating curve demonstrated an area under the curve for the detection of neoplasia of 0.91.51 Such targeted labelling could potentially provide risk stratification beyond histology and in a real-time fashion, enabling intra-procedural decision making or prediction of response to therapy.
Optical coherence tomography (OCT) based technologies also can be utilized to provide in vivo microarchitectural detail. Commercially available systems can be used during an endoscopic procedure to image and to distinguish between Barrett’s esophagus and normal squamous epithelium.52 Moreover, criteria to determine dysplasia are being developed and refined.52 Furthermore, while an increase in microvascular density does correlate with increasing carcinogenesis,53 clinicians typically do not rely on vascular features to diagnose neoplasia in routine H&E fixed tissues. The modality of optical coherence tomography angiography (OCTA) readily enables a detailed view of the vascularity at the microarchitectural level. Features such as irregular branching and heterogeneous vessel size on OCTA may potentially correlate with dysplasia in Barrett’s esophagus.54 The OCT based technologies have broadened into developing a tethered imaging capsule platform that enables cross sectional imaging of the esophagus without the need for endoscopy.55 The optical marker of the lack of layered architecture and presence of glands in the epithelium may allow for a tethered imaging device (similar to the Cytosponge) to screen for patients with Barrett’s esophagus without the need for an endoscopy.
Exploiting the Fifth Sense
The utilization of the often overlooked fifth sense of smell may provide some new biomarkers to explore, and is altogether an innovative break from traditional biomarker development. A computer aided electronic nose can use a chemical interface to detect volatile organic compounds (VOC) exhaled from subjects. The VOC profile in the breath can be altered by disease, and the electronic nose uses a machine learning platform to develop algorithms for differentiating among disease states based on VOC breath patterns. In a pilot study among 122 patients, the electronic nose was able to differentiate benign from dysplastic Barrett’s-associated VOCs using breath samples acquired over five minutes with an accuracy of 81%.56 The potential of this screening method is enticing for the general population given the non-invasive approach using a simple breath test.
SUMMARY
Gastrointestinal society guidelines currently recommended against the routine use of biomarkers in management of Barrett’s esophagus, but we suspect that these recommendations will change in the near future. The success of endoscopic eradication therapy in preventing neoplastic progression of dysplastic Barrett’s esophagus, and in silico models demonstrating the cost-effectiveness of biomarker-based ablation therapy for non-dysplastic Barrett’s esophagus have increased the incentive to find a reliable “Barrett’s biomarker”. Modern “omics” technologies have rapidly increased the identification of putative biomarkers, which can be combined with clinical and environmental factors to generate a “personalized” risk prediction model for Barrett’s esophagus and esophageal adenocarcinoma. At the clinical front, new, non-invasive modes of tissue sampling are being developed and new technologies in in vivo molecular imaging show promise in detecting early disease. The Precision Medicine Initiative partners government regulatory networks with scientists to promote faster translation of biomarker discoveries into the clinic. Such a partnership is pivotal to achieve the goal of moving biomarkers for Barrett’s esophagus from bench to bedside.
Figure 4.
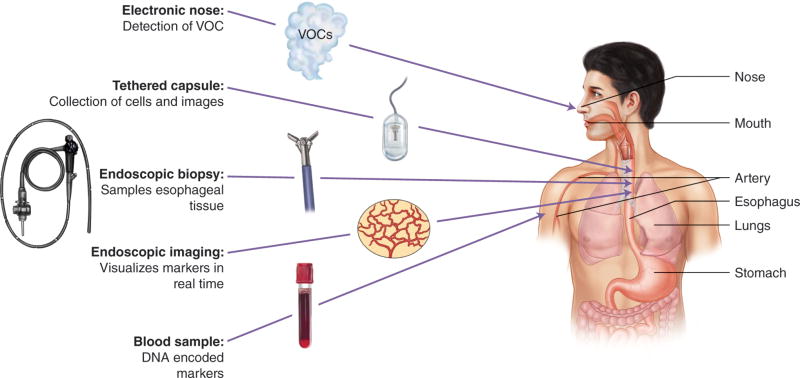
Sources of Biomarkers. The sources for interrogation of biomarkers in Barrett’s esophagus has extended from beyond the acquisition of tissue with endoscopic biopsy to include cell sampling without the need for endoscopy and imaging molecular markers without the need for tissue. Furthermore, molecular markers available in the whole body may be utilized and sources include blood samples which can provide information encoded in DNA and volatile organic compounds (VOCs) which can be interrogated via the novel electronic nose.
KEY POINTS.
The presence of dysplasia is the current gold standard biomarker for cancer risk in Barrett’s esophagus, despite its numerous limitations.
The success of endoscopic therapy in preventing neoplastic progression of dysplastic Barrett’s esophagus has stimulated interest in identification of a reliable “Barrett’s biomarker”.
Precision medicine and new biomarker identification techniques such as “omics” have (r)evolutionized our approach to biomarker development.
Immunostaining for p53 is recommended by the British Society of Gastroenterology as an adjunct to histological assessment of dysplasia in patients with Barrett’s esophagus, essentially meaning that aberrant p53 expression can be used as a biomarker for neoplasia.
Early proof-of-principal studies demonstrate the promise of optical biomarkers to enhance the sensitivity and specificity of neoplasia detection during in vivo imaging for patients with Barrett’s esophagus.
Non-endoscopic tools may enhance screening for the general population to determine who may benefit from an endoscopy.
The US Precision Medicine Initiative, which brings together government regulatory agencies and the scientific community with the common goal of hastening the movement of biomarker discoveries into clinical practice, may hasten identification of a biomarker that can risk-stratify patients with Barrett’s esophagus.
Acknowledgments
Vani J.A. Konda, M.D. has served as a consultant for C2 therapeutics and receives research support from Olympus. Rhonda F. Souza, M.D. has served as a consultant and receives research support from Ironwood Pharmaceuticals.
This work was supported by the National Institutes of Health (R01 DK103598, R01 DK063621, R21 DK111369 to R.F.S.)
We are grateful to Dr. Stuart Jon Spechler for his thoughtful discussions with us and review of this manuscript
References
- 1.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. The New England journal of medicine. 2009;360(22):2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 2.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;311(12):1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 3.Coco DP, Goldblum JR, Hornick JL, et al. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. The American journal of surgical pathology. 2011;35(1):45–54. doi: 10.1097/PAS.0b013e3181ffdd14. [DOI] [PubMed] [Google Scholar]
- 4.Naini BV, Souza RF, Odze RD. Barrett's Esophagus: A Comprehensive and Contemporary Review for Pathologists. The American journal of surgical pathology. 2016;40(5):e45–66. doi: 10.1097/PAS.0000000000000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Human pathology. 2001;32(4):368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 6.Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut. 2012;61(7):970–976. doi: 10.1136/gutjnl-2011-300730. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. The American journal of gastroenterology. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serag HB, Graham DY. Routine polypectomy for colorectal polyps and ablation for Barrett's esophagus are intellectually the same. Gastroenterology. 2011;140(2):386–388. doi: 10.1053/j.gastro.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Bulsiewicz WJ, Kim HP, Dellon ES, et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(6):636–642. doi: 10.1016/j.cgh.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hur C, Choi SE, Rubenstein JH, et al. The cost effectiveness of radiofrequency ablation for Barrett's esophagus. Gastroenterology. 2012;143(3):567–575. doi: 10.1053/j.gastro.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140(3):1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 13.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19(6):1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 14.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23(12):3155–3162. doi: 10.1093/annonc/mds181. [DOI] [PubMed] [Google Scholar]
- 15.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Annals of internal medicine. 2003;138(3):176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 16.Das A, Wells C, Kim HJ, Fleischer DE, Crowell MD, Sharma VK. An economic analysis of endoscopic ablative therapy for management of nondysplastic Barrett's esophagus. Endoscopy. 2009;41(5):400–408. doi: 10.1055/s-0029-1214612. [DOI] [PubMed] [Google Scholar]
- 17.Das A, Callenberg KM, Styn MA, Jackson SA. Endoscopic ablation is a cost-effective cancer preventative therapy in patients with Barrett's esophagus who have elevated genomic instability. Endoscopy international open. 2016;4(5):E549–559. doi: 10.1055/s-0042-103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nature reviews Cancer. 2016;16(8):525–537. doi: 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. American journal of cancer research. 2017;7(5):1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 22.Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA: a cancer journal for clinicians. 2005;55(6):334–351. doi: 10.3322/canjclin.55.6.334. [DOI] [PubMed] [Google Scholar]
- 23.Stachler MD, Taylor-Weiner A, Peng S, et al. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nature genetics. 2015;47(9):1047–1055. doi: 10.1038/ng.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid BJ, Paulson TG, Li X. Genetic Insights in Barrett's Esophagus and Esophageal Adenocarcinoma. Gastroenterology. 2015;149(5):1142–1152. e1143. doi: 10.1053/j.gastro.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nones K, Waddell N, Wayte N, et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nature communications. 2014;5:5224. doi: 10.1038/ncomms6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ek WE, Levine DM, D'Amato M, et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett's esophagus, and gastroesophageal reflux. Journal of the National Cancer Institute. 2013;105(22):1711–1718. doi: 10.1093/jnci/djt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Z, Gay LJ, Strange A, et al. Common variants at the MHC locus and at chromosome 16q24.1 predispose to Barrett's esophagus. Nature genetics. 2012;44(10):1131–1136. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gharahkhani P, Fitzgerald RC, Vaughan TL, et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett's oesophagus: a large-scale meta-analysis. The lancet oncology. 2016;17(10):1363–1373. doi: 10.1016/S1470-2045(16)30240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dura P, van Veen EM, Salomon J, et al. Barrett associated MHC and FOXF1 variants also increase esophageal carcinoma risk. International journal of cancer Journal international du cancer. 2013;133(7):1751–1755. doi: 10.1002/ijc.28160. [DOI] [PubMed] [Google Scholar]
- 30.Buas MF, He Q, Johnson LG, et al. Germline variation in inflammation-related pathways and risk of Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2017;66(10):1739–1747. doi: 10.1136/gutjnl-2016-311622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feagins LA, Zhang HY, Zhang X, et al. Mechanisms of oxidant production in esophageal squamous cell and Barrett's cell lines. American journal of physiology Gastrointestinal and liver physiology. 2008;294(2):G411–417. doi: 10.1152/ajpgi.00373.2007. [DOI] [PubMed] [Google Scholar]
- 32.Spechler SJ, Souza RF. Barrett's esophagus. The New England journal of medicine. 2014;371(9):836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 33.Huo X, Zhang X, Yu C, et al. Aspirin prevents NF-kappaB activation and CDX2 expression stimulated by acid and bile salts in oesophageal squamous cells of patients with Barrett's oesophagus. Gut. 2017 doi: 10.1136/gutjnl-2016-313584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palles C, Chegwidden L, Li X, et al. Polymorphisms near TBX5 and GDF7 are associated with increased risk for Barrett's esophagus. Gastroenterology. 2015;148(2):367–378. doi: 10.1053/j.gastro.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine DM, Ek WE, Zhang R, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett's esophagus. Nature genetics. 2013;45(12):1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai JY, de Dieu Tapsoba J, Buas MF, et al. A newly identified susceptibility locus near FOXP1 modifies the association of gastroesophageal reflux with Barrett's esophagus. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24(11):1739–1747. doi: 10.1158/1055-9965.EPI-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins FS, Varmus H. A new initiative on precision medicine. The New England journal of medicine. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvi MA, Liu X, O'Donovan M, et al. DNA methylation as an adjunct to histopathology to detect prevalent, inconspicuous dysplasia and early-stage neoplasia in Barrett's esophagus. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(4):878–888. doi: 10.1158/1078-0432.CCR-12-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eluri S, Brugge WR, Daglilar ES, et al. The Presence of Genetic Mutations at Key Loci Predicts Progression to Esophageal Adenocarcinoma in Barrett's Esophagus. The American journal of gastroenterology. 2015;110(6):828–834. doi: 10.1038/ajg.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Critchley-Thorne RJ, Davison JM, Prichard JW, et al. A Tissue Systems Pathology Test Detects Abnormalities Associated with Prevalent High-Grade Dysplasia and Esophageal Cancer in Barrett's Esophagus. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(2):240–248. doi: 10.1158/1055-9965.EPI-16-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curvers WL, Peters FP, Elzer B, et al. Quality of Barrett's surveillance in The Netherlands: a standardized review of endoscopy and pathology reports. European journal of gastroenterology & hepatology. 2008;20(7):601–607. doi: 10.1097/MEG.0b013e3282f8295d. [DOI] [PubMed] [Google Scholar]
- 42.Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7(7):736–742. doi: 10.1016/j.cgh.2008.12.027. quiz 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett's esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. The American journal of gastroenterology. 2007;102(6):1154–1161. doi: 10.1111/j.1572-0241.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 44.Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett's oesophagus. Gut. 2013;62(12):1676–1683. doi: 10.1136/gutjnl-2012-303594. [DOI] [PubMed] [Google Scholar]
- 45.Davelaar AL, Calpe S, Lau L, et al. Aberrant TP53 detected by combining immunohistochemistry and DNA-FISH improves Barrett's esophagus progression prediction: a prospective follow-up study. Genes, chromosomes & cancer. 2015;54(2):82–90. doi: 10.1002/gcc.22220. [DOI] [PubMed] [Google Scholar]
- 46.Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ (Clinical research ed) 2010;341:c4372. doi: 10.1136/bmj.c4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross-Innes CS, Debiram-Beecham I, O'Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi-center case-control study. PLoS medicine. 2015;12(1):e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross-Innes CS, Chettouh H, Achilleos A, et al. Risk stratification of Barrett's oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: a cohort study. The lancet Gastroenterology & hepatology. 2017;2(1):23–31. doi: 10.1016/S2468-1253(16)30118-2. [DOI] [PubMed] [Google Scholar]
- 49.Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011;43(10):882–891. doi: 10.1055/s-0030-1256632. [DOI] [PubMed] [Google Scholar]
- 50.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointestinal endoscopy. 2011;74(3):465–472. doi: 10.1016/j.gie.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturm MB, Joshi BP, Lu S, et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first-in-human results. Science translational medicine. 2013;5(184):184ra161. doi: 10.1126/scitranslmed.3004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sauk J, Coron E, Kava L, et al. Interobserver agreement for the detection of Barrett's esophagus with optical frequency domain imaging. Digestive diseases and sciences. 2013;58(8):2261–2265. doi: 10.1007/s10620-013-2625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konda VJ, Hart J, Lin S, et al. Evaluation of microvascular density in Barrett's associated neoplasia. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2013;26(1):125–130. doi: 10.1038/modpathol.2012.146. [DOI] [PubMed] [Google Scholar]
- 54.Lee HC, Ahsen OO, Liang K, et al. Endoscopic optical coherence tomography angiography microvascular features associated with dysplasia in Barrett's esophagus (with video) Gastrointestinal endoscopy. 2017;86(3):476–484. e473. doi: 10.1016/j.gie.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gora MJ, Simmons LH, Queneherve L, et al. Tethered capsule endomicroscopy: from bench to bedside at a primary care practice. Journal of biomedical optics. 2016;21(10):104001. doi: 10.1117/1.JBO.21.10.104001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan DK, Zakko L, Visrodia KH, et al. Breath Testing for Barrett's Esophagus Using Exhaled Volatile Organic Compound Profiling With an Electronic Nose Device. Gastroenterology. 2017;152(1):24–26. doi: 10.1053/j.gastro.2016.11.001. [DOI] [PubMed] [Google Scholar]